Abstract
Background:
Adaptive function and general intellectual function are two important and often correlated domains. While youth with prenatal alcohol exposure frequently demonstrate impairments in both domains, it is not clear whether the relation between these domains is consistent across levels of ability or whether, for example, adaptive function is less affected by intellectual function at higher ability levels. The aim of the current study was to test this relation in youth with and without prenatal alcohol exposure.
Methods:
As part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders, Phase II, subjects with heavy prenatal alcohol exposure (AE) and nonexposed subjects with and without other clinical conditions or concerns (CON) completed a comprehensive neurobehavioral battery. Multiple regression analyses tested the relation between full scale IQ (FSIQ) and overall adaptive function. Interaction terms between Group and each variable were created to formally test for group differences. Three subsequent regression analyses tested which adaptive function domains (Communication, Daily Living Skills, Socialization) significantly contributed to results. Follow-up analyses examined correlations based on IQ range (low IQ<85; high IQ≥85).
Results:
The interaction between FSIQ and Group on overall adaptive function was significant; the relationship between FSIQ and adaptive function was weaker in the AE group than in the CON group. Regarding specific adaptive function domains, the interaction between FSIQ and Group was significant only in the Communication domain. Follow-up analyses showed, within the low IQ range, the correlation between FSIQ and Communication was stronger in the CON group than the AE group. Within the high IQ range, the correlation between FSIQ and Communication was significant only in the CON group.
Conclusions:
Although higher intellectual functioning was associated with better adaptive function ability among controls, this was not found among the alcohol-exposed youth where a general dampening of adaptive ability was noted. Further, the differential relationship between IQ and adaptive function between groups appears to be driven by communication abilities. These findings suggest that level of intellectual functioning of children with prenatal alcohol exposure does not fully account for caregiver-reported communication and overall adaptive function deficits particularly at higher levels of functioning.
Keywords: Fetal alcohol spectrum disorders (FASD), fetal alcohol syndrome (FAS), intellectual functioning, adaptive function
Introduction
Prenatal alcohol exposure can significantly alter development of the fetus and result in a multitude of cognitive and behavioral consequences for the individual (Mattson, Crocker, & Nguyen, 2011; Riley, Infante, & Warren, 2011). Effects due to prenatal alcohol exposure fall along a continuum known as fetal alcohol spectrum disorders (FASD), with fetal alcohol syndrome (FAS) representing one of the more serious consequences. With prevalence estimates as high as 2–5% among school-age children (May et al., 2014; May et al., 2015; May et al., 2018), FASD represents a serious public health concern.
Research has shown that children with FASD are impaired on a wide variety of neurobehavioral domains including general intellectual functioning (Mattson & Riley, 1996; Mattson, Riley, Gramling, Delis, & Jones 1997) and adaptive functioning (Crocker, Vaurio, Riley, & Mattson, 2009; Mattson et al., 2011; Ware et al., 2012). General intellectual functioning includes cognitive abilities such as verbal, nonverbal, and perceptual reasoning and refers to one’s global ability to act purposefully and interact with the environment in a meaningful way (Wechsler, 1944). In terms of general intellectual functioning, children with FASD demonstrate impaired overall IQ with average IQ scores falling within the borderline to low average ranges (Coles et al., 1991; Mattson & Riley, 1996). More severe deficits in intellectual functioning can be seen with greater physical dysmorphology (Streissguth et al., 1991), but these IQ deficits can still be observed across the full spectrum of FASD including those individuals who do not display physical dysmorphology (Mattson et al., 1997).
Adaptive function refers to one’s ability to successfully function independently in everyday life and encompasses tasks such as communication, socialization, and daily living skills (Sparrow, Cicchetti, & Balla, 2005; Whaley, O’Connor, & Gunderson, 2001). Youth with prenatal alcohol exposure have shown deficits in all aspects of adaptive function. For example, youth with FASD have difficulty completing daily living tasks (e.g., toileting, feeding, bathing) and are less likely to be able to successfully live independently (Streissguth et al., 1991; Thomas, Kelly, Mattson, & Riley, 1998). Adaptive function domains that appear to be particularly affected include socialization and communication (Fagerlund et al., 2012). One cross-sectional study found that socialization and communication skills do not improve with age among youth with heavy prenatal alcohol exposure, suggesting that individuals with FASD demonstrate an arrest in development of these skills (Crocker et al., 2009) though longitudinal studies are still needed to clarify this trajectory.
General intellectual function and adaptive function are often correlated. However, the relationship between these two domains has not been explored among the FASD population. Studies have shown that youth with FASD are more impaired in adaptive function domains even as compared to IQ-matched controls with other neurodevelopmental disorders (Fagerlund et al., 2012) suggesting a differential relationship between IQ and adaptive function among youth with FASD. In other neurodevelopmental disorders (i.e., autism spectrum disorder), IQ has been shown to more strongly predict adaptive functioning in lower-functioning individuals while higher-functioning individuals are more limited by specific deficits such as language and verbal memory (Liss et al., 2001). Others have shown that the discrepancy between intellectual functioning and adaptive functioning is smaller among lower-functioning individuals with autism spectrum disorder though this discrepancy increases among higher-functioning individuals (Kanne et al., 2011). Similarly, investigations among the general population have shown the relation between IQ and adaptive functioning to be strongest among those with lower intellectual functioning and the strength of this relationship decreases with increasing IQ (Committee on Disability Determination for Mental Retardation, 2002). As such, we might expect the same effect among youth with FASD: a stronger relation between IQ and adaptive function among lower functioning individuals and a weaker relation between these two domains among higher functioning individuals.
