Abstract
In patients with Barrett’s esophagus (BE), metaplastic columnar mucosa, containing epithelial cells with gastric and intestinal features, replaces esophageal squamous mucosa damaged by gastroesophageal reflux disease. This condition is estimated to affect 5.6% of adults in the United States, and is a major risk factor for esophageal adenocarcinoma. Despite the prevalence and importance of BE, its pathogenesis is incompletely understood and there are disagreements over the cells of origin. We review mechanisms of BE pathogenesis, including transdifferentiation and transcommitment, and discuss potential cells of origin including basal cells of the squamous epithelium, cells of esophageal submucosal glands and their ducts, cells of the proximal stomach, and specialized populations of cells at the esophagogastric junction (residual embryonic cells and transitional basal cells). We discuss the concept of metaplasia as a wound-healing response, and how cardiac mucosa might be the precursor of the intestinal metaplasia of BE. Finally, we discuss shortcomings in current diagnostic criteria for BE that have important clinical implications.
Keywords: gastroesophageal reflux disease, esophago-gastric junction, esophageal adenocarcinoma, metaplasia, progenitor cells
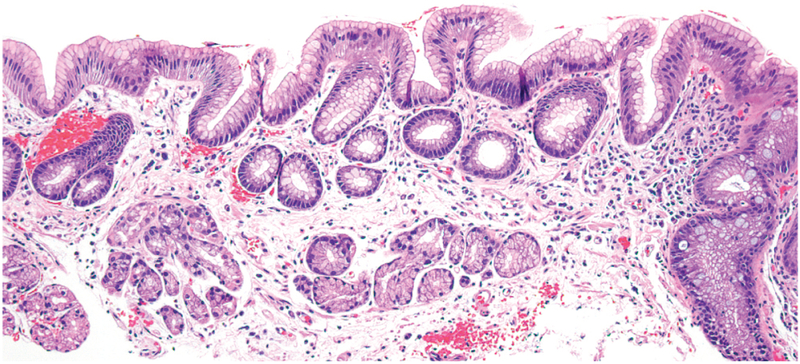
In patients with Barrett’s esophagus (BE), metaplastic columnar mucosa, containing epithelial cells with gastric and intestinal features, replaces esophageal squamous mucosa damaged by gastroesophageal reflux disease (Figure 1).1 United States guidelines state that a diagnosis of BE requires endoscopic identification of columnar mucosa extending at least 1 cm proximal to the esophago-gastric junction and histologic confirmation that the columnar mucosa is intestinal-type.2 There is no consensus on the precise definition of the term metaplasia, which was first used by Rudolf Virchow in 1884 to describe normal tissue in an abnormal location.5 Today, many types of metaplastic tissue, including Barrett’s esophagus (BE), are not considered normal, but pathologic because they are precursors to cancer. Cell biologists often have defined metaplasia as the conversion of 1 differentiated cell type into another, but JM Slack has argued that it is better to define metaplasia as the conversion of 1 tissue type into another, noting that metaplastic tissues comprise multiple disparate types of differentiated cells.6,7 Metaplasias usually develop in patients with chronic tissue injury, and it is widely accepted that the pathogenesis of BE begins with chronic esophageal injury caused by gastroesophaglea reflux disease (GERD). However, it is not clear how columnar mucosa replaces GERD-damaged squamous mucosa. We review potential mechanisms of BE pathogenesis, including transdifferentiation and transcommitment, and discuss potential cells of origin for Barrett’s metaplasia—hypotheses are not mutually exclusive and there could be more than 1 type of BE progenitor cell. We discuss the concept of metaplasia as an initial wound healing response and clinical implications.
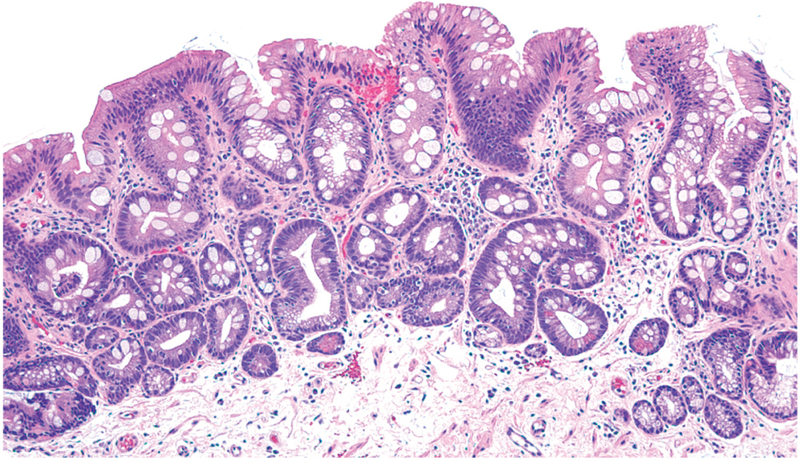
Figure 1.
Barrett’s intestinal metaplasia with mucin-secreting gastric foveolar-type cells and prominent intestinal-type goblet cells. (Photomicrograph provided by Robert Genta, H&E, 20X)
Transdifferentiation
A potential mechanism of BE pathogenesis involves transdifferentiation, in which fully differentiated esophageal squamous cells change into fully differentiated columnar cells—either directly (without undergoing a cell division) or indirectly (via cell division).8 Although differentiated cells once were considered immutable, studies have demonstrated that differentiated cells can be reprogrammed to acquire characteristics of immature progenitor cells.9 Many types of mature cells have the capacity to dedifferentiate into cells with progenitor cell characteristics.10 Transdifferentiation in the esophagus therefore might occur via a 2-stage process of GERD-induced reprogramming, in which mature squamous cells reverse their differentiation to acquire progenitor cell-like plasticity before changing to a columnar phenotype. Although it is conceivable that a squamous cell might change its phenotype without first dedifferentiating (by simultaneously downregulating its squamous genetic program while upregulating a columnar genetic program), it is not clear that such a process occurs naturally in adult tissues.11
In the fundus of mouse stomach injured by infection with Helicobacter pylori or by drugs toxic to parietal cells, the death of parietal cells appears to be accompanied by transdifferentiation of chief cells into proliferative cells that expresses trefoil factor 2 (TFF2, also known as spasmolytic polypeptide).12-15 In mice with acute injury, there is evidence that development of spasmolytic polypeptide-expressing metaplasia (SPEM) occurs when mature chief cells dedifferentiate and re-enter the cell cycle. This is a 3-stage process during which the cells: shut down mTORC1 signaling, which enables autophagy to recycle cellular material for use in the synthesis of new cell structures; begin to express genes associated with metaplasia, such as SOX9 and TFF2; and then reactivate mTORC1 signaling, which enables them to re-enter the cell cycle.16 Jason Mills et al coined the term paligenosis (from the Greek term, return to the regenerative state) for this process, which appears to be a conserved function of many cell types.16 Conceivably, paligenosis in esophageal squamous cells could result in their transdifferentiation into BE metaplastic cells, or into SPEM-like cells that could transition to BE metaplasia through further paligenotic events.17
Through paligenosis, mature cells dedifferentiate and re-enter the cell cycle to repair injured tissues either by regenerating more normal tissue, or by transdifferentiating into a metaplastic tissue that might be more resistant to whatever noxious factor is inducing the chronic injury (GERD in the esophagus). With repeated cycles of injury and repair in chronic inflammatory conditions, cells could undergo multiple rounds of paligenotic dedifferentiation and redifferentiation cycles, progressively acquiring mutations through replicative stress.15 Those mutations might be retained without consequence in quiescent, redifferentiated cells. When the mutated cells re-enter the cell cycle with a subsequent injury, however, they might acquire further mutations that eventually block redifferentiation, leading to clonal expansion and carcinogenesis. This has been called the cyclical hit model of tumorigenesis.15 If metaplastic tissues develop through transdifferentiation, they might undergo multiple cyclical hits, which increase their potential for transformation.
There are no data to directly support the model in which BE develops through transdifferentiation of mature squamous cells. Lineage-tracing studies in mice have not identified such transdifferentiation as a likely etiology for a columnar-lined esophagus.18 Even in mouse stomach, in which there is strong evidence that acute injury results in transdifferentiation of chief cells into SPEM, there is evidence for an alternative mechanism, in which SPEM develops and persists through the abnormal differentiation of stem cells in the isthmus of gastric glands.19 It also is difficult to reconcile the concept of BE development through transdifferentiation with the observation that Barrett’s metaplasia persists even when the GERD that might initiate transdifferentiation through paligenosis is controlled by medical or surgical treatment. Mucosa comprising exclusively uninjured, transdifferentiated cells that have exited the cell cycle could not maintain the multiple cell types present in Barrett’s metaplasia. Although it could be possible that paligenotic dedifferentiation confers stem cell-like self-renewal abilities in addition to the plasticity leading to transdifferentiation, there is no evidence from experiments for this concept. Persistence of BE in the absence of reflux esophagitis is more readily explained by metaplasia developing from reprogramming of an extant stem-like progenitor cell, which is the process of transcommitment.
Transcommitment
Transcommitment is the process in which immature progenitor cells that are able to proliferate and differentiate into different cell types are reprogrammed to alter their normal pattern of differentiation.20 Transcommitment shares late features of transdifferentiation through paligenosis, a process that starts with dedifferentiation of mature cells into progenitor-like cells before they re-differentiate abnormally. In contrast, transcommitment starts with immature progenitor cells that differentiate abnormally, presumably due to abnormal environmental factors like GERD. The development of Barrett’s metaplasia from reprogrammed (transcommitted) progenitor cells could readily account for the different cell types and their persistence even when GERD is controlled.
