Abstract
Pro-inflammatory cytokines produce manifestations of sickness during inflammation, such as malaise and lethargy. They also contribute to effects of inflammation on mood. Anti-inflammatory cytokines counteract damage caused by inflammatory processes and can limit the severity of inflammation. However, very little is known about the role of anti-inflammatory cytokines in sickness and mood changes during immune activation. The purpose of this study was to determine if a prototypical anti-inflammatory cytokine, interleukin 10 (IL-10), can offset sickness behavior and anxiety caused by a pro-inflammatory cytokine, and whether IL-10 itself modifies anxiety. Rodent models of sickness display suppression of behavioral activity that may reflect lethargy or malaise, while models of anxiety display reduced exploration in several tasks. The effects of peripheral single dose of cytokines on open field exploration, social interaction and elevated plus maze (EPM) tests in adult male Sprague-Dawley rats were measured at 30 – 50 min post-treatment. The prototypical pro-inflammatory cytokine IL-1β (1 μg, i.p.) caused a decrease in locomotor activity indicative of sickness behavior, but disproportionately reduced central area exploration in the open field, open arm exploration in the EPM and lowered social interaction. IL-10 (1 μg, i.p.) had no effect on locomotor activity, but itself produced anxiety-like behavior in the open field and EPM. However, rats co-treated with both IL-10 and IL-1β showed locomotor activity, open field, social interaction and EPM behaviors very similar to control groups. This data demonstrate that IL-10 is capable of mitigating the sickness and anxiogenic effects caused by IL-1β, but that immune imbalance toward either a pro-inflammatory or an anti-inflammatory state can produce anxiety. This has importance for understanding the scope of immune changes that produce psychiatric symptoms, and provides preliminary indication that anti-inflammatory cytokines may be potentially useful in treatment of anxiety induced by inflammatory conditions.
Keywords: Anxiety, Cytokines, Interleukin-10, Open field test, Elevated plus maze test
1. INTRODUCTION
There are two major arms of the immune system, the pro-inflammatory and the anti-inflammatory components. An imbalance between these two components to favor either pro-inflammatory or anti-inflammatory state may be harmful. The pro-inflammatory cytokines are responsible for different phenomena underlying the inflammatory response including proliferation, differentiation, stimulation and activation of immune cells (Dinarello, 2000). Agents that produce inflammation induce feelings of malaise and lethargy that are grouped together as sickness behaviors. Sickness behaviors studies in rodents are mediated at the cellular levels by the pro-inflammatory cytokines like interleukin (IL)-1, IL-6 and tumor necrosis factor (TNF)-α (Dantzer, 2009; Howren et al., 2009; Felger and Lotrich, 2013). IL-1β is a classical pro-inflammatory cytokine implicated in the pathogenesis of inflammatory processes as well as diseases with inflammatory and immunological etiology, and is a powerful trigger for malaise and other symptoms of sickness. Blockade of IL-1 activity in vivo with its receptor antagonist, IL-1β antibody, or small molecule inhibitors of IL-1 production (caspase 1 inhibitors) have successfully treated inflammatory and autoimmune conditions in experimental animals as well as in human patients (Cunnane et al., 2001; Bresnihan et al., 2004; Gul et al., 2011; Wang et al., 2015). A recent meta-analysis of clinical trials has shown that pro-inflammatory cytokines have a potentially causal role in depression, and treatment with cytokine inhibitors or antibodies against inflammatory cytokines robustly improve depressive symptoms (Kappelmann et al., 2018). Furthermore, broad-spectrum non-steroidal anti-inflammatory drugs are more effective in treating depression than placebo (Muller et al., 2006; Kohler et al., 2014; Kappelmann et al., 2018). These studies suggest that pro-inflammatory processes may contribute to symptoms of affective disorders, and indicate that blockade of IL-1β cytokine activity can be therapeutic in their treatment. Furthermore, IL-1β produces anxiety in rodent models of affective behavior (Lacosta et al, 1999; Swiergiel and Dunn, 2007).
Endogenous anti-inflammatory cytokines control the immune response by suppressing the impact of the pro-inflammatory cytokines (Dinarello, 2000; Cavaillon, 2001; Moore et al., 2001; Lobo-Silva et al., 2016). IL-10 is a prototypical anti-inflammatory cytokine that acts via several mechanisms to ultimately minimize inflammation both within the periphery and in the central nervous system, and is one of the most potent anti-inflammatory cytokines (Mosser and Zhang, 2008; Zhou et al., 2009; Bijjiga and Martino, 2013; Lobo-Silva et al., 2016). It inhibits the synthesis and secretion of pro-inflammatory cytokines, encourages anti-inflammatory cytokine response and regulates the expression of major histocompatibility complex II and co-stimulatory molecules on antigen presenting cells, thereby suppressing pro-inflammatory immune responses (Moore et al., 2001; Zhou et al., 2009; Bijjiga and Martino, 2013). Thus increasing IL-10 might be a reasonable strategy to combat the sickness-like effects caused by inflammation. In fact, anti-inflammatory cytokines like IL-10, IL-4, and IL-13 limit inflammatory pain reactions by decreasing production of pro-inflammatory cytokines in rodents (Vale et al., 2003), and IL-10 decreases IL-1β production and sickness triggered by lipopolysaccharide (LPS) endotoxin in rodents (Howard et al., 1993; Bluthe et al., 1999; Leon et al., 1999; Vale et al., 2003). This provides support that IL-10 can oppose sickness produced by pro-inflammatory cytokines. However, there is nothing known about effects of IL-10 or other anti-inflammatory cytokines in anxiety or depressive behaviors. Anxiety assays provide a robust measure of affective behaviors in rodents. Therefore, the purpose of this study was to test if IL-10 itself is anxiogenic, and whether IL-10 can suppress the anxiogenic effects of pro-inflammatory cytokines. To accomplish this, in the present study, we examined the effects of peripherally administered IL-10 or IL-1β on sickness behavior and anxiety behavior in rats. We then tested if IL-10 ameliorates the sickness and anxiogenic behavioral effects of IL-1β. This is important as it can uncover whether anxiety induced by immune activation can be triggered by imbalance towards pro- or anti-inflammatory state, aid our understanding of the role of anti-inflammatory cytokines in mood changes associated with immune activation, and might provide a potential therapeutic option in the treatment of sickness and anxiety associated with inflammatory conditions.