Impairments in adaptive functioning can significantly impact these individuals’ ability to live independently as well as impact the lives of caregivers. Investigation into the relationship between intellectual functioning and adaptive functioning among youth with prenatal alcohol exposure can help elucidate possible cognitive bases of functional impairment among these individuals. Identification of cognitive factors (i.e., intellectual functioning) that may potentially influence adaptive function ability could help suggest targets for intervention and assist with treatment planning for these individuals. Additionally, investigation into the pattern of impairment at varying levels of intellectual functioning will provide greater clarity regarding adaptive functioning ability across the full spectrum of alcohol-affected individuals.
The current study had two main aims: 1) investigate the relationship between general intellectual functioning and adaptive functioning among youth with heavy prenatal alcohol exposure to determine whether IQ contributes to observed adaptive function deficits among this population; 2) investigate if the relationship between adaptive function and intellectual functioning differs between lower and higher functioning individuals. We hypothesized that youth with histories of heavy prenatal alcohol exposure would demonstrate impaired adaptive function as compared to nonexposed controls and that the relationship between general intellectual functioning and adaptive functioning would be weaker within the alcohol-exposed group. To determine if the relation differed between lower and higher functioning individuals, we investigated the relationship between IQ and adaptive function among two ranges: low IQ (IQ < 85) and high IQ (IQ ≥ 85). We hypothesized that, within both IQ ranges, the relationship between IQ and adaptive functioning would be stronger in the nonexposed control group than the alcohol-exposed group. Moreover, we hypothesized that, within the alcohol-exposed group, the relationship between adaptive function and IQ would be weaker in the high IQ range than the low IQ range.
Methods
General Methods
Data were collected as part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders, Phase Two (CIFASD II) multisite study. Participants (N = 437) aged 8–16 (M = 12.29) completed a comprehensive battery comprising standardized neuropsychological assessment and dysmorphology evaluations. General CIFASD methodology has been described previously (Mattson et al., 2010). Subjects were tested at five sites: (1) the Center for Behavioral Teratology at San Diego State University, (2) The Marcus Institute at Emory University, (3) the Children’s Hospital at the University of Southern California, (4) the Center on Alcoholism, Substance Abuse and Addictions at the University of New Mexico, and (5) seven different communities throughout North Dakota, South Dakota, and Montana (Northern Plains). Study protocol was equivalent across all sites; testers were trained to administer all assessments in a standardized manner and 10% of all assessments were reviewed for validity. Informed consent and assent were obtained from caregivers and subjects prior to testing and financial incentive for participation was provided. The Institutional Review Board at San Diego State University and other CIFASD sites approved this study.
Subjects
Subjects comprised two groups: youth with histories of heavy prenatal alcohol exposure (AE, n = 163) and nonexposed controls (CON, n = 274). Subjects in the AE group had confirmed histories of heavy prenatal alcohol exposure. Heavy prenatal alcohol exposure was defined as a pattern of heavy or binge drinking in pregnancy evidenced by maternal consumption of >13 drinks per week or >4 drinks per occasion, on average (Jones et al., 2006; Mattson et al., 2010). In cases where direct maternal report was not available, a review of medical, social services, or court records was required. In these instances, subjects were included in the AE group if there was documentation of alcohol abuse or dependence in the biological mother or if exposure was suspected and the child met criteria for FAS. Within the AE group, about 21% of subjects were direct report (i.e., biological mother) and about 79% of subjects were collateral report.
Control subjects were recruited from the same communities as the AE group. Subjects were excluded from the CON group if prenatal alcohol exposure was more than minimal or if information on exposure was unavailable. Minimal exposure was defined as no more than 1 drink per week on average and never more than 2 drinks per occasion. Confirmation of alcohol exposure histories occurred by direct report for about 92% of control subjects and by collateral report for about 8% of control subjects. The larger study from which these data were collected included subjects without histories of prenatal alcohol exposure but with parent-reported behavior concerns or conditions or previous clinical diagnoses of behavior disorders (e.g., attention-deficit/hyperactivity disorder [ADHD]). To obtain a clinically relevant comparison group for the current study, subjects were not excluded from the nonexposed CON group based on the presence clinically significant behavioral problems or previous clinical diagnoses at the time of initial study enrollment unless symptoms interfered with participation, as detailed below. As a result, this group represents a heterogenous comparison sample. Additional exclusion criteria for both groups included: primary language other than English, being adopted from abroad within two years of participation or after the age of 5, history of significant head injury and/or loss of consciousness greater than 30 minutes (no subjects had loss of consciousness greater than 5 minutes), or presence of a severe mental, psychiatric, or physical disability that precluded participation in the study (e.g., autism spectrum disorder, active mania or psychosis, blindness). Presence of symptoms associated with psychiatric conditions was determined using the Computerized Diagnostic Interview Schedule for Children Version IV (C-DISC-4.0; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000), which was conducted in person with the parent while subjects completed testing. Finally, all subjects were examined for the presence of FAS and other alcohol-related diagnoses based upon CIFASD criteria (Jones et al., 2006; Mattson et al., 2010).
Measures
Selected measures from the larger CIFASD II battery were chosen to assess the relation between adaptive function and general cognitive ability among youth with heavy prenatal alcohol exposure. Measures included in this study are described below.
Wechsler Intelligence Scale for Children – Fourth Edition (WISC-IV; Wechsler, 2003).
The WISC-IV is a standardized measure to assess children’s general intellectual functioning. Performance on various subtests results in four index scores (Perceptual Reasoning Index, Verbal Comprehension Index, Processing Speed Index, Working Memory Index), which are combined to provide a global, Full Scale IQ (FSIQ) score. Performance on the four indices and overall FSIQ results in standard scores (M = 100, SD = 15) with lower scores indicating weaker performance. The FSIQ standard score was chosen to measure general intellectual functioning in the current study.
Vineland Adaptive Behavior Scales – Second Edition, Questionnaire (VABS-II; Sparrow, Cicchetti, & Balla, 2005).
