Abstract
Despite a persistent interest in verb processing, data on the neural underpinnings of verb retrieval are fragmentary. The present study is the first to analyze the contributions of both grey and white matter damage affecting verb retrieval through action naming in stroke. We used voxel-based lesion-symptom mapping (VLSM) with an action naming task in 40 left-hemisphere stroke patients. Within the grey matter, we revealed the critical involvement of the left precentral and inferior frontal gyri, insula, and parts of basal ganglia. An overlay of white matter tract probability masks on the VLSM lesion map revealed involvement of left-hemisphere long and short association tracts with terminations in the frontal areas; and several projection tracts. The involvement of these structures is interpreted in the light of existing picture naming models, semantic control processes, and the embodiment cognition framework. Our results stress the importance of both cortico-cortical and cortico-sub-cortical networks of language processing.
Keywords: Picture naming, Verbs, VLSM, Aphasia, White-matter tracts
1. Introduction
A common way to study the neural underpinnings of word retrieval is combining a functional/structural neuroimaging method with a picture naming task. For verb retrieval by an action naming task, however, these combined data are fragmentary. First, studies that inform the majority of contemporary neurolinguistic naming models are mainly based on object naming and noun retrieval. Second, lesion studies of action and verb semantics yield inconsistent results. Finally, white matter substrates of verb processing are still largely understudied. In our experiment, we sought to fill the gaps and assess grey and white matter structures associated with action naming using Voxel-based Lesion Symptom Mapping (VLSM). To narrow down the focus of the research, we only investigated the lexical-semantic stages of naming in this study.
1.1. Neural substrates of the lexical-semantic level of word retrieval
Word retrieval is crucial to language production. Among psycho-linguistic models of word retrieval, the two most influential are the functional model of Levelt and colleagues (Levelt, 1983, 1989; 1992; Levelt et al., 1999) and the interactive activation model of Dell and colleagues (Dell & O’Seaghdha, 1991; Dell, 1986; Foygel and Dell, 2000). Both models make a distinction between lexical-semantic and phonological stages of naming. At the lexical-semantic level in Levelt’s model, the process first involves the activation of a lexical concept which contains meaning to be conveyed. At the lexical selection stage, a lemma is chosen based on the active lexical concept. A lemma is an intermediate unit between the conceptual and the word form levels, and it is where its syntactic properties become available for further grammatical encoding (Levelt et al., 1999). The selection of a phono-logical code happens at a later stage. In Dell’s model, the conceptual representations are mapped onto semantic features from where the activation spreads further to the lexical nodes (words), and defines the choice of position-specific phonemes. The experimental comparison of the models described above, as well as the fine-grained mapping of model stages, are beyond the scope of our study. Following Schwartz et al. (2009), we will combine the concept/semantic features stages with the lemma/word stages. Thus, we will use the term ‘lexical-semantic’ irrespective of the model to refer to the word retrieval stages before and including the access to lemmas/words.
In language pathology, the hallmark of lexical-semantic impairment is semantic errors (e.g. saying dog instead of cat; Cloutman et al., 2009; Duffau et al., 2014; Howard and Gatehouse, 2006; Schwartz et al., 2009). Error types that result in an existing word (e.g. formal (dog > log), unrelated (dog > water) or mixed semantic-formal (dog > frog) errors can be viewed as arising at the lexical-semantic level (Dell et al., 2013; Foygel and Dell, 2000). The deficits at the lexical-semantic level may be a consequence of impaired storage of units or access to them (e.g., Mirman and Britt, 2014). Storage deficits (i.e. deficient semantic representations) are usually associated with neurodegenerative diseases (e.g., the semantic variant of primary progressive aphasia; ibid), although the performance patterns exhibited by some stroke patients can be interpreted in a similar way (Cloutman et al., 2009; DeLeon et al., 2007; Hillis et al., 1990; Howard and Gatehouse, 2006; Tsapkini et al., 2011). The term ‘access’, in turn, can refer to various phenomena. In lexical retrieval models, it can indicate a particular stage of word retrieval as specified by the model, for example, access from the conceptual semantics level to lemmas. In a broader neurocognitive perspective, it can be interpreted as an abnormal delay in the return of the lexical-semantic system to the default state, excessive activation of representations, impaired selection processes, or deficient semantic control (Mirman and Britt, 2014).
Lexical-semantic object naming stages have been mapped to brain regions in temporal, frontal and, to a lesser extent, parietal lobes; using activation as well as lesion methods. The storage deficit is usually associated with the degeneration of bilateral anterior temporal lobes and inferior temporal cortex in the semantic variant of primary progressive aphasia (e.g., Lambon Ralph and Patterson, 2008). However, performance patterns compatible with storage deficits can also be observed in left-hemisphere stroke-related damage to these areas (Cloutman et al., 2009; DeLeon et al., 2007). Some researchers, however, relate uni-lateral left-hemisphere damage to anterior temporal cortices to deficits in word access rather than storage (Mirman et al., 2015a; Schwartz et al., 2009; Walker et al., 2011). Areas in the temporal lobe associated with conceptual preparation, lexical concepts and the conceptually-driven retrieval of lemmas/words are portions of the middle temporal gyrus (Baldo et al., 2013; Cloutman et al., 2009; Dronkers et al., 2004; Indefrey and Levelt, 2004; Indefrey, 2011; Mirman et al., 2015a,b; Schuhmann et al., 2012; Schwartz et al., 2009; Walker et al., 2011); superior temporal gyrus (Price, 2012); anterior-ventral temporal regions (Roelofs, 2014); and posterior inferior temporal cortex and middle fusiform gyrus (Cloutman et al., 2009; DeLeon et al., 2007; Schwartz et al., 2009; Walker et al., 2011). In the frontal lobe, the word/lemma retrieval from semantics has been linked to middle frontal (Dell et al., 2013; Price, 2012; Schwartz et al., 2009; Walker et al., 2011) and inferior frontal gyri (Dell et al., 2013; Mirman and Graziano, 2013; Mirman et al., 2015a,b; Price, 2012; Schwartz et al., 2009; Walker et al., 2011). In the parietal lobe, the temporo-parietal junction (Dell et al., 2013; Price, 2012), including the left angular gyrus (Dell et al., 2013), has also been related to transition from semantics to words/lemmas.
In addition to their role in lexical-semantic processes, fronto-temporo-parietal areas are also thought to be involved in general conceptual processing. For example, in the framework of controlled semantic cognition (Rogers et al., 2015; Lambon Ralph et al., 2017), the modality-specific (e.g. visual, somatosensory, olfactory etc.) conceptual representations are distributed throughout the cortex, and a transmodal semantic hub subserved by the anterior temporal lobe bilaterally carries out the interactions between various modality-specific surface representations (Lambon Ralph and Patterson, 2008; Patterson et al., 2007). The left inferior frontal gyrus, posterior middle temporal gyrus, and inferior parietal cortices (dorsal angular gyrus/intraparietal sulcus) carry out the cognitive control over this network (Jefferies, 2013). On the other hand, Gainotti (2011) proposed a framework of two higher-order convergence zones, one of which specifically supplies the integration of action schemata with visual data in the portions of left fronto-temporo-parietal convexity. This zone supports different aspects of action processing, including action verb semantics. Binder and Desai (2011) propose that modality-specific regions provide input to inferior parietal lobe and ventro-lateral portions of the temporal lobe that serve as convergence zones for abstract representations storage. Modality-specific regions include sensory-motor and visual motion cortices in precentral, postcentral and supragarginal gyri, and posterior inferior temporal areas, respectively. Dorsomedial and inferior parietal cortices, in their turn, govern goal-directed activation and selection of information from temporoparietal cortices. A later review of Pulvermüller (2013) proposed an account where several regions in the perisylvian area (inferior frontal; inferior, anterior, and superior temporal; and inferior parietal cortices) constituted potential semantic hubs for general meaning processing, and left inferior frontal cortex and bilateral fronto-central motor systems were involved in processing of action-related concepts and words.
To summarize, the lexical-semantic deficits emerging in picture naming and related to storage or access problems have been associated with the left superior temporal, middle temporal, and inferior temporal gyri; the middle frontal and inferior frontal gyri; inferior parietal areas and the temporo-parietal junction; and bilateral anterior temporal and inferior temporal cortices. However, the majority of the models mentioned above were mainly substantiated with object naming data. It is an open question whether these areas are equally critical for lexical-semantic stages of action naming.
1.2. Neural substrates of lexical-semantic stages of verb processing
In studies of language pathology, the interest for the neural underpinnings of verb processing originated from verb-noun dissociations at the behavioral level (Cappa and Perani, 2003). To explain the phenomenon, the fronto-temporal dichotomy hypothesis (Crepaldi et al., 2011) postulated that verbs rely mainly upon left inferior frontal areas and nouns mainly upon left temporal areas of the brain (Damasio and Tranel, 1993; Daniele et al., 1994). Since then, many neuroimaging and neuropsychological studies contrasted verbs and nouns in different tasks (for reviews, see Cappa and Perani, 2003; Crepaldi et al., 2013; Crepaldi et al., 2011; Mätzig et al., 2009; Vigliocco et al., 2011). The contemporary evidence does not support the exclusive role of frontal regions in verb processing, nor the account of total segregation between the grammatical classes in the brain (Crepaldi et al., 2013, 2011; Tranel et al., 2001; Vigliocco et al., 2011). It is argued that grammatical class per se is not the organizational principle of word processing in the brain (Vigliocco et al., 2011), although manipulating features specific to verbs can recruit additional brain structures (see, for example, Thompson et al., 2007, an fMRI study where activation in posterior peri-sylvian regions was associated with verb argument structure complexity). Generally, though, it can be argued that the observed differences between verbs and nouns are often mediated by action/verb semantics (Cappa and Perani, 2003; Vigliocco et al., 2011).
The action and verb semantics have been studied extensively in the framework of embodied cognition. The latter postulates that cognitive processes hinge on perception and action (Meteyard et al., 2012). In this vein, action verb semantics might be rooted in the neural substrates related to their visual-motion and motor features (for a review, see Kemmerer, 2015b). The visual-motion features of action verb semantics are subserved by left postero-lateral temporal cortex. The motor semantic representations, in turn, are supported by left premotor and primary motor cortices.
Among the studies supporting the embodied view on action concept processing, the bulk of evidence comes from functional neuroimaging (see Watson et al., 2013; for a meta-analysis), TMS studies (Cappa et al., 2002; Pulvermüller et al., 2005; Tomasino et al., 2008; Vukovic et al., 2017; Willems, Labruna, D’Esposito, Ivry and Casasanto, 2011) or studies of neurodegenerative diseases affecting motor cortex (Bak, 2013; Bak and Chandran, 2012; Bak and Hodges, 2004; Grossman et al., 2008; York et al., 2014). However, the question still remains whether the involvement of visual motion and motor areas in concept processing is automatic, functionally relevant, or triggered by both verbal and non-verbal stimuli, independently of the task (Kemmerer, 2015a, 2015b; Watson et al., 2013). The evidence from lesion studies is mixed as well. Tranel et al. (2001) tested 75 patients with brain lesions, identified 22 that had impaired action naming, and subtracted their lesion overlaps from lesion overlaps of 22 patients with unimpaired action naming. Inferior precentral gyrus was one of the structures related to the action naming deficit. Note, however, that when 19 of the patients with action naming impairment were additionally tested with an action recognition task, they scored significantly better than in the action naming task. For that reason, the authors suggested that the identified deficit was rather due to phonological form retrieval problems than due to impaired action semantics knowledge. Arévalo and colleagues (Arévalo et al., 2012) used VLSM in left hemisphere stroke patients with a picture-word matching judgment task using action and action-associated object stimuli versus neutral stimuli. They found that regions that were necessary for successful task completion included motor and premotor areas, but were not confined to them. Kemmerer and colleagues (Kemmerer et al., 2012) probed conceptual and lexical knowledge of action and verb semantics in a large group of left hemisphere stroke patients (N = 147 for lesion analysis). At least four of their six tasks – picture naming, picture attribute, picture comparison, word-picture matching, word attribute and word comparison – implicated sensori-motor systems.
However, Maieron and colleagues (Maieron et al., 2013) found neither an association between damage to the motor cortex and the action naming task deficits nor a significant functional coupling between motor and other brain areas during an action generation task in a group of neurosurgical patients. Papeo and colleagues (Papeo et al., 2010) observed double dissociations between pantomime imitation and action verb comprehension and production at an individual level in persons with focal damage to the left hemisphere. Their single-case lesion analysis did not confirm a critical involvement of the anterior fronto-parietal sensorimotor networks in action-word comprehension. Saygin and colleagues (Saygin et al., 2004) found an association of motor, premotor and parts of primary somatosensory cortex with a non-linguistic action comprehension task, but not with a linguistic written sentence comprehension task in a group of left hemisphere stroke patients. In Tomasino et al.’s study (Tomasino et al., 2012), neurosurgical patients with damage to precentral and postcentral sulci read action verbs and then had to provide their vividness and frequency ratings. If the lesion affected a part of the cortex related to an action (e.g. hand, leg motor cortex etc.), the reaction time for the corresponding verbs was slowed down for the vividness rating (which requires mental movement simulation) but not for the frequency rating (purely linguistic level). The authors suggested that sensorimotor regions are critically involved in action verb processing only when the corresponding movements are simulated. Overall, the critical role of the motor and visual motion regions in verb processing at the linguistic level must be confirmed by further studies.
1.3. White matter substrates of lexical-semantic stages of object and action naming
Some of the aforementioned lesion studies discuss the connection between white-matter damage and object naming deficits, but particular white matter tracts are rarely specified. The evidence for the involvement of specific white-matter tracts in object/noun lexical-semantic processing comes mainly from direct electrical stimulation in brain tumor patients (Duffau et al., 2005; Duffau et al., 2009; Duffau et al., 2013; Gil-Robles et al., 2013; Mandonnet et al., 2007), neuro-degeneration studies (Catani et al., 2013) or diffusion tensor imaging in healthy subjects (De Zubicaray, Rose and McMahon, 2011).
