Abstract
Beta-site amyloid precursor protein-cleaving enzyme 1 (BACE1) is best known for its role in Alzheimer’s disease amyloid plaque formation, but also contributes to neurodegenerative processes triggered by CNS injury. Here we report that BACE1 is expressed in murine CD4+ T cells and regulates signaling through the T cell receptor. BACE1 deficient T cells have reduced IL-17A expression under Th17 conditions, and reduced CD73 expression in Th17 and inducible Tregs. However, induction of the Th17 and Treg transcription factors RORγt and Foxp3 were unaffected. BACE1-deficient T cells showed impaired pathogenic function in experimental autoimmune encephalomyelitis (EAE). These data identify BACE1 as a novel regulator of T cell signaling pathways that impact autoimmune inflammatory T cell function.
INTRODUCTION
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system initiated by myelin-reactive T cells that produce cytokines that cause direct damage to CNS tissue as well as triggering recruitment and activation of macrophages and myelin-reactive autoantibody-producing B cells. Th17 cells were initially defined as a distinct pro-inflammatory CD4+ T helper cell subset in the mouse model of MS, experimental autoimmune encephalomyelitis (EAE) (1). Although previously considered a Th1-mediated disease, numerous studies have consistently demonstrated the vital contribution of Th17 cells to EAE development (2), including the finding that the majority of IFNγ+ Th1 cells found in the CNS of mice with EAE are in fact derived from the Th17 lineage (3). Clinical trials testing blockade of IL-17A in relapsing-remitting MS are showing promise, supporting the importance of this pathway in MS (4). IL-17A acts on multiple CNS-resident cells to potentiate inflammation. Astrocytes respond to IL-17A by producing chemokines to facilitate recruitment of inflammatory cells such as macrophages and neutrophils (5–9). Likewise, oligodendrocytes contribute to the Th17 inflammatory response (10, 11) and are also induced to undergo apoptosis in response to IL-17A signaling through Act1 (10). IL-17A can also be directly neurotoxic by activating Ca+ flux in neurons (12). Hence, accumulated damage, not only to neurons but also to the cells that support them, impairs future CNS function, leading to permanent disability.
BACE1 is a transmembrane aspartyl protease that was initially identified for its role in cleavage of amyloid precursor protein (APP) to generate amyloidβ peptides that form plaques in Alzheimer’s disease (AD) (13). Blockade of BACE1, either by genetic deletion or chemical inhibitors, greatly reduces amyloid plaque formation in mouse models of AD, and BACE1 inhibitors are now being tested in clinical trials for AD (14). Inflammatory signals, including hypoxia and cytokines such as IL-1 and TNF, contribute to upregulation of BACE1 in AD (15, 16), while non-steroidal anti-inflammatory drugs reduce BACE1 expression and associated plaque burden (17, 18). Furthermore, BACE1 expression increases following damage to the CNS due to ischemia (stroke) (19–22) and traumatic brain injury (23, 24), and BACE1 deficient mice show reduced lesion volume and better recovery following traumatic brain injury (24), although not all studies find the same result (25). Thus it appears that BACE1 may have additional functions in neuroinflammatory or neurodegenerative responses beyond Alzheimer’s disease, although this has not been investigated in MS.
Intriguingly, the IL-23-IL-17A axis has also been found to promote neurodegeneration and impair recovery following brain ischemia (26–28), but any connection between BACE1 and Th17 cells have not been probed. We therefore queried a potential role for BACE1 in the immune system, and report here that BACE1 is expressed in CD4+ T cells and modulates T cell response to TCR ligation, as well as the some effector molecules in Th17 and Treg differentiation. Ultimately, BACE1-deficient T cells were found to have reduced inflammatory capacity in the EAE model, indicating an important functional role for this neurodegenerative protein in the immune system.
MATERIALS AND METHODS
Mice
BACE1−/−(29), C57BL/6, CD45.1+, 2D2, and RAG1−/− mice were purchased from Jackson Labs. Animals were housed and bred under SPF conditions in an AAALAC-approved facility and all animal procedures were approved by the IACUC committee at the University of Pittsburgh.
In vitro CD4+ T cell differentiation
CD4+ T cells from spleens and lymph nodes of naive mice were purified by magnetic separation (Miltenyi Biotec, Germany). T cells were activated by plate-bound anti-CD3 (clone 145-TC11, 5μg/ml; BioXcell) in RPMI medium supplemented with 10% fetal bovine serum, 2mM L-glutamine, 100 U/ml penicillin, 100μg/ml streptomycin, and 50μM 2-β-mercaptoethanol, Hepes and Na pyruvate. For Th17 differentiation, cells were cultured in the presence of recombinant mouse IL-1β (40ng/mL), IL-23 (20ng/ml), IL-6 (100ng/ml), TGFβ1 (10ng/ml); all cytokines from R&D Systems, MN. In all Th0 cell cultures anti-IFN-γ neutralizing antibodies (10μg/ml, BioXcell) were added. For Th1 cultures, IL-12 (PeproTech, NJ) was added at 10ng/ml. For Treg differentiation, T cells were cultured in the presence of recombinant mouse TGFβ1 (20ng/ml), recombinant human IL-2 (100U/mL) and anti-IFN-γ neutralizing antibodies (10μg/ml).
To assess cAMP production, 4 million CD4+ T cells isolated from WT or BACE1−/− mice were stimulated for 30 minutes with 10ug/ml Forskolin (EMD Millipore). cAMP levels in cell lysates were then analyzed using the cAMP Assay kit from Abcam, USA.
EAE induction
Active immunization: mice were immunized subcutaneously with 100μg MOG(35–55) (Bio synthesis, Lewisville, Texas, USA) emulsified in 200μl CFA (Difco Laboratories, Detroit, Michigan, USA) containing 100μg Heat Killed Mycobacterium tuberculosis H37Ra (Difco Laboratories, Detroit, Michigan, USA) distributed in four sites on the flank. 200ng Pertussis toxin (List Biological Laboratories) was given intraperitoneally on day 0 and 2. For RAG−/− transfer experiments, all LN and spleen were harvested from donor C57Bl/6 mice or BACE1−/− mice, and CD4+ cells were isolated by magnetic separation using CD4 microbeads (Miltenyi Biotec). 10–14 million CD4+ cells were transferred intraperitoneally to naïve RAG1−/− recipients, which were immunized the following day to as described above.
Passive transfer of EAE: LN and spleen were harvested from wt or BACE1−/− 2D2 mice and stimulated in vitro according to protocol described by Jager et al (30). Briefly, cells were activated with MOG35–55 (20ug/mL) in the presence TGFβ and IL-6 (5 and 50ng/mL respectively) for 4 days. Cells were washed, split and resuspended in complete RPMI containing IL-2 (10U/mL). After three days of resting, cells were reactivated in 24-well plates with plate-bound αCD3 (1ug/mL) and soluble IL-23 (20ng/mL) for two days before transferring to recipient mice. IL-17A expression was determined by flow cytometry at the end of the first activation stage (Day 4).
Clinical scoring: EAE was assessed according to the following clinical grades: 1: flaccid tail; 2: impaired righting reflex and hindlimb weakness; 3: partial hindlimb paralysis; 4: complete hindlimb paralysis; 5: hindlimb paralysis with partial forelimb paralysis; 6: moribund/dead. Atypical EAE was noted when mice demonstrated advanced ataxia, circling, head tilt with or without classical signs of EAE, these mice were scored as grade 2 if other classical signs of EAE were not present.
Flow cytometry and Imagestream
The following FACS antibodies were purchased from BD Biosciences: CD4 (RM4–5), IL-17A (TC11–18H10), CD25 (7D4). The following were purchased from eBioscience (Invitrogen): RORγt (AKFJS9), Foxp3 (FJK-16s), CD73 (eBIOTY/11.8), IFNγ (XMG1.2), GM-CSF (MP1–22E9). For cytokine analysis, cells were cultured in complete medium (RPMI media containing 10% FCS, supplemented with Pen-Strep, L-Glutamine, HEPES, sodium pyruvate, and 2-ME) with 50 ng/ml PMA and 500 ng/ml ionomycin (Sigma-Aldrich) in the presence of Golgiplug (BD Biosciences) for 3 to 4 hours followed by FACS staining and analysis. For intracellular cytokines staining was performed using Cytofix-cytoperm kit from BD; RORγt and Foxp3 intracellular stains were performed using eBioscience Foxp3 staining kit according to manufacturer’s instructions. Samples were acquired on a BD Fortessa, and analyzed using FlowJo (Treestar).
