Abstract
Cre/loxP technology is an important tool for studying cell type-specific gene functions. Cre recombinase mouse lines, including Agc1-CreERT2, Col2a1-Cre; Col2a1-CreERT2, Shh-Cre, Shh-CreERT2, and Osx-Cre, have been proven to be valuable tools to elucidate the biology of long bones, yet the information for their activity in postnatal intervertebral disc (IVD) tissues was very limited. In this study, we used R26-mTmG fluorescent reporter to systematically analyze cell specificity and targeting efficiency of these six mouse lines in IVD tissues at postnatal growing and adult stages. We found that Agc1-CreERT2 is effective to direct recombination in all components of IVDs, including annulus fibrosus (AF), nucleus pulposus (NP), and cartilaginous endplate (CEP), upon tamoxifen induction at either 2 weeks or 2 months of ages. Moreover, Col2a1-Cre targets most of the cells in IVDs, except for some cells in the outer AF (OAF) and NP. In contrast, the activity of Col2a1-CreERT2 is mainly limited to the IAF of IVD tissues at either stage of tamoxifen injection. Similarly, Shh-Cre directs recombination specifically in all NP cells, whereas Shh-CreERT2 is active only in a few NP cells when tamoxifen is administered at either stage. Finally, Osx-Cre targets cells in the CEP, but not in the NP or AF of IVDs tissues at these two stages. Thus, our data demonstrated that all these Cre lines can direct recombination in IVD tissues at postnatal stages with different cell type specificity and/or targeting efficiency, and can, therefore, serve as valuable tools to dissect cell type-specific gene functions in IVD development and homeostasis.
Keywords: annulus fibrosus, Cre recombinase, intervertebral disc, nucleus pulposus
1 |. INTRODUCTION
Low back pain is a common musculoskeletal problem, with its lifetime incidence reaching as high as 84% (Risbud & Shapiro, 2014). As the leading cause of musculoskeletal disabilities, low back pain can bring devastating consequences to the patients, and add enormous socioeconomic burdens to the societies (Bedore et al., 2013; Wang et al., 2012). Although it is well accepted that low back pain is primarily caused by degeneration of intervertebral disc (IVD), no disease-modifying therapeutic approach is currently available, largely due to our limited understanding of the molecular mechanisms underlying IVD homeostasis and degeneration.
The mouse has emerged as a valuable animal model to study IVD biology, since IVDs in mouse and human have the similar structure, and exhibit similar age-related histological changes (Zhang et al., 2018). As a specialized structure located between two adjacent vertebral bodies, IVD has three major components in mice, including the nucleus pulposus (NP), the annulus fibrosus (AF), and the cartilaginous endplate (CEP) (Zheng et al., 2018). Each of these components contains distinct cell types that are believed to express specific matrix components, as well as signal molecules and growth factors to delicately regulate IVD development and homeostasis. Disruption of these regulations may cause IVD degeneration (Bedore, Quesnel, Quinonez, Seguin, & Leask, 2016). However, efforts to identify the contribution of the specific cell type to the disease development are hindered by the limited tools that can target specific cell type populations within the IVDs.
Cre/LoxP technology is an important genetic tool that enables gene deletion or overexpression in a cell type-specific and/or temporal-controlled manner, and has made significant contributions to the understanding of roles of various genes under both physiological and pathological conditions in mice (Henry et al., 2009; Lim, Burclaff, He, Mills, & Long, 2017). In this system, expression of Cre recombinase is driven by a cell type-specific promoter/enhancer, therefore allowing for cell type-specific targeting. Moreover, the temporal control can be achieved by the approach of tamoxifen-inducible Cre recombinase, commonly known as the CreERT2 system that is a fusion protein of Cre with a mutated ligand-binding domain of estrogen receptor (Leone et al., 2003). In this modified Cre system, CreERT2 fusion protein is translocated to the nucleus and subsequently directs recombination only upon tamoxifen induction (Hayashi & McMahon, 2002; Leone et al., 2003). In recent decades, a number of Cre and CreERT2 mouse lines have been generated for targeting osteoblast or chondrocyte lineage cells in long bones (Chen et al., 2007; Chen, Li, Xie, Wang, & Chen, 2014b; Harfe et al., 2004; Henry et al., 2009; Long, Zhang, Karp, Yang, & McMahon, 2001; Rodda & McMahon, 2006), and have been proven to be valuable tools to study the biology of bone and cartilage (Chen & Long, 2018; Long, 2011; Long & Ornitz, 2013). Although several studies indicated that at least some of these Cre lines also targeted the disc cells (Chen et al., 2007; Choi & Harfe, 2011; Henry et al., 2009), these tools have not been widely used to study IVD development and homeostasis, partly due to the lack of a detailed and systematic analysis of their targeting specificity and efficiency in IVDs tissues at postnatal stages.