The VABS-II is a caregiver-completed questionnaire that covers three domains of adaptive functioning (Communication, Daily Living Skills, and Socialization). Adaptive functioning refers to one’s ability to successfully meet everyday demands (e.g., maintaining hygiene, communicate with others, interact appropriately in social situations). Responses from these three domains are combined into an overall Adaptive Behavior Composite. The VABS-II provides standard scores for each domain and the overall composite (M = 100, SD = 15). The Adaptive Behavior Composite standard score was used in analyses with lower scores indicating weaker performance. Follow-up analyses investigated the relation between the Communication, Daily Living Skills, and Socialization standard scores and FSIQ.
Statistical Analyses
SPSS statistical software v.24 was used for all analyses. Demographic data were analyzed using Pearson’s chi-square (categorical data) or univariate analysis of variance (ANOVA; continuous data) techniques. Group differences on all variables (FSIQ, Communication, Socialization, Daily Living Skills, Adaptive Behavior Composite) were tested using independent-samples t-tests. Multiple regression analyses tested the relation between FSIQ and adaptive function as measured by the Adaptive Behavior Composite from the VABS-II. Interaction terms between Group and FSIQ were created to formally test for group differences on each variable. Three separate multiple regression analyses were then conducted to determine which adaptive function domains (Communication, Daily Living Skills, Socialization) significantly contributed to overall results. Finally, groups were then split into two IQ ranges: low IQ (FSIQ < 85) and high IQ (FSIQ ≥ 85). An IQ of 85 is equivalent to one standard deviation below the population mean and corresponds with the lower limit of the average range. As such, subjects falling within the low IQ subgroup had IQ scores below average or lower and subjects falling within the high IQ subgroup had IQ scores in the average range or higher. For our interpretive purposes, those subjects in the low IQ range can be described as lower functioning and those subjects in the high IQ range can be described as higher functioning. Analyses examined the correlations between FSIQ and significant adaptive function domains based on these low and high IQ ranges to determine if relationships differed with varying levels of IQ.
Evaluation of Covariates
Potential covariates were evaluated for inclusion in the analyses. An alpha level of p < .05 was used to determine appropriateness as a covariate and the homogeneity of regression assumption was tested by examining the interaction between each variable and Group. Six potential covariates were tested based on their potential relationship to the dependent variable (adaptive function): age, sex, site, race, ethnicity, and presence of ADHD. Although significant relations with Group were noted for age (p = .029), sex (p = .018), race (p = .020), and site (p = .035), none of these variables showed a significant linear relation with the dependent variable (VABS-II Adaptive Behavior Composite). Further, ethnicity did not show a significant relation with the dependent variable (p = .552). While ADHD showed a significant relation with the dependent variable (p < .001), an interaction effect was noted with Group (p = .041). As such, no covariates were included in subsequent analyses.
Results
Demographic Data
Groups did not significantly differ on sex (p = .573) or handedness (p = .158). Groups significantly differed on age (p = .026), race (p = .043), ethnicity (p = .046), FSIQ (p < .001), rate of research diagnosis of ADHD (p < .001), and site (p = .002). Specifically, the AE group was significantly older (M = 12.6, SD = 2.35) than the CON group (M = 12.1, SD = 2.57). The AE group also had significantly fewer white subjects (n = 87, 53.4%) than the CON group (n = 174, 64.2%) and had significantly fewer Hispanic subjects (n = 22, 13.4%) than the CON group (n = 62, 22.9%). Furthermore, the AE group had significantly lower FSIQ scores (M = 84.3, SD = 16.40) than the CON group (M = 100.0, SD = 17.81) as well as higher rates of ADHD diagnoses (n = 94, 57.7%) than the CON group (n = 97, 35.8%). Certain sites recruited more subjects than others. See Table 1 for complete demographic information by group.
Table 1.
Demographic information by group.
| Variable | AE n = 163 |
CON n = 271 |
p |
|---|---|---|---|
| Sex [n (% Female)] | 67 (41.1) | 104 (38.4) | .573 |
| Age [Mean (SD)] | 12.6 (2.35) | 12.1 (2.57) | .019 |
| Race [n (% White)] | 87 (53.4) | 174 (64.2) | .043 |
| Ethnicity [n (% Hispanic)] | 22 (13.5) | 62 (22.9) | .046 |
| Handedness [n (% Right)] | 141 (86.5) | 247 (91.1) | .158 |
| FSIQ [Mean (SD)] | 84.3 (16.40) | 100.0 (17.81) | <.001 |
| ADHD [n (%)] | 94 (57.7) | 97 (35.8) | <.001 |
| FAS [n (%)] | 39 (23.9) | 0 (0.0) | <.001 |
| CIFASD Site [n (%)] | .002 | ||
| Atlanta | 30 (18.4) | 53 (19.6) | |
| Los Angeles | 28 (17.2) | 21 (7.7) | |
| Northern Plains | 22 (13.5) | 34 (12.5) | |
| New Mexico | 11 (6.7) | 47 (17.3) | |
| San Diego | 72 (44.2) | 116 (42.8) |
Note: Full Scale IQ (FSIQ), a measure of general intellectual functioning, was measured using the Wechsler Intelligence Scale for Children – Fourth Edition (WISC-IV).
Neurobehavioral Data
Group performances on all neurobehavioral variables are presented in Table 2. There was a significant difference in all neurobehavioral scores for the AE and CON groups (ps < .001) as has been reported previously (Crocker et al., 2009; Mattson et al., 1997; Streissguth et al., 1991; Ware et al., 2012). The CON group performed significantly better than the AE group on the VABS-II Adaptive Behavior Composite (t(380) = −10.43, p < .001), the VABS-II Communication domain (t(396) = −11.206, p < .001 ), the VABS-II Daily Living Skills domain (t(391) = −8.440, p < .001), the VABS-II Socialization domain (t(392) = −7.850, p < .001), and the WISC-IV FSIQ (t(431) = −9.337, p < .001). Results from regression analyses are described below.
Table 2.