For imagestream analysis, cells were stained with anti-CD4, then fixed overnight before staining with anti-BACE1 (D10E5 rabbit mAb; Cell Signaling) in permeabilization buffer containing 2% BSA, followed by secondary antibody labeling using FITC-conjugated or PE-conjugated donkey-anti-rabbit. Samples were acquired on an ImageStreamX MkII imaging cytometer, at 60X magnification, with low flow rate/high sensitivity using INSPIRE software. Data analyzed using IDEAS 6.2 software according to the manufacture guidelines.
ELISA
IL-17A and IFNγ production was analyzed using Ready-Set-Go kits (eBioscience) in culture supernatants from in vitro T cell differentiation (as described above), or supernatants from ex vivo stimulated cultures as follows: dLN were isolated from mice with EAE and single cell suspensions obtained, cells were cultured for indicated times with 50ug/ml MOG(35–55) and 10ng/ml IL-12 (Peprotech) to promote IFNγ or 20ng/ml IL-23 (R&D Systems) to promote IL-17 production.
qRT-PCR
RNA was isolated using an RNeasy Mini Kit (QIAGEN) and converted to cDNA using a High Capacity cDNA Reverse Transcription Kit (Applied Bioscences). Gene expression was quantified using Excella SYBR Mastemix Rox (WWMP) with RT2 qPCR primers (QIAGEN – Gapdh-PPM02946E, Il17a-PPM03023A, Rorc-PPM25095A, Il23r-PPM33761A, Tbx21-PPM03727A, Foxp3-PPM05497F) in a 7300 Real Time PCR System (Applied Byosystems).
Western blotting
Western blots were performed using antibodies from Cell Signaling Technology: phospho-473 Akt (C67E7), Pten (D4.3) and β-actin (D6A8). T cells for western blot analysis were stimulated with soluble anti-CD3 and anti-CD28 crosslinked by streptavidin, in presence of Th17-inducing cytokines.
Statistics
ONE-WAY ANOVA (for multiple groups) or Student’s t-test were performed for experiments with parametric values (such as FACS %); Mann-Whitney test test was performed for EAE experiments, analyzing scores for each day separately. P values are shown as * = p <0.05, ** = p < 0.01, and *** =p < 0.001, where statistical significance was found, and all data are represented as means + standard deviation (s.d.).
RESULTS
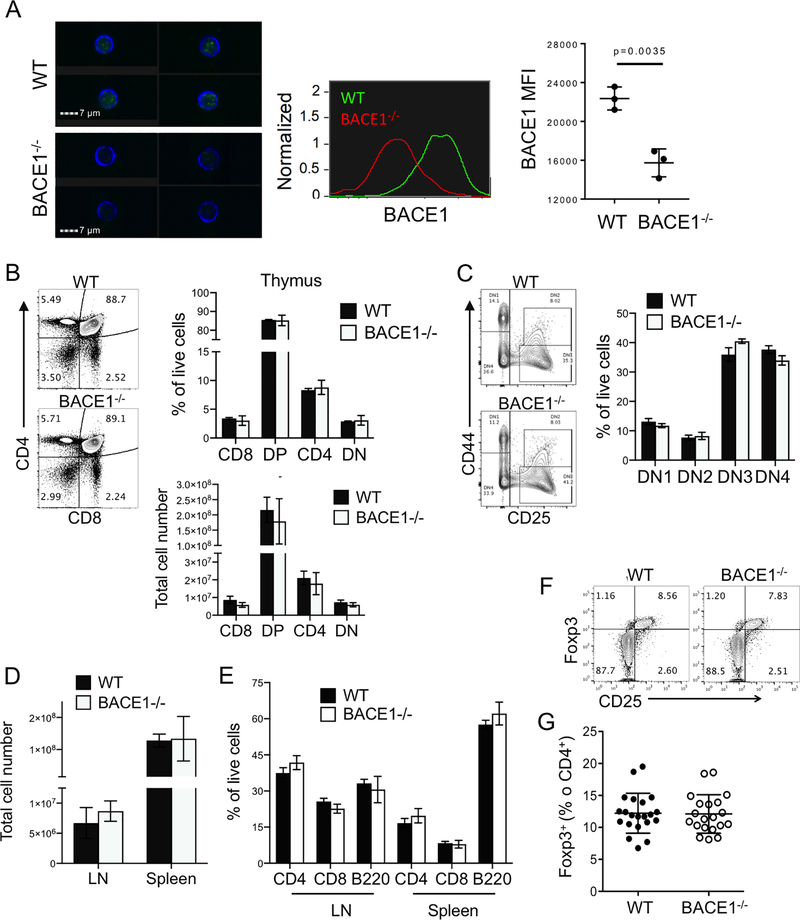
RNA sequencing analysis of different immune populations by the Immgen consortium (31) indicated widespread expression of BACE1 throughout the immune system (Supplementary Figure SF1). Of the T cell populations tested, double-negative thymocytes showed the highest expression of BACE1, but all mature T cells appear to maintain BACE1 (SF1). We confirmed expression of BACE1 protein in mature CD4+ T cells by Imagestream, using cells from BACE1−/− mice (29) as controls (Fig 1A). BACE1−/− had normal numbers and proportions of thymic CD4+, CD8+, double-positive and double-negative thymocytes (Fig 1B). Likewise, ratios of the four stages of double-negative thymocyte development (defined by expression of CD44 and CD25) were unaffected by BACE1 deficiency (Fig 1C). Numbers of cells in peripheral LN and spleen (Fig 1D) and frequencies of mature CD4+ T cells, CD8 T cells and B220+ B cells were similar in WT and BACE1−/− mice (Fig 1E). Finally, BACE1−/− mice had normal CD25 expression on peripheral Foxp3+ regulatory T cells (Fig 1F), as well as normal frequencies of Tregs in peripheral LN (Fig 1G). Although we have not analyzed T cell receptor repertoire in these mice, we can conclude that BACE1 does not alter absolute numbers in thymic generation or maintenance of mature T cells.
Figure 1: BACE1−/− T cells develop at normal frequencies.
A: Expression of BACE1 in mature WT and BACE1−/− T cells analyzed by Imagestream, data representative of 3 independent experiments with 3 mice/group. B: Numbers and proportions of CD8+, CD4+, CD8+CD4+double-positive (DP) and CD8−CD4−double-negative (DN) thymocyte populations in 6 week old WT and BACE1−/− thymus analyzed according to representative FACS plot. C: Frequencies of thymus DN populations gated as shown in representative FACS plot. D: Numbers of cells in cutaneous LN and spleen. E: Proportions of live CD4+ T cells, CD8+ T cells and B220+ B cells in those LN and spleen. F: Representative FACS plots of Foxp3 and CD25 expression in live CD4+ T cells from peripheral LN. G: Frequencies of Foxp3+ cells in CD4+ T cells from WT and BACE1−/− mice. Graphs show mean +/− S.D. of 3–4 mice/group representative of 3–4 independent experiments except F is pooled from 3 experiments.