The two white matter bundles systematically implicated in semantic processing and lexical retrieval are the inferior fronto-occipital fasciculus (De Zubicaray et al., 2011; Duffau et al., 2005, 2013; Gil-Robles et al., 2013; Mandonnet et al., 2007) and the uncinate fasciculus (Catani et al., 2013; De Zubicaray et al., 2011). The inferior fronto-occipital fasciculus connects the ventral occipital regions with orbito-frontal cortices, and the uncinate fasciculus bridges temporo-polar and orbito-frontal cortices (Catani and Thiebaut de Schotten, 2008). Deficits at the lexical-semantic level can be related to damage to both these tracts. For the uncinate fasciculus, the inconsistency of current data challenges its critical role in lexico-semantic processing (Duffau et al., 2009, 2013; Mandonnet et al., 2007; Von Der Heide, Skipper, Klobusicky, & Olson, 2013). However, Duffau and his colleagues (Duffau et al., 2014) accommodate the existing evidence of object naming in awake neurosurgery in a hodotopical arrangement of Levelt’s lexical retrieval model. By analogy to the auditory linguistic information processing dual-route model (Hickok and Poeppel, 2007; Saur et al., 2008), two processing streams for picture naming are postulated, with the ventral stream corresponding to the processing of meaning. The ventral stream consists of two routes, a direct one via the inferior fronto-occipital fasciculus and an indirect one via the anterior part of the inferior longitudinal fasciculus and the uncinate fasciculus. The direct route bridges occipital and temporo-basal associative cortices where visual information is processed, and temporal and parietal associative cortices, where auditory information is processed, with pre-frontal areas where top-down control of amodal information is performed. The indirect route connects regions that support semantic processing such as posterior occipito-temporal junction and orbito-frontal cortex, and also has a relay in the temporal pole. When the indirect pathway is damaged, the direct pathway can functionally compensate for it (Duffau et al., 2013, 2014).
The aforementioned studies were based primarily on object and noun processing. As for verb processing at the lexical-semantic level, the studies exploring its complex neural architectures are scarce. Direct electrical stimulation evidence for verb processing summarized in a recent review (Rofes and Miceli, 2014) is in line with noun processing studies: the white-matter tracts that cause semantic paraphasias upon stimulation are the inferior fronto-occipital fasciculus, inferior long-itudinal fasciculus and uncinate fasciculus of the language-dominant hemisphere (Bello et al., 2007, 2008).
To summarize, left inferior fronto-occipital, inferior longitudinal, and uncinate fasciculi are expected to be involved in the lexical-semantic processing of verbs. At any rate, given the scarceness of the empirical data, the white-matter tracts supporting verb processing warrant further investigation.
1.4. The present study
Voxel-based lesion-symptom mapping, or VSLM (Bates et al., 2003) is a statistical method of establishing neural foundations of a behavioral function in patients with brain lesions. A large group of brain-injured individuals perform a behavioral test on a cognitive function of interest, their structural neuroimaging (MRI) data are collected, and a statistical test is applied at each voxel in common stereotaxic space, relating the presence or absence of the lesion to the behavioral results at the group level (Baldo et al., 2012). The dependent variable of interest can further be refined by partialing out other behavioral variables, thus deriving the maps of crucial brain regions from the variance in the main behavioral score unaccounted for by the additional variables. Various designs were applied in previous VSLM-based studies to specifically tap into specific lexical-semantic stages of naming: for example, scoring of minor phonological errors and conduite d’approche as correct (Campanella, D’Agostini, Skrap and Shallice, 2010), using speech fluency as a covariate in a VLSM analysis of naming (Baldo et al., 2013), or mapping semantic errors in naming while factoring out scores for non-verbal and verbal semantic comprehension (Schwartz et al., 2009; Walker et al., 2011).
To our knowledge, the only published VLSM study that directly engaged action naming score as a dependent variable, contrasting it to object naming, was conducted by Piras and Marangolo (2007). Consistent with the fronto-temporal dichotomy hypothesis, they revealed that action, but not object naming was correlated with damage to inferior frontal areas. The areas common for both tasks were found in superior and middle temporal areas, and object naming relied upon more posterior middle temporal regions. Another study, that of Campana and colleagues (Campana et al., 2015), employed action naming as one of the tasks to measure language recovery after transcranial direct current stimulation over the left inferior frontal gyrus. The amount of improvement in people with non-fluent aphasia was used as a dependent variable in a VLSM analysis. The analysis revealed that the improvement negatively correlated with the damage to a number of cortical and subcortical structures, involving the Rolandic operculum, the inferior frontal, precentral, postcentral gyri, insula, anterior superior gyrus and transverse temporal gyrus; putamen and globus pallidus; and also superior and inferior longitudinal fasciculi. However, sample sizes in these studies were small: 16 participants in (Piras and Marangolo, 2007) and 20 in (Campana et al., 2015); and no multiple comparison correction method was reported (Piras and Marangolo, 2007) or applied (Campana et al., 2015). This warrants confirmation of the results in a more methodologically robust experiment. It is also noteworthy that in Piras and Marangolo (2007), phonological errors were taken into account, and Campana et al. (2015) did not specify their scoring procedures. Thus, a more specific scoring is required to verify whether those regions are involved in lexical-semantic stages of action naming.
Our study was designed to reveal grey and white matter correlates of lexical-semantic stages of action naming. We used VLSM in 40 left-hemisphere stroke patients with aphasia, using an action naming task. To specifically tap into the lexical-semantic stages of action naming, we performed the VLSM analysis as follows. We used a scoring system that took into account errors that most probably arose at lexical-semantic stages, that is errors resulting in a recognizable existing word, and disregarded other errors. However, one can argue that analyzing just one error type is not enough to rule out other functional sources of the naming deficit. One of the arguments is that lexical errors, and semantic errors in particular, can also arise at post-semantic levels, at the stages of mapping meanings onto phonological forms. This argument is supported by reports of patients who had selective production deficits in oral modality but not in written modality (Caramazza and Hillis, 1990; Rapp et al., 1997) and vice versa (Hillis et al., 1999; Hillis et al., 2002). To control for possible phonological deficits, we used phonological error rate as a covariate. The significant VLSM map was overlaid with grey and white matter brain atlases to formally assess the affected structures at the group level.
2. Material and methods
2.1. Participants
Forty premorbidly right-handed native speakers of Russian with language and speech disorders (aphasia and/or dysarthria) due to stroke were recruited from the inpatient units of the Center for Speech Pathology and Neurorehabilitation, Moscow, Russia. There were 21 females; the age ranged from 33 to 78 years (M = 51.65, SD = 10.97), and the education ranged from secondary school (typically 10 years) to a university degree (typically 15 years). Thirty-nine patients had a clinical diagnosis of a single ischemic or hemorrhagic symptomatic stroke in the left hemisphere; one patient (Patient 21) had recurrent ischemic strokes in the left hemisphere with 50 days between events. The time post onset calculated as time between the last stroke and the MRI acquisition date ranged from 3 to 146 months (M = 24.88, SD = 28.15). None of the patients had any history of alcohol and drug abuse (as per indication in the official medical record) or had been diagnosed with neurological or psychiatric disease before the stroke. All the patients were administered standard comprehensive neuropsycho-logical assessment upon admission and were diagnosed with aphasia (N = 38) or dysarthria (N = 2) as their primary speech-language disorder. The aphasia type was established within the framework of Luria’s classification system (Akhutina, 2016; Luria, 1966). The severity of aphasia was determined based on the Quantitative Assessment of Speech in Aphasia (QASA; Tsvetkova et al., 1981). The total score (the sum of comprehension and production subtest scores; maximum 300 points) ranged from 141.5 (moderate-to-severe) to 297 (mild). See Appendix A for the demographic, clinical and neuropsychological data. The content and amount of the speech therapy that the patients had received before the recruitment in the study during their stay in the clinic was not controlled for. All the patients gave informed consent prior to participation; the study was approved by the Committee on Interuniversity Surveys and Ethical Assess of Empirical Research of the National Research University Higher School of Economics.
2.2. MRI acquisition
The MRI anatomical brain images were acquired on the same 1.5 T S Magnetom Avanto scanner. For all patients, three sequences (high-resolution T1, T2-weighted, and fluid-attenuated inversion recovery (FLAIR) images) were acquired with the following parameters: (i) for T1: repetition time = 1900 ms, echo time = 3.37 ms (2.93 ms in Patients 25 and 35, 2.91 ms in Patient 36), field of view = 256*256 mm (250*250 mm in Patients 25, 35 and 36), slice thickness = 1 mm, 176 transverse (sagittal in Patients 25, 35 and 36) slices; (ii) for T2: repetition time = 5000 ms (4000 ms for Patients 28, 35, 36, and 38), echo time = 93 ms, field of view = 208*230 mm, slice thickness = 4 mm (5 mm for Patients 15, 17, 28, 33, 35, 36, and 38), 28 (22 for Patients 15, 17, 28, 33, 35, 36, and 38) transverse slices; (iii) for FLAIR: repetition time = 9000 ms, echo time = 89 ms, field of view = 201*230 ms, slice thickness = 4 mm (5 mm for Patients 15, 17, 33, 35, 36, and 38), 28 (22 for Patients 15, 17, 33, 35, 36, and 38) transverse slices.
2.3. MRI preprocessing
Preprocessing was performed in the SPM8 software (Version 6313, http://www.fil.ion.ucl.ac.uk/spm; last accessed on April 9, 2018). T1, T2 and FLAIR images of each patient were manually reoriented to ACPC plane, the T1 was resliced to the MNI152 template with 1 mm3 resolution using 4-th degree B-spline transformation, and T2 and FLAIR were co-registered and resliced to the new T1 using trilinear transformation.
2.4. Lesion analysis
The lesion masks were manually delineated using MRIcron (Rorden and Brett, 2000) and ITK-snap (Yushkevich et al., 2006; Version 3.4.0-rc1, www.itksnap.org; last accessed on April 9, 2018) based on the visual inspection of the preprocessed T1, T2 and FLAIR anatomical scans. To identify the lesion boundaries, we delineated tissue damage visible on the T1 and then used T2 and FLAIR images to verify the lesion and expand it by including adjoining gliosis and hemosiderin. Any visible lesions, albeit unrelated to the major lesion, were delineated. The lesion masks were then normalized to the MNI152 1 mm3 template using an original MATLAB script that employed cost-function masking (Brett et al., 2001) to avoid lesion-related distortions.
The alignment between the normalized lesion files and the lesion in the native space was visually inspected by at least two authors experienced in identification of stroke lesions (Yu.A, M.V.I. or N.F.D) by comparing the overlay of the normalized image to the MNI template to the overlay of the lesion mask on the T1 in the patient’s native space. The cases of misalignment (lesion masks inside the ventricles or outside the meninges, lack of or spurious lesion masks in the cortical and subcortical grey and white matter structures) were manually corrected directly in the normalized lesion mask file using ITK-snap software.
2.5. Action naming
2.5.1. Materials
Eighty black-and-white drawings of actions corresponding to two-argument verbs taken from the database of action pictures and their relevant psycholinguistic parameters (Akinina et al., 2015) were used as stimuli for the action naming experiment. All the selected pictures had high (M = 87.39, range = 71–99%) name agreement (the number of participants per hundred who elicited the most frequent response during the normative picture naming study), and the corresponding verbs were highly imageable (maximum 2.09 on a five-point scale where 1 refers to the most imageable verbs). As there is evidence that instrumentality of a verb – that is, the obligatory use of the instrument required to perform an action (Jonkers and Bastiaanse, 1996) – can influence the behavioral performance (Malyutina et al., 2014) and related neural substrates (e.g., Malyutina et al., 2016), the verbs were balanced on instrumentality (there were 40 instrumental, 40 non-instrumental verbs). Although the stimuli were not explicitly controlled for the body parts typically used for the action, most of the pictures corresponded to hand-related actions. The stimuli were split into two lists that did not differ on number of instrumental verbs, name agreement, subjective visual complexity, image agreement, action familiarity, subjective age of acquisition, imageability, frequency or length in syllables. The descriptive statistics of the stimuli parameters are presented in Table 1.
Table 1.
Descriptive statistics of the action naming stimuli.
| Parameters | Mean | St. Dev. | Min | Max |
|---|---|---|---|---|
| Name agreement, % | 87.39 | 8.48 | 71 | 99 |
| Subjective visual complexity (scale 1–5) | 2.78 | 0.41 | 1.82 | 3.79 |
| Image agreement (scale 1–5) | 4.02 | 0.71 | 2.17 | 4.96 |
| Age of acquisition (scale 1–5) | 1.97 | 0.44 | 1.19 | 3.14 |
| Imageability (scale 1–5) | 1.27 | 0.21 | 1.02 | 2.09 |
| Action familiarity (scale 1–5) | 3.49 | 0.70 | 1.90 | 4.89 |
| Lexical frequency (ipm) | 13.27 | 16.12 | 0.50 | 85.70 |
| Length (syllables) | 3.00 | 1.10 | 1 | 5 |
Note. Name agreement, % - percentage of the verb most frequently used as a name; subjective visual complexity – subjectively assessed number of lines and details on a five-point scale (1 refers to the simplest pictures); image agreement – rate of match between the mental image corresponding to a verb and the action picture on a five-point scale (1 refers to the worst match); age of acquisition – subjective rate of the age of acquisition of a word on a five-point scale (1 refers to the interval 0–3 years, 5 refers to the interval 9–12 years); imageability – rate of how easy it is to imagine the action denoted by the verb (1 refers to the verbs that are the easiest to imagine); action familiarity – subjective rate of exposure to the action (1 refers to the least familiar actions); lexical frequency (imp) – lemma frequency per million based on (Lyashevskaya and Sharov, 2009).
2.5.2. Procedure
The presentation of the stimuli was programmed in the E-Prime 2.0 (Release Candidate 2.0.8.90) software (Psychology Software Tools, Inc., 2012). The action pictures were presented in the center of the screen one by one in a fixed pseudo-randomized order; the next trial was triggered by the experimenter. The patients received the following instruction: “You will see a picture. Please name in one word what the character/characters is doing in the picture”. All the responses were audio-recorded; at the same time, the experimenter noted given responses and relevant comments in a paper protocol. Both lists were presented to each participant; the order of lists was counterbalanced across the participants; the two lists were presented in one or two sessions depending on a participant’s level of fatigue. Each list was preceded by five training trials during which the experimenter could give feedback to make sure the patients understood the task correctly. In the experimental trials, giving meaningful cues (such as phonological/semantic cues or negative feedback) was not allowed. However, an experimenter could provide such a cue if he could see that a patient was obviously unable to retrieve a verb before proceeding to the next trial, in order to maintain his/her motivation; the responses given after the cue were not scored.
The time between the verb naming test and the MRI acquisition did not exceed 35 days (it was performed either prior or after the scanning) except for the Patient 33, who was tested 15 months after MRI acquisition upon another admission to the Center for Speech Pathology and Neurorehabilitation. The medical records did not indicate any occur-rence of cerebrovascular incidents between the MRI acquisition and behavioral testing time points that could have influenced the brain-function relationship.
2.5.3. Scoring
The aim of our study was to pinpoint the lexical-semantic stages of action naming. This determined our choice of the action naming scoring procedure. For the main dependent variable, we scored all responses resulting in an incorrect lexical unit as incorrect because they could arise at the lexical-semantic level (see Section 1.1). Other errors, such as phonemic errors resulting in a non-word, morphological inflection errors etc., were disregarded; and these responses were scored as correct. The phonological errors were analyzed separately to be used as a covariate in the analysis. By defining our variables in this way, we could evaluate the neural substrates of lexical-semantic stages of action naming, discounting additional regions that might subserve phonological form access, encoding, and articulation.
