Abstract
Background:
Negative outcomes related to prematurity may lead to maternal distress. Mothers of premature/Low-Birth-Weight infants report increased posttraumatic stress (50%) and depressive symptoms (63%) compared to mothers of full-term infants. Low-income, minority mothers with greater posttraumatic stress and depression have an increased risk for premature/Low-Birth-Weight delivery compared to their white counterparts. Variations in the neuropeptide oxytocin is implicated in lactation, perinatal depression, and maternal behavior.
Purpose:
To examine the associations among posttraumatic stress, depressive symptoms, and oxytocin in a pilot sample of minority mothers with premature/ Low-Birth-Weight infants in the Neonatal-ICU (NICU).
Methods:
This study employed a descriptive, correlational pilot design of eight minority, low-income mothers with premature/Low-Birth-Weight infant. Participants answered questionnaires pertaining to posttraumatic stress, depression, lactation, and demographics and oxytocin was measured. This is a sub-study which added oxytocin values.
Results:
Four participants had elevated depressive symptoms and five supplied breastmilk. Women who provided breastmilk had lower depressive (t=3.03,p=.023) and posttraumatic stress (t=3.39,p=.015) symptoms compared to women not supplying breastmilk. Women with elevated posttraumatic stress had higher levels of depressive symptoms (r(8)=0.8,p=0.006) and lower levels of oxytocin (r(8)=0.77,p=0.026).
Implications for Practice:
These results are congruent with previous literature on providing breastmilk and maternal mental health. In addition, we found a possible relationship between postpartum posttraumatic stress and oxytocin in minority women with premature/Low-Birth-Weight infants. NICU Nurses should encourage lactation and assess mothers for posttraumatic stress and depressive symptoms.
Implications for Research:
Research is needed to identify the biological milieu associated with posttraumatic stress and depression in at-risk mothers.
Keywords: Oxytocin, postpartum posttraumatic stress, postpartum depression
In the United States in 2015, 9.57% of births were premature (before 37th completed weeks gestation) and 8.07% of infants were born low birth weight (less than 2,500 grams).1,2 In 2015, 13.41% of African American and 9.14% of Hispanic women delivered preterm infants compared to 8.88% of non-Hispanic white women.2 Premature and LBW infants are at higher risk of developmental delays including poor growth, behavioral problems, and cognitive delays.3 Further, the consequences of prematurity may continue into adulthood with increased rates of hospitalization and chronic disease.4
The negative outcomes related to prematurity may also lead to maternal emotional distress. Indeed, mothers of premature and LBW infants have higher incidence of postpartum posttraumatic stress and elevated postpartum depressive symptoms related to the birthing and postpartum experience compared to mothers of healthy full-term infants.1,5 Over 50% of mothers of premature and LBW infants reported elevated postpartum posttraumatic stress symptoms1 and up to 63% reported elevated depressive symptoms.5 Postpartum posttraumatic stress has been reported to occur simultaneously with postpartum elevated depressive symptoms in a majority of cases (85%).6 Though it is recommended that women be screened for perinatal depression, currently this screening is not a required. Unfortunately, elevated maternal postpartum posttraumatic stress and depressive symptoms place premature infants at a greater risk for altered growth, development, and challenges for mother-infant interactions than other premature infants.7
Posttraumatic Stress Disorder (PTSD) is a specific type of anxiety disorder affecting persons exposed to traumatic events.8 Elevated posttraumatic stress symptoms include 1) re-experiencing the event through thoughts, 2) avoidance of stimuli related to the event, and 3) increased arousal since the event. Posttraumatic stress symptoms after childbirth may be related to a traumatic childbirth experience or to a previous traumatic event in the mother’s life.9 Women with premature births are at a higher risk of developing postpartum posttraumatic stress symptoms due to childbirth than mothers of full-term infants. In fact, up to 20% of mothers with premature infants have been identified as having postpartum posttraumatic stress disorder.10 Mothers with postpartum posttraumatic stress have difficulty with mother-infant interactions.11 Postpartum posttraumatic stress in mothers of premature infants has been identified as a risk factor for infant sleeping problems up to 18 months when compared to premature infants whose mothers did not report elevated postpartum posttraumatic stress.12 Postpartum posttraumatic stress was also more likely to be elevated in mothers who experienced pre-eclampsia or a premature rupture of membranes up to 15 months postpartum compared to healthy postpartum mothers.13 The strongest predictor of postpartum posttraumatic stress disorder was the amount of physical symptoms women experience postpartum and the presence of perinatal depressive symptoms.14
Perinatal depression is common and debilitating, with consequences for mother and child. Almost one in five women report elevated perinatal depressive symptoms and as many as 12.7% of pregnant women experience at least one major depressive episode during pregnancy.15 Perinatal depression can lead to maladaptive mothering behaviors.16 Mothers with perinatal depression engage in fewer interactive behaviors with their infants and may have insecure attachment with their infants.16 This leads to delays in infant development and when most severe, failure to thrive.17 Perinatal depression is not only more common in low-income mothers,18 but the consequences for their children are magnified. For example, in lower socioeconomic communities, mothers with perinatal depression (compared to mothers without depression) are more likely to have their child hospitalized, use corporal punishment,18 and provide fewer preventive health measures for their children.19
Oxytocin is a neuropeptide hormone released in the brain and the periphery. Parvocellular neurons (PVN) release oxytocin within the brain while stimuli of PVN and supraoptic nucleus (SON) result in oxytocin release in the periphery via the posterior pituitary gland. In addition to the posterior pituitary, other peripheral organs may also release oxytocin (uterus, placenta, amnion, corpus luteum, testis, and heart). Oxytocin is released in response various stimuli including social interactions, vaginal stimulation, labor progression, and lactation.20 Increase in oxytocin release causes cells around the alveoli and milk ducts to contract during the letdown reflex of lactation.21 Animal model research on oxytocin has established a pulsatile or episodic oxytocin release during pregnancy and non-pregnant states in addition to a short half-life of 3–5 minutes.22,23 The combination of an episodic release and short half-life contribute to a wide range of reported pregnancy and postpartum oxytocin plasma values (10 pg/ml to 4000 pg/ml).24 Oxytocin is essential to the initiation of nurturing maternal behaviors and bonding between mother and child.25 Low levels of oxytocin are linked to less touch between mothers and infants;26 whereas high levels of oxytocin are associated with increased gaze, vocalization, positive affect, and touch.24 Altered levels of oxytocin may play a role in the development and/or exacerbation of depressive symptoms.27–29 In non-pregnant individuals, lower oxytocin levels are associated with major depression, as well as depressive symptoms.28 Perinatal depression and negative affect are associated with lower levels of oxytocin, while low oxytocin levels in mid-pregnancy predicted depressive symptoms at 2 weeks postpartum.29 In low-income African American women, women with low levels of oxytocin during pregnancy reported more depressive symptoms.27 Current research on oxytocin suggests oxytocin may be a biologic marker to identify individuals at risk for mental health disorders such as perinatal depression.27 As mentioned above, maternal mental health has extensive maternal and infant sequalae. NICU nurses have limited resources for mothers at risk or for those experiencing a mental health disorders. One of the advantages of identifying a biomarker (such as oxytocin) for maternal mental health would be a more efficient allocation of resources for mothers at risk. For example, if all mothers had their blood drawn after delivery for a biomarker (such as oxytocin), then those mothers at risk would receive extra services. Healthy mothers, without mental health disorders, provide infants with the needed stimulation, affection, and care to promote health, growth and development.7
To our knowledge oxytocin has not been previously studied with traumatic childbirth and postpartum posttraumatic stress though a relationship between the two has been hypothesized in the literature.30 Given the extensive effects of postpartum posttraumatic stress and perinatal depression on maternal-child health, identifying the biological pathways that bridge perinatal experience to future postpartum psychopathology is critical.31 We propose that low oxytocin may be involved in the constellation of biological events placing women at risk for postpartum posttraumatic stress and elevated depressive symptoms. The purpose of this pilot study was to examine the associations among postpartum posttraumatic stress, postnatal depressive symptoms, and oxytocin levels in a small sample of low income, minority mothers with a premature, low birthweight infant in the Neonatal Intensive Care Unit (NICU).