2. EXPERIMENTAL PROCEDURES
2.1. Animal subjects
Adult male Sprague-Dawley rats (Envigo, Indianapolis, IN) were obtained at post-natal day 59-63 and were housed 2 – 3 per cage in the climate-controlled Biological Resource Facility at Rosalind Franklin University of Medicine and Science with ad libitum access to food and water. Lights in the housing room were on a reversed 12 h light / dark schedule (light off: 07:00–19:00). All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Rosalind Franklin University of Medicine and Science and complied with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
2.2. Treatment groups
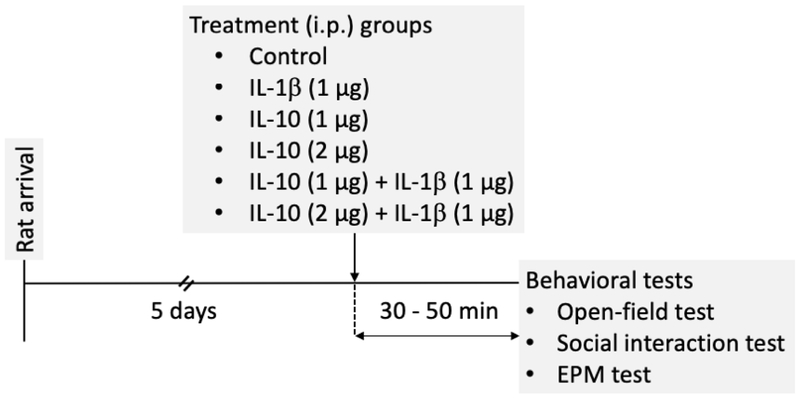
After habituation in the animal facility for at least 5 days, rats were randomly assigned to four different groups: (a) control (0.9 % saline, i.p.) (b) IL-10-treated (1 μg, i.p.) (c) IL-1β-treated (1 μg; i.p.), and (d) co-treated with IL-10 and IL-1β (1 μg each, i.p.). Recombinant rat IL-1β and IL-10 were purchased from GenScript (Piscataway, NJ). One aim of these experiments was to determine whether IL-10 could reverse sickness-like behaviors caused by IL-1β. Therefore, it was important to find a dose of IL-10 that itself did not cause sickness-like behaviors. A previous study demonstrated that IL-10 at a dose of 300 ng administered by intracerebroventricular (i.c.v.) route was enough to exert anti-inflammatory effects within the brain in rats (Bluthe et al., 1999). While only a fraction of peripherally administered IL-10 will enter the brain, this provided some initial dose guidance for our preliminary studies. Our preliminary experiments using approximately a 3-fold dose of IL-10 (1 μg i.p.) did not cause any effect on locomotion that would indicate sickness-like behavior at our tested time point (30 – 50 min post-treatment). Therefore this dose of IL-10 (1 μg) was chosen for i.p. administration. A second dose of IL-10 (2 μg) was included to verify dose-dependency and test whether higher doses similarly interact with IL-1β. On the other hand, IL-1β at 1 μg, i.p. caused significant reduction of locomotion consistent with sickness-like behavior in preliminary experiments at 30 min post-treatment, and consistent with other studies, has significant impact on the inflammatory state at this time range when injected at this dose (Lacosta et al., 1998; Swiergiel and Dunn, 2002; Swiergiel and Dunn, 2007; Munshi and Rosenkranz, 2018). For the rats co-treated with the two cytokines, IL-1β was injected immediately after IL-10 injection at two separate i.p. injection sites. All behavioral tests were performed at 30 – 50 min post-treatment (Figure 1). A total of 168 rats were used in the study (34 for all three behavioral tests in the order of (1) open field, (2) social interaction and (3) elevated plus maze tests; 26 for open field tests only; 27 for social interaction tests only; 28 for elevated plus maze tests only and 53 novel rats for social interaction tests).
Figure 1: Timeline of treatment interventions and behavioral tests.
Adult male Sprague-Dawley rats were treated with 250 μL of saline (control), IL-10 (1 μg or 2 μg), IL-1β (1 μg) or cotreated with IL-10 (1 μg or 2 μg) and IL-1β (1 μg) by i.p. routes. The rats were examined for open field behaviors at 30 – 50 min post-treatment.
2.3. Open field test
The open field test (in a black opaque, 24 in. × 35 in. open field) was performed in a dimly lit room (20-25 lx) with computer-generated white noise (65 – 70 dB) for 5 min at 30 – 50 min posttreatment. Behavioral recordings were obtained using IR-sensitive cameras (Fire-i, Unibrain, San Ramon, CA) connected to a computer (Dell E6500, Round Rock, TX) and were saved for off-line analysis using ANY-Maze version 4.99 z (Wood Dale, IL). The field was thoroughly cleaned with 70% ethanol between rats. All quantification of exploration was performed by software, blind to conditions. Central area exploration was measured as an index of anxiety-like behavior and total distance traveled was used as a measure of locomotor activity. The locomotor activity was used to gauge sickness-like behavior.
2.4. Social interaction test
The social interaction test was performed in an open field (conditions as above). The test subject was placed first in the open field. A novel adult male Sprague-Dawley rat, weighing within 50 g of the test rat, was then introduced in the field and the rats were allowed to interact freely with each other for 5 min. The novel rat had previously been acclimated to this open field for at least 10 min. The test rats were marked with black ink for identification during the tests and subsequent analysis. Social contacts were defined by conspecific sniffing, pushing of head or snout under the conspecific’s body, chasing, crawling over (or under), boxing or wrestling (File and Hyde, 1978). The number of times the test rat contacted the novel rat were quantified from the recorded video by a trained rater. The total time of interaction was also quantified during a separate video replay using a digital stop-watch. The rater was blind to treatment conditions and showed > 80 % consistency in tabulations before all final data were collected.
2.5. Elevated plus maze test
The EPM (Scientific Designs, Pittsburgh, PA) consisted of four arms: two open arms (width × length: 5 in. × 20 in.) and two closed arms (width × length × wall height: 5 in. × 20 in. × 18 in.), with a central junction (5 in. × 5 In.). Each arm was attached to a leg stand, elevated 32 in. from the ground. The rats were individually placed at the junction of the four arms, facing the open arm and opposite the experimenter. Rat behavior was recorded for 5 min with video-tracking ANY-Maze version 4.99 z software (Wood Dale, IL) and was saved for future analysis on a computer (Dell E6500, Round Rock, TX). All quantification of exploration was performed by software, blind to conditions. The time spent on open arms was measured and used as an index of anxiety-like behavior. Additionally, the number of total arm entries and total distance traveled in EPM were measured to use as an indicator of locomotor activity.