The patients’ responses were scored as follows. A response was scored as correct if a patient, at any point of his/her response, could produce an identifiable verb that was named by at least one participant in the norming study (Akinina et al., 2015). Different morphological forms of the verb (such as using infinitive or improper personal form instead of the target 3d person form), and verbs produced within a full sentence were also scored as correct. Word form errors (e.g., pynisosit ‘vacuum cleans’ instead of pylisosit) were not taken into account unless they resulted in another existing verb (a mixed error). Verbs that were not produced during the normative procedure, including the mixed errors, and nouns (either referring to the action itself or the arguments of a verb) were scored as incorrect. If a patient failed to produce a response related to the picture, such responses were scored as ‘no response’ (NR). These included gestures and onomatopoeia, meta-linguistic comments of a patient (e.g., “I can’t remember the word”, “Let’s proceed”) and false starts. In order to eliminate the effect of possible recognition deficits on naming accuracy the trials with visual recognition problems (VRP) were identified. A response was categorized as VRP if the patient did not produce any completed verbal response related to the task and (i) either gave a meta-comment that he couldn’t recognize the picture fully or partially (e.g., “What’s this, I don’t understand”), or (ii) the experimenter noted in the protocol that the patient had recognition problems. Trials lost due to a technical error were coded as “no data” (ND). Utterances pronounced after an experimenter’s phonological, semantic or other meaningful prompt were not scored; in such cases the score was given based on what the patient had said before the prompt. The total naming accuracy was calculated as the proportion of correct responses to all given complete verbal responses (i.e., excluding ND, NR, and VRP). This was done to ascertain that we assessed the lexical-semantic stages of processing, since there is evidence that anomias can result from breakdown at post-lexical stages, as well (Fama et al., 2017).
The phonological error analysis was performed as follows. For each complete verbal response, we marked the presence of a phonological error, defined as phoneme omission, substitution, insertion etc., related to the target word or to the verb (or noun) that was scored as the final lexical response of the patient; including both existing word and non-word errors. Phonological errors in other words of the utterance (when the patient, for example, used a correct verb in a sentence context and made an error in one of the arguments) were not scored. Dysarthric errors (slurred, slowed, choppy or effortful pronunciation) were not included either. The phonological error rate was calculated as the proportion of responses where a phonological error is present to all given complete verbal responses (excluding ND, NR, and VRP).
2.6. VLSM analysis
To establish the brain areas pertaining to action naming, VLSM analysis (Bates et al., 2003; Version 2.55, http://aphasialab.org/vlsm; last accessed on April 18, 2019) was performed with the naming accuracy as the main dependent variable. In this variant of VLSM, for each voxel, linear regression (a parametric test) is performed, comparing behavioral scores in participants with and without a lesion in that voxel. Patient age, lesion volume (calculated automatically by the VLSM analysis software), and phonological error rate were used as covariates in order to factor out their possible effects. Only voxels that were lesioned in more than 10% (N = 4) of the patients entered the analysis. To determine significant voxels, we first implemented a commonly used voxel-wise threshold of p < .005 (Binder et al., 2016; Pillay et al., 2017; Pillay et al., 2014; Wilson et al., 2010). Next to properly correct for multiple comparisons, we used permutations (N = 1000) and cluster size thresholding (p < .05) (similar to the correction implemented in Ivanova et al., 2018). Permutation-based thresholding is a non-parametric type of FWER method that provides a reliable correction for multiple comparisons in lesion mapping approaches (Kimberg et al., 2007). Cluster-size corrections take into account the anatomical continuity of the lesion and control the rate of false positive clusters rather than individual voxels (Karnath et al., 2018). Permutations of data are performed to compute the null distribution of cluster sizes that survive the voxel-wise threshold and use this distribution to determine a minimum cluster size that would occur by chance in less than 5% of cases (p < .05).
To identify the critical grey-matter regions revealed by VLSM analysis, we binarized the resulting VLSM map using ImCalc function in SPM12 (Version 7219, http://www.fil.ion.ucl.ac.uk/spm; last accessed on April 18, 2019) and overlaid it on the Automated Anatomical Labeling (AAL) template using Batch Descriptives function in MRIcron (Version 2 May, 2016, https://www.nitrc.org/projects/mricron; last accessed on April 7, 2019).
To formally assess white matter tract involvement, we used a simple atlas-based overlay approach. Tract probability maps obtained from healthy controls (Rojkova et al., 2016) were overlaid upon the binarized VLSM map for action naming using the Tractotron software (Foulon et al., 2018; as a part of BCBtoolkit Version 4.1.0, http://www.bcblab.com/BCB/Software.html; last accessed on April 7, 2019). This tool allowed us to estimate the probability of disconnection for a given tract, where the probability corresponds to the affected voxel with the highest percent value of participants with the tract going through this voxel. Though Tractotron was initially designed to assess individual patient data, we used it as an atlas tool to formally identify the affected tracts in a VLSM map derived from a group of patients, similarly to using a grey matter atlas (e.g., AAL) to assess grey matter involvement. The probability of disconnection of a given tract = p in this case meant that there were voxels in MNI (Montreal Neurological Institute) space where p proportion of participants in the healthy group (Rojkova et al., 2016) had this tract, which was included in the VLSM map (probability of being affected). To verify the suggested tracts disruption and locate its site, the tracts were later visually inspected by overlaying the VLSM map with the masks of each tract in MRICroGL64 software (Version v1.0.20180623; http://www.mccauslandcenter.sc.edu/mricrogl/home; last accessed on April 18, 2019), with a tract mask probability threshold > .7.
3. Results
3.1. Action naming results
A total of three trials were lost due to technical errors (ND). The number of NR for each patient ranged from 0 to 21 (M = 1.65, SD = 3.61, Mdn = 0). The proportion of correct responses to all trials(i.e. including ND, NR, and VRP) ranged from 0.25 to 0.98 (M = .83, SD = .16, Mdn = .87). Naming accuracy ranged from 0.34 to 0.98 (M = .84, SD = .15, Mdn = .87).
In the phonological error rate analysis, one more trial could not be scored due to technical reasons (it was not audio-recorded). It was classified as ND. The phonological error rates ranged from 0 to 0.22 (M = .03, SD = .05, Mdn = .01).
The naming accuracy scores and phonological error rates for each patient were given in Appendix A.
3.2. VLSM results
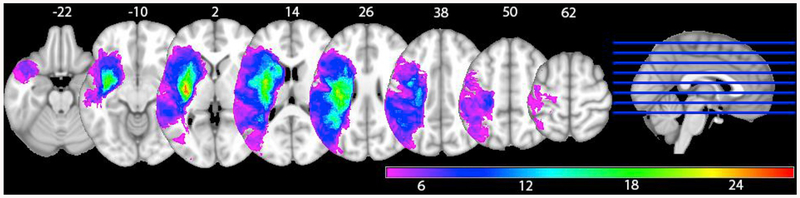
Fig. 1 shows the lesion overlay map with a minimum of four patients per voxel, thus representing the voxels eventually entered into the VLSM analysis. The overlap size ranges from four patients (in purple) to 27 (in red).
Fig. 1.
Lesion overlay map of the voxels entered into the analysis (equal or more than four patients per voxel)
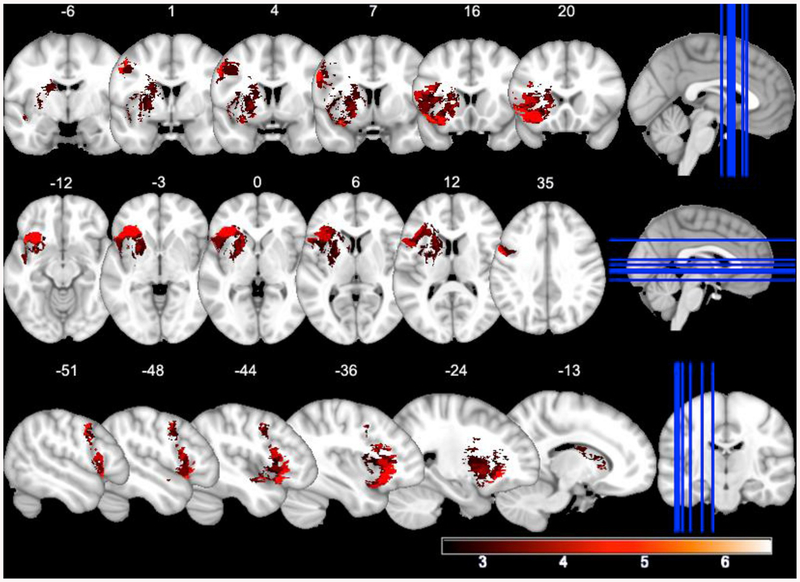
The VLSM analysis of naming accuracy and lesion size, patient age and phonological error rate as covariates with a voxel-wise threshold p < .005 corrected with the permutations (N = 1000) and cluster size method revealed one significant (corrected p = .002) cluster (volume = 27305 voxels) with T-values ranging from 2.72 to 6.18. The peak MNI coordinates were (−45 0–6), and the center coordinates were (−33 14 6) (see Fig. 2).
Fig. 2.
VLSM-map for action naming. Higher T-values appear in lighter shades of red. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Grey and white matter involvement
The significant cluster involved regions in the frontal and insular areas, and portions of the basal ganglia. The analysis of grey matter involvement revealed that the affected left hemisphere structures (> 5% damaged) were inferior frontal gyrus (pars triangularis, opercularis and orbitalis, extending to the cortex and deep into the white matter); portions of the most anterior part of the insula; a small portion of the precentral gyrus at the level of the middle frontal and inferior frontal gyri; large parts of the basal ganglia – putamen, caudate nucleus and globus pallidus. The raw percentage and number of voxels of each affected left hemisphere structure is presented in Table 2.
Table 2.
Percent and number of voxels of structures intersecting with the VLSM map.
| AAL grey matter structure | Percent affected | N voxels affected |
|---|---|---|
| Putamen | 55.90 | 4439 |
| Insula | 40.70 | 6120 |
| Inferior frontal gyrus, pars opercularis | 26.30 | 2179 |
| Caudate nucleus | 24.80 | 1902 |
| Globus pallidus | 21.50 | 491 |
| Inferior frontal gyrus, pars orbitalis | 13.40 | 1826 |
| Inferior frontal gyrus, pars triangularis | 10.80 | 2171 |
| Precentral gyrus | 7.50 | 2109 |
| Superior temporal pole | 3.50 | 361 |
| Olfactory cortex | 3.10 | 71 |
| Amygdala | 2.00 | 34 |
| Superior temporal gyrus | 1.40 | 263 |
| Superior frontal gyrus, orbital part | 1.00 | 77 |
| Rolandic operculum | 0.40 | 32 |
| Thalamus | 0.20 | 16 |
| Middle frontal gyrus | 0.00 | 1 |
The analysis of white matter involvement showed an intersection of the VLSM map with a number of association, projection and commissural fibers. Further, we will only report and discuss the association and projection tracts with the highest probability of disconnection, choosing the cut-off threshold of 80%. All the tracts that intersected with the VLSM map are presented in Appendix B.
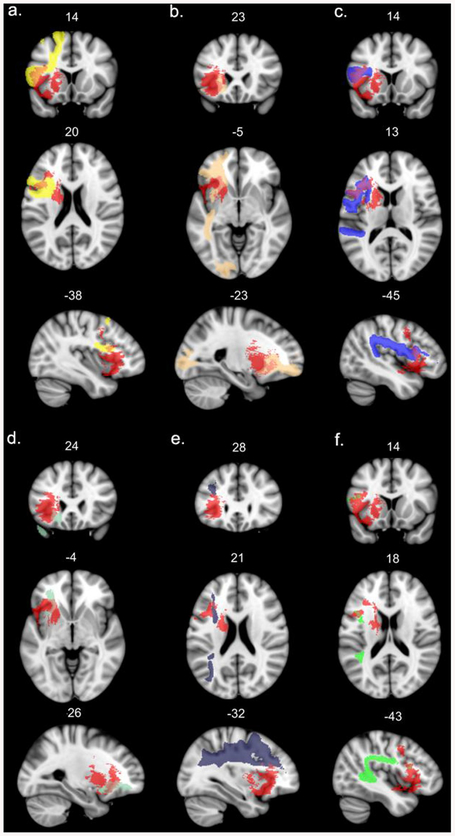
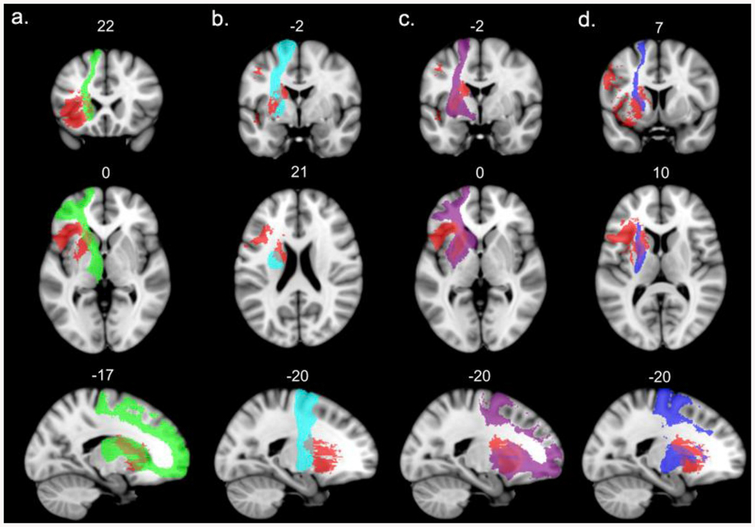
The association fibers were the frontal aslant tract, inferior fronto-occipital fasciculus, superior longitudinal fasciculi II and III, uncinate fasciculus, long segment of the arcuate fasciculus, fronto-orbital polar and frontal inferior longitudinal tracts, and the fronto-insular tract 4. The projection fibers were anterior thalamic projections, cortico-spinal, fronto-striatal and fronto-pontine tracts. The overlays of these tracts on the binarized VLSM map are presented in Figs. 3 and 4 (association tracts), and Fig. 5 (projection tracts). For purposes of visualization, we adjusted the lower probability threshold for each tract mask at 0.7 and binarized the files.
Fig. 3.