Methods
Design
This study employed a descriptive, correlational pilot design of minority, low-income mothers with premature, low birth-weight infant cared for in the NICU. This study was approved by the Institutional Review Boards of recruiting hospitals and academic institutions. Data for this analysis were collected through mother report, infant medical record review, and blood samples. Eight low-income minority mothers of premature infants in the NICU were included. This is a sub-study of a larger study32 in which participants in the sub-study provided blood samples for oxytocin plasma analysis.
Setting
Participants were recruited from two level IIIB Neonatal Intensive Care Units (NICU) in teaching trauma centers in the Midwest. These hospitals were community-based inner-city medical centers serving underserved and uninsured populations. Being served by these hospitals were used as a proxy for low maternal income. One hospital was a county trauma center serving only the uninsured. This hospital has a 52-bed NICU with 60% African American and 30% Hispanic patients. The second hospital has a 24-bed NICU and served primarily a low income Hispanic community.
Sample
The convenience sample included 8 mothers of low birth-weight, preterm infants who were concurrently enrolled in a larger longitudinal study.32 Due to the pilot nature of the study, we included English-speaking mothers without a current mental health diagnosis, whose infants were clinically stable and did not have a congenital neurological problems or symptoms of substance abuse. We excluded mothers younger than 18, those who had ongoing critical illness (e.g., Human Immunodeficiency Virus, seizure disorders, etc.), or a current diagnosis of major depression, psychosis, or bipolar disease. We also excluded mothers of infants currently receiving mechanical ventilation, as the burden on these mothers was deemed high.
Procedures.
The Institutional Review Boards at each clinical site and the university approved this study. Healthcare staff in the NICU notified the research team when a new mother and her infant met inclusion criteria including clinical stability of her infant. A member of the research team approached eligible new mothers at their infants’ bedside to explain the research study and obtain informed consent. The participants completed the questionnaire in a private room separate from the NICU and had a heparin lock IV placed in their forearms by a registered nurse. Four serial blood samples were taken every 20 minutes for one hour through the heparin lock IV. Multiple samples were taken to determine if oxytocin values were stable in non-breastfeeding postpartum mothers exposed to stimuli such as intravenous placement or visual stimuli of infant at the bedside. Sample 1 was taken immediately after intravenous placement. Samples 1 and 2 were obtained in a separate room away from the mother’s infant. No stimuli were present that might affect oxytocin levels (such as pictures of loved ones, baby toys/dolls, or pregnancy/baby related magazines). Samples 3 and 4 were obtained at the infant’s bedside. All blood was drawn into an ethylenediaminetetracetic acid (EDTA) sterile tube (10 ml) and placed on ice. The samples were transported on ice, centrifuged (1500 × g, 15 minutes), and aliquoted within 2 hours. Once plasma was aliquoted, the samples were stored at −80°C for batch analysis. All assays were conducted according to manufacturer specifications without extraction. Women received $10 reimbursement for their time involved with completion of the study. Finally, a member of the research team reviewed the infant’s medical records for birth data.
Measures
The socio-demographic questionnaire included data about maternal characteristics of age, race, and education from self-report. In addition, maternal birth records were reviewed for infant birth weight, severity of infant illness, and if mothers provided breastmilk during the infants NICU admission. Severity of the infant’s illness was calculated using the Neurobiologic Risk Score (NBRS).27 The NBRS consists of 7-items which assess infant brain injury and NBRS scores relate to future development at 6, 15, and 24 months. Injury severity is ranked on a 4-point geometric grade (0, 1, 2, 4). The NBRS scores correlate with neurologic examinations and the Bayley II mental and psychomotor developmental indices.33 Scores ≤ 4 are considered low risk, 5 – 7 are considered intermediate risk, and ≥ 8 are considered high risk.34 The Cronbach’s alpha was 0.71.
Postpartum posttraumatic stress symptoms were assessed using the Perinatal Post-Traumatic Stress Disorder Questionnaire (PPQ). The PPQ is a 14-item yes/no questionnaire with statements concerning the mother having upsetting memories of giving birth, avoiding thinking about the baby’s hospital stay, having inability to remember parts of the hospital stay, and reporting difficulty feeling loved. A score of 6 or greater represents significant postpartum posttraumatic stress symptoms and at risk for postpartum posttraumatic stress disorder. The PPQ has been used with black and Hispanic minority samples.35 Cronbach’s alpha for the PPQ was 0.79.
