Abstract
Indocyanine green (ICG) is a clinically-approved near infrared (NIR) dye used for optical imaging. The dye is only slightly soluble in water and is prone to aggregation in saline solutions, so that alternative formulations can improve photophysical performance. Numerous nanoscale formulations of ICG have been described in the literature, but we sought to develop an approach that does not require additional purification steps. Pre-formed liposomes incorporating 45 mol. % of the cationic lipid 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) rapidly bind ICG, resulting in enhanced NIR optical properties. ICG binding is dependent on the amount of DOTAP incorporated in the liposomes. A dye-to-lipid mass ratio of [0.5:25] is sufficient for full complexation, without additional purification steps following mixing. NIR absorption, fluorescence intensity, and photoacoustic signals are increased for the liposome-bound dye. Not only is the optical character enhanced by simple mixing of ICG with liposomes, but retention in 4T1 mammary tumors is observed following intratumor injection, as assessed by fluorescence and photoacoustic imaging. Subsequent photothermal therapy with 808 nm laser irradiation is effective and results in tumor ablation without regrowth for at least 30 days. Thus, ICG optical properties and photothermal ablation outcomes can be improved by mixing the dye with pre-formed DOTAP liposomes in conditions that result in full dye-binding to the liposomes.
Graphical Abstract

simple mixing of ICG with DOTAP liposomes results in full dye binding to the liposomes and enhanced ICG optical properties
Introduction
Indocyanine green (ICG) is an FDA-approved near infrared (NIR) dye that has been applied clinically for optical imaging in surgery and oncology.1, 2 The long absorption wavelength (~800 nm) allows for optical imaging in the NIR window, in which light can penetrate deeper into tissues. ICG has aqueous solubility in the low mg/mL range, which is how the dye is administered clinically. This solubility range is considered to be only slightly soluble. In saline solutions, ICG is especially prone to aggregation, resulting in suboptimal spectral properties.
Given the clinical relevance of ICG, a wide variety of approaches have been developed to formulate the dye with enhanced properties.3 Liposomes have been used in numerous cases to formulate ICG and other cyanine dyes for optical imaging and phototherapy.4–10 In some cases, drugs have been combined with ICG liposomes for monitoring drug delivery.11, 12 ICG has also been used to trigger cargo release from liposomes.13, 14 Many other formulations of ICG have been developed including, for example, those based on metallic nanoparticles15, silica nanoparticles16, 17, polymer nanoparticles18, 19, iron oxide nanoparticles20, nanotubes21, albumin22, and even virus-like particles23. Such formulations have advantageous properties as theranostic nanomaterials which integrate optical imaging and therapy.24, 25
1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) is a synthetic unsaturated cationic lipid which has been incorporated in liposomes in the EndoTAG taxane formulation undergoing clinical trials.26 Cationic DOTAP liposomes have altered properties compared to neutral ones, and generally have short circulation and rapidly bind blood vessels following intravenous administration.27 Notably, DOTAP liposomes have previously been used to encapsulate ICG for assessing lymphatic tracking and biodistribution in immunization studies28 as well as imaging choroidal angiogenesis.29
Contrast-enhanced photothermal therapy (PTT) has been demonstrated for a wide range of materials, beyond those that incorporate ICG. These include inorganic material such as gold nanoparticles30, carbon nanotubes31, graphene32, and many others33. Organic nanomaterials have also been explored extensively for PTT.34–36 PTT is an ablative technique that uses NIR lasers to heat and destroy tumors which have accumulated NIR contrast agents. Intratumoral injection of theranostic probes has potential to be used for precise tumor targeting without high systemic toxicity. An optimized liposomal ICG formulation has been shown to be effective for PTT following intratumor injection.37 This approach was extended to incorporate a chemotherapy agent along with the ICG,38 for chemophototherapy.39 ICG has also been loaded into therapeutic hydrogels for intratumoral injection.40 Liposomes with imaging contrast agents loaded can be used to guide intratumoral injections and plan phototherapy.41
In typical ICG nanoscale formulations, ICG is incorporated early in the procedure and then free ICG is removed by techniques such as dialysis or gel filtration, leaving only the entrapped or bound dye. However, such purification adds some additional complexity to the formulation process. In theory, use of a mixing approach which results in full ICG complexation without additional purification could be a simpler approach. In this study, we show that pre-formed DOTAP liposomes can be mixed with an ICG solution to achieve full dye binding, improved optical properties and enhanced PTT.