Left hemisphere long association white matter tracts affected by the lesion (probability of disconnection by the VLSM map > 80%). The significant VLSM cluster is shown in red. For the sake of visualization, the probabilistic masks of the tracts are thresholded at minimum 0.7. a – frontal aslant tract, b – inferior fronto-occipital fasciculus, c – superior longitudinal fasciculus III, d – uncinate fasciculus, e − superior longitudinal fasciculus II, f – long segment of the arcuate fasciculus. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
Left hemisphere short intralobar association white matter tracts affected by the lesion (probability of disconnection by the VLSM map > 80%). The significant VLSM cluster is shown in red. For the sake of visualization, the probabilistic masks of the tracts are thresholded at minimum 0.7. a – frontal orbito-polar tract, b – frontal inferior longitudinal tract, c – fronto-insular tract4. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
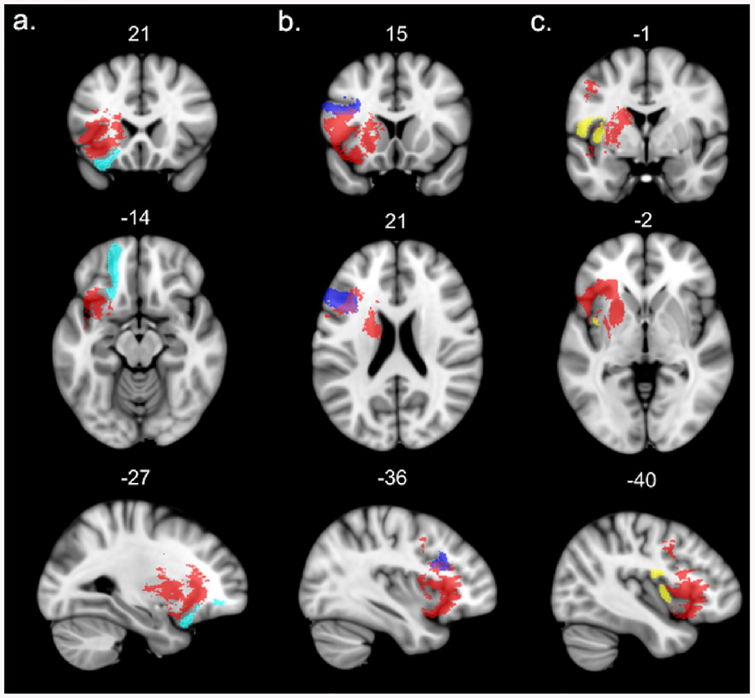
Fig. 5.
Left hemisphere projection white matter tracts affected by the lesion (probability of disconnection by the VLSM map = 100%). The significant VLSM cluster is shown in red. For the sake of visualization, the probabilistic masks of the tracts are thresholded at minimum 0.7. a – anterior thalamic projections, b – cortico-spinal tract, c – fronto-striatal projection, d – fronto-pontine projections. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
4.1. Grey matter cortical findings
At the grey matter level, we found that lesions to portions of the precentral gyrus, inferior frontal gyrus, anterior insula, and parts of the basal ganglia – putamen, caudate nucleus and globus pallidus – affected lexical-semantic stages of action naming. We will discuss grey matter cortical findings here. The involvement of the basal ganglia will be discussed in Section 4.3.
The involvement of the precentral gyrus in action naming complies with the embodiment hypothesis. This result is in line with previous lesion studies that demonstrated premotor and motor area involvement in action semantics processing in tasks with verbal stimuli (Arévalo et al., 2012; Kemmerer et al., 2012). Given the inconsistency of the data for motor and premotor cortices coming from studies implying different methods (Kemmerer, 2015a; Watson et al., 2013), including lesion studies (Maieron et al., 2013; Papeo et al., 2010; Saygin et al., 2004), our results present a valuable contribution. For motor cortices specifically, the question is also open whether their involvement is a corollary, a side effect or a requirement for action word processing (e.g., Tomasino et al., 2008). Our results suggest that the latter might be the case, in that the precentral gyrus is part of a network of areas that contribute to action naming. In the comprehension domain, some researchers argue that understanding action-related words requires simulation of these actions in one’s own motor system (Kemmerer et al., 2012). Others, however, suggest that motor activation is not necessary, but rather helps word processing (Boulenger et al., 2006), and this might be a strategy used by some participants but not others (Arévalo et al., 2012). Similar logic may apply to the production of action words. It is also unclear whether impaired action naming is due to a storage deficit (the physical damage to parts of representations of action semantics) or an access deficit. Gainotti (2011), for example, argued that selective deficits in naming and the understanding of action verbs in patients with lesions to frontal, temporal and parietal regions are caused by damage to components of action schemata representations. Alternatively, the intact premotor and motor cortices can help access the relevant lemma/word. Their additional recruitment may prompt supplementary activation from the semantic system that helps successful lexical retrieval: the ability to self-cue by additionally activating motor cortices (e.g. by openly or covertly simulating the action) may lead to an increase in accuracy.
As for the inferior frontal gyrus, different neurolinguistic lexical retrieval models relate it to different stages of naming. Thus, the combination of spatial and chronometric data from neuroimaging and behavioral studies (Indefrey and Levelt, 2004; Indefrey, 2011), supported by computational data (Roelofs, 2014), provides evidence in favor of its involvement in syllabification rather than lexical-semantic processing. In this regard, our results disagree with that neuroanatomical model of naming, especially because even after phonological errors had been regressed out, the inferior frontal gyrus still held as a critical region for lexical errors. In a similar vein, Duffau et al. (2014) claim that the orbito-frontal part of the inferior frontal cortex belongs to the ventral semantic stream, based on the intraoperative evidence that stimulation of this region during awake neurosurgery can elicit errors in tasks involving semantics (Bello et al., 2007; Duffau et al., 2005). Dell et al. (2013) also found involvement of the inferior frontal gyrus, among other regions, in their VLPM analysis of the s-parameter.
Essentially, the involvement of the inferior frontal gyrus is in line with semantic processing models that claim its role in semantic control (Binder and Desai, 2011; Jefferies, 2013; Lambon Ralph and Patterson, 2008; Lambon Ralph et al., 2017; Pulvermüller, 2013). Indeed, neuroimaging and TMS studies have shown that the left inferior frontal gyrus is responsible for competitive selection and conflict resolution (Badre, Poldrack, Paré-blagoev, Insler and Wagner, 2005; Bedny et al., 2008; Hirshorn and Thompson-Schill, 2006; Kan and Thompson-Schill, 2004a, 2004b; Mirman and Graziano, 2013; Noppeney et al., 2004; Novick et al., 2009; Novick et al., 2005; Schnur et al., 2009; Snyder et al., 2007; Thompson-Schill et al., 1997, 1998; 1999; Thompson-Schill et al., 2005), semantic retrieval (Badre et al., 2005; Krieger-Redwood and Jefferies, 2014; Noppeney et al., 2004; Wagner et al., 2001; Whitney et al., 2011) and inhibitory control (Cardillo et al., 2004). Some of these studies implied lexical-semantic tasks such as picture naming (Kan and Thompson-Schill, 2004a; Mirman and Graziano, 2013; Novick et al., 2009), semantic relatedness (Badre et al., 2005; Bedny et al., 2008; Snyder et al., 2007; Whitney et al., 2011) tasks; and verb-to-noun generation (Thompson-Schill et al., 1998). Generally, the effects were observed in conditions when selection and control demands were increased. Detrimental effect of damage to the left inferior frontal gyrus for semantic control was confirmed by lesion studies (Mirman and Graziano, 2013; Novick et al., 2009; Schnur et al., 2009; Thompson-Schill et al., 1998).
For verbs, cognitive control load may be increased by their specific lexical properties, such as multiple meanings or rich morphosyntactic information (Thompson-Schill et al., 2005). In our stimulus set, both premises are true. First, the average percentage of name agreement in the norming study (Akinina et al., 2015) is lower than 100 (M = 87.39, SD = 8.48; see Table 1), which indicates that participants needed to choose between the word/lemma alternatives during the task, which, in turn, could increase the semantic control load. Second, verbs in Russian require morphological markers, which means that one morphological form had to be chosen from the verb inflection paradigm in order to complete the task. Thompson-Schill et al. (2005) argue that verb processing in patients with damage to portions of inferior frontal gyrus is impaired only under conditions of high conflict between stimuli (Thompson-Schill et al., 1998). An additional controlled VLSM experiment with an action naming task, where degree of conflict is varied, would be a fruitful direction for future research.
There is another potential explanation of the inferior frontal gyrus involvement in action naming. Several lesion and TMS studies have shown that portions of the inferior frontal gyrus can be directly involved in tasks that probe action semantics knowledge in non-verbal tasks (Cattaneo et al., 2010; Clerget et al., 2009; Kemmerer et al., 2012; Tranel et al., 2003; Urgesi et al., 2014). In this vein, the inferior frontal gyrus could be involved in representation of certain aspects of action concepts. Its role, therefore, could be interpreted in the embodiment framework. In our study, however, with action naming data only, it is impossible to determine whether it is action concepts, lexical retrieval, or retrieval/manipulation of semantic information that are impaired in our patient cohort (see also Tranel et al., 2008).
The left insula has less frequently been discussed in context of language processing. Generally, it may be involved in language production and comprehension, according to lesion (Ardila, 1999) and activation (Oh et al., 2014) studies. In particular, a small portion of the anterior insula is associated with motor coordination of complex speech movements (Dronkers, 1996) and fluency (Bates et al., 2003; Borovsky et al., 2007). Interestingly, in studies that reported fluency deficits, fluency measures included tasks that inherently involved word retrieval: scores from the Western Aphasia Battery (Kertesz, 1982) that reflect articulatory, word-finding and sentence production competence in (Bates et al., 2003), and number of tokens in an interview in (Borovsky et al., 2007). Hence, maybe fluency deficits are partially related to word retrieval problems. Interestingly, portions of the insula appear in VLSM-maps for naming (Baldo et al., 2013; Banerjee et al., 2015; Goldenberg and Randerath, 2015; Piras and Marangolo, 2007), but might be filtered out when fluency measures are taken into account (Baldo et al., 2013). The anterior insula was also related to deficits in all tasks probing production and comprehension, including action naming, in a large-scale study of action semantics and verb processing in stroke patients (Kemmerer et al., 2012). Overall, the role of the insula in single word retrieval and in verb processing, as well as interaction of these processes with fluency measures, requires further investigation.
Generally, our cortical findings in the frontal regions correspond well to the results of other lesion studies of action naming (Kemmerer et al., 2012; Piras and Marangolo, 2007; Tranel et al., 2001, 2008): damage to the inferior frontal gyrus was associated with action naming deficits in all of these studies, and involvement of precentral gyrus and insula was reported in two of them (Tranel et al., 2001; Kemmerer et al., 2012). But contrary to our expectations (and in discrepancy with abovementioned studies), we did not find significant results in temporal or parietal areas. Lexical-semantic processing has been associated with temporal and parietal areas, and parietal areas are also a part of sensorimotor network relevant to language processing in embodiment frameworks. However, parts of these regions might have poor coverage in our sample. Thus, we cannot draw any inference about whether this is a true or a false negative finding. Additionally, temporo-parietal regions might be involved in processing of argument structure information (Thompson et al., 2007). However, at the single verb level, in contrast to the sentence level, verb argument structure might not be actively processed (Malyutina and den Ouden, 2017), hence damage to temporo-parietal regions might not be critical to action naming. To test the latter hypothesis, further VLSM experiments with design varying single and sentence level tasks and argument complexity are warranted.
4.2. White matter findings: association fibers and cortico-cortical networks
The overlay analysis of the white matter revealed intersections with a number of association tracts. These were inferior fronto-occipital, uncinate, aslant, superior longitudinal fasciculi II and III, long segment of the arcuate fasciculus, frontal orbito-polar and frontal inferior longitudinal tracts, and the fronto-insular tract 4. There are several long association fibers that are thought to subserve picture naming and verb processing. The involvement of the inferior fronto-occipital and uncinate tracts in action naming is in line with existing research (Bello et al., 2007, 2008; Catani et al., 2013; De Zubicaray et al., 2011; Duffau et al., 2005, 2013; Gil-Robles et al., 2013; Mandonnet et al., 2007). These tracts comprise direct (inferior fronto-occipital fasciculus) and a part of indirect (uncinate fasciculus) pathways of the ventral stream of visual information processing during picture naming (Duffau et al., 2013, 2014). Thus, the disruption of both tracts hampers the information flow from visual areas to the inferior frontal gyrus which is involved in language semantics, causing semantic paraphasias. In action naming, the role of the inferior fronto-occipital and uncinate tracts was previously supported by direct electrical stimulation studies (Bello et al., 2008, 2007). Our results concur with evidence.
The long segment of the arcuate fasciculus (the “classic” arcuate fasciculus) bridges the frontal and temporal lobes (Catani, Jones, & ffytche, 2005), and is traditionally associated with phonological functions (Catani et al., 2005; Duffau et al., 2014). Thus, its involvement in the lexical-semantic levels of naming was not expected. This result could most probably be explained by proximity of this tract to other tracts, especially in its frontal landing zones. BA44 hosts terminations of not only the long segment of the arcuate fasciculus, but also of the frontal aslant tract and superior longitudinal fasciculus III (Rojkova et al., 2016). Note also that the probability of its being damaged is lower (82%) than for other tracts. Because the method that we used for white matter fiber analysis can only detect intersections with different portions of tract probability maps and does not track the fibers along their course, our finding may require further evaluation.
On the other hand, the frontal aslant tract and superior longitudinal fasciculi II and III are not commonly discussed in the context of picture naming. The frontal aslant tract connects supplementary and pre-supplementary motor areas of the superior frontal gyrus to the posterior portions of the inferior frontal gyrus (Catani et al., 2012). The left frontal aslant tract has been associated with verbal fluency, speech initiation and spontaneity (Catani et al., 2013; Fujii et al., 2015; Kinoshita et al., 2015; Vassal et al., 2014), and speech fluency in stuttering (Kronfeld-Duenias et al., 2016); its more specific language functions are still obscure. One recent study (Sierpowska et al., 2015) described a patient who underwent brain tumor resection at the level of the left frontal aslant tract. In a verb to noun generation task during electrical stimulation, she made over regularization errors, producing non-existent verbs using a standard verb derivation model. She also had problems with a reverse task, noun to verb generation, at the follow-up exam. Sierpowska et al. (2015) hypothesized that surgical damage to inferior connections of the frontal aslant tract affected proper functioning of the inferior frontal gyrus which is necessary for lexical retrieval and semantic knowledge control. These results are in line with our findings about the left frontal aslant tract and inferior frontal gyrus involvement in the lexical retrieval component of action naming. In addition, Budisavljevic et al. (2017) have demonstrated that micro-structural properties of the bilateral frontal aslant tract correlate with characteristics of visually guided hand movements. If this tract also pertains to the motor control network, its involvement in action naming can be interpreted as yet another piece of evidence of the embodied verb and action processing in the brain.
