Abstract
The thymus is critical for the establishment of the adaptive immune system and the development of a diverse T cell repertoire. T cell development depends upon cell-cell interactions with epithelial cells in the thymus. The thymus is composed of two different types of epithelial cells; cortical and medullary epithelial cells. Both of these express, and critically depend on, the transcription factor Foxn1. Foxn1 is also expressed in the hair follicle, and disruption of Foxn1 function in mice results in severe thymic developmental defects and the hairless (nude) phenotype. Despite its importance, little is known about the direct regulation of Foxn1 expression. In this study, we identify a cis-regulatory element critical for expression of Foxn1 in mouse thymic epithelial cells, but dispensable for expression in hair follicles. Analysis of chromatin accessibility, histone modifications, and sequence conservation identified regions within the first intron of Foxn1 that possessed the characteristics of regulatory elements. Systematic knockout of candidate regions lead us to identify a 1.6kb region that, when deleted, results in a near total disruption of thymus development. Interestingly, Foxn1 expression and function in the hair follicle were unaffected. RNA-FISH showed a near complete loss of Foxn1 mRNA expression in the embryonic thymic bud. Our studies have identified a genomic regulatory element with thymic-specific control of Foxn1 gene expression.
Introduction
The thymus is essential for T cell development. The thymus recruits lymphoid progenitors from the bone marrow that settle within the thymus and give rise to T cell progeny. T cell development requires interactions of T cell precursors with multiple cell types, including dendritic and epithelial cells. Two thymic epithelial cell (TEC) subsets play distinct roles in T cell development (1–6). Interactions with cortical TEC (cTEC) result in positive selection, after which selected T cell precursors migrate to the medulla. Subsequently, high affinity interactions with self-peptide processed and presented by medullary TEC (mTEC) or presented by dendritic cells mediate negative selection or differentiation into regulatory T cells (Tregs) (5, 7). This critical process prevents the development of self-reactive T cells that would result in autoimmune syndromes. Naïve T cells that pass both positive and negative selection emigrate from the thymus into the periphery to protect the host. A fully formed and functional thymus and its TEC compartments are therefore critical to the development of a self-tolerant and diverse T cell repertoire.
Foxn1 was first discovered by a spontaneous mutation in the third exon of Foxn1 resulting in the nude mouse (8, 9). Foxn1 is expressed in the hair follicle and in TEC and, consequently, the nude mouse is hairless and possesses a rudimentary thymus that is non-functional (10, 11). The thymus is endodermally derived and arises from the third pharyngeal pouch. Foxn1 expression initiates as early as E9.5 in the third pharyngeal pouch in the mouse embryo and precedes the differentiation of the thymus epithelium (8). Both TEC types derive from a common bipotent progenitor (12, 13). While these progenitor cells are maintained in the nude mouse, differentiation of these precursor cells into the cTEC and mTEC lineage is blocked (13, 14).
Foxn1 is important postnatally for the maintenance of thymus function. Foxn1 is not only critical for the expansion and differentiation of TEC, but also for inducing and maintaining the expression of genes critical for the development of T cells, including Dll4, Cxcl12, and Ccl25 (15). Declining Foxn1 expression is thought to contribute to thymic involution; the age-related reduction of thymus size and the reduction of naïve T cell output (16–19). Postnatal expression of Foxn1 is critical for the maintenance of TEC, and overexpression in older mice can reverse age-related thymic involution (16, 17, 20, 21). Mutations in the human FOXN1 gene result in similar phenotypes wherein the patient exhibits congenital alopecia and severe combined immunodeficiency syndrome (22). Study of the regulation of Foxn1 expression is therefore important to the identification of disease-related variants in humans, and as a way to further understand the loss of TEC populations with age, leading to the decline of thymus function.
Gene regulation is controlled by both proximal and distal cis-regulatory elements (REs). Active genomic REs can be characterized by histone modifications including acetylated lysine 27 of histone 3 (H3K27ac), methylated lysine 4 of histone 3 (H3K4me1), and chromatin accessibility (23, 24). These elements are also highly conserved (25). Through the examination of chromatin characteristics consistent with active REs, we have identified a highly conserved 1.6kb region of the 14.5kb first intron of Foxn1 that is absolutely critical for Foxn1 expression in TECs. Deletion of this element results in the complete abrogation of thymus development and T cell development. Interestingly, this region is not required for hair morphogenesis and Foxn1 expression in keratinocytes is unaffected. We have therefore identified the first thymus-specific RE essential for expression of Foxn1 in TEC.
Materials and Methods
Mice
Nude mice were purchased from The Jackson Laboratory (Stock No: 000819). gRNAs used to generate the RE knockout mice were designed using the crispr.mit.edu website. The injection strategy to generate each mutant mouse is outlined in Figure S4B. The gRNA targeting sequence (Fig. S4C) was cloned into a plasmid containing the full gRNA backbone and T7 promoter. gRNAs were transcribed using the MEGAshortscript T7 Transcription Kit (ThermoFisher Scientific). Microinjections to generate knockout mice by CRISPR-Cas9 were performed by the Transgenics/Cryopreservation – Laboratory Animal Sciences Program NCI core on a C57BL/6NCr background. The genomic coordinates of each deletion are provided in Figure S4D.
Mice used in flow cytometry experiments were 4–8 weeks old and of either sex. The ages of embryonic mice are specified with E0.5 being noon of the day of the discovered plug. Animal procedures were approved by relevant National Institutes of Health Animal Care and Use Committees.
Tissue preparation
Thymus and spleen were dissected into RPMI (ThermoFisher Scientific) containing 5% NCS (Atlanta Biologicals) and mechanically teased with forceps to acquire a single-cell suspension. Splenocytes were treated with ACK Lysing Buffer (Lonza) for 3 minutes on ice to eliminate red blood cells. When analyzing thymic epithelial cells, single-cell thymic suspensions were enzymatically digested with Liberase TM (63ug/ml; Roche) and DNase I (20ug/ml; Roche) for 40 minutes shaking at 37°C. Following digestion, epithelial cells were enriched by centrifugation in a Percoll (GE) gradient. The cell suspensions were then further processed and stained as described below.
Epidermal cell suspensions were prepared from mouse back skin. Hairs were shaved, and skin samples were placed in PBS on ice. After mechanically removing subcutaneous tissue, samples were floated on 10 ml of 0.15% trypsin and 0.75 mM EDTA (a mixture of 5 ml of 0.25% Trypsin-1mM EDTA and 5 ml of 0.05% Trypsin-0.5mM EDTA) (Gibco) and were incubated at 37°C for 45 minutes. The epidermis was then gently scraped off with forceps into PBS containing 2% FBS. Cell suspensions were further mechanically dissociated with a 50 ml syringe (Covidien) and then filtered through a sterile 100 μm cell strainer (BD). The cells were washed with PBS containing 2% FBS and filtered through a 40 μm cell strainer into a new 50 ml conical tube. Hair follicles in anagen phase penetrate through the dermal layer and extend into the hypodermis and can be macroscopically identified from the hypodermal side of the skin. Growing portions of anagen hair follicles were dissected out with scissors and incubated for 30 minutes in a mixture of 0.15% trypsin, 0.75 mM EDTA and 1 mg/mL of Liberase TM (Roche).
Livers were mechanically dissociated through 70 μm filters and centrifuged at 400 g for 5 minutes. Following aspiration of the supernatant, the pellet was resuspended in 10 ml of 37.5% Percoll and then centrifuged at 800 g for 10 minutes. The supernatant was aspirated and the cells were resuspended in complete RPMI containing 10% FBS.
For the isolation of lung lymphocytes, pairs of lungs were diced with a razor blade and incubated in 2 ml of pre-warmed RPMI containing 0.05% DNase I (Sigma) and 0.2% collagenase IV (Sigma) in a 37°C water bath for 20 minutes. The digested tissue was passed through a 70 μm filter and centrifuged at 400 g for 5 minutes. The cells were resuspended in 5 ml of 37.5% Percoll and centrifuged at 800 g for 10 minutes. The supernatant was aspirated and the cells were resuspended in complete RPMI containing 10% FBS. The cell suspensions were then further processed and stained as described below.