Results and Discussion
ICG binds to liposomes that contain DOTAP
A stock solution of ICG was formed at 1 mg/mL in phosphate buffered saline (PBS). When ICG was subsequently diluted in a 1:1 volume ratio with a DOTAP liposome formulation, enhanced NIR absorption was observed compared to the same dilution in PBS (Figure 1A). The cationic liposomes incorporated 45 mol. % DOTAP, 50 mol. % cholesterol and 5 mol. % polyethylene-glycol 2000 distearoyl phosphatidylethanolamine (PEG-DSPE). The nature of the absorption increase is likely due to enhanced solubility of the ICG in the lipophilic environment compared to saline solution, as ICG absorption is known to be enhanced with lipids.42 As shown in Figure 1B, NIR photoacoustic intensity was greater for ICG when mixed with DOTAP liposomes. This was expected, due to the greater NIR absorption in the colloidal solution, which is responsible for conversion of light into sound. Likewise, Figure 1C, shows that the florescence increases when ICG is mixed with DOTAP liposomes. In the saline solution, the ICG likely is partially aggregated with quenched fluorescence so minimal fluorescent emission signal is apparent. Mixing ICG with DOTAP liposomes results in better dye solubilization, leading to higher fluorescence.
Figure 1. ICG binds to DOTAP liposomes for enhanced optical properties:

A) Calculated absorption (absorption multiplied by the dilution factor, which was 100) of 0.5 mg/mL ICG in saline, with or without DOTAP liposomes (25 mg/mL total lipid). B) Photoacoustic signal of ICG with or without mixing with DOTAP liposomes. C) Fluorescence of ICG with increasing amounts of DOTAP liposomes. The volume ratios are shown for mixing 1 mg/mL ICG with 50 mg/mL liposomes. D) Agarose gel electrophoresis of ICG, or ICG mixed with DOTAP liposomes or DSPC liposomes. Liposomes size (E) and polydispersity (F) upon incubation with ICG for indicated times. G) Negative stained transmission electron microscopy of DOTAP liposomes complexed with ICG. A 100 nm scale bar is shown. H) Schematic representation of the enhancement of ICG photophysical properties (shown by green color) upon mixing and binding to DOTAP liposomes.
To confirm the nature of the enhanced optical properties was due to dye binding to the liposomes, an electrophoresis gel shift assay was carried out (Figure 1D). ICG was mixed with liposomes for a final concentration of 0.5 mg/mL ICG and 25 mg/mL liposomes. Free ICG is negatively charged and therefore moved towards the cathode. In contrast, cationic DOTAP liposomes completely bound all the free ICG at that liposome and dye ratio. As a result, no free dye migrated. The third well was loaded with ICG that was mixed with neutral liposomes formed from the saturated lipid distearoylphosphatidylcholine (DSPC). In this case, ICG migrated towards the cathode than similarly to the free dye. Since liposomes migrate slowly in the gel, the lack of dye near the loading well indicates minimal dye bound the DSPC liposomes. The electrophoresis result provides direct proof of ICG binding to DOTAP liposomes. Mixing ICG with DSPC liposomes also did not provide for as strong absorption as DOTAP liposomes (Figure S1 in the Supporting Information). Interestingly, liposomes formed from DOTAP or another unsaturated lipid, dipalmitoylphosphatidylcholine (DOPC), both provided for similarly strong ICG absorption, suggesting that ICG interaction with liposomes may be enhanced by the presence unsaturated lipids. This is in agreement with a prior study that found that compared to DSPC, a DOPC-based formulation, also containing 5 mol. % PEG, was optimal for fluorescence imaging and PTT.37
The size of DOTAP liposomes was assessed 30 and 60 minutes following mixing with ICG to induce dye binding (Figure 1E). No major changes in particle size were induced by ICG binding. Liposome sizes remained close to 100 nm. Likewise, the liposomes did not increase in polydispersity upon ICG binding (Figure 1F). The morphology of the liposomes remained intact after ICG binding (Figure 1G). These data show that ICG binding to DOTAP liposomes does not induce liposome aggregation. A simple schematic of ICG activation is presented in Figure 1H, in which ICG is mixed with DOTAP liposomes, resulting in enhanced optical properties upon dye binding.
