Abstract
Aphasia typically is associated with comparable difficulties in written and spoken modalities of language expression and comprehension. In contrast, auditory verbal agnosia is the disproportionate difficulty comprehending spoken compared to written language, also typically greater than difficulties with spoken and written language expression, in the absence of a primary sensory deficit. The terms pure word deafness and auditory verbal agnosia are often used synonymously. However, the broader term of auditory agnosia more accurately reflects difficulty processing both speech and non-speech sounds whereas individuals with auditory verbal agnosia (pure word deafness) have preserved processing of environmental sounds. Auditory agnosia is reported in the stroke literature, but rarely reported in progressive neurologic disorders. Here, we report a case of a woman who presented with what is best described as a prominent auditory deficit in the context of an initially unclassifiable, or mixed, primary progressive aphasia (PPA) with accompanying apraxia of speech. Her clinical presentation shared features with auditory agnosia, although sensory functioning was not formally assessed. We report clinical and neuroimaging data spanning 6 years and subsequent autopsy results. She presented at 65 years of age, 5 years post onset of symptoms that included insidious and progressive difficulties thinking of words, constructing sentences, pronouncing words, and understanding instructions. She had disproportionate difficulty with comprehension of spoken compared to written language. She eventually developed features of the nonfluent/ agrammatic variant of PPA, as well as an apraxia of speech. Imaging with [18F]-fluorodeoxyglucose (FDG)-PET revealed progression of bilateral (left greater than right) hypometabolism involving the frontal, temporal (predominantly the lateral superior gyrus), and parietal lobes, that eventually included the supplementary motor area, anterior cingulate, and caudate. Autopsy revealed pathological lesions consistent with corticobasal degeneration.
Keywords: primary progressive aphasia, auditory agnosia, frontotemporal dementia, PET, corticobasal degeneration
1.0. Introduction
Aphasia typically is associated with parallel difficulties in written and spoken modalities of language expression and comprehension. In contrast, auditory verbal agnosia reflects disproportionate difficulty comprehending spoken compared to written language that cannot be explained by hearing loss. The terms pure word deafness and auditory verbal agnosia are often used synonymously. However, the broader term of auditory agnosia more accurately reflects difficulty processing both speech and non-speech sounds whereas individuals with auditory verbal agnosia (pure word deafness) have preserved processing of environmental sounds [see (Slevc & Shell, 2015) for a recent review]. Auditory agnosia, as reported in the stroke literature, is associated with bilateral putamen hemorrhages (Sugiura & Torii, 2017). Auditory verbal agnosia (pure word deafness) is also reported in the stroke literature and associated with bilateral lesions (Auerbach, Allard, Naeser, Alexander, & Albert, 1982; Burns, 2004; Coslett, Brashear, & Heilman, 1984; Engelien et al., 1995), but also unilateral infarcts (Maffei et al., 2017; Poeppel, 2001). Both auditory verbal agnosia (pure word deafness) and auditory agnosia are rarely reported in progressive neurologic disorders, with fewer than ten total reported cases in the literature (Ceccaldi, Soubrouillard, Poncet, & Lecours, 1996; Iizuka, Suzuki, Endo, Fujii, & Mori, 2007; Jorgens et al., 2008; Kuramoto et al., 2002; Mesulam, 1982; Otsuki, Soma, Sato, Homma, & Tsuji, 1998; Rapcsak, Kentros, & Rubens, 1989), and one additional report of degenerative environmental sound agnosia alone (Uttner et al., 2006). Pathology has not been reported in these cases and they have spanned a broad spectrum of clinically-defined neurologic diseases, including Alzheimer’s disease dementia, frontotemporal dementia, and primary progressive aphasia (PPA).
PPA is a neurodegenerative syndrome characterized by varying combinations of progressive language impairments (Mesulam, 1982, 2001). The current consensus criteria for diagnosis (Gorno-Tempini et al., 2011) reflect the broad diagnosis of PPA and three clinical variants, including 1) the nonfluent/ agrammatic variant (agPPA) characterized by grammatical errors in speech and writing, difficulty with comprehension of grammatically complex information, and often accompanied by apraxia of speech, 2) the semantic variant characterized by loss of knowledge about the meaning of words and associated reduced word comprehension, and 3) the logopenic variant characterized by word retrieval problems, poor sentence repetition, phonological errors, and difficulty with comprehension associated with reduced working memory. Research has demonstrated that 16–31% of patients who meet root criteria for PPA are not classifiable as one of the aforementioned variants (Gil-Navarro et al., 2013; Harris et al., 2013; Mesulam, Wieneke, Thompson, Rogalski, & Weintraub, 2012; Wicklund et al., 2014).
Each of the aforementioned clinical presentations is associated with typical patterns on MRI and PET imaging, as outlined in the diagnostic consensus criteria (Gorno-Tempini et al., 2011). Briefly, agPPA is associated with predominant left posterior fronto-insular atrophy and hypometabolism. The presence of AOS is associated with involvement of the supplementary motor and premotor regions. The semantic variant is associated with predominant anterior temporal lobe involvement. Diagnosis of the logopenic variant is supported by predominant left posterior perisylvian or parietal atrophy.
In this paper, we report a case report of a woman who had an unusual constellation of symptoms that is best described as a prominent auditory deficit in the context of an initially unclassifiable PPA. Her clinical presentation shared features with auditory agnosia, although sensory functioning was not formally assessed, and therefore a fully confirmed diagnosis of such could not be made. We describe clinical and neuroimaging data spanning 6 years and subsequent autopsy results. She eventually developed features of the nonfluent/agrammatic variant of PPA, as well as an apraxia of speech (AOS). While she never met criteria for a clinical diagnosis of corticobasal syndrome, she had pathological lesions consistent with corticobasal degeneration (CBD) at autopsy. This case demonstrates that prominent, disproportionate auditory deficits in the context of agPPA with AOS can be associated with CBD pathology.
2.0. Case Report
The patient was a right handed, Caucasian female, with 12 years of education. At visit 1, she was 65-years old and reported a five-year history of insidious, progressive difficulties thinking of words, constructing sentences, pronouncing words, and understanding instructions. At initial exam, she presented with phonological errors, agrammatic spoken and written language, and lacked specificity in spoken language. She had an equivocal non-verbal oral apraxia (NVOA). Her speech was both fluent, meeting some but not all criteria for logopenic PPA, and nonfluent, again meeting some but not all criteria for agPPA. She did not present with unequivocal evidence of apraxia of speech (AOS); while some sound level errors may have been apraxic in nature, most, if not all sound level errors were judged to be phonological at visit 1. Given the above, she was given a diagnosis of PPA-Unclassifiable.