2.6. Data analysis
Data analysis was done using Microsoft Excel and GraphPad Prism software version 8.0 (La Jolla, CA). The number of rats initially chosen was based on the power needed to uncover anxiogenic and sickness effects of IL-1β in a previous study (Munshi and Rosenkranz, 2018). Data were tested for outliers (1.5 times the inter-quartile range), which were removed as indicated in the text. Two rats were excluded from the study due to unforeseen technical problem during video capture as indicated in the text. Comparison among three or more groups was done by analysis of variance (ANOVA). Because multiple doses of IL-10 were used, a mixed-effects model was tested. Significance in ANOVA was followed by Holm-Sidak's post hoc test and separate analysis for differences between IL-10 doses (two-tailed unpaired t-test). Statistical significance was set at p < 0.05.
3. RESULTS
3.1. Effect of IL-10 on open field behavior.
Open field behavior was measured from groups 30 – 50 min after treatment with saline (N = 13), IL-10 (1 μg, N = 9; 2 μg N = 6), IL-1β (N = 10), or IL-10 and IL-1β co-treatment (1 μg IL-10, N = 16; 2 μg IL-10, N = 6).
3.1.1. Distance traveled.
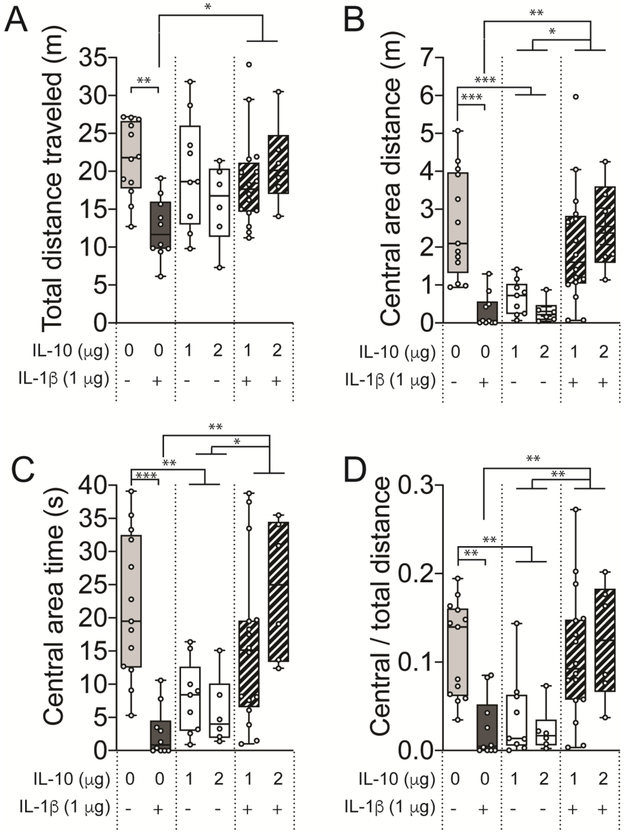
There was a significant effect of treatment on the total distance traveled in the open field (Figure 2A; F(3,44) = 6.557, p = 0.0017, one-way mixed effects ANOVA). IL-1β decreased the total distance traveled compared to the control (p < 0.01, t = 4.342, DF = 44, Holm-Sidak’s post hoc test). IL-10 treatment alone had no effect on the total distance traveled compared to the control (p > 0.05, t = 1.396, DF = 44, Holm-Sidak’s post hoc test), with no significant difference between doses of IL-10 (p > 0.05, t = 1.163, DF = 13, two-tailed unpaired t-test). However, co-treatment with IL-10 and IL-1β reduced the effect of IL-1β alone, as evidenced by increase in total distance traveled compared to the IL-1β treated group (p < 0.05, t = 3.204, DF = 44, Holm-Sidak’s post hoc test). There was no difference in the distance traveled between the control and the co-treatment group (p > 0.05, t = 1.469, DF = 44, Holm-Sidak’s post hoc test). This is consistent with sickness-inducing effects of IL-1β that are mitigated by IL-10.
Figure 2: IL-10 causes anxiety-like behavior and protects against IL-1β-induced sicknesslike behavior in open field test.
Adult male Sprague-Dawley rats were treated with 250 μL of saline (control), IL-10 (1 μg), IL-1β (1 μg) or both IL-10 and IL-1β (1 μg each, co-treatment) by i.p. routes. The rats were examined for open field behaviors at 30 – 50 min post-treatment. (A) Total distance traveled was unaffected in the IL-10 and co-treatment groups, while it was significantly reduced in the IL-1β group. (B) Central area distance and (D) Central / Total distance was significantly reduced in the IL-10 as well as in the IL-1β treated groups, while it was rescued by co-treatment of IL-10 and IL-1β. (C) Time spent in the central area was significantly reduced in IL-10 as well as in the IL-1β treated group. *p < 0.05, **p < 0.01, ***p < 0.001. Holm-Sidak’s post hoc test after significance in one-way ANOVA. N = 6 – 16 rats per group. Plots show mean ± Tukey. “+” indicates presence, “−” indicates absence.
3.1.2. Central area exploration.
Exploration of the central area of the open field is used as a measure of anxiety-like behavior. There was a significant effect of treatment on the central area distance traveled (Figure 2B; F(3,44) = 10.98, p < 0.0001, one-way mixed effects ANOVA). IL-1β decreased central distance traveled compared to the control (p < 0.001, t = 4.245, DF = 44, Holm-Sidak’s post hoc test). IL-10 treatment alone also reduced the central area distance traveled compared to the control (p < 0.001, t = 4.890, DF = 44, Holm-Sidak’s post hoc test), with no difference between IL-10 doses (p > 0.05, t = 0.1115, DF = 13, two-tailed unpaired t-test). Co-treatment with IL-10 and IL-1β increased the central area distance traveled compared to IL-1β (p < 0.01, t = 3.588, DF = 44, Holm-Sidak’s post hoc test) or compared to IL-10 (p < 0.05, t = 2.953, DF = 44, Holm-Sidak’s post hoc test). There was no difference in the distance traveled between the control and the co-treatment group (p > 0.05, t = 1.634, DF = 44, Holm-Sidak’s post hoc test). This suggests that IL-1β or IL-10 is anxiogenic, but have opposing actions when combined.