Group performance on neurobehavioral variables.
| Neurobehavioral Variable [Mean (SD)] |
AE (n = 163) |
CON (n = 274) |
p |
|---|---|---|---|
| VABS-II Adaptive Behavior Composite | 83.4 (18.00) | 103.6 (17.81) | <.001 |
| VABS-II Communication | 82.2 (15.17) | 101.1 (17.78) | <.001 |
| VABS-II Socialization | 85.0 (21.86) | 102.1 (19.15) | <.001 |
| VABS-II Daily Living Skills | 89.2 (20.15) | 106.6 (19.03) | <.001 |
| WISC-IV FSIQ | 84.3 (16.40) | 100.0 (17.81) | <.001 |
Note: Groups significantly differed on all measures. Groups comprised alcohol-exposed (AE) or nonexposed control (CON) subjects. Communication was measured by the Communication standard score from the Vineland Adaptive Behavior Scales – Second Edition (VABS-II); Socialization was measured by the Socialization standard score from the VABS-II; Daily Living Skills was measured by the Daily Living Skills standard score from the VABS-II; the Adaptive Behavior Composite standard score from the VABS-II combines the three domain specific scores (Communication, Socialization, Daily Living Skills); general intelligence was measured by the Full Scale IQ (FSIQ) standard score from the Wechsler Intelligence Scale for Children – Fourth Edition (WISC-IV).
Multiple Regression Analyses
Results from regression analyses are presented in Table 3. Overall, the interaction with FSIQ and Group was significant on the Adaptive Behavior Composite with subsequent analyses showing the interaction to be significant only within the Communication domain. The Adaptive Behavior Composite regression model was significant (F(3, 380) = 61.993; p < .001; R2 = .329). The interaction between FSIQ and Group was significant on the Adaptive Behavior Composite score (p = .045). Specifically, the relation between Adaptive Behavior Composite and FSIQ was stronger in the CON group (R2 = 0.204) than in the AE group (R2 = 0.047). Subsequent analyses showed that the Communication regression model was significant (F(3, 396) = 87.863; p < .001; R2 = .400) with a significant interaction between FSIQ and Group (p = .024). Specifically, the relation between Communication and FSIQ was stronger in the CON group (R2 = 0.282) than in the AE group (R2 = 0.111). The Daily Living Skills regression model was significant (F(3, 391) = 34.096; p < .001; R2 = .207), but the interaction between FSIQ and Group was not significant (p = .275). Upon removing the interaction term, the Daily Living Skills regression model remained significant (F(2, 392) = 47.501; p < .001; R2 = .196) with significant main effects of Group (p < .001) and FSIQ (p < .001). Finally, the Socialization regression model was significant (F(3, 392) = 33.832; p < .001; R2 = .206) and the interaction between FSIQ and Group approached significance (p = .063). Upon removing the interaction term, the Socialization regression model remained significant (F(2, 391) = 49.941; p < .001; R2 = .204) with significant main effects of Group (p < .001) and FSIQ (p < .001).
Table 3.
Multiple regression results.
| MODEL | Βa | SEa | p | R2 | Constant (SE) |
|---|---|---|---|---|---|
| Adaptive Behavior Composite | < .001 | 0.329 | 63.341 (7.705) | ||
| Group | −5.304 | 9.793 | .588 | ||
| FSIQ | .239 | .090 | .008 | ||
| Group x FSIQ | .217 | .108 | .045 | ||
| Communication | < .001 | 0.400 | 56.033 (6.446) | ||
| Group | −6.386 | 8.285 | .441 | ||
| FSIQ | .309 | .075 | < .001 | ||
| Group x FSIQ | .205 | .091 | .024 | ||
| Daily Living Skills | < .001 | 0.207 | 73.417 (8.189) | ||
| Group | 1.795 | 10.514 | .864 | ||
| FSIQ | .188 | .095 | .048 | ||
| Group x FSIQ | .126 | .115 | .275 | ||
| Socialization | < .001 | 0.206 | 72.511 (8.420) | ||
| Group | −7.524 | 10.826 | .487 | ||
| FSIQ | .150 | .098 | .126 | ||
| Group x FSIQ | .221 | .118 | .063 |
Note:
Unstandardized coefficients. Groups comprised alcohol-exposed (AE) or nonexposed control (CON) subjects. Communication was measured by the Communication standard score from the Vineland Adaptive Behavior Scales – Second Edition (VABS-II); Socialization was measured by the Socialization standard score from the VABS-II; Daily Living Skills was measured by the Daily Living Skills standard score from the VABS-II; the Adaptive Behavior Composite standard score from the VABS-II combines the three domain specific scores (Communication, Socialization, Daily Living Skills); general intelligence was measured by the Full Scale IQ (FSIQ) standard score from the Wechsler Intelligence Scale for Children – Fourth Edition (WISC-IV). Significant effects indicated with bold font.
Low and High IQ Follow-up
Follow-up correlation analyses tested the relationship between IQ and Communication as this was the only domain with a significant Group x IQ interaction. Adaptive behavior scores by group and IQ range are presented in Table 4 and follow-up correlation analyses are presented in Table 5. Follow-up analyses indicated that within the low IQ subgroup, the correlation between FSIQ and Communication was stronger in the CON group (r = .418) than the AE group (r = .337), though both were significant (ps ≤ .004). Furthermore, average Communication scores did not differ (p = .641) between the CON (M = 87.62) and AE (M = 88.42) groups within this subgroup. Within the high IQ range, the correlation between FSIQ and Communication was significant in the CON group (r = .445; p < .001) but not in the AE group (r = −.005; p = .963) and average Communication scores were significantly higher (p = .018) in the CON group (M = 106.67) than the AE group (M = 92.26). See Figure 1).
Table 4.