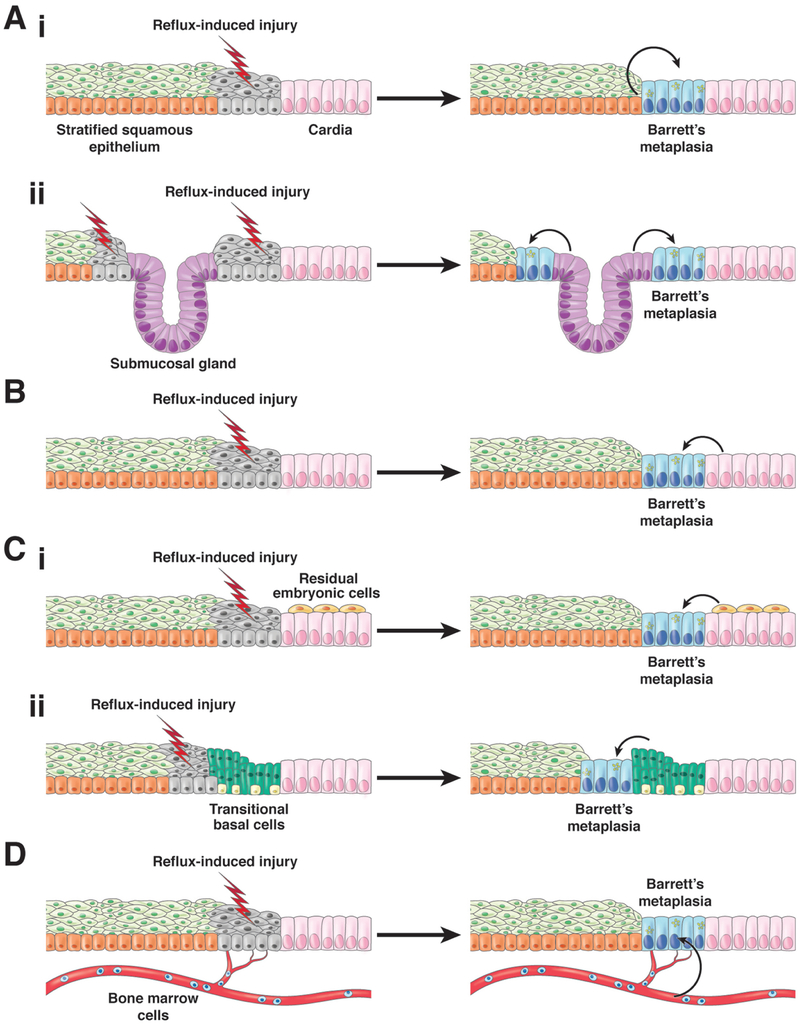
We do not know which progenitor cells give rise to Barrett’s metaplasia, but there are 4 categories of candidates (Figure 2). These include progenitor cells native to the esophagus, including basal cells of the squamous epithelium or cells of esophageal submucosal glands and their ducts; progenitor cells native to the proximal stomach (the gastric cardia) that migrate into the esophagus to repair reflux-damaged squamous epithelium; specialized populations of cells at the esophago-gastric junction (EGJ) that migrate into the esophagus to replace reflux-damaged squamous epithelium; and bone marrow progenitor cells transported through the blood to the esophagus to replace reflux-damaged squamous epithelium (see Table 1).
Figure 2.
Proposed cells of origin for BE. 1) Cells native to the esophagus including (1a) squamous epithelial cells that undergo reflux-induced transdifferentiation or transcommitment to produce the columnar cells of Barrett’s metaplasia and (1b) Progenitor cells in esophageal submucosal glands and/or their ducts. 2) Progenitor cells in the gastric cardia. 3) Specialized populations of cells at the esophago-gastric junction migrate into the reflux-damaged esophagus including (3a) residual embryonic cells (RECs) or (3b) transitional basal cells (TBCs). 4) Circulating bone marrow cells. For all of these proposed progenitor cells, reflux-induced injury to the esophageal squamous mucosa is assumed to initiate the metaplastic process, perhaps by stimulating progenitor cell migration into the damaged esophagus via a wound-healing process. In addition, reflux is assumed to induce the transcommitment of the progenitor cells to produce the multiple columnar cell types of Barrett’s metaplasia. (Figure modified from Jiang et al.17)
Table 1.
Proposed Progenitor Cells and Studies Supporting Their Participation in BE Pathogenesis
| Esophageal Squamous Epithelium | |||
|---|---|---|---|
| Model | Human or Animal | Study Findings | References |
| Biopsy tissue | Human | Scanning electron micrographs of biopsies at the squamous–BE junction revealed a distinct cell with squamous and columnar features. |
40Shields 1993 41Sawhney 1996 |
| Reflux esophagitis induced by esophago-jejunostomy | Rat | Esophago-jejunostomy resulted in esophageal ulceration with squamous cells at the proximal ulcer edges showing decreased SOX2 (squamous cell gene) and increased SOX9 (columnar cell gene). Distally, however, columnar-lined esophagus developed through proximal migration of jejunal cells in a wound-healing response. | 42Agoston 2018 |
| Cell culture | Human NES-B3T and NES-B10T cells (telomerase-immortalized esophageal squamous cell lines from patients with BE) | Exposure to acid, bile salts, or nitric oxide resulted in decreased expression of squamous cell factors (p63, SOX2), increased expression of columnar cell factors (SOX9, CDX2, FOXA2), and activation of upstream signaling pathways (Hedgehog, BMP4). Long-term exposure (more than 30 weeks) of NES-B10T to acid and bile salts caused columnar cell-like morphologic changes. |
43Asanuma 2016 44Minacapelli 2017 46Wang 2014 |
| Cell culture | Human HET-1A cells (SV40 transfected human esophageal cells) | Cells treated with BMP4 or transfected with constitutively-active BMPR1A showed upregulated SOX9 expression. | 45Wang 2010 |
| Cell Culture | Human Primary culture of esophageal cells from patients without Barrett’s or esophagitis | Exposure to acid and bile salts increased BMP4 expression. Treatment with BMP4 increased CDX2 protein expression. | 47Zhou 2009 |
| ESMGs | |||
| Esophagectomy and autopsy tissue | Human | Esophagectomy and autopsy specimens show ESMGs associated with both squamous islands and areas of Barrett’s metaplasia. ESMGs can express Krt7, P63 and SOX9, and ESMGs associated with Barrett’s metaplasia can demonstrate necrotizing sialometaplasia-like change/acinar ductal metaplasia. |
53Coad 2005 58Braxton 2014 59Garman 2015 60Gonzalez 2016 |
| Esophagectomy and biopsy tissue, genomic assessment used for clonality studies | Human | In some patients with BE,ESMGs and their ducts share p16/CDKN2A mutations with overlying Barrett’s metaplasia, and a patient has been described with shared mitochondrial DNA mutations in both squamous and Barrett’s epithelium suggesting that ESMGs harbor plastic progenitor cells that can give rise either to squamous or BE. |
54Paulson 2006 56Leedham 2008 55Nicholson 2012 |
| Rings of esophageal epithelium excised, reflux esophagitis induced by cardioplasty and pentagastrin administration | Canine | Columnar-lined esophagus developed above ESMGs. Proliferation in ESMGs and ducts increased with surgically induced reflux. |
61Gillen 1988 62Li 1994 63van Nieuwenhove 1998 |
| Esophageal injury by radiofrequency ablation | Porcine | Ablation injury was associated with ESMG proliferation, acinar ductal metaplasia, and increased SOX9 and KRT7 expression in ESMGs. | 64Kruger 2017 |
| 3D culture of ESMG cells | Porcine | ESMG cells grown in 3-dimensional culture produce 2 distinct phenotypes of spheroids:1 solid with squamous markers (p63) and 1 hollow with BE markers (KRT7). | 65von Furstenberg 2017 |
| Gastric Cardiac Mucosa | |||
| Transgenic mice (mixed 129/SvEv and C57BL/6 background) with inflammation induced by esophageal IL1B expression, with lineage-tracing and 3-dimensional cell culture | Mouse | Gastric cardiac mucosa expands and contributes to Barrett’s-like changes at the squamo-columnar junction. Bile acid and hypergastrinemia promote metaplasia and dysplasia. |
68Quante 2012 72Lee 2017 |
| Specialized Populations of Cells at the EGJ – Residual Embryonic Cells | |||
| Transgenic mice (mixed 129/SvEv and C57BL/6 background) with inflammation induced by esophageal diphtheria toxin expression, and mice with p63 deletion (lineage tracing not used) | Mouse | A normally-quiescent population of residual embryonic cells (p63-negative, CAR4+, KRT7+) at the EGJ contributes to Barrett’s-like changes in embryos with p63 deletion and in adults with diphtheria toxin-induced squamous cell injury. | 73Wang 2011 |
| Specialized Populations of Cells at the EGJ – Transitional Basal Cells | |||
| Cell culture, esophago-duodenal anastomosis and genetic mouse models (mixed 129/SvEv and C57BL/6 background) with lineage tracing | Mouse and human | Transitional basal cells (KRT5+, p63+, KRT7+) at the EJG contribute to Barrett’s-like changes in 3-dimensional organoids and at the squamo-columnar junction. | 18Jiang 2017 |
| Circulating Bone Marrow Cells | |||
| Transplantation of bone marrow from male rats to female rats, followed by esophago-jejunostomy to produce columnar-lined esophagus | Rat | Y chromosome found in epithelial cells of columnar-lined esophagus of female rats. | 82Sarosi 2008 |
| Transplant of beta-galactosidase-expressing bone marrow cells, followed by esophago-jejunostomy to produce columnar-lined esophagus | Mouse | Beta-galactosidase-expressing epithelial cells found in columnar-lined esophagus. | 83Hutchinson 2011 |
| Case report of a male patient who received a bone marrow transplant from a female | Human | Patient later developed an esophageal tumor with cells containing 2 X and no Y chromosomes | 83Hutchinson 2011 |
Since the intestinal-type cells that characterize Barrett’s metaplasia are not normally found in the esophagus, stomach, or bone marrow, reprogramming would be required for any of these progenitor cell candidates to give rise to Barrett’s metaplasia. GERD is presumed to be the factor that induces the reprogramming needed for the transcommitment of these progenitor cells. It is important to note that hypotheses about the cell of origin are not mutually exclusive— there could be more than 1 type of Barrett’s progenitor cell. Furthermore, in addition to giving rise to BE initially, any of these potential progenitor cells might give rise to the recurrences of Barrett’s metaplasia that can develop months to years after initially successful endoscopic eradication therapy.21
During injury and repair, tissues often reactivate early developmental signaling pathways. Progenitor cells in an adult organ are assumed to retain the genotype of the more primitive embryonic progenitor cells from which they arose.22 Consequently, if metaplasias develop from GERD-induced reprogramming of progenitor cells in adult organs, the new cell types that develop are likely to reflect the differentiation potential of those embryonic progenitor cells. It is therefore important to consider the embryological development of the esophagus and stomach.
Embryology of the esophagus and stomach
The esophagus and stomach develop from the foregut portion of the primitive endodermal digestive tube.23-25 The respiratory tube that gives rise to the trachea and lungs forms as an outgrowth of the digestive tube, separating from it at around 4 weeks of gestation in humans and during embryonic (E) days 9.5–11.5 in mice.26 In mice, multiple signaling pathways (such as those involving SHH, BMP, and WNT) and transcription factors (such as SOX2 and FOXF1) mediate this separation process.27-32 Just before separation of the trachea from esophagus in the anterior foregut, these signaling molecules and transcription factors adopt a dorsal–ventral expression pattern that leads to differences in cell differentiation along the dorsal–ventral axis.26,33 Disruption of this patterns results in esophageal atresia, a relatively common birth defect (1/3000) in which the proximal portion of the esophagus ends as a blind sac, often accompanied by tracheo-esophageal fistula.26-32 Some of these signaling molecules and transcription factors have important roles in subsequent esophageal morphogenesis,32,34 and their reactivation has been observed in biopsies of Barrett’s metaplasia.
