Abstract
Age-related cognitive decline has been associated with proinflammatory cytokines, yet the precise relationship between cognitive decline and cytokine load remains to be elucidated. β-caryophyllene (BCP) is a cannabinoid receptor 2 (CB2) agonist with established anti-inflammatory effects that is known to improve memory and increase lifespan. It is of interest to explore the potential of BCP to reduce age-related cognitive decline and proinflammatory cytokine load. In this study, we assessed changes in circulating cytokines across the lifespan, memory performance in young and aged mice, and the effects of BCP on memory function and cytokine load. The plasma levels of 12 cytokines were assessed in male Swiss-Webster mice at 3, 12, and 18 months of age using multiplexed flow cytometry. Working memory was compared in 3 and 12 month-old mice using spontaneous alternations. A dose-response function (100–300 mg/kg, subchronic administration) for BCP-induced memory restoration was determined in 3 and 12 month-old mice. Finally, the effects on cytokine levels of the peak memory enhancing dose of BCP was assessed in 18 month-old mice. Circulating levels of several cytokines significantly increased with age. Multilinear regression analysis showed that IL-23 levels were most strongly associated with age. Aged mice showed deficits in working memory and higher levels of IL-23, both of which were reversed by BCP treatment. BCP appears to reverse age-associated impairments in memory and modulates cytokine production. IL-23 may play a significant role in the aging process, and future research should determine whether it has utility as a biomarker for novel anti-aging therapeutics.
Introduction
As the world population rapidly ages, it is important for measures to be taken to ensure quality of life as well as longevity in the middle aged to elderly. The literature provides evidence that aging provokes heightened inflammation throughout many organ systems, including the brain [1, 2]. Increased brain inflammation, or neuroinflammation, can sensitize the elderly brain to adverse effects, such as an increased vulnerability to the negative effects of stress [1]. The idea that aging is associated with a progressive decline in the ability to cope with stressors and a progressive increase in the whole body load of proinflammatory cytokines has been termed “inflamm-aging” [3]. It has been argued that inflamm-aging is driven by immunosenescence [4] and may be a key component of the etiology and progression of many aging-related diseases, such as atherosclerosis, heart disease, and type II diabetes. Inflamm-aging could have specific relevance for aging-related brain diseases like Alzheimer’s disease [5]. Despite the extensive research on the importance of inflamm-aging, we do not yet have a clear understanding of the specific proinflammatory cytokines that accelerate aging. A better characterization of these cytokines may provide novel biomarkers for the development of interventions to slow or reverse inflamm-aging.
Previous studies have related aging to levels of proinflammatory cytokines circulating in the blood. Perhaps the most well characterized cytokines in the context of aging are tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6). Increased circulating levels of TNF-α are associated with dementia [6] and predictive of mortality [7] in centenarians. The levels of circulating TNF-α and IL-6 are elevated and predictive of mortality in octogenarians [8, 9]. Elevated levels of IL-6, C-reactive protein, and the endogenous protein antagonist of the IL-1 receptor have been reported in a sample of more than 1000 participants aged 65 years and older, and were associated with declines in physical performance [10]. A causal relationship between cytokine load and exacerbated aging remains to be established, yet it has been shown that the presence of these proinflammatory cytokines can induce DNA damage [11], impair autophagic cleansing of tissue [12], induce oxidative stress [12, 13], accelerate stem cell aging [14], and trigger other processes that suggest that proinflammatory cytokines accelerate aging [15]. Additional research is necessary to fully characterize the precise changes in cytokine load associated with aging.
As humans age, an important determinant of quality of life is cognitive function. The progressive increase in circulating proinflammatory cytokines in inflamm-aging has been associated with age-related cognitive decline [16] as well as enhanced neuroinflammation, neurodegeneration, and brain release of cytokines by the microglia that act as resident phagocytic inflammatory cells in the brain [17]. Microglial cells are vital in recruiting these inflammatory mediators and activated microglial cells are neuropathological hallmarks in many neurodegenerative diseases [18, 19]. In animal models, neuroinflammation has been closely tied to impaired function of the hippocampus, an area of the brain critical for spatial working memory, as well as disruption of hippocampal-dependent cognitive tasks. Glial activation and cytokine production following exposure to lipopolysaccharide endotoxins impair spatial working memory as indexed by spontaneous alternation performance in the hippocampal-dependent T-maze task [20] and disrupts long-term potentiation in hippocampal synapses [21, 22]. In line with the depth in which they have been studied in the context of aging, TNF-α are IL-1β are two cytokines with well characterized roles in neuroinflammation-associated cognitive impairments. For example, acute application of IL-1β and TNF-α impairs hippocampal synaptic plasticity [23–25]. Likewise, in a mouse model of accelerated senescence, IL-1β levels are elevated in the hippocampus and TNF-α and IL-6 levels are elevated in the hippocampus and cortex as compared to control mice [26]. Few studies have examined the relationship between other proinflammatory cytokines and aging. The identification of cytokines specifically associated with aging may provide new targets for the amelioration of age-related neuroinflammation and cognitive decline, which is of great interest.
As outlined in this paragraph, a series of exciting recent studies has supported the endocannabinoid system as a promising target for the treatment of neuroinflammation and age-related cognitive decline, because activation of CB2 receptors may both ameliorate neuroinflammation and engender procognitive effects. Cannabinoid receptors are G-protein coupled receptors that are classified as two basic types: CB1 and CB2 receptors. CB1 receptors are mainly found in the brain where they mediate the psychoactive effects of cannabinoids that activate them. CB2 receptors are predominantly located in peripheral tissues and immune cells [27] but have been recently found to be present in the brain [28]. Mice that genetically lack CB2 receptors recapitulate the effects of aging, in that they exhibit impaired memory consolidation and reduced hippocampal synapses. Likewise, in the same study, wild-type mice showed impaired memory consolidation following administration of the CB2 receptor antagonist AM630 and enhanced memory consolidation following administration of the CB2 receptor agonist JWH133 [29]. Similarly, endocannabinoid enzyme inhibitors and synthetic agonists attenuate neuroinflammation and have procognitive effects in hippocampal-dependent tasks through activity at CB2 receptors [30–32]. The combination of reduced neuroinflammation and procognitive effects following activation of the CB2 receptor makes it a compelling target for treating age-related cognitive decline.
β-caryophyllene (BCP) is a naturally occurring phytocannabinoid that is a selective CB2 receptor agonist [33], and has been reported to attenuate oxidative stress, neuroinflammation, and glial activation in several model systems [34–36]. BCP seems to be particularly attractive as an agent to reverse inflamm-aging and associated cognitive decline. It is generally regarded as tolerable, safe, and non-toxic and has been approved by the US Food and Drug Administration and European Food and Safety Authority as a food additive. BCP has particular relevance for aging as it increases lifespan by 22% in round worms [37]. BCP also decreases cognitive deficits, inflammation in the hippocampus, and mRNA levels of IL-1β and TNF-α in the cortex of the APP/PS1 transgenic mouse model of Alzheimer’s disease [38]. To build upon this literature, in this study, we used multiplexed flow cytometry to assess changes in circulating cytokines across the life-span in wild-type mice to discover which cytokines are most closely associated with aging. We then determined whether BCP can reverse cognitive deficits in aged male mice and whether improved cognitive function is associated with decreased levels of aging-related cytokines.