Adaptive behavior scores by group and IQ range.
| AE | CON | |||
|---|---|---|---|---|
| IQ RANGE | Low IQ | High IQ | Low IQ | High IQ |
| VABS-II Communication | 88.4 (12.20) | 92.3 (10.67) | 87.6 (19.00) | 106.7 (14.46) |
| VABS-II Socialization | 85.8 (24.50) | 84.2 (19.16) | 95.4 (21.21) | 104.0 (18.13) |
| VABS-II Daily Living Skills | 87.7 (21.66) | 90.7 (18.64) | 100.5 (22.54) | 108.4 (17.54) |
Note: Data are presented as Mean (Standard Deviation) standard score on the Vineland Adaptive Behavior Scales – Second Edition (VABS-II). Groups comprised alcohol-exposed (AE) or nonexposed control (CON) subjects and are classified as within low IQ (FSIQ < 85) and high IQ (FSIQ ≥ 85) ranges based on the Wechsler Intelligence Scale for Children – Fourth Edition (WISC-IV).
Table 5.
Correlation results between Communication scores and FSIQ by IQ range.
Note:
significant at the p < .05 level. Groups comprised alcohol-exposed (AE) or nonexposed control (CON) subjects. Communication was measured by the Communication standard score from the Vineland Adaptive Behavior Scales – Second Edition (VABS-II) and general intelligence was measured by the Full Scale IQ (FSIQ) standard score from the Wechsler Intelligence Scale for Children – Fourth Edition (WISC-IV). Correlation analyses tested the relation between Communication and FSIQ within low IQ (FSIQ < 85) and high IQ (FSIQ ≥ 85) ranges among AE and CON. Significant effects indicated with bold font.
Figure 1.
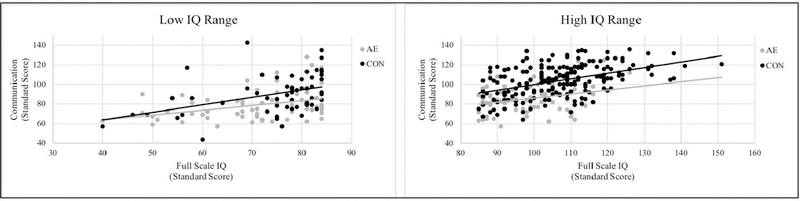
Correlation between IQ and Communication scores by IQ range. Groups consisted of youth with heavy prenatal alcohol exposure (AE) and nonexposed control (CON) subjects. Within the low IQ range (FSIQ < 85), the correlation between IQ and Communication was significant in both groups though this relationship was stronger in the CON group than the AE group. Within the high IQ range (FSIQ ≥ 85), the correlation between IQ and Communication was significant only in the CON group. IQ was measured with the Full Scale IQ standard score from the Wcchslcr Intelligence Scale for Children – Fourth Edition (WISC-IV) and communication was measured with the Vineland Adaptive Behavior Scales – Second Edition (VABS-II) Communication standard score.
Discussion
We aimed to determine whether the relationship between adaptive functioning and intellectual functioning differed among youth with heavy prenatal alcohol exposure as compared to nonexposed controls. Our findings indicated that the relation between adaptive functioning and IQ differed between groups. In general, higher intellectual functioning was more strongly associated with higher adaptive functioning among nonexposed controls than alcohol-exposed youth. This differential relation between IQ and adaptive functioning appears to be driven by communication abilities among alcohol-exposed youth as the interaction between Group and adaptive function was significant only in the Communication domain of the VABS-II. We also aimed to determine if the relationship between IQ and significant adaptive function domains (i.e., Communication) varied between lower and higher functioning individuals. Within the low IQ subgroup, the CON and AE groups showed similar communication ability and similar correlations between Communication and IQ. Within the high IQ subgroup, the relationship between IQ and Communication was only significant in the CON group. Additionally, between the low and high IQ ranges, the CON group showed a greater increase in Communication scores than the AE group. Findings suggest that caregiver-reported adaptive function deficits appear to be due to communication impairment among youth with histories of heavy prenatal alcohol exposure and are not fully explained by lower IQ scores. In fact, it appears that communication skills are relatively more impaired among the higher functioning subgroup of youth with prenatal alcohol exposure as the discrepancy between IQ and adaptive function was larger than in (1) controls with similar IQ scores and (2) alcohol-exposed youth with lower overall functioning. The interaction between IQ and Group was not significant in the Socialization or Daily Living Skills domains. As in our previous study, these findings were consistent for both females and males, as observed patterns did not differ by sex. Likewise, age did not impact our results (Panczakiewicz et al., 2016).
In the current study, communication abilities of alcohol-exposed youth correlated with IQ only at lower levels of IQ (<85). The relation was consistent across groups at this IQ level. At higher levels of IQ (≥85), however, communication correlated with IQ only in the CON group. As IQ scores increased, Communication scores likewise increased in the CON group but not the AE group. These findings are consistent with previous studies of other developmental disorders suggesting that with increasing IQ, adaptive function does not relate as strongly to IQ (Crocker et al., 2009; Liss et al., 2001; Committee on Disability Determination for Mental Retardation, 2002). The lack of a relationship between IQ and adaptive ability resulted in consistent adaptive behavior deficits in the AE group across levels of IQ and suggests a general dampening of adaptive function in this population (see Table 4) that is independent of overall ability level. A similar pattern has been seen among other neurodevelopmental disorders (Liss et al., 2001; Committee on Disability Determination for Mental Retardation, 2002) and our findings extend this knowledge to youth with prenatal alcohol exposure.
The explanation for these results is not entirely clear. Youth with heavy prenatal alcohol exposure demonstrate deficits in numerous domains of cognitive and behavioral functioning (Mattson & Riley, 1998; Mattson et al., 2011) which may impact adaptive functioning (Ware et al., 2012). Perhaps at higher levels of general intellectual ability, deficits in other cognitive domains are contributing to adaptive function ability to a greater degree than IQ, resulting in lower adaptive ability. It is not clear whether other functional domains (e.g., executive function) have the same differential relation to IQ as observed here. Further study is warranted to understand the relations between neuropsychological and behavioral functioning and overall intellectual ability. Such studies will provide greater clarity regarding the domains contributing to functional impairment across all levels of ability among this population.