In this study, we aimed to systematically analyze recombination directed by Agc1-CreERT2, Col2a1-Cre, Col2a1-CreERT2, Shh-Cre, Shh-CreERT2, and Osx-Cre mice, in IVDs at postnatal growing and adult stages, by employing R26-mTmG, a robust and sensitive dual fluorescent Cre reporter mouse. We found that all these Cre lines can mediate recombination in IVD cells at these two postnatal stages with different cell type specificity and/or targeting efficiency. Thus, our study has provided critical insights into the utilization of these genetic tools, either alone or in combination, to dissect the cellular and molecular mechanisms underlying IVD development and homeostasis.
2 |. MATERIALS AND METHODS
2.1 |. Mouse lines and breeding
Mouse lines used in this study, including Col2a1-Cre (Long et al., 2001), Col2a1-CreERT2 (Chen et al., 2007, Chen et al., 2014b), Osx-Cre (Rodda & McMahon, 2006), Agc1-CreERT2 (Henry et al., 2009), Shh-Cre (Harfe et al., 2004), Shh-CreERT2 (Harfe et al., 2004), and R26-mTmG mice (Muzumdar, Tasic, Miyamichi, Li, & Luo, 2007), were described previously. Col2a1-Cre, Col2a1-CreERT2, and Agc1-CreERT2 mice were provided by Dr. Di Chen (Rush University, Chicago). Osx-Cre and Shh-CreERT2 mice were gifts from Dr. Ting Chen (Beijing Institute of Biological Sciences, Beijing, China) and Dr. Xiaochun Bai (Southern Medical University, Guangzhou, China), respectively. Shh-Cre and R26-mTmG mice were purchased from Jackson Laboratories. To map Cre target cells in IVD tissues at postnatal stages, the above Cre mouse lines were separately crossed with R26-mTmG mice to get double heterozygous mice. For conventional Cre mouse lines (Col2a1-Cre, Shh-Cre, and Osx-Cre), the double heterozygous were killed at 2 weeks and 2 months of ages. For tamoxifen-inducible Cre mouse lines (Agc1-CreERT2, Col2a1-CreERT2, and Shh-CreERT2), the double heterozygous mice were subjected to tamoxifen or vehicle (corn oil) administration before analysis. All mice were housed in a specific pathogen-free facility with controlled temperature and humidity at a 12-hr day/night cycle. All mice had free access to standard chow diet and sterilized water, and were monitored daily for health status. No adverse events were observed throughout the experiments. All animal protocols in this study were reviewed and approved by the Animal Studies Committee at Soochow University and all experimental methods and procedures were carried out in accordance with the approved guidelines.
2.2 |. Tamoxifen administration
Tamoxifen (T5684; Sigma, St. Louis, MI) was dissolved in corn oil at a concentration of 10 mg/ml. Two-week-old or two-month-old double heterozygous mice (Col2a1-CreERT2; R26-mTmG, or Agc1-CreERT2; R26-mTmG, or Shh-CreERT2; R26-mTmG mice) were randomly divided into two groups (three mice per group for each genotype). Group I and II mice were injected intraperitoneally with tamoxifen (100 μg per gram of body weight) and corn oil once daily for 5 consecutive days, respectively. All these mice were harvested one day after the last injection.
2.3 |. Sample preparation
Cryojane sections were prepared as previously reported (Chen & Long, 2013; Chen et al., 2014a). Briefly, IVD samples were isolated and fixed in freshly made 4% paraformaldehyde overnight at 4°C. After three washes in PBS (5 min each), the fixed IVDs were decalcified in 14% EDTA for 3 days at 4°C on a rocker. After decalcification, IVDs were cryoprotected in 15% sucrose in PBS for 2 hr and 30% sucrose in PBS overnight at 4°C. Samples were then snap-frozen in OCT embedding medium. Frozen tissue blocks were sectioned at a thickness of 8 μm using a Leica CM3050S cryostat and the CryoJane Tape Transfer System (Leica, Buffalo Grove, IL). IVD sections were mounted onto adhesive coated slides (Leica), and stored at −20°C until they were analyzed.