Materials and Methods
Animals
Male Swiss-Webster mice (CFW; Charles River Laboratories, Inc.; Wilmington, MA) served as the subjects of these experiments. Swiss-Webster mice were chosen for these studies because these mice are a general purpose strain that has been used extensively to study behavior, physiology, and neurochemistry [39–43]. Mice were housed in groups of 2–3 mice and were given food (Laboratory Rodent Diet #5001, PMI Feeds, Inc., St. Louis, MO, USA) and water ad libitum. The temperature of the facility was maintained at 22–23.5 °C on a 12 hour light/12 hour dark cycle. Mice were routinely handled prior to the initiation of experimental data collection to minimize stress. A total of 21 mice were used to complete all experiments. Mice were maintained for at least two weeks after arrival in the vivarium prior to testing. All studies were carried out in accordance with the Guide for Care and Use of Laboratory animals as adopted and promulgated by the National Institutes of Health, and experimental protocols were approved by the Institutional Animal Care and Use Committee at Mercer University. Cytokine load was assessed in separate groups of mice at 3, 12, and 18 months of age as described below. Hippocampal spatial working memory was assessed in the mice at 3 and 12 months of age using the Y-maze task as described below. Mice were assessed repeatedly to determine whether a repeated measures design could be utilized with the Y-maze task in young and aged mice. These young and aged mice then underwent dosing with BCP across a range of ascending doses to assess working memory before and after treatment. The very aged 18-month-old mice underwent dosing with the most effective dose of BCP in the working memory study to assess its impact on circulating cytokine load.
Drugs and Dosing Regimen
BCP was commercially purchased (Sigma Aldrich, St. Louis, MI) and diluted to the desired concentrations with olive oil and administered by intraperitoneal (IP) administration. Each mouse was administered BCP on a Monday/Wednesday/Friday dosing schedule for one week with the Friday dosing being an acute dose administered 30 minutes prior to beginning the Y-maze test. The doses given were 100 mg/kg, 178 mg/kg and 300 mg/kg. All injection volumes across the doses were matched to each other and the vehicle volume through serial dilution of the stock of BCP, and all injections were less 0.6 ml per mouse. These doses and the dosing regimen were chosen based on previous studies [18, 36]. Between each dosing regimen, a washout period was observed for one week. After the wash out period, Y-maze performance was measured to ensure there were no lasting effects of BCP before the next treatments were initiated. The dosing regimens used to assess the effects of BCP on cognition and cytokine load were identical. As 178 mg/kg was the most effective dose of BCP in the memory study, it was also used for the cytokine study.
Cognitive Assessment
The use of spontaneous alternations in the Y-maze has been proposed to measure hippocampal-dependent spatial working memory [44]. Moreover, cytokines may impair memory by disrupting hippocampal synaptic plasticity [23–25] and CB2 agonists may reverse memory loss by restoring hippocampal synaptic plasticity [30–32]. The methods we used for this test are described in brief as they have been previously reported [39]. Each mouse was examined in the Y-maze for 10 minutes, and the sequence of arm entries and total arm entries were recorded. Following each test the maze was cleaned and wiped down with Decon 0.01%. Our primary measure of working memory was spontaneous alternations, calculated using the formula below:
This task is typically used for single acute assessments of working memory, often with a between subjects group design. However, given the time and logistical challenges of aging studies, and our desire to test a range of doses of BCP because the appropriate dose range for anti-aging effects was not previously known, we aimed to use a repeated-measures design for this memory study. Therefore, prior to beginning the drug dosing, we assessed performance in the Y-maze once per week (on Fridays) for 3 weeks in the 3- and 12-month-old mice to ensure performance was not altered by repeated testing.
Circulating Cytokine Analysis
Blood collection was performed humanely through submandibular bleeding [45]. Blood was collected from treatment naïve mice at the 3-, 12-, and 18-month time points. Blood was collected before and after treatment in the mice at 18 months. Each mouse was bled prior to treatments with a 2-week minimum recovery period prior to initiation of the treatment. For the treatment group, samples were taken 30 minutes after the final 178mg/kg BCP injection to match the memory study. Approximately 250 μL of whole blood was transferred into BD microcontainer tubes coated with 1 mg EDTA and was immediately chilled and subsequently centrifuged at 4200 RPM for 15 minutes at 4°C. Subsequently, plasma was collected, aliquoted, and at −80°C until analysis was performed. Cytokine concentrations were assessed using flow cytometry with bead-based multiplex immunoassay (Legendplex™, Biolegend, San Diego, CA) according to the manufacturer’s protocol. The results were read on a FACSAria II flow cytometer (BD Biosciences, San Jose CA) and the data were evaluated with the Legendplex™ data analysis software (VigeneTech, San Diego, CA). This multiplexed method provided data on 12 cytokines in total. These cytokines included TNF-α, IL-1β, and IL-6, which have been previously associated with aging. It also provided data on cytokines with a less well established relationship with aging, including IL-1α, monocyte chemoattractant protein-1 (MCP-1), IL-10, IL-17, IL-23, IL-27, interferon (IFN) - beta (IFNβ), IFNγ, and granulocytemacrophage colony-stimulating factor (GM-CSF).
Data Analysis
The circulating cytokine data were initially analyzed by one-way analysis of variance (ANOVA) for group differences at each of the ages. Linear regression analysis was then used to assess the association between age and cytokine level. To provide an integrated assessment of the relationship between cytokine level and age, all 12 cytokines were analyzed using multilinear regression analysis. Prior to the regression analysis, collinearity between cytokines was assessed based on the method of variable inflation factor (VIF) [46]. Briefly, a univariate elimination of cytokines was implemented based on the VIF value. The cytokine with the greatest VIF was removed first and new VIF values were generated. This process was repeated until a VIF value of < 5 was attained for all remaining cytokines. The final set of cytokines was included in the multilinear regression analysis. This analysis was performed in the R statistical software package (version 3.4.3). The dose-response function for the effects BCP on memory was assessed by repeated measures one-way ANOVA, with post-hoc comparisons by Dunnett’s test in comparison to vehicle. Treatment effects on cytokine levels were assessed by paired t-test, and corrected for multiple comparisons by the Bonferroni method to maintain the probability of making a type 1 error at 5%. All treatment analyses and graphical data presentations were created using GraphPad Prism (La Jolla, CA).
Results
Relationship Between Circulating Cytokines And Aging
Table 1 shows the results of one-way ANOVA for group differences in cytokine levels in 3-, 12-, and 18-month-old mice. To evaluate the association between cytokines and aging, multilinear regression was conducted. However, to minimize the chances for bias due to collinearity, we first investigated the presence of collinearity between cytokines using the VIF method. Cytokines were removed individually in a repeated manner in order to reach cytokines with a VIF < 5. As a result, seven cytokines (IL-23, IL-1α, INFγ, IL-1β, IL-6, IL-17α and GM-CSF) were retained and included in the multilinear regression (Table 2). Two cytokines, IL-23 and IL-1β, were identified to have a significant association with aging as evident by their p-values.