Flow cytometry
Thymocyte and splenocyte cell suspensions were incubated with a mix of purified rat, mouse, and hamster IgG before addition of specific antibodies. Thymocytes and splenocytes were stained and analyzed in FACS buffer (PBS containing 0.1% sodium azide, 1 mM EDTA, and 0.5% BSA). Thymic epithelial cell preps were analyzed in MACS buffer (PBS containing 2 mM EDTA and 0.5% FCS). Antibodies specific for Kit (2B8), CD25 (PC61.5), CD45.2 (104), CD4 (GK1.5), CD8α (53–6.72), TCRβ (H57), Ly51 (6C3), EpCAM (G8.8), CD80 (16–10A1), MHCII (M5/114.15.2), were from eBioscience. Biotinylated UEA-1 (B-1065) was from Vector Labs, and Streptavidin (25–4317-82) was from Invitrogen. Thymocyte lineage cocktail was a mix of the following antibodies from eBioscience: B220 (RA3–6B2), CD19 (1D3), Mac-1(M1/70), Gr-1 (8C5), CD11c (N418), Ter119 (TER119), and NK1.1 (PK136). Splenocyte lineage cocktail was the same as thymocyte but did not include antibodies against CD19 or B220. For intracellular staining, cells were first stained for cell surface molecules, permeabilized using the eBioscience’s transcription factor staining buffer set (cat: 00–5523-00) according to the manufacturer’s instructions and then stained with antibodies specific to Aire (5H12) or FoxP3 (FJK-16s).
Epidermal cell suspensions harvested from telogen skin were stained with antibodies against CD45.2 (104), EpCAM (G8.8), Sca-1 (D7), and CD34 (RAM34). CD45-Sca-1-EpCAM+ cells contain keratinocytes from the isthmus and bulge and excludes those from the interfollicular epidermis and infundibulum. Anagen cell suspensions were sorted based on the same cell-surface phenotype.
Liver and lung cell suspensions were stained with Thy1.2, CD45.2 (104), TCRγδ (GL3), TCRβ (H57), RORγt, mMR1 5-OP-RU tetramer, mCD1d PBS57 tetramer, TCR Vγ4 (UC3–10A6), and TCR Vγ6 (17D1).
Samples were acquired on either a BD LSRFortessa or BD CantoII and analyzed using FlowJo software (Tree Star). Cells were sorted using a BD FACSAria flow cytometer. Absolute cell numbers were calculated using a BD Accuri C6 PLUS flow cytometer.
ATAC-Seq
ATAC-Seq was performed as described previously (26) with minor modifications. Cells were sorted, and 50,000 cells were pelleted (500 g, 4°C) and washed with PBS followed by incubation in 50 μl of cold lysis buffer (10 mM TRIS-HCl, pH 7.4, 10 mM NaCl, 3 mM MgCl2, 0.1% Igepal CA-630) for 15 minutes while centrifuging (500 g, 4°C). Pelleted nuclei were resuspended in transposition reaction mix with Tn5 Transposase (Illumina FC-121–1030) and incubated for 45 minutes with gentle shaking at 37°C. Tagmented DNA was purified using SPRIselect beads (Beckman Coulter B23317) and amplified by PCR. Resulting libraries were size-selected and purified again by SPRIselect beads. Libraries were sequenced by single-read (75 cycles) or paired-end (150 cycles), on the Illumina NextSeq Series System.
Reads were trimmed to remove adapters, sequencing primers or any such unwanted sequences. Trimmed reads were aligned to mm10 blacklisted regions and only reads not aligning to the blacklisted regions were included for further analysis. These reads were aligned to mm10 genome using bowtie2 aligner to generate a BAM alignment file. Duplicates arising due to PCR artifacts were marked and removed using Picardtools package from the Broad Institute. Custom R scripts were run to correct for differences in sequencing depths across samples applying Cyclic Loess normalization. Resulting normalized bed files were used to call peaks using macs2 with ENCODE recommended parameters for ATAC-Seq data. ATAC-Seq data is accessible https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE131595. Transcription factor binding motifs located in peaks indicating open chromatin were annotated using HOMER (27).
qRT-PCR
Tissue was flash frozen and stored at −80°C until RNA was extracted using a Qiagen RNeasy Mini or Micro Extraction kit depending on cell quantity. Reverse transcription was performed using the SuperScript VILO cDNA Synthesis Kit (Invitrogen). qPCR was performed on a StepOnePlus Real-Time PCR System (Applied Biosystems) using Taqman probes designed against Foxn1 (Mm00433948; Applied Biosystems) and Gapdh (Mm99999915; Applied Biosystems). Results were analyzed using the ΔΔcT method.
RNA-Scope
Embryos were dissected at the indicated ages and fixed in freshly prepared 4% PFA for 24–36 hours at room temperature and then processed into paraffin blocks. Embryos were faced until the thymic bud was visible, and then 5 micron sections were cut serially and mounted onto positively charged glass microscope slides. Slides were stained with the RNAScope® Multiplex Fluorescent V2 Assay (ACDBio.) following the manufacturer’s instructions with 30 minute incubations for both the Target Retrieval and Protease Plus digestion steps. Embryo sections were hybridized with either RNAScope 3-plex negative control (320871; ACDBio.), 3-plex positive-Mm control probe (320881; ACDBio), or Foxn1-Mm probes (482021-C2; ACDBio.). Slide images were acquired at a 20X magnification using an Aperio FL scanner.
Thymus section analysis
Thymus tissues were fixed with 4% (g/vol) PFA and embedded in OCT compound (Sakura Finetek). Frozen thymuses were sliced into 10 micron thick sections and stained with eFluor660-conjugated anti-Aire antibody (Invitrogen, clone 5H12) and rabbit antibody specific for beta5t (28) followed by AlexaFluor488-conjugated anti-rabbit IgG antibody (Invitrogen). Sections were also stained for the reactivity with UEA-1 (Vector Laboratories). Images were visualized and analyzed with an ECLIPSE Ti2 (Nikon) confocal laser scanning microscope.
Statistics
Statistical significance was performed with GraphPad Prism. Differences between groups of mice were determined by one-way ANOVA with multiple comparisons.
Results
The first intron of Foxn1 exhibits the characteristics of active REs
Promoter elements of Foxn1 have been used previously to generate several transgenic animals (29–31). One transgene includes the 28kb region of DNA from the Slc13a2 gene located 5’ of the Foxn1 promoter to the second exon of Foxn1 (Fig. 1A, gray bar)(29). By reporter analysis, this transgene faithfully mimics endogenous Foxn1 expression in TEC (29), indicating that regulatory elements directing Foxn1 expression in TEC are likely to be located within this 28kb region.
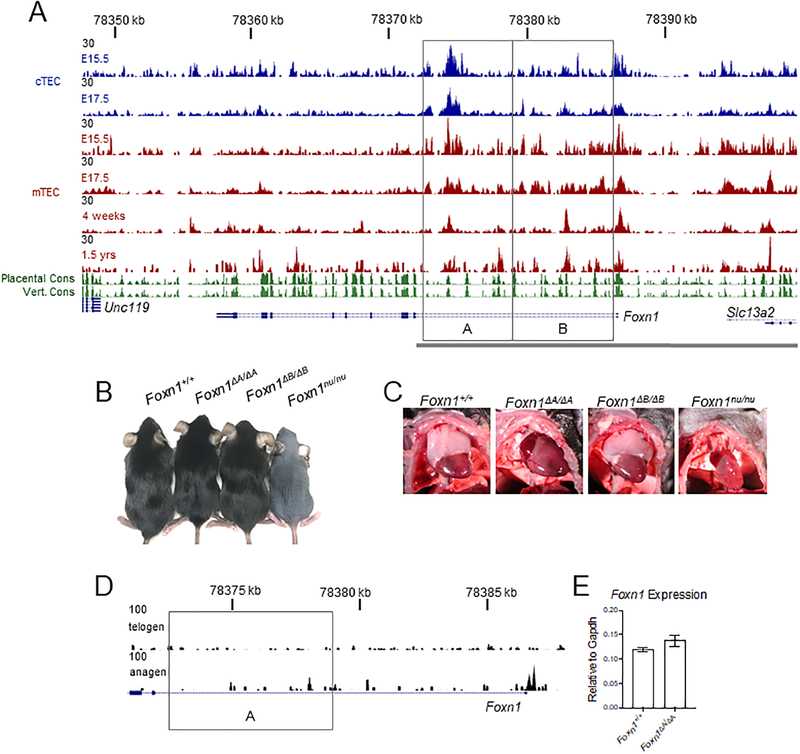
Figure 1.