At visit 2 (6 years post symptom onset), her language impairments had progressed. There was unequivocal evidence of NVOA and AOS, less severe than her now marked agrammatic aphasia. She and her husband denied reduced comprehension of speech but difficulties were evident throughout the exam. She reported increased spelling errors but writing still facilitated expressive communication. She had a stereotypic speech pattern whereby she repeated the phrase “yes I am” or “yes it is” in place of, or at the end of, a response to questions. She had begun therapy following the first visit and was working with a speech-language pathologist two to three times monthly. At this time, a tauopathy was the suspected underlying pathology, given its association with degenerative speech and language disorders, but she had no other symptoms suggestive of progressive supranuclear palsy or corticobasal syndrome.
At visit 3 (7 years post symptom onset), she presented with limited verbal output and communicated primarily via writing. She had yes/no reversals. It was at this visit that a marked prominent auditory deficit was noted, as she had significant difficulty understanding spoken language that was disproportionate to any deficits in comprehending written language. For example, during neurologic testing, she could not respond to the question “how are two things alike?” but could easily respond to the written question. Although pure tone hearing tests were not completed, the patient’s family reported she startled to noise (quite easily, in fact), responded appropriately to the doorbell and phone ringing, responded to the sound of a conversational voice (i.e. turned to respond to her name), and maintained focus when conversation partners were talking. She relied on closed captioning while watching television. During assessment, she demonstrated intact word knowledge and comprehension, as she accurately identified 48/48 written words, but only 4/12 spoken words. She also had difficulty recognizing environmental sounds (she recognized 2/10 common sounds, correctly identifying the neigh of a horse and the sound made by a gong) and common tunes (she recognized 0/5 tunes). Overall, we observed a deficit in the auditory input lexicon but not in the orthographic input lexicon, which was intact according to the patient’s performance on the subtests of the Western Aphasia Battery- Revised (WAB; see Table 3). The pattern of performance is similar to that seen in patients with an auditory agnosia; however, given the lack of formal audiological assessment, a fully confirmed diagnosis of auditory agnosia could not be made. At this time, there was evidence of mild impairment in mental flexibility.
Table 3.
Speech and language data for each visit. Note: Maximum score noted in row header, when applicable; aphasia severity rating, where 1 = mild and 4 = severe; WAB-AQ = Western Aphasia Battery- Revised Aphasia Quotient; WAB Recognition = Recognition section of the Auditory Verbal Comprehension subtest of the WAB; PPTT = Pyramids and Palm Trees Test; BNT = Boston Naming Test, short form; NVOA = Non-verbal oral apraxia; MSD = Motor Speech Disorders Severity Rating; ASRS= Apraxia of Speech Rating Scale; AES = Articulatory Error Score; AOS = Apraxia of Speech, severity rating, where 1 = mild and 4 = severe; DNT = did not test; CND = could not determine, although test was attempted.
| Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 5 | |
|---|---|---|---|---|---|
| Diagnosis | PPA-U | Agrammatic aphasia with AOS | Agrammatic aphasia with AOS and prominent auditory deficit | Agrammatic aphasia with AOS and prominent auditory deficit | Agrammatic aphasia with AOS and prominent auditory deficit |
| Aphasia severity (/4) | 2 | 3 | 3 | 3 | 4 |
| WAB-AQ (/100) | 72.4 | 59 | 58.6 | CND | 14.8 |
| WAB Word Recognition (auditory) | 57/60 | 57/60 | 4/12 | 4/60 | 0/18 |
| WAB Word Recognition (written) | DNT | DNT | 48/48 | 58/60 | DNT |
| WAB Reading comprehension (/40) | 40 | 28 | 20 | DNT | 10 |
| WAB Reading commands (/20) | 15 | 14 | 9 | 6/10 | 1 |
| WAB Writing output (/34) | 34 | 25.5 | 26 | DNT | 10 |
| WAB Reading Irregular words (/10) | 4 | 2 | 0 | DNT | DNT |
| WAB Reading non-words (/10) | 6 | 6 | 0 | DNT | DNT |
| WAB Writing Irregular words (/10) | 7 | 8 | 2 | DNT | DNT |
| WAB Writing non-words (/10) | 2 | 3 | 3 | DNT | DNT |
| Token Test (/22) | 4 | 1 | DNT | CND | DNT |
| PPTT (/52) | 51 | 50 | 50 | DNT | DNT |
| BNT (/15) | 11 | 10 | 9 | 5 | 2 |
| NVOA (132) | 29 | 15 | 4 | DNT | 0 |
| MSD (/10) | 10 | 5 | 3 | 1 | 1 |
| ASRS (/52) | 13 | 15 | 17 | DNT | DNT |
| AES Total | 70.8 | 78.8 | 100 | DNT | DNT |
| AOS severity (/4) | 0 | 2 | 2 | 2 | 4 |
At visit 4 (8.5 years post symptom onset), she did not respond to spoken questions; therefore, all test stimuli were presented in writing. On the auditory recognition subtest of the WAB, she accurately responded to 4/60 items when verbalized, but 58/60 item when written. She had difficulty recognizing common sounds (2/10 accurate). At this time, she associated the neigh of a horse with an elephant, both a helicopter and a fire engine with a train, and a lion with an owl. Her AOS had progressed. She was essentially mute and communicated entirely through writing, which was difficult to interpret secondary to paraphasic errors and agrammatism. Behaviorally, she was impatient and somewhat impulsive. Her yes/no reversals persisted, although she communicated accurately with thumbs up and down for “yes” and “no.” Cognitive testing was difficult to complete. For instance, she did not respond to orientation questions when asked verbally; when asked for place orientation via written question, she responded “Montana” in place of the accurate answer “Minnesota.” Whether this was a paraphasic error or true lack of orientation was unclear. She had also developed an unusual breathing pattern of uncertain significance, best described as a shallow, deep inspiration with a stereotypic output “ne-haum” (see Supplementary Videos).
At the final visit (visit 5; 9.5 years post symptom onset), she had limited intentional phonation and produced essentially no meaningful speech. Writing was largely uninterpretable secondary to the severity of agrammatism and paraphasias. Despite these limitations, she did many things independently. It was difficult to administer standardized tests but she was able to match colors and objects to written words. She identified 0/18 spoken words; written comprehension was not assessed secondary to fatigue. Again, it was difficult to discern if she was fully oriented; when given a calendar and asked to point to the current day, she was unable to do so. When shown the current date and asked if it was accurate she wrote the word “speech,” suggesting reduced comprehension and, perhaps also, further cognitive decline.