Table 1:
Differences in circulating cytokines in Swiss-Webster mice at 3, 12, and 18 months of age. Group differences in cytokine level across age were analyzed by one-way analysis of variance followed by the appropriate post hoc tests. The association between age and cytokine level was further evaluated by determining how well it fit a linear or an exponential increase function. There were significant increases in circulating TNF-α, MCP-1, IL-10, IL-23, IL-27, IFNβ, and IFNγ levels. There was a significant decrease in circulating IL-1β levels. The cytokine levels represent picograms of cytokine protein per milliliter of plasma.
| Cytokine | 3 Months (Mean ± SEM) | 12 Months (Mean ± SEM) | 18 Months (Mean ± SEM) | Group Difference (F value) | Group Difference (F value) |
|---|---|---|---|---|---|
| TNF-α | 6.59 +/− 3.17 | 20.03 +/− 0.73 | 39.11 +/− 5.06 | 19.47 | P < 0.0001 |
| IL-1β | 86.68 +/− 3.17 | 20.03 +/− 0.73 | 39.11 +/− 5.06 | 80.87 | P < 0.0001 |
| IL-6 | 14.59 +/− 1.54 | 37.30 +/− 15.04 | 20.52 +/− 1.07 | 2.85 | P = 0.08 |
| MCP-1 | 6.28 +/− 0.85 | 13.55 +/− 0.33 | 17.87 +/− 1.56 | 27.85 | P < 0.0001 |
| IL-1α | 41.71 +/− 14.51 | 57.80 +/− 17.05 | 20.58 +/− 2.19 | 2.25 | P = 0.13 |
| IL-10 | 21.81 +/− 3.59 | 180.30 +/− 10.04 | 138.90 +/− 8.24 | 126.80 | P < 0.0001 |
| IL-17 | 21.28 +/− 3.39 | 21.29 +/− 1.04 | 21.71 +/− 2.11 | 0.01 | P = 0.99 |
| IL-23 | 1.86 +/− 0.47 | 34.89 +/− 1.11 | 372.60 +/− 40.97 | 62.80 | P < 0.0001 |
| IL-27 | 53.45 +/− 11.38 | 110.30 +/− 4.08 | 480.40 +/− 17.84 | 298.60 | P < 0.0001 |
| IFNβ | 67.45 +/− 3.45 | 330.60 +/− 12.26 | 271.10 +/− 10.26 | 333.1 | P < 0.0001 |
| IFNγ | 5.30 +/− 2.31 | 27.14 +/− 1.60 | 30.62 +/− 4.48 | 17.98 | P < 0.0001 |
| GM-CSF | 30.01 +/− 11.27 | 46.70 +/− 1.26 | 45.66 +/− 2.48 | 1.59 | P = 0.23 |
Table 2: Multilinear Regression Analysis.
To evaluate the association between cytokines and aging, a multilinear regression was conducted. The R2 of the regression model was 0.935.
| Cytokine | Regression Coefficient | Standard Error | P value |
|---|---|---|---|
| IL-23 | 0.017116 | 0.003049 | P < 0.0001 |
| IL-1α | 0.000185 | 0.005611 | 0.9741 |
| IFNγ | 0.047750 | 0.049504 | 0.3491 |
| IL-1β | −0.146936 | 0.030955 | P < 0.0001 |
| IL-6 | 0.004026 | 0.024075 | 0.8692 |
| IL-17 | 0.035373 | 0.088869 | 0.6958 |
| GM-CSF | −0.034712 | 0.030023 | 0.2645 |
Effects of BCP on Working Memory
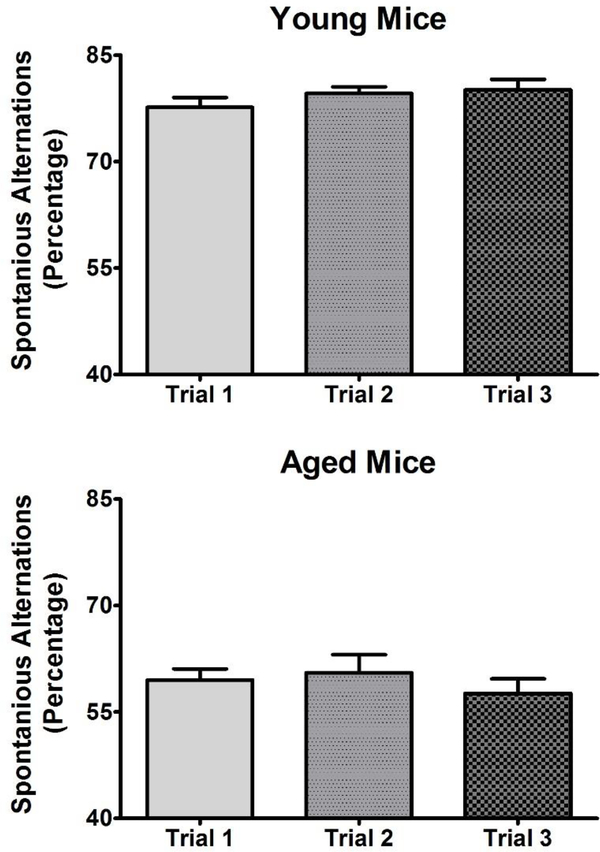
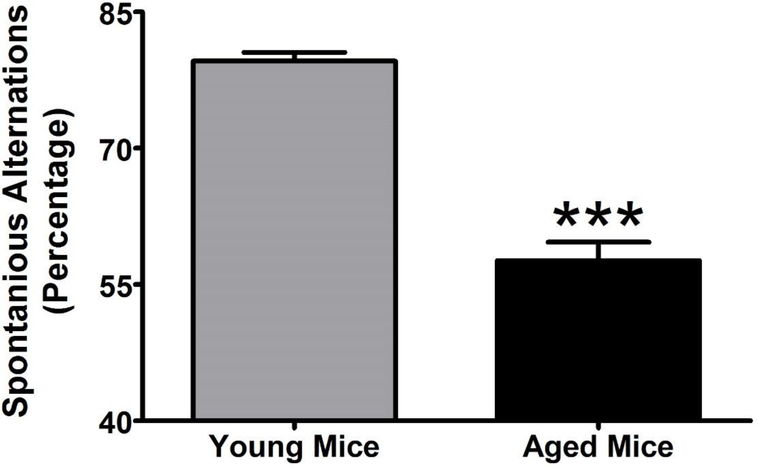
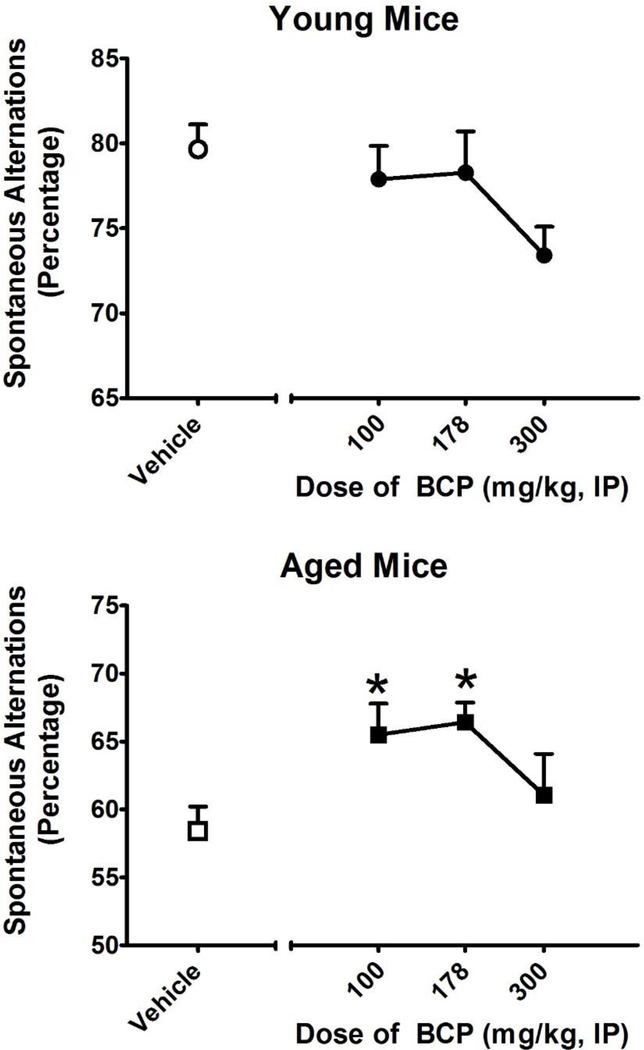
To assess the applicability of a repeated-measures design to the study of spontaneous alternations in the Y-maze in young and aged mice, we first determined whether performance changes (either improves or degrades) with repeated testing. As shown in Figure 1, changes in performance were assessed once per week for three weeks in young (N = 8) and aged (N = 7) mice. One-way repeated-measures ANOVA revealed no significant difference in performance across this testing schedule in young (F2,21 = 1.327; p = 0.287) or aged (F2,18 = 0.199; p = 0.821) mice. We then compared performance between the young and aged mice (Figure 2). Student’s