The superior longitudinal fasciculus connects temporo-parietal and frontal regions, with its second subcomponent occupying white matter above the insula and running from the angular gyrus to the prefrontal cortex, and the third subcomponent extending laterally and bridging the supramarginal and inferior frontal gyri (Makris et al., 2005). Their linguistic function is yet to be determined, although it has been suggested that the superior longitudinal fasciculus III plays a role in articulation (Duffau et al., 2014; Makris et al., 2005). One recent study (Parlatini et al., 2017) demonstrated that the superior longitudinal fasciculi II and III are related to a broader range of functions. In their study, a meta-analysis of functional MRI activation maps was performed for different cognitive functions, and the contribution of different superior long-itudinal fasciculus branches to these maps was quantified. The superior longitudinal fasciculus II subserved spatial/motor functions, including mental imagery and motor sequences, and both II and III contributed to non-spatial/motor functions network, which included, among others, motor neurons, semantic processing and response inhibition. As these functions are also relevant to action naming, our findings are in line with this study and add to the discussion of the purported role of the superior longitudinal fasciculi II and III.
Finally, fronto-orbital polar, frontal inferior longitudinal, and fronto-insular tract 4 are a group of short U-shaped intra-lobar fibers. The fronto-orbital polar tract connects the posterior orbito-polar areas to the frontal pole (Thiebaut de Schotten, Dell’Aqua, Valabregue and Catani et al., 2012), the frontal inferior longitudinal tract projects from the precentral gyrus to the ventral middle frontal gyrus and superior portions of inferior frontal gyrus (Catani et al., 2012), and fronto-insular tract 4 is a part of fronto-insular system (Catani et al., 2012), where the 4th bundle specifically links the precentral gyrus to the anterior insula (Rojkova et al., 2016). These tracts have only recently been described in humans (Thiebaut de Schotten et al., 2012; Catani et al., 2012; Cerliani et al., 2012), and their functions in language and other cognitive processes are yet to be established.
4.3. Cortico-subcortical networks in action naming
In addition to association fibers, we identified damage to portions of the basal ganglia - putamen, caudate nucleus and globus pallidus; and to a number of projection fibers: anterior thalamic projections, cortico-spinal, fronto-striatal, and fronto-pontine tracts. A thorough discussion of each of these structures goes beyond the scope of our study. However, these results indicate that cortico-subcortical networks can be implicated in action naming on par with cortico-cortical networks.
The traditionally established and extensively studied function of the basal ganglia and the cortico-subcortical networks is motor control (Watkins and Jenkinson, 2016). However, research on their possible role in cognition has recently emerged. Specifically, it has been suggested that they play a role in cognitive control consisting of enhancing and suppressing relevant activities (Crosson et al., 2003, 2007), which can also manifest in language tasks such as lexical ambiguity resolution (Chenery et al., 2008; Ketteler et al., 2008; Ketteler et al., 2014), suppressing acceptable semantic alternatives during inflection of novel verbs (Longworth et al., 2005), or inhibiting previously activated responses in picture naming (Gil-Robles et al., 2005). Other researchers (Ullman, 2006) hypothesized the existence of basal ganglia – thalamocortical circuitry that projects to and loops back from Broca’s area, and serves in retrieval of lexical and semantic information stored in declarative memory, and for the acquisition and real-time expression of motor and cognitive skills.
Although not consistently, isolated damage to the basal ganglia can give rise to aphasic symptoms (see Radanovic and Mansur, 2017, for a review). Radanovic and Mansur (2017) summarize that out of 180 reported cases of acute stage patients with left basal ganglia damage, almost a half (46.6%) had naming deficits. However, the role of the basal ganglia and their subdivisions in naming is still unclear, partially due to the inconsistency of empirical evidence. For instance, in VLSM naming studies on patients with focal brain lesions, the involvement of basal ganglia is usually not found and/or not reported and discussed (Baldo et al., 2013; Campanella et al., 2010; Dell et al., 2013; Mirman et al., 2015a,b; Piras and Marangolo, 2007; Schwartz et al., 2009; Walker et al., 2011). Similarly, the basal ganglia are not integrated into the majority of existing neurolinguistic picture naming and semantic processing models (Price, 2012; Indefrey and Levelt, 2004; Indefrey, 2011; Jefferies, 2013). Duffau et al. (2014), though, posited their role in cognitive control during picture naming, based on the findings that direct electrical current stimulation of the head of the dominant caudate nucleus elicits perseverations (Gil-Robles et al., 2005). Our findings of the involvement of the caudate nucleus in action naming are in line with this model.
The relation between the basal ganglia and verb processing in patients with focal brain lesions has also rarely been focused upon (Cappa and Perani, 2003; Crepaldi et al., 2011; Kemmerer, 2015b; Kemmerer et al., 2012; Mätzig et al., 2009; Vigliocco et al., 2011). However, evidence from studies of language in Parkinson’s disease (PD) suggests that basal ganglia might be involved in action semantics processing. PD is associated with basal ganglia malfunction, clinically manifests in motor disorders, but might also affect cognitive abilities (e.g., Rodriguez-Oroz et al., 2009). In this vein, studies of language function in such patients gave rise to a new body of evidence for the embodied cognition theory. Thus, patients with PD have poor action-word processing in a variety of tasks (see Cardona et al., 2013, for a review). Additionally, Bocanegra et al. (2015) disentangled the executive function deficits from action-verb production and action semantics deficits in a group of patients with PD, and Fernandino et al. (2013) showed that the action verb deficit in PD is selective compared to abstract verb processing. Therefore, damage to the basal ganglia might have disrupted action naming in our patient group by destroying areas pertaining to action semantics processing networks.
On the other hand, the evidence from the literature is still conflicting and inconclusive. For example, in (Bocanegra et al., 2015), the production of non-action verbs was not tested, and Colman et al. (2009) found that in sentence context, verb production deficits are related to executive disfunction in patients with PD. In (Fernandino et al., 2013), the absolute differences in patients’ accuracy in the semantic similarity judgment task were small both between the action and abstract verb groups (M = 95.5% versus 97.5%, respectively), and compared to neurologically intact individuals (M = 96.7% for action and 96.9% for abstract verbs). The reaction times, on the other hand, were equally delayed in PD patients for both verb groups in comparison to neurologically intact individuals. Kemmerer, Miller, MacPherson, Huber, and Tranel (2013) obtained similar results: the difference in semantic similarity judgements was observed not between action and non-action verbs, but between the reaction times in PD and neurologically intact groups. These results are not easily interpretable within the embodied cognition framework. Overall, the role of the basal ganglia and corticosubcortical networks in naming and verb and action processing is yet to be studied systematically, but it should not be overlooked.
4.4. Limitations and further directions
Our study has several methodological and conceptual limitations. From the methodological point of view, it could benefit from a larger patient sample with more various lesion locations, for example with better coverage of the temporal and parietal areas. Unfortunately, incomplete coverage did not allow us to test existing neuroanatomical picture naming (Indefrey and Levelt, 2004; Indefrey, 2011; Roelofs, 2014) and semantic processing (Binder and Desai, 2011; Gainotti, 2011; Lambon Ralph et al., 2017; Pulvermüller, 2013) accounts that attribute specific functions to different regions in these posterior areas. The analysis of white matter involvement could be strengthened by using diffusion data, which is a standard non-invasive technique for studying structural connectivity of the brain.
Conceptually, the study only focused on the neural bases of oral action naming. Hence, we cannot claim that our results are specific to action naming and not related to lexical retrieval in general, and that they would hold in the written modality. Finding dissociations between neural substrates of oral and written action and object naming using VLSM would be an informative addition to the field of lexical retrieval studies.
This particular experiment could not pinpoint the contribution of semantic control to patients’ performance. For instance, the materials were not controlled for cognitive load (e.g. the number of lexical competitors), and features of deficient semantic control (consistency of the deficit, accompanying executive dysfunction etc.; Jefferies, 2013) were not measured. Additional experiments that use materials employing action semantics in tasks where semantic control requirements are directly manipulated, measuring consistency of the deficit, and additionally assessing executive dysfunction could help disentangle deficits in action semantics representations and executive control over semantic processing.
More advanced methods could potentially provide more of a network approach that would extend beyond the current analysis of the affected anatomical structures. Several state-of-the-art techniques based on VLSM have recently been proposed, such as multimodal imaging (composite analysis of structural lesion data and diffusion or resting state functional MRI) or a combination of VLSM and normative connectome data (Karnath et al., 2018). Applying these techniques with behavioral data could shed more light on the functional organization of networks supporting action naming.
Finally, as with all studies, some of our findings could have several alternative interpretations. For instance, our data do not disentangle word/lemma retrieval and action semantics processing. Segregation of the suggested roles of discovered regions in controlled experiments, for example, by employing action word/concept comprehension tasks on par with production tasks, is a promising direction for further research.
Acknowledgements
We would like to thank our colleagues and collaborators for their invaluable contributions to this paper: Svetlana Malyutina for her helpful comments on the text, Anna Yurchenko for her assistance with lesion data processing, Roelien Bastiaanse for her suggestions regarding the revisions of the paper, and all those who assisted with behavioral data pre-processing (Anna Kotova, Tatiana Rylko, Anastasia Novikova, Julia Edeleva, Grigory Ignatyev, Valeriya Garkavaya, Maria Melnikova, Maria Grabovskaya, Viktoriya Silayeva, Anna Vechkaeva, and Olga Rudina). We also thank the anonymous reviewers for their constructive feedback that helped to improve this work immensely. Finally, we thank our research participants for taking part in our experiment and making this study possible.
Funding
The study has been funded by the Center for Language and Brain NRU Higher School of Economics, RF Government grant, ag. №14.641.31.0004. The contribution of NFD and MVI was also supported by NIH/NIDCD grant 1 R01 DC016345; NFD was additionally supported by a Department of Veterans Affairs CSR&D Research Career Scientist Award. The contribution of AUT was covered by a grant from the U.S. Department of Veterans Affairs, Office of Research and Development CSR &D Program. The contents reported within do not represent the views of the Department of Veterans Affairs or the United States Government.
Appendix A
Demographic, clinical, neuropsychological, and behavioral data of the patients
| ID | Age | Sex | Education, estimated years |
Post Onset, months |
Type of stroke | MRI localization | Neuropsychological diagnosis, speech |
Neuropsychological diagnosis, other higher cognitive functions |
QASA, total |
QASA - C | QASA - P | Severity | Action Naming Score |
Phonological Error Rate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PI | 55 | f | 13 | 100 | hemorrhagic | LH | efferent motor aphasia | dynamic manual apraxia | 269.5 | 138.5 | 131 | mild | .76 | 0 |
| P2 | 47 | f | 12.5 | 19 | ischemic | distribution of LMCA central and cortical branches | acoustic-mnestic aphasia | 251 | 135 | 116 | moderate- mild |
.82 | .01 | |
| P3 | 78 | m | 15 | 5 | hemorrhagic | LH | acoustic-mnestic aphasia | kinesthetic manual apraxia, dynamic manual apraxia | 174.5 | 85.5 | 89 | moderate- severe |
.76 | .04 |
| P4 | 40 | m | 13 | 26 | hemorrhagic | LH | spastic-paretic dysarthria | ND | ND | ND | .98 | 0 | ||
| P5 | 70 | f | 15 | 16 | ischemic | LMCA distribution | sensory aphasia, acoustic-mnestic aphasia; spastic-paretic dysarthria | dynamic manual apraxia | 181.5 | 99.5 | 82 | moderate | .7 | .01 |
| P6 | 40 | f | 15 | 12 | hemorrhagic | left temporal lobe | acoustic-mnestic aphasia | 288.5 | 143.5 | 145 | mild | .85 | .03 | |
| P 7 | 50 | m | 13 | 59 | ischemic | LMCA distribution | efferent motor aphasia, spastic- paretic dysarthria | dynamic manual apraxia | 270 | 149 | 121 | mild | .98 | .08 |
| P8 | 57 | f | 13 | 23 | ischemic | distribution of LMCA cortical branches | efferent-afferent motor aphasia | 291.5 | 150 | 141.5 | mild | .96 | .1 | |
| P9 | 43 | m | 15 | 10 | hemorrhagic | deep sections of left fronto- temporal region | spastic-paretic dysarthria | ND | ND | ND | .96 | 0 | ||
| P10 | 50 | m | 10 | 5 | ischemic | LMCA distribution | acoustic-mnestic aphasia | 269 | 142 | 127 | mild | .84 | 0 | |
| Pll | 39 | f | 13 | 48 | ischemic | LMCA distribution | efferent motor aphasia, spastic- paretic dysarthria | 285 | 143 | 142 | mild | .96 | .01 | |
| P12 | 48 | m | 15 | 49 | ischemic | LMCA distribution | efferent-afferent motor aphasia | dynamic manual apraxia | 225.5 | 146.5 | 79 | moderate- mild |
.97 | .19 |
| P13 | 59 | f | 15 | 36 | hemorrhagic | left temporal lobe | acoustic-mnestic aphasia, sensory aphasia | 283 | 143 | 140 | mild | .95 | 0 | |
| P14 | 66 | f | 10 | 36 | hemorrhagic | left fronto-parietal region | efferent motor aphasia, spastic- paretic dysarthria | dynamic manual apraxia, constructive apraxia; domain-general memory impairment | 278 | 147.5 | 130.5 | mild | .95 | .01 |
| P15 | 50 | m | 15 | 51 | ischemic | LMCA distribution, lacune in right frontal lobe | efferent motor aphasia, amnestic aphasia | dynamic manual apraxia | 236 | 135 | 101 | moderate- mild |
.94 | .04 |
| P16 | 63 | f | 10 | 17 | ischemic | LMCA distribution | efferent motor aphasia | 271.5 | 146 | 125.5 | mild | .92 | .06 | |
| PI 7 | 68 | f | 15 | 3 | ischemic | distribution of the LMCA cortical branches, lacune in left frontal lobe | sensory aphasia | constructive apraxia, dynamic manual apraxia | 242.5 | 128.5 | 114 | moderate- mild |
.83 | 0 |
| P18 | 54 | f | 13 | 12 | hemorrhagic | left basal ganglia, changes in right parietal lobe | acoustic-mnestic aphasia, dynamic aphasia | 264 | 138.5 | 125.5 | mild | .86 | .01 | |
| P19 | 50 | m | 13 | 29 | ischemic | LMCA distribution | efferent motor aphasia, spastic-rigid dysarthria | 232 | 130 | 102 | moderate- mild |
.64 | .13 | |
| P20 | 41 | f | 15 | 28 | ischemic | LMCA distribution | acoustic-mnestic aphasia, efferent motor aphasia | dynamic manual apraxia | 264.5 | 143 | 121.5 | mild | .84 | 0 |
| P21 | 67 | m | 15 | 10 | ischemic | LMCA distribution | efferent-afferent motor aphasia | dynamic manual apraxia | 209 | 121.5 | 87.5 | moderate | .62 | .04 |
| P22 | 42 | f | 15 | 14 | ischemic | LH | efferent motor aphasia | 297 | 147 | 150 | mild | .96 | 0 | |
| P23 | 51 | f | 15 | 52 | ischemic | LMCA distribution | dynamic aphasia, efferent-afferent motor aphasia | dynamic manual apraxia | 183 | 125 | 58 | moderate | .34 | 0 |
| P24 | 56 | m | 15 | 10 | hemorrhagic | left thalamus | subcortical aphasia, acoustic- mnestic type | 278 | 148 | 130 | mild | .97 | 0 | |
| P25 | 53 | m | 15 | 37 | hemorrhagic | left temporo-parieto-occipital region | sensory aphasia | mild attention impairment | 216.5 | 129.5 | 87 | moderate | .89 | 0 |
| P26 | 54 | m | 10 | 6 | ischemic | distribution of LMCA central branches | efferent-afferent motor aphasia | kinesthetic manual apraxia, do- main-general memory impairment | 217 | 131.5 | 85.5 | moderate | .71 | 0 |
| P27 | 73 | f | 15 | 4 | ischemic | LMCA distribution | acoustic-mnestic aphasia, sensory aphasia | 141.5 | 59 | 82.5 | moderate- severe |
.86 | .04 | |
| P28 | 50 | m | 13 | 26 | ischemic | LMCA distribution | sensory aphasia | 249.5 | 136 | 113.5 | moderate- mild |
.94 | 0 | |
| P29 | 44 | f | 15 | 9 | ischemic | left temporo-parietal region | efferent-afferent motor aphasia | dynamic manual apraxia | 231 | 142 | 89 | moderate- mild |
.68 | 0 |
| P30 | 33 | f | 15 | 26 | ischemic | lentiform nucleus, left frontal lobe | efferent motor aphasia | dynamic manual apraxia | 278.5 | 150 | 128.5 | mild | .95 | .04 |
| P31 | 34 | f | 15 | 7 | ischemic | left fronto-parieto-occipital resion | semantic aphasia | 242.5 | 124.5 | 118 | moderate- mild |
.95 | 0 | |
| P32 | 44 | m | 13 | 19 | ischemic | left temporo-parietal region | acoustic-mnestic aphasia, sensory aphasia | 234.5 | 114 | 120.5 | moderate- mild |
.91 | .01 | |
| P33 | 48 | m | 10 | 9 | ischemic | LMCA distribution | efferent-afferent motor aphasia, dynamic aphasia | dynamic manual apraxia | 189 | 103.5 | 85.5 | moderate | .42 | .22 |
| P34 | 68 | f | 10 | 15 | ischemic | left fronto-temporal region | efferent-afferent motor aphasia | dynamic manual apraxia | 255 | 138 | 117 | moderate- mild |
.78 | .05 |
| P35 | 55 | m | 13 | 5 | hemorrhagic | left fronto-temporal region | sensory aphasia; spastic-paretic dysarthria | 187.5 | 100 | 87.5 | moderate | .94 | .03 | |
| P36 | 44 | m | 13 | 6 | ischemic | left temporo-parietal region | sensory aphasia | 196 | 110 | 86 | moderate | .76 | .07 | |
| P37 | 40 | f | 13 | 4 | hemorrhagic | left fronto-temporal region | efferent motor aphasia | dynamic manual apraxia, kinesthetic manual apraxia | 250 | 128 | 122 | moderate- mild |
.94 | .01 |
| P38 | 52 | f | 13 | 3 | ischemic | left fronto-parieto-temporal region | acoustic-mnestic aphasia, semantic aphasia | cognitive deficits | 244 | 123.5 | 120.5 | moderate- mild |
.79 | 0 |
| P39 | 53 | m | 10 | 3 | hemorrhagic | left putamen; lacune in left frontal lobe | sensory aphasia, acoustic-mnestic aphasia, spastic-paretic dysarthria | dynamic manual apraxia | 236.5 | 132.5 | 104 | moderate- mild |
.98 | 0 |
| P40 | 37 | m | 15 | 146 | ischemic | left fronto-temporal region | efferent-afferent motor aphasia, acoustic-mnestic aphasia; spastic- paretic dysarthria | 255.5 | 138.5 | 117 | moderate- mild |
.75 | 0 |
Note: education information in years was not available and was estimated according to typical education duration as follows: secondary school=10 years, secondary professional=13 years, incomplete higher=12.5 years, higher=15 years; lesion localization is derived from the radiologists’ clinical report; QASA – C=QASA – Comprehension subtest score; QASA – P=QASA – Production score; LH=left hemisphere; LMCA=left middle cerebral artery; ND=no data.
