Abstract
Research on cardiac autonomic function in major depressive disorder (MDD) has predominantly examined cardiac vagal control and adopted a model of reciprocal autonomic balance. A proposed bivariate autonomic continuum uses cardiac autonomic balance (CAB) and cardiac autonomic regulation (CAR) models, derived from normalized values of respiratory sinus arrhythmia and pre-ejection period, to more adequately index patterns of autonomic control. The purpose of this study was to assess resting levels of CAB and CAR among young adults with and without a current diagnosis of major depression. One hundred forty-two young adults (n = 65 MDD, n = 77 healthy controls; 20.8 ± 2.6 years) completed a structured diagnostic interview, cardiovascular assessment, and a maximal aerobic fitness test. The findings revealed that CAB, but not CAR, significantly predicted current MDD status (OR = 0.70, 95% CI [0.53, 0.93]), an effect that remained after controlling for aerobic fitness and body mass index. Although CAB was found to be a significant predictor of current MDD status among a sample of young adults, there remained substantial variation in autonomic control that was not captured by the traditional model of reciprocal autonomic balance.
Keywords: autonomic nervous system, cardiac autonomic balance, depression, physical health
1 |. INTRODUCTION
Major depressive disorder (MDD) is one of the most common mental health disorders with an estimated 10–16 million U.S. adults experiencing at least one major depressive episode each year (Ahrnsbrak, Bose, Hedden, Lipari, & Park-Lee, 2017; National Institute of Mental Health, 2019). Many people experience their first major depressive episode during emerging adulthood, and approximately two thirds of MDD patients experience recurring episodes or a chronic disease course (Kessler et al., 2005, 2010; Paykel, Brugha, & Fryers, 2005). This burden has led investigators to examine potential treatment targets as well as novel diagnostic and symptom-based predictors of MDD. One promising area relates to autonomic dysfunction in depression. Dysregulation in parasympathetic and sympathetic branches of the autonomic nervous system (ANS), often indexed by alterations in heart rate variability (HRV), has been implicated in a number of physical and mental health conditions, including the generation and maintenance of depressive episodes (Carney & Freedland, 2009; Quintana, Guastella, McGregor, Hickie, & Kemp, 2013; Thayer, Yamamoto, & Brosschot, 2010). However, most studies to date have focused solely on cardiac vagal control (e.g., Beauchaine, 2015; Rottenberg, 2007).
Cardiac vagal control has been assessed primarily using high-frequency (HF) HRV or respiratory sinus arrhythmia (RSA) as a noninvasive method that provides a window into the dynamic influence of myelinated vagal efferent pathways emerging from the nucleus ambiguus of the brainstem to the sinoatrial node of the heart. RSA is a naturally occurring rhythm in the heart rate (HR) pattern that occurs at approximately the frequency of spontaneous breathing due to respiratory cycles of inspiration and expiration (Berntson, Cacioppo, & Quigley, 1993; Porges, 2007) and reflects parasympathetic autonomic control. In line with polyvagal theory (Porges, 1995, 2007), greater resting HRV and enhanced cardiac vagal control are hypothesized to reflect a dynamic and adaptive psychophysiological state, while lower HRV and reduced cardiac vagal control reflect psychological inflexibility (i.e., poor physiological, emotional, cognitive, and behavioral regulation) and vulnerability to adverse health consequences.
The existing literature on resting RSA in MDD is mixed, although findings from several meta-analyses suggest that individuals with MDD exhibit lower resting RSA relative to controls. A meta-analysis of 13 studies including 312 depressed and 374 nondepressed individuals revealed a small-to-moderate overall effect size (d = 0.332), indicating significantly reduced resting cardiac vagal control (i.e., reduced frequency- and time-domain measures) among individuals with MDD (see Rottenberg, 2007). In a meta-analysis of 18 studies, Kemp et al. (2010) examined differences between 673 depressed and 407 healthy comparison participants. Findings from this review indicated significantly lower HF HRV (n = 14 studies; g = −0.293) and a greater low-frequency (LF)/HF ratio (n = 5 studies; g = 0.633) among depressed patients, but no significant difference in LF HRV (n = 9 studies; g = −0.101) between depressed and control subjects. These findings were interpreted as a possible reduction in vagally mediated autonomic control and disinhibition of sympathetic influences. However, LF HRV has been shown to reflect a combination of both sympathetic and parasympathetic influences, and the LF/HF ratio has been largely discredited as a measure of autonomic or sympathovagal balance (Berntson, 2019; Berntson et al., 1997; Eckberg, 1997; Reyes del Paso, Langewitz, Mulder, van Roon, & Duschek, 2013; Sherwood et al., 1990).
In addition to the nearly exclusive focus on the parasympathetic branch of the ANS, researchers also have tended to adopt a conceptual model of reciprocal autonomic balance. That is, differences in resting HRV (or HF HRV) between depressed patients and healthy controls have been interpreted to reflect balanced fluctuations in both sympathetic and parasympathetic contributions to autonomic control, such that observed increases in vagal control also imply decreases in sympathetic control. For instance, Kemp et al. (2010) stated that a higher LF/HF ratio in MDD patients may reflect “… an increase in sympathetic activity and a reciprocal decrease in parasympathetic activity…” (italics added, p. 1067). The lack of research focus on sympathetic contributions to autonomic control is troubling given that the two branches of the ANS can vary independently and even coactively (Berntson, 2019; Berntson, Norman, Hawkley, & Cacioppo, 2008). Pre-ejection period (PEP), the time between electrical depolarization of the left ventricle and the beginning of ventricular ejection, has been proposed as an index of cardiac sympathetic activity (Ahmed, Levinson, Schwartz, & Ettinger, 1972; Berntson, Lozano, Chen, & Cacioppo, 2004; Berntson, Quigley, & Lozano 2007; Cacioppo et al., 1994; Harris, Schoenfeld, & Weissler, 1967; Larkin & Kasprowicz, 1986; Newlin & Levenson, 1979; Schachinger, Weinbacher, Kiss, Ritz, & Langewitz, 2001) and beta-adrenergic influences on myocardial contractility (Newlin & Levenson, 1979). PEP is derived from noninvasive measures of impedance cardiography (ICG) and permits researchers to examine less well-studied relationships of the ANS branches (e.g., independence or coactivation) in order to better understand the full range of adaptive regulatory functioning and potential autonomic dysfunction in depression.
Based on evidence that autonomic branches are not invariably reciprocally controlled, Berntson and colleagues (2008) examined cardiac autonomic balance (CAB) and cardiac autonomic regulatory (CAR) capacity models as predictors of health status, myocardial infarction, and diabetes among 229 adults between the ages of 50 and 67 years old. CAB was quantified as the difference between normalized values of parasympathetic (HF HRV) and sympathetic (PEP) control, while CAR was quantified as the sum of the normalized values of HF HRV and PEP to represent overall coactivation or coinhibition of the two ANS branches. CAB emerged as a significant negative predictor of diabetes (OR = 0.61; r(38) = −.29), whereas CAR was positively associated with overall health status, r(220) = .15, and a negative predictor of the prior occurrence of myocardial infarction (OR = 0.11; r(9) = −.26). These findings suggest that a reciprocal autonomic balance model may not capture the full range of autonomic patterns across health conditions, including coinhibition and coactivation of the two ANS branches.