The human embryonic esophagus is lined by a stratified columnar epithelium that changes into a simple, ciliated columnar epithelium between 8 and 10 weeks of gestation.23-25 At 5 months gestation, squamous epithelium begins to replace the ciliated columnar epithelium that lines the fetal esophagus. Squamous epithelium appears first in the middle esophagus, and then extends proximally and distally until, at birth, the esophagus is lined almost entirely by squamous epithelium.32 A similar developmental process occurs in the mouse esophagus.35-38 Signaling molecules such as BMP7 and its inhibitor Noggin are enriched in the columnar epithelium lining the early embryonic mouse esophagus.34,39 In Noggin null (Nog−/−) mice, the esophagus fails to separate from the early foregut, and approximately 70% of these mice develop esophageal atresia with tracheo-esophageal fistula. Interestingly, the esophagus does separate from the trachea in the other 30% of Nog−/− mice, but the esophageal epithelium comprises columnar and squamous cells.28 So, Noggin appears to be required for development of normal esophageal squamous epithelium. In support of this concept, incubation of human embryonic stem cells with Noggin promotes their commitment to an esophageal cell fate.39
Using tissue explants from E11.5 mouse embryos to study the mechanisms of the transition from simple columnar to esophageal squamous epithelium, Yu et al identified some epithelial cells that simultaneously expressed columnar and squamous cytokeratins.37 In studies that used immunofluorescence to identify columnar cells that express keratin 8 (KRT8) and squamous cells with KRT14, KRT8+ columnar cells were found to express KRT14 as the esophagus developed. This switch in protein expression occurred in the absence of cell division or cell death, and was caused by silencing of Krt8 via de novo methylation in the promoter region.37 This observation provides evidence that direct transdifferentiation (without a dedifferentiation event) can occur during embryonic development, but it is not clear that a similar process occurs in adults. Nevertheless, a reversal of this process in the adult esophagus might result in columnar metaplasia.
Could Cells Native to the Esophagus Provide BE progenitor cells?
There are several lines of evidence that could support either transdifferentiation of esophageal squamous cells, through paligenosis, or transcommitment of esophageal progenitor cells in the pathogenesis of Barrett’s metaplasia. For example, scanning electron microscopy of biopsy specimens taken from the junction between squamous and Barrett’s epithelium revealed a distinct cell type, with prominent intercellular ridges (a feature of squamous cells) and microvilli (a feature of columnar cells).40,41 These distinctive cells might have developed via reprogramming of pluripotent progenitor cells in the squamous epithelium. In rats with ulcerative reflux esophagitis, researchers found the nuclei of proliferative esophageal basal cells located adjacent to esophageal ulcerations to have decreased levels of SOX2 (a marker of basal progenitor cells in adult squamous esophagus) and increased levels of SOX9 (a marker of progenitor cells in adult intestine, liver, pancreas, and gastric corpus that also is expressed in the embryonic esophagus), compared to non-ulcerated tissue in the same esophagus.42 Reflux esophagitis can therefore reprogram squamous progenitor cells to express columnar genes, but those cells retained their squamous morphology and would therefore not be considered metaplastic.
In support of a role for reprogramming of progenitor cells by GERD in the development of BE, telomerase-immortalized human esophageal squamous cells exposed in vitro to acid, bile salts or nitric oxide (a toxic molecule formed when dietary nitrate encounters acid in refluxed gastric juice) down-regulate expression of transcription factors involved in squamous cell differentiation such as p63 and SOX2,43,44 and up-regulate expression of columnar and intestinal transcription factors such as SOX9, CDX2, and FOXA2.44-46 Acid and bile salts also can activate signaling pathways in esophageal squamous cells (such as hedgehog and BMP4) that control activity of transcription factors that regulate development and cell phenotypes.45,47 Furthermore, prolonged exposure of telomerase-immortalized esophageal squamous cells with acidic bile salts produces alterations in morphology characteristic of columnar cells.44 However, these in vitro manipulations of squamous cells have not resulted in their transformation into goblet cells, which are typically found in Barrett’s metaplasia.
Esophageal submucosal glands
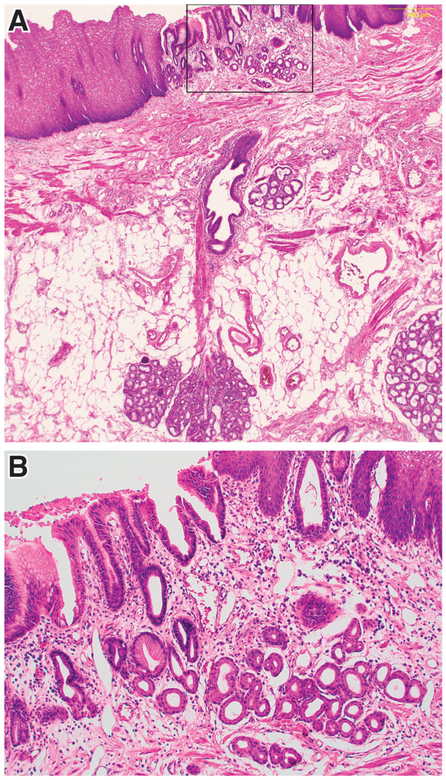
Endoscopists often observe discrete islands of squamous epithelium within a field of Barrett’s metaplasia or, conversely, islands of columnar epithelium proximal to the squamo-columnar junction (SCJ). A prospective study of 555 patients (59% with BE) evaluated by endoscopy found columnar islands in 34% of cases.48 In a study of esophagectomy specimens from 131 patients with esophageal squamous cell carcinoma in Japan, columnar epithelial islands were identified in 57% of specimens.49 It is noteworthy that in patients with BE, columnar islands often are located a considerable distance proximal to the Barrett’s segment, so these islands are likely to emerge due to an etiology other than direct extension of columnar metaplasia.48 One potential source of these columnar or squamous islands is the esophageal submucosal glands (ESMGs) (Figure 3).
Figure 3.
In a patient with BE who had an island of columnar epithelium in squamous mucosa, an ESMG and duct is present directly beneath the columnar island. A) Low-power image showing the ESMG, duct and overlying columnar island (H&E, 10X). B) Higher-power image of the columnar island enclosed by the rectangle in Figure 3A (H&E, 20X). (Photomicrographs provided by Dr. Shannon J. McCall)
ESMGs protect the esophagus by producing acid-neutralizing bicarbonate, mucins, and antimicrobial lysozyme.50 Some ESMGs form during the late stages of fetal development, but most develop in the post-natal period. They are found throughout the human adult esophagus and are characterized by mucinous acini and less frequent serous cells.50 ESMGs drain into excretory ducts lined by cuboidal cells that transition to squamous epithelium before emptying into the esophageal lumen. Cells from ESMGs and their ducts can respond to esophageal injury by proliferating and replacing the damaged surface cells.51
A mapping study of esophagectomy specimens from 7 patients with esophageal adenocarcinoma identified ESMGs beneath squamous and Barrett’s epithelium, with the greatest ESMG density found in the transition zone between the 2 epithelia.52 To provide a more complete evaluation of ESMGs, their tortuous ducts, and their relationship with the overlying epithelium, Coad et al carefully examined esophagectomy specimens from 14 patients with BE-associated neoplasia (reorienting and re-embedding specimens when necessary).53 Each of 15 squamous islands identified in Barrett’s metaplasia was in continuity with an ESMG duct, suggesting that the squamous islands arose from the ducts. The investigators also found ESMG ducts in continuity with multilayered epithelium, a distinctive epithelial type thought to represent an early stage in the development of Barrett’s metaplasia in which a basal layer of squamous-type cells underlies a superficial layer of mucus-secreting columnar cells (Supplemental Figure 1). In addition, 21 gland ducts were observed to open onto the surface of Barrett’s epithelium—in some cases with duct cells transitioning from cuboidal to simple columnar phenotype (reminiscent of cells lining the embryonic esophagus) before reaching the BE surface.
Several important clonality studies have expanded upon the morphometric studies associating ESMG gland ducts with overlying Barrett’s metaplasia and squamous islands. In a study of 20 patients, squamous islands surrounded by Barrett’s metaplasia were isolated by microdissection and subjected to genetic analyses.54 In most cases, squamous islands and the ESMGs beneath them did not share mutations in p16/CDKN2A or p53 with the surrounding Barrett’s metaplasia. In 1 patient, however, a squamous island shared a p16/CDKN2A mutation with the surrounding Barrett’s metaplasia, indicating a common progenitor for both epithelial types. A study that used non-pathogenic mitochondrial DNA mutations as clonal markers found mitochondrial mutations that were shared between squamous and Barrett’s epithelium in 1 patient, supporting the concept that squamous and Barrett’s epithelium can derive from the same progenitor cell.55 In another study, biopsy and esophagectomy samples were used to compare clonality of ESMG ducts and overlying squamous and Barrett’s epithelia.56 Consistent with finding from other studies, squamous islands often were found above ESMGs and ducts, and in 1 case the surrounding BE tissue contained a mutation in p53 not found in the squamous island or ESMG, indicating different cells of origin for the Barrett’s metaplasia and squamous island. However, in 1 of 3 cases in which an ESMG duct opened onto Barrett’s metaplasia, the ESMG, its squamous ductal epithelium, and the Barrett’s metaplasia all had the same p16/CDKN2A mutation.
Taken together, findings from these studies indicate that ESMGs and/or their ducts contain plastic progenitor cells capable of differentiating either into squamous or Barrett’s epithelium. A recent study that used single-cell RNA sequencing to compare gene expression profiles among cells of the esophagus, stomach, and duodenum for 13 patients with BE provides correlative evidence to support this concept.57 The profiles of BE cells that expressed the developmental gene LEFTY1 and the stem cell-associated gene OLFM4 overlapped with those of putative ESMG cells, but not with gene expression profiles of gastric or duodenal cells.
Some studies have found ESMGs to contain mixed clonal populations of cells, indicating more than 1 stem/progenitor cell per ESMG.55 Mutations in these progenitors could lead to clonal populations of abnormal cells within ESMGs and their overlying epithelium. Phenotypic alterations in ESMGs have been reported wherein the normal mucinous acini are replaced by metaplastic ductal cells—a process referred to as either necrotizing sialometaplasia-like change or acinar ductal metaplasia.55,58,59 Acinar ductal metaplasia in ESMGs has been observed at the EGJ and under squamous and Barrett’s epithelia, but it appears to be associated most often with Barrett’s metaplasia.58 A study of esophagectomy specimens from patients with high-grade dysplasia or adenocarcinoma and from controls, collected at autopsy, found that the proportion of non-mucinous acini in ESMGs increased with their degree of inflammation, and that acinar ductal metaplasia was strongly associated with esophageal neoplasia.59 When the type of overlying epithelium was associated with the proportion of underlying ESMGs that had acinar ductal metaplasia, squamous islands had the lowest rate of acinar ductal metaplasia (5.4%), with much higher rates found in ESMGs beneath ulcers (54.4%) and adenocarcinomas (37.3%). These findings support findings from clonality studies and indicate that squamous islands arise from normal, healthy ESMGs, whereas overlying Barrett’s metaplasia and cancer are associated with abnormal, inflamed ESMGs that have the ductal phenotype.