ICG binds to liposomes that contain DOTAP
Given that ICG bound to unsaturated DOTAP liposomes, relative to saturated DSPC liposomes, we assessed ICG binding as a function of the amount of DOTAP present. Cholesterol and DSPE-PEG were kept constant at 50% to 5%, respectively, while DOTAP was titrated in place of DSPC. As shown in Figure 2A, as DOTAP content was increased, ICG binding increased, and full ICG binding was observed with 45 mol. % DOTAP. Next, using 45 mol. % DOTAP liposomes, the amount of liposomes was varied to examine the relation with ICG binding (Figure 2B). Full binding of 0.5 mg/mL ICG was observed with 25 mg/mL lipid. Since the lipid formulation also comprised DSPE-PEG, we assessed whether PEG was essential for ICG binding. As shown in Figure 2C, full binding was achieved with or without DOTAP liposome PEGylation.
Figure 2. Characterizing ICG binding to DOTAP liposomes:
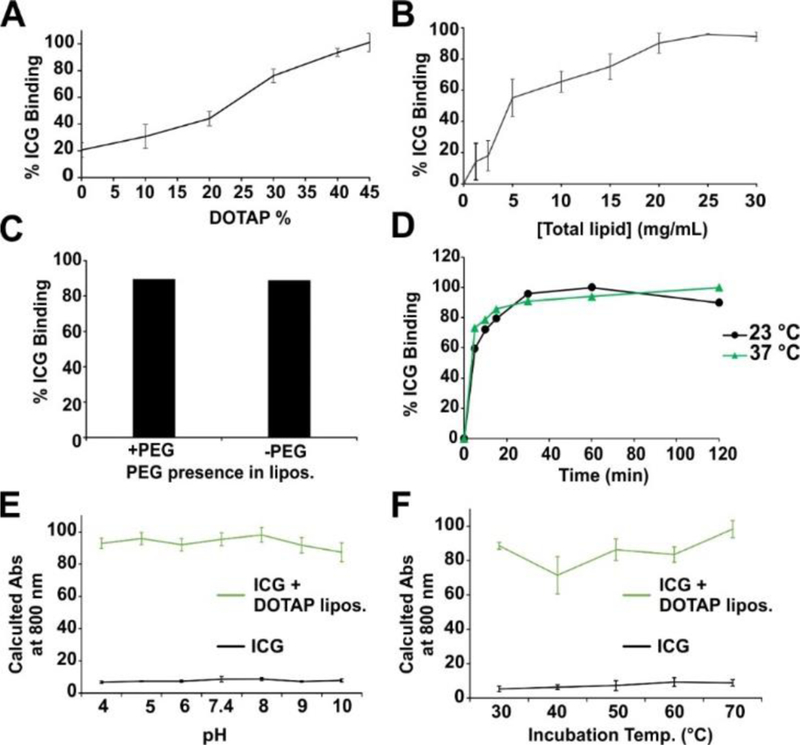
A) ICG binding to liposomes containing variable amounts of DOTAP. B) ICG binding to DOTAP liposomes (45 mol. % DOTAP) with varying liposome concentration. C) Binding of ICG to DOTAP liposomes with or without 5 mol. % PEG-DSPE included. D) Kinetics of ICG binding to DOTAP liposomes at indicated temperatures. Calculated absorption values of ICG at 800 nm with or without DOTAP liposomes following incubation at varying pH (E) or elevated temperatures (F). Error bars reflect mean +/− std. dev. for n=3 samples.
The binding kinetics between ICG and DOTAP liposomes was compared at 23 and 37 °C (Figure 2D). No major differences in binding were observed at these temperatures. Most of the binding was complete in less than 30 minutes. The stability of ICG complexed with DOTAP liposomes was inspected in varying pH and temperature conditions. Based on the increased absorbance at 800 nm compared to the free dye, ICG remained stably bound to DOTAP liposomes in a wide pH range (Figure 2E). Likewise, after ICG or liposome-bound ICG was incubated for 30 minutes at elevated temperatures, no decrease in NIR absorption was observed, showing thermostable binding of the dye to the liposomes.