In the only study to date to examine CAB in depression, Bylsma et al. (2015) found increases in CAB during a series of physical (handgrip challenge, forehead cold pressor) and psychological (sad film, unsolvable puzzle) stressors among youth with juvenile onset depression, reflecting relative parasympathetic activation and/or sympathetic withdrawal. In contrast, healthy controls showed decreases in CAB during these tasks, reflecting a pattern of relative sympathetic activation and/or parasympathetic withdrawal. Importantly, no studies to date have examined resting levels of CAB or CAR among individuals with depression. In addition, the mixed findings related to resting RSA in depression may be due to failure to control for coexisting health indicators (e.g., cardiorespiratory fitness, body mass index [BMI]) that may be related to both depression and cardiac vagal control (Bylsma, Salomon, Taylor-Clift, Morris, & Rottenberg, 2014; Rottenberg, Clift, Bolden, & Salomon, 2007). For instance, resting levels of autonomic activity have been shown to correlate with BMI (El-Sheikh, Keller, & Erath, 2007; Molfino et al., 2009; Yaptangco, Crowell, Baucom, Bride, & Hansen, 2015) and cardiorespiratory fitness (Hatfield et al., 1998; Joyner & Green, 2009). Rottenberg et al. (2007) found that individuals with depression had lower resting RSA relative to healthy controls; however, the group effect was no longer significant after controlling for physical health variables (BMI, total calories, sleep quality) and respiratory parameters. In a subsequent study (Bylsma et al., 2014), individuals with MDD exhibited lower resting RSA during paced breathing relative to healthy controls, but this group effect was no longer significant after controlling for age, gender, waist circumference, and physical activity. The MDD group also had blunted RSA fluctuation to a speech stressor task relative to healthy controls, and this effect survived covariation for waist circumference and physical activity, but not sleep quality. Although physical activity has previously been associated with resting vagal activity (Mølgaard, Hermansen, & Bjerregaard, 1994) and individuals with depression tend to be less physically active (Hollenberg, Haight, & Tager, 2003), these studies have generally relied on self-report measures of leisure-time physical activity. It therefore remains unknown whether cardiorespiratory fitness influences autonomic balance in depression, which is important considering that cardiorespiratory fitness, as an objective measure, is inversely related to depression symptom severity (Papasavvas, Bonow, Alhashemi, & Micklewright, 2016).
The present study addressed this knowledge gap by comparing resting CAB and CAR between young adults with and without a current diagnosis of MDD. Patients with MDD have largely demonstrated reduced parasympathetic (Kemp et al., 2010; Rottenberg, 2007) and elevated sympathetic cardiac control (Barton et al., 2007; Carney & Freedland, 2017). We therefore hypothesized that CAB would serve as a better predictor than CAR at detecting a current diagnosis of MDD. The present study also examined the sensitivity, specificity, and positive and negative predictive value of CAB, as well as CAR, for detecting MDD. We hypothesized that CAB would provide more adequate sensitivity and positive predictive value relative to CAR in detecting current MDD status. We additionally characterized key demographic and physical health characteristics (cardiorespiratory fitness, BMI) among individuals whose autonomic patterns differed across a bivariate autonomic space bounded by sympathetic and parasympathetic axes as outlined by Berntson and colleagues (2008).
2 |. METHOD
2.1 |. Study sample
This study was a retrospective analysis of data collected as part of two studies that examined the effects of aerobic exercise as either a stand-alone (Olson, Brush, Ehmann, & Alderman, 2017) or combined treatment intervention (Alderman, Olson, Brush, & Shors, 2016) for cognitive control deficits in MDD. Recruitment for these studies included young adults with and without a current diagnosis of MDD. Data presented herein were obtained during the initial screening and baseline assessments prior to study randomization. Inclusion criteria for the parent studies consisted of men and women between the ages of 18–30 years old who were physically able to complete an assessment of cardiorespiratory fitness, which was determined by the physical activity readiness questionnaire (PAR-Q; Thomas, Reading, & Shephard, 1992). Briefly, the PAR-Q is used to screen for cardiovascular symptoms (e.g., chest pain) or musculoskeletal problems that would preclude someone from engaging in physical activity. No subject endorsed any item on the PAR-Q, and all were able to complete the fitness assessment with no adverse events. Exclusion criteria included any history or presence of bipolar spectrum disorder, schizophrenia, self-injurious behavior or suicidal ideation, or neurological disorders or injuries resulting in a loss of consciousness. The final sample size was 142 participants. Informed consent was obtained from all participants, and the study was approved by the university’s Institutional Review Board.
2.2 |. Procedures
During the study session, participants completed a structured diagnostic interview, a cardiovascular assessment, and a maximal graded exercise test for the determination of cardiorespiratory fitness (VO2peak). Participants were asked to refrain from exercise for 24 hr and to avoid any caffeine for 3–4 hr prior to testing. Following informed consent procedures, participants completed health history and demographic forms as well as the PAR-Q to ensure safety for the cardiorespiratory fitness assessment. Height and weight were then measured for the calculation of BMI using a stadiometer and scale (Healthometer 500 KL Fitness Scale, Healthometer Professional, McCook, IL). Participants were then escorted to a sound-attenuated room for electrophysiological testing. Participants were fitted with disposable snap electrodes for electrocardiography (ECG) and ICG recordings and were instructed to relax during this time period. A “vanilla” task (Jennings, Kamarck, Stewart, Eddy, & Johnson, 1992) was then presented on a 17″ Dell laptop using E-Prime Professional version 2.0 software (Psychology Software Tools, Pittsburgh, PA), with the center of the laptop screen being situated approximately 60 cm from the participant’s head at eye level. During the task, participants passively viewed slides of rectangles that changed color every 5 s. Participants were instructed to silently count the number of blue rectangles they saw during the 5-min stimulus presentation and were subsequently asked at the end of the task to recall how many blue rectangles they counted. The vanilla task is a minimally demanding cognitive task that has been validated as a reliable method for assessing resting cardiovascular function that helps to standardize resting cognitive activity both within and across participants (Jennings et al., 1992). Verbal and written instructions were provided prior to the start of data collection, and cardiovascular responses were assessed continuously throughout the 5-min presentation. Following the cardiovascular assessment, all electrodes were removed, and participants were escorted to an exercise testing area to complete a cardiorespiratory fitness assessment on a motor-driven treadmill. Following the cardiorespiratory fitness test, participants were provided time to cool down and were provided further instructions for the intervention studies.
2.3 |. Diagnostic assessment
Presence of a current major depressive episode was assessed using the Mini-International Neuropsychiatric Interview (MINI; Sheehan et al., 1998). The MINI is a brief, structured interview that has established validity and reliability in making diagnoses of Diagnostic and Statistical Manual of Mental Disorders, 4th and 5th editions (DSM-IV, DSM-5) and International Classification of Diseases-10 (ICD-10) psychiatric disorders (Sheehan et al., 1998). All interviewers received supervised training in the use of the MINI and had previous experience in administering structured clinical interviews with psychiatric patients.
2.4 |. Measures
2.4.1 |. Clinical symptoms
Depressive symptoms were assessed using the Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996); anxiety symptoms were measured using the Beck Anxiety Inventory (BAI; Beck, Epstein, Brown, & Steer, 1988). The BDI-II and BAI are 21-item self-report inventories that assess depression and anxiety symptom severity over the past 2 weeks, respectively. For both scales, each item is scored on a 4-point scale (0–3), with a total score ranging between 0–63. Higher scores reflect the presence of greater symptomatology. In the present sample, BDI-II and BAI scores both demonstrated high internal consistency (BDI-II: α = 0.92; BAI: α = 0.85).
2.4.2 |. Cardiorespiratory fitness
Cardiorespiratory fitness (VO2peak) was assessed using a modified Bruce protocol (American College of Sports Medicine, 2018) on a motor-driven treadmill, which involved increasing the speed and grade of the treadmill every 2 min until volitional exhaustion was reached. A Polar HR monitor was used to record heart rate throughout the test. VO2peak was calculated from direct expired gas exchange data using a computerized metabolic system (Parvo Medics True Max 2400 Metabolic Cart, ParvoMedics, Inc., Sandy, UT). VO2peak was defined as the maximal rate of oxygen consumption per kilogram of body weight (mL*kg−1*min−1) when at least three of the following criteria were met: (a) a plateau in oxygen consumption corresponding to an increase of less than 150 ml in oxygen uptake despite a progressive increase in workload, (b) maximal heart rate within 10 beats per min (bpm) of age-predicted maximal values (220 bpm—age in years), (c) a respiratory exchange ratio greater than 1.10, or (d) a rating of perceived exertion greater than or equal to 17. Upon completion of the assessment, participants cooled down for 5 min at a self-selected pace at a 0% grade.