BACE1 deficient T cells have altered cAMP and PTEN regulation
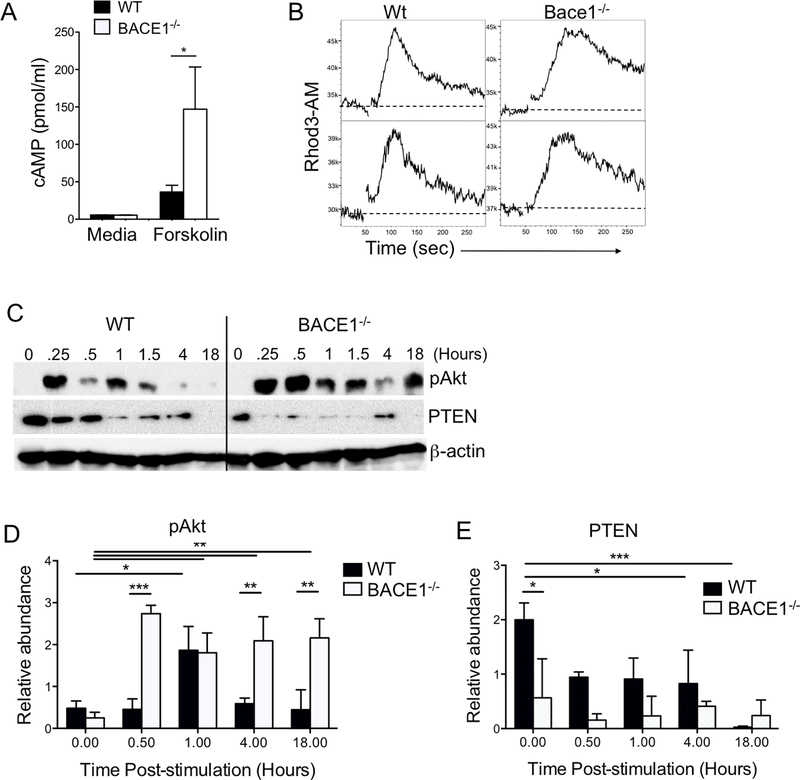
Separately from its proteolyic functions on APP, BACE1 has been reported to regulate adenylyl cyclase to decrease cAMP production in neurons (32). Accordingly, we tested the cAMP response to forskolin-mediated adenylyl cyclase activation. Indeed, BACE1−/− T cells showed a significant increase in cAMP production compared to WT T cells (Fig 2A). In T cells, cAMP acts as a second messenger to activate multiple pathways that can impact T cell receptor signaling (33). One of the first events in TCR signaling is activation of store-operated calcium entry (SOCE) leading to Calcium flux. Following TCR/CD28 stimulation, WT and BACE1−/− T cells showed a similar rapid increase of intracellular Calcium, however BACE1−/− T cells consistently showed a slower loss of intracellular Calcium, leading to a more sustained Calcium flux (Fig 2B). Similarly, BACE1−/− T cells showed enhanced and sustained Akt activation responses compared to WT T cells following stimulation under Th17 conditions (Fig 2C,D). This led us to examine PTEN, the key lipid phosphatase regulator of PI3K. Corresponding to exaggerated Akt activation, PTEN expression was reduced in BACE1−/− T cells compared to WT, even in unstimulated T cells (Fig 2C,E). These results suggest that BACE1 negatively regulates T cell signaling, including during Th17 differentiation.
Figure 2: Altered cAMP and TCR signaling in BACE1−/− T cells.
A: Intracellular cAMP concentration in WT and BACE1−/− T cells after 30 minutes stimulation with Forskolin or media only control, mean +/− S.D. of pooled data from 3 experiments with 2–3 replicates. B: Calcium flux assessed in Rhod3AM-labelled WT and BACE1−/− T cells, activated with anti-CD3/CD28 at 50 seconds, T cell responses from two individual mice per group shown, representative of three independent experiments. C: Immunoblots of pAkt(Ser473), PTEN and β-actin following stimulation of WT and BACE1−/− T cells for indicated time periods. D: Densitometry of pAkt relative to β-actin, mean +/− S.D. data pooled from 3 experiments. E: Densitometry of PTEN relative to β-actin, mean +/− S.D. data pooled from 3 experiments.
BACE1 deficient Th17 cells have impaired IL-17 production
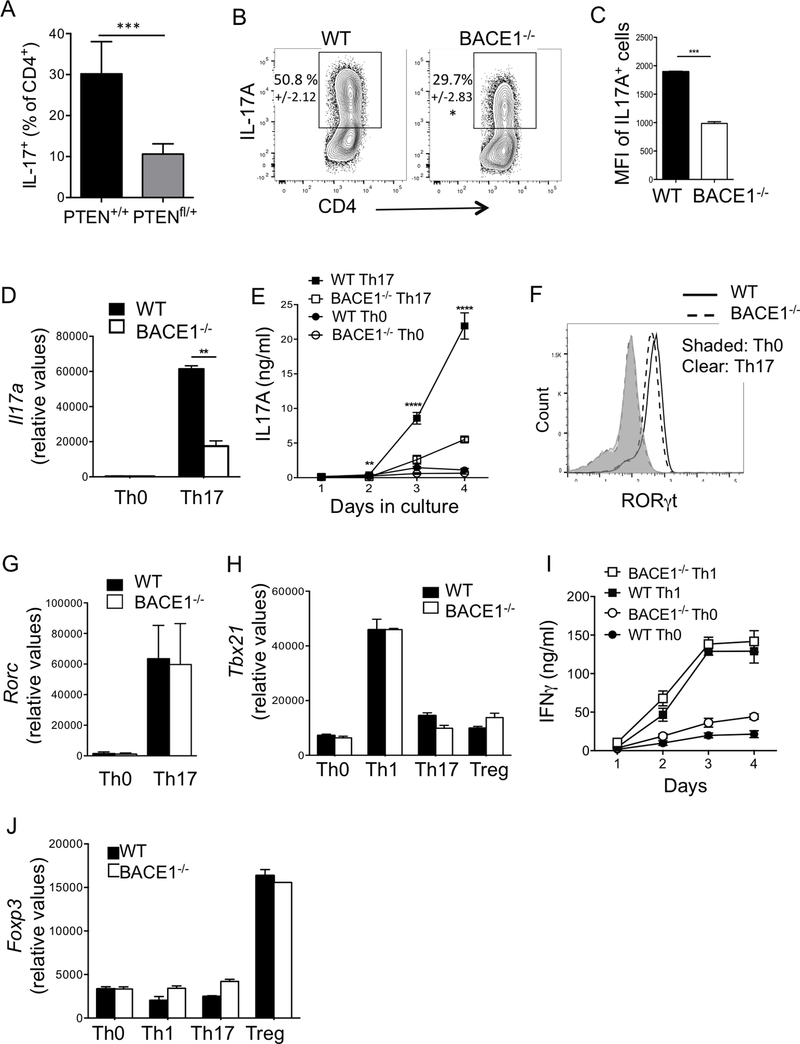
PTEN deletion was recently shown to impair Th17 differentiation (34). We determined whether low PTEN expression could affect Th17 development by stimulating CD4-Cre/PTENfl/+ cells, which have reduced but not complete knockdown of PTEN expression. Indeed, PTENfl/+ Th17 cells had significantly fewer IL-17-producing Th17 cells compared to PTEN+/+ controls (Fig 3A). Given that BACE1−/− T cells had reduced PTEN expression, we tested their capacity to differentiate into Th17 cells. BACE1−/− Th17 cells had significantly reduced frequencies of IL-17A-producing cells compared to WT cells, although IL-17+ cell frequencies varied between experiments, typically BACE1−/− T cells had one third to half the frequency of IL-17+ cells compared to WT Th17 cells (Fig 3B). It was clear that BACE1−/− Th17 cells also produced less IL-17A on a per cell basis, as demonstrated by the geometric mean fluorescence intensity (GMFI) of IL-17A+ cells in these cultures (Fig 3C). Il17a gene expression and secreted IL-17A protein were also significantly decreased in BACE1−/− Th17 cultures versus WT controls (Fig 3D,E). Presence or absence of IL-23 or IL-1 had no additional effects on the IL-17A defect in BACE1−/− cells (data not shown). Although IL-17 was decreased, BACE1−/− T cells cultured under Th17-differentiating conditions expressed normal levels of the Th17 master transcription factor RORγt (Fig 3F,G).
Figure 3: T helper subset differentiation in BACE1−/− T cells.
A: CD4-cre/PTEN+/+ (WT control) and CD4-cre/PTENfl/+ T cells were cultured under Th17 conditions for 3 days and IL-17A+ cells assessed by flow cytometry. B: WT or BACE1−/− CD4+ T cells were differentiated under Th17 conditions and intracellular IL-17A analyzed by flow cytometry on indicated days of culture, mean +/−S.D. indicated. C: Mean fluorescence intensity of IL-17A, gated on IL-17A+ cells, analyzed by flow cytometry on day 3 of culture under Th17 differentiating conditions. D: Gene expression of Il17a in Th0 and Th17 cells on day 3 of culture, normalized to Gapdh. E: IL-17A in culture supernatants analyzed by ELISA at indicated times, IL-17A levels reflect accumulated cytokine from start of culture. F: RORγt protein expression analyzed by flow cytometry on day 3 of indicated cultures. G: Rorc gene expression in T cells cultured under indicated differentiation conditions for two days, normalized to Gapdh. H: Tbx21 (Tbet) gene expression in T cells cultured under indicated differentiation conditions for two days, normalized to Gapdh. I: IFNγ production in Th0 and Th1 cells, analyzed by ELISA at indicated times, IFNγ levels reflect accumulated cytokine from start of culture J: Foxp3 gene expression in T cells cultured under indicated differentiation conditions for two days, normalized to Gapdh. Data indicate mean +/− S.D. of 2–3 replicates representative of at least 4 experiments.