Similar to central area distance, the time spent exploring the central area was sensitive to cytokine treatments (Figure 2C; F(3,44) = 10.59, p < 0.0001, one-way mixed effects ANOVA). IL-1β decreased central area time compared to the control (p < 0.001, t = 5.081, DF = 44, Holm-Sidak’s post hoc test). IL-10 treatment alone also reduced the central area time compared to the control (p < 0.01, t = 3.664, DF = 44, Holm-Sidak’s post hoc test), with no difference between IL-10 doses (p > 0.05, t = 0.757, DF = 13, two-tailed unpaired t-test). Co-treatment with IL-10 and IL-1β increased the central area time compared to IL-1β (p < 0.01, t = 3.687, DF = 44, Holm-Sidak’s post hoc test) or compared to IL-10 (p < 0.05, t = 2.852, DF = 44, Holm-Sidak’s post hoc test). There was no difference in the central area time between the control and the co-treatment group (p > 0.05, t = 1.731, DF = 44, Holm-Sidak’s post hoc test).
Central area distance and central area time can be an index of anxiety. However, reliability is decreased if there is a global decrease in exploration that may contribute to decreased exploration in the central area, as seen here (total distance traveled). Therefore, the ratio of central to total distance was quantified (central area distance ÷ total distance traveled) to determine if there was a disproportionate decrease in central area exploration.
There was a significant effect of cytokine treatment on the ratio of central distance to total distance traveled (Figure 2D; F(3,44) = 10.25, p < 0.0001, one-way mixed effects ANOVA). IL-1β decreased the relative central area exploration compared to control (p < 0.01, t = 4.394, DF = 44, Holm-Sidak’s post hoc test) as did IL-10 compared to control (p < 0.01, t = 4.019, DF = 44, Holm-Sidak’s post hoc test), with similar effects of IL-10 doses (p > 0.05, t = 0.7570, df = 13, two-tailed unpaired t-test). However, co-treatment increased the relative central area exploration compared to IL-1β (p < 0.01, t = 3.965, DF = 44, Holm-Sidak’s post hoc and compared to IL-10 (p < 0.01, t = 3.586, DF = 42, Holm-Sidak’s post hoc test). There was no difference in the distance traveled between the control and the co-treatment group (p > 0.05, t = 0.6471, DF = 44, Holm-Sidak’s post hoc test). This indicates that IL-1β and IL-10 exerted anxiogenic effect in the open field, even when accounting for decreased locomotion induced by IL-1β. Overall, these results indicate that, while only IL-1β produced sickness effects, either IL-1β or IL-10 was anxiogenic in the open field, but their effects were offset by IL-1β + IL-10 co-treatment.
3.2. Effect of IL-10 on social interaction.
Social interaction between cytokine-treated and conspecifics was tested in an open field and measured from groups at 30 – 50 min after treatment with saline (N = 15, one outlier removed), IL-10 (1 μg, N = 10; 2 μg, N = 6), IL-1β (N = 7), or IL-10 and IL-1β co-treatment (1 μg IL-10, N = 16; 2 μg IL-10, N = 6).
3.2.1. Total number of social contacts.
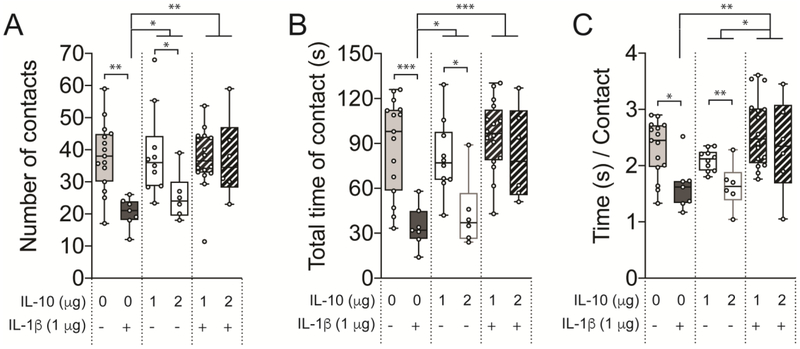
Cytokine treatment impacted the number of social contacts (Figure 3A; F(3,44) = 5.995, p = 0.0016, one-way mixed effects ANOVA). IL-1β treatment decreased the number of social contacts compared to control (p < 0.01, t = 3.845, DF = 44, Holm-Sidak’s post hoc test) or compared to IL-10 (p < 0.05, t = 2.901, DF = 44). However, IL-10 itself showed dose-dependency, such that the higher dose (2 μg) had greater impact on the number of contact (p < 0.05, t = 2.216, DF = 13, two-tailed unpaired t-test). The suppressive effect of IL-1β was reversed by co-treatment with IL-1β + IL-10 (p < 0.01, t = 3.937, DF = 44, Holm-Sidak’s post hoc test). There was no difference among the control, IL-10 treated group and co-treatment group (p > 0.05; Holm-Sidak’s post hoc test). This is consistent with socially anxiogenic effect of IL-1β that is mitigated by IL-10.
Figure 3: IL-10 has no effect on social interaction but its co-treatment protects against the IL-1β-induced reduction of social interaction.
Adult male Sprague-Dawley rats were treated with 250 μL of saline (control), IL-10 (1 μg), IL-1β (1 μg) or both IL-10 and IL-1β (1 μg each, co-treatment) by i.p. routes. The rats were allowed to socially interact for 5 min with a novel rat in an open chamber at 30 – 50 min post-treatment. (A) The number of interactions was unaffected in the IL-10, was significantly reduced in the IL-1β group, while rescued by cotreatment of IL-10 and IL-1β treated group. (B) Total time of interaction was only significantly reduced in the IL-1β treated group, while it was rescued by co-treatment of IL-10 and IL-1β. (C) Time / contact was significantly reduced in the IL-1β treated group only. *p < 0.05, **p < 0.01, ***p < 0.001. Holm-Sidak’s post hoc test after significance in one-way ANOVA or two-tailed unpaired t-test between doses of IL-10. N = 6 – 16 rats per group. Plots show mean ± Tukey. “+” indicates presence, “−” indicates absence.