t-test revealed that aged mice exhibited significantly (T13 = 10.16; p < 0.001) fewer spontaneous alternations than young mice in the Y-maze task. Moreover, BCP treatment significantly increased the number of spontaneous alternations in aged mice (F2,21 =4.79; p<0.05) but did not affect the performance of young mice (Figure 3). Dunnett’s post-hoc test revealed that aged mice exhibited significantly more spontaneous alternations at 100 and 178 mg/kg. As a control for behavioral disruption and decreased locomotor activity, we further examined the effects of BCP on the number of arms explored in the Y-maze over the 10-minute session. One-way repeated-measures ANOVA revealed a significant main effect of BCP treatment on arm entries in the young (F2,21 = 7.572; p < 0.001) but not the aged (F2,18 = 0.921; p = 0.450) mice. Dunnett’s post-hoc test revealed that young mice exhibited significantly fewer arm entries at 300 mg/kg.
Figure 1:
To ensure repeated measures did not alter Y-maze performance, the mice were examined three times on a once per week schedule following the same protocol to determine if there were any chances in performance. Each mouse was exposed to the Y-maze for 10 minutes after a 15-minute acclimation period to the testing room. For each 10-minute test, spontaneous alternations were calculated and averaged for each group. All values represent the mean ± SEM. N = 7–8 per group. There was no significant difference in the from the first to third trial in either the 3 month or 12 month mice indicating that his is a stable phenotype that is amenable to repeated measures designs.
Figure 2:
Base line performance in young (3 months of age) and aged (12 months of age) mice. Each mouse was exposed to the Y-maze for 10 minutes after a 15-minute acclimation period to the testing room. For each 10-minute test, spontaneous alternations were calculated and averaged for each group. All values represent the mean + SEM. N = 7–8 per group; ** = p < 0.001 as assessed by unpaired t-test.
Figure 3:
Effects of BCP treatment on spontaneous alternations in the Y-maze in young (3 months of age; TOP) and aged (12 months of age; BOTTOM) mice. Spontaneous alternations in the Y-maze are used to measure hippocampal-dependent spatial working memory. BCP was administered every other day for 1 week with the last dose administered 30 minutes before testing. Each mouse was exposed to the Y-maze for 10 minutes after a 15-minute acclimation period to the testing room. For each 10-minute test, spontaneous alternations were calculated and averaged for each group. All values represent the mean ± SEM. N= 7–8 per group; *= p < 0.05 as assessed by Dunnett’s post-hoc test.
Effects of BCP on Circulating Cytokines
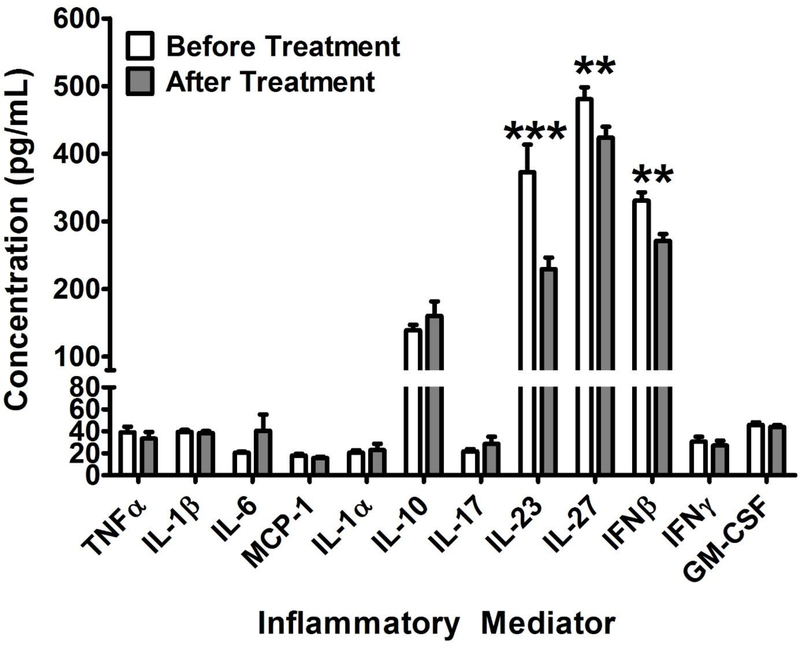
After analyzing the relationship between aging and cytokine levels, the effects of BCP were assessed by measuring cytokine levels before and after treatment. As seen in Figure 4, paired t-test revealed that BCP treatment significantly reduced circulating levels of IL-23 (T8 = 3.88; p < 0.001), IL-27 (T8 = 2.92; p < 0.01) and IFN-β (T8 = 4.17; p < 0.01) in 18 month old mice.