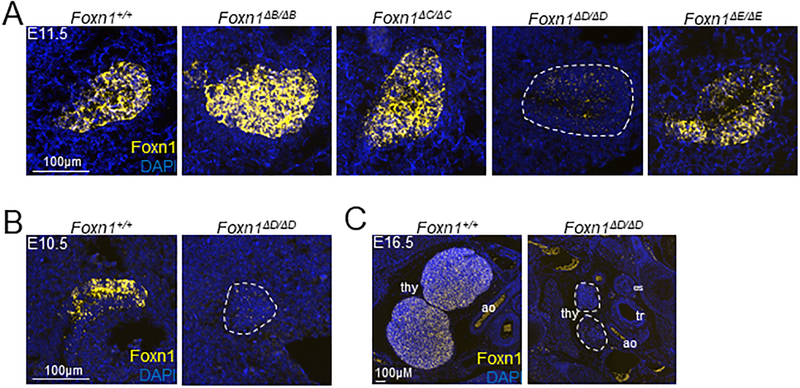
TEC specific chromatin accessibility identifies a functional regulatory element. (A) 50,000 cTEC (CD45-EpCAM+Ly51+UEA-1-) and mTEC (CD45-EpCAM+Ly51-UEA-1+) were sorted for ATAC-Seq at the indicated ages. Conservation (PhastCons) tracks of placental and vertebrate animals are shown from the UCSC Genome Browser. Knockouts of the first intron were done by CRISPR-Cas9. The resulting knockouts are indicated by the gray boxes and regions are identified as A and B. Mice resulting from the deletion of the A or B regions were designated Foxn1ΔA and Foxn1ΔB respectively. The gray bar represents the 28kb of DNA used to drive reporter expression in TECs in a previously described transgenic mouse (29). (B) Gross fur coat morphology of intron knockouts aged 6–8 weeks and Foxn1nu/nu mice. (C) Images of the thymus of intron knockout mice and the Foxn1nu/nu mouse. (D) 50,000 cells from the hair follicle (CD45-Sca-1-EpCAM+) were sorted for ATAC-Seq. The gray box indicates the deleted region in the Foxn1ΔA/ΔA mouse. (E) Foxn1 expression in sorted hair follicle cells in anagen (4–5wk old mice) was measured by qRT-PCR. The bar plot represents mean ± SEM for n = 3–4 mice per genotype. ANOVA was performed to determine statistical significance.
We assessed chromatin accessibility, an indicator of active REs, using assay for transposase-accessible chromatin by sequencing (ATAC-Seq)(26). Standard ATAC-Seq protocols require only 50,000 cells making this a feasible method of testing chromatin characteristics in both cTEC and mTEC. We sorted TEC identified as EpCAM+CD45- and acquired a sufficient number of both cTEC (Ly51+UEA-1-) and mTEC (Ly51-UEA-1+) from embryonic stages (Fig. S1A). cTEC are difficult to isolate at post-natal stages and therefore only mTEC were used for ATAC-Seq at later stages (Fig. 1A)(32). We found that regions of accessible chromatin are present throughout the first intron of Foxn1 (Fig. 1A). The 3’ side of the intron (box “A”) shows a large peak, indicating accessible chromatin, in embryonic stages of both cTEC and mTEC (Fig. 1A). The 5’ side of the intron (box “B”) contains a peak indicating accessible chromatin in mTEC (Fig. 1A).
We next compared results from ATAC-Seq to conserved elements identified by the PhastCons algorithm on the UCSC Genome Browser. Two conservation tracks (green) represent conservation across placental animals and vertebrates with peaks in the track indicating high levels of conservation (Fig. 1A). Each peak of accessible chromatin in the first intron of Foxn1 corresponds to PhastCons elements further suggesting that critical REs are located in this region (Fig. 1A).
In addition, we examined previously published ChIP-Seq data for histone modifications generated from mTEC collected from 4–6-week-old mice (33). We examined H3K4me1 (enriched at poised and active enhancers), H3K27ac (enriched at active enhancers), and H3K27me3 (linked to inactive gene expression) (24, 34). We found the first intron of Foxn1 to be enriched for H3K4me1 and H3K27ac and devoid of signal for H3K27me3 in TECs (Fig. S2A). This epigenetic profile is indicative of active regulatory elements in the first intron of Foxn1.
CTCF binds to DNA and helps to form topologically associated domains (TADs). These TADs are characterized by enhanced interactions between loci within a TAD and help to facilitate enhancer-promoter interactions (35). CTCF ChIP-Seq data available from ENCODE/LICR through the UCSC Genome Browser shows consistent CTCF binding across many cell types at the Foxn1 locus (Fig. S2B). Specifically, CTCF binding is located at the 5’ end of the Foxn1 gene and on the last exon of Slc13a2, the gene immediately 5’ of Foxn1 (Fig. S2B). This suggests that REs critical for Foxn1 expression are located in close proximity to the Foxn1 promoter.
Knockout of regions of the first intron of Foxn1 severely disrupt thymus development, but cause no defects in the skin
The previous observations together strongly suggested the presence of active REs within the first intron of Foxn1. This informed the generation of knockout animals by CRISPR/Cas9 in which each half of the first intron (shown as boxes labeled A and B) was deleted separately (Fig. 1A). The resulting homozygous knockout animals were designated Foxn1ΔA/ΔA or Foxn1ΔB/ΔB based on the region deleted (Fig. 1A). While total inactivation of Foxn1 results in hairlessness exhibited by Foxn1nu/nu mice, both of the intron knockout mutants had a normal fur coat (Fig. 1B). Examination of the thymus, however, showed a striking phenotype as Foxn1ΔA/ΔA mice contained no apparent thymus, and appeared indistinguishable from Foxn1nu/nu mice in which Foxn1 function is totally disrupted (Fig. 1C). Unlike the Foxn1ΔA/ΔA mouse, the Foxn1ΔB/ΔB mouse had no gross anatomical thymic defect (Fig. 1C).
Due to the apparent TEC specific phenotype of this knockout, we were curious if the Foxn1 expressing cells of the hair follicles exhibited a chromatin accessibility landscape similar to TEC. Foxn1 is expressed in the anagen (growth phase) portion of hair follicles and is not expressed in telogen (resting phase) hair follicles (36). We sorted CD45-Sca-1-EpCAM+ cells from the epidermis which contains the upper portion (isthmus) and the bulge region from telogen hair follicles (37), as well as from dissected anagen hair follicles, and examined chromatin accessibility by ATAC-Seq (Fig. 1D, Fig. S1B). Telogen hair follicle keratinocytes exhibited inaccessible chromatin in the first intron and promoter of Foxn1 while anagen hair follicle keratinocytes showed accessible chromatin at the promoter; consistent with active gene expression (Fig. 1D). We found that unlike TEC, the first intron of Foxn1 in hair follicle keratinocytes is not accessible (Fig. 1D). These data suggest that REs critical for Foxn1 expression in hair follicle keratinocytes are not located in the first intron of Foxn1.
We also examined Foxn1 expression by qRT-PCR with RNA extracted from sorted keratinocytes from the anagen portion of hair follicles. Consistent with normal hair growth, Foxn1 expression was unchanged in Foxn1ΔA/ΔA mice compared to Foxn1+/+ (Fig. 1E). These data show TEC specific regions of accessible chromatin in the first intron of Foxn1 that are critically important for the expression of Foxn1 in the thymus, but unnecessary for Foxn1 expression in the skin.