Optical imaging following intratumoral injection
To visualize the tumor uptake of free ICG compared to ICG mixed with DOTAP liposomes, mice bearing 4T1 orthotopic mammary tumors were used. When the average volume reached approximately 75 mm3, tumors were directly injected with ICG with or without DOTAP liposomes. 24 hours after intratumoral injection, the dye that had been mixed with DOTAP liposomes prior to injection showed superior retention and distribution in the tumors, compared to the free dye (Figure 3A). Several of the 5 mice injected with the free dye had no signal remaining in the tumor 24 hours following injection and the other mice in that group had relatively weak signal. In contrast, all the mice injected with ICG complexed with DOTAP retained a strong fluorescence signal in the tumor. Further research is required to better elucidate the mechanism of enhanced tumor retention of the DOTAP formulation, but it is likely that the larger size of the liposomal ICG may have retarded drainage of the small molecule ICG from the tumor. Furthermore, the cationic character of the liposomes may have led to more interaction with various cell membranes in the tumor, which carry a mild anionic charge. Incubation of ICG with 4T1 cells in vitro revealed that, compared to the free dye, ICG mixed with DOTAP liposomes was uptaken more avidly (Figure S2 in the Supporting Information).
Figure 3. NIR fluorescence and photoacoustic tumor imaging of ICG with or without mixing with DOTAP liposomes.
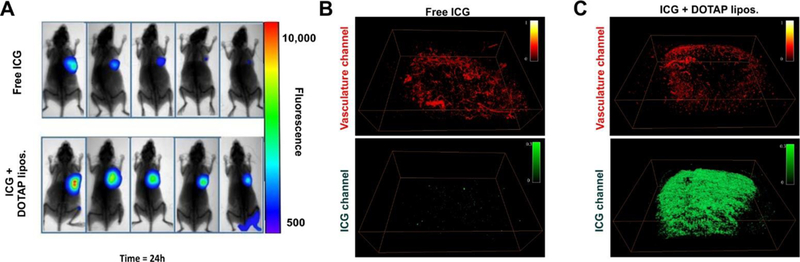
A) Fluorescence imaging of 4T1-bearing mice 24 hours after intratumoral injection of ICG with or without complexing with DOTAP liposomes. Representative photoacoustic image of free ICG (B) or ICG complexed with DOTAP liposomes (C), 24 hours after intratumoral injection. The top image in red shows the tumor blood vessels and the bottom image in green shows ICG distribution in the tumor.
Figure 3B shows a representative photoacoustic tomography image of a tumor 24 hours after injection with free ICG. The blood vessels of the tumor are shown in red (top image) and are based on endogenous absorption from hemoglobin. The ICG signal is shown in green (bottom image). Minimal ICG signal is present in the free ICG-injected mice. Figure 3C shows a representative photoacoustic image of a tumor injected with ICG complexed with DOTAP liposomes. Whereas the tumor blood vasculature appears comparable to the free ICG injection, the photoacoustic contrast image reveals greatly stronger ICG signal. The photoacoustic image reveals that the ICG is relatively well-distributed throughout the tumor volume.
Photothermal therapy
Under 808 nm irradiation, ICG with and without mixing with DOTAP liposomes resulted in production of photothermal heating (Figure 4A). Owing to the higher NIR absorption, liposome-bound ICG was more effective at photothermal transduction. Tumor cells are thermally damaged at temperatures above 40°C and coagulative necrosis and ablation occurs at temperatures above 60 °C with rapid denaturing of proteins and cellular membrane collapse. As seen in Figure 4B, ICG mixed with DOTAP liposomes exposed to 1000 mW/cm2 laser power the temperature plateaued at ~70 °C after a few minutes of irradiation, whereas a lower fluence rate produced less heating.
Figure 4. Photothermal therapy with ICG and DOTAP liposomes.

A) Temperature of ICG solutions under 808 nm laser irradiation. B) Temperature of ICG mixed with DOTAP liposomes at two different laser fluence rates. C) Mice were inoculated with orthotopic 4T1 tumors on day 0, then on day 7 were injected intratumorally with ICG (25 µg). 24 hours later, tumors were treated with an 808 nm laser (0.75 W/cm2, 8 min). n=6–7 mice per group. D) The body weight of mice over the course of treatment E) Survival for indicated groups. Arrow shows the time of the single PTT treatment.