Following her final visit, behavioral changes became more pervasive. She continued to do well physically, but developed myoclonic movements in the lower extremities. She could no longer read 6 months following her final visit.
She passed away at 70 years of age, 8 months after the final visit and 10 years after the initial onset of symptoms. Autopsy revealed a tauopathy with astrocytic plaques and many threads in the white matter consistent with CBD pathology.
3.0. Methods
She underwent a standard protocol of neurological, neuropsychological, speech, and language assessments, and [18F] fluorodeoxyglucose (FDG) PET at each visit, a 3T volumetric head MRI at visits 1 and 2, and a Pittsburgh Compound B (PiB) PET scan during visit 1. She was seen for two studies funded by the National Institutes of Health and provided written consent for participation. The study was approved by the Institutional Review Board at Mayo Clinic.
3.1. Clinical assessments
The neurological evaluations (performed by KAJ) included the Mini-Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, 1975) and Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005) to index general cognition; the short version of the Neuropsychiatric Inventory Questionnaire (NPI-Q) (Kaufer et al., 2000) to assess behavioral and psychiatric features; the Frontal Assessment Battery (FAB) (Dubois, Slachevsky, Litvan, & Pillon, 2000) and the Frontal Behavioral Inventory (FBI) (Kertesz, Davidson, & Fox, 1997) to evaluate executive dysfunction; the WAB (Kertesz, 2007) apraxia rating scale to judge limb and non-verbal oral apraxia; and the Movement Disorder Society Sponsored revision of the Unified Parkinson’s disease Rating Scale III (MDS-UPDRS III) (Goetz et al., 2008) to assess the presence, nature, and severity of parkinsonism.
The neuropsychological test battery (overseen by MMM) included the Auditory Verbal Learning Test (Rey, 1964) to assess verbal memory; Trail Making Test (TMT) A and B (Spreen & Strauss, 1998) to evaluate processing speed and mental flexibility, respectively; the incomplete letters and cube subtests of the Visual Object and Space Perception Battery (VOSP) (Warrington & James, 1991) to index visuoperceptual and visuospatial processing; and the Rey-Osterrieth Complex Figure test (Rey-O) to evaluate visual constructional ability (Osterrieth, 1944; Rey, 1964). In visit 3, the AVLT was administered via a PowerPoint presentation and she written instructions for TMT A and B and VOSP subtests were provided.
The speech and language assessments were performed by one of two speech-language pathologists (JRD or HMC). The WAB (Kertesz, 2007) served as the primary measure of global language ability. Supplementary reading and writing tasks from Part 2 of the WAB were also administered. The Token Test, Part V (De Renzi & Vignolo, 1962) served as a measure of syntactic comprehension, and the 15-item Boston Naming Test (Lansing, Ivnik, Cullum, & Randolph, 1999) as a measure of confrontation naming ability. The composite of the aforementioned tests was utilized in the judgment of aphasia severity (1 = mild; 4 = severe).
Assessment of speech function included the sample elicited in the above language tasks and several supplementary motor speech tasks that included vowel prolongation, speech alternating motion rates (e.g. rapid repetitions of “puh,” “tuh,” and “kuh”), and speech sequential motion rates (e.g. rapid repetitions of “puh tuh kuh”). A full examination of the oral mechanism, including assessment of strength, tone, and range of motion of the articulators was conducted. The presence, nature, and severity of apraxia of speech was indexed with a global judgment of severity (1 = mild; 4 = severe) and further quantified with the Apraxia of Speech Rating Scale (ASRS) (Duffy, Strand, & Josephs, 2014). The Articulatory Error Score (AES) was also calculated; this was derived from the proportion of incorrectly produced words on the supplementary speech tasks. This included three repetitions of 13 words plus one repetition of three sentences for a total of 56 words. NVOA was rated based on accuracy of responses to eight items. The severity of functional impairment associated with the motor speech disorder was quantified using the Motor Speech Disorder (MSD) scale (Yorkston, Strand, Miller, Hillel, & Smith, 1993). Clinical diagnoses (e.g. PPA or AOS) were made according to performance on behavioral testing, blinded to neuroimaging results.
3.2. Neuroimaging assessments
She underwent a standardized imaging protocol on a 3T GE scanner at visits 1 and 2. A 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) T1-weighted sequence was acquired (TR/TE/T1, 2300/3/900 ms; flip angle 8°, 26-cm field of view (FOV); 256 × 256 in-plane matrix; phase FOV = 0.94, slice thickness = 1.2 mm, in-plane resolution = 1.0 mm). All MPRAGE images underwent pre-processing correction for gradient non-linearity and intensity non-uniformity. All scans were registered using nine degrees of freedom rigid-body registration with differential bias correction (Gunter, Senjem, Vemuri, & Jack, 2012). To assess regional atrophy on each MRI scan we calculated grey matter volume for 91 regions across the brain used the automated anatomical labelling atlas (Tzourio-Mazoyer et al., 2002), and calculated age and total intracranial volume adjusted Z scores for each region using a control cohort, as previously described in detail (Vemuri et al., 2011). We considered a region atrophic if Z ≥1.
FDG-PET scans were acquired at each visit, using a PET/CT scanner (GE Healthcare, Milwaukee, Wisconsin) as previously described (Josephs et al., 2012). Individual patterns of hypometabolism were assessed using the clinical tool of 3-dimensional stereotactic surface projections (SSP) (Minoshima, Frey, Koeppe, Foster, & Kuhl, 1995). Activity was normalized to the pons and compared with an age-segmented normative database, yielding a 3-dimensional SSP z score image. FDG-PET analyses were completed with the CortexID software package (GE Healthcare, Waukesha, Wisconsin).
At visit 1, PiB-PET was acquired. She was injected with PiB and after a 40–60 minute uptake period a 20 minute PiB scan was obtained consisting of four 5-minute dynamic frames following a low dose CT transmission scan. Standard corrections were applied. Emission data was reconstructed into a 256 × 256 matrix with a 30-cm FOV (Pixel size = 1.0mm, slice thickness = 1.96mm). A global PiB standard uptake value ratio (SUVR) was generated; a cut-point of 1.42 was used to establish Aβ positivity, as previously described (Jack et al., 2008).