While PTEN has been reported to enhance Th17 differentiation by limiting IL-2 and IFNγ, we did not see a consistent change in IL-2 or IFNγ production by flow cytometry or ELISA (data not shown). Similarly, proliferation of BACE1−/− cells was similar to WT T cells (data not shown). Similarly, BACE1 deficiency had no effect on Tbx21 gene expression (Fig 3H) or IFNγ production (Fig 3I) in Th1 cells. Similar to in vivo Tregs, induction of Foxp3 gene or Foxp3 protein expression by TGFβ was unaffected by BACE1 deficiency (Fig 3J and data not shown). Compared to other T cell subsets, Th17 cells seem particularly prone to conversion to other helper T cell phenotypes, particularly Th1 or T regulatory cells (35). However, these data demonstrate that BACE1−/− cells cultured under Th17 conditions did not upregulate either Tbx21 (Fig 3H) or Foxp3 expression (Fig 3J). Hence we concluded that BACE1 affects Th17 differentiation leading to reduced production of IL-17A, but does not alter regulation of T helper subset master transcription factors or conversion to Th1 or Treg cell phenotypes.
CD73 expression is regulated by BACE1 in Th17 and Treg cells
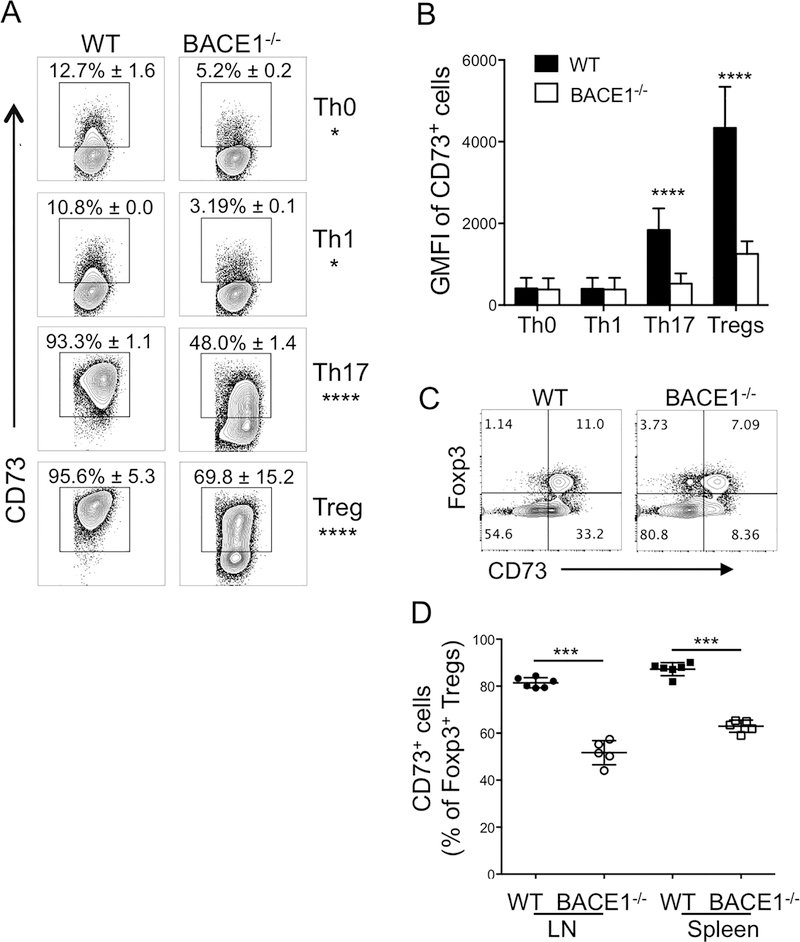
Preliminary gene expression profiling of WT and BACE1−/− Th17 cells confirmed that IL-17A was reduced in absence of BACE1, and that surprisingly few genes were changed in BACE1−/− Th17 cells (data not shown). One clearly Th17-assocated gene other than IL-17A found to be significantly downregulated in BACE1−/− Th17 cells was Nt5e, encoding ecto-5’-nucleotidase or CD73 (36, 37). CD73 expression is regulated by several pathways, including purinergic signaling through the second messenger intracellular cAMP (38). We confirmed that both the frequency of CD73-expressing cells and the amount of CD73 protein expressed per cell were increased on WT Th17 cells compared to Th0 and Th1 cells, and CD73 expression was significantly reduced on BACE1−/− Th17 cells (Fig 4A,B). CD73 acts on the purinergic pathway, converting extracellular AMP to adenosine, which is thought to play an immunoregulatory role in cancer (39). As expected, in vitro-generated regulatory T cells expressed high levels of CD73, which were partially dependent on BACE1 (Fig 4A,B). In vivo, BACE1−/− Tregs showed a partial but significant decrease in CD73 expression by Tregs, as well as reduced CD73 expression by Foxp3− CD4+ T cells (Fig 4C,D), supporting a role for BACE1 in promoting T cell expression of the immunoregulatory enzyme CD73.
Figure 4: CD73 expression is reduced on BACE1−/− Th17 and Treg cells.
A: WT and BACE1−/− T cells were cultured under indicated conditions for 4 days and CD73 expression assessed by flow cytometry on live CD4+ cells, representative FACS plots showing mean +/−S.D. of 4 experiments with 2 replicates each. B: geometric mean fluorescence intensity of CD73 expression on CD73+ cells gated as in A, data show mean +/− S.D. of 4 experiments with 2 replicates/experiment. C: Representative FACS plots showing Foxp3 and CD73 expression in peripheral LN CD4+ T cells from naïve WT and BACE1−/− mice. D: frequencies of CD73+ cells out of Foxp3+CD4+ cells from naïve WT and BACE1−/− mice, mean +/−S.D. of 3 experiments.
BACE1 deficiency in CNS versus T cells differentially affects susceptibility to EAE
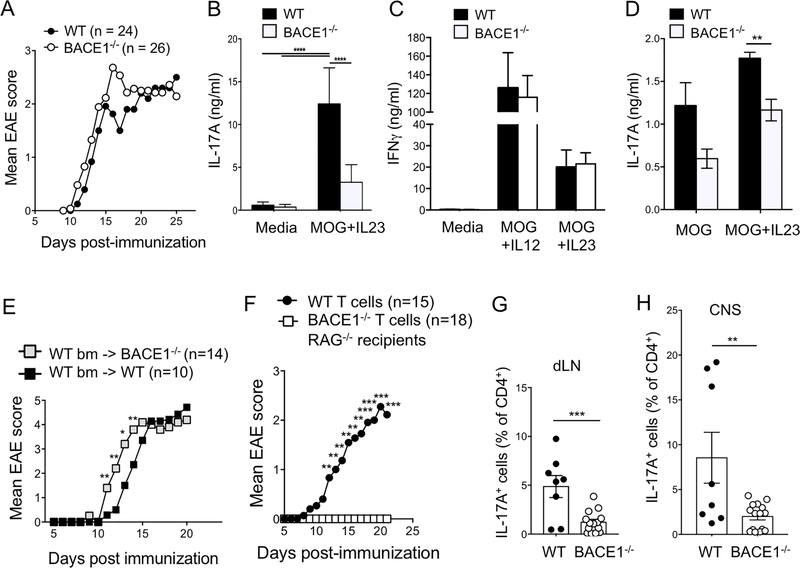
The data so far indicate that BACE1−/− T cells have limited but potentially important defects in expression of effector molecules associated with inflammation (IL-17) and immunoregulation (CD73). We therefore investigated the contribution of BACE1 to CNS inflammation in the animal model of multiple sclerosis, EAE. BACE1−/− and WT controls were immunized with MOG(35–55) in complete Freund’s adjuvant (CFA). Incidence and clinical severity of EAE were similar between WT and BACE1−/− mice (Fig 5A). However, ex vivo stimulation of dLN Th17 cells with MOG(35–55) and IL-23 for three days demonstrated reduced IL-17 response on day 8 post-immunization (Fig 5B), similar to the in vitro differentiation results. In contrast, IFNγ production under Th1 or Th17 conditions was unaffected in BACE1−/− dLN (Fig 5C). IL-17 production after 18hr culture with MOG(35–55) and IL-23 still showed a significant defect in BACE1−/− cells (Fig 5D), suggesting that these affects were unlikely to be due to differences in the in vitro proliferation or survival of BACE1−/− T cells responding to antigen.
Figure 5: Differential roles for BACE1 in CNS and Th17 cells during EAE.