Tumors were injected with ICG and subjected to laser irradiation 24 hours later. Several reports have suggested that photodynamic therapy, along with PTT, contributes to ICG mechanism of action, which is possible in this case.9, 43–45 As shown in Figure 4C, the tumor volumes of three controls had little difference between them: 1) Untreated; 2) Laser alone; and 3) ICG mixed with DOTAP liposomes without laser. The tumor volumes of these groups increased steadily over the study period. The ICG with laser treatment group had a slight impact on tumor growth, as this treatment likely resulted in some minor heating. In contrast to the other groups, ICG with DOTAP liposomes and laser treatment resulted in tumor ablation with a single laser treatment due to the increased photothermal properties of complexed ICG as well as improved dye retention in the tumor. ICG and DOTAP liposome treatment had low systemic toxicity, as seen in Figure 4D, as the mice were able to increase or maintain their body weight. However, additional toxicity studies are warranted. Due to the enhanced photothermal effect, mice phototreated with ICG and DOTAP liposomes displayed 100% survival at 40 days post tumor inoculation (Figure 4E).
Conclusion
In this work, we demonstrated that simple mixing of ICG with DOTAP liposomes can result in full dye binding to the liposomes and enhanced ICG optical properties. Greater NIR fluorescence and photoacoustic signal generation can be used for improved contrast imaging. Tumor retention also appeared superior following intratumoral injection of ICG complexed with DOTAP liposomes. These factors resulted in effective photothermal ablation of orthotopic mammary tumors in mice. Taken together, these results demonstrate a simple route to improving the efficacy of ICG by simply mixing the dye with pre-formed liposomes.
Experimental
Materials
Indocyanine green (ICG) was obtained from Chem-Impex International Inc. Lipids were purchased from CordenPharma and included 2,3-Dioleoyloxy-propyl-trimethyammoniumchloride (DOTAP, # LP-R4-117), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC, # LP-R4-076), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(methoxy(PEG)-2000 (MPEG-2000-DSPE, # LP-R4-039), and 1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC, # LP-R4-070). Cholesterol was purchased from Nu-Chek Prep, Inc. (#CH-800-A28-Z). Other chemicals were from Sigma. The 4T1 cell line (BALB/c mouse breast carcinoma) was kindly provided by Bo Huang (Peking Union Medical College, Beijing, China). Six- to eight-week-old female BALB/c mice were obtained from HBCDC (Wuhan, China).
Liposomes
Lipids were dissolved in chloroform and dispensed in a glass test tube and the chloroform was evaporated by nitrogen gas flow. The molar ratio of Chol:DOTAP:DSPE-PEG was 10:9:1. 1 mL of PBS (150 mM NaCl, 10 mM phosphate buffer) at 60 °C was added and the lipids were sonicated for 30 min in a water bath at 60 °C. DSPC and DOPC liposomes were formed in the same way, by substituting those lipids for DOTAP. The size and polydispersity of DOTAP liposomes was determined by dynamic light scattering in a NanoBrook 90 plus PALS instrument. Electron microscopy was carried out with a JEM-2010 electron microscope with liposomes that were placed on a grid and negative stained with uranyl acetate.
ICG binding to liposomes
A 1 mg/mL ICG solution was mixed with liposomes, to generate a final concentration of 0.5 mg/mL ICG and 25 mg/mL total lipid. This mixture was incubated for 1 hr at 37 °C with shaking. Samples were diluted 100 fold and absorbance was measured with a PerkinElmer Lambda 365 UV-Vis Spectrophotometer. Binding could be quantified by measuring absorption after passing samples through a 0.2 µm nylon syringe filter (Agilent # 5190–5269), which we found adsorbs free ICG. For gel electrophoresis, a 2% agarose gel was used with Tris, acetic acid and EDTA (TAE) buffer. To visualize the fluorescence of ICG the samples were imaged using an IVIS Lumina imager. For pH stability the ICG samples were diluted by 100 fold into phosphate buffer of the indicated pH, incubated for 30 min at room temperature prior to measurement. For temperature stability, the samples were incubated for 30 min at the indicated temperatures, then rapidly diluted 100 fold into a PBS and absorbance measurement. For in vitro uptake of liposomes 4T1 cells (4×105 cells/well) were seeded into a 6-well plate with 2 mL RPMI 1640 medium for 12 h. Then, 5 μL of ICG solution (containing 0.5 mg/mL ICG) with or without DOTAP liposomes was added. Cells were harvested at 6 or 12 h, washed with PBS, and ICG was detected with flow cytometry.