4.0. Results
4.1. Neurological
Results of neurological testing are reported in Table 1. She showed a steady decline on tests of global cognitive functioning (MMSE and MOCA). Over time, there were increased neuropsychiatric symptoms, including disinhibition, agitation, anxiety, and irritability (assessed with the NPI-Q). There was severe executive dysfunction (indexed with the FAB) at visit 4 that was not previously reported. With some fluctuations over time, she ultimately showed moderate behavioral dyscontrol (evaluated with the FBI). She developed difficulty recognizing environmental sounds and common tunes, despite seemingly adequate hearing. At visit 1, she did not have any evidence of limb apraxia, but it was detected at visit 2 and worsened by visit 4. She did not present with, nor develop, any evidence of parkinsonism.
Table 1.
Demographic information and neurologic data for each visit. Note: Maximum score noted in each row header. MMSE = The Mini Mental State Examination; MOCA = Montreal Cognitive Assessment; NPI-Q = Neuropsychiatric Inventory Questionnaire; FAB = Frontal Assessment Battery; FBI = Frontal Behavioural Inventory; Western Aphasia Battery- Revised (WAB) praxis subtest; MDS-UPDRS III = the Movement Disorders Society-sponsored version of the Unified Parkinson’s Disease Rating Scale motor subsection; DNT = did not test.
| Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 5 | |
|---|---|---|---|---|---|
| Age (years) | 65 | 66 | 67 | 69 | 70 |
| Disease duration (years since symptom onset) | 5 | 6 | 7 | 8.5 | 9.5 |
| MMSE (/30) | 29 | 29 | 24 | 12 | DNT |
| MoCA (/30) | 24 | 23 | 20 | 16 | DNT |
| NPI-Q (/36) | 1 | 1 | 0 | 6 | DNT |
| FAB (/18) | 18 | 18 | 17 | 4 | DNT |
| FBI (/72) | 15 | 11 | 7 | 18 | 30 |
| WAB-apraxia (/60) | 59 | 54 | 54 | 51 | DNT |
| MDS-UPDRS III (/132) | 0 | 6 | 6 | 9 | 7 |
| Environmental sounds | DNT | DNT | 2/10 | 2/10 | DNT |
| Musical tunes | DNT | DNT | 0/5 | DNT | DNT |
4.2. Neuropsychological
Results of neuropsychological testing are presented in Table 2. She did not receive testing at visits 4 and 5 secondary to fatigue and severity of speech and language difficulties. There were mild fluctuations in test scores across visits 1–3, but, overall, testing suggested average or above average short- and long-term verbal memory. Visuomotor speed (Trail Making Test A) was normal, but she demonstrated low average to mildly impaired speeded divided attention/cognitive flexibility (Trail Making Test B). VOSP testing was normal, suggesting preserved visuoperceptual and visuospatial functioning at the time testing was conducted. Her performance on the Rey-O was also normal when tested, consistent with intact visuoconstructional ability.
Table 2.
Neuropsychological testing for each visit. Note: Scores for Auditory Verbal Learning Test (AVLT) short-term, long-term retention, and recognition, Trail Making Test (TMT) A and B, and Rey-O Complex Figure test are Mayo’s Older Americans Normative Studies (MOANS) scores (Machulda et al., 2007; Steinberg, Bieliauskas, Smith, & Ivnik, 2005). Cube Analysis and Incomplete Letters of the Visual Object and Space Perception Battery (VOSP) are presented in raw scores (maximum scored noted in row header). DNT = did not test.
| Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 5 | |
|---|---|---|---|---|---|
| AVLT Short Term Retention | 10 | 12 | 14 | DNT | DNT |
| AVLT Long Term Retention | 11 | 13 | 14 | DNT | DNT |
| AVLT recognition | 9 | 10 | 12 | DNT | DNT |
| TMTA | 9 | 9 | 10 | DNT | DNT |
| TMTB | 7 | 7 | 5 | DNT | DNT |
| Rey-O | 10 | 10 | 11 | DNT | DNT |
| VOSP letters (/20) | 19 | 19 | 18 | DNT | DNT |
| VOSP cubes (/10) | 9 | 8 | 10 | DNT | DNT |
4.3. Language
For results of language testing, see Table 3. See Figures 1, 2, and 3 for writing samples. At visit 1, she had a moderate aphasia, supported by a WAB-AQ score of 72.4. She did not meet classification for any variant of PPA (Gorno-Tempini et al., 2011) . While she resembled patients with logopenic PPA given her prominent phonological errors and reduced comprehension, she also resembled patients with nonfluent/agrammatic PPA, given her frank agrammatism in writing; therefore, she was given a diagnosis of PPA-Unclassifiable. At visit 2, she met criteria for the nonfluent/agrammatic variant of PPA, with a marked aphasia (WAB-AQ of 59) and noticeable agrammatism in writing. At visit 3, she had a stable marked aphasia and a prominent, disproportionate auditory deficit, atypical for PPA. The pattern of performance is similar to that seen in patients with an auditory agnosia; however, given the lack of formal audiological assessment, a fully confirmed diagnosis of auditory agnosia could not be made. Upon review, it may be the case this disproportionate difficulty was present and missed at the first or second visit, given her propensity to ask for repetition of verbal questions; it would not have influenced her overall diagnosis. The observation of a prominent auditory deficit was supported by the disproportionate difficulty with comprehension of spoken compared to written words (see Table 3), again despite seemingly adequate hearing (although it was not formally assessed). See Supplementary Videos for examples of language expression and comprehension; the videos provide a demonstration of the discrepancy of deficits. Semantic access (tested via the Pyramids and Palm Trees Test) remained relatively intact through visit 3, after which the test was not administered. Confrontation naming was mildly reduced at visit 1 and severely impaired by visit 5. At visits 4 and 5, she was markedly-severely impaired and many tests were not administered or could not be validly scored. The decline of overall language function is consistent with the broader clinical presentation of agPPA.
Figure 1.
Written picture descriptions from each visit, describing a scene from the Western Aphasia Battery- Revised. Note: Brackets (e.g. [We return the lake.]), along with values (e.g. 4.5) were added by the examiner during scoring.
Figure 2.
Written irregular words from each visit (from Part 2 of the Western Aphasia Battery-Revised). Target words were: yacht, guide, ache, debt, knife, bargain, biscuit, magician, and courageous.
Figure 3.
Written regular non-words from each visit (from Part 2 of the Western Aphasia Battery- Revised). Target words were: fess, munt, feet, dosh, shab, aponster, poliket, aldinger, limponit, and globter.