2.4 |. Immunofluorescence staining
Immunofluorescence staining was performed to enhance GFP signals as described previously (Chen et al., 2014a). Briefly, frozen sections were incubated with 5% goat serum in PBS to prevent nonspecific binding and then incubated with a chicken polyclonal GFP antibody (1:2,500; Abcam, Cambridge, MA) in a humidified chamber overnight at 4°C. After three washes in PBST, sections were incubated with Alexa Fluor® 488-conjugated goat anti-chicken antibody (1:250; Life Technologies, Grand Island, NY) for 1 hr. Sections were mounted with VECTASHIELD Mounting Medium and imaged with a fluorescence microscope. The primary and secondary experimental outcomes were assessing IVD components that exhibited GFP signals, and the percentage of GFP-positive cells in these structures, respectively.
2.5 |. Statistical analysis
All quantitative results were shown as mean ± standard deviation. Student’s t test was used to determine the statistical difference between two groups. Any difference with a p value < 0.05 was considered as statistically significant. For each test, the experimental unit was a single mouse. The quantitative results were calculated from three independent mice for each group.
3 |. RESULTS
3.1 |. Agc1-CreERT2 is active in all cell types of postnatal IVDs
Agc1-CreERT2 (also known as aggrecan-CreERT2) is a knock-in mouse line expressing a tamoxifen-inducible Cre recombinase (CreERT2) from the endogenous aggrecan locus (Henry et al., 2009). Initial characterization of this mouse line using R26R reporter detected inducible expression of Cre recombinase in IVDs of 6-month-old mice (Henry et al., 2009). To further determine the cell type specificity and targeting efficiency of Agc1-CreERT2 in IVD tissues at postnatal growing and adult stages, we generated 2-week-old or 2-month-old Agc1-CreERT2; R26-mTmG mice and treated these mice with either corn oil or tamoxifen for 5 consecutive days (n =3 independent mice for each group). We harvested IVDs 1 day after the last treatment. GFP staining of Cryojane sections revealed abundant GFP-positive cells in IVDs in the mice administered with tamoxifen (Figure 1a,b). In contrast, IVDs from corn oil-injected mice exhibited little to no GFP signal (Supporting Information Figure S1). A closer examination of magnified images from tamoxifen-treated mice revealed that all the cells in the NP and all the chondrocytes within the growth plate of the vertebral body expressed GFP when tamoxifen was injected at either 2 weeks or 2 months of age (Figure 1c–f). Similarly, 47.1 ± 4% and 57.3 ± 4.3% of GFP-positive cells were detected in the CEP in 2-week-old and 2-month-old mice, respectively (Figure 1c,d). Moreover, GFP-labeled cells were observed in both OAF and IAF of