Little is known about gene expression changes in ESMG acinar ductal metaplasia. However, Gonzalez et al identified SOX9 in ESMGs of esophageal specimens collected at autopsy from patients without BE.60 SOX9 is expressed in BE tissues, and also in acinar ductal metaplasias in the pancreas (a pre-malignant condition).10,45
Although findings from human studies support a model in which ESMGs are a source of precursor cells for Barrett’s metaplasia, those studies were cross-sectional and observational. Studies of model systems are needed to confirm that ESMGs can produce Barrett’s metaplasia. However, mice and rats do not have ESMGs. Studies of larger animals with ESMGs have, however, generated compelling data associating ESMGs with columnar metaplasia. Researchers induced acute esophageal injury in dogs by excising a pair of 2 cm circumferential rings of normal squamous epithelium from the distal esophagus, leaving an intact 2 cm ring of normal squamous epithelium in between the 2 areas of injury.61 When GERD was induced by creating a hiatal hernia and administering pentagastrin, one-third of the dogs developed columnar epithelium in the upper excised ring, despite its separation from the EGJ. In addition, epithelial regeneration in the ulcerated areas appeared to emerge from ESMG ducts. In a follow-up study, columnar epithelium alone formed in 70% of dogs with surgically induced GERD and injury to the distal esophagus.62 When GERD was controlled in those same dogs, with an anti-reflux procedure and acid suppression, squamous islands formed within columnar patches, and areas of squamous and columnar healing were found to be in continuity with ESMG ducts.
A separate study of dogs demonstrated that ESMGs and their ducts are quiescent under normal conditions, exhibiting no mitoses and incorporating little to no bromodeoxyuridine (a thymidine analog used to label proliferating cells making new DNA).63 After the surgical induction of reflux, however, cell proliferation increased significantly in esophageal squamous epithelium, as well as in ESMGs and their ducts. ESMG lower excretory ducts developed a labeling index of 3.37% after reflux-inducing surgery, compared with 0.11% in controls, whereas the ESMGs themselves had a labeling index of 0.35% with reflux, compared to no labeling in controls. Together, these studies demonstrate that GERD induces a proliferative response in cells of the ESMGs and their ducts, and that these cells can repopulate injured esophageal epithelium.
Porcine esophagus also contains ESMGs and, as in dogs, normally quiescent porcine ESMGs proliferate following esophageal injury.64 Porcine ESMGs placed in 3-dimensional culture also proliferated and single cells derived from these ESMGs formed 2 types of spheroids, which recapitulated the squamous and columnar phenotypes associated with ESMGs in the human and dog studies.65 One spheroid type had a solid, squamous appearance and expressed squamous markers such as p63, whereas the other was hollow and expressed columnar cell markers found in Barrett’s metaplasia, including AGR2, MUC13, KRT18, MUC1, KRT8, and SOX9. Research is underway in pigs to elucidate factors that affect expression of squamous vs columnar markers. Lineage-tracing studies in animals with ESMGs, and studies that isolate ESMGs to directly interrogate their transcriptional profiles are needed to confirm the theory that ESMGs are progenitors of BE.
Stomach Cells as BE Progenitor Cells
Although Barrett’s metaplasia is considered intestinal, because it contains goblet cells and expresses some intestinal markers, it also contains gastric-type cells that express gastric proteins, such as claudin18 and TFF2.66,67 Quante et al found that Lgr5+ epithelial progenitor cells in the gastric cardia might be cells of origin for Barrett’s-like metaplasia in mice with chronic inflammation in the esophagus and forestomach.68 In these mice, an Epstein-Barr virus promoter (ED-L2) promotes expression of the inflammatory cytokine interleukin 1 beta (IL1B) in the stratified squamous epithelium of the esophagus and forestomach (L2-IL1B mice). The investigators found that Lgr5-CreER-labeled cells in the mouse gastric cardia migrated into neighboring inflamed squamous territory, where they underwent BE-like phenotypic changes. The L2-IL1B mice developed chronic epithelial inflammation and thickening in the esophagus and forestomach. However, at ages of 12–15 months, expression of proteins associated with BE (TFF2, MUC5AC, and PAS) were observed only at the SCJ.68 At 20–22 months, 2 of 9 mice developed high-grade dysplasia or intramucosal carcinoma. Furthermore, development of Barrett’s metaplasia, high-grade dysplasia, and intramucosal carcinoma accelerated when mice were given water containing an unconjugated bile acid (0.2% deoxycholate). The Barrett’s-like epithelium also had evidence for activation of Notch signaling, with increased levels of Notch 1 and its ligand, DLL1. Interestingly, L2-IL1B mice with disruption of IL6 do not develop metaplasia or dysplasia, indicating that IL6 mediates these processes in this model.37 Levels of IL1B and IL6 also are increased in biopsies of Barrett’s metaplasia from patients.69,70 Among patients with GERD, polymorphisms in the IL1 receptor antagonist (IL1RA) have been linked to increased esophageal levels of IL1B and increased risk of BE.71
Barrett’s metaplasia specimens express the gastrin receptor (CCK2R). Lee et al demonstrated that hypergastrinemia can accelerate the progression of Barrett’s-like metaplasia in L2-IL1B mice.72 They found that inflammation of squamous epithelium in these mice was accompanied by expansion of CCK2R+ cardia progenitor cells at the SCJ—a finding they confirmed by lineage-tracing experiments, using CCK2R-CreER mice. Notably, approximately 85% of CCK2R+ cells in the cardia were Lgr5-negative, indicating that 2 distinct progenitor cell populations in the gastric cardia contribute to Barrett’s-like metaplasia in these mice. Furthermore, protracted lineage-tracing experiments found that these 2 progenitor cell populations can interconvert. This study also showed that gastrin can promote the expansion of CCK2R+ cardia progenitor cells in 3-dimensional organoids; the CCK2R inhibitor YF476 blocked the growth of organoids and the progression of Barrett’s-like metaplasia in L2-IL1B mice.
These findings indicate that the gastric mucosa could be a source of the cells of origin for BE. Although bona fide intestinal differentiation (with goblet cells) was not observed in L2-IL1B mice, extensive expansion of the cardia epithelium (based on expression of TFF2 and MUC5AC, and on PAS staining) was found. This could indicate a transitional stage in the development of BE with intestinal metaplasia. Studies of mice with longer exposures to IL1B could provide further insights into BE pathogenesis.
Residual Embryonic Cells
Wang et al proposed that a small population of epithelial cells at the SCJ, established during embryonic development, persists into adulthood and might provide progenitor cells for Barrett’s metaplasia.73 In mice with esophageal injury, these quiescent residual embryonic cells (RECs) could be stimulated to expand into squamous territory and give rise to BE. RECs express KRT7, as do epithelial cells of Barrett’s metaplasia,74 and do not express the squamous cell markers KRT5, KRT14, or p63. The researchers used Krf14-CreER;R26-DTA mice, in which the esophageal squamous epithelium expresses diphtheria toxin A after tamoxifen injection, to study esophageal injury. In these mice, tamoxifen administration results in the destruction of KRT14+ basal cells and their derivatives by diphtheria toxin. Following tamoxifen administration, KRT7+ RECs were observed to expand proximally to replace damaged squamous cells. This is similar to what happens in the in L2-IL1B mice–as in those mice, the Krt14-CreER;R26-DTA mice did not develop bona fide intestinal metaplasia with goblet cells.
Wang et al also showed that deletion of p63 promotes the development of Barrett’s-like epithelium in the mouse forestomach.73 Microarray analysis of the forestomach revealed that the gene expression patterns in embryos of mice with full-length Trp63 (wild-type) or Trp63−/− mice differed by E18; forestomach tissues from Trp63−/− mice upregulated genes that are normally expressed in intestine, such as Cdx2 and Villin1. The authors studied expression of p63 at different embryonic stages of foregut development and found that p63-positive cells in wild-type mice spread from the esophagus to the forestomach, eventually replacing the CAR4+, KRT7+ simple columnar epithelium that lines the early embryonic forestomach. Wang et al showed that expansion of the p63-positive cells terminated at the SCJ, where the RECs were located. In p63−/− mice, in contrast, the CAR4+, KRT7+ columnar forestomach epithelium was not replaced by p63-positive squamous epithelium. Instead, the CAR4+, KRT7+ columnar cells remained and acquired Barrett’s-like features. Consistently, in adult mice with esophageal injury, caused by expression of diphtheria toxin in squamous cells, KRT7+ RECs that did not express CAR4 expanded into squamous territory, so there might be 2 different types of KRT7-positive RECs. Jiang et al also investigated phenotypic changes in the forestomach of p63−/− mice.18 Surprisingly, their lineage-tracing experiments, using a p63-CreER knock-in mice, found that the simple columnar cells of the forestomach (so-called Barrett’s cells in the REC model) originated from p63-positive progenitor cells.18
Transitional basal cells
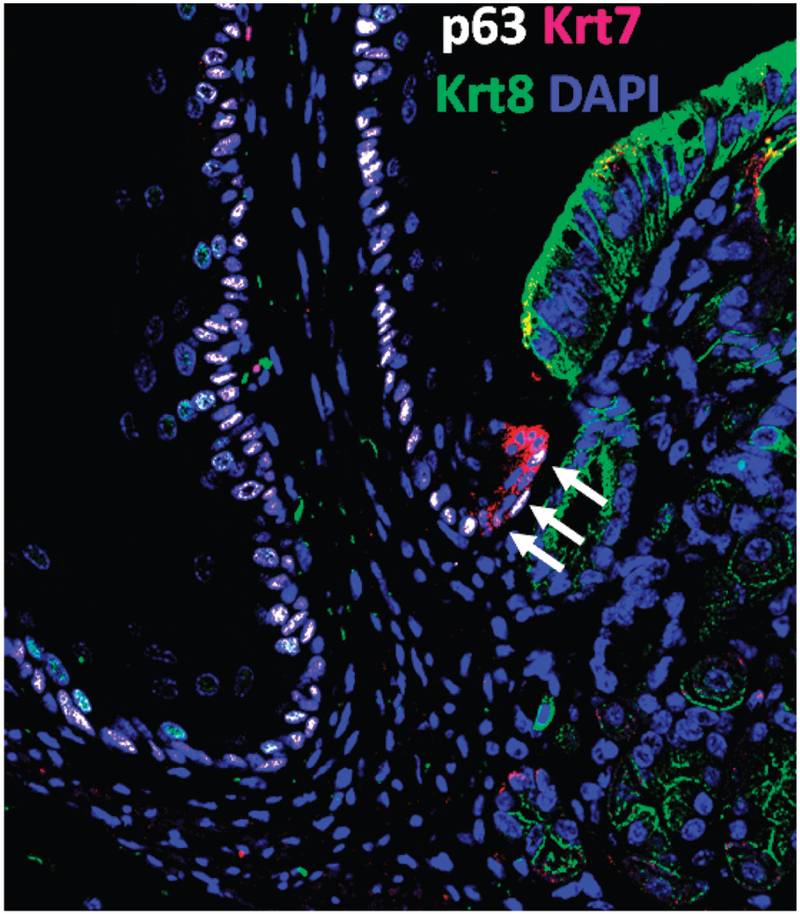
Jiang et al identified a population of cells, different from RECs, at the transitional zone of the SCJ in mice and humans that might give rise to BE.18 They found that this transitional epithelium was maintained by a population of progenitor cells they called transitional basal cells (TBCs). The TBCs expressed squamous markers (KRT5, KRT14, and p63) and a columnar cytokeratin found in Barrett’s metaplasia (KRT7+), in contrast to their neighboring squamous basal cells (KRT5+, KRT14+, p63-positive, KRT7-negative) and cardia mucosal progenitor cells (KRT5-negative, KRT14-negative, p63-negative, KRT7-negative) (Figure 4). After the authors created an esophago-jejunostomy to induce bile reflux, they found that TBCs proliferated and differentiated into columnar epithelium that expressed genes expressed by intestinal cells, such as Cdx2. Overexpression of Cdx2 in TBCs also promoted their differentiation into intestinal-type epithelial cells.