Figure 4:
The effects of BCP administered at 178 mg/kg on circulating cytokine levels in very aged mice (18 months of age). As in the memory study, BCP was administered every other day for 1 week with the last dose administered 30 minutes before blood samples were collected. Multiplexed flow cytometry was utilized to measure cytokine levels. Data are presented as the mean ± SEM; * = p<0.05, ** = p<0.01, *** = p<0.001 as assessed by students t-test for before and after treatment.
Discussion
The major findings of the present study are that: 1) IL-23 appears to be the cytokine (among those that we have studied) most strongly associated with aging, and 2) BCP both reverses age-related cognitive deficits and decreases circulating levels of IL-23. These data establish that there is a cognitive deficit in aged mice that have undergone natural age-related decline. This builds upon studies that have used toxin or genetic manipulations to accelerate the aging process. As such, this model may have translational relevance for the age-related decline faced by all humans. This study corroborates previous studies showing that aging is associated with increases in TNF-α and IL-6 levels, but also reports the novel finding, based on multilinear regression analysis, that IL-23 is the cytokine most closely associated with aging. The further findings that BCP both improves cognitive performance and decreases circulating IL-23 are suggestive of some causal relationship between IL-23 and cognitive decline, but this remains to be determined. Overall, our findings provide evidence of a novel immune pathway that may drive the aging process and a novel mechanism through which BCP, and perhaps other CB2 receptor agonists, may act to reverse age-related cognitive decline.
Neuroinflammation can lead to impairments in attention, mood, memory, decision making, problem solving, and motor function, all of which can significantly impact daily activities. Targeting neuroinflammation has been shown to be a viable option in combating age-related cognitive decline [2]. Nonsteroidal anti-inflammatory drugs (NSAIDS) improve declines in hippocampal-dependent memory and reverse increases in cytokine load in middle-aged rats [47] as well as reverse loss of hippocampal synaptic plasticity and hippocampal-dependent memory in mouse models of Alzheimer’s disease [48, 49]. Anti-inflammatory agents such as phenolic compounds found in fruits and vegetables, minocycline, resveratrol, and dietary flavonoids have all been reported to have benefits for aging [17]. The present study builds upon this literature as BCP has established anti-inflammatory properties. However, the present study, to the best of our knowledge, is also the first to associate the beneficial effects of an anti-aging compound to decreased concentrations of IL-23. In combination with the previously reported data that BCP may extend the lifespan [37], there is now an emerging set of convergent data that BCP specifically, and the CB2 receptor in general, is deserving of further study in the context of aging.
There are several additional lines of research that support additional study of BCP and the CB2 receptor in aging and age-related cognitive decline. It has been reported that the progressive increase in proinflammatory cytokines in inflamm-aging is associated with age-related cognitive decline [16]. Moreover, modifiable lifestyle factors such as improved sleep integrity and reduced adiposity are known to reduce proinflammatory cytokine load and protect cognitive function [16]. It has likewise been shown that stimulation of CB2 receptors improves diabetic insulin resistance due to adiposity [50] and improves sleep quality [51]. The convergence of these phenomena suggests that common mechanisms may be at work. In studies of cognitive function, the fatty-acid amide-hydrolase (FAAH) inhibitor URB597 reversed neuroinflammation and improved deficits in both short- and long-term visual recognition memory in rats that had been exposed to ethanol [32]. Pharmacological inhibition of monoacylglycerol lipase (MAGL) immediately after training of the inhibitory avoidance task enhances memory consolidation through activation of CB2 receptors [31]. Likewise, the synthetic cannabinoid agonist WIN55,212–2 and the FAAH inhibitor URB597 both enhance memory consolidation in the inhibitory avoidance task through a mechanism found to involve CB2 receptors [30].
We report in this study that BCP increased spontaneous alternation behavior at 100 and 178 mg/kg in the aged mice. We chose in the present study to utilize Y-maze assessments of working memory as this task selectively depends upon the hippocampus [44], cytokines may impair memory by disrupting hippocampal synaptic plasticity [23–25], and CB2 agonists may reverse memory loss by restoring hippocampal synaptic plasticity [30–32]. However, the Y-maze task uses within session learning and memory that is sensitive to environmental conditions, and the literature above documents that CB2 agonists also improve inhibitory and visual recognition memory. Future studies should extend our findings to additional learning and memory paradigms as the combination of potentially addressing pathophysiological changes that drive age-related decline and direct procognitive activity make the CB2 receptor a compelling target for age-related cognitive decline. It is notable that the 300 mg/kg dose did not show a similar effect to the other doses in the Y-maze. It did not significantly improve performance in the aged mice, and seemed to produce a modest impairment in young mice. We have not specifically determined the reason for this difference. There are many other examples of drugs that produce what has been termed an inverted-U shaped dose-response function in behavioral studies [52]. For other drug classes, this has been associated with the recruitment of additional pharmacological targets as well as non-specific behavior disrupting effects [52–55]. The 300 mg/kg dose of BCP reduced the number of alleys that young but not aged mice explored, suggesting that some behavioral disruption may have occurred.
We propose that BCP, and perhaps other CB2 agonists, may be in a unique position to slow or reverse age-related cognitive decline because they reduce levels of IL-23, which we have found to be the cytokine most strongly associated with aging. Our findings that 1) BCP appears to be more effective in aged mice than young mice, 2) IL-23 appears to only be elevated in aged mice, and 3) that BCP reduces IL-23 levels support this proposition. Previous studies also support an association between IL-23 and aging, as well as the capacity of CB2 receptor agonists to influence IL-23 levels. IL-23 is a member of the IL-12 family of cytokines that are released by brain microglial cells to promote and maintain cell-mediated inflammatory responses, including the release of TNF-α. In an AD mouse model, it has been shown that reduced IL-12/IL-23 signaling corresponds with a decreased plaque load and improved cognitive function [56]. CB2 receptors are known to be expressed on phagocytic cells and modulate the release of cytokines. In the brain, the IL-12/IL-23 pathway has been associated with the pathogenesis of multiple sclerosis. It has also been shown that anandamide works, at least partially, through CB2 receptors expressed on brain phagocytic microglial cells, and their associated ERK½ and JNK pathways, to inhibit IL-23 release [57]. This has been corroborated in similar studies [58, 59]. It is interesting to note that these studies also report that the anti-inflammatory cytokine IL-10 modulates IL-12/IL-23 signaling. We found that IL-10 levels were also raised in aged animals, perhaps as a compensatory response to the elevated level of IL-23. The roles of inflammatory signaling and inflammatory pathways in aging remains complicated and not particularly well understood. Beyond the complexity of the number of potential molecules and pathways that may be involved, individual differences in the response to cytokines such as IL-10 and TNF-α can play an important role in the final outcome of inflammation [60]. Nevertheless, the present study and its supporting literature suggest that this system is worthy of further study in the search for treatments to mitigate, slow, or reverse the symptoms of aging. It is also of interest that we did not find an age-related increase in IL-1β mice that underwent natural ageing, which lies in contrast to the elevations in IL-1β reported in genetic mouse model of accelerated senescence [26] or Alzheimer’s disease [38]. This suggests that IL-1β levels could, at least in mice, differentiate natural age-related decline from aging accelerated by pathophysiology.