Postpartum depressive symptoms were measured using the Center for Epidemiologic Studies - Depression Scale (CES-D). The CES-D consists of 20 items measuring depressive symptoms (e.g., bothered by things more than usual, felt lonely) rated on a 4-point Likert scale (0 = rarely or none of the time and 3 = most or all of the time) with scores ranging from 0 – 60. A score greater than 16 indicates clinically elevated depressive symptoms. The CES-D was not developed to assess postpartum women, yet in the general population it is widely used to screen for Major Depressive Disorder.18 The CES-D has been used with Hispanic and black samples.36,37 In postpartum women, and specifically mothers of premature infants, the CES-D had internal consistency of 0.82 – 0.91, a sensitivity of 60%, and a specificity of 92%.5,38 The Cronbach’s alpha was 0.90.
State anxiety was measured using the state subscale of the State-Trait Anxiety Inventory (STAI). The STAI has been validated in many languages and used with diverse populations including black and Hispanic samples.39 The state subscale consisted of 20 items rated on a 4-point Likert scale (1 = not at all and 4 = very much so) and included topics such as the degree to which the mother currently felt happy, calm, comfortable, jittery, upset, and confused. A higher score represents higher levels of anxiety. Scores greater than 40 have been suggested to indicate clinically significant anxiety.40 The Cronbach’s alpha was 0.93.
Blood samples were analyzed for oxytocin. Plasma oxytocin was analyzed in duplicate using enzyme-linked immunoassay (ELISA) and methods were according to the Enzo Life Sciences Oxytocin ELISA kit Product Manual.41 Oxytocin detection levels were 15.6 pg/ml to 1000 pg/ml. The intra-assay coefficient of variability was 9.7% to 30% and an inter-assay coefficient of variability was 4.6% to 8.6%.
Data Analysis.
First, descriptive statistics were computed to describe the sample. Analysis of Variance (ANOVA) was used to determine if there was a difference in oxytocin values across the four times points. Pearson r correlation coefficients were used to examine the unadjusted relationships among variables. Oxytocin is expected to have a wide range of normal values and will be divided into categories (high and low values) or a cutoff (usually 1000pg/ml) will be utilized to account for skewed data. Due to the pilot nature of the study with 8 participants, the results will be mainly descriptive with a significance reported for if p < 0.1.42
Results
Sample Characteristics.
The convenience sample for this pilot consisted of eight women concurrently enrolled in a larger longitudinal study. Participants were enrolled during the first three months postpartum, while her infant was cared for in the NICU. Variations on enrollment time greatly depended on the clinical stability of the participants infant. More specifically, five (62.5%) participants were enrolled in their first postpartum month, two (25%) participants were enrolled by month two, and one (12.5%) participant was enrolled by month three. Five of the eight mothers provided breastmilk during their infant’s NICU admission. Mothers that provided breastmilk to their infants were enrolled in the study between day 10 – 57 postpartum (M = 22.6 days, SD = 19.4), while mothers that did not provide breastmilk to her infant was enrolled between day 27 – 74 postpartum (M = 49, SD = 23.6). Infants birth weight mean was 1168.4 grams (range 760 to 1780 grams, SD 313.9). Women had a mean age of 22.5 years (range 18 to 37 years of age) and a mean gestational age at birth of 29 weeks. One infant was severely at risk for future developmental delays, one infant at moderate risk, and 6 at low risk according to the NBRS. Three women lived with the father of the baby, four women were multi-gravida, five women had some college and six women had an annual income of less than $10,000. Women reported moderate levels of anxiety (M = 42, SD = 16, range 23 – 63). Four women had elevated depressive symptoms (score ≥ 16 on CESD), and two of these women had elevated postpartum posttraumatic stress scores (score ≥ 6 on PPQ) as well (see Table 1).
Table 1.
Sample Characteristics
| Mean (SD) | |
|---|---|
| Maternal age (years) | 22.5 (6.2) |
| Infant gestational birth age (weeks) | 29 (2.6) |
| Provided Breastmilk | 62.5%(n=5) |
| Days postpartum (10–74 days) | 32.7 (22) |
|
Race/Ethnicity
% Black % Hispanic |
50% (n = 4) 50% (n = 4) |
| % Live with father of the baby | 37.5%(n = 3) |
| Infant Illness Severity (NBRS) | 3.25 (4.2) |
| State Anxiety (STAI) | 42 (16) |
|
Posttraumatic Stress (PPQ)
% Score greater than 6 |
4.3 (3.2) 25% (n = 2) |
|
Depressive Symptoms (CESD) % Score greater than 16 |
18.8 (14.1) 50% (n = 4) |
NBRS = Neonatal Neurobiologic Risk Score. STAI = State Trait Anxiety Inventory. PPQ = Postpartum Posttraumatic stress Questionnaire. CESD = Center for Epidemiology Studies – Depressive Scale
The median oxytocin value was 309 (range 103–4000 pg/ml), with one blood draw was not possible as the participant was holding her infant during a feeding and did not feel comfortable moving her arm to allow access to the heparin lock (see table 2). Oxytocin values had a Weibull Distribution with values severely shifted to the right and a skewness of 1.5 which is considered highly skewed (highly skewed: N ≈ 10, skewness > 0.96).43 This suggests the normal wide range of oxytocin, in which there is a great variation between normal and high levels due to the phenomenon of oxytocin firing pulses presenting as spikes in plasma levels.44 For example, participant #7 had an oxytocin spike at time 3. She had the following oxytocin levels; time 1 = 181pg/ml, time 2 = 236pg/ml, time 3 = 2100pg/ml, and time 4 = 190pg/ml. An oxytocin spike is a level of oxytocin 10 times greater than the average of a participant’s other oxytocin values. At time 3, participant #7 had a value of 2100pg/ml while the mean of time 1, 2, and 4 = 202.3pg/ml. Therefore because 2100 is greater than 10 × 202.3, it is assumed that this was an oxytocin spike. When accessing oxytocin with only one sample, it is difficult to determine if a high value is an oxytocin spike or if that individual excretes large amounts of oxytocin. No significant differences in oxytocin levels were found between the four time points per the ANOVA and all time 1 values were not oxytocin spikes. Therefore, the remaining analyses were conducted only with data from the first collection point with oxytocin data adjusted as described in the data analysis section. In addition, due to the high skewness of the data, oxytocin values that were greater than 1,000pg/ml (2448pg/ml and 3080pg/ml) were relabeled at 1,000pg/ml. This changed the median of oxytocin to 292 pg/ml (range 103 – 1000 pg/ml).
Table 2.