Appendix B
White-matter tracts (Rojkova et al., 2016) that intersected with the VLSM map
| White-matter tract | Probability of being affected |
|---|---|
| Anterior thalamic projections | 1 |
| Corpus callosum | 1 |
| Cortico-spinal tract | 1 |
| Frontal aslant tract | 1 |
| Frontal commissural fibers | 1 |
| Fronto-striatal projections | 1 |
| Inferior fronto-occipital fasciculus | 1 |
| Fronto-pontine projections | 1 |
| Superior londgitudinal fasciculus III | 1 |
| Uncinate | 1 |
| Superior londgitudinal fasciculus II | .99 |
| Frontal orbito-polar tract | .98 |
| Frontal inferior longitudinal tract | .96 |
| Anterior commissure | .9 |
| Fronto-insular tract 4 | .9 |
| Arcuate long segment | .82 |
| Fronto-insular tract 3 | .68 |
| Arcuate anterior segment | .64 |
| Cingulum | .64 |
| Fronto-insular tract 5 | .6 |
| Frontal superior longitudinal tract | .58 |
| Inferior longitudinal tract | .5 |
| Hand inferior U-shaped tract | .49 |
| Fornix | .34 |
| Fronto-insular tract 2 | .32 |
| Cingulum anterior | .3 |
| Face U-shaped tract | .27 |
| Superior londgitudinal fasciculus I | .22 |
| Fronto-insular tract 1 | .16 |
Note. All structures’ labels refer to the left hemisphere.
Footnotes
Declarations of interest
None.
References
- Akhutina T, 2016. Luria’s classification of aphasias and its theoretical basis. Aphasiology 30 (8), 878–897. 10.1080/02687038.2015.1070950. [DOI] [Google Scholar]
- Akinina Y, Malyutina S, Ivanova M, Iskra E, Mannova E, Dragoy O, 2015. Russian normative data for 375 action pictures and verbs. Behav. Res. Methods 47 (3), 691–707. 10.3758/s13428-014-0492-9. [DOI] [PubMed] [Google Scholar]
- Ardila A, 1999. The role of insula in language: an unsettled question. Aphasiology 13(1), 79–87. 10.1080/026870399402334. [DOI] [Google Scholar]
- Arévalo AL, Baldo JV, Dronkers NF, 2012. What do brain lesions tell us about theories of embodied semantics and the human mirror neuron system? Cortex 48 (2), 242–254. 10.1016/j.cortex.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Paré-blagoev EJ, Insler RZ, Wagner AD, 2005. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron 47, 907–918. 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Bak TH, 2013. The neuroscience of action semantics in neurodegenerative brain diseases. Curr. Opin. Neurol 26 (6), 671–677. 10.1097/WCO.0000000000000039. [DOI] [PubMed] [Google Scholar]
- Bak TH, Chandran S, 2012. What wires together dies together: verbs, actions and neurodegeneration in motor neuron disease. Cortex 48 (7), 936–944. 10.1016/j.cortex.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Bak TH, Hodges JR, 2004. The effects of motor neurone disease on language: further evidence. Brain Lang. 89 (2), 354–361. 10.1016/S0093-934X(03)00357-2. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Arévalo A, Patterson JP, Dronkers NF, 2013. Grey and white matter correlates of picture naming: evidence from a voxel-based lesion analysis of the Boston Naming Test. Cortex 49 (3), 658–667. 10.1016/j.cortex.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo JV, Wilson SM, Dronkers NF, 2012. Uncovering the neural substrates of language: a voxel-based lesion symptom mapping approach In: Mostofsky D & Faust M (Ed.), The Handbook of the Neuropsychology of Language. Wiley-Blackwell, pp. 582–594. 10.1002/9781118432501.ch28. [DOI] [Google Scholar]
- Banerjee P, Leu K, Harris RJ, Cloughesy TF, Lai A, Nghiemphu PL, et al. , 2015. Association between lesion location and language function in adult glioma using voxel-based lesion-symptom mapping. Neuroimage: Clinic 9, 617–624. 10.1016/j.nicl.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF, 2003. Voxel-based lesion-symptom mapping. Nat. Neurosci 6 (5), 448–450. 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Bedny M, McGill M, Thompson-Schill SL, 2008. Semantic adaptation and competition during word comprehension. Cerebr. Cortex 18 (11), 2574–2585. 10.1093/cercor/bhn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello L, Gallucci M, Ph D, Carrabba G, Gaini SM, 2007. Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas. Neurosurgery 60 (1), 67–82. 10.1227/01.NEU.0000249206.58601.DE. [DOI] [PubMed] [Google Scholar]
- Bello L, Gambini A, Castellano A, Carrabba G, Acerbi F, Fava E, et al. , 2008. Motor and language DTI Fiber Tracking combined with intraoperative subcortical mapping for surgical removal of gliomas. Neuroimage 39 (1), 369–382. 10.1016/j.neuroimage.2007.08.031. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, 2011. The neurobiology of semantic memory. Trends Cognit. Sci. 15 (11), 527–536. 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Pillay SB, Humphries CJ, Gross WL, Graves WW, Book DS, 2016. Surface errors without semantic impairment in acquired dyslexia: a voxel-based lesion-symptom mapping study. Brain 139 (5), 1517–1526. 10.1093/brain/aww029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocanegra Y, García AM, Pineda D, Buriticá O, Villegas A, Lopera F, et al. , 2015. Syntax, action verbs, action semantics, and object semantics in Parkinson’s disease: dissociability, progression, and executive influences. Cortex 69, 237–254. 10.1016/j.cortex.2015.05.022. [DOI] [PubMed] [Google Scholar]
- Borovsky A, Saygin AP, Bates E, Dronkers N, 2007. Lesion correlates of conversational speech production deficits. Neuropsychologia 45 (11), 2525–2533. 10.1016/j.neuropsychologia.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenger V, Roy AC, Paulignan Y, Deprez V, Jeannerod M, Nazir TA, 2006. Cross-talk between language processes and overt motor behavior in the first 200 msec of processing. J. Cogn. Neurosci 18 (10), 1607–1615. 10.1162/jocn.2006.18.10.1607. [DOI] [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J, 2001. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage 14 (2), 486–500. 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- Budisavljevic S, Dell’Acqua F, Djordjilovic V, Miotto D, Motta R, Castiello U, 2017. The role of the frontal aslant tract and premotor connections in visually guided hand movements. Neuroimage 146, 419–428. 10.1016/j.neuroimage.2016.10.051. [DOI] [PubMed] [Google Scholar]
- Campana S, Caltagirone C, Marangolo P, 2015. Combining Voxel-based lesion-symptom mapping (VLSM) with A-tDCS language treatment: predicting outcome of recovery in nonfluent chronic aphasia. Brain Stimulation 8 (4), 769–776. 10.1016/j.brs.2015.01.413. [DOI] [PubMed] [Google Scholar]
- Campanella F, D’Agostini S, Skrap M, Shallice T, 2010. Naming manipulable objects: anatomy of a category specific effect in left temporal tumours. Neuropsychologia 48(6), 1583–1597. 10.1016/j.neuropsychologia.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Cappa SF, Perani D, 2003. The neural correlates of noun and verb processing. J. Neurolinguistics 16 (2–3), 183–189. 10.1016/S0911-6044(02)00013-1. [DOI] [Google Scholar]
- Cappa SF, Sandrini M, Rossini PM, Sosta K, Miniussi C, 2002. The role of the left frontal lobe in action naming. Neurology 59, 720–723. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Hillis AE, 1990. Where do semantic errors come from? Cortex 26 (1),95–122. 10.1016/S0010-9452(13)80077-9. [DOI] [PubMed] [Google Scholar]
- Cardillo ER, Aydelott J, Matthews PM, Devlin JT, 2004. Left inferior prefrontal cortex activity reflects inhibitory rather than facilitatory priming. J. Cogn. Neurosci 16 (9), 1552–1561. 10.1162/0898929042568523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona JF, Gershanik O, Gelormini-Lezama C, Houck AL, Cardona S, Kargieman L, et al. , 2013. Action-verb processing in Parkinson’s disease: new pathways for motor-language coupling. Brain Struct. Funct 218 (6), 1355–1373. 10.1007/s00429-013-0510-1. [DOI] [PubMed] [Google Scholar]
- Catani M, Dell’Acqua F, Vergani F, Malik F, Hodge H, Roy P, et al. , 2012. Short frontal lobe connections of the human brain. Cortex 48 (2), 273–291. 10.1016/j.cortex.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH, 2005. Perisylvian language networks of the human brain. Ann. Neurol 57 (1), 8–16. 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam MM, Jakobsen E, Malik F, Martersteck A, Wieneke C, et al. , 2013. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain 136 (8), 2619–2628. 10.1093/brain/awt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M, 2008. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44 (8), 1105–1132. 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Sandrini M, Schwarzbach J, 2010. State-dependent TMS reveals a hierarchical representation of observed acts in the temporal, parietal, and premotor cortices. Cerebr. Cortex 20 (9), 2252–2258. 10.1093/cercor/bhp291. [DOI] [PubMed] [Google Scholar]
- Cerliani L, Thomas RM, Jbabdi S, Siero JCW, Nanetti L, Crippa A, et al. , 2012. Probabilistic tractography recovers a rostrocaudal trajectory of connectivity variability in the human insular cortex. Hum. Brain Mapp. 33 (9), 2005–2034. 10.1002/hbm.21338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenery HJ, Angwin AJ, Copland DA, 2008. The basal ganglia circuits, dopamine, and ambiguous word processing: a neurobiological account of priming studies in Parkinson’s disease. J. Int. Neuropsychol. Soc 14 (3), 351–364. 10.1017/S1355617708080491. [DOI] [PubMed] [Google Scholar]
- Clerget E, Winderickx A, Fadiga L, Olivier E, 2009. Role of Broca’s area in encoding sequential human actions: a virtual lesion study. Neuroreport 20 (16) Retrieved from. https://journals.lww.com/neuroreport/Fulltext/2009/10280/Role_of_Broca_s_area_in_encoding_sequential_human.20.aspx. [DOI] [PubMed] [Google Scholar]
- Cloutman L, Gottesman R, Chaudhry P, Davis C, Kleinman T, Pawlak M, et al. , 2009. Where (in the brain) do semantic errors come from? Cortex 45 (5), 641–649. 10.1016/j.cortex.2008.05.013.Where. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman KSF, Koerts J, van Beilen M, Leenders KL, Post WJ, Bastiaanse R, 2009. The impact of executive functions on verb production in patients with Parkinson’s disease. Cortex 45 (8), 930–942. 10.1016/j.cortex.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Crepaldi D, Berlingeri M, Cattinelli I, Borghese NA, Luzzatti C, Paulesu E, 2013. Clustering the lexicon in the brain: a meta-analysis of the neurofunctional evidence on noun and verb processing. Front. Hum. Neurosci 7, 1–15. 10.3389/fnhum.2013.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepaldi D, Berlingeri M, Paulesu E, Luzzatti C, 2011. A place for nouns and a place for verbs? A critical review of neurocognitive data on grammatical-class effects. Brain Lang. 116 (1), 33–49. 10.1016/j.bandl.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Crosson B, Benefield H, Cato MA, Sadek JR, Moore AB, Wierenga CE, et al. , 2003. Left and right basal ganglia and frontal activity during language generation: contributions to lexical, semantic, and phonological processes. J. Int. Neuropsychol. Soc.: JINS 9 (7), 1061–1077. 10.1017/S135561770397010X. [DOI] [PubMed] [Google Scholar]
- Crosson B, McGregor K, Gopinath KS, Conway TW, Benjamin M, Chang Y-L,et al. , 2007. Functional MRI of language in aphasia: a review of the literature and the methodological challenges. Neuropsychol. Rev 17 (2), 157–177. 10.1007/s11065-007-9024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, 1993. Nouns and verbs are retrieved with differently distributed neural systems. Proceedings of the National Academy of Sciences of the United States of America 90 (11), 4957–4960. 10.1073/pnas.90.11.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele A, Giustolisi L, Silveri MC, Colosimo C, Gainotti G, 1994. Evidence for a possible neuroanatomical basis for lexical processing of nouns and verbs. Neuropsychologia 32 (11), 1325–1341. 10.1016/0028-3932(94)00066-2. [DOI] [PubMed] [Google Scholar]
- De Zubicaray GI, Rose SE, McMahon KL, 2011. The structure and connectivity of semantic memory in the healthy older adult brain. Neuroimage 54 (2), 1488–1494. 10.1016/j.neuroimage.2010.08.058. [DOI] [PubMed] [Google Scholar]
- DeLeon J, Gottesman RF, Kleinman JT, Newhart M, Davis C, Heidler-Gary J,et al. , 2007. Neural regions essential for distinct cognitive processes underlying picture naming. Brain 130 (5), 1408–1422. 10.1093/brain/awm011. [DOI] [PubMed] [Google Scholar]
- Dell GS, 1986. A spreading-activation theory of retrieval in sentence production. Psychol. Rev 93 (3), 283–321. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/3749399. [PubMed] [Google Scholar]
- Dell GS, O’Seaghdha PG, 1991. Mediated and convergent lexical priming in language production: a comment on Levelt et al. (1991). Psychol. Rev 98 (4), 604–614. 10.1037/0033-295X.98.4.604. [DOI] [PubMed] [Google Scholar]
- Dell GS, Schwartz MF, Nozari N, Faseyitan O, Branch Coslett H, 2013. Voxel-based lesion-parameter mapping: identifying the neural correlates of a computational model of word production. Cognition 128 (3), 380–396. 10.1016/j.cognition.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF, 1996. A new brain region for coordinating speech articulation. Nature 384, 159 Retrieved from. 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD Jr., Redfern BB, Jaeger JJ, 2004. Lesion analysis of the brain areas involved in language comprehension. Cognition 92 (1–2), 145–177. 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio-Mazoyer N, Capelle L, 2005. New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain 128 (4), 797–810. 10.1093/brain/awh423. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Moritz-Gasser S, Mandonnet E, 2009. Is the left uncinate fasciculus essential for language?: a cerebral stimulation study. J. Neurol 256 (3), 382–389. 10.1007/s00415-009-0053-9. [DOI] [PubMed] [Google Scholar]