Agc1-CreERT2; R26-mTmG mice, but their numbers decreased when tamoxifen was administered in older mice (Figure 1g,h). Nearly 100% of IAF and OAF cells were GFP-positive when tamoxifen was injected at 2 weeks of age, whereas these numbers dropped to 56 ± 4% (n = 3) and 35± 5% (n = 3) respectively, when tamoxifen was injected at 2 months of age (Figure 1g,h). Taken together, our data confirmed the activity of Agc1-CreERT2 and determined its cell type specificity and targeting efficiency in IVD tissues at postnatal stages.
FIGURE 1.
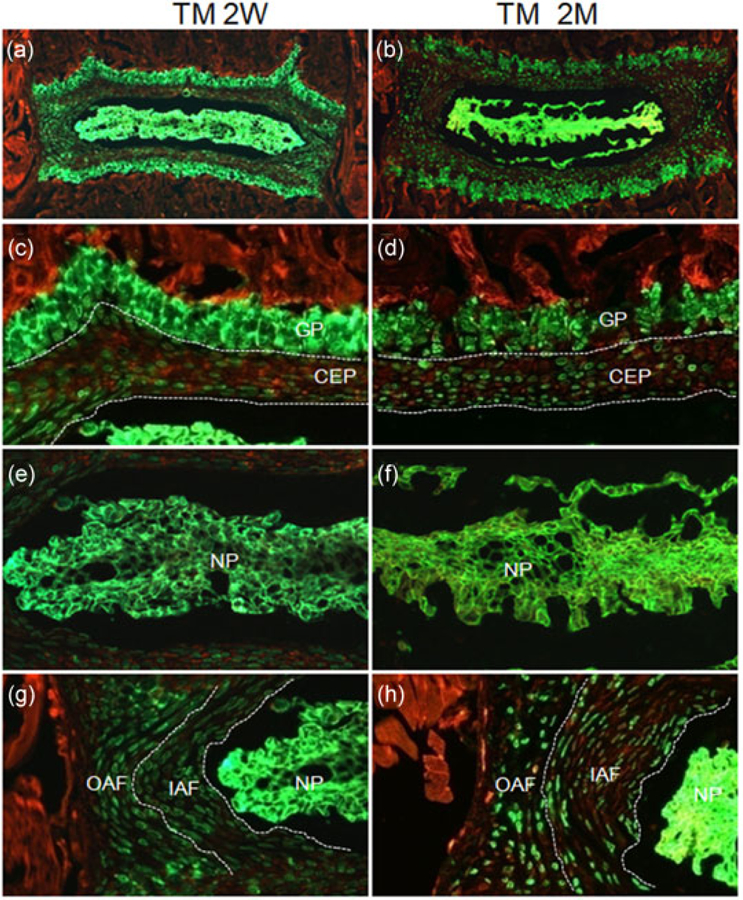
Agc1-CreERT2 is active in all cell types of IVD tissues at postnatal stages. Agc1-CreERT2; R26-mTmG mice were treated with tamoxifen at 2 weeks (TM 2W) or 2 months (TM 2M) of ages. (a,b) Fluorescence images of EGFP/ mTomato on intervertebral disc sections from TM 2W (a) and TM 2M (b) mice. (c–h) Higher magnification EGFP/mTomato images for CEP/GP (c,d), NP (e,f), and AF (g, h) from TM 2W (c,e,g) and TM 2M (d,f,h) mice. Red: membrane-targeted tdTomato; Green: membrane-targeted EGFP. CEP: cartilaginous endplate; GP: growth plate cartilage; IAF: inner annulus fibrosus; IVD: intervertebral disc; NP: nucleus pulposus; OAF: outer annulus fibrosus. Images shown were representative results from three independent mice analyzed [Color figure can be viewed at wileyonlinelibrary.com]
3.2 |. Col2a1-Cre mediates recombination in the majority of cells in IVD tissues at postnatal stages
Col2a1-Cre is a transgenic mouse line in which the expression of Cre recombinase is under the control of a type II collagen promoter (Col2a1; Long et al., 2001). Col2a1-Cre was shown to target chondrocytes and osteoblastic lineage cells (including osteocytes, osteoblasts, and their precursors/progenitors) in long bones (Nagao, Cheong, & Olsen, 2016). To characterize its activity in IVDs, we generated Col2a1-Cre; R26-mTmG compound heterozygous mice, and harvested IVDs at 2 weeks and 2 months of ages. GFP staining showed that IVD sections from Col2a1-Cre; R26-mTmG mice contained many GFP-positive cells (Figure 2a,b). Examination of higher magnification images revealed that the pattern and level of GFP expression were similar between 2-week-old and 2-month-old mice. First, all the cells in the CEP and all the chondrocytes within the GP were GFP-positive (n = 3) (Figure 2c,d). Secondly, about 73% of cells in the IAF expressed GFP at both ages (Figure 2e), whereas only 27.7% and 10.6% cells in the OAF were GFP-positive at 2 weeks and 2 months of ages, respectively (Figure 2e). Finally, about 75% of NP cells, are GFP-positive at either age (Figure 2g,h). Collectively, our data demonstrated that Col2a1-Cre targets most of the cells in postnatal IVDs, except for some cells in the OAF and NP.
FIGURE 2.