4.4. Speech
For results of speech testing, see Table 3. At visit 1, she had neither dysarthria nor apraxia of speech (AOS); she had an emerging non-verbal oral apraxia. Despite preserved motor speech, she had an elevated articulatory error score, reflective of prominent phonological errors. At visit 2, she had a moderate AOS and marked-severe NVOA. Her AOS was characterized as Phonetic predominant (Utianski, Duffy, et al., 2018). The severity of AOS remained stable at visits 3 and 4, and was presumed severe at her final visit, when she was essentially mute. See Supplementary Videos for examples of speech production.
4.5. MRI
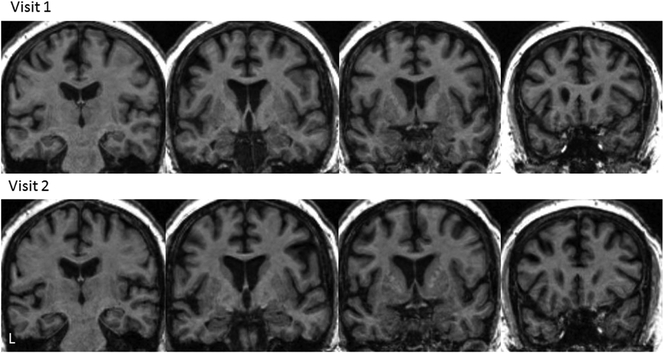
MRIs were acquired at visits 1 and 2 only, secondary to confusion and agitation at subsequent visits. At visit 1, the brain MRI was read as normal. At visit 2, mild leukoaraiosis was noted. On re-review, there was some evidence of left superior temporal atrophy; however, in our regional analysis, none of the regional volumes were greater than 1 (or 0.5) Z-score from controls, demonstrating normal volume for the patient’s age. The two MRI scans are shown in Figure 4.
Figure 4.
T1 brain MRI images for visits 1 and 2. Note: L = left, indicating anatomical orientation.
4.6. FDG-PET
See Figure 5 for FDG-PET Cortex-IDs at each visit. FDG-PET at visit 1 showed mild decreased FDG uptake in the medial and lateral frontal, lateral temporal (predominantly superior gyrus), and medial and lateral parietal lobes bilaterally, greater on the left. The cortex ID images indicated a more focal area of hypometabolism in the left inferior frontal lobe. At visit 2, there was diffuse decreased activity throughout the cerebral cortex with more prominent decreased activity in the left frontal and superior temporal lobes, including hypometabolism in the left supplementary motor area (SMA). Visit 3 images were similar to those acquired at visit 2, with slight progression noted between visits. At visit 4, there was again interval progression of the hypometabolism, predominantly involving the mid and posterolateral aspect of the left frontal lobe and the superior aspect of the left temporal lobe. There was minor bilateral parietal, occipital and lateral right frontal hypometabolism. Additionally, there was moderate hypometabolism in the anterior cingulate region and mild left caudate hypometabolism. Visit 5 revealed no significant changes from visit 4.
Figure 5.
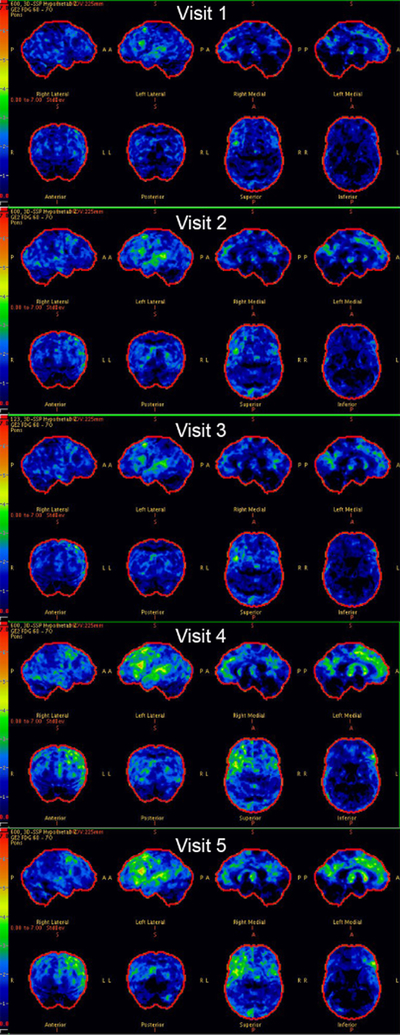
Serial FDG-PET scans for this patient. Cortex-ID z-score maps are shown for each visit.
4.7. PiB PET
At visit 1, global PiB ratio was 1.30, consistent with the absence of beta-amyloid and hence absence of Alzheimer’s disease. PiB PET testing was not repeated at subsequent visits.
4.8. Pathology
At autopsy, she had astrocytic plaques and threads in the white matter consistent with CBD pathology.
5.0. Discussion and Conclusions
This report has detailed the neurological, speech-language, neuropsychological, and neuroimaging findings that describe the evolution of a patient who originally presented with unclassifiable PPA with some features of both agrammatic and logopenic PPA. She subsequently developed apraxia of speech and a prominent auditory deficit, and died with autopsy confirmed CBD. This case demonstrates that prominent auditory deficits, or what some might refer to as suspected auditory agnosia, in the context of agrammatic PPA with AOS can be associated with CBD pathology.
At initial presentation, the patient did not fulfill criteria for a single variant of PPA (Gorno-Tempini et al., 2011), but clearly presented with a progressive language disorder and met root criteria for PPA, as opposed to another form of dementia. Her initial constellation of symptoms was viewed as a mix of logopenic PPA and agPPA, and therefore she was given a diagnosis of PPA-Unclassifiable. Her initial difficulties were thinking of words, constructing sentences, and understanding instructions. She demonstrated difficulty with comprehension of syntactically complex information and demonstrated frank agrammatism in writing, consistent with the overall diagnosis of agrammatic PPA. She developed AOS and NVOA, which were both detected at visit 2, 6 years post symptom onset. Over time, she developed increased difficulty understanding spoken versus written language and difficulty perceiving environmental sounds and common musical tunes. This prominent auditory deficit was noted at visit 3; the Supplementary Videos provide a clear demonstration of her disproportionate difficulty with auditory versus written comprehension. Of course, the prominent auditory deficits were in the context of an otherwise progressive disorder of language, and the other aspects of language also declined over time.
Across visits, there was evidence of decline in general cognition increased neuropsychiatric symptoms and behavioral dyscontrol, executive dysfunction, and mildly impaired mental flexibility by visit 3. Overall, neuropsychological functioning was difficult to assess as her speech, language, and auditory comprehension difficulties progressed. Testing, when possible, indicated normal short- and long-term verbal memory, motor speed, and visuospatial functioning. At her final visit, only several months before she passed away, she did not have parkinsonism or vertical supranuclear palsy, nor was she falling. Hence, she did not meet criteria for either corticobasal syndrome or progressive supranuclear palsy, which was surprising given that she had CBD at autopsy.