Raw Oxytocin Values
| Subject number | Oxytocin T1 pg/ml |
Oxytocin T2 pg/ml |
Oxytocin T3 pg/ml |
Oxytocin T4 pg/ml |
|---|---|---|---|---|
| 1 | 189 | 276 | 335 | 240 |
| 2 | 3080 | 2002 | 1950 | 1698 |
| 3 | 198 | 216 | 157.2 | 130 |
| 4 | 2448 | 4000 | 2697 | 1798 |
| 5 | 343 | 380 | 382 | 326 |
| 6 | 292 | 426 | 234 | 278 |
| 7 | 181 | 236 | *2100 | 190 |
| 8 | 103 | 122 | † | 152 |
| Mean | 854 | 964 | 1122 | 601 |
| Std Dev | 1192 | 1391 | 1080 | 711 |
| Median | 245 | 328 | 382 | 259 |
T1 and T2 blood samples were drawn in a private room away from the participant’s infant. T3 and T4 blood was drawn at the infant’s bedside.
Oxytocin spike (a level of oxytocin 10 times greater than a participants other values).
Participant was holding her infant and blood draw was not possible at this time.
Posttraumatic Stress, Depressive Symptoms, Anxiety, Breastmilk, and Oxytocin
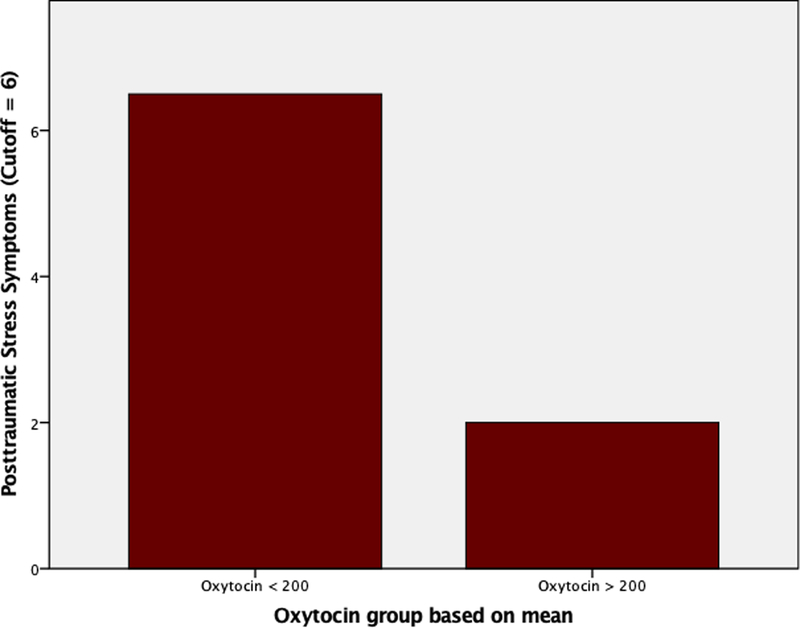
Associations or inferences suggested below are speculative due to the small sample size and large sample and variable variations. All findings presented need further studies to confirm. Women with elevated postpartum posttraumatic stress symptoms had high levels of depressive symptoms (r(8) = 0.80, p = 0.006), low levels of oxytocin (r(8) = −0.77, p = 0.026), and high levels of anxiety (r(8) = 0.63, p = 0.093). Women with PPQ scores < 6, which represents “not at risk” for postpartum posttraumatic stress disorder, had median oxytocin levels of 317 (range 188 to 1000 pg/ml), whereas women with PPQ ≥ 6, which represents “at risk” for postpartum posttraumatic stress disorder, had oxytocin median levels of 142 (range 103 to 181 pg/ml) (see figure 1). Depressive symptoms were directly correlated with postpartum posttraumatic stress (r(8) = 0.80, p = 0.006) and levels of anxiety (r(8) = 0.65, p = 0.081). Depressive symptoms were not correlated with oxytocin (r(8) = −0.226, p = 0.590). Providing breastmilk was related to decreased PPQ scores (t=3.39,p=.015) and decreased depressive symptoms (t=3.03,p=.023) compared to women who did not provide breastmilk. However, there was no difference in oxytocin values between women who provided breastmilk and those that did not. Anxiety level was the only psychological measure correlated with infant birth-weight (r(8) = 0.64, p = 0.088) and severity of infant illness (r(8) = 0.66, p = 0.077).
Figure 1:
Posttraumatic Stress Symptoms in Women with Low (<200 pg/ml) or High (>200 pg/ml) Oxytocin Level.
Discussion
In our pilot sample of urban, minority, low-income mothers with premature infants in the NICU, two participants with higher levels of postpartum posttraumatic stress had lower levels of plasma oxytocin. Though this is a novel finding, due to the pilot nature of our study and the vast range of potential oxytocin values, this finding is speculative and further investigation is needed. Recent literature has suggested low oxytocin may be a relevant biomarker to determine an individual’s acute risk of mental health disorders. Due to the unique acute postpartum period, oxytocin has been studied in relation to mother/child interactions,45 maternal depressive symptoms,27,46 and effects of maternal oxytocin on children’s oxytocin and social reciprocity.47 Limited data is available on the relationship between plasma oxytocin values and posttraumatic stress. Seng et al. (2013) found pregnant women with PTSD had greater dissociative symptoms and greater oxytocin levels.48 Similarly, men and non-pregnant women who experienced early childhood maltreatment had greater peripheral oxytocin levels in adulthood.49 Though these findings appear contradictory to our findings, posttraumatic stress in the previous studies was not examined in relation to childbirth and results included both men and women. However, congruent with our findings, both studies suggest a dysregulated oxytocin system in relation to posttraumatic stress. Researchers have suggested oxytocin as a potential treatment for posttraumatic stress.50 Olff et al (2010) made this suggestion based on the knowledge that oxytocin can reduce fear and increase social interactions. Koch et al (2016), recently conducted a study on the effect of intranasal oxytocin on amygdala function in particpants with posttraumatic stress finding oxytocin decreased anxiety and nervousness.51 Oxytocin thus far shows a possibility as a treatment modality for those with posttraumatic stress, though continued research is needed specifically with postpartum women.