Foxn1ΔA/ΔA mice lack thymic function and do not generate T cells
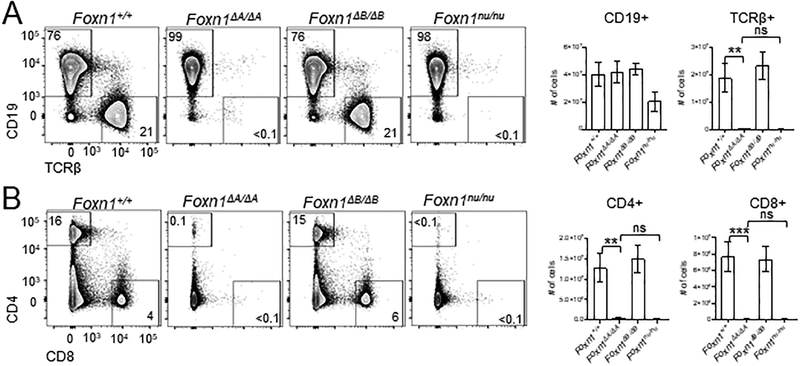
Foxn1nu/nu mice have an almost complete absence of peripheral T cells due to a maturational arrest of TEC development that renders the thymus unable to support T cell development (38). As a readout to determine if any thymic function remains in Foxn1ΔA/ΔA mice and to assess for potential functional thymic defects in Foxn1ΔB/ΔB mice, we examined peripheral T cell populations in the spleen (Fig. 2A and B). We assessed the abundance of T cells in the periphery by staining splenocytes with antibodies against TCRβ, CD4, and CD8 followed by flow cytometric analysis. In addition, we stained splenocytes for CD19 to identify extrathymic derived B cells as a control population that should be present in mice lacking a functional thymus. As predicted, numbers of CD19+ B cells in all mutants were not significantly different compared to Foxn1+/+ controls (Fig. 2A). Foxn1ΔA/ΔA mice had almost no TCRβ+ T cells compared to Foxn1+/+ mice (Fig. 2A). The loss of TCRβ+ cells in the Foxn1ΔA/ΔA mouse was as complete as that of the Foxn1nu/nu mouse by overall cell numbers (Fig. 2A). There was no change in the overall number of TCRβ+ cells in Foxn1ΔB/ΔB mice (Fig. 2A). Single-positive CD4+ (SP4) and CD8+ (SP8) cell proportions and cell numbers were normal in Foxn1ΔB/ΔB mice whereas SP4 and SP8 cells were nearly absent from Foxn1ΔA/ΔA mice (Fig. 2B).
Figure 2.
Foxn1ΔA/ΔA mice lack T cells. Flow cytometry was used to analyze T cell populations in the spleen as a readout of thymus function. (A) Representative flow cytometry plots of Lin- splenocytes comparing intron knockout mice to Foxn1nu/nu mice. Numbers show the percentage of cells in each gate. Total numbers of CD19+ cells and TCRβ+ cells per spleen calculated from DAPI- live cells. (B) Representative flow cytometry plots showing DAPI-Lin- CD4+ and CD8+ populations in the spleen. Total number of CD4+ and CD8+ cells per spleen was calculated from DAPI- live cells. All bar plots show mean ± SEM for n = 4–7 mice (5–8weeks old) per genotype. ANOVA was performed to determine statistical significance. ** p < 0.01, *** p < 0.001.
Foxn1ΔB/ΔB mice have mild TEC defects, but have normal T cell populations
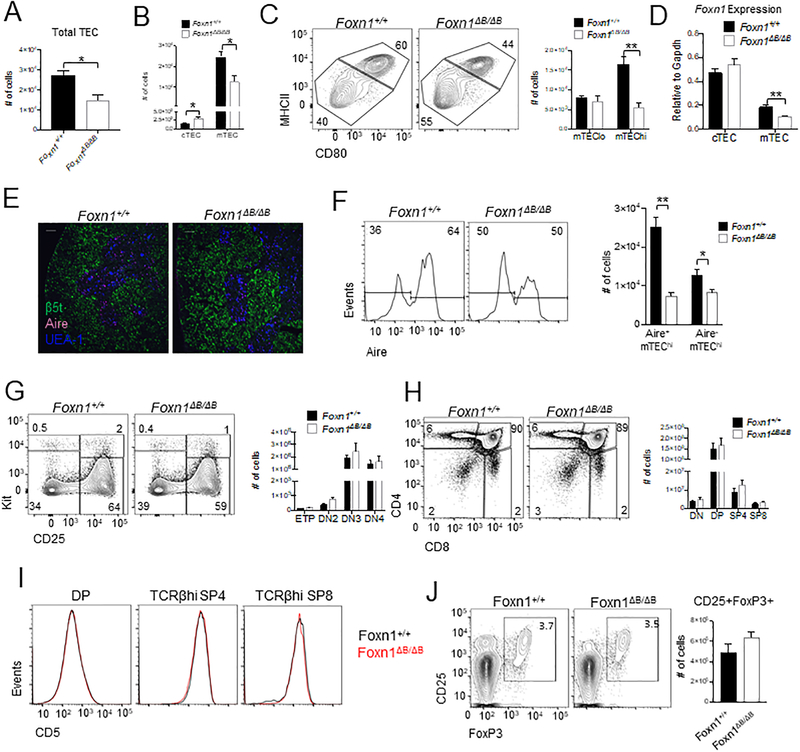
There were no obvious gross anatomical thymus defects or changes in T cells in the periphery; however, we wanted to further examine the potential for TEC specific defects in Foxn1ΔB/ΔB mice. We found that Foxn1ΔB/ΔB mice had reduced overall TEC numbers (Fig. 3A). This reduction was specific to the mTEC population, with the number of cTEC in the Foxn1ΔB/ΔB mouse displaying a slight increase (Fig. 3B). We further examined the mTEC population of the Foxn1ΔB/ΔB mouse and found fewer MHCII+CD80+ (mTEChi) vs MHCIIloCD80- (mTEClo) cells (Fig. 3C). This skewing in the frequency of mTEChi vs lo populations was due to a significant reduction in the numbers of mTEChi in the Foxn1ΔB/ΔB thymus, whilst mTEClo numbers remained unchanged compared to Foxn1+/+ mice (Fig. 3C). We next examined Foxn1 expression by qRT-PCR in sorted cTEC and mTEC from 6–8-week-old mice. Foxn1 expression was unchanged in the cTEC of Foxn1ΔB/ΔB mice (Fig. 3D). Consistent with a loss of mTEC numbers, Foxn1 expression was significantly lower in mTEC (Fig. 3D). This specific loss of MHCII+CD80+ mTEChi cells is consistent with previous reports of a hypomorphic phenotype resulting from reduced Foxn1 expression in TEC (15).
Figure 3.
Foxn1ΔB/ΔB mice have mild mTEC defects but no apparent defect in T cell populations or cell numbers. Absolute numbers of total TEC (A) and cTEC and mTEC (B). (C) Representative flow plots of CD45-EpCAM+Ly51-UEA-1+ gated mTECs showing MHCIIhiCD80+ mTEChi vs MHCIIloCD80- mTEClo and calculations of overall cell numbers. (D) EpCAM+CD45- cTEC (Ly51+UEA-1-) and mTEC (Ly51-UEA-1-) were sorted and Foxn1 expression was measured by qRT-PCR. (E) Immunofluorescence analysis of thymic sections from indicated mice for β5t (green), Aire (magenta), and UEA-1 (blue). Scale bars indicate 100 μm. (F) Flow cytometry analysis of Aire expression in mTEChi (EpCAM+CD45-UEA-1+Ly51-MHCIIhiCD80+). (G) Lin-TCRβ-CD4-CD8- gated representative flow cytometry plots and overall cell numbers for ETPs (KithiCD25-), DN2 (KithiCD25+), DN3 (Kit-CD25+), and DN4 (Kit-CD25-). (H) Lin- gated thymocytes showing CD4 and CD8 profiles and overall cell numbers for each population. (I) Flow cytometry of thymocytes showing CD5 expression on DAPI- DP, TCRβhi SP4 and TCRβhi SP8 cells (J) Flow cytometry analysis and quantification of Tregs (CD4+CD8-TCRβ+CD25+FoxP3+) in the thymus. All bar plots show mean ± SEM for n = 4–7 mice (6–8 weeks old) per genotype. ANOVA was performed to determine statistical significance. *p < 0.05, **p < 0.01.
To further investigate the loss of mTEC in Foxn1ΔB/ΔB mice we examined overall thymic architecture by immunofluorescent staining of cTEC by β5t and mTEC by UEA-l (Fig. 3E). Thymic architecture appeared normal, with well-defined boundaries between the cortex and medulla (Fig. 3E). As the Foxn1ΔB/ΔB thymus had a specific loss of mTEChi cells, which contain the Aire expressing mTEC (5), we examined if the Foxn1ΔB/ΔB mice had altered Aire expression. Confocal microscopy confirmed the presence and normal localization of Aire+ cells to medullary regions (Fig. 3E). Flow cytometric analysis revealed a significant reduction in the frequency and number of Aire+ cells within the already reduced mTEChi mTEC population in Foxn1ΔB/ΔB mice (Fig. 3F).