While MRIs were not available past visit 2, there were no apparent abnormalities at that time, despite the severity of her clinical impairments. FDG-PET scans were far more revealing. Spanning the five visits, her FDG-PET scan showed progression of decreased FDG uptake in the frontal, lateral temporal, and parietal lobes bilaterally, greater on the left, with markedly decreased uptake in the mid and posterolateral aspect of the left frontal and superior aspect of the left temporal lobes. This is consistent with the patterns of atrophy (Amici et al., 2007; Botha et al., 2015; Gorno-Tempini, Murray, Rankin, Weiner, & Miller, 2004; K.A. Josephs et al., 2006), hypometabolism (Josephs, Duffy, Fossett, & et al., 2010), and tau tracer uptake (Josephs et al., 2018; Utianski, Whitwell, et al.) associated with agPPA in past studies. Additionally, involvement of the SMA is consistent with the presence of AOS (K.A. Josephs et al., 2006; Josephs et al., 2013; Josephs et al., 2012; Whitwell et al., 2013), both of which were noted at visit 2. Past research has demonstrated an association between hypometabolism in the superior temporoparietal regions and auditory agnosia of sounds and words (Vignolo, 2003), which could account for the patient’s prominent auditory deficit. While there was some involvement of the caudate, there were no detectable clinical correlates to this imaging finding, namely parkinsonism. Hypometabolism in the striatum has been documented in other cases of autopsy-proven corticobasal degeneration (Josephs et al., 2016; Josephs et al., 2008; Zalewski et al., 2014). We can ascertain from the current study, along with past research, that the aphasia reflects the apparent involvement of the language network, the AOS and NVOA reflect the involvement of the premotor and motor cortices, and the prominent auditory deficits is reflective of involvement of the posterior superior temporal cortices.
The exact underlying pathology was unclear throughout follow-up as she did not develop a clear, single variant of PPA or other neurologic syndrome. Specifically, she did not develop clinical findings that met criteria for corticobasal syndrome. However, the presence of AOS is highly suggestive of a 4 repeat tauopathy and hence this class of pathology was suspected. Alzheimer’s disease was not a concern given the results of her PiB PET scan. At autopsy, it was determined that she had CBD pathology. While there have been some cases of Pick’s disease (Hodges et al., 2004) or TDP-43 pathology associated with agPPA (Deramecourt et al., 2010; Mesulam et al., 2014; Spinelli et al., 2017), most pathological studies have reported a 4R-tauopathy, either CBD or PSP, as the underlying pathology associated with this clinical presentation (Josephs et al., 2011; K. A. Josephs et al., 2006; Mesulam et al., 2014; Mesulam et al., 2008) when AOS is present. Therefore, this case is consistent with the majority of other reported cases of agPPA with AOS who have come to autopsy.
While the clinical presentation may not have informed the underlying pathology and overall diagnostic categorization, identifying this disproportionate auditory deficit certainly impacted our recommendations for improved communication. Further, the patient demonstrated a deficit in the auditory input lexicon but not in the orthographic input lexicon. This is quite atypical in progressive neurological disorders. The presentation and neuroimaging of this patient may provide support for modality-specific input lexicons and potential anatomical correlates. The aforementioned clinical-anatomical associations will inform future studies.
Inherent in any case study are limitations. The absence of audiological findings to rule out a primary sensory deficit is not ideal; however, the prominent auditory deficit was unequivocal to the physicians and consultants examining the patient. We can therefore only describe, rather than explain, the clinical presentation. Further, the patient did also not undergo any formal test assessing music and non-environmental sound perception. Informal testing was administered once the clinical suspicion of auditory deficits arose, but baseline testing was unavailable. In addition, secondary to the nature of the research protocol, not all tests were administered in both modalities (spoken and written).
In summary, longitudinal assessment and observation of this patient provided invaluable information about the clinical emergence and evolution of a progressive, prominent auditory deficit in the context of PPA and AOS. Accurate identification of a prominent auditory deficit, which is atypical in PPA, is important for clinicians to provide appropriate education and therapy for affected patients and their families. It may also inform future studies of modality-specific input lexicon deficits.
Supplementary Material
6.0. Acknowledgments
We extend our gratitude to this patient and her family for their time and dedication to our research program.
This study was funded by the National Institutes of Health (NIH), National Institute on Deafness and Other Communication Disorders grants R01 DC010367 (PI: Josephs), R01 DC012519 (PI: Whitwell), and R01 DC014942 (PI: Josephs).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
The authors report no conflicts of interest.
Contributor Information
Joseph R. Duffy, Email: jduffy@mayo.edu.
Heather M. Clark, Email: clark.heather1@mayo.edu.
Mary M. Machulda, Email: machulda.mary@mayo.edu.
Dennis W. Dickson, Email: dickson.dennis@mayo.edu.
Jennifer L. Whitwell, Email: whitwell.jennifer@mayo.edu.
Keith A. Josephs, Email: josephs.keith@mayo.edu.