We did not find a relationship between oxytocin values and postpartum depression or oxytocin and providing breastmilk. This lack of a relationship is attributed to the pilot nature of this study. Oxytocin is known to directly effect breastmilk production and letdown.52 In addition, our previous published data,53 other researchers,29 and our hypothesis all suggested oxytocin values and postpartum depression would be correlated. Additionally, these result may be due to the small sample size, wide range of oxytocin, varying days postpartum, and the selected population of NICU mothers of low birthweight infants. The relationships among oxytocin, providing breastmilk, and postpartum depression should continue to be studied in mothers with a low birthweight infant.
Oxytocin in our sample included a large range of values (103pg/ml – 4000pg/ml). Large ranges of oxytocin are consistent with other published data. Feldman et al (2007) reported a wide range of oxytocin values from 11pmol – 3,648pmol in pregnancy and postpartum women.24 Similarly, Feldman et al (2007) had a repeated measures design (measuring oxytocin levels in the first trimester, third trimester, and postpartum) also noting that the mean at all three time points was not significantly different.24 Skrundz et al (2011) reported a range of pregnacy oxytocin values from 14 pg/ml to 246 pg/ml. The procedures for sampling and preparation of the plasma sample are varied from this study (heparin tube instead of ethylenediaminetetracetic acid (EDTA).29 Though the oxytocin range is different, oxytocin values still vary by 10 times. Gouin et al (2009) measures oxytocin values in men and women not during pregnancy or postpartum and reports a range of oxytocin values from 100 pg/ml to 1200 pg/ml. We conclude that our wide range of oxytocin is comparable to other published data with reasonable variations.
Prevalence rates of mothers providing breastmilk, postpartum posttraumatic stress symptoms, and postpartum depressive symptoms in our sample are similar to previous studies of low-birthweight infants in the NICU, though our sample is smaller. Five out of eight mothers (63%) in our sample of minority low-income women provided breastmilk. The Center For Disease Control reports 64% of black mothers with full term infants initiate breastfeeding, which is similar to our findings. Fifty percent (n = 4) of women in our study experienced elevated postpartum depressive symptoms and two of these women also experienced elevated postpartum posttraumatic stress symptoms. Postpartum posttraumatic stress has been reported to occur simultaneously with postpartum elevated depressive symptoms in a majority of cases (85%).6 Postpartum posttraumatic stress symptoms in previous studies have been identified in 18% of women (n = 1373) with term gestational deliveries14 and up to 53% of mothers (n = 30) with premature infants.1 Specifically, most mothers of high risk premature infants (such as low income mothers with low-birth-weight infants) continue to experience at least one symptom of postpartum posttraumatic stress months after birth.1 Maternal postpartum posttraumatic stress increases infant reactivity and decreases an infant’s ability to self-regulate.54 Gavin et al. (2005), conducting a meta-analysis of 28 studies, found that 19% of women experienced postpartum depressive symptoms regardless of gestational age.15 However, other researchers have found that 39% of mothers (n = 67) with NICU infants55 and 48% of low-income mothers (n = 774)18 had elevated postpartum depressive symptoms. Mothers with postpartum depressive symptoms exhibit more unresponsive and inattentive parenting as well as more intrusive and punitive parenting compared to mothers without depressive symptoms, resulting in children with greater risk for emotional or behavioral problems.56 Our results suggest the possibility of urban, minority, low-income postpartum women with premature infants in NICU may have particularly high rates of postpartum posttraumatic stress and depressive symptoms, which may impact parenting and infant outcomes.
Limitations
This study had several limitations. This was a pilot study and the sample size was small. Therefore, the results cannot be generalized to other samples or settings. Future research needs to include larger sample sizes to examine the relationship between postpartum posttraumatic stress and oxytocin regulation. Data were collected one day during the postpartum period, which varied dramatically between participants. This variation was primarily due to infants clinical stability. In the best interest of the mother, we did not approach mothers for enrollment until her infant was in stable condition. Therefore, we cannot determine whether the oxytocin system is dysregulated or blunted in postpartum posttraumatic stress. Longitudinal data with multiple measures across the pregnancy and postpartum period would provide a better understanding of the relationship between postpartum posttraumatic stress and oxytocin regulation. Multiple data points would provide a better oxytocin norm accounting for individual differences. With only one day of oxytocin measures, it is difficult to rule out if a participant experienced an increase in stress or social interactions affecting oxytocin on that one day. Finally, we are unable to control for confounding variables such as infant illness, varying gestational age at delivery, and varying postpartum time of data collection. An ideal study would include a larger sample, longitudinal oxytocin data, a more homogenous postpartum infant age and prognosis, as well as questionnaires pertaining to lifetime trauma to differentiate between birthing trauma.
Implications and Recommendations
The results of this pilot study suggest that postpartum posttraumatic stress and depressive symptoms may be prevalent in mothers with infants in the NICU. In our pilot study, postpartum posttraumatic stress and depressive symptoms were not related the severity of the infant’s illness. This may suggest that mothers with low birth weight infants in the NICU view their infants as ill, though healthcare providers may categorize these infants with different degrees of illness. NICU nurses should encourage all mothers to provide breastmilk for their infants to improve infant health and maternal mental health. NICU nurses can support mothers lactation by making a private room with the necessary equipment available for mothers to provide breastmilk. Nurses who care for infants in NICU should be aware that mothers of premature infants might experience postpartum posttraumatic stress and depressive symptoms. Women may report upsetting memories of giving birth, being unable to remember parts of the postpartum hospitalization, or being bothered by things more than usual or feeling lonely. They may avoid talking about infant’s hospitalization. These behaviors may be warning signs of postpartum posttraumatic stress and/ or depressive symptoms. One qualitative study noted postpartum mothers stating they needed validation and support for birth trauma they have experience.57 NICU nurses should validate postpartum women’s feelings and encourage women to talk with their health care providers about their feelings. Researchers have suggested all mothers should be encouraged by healthcare providers to discuss their birth experience in order to clarify events and identify women at increased risk for postpartum posttraumatic stress.58,59
In addition nurses can screen new mothers at risk for postpartum posttraumatic stress by using verified screening scales.60 These screening instruments are easy to administer, cost effective, and can identify women with postpartum posttraumatic stress early so they can receive proper treatment. However, we acknowledge that screening mothers for postpartum posttraumatic stress and/ or depressive symptoms may be difficult for NICU providers given limited resources, the time required, and current hospital policies.