Figure 4.
Transitional basal cells at the mouse squamo-columnar junction. The arrows point to transitional basal cells (p63+ Krt7+ Krt8-negative) located at the squamo-columnar junction. Note the neighboring squamous cells (p63+, Krt7-negative, Krt8-negative) on the left and the columnar gastric cells (p63-negative, Krt7-negative, Krt8+) on the right (see details in Jiang et al17).
Lineage-tracing experiments in Krt5-CreER mice or Krt7-CreER mice confirmed that TBCs were a cell of origin for Barrett’s-like epithelium. In contrast, ectopic expression of Cdx2 in squamous basal cells of the forestomach or esophagus did not convert them into Barrett’s-like cells—an observation consistent with findings by Kong et al.75 Importantly, multilayered epithelium was also observed at the SCJ during the progression towards Barrett’s metaplasia with goblet cells, recapitulating observations in human patients with chronic GERD.76,77 The squamous cells comprising the basal layer of multilayered epithelium express p63, but p63 is rarely detected in Barrett’s metaplasia or in esophageal adenocarcinomas (EACs).18,78,79 Little is known about the events leading to the loss of p63 as TBCs transition into multilayered epithelium and intestinal metaplasia. Elucidation of those pathways might provide insights into the pathogenesis of BE. Studies of immortalized squamous cells or esophageal squamous cancer cell lines found that bile acid exposure reduces expression of p6380, but it is not clear whether bile acid has similar effects in TBCs.
Circulating bone marrow cells
In 2004, Epperly et al reported that radiation injury of mouse esophagus could be repaired by transplanted bone marrow cells.81 Noting this, Sarosi et al investigated whether bone marrow cells could repair reflux-induced esophageal injury and contribute to esophageal metaplasia.82 The authors administered a lethal dose of radiation to female rats to destroy their bone marrow, and then transplanted bone marrow cells from male rats. Ten days later, they performed esophago-jejunostomy to induce reflux esophagitis with columnar-lined esophagus, and the esophagus was collected 8 weeks later. Fluorescence in situ hybridization analysis of esophageal tissues from bone marrow recipients (female rats) revealed Y chromosome-containing squamous and columnar epithelial cells lining the distal esophagus. Cells of bone marrow origin can therefore contribute to esophageal healing and metaplasia in rats.
Hutchinson et al performed a similar experiment in mice that were irradiated, transplanted with bone marrow cells that expressed β-galactosidase, and then subjected to esophago-jejunostomy.83 Twenty-weeks after surgery, 4 of 12 surviving mice had developed esophageal columnar metaplasia with glands containing epithelial cells that expressed beta-galactosidase. In this same report, the investigators described a man with acute myeloid leukemia who received a bone marrow transplant from his sister and developed an adenosquamous tumor of the distal esophagus 10 years later. Fluorescence in situ hybridization experiments showed that the tumor contained cells with 2 X chromosomes and no Y chromosomes, indicating that bone marrow-derived cells from the sister contributed to the esophageal tumor. Although these findings are intriguing, it is not clear that bone marrow-derived cells contribute to the development of esophageal metaplasia outside of the extreme conditions described in these reports.
Barrett’s Metaplasia as a Wound-healing Process
Regardless of which progenitor cells give rise to BE, the metaplastic process is assumed to be initiated by esophageal injury from GERD. Reflux esophagitis often leads to esophageal ulceration that is bordered proximally by squamous epithelium and distally by gastric epithelium, with ESMGs and their ducts lying underneath the ulcer crater. Cells from any of these locations might contribute to esophageal re-epithelization initially via a wound healing process, with GERD-induced reprogramming resulting in metaplasia. Agoston et al studied the similarities between the wound healing response and development of a columnar-lined esophagus in rats after esophago-jejunostomy.42
Esophago-jejunostomy induced large areas of ulceration in the squamous-lined distal esophagus of rats, with ulcers starting at the esophago-jejunal anastomosis and progressing proximally up the esophagus.42 The squamous epithelium at the proximal edge of these ulcerations was infiltrated by inflammatory cells and there were signs of basal cell hyperplasia and spongiosis, typical of reflux esophagitis. At the distal end of the ulceration at the esophago-jejunal anastomosis, the ulcer bed was re-epithelialized by neoglandular epithelium that appeared to originate from budding, immature-appearing crypts arising directly from the base of the native jejunal crypts. Budding intestinal cells extended into mesenchyme underneath the ulcer bed, sometimes growing around islands of squamous epithelium. The neoglandular epithelium was morphologically and molecularly similar to the native jeujnal crypt epithelium from which it arose.
To determine if native progenitor cells of the jejunum were involved in re-epithelialization of esophageal ulcers developing after esophago-jejunostomy, the investigators performed immunohistochemical analysis for PDX1, a foregut transcription factor that has been used as an index of esophageal columnar metaplasia that develops via reprogramming of progenitor cells.84 The intensity of PDX1 staining was similar in neoglandular and native jejunal epithelia, indicating that the columnar-lined esophagus in these rats did not develop via reprogramming of jejunal progenitor cells. Rather, the esophageal intestinal metaplasia developed via a wound healing process, in which jejunal progenitor cells proliferated and migrated into the reflux-damaged esophagus. Although increased proliferation was seen in squamous cells at the proximal border of the esophageal ulcers, those cells were unable to re-epithelialize the ulcers in the setting of ongoing reflux esophagitis. In contrast, the length of neoglandular epithelium at the distal ulcer border increased linearly over time. This observation indicates that, with ongoing reflux, neoglandular cells have a proliferative advantage over their squamous counterparts—a concept supported by earlier studies of co-cultures of BE and esophageal squamous cell lines. These experiments showed that BE cells have a proliferative advantage over squamous cells in an intermittently acidic environment.85 Furthermore, after esophagectomy with esophagogastrostomy, the remnant esophagus develops a columnar lining that increases progressively in length over time, correlating with the severity of acid reflux documented by esophageal pH monitoring.86
Development of columnar-lined esophagus in the rodent esophago-jejunostomy model begins with jejunal cells migrating into mesenchyme underneath esophageal ulcer beds.42 Epithelial-mesenchymal transition (EMT), the process in which epithelial cells tethered to one another by adhesion molecules like E-cadherin morph into mesenchymal-like cells with the ability to migrate, is part of wound healing.87 The development of EMT in jejunal cells at the ulcer edge might cause the neoglandular epithelium to extend into the mesenchyme and grow around squamous islands. As early supporting evidence of a role for EMT in these processes, Agoston et al found that the advancing front of rat neoglandular epithelium contained spindleshaped, mesenchymal-appearing cells that expressed both the mesenchymal marker TWIST1 and the epithelial marker E-cadherin.42 Whether the progenitor cell for BE originates from ESMGs or their ducts, the gastric cardia, or specialized cell populations at the SCJ (RECs or TBCs), these findings indicate that progenitors might migrate into the damaged esophagus through a wound-healing process involving EMT. Under conditions of ongoing reflux, these progenitor cells likely have a competitive advantage over native squamous cells, enabling them to re-epithelialize the ulcerated squamous esophagus. Ongoing reflux also is likely to cause the reprogramming of these progenitors that results in the development of the abnormal columnar lining of BE. Although it is conceivable that refluxed acid and bile could induce transdifferentiation or transcommitment of squamous epithelial cells without inflicting a physical wound, it seems more likely that an esophageal wound would initiate these processes as well.
Cardiac Mucosa, Intestinal Metaplasia with Goblet Cells, and BE
The gastric cardia is believed to be lined, to a variable extent, by cardiac mucosa—a glandular lining comprising mucus-secreting, gastric foveolar-type cells with no goblet cells and few or no parietal cells (Figure 5). However, there is considerable indirect evidence to support a hypothesis, proposed by P Chandrasoma in 1997, that cardiac mucosa is not normal but an acquired, GERD-induced metaplasia, and that cardiac mucosa is the precursor of intestinal metaplasia in BE.88,89 For example, esophagectomy with gastric pull-up reconstruction often results in severe reflux esophagitis in the esophageal remnant, which frequently acquires a columnar lining with cardiac mucosa first, followed years later by intestinal metaplasia.86,90 Patients with GERD have a greater extent of cardiac mucosa than patients without GERD, and the magnitude of that extent appears to be an index of GERD severity.91,92 Although a narrow strip of cardiac mucosa (usually <3 mm in extent) can be found at the esophago-gastric junction (EGJ) in most healthy individuals, 89 this observation does not establish that cardiac mucosa is normal. Autopsies of young men who died of trauma have revealed evidence of atherosclerosis in more than 75%, but atherosclerosis is not considered a normal condition.93 The distal-most, squamous-lined esophagus of healthy individuals regularly experiences intense acid exposure that might result in squamous to cardiac mucosal metaplasia.94
Figure 5.