A: WT and BACE1−/− mice were immunized to induce EAE, and clinical scores monitored, data pooled from 5 experiments. B: Day 8 post-immunization, dLN cells were stimulated with MOG(35–55) + IL-23 for three days then secreted IL-17A analyzed by ELISA. C: Day 8 post-immunization, dLN cells were stimulated with MOG(35–55) + IL-12 or MOG(35–55) + IL-23 for three days, then secreted IFNγ analyzed by ELISA. D: Day 12 post-immunization, dLN cells were stimulated for 18hr with MOG(35–55) +/− IL-23, then secreted IL-17A analyzed by ELISA. B-D data pooled from 3 experiments. E: WT bone marrow was transferred into irradiated WT or BACE1−/− recipients and EAE was induced following 8 weeks reconstitution, data pooled from two experiments. F: WT or BACE1−/− CD4+ T cells were transferred into RAG1−/− recipients, EAE was induced the following day and clinical signs were monitored, data pooled from 3 experiments. G: IL-17A+ cells analyzed by flow cytometry in dLN on day 12 post-EAE induction following PMA/ionomycin stimulation. H: IL-17A+ cells analyzed by flow cytometry in CNS on day 12 post-EAE induction following PMA/ionomycin stimulation. Data in G,H pooled from two experiments, each point represents an individual mouse.
These data suggested the possibility of different roles for BACE1 in CNS-resident cells versus immune cells for determining EAE susceptibility. To test the role of BACE1 in CNS-resident cells in EAE susceptibility, we generated bone marrow chimeras in which irradiated WT or BACE1−/− recipients were reconstituted with WT bone marrow, thus restricting BACE1 deficiency to the non-immune compartment. BACE−/− recipients of WT bone marrow had significantly earlier onset of EAE disease signs compared to controls (Fig 5E), suggesting that BACE1 deficiency in non-immune cells in the CNS causes increased susceptibility to CNS damage. To directly test the requirement for BACE1 in T cell function in vivo, RAG1−/− recipients (lacking T cells) were reconstituted with WT or BACE1−/− T cells before induction of EAE. Mice with T cells that lacked BACE1 (but all other cells including CNS were BACE1-sufficient) were resistant to EAE induction (Fig 5F). Accordingly, IL-17 production was reduced in transferred BACE1−/− T cells in dLN and CNS (Fig 5G,H).
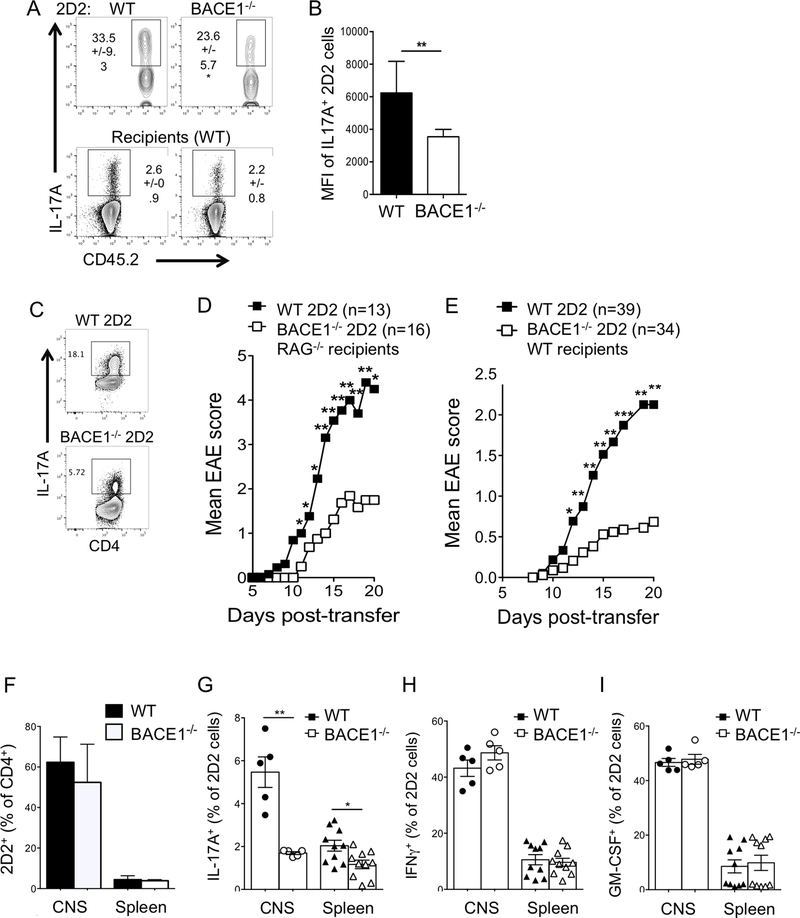
To confirm the Th17 cell-intrinsic requirement for BACE1 in vivo, BACE1−/− 2D2 cells were transferred into CD45.1+ WT recipients and tracked by expression of CD45.2 following immunization with MOG(35–55) in CFA. BACE1−/− 2D2 cells again showed a clear reduction in frequencies of IL-17A producers (Fig 6A), while Th17 responses were not different in recipients of WT or BACE1−/− 2D2 cells (Fig 6A). Of the remaining BACE1−/− cells that did produce IL-17A, mean level of IL-17A protein per cell was reduced (Fig 6B). To further test the role of BACE1 in autoimmune Th17 cells, WT and BACE1−/− 2D2 cells, bearing transgenic T cell receptors reactive to MOG(35–55), were differentiated under Th17 conditions in vitro, and IL-17A production was confirmed (Fig 6C). Upon transfer to RAG−/− naïve hosts, BACE1−/− 2D2 Th17 cells were significantly impaired in their ability to induce EAE (Fig 6D, Table 1). Similar results were observed after transfer of 2D2 Th17 cells into WT recipients (Fig 6E, Table 2). It was particularly striking that many recipients of WT 2D2 cells developed signs of atypical EAE, including severe ataxia and circling behavior, with higher rates of mortality, but these atypical signs were not apparent in recipients of BACE1−/− 2D2 cells (Table 2). This form of atypical EAE has previously been associated with a strong Th17 response (7). Frequencies of WT and BACE1−/− 2D2 cells were similar after transfer (Fig 6F). We did not analyze frequencies of 2D2 cells at late timepoints of EAE, so cannot rule out long-term survival or proliferation defects in BACE1−/− T cells. After EAE onset, IL-17 production as a proportion of 2D2 cells was significantly reduced in BACE1−/− 2D2 compared to WT 2D2 in CNS and spleen, while IFNγ and GM-CSF were unaffected (Fig 6G–I). Taken together, these results demonstrate that BACE1-deficient T cells have impaired pro-inflammatory functions resulting in reduced pathogenicity.
Figure 6: BACE1−/− Th17 cells have reduced pathogenicity in vivo.
A: CD45.2+ WT or BACE1−/− 2D2 cells were transferred into CD45.1+ recipients, which were then immunized with MOG(35–55) in CFA. IL-17 production in CD45.2+ 2D2 cells was analyzed following PMA/ionomycin stimulation in dLN on day 8, mean +/− S.D. shown, B: mean fluorescence intensity of IL-17+ cells in 2D2 cells analyzed in A. Data in A,B pooled from 2 independent experiments with 3–4 mice per group. C: WT and BACE1−/− 2D2 Th17 cells were activated in vitro for passive transfer of EAE, IL-17A expression assessed by flow cytometry on day 4 of culture; D: EAE clinical scores following transfer of WT or BACE1−/− 2D2 Th17 cells into RAG−/− recipients (see also Table 1); E: EAE clinical scores following transfer of WT or BACE1−/− 2D2 Th17 cells into WT recipients (see also Table 2); data in D,E pooled from 5 experiments. F-I: WT and BACE1−/− Th17 cells were generated and transferred into WT recipients as described in C,E, 2D2 cells and cytokine production analyzed by flow cytometry following PMA/ionomycin stimulation in live CD4+ Vβ11+ cells in CNS and spleen after EAE onset (day 12–14), data pooled from independent experiments (n=5 for CNS, n=10 for spleen).