2.5 |. Electrophysiological recording
ECG signals were acquired according to recommendations by Jennings et al. (1981) using a modified limb Lead II configuration with Ag/AgCl electrodes placed on the left and right lowest floating ribs and the right clavicle. ICG signals were recorded using a standard tetrapolar electrode configuration (fully encircling the neck and torso) outlined by Sherwood et al. (1990). Both ECG and ICG signals were digitized at 500 Hz and filtered through a MindWare BioNex system and BioLab software. All cardiovascular data were first visually inspected online by researchers masked to participant diagnosis. Then, artifact detection and correction procedures were performed. The 5-min epoch of data was averaged, and CAB and CAR were calculated based on procedures described in Berntson et al. (2008). The ICG thoracic impedance signal (Z0) was used to quantify respiratory rate and depth according to established methods (Ernst, Litvack, Lozano, Cacioppo, & Berntson, 1999). Spectral analysis can identify the variation in the Z0 signal caused by respiration and yields scores highly similar to spirometric respiration measures (de Geus, Willemsen, Klaver, & van Doornen, 1995; Ernst, Litvack, Lozano, Cacioppo, & Bernston, 1999; Houtveen, Groot, & de Geus, 2006). Because respiratory rate and depth can influence RSA (Ernst et al., 1999), preliminary analyses were conducted to ensure respiration did not correlate with the derived measures of CAB and CAR. No significant correlations were observed, and, additionally, no differences in these respiratory parameters between the MDD and control groups were observed. In addition, respiratory rate and amplitude were not related to clinical symptom measures of depression and anxiety; therefore, these respiratory measures were not considered further in the analyses.
2.5.1 |. RSA and LF/HF ratio
RSA was derived using MindWare HRV version 3.0.17 software (MindWare Technologies, Ltd., Gahanna, OH). Data were cleaned offline by manually correcting software-identified inappropriately placed R peaks, according to the IBI Min/Max and the MAD/MED artifact detection algorithms implemented in the software (Berntson, Quigley, Jang, & Boysen, 1990). From the ECG, the R-R interbeat interval (IBI) series was converted into time series data with a 4 Hz resolution (with interpolation), linearly de-trended and end tapered using a Hamming windowing function and submitted to a fast Fourier transform. In the present study, RSA was calculated as the natural-logged (ln) spectral power value in the HF bandwidth (Berntson et al., 1997). For purposes of comparison with previous studies, the LF/HF ratio was also computed using the ratio of LF (0.04–0.15 Hz) and HF (0.15–0.40 Hz) values.
2.5.2 |. Pre-ejection period
PEP was derived from the ICG signal using MindWare IMP version 3.0.17 software (MindWare Technologies) and was quantified from the dZ/dt signal. Specifically, the time interval (in milliseconds) from the onset of the ECG Q wave (onset of ventricular depolarization) to the B point (opening of the aortic valve) of the dZ/dt wave was used to derive PEP (Sherwood et al., 1990). The max slope method was used to automatically place the B point, which was later manually adjusted based on visual inspection similar to previous recommendations (see Lozano et al., 2007).
2.5.3 |. CAB and CAR
Using RSA and PEP as measures of parasympathetic and sympathetic cardiac control, respectively, an index of CAB was derived as the difference between RSA and PEP. In order to combine the different measurement scales of RSA and PEP into a single index of CAB, each variable was first normalized by transforming raw values to z scores. Given that greater sympathetic activity is associated with shorter PEP values, PEP was multiplied by −1 for ease in interpreting values (i.e., higher −zPEP values indicate more sympathetic activation, just as higher zRSA values indicate more parasympathetic activation; Berntson et al., 2008). CAB was calculated using the formula CAB = zRSA − (−zPEP). Higher CAB values are therefore indicative of reciprocal parasympathetic control, whereas lower CAB values reflect reciprocal sympathetic control. CAR, on the other hand, was calculated using the formula CAR = zRSA + (−zPEP). Thus, higher CAR reflects coactivation, whereas lower CAR reflects coinhibition of both autonomic branches. Additionally, a −zPEP/zRSA ratio was computed to estimate the overall contributions of sympathetic and parasympathetic cardiac control under resting conditions.
2.6 |. Data analysis
Descriptive statistics were conducted, and independent samples t tests were used to assess group differences (MDD, control) in demographic, symptom, and autonomic measures (HR, RSA, PEP, CAB, CAR, LF/HF, −zPEP/zRSA). Bivariate Pearson correlations were performed to examine individual differences in depression symptom severity and the autonomic measures. Binary logistic regression analyses were performed to test the predictive utility of CAB and CAR, as well as RSA and PEP, in detecting a current major depressive episode. Per convention (see Berntson et al., 2008), CAB and CAR measures are a composite of standardized variables (zRSA and zPEP); therefore, the mean for each variable was 0 and the standard deviation was 1. In the post hoc analyses of covariance (ANCOVAs) and planned logistic regression analyses, BMI and cardiorespiratory fitness (VO2peak) served as continuous covariates to control for the potential influence of these physical health variables on parasympathetic and sympathetic cardiac control (Alderman & Olson, 2014; Berntson et al., 2008). Exploratory one-way analyses of variance (ANOVAs) were conducted along with post hoc Fisher’s least significant difference (LSD) contrasts to classify group differences in autonomic balance and regulation. These subgroups were created by using zRSA and zPEP as criteria for classifying individuals into groups exhibiting coactivation (zRSA > 0 and–zPEP > 0), coinhibition (zRSA < 0 and–zPEP < 0), reciprocal parasympathetic (zRSA > 0 and–zPEP < 0), and reciprocal sympathetic cardiac control (zRSA < 0 and–zPEP > 0). A moderated ordinary least squares (OLS) regression analysis was conducted using the PROCESS software for SPSS version 3.0 (Hayes, 2013) to address the extent to which differences in cardiorespiratory fitness moderate the relationship between CAB and depressive symptom severity. The same moderated OLS regression analysis was also conducted for CAR (i.e., substituting CAR into the regression model in place of CAB). Lastly, sensitivity, specificity, and positive and negative predictive values were calculated for significant logistic regression models to determine the diagnostic utility of CAB or CAR as predictors of current major depression. All analyses were conducted in SPSS version 25 (IBM Corp., Armonk, NY) and R version 3.5.2 (R Core Team, 2013) using a two-tailed α level of .05.
3 |. RESULTS
3.1 |. Participant characteristics
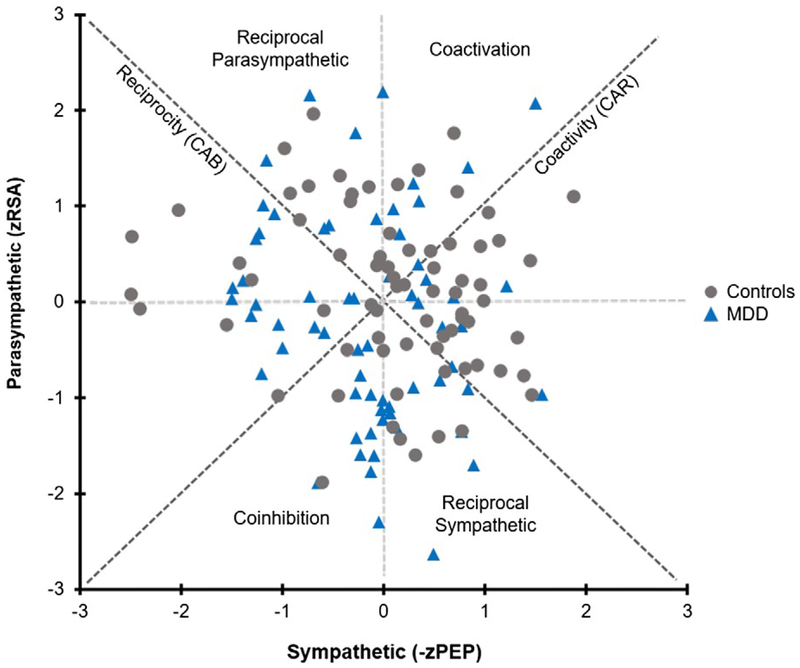
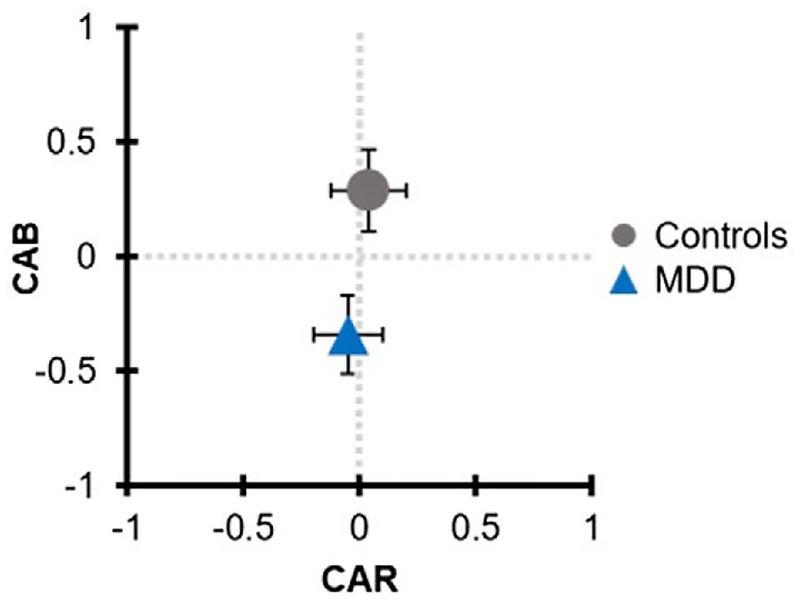
There was no significant difference between groups in the ratio of male to female, χ2(1, N = 142) = 3.01, p > 0.05. There also were no significant between-group differences in age, BMI, or VO2peak, ps > 0.05. As anticipated, participants with MDD reported higher depressive and anxiety symptoms relative to nondepressed controls, ps < 0.05. Independent samples t tests revealed significant between-group differences in HR, t(140) = −3.5, p < 0.01, d = 0.59, RSA, t(140) = 2.2, p < 0.05, d = 0.32, and CAB, t(140) = 2.8, p < 0.01, d = 0.48. After controlling for BMI and VO2peak, post hoc ANCOVAs indicated that group differences in HR, F(1, 138) = 10.3, p < 0.01, , and CAB, F(1, 138) = 6.6, p < 0.05, , remained significantly different, while the effect of group was marginally significant for RSA, F(1, 138) = 3.7, p = 0.055, . No other significant group differences emerged for the other autonomic measures (PEP, CAR, LF/HF, −zPEP/zRSA), ps > 0.05. See Table 1 for participant characteristics as well as a summary of autonomic and respiratory variables by depression status. Figures 1 and 2 display the sample distribution within the bivariate autonomic space.