Col2a1-Cre mediates recombination in the majority of cells in IVD tissues at postnatal stages. (a,b) Fluorescence images of EGFP/mTomato on intervertebral disc sections from 2-week-old (a) and 2-month-old (b) Col2a1-Cre; R26-mTmG mice. (c–h) Higher magnification EGFP/mTomato images for CEP/GP (c,d), AF (e,f), and NP (g,h) from 2-week-old (c,e,g) and 2-month-old (d,f,h) Col2a1-Cre; R26-mTmG mice. Red: membrane-targeted tdTomato; Green: membrane-targeted EGFP. CEP: cartilaginous endplate; GP; growth plate cartilage; IAF: inner annulus fibrosus; IVD: intervertebral disc; NP: nucleus pulposus; OAF: outer annulus fibrosus. Images shown were representative results from three independent mice analyzed [Color figure can be viewed at wileyonlinelibrary.com]
3.3 |. Col2a1-CreERT2 is leaky, and mainly targets cells in the IAF of IVD tissues at postnatal stages
Col2a1-CreERT2 is a tamoxifen-inducible version of Col2a1-Cre in which the Cre recombinase was fused to a mutated ligand-binding domain of estrogen receptor (ERT2) as a fusion protein (CreERT2) (Chen et al., 2007). In Col2a1-CreERT2 mice, the expression of CreERT2 is regulated by Col21a1 promoter, whereas its nuclear translocation and subsequent Cre-mediated recombination is dependent on the tamoxifen induction. To determine the cell type specificity and targeting efficiency of Col2a1-CreERT2 in IVD tissues at postnatal stages, we administered either corn oil or tamoxifen into 2-week-old or 2-month-old Col2a1-CreERT2; R26-mTmG mice, and harvested IVD tissues 1 day after the last injection. Visualization of Cryojane sections revealed that the numbers of GFP-labeled cells in IVD tissues from Col2a1-CreERT2; R26-mTmG mice decreased with ages when tamoxifen was administered (Figure 3a,b). Specifically, GFP expression was present in nearly all chondrocytes within the GP and all cells in the IAF of Col2a1-CreERT2; R26-mTmG mice when tamoxifen was injected at 2 weeks of age (Figure 3c,e). However, only 54 ± 4% (n = 3) of growth plate chondrocytes and 30± 3% (n = 3) of cells in the IAF were GFP-positive when tamoxifen was injected at 2 months of age (Figure 3d,f). Interestingly, about 99% OAF cells were GFP-negative in both 2-week-old and 2-month-old mice (Figure 3c,d). Moreover, only 6.7 ± 3% and 5.2 ± 0.7% of cells in the CEP exhibited GFP expression in mice at 2 weeks and 2 months of ages, respectively (Figure 3e,f). Surprisingly, cells in the NP did not show any GFP signal regardless of ages when tamoxifen was injected (Figure 3g,h). To determine the leakage expression of Cre recombinase in Col2a1-CreERT2 mice, we also examined IVD sections from corn oil-treated Col2a1-CreERT2; R26-mTmG mice. As shown in Supporting Information Figure S1, about 5.1% cells in the IAF and 4.2% cells in the CEP exhibited GFP expression, indicating the leakiness for Cre expression in these mice. Taken together, our data demonstrated that Col2a1-CreERT2 mice exhibit the leakage expression of Cre recombinase, and mainly target cells in the IAF of IVD tissues at postnatal stages.
FIGURE 3.
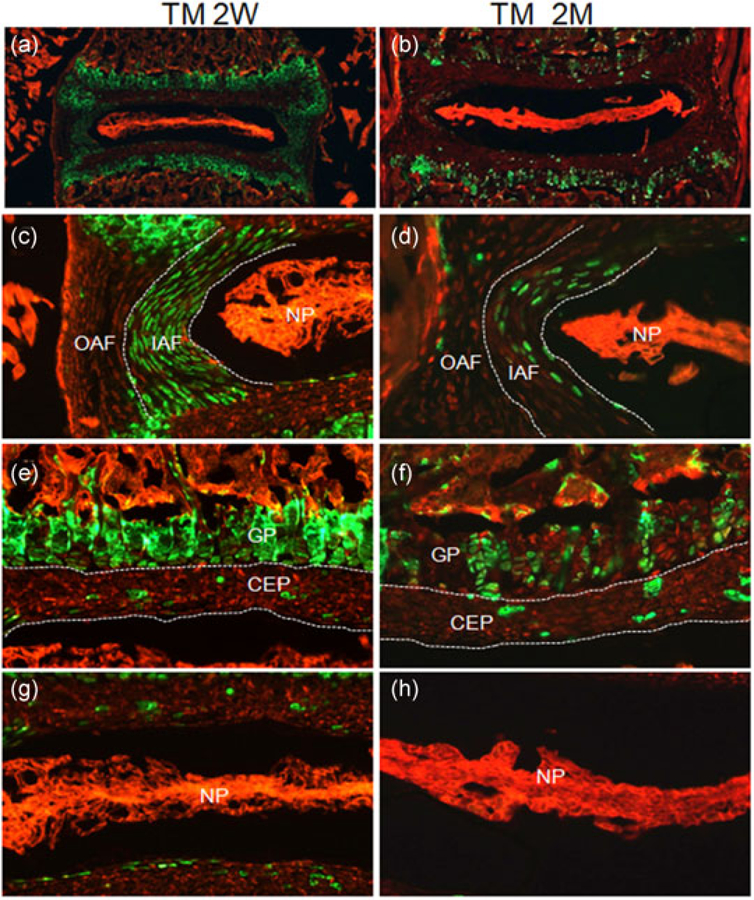
Col2a1-CreERT2 mainly targets cells in the IAF of IVD tissues at postnatal stages. Col2a1-CreERT2 mice were treated with tamoxifen at 2 weeks (TM 2W) or 2 months (TM 2M) of ages. (a,b) Fluorescence images of EGFP/ mTomato on intervertebral disc sections from TM 2W (a) and TM 2M (b) Col2a1-CreERT2; R26-mTmG mice. (c–h) Higher magnification EGFP/mTomato images for AF (c,d), CEP/GP (e,f), and NP (g,h) from TM 2W (c,e,g) and TM 2M (d,f,h) Col2a1-CreERT2; R26-mTmG mice. Red: membrane-targeted tdTomato; Green: membrane-targeted EGFP. CEP: cartilaginous endplate; GP: growth plate cartilage; IAF: inner annulus fibrosus; IVD: intervertebral disc; NP: nucleus pulposus; OAF: outer annulus fibrosus. Images shown were representative results from three independent mice analyzed [Color figure can be viewed at wileyonlinelibrary.com]