Table 1:
Th17 2D2 cell passive transfer of EAE into RAG−/− recipients is reduced in absence of BACE1. Data pooled from 4 independent experiments.
| WT 2D2 | BACE1−/− 2D2 | P value (Fishers) | |
|---|---|---|---|
| Number of recipients | 13 | 16 | |
| Incidence | 13 (100%) | 9 (56%) | 0.0084 (**) |
| Death | 3 (23%) | 0 (0%) | 0.0783 |
Table 2:
Th17 2D2 cell passive transfer of EAE into WT recipients is reduced in absence of BACE1. Data pooled from 6 experiments.
| B6 recipients | WT 2D2 | BACE1−/− 2D2 | P value (Fisher’s) |
|---|---|---|---|
| Number of recipients | 39 | 34 | |
| Incidence | 26 (67%) | 11 (32%) | 0.0049 (**) |
| Death | 8 (21%) | 1 (3%) | 0.0316 (*) |
| Atypical EAE | 19 (49%) | 2 (6%) | < 0.0001 (****) |
DISCUSSION
Our data indicate a role for the CNS-associated protein BACE1 in CD4+ T cells. BACE1 regulates baseline cAMP responses, Calcium signaling and PTEN levels with resultant effects on Th17 and Treg differentiation. Although there was not a complete block in Th17 development, the defects in BACE1−/− T cells were sufficient to strongly impair their capacity to induce disease in the EAE model. Typically, defects in IL-17A production that are observed during early stages of Th17 development are also accompanied by broader defects in the Th17 program including reduced expression of RORγt. However, there is precedent for modulation of IL-17A independently of other Th17 factors downstream of RORγt. Serum amyloid A produced by inflamed epithelium strongly promotes production of IL-17A by RORγt+ effector Th17 cells in epithelial tissues (40). Closer to the initial activation events for Th17 differentiation, PKCα acts as a signaling intermediary to promote TGFβ-mediated SMAD activation, and deficiency results in impaired IL-17A but not IL-17F production by Th17 cells (41). Similarly, TGFβ-regulated inhibition of the transcriptional repressor Gfi1 is required for Th17 development, and Gfi1 deficiency or overexpression has a marked effect on IL-17A but comparatively minor effects on RORγt and IL-17F (36, 42). Strikingly, Gfi1 repression is also required for CD73 expression (36), which we found to be co-regulated with IL-17A in BACE1−/−, making Gfi1 a good candidate to mediate the effects of BACE1. However, we did not observe any difference in Gfi1 expression between BACE1−/− and WT cells (data not shown), suggesting that Gfi1 is not responsible for defective IL-17A and CD73 expression in BACE1−/− cells. It is interesting to note that Akt activation by T cell receptor engagement was also found to inhibit Gfi1 (43), as BACE1−/− cells demonstrated heightened Akt activity.
Aside from activation of Akt, TCR ligation results in Lck-mediated ZAP70 phosphorylation that in turn activates the LAT signaling complex including the TEC kinase Itk that then activates PLCγ1 (44). Itk−/− Th17 cells show strikingly similar defects to BACE1−/− cells, with reduced IL-17A but not RORγt or IL-17F upon in vitro differentiation (45). However, Itk−/− cells have reduced PI3K activation, reduced Calcium signaling and retain high PTEN expression following activation (46), while BACE1−/− T cells have increased Akt activation concomitant with reduced PTEN expression. Therefore, it seems that altering T cell receptor signaling either above or below the optimal threshold contributes to defects in Th17 cells that, although subtle, may impact their function. Itk−/− cells are also more prone to converting to Treg cells under Th17 differentiating conditions (46), as high PTEN promotes Tregs (47, 48), in contrast to BACE1−/− Th17 cells which showed no increase in Foxp3 expression. In this regard it is interesting to note that BACE1−/− Tregs showed reduced CD73 expression, since Treg instability has previously been associated with reduced PTEN localization to TCR that resulted in reduced CD73 expression and Treg function in tumor settings (49). Hence reduced CD73 corresponds well with reduced PTEN expression. We did query whether CD73 expression contributes to the Th17 phenotype of BACE1−/− cells, but found that CD73 is not required for IL-17 production by Th17 cells and is dispensable for EAE induction (50). Furthermore, addition of adenosine to Th17 cultures did not restore IL-17A production in BACE1−/− Th17 cells (data not shown). Thus we conclude that CD73 is dysregulated concomitantly with IL-17 in BACE1-deficient T cells, rather than being an upstream regulator of IL-17A responsible for the effects of BACE1.
A recent report demonstrated that deletion of PTEN in developing Th17 cells inhibited Th17 differentiation (34), and our data confirmed that reduced PTEN inhibits IL-17 production. Regulation of PTEN is complex, with a plethora of mechanisms targeting mRNA transcription and translation as well as degradation of mature protein, for example through ubiquitination (51). The relationship between cAMP and PTEN expression has not been well studied in T cells, but there is evidence for direct downregulation of PTEN protein by cAMP in glial cells and thyroid cells (52, 53). cAMP also activates PKA, which can feed-forward to activate PI3K (54, 55), and since activation of PI3K signaling pathways negatively regulates PTEN levels (56) this provides an indirect mechanism by which increased cAMP could lead to dysregulation of PTEN. It is also feasible that cAMP activation of PKA leads to inhibition of the downstream mediators of Ca2+ activation, such as NFAT. However, we could not find evidence to support defective NFAT nuclear localization in BACE1−/− T cells (data not shown). Furthermore, cAMP induced following TCR engagement has been shown to negatively regulate TCR signaling through Lck inhibition (57) or PKA-Csk activation (58, 59), which would ultimately reduce Ca2+ flux if unchecked. Since PTEN is reduced at baseline in BACE1−/− T cells, we hypothesize that in naïve CD4+ T cells, cAMP and pAkt induced by survival signals through growth factor receptors, chemokines and TCR ‘tickling’ by MHC all contribute to alterations in PTEN that are negatively regulated by BACE1, hence setting the threshold for outcome of eventual effector cell differentiation.
It was intriguing that the global BACE1 knockout mice did not reveal the defect in Th17 pathogenicity that was demonstrated when BACE1 deficiency was restricted to T cells in either active or passive EAE. It is possible that other cytokines, such as IFNγ or GMCSF, are sufficient to induce disease when presented with a CNS that is already suffering some physiological defects, or when occurring in sufficient numbers to override the IL-17A defect. This premise is supported by the finding that WT recipients of BACE1−/− 2D2 cell transfers (arguably at higher cell numbers than would occur physiologically following immunization) were not completely protected from EAE induction. However, the clinical characteristics of EAE were different, and IL-17A-producing capability corresponded to increased incidence of atypical EAE as has previously been reported (7–9, 60). We did not further verify ratios of neutrophils versus macrophages, which are indicators of the Th17 to Th1 ratios (7–9), nevertheless the body of evidence from the active and passive EAE models supports the loss of pathogenecity when BACE1 is specifically lacking in T cells.
One outstanding question is whether the proteolytic activities of BACE1 are required for its IL-17A-promoting effects, since these are the current target of Alzheimer’s disease therapy. In neuronal cells, BACE1 regulates cAMP through non-proteolytic interactions with adenylyl cyclase (32), making it likely that at least some of the effects in T cells could be through this non-canonical BACE1 function. Similarly, the precise mechanisms by which BACE1 contributes to neuroinflammation and degeneration following injury are still not fully clear. Many studies have used BACE1 deficient animals to address the role of BACE1 in determining outcomes of CNS injury. Intriguingly, IL-17A has also been reported to increase following CNS injury (26–28), often produced by γδ T cells, which rapidly enter the site of damage. IL-17A producing γδ T cells also accompany myelin-reactive Th17 cells during the early phases of EAE and contribute to inflammation (61). IL-17A has been shown to promote neuronal cell death post-stroke, and blocking IL-17A reduces lesion size and enhances functional recovery in rodent models (26–28). Recently, IL-17A-producing γδ T cells that exacerbate damage following CNS ischemia were found to be programmed by gut microbiota (26), corresponding to a rapidly growing body of evidence that the microbiome sets the rheostat for immune responses, and particularly Th17 and Treg responses, throughout the body (62, 63). Our findings that BACE1 regulates IL-17A production therefore cast a new light on previous findings on outcomes of CNS injury in BACE1−/− animals, since effects on both CNS cells and immune cells, particularly IL-17A producing cells, may have contributed to these observed outcomes. The finding that BACE1 deficient T cells demonstrate enhanced AC-stimulated production of cAMP corresponds to findings in neurons. It would be therefore also be interesting to determine whether CNS neurons in BACE1 deficient mice also have defects in the PTEN, Akt and PLCγ pathways, or indeed CD73 expression, and whether these contribute to BACE1-mediated effects in healthy and diseased brain.