- Duffau H, Herbet G, Moritz-Gasser S, 2013. Toward a pluri-component, multimodal, and dynamic organization of the ventral semantic stream in humans: lessons from stimulation mapping in awake patients. Front. Syst. Neurosci 7, 44 10.3389/fnsys.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H, Moritz-Gasser S, Mandonnet E, 2014. A re-examination of neural basis of language processing: proposal of a dynamic hodotopical model from data provided by brain stimulation mapping during picture naming. Brain Lang. 131, 1–10. 10.1016/j.bandl.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Fama ME, Hayward W, Snider SF, Friedman RB, Turkeltaub PE, 2017. Subjective experience of inner speech in aphasia: preliminary behavioral relationships and neural correlates. Brain Lang. 164, 32–42. 10.1016/j.bandl.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandino L, Conant LL, Binder JR, Blindauer K, Hiner B, Spangler K, Desai RH, 2013. Parkinson’s disease disrupts both automatic and controlled processing of action verbs. Brain Lang. 127 (1), 65–74. 10.1016/j.bandl.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulon C, Cerliani L, Kinkingnéhun S, Levy R, Rosso C, Urbanski M, et al. , 2018. Advanced lesion symptom mapping analyses and implementation as BCBtoolkit. GigaScience 7 (3) giy004–giy004. Retrieved from. 10.1093/gigascience/giy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foygel D, Dell GS, 2000. Models of impaired lexical access in speech production. J.Mem. Lang 43 (2), 182–216. 10.1006/jmla.2000.2716. [DOI] [Google Scholar]
- Fujii M, Maesawa S, Motomura K, Futamura M, Hayashi Y, Koba I, Wakabayashi T, 2015. Intraoperative subcortical mapping of a language-associated deep frontal tract connecting the superior frontal gyrus to Broca’s area in the dominant hemisphere of patients with glioma. J. Neurosurg 122 (6), 1390–1396. https://doi.org/10.317-014.10.JNS14945. [DOI] [PubMed] [Google Scholar]
- Gainotti G, 2011. The organization and dissolution of semantic-conceptual knowledge: is the “amodal hub” the only plausible model? Brain Cogn. 75 (3), 299–309. 10.1016/j.bandc.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Gil-Robles S, Carvallo A, Jimenez MDM, Gomez Caicoya A, Martinez R, Ruiz-Ocaña C, Duffau H, 2013. Double dissociation between visual recognition and picture naming: a study of the visual language connectivity using tractography and brain stimulation. Neurosurgery 72 (4), 678–686. 10.1227/NEU.0b013e318282a361. [DOI] [PubMed] [Google Scholar]
- Gil-Robles S, Gatignol P, Capelle L, Mitchell M, Duffau H, 2005. The role of dominant striatum in language: a study using intraoperative electrical stimulations. J. Neurol. Neurosurg. Psychiatry 76 (7), 940–946. 10.1136/jnnp.2004.045948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg G, Randerath J, 2015. Shared neural substrates of apraxia and aphasia. Neuropsychologia 75, 40–49. 10.1016/j.neuropsychologia.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Grossman M, Anderson C, Khan A, Avants B, Elman L, McCluskey L, 2008. Impaired action knowledge in amyotrophic lateral sclerosis. Neurology 71 (18), 1396–1401. 10.1212/01.wnl.0000319701.50168.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D, 2007. The cortical organization of speech processing. Nat. Rev. Neurosci. 8 (5), 393–402. 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Rapp BC, Caramazza A, 1999. When a rose is a rose in speech but a tulip in writing. Cortex 35 (3), 337–356. 10.1016/S0010-9452(08)70804-9. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Rapp B, Romani C, Caramazza A, 1990. Selective impairment of semantics in lexical processing. Cogn. Neuropsychol 7 (3), 191–243. 10.1080/02643299008253442. [DOI] [Google Scholar]
- Hillis AE, Wityk RJ, Barker PB, Caramazza A, 2002. Neural regions essential for writing verbs. Nat. Neurosci 6, 19 Retrieved from. 10.1038/nn982. [DOI] [PubMed] [Google Scholar]
- Hirshorn EA, Thompson-Schill SL, 2006. Role of the left inferior frontal gyrus in covert word retrieval: neural correlates of switching during verbal fluency. Neuropsychologia 44 (12), 2547–2557. 10.1016/j.neuropsychologia.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Howard D, Gatehouse C, 2006. Distinguishing semantic and lexical word retrieval deficits in people with aphasia. Aphasiology 20 (9), 921–950. 10.1080/02687030600782679. [DOI] [Google Scholar]
- Indefrey P, 2011. The spatial and temporal signatures of word production components. Front. Psychol 2 (255), 1–16. 10.1016/j.cognition.2002.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJM, 2004. The spatial and temporal signatures of word production components. Cognition 92 (1–2), 101–144. 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Ivanova MV, Dragoy OV, Kuptsova SV, Yu Akinina, S., Petrushevskii AG, Fedina ON, et al. , 2018. Neural mechanisms of two different verbal working memory tasks: a VLSM study. Neuropsychologia 115, 25–41. 10.1016/j.neuropsychologia.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies E, 2013. The neural basis of semantic cognition: converging evidence from neuropsychology, neuroimaging and TMS. Cortex 49 (3), 611–625. 10.1016/j.cortex.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Jonkers R, Bastiaanse R, 1996. The influence of instrumentality and transitivity on action naming in Broca’s and anomic aphasia. Brain Lang. 55, 50–53. [DOI] [PubMed] [Google Scholar]
- Kan IP, Thompson-Schill SL, 2004a. Effect of name agreement on prefrontal activity during overt and covert picture naming. Cognit. Affect Behav. Neurosci 4 (1), 43–57. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/15259888. [DOI] [PubMed] [Google Scholar]
- Kan IP, Thompson-Schill SL, 2004b. Selection from perceptual and conceptual representations. Cognit. Affect Behav. Neurosci 4 (4), 466–482. 10.3758/CABN.4.4.466. [DOI] [PubMed] [Google Scholar]
- Karnath H, Sperber C, Rorden C, 2018. Mapping human brain lesions and their functional consequences. Neuroimage 165, 180–189. 10.1016/J.NEUROIMAGE.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmerer D, 2015a. Are the motor features of verb meanings represented in the pre-central motor cortices? Yes, but within the context of a flexible, multilevel architecture for conceptual knowledge. Psychonomic Bull. Rev 22 (4), 1068–1075. 10.3758/s13423-014-0784-1. [DOI] [PubMed] [Google Scholar]
- Kemmerer D, 2015b. Visual and motor features of the meanings of action verbs: a cognitive neuroscience perspective In: de Almeida RG, Manouilidou C (Eds.), Cognitive Science Perspectives on Verb Representation and Processing. Springer International Publishing, Cham, pp. 189–212. 10.1007/978-3-319-10112-5_9. [DOI] [Google Scholar]
- Kemmerer D, Miller L, MacPherson M, Huber J, Tranel D, 2013. An investigation of semantic similarity judgments about action and non-action verbs in Parkinson’s disease: implications for the Embodied Cognition Framework. Front. Hum. Neurosci 7, 146 10.3389/fnhum.2013.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmerer D, Rudrauf D, Manzel K, Tranel D, 2012. Behavioral patterns and lesion sites associated with impaired processing of lexical and conceptual knowledge of actions. Cortex 48 (7), 826–848. Behavioral. 10.1016/j.cortex.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, 1982. Western Aphasia Battery. Grune & Stratton, New York. [Google Scholar]
- Ketteler D, Kastrau F, Vohn R, Huber W, 2008. The subcortical role of language processing. High level linguistic features such as ambiguity-resolution and the human brain; an fMRI study. Neuroimage 39 (4), 2002–2009. 10.1016/j.neuroimage.2007.10.023.. [DOI] [PubMed] [Google Scholar]
- Ketteler S, Ketteler D, Vohn R, Kastrau F, Schulz JB, Reetz K, Huber W, 2014. The processing of lexical ambiguity in healthy ageing and Parkinson׳s disease: role of cortico-subcortical networks. Brain Res. 1581, 51–63. 10.1016/j.brainres.2014.06.030. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Coslett HB, Schwartz MF, 2007. Power in Voxel-based lesion-symptom mapping. J. Cogn. Neurosci 19 (7), 1067–1080. 10.1162/jocn.2007.19.7.1067. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, de Champfleur NM, Deverdun J, Moritz-Gasser S, Herbet G, Duffau H, 2015. Role of fronto-striatal tract and frontal aslant tract in movement and speech: an axonal mapping study. Brain Struct. Funct 220 (6), 3399–3412. 10.1007/s00429-014-0863-0. [DOI] [PubMed] [Google Scholar]
- Krieger-Redwood K, Jefferies E, 2014. TMS interferes with lexical-semantic retrieval in left inferior frontal gyrus and posterior middle temporal gyrus: evidence from cyclical picture naming. Neuropsychologia 64, 24–32. 10.1016/j.neuropsychologia.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Kronfeld-Duenias V, Amir O, Ezrati-Vinacour R, Civier O, Ben-Shachar M, 2016. The frontal aslant tract underlies speech fluency in persistent developmental stuttering. Brain Struct. Funct 221 (1), 365–381. 10.1007/s00429-014-0912-8. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Patterson K, 2008. Generalization and differentiation in semantic memory. Ann. N. Y. Acad. Sci 1124, 61–76. 10.1196/annals.1440.006. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Jefferies E, Patterson K, Rogers T, 2017. The neural and computational bases of semantic cognition. Nat. Rev. Neurosci 18 (1), 42–55. 2017. 10.1038/nrn.2016.150. [DOI] [PubMed] [Google Scholar]
- Levelt WJM, 1983. Monitoring and self-repair in speech. Cognition 14 (1), 41–104. 10.1016/0010-0277(83)90026-4. [DOI] [PubMed] [Google Scholar]
- Levelt WJM, 1989. Speaking: from Intention to Articulation. MIT Press, Cambridge,Mass. [Google Scholar]
- Levelt WJM, 1992. Accessing words in speech production: stages, processes and representations. Cognition 42 (1), 1–22. 10.1016/0010-0277(92)90038-J. [DOI] [PubMed] [Google Scholar]
- Levelt WJ, Roelofs a, Meyer a S., 1999. A theory of lexical access in speech production. Behav. Brain Sci. 22 (1), 1–38. discussion 38–75. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/11301520. [DOI] [PubMed] [Google Scholar]
- Longworth CE, Keenan SE, Barker RA, Marslen-Wilson WD, Tyler LK, 2005. The basal ganglia and rule-governed language use: evidence from vascular and degenerative conditions. Brain 128 (3), 584–596. 10.1093/brain/awh387. [DOI] [PubMed] [Google Scholar]
- Luria AR, 1966. Higher Cortical Functions in Man B. Haigh, Trans. Basic Books, New York, NY: (Original work published 1962). [Google Scholar]
- Lyashevskaya ON, Sharov SA, 2009. Chastotnyj Slovar’sovremennogo Russkogo Jazyka (Na Materialakh Natsional’nogokorpusa Russkogo Jazyka) [Frequency Vocabulary of ModernRussian (Based on the Materials of Russian National Corpus]. Azbukovnik, Moscow, Russia: Retrieved from. http://dict.ruslang.ru/freq.php. [Google Scholar]
- Maieron M, Marin D, Fabbro F, Skrap M, 2013. Seeking a bridge between language and motor cortices: a PPI study. Front. Hum. Neurosci 7, 1–20. 10.3389/fnhum.2013.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS,Pandya DN, 2005. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cerebr. Cortex 15 (6), 854–869. 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Malyutina S, den Ouden D-B, 2017. Task-dependent neural and behavioral effects of verb argument structure features. Brain Lang. 168, 57–72. 10.1016/j.bandl.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Malyutina S, Dragoy O, Ivanova M, Laurinavichyute A, Petrushevsky A, Meindl T, 2016. Fishing is not wrestling: neural underpinnings of the verb instrumentality effect. J. Neurolinguistics 40, 37–54. 10.1016/j.jneuroling.2016.03.002. [DOI] [Google Scholar]
- Malyutina S, Iskra E, Sevan D, Dragoy O, 2014. The effects of instrumentality and name relation on action naming in Russian speakers with aphasia. Aphasiology 28(10), 1178–1197. 10.1080/02687038.2014.910589. [DOI] [Google Scholar]
- Mandonnet E, Nouet A, Gatignol P, Capelle L, Duffau H, 2007. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain 130(3), 623–629. 10.1093/brain/awl361. [DOI] [PubMed] [Google Scholar]