We then examined if this significant loss of Aire+ mTEChi cells resulted in disruptions in T cell development in the thymus of Foxn1ΔB/ΔB mice. T cell precursors enter the thymus and begin to differentiate in the cortex. CD4-CD8- double-negative (DN) early thymic precursors (ETPs) progress through four stages of development before becoming CD4+CD8+ double-positive (DP) and migrating to the medulla. ETP (defined as Kit+CD25-CD4-CD8-TCRβ- lineage- (Lin-) whereby Lin- is the absence of TER119, CD11c, CD11b, CD19, B220, Gr1, Nk1.1, and Mac1), DN2 (Lin- TCRβ-Kit+CD25+), DN3 (Lin- TCRβ-Kit-CD25+), and DN4 (Lin- TCRβ-Kit-CD25-) cells showed no change in overall proportions or absolute cell number (Fig. 3G). We then examined the CD4 versus CD8 cell profile and found normal frequencies and numbers of DN, DP, SP4 and SP8 cells (Fig. 3H). We also examined CD5 expression on DP, SP4 and SP8 cells to assess TCR signaling intensity and found no difference between Foxn1ΔB/ΔB mice and Foxn1+/+ mice (Fig. 3I)(39). Due to the loss of Aire+ mTEChi and the potential for autoimmune disease, we examined thymic Tregs. Foxn1ΔB/ΔB mice had normal populations of CD25+FoxP3+ Tregs by percentage and overall cell number (Fig. 3J). Thus, despite a significant reduction in the number of Aire+ mTEChi cells, we found no changes in the overall proportions or numbers of developing T cells, including Tregs, in the thymus; and no changes in the number of T cells in the periphery of Foxn1ΔB/ΔB mice.
A ~1.6kb region within the Foxn1A region is critical for thymus development
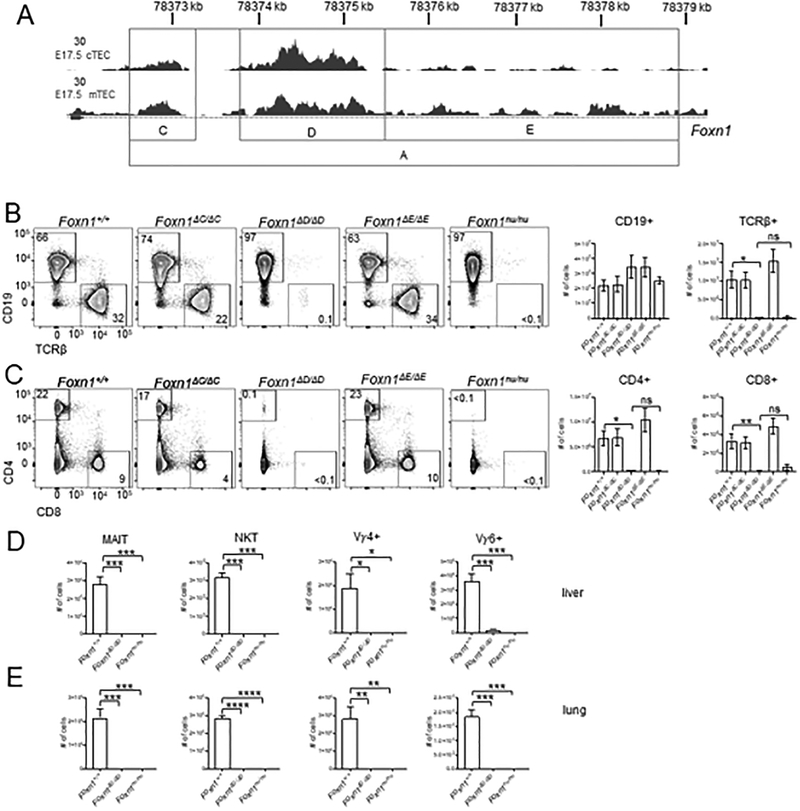
Our initial knockouts of the first intron of Foxn1 removed very broad regions of DNA. Within the Foxn1A region, which is absolutely critical for thymus development, we identified multiple individual elements of accessible chromatin (Fig. 4A). We generated a new cohort of knockout mice based on the ATAC-Seq data in an effort to identify a specific region critical for Foxn1 expression in TEC. Each element of accessible chromatin within the Foxn1A region was removed individually and we designated these Foxn1ΔC, Foxn1ΔD, and Foxn1ΔE (Fig. 4A). Consistent with the Foxn1ΔA/ΔA mouse, all of these new mutant animals had normal fur coats (Fig. S3A). While the Foxn1ΔC/ΔC and Foxn1ΔE/ΔE mutants had a gross anatomically normal thymus, the Foxn1ΔD/ΔD mouse lacked a visible thymus (Fig. S3B).
Figure 4.
Foxn1ΔD/ΔD mice have severely reduced T cells in the periphery. (A) gRNAs were designed to target potential regulatory elements within the A region of the first intron identified by ATAC-Seq. ATAC-Seq data shown is of E17.5 cTEC and mTEC. The gray boxes indicate regions that were deleted within the A region. The resulting mice were designated Foxn1ΔC, Foxn1ΔD, and Foxn1ΔE. (B) Representative flow cytometry plots showing Lin- gated CD19+ B cells and TCRβ+ T cells from the spleen. Total cell numbers were calculated from all DAPI- live cells per spleen. (C) Flow cytometric analysis of DAPI-Lin- CD4+ and CD8+ cells in the spleen. Total cell numbers were calculated from DAPI- live cells. Absolute cell numbers in the liver (D) and lung (E) of MAIT cells (CD45+Thy1.2+TCRγδ-TCRβ+mMR1 5-OP-RU tetramer+), NKT (CD45+Thy1.2+ TCRγδ-TCRβ+mCD1dPBS57 tetramer+), and γδ T cell populations (CD45+Thy1.2+ TCRγδ+ROR γt+) Vγ4+ and Vγ6+ (CD45+Thy1.2+ TCRγδ+ROR γt+ Vγ4-). All absolute cell counts are shown as mean ± SEM for n = 3–8 mice (4–8 weeks old) per genotype. ANOVA was performed to determine statistical significance. *p < 0.05, **p < 0.01, *** p < 0.001, **** p < 0.0001.
We next wanted to further characterize the Foxn1ΔD/ΔD mouse to determine if disrupted thymus development and corresponding T cell defects were as severe as the Foxn1nu/nu mouse. Similar to Foxn1nu/nu and Foxn1ΔA/ΔA mice, Foxn1ΔD/ΔD mice contained almost no TCRβ+ T cells in the spleen (Fig. 4B). Quantification of absolute numbers of TCRβ+ cells showed no difference between the Foxn1nu/nu and Foxn1ΔD/ΔD mice, whereas overall numbers of CD19+ B cells were unchanged for all genotypes (Fig. 4B). The Foxn1ΔC/ΔC and Foxn1ΔE/ΔE mice were indistinguishable from Foxn1+/+ mice and had normal populations of TCRβ+ cells in the spleen (Fig. 4B). Foxn1ΔC/ΔC and Foxn1ΔE/ΔE mice contained normal profiles and absolute numbers of TCRβ+ SP4 and SP8 cells compared to Foxn1+/+ mice (Fig. 4C). Foxn1ΔD/ΔD mice had a near complete loss of SP4 and SP8 cells similar to Foxn1nu/nu mice (Fig. 4C).
Non-conventional T cell subsets are also dependent on the thymus for development (40–42) and we therefore examined these populations as further confirmation of loss of thymus function. Indeed, MAIT, NKT, Vγ4+, and Vγ6+ cells were absent in both the liver (Fig. 4D) and the lung (Fig. 4E) of Foxn1ΔD/ΔD mice. Therefore, the elements most critical for thymus development and subsequently T cell development in the Foxn1A region are located within the 1.6kb Foxn1D region.
In contrast to Foxn1nu/nu mice, Foxn1ΔD/ΔD mice have healthy body weights (Fig. S3C). Foxn1ΔD/ΔD mice are also born in normal Mendelian ratios (Fig. S3D). Overall, Foxn1ΔD/ΔD mice are more robust and easier to maintain than Foxn1nu/nu mice while still exhibiting thymic defects and T cell developmental defects as extreme as Foxn1nu/nu mice.