The observed differential relation between IQ and adaptive function appeared to be driven by communication abilities within the alcohol-exposed group though the alcohol-exposed group was rated as significantly more impaired than the nonexposed group on all adaptive function domains. That communication is one of the most impacted domains of adaptive function among youth with prenatal alcohol exposure is supported by previous studies (Crocker et al., 2009; Fagerlund et al., 2012; Streissguth, Barr, Sampson, & Bookstein, 1994) and is reflected in our findings. Socialization deficits have also been reported among this population (Fagerlund et al., 2012; Streissguth et al., 1991; Thomas et al., 1998; Whaley et al., 2001). However, in the AE group in the current study, deficits in socialization appeared to be better accounted for by intellectual ability than were communication deficits as the relationship between socialization and IQ did not differ based on group (AE, CON). Similarly, the relationship between daily living skills and intellectual functioning did not differ based on group (AE, CON) suggesting IQ likewise accounts for daily living skills among youth with prenatal alcohol exposure. Longitudinal studies in other neurodevelopmental disorders (e.g., autism spectrum disorders) have shown improvement in caregiver-reported symptoms among individuals with less impaired communication skills (Bal, Kim, Fok, & Lord, 2018) suggesting improved functioning over time is closely related to better communication skills. As the current findings suggest communication deficits are driving impaired adaptive function skills among youth with prenatal alcohol exposure, interventions targeted at improving communication abilities may be most helpful in alleviating functional deficits experienced by this population.
As evidenced through these findings, deficient adaptive behavior, specifically communication ability, among youth with FASD is likely due to a multitude of factors and is not fully accounted for by general intellectual functioning. The current study was limited in ability to measure home environment, though it is likely differences exist between groups. Importantly, a majority of alcohol-exposed youth, at least in our studies, does not reside with their biological families and likely experience a greater number of home placements, which can infer greater risk for adaptive function deficits. Similarly, exposure to traumatic experiences among the alcohol-exposed group (Jester, Jacobson, Sokol, Tuttle, & Jacobson, 2000) may result in more significant neurodevelopmental deficits (Henry, Sloane, & Black-Pond, 2007). While one study in particular found no association with home placement on adaptive behavior among alcohol-exposed youth (Whaley et al., 2001), further investigation into the possible contribution of living environment as well as other behavioral and cognitive domains on adaptive function among this population is warranted.
Psychiatric disorders (e.g., ADHD) are highly prevalent among individuals with heavy prenatal alcohol exposure (Burd, Klug, Martsolf, & Kerbeshian, 2003; Fryer, McGee, Matt, Riley, & Mattson, 2007; Landgren, Svensson, Strömland, & Andersson Grönlund, 2010; O’Connor & Paley, 2009; Rasmussen et al., 2010) and may have contributed to our results. However, subjects with other psychiatric or behavioral disorders were not excluded from the control group to provide a clinically relevant heterogeneous comparison group. As such, the nonexposed group comprised nonexposed typically developing controls as well as nonexposed subjects with other disorders such as ADHD, learning disorders, and psychiatric disorders (e.g., major depressive disorder, anxiety disorder) strengthening the role of prenatal alcohol exposure in the observed results. In addition, ADHD was investigated as a covariate but was found to not be appropriate due to its interaction with Group. In a previous study, we examined the relative contribution of prenatal alcohol exposure and ADHD on adaptive behavior in a 2 (AE vs. Non-AE) x 2 (ADHD vs. Non-ADHD) design. The effects of ADHD on communication were stronger in the nonexposed subjects than in the alcohol-exposed subjects and conversely the effects of alcohol exposure were stronger in the subjects without ADHD than in the subjects with ADHD. Although IQ was not addressed in that study, the results support the current findings suggesting that deficits in adaptive behavior, and in particular communication deficits, occur in youth with histories of prenatal alcohol exposure and are not accounted for by IQ or ADHD.
Limitations
Several limitations should be considered regarding these findings. First, we relied on parent-report of adaptive function abilities. Results from the current study are consistent with previous studies in showing group differences on all domains of the VABS-II suggesting this test is sensitive to functional difficulties seen within this population (Crocker et al., 2009; Ware et al., 2012). Our results are predicated on the validity of our measures. Performance on any neurobehavioral measure may be influenced by a multitude of factors. We selected well validated measures that have been used extensively both clinically and in research and we controlled for extraneous variability both by our highly controlled research methods and statistically. While we are confident in our results, replication is warranted. In addition, future studies should consider supplementing parent-report measures with direct assessment of adaptive function skills. Additional concern may include use of such questionnaires with caregivers of lower educational levels, however, completion of the VABS-II requires a reading level roughly equivalent to the fifth grade (Sparrow et al., 2005). Information regarding parental education and socioeconomic status (SES) was not available for the current study. Future studies should include measures of parental education or SES to clarify possible contribution of caregiver-reporting styles to adaptive function deficits. Additionally, validity indicators embedded in the VABS-II questionnaire revealed invalid reporting for less than 1% of our sample, suggesting reliable reporting by parents and caregivers.
As the focus of the current study was on the relationship between IQ and adaptive function, other cognitive measures were not included. Future studies should expand upon those measures included here to investigate additional cognitive contributors to adaptive function deficits within this population (Ware et al., 2012). Other concerns may include further relevant information important for adaptive function and communication abilities that was not available for the current study. Information regarding developmental histories (e.g., early interventions, delayed language development, daily adaptive function demands) will be helpful in further disentangling and clarifying the relationship between IQ and adaptive function as well as the bases of adaptive function deficits observed in the FASD population. While the age range of subjects for the current study is broad (i.e., 8–16 years), it is not known whether our results would generalize to younger or older subjects (Crocker et al., 2009). Future studies should consider expanding this range to investigate the relation between IQ and adaptive function across a wider spectrum of ages.