Future studies should extend the findings reported in this manuscript. In addition to a chronic increase in cytokine load, inflamm-aging has been associated with immunosenescense, or long-term declines in immune system responsivity [61]. The immune system provides a healthy balance within the body to promote optimal functioning. Either over or under activity of this system can lead to deleterious consequences. We have focused on cytokine load in the current study for the reasons presented. However, future research should extend these studies to examine immunosenescense. Likewise, it is possible that sex may have important influences on age-related inflammatory systems, as it has been reported in humans that TNF-α is associated with male but not female mortality [8]. There could also be species dependent differences in inflamm-aging. As with humans and mice, aged horses show increased levels of TNF-α; however, they also shown increased levels of IL-15 and IL-18, both of which are poorly documented in humans and mice [62]. In the current study, we only examined male Swiss-Webster mice, and so, future research should examine whether our reported findings generalize across sex and species. In the present study, only systemic levels of IL-23 were determined. Unlike the known transport of TNF-α [63], IL-1 [64], and IL-6 [65] across the blood-brain barrier (BBB), the brain bioavailability of IL-23 is not particularly well understood. Moreover, it has been documented that only central levels of IL-23 drive the progression of experimental autoimmune encephalomyelitis in mice using viral-mediated IL-23 gene transfer to the brain or the periphery, demonstrating the importance of determining central levels of IL-23 in future studies [66]. Despite this, it has also been reported that elevated levels of TNF-α and IL-23 are associated with BBB disruption [67], IL-23 can act through its receptor to damage the neurovascular unit [68], disruption of the BBB can facilitate brain transport of cytokines [69], and there is crosstalk between IL-23 and TNF-α [70]. It is possible that age-associated increases in systemic levels of IL-23 could lead to increased brain infiltration of itself and other inflammatory cytokines through chronic and perhaps progressive disruption of the BBB.
Conclusions
In the present study, it was shown that IL-23 is the cytokine most closely related to aging in mice. BCP decreases IL-23, a cytokine belonging to the IL-12 family, and appears to reverse age-related cognitive decline in hippocampal dependent working memory. BCP appears to be worthy of further study as a treatment to ameliorate the effects of aging through inhibition of the IL-12 cytokine family.
Highlights.
Age-related cognitive decline has been associated with proinflammatory cytokines, but the precise relationship between cognitive decline and cytokine load remains to be elucidated.
Several proinflammatory cytokines significantly increased with age, but multilinear regression analysis showed that IL-23 levels were the most strongly associated with age.
Aged mice showed deficits in working memory in the hippocampal-dependent Y-maze task. The cannabinoid 2 receptor agonist β-caryophyllene improved working memory and decreased
IL-23 levels in aged mice.
BCP appears to reverse age-associated impairments in memory and IL-23 may play a significant role in the aging process.
Acknowledgements
These studies were supported by the National Institutes of Health [NS100512] and by funding from the Mercer University College of Pharmacy to KSM.
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sparkman NL, Johnson RW, Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress, Neuroimmunomodulation 15(4–6) (2008) 323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sartori AC, Vance DE, Slater LZ, Crowe M, The impact of inflammation on cognitive function in older adults: implications for healthcare practice and research, The Journal of neuroscience nursing : journal of the American Association of Neuroscience Nurses 44(4) (2012) 206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G, Inflamm-aging. An evolutionary perspective on immunosenescence, Ann N Y Acad Sci 908 (2000) 244–54. [DOI] [PubMed] [Google Scholar]
- [4].Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM, Franceschi C, Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes?, Frontiers in immunology 8 (2017) 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xia S, Zhang X, Zheng S, Khanabdali R, Kalionis B, Wu J, Wan W, Tai X, An Update on Inflamm-Aging: Mechanisms, Prevention, and Treatment, Journal of immunology research 2016 (2016) 8426874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhoj P, Pedersen BK, A high plasma concentration of TNF-alpha is associated with dementia in centenarians, J Gerontol A Biol Sci Med Sci 54(7) (1999) M357–64. [DOI] [PubMed] [Google Scholar]
- [7].Bruunsgaard H, Andersen-Ranberg K, Hjelmborg J, Pedersen BK, Jeune B, Elevated levels of tumor necrosis factor alpha and mortality in centenarians, The American journal of medicine 115(4) (2003) 278–83. [DOI] [PubMed] [Google Scholar]
- [8].Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jorgensen T, Pedersen BK, Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people, Clinical and experimental immunology 132(1) (2003) 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Roubenoff R, Parise H, Payette HA, Abad LW, D’Agostino R, Jacques PF, Wilson PW, Dinarello CA, Harris TB, Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study, The American journal of medicine 115(6) (2003) 429–35. [DOI] [PubMed] [Google Scholar]
- [10].Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, Guralnik JM, Ferrucci L, Inflammatory markers and physical performance in older persons: the InCHIANTI study, J Gerontol A Biol Sci Med Sci 59(3) (2004) 242–8. [DOI] [PubMed] [Google Scholar]
- [11].Bonafe M, Storci G, Franceschi C, Inflamm-aging of the stem cell niche: breast cancer as a paradigmatic example: breakdown of the multi-shell cytokine network fuels cancer in aged people, BioEssays : news and reviews in molecular, cellular and developmental biology 34(1) (2012) 40–9. [DOI] [PubMed] [Google Scholar]
- [12].Salminen A, Kaarniranta K, Kauppinen A, Inflammaging: disturbed interplay between autophagy and inflammasomes, Aging 4(3) (2012) 166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].De la Fuente M, Miquel J, An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflamm-aging, Curr Pharm Des 15(26) (2009) 3003–26. [DOI] [PubMed] [Google Scholar]
- [14].Jones DL, Rando TA, Emerging models and paradigms for stem cell ageing, Nature cell biology 13(5) (2011) 506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Murray CA, Lynch MA, Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation, J Neurosci 18(8) (1998) 2974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Atienza M, Ziontz J, Cantero JL, Low-grade inflammation in the relationship between sleep disruption, dysfunctional adiposity, and cognitive decline in aging, Sleep medicine reviews 42 (2018) 171–183. [DOI] [PubMed] [Google Scholar]