In Vivo Imaging
All animal studies were performed in compliance with protocols approved by the Hubei Provincial Animal Care and Use Committee, following the experimental guidelines of the Animal Experimentation Ethics Committee of the Huazhong University of Science and Technology (HUST, Wuhan, China). 5×105 4T1 cells in 50 μL PBS were inoculated into the right second breast pad of female BALB/c mice. When the average tumor volume reached approximately 75 mm3, mice were allocated into two groups randomly. Mice in the ICG group were intratumorally injected with 50 μL of free ICG solution (containing 0.25 mg/mL ICG), and mice in the ICG-LIP group were intratumorally injected with 50 μL of ICG-LIP (containing 0.25 mg/mL ICG). For the semiquantitative fluorescence analysis of ICG at 0, 6, and 24 h after injection, a Spectral Instruments Imaging Optical Imaging Platform equipped with an excitation filter (750 nm) and emission filter (790 nm) was used to collect ICG fluorescence signals. For photoacoustic imaging, 4T1 murine tumor-bearing female BALB/c mice were intratumorally injected with free ICG solution (50 μL of 0.5 mg/mL ICG) or ICG with DOTAP liposomes (50 μL of 0.5 mg/mL ICG). PAT imaging was carried out 24 h after injection. Mice were scanned using a multispectral optoacoustic tomography (MSOT inVision 128, iThera Medical GmbH, Germany). Photoacoustic signals of blood vessels and ICG were recorded at excitation wavelengths of 532 and 710 nm, respectively.
Photothermal Therapy
In vitro photothermal measurements were carried out with a 1 mL sample volume in microcentrifuge tubes which were exposed to 808 nm laser irradiation. Temperatures were recorded with an infrared FLIR thermal camera. For in vivo studies, female BALB/c mice were inoculated in the mammary fat pad with 4T1 cells and on day 7, when tumor volumes reached approximately 75 mm3, mice were allocated randomly into the following five groups (6–7 mice in each group): (a) Untreated, (b) Laser alone, (c) ICG solution alone (50 μL, containing 0.5 mg/mL ICG), (d) ICG with DOTAP liposomes alone (50 μL, containing 0.5 mg/mL ICG) and (e) ICG with DOTAP liposomes (50 μL, containing 0.5 mg/mL ICG) with laser treatment. The samples were intratumorally injected. 24 h after injection, the tumors of groups (b) and (e) were irradiated with an 808 nm laser (0.75 W/cm2, 8 min). The tumor size was measured using a caliper every other day. The mice were sacrificed when tumor volume exceeded 1000 mm3.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (R01EB017270 and DP5OD017898), the National Science Foundation (1555220), a Brazilian CAPES Science without Borders scholarship (D.M.) and a Fulbright Scholarship (M.T.M.).
Footnotes
Electronic Supplementary Information (ESI) available: [ICG spectra and cellular uptake]. See DOI: 10.1039/x0xx00000x
Conflicts of Interest
The authors declare no competing interests.