3.2.2. Time of social contacts.
Cytokine treatment had an effect on the total time of social contact (Figure 3B; F(3,44) = 10.26, p < 0.001, one-way mixed effects ANOVA). IL-1β treatment decreased the total social contact time compared to control (p < 0.001, t = 4.675, DF = 44, Holm-Sidak’s post hoc test) or compared to IL-10 (p < 0.05, t = 2.762, DF = 44). While overall, IL-10 did not significantly decrease the time of social contact (p > 0.05, t = 1.907), the higher dose of IL-10 (2 μg) decreased social contact time compared to the lower dose (p = 0.0104, t = 2.958, DF = 13, two-tailed unpaired t-test). Co-treatment with IL-1β + IL-10 increased total social contact time compared to IL-1β (p < 0.001, t = 5.163, DF = 44, Holm-Sidak’s post hoc test). There was no difference in the total time of contacts among the control, IL-10 treated group and co-treatment group (p > 0.05; Holm-Sidak’s post hoc test).
Similarly, cytokine treatment had an effect on duration of each social contact (time/contact; Figure 3C; F(3,44) = 6.368, p = 0.0011, one-way mixed effects ANOVA). IL-1β treatment decreased the time/contact compared to control (p < 0.05, t = 3.001, DF = 44, Holm-Sidak’s post hoc test). While IL-10 alone, overall, did not significantly decrease the duration of social contacts (p > 0.05, t = 1.793, DF = 44), the higher dose of IL-10 (2 μg) more greatly decreased the duration of social contacts compared to the lower dose (p < 0.01, t = 3.108, DF = 13, two-tailed unpaired t-test), such that no significant difference emerged between IL-10 and IL-1β treatment (p > 0.05, t = 1.302, DF = 44). The suppressive effect of IL-1β was reversed by co-treatment with IL-1β + IL-10 (p < 0.01, t = 3.946, DF = 44, Holm-Sidak’s post hoc test), as was the suppressive effect of IL-10 alone (p < 0.05, t = 2.844, DF = 44, Holm-Sidak’s post hoc test). There was no difference in the time per contact among the control and co-treatment group (p > 0.05; Holm-Sidak’s post hoc test). This suggests that IL-1β produces social anxiety but IL-10 does not, and IL-10 can reduce the socially anxiogenic actions of IL-1β.
3.3. Effect of IL-10 on elevated plus maze test.
EPM activity was measured from groups at 30 – 50 min post-treatment. After removal of 4 data points (2 outliers and 2 for technical problem during video capture), the groups were saline (N = 13), IL-10 (1 μg, N = 9; 2 μg, N = 6), IL-1β (N = 10), or IL-10 and IL-1β co-treatment (1 μg IL-10, N = 14; 2 μg IL-10, N = 6).
3.3.1. Effect on locomotion.
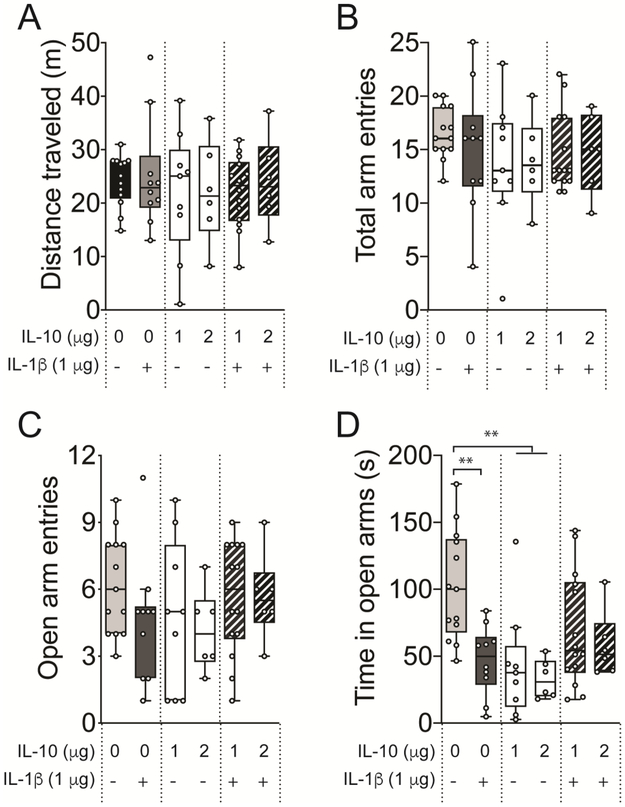
There was no effect of cytokine treatment on total distance traveled in EPM (Figure 4A; F(3,44) = 0.5337, p = 0.6616, one-way mixed effects ANOVA) or total arm entries (Figure 4B; F(3,44) = 0.9887, p = 0.4069, one-way mixed effects ANOVA). It also had no significant effect on open arm entries (Figure 3C; F(3,44) = 0.8517, p = 0.4732, one-way mixed effects ANOVA). Thus, locomotion in the EPM was not affected. IL-1β caused no significant effect on total number of arm entries, although visual inspection indicates a possible trend towards reduced open arm entries after treatment with IL-1β (Figure 4C; t = 1.560, p > 0.05) compared to control.
Figure 4: IL-10 causes anxiety-like behavior in elevated plus maze.
Adult male Sprague-Dawley rats were treated with 250 μL of saline (control), IL-10 (1 μg), IL-1β (1 μg) or both IL-10 and IL-1β (1 μg each, co-treatment) by i.p. routes. The rats were examined in elevated plus maze 30 – 50 min post-treatment. (A) Total arm entries, (B) open arm entries, (C) closed arm entries, and (D) total distance traveled in EPM were not affected among the treatment groups. (E) Time spent in open arm was significantly reduced in the IL-10 treated group, and a trend towards a reduced time spent in the open arm by IL-1β treatment is seen. (F) Time spent in closed arms was not affected among the treatment groups. **p < 0.01. Holm-Sidak’s post hoc test after significance in one-way ANOVA. N = 6 – 14 rats per group. Plots show mean ± Tukey. “+” indicates presence, “−” indicates absence.