Cardiac mucosa comprised exclusively of mucus-secreting cells and glands. (Photomicrograph provided by Robert Genta, H&E, 20X)
Although cardiac mucosa lacks the goblet cells required for a histologic diagnosis of intestinal metaplasia, cardiac mucosa has other intestinal features, including expression of intestinal-type acidic mucins and proteins such as villin and CDX2.95,96 Furthermore, cardiac mucosa can develop DNA content abnormalities similar to those of intestinal metaplasia with goblet cells.97 Nevertheless, and despite some contradictory data, cardiac mucosa appears to have minimal risk for malignant transformation until it develops goblet cells.98,99 That is why American gastroenterology societies require an esophageal biopsy showing intestinal metaplasia for a diagnosis of BE, and reject that diagnosis if biopsies show only cardiac mucosa.2,100,101 In contrast, the British Society of Gastroenterology accepts a diagnosis of BE when esophageal biopsies show only cardiac mucosa.102
McColl et al have proposed that cardiac mucosa develops from a limited form of GERD that induces columnar metaplasia only in a short segment of the distalmost esophagus.103-105 Their proposal is based on a series of studies involving a group of healthy volunteers without GERD symptoms who had normal results from conventional esophageal pH monitoring studies with the pH electrode positioned 5 cm above the lower esophageal sphincter (LES). Using a series of closely spaced pH electrodes within the LES, however, 27 subjects with central obesity had greater proximal extension of gastric acid within the LES than 24 subjects with smaller waist circumferences, such that the distalmost, squamous-lined esophagus of the obese subjects experienced protracted acid exposure.103 Compared to the non-obese volunteers, the obese subjects had a significantly greater length of cardiac mucosa (2.5 vs 1.8 mm) in biopsies collected across the SCJ. Furthermore, 15 healthy volunteers with hiatal hernias detected by endoscopy and/or magnetic resonance imaging had significantly longer durations of postprandial intra-sphincteric acid reflux than 15 subjects without hiatal hernias, and those with hiatal hernias had a significantly longer extent of cardiac mucosa (3.5 vs 2.5 mm).105 These observations support the hypothesis that cardiac mucosa is a GERD-induced metaplasia and, potentially, the precursor of intestinal metaplasia that predisposes to malignancy. Any of the potential BE progenitor cells discussed might first give rise to cardiac mucosa before transforming into intestinal metaplasia with goblet cells. Such a process could well account for the high prevalence of the condition called intestinal metaplasia at the esophago-gastric junction (IM at the EGJ).
Studies in which biopsies were taken at the SCJ of random patients in general endoscopy units have found IM at the EGJ in 9% to 36% of patients, and most of those studies found no association between IM at the EGJ and GERD symptoms or H pylori infection.106 It seems highly likely that a limited, asymptomatic form of GERD involving only the distalmost esophagus causes the development of IM at the EGJ, perhaps through a cardiac mucosa intermediate, from the same progenitor cells that give rise to longer segments of Barrett’s metaplasia in patients with more severe forms of GERD. For patients with BE, numerous studies have found the risk of cancer development to vary with the extent of metaplastic mucosa (length of the BE segment).2,102 The risk of cancer in patients with IM only at the EGJ is therefore expected to be small—an expectation confirmed in a study that found the cumulative risk of progression to high-grade dysplasia or esophageal adenocarcinoma at 10 yrs to be 7%, in 355 patients with BE (0.8% per year), but in none of 86 patients with IM at the EGJ.107 However, this was a retrospective study, with limitations precluding the conclusion that IM at the EGJ does not increase risk of cancer. For example, only 59 of the 86 patients with IM at the EGJ had a subsequent endoscopy to assess neoplastic progression, and low-grade dysplasia was noted in 7 patients, albeit without progression. Considering the prevalence of IM at the EGJ in the general population, even a small increase in risk could account for the development of many EACs.
Our review of BE reveals shortcomings that have important implications for extant clinical definitions of the disorder. The most recent guidelines from the British Society of Gastroenterology and the American College of Gastroenterology require at least 1 cm of esophageal metaplasia for a diagnosis of BE—a restriction based primarily on the assumption of negligible cancer risk for patients with shorter segments of cardiac mucosa and intestinal metaplasia.2,102 For practical purposes, this seems a reasonable restriction that avoids the expense, inconvenience, procedure-related risks, and anxiety associated with a diagnosis of BE. Scientifically, however, the restriction is entirely arbitrary, since metaplastic esophageal segments <1 cm in length almost certainly develop from the same underlying mechanism (GERD, albeit limited) and from the same progenitor cells that give rise to long-segment BE and, on a millimeter for millimeter basis, probably have similar malignant predisposition.
A study of patients with newly diagnosed EAC found that tumors that developed from short segments of Barrett’s metaplasia were even more aggressive than those arising from longer segments.108 In that study, approximately 50% of patients with newly diagnosed EACs had no endoscopic evidence for Barrett’s metaplasia, grossly or by endoscopic biopsy, and no evidence of Barrett’s metaplasia in the resected esophagus. Compared to patients who did have Barrett’s metaplasia identified at the time of tumor diagnosis, the patients without Barrett’s metaplasia had more advanced tumors and shorter survival times, even after adjustments for cancer stage. The authors proposed that EACs in patients without BE at the time of cancer diagnosis might have developed from small, easily overlooked areas of metaplastic epithelium that suddenly acquired genomic instability and underwent carcinogenesis.108
For reasons that remain unexplained, BE appears to develop to its full extent all at once. Barrett’s metaplasia is not observed to progress in extent over time,109,110and endoscopically identified short segments of Barrett’s metaplasia rarely, if ever evolve into long segments.111 If, as McColl et al propose, most EGJ tumors arise from millimeter-long segments of metaplastic mucosa at the EGJ in individuals without GERD symptoms,103-105 then our screening and surveillance policies for BE will have little effect in preventing esophageal cancer. Not only will most of those at risk not be screened, because they have no GERD symptoms, but screening will not recognize their few millimeters of esophageal metaplasia as BE that warrants endoscopic surveillance or other intervention. Although we can offer no simple solutions to this conundrum, clinicians should be aware of the limitations imposed by our incomplete understanding of BE pathogenesis. Further research on this fascinating metaplastic process is warranted and eagerly awaited.
Supplementary Material
Multilayered epithelium with squamoid-appearing cells in the basal layers, and overlying columnar mucus-secreting cells. (Photomicrograph provided by Robert Genta, H&E, 40X)
Acknowledgments
Grant Support: This work was supported by the National Institutes of Health (DK113144, DK100342, DK120650 to J.Q.; DK098528, DK118022 to K.S.G.; DK63621, DK103598, K11139 to R.F.S. and S.J.S.) and by Baylor Scott and White Research Institute (R.F.S. and S.J.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spechler SJ, Souza RF. Barrett’s esophagus. N Engl J Med 2014; 371:836–45. [DOI] [PubMed] [Google Scholar]
- 2.Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus. Am J Gastroenterol 2016; 111:30–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayeck TJ, Kong CY, Spechler SJ, Gazelle GS, Hur C. The prevalence of Barrett’s esophagus in the US: estimates from a simulation model confirmed by SEER data. Dis Esoph 2010; 23:451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thrift AP. Barrett’s esophagus and esophageal adenocarcinoma: How common are they really? Dig Dis Sci 2018; 63:1988–96. [DOI] [PubMed] [Google Scholar]
- 5.Virchow R Ueber metaplasie: Vortrag, gehalten auf dem internationalen medicinischen Congress in Kopenhagen. Virchows Archiv 1884; 97:410–30. [Google Scholar]
- 6.Tosh D, Slack JM. How cells change their phenotype. Nat Rev Mol Cell Biol 2002; 3:187–94. [DOI] [PubMed] [Google Scholar]
- 7.Slack JM. Metaplasia and transdifferentiation: from pure biology to the clinic. Nat Rev Mol Cell Biol 2007; 8:369–78. [DOI] [PubMed] [Google Scholar]
- 8.Wang DH. The esophageal squamous epithelial cell-still a reasonable candidate for the Barrett's esophagus cell of origin? Cell Mol Gastroenterol Hepatol 2017; 4:157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131:861–72. [DOI] [PubMed] [Google Scholar]
- 10.Mills JC, Sansom OJ. Reserve stem cells: Differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract. Sci Signal 2015; 8(385):re8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jopling C, Boue S, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat Rev Mol Cell Biol 2011; 12:79–89. [DOI] [PubMed] [Google Scholar]
- 12.Goldenring JR, Nam KT. Oxyntic atrophy, metaplasia, and gastric cancer. Prog Mol Biol Transl Sci 2010; 96:117–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldenring JR, Nam KT, Mills JC. The origin of pre-neoplastic metaplasia in the stomach: chief cells emerge from the Mist. Exp Cell Res 2011; 317:2759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radyk MD, Burclaff J, Willet SG, Mills JC. Metaplastic cells in the stomach arise, independently of stem cells, via dedifferentiation or transdifferentiation of chief cells. Gastroenterology 2018; 154:839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sáenz JB, Mills JC. Acid and the basis for cellular plasticity and reprogramming in gastric repair and cancer. Nat Rev Gastroenterol Hepatol 2018; 15:257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willet SG, Lewis MA, Miao ZF, Liu D, Radyk MD, Cunningham RL, Burclaff J, Sibbel G, Lo HG, Blanc V, Davidson NO, Wang ZN, Mills JC. Regenerative proliferation of differentiated cells by mTORC1-dependent paligenosis. EMBO J 2018; 37 pii: e98311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin RU, Mills JC. Are gastric and esophageal metaplasia relatives? the case for Barrett's stemming from SPEM. Dig Dis Sci 2018; 63:2028–2041. [DOI] [PubMed] [Google Scholar]