References
- Amici S, Ogar J, Brambati SM, Miller BL, Neuhaus J, Dronkers NL, & Gorno-Tempini ML (2007). Performance in specific language tasks correlates with regional volume changes in progressive aphasia. Cogn Behav Neurol, 20(4), 203–211. doi: 10.1097/WNN.0b013e31815e6265 [DOI] [PubMed] [Google Scholar]
- Auerbach SH, Allard T, Naeser M, Alexander MP, & Albert ML (1982). Pure word deafness. Analysis of a case with bilateral lesions and a defect at the prephonemic level. Brain, 105(Pt 2), 271–300. [DOI] [PubMed] [Google Scholar]
- Botha H, Duffy JR, Whitwell JL, Strand EA, Machulda MM, Schwarz CG, … Josephs KA (2015). Classification and clinicoradiologic features of primary progressive aphasia (PPA) and apraxia of speech. Cortex, 69, 220–236. doi: 10.1016/j.cortex.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns MS (2004). Clinical Management of Agnosia. Topics in Stroke Rehabilitation, 11(1), 1–9. doi: 10.1310/N13K-YKYQ-3XX1-NFAV [DOI] [PubMed] [Google Scholar]
- Ceccaldi M, Soubrouillard C, Poncet M, & Lecours AR (1996). A case reported by Serieux: The first description of a “primary progressive word deafness?”. Classic cases in neuropsychology, 45–52. [Google Scholar]
- Coslett HB, Brashear HR, & Heilman KM (1984). Pure word deafness after bilateral primary auditory cortex infarcts. Neurology, 34(3), 347–352. [DOI] [PubMed] [Google Scholar]
- De Renzi E, & Vignolo LA (1962). The token test: A sensitive test to detect receptive disturbances in aphasics. Brain, 85, 665–678. [DOI] [PubMed] [Google Scholar]
- Deramecourt V, Lebert F, Debachy B, Mackowiak-Cordoliani MA, Bombois S, Kerdraon O, … Pasquier F (2010). Prediction of pathology in primary progressive language and speech disorders. Neurology, 74(1), 42–49. [DOI] [PubMed] [Google Scholar]
- Dubois B, Slachevsky A, Litvan I, & Pillon B (2000). The FAB: a Frontal Assessment Battery at bedside. Neurology, 55(11), 1621–1626. [DOI] [PubMed] [Google Scholar]
- Duffy JR, Strand EA, & Josephs KA (2014). Motor Speech Disorders Associated with Primary Progressive Aphasia. Aphasiology, 28(8–9), 1004–1017. doi: 10.1080/02687038.2013.869307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelien A, Silbersweig D, Stern E, Huber W, Doring W, Frith C, & Frackowiak RSJ (1995). The functional anatomy of recovery from auditory agnosia - A PET study of sound categorization in a neurological patient and normal controls. Brain, 118(6), 1395–1409. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Gil-Navarro S, Lladó A, Rami L, Castellví M, Bosch B, Bargalló N, … Sánchez-Valle R (2013). Neuroimaging and biochemical markers in the three variants of primary progressive aphasia. Dementia and geriatric cognitive disorders, 35(1–2), 106–117. doi: 10.1159/000346289 [DOI] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, … LaPelle N (2008). Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord, 23(15), 2129–2170. doi: 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini M, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SE, & Manes F (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M, Murray R, Rankin K, Weiner M, & Miller B (2004). Clinical, cognitive and anatomical evolution from nonfluent progressive aphasia to corticobasal syndrome: a case report. Neurocase, 10, 426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter JL, Senjem ML, Vemuri P, & Jack CR Jr. (2012). Comparison of mask-based differences, boundary shift integral and symmetric normalization jacobian integration. Paper presented at the MICCAI 2012 workshop on novel imaging biomarkers for Alzheimer’s disease and related disorders (NIBAD’12). [Google Scholar]
- Harris JM, Gall C, Thompson JC, Richardson AMT, Neary D, du Plessis D, … Jones M (2013). Classification and pathology of primary progressive aphasia. Neurology, 81(21), 1832–1839. doi: 10.1212/01.wnl.0000436070.28137.7b [DOI] [PubMed] [Google Scholar]
- Hodges J, Rhys DR, H XJ, Barney C, Melissa B, H BT, … M. HG (2004). Clinicopathological correlates in frontotemporal dementia. Annals of Neurology, 56(3), 399–406. doi: 10.1002/ana.20203 [DOI] [PubMed] [Google Scholar]
- Iizuka O, Suzuki K, Endo K, Fujii T, & Mori E (2007). Pure word deafness and pure anarthria in a patient with frontotemporal dementia. Eur J Neurol, 14(4), 473–475. doi: 10.1111/j.1468-1331.2007.01671.x [DOI] [PubMed] [Google Scholar]
- Jack CR Jr., Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, … Petersen RC (2008). 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain, 131(Pt 3), 665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgens S, Biermann-Ruben K, Kurz MW, Flugel C, Daehli Kurz K, Antke C, … Schnitzler A (2008). Word deafness as a cortical auditory processing deficit: a case report with MEG. Neurocase, 14(4), 307–316. doi: 10.1080/13554790802363738 [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Fossett TR, & et al. (2010). Fluorodeoxyglucose f18 positron emission tomography in progressive apraxia of speech and primary progressive aphasia variants. Archives of Neurology, 67(5), 596–605. doi: 10.1001/archneurol.2010.78 [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand E, Whitwell J, Layton K, Parisi J, … Petersen RC (2006). Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain, 129, 1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Lowe VJ, … Whitwell JL (2013). Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology, 81(4), 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, … Whitwell JL (2012). Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain, 135(5), 1522–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Hodges JR, Snowden JS, Mackenzie IR, Neumann M, Mann DM, & Dickson DW (2011). Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol, 122(2), 137–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Petersen RC, Knopman DS, Boeve BF, Whitwell JL, Duffy JR, … Dickson DW (2006). Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology, 66(1), 41–48. doi: 10.1212/01.wnl.0000191307.69661.c3 [DOI] [PubMed] [Google Scholar]
- Josephs KA, R. MP, Hugo B, G. SC, R. DJ, M. CH, … L. WJ (2018). [18F]AV-1451 tau-PET and primary progressive aphasia. Annals of Neurology, 83(3), 599–611. doi:: 10.1002/ana.25183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell J, Tacik P, Duffy J, Senjem M, Tosakulwong N, … Murray M (2016). [18F]AV-1451 tau-PET uptake does correlate with quantitatively measured 4R-tau burden in autopsy-confirmed corticobasal degeneration. Acta Neuropathol, 931–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Dickson DW, Boeve BF, Knopman DS, Petersen RC, … Jack CR (2008). Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging, 29(2), 280–289. doi: 10.1016/j.neurobiolaging.2006.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, … DeKosky ST (2000). Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci, 12(2), 233–239. [DOI] [PubMed] [Google Scholar]
- Kertesz A (2007). Western Aphasia Battery (Revised). San Antonio, TX: PsychCorp. [Google Scholar]