3.3.2. Effect on anxiety-like behavior.
There was a significant effect of cytokine treatment on the time spent in open arms of EPM (Figure 4D; F(3,44) = 5.991, p = 0.0016, one-way mixed effects ANOVA). IL-1β or IL-10 caused decreases in the time spent in open arms compared to controls (IL-1β p < 0.01, t = 3.621, DF = 44; IL-10 p < 0.01, t = 3.526, DF = 44, Holm-Sidak’s post hoc test), with similar suppressive effects of both doses of IL-10 (p < 0.05, t = 0.5800, DF = 13). The suppressive effects of IL-1β were partly reversed by co-treatment with IL-1β + IL-10, such that control and co-treatment were not different (p > 0.05, Holm-Sidak’s post hoc test). This indicates that either IL-1β or IL-10 is anxiogenic in the EPM, and that IL-10 reduces these anxiogenic effects of IL-1β.
4. DISCUSSION
In this study, we examined how peripherally administered anti-inflammatory cytokine IL-10 affects behavioral changes seen during inflammation and sickness. Our findings suggest two salient features of peripherally administered IL-10 effects in adult male rats: (a) it prevents the development of sickness-like and anxiety-like behaviors when co-exposed to IL-1β, and (b) it can cause anxiety-like behavior by itself (Table 1).
Table 1: Comparison of the behavioral effects after treatment with IL-10, IL-1β, and cotreatment of IL-10 and IL-1β.
The table summarizes the effect of IL-10, IL-1β and co-treatment of IL-10 and IL-1β on the open field, elevated plus, and social interaction tests. “+” indicates significantly increased effect, while “−” indicates no difference in effect compared to respective control.
| IL-1β | IL-10 (1 μg) |
IL-10 (2 μg) |
Co-treatment (1 μg IL-10 + IL-1β) |
Co-treatment (2 μg IL-10 + IL-1β) |
|
|---|---|---|---|---|---|
| Anxiety-like behavior in OFT | + | + | + | - | - |
| Anxiety-like behavior in social interaction | + | - | + | - | - |
| Anxiety-like behavior in EPM | + | + | + | - | - |
IL-10 blocks sickness effects of IL-1β.
The current results add to the existing evidence that IL-10 can mitigate the impact of inflammatory cytokines. IL-10 reduces fever, a key sign of inflammation, associated with both gram-positive (Staphylococcus aureus) and gram-negative (Escherichia coli LPS) infection in adult Sprague-Dawley rats (Cartmell et al., 2003). In addition, IL-10 reduces sickness-like and weight loss effects of centrally administered endotoxin LPS (Bluthe et al., 1999), and reverses sickness, fever, and locomotor effects of peripherally administered LPS (Bluthe et al., 1999; Nava et al, 1997). The current experiments were designed to uncover anxiogenic effects of IL-10, and effects that can oppose IL-1β, and were not designed to assess sickness-inducing effects of IL-10. Indeed, in these experiments, a dose of IL-10 was specifically chosen because it did not produce acute sickness behaviors. One study that did not find a similar result used a much higher dosing of IL-10 (130 μg/kg) and at a much later time point (6 h; Harvey et al., 2006). Therefore, it is important to appreciate that effects of IL-10 may be time limited, and higher doses may be capable of inducing sickness.
IL-10 blocks anxiogenic effects of IL-1β.
A novel finding from the current study is the ability of IL-10 to block acute anxiogenic effects of IL-1β. Previous studies from IL-10 knock-out mice suggest that IL-10 may provide protective effects against anxiety (Mesquita et al, 2008), though others suggest that a high dose of IL-10 may impair habituation and produce anxiety in the open field (Harvey et al, 2006). Surprisingly, we found that IL-10 produces anxiety in the elevated plus maze and the open field, and in the social interaction test at higher doses, independent of effects on locomotion. It is not clear why higher doses of IL-10 were required to induce anxiety in the social interaction test, though data suggest shorter duration of social contacts even after the lower dose of IL-10. This might not translate into less overall social interaction because, unlike EPM and open field, the exploration opportunity is brought directly to the rat when the conspecific rat initiates social interactions in this assay. Alternatively, social anxiety may be a distinct construct that utilizes neural circuitry that is less sensitive to IL-10. Despite anxiogenic effects of IL-10, it was able to mitigate the anxiogenic effects of IL-1β in all assays. While one important previous study suggested the ability of IL-10 to reduce anxiogenic effects of LPS in the open field (Bluthe et al., 1999), the current results significantly extend the field because that prior study was limited to one anxiety assay, used a very high dose of IL-10, and IL-10 alone produced increased activity in the open field that could have been interpreted to simply be offsetting the behavioral effects of LPS. Because IL-10 alone was not anxiolytic in the current study, it would be difficult to explain these results as IL-10 behavioral effects offsetting IL-1β behavioral effects.
IL-10 antagonism of IL-1β behavioral effects.
Initial experiments using 1 μg IL-10 found that it was capable of seemingly mitigating the anxiogenic effects of IL-1β. One possible explanation of this, particularly in measures where IL-10 alone had minimal effect, is that IL-10 simply produces a behavior that interferes with measurement of anxiety. While this conclusion would only be partly supported from our results, we also tested a higher dose of IL-10 that itself produced clear anxiogenic effects. This demonstrates a dose-dependency of the effects of IL-10. But more importantly, from our results, it is clear that not only IL-10 can block the effects of IL1β, but it appears that IL-1β is also able to block the anxiogenic effects of IL-10. This hints at counteracting effects of IL-10 and IL1β, wherein either alone can induce anxiety, but cancel each other when together. There are some hints to possible mechanisms for this. IL-10 reduces the effects of pro-inflammatory cytokines by inhibiting their synthesis and action in the periphery and in the brain (de Waal et al., 1991; Cassatella et al., 1993; Enk et al., 1994; Knoblach and Faden, 1998; Bluthe et al., 1999; Pahan et al., 2000). It is not entirely clear how acute IL-10 antagonizes the acute effects of IL-1β seen in the present study, but it may include one or more of the following mechanisms: (1) downregulating the pro-inflammatory cytokine IL-1R1 receptors (Kelly et al., 2001), (2) inducing naturally occurring antagonist of IL-1 receptor (IL-1ra) (Jenkins et al., 1994), (3) reducing the immune stimulatory effects of IL-1β by decreasing the synthesis of pro-inflammatory cytokines via inhibition of NF-kB activated gene transcription (Driessler et al., 2004) or via inhibition of transcription of inflammatory target genes without involving NF-kB activation (Murray, 2005), (4) decreasing expression of IL-1β by decreasing the activation of NLRP3 inflammasome and caspase-8 (Gurung et al., 2015), (5) decreasing MHC Class II expression on APCs, thereby decreasing CD4+ T-cell response (Mittal et al., 2015), and (6) abolishing the IL-1β-induced inhibition of glutamate release and long-term potentiation and by reversing the IL-1β-induced stimulation of c-Jun activated protein kinase activity in hippocampal synapses (Kelly et al., 2001). It is important to note that although it is widely accepted that IL-10 suppresses the effects of pro-inflammatory cytokines, it can also act as an immunostimulatory cytokine during endotoxemia, thereby increasing the production of pro-inflammatory cytokines (Lauw et al., 2000); however, this is unlikely to underlie effects in our present study because IL-10 is not aggravating, but counteracting, the behavioral effect of IL-1β.