TABLE 1.
Demographic and clinical characteristics
| MDD (n = 65) | Controls (n = 77) | Overall (n = 142) | |
|---|---|---|---|
| Characteristics | |||
| Gender, male, n (%) | 12 (18.5%) | 24 (31.2%) | 36 (25.4%) |
| Age (years) | 20.7 (2.6) | 20.8 (2.5) | 20.8 (2.6) |
| BMI (kg/m2) | 23.1 (3.5) | 23.5 (4.2) | 23.3 (3.9) |
| VO2peak (mL*kg−1*min−1) | 36.9 (7.0) | 38.7 (8.7) | 37.9 (8.0) |
| BDI-II scorec | 24.1 (8.6) | 8.8 (7.1) | 15.8 (10.9) |
| BAI scorec | 13.4 (8.2) | 7.5 (7.3) | 10.3 (8.2) |
| Comorbidities, n (%)# | 16 (24.6%) | ||
| Autonomic measures | |||
| HR (bpm)b | 78.1 (10.2) | 72.5 (8.7) | 75.0 (9.8) |
| RSA (ms2)a | 6.2 (1.0) | 6.5 (0.8) | 6.4 (1.0) |
| PEP (ms) | 133.1 (13.3) | 137.8 (21.0) | 135.7 (18.0) |
| CABb | −0.3 (1.2) | 0.3 (1.4) | 0.0 (1.3) |
| CAR | −0.1 (1.4) | 0.0 (1.6) | 0.0 (1.5) |
| LF/HF | 2.6 (2.6) | 2.3 (1.7) | 2.4 (2.1) |
| -zPEP/zRSA | 0.8 (10.0) | −1.5 (11.8) | −0.4 (10.9) |
| Respiratory parameters | |||
| Respiratory rate (cycles/min) | 16.5 (3.5) | 15.7 (3.4) | 16.1 (3.5) |
| Respiratory depth | 0.04 (0.07) | 0.03 (0.07) | 0.04 (0.07) |
Note. Values are mean (SD), unless otherwise indicated.
Abbreviations: BAI, Beck Anxiety Inventory; BDI-II, Beck Depression Inventory, 2nd ed.; BMI, body mass index; CAB, cardiac autonomic balance; CAR, cardiac autonomic regulation; HR, heart rate; LF/HF, low frequency/high frequency ratio; MDD, major depressive disorder; PEP, pre-ejection period; RSA, respiratory sinus arrhythmia; VO2peak, peak oxygen consumption; −zPEP/zRSA, ratio of the inverse normalized pre-ejection period to normalized respiratory sinus arrhythmia; cycles/min, respiratory cycles per minute.
p < 0.05
p < 0.01
p < 0.001.
Generalized anxiety disorder (n = 9), substance-related disorders (n = 2), post-traumatic stress disorder (n = 1), panic disorder with agoraphobia (n = 1), social anxiety disorder (n = 1); two participants had two of these comorbidities.
FIGURE 1.
Distribution of normalized RSA (zRSA) and PEP (−zPEP) scores across the sample of young adults with and without a current diagnosis of MDD. High and low CAB scores are represented by the reciprocal parasympathetic and reciprocal sympathetic quadrants, respectively, while high and low CAR scores are represented by the coactivation and coinhibition quadrants. Although CAB was a significant predictor of current MDD, substantial variation remained that was not captured by the traditional model of reciprocal autonomic control
FIGURE 2.
CAB and CAR values in individuals with and without current MDD. Data points and caps illustrate mean and standard errors for CAB and CAR by diagnostic group. Individuals with MDD had significantly lower CAB scores, reflective of reciprocal sympathetic dominance. No significant between-group differences were found for CAR
3.2 |. Subgroup analyses
To further understand whether the subgroups (as indicated by the four quadrants in Figure 1) differed in key demographic and physical health characteristics, we split the sample into four subgroups, based on sympathetic (−zPEP) and parasympathetic (zRSA) activity within a bipolar autonomic space. Overall, 35 participants exhibited autonomic coinhibition, 37 showed autonomic coactivation, 37 displayed greater parasympathetic predominance (i.e., reciprocal para-sympathetic cardiac control), and 33 demonstrated greater sympathetic predominance (i.e., reciprocal sympathetic cardiac control). Findings from exploratory one-way ANOVAs indicated significant differences in VO2peak, F(3, 138) = 2.74, p < 0.05, , among the four groups. In terms of VO2peak, post hoc Fisher’s LSD exploratory analyses indicated significant differences between individuals exhibiting coinhibition (VO2peak = 36.9 ± 7.6 mL/kg/min) relative to those demonstrating parasympathetic dominance (VO2peak = 40.9 ± 9.9 mL/kg/min) and between those with parasympathetic dominance and those demonstrating sympathetic dominance, (VO2peak = 35.8 ± 6.2 mL/kg/min). The post hoc Fisher’s LSD exploratory analysis between those with parasympathetic dominance and those demonstrating coactivation (VO2peak = 37.7 ± 7.0 mL/kg/min) was not significant (p = 0.083). No significant differences in age, gender, BMI, depressive or anxiety symptoms were observed between the four groups, all ps > 0.05.
3.3 |. Correlation analyses
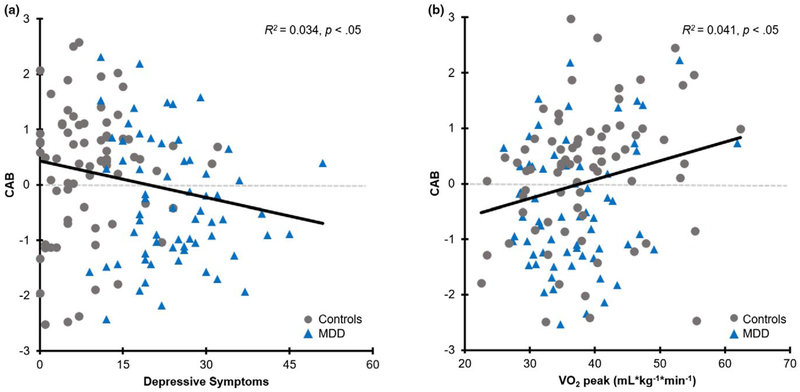
Bivariate Pearson correlations indicated that depressive symptoms were significantly associated with HR, r(140) = 0.19, RSA, r(140) = −0.22, and CAB, r(140) = −0.18, ps < 0.05. There was also a significant negative relationship between depressive symptoms and VO2peak, r(140) = −0.18, p < 0.05, but no significant relationship between depressive symptoms and BMI, r(140) = 0.03, p = 0.70. Although VO2peak and BMI were not correlated and all other correlations between depressive symptoms and autonomic measures were nonsignificant (ps > 0.05), there was a significant positive relationship between VO2peak and CAB, r(140) = 0.20, p < 0.05. Despite a significant relationship between depressive and anxiety symptoms, r(140) = 0.50, p < 0.001, no significant relationships were found between anxiety symptoms and any of the autonomic measures, ps > 0.05. There was, however, a significant negative relationship between anxiety symptoms and VO2peak, r(140) = −0.18, p < 0.05. Figure 3 depicts the significant relationships between depressive symptoms and CAB, and VO2peak and CAB.