Foxn1ΔC/ΔC and Foxn1ΔE/ΔE mice do not have TEC or T cells defects
Because of the severity of the Foxn1ΔA/ΔA mutation and the potential for mild TEC specific phenotypes such as in the Foxn1ΔB/ΔB mice, we further investigated the potential thymus phenotypes of the Foxn1ΔC/ΔC and Foxn1ΔE/ΔE mice. Foxn1ΔC/ΔC and Foxn1ΔE/ΔE mice had normal overall TEC numbers (Fig. S3E) and showed no difference in cTEC or mTEC numbers (Fig. S3F). Foxn1ΔC/ΔC and Foxn1ΔE/ΔE mice also had a normal profile of MHCIIloCD80- mTEClo and MCHIIhiCD80+ mTEChi cells and normal numbers of mTEChi and lo cells compared to Foxn1+/+ mice (Fig. S3G).
To assess potential functional phenotypes, we examined T cell development in the thymus of the Foxn1ΔC/ΔC and Foxn1ΔE/ΔE mutants (Fig. S3H and S3I). There was no alteration of any DN stage cell population or overall numbers of each of the DN subtypes (Fig. S3H). Examination of total DN, and the more mature DP, SP4, and SP8 subsets showed that frequencies were the same as Foxn1+/+ thymocytes and absolute cell numbers were also unchanged (Fig. S3I). These data show that the thymus phenotype resulting in the Foxn1ΔA/ΔA mice can be entirely attributed to the Foxn1D region of the first intron.
Foxn1 expression is dramatically decreased in the Foxn1ΔD/ΔD embryonic thymus
Foxn1 expression can be detected by RT-PCR as early as E9.5 in the third pharyngeal pouch of the mouse embryo (8). We used a fluorescent in situ hybridization (FISH) method called RNAScope (ACDBio) to examine Foxn1 expression in the developing embryo of Foxn1+/+ control and intron knockout mice. We examined each mutant mouse at E11.5 and found fluorescent signal from RNA probes designed against Foxn1 mRNA was readily detected in the Foxn1+/+ control, Foxn1ΔB/ΔB, Foxn1ΔC/ΔC, and Foxn1ΔE/ΔE animals, whereas expression in the Foxn1ΔD/ΔD mutant embryo was almost completely absent (Fig. 5A). We also examined Foxn1+/+ control and Foxn1ΔD/ΔD embryos at E10.5, soon after Foxn1 is first expressed. Foxn1 expression in the Foxn1+/+ embryo was apparent throughout the epithelium but Foxn1 expression was absent in the Foxn1ΔD/ΔD thymic bud (Fig. 5B). At E16.5, Foxn1 expression was detected throughout both lobes of the thymus in the Foxn1+/+ embryo; however, no Foxn1 expression was detected in the Foxn1ΔD/ΔD embryonic thymus (Fig. 5C). The thymic buds were present but are greatly reduced in size (Fig. 5C). Overall, we detected little to no expression of Foxn1 in the Foxn1ΔD/ΔD embryonic thymus and thymus development was severely disrupted by E16.5 in the Foxn1ΔD/ΔD mutant embryo.
Figure 5.
Foxn1 expression is absent in the Foxn1ΔD/ΔD embryonic thymus. Foxn1 mRNA was visualized using fluorescent in situ hybridization. (A) Sagittal sections were taken of E11.5 embryos for each genotype. (B) Sagittal sections were taken of E10.5 control and Foxn1ΔD/ΔD embryos. (C) Transverse sections were taken of E16.5 embryos. ao = aorta, tr = trachea, es = esophagus. White dotted outline indicates the thymus in Foxn1ΔD/ΔD mice.
The Foxn1 intronic RE is specific to Foxn1 and functions in cis
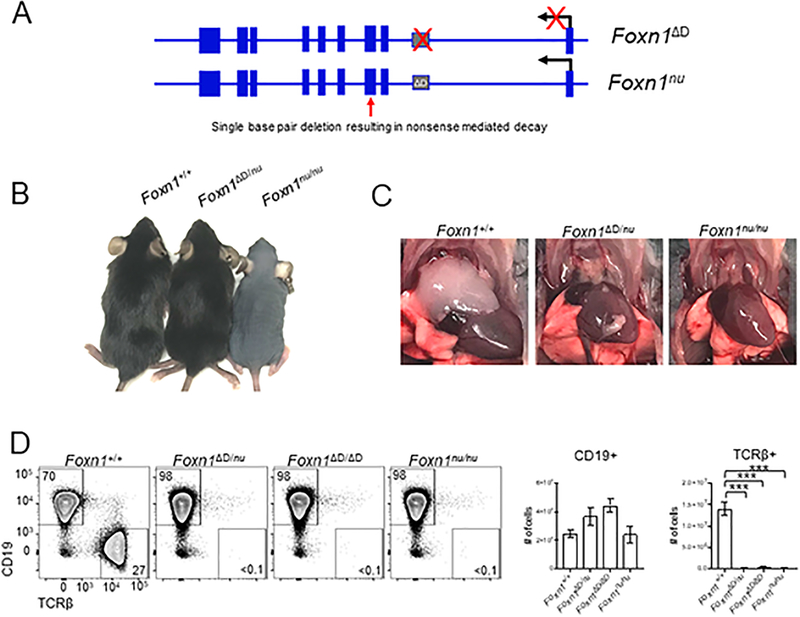
To determine whether the intronic regulatory element functions in cis or trans we bred the Foxn1ΔD/ΔD mouse to the Foxn1nu/nu. The Foxn1nu/nu mutation is a single base pair substitution in the third exon that results in a nonsense mutation (Fig. 6A)(8). The first intron of the Foxn1nu/nu mouse is intact and the exons of the Foxn1ΔD/ΔD mouse are normal (Fig. 6A). If the RE functions in trans, the RE from the nude allele should be able to promote the expression of the Foxn1 gene from the Foxn1ΔD allele in the thymus. Foxn1ΔD/nu mice have normal fur growth (Fig. 6B) but lack a normal thymus (Fig. 6C). We assessed peripheral T cell numbers by flow cytometry and found almost no TCRβ+ cells in the spleen of Foxn1ΔD/nu mouse and no difference in cell number between the Foxn1ΔD/nu, Foxn1ΔD/ΔD, and Foxn1nu/nu mice (Fig. 6D). CD19+ B cell numbers were unchanged in all mutants (Fig. 6D).
Figure 6.
The Foxn1D RE is specific to Foxn1 and functions in cis. (A) Comparison of the mutations of the Foxn1 gene in Foxn1ΔD/ΔD and Foxn1nu/nu mice. (B) Images of Foxn1+/+, Foxn1ΔD/nu, and Foxn1nu/nu mice showing normal overall fur coat in the Foxn1ΔD/nu mouse. (C) The Foxn1ΔD/nu mouse lacks a normal appearing thymus just like the Foxn1nu/nu mouse. (D) Flow cytometric analysis was used to examine TCRβ+ cells in the spleen. Total cell numbers were calculated from DAPI- cells. There is a significant loss of TCRβ+ cells in the Foxn1ΔD/nu mouse however there is no difference in the overall number of TCRβ+ cells in the Foxn1ΔD/nu mouse compared to the Foxn1ΔD/ΔD and Foxn1nu/nu mice. Total cell numbers were calculated from DAPI- live cells and shown as mean ± SEM for n = 3–4 mice (4–8 weeks old) per genotype. ANOVA was performed to determine statistical significance. *** p < 0.001.
Altogether these data show that the Foxn1D regulatory element functions in cis and is specific to the regulation of Foxn1 expression. The intact Foxn1D regulatory element on the nude allele is not sufficient or capable of functioning in trans to induce expression of Foxn1 from the Foxn1D allele. Both the Foxn1ΔD/+ and Foxn1nu/+ mice have a thymus and essentially normal T cell populations. Therefore, we would expect that if the Foxn1ΔD mutation affected any other gene, possibly an upstream regulator of Foxn1, we would not see the thymus development phenotype observed in the Foxn1ΔD/nu mouse. The Foxn1ΔD mutation and the Foxn1nu mutation function in concert to totally disrupt Foxn1 expression and function.