References
- 1.Polom K, Murawa D, Rho Y.-s., Nowaczyk P, Hünerbein M and Murawa P, Cancer, 2011, 117, 4812–4822. [DOI] [PubMed] [Google Scholar]
- 2.Marshall MV, Rasmussen JC, Tan IC, Aldrich MB, Adams KE, Wang X, Fife CE, Maus EA, Smith LA and Sevick-Muraca EM, Open Surg. Oncol. J, 2010, 2, 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheng Z, Hu D, Xue M, He M, Gong P and Cai L, Nano-Micro Lett, 2013, 5, 145–150. [Google Scholar]
- 4.Beziere N, Lozano N, Nunes A, Salichs J, Queiros D, Kostarelos K and Ntziachristos V, Biomaterials, 2015, 37, 415–424. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Li N, Liu Y, Ji B, Wang Q, Wang M, Dai K and Gao D, Nanomed. Nanotechnol. Biol. Med, 2016, 12, 2019–2029. [DOI] [PubMed] [Google Scholar]
- 6.Jeong HS, Lee CM, Cheong SJ, Kim EM, Hwang H, Na KS, Lim ST, Sohn MH and Jeong HJ, J. Liposome Res, 2013, 23, 291–297. [DOI] [PubMed] [Google Scholar]
- 7.Proulx ST, Luciani P, Derzsi S, Rinderknecht M, Mumprecht V, Leroux JC and Detmar M, Cancer Res, 2010, 70, 7053–7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mikada M, Sukhbaatar A, Miura Y, Horie S, Sakamoto M, Mori S and Kodama T, Cancer Sci, 2017, 108, 846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan F, Wu H, Liu H, Deng Z, Liu H, Duan W, Liu X and Zheng H, J. Controlled Release, 2016, 224, 217–228. [DOI] [PubMed] [Google Scholar]
- 10.Miranda D, Huang H, Kang H, Zhan Y, Wang D, Zhou Y, Geng J, Kilian HI, Stiles W, Razi A, Ortega J, Xia J, Choi HS and Lovell JF, Theranostics, 2019, 9, 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lozano N, Al-Ahmady ZS, Beziere NS, Ntziachristos V and Kostarelos K, Int. J. Pharm, 2015, 482, 2–10. [DOI] [PubMed] [Google Scholar]
- 12.Xue X, Fang T, Yin L, Jiang J, He Y, Dai Y and Wang D, Drug Deliv, 2018, 25, 1826–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lajunen T, Nurmi R, Wilbie D, Ruoslahti T, Johansson NG, Korhonen O, Rog T, Bunker A, Ruponen M and Urtti A, J. Controlled Release, 2018, 284, 213–223. [DOI] [PubMed] [Google Scholar]
- 14.Lajunen T, Kontturi L-S, Viitala L, Manna M, Cramariuc O, Róg T, Bunker A, Laaksonen T, Viitala T, Murtomäki L and Urtti A, Mol. Pharm, 2016, 13, 2095–2107. [DOI] [PubMed] [Google Scholar]
- 15.Han L, Zhang Y, Chen X-W, Shu Y and Wang J-H, J. Mater. Chem. B, 2016, 4, 105–112. [DOI] [PubMed] [Google Scholar]
- 16.Ferrauto G, Carniato F, Di Gregorio E, Tei L, Botta M and Aime S, Nanoscale, 2017, 9, 99–103. [DOI] [PubMed] [Google Scholar]
- 17.Sharma P, Bengtsson NE, Walter GA, Sohn H-B, Zhou G, Iwakuma N, Zeng H, Grobmyer SR, Scott EW and Moudgil BM, Small, 2012, 8, 2856–2868. [DOI] [PubMed] [Google Scholar]
- 18.Zheng M, Yue C, Ma Y, Gong P, Zhao P, Zheng C, Sheng Z, Zhang P, Wang Z and Cai L, ACS Nano, 2013, 7, 2056–2067. [DOI] [PubMed] [Google Scholar]
- 19.Saxena V, Sadoqi M and Shao J, J. Photochem. Photobiol. B: Biol, 2004, 74, 29–38. [DOI] [PubMed] [Google Scholar]
- 20.Ma Y, Tong S, Bao G, Gao C and Dai Z, Biomaterials, 2013, 34, 7706–7714. [DOI] [PubMed] [Google Scholar]
- 21.Wang G, Zhang F, Tian R, Zhang L, Fu G, Yang L and Zhu L, ACS Appl. Mater. Interfaces, 2016, 8, 5608–5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheng Z, Hu D, Zheng M, Zhao P, Liu H, Gao D, Gong P, Gao G, Zhang P, Ma Y and Cai L, ACS Nano, 2014, 8, 12310–12322. [DOI] [PubMed] [Google Scholar]