FIGURE 3.
Relationships between (a) depressive symptoms and CAB, and (b) cardiorespiratory fitness (VO2peak) and CAB
3.4 |. Binary logistic regression analyses
Separate binary logistic regression analyses were performed to examine the predictive utility of CAB and CAR in detecting a current major depressive episode. CAB was a significant predictor of current major depression, χ2(3) = 9.2, p < 0.05, explaining 8.4% (Nagelkerke R2) of the total variance. The model correctly identified 64.1% of the cases. After holding BMI and VO2peak constant, CAB remained a significant negative predictor of a current major depressive episode, β = −0.35, Wald = 6.21, p < 0.05, OR = 0.70, 95% CI [0.53, 0.93]. That is, for every one unit increase in CAB, the odds of having a current major depressive episode (vs. a nondiagnosis) decreased by a factor of 0.70. Thus, at a group level, low CAB (reciprocal sympathetic) best characterized individuals with a current major depressive episode. Conversely, the binary logistic regression model assessing CAR as a predictor of a current major depressive episode was nonsignificant, χ2 (3) = 2.6, p = 0.45. Thus, CAR was a nonsignificant predictor of current major depression, β = −0.04, Wald = 0.11, p = 0.74, OR = 0.96, 95% CI [0.77, 1.21]. To understand whether each autonomic branch served as independent predictors of the presence of a current major depressive episode, RSA and PEP were substituted into the same binary logistic regression analyses as CAB and CAR. After accounting for BMI and VO2peak, RSA was a marginally significant negative predictor of current major depressive episode, β = −0.36, Wald = 3.65, p = 0.056, OR = 0.70, 95% CI [0.49, 1.01], while PEP was a nonsignificant predictor, β = −0.01, Wald = 2.05, p = 0.15, OR = 0.99, 95% CI [0.56, 1.09]. Thus, for every one unit increase in RSA, the odds of having a current major depressive episode (vs. a nondiagnosis) decreased by a factor of 0.70, and at a group level, individuals with a current major depressive episode had lower resting levels of RSA.
Given that CAB emerged as a significant negative predictor, we explored its sensitivity and specificity as well as positive and negative predictive value in detecting the presence of a current major depressive episode. Overall, CAB provided moderate sensitivity, specificity, and positive predictive value, while demonstrating high negative predictive value (see Table 2). CAR was a nonsignificant predictor of the presence of current major depression; therefore, the sensitivity and specificity analyses were not conducted for CAR.
TABLE 2.
Sensitivity, specificity, positive predictive value, and negative predictive value (with 95% confidence intervals) for CAB predicting a current major depressive episode at a 46% prevalence rate
| CAB | Predictive values | ||
|---|---|---|---|
| MDD present | MDD absent | ||
| Test positive | 34 | 31 | PPV: 0.52 (0.40, 0.65) |
| Test negative | 20 | 57 | NPV: 0.74 (0.63, 0.83) |
| Totals | 54 | 88 | |
| Sensitivity: 0.63 (0.49, 0.76) | Specificity: 0.65 (0.54, 0.75) | ||
Abbreviations: CAB, cardiac autonomic balance; MDD, major depressive disorder; NPV, negative predictive value; PPV, positive predictive value.
3.5 |. Moderated regression analysis
Individual differences in VO2peak were examined as moderators of the relationship between CAB and depressive symptom severity because VO2peak was significantly associated with both CAB and depressive symptoms. Results of the moderation analysis revealed a nonsignificant interaction between VO2peak and CAB on depressive symptoms, F(1, 138) = 0.2, p = 0.68, ΔR2 = 0.001, indicating that cardiorespiratory fitness did not moderate the relationship between CAB and depressive symptoms.
4 |. DISCUSSION
The purpose of this study was to assess resting cardiac autonomic balance (CAB), an index of dual-branch cardiac function, and cardiac autonomic regulation (CAR), which reflects overall cardiac regulatory capacity, among young adults with and without a current diagnosis of major depression. Given the large number of studies indicating reduced cardiac vagal (Kemp et al., 2010; Rottenberg, 2007) and elevated sympathetic cardiac control in depression (Barton et al., 2007; Carney & Freedland, 2017), it was hypothesized that CAB would serve as a better predictor of current MDD status relative to CAR, independent of the coexisting physical health variables of cardiorespiratory fitness and BMI. At a group level, findings revealed that CAB best predicted the presence of a current major depressive episode, independent of fitness and BMI. CAB also provided moderate positive predictive value for a current major depressive episode, such that every one unit increase in CAB reduced the odds of current MDD by 0.70. However, the sample was characterized by substantial variability, such that approximately half of the sample fit a profile of reciprocal autonomic balance (CAB), while the other half of the sample fit a profile of CAR (coactivation or coinhibition of both ANS branches).
Our findings are consistent with prior meta-analytic data demonstrating lower parasympathetic (or cardiac vagal) control in depression (Kemp et al., 2010; Rottenberg, 2007). Lower RSA is thought to contribute to poor physiological, emotional, cognitive, and behavioral regulation and may confer vulnerability to psychopathology (Koenig, Kemp, Beauchaine, Thayer, & Kaess, 2016). In line with this hypothesis, overall depressive symptoms in this sample of 65 currently depressed and 77 never depressed healthy control participants were significantly associated with resting RSA levels. However, these findings only reflect cardiac vagal control and do not include the relative contributions of sympathetic activity to overall autonomic control. Furthermore, relying exclusively on HRV metrics (rather than PEP from ICG) has often led to premature conclusions that, for instance, decreased HRV necessarily implies reciprocal sympathetic (and reduced cardiac vagal) control. High parasympathetic activity at rest is generally considered adaptive and associated with positive health states and reduced susceptibility to health risk (Porges, 1997; Thayer & Sternberg, 2006), whereas high sympathetic activity at rest has been interpreted to reflect elevated physiological arousal and serves as a psychophysiological risk factor for future cardiovascular events (Hohnloser, 2005; Schwartz, La Rovere, & Vanoli, 1992). It has also been suggested that high parasympathetic activity may serve to buffer the negative consequences of elevated sympathetic cardiac control (Dyavanapalli, Dergacheva, Wang, & Mendelowitz, 2016), underscoring the need to examine the influence of both autonomic branches. Moreover, there even may be synergistic benefits of coactive resting sympathetic and parasympathetic activity (Berntson, 2019). Our findings extend this prior work by examining RSA in the context of sympathetic activity and suggest that sympathetic predominance (i.e., lower CAB), but neither coactivation nor coinhibition (as indexed by CAR), predicted current major depression.