- 18.Jiang M, Li H, Zhang Y, et al. Transitional basal cells at the squamous-columnar junction generate Barrett’s oesophagus. Nature 2017;550:529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayakawa Y, Fox JG, Wang TC. Isthmus Stem Cells Are the Origins of Metaplasia in the Gastric Corpus. Cell Mol Gastroenterol Hepatol 2017; 4:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang DH, Souza RF. Transcommitment: Paving the way to Barrett's metaplasia. Adv Exp Med Biol 2016; 908:183–212. [DOI] [PubMed] [Google Scholar]
- 21.Guthikonda A, Cotton CC, Madanick RD, Spacek MB, Moist SE, Ferrell K, Dellon ES, Shaheen NJ. Clinical outcomes following recurrence of intestinal metaplasia after successful treatment of Barrett's esophagus with radiofrequency ablation. Am J Gastroenterol 2017; 112:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen CN, Burke ZD, Tosh D. Transdifferentiation, metaplasia and tissue regeneration. Organogenesis 2004; 1:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johns BAE. Developmental changes in the oesophageal epithelium in man. J Anat 1952; 86:431–9. [PMC free article] [PubMed] [Google Scholar]
- 24.DeNardi FG, Riddell RH. The normal esophagus. Am J Surg Pathol 1991; 15:296–309. [DOI] [PubMed] [Google Scholar]
- 25.De Hertogh G, Van Eyken P, Ectors N, Geboes K. On the origin of cardiac mucosa: a histological and immunohistochemical study of cytokeratin expression patterns in the developing esophagogastric junction region and stomach. World J Gastroenterol 2005; 11:4490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Que J The initial establishment and epithelial morphogenesis of the esophagus: a new model of tracheal-esophageal separation and transition of simple columnar into stratified squamous epithelium in the developing esophagus. Wiley Interdiscip Rev Dev Biol 2015; 4:419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Litingtung Y, Lei L, Westphal H, et al. Sonic hedgehog is essential to foregut development. Nat Genet 1998;20:58–61. [DOI] [PubMed] [Google Scholar]
- 28.Que J, Choi M, Ziel JW, et al. Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation 2006;74:422–37. [DOI] [PubMed] [Google Scholar]
- 29.Mahlapuu M, Enerback S, Carlsson P. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development 2001;128:2397–406. [DOI] [PubMed] [Google Scholar]
- 30.Motoyama J, Liu J, Mo R, et al. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet 1998;20:54–7. [DOI] [PubMed] [Google Scholar]
- 31.Harris-Johnson KS, Domyan ET, Vezina CM, et al. beta-Catenin promotes respiratory progenitor identity in mouse foregut. Proc Natl Acad Sci U S A 2009;106:16287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Que J, Okubo T, Goldenring JR, et al. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development 2007;134:2521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell 2010;18:8–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez P, Da Silva S, Oxburgh L, et al. BMP signaling in the development of the mouse esophagus and forestomach. Development 2010;137:4171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daniely Y, Liao G, Dixon D, et al. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol 2004;287:C171–81. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Jiang M, Kim E, et al. Development and stem cells of the esophagus. Semin Cell Dev Biol 2017; 66:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu WY, Slack JM, Tosh D. Conversion of columnar to stratified squamous epithelium in the developing mouse oesophagus. Dev Biol 2005;284:157–70. [DOI] [PubMed] [Google Scholar]
- 38.Menard D Morphological studies of the developing human esophageal epithelium. Microsc Res Tech 1995;31:215–25. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Yang Y, Jiang M, et al. 3D modeling of esophageal development using human PSC-derived basal progenitors reveals a critical role for notch signaling. Cell Stem Cell. 2018. September 13. pii: S1934–5909(18)30394–1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shields HM, Zwas F, Antonioli DA, et al. Detection by scanning electron microscopy of a distinctive esophageal surface cell at the junction of squamous and Barrett’s epithelium. Dig Dis Sci 1993; 38:97–108. [DOI] [PubMed] [Google Scholar]
- 41.Sawhney RA, Shields HM, Allan CH, et al. Morphological characterization of the squamocolumnar junction of the esophagus in patients with and without Barrett’s epithelium. Dig Dis Sci 1996; 41:1088–98. [DOI] [PubMed] [Google Scholar]
- 42.Agoston AT, Pham TH, Odze RD, Wang DH, Das KM, Spechler SJ, Souza RF. Columnar-lined esophagus develops via wound repair in a surgical model of reflux esophagitis. Cell Mol Gastroenterol Hepatol 2018; 6:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asanuma K, Huo X, Agoston A, Zhang X, Yu C, Cheng E, Zhang Q, Dunbar KB, Pham TH, Wang DH, Iijima K, Shimosegawa T, Odze RD, Spechler SJ, Souza RF. In oesophageal squamous cells, nitric oxide causes S-nitrosylation of Akt and blocks SOX2 (sex determining region Y-box 2) expression. Gut 2016; 65:1416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minacapelli CD, Bajpai M, Geng X, Cheng CL, Chouthai AA, Souza R, Spechler SJ, Das KM. Barrett’s metaplasia develops from cellular reprograming of esophageal squamous epithelium due to gastroesophageal reflux. Am J Physiol Gastrointest Liver Physiol 2017; 312:G615–G622. [DOI] [PubMed] [Google Scholar]
- 45.Wang DH, Clemons NJ, Miyashita T, Dupuy AJ, Zhang W, Szczepny A, Corcoran-Schwartz IM, Wilburn DL, Montgomery EA, Wang JS, Jenkins NA, Copeland NA, Harmon JW, Phillips WA, Watkins DN. Aberrant epithelial-mesenchymal Hedgehog signaling characterizes Barrett’s metaplasia. Gastroenterology 2010; 138:1810–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang DH, Tiwari A, Kim ME, Clemons NJ, Regmi NL, Hodges WA, Berman DM, Montgomery EA, Watkins DN, Zhang X, Zhang Q, Jie C, Spechler SJ, Souza RF. Hedgehog signaling regulates FOXA2 in esophageal embryogenesis and Barrett’s metaplasia. J Clin Invest 2014; 124:3767–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou G, Sun YG, Wang HB, Wang WQ, Wang XW, Fang DC. Acid and bile salt up-regulate BMP4 expression in human esophageal epithelium cells. Scand J Gastroenterol 2009; 44:926–32. [DOI] [PubMed] [Google Scholar]
- 48.Epstein JA, Cosby H, Falk GW, Khashab MA, Kiesslich R, Montgomery EA, Wang JS, Canto MI. Columnar islands in Barrett's esophagus: Do they impact Prague C&M criteria and dysplasia grade? J Gastroenterol Hepatol 2017; 32:1598–1603. [DOI] [PubMed] [Google Scholar]
- 49.Nakanishi Y, Saka M, Eguchi T, Sekine S, Taniguchi H, Shimoda T. Distribution and significance of the oesophageal and gastric cardiac mucosae: a study of 131 operation specimens. Histopathology 2007; 51:515–9. [DOI] [PubMed] [Google Scholar]
- 50.Abdulnour-Nakhoul S, Nakhoul NL, Wheeler SA, Haque S, Wang P, Brown K, et al. Characterization of esophageal submucosal glands in pig tissue and cultures. Dig Dis Sci 2007; 52:3054–65. [DOI] [PubMed] [Google Scholar]
- 51.Wright NA. Migration of the ductular elements of gut-associated glands gives clues to the histogenesis of structures associated with responses to acid hypersecretory state: the origins of "gastric metaplasia" in the duodenum of the specialized mucosa of barrett's esophagus and of pseudopyloric metaplasia. Yale J Biol Med 1996; 69:147–53. [PMC free article] [PubMed] [Google Scholar]
- 52.Lorinc E, Oberg S. Submucosal glands in the columnar-lined oesophagus: evidence of an association with metaplasia and neosquamous epithelium. Histopathology 2012; 61:53–8. [DOI] [PubMed] [Google Scholar]
- 53.Coad RA, Woodman AC, Warner PJ, Barr H, Wright NA, Shepherd NA. On the histogenesis of Barrett’s oesophagus and its associated squamous islands: a three-dimensional study of their morphological relationship with native oesophageal gland ducts. J Pathol 2005; 206:388–94. [DOI] [PubMed] [Google Scholar]
- 54.Paulson TG, Xu L, Sanchez C, Blount PL, Ayub K, Odze RD, et al. Neosquamous epithelium does not typically arise from Barrett’s epithelium. Clin Cancer Res. 2006; 12:1701–6. [DOI] [PubMed] [Google Scholar]
- 55.Nicholson AM, Graham TA, Simpson A, Humphries A, Burch N, Rodriguez-Justo M, et al. Barrett’s metaplasia glands are clonal, contain multiple stem cells and share a common squamous progenitor. Gut 2012; 61:1380–9. [DOI] [PubMed] [Google Scholar]
- 56.Leedham SJ, Preston SL, McDonald SA, Elia G, Bhandari P, Poller D, et al. Individual crypt genetic heterogeneity and the origin of metaplastic glandular epithelium in human Barrett’s oesophagus. Gut 2008; 57:1041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Owen RP, White MJ, Severson DT, et al. Single cell RNA-seq reveals profound transcriptional similarity between Barrett's oesophagus and oesophageal submucosal glands. Nat Commun 2018; 9:4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Braxton DR, Nickleach DC, Liu Y, Farris AB 3rd. Necrotizing sialometaplasia-like change of the esophageal submucosal glands is associated with Barrett’s esophagus. Virchows Arch. 2014;465(2):135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garman KS, Kruger L, Thomas S, Swiderska-Syn M, Moser BK, Diehl AM, et al. Ductal metaplasia in oesophageal submucosal glands is associated with inflammation and oesophageal adenocarcinoma. Histopathology. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gonzalez G, Huang Q, Mashimo H. Characterization of oncocytes in deep esophageal glands. Dis Esophagus. 2016; 29:670–80. [DOI] [PubMed] [Google Scholar]
- 61.Gillen P, Keeling P, Byrne PJ, West AB, Hennessy TP. Experimental columnar metaplasia in the canine oesophagus. Br J Surg. 1988;75(2):113–5. [DOI] [PubMed] [Google Scholar]
- 62.Li H, Walsh TN, O'Dowd G, Gillen P, Byrne PJ, Hennessy TP. Mechanisms of columnar metaplasia and squamous regeneration in experimental Barrett’s esophagus. Surgery. 1994;115(2):176–81. [PubMed] [Google Scholar]
- 63.Van Nieuwenhove Y, Willems G. Gastroesophageal reflux triggers proliferative activity of the submucosal glands in the canine esophagus. Dis Esophagus. 1998;11(2):89–93. [DOI] [PubMed] [Google Scholar]
- 64.Kruger L, Gonzalez LM, Pridgen TA, McCall SJ, von Furstenberg R, Harnden I, et al. Ductular and proliferative response of esophageal submucosal glands in a porcine model of esophageal injury and repair. Am J Physiol Gastrointest Liver Physiol. 2017:ajpgi 00036 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.von Furstenberg R, Li J, Stolarchuk C, Feder R, Campbell A, Kruger L, et al. Porcine Esophageal Submucosal Gland Culture Model Shows Capacity for Proliferation and Differentiation. Cellular and Molecular Gastroenterology and Hepatology. 2017;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jovov B Claudin-18: a dominant tight junction protein in Barrett’s esophagus and likely contributor to its acid resistance. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G1106–G1113. [DOI] [PubMed] [Google Scholar]