Women that are identified as having postpartum posttraumatic stress or depression may also benefit from telehealth services and other nursing interventions. In a meta-analysis of 13 telehealth posttraumatic stress treatment studies, participants reported a reduction of posttraumatic stress symptoms for those participating in the telehealth intervention versus control particpants on a waitlist.61 NICU nurses also have a major role in teaching mothers with infants in the NICU interventions to reduce parental stress such as kangaroo care,62 infant massage,63 support and education,64 and home nurse visits.65
We also found that postpartum posttraumatic stress symptoms were related to dysregulated oxytocin release. It is possible in the future that maternal oxytocin levels may be drawn postpartum to assess the maternal risk of mental health disorders. If women at risk could be identified by an objective blood test, resources could target these at risk women. This may result in a decreased incidence of postpartum mental health with the goal of improving maternal health and infant health, growth, and development. However, at this time there is no recommendation for measuring oxytocin for women with infants in NICU. In addition, the methodology for measuring oxytocin in research is under debate.66 The current gold standard for oxytocin measurement is an invasive blood sampling, though currently validation studies for salivary oxytocin in animal models is underway.67 Future larger studies need to confirm this pilot study findings before recommendations for an invasive universal oxytocin screening for women with infants in NICU are implemented. Further research on the identification of biomarkers to detect women at risk for postpartum posttraumatic stress symptoms may aid in identification and treatment of these women.
Conclusion
Mother’s mental health status and parenting are crucial to infant’s health, growth, and development. In summary, our pilot study identified 50% (n = 4) of our low-income urban mothers of low-birth-weight infants at risk for depressive symptoms and half (n = 2) of these mothers were also at risk for postpartum posttraumatic stress. Women who provided breastmilk while their infant was in the NICU reported less depressive and posttraumatic stress symptoms, however providing breastmilk was not related to oxytocin levels. Women with higher levels of postpartum posttraumatic stress symptoms also had more depressive symptoms and lower levels of oxytocin. Postpartum posttraumatic stress and depressive symptoms were not related to infant severity when comparing women who all had a low-birth-weight infant in the NICU. All mothers with a low-birth-weight infant are at risk for postpartum posttraumatic stress and depressive symptoms and need screening, interventions, and mental health education. Postpartum posttraumatic stress and depression should be a major concern for healthcare providers taking care of infants in the NICU as a mother’s ability to care for her infant is affected by her mental health status. As research progresses in this area, biological factors such as oxytocin may become available to aid in identification of women at risk and diagnosis of maternal mental health disorders providing efficient use of preventive resources and early treatment to improve the health of infants in the NICU.
| Summary of Recommendations for Practice and Research | |
|---|---|
| What we know: | African American and Hispanic mothers have increased rates of premature birth compared to their white counterparts. Mothers of premature and LBW infants have increased rates of postpartum depression and posttraumatic stress. Oxytocin is a neuropeptide that has been implicated in perinatal depression, maternal behavior, lactation and regulation of stress responses. |
| What needs to be studied: | Biological markers that may identify women at risk for postpartum posttraumatic stress. Oxytocin in relation to postpartum posttraumatic stress. Postpartum posttraumatic stress in minority mothers of premature and LBW infants. |
| What can we do today: | Assess mothers of premature and LBW infants for symptoms of both postpartum depression and posttraumatic stress. Have referral protocols in place for maternal mental health care in the NICU. Provide resources for lactation and positive parenting to mothers, especially those with depression and/or posttraumatic stress. |
What This Study Adds:
Provides possibility of oxytocin as a biological marker of postpartum posttraumatic stress.
Importance of assessing maternal mental health and lactation in the NICU.
Addressing the increased incidence of posttraumatic stress in mothers of premature, low birth weight infants
Acknowledgments
Conflicts of Interest and Source of Funding: This study was supported by National Institutes of Health grants NR 09418 (to Dr. Holditch-Davis), NR 010176 (to Dr. Garfield). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The work for this manuscript occurred in Chicago, IL at the University of Illinois at Chicago, John H. Stroger Medical Center, and Mount Sinai Medical Center.
Contributor Information
Lindsey Garfield, Assistant Professor, Marcella Niehoff School of Nursing, Loyola University Chicago, Chicago, Illinois.
Diane Holditch-Davis, Marcus Hobbs Distinguished Professor of Nursing, Associate Dean for Research Affairs, Duke School of Nursing, Durham, North Carolina.
C. Sue Carter, Executive Director of the Kinsey Institute, Rudy Professor of Biology, Indiana University, Bloomington, IN.
Barbara L. McFarlin, Associate Professor, Department Head for Women, Children, and Family Health, Sciences, University of Illinois, Chicago, Illinois.
Julia S. Seng, Professor, Department of Systems, Populations, and Leadership, University of Michigan School of Nursing, Ann Arbor, Michigan.
Carmen Giurgescu, Associate Professor, The Ohio State University, Columbus, OH.
Rosemary White-Traut, Professor Emerita, University of Illinois at Chicago, Director of Nursing Research, Children’s Hospital of Wisconsin, Adjunct Professor of Pediatrics, Medical College of Wisconsin, Milwaukee, WI.