3.4 |. Shh-Cre mediates recombination in all NP cells of IVD tissues at postnatal stages
Shh-Cre (also known as Shh::GFPCre) is a knock-in mouse line in which a GFP::Cre fusion gene is targeted into the Shh locus so that the expression of Cre recombinase is turned on in cells that normally express Shh (Harfe et al., 2004). Previous fate-mapping studies showed Shh-Cre specifically targeted NP cells in the IVDs of mouse embryos and the aged mice (Choi, Cohn, & Harfe, 2008), which is consistent with the fact that Shh is expressed in notochord where nucleus pulpous tissues are derived. To further characterize cell type specificity and targeting efficiency of Shh-Cre in IVD tissues at postnatal stages, we generated Shh-Cre; R26-mTmG compound heterozygous mice, and harvested IVDs at 2 weeks and 2 months of ages. A thorough examination of IVD sections from three independent mice for each stage demonstrated that 100% NP cells were labeled by GFP (Figure 4). Interestingly, we also noticed a few GFP-positive cells in the GP and CEP (Figure 4c,d), which probably represent notochordal remnants as previously suggested (Choi et al., 2008). In contrast, all other IVD tissues did not show any GFP signal (Figure 4). Together, our data indicated that Shh-Cre drives recombination specifically in the NP cells of IVD tissues at postnatal stages.
FIGURE 4.

Shh-Cre mediates recombination in all NP cells of IVD tissues at postnatal stages. (a,b) Fluorescence images of EGFP/mTomato on intervertebral disc sections from 2-week-old (a) and 2-month-old (b) Shh-Cre; R26-mTmG mice. (c–h) Higher magnification EGFP/mTomato images for CEP/GP (c,d), AF (e,f) and NP (g,h) from 2-week-old (c,e,g) and 2-month-old (d,f,h) Shh-Cre; R26-mTmG mice. Red: membrane-targeted tdTomato; Green: membrane-targeted EGFP. CEP: cartilaginous endplate; GP: growth plate cartilage; IAF: inner annulus fibrosus; IVD: intervertebral disc; NP: nucleus pulposus; OAF: outer annulus fibrosus. Arrows point to cells that likely represents notochordal remnants in the CEP/CP. Images shown were representative results from three independent mice analyzed [Color figure can be viewed at wileyonlinelibrary.com]
3.5 |. Shh-CreERT2 specifically targets a few cells located at the NP in IVD tissues at postnatal stages
Shh-CreERT2 is a tamoxifen-inducible Cre mouse line in which the gene encoding the CreERT2 fusion protein has been inserted into the Shh locus so that expression of Cre recombinase is controlled by endogenous Shh locus, and its nuclear translocation and subsequent Cre-mediated recombination, are induced by tamoxifen (Harfe et al., 2004). Shh-CreERT2 has been shown to specifically target the NP cells when induced at early embryonic stages (E8.5-E11.5; Choi & Harfe, 2011). To determine the activity of Shh-CreERT2 in IVD tissues at postnatal stages, we administered either corn oil or tamoxifen into 2-week-old or 2-month-old Shh-CreERT2; R26-mTmG mice, and harvested IVD tissues 1 day after the last injection. GFP staining of Cryojane sections showed that only a few cells are GFP-positive in IVDs from the tamoxifen-injected mice (Figure 5a,b). Specifically, an average of 1.6% GFP-positive cells (1.6 ± 0.2%, n = 3) were detected in each IVD section of Shh-CreERT2; R26-mTmG mice that were injected with tamoxifen at 2 weeks of age (Figure 5c,e,g), whereas only 0.5% GFP-positive cells (0.5 ± 0.3%, n = 3) appeared in each IVD section when tamoxifen was given at 2 months of age (Figure 5d,f,g). Interestingly, all GFP-labeled cells are within the NP tissues that are immediately underneath the CEP. To determine the leakage expression of Cre recombinase in Shh-CreERT2 mice, we also examined IVD sections from three corn oil-treated Shh-CreERT2; R26-mTmG mice and no GFP-positive cells were observed (data not shown), indicating no leakiness for Cre expression in these mice. Together, our data indicated that Shh-CreERT2 specifically targets NP cells with low targeting efficiency in IVD tissues at postnatal stages.