- [17].Sama DM, Norris CM, Calcium dysregulation and neuroinflammation: discrete and integrated mechanisms for age-related synaptic dysfunction, Ageing research reviews 12(4) (2013) 982–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yang M, Lv Y, Tian X, Lou J, An R, Zhang Q, Li M, Xu L, Dong Z, Neuroprotective Effect of beta-Caryophyllene on Cerebral Ischemia-Reperfusion Injury via Regulation of Necroptotic Neuronal Death and Inflammation: In Vivo and in Vitro, Front Neurosci 11 (2017) 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T, Inflammation and Alzheimer’s disease, Neurobiology of aging 21(3) (2000) 383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hauss-Wegrzyniak B, Vannucchi MG, Wenk GL, Behavioral and ultrastructural changes induced by chronic neuroinflammation in young rats, Brain Res 859(1) (2000) 157–66. [DOI] [PubMed] [Google Scholar]
- [21].Hauss-Wegrzyniak B, Lynch MA, Vraniak PD, Wenk GL, Chronic brain inflammation results in cell loss in the entorhinal cortex and impaired LTP in perforant path-granule cell synapses, Experimental neurology 176(2) (2002) 336–41. [DOI] [PubMed] [Google Scholar]
- [22].Kelly A, Vereker E, Nolan Y, Brady M, Barry C, Loscher CE, Mills KH, Lynch MA, Activation of p38 plays a pivotal role in the inhibitory effect of lipopolysaccharide and interleukin-1 beta on long term potentiation in rat dentate gyrus, The Journal of biological chemistry 278(21) (2003) 19453–62. [DOI] [PubMed] [Google Scholar]
- [23].Tancredi V, D’Arcangelo G, Grassi F, Tarroni P, Palmieri G, Santoni A, Eusebi F, Tumor necrosis factor alters synaptic transmission in rat hippocampal slices, Neurosci Lett 146(2) (1992) 176–8. [DOI] [PubMed] [Google Scholar]
- [24].Bellinger FP, Madamba S, Siggins GR, Interleukin 1 beta inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus, Brain Res 628(1–2) (1993) 227–34. [DOI] [PubMed] [Google Scholar]
- [25].Katsuki H, Nakai S, Hirai Y, Akaji K, Kiso Y, Satoh M, Interleukin-1 beta inhibits long-term potentiation in the CA3 region of mouse hippocampal slices, Eur J Pharmacol 181(3) (1990) 323–6. [DOI] [PubMed] [Google Scholar]
- [26].Tha KK, Okuma Y, Miyazaki H, Murayama T, Uehara T, Hatakeyama R, Hayashi Y, Nomura Y, Changes in expressions of proinflammatory cytokines IL-1beta, TNF-alpha and IL-6 in the brain of senescence accelerated mouse (SAM) P8, Brain Res 885(1) (2000) 25–31. [DOI] [PubMed] [Google Scholar]
- [27].Munro S, Thomas KL, Abu-Shaar M, Molecular characterization of a peripheral receptor for cannabinoids, Nature 365(6441) (1993) 61–5. [DOI] [PubMed] [Google Scholar]
- [28].Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR, Cannabinoid CB2 receptors: immunohistochemical localization in rat brain, Brain Res 1071(1) (2006) 10–23. [DOI] [PubMed] [Google Scholar]
- [29].Garcia-Gutierrez MS, Ortega-Alvaro A, Busquets-Garcia A, Perez-Ortiz JM, Caltana L, Ricatti MJ, Brusco A, Maldonado R, Manzanares J, Synaptic plasticity alterations associated with memory impairment induced by deletion of CB2 cannabinoid receptors, Neuropharmacology 73 (2013) 388–96. [DOI] [PubMed] [Google Scholar]
- [30].Ratano P, Palmery M, Trezza V, Campolongo P, Cannabinoid Modulation of Memory Consolidation in Rats: Beyond the Role of Cannabinoid Receptor Subtype 1, Frontiers in pharmacology 8 (2017) 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ratano P, Petrella C, Forti F, Passeri PP, Morena M, Palmery M, Trezza V, Severini C, Campolongo P, Pharmacological inhibition of 2-arachidonoilglycerol hydrolysis enhances memory consolidation in rats through CB2 receptor activation and mTOR signaling modulation, Neuropharmacology 138 (2018) 210–218. [DOI] [PubMed] [Google Scholar]
- [32].Rivera P, Fernandez-Arjona MDM, Silva-Pena D, Blanco E, Vargas A, Lopez-Avalos MD, Grondona JM, Serrano A, Pavon FJ, Rodriguez de Fonseca F, Suarez J, Pharmacological blockade of fatty acid amide hydrolase (FAAH) by URB597 improves memory and changes the phenotype of hippocampal microglia despite ethanol exposure, Biochem Pharmacol 157 (2018) 244–257. [DOI] [PubMed] [Google Scholar]
- [33].Gertsch J, Leonti M, Raduner S, Racz I, Chen JZ, Xie XQ, Altmann KH, Karsak M, Zimmer A, Beta-caryophyllene is a dietary cannabinoid, Proc Natl Acad Sci U S A 105(26) (2008) 9099–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Aso E, Ferrer I, CB2 Cannabinoid Receptor As Potential Target against Alzheimer’s Disease, Front Neurosci 10 (2016) 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Klauke A-L, Racz I, Pradier B, Markert A, Zimmer A, Gertsch J, Zimmer A, The cannabinoid CB2 receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain, European Neuropsychopharmacology 24(4) (2014) 608–620. [DOI] [PubMed] [Google Scholar]
- [36].Ojha S, Javed H, Azimullah S, Haque ME, beta-Caryophyllene, a phytocannabinoid attenuates oxidative stress, neuroinflammation, glial activation, and salvages dopaminergic neurons in a rat model of Parkinson disease, Molecular and cellular biochemistry 418(1–2) (2016) 59–70. [DOI] [PubMed] [Google Scholar]
- [37].Pant A, Mishra V, Saikia SK, Shukla V, Asthana J, Akhoon BA, Pandey R, Beta-caryophyllene modulates expression of stress response genes and mediates longevity in Caenorhabditis elegans, Experimental gerontology 57 (2014) 81–95. [DOI] [PubMed] [Google Scholar]
- [38].Cheng Y, Dong Z, Liu S, beta-Caryophyllene ameliorates the Alzheimer-like phenotype in APP/PS1 Mice through CB2 receptor activation and the PPARgamma pathway, Pharmacology 94(1–2) (2014) 1–12. [DOI] [PubMed] [Google Scholar]
- [39].Ray A, Chitre NM, Daphney CM, Blough BE, Canal CE, Murnane KS, Effects of the second-generation “bath salt” cathinone alpha-pyrrolidinopropiophenone (alpha-PPP) on behavior and monoamine neurochemistry in male mice, Psychopharmacology (Berl) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Murphy TJ, Murnane KS, The serotonin 2C receptor agonist WAY-163909 attenuates ketamine-induced hypothermia in mice, Eur J Pharmacol 842 (2019) 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Murnane KS, Perrine SA, Finton BJ, Galloway MP, Howell LL, Fantegrossi WE, Effects of exposure to amphetamine derivatives on passive avoidance performance and the central levels of monoamines and their metabolites in mice: correlations between behavior and neurochemistry, Psychopharmacology (Berl) 220(3) (2012) 495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Oppong-Damoah A, Zaman RU, D’Souza MJ, Murnane KS, Nanoparticle encapsulation increases the brain penetrance and duration of action of intranasal oxytocin, Horm Behav 108 (2019) 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Murnane KS, Guner OF, Bowen JP, Rambacher KM, Moniri NH, Murphy TJ, Daphney CM, Oppong-Damoah A, Rice KC, The adrenergic receptor antagonist carvedilol interacts with serotonin 2A receptors both in vitro and in vivo, Pharmacol Biochem Behav (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Walker DL, Gold PE, Intrahippocampal administration of both the D- and the L-isomers of AP5 disrupt spontaneous alternation behavior and evoked potentials, Behav Neural Biol 62(2) (1994) 151–62. [DOI] [PubMed] [Google Scholar]