Additional potential confounds to consider include maternal smoking or use of other drugs (e.g., cocaine, methamphetamine) in pregnancy. Our studies are retrospective in nature and as such we do not have specific information regarding maternal use of other drugs and cannot include these in our analyses. To meet inclusion criteria for our AE group, we require sufficient documentation that the primary in utero exposure substance is alcohol. Though alcohol is considered to be one of the most significantly detrimental teratogens beyond other drugs of abuse, we cannot rule out the effects of other teratogen use during pregnancy. Along those lines, historical information regarding stimulant or other medication usage was not available. All subjects were asked to refrain from medication use on the day of testing though possible cumulative effects related to medication cannot be fully ruled out. Concern may exist regarding potential differences in training, testing environments, and other factors across sites. Per CIFASD study design, all sites followed the same testing protocol and procedures to ensure cross-site validity were followed. As such, we can be confident that these possible differences across site are not significantly contributing to our findings.
Conclusions
Youth with heavy prenatal alcohol exposure evidence significant impairment on adaptive functioning measures, as reported by caregivers. Adaptive function tends to strongly relate to intellectual functioning among other neurodevelopmental disorders, yet this relationship has not been explored among youth with heavy prenatal alcohol exposure. Our findings suggest that adaptive function and IQ are not as strongly related among alcohol-exposed youth as compared to their nonexposed counterparts, suggesting other cognitive or behavioral domains may contribute more to adaptive function deficits experienced by this population. Although impairments in intellectual functioning are common and important, they do not sufficiently explain the observed deficits in adaptive behavior, which occur across the range of overall ability level.
This differential relation between adaptive behavior and IQ is primarily due to performance in the communication domain; the correlation between IQ and communication became weaker with increasing IQ and this relationship was not significant in the high IQ range among alcohol-exposed youth. Impaired adaptive function and communication abilities may prevent these individuals from functioning independently and impact academic, social, and occupational domains. Further elucidation of factors contributing to adaptive function deficits of youth with prenatal alcohol exposure will provide clarity regarding the etiology of functional deficits and suggest targets for clinical intervention.
Acknowledgements:
The authors thank the families who graciously participate in our studies. The authors have no financial or other conflicts of interest. All or part of this work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Additional information about CIFASD can be found at www.cifasd.org.
Research described in this paper was supported by NIAAA grant number U01 AA014834. Additional support was provided by U24 AA014811, U24 AA014815, and F31 AA 025256.
References
- Bal VH, Kim S-H, Fok M, & Lord C (2018). Autism spectrum disorder symptoms from ages 2 to 19 years: Implications for diagnosing adolescents and young adults. International Society for Autism Research [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Burd L, Klug MG, Martsolf JT, & Kerbeshian J (2003). Fetal alcohol syndrome: Neuropsychiatric phenomics. Neurotoxicology and Teratology, 25(6), 697–705. doi: 10.1016/j.ntt.2003.07.014 [DOI] [PubMed] [Google Scholar]
- Coles CD, Brown RT, Smith IE, Platzman KA, Erickson S, & Falek A (1991). Effects of prenatal alcohol exposure at school age. I. Physical and cognitive development. Neurotoxicology and Teratology, 13(4), 357–367. [DOI] [PubMed] [Google Scholar]
- Crocker N, Vaurio L, Riley EP, & Mattson SN (2009). Comparison of adaptive behavior in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Alcoholism: Clinical and Experimental Research, 33(11), 2015–2023. doi: 10.1111/j.1530-0277.2009.01040.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerlund A, Ase F, Autti-Ramo I, Ilona A-R, Kalland M, Mirjam K, … Marit K (2012). Adaptive behaviour in children and adolescents with foetal alcohol spectrum disorders: a comparison with specific learning disability and typical development. European Child & Adolescent Psychiatry, 21(4), 221–231. doi: 10.1007/s00787-012-0256-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, McGee CL, Matt GE, Riley EP, & Mattson SN (2007). Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics, 119(3), E733–E741. doi: 10.1542/peds.2006-1606 [DOI] [PubMed] [Google Scholar]
- Henry J, Sloane M, & Black-Pond C (2007). Neurobiology and neurodevelopmental impact of childhood traumatic stress and prenatal alcohol exposure. Language, Speech, and Hearing Services in Schools, 38(2), 99–108. doi: 10.1044/0161-1461(2007/010) [DOI] [PubMed] [Google Scholar]
- Jester JM, Jacobson SW, Sokol RJ, Tuttle BS, & Jacobson JL (2000). The influence of maternal drinking and drug use on the quality of the home environment of school-aged children. Alcoholism: Clinical and Experimental Research, 24(8), 1187–1197. [PubMed] [Google Scholar]
- Jones KL, Robinson LK, Bakhireva LN, Marintcheva G, Storojev V, Strahova A, … Chambers CD (2006). Accuracy of the diagnosis of physical features of fetal alcohol syndrome by pediatricians after specialized training. Pediatrics, 118(6), E1734–E1738. doi: 10.1542/peds.2006-1037 [DOI] [PubMed] [Google Scholar]
- Kanne SM, Gerber AJ, Quirmbach LM, Sparrow SS, Cicchetti DV, & Saulnier CA (2011). The role of adaptive behavior in autism spectrum disorders: Implications for functional outcome. Journal of Autism and Developmental Disorders, 41(8), 1007–1018. doi: 10.1007/s10803-010-1126-4 [DOI] [PubMed] [Google Scholar]