From a therapeutic point of view, these data suggest that blocking BACE1 has the potential to target both inflammation and neurodegeneration, prompting further investigation of the role of BACE1 in neuroinflammation provoked by CNS injury, be it autoimmune, traumatic or ischemic. The finding that the same molecule can have very different effects and outcomes on disease depending on the cell type targeted is a recurring theme in immunology: for example, STAT3 deletion in all CD4+ T cells renders mice resistant to Th17 induction and associated inflammation (64), while STAT3 deletion in Foxp3+ regulatory T cells results in spontaneous development of Th17-associated autoimmune disease (65). Another example is CD47: blockade of CD47 has completely opposite effects on EAE development depending on the cell type targeted (immune cells versus CNS) and timing of blockade (66). It will be interesting in future studies to conditionally delete BACE1 in specific T cell populations (for example Tregs versus Th17 cells) and also in other immune cells to determine additional roles in immune function including inflammatory disease, infection control and tumor eradication.
Supplementary Material
KEY POINTS.
BACE1 is expressed in CD4+ T cells and regulates signaling events during activation
BACE1-deficient T cells show reduced IL-17A expression in vitro
BACE1−/− Th17 cells have impaired pathogenicity in EAE
Acknowledgements
We thank Sarah Gaffen, Partha Biswas, Louise D’Cruz, Anuradha Ray, and Amanda Poholek for suggestions and helpful advice. GEH was a graduate student in the Immunology graduate program at the University of Pittsburgh.
Funding for this study provided by NIH AI110822 (to MJM), NIH AI103022 (to LK), IR was supported by NIH AI089443 T32 training grant, and this work benefitted from ImageStreamX MARKII grant NIH 1S10OD019942-01
References
- 1.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, and Cua DJ 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 201: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, and Mills KH 2010. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clinical and experimental immunology 162: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, and Stockinger B 2011. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol 12: 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel DD, and Kuchroo VK 2015. Th17 Cell Pathway in Human Immunity: Lessons from Genetics and Therapeutic Interventions. Immunity 43: 1040–1051. [DOI] [PubMed] [Google Scholar]
- 5.Kang Z, Altuntas CZ, Gulen MF, Liu C, Giltiay N, Qin H, Liu L, Qian W, Ransohoff RM, Bergmann C, Stohlman S, Tuohy VK, and Li X 2010. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity 32: 414–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, Kordula T, Zhang QW, Vallance B, Swaidani S, Aronica M, Tuohy VK, Hamilton T, and Li X 2007. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nature immunology 8: 247–256. [DOI] [PubMed] [Google Scholar]
- 7.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, and Goverman JM 2008. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med 14: 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Holdbrooks AT, Meares GP, Buckley JA, Benveniste EN, and Qin H 2015. Preferential Recruitment of Neutrophils into the Cerebellum and Brainstem Contributes to the Atypical Experimental Autoimmune Encephalomyelitis Phenotype. J Immunol 195: 841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroenke MA, Carlson TJ, Andjelkovic AV, and Segal BM 2008. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med 205: 1535–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang Z, Wang C, Zepp J, Wu L, Sun K, Zhao J, Chandrasekharan U, DiCorleto PE, Trapp BD, Ransohoff RM, and Li X 2013. Act1 mediates IL-17-induced EAE pathogenesis selectively in NG2+ glial cells. Nat Neurosci 16: 1401–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodgers JM, Robinson AP, Rosler ES, Lariosa-Willingham K, Persons RE, Dugas JC, and Miller SD 2015. IL-17A activates ERK½ and enhances differentiation of oligodendrocyte progenitor cells. Glia 63: 768–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siffrin V, Radbruch H, Glumm R, Niesner R, Paterka M, Herz J, Leuenberger T, Lehmann SM, Luenstedt S, Rinnenthal JL, Laube G, Luche H, Lehnardt S, Fehling HJ, Griesbeck O, and Zipp F 2010. In vivo imaging of partially reversible th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity 33: 424–436. [DOI] [PubMed] [Google Scholar]
- 13.Vassar R 2004. BACE1: the beta-secretase enzyme in Alzheimer’s disease. Journal of molecular neuroscience : MN 23: 105–114. [DOI] [PubMed] [Google Scholar]
- 14.Vassar R 2014. BACE1 inhibitor drugs in clinical trials for Alzheimer’s disease. Alzheimer’s research & therapy 6: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao J, O’Connor T, and Vassar R 2011. The contribution of activated astrocytes to Abeta production: implications for Alzheimer’s disease pathogenesis. J Neuroinflammation 8: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto M, Kiyota T, Horiba M, Buescher JL, Walsh SM, Gendelman HE, and Ikezu T 2007. Interferon-gamma and tumor necrosis factor-alpha regulate amyloid-beta plaque deposition and beta-secretase expression in Swedish mutant APP transgenic mice. The American journal of pathology 170: 680–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sastre M, Dewachter I, Rossner S, Bogdanovic N, Rosen E, Borghgraef P, Evert BO, Dumitrescu-Ozimek L, Thal DR, Landreth G, Walter J, Klockgether T, van Leuven F, and Heneka MT 2006. Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proceedings of the National Academy of Sciences of the United States of America 103: 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang X, Wang Q, Hand T, Wu L, Breyer RM, Montine TJ, and Andreasson K 2005. Deletion of the prostaglandin E2 EP2 receptor reduces oxidative damage and amyloid burden in a model of Alzheimer’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience 25: 10180–10187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen Y, Onyewuchi O, Yang S, Liu R, and Simpkins JW 2004. Increased beta-secretase activity and expression in rats following transient cerebral ischemia. Brain research 1009: 1–8. [DOI] [PubMed] [Google Scholar]
- 20.Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, Staufenbiel M, Huang LE, and Song W 2006. Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. Proceedings of the National Academy of Sciences of the United States of America 103: 18727–18732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guglielmotto M, Aragno M, Autelli R, Giliberto L, Novo E, Colombatto S, Danni O, Parola M, Smith MA, Perry G, Tamagno E, and Tabaton M 2009. The up-regulation of BACE1 mediated by hypoxia and ischemic injury: role of oxidative stress and HIF1alpha. Journal of neurochemistry 108: 1045–1056. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Zhou K, Wang R, Cui J, Lipton SA, Liao FF, Xu H, and Zhang YW 2007. Hypoxia-inducible factor 1alpha (HIF-1alpha)-mediated hypoxia increases BACE1 expression and beta-amyloid generation. The Journal of biological chemistry 282: 10873–10880. [DOI] [PubMed] [Google Scholar]
- 23.Blasko I, Beer R, Bigl M, Apelt J, Franz G, Rudzki D, Ransmayr G, Kampfl A, and Schliebs R 2004. Experimental traumatic brain injury in rats stimulates the expression, production and activity of Alzheimer’s disease beta-secretase (BACE-1). J Neural Transm 111: 523–536. [DOI] [PubMed] [Google Scholar]
- 24.Loane DJ, Pocivavsek A, Moussa CE, Thompson R, Matsuoka Y, Faden AI, Rebeck GW, and Burns MP 2009. Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nature medicine 15: 377–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mannix RC, Zhang J, Park J, Lee C, and Whalen MJ 2011. Detrimental effect of genetic inhibition of B-site APP-cleaving enzyme 1 on functional outcome after controlled cortical impact in young adult mice. Journal of neurotrauma 28: 1855–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, Iadecola C, and Anrather J 2016. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med 22: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gelderblom M, Weymar A, Bernreuther C, Velden J, Arunachalam P, Steinbach K, Orthey E, Arumugam TV, Leypoldt F, Simova O, Thom V, Friese MA, Prinz I, Holscher C, Glatzel M, Korn T, Gerloff C, Tolosa E, and Magnus T 2012. Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood 120: 3793–3802. [DOI] [PubMed] [Google Scholar]
- 28.Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, Iwakura Y, and Yoshimura A 2009. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nature medicine 15: 946–950. [DOI] [PubMed] [Google Scholar]
- 29.Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, and Wong PC 2001. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat Neurosci 4: 233–234. [DOI] [PubMed] [Google Scholar]