- [45].Golde WT, Gollobin P, Rodriguez LL, A rapid, simple, and humane method for submandibular bleeding of mice using a lancet, Lab animal 34(9) (2005) 39–43. [DOI] [PubMed] [Google Scholar]
- [46].Akinwande MO, Dikko HG, Samson A, Variance Inflation Factor: As a Condition for the Inclusion of Suppressor Variable(s) in Regression Analysis, Open Journal of Statistics Vol.05No.07 (2015) 14. [Google Scholar]
- [47].Casolini P, Catalani A, Zuena AR, Angelucci L, Inhibition of COX-2 reduces the age-dependent increase of hippocampal inflammatory markers, corticosterone secretion, and behavioral impairments in the rat, J Neurosci Res 68(3) (2002) 337–43. [DOI] [PubMed] [Google Scholar]
- [48].Kotilinek LA, Westerman MA, Wang Q, Panizzon K, Lim GP, Simonyi A, Lesne S, Falinska A, Younkin LH, Younkin SG, Rowan M, Cleary J, Wallis RA, Sun GY, Cole G, Frautschy S, Anwyl R, Ashe KH, Cyclooxygenase-2 inhibition improves amyloid-beta-mediated suppression of memory and synaptic plasticity, Brain 131(Pt 3) (2008) 651–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bachstetter AD, Norris CM, Sompol P, Wilcock DM, Goulding D, Neltner JH, St Clair D, Watterson DM, Van Eldik LJ, Early stage drug treatment that normalizes proinflammatory cytokine production attenuates synaptic dysfunction in a mouse model that exhibits age-dependent progression of Alzheimer’s disease-related pathology, J Neurosci 32(30) (2012) 10201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Youssef DA, El-Fayoumi HM, Mahmoud MF, Beta-caryophyllene alleviates diet-induced neurobehavioral changes in rats: The role of CB2 and PPAR-gamma receptors, Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 110 (2019) 145–154. [DOI] [PubMed] [Google Scholar]
- [51].Calik MW, Carley DW, Effects of Cannabinoid Agonists and Antagonists on Sleep and Breathing in Sprague-Dawley Rats, Sleep 40(9) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Fantegrossi WE, Murnane KS, Reissig CJ, The behavioral pharmacology of hallucinogens, Biochem Pharmacol 75(1) (2008) 17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Murnane KS, The renaissance in psychedelic research: What do preclinical models have to offer, Prog Brain Res 242 (2018) 25–67. [DOI] [PubMed] [Google Scholar]
- [54].Murnane KS, Serotonin 2A receptors are a stress response system: implications for post-traumatic stress disorder, Behavioural pharmacology (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Canal CE, Murnane KS, The serotonin 5-HT2C receptor and the non-addictive nature of classic hallucinogens, Journal of psychopharmacology (Oxford, England) 31(1) (2017) 127–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Vom Berg J, Prokop S, Miller KR, Obst J, Kalin RE, Lopategui-Cabezas I, Wegner A, Mair F, Schipke CG, Peters O, Winter Y, Becher B, Heppner FL, Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease-like pathology and cognitive decline, Nature medicine 18(12) (2012) 1812–9. [DOI] [PubMed] [Google Scholar]
- [57].Correa F, Docagne F, Mestre L, Clemente D, Hernangomez M, Loria F, Guaza C, A role for CB2 receptors in anandamide signalling pathways involved in the regulation of IL-12 and IL-23 in microglial cells, Biochem Pharmacol 77(1) (2009) 86–100. [DOI] [PubMed] [Google Scholar]
- [58].Correa F, Hernangomez-Herrero M, Mestre L, Loria F, Docagne F, Guaza C, The endocannabinoid anandamide downregulates IL-23 and IL-12 subunits in a viral model of multiple sclerosis: evidence for a cross-talk between IL-12p70/IL-23 axis and IL-10 in microglial cells, Brain, behavior, and immunity 25(4) (2011) 736–49. [DOI] [PubMed] [Google Scholar]
- [59].Correa F, Mestre L, Docagne F, Guaza C, Activation of cannabinoid CB2 receptor negatively regulates IL-12p40 production in murine macrophages: role of IL-10 and ERK½ kinase signaling, British journal of pharmacology 145(4) (2005) 441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lio D, Scola L, Crivello A, Colonna-Romano G, Candore G, Bonafe M, Cavallone L, Marchegiani F, Olivieri F, Franceschi C, Caruso C, Inflammation, genetics, and longevity: further studies on the protective effects in men of IL-10 −1082 promoter SNP and its interaction with TNF-alpha −308 promoter SNP, Journal of medical genetics 40(4) (2003) 296–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].De Martinis M, Franceschi C, Monti D, Ginaldi L, Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity, FEBS letters 579(10) (2005) 2035–9. [DOI] [PubMed] [Google Scholar]
- [62].Adams AA, Breathnach CC, Katepalli MP, Kohler K, Horohov DW, Advanced age in horses affects divisional history of T cells and inflammatory cytokine production, Mechanisms of ageing and development 129(11) (2008) 656–64. [DOI] [PubMed] [Google Scholar]
- [63].Gutierrez EG, Banks WA, Kastin AJ, Murine tumor necrosis factor alpha is transported from blood to brain in the mouse, Journal of neuroimmunology 47(2) (1993) 169–76. [DOI] [PubMed] [Google Scholar]
- [64].Banks WA, Kastin AJ, Gutierrez EG, Interleukin-1 alpha in blood has direct access to cortical brain cells, Neurosci Lett 163(1) (1993) 41–4. [DOI] [PubMed] [Google Scholar]
- [65].Banks WA, Kastin AJ, Gutierrez EG, Penetration of interleukin-6 across the murine blood-brain barrier, Neurosci Lett 179(1–2) (1994) 53–6. [DOI] [PubMed] [Google Scholar]
- [66].Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD, Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain, Nature 421(6924) (2003) 744–8. [DOI] [PubMed] [Google Scholar]
- [67].Cheng Y, Desse S, Martinez A, Worthen RJ, Jope RS, Beurel E, TNFalpha disrupts blood brain barrier integrity to maintain prolonged depressive-like behavior in mice, Brain, behavior, and immunity 69 (2018) 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wang M, Zhong D, Zheng Y, Li H, Chen H, Ma S, Sun Y, Yan W, Li G, Damage effect of interleukin (IL)-23 on oxygen-glucose-deprived cells of the neurovascular unit via IL-23 receptor, Neuroscience 289 (2015) 406–16. [DOI] [PubMed] [Google Scholar]
- [69].Pan W, Banks WA, Kennedy MK, Gutierrez EG, Kastin AJ, Differential permeability of the BBB in acute EAE: enhanced transport of TNT-alpha, The American journal of physiology 271(4 Pt 1) (1996) E636–42. [DOI] [PubMed] [Google Scholar]
- [70].Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, Lucian L, Geissler R, Brodie S, Kimball AB, Gorman DM, Smith K, de Waal Malefyt R, Kastelein RA, McClanahan TK, Bowman EP, IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis, The Journal of experimental medicine 203(12) (2006) 2577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]