- 67.Lavery DL, Nicholson AM, Poulsom R, et al. The stem cell organisation, and the proliferative and gene expression profile of Barrett’s epithelium, replicates pyloric-type gastric glands. Gut 2014;63:1854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quante M, Bhagat G, Abrams JA, et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell 2012;21:36–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fitzgerald RC. Inflammatory gradient in Barrett's oesophagus: implications for disease complications. Gut 2002;51:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dvorakova K, Payne CM, Ramsey L, et al. Increased expression and secretion of interleukin-6 in patients with Barrett's esophagus. Clin Cancer Res 2004;10:2020–8. [DOI] [PubMed] [Google Scholar]
- 71.Gough MD, Ackroyd R, Majeed AW, et al. Prediction of malignant potential in reflux disease: are cytokine polymorphisms important? Am J Gastroenterol 2005;100:1012–8. [DOI] [PubMed] [Google Scholar]
- 72.Lee Y, Urbanska AM, Hayakawa Y, et al. Gastrin stimulates a cholecystokinin-2-receptor-expressing cardia progenitor cell and promotes progression of Barrett's-like esophagus. Oncotarget 2017;8:203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang X, Ouyang H, Yamamoto Y, et al. Residual embryonic cells as precursors of a Barrett’s-like metaplasia. Cell 2011;145:1023–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cabibi D, Fiorentino E, Pantuso G, et al. Keratin 7 expression as an early marker of reflux-related columnar mucosa without intestinal metaplasia in the esophagus. Med Sci Monit 2009;15:CR203–210. [PubMed] [Google Scholar]
- 75.Kong J, Crissey MA, Funakoshi S, et al. Ectopic Cdx2 expression in murine esophagus models an intermediate stage in the emergence of Barrett’s esophagus. PLoS One 2011. ;6:e18280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen X, Qin R, Liu B, et al. Multilayered epithelium in a rat model and human Barrett’s esophagus: similar expression patterns of transcription factors and differentiation markers. BMC Gastroenterol 2008;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shields HM, Rosenberg SJ, Zwas FR, et al. Prospective evaluation of multilayered epithelium in Barrett's esophagus. Am J Gastroenterol 2001;96:3268–73. [DOI] [PubMed] [Google Scholar]
- 78.Glickman JN, Yang A, Shahsafaei A, et al. Expression of p53-related protein p63 in the gastrointestinal tract and in esophageal metaplastic and neoplastic disorders. Hum Pathol 2001;32:1157–65. [DOI] [PubMed] [Google Scholar]
- 79.DiMaio MA, Kwok S, Montgomery KD, et al. Immunohistochemical panel for distinguishing esophageal adenocarcinoma from squamous cell carcinoma: a combination of p63, cytokeratin 5/6, MUC5AC, and anterior gradient homolog 2 allows optimal subtyping. Hum Pathol 2012;43:1799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roman S, Petre A, Thepot A, et al. Downregulation of p63 upon exposure to bile salts and acid in normal and cancer esophageal cells in culture. Am J Physiol Gastrointest Liver Physiol 2007;293:G45–53. [DOI] [PubMed] [Google Scholar]
- 81.Epperly MW, Guo H, Shen H, Niu Y, Zhang X, Jefferson M, Sikora CA, Greenberger JS. Bone marrow origin of cells with capacity for homing and differentiation to esophageal squamous epithelium. Radiat Res 2004; 162:233–40. [DOI] [PubMed] [Google Scholar]
- 82.Sarosi G, Brown G, Jaiswal K, Feagins LA, Lee E, Crook TW, Souza RF, Zou YS, Shay JW, Spechler SJ. Bone marrow progenitor cells contribute to esophageal regeneration and metaplasia in a rat model of Barrett’s esophagus. Dis Esophagus 2008; 21:43–50. [DOI] [PubMed] [Google Scholar]
- 83.Hutchinson L, Stenstrom B, Chen D, Piperdi B, Levey S, Lyle S, Wang TC, Houghton J. Human Barrett’s adenocarcinoma of the esophagus, associated myofibroblasts, and endothelium can arise from bone marrow-derived cells after allogeneic stem cell transplant. Stem Cells Dev 2011; 20:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Terabe F, Aikou S, Aida J, et al. Columnar Metaplasia in Three Types of Surgical Mouse Models of Esophageal Reflux. Cell Mol Gastroenterol Hepatol 2017; 4:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Merlo LM, Kosoff RE, Gardiner KL, et al. An in vitro co-culture model of esophageal cells identifies ascorbic acid as a modulator of cell competition. BMC Cancer 2011; 11:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oberg S, Johansson J, Wenner J, et al. Metaplastic columnar mucosa in the cervical esophagus after esophagectomy. Ann Surg 2002; 235:338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119:1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chandrasoma P Pathophysiology of Barrett's esophagus. Semin Thorac Cardiovasc Surg 1997; 9:270–8. [PubMed] [Google Scholar]
- 89.Spechler SJ. Cardiac Metaplasia: Follow, Treat, or Ignore? Dig Dis Sci 2018; 63:2052–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dunn LJ, Burt AD, Hayes N, Griffin SM. Columnar metaplasia in the esophageal remnant after esophagectomy: A common occurrence and a valuable insight into the development of Barrett esophagus. Ann Surg 2016; 264:1016–1021. [DOI] [PubMed] [Google Scholar]
- 91.Chandrasoma P, Makarewicz K, Wickramasinghe K, Ma Y, Demeester T. A proposal for a new validated histological definition of the gastroesophageal junction. Hum Pathol 2006; 37:40–7. [DOI] [PubMed] [Google Scholar]
- 92.Chandrasoma P, Wijetunge S, Demeester SR, Hagen J, Demeester TR. The histologic squamo-oxyntic gap: an accurate and reproducible diagnostic marker of gastroesophageal reflux disease. Am J Surg Pathol 2010; 34:1574–81. [DOI] [PubMed] [Google Scholar]
- 93.Joseph A, Ackerman D, Talley JD, Johnstone J, Kupersmith J. Manifestations of coronary atherosclerosis in young trauma victims--an autopsy study. J Am Coll Cardiol 1993; 22:459–67. [DOI] [PubMed] [Google Scholar]
- 94.Fletcher J, Wirz A, Young J, Vallance R, McColl KE. Unbuffered highly acidic gastric juice exists at the gastroesophageal junction after a meal. Gastroenterology 2001; 121:775–83. [DOI] [PubMed] [Google Scholar]
- 95.Ellison E, Hassall E, Dimmick JE. Mucin histochemistry of the developing gastroesophageal junction. Pediatr Pathol Lab Med 1996; 16:195–206. [PubMed] [Google Scholar]
- 96.Hahn HP, Blount PL, Ayub K, Das KM, Souza R, Spechler S, Odze RD. Intestinal differentiation in metaplastic, nongoblet columnar epithelium in the esophagus. Am J Surg Pathol 2009; 33:1006–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu W, Hahn H, Odze RD, Goyal RK. Metaplastic esophageal columnar epithelium without goblet cells shows DNA content abnormalities similar to goblet cell-containing epithelium. Am J Gastroenterol 2009; 104:816–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Westerhoff M, Hovan L, Lee C, Hart J. Effects of dropping the requirement for goblet cells from the diagnosis of Barrett’s esophagus. Clin Gastroenterol Hepatol 2012; 10:1232–6. [DOI] [PubMed] [Google Scholar]
- 99.Khandwalla HE, Graham DY, Kramer JR, Ramsey DJ, Duong N, Green LK, El-Serag HB. Barrett's esophagus suspected at endoscopy but no specialized intestinal metaplasia on biopsy, what's next? Am J Gastroenterol 2014; 109:178–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.American Gastroenterological Association, Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011; 140:1084–91. [DOI] [PubMed] [Google Scholar]
- 101.ASGE Standards of Practice Committee, Evans JA, Early DS, Fukami N, Ben-Menachem T, Chandrasekhara V, Chathadi KV, Decker GA, Fanelli RD, Fisher DA, Foley KQ, Hwang JH, Jain R, Jue TL, Khan KM, Lightdale J, Malpas PM, Maple JT, Pasha SF, Saltzman JR, Sharaf RN, Shergill A, Dominitz JA, Cash BD; Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy. The role of endoscopy in Barrett's esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc 2012; 76:1087–94. [DOI] [PubMed] [Google Scholar]
- 102.Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, Trudgill N, Patel P, Kaye PV, Sanders S, O'Donovan M, Bird-Lieberman E, Bhandari P, Jankowski JA, Attwood S, Parsons SL, Loft D, Lagergren J, Moayyedi P, Lyratzopoulos G, de Caestecker J; British Society of Gastroenterology. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut 2014; 63:7–42. [DOI] [PubMed] [Google Scholar]
- 103.Robertson EV, Derakhshan MH, Wirz AA, Lee YY, Seenan JP, Ballantyne SA, Hanvey SL, Kelman AW, Going JJ, McColl KE. Central obesity in asymptomatic volunteers is associated with increased intrasphincteric acid reflux and lengthening of the cardiac mucosa. Gastroenterology 2013; 145:730–9. [DOI] [PubMed] [Google Scholar]
- 104.Derakhshan MH, Robertson EV, Lee YY, Harvey T, Ferrier RK, Wirz AA, Orange C, Ballantyne SA, Hanvey SL, Going JJ, Kelman AW, McColl KE. In healthy volunteers, immunohistochemistry supports squamous to columnar metaplasia as mechanism of expansion of cardia, aggravated by central obesity. Gut 2015; 64:1705–14. [DOI] [PubMed] [Google Scholar]
- 105.Robertson EV, Derakhshan MH, Wirz AA, Mitchell DR, Going JJ, Ballantyne SA, McColl KEL. Hiatus hernia in healthy volunteers is associated with intrasphincteric reflux and cardiac mucosal lengthening without traditional reflux. Gut 2016. [DOI] [PubMed] [Google Scholar]
- 106.Spechler SJ. The columnar lined oesophagus: a riddle wrapped in a mystery inside an enigma. Gut 1997; 41:710–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jung KW, Talley NJ, Romero Y, et al. Epidemiology and Natural History of Intestinal Metaplasia of the Gastroesophageal Junction and Barrett’s Esophagus: A Population-Based Study. Am J Gastroenterol 2011; 106:1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sawas T, Killcoyne S, Iyer PG, Wang KK, Smyrk TC, Kisiel JB, Qin Y, Ahlquist DA, Rustgi AK; OCCAMS Consortium, Costa RJ, Gerstung M, Fitzgerald RC, Katzka DA. Identification of prognostic phenotypes of esophageal adenocarcinoma in two independent cohorts. Gastroenterology. 2018. August 27. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cameron AJ, Lomboy CT. Barrett’s esophagus: age, prevalence, and extent of columnar epithelium. Gastroenterology 1992; 103:1241–1245. [DOI] [PubMed] [Google Scholar]
- 110.Moawad FJ, Young PE, Gaddam S, Vennalaganti P, Thota PN, Vargo J, Cash BD, Falk G, Sampliner RE, Lieberman D, Sharma P. Barrett’s oesophagus length is established at the time of initial endoscopy and does not change over time: results from a large multicentre cohort. Gut 2015; 64:1874–80. [DOI] [PubMed] [Google Scholar]
- 111.Benipal P, Garewal HS, Sampliner RE, Martinez P, Hayden CW, Fass R. Short segment Barrett’s esophagus: relationship of age with extent of intestinal metaplasia. Am J Gastroenterol 2001; 96:3084–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multilayered epithelium with squamoid-appearing cells in the basal layers, and overlying columnar mucus-secreting cells. (Photomicrograph provided by Robert Genta, H&E, 40X)