In order to understand individual characteristics associated with risk for mental and physical health comorbidities, subgroup analyses were conducted to examine participants whose resting autonomic activity placed them in each of the four quadrants of the bivariate autonomic space (Berntson, 2019; Berntson et al., 2008). Approximately equal numbers of participants were characterized by resting RSA and PEP values that placed them within the four quadrants of coin-hibition, coactivation, relative parasympathetic, and relative sympathetic predominance. This sample heterogeneity in autonomic balance would not be uncovered in traditional investigations of RSA (or PEP) in depression, potentially obscuring the diagnostic and prognostic utility of measures of autonomic balance. Interestingly, the highest levels of cardio-respiratory fitness were observed among participants demonstrating resting parasympathetic predominance (i.e., increased RSA, decreased PEP). In turn, participants with sympathetic predominance (i.e., increased PEP, decreased RSA) had the lowest cardiorespiratory fitness levels. Notably, 66.7% of the participants characterized by sympathetic predominance had current MDD, while only 45.9% of participants characterized by coactivation had current MDD. In terms of the other quadrants, 40% of individuals with MDD exhibited coinhibition, while 32.4% of individuals with MDD demonstrated parasympathetic predominance. Future studies should continue to examine autonomic contributions to depression using both CAB and CAR models of autonomic regulation and determine if a subgroup of patients with depression (e.g., coinhibition vs. sympathetic dominance) is at greater risk for physical and mental health comorbidities. For instance, Carney and Freedland (2009) argued that low HRV and other markers of autonomic dysfunction in depressed patients are likely to contribute to an elevated risk among patients with coronary heart disease. A broader approach to ANS function beyond the traditional autonomic balance model is likely to advance both descriptive and intervention-based research in this area.
Previous studies have demonstrated an inverse relationship between aerobic fitness and depressive symptom severity (Hollenberg et al., 2003; Martinsen, Strand, Paulsson, & Kaggestad, 1989; Papasavvas et al., 2016). In this study, depressive symptoms were correlated with aerobic fitness (VO2peak) such that higher fit individuals reported lower symptoms of depression. Moreover, cardiorespiratory fitness and CAB were positively correlated, suggesting that higher fitness levels were associated with greater resting CAB. Cardiorespiratory fitness, however, did not moderate the relationship between CAB and BDI-II scores, while CAB continued to predict a diagnosis of a major depressive episode even after controlling for cardiorespiratory fitness and BMI. Relatedly, Byrne, Fleg, Vaitkevicius, Wright, and Porges (1996) failed to find a significant contribution of cardiorespiratory fitness or BMI on RSA assessed in supine, seated, or standing postures in healthy normotensive adult men from the Baltimore Longitudinal Study on Aging. Despite a very large number of studies examining the effects of aerobic and resistance exercise on depression (Cooney et al., 2013; Gordon et al., 2018; Rethorst & Trivedi, 2013), there are remarkably many fewer published studies that examined the association between cardiorespiratory fitness and depression (Sui et al., 2009). Although fitness did not significantly moderate relationships between CAB and MDD status or symptom severity, it is possible that the effects of aerobic exercise on depression are mediated by changes in cardiac autonomic control. Indeed, autonomic control has been suggested as a potential treatment target for intervention and has shown some success in predicting successful antidepressant treatment response (Balogh, Fitzpatrick, Hendricks, & Paige, 1993; Chambers & Allen, 2002). Future intervention studies are needed to directly address this possibility.
Several limitations to this study should be noted. First, ANS function was measured only at rest and not also in response to a physical and/or psychological stressor, which may provide more nuance to the analyses. Although a large body of research has examined resting levels of cardiac vagal control (e.g., Rottenberg, 2007), and to a lesser extent sympathetic activity (Fiskum et al., 2018), this study adds to the extant literature by examining a broader pattern of potential autonomic dysfunction in depression within a bivariate autonomic space. More recent research has examined autonomic flexibility in response to physical and psychological stressors (Bylsma et al., 2015; Daches et al., 2017). For instance, Bylsma et al. found abnormalities in CAB reactivity to laboratory stressors among a sample with a history of juvenile onset depression, which were not explained by current depression. In addition, no differences in resting CAB or CAR were found among the pro-bands relative to controls. The current findings in combination with Bylsma et al. suggest that differences in resting CAB may normalize once depression is remitted, but that CAB reactivity to physical and psychological stressors may not fully normalize, highlighting the potential for examining both resting and reactive levels of CAB using longitudinal research designs.
Second, this was a cross-sectional study, and it is not possible to infer causality. It is also not known if disruptions in autonomic balance precede the first onset of depression; thus, prospective study designs are needed to examine this possibility. Such approaches may help to determine whether CAB is a potential vulnerability marker for the development of a future depressive episode and whether deficits in CAB can be remediated following successful treatment or during remission. In addition, approximately 25% of the sample had comorbid depression and anxiety, although symptoms of anxiety did not relate to any of the autonomic measures. Previous research has demonstrated reduced HRV and cardiac vagal tone in anxiety (e.g., Chalmers, Quintana, Abbott, & Kemp, 2014; Friedman, 2007), suggesting that individuals with comorbid depression and anxiety may have further reductions in CAB. The lower rates and less severe anxiety levels reported in this sample may account for the lack of a significant relationship between anxiety and resting CAB or CAR. Future studies are warranted to examine depression versus anxiety as well as co-morbid diagnoses on CAB and CAR.
Lastly, participants with MDD in this study sample represented a relatively pure depressed sample, without co-occurring anxiety or other mental health disorders. Although these findings indicate that CAB demonstrates moderate specificity with MDD, it is difficult to confirm whether CAB is specific to depression (i.e., one-to-one relationship) or is also related to other disease states (i.e., one-to-many relationship; Cacioppo & Tassinary, 1990) without the inclusion of individuals with other neuropsychiatric conditions or coexisting health conditions. Therefore, future research should include more heterogeneous samples consisting of a variety of health conditions (e.g., similar to diabetes and myocardial infarction from Berntson et al., 2008) to address the role of CAB as a potential vulnerability marker of disease.
In summary, our findings suggest that resting CAB may be a useful index to predict current MDD and may be a useful psychophysiological index that is sensitive to psychopathology or depressive symptomatology. Furthermore, CAB was a significant negative predictor of MDD, even after accounting for the physical health characteristics of cardio-respiratory fitness and BMI. CAB demonstrated moderate sensitivity and specificity and was related to severity of depression, highlighting the utility of CAB as a potential psychophysiological marker of MDD. The current findings, along with previous studies (e.g., Berntson, 2019; Berntson et al., 2008), suggest that autonomic balance models may not capture the full picture of autonomic response patterns in depression and other health conditions (e.g., diabetes, myocardial infarction). CAB was a significant predictor of current MDD status among a sample of young adults, although substantial variation in autonomic control remained that was not captured by the traditional model of reciprocal autonomic control. Future research incorporating similar autonomic indices across the bivariate autonomic space may help to advance understanding of heterogeneity in the diagnosis, clinical course, and treatment approaches for depression.