- 23.Shan W, Chen R, Zhang Q, Zhao J, Chen B, Zhou X, Ye S, Bi S, Nie L and Ren L, Adv. Mater, 2018, 30, 1707567. [DOI] [PubMed] [Google Scholar]
- 24.Cheng L, Wang C, Feng L, Yang K and Liu Z, Chem. Rev, 2014, 114, 10869–10939. [DOI] [PubMed] [Google Scholar]
- 25.Huang H and Lovell JF, Adv. Funct. Mater, 2017, 27, 1603524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fasol U, Frost A, Büchert M, Arends J, Fiedler U, Scharr D, Scheuenpflug J and Mross K, Ann. Oncol, 2012, 23, 1030–1036. [DOI] [PubMed] [Google Scholar]
- 27.Luo D, Geng J, Li N, Carter KA, Shao S, Atilla-Gokcumen GE and Lovell JF, Mol. Cancer Ther, 2017, 16, 2452–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhuang Y, Ma Y, Wang C, Hai L, Yan C, Zhang Y, Liu F and Cai L, J. Control. Release, 2012, 159, 135–142. [DOI] [PubMed] [Google Scholar]
- 29.Hua J, Gross N, Schulze B, Michaelis U, Bohnenkamp H, Guenzi E, Hansen LL, Martin G and Agostini HT, Mol. Vis, 2012, 18, 1045–1054. [PMC free article] [PubMed] [Google Scholar]
- 30.Huang X and El-Sayed MA, J. Adv. Res, 2010, 1, 13–28. [Google Scholar]
- 31.Kam NWS, O’Connell M, Wisdom JA and Dai H, Proc. Natl. Acad. Sci. U. S. A, 2005, 102, 11600–11605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang K, Zhang S, Zhang G, Sun X, Lee S-T and Liu Z, Nano Lett, 2010, 10, 3318–3323. [DOI] [PubMed] [Google Scholar]
- 33.Bao Z, Liu X, Liu Y, Liu H and Zhao K, Asian J. Pharm. Sci, 2016, 11, 349–364. [Google Scholar]
- 34.Lovell JF, Jin CS, Huynh E, Jin H, Kim C, Rubinstein JL, Chan WCW, Cao W, Wang LV and Zheng G, Nat. Mater, 2011, 10, 324–332. [DOI] [PubMed] [Google Scholar]
- 35.Lyu Y, Fang Y, Miao Q, Zhen X, Ding D and Pu K, ACS Nano, 2016, 10, 4472–4481. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Hong H, Sun B, Carter K, Qin Y, Wei W, Wang D, Jeon M, Geng J, Nickles RJ, Chen G, Prasad PN, Kim C, Xia J, Cai W and Lovell JF, Nanoscale, 2017, 9, 3391–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon H-J, Lee H-S, Lim J-Y and Park J-H, ACS Appl. Mater. Interfaces, 2017, 9, 5683–5691. [DOI] [PubMed] [Google Scholar]
- 38.Yoon H-J, Lee H-S, Jung J-H, Kim HK and Park J-H, ACS Applied Materials & Interfaces, 2018, 10, 6118–6123. [DOI] [PubMed] [Google Scholar]
- 39.Luo D, Carter KA, Miranda D and Lovell JF, Adv. Sci, 2017, 4, 1600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin H, Zhao G, Hu J, Ren Q, Yang K, Wan C, Huang A, Li P, Feng J-P, Chen J and Zou Z, ACS Appl. Mater. Interfaces, 2017, 9, 25755–25766. [DOI] [PubMed] [Google Scholar]
- 41.Miranda D, Carter K, Luo D, Shao S, Geng J, Li C, Chitgupi U, Turowski SG, Li N, Atilla-Gokcumen GE, Spernyak JA and Lovell JF, Adv. Healthc. Mater, 2017, 6, 1700253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kraft JC and Ho RJY, Biochemistry, 2014, 53, 1275–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirata C, Kaneko J, Inagaki Y, Kokudo T, Sato M, Kiritani S, Akamatsu N, Arita J, Sakamoto Y, Hasegawa K and Kokudo N, Sci. Rep, 2017, 7, 13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng K, Hou Z, Deng X, Yang P, Li C and Lin J, Adv. Funct. Mater, 2015, 25, 7280–7290. [Google Scholar]
- 45.Song W, Li Y, Wang Y, Wang D, He D, Chen W, Yin W and Yang W, J. Biomed. Nanotechnol, 2017, 13, 1115–1123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