Discussion
Foxn1 is essential for the development of the thymus and ultimately, the adaptive immune system. Herein, we have identified the first genomic RE critical for Foxn1 expression. This RE is remarkably tissue-specific as there is an almost total loss of expression of Foxn1 in the thymic epithelium while leaving Foxn1 expression and function in the skin unaffected. Loss of this RE results in defective thymus development and function and a near complete loss of T cells in the periphery. The Foxn1D RE is specific for Foxn1 expression as shown by the Foxn1ΔD/nu compound mutant mouse. The Foxn1ΔB/ΔB mouse identified a broader region of DNA containing a RE(s) specific for Foxn1 expression in mTEC. However, this RE is significantly weaker than the Foxn1D RE and despite a reduction in the overall number of mTEChi cells, there were no identified T cell defects in the Foxn1ΔB/ΔB mouse.
We believe the Foxn1ΔD/ΔD mouse to be a good model to study thymus independent immune cell development. These animals are generally healthier than the nude mouse, breed like WT mice, and maintain Foxn1 expression in the skin allowing for the study of immune cells that reside in the skin. The Foxn1ΔD/ΔD mice breed as homozygotes unlike the traditional nude mice which are known to have a poor capacity for breeding. This provides a robust and more easily accessible model to study thymus-related immunological questions.
Transcription factors can bind to the promoter, genomic regulatory elements, or both, to control gene expression. Studies have identified candidate regulators of Foxn1 expression as several transcription factor knockouts result in mice lacking a functional thymus (6). Pax family genes are expressed in the endoderm and knockout of Pax1 results in a hypoplastic thymus while knockout of Pax9 results in athymia (43, 44). Loss of Hoxa3, Eya1, or Six1 results in severe thymic developmental defects (45–48). Signals from the surrounding endothelium and mesenchyme including Bmp4 and Wnt signaling have been implicated in regulation of Foxn1 expression (49, 50). While many of these factors have been implicated in Foxn1 expression, none have been shown to directly bind to Foxn1 REs to induce Foxn1 expression in the early embryo. Several transcription factor binding motifs for Pax1, Six1, and Smads are present in the Foxn1 first intron regulatory element (Fig. S4A); however, additional studies are needed to determine which of these transcription factors interact with this intronic element to promote Foxn1 expression in TEC.
In humans, FOXN1 mutations result in congenital alopecia, nail dystrophy and severe T cell lymphopenia (22). The Foxn1D RE exhibits a high level of sequence conservation with the human FOXN1 intron. BLAT of the Foxn1D RE sequence to the human genome identifies a conserved region in the first intron of the FOXN1 gene with 89.7% identity (UCSC Genome Browser BLAT). This suggests that the Foxn1 RE we have identified in mouse could be critically important for FOXN1 expression in human TEC.
Genome-wide association studies (GWAS) have shown that a large proportion of variants likely to cause human disease are located outside of the protein coding domains (51, 52). There is potential for this study to inform genomic screening assays for mutations in human FOXN1 REs that currently remain undiagnosed. Whole exome sequencing and T cell receptor excision circle (TREC) count assays are performed on patients with T cell lymphopenia (53). It is likely that in some patients, FOXN1 expression is disrupted due to mutations in the REs rather than the exons. Further study of this RE and its conserved human RE is therefore relevant for the proper identification and treatment of immunodeficiency disorders resulting from aberrant FOXN1 expression in human patients.
Supplementary Material
Key Points.
Regulatory elements critical for Foxn1 expression are in the first intron of Foxn1.
These elements are necessary for Foxn1 expression in thymic epithelial cells.
These elements are not required for Foxn1 expression in the skin.
Acknowledgements
We thank the CCR Sequencing Facility and CCR Flow Cytometry Core Facility for technical support; the NCI Pathology/Histotechnology Laboratory specifically Andrew Warner, Jennifer Matta, and Tamara Morgan; the Confocal Microscopy and Digital Imaging Core, Experimental Immunology Branch, National Cancer Institute specifically Jan Wisniewski; the Transgenics/Cryopreservation – Laboratory Animal Sciences Program; and the CCR Collaborative Bioinformatics group including Maggie Cam and Vishal Koparde.
This work was supported by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research, National Institutes of Health.
References
- 1.Shah DK, and Zuniga-Pflucker JC. 2014. An overview of the intrathymic intricacies of T cell development. J Immunol 192: 4017–4023. [DOI] [PubMed] [Google Scholar]
- 2.Cowan JE, Jenkinson WE, and Anderson G. 2015. Thymus medulla fosters generation of natural Treg cells, invariant gammadelta T cells, and invariant NKT cells: what we learn from intrathymic migration. Eur J Immunol 45: 652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson G, and Jenkinson EJ. 2001. Lymphostromal interactions in thymic development and function. Nat Rev Immunol 1: 31–40. [DOI] [PubMed] [Google Scholar]
- 4.Manley NR, Richie ER, Blackburn CC, Condie BG, and Sage J. 2011. Structure and function of the thymic microenvironment. Front Biosci (Landmark Ed) 16: 2461–2477. [DOI] [PubMed] [Google Scholar]
- 5.Anderson G, and Takahama Y. 2012. Thymic epithelial cells: working class heroes for T cell development and repertoire selection. Trends Immunol 33: 256–263. [DOI] [PubMed] [Google Scholar]
- 6.Abramson J, and Anderson G. 2017. Thymic Epithelial Cells. Annu Rev Immunol 35: 85–118. [DOI] [PubMed] [Google Scholar]
- 7.Perry JSA, Lio CJ, Kau AL, Nutsch K, Yang Z, Gordon JI, Murphy KM, and Hsieh CS. 2014. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity 41: 414–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nehls M, Pfeifer D, Schorpp M, Hedrich H, and Boehm T. 1994. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature 372: 103–107. [DOI] [PubMed] [Google Scholar]
- 9.Nehls M, Kyewski B, Messerle M, Waldschutz R, Schuddekopf K, Smith AJ, and Boehm T. 1996. Two genetically separable steps in the differentiation of thymic epithelium. Science 272: 886–889. [DOI] [PubMed] [Google Scholar]
- 10.Pantelouris EM 1968. Absence of thymus in a mouse mutant. Nature 217: 370–371. [DOI] [PubMed] [Google Scholar]
- 11.Flanagan SP 1966. ‘Nude’, a new hairless gene with pleiotropic effects in the mouse. Genet Res 8: 295–309. [DOI] [PubMed] [Google Scholar]
- 12.Bennett AR, Farley A, Blair NF, Gordon J, Sharp L, and Blackburn CC. 2002. Identification and characterization of thymic epithelial progenitor cells. Immunity 16: 803–814. [DOI] [PubMed] [Google Scholar]
- 13.Alves NL, Takahama Y, Ohigashi I, Ribeiro AR, Baik S, Anderson G, and Jenkinson WE. 2014. Serial progression of cortical and medullary thymic epithelial microenvironments. Eur J Immunol 44: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bleul CC, Corbeaux T, Reuter A, Fisch P, Monting JS, and Boehm T. 2006. Formation of a functional thymus initiated by a postnatal epithelial progenitor cell. Nature 441: 992–996. [DOI] [PubMed] [Google Scholar]
- 15.Zuklys S, Handel A, Zhanybekova S, Govani F, Keller M, Maio S, Mayer CE, Teh HY, Hafen K, Gallone G, Barthlott T, Ponting CP, and Hollander GA. 2016. Foxn1 regulates key target genes essential for T cell development in postnatal thymic epithelial cells. Nat Immunol 17: 1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Xiao S, and Manley NR. 2009. Foxn1 is required to maintain the postnatal thymic microenvironment in a dosage-sensitive manner. Blood 113: 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbeaux T, Hess I, Swann JB, Kanzler B, Haas-Assenbaum A, and Boehm T. 2010. Thymopoiesis in mice depends on a Foxn1-positive thymic epithelial cell lineage. Proc Natl Acad Sci U S A 107: 16613–16618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng L, Guo J, Sun L, Fu J, Barnes PF, Metzger D, Chambon P, Oshima RG, Amagai T, and Su DM. 2010. Postnatal tissue-specific disruption of transcription factor FoxN1 triggers acute thymic atrophy. J Biol Chem 285: 5836–5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun L, Guo J, Brown R, Amagai T, Zhao Y, and Su DM. 2010. Declining expression of a single epithelial cell-autonomous gene accelerates age-related thymic involution. Aging Cell 9: 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zook EC, Krishack PA, Zhang S, Zeleznik-Le NJ, Firulli AB, Witte PL, and Le PT. 2011. Overexpression of Foxn1 attenuates age-associated thymic involution and prevents the expansion of peripheral CD4 memory T cells. Blood 118: 5723–5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bredenkamp N, Nowell CS, and Blackburn CC. 2014. Regeneration of the aged thymus by a single transcription factor. Development 141: 1627–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adriani M, Martinez-Mir A, Fusco F, Busiello R, Frank J, Telese S, Matrecano E, Ursini MV, Christiano AM, and Pignata C. 2004. Ancestral founder mutation of the nude (FOXN1) gene in congenital severe combined immunodeficiency associated with alopecia in southern Italy population. Ann Hum Genet 68: 265–268. [DOI] [PubMed] [Google Scholar]