- 30.Jager A, Dardalhon V, Sobel RA, Bettelli E, and Kuchroo VK 2009. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol 183: 7169–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heng TS, Painter MW, and C. Immunological Genome Project 2008. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol 9: 1091–1094. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Huang X, Zhang YW, Rockenstein E, Bu G, Golde TE, Masliah E, and Xu H 2012. Alzheimer’s beta-secretase (BACE1) regulates the cAMP/PKA/CREB pathway independently of beta-amyloid. J Neurosci 32: 11390–11395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arumugham VB, and Baldari CT 2017. cAMP: a multifaceted modulator of immune synapse assembly and T cell activation. J Leukoc Biol 101: 1301–1316. [DOI] [PubMed] [Google Scholar]
- 34.Kim HS, Jang SW, Lee W, Kim K, Sohn H, Hwang SS, and Lee GR 2017. PTEN drives Th17 cell differentiation by preventing IL-2 production. J Exp Med 214: 3381–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou L, Chong MM, and Littman DR 2009. Plasticity of CD4+ T cell lineage differentiation. Immunity 30: 646–655. [DOI] [PubMed] [Google Scholar]
- 36.Chalmin F, Mignot G, Bruchard M, Chevriaux A, Vegran F, Hichami A, Ladoire S, Derangere V, Vincent J, Masson D, Robson SC, Eberl G, Pallandre JR, Borg C, Ryffel B, Apetoh L, Rebe C, and Ghiringhelli F 2012. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity 36: 362–373. [DOI] [PubMed] [Google Scholar]
- 37.Doherty GA, Bai A, Hanidziar D, Longhi MS, Lawlor GO, Putheti P, Csizmadia E, Nowak M, Cheifetz AS, Moss AC, and Robson SC 2012. CD73 is a phenotypic marker of effector memory Th17 cells in inflammatory bowel disease. Eur J Immunol 42: 3062–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narravula S, Lennon PF, Mueller BU, and Colgan SP 2000. Regulation of endothelial CD73 by adenosine: paracrine pathway for enhanced endothelial barrier function. J Immunol 165: 5262–5268. [DOI] [PubMed] [Google Scholar]
- 39.Antonioli L, Yegutkin GG, Pacher P, Blandizzi C, and Hasko G 2016. Anti-CD73 in cancer immunotherapy: awakening new opportunities. Trends Cancer 2: 95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sano T, Huang W, Hall JA, Yang Y, Chen A, Gavzy SJ, Lee JY, Ziel JW, Miraldi ER, Domingos AI, Bonneau R, and Littman DR 2015. An IL-23R/IL-22 Circuit Regulates Epithelial Serum Amyloid A to Promote Local Effector Th17 Responses. Cell 163: 381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meisel M, Hermann-Kleiter N, Hinterleitner R, Gruber T, Wachowicz K, Pfeifhofer-Obermair C, Fresser F, Leitges M, Soldani C, Viola A, Kaminski S, and Baier G 2013. The kinase PKCalpha selectively upregulates interleukin-17A during Th17 cell immune responses. Immunity 38: 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu J, Davidson TS, Wei G, Jankovic D, Cui K, Schones DE, Guo L, Zhao K, Shevach EM, and Paul WE 2009. Down-regulation of Gfi-1 expression by TGF-beta is important for differentiation of Th17 and CD103+ inducible regulatory T cells. J Exp Med 206: 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurebayashi Y, Nagai S, Ikejiri A, Ohtani M, Ichiyama K, Baba Y, Yamada T, Egami S, Hoshii T, Hirao A, Matsuda S, and Koyasu S 2012. PI3K-Akt-mTORC1-S6K½ axis controls Th17 differentiation by regulating Gfi1 expression and nuclear translocation of RORgamma. Cell Rep 1: 360–373. [DOI] [PubMed] [Google Scholar]
- 44.Andreotti AH, Schwartzberg PL, Joseph RE, and Berg LJ 2010. T-cell signaling regulated by the Tec family kinase, Itk. Cold Spring Harb Perspect Biol 2: a002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez-Rodriguez J, Sahu N, Handon R, Davidson TS, Anderson SM, Kirby MR, August A, and Schwartzberg PL 2009. Differential expression of interleukin-17A and −17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity 31: 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gomez-Rodriguez J, Wohlfert EA, Handon R, Meylan F, Wu JZ, Anderson SM, Kirby MR, Belkaid Y, and Schwartzberg PL 2014. Itk-mediated integration of T cell receptor and cytokine signaling regulates the balance between Th17 and regulatory T cells. J Exp Med 211: 529–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shrestha S, Yang K, Guy C, Vogel P, Neale G, and Chi H 2015. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol 16: 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM, Townamchai N, Gerriets VA, Rathmell JC, Sharpe AH, Bluestone JA, and Turka LA 2015. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol 16: 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, Workman CJ, and Vignali DA 2013. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 501: 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hernandez-Mir G, and McGeachy MJ 2017. CD73 is expressed by inflammatory Th17 cells in experimental autoimmune encephalomyelitis but does not limit differentiation or pathogenesis. PLoS One 12: e0173655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song MS, Salmena L, and Pandolfi PP 2012. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol 13: 283–296. [DOI] [PubMed] [Google Scholar]
- 52.Sugimoto N, Miwa S, Ohno-Shosaku T, Tsuchiya H, Hitomi Y, Nakamura H, Tomita K, Yachie A, and Koizumi S 2011. Activation of tumor suppressor protein PTEN and induction of apoptosis are involved in cAMP-mediated inhibition of cell number in B92 glial cells. Neurosci Lett 497: 55–59. [DOI] [PubMed] [Google Scholar]
- 53.Tell G, Pines A, Arturi F, Cesaratto L, Adamson E, Puppin C, Presta I, Russo D, Filetti S, and Damante G 2004. Control of phosphatase and tensin homolog (PTEN) gene expression in normal and neoplastic thyroid cells. Endocrinology 145: 4660–4666. [DOI] [PubMed] [Google Scholar]
- 54.Cosentino C, Di Domenico M, Porcellini A, Cuozzo C, De Gregorio G, Santillo MR, Agnese S, Di Stasio R, Feliciello A, Migliaccio A, and Avvedimento EV 2007. p85 regulatory subunit of PI3K mediates cAMP-PKA and estrogens biological effects on growth and survival. Oncogene 26: 2095–2103. [DOI] [PubMed] [Google Scholar]
- 55.Ciullo I, Diez-Roux G, Di Domenico M, Migliaccio A, and Avvedimento EV 2001. cAMP signaling selectively influences Ras effectors pathways. Oncogene 20: 1186–1192. [DOI] [PubMed] [Google Scholar]
- 56.Hawse WF, Sheehan RP, Miskov-Zivanov N, Menk AV, Kane LP, Faeder JR, and Morel PA 2015. Cutting Edge: Differential Regulation of PTEN by TCR, Akt, and FoxO1 Controls CD4+ T Cell Fate Decisions. J Immunol 194: 4615–4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tamir A, Granot Y, and Isakov N 1996. Inhibition of T lymphocyte activation by cAMP is associated with down-regulation of two parallel mitogen-activated protein kinase pathways, the extracellular signal-related kinase and c-Jun N-terminal kinase. J Immunol 157: 1514–1522. [PubMed] [Google Scholar]
- 58.Bjorgo E, Solheim SA, Abrahamsen H, Baillie GS, Brown KM, Berge T, Okkenhaug K, Houslay MD, and Tasken K 2010. Cross talk between phosphatidylinositol 3-kinase and cyclic AMP (cAMP)-protein kinase a signaling pathways at the level of a protein kinase B/beta-arrestin/cAMP phosphodiesterase 4 complex. Mol Cell Biol 30: 1660–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tasken K, and Ruppelt A 2006. Negative regulation of T-cell receptor activation by the cAMP-PKA-Csk signalling pathway in T-cell lipid rafts. Front Biosci 11: 2929–2939. [DOI] [PubMed] [Google Scholar]
- 60.Pierson E, Simmons SB, Castelli L, and Goverman JM 2012. Mechanisms regulating regional localization of inflammation during CNS autoimmunity. Immunol Rev 248: 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, and Mills KH 2009. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31: 331–341. [DOI] [PubMed] [Google Scholar]
- 62.Wekerle H 2017. Brain Autoimmunity and Intestinal Microbiota: 100 Trillion Game Changers. Trends Immunol 38: 483–497. [DOI] [PubMed] [Google Scholar]
- 63.Hand TW, Vujkovic-Cvijin I, Ridaura VK, and Belkaid Y 2016. Linking the Microbiota, Chronic Disease, and the Immune System. Trends Endocrinol Metab 27: 831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, and Dong C 2007. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem 282: 9358–9363. [DOI] [PubMed] [Google Scholar]
- 65.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, and Rudensky AY 2009. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 326: 986–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han MH, Lundgren DH, Jaiswal S, Chao M, Graham KL, Garris CS, Axtell RC, Ho PP, Lock CB, Woodard JI, Brownell SE, Zoudilova M, Hunt JF, Baranzini SE, Butcher EC, Raine CS, Sobel RA, Han DK, Weissman I, and Steinman L 2012. Janus-like opposing roles of CD47 in autoimmune brain inflammation in humans and mice. J Exp Med 209: 1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.