The site of interaction between IL-10 and IL-1β is not clear. However, peripheral IL-10 is able to cross the blood-brain barrier and act on the central nervous system to reduce the synthesis and action of pro-inflammatory cytokine in the brain (Di Santo et al., 1997), and centrally administered IL-10 reduced the effects of peripherally administered LPS, thereby suggesting a central site of IL-10 action (Bluthe et al., 1999). This is supported by findings that IL-10 and IL-1β both also produce rapid changes in CNS neurotransmission (Dunn, 2006; Skelly et al, 2013; Zhu et al, 2006), and IL-1β produces rapid changes of neuronal activity (Munshi and Rosenkranz, 2018).
One limitation of the study is that, we did not address the possible sites of action of the cytokines IL-10 and IL-1β. In addition, we did not measure the levels of IL-10 and IL-1β in the CSF, although this approach has its own limitations. Injected cytokines themselves can activate elements of the immune system and increase levels of IL-10 or IL-1β, and they can impact the CNS even without influencing CNS immune status (e.g. by activating the subdiaphragmatic vagus nerve). Therefore, we have been conservative about our interpretation of where the cytokines may be acting and interacting.
A range of previous studies link pro-inflammatory status with anxiety and depression. An important conclusion that can be drawn from the current results is that imbalance of the peripheral immune system towards either pro-inflammatory or anti-inflammatory states can produce anxiety. Furthermore, our data suggest that IL-10 might be beneficial in mitigating the effects of anxiety behavior caused by a pro-inflammatory state, and this might provide a strong rationale to target activation of the IL-10 signaling pathway in experimental models with a goal to develop novel therapeutic molecules in the treatment of inflammation-induced sickness and anxiety conditions.
HIGHLIGHTS:
IL-1β caused sickness-like behavior and anxiety-like behavior.
IL-10 alone did not cause acute sickness-like behavior.
IL-10 alone caused anxiety-like behavior in the open field, social interaction, and elevated plus maze tests.
IL-10 co-administration blocked the acute sickness-like and anxiogenic effects caused by IL-1β.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Drs. Anthony R. West, Janice H. Urban, Grace E. Stutzmann, Robert A. Marr and Gloria E. Meredith for helpful discussion. This study was presented by the authors at the Annual Meeting of the Society for Neuroscience (San Diego, 2018).
FUNDING
This study was supported by the National Institutes of Health grants MH084970 and MH109484. The funding body had no role in the design of the study, collection and analysis of data and the decision to publish.
Footnotes
DISCLOSURE
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bijjiga E, Martino AT (2013) Interleukin 10 (IL-10) Regulatory Cytokine and its Clinical Consequences. J Clin Cell Immunol S1 10.4172/2155-9899.S1-007 [DOI] [Google Scholar]
- Bluthe RM, Castanon N, Pousset F, Bristow A, Ball C, Lestage J, Michaud B, Kelley KW, Dantzer R (1999) Central injection of IL-10 antagonizes the behavioural effects of lipopolysaccharide in rats. Psychoneuroendocrinology 24:301–311. [DOI] [PubMed] [Google Scholar]
- Bresnihan B, Newmark R, Robbins S, Genant HK (2004) Effects of anakinra monotherapy on joint damage in patients with rheumatoid arthritis. extension of a 24-week randomized, placebo-controlled trial. J Rheumatol 31:1103–1111. [PubMed] [Google Scholar]
- Cartmell T, Ball C, Bristow AF, Mitchell D, Poole S (2003) Endogenous interleukin-10 is required for the defervescence of fever evoked by local lipopolysaccharide-induced and staphylococcus aureus-induced inflammation in rats. J Physiol 549:653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassatella MA, Meda L, Bonora S, Ceska M, Constantin G (1993) Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J Exp Med 178:2207–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillon JM (2001) Pro- versus anti-inflammatory cytokines: Myth or reality. Cell Mol Biol 47:695–702. [PubMed] [Google Scholar]
- Cunnane G, Madigan A, Murphy E, FitzGerald O, Bresnihan B (2001) The effects of treatment with interleukin-1 receptor antagonist on the inflamed synovial membrane in rheumatoid arthritis. Rheumatology (Oxford) 40:62–69. [DOI] [PubMed] [Google Scholar]
- Dantzer R (2009) Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am 29: 247–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE (1991) Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of IL-10 produced by monocytes. J Exp Med 174:1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Santo E, Adami M, Bertorelli R, Ghezzi P (1997) Systemic interleukin 10 administration inhibits brain tumor necrosis factor production in mice. Eur J Pharmacol 336:197–202. [DOI] [PubMed] [Google Scholar]
- Dinarello CA (2000) Proinflammatory cytokines. Chest 118:503–508. [DOI] [PubMed] [Google Scholar]
- Driessler F, Venstrom K, Sabat R, Asadullah K, Schottelius AJ (2004) Molecular mechanisms of interleukin-10-mediated inhibition of NF-kappaB activity: A role for p50. Clin Exp Immunol 135:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enk AH, Saloga J, Becker D, Mohamadzadeh M, Knop J (1994) Induction of hapten-specific tolerance by interleukin 10 in vivo. J Exp Med 179:1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Lotrich FE (2013) Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 246: 199–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Hyde JR (1978) Can social interaction be used to measure anxiety? Br J Pharmacol 62:19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gul A, Tugal-Tutkun I, Dinarello CA, Reznikov L, Esen BA, Mirza A, Scannon P, Solinger A (2012) Interleukin-1beta-regulating antibody XOMA 052 (gevokizumab) in the treatment of acute exacerbations of resistant uveitis of behcet's disease: An open-label pilot study. Ann Rheum Dis 71:563–566. [DOI] [PubMed] [Google Scholar]