- 23.Visel A, Blow MJ, Li Z, Zhang T, Akiyama JA, Holt A, Plajzer-Frick I, Shoukry M, Wright C, Chen F, Afzal V, Ren B, Rubin EM, and Pennacchio LA. 2009. ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature 457: 854–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, Hanna J, Lodato MA, Frampton GM, Sharp PA, Boyer LA, Young RA, and Jaenisch R. 2010. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci U S A 107: 21931–21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noonan JP, and McCallion AS. 2010. Genomics of long-range regulatory elements. Annu Rev Genomics Hum Genet 11: 1–23. [DOI] [PubMed] [Google Scholar]
- 26.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, and Greenleaf WJ. 2013. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods 10: 1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, and Glass CK. 2010. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38: 576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murata S, Sasaki K, Kishimoto T, Niwa S, Hayashi H, Takahama Y, and Tanaka K. 2007. Regulation of CD8+ T cell development by thymus-specific proteasomes. Science 316: 1349–1353. [DOI] [PubMed] [Google Scholar]
- 29.Bleul CC, and Boehm T. 2005. BMP signaling is required for normal thymus development. J Immunol 175: 5213–5221. [DOI] [PubMed] [Google Scholar]
- 30.Cunliffe VT, Furley AJ, and Keenan D. 2002. Complete rescue of the nude mutant phenotype by a wild-type Foxn1 transgene. Mamm Genome 13: 245–252. [DOI] [PubMed] [Google Scholar]
- 31.Kurooka H, Segre JA, Hirano Y, Nemhauser JL, Nishimura H, Yoneda K, Lander ES, and Honjo T. 1996. Rescue of the hairless phenotype in nude mice by transgenic insertion of the wild-type Hfh11 genomic locus. Int Immunol 8: 961–966. [DOI] [PubMed] [Google Scholar]
- 32.Sakata M, Ohigashi I, and Takahama Y. 2018. Cellularity of Thymic Epithelial Cells in the Postnatal Mouse. J Immunol 200: 1382–1388. [DOI] [PubMed] [Google Scholar]
- 33.Bansal K, Yoshida H, Benoist C, and Mathis D. 2017. The transcriptional regulator Aire binds to and activates super-enhancers. Nat Immunol 18: 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, Van Calcar S, Qu C, Ching KA, Wang W, Weng Z, Green RD, Crawford GE, and Ren B. 2007. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet 39: 311–318. [DOI] [PubMed] [Google Scholar]
- 35.Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, Hu M, Liu JS, and Ren B. 2012. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485: 376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee D, Prowse DM, and Brissette JL. 1999. Association between mouse nude gene expression and the initiation of epithelial terminal differentiation. Dev Biol 208: 362–374. [DOI] [PubMed] [Google Scholar]
- 37.Nagao K, Kobayashi T, Moro K, Ohyama M, Adachi T, Kitashima DY, Ueha S, Horiuchi K, Tanizaki H, Kabashima K, Kubo A, Cho YH, Clausen BE, Matsushima K, Suematsu M, Furtado GC, Lira SA, Farber JM, Udey MC, and Amagai M. 2012. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat Immunol 13: 744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blackburn CC, Augustine CL, Li R, Harvey RP, Malin MA, Boyd RL, Miller JF, and Morahan G. 1996. The nu gene acts cell-autonomously and is required for differentiation of thymic epithelial progenitors. Proc Natl Acad Sci U S A 93: 5742–5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, and Love PE. 1998. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med 188: 2301–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, Bendelac A, Bonneville M, and Lantz O. 1999. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med 189: 1907–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benlagha K, Wei DG, Veiga J, Teyton L, and Bendelac A. 2005. Characterization of the early stages of thymic NKT cell development. J Exp Med 202: 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fahl SP, Coffey F, and Wiest DL. 2014. Origins of gammadelta T cell effector subsets: a riddle wrapped in an enigma. J Immunol 193: 4289–4294. [DOI] [PubMed] [Google Scholar]
- 43.Wallin J, Eibel H, Neubuser A, Wilting J, Koseki H, and Balling R. 1996. Pax1 is expressed during development of the thymus epithelium and is required for normal T-cell maturation. Development 122: 23–30. [DOI] [PubMed] [Google Scholar]
- 44.Peters H, Neubuser A, Kratochwil K, and Balling R. 1998. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev 12: 2735–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zou D, Silvius D, Davenport J, Grifone R, Maire P, and Xu PX. 2006. Patterning of the third pharyngeal pouch into thymus/parathyroid by Six and Eya1. Dev Biol 293: 499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu PX, Zheng W, Laclef C, Maire P, Maas RL, Peters H, and Xu X. 2002. Eya1 is required for the morphogenesis of mammalian thymus, parathyroid and thyroid. Development 129: 3033–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manley NR, and Capecchi MR. 1995. The role of Hoxa-3 in mouse thymus and thyroid development. Development 121: 1989–2003. [DOI] [PubMed] [Google Scholar]
- 48.Manley NR, and Capecchi MR. 1998. Hox group 3 paralogs regulate the development and migration of the thymus, thyroid, and parathyroid glands. Dev Biol 195: 1–15. [DOI] [PubMed] [Google Scholar]
- 49.Wertheimer T, Velardi E, Tsai J, Cooper K, Xiao S, Kloss CC, Ottmuller KJ, Mokhtari Z, Brede C, deRoos P, Kinsella S, Palikuqi B, Ginsberg M, Young LF, Kreines F, Lieberman SR, Lazrak A, Guo P, Malard F, Smith OM, Shono Y, Jenq RR, Hanash AM, Nolan DJ, Butler JM, Beilhack A, Manley NR, Rafii S, Dudakov JA, and van den Brink MRM. 2018. Production of BMP4 by endothelial cells is crucial for endogenous thymic regeneration. Sci Immunol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balciunaite G, Keller MP, Balciunaite E, Piali L, Zuklys S, Mathieu YD, Gill J, Boyd R, Sussman DJ, and Hollander GA. 2002. Wnt glycoproteins regulate the expression of FoxN1, the gene defective in nude mice. Nat Immunol 3: 1102–1108. [DOI] [PubMed] [Google Scholar]
- 51.Rickels R, and Shilatifard A. 2018. Enhancer Logic and Mechanics in Development and Disease. Trends Cell Biol 28: 608–630. [DOI] [PubMed] [Google Scholar]
- 52.Sakabe NJ, Savic D, and Nobrega MA. 2012. Transcriptional enhancers in development and disease. Genome Biol 13: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwan A, Abraham RS, Currier R, Brower A, Andruszewski K, Abbott JK, Baker M, Ballow M, Bartoshesky LE, Bonilla FA, Brokopp C, Brooks E, Caggana M, Celestin J, Church JA, Comeau AM, Connelly JA, Cowan MJ, Cunningham-Rundles C, Dasu T, Dave N, De La Morena MT, Duffner U, Fong CT, Forbes L, Freedenberg D, Gelfand EW, Hale JE, Hanson IC, Hay BN, Hu D, Infante A, Johnson D, Kapoor N, Kay DM, Kohn DB, Lee R, Lehman H, Lin Z, Lorey F, Abdel-Mageed A, Manning A, McGhee S, Moore TB, Naides SJ, Notarangelo LD, Orange JS, Pai SY, Porteus M, Rodriguez R, Romberg N, Routes J, Ruehle M, Rubenstein A, Saavedra-Matiz CA, Scott G, Scott PM, Secord E, Seroogy C, Shearer WT, Siegel S, Silvers SK, Stiehm ER, Sugerman RW, Sullivan JL, Tanksley S, Tierce M. L. t., Verbsky J, Vogel B, Walker R, Walkovich K, Walter JE, Wasserman RL, Watson MS, Weinberg GA, Weiner LB, Wood H, Yates AB, Puck JM, and Bonagura VR. 2014. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA 312: 729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.