Funding information
National Institute on Drug Abuse, Grant/Award Number: R34DA043751; National Institute on Alcohol Abuse and Alcoholism, Grant/Award Number: K02AA025123 and K24AA021778
REFERENCES
- Ahmed SS, Levinson GE, Schwartz CJ, & Ettinger PO (1972). Systolic time intervals as measures of the contractile state of the left ventricular myocardium in man. Circulation, 46(3), 559–571. 10.1161/01.cir.46.3.559 [DOI] [PubMed] [Google Scholar]
- Ahrnsbrak R, Bose J, Hedden SL, Lipari RN, & Park-Lee E (2017). Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health. Retrieved from Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration website: https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2016/NSDUH-FFR1-2016.htm
- Alderman BL, & Olson RL (2014). The relation of aerobic fitness to cognitive control and heart rate variability: A neurovisceral integration study. Biological Psychology, 99, 26–33. 10.1016/j.biopsycho.2014.02.007 [DOI] [PubMed] [Google Scholar]
- Alderman BL, Olson RL, Brush CJ, & Shors TJ (2016). MAP training: Combining meditation and aerobic exercise reduces depression and rumination while enhancing synchronized brain activity. Translational Psychiatry, 6, e726 10.1038/tp.2015.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Sports Medicine. (2018). ACSM’s health-related physical fitness assessment manual. Philadelphia. PA: Lippincott Williams & Wilkins. [Google Scholar]
- Balogh S, Fitzpatrick DF, Hendricks SE, & Paige SR (1993). Increases in heart rate variability with successful treatment in patients with major depressive disorder. Psychopharmacology Bulletin, 29(2), 201–206. [PubMed] [Google Scholar]
- Barton DA, Dawood T, Lambert EA, Esler MD, Haikerwal D, Brenchley C, … Lambert GW (2007). Sympathetic activity in major depressive disorder: Identifying those at increased cardiac risk? Journal of Hypertension, 25(10), 2117–2124. 10.1097/HJH.0b013e32829baae7 [DOI] [PubMed] [Google Scholar]
- Beauchaine TP (2015). Respiratory sinus arrhythmia: A transdiagnostic biomarker of emotion dysregulation and psychopathology. Current Opinions in Psychology, 3, 43–47. 10.1016/j.copsyc.2015.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, & Steer RA (1988). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893–897. 10.1037//0022-006x.56.6.893 [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the beck depression inventory–II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Berntson GG (2019). Presidential address 2011: Autonomic modes of control and health. Psychophysiology, 56(1), e13306 10.1111/psyp.13306 [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT Jr., Eckberg DL, Grossman P, Kaufmann PG, Malik M, … van der Molen MW (1997). Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology, 34(6), 623–648. 10.1111/j.1469-8986.1997.tb02140.x [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, & Quigley KS (1993). Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology, 30(2), 183–196. 10.1111/j.1469-8986.1993.tb01731.x [DOI] [PubMed] [Google Scholar]
- Berntson GG, Lozano DL, Chen YJ, & Cacioppo JT (2004). Where to Q in PEP. Psychophysiology, 41(2), 333–337. 10.1111/j.1469-8986.2004.00156.x [DOI] [PubMed] [Google Scholar]
- Berntson GG, Norman GJ, Hawkley LC, & Cacioppo JT (2008). Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology, 45(4), 643–652. 10.1111/j.1469-8986.2008.00652.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Quigley KS, Jang JF, & Boysen ST (1990). An approach to artifact identification: Application to heart period data. Psychophysiology, 27(5), 586–598. 10.1111/j.1469-8986.1990.tb01982.x [DOI] [PubMed] [Google Scholar]
- Berntson GG, Quigley KS, & Lozano D (2007). Cardiovascular psychophysiology. Handbook of Psychophysiology, 3, 182–210. 10.1017/9781107415782.009 [DOI] [Google Scholar]
- Bylsma LM, Salomon K, Taylor-Clift A, Morris BH, & Rottenberg J (2014). RSA reactivity in current and remitted major depressive disorder. Psychosomatic Medicine, 76(1), 66–73. 10.1097/psy.0000000000000019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma LM, Yaroslavsky I, Rottenberg J, Jennings JR, George CJ, Kiss E, … Kovacs M (2015). Juvenile onset depression alters cardiac autonomic balance in response to psychological and physical challenges. Biological Psychology, 110, 167–174. 10.1016/j.biopsycho.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne EA, Fleg JL, Vaitkevicius PV, Wright J, & Porges SW (1996). Role of aerobic capacity and body mass index in the age-associated decline in heart rate variability. Journal of Applied Physiology, 81(2), 743–750. 10.1152/jappl.1996.81.2.743 [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Binkley PF, Quigley KS, Uchino BN, & Fieldstone A (1994). Autonomic cardiac control. II. Noninvasive indices and basal response as revealed by autonomic blockades. Psychophysiology, 31(6), 586–598. 10.1111/j.1469-8986.1994.tb02351.x [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, & Tassinary LG (1990). Inferring psychological significance from physiological signals. American Psychologist, 45(1), 16–28. 10.1037//0003-066x. [DOI] [PubMed] [Google Scholar]
- Carney RM, & Freedland KE (2009). Depression and heart rate variability in patients with coronary heart disease. Cleveland Clinic Journal of Medicine, 76(Suppl 2), S13–S17. 10.3949/ccjm.76.s2.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RM, & Freedland KE (2017). Depression and coronary heart disease. Nature Reviews Cardiology, 14(3), 145–155. 10.1038/nrcardio.2016.181 [DOI] [PubMed] [Google Scholar]
- Chalmers JA, Quintana DS, Abbott MJ, & Kemp AH (2014). Anxiety disorders are associated with reduced heart rate variability: A meta-analysis. Frontiers in Psychiatry, 5, 80 10.3389/fpsyt.2014.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AS, & Allen JJ (2002). Vagal tone as an indicator of treatment response in major depression. Psychophysiology, 39(6), 861–864. 10.1111/1469-8986.3960861 [DOI] [PubMed] [Google Scholar]
- Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, … Mead GE (2013). Exercise for depression. Cochrane Database of Systematic Reviews, 9, CD004366 10.1111/jebm.12076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daches S, Kovacs M, George CJ, Yaroslavsky I, Kiss E, Vetro A, … Rottenberg J (2017). Childhood adversity predicts reduced physiological flexibility during the processing of negative affect among adolescents with major depression histories. International Journal of Psychophysiology, 121, 22–28. 10.1016/j.ijpsycho.2017.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Geus EJ, Willemsen GH, Klaver CH, & van Doornen LJ (1995). Ambulatory measurement of respiratory sinus arrhythmia and respiration rate. Biological Psychology, 41(3), 205–227. 10.1016/0301-0511(95)05137-6 [DOI] [PubMed] [Google Scholar]
- Dyavanapalli J, Dergacheva O, Wang X, & Mendelowitz D (2016). Parasympathetic vagal control of cardiac function. Current Hypertension Reports, 18(3), 22 10.1007/s11906-016-0630-0 [DOI] [PubMed] [Google Scholar]
- Eckberg DL (1997). Sympathovagal balance: A critical appraisal. Circulation, 96(9), 3224–3232. 10.1161/01.cir.98.23.2643 [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Keller PS, & Erath SA (2007). Marital conflict and risk for child maladjustment over time: Skin conductance level reactivity as a vulnerability factor. Journal of Abnormal Child Psychology, 35(5), 715–727. 10.1007/s10802-007-9127-2 [DOI] [PubMed] [Google Scholar]
- Ernst JM, Litvack DA, Lozano DL, Cacioppo JT, & Berntson GG (1999). Impedance pneumography: Noise as signal in impedance cardiography. Psychophysiology, 36(3), 333–338. 10.1017/s0048577299981003 [DOI] [PubMed] [Google Scholar]
- Fiskum C, Andersen TG, Bornas X, Aslaksen PM, Flaten MA, & Jacobsen K (2018). Non-linear heart rate variability as a discriminator of internalizing psychopathology and negative affect in children with internalizing problems and healthy controls. Frontiers in Physiology, 9, 561 10.3389/fphys.2018.00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman BH (2007). An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology, 74(2), 185–199. 10.1016/j.biopsycho.2005.08.009 [DOI] [PubMed] [Google Scholar]
- Gordon BR, McDowell CP, Hallgren M, Meyer JD, Lyons M, & Herring MP (2018). Association of efficacy of resistance exercise training with depressive symptoms: Meta-analysis and meta-regression analysis of randomized clinical trials. JAMA Psychiatry, 75(6), 566–576. 10.1001/jamapsychiatry.2018.0572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris WS, Schoenfeld CD, & Weissler AM (1967). Effects of adrenergic receptor activation and blockade on the systolic preejection period, heart rate, and arterial pressure in man. Journal of Clinical Investigation, 46(11), 1704–1714. 10.1172/jci105661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield BD, Spalding TW, Santa Maria DL, Porges SW, Potts JT, Byrne EA, … Mahon AD (1998). Respiratory sinus arrhythmia during exercise in aerobically trained and untrained men. Medicine & Science in Sports & Exercise, 30(2), 206–214. 10.1097/00005768-199802000-00006 [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: The Guilford Press. [Google Scholar]