References
- 1.Holditch-Davis D, Bartlett TR, Blickman AL, Miles MS. Posttraumatic stress symptoms in mothers of premature infants. J Obstet Gynecol Neonatal Nurs. 2003;32(2):161–171. [DOI] [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: Final Data for 2015. Natl Vital Stat Rep. 2017;66(1):1. [PubMed] [Google Scholar]
- 3.Allen MC. Neurodevelopmental outcomes of preterm infants. Curr Opin Neurol. 2008;21(2):123–128. [DOI] [PubMed] [Google Scholar]
- 4.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–269. [DOI] [PubMed] [Google Scholar]
- 5.Miles MS, Holditch-Davis D, Schwartz TA, Scher M. Depressive symptoms in mothers of prematurely born infants. J Behav Pediatr. 2007;28(1):36–44. [DOI] [PubMed] [Google Scholar]
- 6.White T, Matthey S, Boyd K, Barnett B. Postnatal depression and post-traumatic stress after childbirth: Prevalence, course and co-occurrence. J Reprod Infant Psychol. 2006;24(2):107–120. [Google Scholar]
- 7.Miceli PJ, Goeke-Morey MC, Whitman TL, Kolberg KS, Miller-Loncar C, White RD. Brief report: birth status, medical complications, and social environment: individual differences in development of preterm, very low birth weight infants. J Pediatr Psychol. 2000;25(5):353–358. [DOI] [PubMed] [Google Scholar]
- 8.Seng JS. Acknowledging posttraumatic stress effects on health. A nursing intervention model. Clin Nurse Spec. 2003;17(1):34–41; [DOI] [PubMed] [Google Scholar]
- 9.Grekin R, O’Hara MW. Prevalence and risk factors of postpartum posttraumatic stress disorder: a meta-analysis. Clin Psychol Rev. 2014;34(5):389–401. [DOI] [PubMed] [Google Scholar]
- 10.Ahlund S, Clarke P, Hill J, Thalange NK. Post-traumatic stress symptoms in mothers of very low birth weight infants 2–3 years post-partum. Arch Womens Ment Health. 2009;12(4):261–264. [DOI] [PubMed] [Google Scholar]
- 11.Forcada-Guex M, Borghini A, Pierrehumbert B, Ansermet F, Muller-Nix C. Prematurity, maternal posttraumatic stress and consequences on the mother-infant relationship. Early Hum Dev. 2011;87(1):21–26. [DOI] [PubMed] [Google Scholar]
- 12.Pierrehumbert B, Nicole A, Muller-Nix C, Forcada-Guex M, Ansermet F. Parental post-traumatic reactions after premature birth: implications for sleeping and eating problems in the infant. Arch Dis Child Fetal Neonatal Ed. 2003;88(5):F400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stramrood CA, Wessel I, Doornbos B, et al. Posttraumatic stress disorder following preeclampsia and PPROM: a prospective study with 15 months follow-up. Reprod Scis. 2011;18(7):645–653. [DOI] [PubMed] [Google Scholar]
- 14.Beck CT, Gable RK, Sakala C, Declercq ER. Posttraumatic stress disorder in new mothers: results from a two-stage U.S. national survey. Birth. 2011;38(3):216–227. [DOI] [PubMed] [Google Scholar]
- 15.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: A systematic review of prevalence and incidence. Obstet Gynecol. 2005;106(5):1071–1083. [DOI] [PubMed] [Google Scholar]
- 16.Poehlmann J, Fiese BH. The interaction of maternal and infant vulnerabilities on developing attachment relationships. Dev Psychopathol. 2001;13:1–11. [DOI] [PubMed] [Google Scholar]
- 17.Lyons-Ruth K, Zoll D, Connell D, Grunebaum HU. The depressed mother and her one-year-old infant: environment, interaction, attachment, and infant development. New Dir Child Adolesc Dev. 1986;34:61–82. [DOI] [PubMed] [Google Scholar]
- 18.Chung EK, McCollum KF, Elo IT, Lee HJ, Culhane JF. Maternal depressive symptoms and infant health practices among low-income women. Pediatrics. 2004;113(6):523–529. [DOI] [PubMed] [Google Scholar]
- 19.Minkovitz CS, Strobino D, Scharfstein D, et al. Maternal Depressive Symptoms and Children’s Receipt of Health Care inteh First 3 Years of Life. Pediatrics. 2005;115(2):306–314. [DOI] [PubMed] [Google Scholar]
- 20.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81(2). [DOI] [PubMed] [Google Scholar]
- 21.Renfrew MJ, Lang S, Woolridge M. Oxytocin for promoting successful lactation. Cochrane Database Syst Rev. 2000(2): [DOI] [PubMed] [Google Scholar]
- 22.Mitchell MD, Kraemer DL, Brennecke SP, Webb R. Pulsatile release of oxytocin during the estrous cycle, pregnancy and parturition in sheep. Biol Reprod. 1982;27(5):1169–1173. [DOI] [PubMed] [Google Scholar]
- 23.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81(2):629–683. [DOI] [PubMed] [Google Scholar]
- 24.Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol Sci. 2007;18(11):965–970. [DOI] [PubMed] [Google Scholar]
- 25.Heinrichs M, Domes G. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Prog Brain Res. 2008;170:337–350. [DOI] [PubMed] [Google Scholar]
- 26.Feldman R, Zagoory-Sharon O, Weisman O, et al. Sensitive Parenting is Associated with Plasma Oxytocin and Polymorphisms in the OXTR and CD38 Genes. Biol Psychiatry 2012;72(3):175–181. [DOI] [PubMed] [Google Scholar]
- 27.Garfield L, Giurgescu C, Carter CS, et al. Depressive symptoms in the second trimester relate to low oxytocin levels in African-American women: a pilot study. Arch Womens Ment Health. 2015;18(1):123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scantamburlo G, Hansenne M, Fuchs S, et al. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology. 2007;32(4):407–410. [DOI] [PubMed] [Google Scholar]
- 29.Skrundz M, Bolten M, Nast I, Hellhammer DH, Meinlschmidt G. Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology. 2011;36(9):1886–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seng JS. Posttraumatic oxytocin dysregulation: is it a link among posttraumatic self disorders, posttraumatic stress disorder, and pelvic visceral dysregulation conditions in women? J Trauma Dissociation. 2010;11(4):387–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298(14):1685–1687. [DOI] [PubMed] [Google Scholar]
- 32.Holditch-Davis D, White-Traut RC, Levy JA, O’Shea TM, Geraldo V, David RJ. Maternally administered interventions for preterm infants in the NICU: effects on maternal psychological distress and mother-infant relationship. Infant Behav Dev. 2014;37(4):695–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brazy JE, Eckerman CO, Oehler JM, Goldstein RF, O’Rand AM. Nursery Neurobiologic Risk Score: important factor in predicting outcome in very low birth weight infants. J Pediatr. 1991;118(5):783–792. [DOI] [PubMed] [Google Scholar]
- 34.Brazy JE, Goldstein RF, Oehler JM, Gustafson KE, Thompson RJ Jr. Nursery neurobiologic risk score: levels of risk and relationships with nonmedical factors. J Dev Behav Pediatr. 1993;14(6):375–380. [PubMed] [Google Scholar]