- Gurung P, Li B, Subbarao Malireddi RK, Lamkanfi M, Geiger TL, Kanneganti TD (2015) Chronic TLR stimulation controls NLRP3 inflammasome activation through IL-10 mediated regulation of NLRP3 expression and caspase-8 activation. Sci Rep 5:14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey D, Smith R, English K, Mahon B, Commins S (2006) Interleukin-10 (IL-10) but not lipopolysaccharide (LPS) produces increased motor activity and abnormal exploratory patterns while impairing spatial learning in Balb/c mice. Physiol Behav 87:842–847 [DOI] [PubMed] [Google Scholar]
- Howard M, Muchamuel T, Andrade S, Menon S (1993) Interleukin 10 protects mice from lethal endotoxemia. J Exp Med 177:1205–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J (2009) Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom Med 71:171–186. [DOI] [PubMed] [Google Scholar]
- Jenkins JK, Malyak M, Arend WP (1994) The effects of interleukin-10 on interleukin-1 receptor antagonist and interleukin-1 beta production in human monocytes and neutrophils. Lymphokine Cytokine Res 13:47–54. [PubMed] [Google Scholar]
- Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM (2018) Antidepressant activity of anti-cytokine treatment: A systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry 23:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A, Lynch A, Vereker E, Nolan Y, Queenan P, Whittaker E, O'Neill LA, Lynch MA (2001) The anti-inflammatory cytokine, interleukin (IL)-10, blocks the inhibitory effect of IL-1 beta on long term potentiation. A role for JNK. J Biol Chem 276:45564–45572. [DOI] [PubMed] [Google Scholar]
- Knoblach SM, Faden AI (1998) Interleukin-10 improves outcome and alters proinflammatory cytokine expression after experimental traumatic brain injury. Exp Neurol 153:143–151. [DOI] [PubMed] [Google Scholar]
- Kohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, Krogh J (2014) Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: A systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 71:1381–1391. [DOI] [PubMed] [Google Scholar]
- Lacosta S, Merali Z, Anisman H (1998) Influence of interleukin-1beta on exploratory behaviors, plasma ACTH, corticosterone, and central biogenic amines in mice. Psychopharmacology 137:351–361. [DOI] [PubMed] [Google Scholar]
- Lacosta S, Merali Z, Anisman H (1999) Behavioral and neurochemical consequences of lipopolysaccharide in mice: anxiogenic-like effects. Brain Res. 818:291–303. [DOI] [PubMed] [Google Scholar]
- Lauw FN, Pajkrt D, Hack CE, Kurimoto M, van Deventer SJ, van der Poll T (2000) Proinflammatory effects of IL-10 during human endotoxemia. J Immunol 165:2783–2789. [DOI] [PubMed] [Google Scholar]
- Leon LR, Kozak W, Rudolph K, Kluger MJ (1999) An antipyretic role for interleukin-10 in LPS fever in mice. Am J Physiol 276:R81–9. [DOI] [PubMed] [Google Scholar]
- Lobo-Silva D, Carriche GM, Castro AG, Roque S, Saraiva M (2016) Balancing the immune response in the brain: IL-10 and its regulation. J Neuroinflammation 13:297-016-0763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita AR, Correia-Neves M, Roque S, Castro AG, Vieira P, Pedrosa J, Palha JA, Sousa N (2008) IL-10 modulates depressive-like behavior. J Psychiatr Res 43:89–97. [DOI] [PubMed] [Google Scholar]
- Mittal SK, Cho KJ, Ishido S, Roche PA (2015) Interleukin 10 (IL-10)-mediated immunosuppression: MARCH-1 induction regulates antigen presentation by macrophages but not dendritic cells. J Biol Chem 290:27158–27167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A (2001) Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19:683–765. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Zhang X (2008) Interleukin-10: New perspectives on an old cytokine. Immunol Rev 226:205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, Spellmann I, Hetzel G, Maino K, Kleindienst N, Moller HJ, Arolt V, Riedel M (2006) The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: Results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry 11:680–684. [DOI] [PubMed] [Google Scholar]
- Munshi S, Rosenkranz JA (2018) Effects of Peripheral Immune Challenge on In Vivo Firing of Basolateral Amygdala Neurons in Adult Male Rats. Neuroscience 390:174–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ (2005) The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc Natl Acad Sci 102:8686–8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (2011) Guide for the Care and Use of Laboratory Animals. National Academies Press. [PubMed] [Google Scholar]
- Navá F, Calapai G, Facciola G, Cuzzocrea S, Marciano MC, De Sarro A, Caputi AP (1997) Effects of interleukin-10 on water intake, locomotory activity, and rectal temperature in rat treated with endotoxin. Int J immunopharmacol 19:31–38 [DOI] [PubMed] [Google Scholar]
- Pahan K, Khan M, Singh I (2000) Interleukin-10 and interleukin-13 inhibit proinflammatory cytokine-induced ceramide production through the activation of phosphatidylinositol 3-kinase. J Neurochem 75:576–582. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Dunn AJ (2002) Distinct roles for cyclooxygenases 1 and 2 in interleukin-1-induced behavioral changes. J Pharmacol Exp Ther 302:1031–1036. [DOI] [PubMed] [Google Scholar]
- Swiergiel AH, Dunn AJ (2007) Effects of interleukin-1beta and lipopolysaccharide on behavior of mice in the elevated plus-maze and open field tests. Pharmacol Biochem Behav 86:651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale ML, Marques JB, Moreira CA, Rocha FA, Ferreira SH, Poole S, Cunha FQ, Ribeiro RA (2003) Antinociceptive effects of interleukin-4, −10, and −13 on the writhing response in mice and zymosan-induced knee joint incapacitation in rats. J Pharmacol Exp Ther 304:102–108. [DOI] [PubMed] [Google Scholar]
- Wang CC, Li H, Zhang M, Li XL, Yue LT, Zhang P, Zhao Y, Wang S, Duan RN, Li YB, Duan RS (2015) Caspase-1 inhibitor ameliorates experimental autoimmune myasthenia gravis by innate dendric cell IL-1-IL-17 pathway. J Neuroinflammation 12:118-015-0334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Peng X, Insolera R, Fink DJ, Mata M (2009) IL-10 promotes neuronal survival following spinal cord injury. Exp Neurol 220:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]