FIGURE 5.

Shh-CreERT2 specifically targets a few cells located at the NP in IVD tissues at postnatal stages. Shh-CreERT2 mice were treated with tamoxifen at 2 weeks (TM 2W) or 2 months (TM 2M) of ages. (a,b) Fluorescence images of EGFP/mTomato on intervertebral disc sections from TM 2W (a) and TM 2M (b) Shh-CreERT2; R26-mTmG mice. (c–h) Higher magnification EGFP/mTomato images for CEP/GP (c,d), AF (e,f), and NP (g,h) from TM 2 W (c,e,g) and TM 2 M (d,f,h) Shh-CreERT2; R26-mTmG mice. Red: membrane-targeted tdTomato; Green: membrane-targeted EGFP. CEP, cartilaginous endplate; GP, growth plate cartilage; IAF, inner annulus fibrosus; IVD: intervertebral disc; NP, nucleus pulposus; OAF, outer annulus fibrosus. Arrows point to GFP-positive NP cells that locate at the border between the NP and CEP. Images shown were representative results from three independent mice analyzed [Color figure can be viewed at wileyonlinelibrary.com]
3.6 |. Osx-Cre targets cells in the CEP of IVDs tissues at postnatal stages
Osx-Cre (also known as Osx1-GFP::Cre and Sp7-Cre) is a BAC transgenic mouse line in which expression of a GFP::Cre fusion protein is under the control of the Osterix gene regulatory elements (Rodda & McMahon, 2006). Osx-Cre was shown to target multiple cell types in long bones in mice at the postnatal stage, including osteoblasts, osteocytes, and prehypertrophic chondrocytes, as well as stromal cells, adipocytes, and perivascular cells in the bone marrow (Chen et al., 2014a; Liu et al., 2013; Mizoguchi et al., 2014). To characterize the potential targeting of IVDs by Osx-Cre in postnatal mice, we generated Osx-Cre; R26-mTmG mice compound heterozygous mice, and harvested IVDs at 2 weeks and 2 months of ages. GFP staining detected some GFP-positive cells in IVD sections in Osx-Cre; R26-mTmG mice (Figure 6a,b). Examination of higher magnification images revealed that IVDs from both 2-week-old and 2-month-old mice exhibited a similar pattern and level of GFP expression (Figure 6C–H). First, some prehypertrophic and hypertrophic chondrocytes within the growth plate of vertebral bodies were GFP-positive in both 2-week-old and 2-month-old mice (Figure 6e,f), consistent with the previous reports of its similar activity in long bones (Chen et al., 2014a; Liu et al., 2013). Secondly, 28.9 ± 6% and 47.8 ± 2.5% cells in the CEP were GFP-positive at 2 weeks and 2 months of ages, respectively (Figure 6e,f). Finally, the NP and AF tissues did not exhibit any GFP signal at either age (Figure 6c,d,g,h). Collectively, our data indicated that Osx-Cre targets cells in the CEP of IVD tissues as well as some prehypertrophic and hypertrophic chondrocytes within the GP at postnatal stages.
FIGURE 6.