- Hohnloser SH (2005). Ventricular arrhythmias: Antiadrenergic therapy for the patient with coronary artery disease. Journal of Cardiovascular Pharmacology and Therapeutics, 10(Suppl 1), S23–S31. 10.1177/10742484050100i404 [DOI] [PubMed] [Google Scholar]
- Hollenberg M, Haight T, & Tager IB (2003). Depression decreases cardiorespiratory fitness in older women. Journal of Clinical Epidemiology, 56(11), 1111–1117. 10.1016/s0895-4356(03)00167-7 [DOI] [PubMed] [Google Scholar]
- Houtveen JH, Groot PF, & de Geus EJ (2006). Validation of the thoracic impedance derived respiratory signal using multilevel analysis. International Journal of Psychophysiology, 59(2), 97–106. 10.1016/j.ijpsycho.2005.02.003 [DOI] [PubMed] [Google Scholar]
- Jennings JR, Berg WK, Hutcheson JS, Obrist P, Porges S, & Turpin G (1981). Committee report. Publication guidelines for heart rate studies in man. Psychophysiology, 18(3), 226–231. 10.1111/j.1469-8986.1981.tb03023.x [DOI] [PubMed] [Google Scholar]
- Jennings JR, Kamarck T, Stewart C, Eddy M, & Johnson P (1992). Alternate cardiovascular baseline assessment techniques: Vanilla or resting baseline. Psychophysiology, 29(6), 742–750. 10.1111/j.1469-8986.1992.tb02052.x [DOI] [PubMed] [Google Scholar]
- Joyner MJ, & Green DJ (2009). Exercise protects the cardiovascular system: Effects beyond traditional risk factors. Journal of Physiology, 587(Pt 23), 5551–5558. 10.1113/jphysiol.2009.179432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, & Gatt JM (2010). Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biological Psychiatry, 67(11), 1067–1074. 10.1016/j.biopsych.2009.12.012 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, … Zaslavsky AM (2005). Prevalence and treatment of mental disorders, 1990 to 2003. New England Journal of Medicine, 352(24), 2515–2523. 10.1056/nejmsa043266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, … Williams DR (2010). Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. British Journal of Psychiatry, 197(5), 378–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig J, Kemp AH, Beauchaine TP, Thayer JF, & Kaess M (2016). Depression and resting state heart rate variability in children and adolescents—A systematic review and meta-analysis. Clinical Psychology Review, 46, 136–150. 10.1016/j.cpr.2016.04.013 [DOI] [PubMed] [Google Scholar]
- Larkin KT, & Kasprowicz AL (1986). Validation of a simple method of assessing cardiac preejection period: A potential index of sympathetic nervous system activity. Perceptual and Motor Skills, 63(1), 295–302. 10.2466/pms.1986.63.1.295 [DOI] [PubMed] [Google Scholar]
- Lozano DL, Norman G, Knox D, Wood BL, Miller BD, Emery CF, & Berntson GG (2007). Psychophysiology, 44(1), 113–119. 10.1111/j.1469-8986.2006.00468.x [DOI] [PubMed] [Google Scholar]
- Martinsen EW, Strand J, Paulsson G, & Kaggestad J (1989). Physical fitness level in patients with anxiety and depressive disorders. International Journal of Sports Medicine, 10(1), 58–61. 10.1055/s-2007-1024876 [DOI] [PubMed] [Google Scholar]
- Molfino A, Fiorentini A, Tubani L, Martuscelli M, Rossi Fanelli F, & Laviano A (2009). Body mass index is related to autonomic nervous system activity as measured by heart rate variability. European Journal of Clinical Nutrition, 63(10), 1263–1265. 10.1038/ejcn.2009.35 [DOI] [PubMed] [Google Scholar]
- Mølgaard H, Hermansen K, & Bjerregaard P (1994). Spectral components of short-term RR interval variability in healthy subjects and effects of risk factors. European Heart Journal, 15(9), 1174–1183. 10.1093/oxfordjournals.eurheartj.a060650 [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health. (2019). Major depression among adults. Retrieved from https://www.nimh.nih.gov/health/statistics/prevalence/major-depression-among-adults/
- Newlin DB, & Levenson RW (1979). Pre-ejection period: measuring beta-adrenergic influences upon the heart. Psychophysiology, 16(6), 546–553. 10.1111/j.1469-8986.1979.tb01519.x [DOI] [PubMed] [Google Scholar]
- Olson RL, Brush CJ, Ehmann PJ, & Alderman BL (2017). A randomized trial of aerobic exercise on cognitive control in major depression. Clinical Neurophysiology, 128(6), 903–913. 10.1016/j.clinph.2017.01.023 [DOI] [PubMed] [Google Scholar]
- Papasavvas T, Bonow RO, Alhashemi M, & Micklewright D (2016). Depression symptom severity and cardiorespiratory fitness in healthy and depressed adults: A systematic review and meta-analysis. Sports Medicine, 46(2), 219–230. 10.1007/s40279-015-0409-5 [DOI] [PubMed] [Google Scholar]
- Paykel ES, Brugha T, & Fryers T (2005). Size and burden of depressive disorders in Europe. European Neuropsychopharmacology, 15(4), 411–423. 10.1016/j.euroneuro.2005.04.008 [DOI] [PubMed] [Google Scholar]
- Porges SW (1995). Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology, 32(4), 301–318. 10.1111/j.1469-8986.1995.tb01213.x [DOI] [PubMed] [Google Scholar]
- Porges SW (1997). Emotion: An evolutionary by-product of the neural regulation of the autonomic nervous system. Annals of the New York Academy of Sciences, 807, 62–77. 10.1111/j.1749-6632.1997.tb51913.x [DOI] [PubMed] [Google Scholar]
- Porges SW (2007). The polyvagal perspective. Biological Psychology, 74(2), 116–143. 10.1016/j.biopsycho.2006.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana DS, Guastella AJ, McGregor IS, Hickie IB, & Kemp AH (2013). Heart rate variability predicts alcohol craving in alcohol dependent outpatients: Further evidence for HRV as a psychophysiological marker of self-regulation. Drug and Alcohol Dependence, 132(1–2), 395–398. 10.1016/j.drugalcdep.2013.02.025 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2013). R: A language and environment for statistical computing [Computer software]. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from http://www.R-project.org/ [Google Scholar]
- Rethorst CD, & Trivedi MH (2013). Evidence-based recommendations for the prescription of exercise for major depressive disorder. Journal of Psychiatric Practice, 19(3), 204–212. 10.1097/01.pra.0000430504.16952.3e [DOI] [PubMed] [Google Scholar]
- Reyes del Paso GA, Langewitz W, Mulder LJ, van Roon A, & Duschek S (2013). The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: A review with emphasis on a reanalysis of previous studies. Psychophysiology, 50(5), 477–487. 10.1111/psyp.12027 [DOI] [PubMed] [Google Scholar]
- Rottenberg J (2007). Cardiac vagal control in depression: A critical analysis. Biological Psychology, 74(2), 200–211. 10.1016/j.biopsycho.2005.08.010 [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Clift A, Bolden S, & Salomon K (2007). RSA fluctuation in major depressive disorder. Psychophysiology, 44(3), 450–458. 10.1111/j.1469-8986.2007.00509.x [DOI] [PubMed] [Google Scholar]
- Schachinger H, Weinbacher M, Kiss A, Ritz R, & Langewitz W (2001). Cardiovascular indices of peripheral and central sympathetic activation. Psychosomatic Medicine, 63(5), 788–796. 10.1097/00006842-200109000-00012 [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, La Rovere MT, & Vanoli E (1992). Autonomic nervous system and sudden cardiac death. Experimental basis and clinical observations for post-myocardial infarction risk stratification. Circulation, 85(1 Suppl), I77–91. [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59(Suppl 20), 22–33; quiz, 34–57. [PubMed] [Google Scholar]
- Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, & van Doornen LJ (1990). Methodological guidelines for impedance cardiography. Psychophysiology, 27(1), 1–23. 10.1111/j.1469-8986.1990.tb02171.x [DOI] [PubMed] [Google Scholar]
- Sui X, Laditka JN, Church TS, Hardin JW, Chase N, Davis K, & Blair SN (2009). Prospective study of cardiorespiratory fitness and depressive symptoms in women and men. Journal of Psychiatric Research, 43(5), 546–552. 10.1016/j.jpsychires.2008.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, & Sternberg E (2006). Beyond heart rate variability: Vagal regulation of allostatic systems. Annals of the New York Academy of Sciences, 1088, 361–372. 10.1196/annals.1366.014 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Yamamoto SS, & Brosschot JF (2010). The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International Journal of Cardiology, 141(2), 122–131. 10.1016/j.ijcard.2009.09.543 [DOI] [PubMed] [Google Scholar]
- Thomas S, Reading J, & Shephard RJ (1992). Revision of the physical activity readiness questionnaire (PAR-Q). Canadian Journal of Sports Sciences, 17(4), 338–345. [PubMed] [Google Scholar]
- Yaptangco M, Crowell SE, Baucom BR, Bride DL, & Hansen EJ (2015). Examining the relation between respiratory sinus arrhythmia and depressive symptoms in emerging adults: A longitudinal study. Biological Psychology, 110, 34–41. 10.1016/j.biopsycho.2015.06.004 [DOI] [PubMed] [Google Scholar]