- Kertesz A, Davidson W, & Fox H (1997). Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Canadian Journal of Neurological Sciences, 24(1), 29–36. [DOI] [PubMed] [Google Scholar]
- Kuramoto S, Hirano T, Uyama E, Tokisato K, Miura M, Watanabe S, & Uchino M (2002). [A case of slowly progressive aphasia accompanied with auditory agnosia]. Rinsho shinkeigaku = Clinical neurology, 42(4), 299–303. [PubMed] [Google Scholar]
- Lansing AE, Ivnik RJ, Cullum CM, & Randolph C (1999). An empirically derived short form of the Boston naming test. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists, 14(6), 481–487. [PubMed] [Google Scholar]
- Machulda MM, Ivnik RJ, Smith GE, Ferman TJ, Boeve BF, Knopman D, … Tangalos EG (2007). Mayo’s Older Americans Normative Studies: Visual Form Discrimination and copy trial of the Rey-Osterrieth Complex Figure. J Clin Exp Neuropsychol, 29(4), 377–384. doi: 10.1080/13803390600726803 [DOI] [PubMed] [Google Scholar]
- Maffei C, Capasso R, Cazzolli G, Colosimo C, Dell’Acqua F, Piludu F, … Miceli G (2017). Pure word deafness following left temporal damage: Behavioral and neuroanatomical evidence from a new case. Cortex, 97, 240–254. doi: 10.1016/j.cortex.2017.10.006 [DOI] [PubMed] [Google Scholar]
- Mesulam MM (1982). Slowly progressive aphasia without generalized dementia. Ann Neurol, 11(6), 592–598. [DOI] [PubMed] [Google Scholar]
- Mesulam MM (2001). Primary progressive aphasia. Ann Neurol, 49(4), 425–432. [PubMed] [Google Scholar]
- Mesulam MM, Weintraub S, Rogalski EJ, Wieneke C, Geula C, & Bigio EH (2014). Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia. Brain, 137(Pt 4), 1176–1192. doi: 10.1093/brain/awu024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, … Bigio EH (2008). Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol, 63(6), 709–719. doi: 10.1002/ana.21388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Wieneke C, Thompson C, Rogalski E, & Weintraub S (2012). Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain, 135(5), 1537–1553. doi: 10.1093/brain/aws080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima S, Frey KA, Koeppe RA, Foster NL, & Kuhl DE (1995). A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med, 36(7), 1238–1248. [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, … Chertkow H (2005). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc, 53(4), 695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Osterrieth P (1944). Le test de copie d’une figure complex: Coontribution a l’etude de la perception et de la memoire. Archives de Psychologie, 30, 286–356. [Google Scholar]
- Otsuki M, Soma Y, Sato M, Homma A, & Tsuji S (1998). Slowly progressive pure word deafness. European Neurology, 39(3), 135–140. doi: 10.1159/000007923 [DOI] [PubMed] [Google Scholar]
- Poeppel D (2001). Pure word deafness and the bilateral processing of the speech code. Cognitive Science, 25(5), 679–693. doi: 10.1207/s15516709cog2505_3 [DOI] [Google Scholar]
- Rapcsak SZ, Kentros M, & Rubens AB (1989). Impaired recognition of meaningful sounds in Alzheimer’s disease. Archives of Neurology, 46(12), 1298–1300. [DOI] [PubMed] [Google Scholar]
- Rey A (1964). L’examen clinique en psychologie. Paris: Presses Universitaires de France. [Google Scholar]
- Slevc LR, & Shell AR (2015). Auditory agnosia. Handb Clin Neurol, 129, 573–587. doi: 10.1016/b978-0-444-62630-1.00032-9 [DOI] [PubMed] [Google Scholar]
- Spinelli EG, Mandelli ML, Miller ZA, Santos-Santos MA, Wilson SM, Agosta F, … Gorno-Tempini ML (2017). Typical and atypical pathology in primary progressive aphasia variants. Ann Neurol, 81(3), 430–443. doi: 10.1002/ana.24885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, & Strauss E (1998). Compendium of Neuropsychological tests, second edition: administration, norms and commentary. New York: Oxford University Press. [Google Scholar]
- Steinberg BA, Bieliauskas LA, Smith GE, & Ivnik RJ (2005). Mayo’s Older Americans Normative Studies: Age- and IQ-Adjusted Norms for the Trail-Making Test, the Stroop Test, and MAE Controlled Oral Word Association Test. Clinical Neuropsychology, 19(3–4), 329–377. doi: 10.1080/13854040590945210 [DOI] [PubMed] [Google Scholar]
- Sugiura T, & Torii T (2017). Auditory agnosia caused by bilateral putamen haemorrhage. BMJ Case Rep, 2017. doi: 10.1136/bcr-2017-222535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, … Joliot M (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15(1), 273–289. doi: 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Utianski RL, Duffy JR, Clark HM, Strand E, Botha H, Schwarz CG, … Josephs KA (2018). Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain and Language, 184, 54–65. doi: 10.1016/j.bandl.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski RL, Whitwell JL, Schwarz CG, Duffy JR, Botha H, Clark HM, …Josephs KA (2018). Tau Uptake in Agrammatic Primary Progressive Aphasia with and without Apraxia of Speech. European Journal of Neurology. doi: 10.1111/ene.13733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttner I, Mottaghy FM, Schreiber H, Riecker A, Ludolph AC, & Kassubek J (2006). Primary progressive aphasia accompanied by environmental sound agnosia: a neuropsychological, MRI and PET study. Psychiatry Res, 146(2), 191–197. doi: 10.1016/j.pscychresns.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Vemuri P, Simon G, Kantarci K, Whitwell JL, Senjem ML, Przybelski SA, … Jack CR (2011). Antemortem Differential Diagnosis of Dementia Pathology using Structural MRI: Differential-STAND. Neuroimage, 55(2), 522–531. doi: 10.1016/j.neuroimage.2010.12.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignolo L, (2003). Music Agnosia and Auditory Agnosia. Ann N Y Acad Sci, 999(1), 50–57. doi: 10.1196/annals.1284.005 [DOI] [PubMed] [Google Scholar]
- Warrington EK, & James M (1991). The visual object and space perception battery. Bury St Edmonds, UK: Thames Valley Test Company. [Google Scholar]
- Whitwell JL, Duffy JR, Strand EA, Machulda MM, Senjem ML, Gunter JL, …Josephs KA (2013). Neuroimaging comparison of primary progressive apraxia of speech and progressive supranuclear palsy. European Journal of Neurology, 20(4), 629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicklund MR, Duffy JR, Strand EA, Machulda MM, Whitwell JL, & Josephs KA (2014). Quantitative Application of the PPA Consensus Criteria. Neurology, 82, 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorkston K, Strand E, Miller R, Hillel A, & Smith K (1993). Speech deterioration in amyotrophic lateral sclerosis: Implications for the timing of intervention. Journal of Medical Speech-Language Pathology, 1(1), 35–46. [Google Scholar]
- Zalewski N, Botha H, Whitwell JL, Lowe V, Dickson DW, & Josephs KA (2014). FDG-PET in pathologically confirmed spontaneous 4R-tauopathy variants. J Neurol, 261(4), 710–716. doi: 10.1007/s00415-014-7256-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.