- Landgren M, Svensson L, Strömland K, & Andersson Grönlund M (2010). Prenatal alcohol exposure and neurodevelopmental disorders in children adopted from eastern Europe. Pediatrics, 125(5), E1178–E1185. doi: 10.1542/peds.2009-0712 [DOI] [PubMed] [Google Scholar]
- Liss M, Harel B, Fein D, Allen D, Dunn M, Feinstein C, … Rapin I (2001). Predictors of correlates of adaptive functioning in children with developmental disorders. Journal of Autism and Developmental Disorders, 31(2), 219–230. [DOI] [PubMed] [Google Scholar]
- Mattson S, & Riley E (1998). A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcoholism: Clinical and Experimental Research, 22(2), 279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x [DOI] [PubMed] [Google Scholar]
- Mattson S, Riley E, Gramling L, Delis D, & Jones K (1997). Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. Journal of Pediatrics, 131(5), 718–721. doi: 10.1016/S0022-3476(97)70099-4 [DOI] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, & Nguyen TT (2011). Fetal alcohol spectrum disorders: Neuropsychological and behavioral features. Neuropsychology Review, 21(2), 81–101. doi: 10.1007/s11065-011-9167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Foroud T, Sowell ER, Jones KL, Coles CD, Fagerlund Å, … the CIFASD. (2010). Collaborative initiative on fetal alcohol spectrum disorders: Methodology of clinical projects. Alcohol, 44(7–8), 635–641. doi: 10.1016/j.alcohol.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, & Riley EP (1996). Brain anomalies in fetal alcohol syndrome. In Abel EL (Ed.), Fetal Alcohol Syndrome: From Mechanism to Prevention (pp. 51–68). Boca Raton: CRC Press. [Google Scholar]
- Mattson SN, & Riley EP (1997). Neurobehavioral and neuroanatomical effects of heavy prenatal exposure to alcohol. In Ann Streissguth E, Jonathan Kanter E, & et al. (Eds.), The Challenge of Fetal Alcohol Syndrome: Overcoming Secondary Disabilities (pp. 3–14). Seattle: University of Washington Press. [Google Scholar]
- May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, … Hoyme HE (2014). Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics, 134(5), 855–866. doi: 10.1542/peds.2013-3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, … Hoyme HE (2018). Prevalence of fetal alcohol spectrum disorders in 4 US communities. The Journal of the American Medical Association, 319(5), 474–482. doi: 10.1001/jama.2017.21896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Keaster C, Bozeman R, Goodover J, Blankenship J, Kalberg WO, … Hoyme HE (2015). Prevalence and characteristics of fetal alcohol syndrome and partial fetal alcohol syndrome in a Rocky Mountain Region City. Drug and Alcohol Dependence, 155, 118–127. doi: 10.1016/j.drugalcdep.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MJ, & Paley B (2009). Psychiatric conditions associated with prenatal alcohol exposure. Developmental Disabilities Research Reviews, 15(3), 225–234. doi: 10.1002/ddrr.74 [DOI] [PubMed] [Google Scholar]
- Panczakiewicz AL, Glass L, Coles CD, Kable JA, Sowell ER, Wozniak JR, … the CIFASD. (2016). Neurobehavioral deficits consistent across age and sex in youth with prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research, 40(9). doi: 10.1111/acer.13153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen C, Benz J, Pei J, Andrew G, Schuller G, Abele-Webster L, … Lord L (2010). The impact of an ADHD co-morbidity on the diagnosis of FASD. The Canadian Journal of Clinical Pharmacology, 17(1), E165–E176. [PubMed] [Google Scholar]
- Committee on Disbility Determination for Mental Retardation (2002). Mental Retardation: Determining Eligibility for Social Security Benefits (Reschly DJ, Myers TG, & Hartel CR Eds.). Washington, D.C.: National Academy Press. [PubMed] [Google Scholar]
- Riley EP, Infante MA, & Warren KR (2011). Fetal alcohol spectrum disorders: An overview. Neuropsychology Review, 21(2), 73–80. doi: 10.1007/s11065-011-9166-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, & Schwab-Stone ME (2000). NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry, 39(1), 28–38. doi: 10.1097/00004583-200001000-00014 [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, & Balla DA (2005). Vineland Adaptive Behavior Scales, Second Edition: Survey forms manual Circle Pines, MN: AGS. [Google Scholar]
- Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, & Smith DF (1991). Fetal alcohol syndrome in adolescents and adults. Journal of the American Medical Association, 265(15), 1961–1967. [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD, & Bookstein FL (1994). Prenatal alcohol and offspring development: The first fourteen years. Drug and Alcohol Dependence, 36, 89–99. [DOI] [PubMed] [Google Scholar]
- Thomas S, Kelly S, Mattson S, & Riley E (1998). Comparison of social abilities of children with fetal alcohol syndrome to those of children with similar IQ scores and normal controls. Alcoholism: Clinical and Experimental Research, 22(2), 528–533. doi: 10.1097/00000374-199804000-00034 [DOI] [PubMed] [Google Scholar]
- Ware AL, Crocker N, O’Brien JW, Deweese BN, Roesch SC, Coles CD, … Mattson SN (2012). Executive function predicts adaptive behavior in children with histories of heavy prenatal alcohol exposure and attention-deficit/hyperactivity disorder. Alcoholism: Clinical and Experimental Research, 36(8), 1431–1441. doi: 10.1111/j.1530-0277.2011.01718.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1944). The measurement of adult intelligence (3rd ed.). Baltimore: Williams & Wilkins. [Google Scholar]
- Wechsler D (2003). Manual for the Wechsler Intelligence Scale for Children-Fourth Edition San Antonio: Pearson. [Google Scholar]
- Whaley SE, O’Connor MJ, & Gunderson B (2001). Comparison of the adaptive functioning of children prenatally exposed to alcohol to a nonexposed clinical sample. Alcoholism: Clinical and Experimental Research, 25(7), 1018–1024. doi: 10.1111/j.1530-0277.2001.tb02311.x [DOI] [PubMed] [Google Scholar]