- 35.Vanderbilt D, Bushley T, Young R, Frank DA. Acute Posttraumatic Stress Symptoms Among Urban Mothers With Newborns in the Neonatal Intensive Care Unit: A Preliminary Study. J Dev Behav Pediatr. 2009;30(1):50–56. [DOI] [PubMed] [Google Scholar]
- 36.McCabe BE, Vermeesch AL, Hall RF, Peragallo NP, Mitrani VB. Acculturation and the Center For Epidemiological Studies-Depression Scale for Hispanic women. Nurs Res. 2011;60(4):270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holditch-Davis D, Miles MS, Weaver MA, et al. Patterns of distress in African-American mothers of preterm infants. J Dev Behav Pediatr. 2009;30(3):193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boyd RC, Le HN, Somberg R. Review of screening instruments for postpartum depression. Arch Womens Ment Health. 2005;8(3):141–153. [DOI] [PubMed] [Google Scholar]
- 39.Novy DM, Nelson DV, Goodwin J, Rowzee RD. Psychometric comparability of the STate-Trait Anxieyt INventory for differenct ethnic suppopulations. Psychol Assess. 1993;5(3):343–349. [Google Scholar]
- 40.Knight RG, Waal-Manning HJ, Spears GF. Some norms and reliability data for the State--Trait Anxiety Inventory and the Zung Self-Rating Depression scale. Br J Clin Psychol. 1983;22 (Pt 4):245–249. [DOI] [PubMed] [Google Scholar]
- 41.Oxytocin ELISA Kit Product Manual. In: Enzo Life Sciences. [Google Scholar]
- 42.Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10(2):307–312. [DOI] [PubMed] [Google Scholar]
- 43.Doane DP, Seward LE. Measuring Skewness: A forgotten statistic? J Stat Educ. 2011;19(2):1–18. [Google Scholar]
- 44.Armstrong WE, Wang L, Li C, Teruyama R. Performance, properties and plasticity of identified oxytocin and vasopressin neurones in vitro. J Neuroendocrinol. 2010;22(5):330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biol psychiatry. 2010;68(4):377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kimmel M, Clive M, Gispen F, et al. Oxytocin receptor DNA methylation in postpartum depression. Psychoneuroendocrinology. 2016;69:150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feldman R, Gordon I, Influs M, Gutbir T, Ebstein RP. Parental oxytocin and early caregiving jointly shape children’s oxytocin response and social reciprocity. Neuropsychopharmacology. 2013;38(7):1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seng J, Miller J, Sperlich M, et al. Exploring dissociation and oxytocin as pathways between trauma exposure and trauma-related hyperemesis gravidarum: a test-of-concept pilot. J Trauma Dissociation. 2013;14(1):40–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olff M, Frijling JL, Kubzansky LD, et al. The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. 2013;38(9):1883–1894. [DOI] [PubMed] [Google Scholar]
- 50.Van der Kolk BA. The body keeps the score: memory and the evolving psychobiology of posttraumatic stress. Harv Rev Psychiatry. 1994;1(5):253–265. [DOI] [PubMed] [Google Scholar]
- 51.Koch SB, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Intranasal Oxytocin Normalizes Amygdala Functional Connectivity in Posttraumatic Stress Disorder. Neuropsychopharmacology. 2016;41(8):2041–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grewen KM, Davenport RE, Light KC. An investigation of plasma and salivary oxytocin responses in breast- and formula-feeding mothers of infants. Psychophysiology. 2010;47(4):625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garfield L, Giurgescu C, Carter CS, et al. Depressive symptoms in the second trimester relate to low oxytocin levels in African-American women: a pilot study. Arch Womens Ment Health. 2015;18(1):123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bosquet Enlow M, Kitts RL, Blood E, Bizarro A, Hofmeister M, Wright RJ. Maternal posttraumatic stress symptoms and infant emotional reactivity and emotion regulation. Infant behav Dev. 2011;34(4):487–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cho J, Holditch-Davis D, Miles MS. Effects of maternal depressive symptoms and infant gender on the interactions between mothers and their medically at-risk infants. J Obstet Gynecol Neonatal Nurs. 2008;37(1):58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gelfand DM, Teti DM. The effects of maternal depression on children. Clin Psychol Rev. 1990;10:329–353. [Google Scholar]
- 57.Coates R, Ayers S, de Visser R. Women’s experiences of postnatal distress: a qualitative study. BMC Pregnancy Childbirth. 2014;14:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anderson C, Logan D. Impact of traumatic birth experience on Latina adolescent mothers. Issues Ment Health Nurs. 2010;31(11):700–707. [DOI] [PubMed] [Google Scholar]
- 59.Stone HL. Post-traumatic stress disorder in postpartum patients: what nurses can do. Nurs Womens Health. 2009;13(4):284–291. [DOI] [PubMed] [Google Scholar]
- 60.Hynan MT, Mounts KO, Vanderbilt DL. Screening parents of high-risk infants for emotional distress: rationale and recommendations. J Perinatol. 2013;33(10):748–753. [DOI] [PubMed] [Google Scholar]
- 61.Sloan DM, Gallagher MW, Feinstein BA, Lee DJ, Pruneau GM. Efficacy of telehealth treatments for posttraumatic stress-related symptoms: a meta-analysis. Cogn Behav Ther. 2011;40(2):111–125. [DOI] [PubMed] [Google Scholar]
- 62.Cho ES, Kim SJ, Kwon MS, et al. The Effects of Kangaroo Care in the Neonatal Intensive Care Unit on the Physiological Functions of Preterm Infants, Maternal-Infant Attachment, and Maternal Stress. J Pediatr Nurs. 2016;31(4):430–438. [DOI] [PubMed] [Google Scholar]
- 63.Feijo L, Hernandez-Reif M, Field T, Burns W, Valley-Gray S, Simco E. Mothers’ depressed mood and anxiety levels are reduced after massaging their preterm infants. Infant Behav Dev. 2006;29(3):476–480. [DOI] [PubMed] [Google Scholar]
- 64.Turan T, Basbakkal Z, Ozbek S. Effect of nursing interventions on stressors of parents of premature infants in neonatal intensive care unit. J Clin Nurs. 2008;17(21):2856–2866. [DOI] [PubMed] [Google Scholar]
- 65.Kaaresen PI, Ronning JA, Ulvund SE, Dahl LB. A randomized, controlled trial of the effectiveness of an early-intervention program in reducing parenting stress after preterm birth. Pediatrics. 2006;118(1):e9–19. [DOI] [PubMed] [Google Scholar]
- 66.Horvat-Gordon M, Granger DA, Schwartz EB, Nelson VJ, Kivlighan KT. Oxytocin is not a valid biomarker when measured in saliva by immunoassay. Physiol Behav. 2005;84(3):445–448. [DOI] [PubMed] [Google Scholar]
- 67.MacLean EL, Gesquiere LR, Gee N, Levy K, Martin WL, Carter CS. Validation of salivary oxytocin and vasopressin as biomarkers in domestic dogs. J Neurosci Methods. 2018;293:67–76. [DOI] [PubMed] [Google Scholar]