- Mätzig S, Druks J, Masterson J, Vigliocco G, 2009. Noun and verb differences in picture naming: past studies and new evidence. Cortex 45 (6), 738–758. 10.1016/j.cortex.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Meteyard L, Cuadrado SR, Bahrami B, Vigliocco G, 2012. Coming of age: a review of embodiment and the neuroscience of semantics. Cortex 48 (7), 788–804. 10.1016/j.cortex.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Mirman D, Britt AE, 2014. What we talk about when we talk about access deficits. Phil. Trans. Biol. Sci 369 (1634), 20120388 10.1098/rstb.2012.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Chen Q, Zhang Y, Wang Z, Faseyitan OK, Coslett HB, Schwartz MF, 2015a. Neural organization of spoken language revealed by lesion–symptom mapping. Nat. Commun 6, 6762 10.1038/ncomms7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Graziano KM, 2013. The neural basis of inhibitory effects of semantic and phonological neighbors in spoken word production. J. Cogn. Neurosci 25 (9), 1504–1516. 10.1162/jocn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Zhang Y, Wang Z, Coslett HB, Schwartz MF, 2015b. The ins and outs of meaning: behavioral and neuroanatomical dissociation of semantically-driven word retrieval and multimodal semantic recognition in aphasia. Neuropsychologia 76, 208–219. 10.1016/j.neuropsychologia.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppeney U, Phillips J, Price C, 2004. The neural areas that control the retrieval and selection of semantics. Neuropsychologia 42 (9), 1269–1280. 10.1016/j.neuropsychologia.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Novick JM, Kan IP, Thompson-Schill SL, 2009. A case for conflict across multiple domains: memory and language impairments following damage to ventrolateral prefrontal cortex. Cogn. Neuropsychol 26 (6), 527–567. 10.1080/02643290903519367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, Thompson-Schill SL, 2005. Cognitive control and parsing: reexamining the role of Broca’s area in sentence comprehension. Cognit. Affect Behav. Neurosci 5 (3), 263–281. Retrieved from. http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2005-99024-262&site=ehost-live. [DOI] [PubMed] [Google Scholar]
- Oh A, Duerden EG, Pang EW, 2014. The role of the insula in speech and language processing. Brain Lang. 135, 96–103. 10.1016/j.bandl.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papeo L, Negri GAL, Zadini A, Rumiati RI, 2010. Action performance and action-word understanding: evidence of double dissociations in left-damaged patients. Cogn. Neuropsychol 27 (5), 428–461. 10.1080/02643294.2011.570326. [DOI] [PubMed] [Google Scholar]
- Parlatini V, Radua J, Dell’Acqua F, Leslie A, Simmons A, Murphy DG, et al. , 2017. Functional segregation and integration within fronto-parietal networks. Neuroimage 146, 367–375. 10.1016/j.neuroimage.2016.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT, 2007. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat. Rev. Neurosci 8 (12), 976–987. 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Pillay SB, Binder JR, Humphries C, Gross WL, Book DS, 2017. Lesion localization of speech comprehension deficits in chronic aphasia. Neurology 88 (10), 970–975. 10.1212/WNL.0000000000003683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay SB, Stengel BC, Humphries C, Book DS, Binder JR, 2014. Cerebral localization of impaired phonological retrieval during rhyme judgment. Ann. Neurol 76(5), 738–746. 10.1002/ana.24266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras F, Marangolo P, 2007. Noun-verb naming in aphasia: a voxel-based lesion-symptom mapping study. Neuroreport 18 (14), 1455–1458. 10.1097/WNR.0b013e3282ef6fc9. [DOI] [PubMed] [Google Scholar]
- Price CJ, 2012. A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage 62 (2), 816–847. 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermüller F, 2013. How neurons make meaning: brain mechanisms for embodied and abstract-symbolic semantics. Trends Cognit. Sci 17 (9), 458–470. 10.1016/j.tics.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Hauk O, Nikulin VV, Ilmoniemi RJ, 2005. Functional links between motor and language systems. Eur. J. Neurosci 21 (3), 793–797. 10.1111/j.1460-9568.2005.03900.x. [DOI] [PubMed] [Google Scholar]
- Radanovic M, Mansur LL, 2017. Aphasia in Vascular Lesions of the Basal Ganglia: A Comprehensive Review. Brain and Language. Elsevier Inc. 10.1016/j.bandl.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Rapp B, Benzing L, Caramazza A, 1997. The autonomy of lexical orthography. Cogn. Neuropsychol 14 (1), 71–104. 10.1080/026432997381628. [DOI] [Google Scholar]
- Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, Obeso JA, 2009. Initial clinical manifestations of Parkinson’s disease: features and patho-physiological mechanisms. Lancet Neurol. 8 (12), 1128–1139. 10.1016/S1474-4422(09)70293-5. [DOI] [PubMed] [Google Scholar]
- Rofes A, Miceli G, 2014. Language mapping with verbs and sentences in awake surgery: a review. Neuropsychol. Rev 24, 185–199. 10.1007/s11065-014-9258-5. [DOI] [PubMed] [Google Scholar]
- Roelofs A, 2014. ScienceDirect A dorsal-pathway account of aphasic language production: the WEAVER ++/ARC model. Cortex 59, 33–48. 1874. 10.1016/j.cortex.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Patterson K, Jefferies E, Lambon Ralph MA, 2015. Disorders of representation and control in semantic cognition: effects of familiarity, typicality, and specificity. Neuropsychologia 76, 220–239. 10.1016/j.neuropsychologia.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojkova K, Volle E, Urbanski M, Humbert F, Dell’Acqua F, Thiebaut de Schotten M, 2016. Atlasing the frontal lobe connections and their variability due to age and education: a spherical deconvolution tractography study. Brain Struct. Funct 221 (3), 1751–1766. 10.1007/s00429-015-1001-3. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M, 2000. Stereotaxic display of brain lesions. Behav. Neurol 12 (4),191–200. 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry M-S, et al. , 2008. Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences of the United States of America 105 (46), 18035–18040. 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin AP, Wilson SM, Dronkers NF, Bates E, 2004. Action comprehension in aphasia: linguistic and non-linguistic deficits and their lesion correlates. Neuropsychologia 42 (13), 1788–1804. 10.1016/j.neuropsychologia.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Schnur TT, Schwartz MF, Kimberg DY, Hirshorn E, Coslett HB, Thompson-Schill SL, 2009. Localizing interference during naming: convergent neuroimaging and neuropsychological evidence for the function of Broca’s area. Proc. Natl. Acad. Sci. Unit. States Am 106 (1), 322–327. 10.1073/pnas.0805874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmann T, Schiller NO, Goebel R, Sack AT, 2012. Speaking of which: dissecting the neurocognitive network of language production in picture naming. Cerebr. Cortex 22 (3), 701–709. 10.1093/cercor/bhr155. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS,Coslett HB, 2009. Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia. Brain 132 (12), 3411–3427. 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierpowska J, Gabarrós A, Fernandez-Coello A, Camins A, Castañer S, Juncadella M, et al. , 2015. Morphological derivation overflow as a result of disruption of the left frontal aslant white matter tract. Brain Lang. 142, 54–64. 10.1016/j.bandl.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Snyder HR, Feigenson K, Thompson-Schill SL, 2007. Prefrontal cortical response to conflict during semantic and phonological tasks. J. Cogn. Neurosci 19 (5), 761–775. 10.1162/jocn.2007.19.5.761. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell’Acqua F, Valabregue R, Catani M, 2012. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex 48 (1), 82–96. 10.1016/j.cortex.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Bonakdarpour B, Fix SC, Blumenfeld HK, Parrish TB, Gitelman DR, Mesulam M-M, 2007. Neural correlates of verb argument structure processing. J. Cogn. Neurosci 19 (11), 1753–1767. 10.1162/jocn.2007.19.11.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Bedny M, Goldberg RF, 2005. The frontal lobes and the regulation of mental activity. Curr. Opin. Neurobiol 15 (2), 219–224. 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ, 1997. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences of the United States of America 94(26), 14792–14797. Retrieved from. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC25116/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Kan IP, 1999. Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron 23 (3), 513–522. 10.1016/S0896-6273(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D’Esposito M, Kan IP, Knight RT, 1998. Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proceedings of the National Academy of Sciences of the United States of America 95 (26), 15855–15860. Retrieved from. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC28134/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasino B, Ceschia M, Fabbro F, Skrap M, 2012. Motor simulation during action word processing in neurosurgical patients. J. Cogn. Neurosci 24 (3), 736–748. 10.1162/jocn_a_00168. [DOI] [PubMed] [Google Scholar]
- Tomasino B, Fink GR, Sparing R, Dafotakis M, Weiss PH, 2008. Action verbs and the primary motor cortex: a comparative TMS study of silent reading, frequency judgments, and motor imagery. Neuropsychologia 46 (7), 1915–1926. 10.1016/j.neuropsychologia.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Tranel D, Adolphs R, Damasio H, Damasio AR, 2001. A neural basis for the retrieval of words for actions. Cogn. Neuropsychol 18 (7), 655–674. 10.1080/02643290126377. [DOI] [PubMed] [Google Scholar]
- Tranel D, Manzel K, Asp E, Kemmerer D, 2008. Naming dynamic and static actions: neuropsychological evidence. J. Physiol. Paris 102 (1–3), 80–94. 10.1016/j.jphysparis.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Kemmerer D, Adolphs R, Damasio H, Damasio AR, 2003. Neural correlates of conceptual knowledge for actions. Cogn. Neuropsychol 20 (3–6), 409–432. 10.1080/02643290244000248. [DOI] [PubMed] [Google Scholar]
- Tsapkini K, Frangakis CE, Hillis AE, 2011. The function of the left anterior temporal pole: evidence from acute stroke and infarct volume. Brain 134 (10), 3094–3105. 10.1093/brain/awr050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkova LS, Akhutina TV, Pylaeva NM, 1981. Metodika Otsenki Rechi Pri Afazii [Method for Speech Evaluation in Aphasia]. Moscow University Press, Moscow. [Google Scholar]
- Ullman MT, 2006. Is Broca’s area part of a basal ganglia thalamocortical circuit? Cortex 42 (4), 480–485. 10.1016/S0010-9452(08)70382-4. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Candidi M, Avenanti A, 2014. Neuroanatomical substrates of action perception and understanding: an anatomic likelihood estimation meta-analysis of lesion-symptom mapping studies in brain injured patients. Front. Hum. Neurosci 8, 344 10.3389/fnhum.2014.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassal F, Boutet C, Lemaire JJ, Nuti C, 2014. New insights into the functional significance of the frontal aslant tract: an anatomo-functional study using intraoperative electrical stimulations combined with diffusion tensor imaging-based fiber tracking. Br. J. Neurosurg 28 (5), 685–687. 10.3109/02688697.2014.889810. [DOI] [PubMed] [Google Scholar]
- Vigliocco G, Vinson DP, Druks J, Barber H, Cappa SF, 2011. Nouns and verbs in the brain: a review of behavioural, electrophysiological, neuropsychological and imaging studies. Neurosci. Biobehav. Rev 35 (3), 407–426. 10.1016/j.neubiorev.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR, 2013. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain 136 (6), 1692–1707. 10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukovic N, Feurra M, Shpektor A, Myachykov A, Shtyrov Y, 2017. Primary motor cortex functionally contributes to language comprehension: an online rTMS study. Neuropsychologia 96, 222–229. 10.1016/j.neuropsychologia.2017.01.025. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Bjork RA, Schacter DL, 2001. Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral prefrontal cortex. Neuroimage 14 (6), 1337–1347. 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- Walker GM, Schwartz MF, Kimberg DY, Faseyitan O, Brecher A, Dell GS, Coslett HB, 2011. Support for anterior temporal involvement in semantic error production in aphasia: new evidence from VLSM. Brain Lang. 117 (3), 110–122. 10.1016/j.bandl.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Jenkinson N, 2016. The anatomy of the basal ganglia In: Hickok G, Small SL (Eds.), Neurobiology of Language. Academic Press, San Diego, pp. 85–94. 10.1016/B978-0-12-407794-2.00008-0. [DOI] [Google Scholar]
- Watson CE, Cardillo ER, Ianni GR, Chatterjee A, 2013. Action concepts in the brain: an activation likelihood estimation meta-analysis. J. Cogn. Neurosci 25 (8), 1191–1205. 10.1162/jocn. [DOI] [PubMed] [Google Scholar]
- Whitney C, Kirk M, O’Sullivan J, Lambon Ralph MA, Jefferies E, 2011. The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cerebr. Cortex 21 (5), 1066–1075. 10.1093/cercor/bhq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems RM, Labruna L, D’Esposito M, Ivry R, Casasanto D, 2011. A functional role for the motor system in language understanding: evidence from theta-burst transcranial magnetic stimulation. Psychol. Sci 22 (7), 849–854. 10.1177/0956797611412387. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, et al. , 2010. Connected speech production in three variants of primary progressive aphasia. Brain 133 (7), 2069–2088. 10.1093/brain/awq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York C, Olm C, Boller A, McCluskey L, Elman L, Haley J, et al. , 2014. Action verb comprehension in amyotrophic lateral sclerosis and Parkinson’s disease. J. Neurol 261 (6), 1073–1079. 10.1007/s00415-014-7314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Cody Hazlett H, Gimpel Smith R, Ho S, Gee JC, Gerig G, 2006. User-Guided {3D} Active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31 (3), 1116–1128. [DOI] [PubMed] [Google Scholar]