Osx-Cre targets cells in the CEP of IVD tissues at postnatal stages. (a, b) Fluorescence images of EGFP/mTomato on intervertebral disc sections from 2-week-old (a) and 2-month-old (b) Osx-Cre; R26-mTmG mice. (c–h) Higher magnification EGFP/mTomato images for AF (c,d), CEP/GP (e,f), and NP (g,h) from 2-week-old (c,e,g) and 2-month-old (d,f,h) Osx-Cre; R26-mTmG mice. Red: membrane-targeted tdTomato; Green: membrane-targeted EGFP. CEP: cartilaginous endplate; GP: growth plate cartilage; NP: nucleus pulposus; IAF: inner annulus fibrosus; OAF: outer annulus fibrosus. Images shown were representative results from three independent mice analyzed [Color figure can be viewed at wileyonlinelibrary.com]
4 |. DISCUSSION
Low back pain is mainly caused by IVD degeneration, a degenerative disease initiated in the postnatal life. The Cre/LoxP technology has significantly advanced our understanding of gene functions in development and disease of various tissues. In recent years, several Cre mouse lines have been generated to target specific cell types in long bones. However, the information for their activity in IVD tissues at the postnatal stages was very limited, which prevented the utilization of these genetic tools to study IVD homeostasis and degeneration. In this study, we systematically assessed recombination specificity and efficiency of Agc1-CreERT2, Col2a1-Cre, Col2-CreERT2, Shh-Cre, Shh-CreERT2, and Osx-Cre mice in the IVDs of both 2-week-old and 2-month-old mice, and provided the first detailed description of their activity in postnatal IVDs. Our data will facilitate the utilization of these Cre lines to study IVD biology.
Our data clearly showed that Col2a1-Cre targets the majority of cells in the NP. In contrast, we did not detect any GFP expression in NP cells of Col2a1-CreERT2; R26-mTmG mice when induced by tamoxifen at either 2 weeks or 2 months of age. This finding is consistent with the previous report using Col2a1-CreERT2; R26-lacZ mice (Jin et al., 2011). The reason behind the discrepancy between Col2a1-Cre and Col2a1-CreERT2 is still not clear. One possibility is that Col2a1 gene only turns on at an early embryonic stage but not the postnatal stage in NP cells. Alternatively, it is possible that Col2a1 is normally expressed in postnatal NP cells, but is expression is lost due to the positional effect of a transgene, a common phenomenon that the expression level or pattern of a transgene is altered by its integration site in the chromosome.
Our data showed that Osx-Cre-targeted cells include cells in the CEP and GP, but not in the NP or AF tissues in both 2-week-old and 2-month-old mice. Therefore, Osx-Cre could be used for targeting CEP cells without affecting NP and AF cells. Moreover, our direct visualization of IVDs from postnatal Osx-Cre mice detected GFP-positive cells specifically in the CEP and GP, likely reflecting the endogenous expression of Osx in these cells (data not shown). Many studies used extracellular matrix(ECM)-related genes (such as Sox9, Col2a1, and Agc1) as markers to assess phenotypes of NP cells. However, these genes are also expressed by chondrocytes, therefore not specific markers for either NP cells or chondrocytes. Since Osx is expressed in prehypertrophic and early hypertrophic chondrocytes, but not in NP or AF cells, Osx may be used as a marker to distinguish between NP cells and chondrocytes, when used in combination with these ECM-related genes.
Our data showed that Col2a1-CreERT2 mainly targets cells in the IAF of IVD tissues at postnatal stages. In contrast, none of these six Cre mouse lines enables specific targeting of cells in the OAF of postnatal IVDs. Interestingly, Bedore et al., 2016 recently characterized Cre activity of Col1a2-CreER, a transgenic mouse line in which expression of a CreER fusion protein is driven by the regulatory elements of the type I collagen gene (Cola2), in IVD tissues (Ponticos et al., 2004). They showed that Col1a2-CreER mediated recombination specifically in the OAF, but not in the IAF or NP, when induced by tamoxifen at 3 weeks of ages and analyzed 100 days later (Bedore et al., 2016). Thus, Col2a1-CreERT2 and Col1a2-CreER may be useful for delineating IAF- and OAF-specific gene functions in IVD development and homeostasis, respectively.
Our studies also added some important insights into the embryonic origin of the NP tissues and the heterogeneity of their resident cells. On one hand, our detailed analysis of IVDs from Shh-Cre; R26-mTmG mice revealed that the postnatal NP is entirely derived from Shh-expressing cells, supporting previous observations that all NP cells were descendants of Shh-positive notochord cells. Our results were also in line with a previous study that traced notochord-derived cells using Noto-Cre,a knock-in mouse generated by targeting Cre into the Noto (a notochord-specific homeobox transcription factor) locus (McCann, Tamplin, Rossant, & Seguin, 2012). Like Shh-Cre, Noto-Cre was found to specifically target all cells within the NP of postnatal IVDs (McCann et al., 2012). On the other hand, our fate-mapping of Col2a1-Cre-labeled cells indicated that the NP cells are not homogenous, and can be divided into two populations based on whether they are targeted by Col2a1-Cre. Earlier studies suggested that there were two distinct cell populations residing in the NP, including larger notochordal cells and smaller chondrocyte-like cells (Zhang et al., 2018). It will be interesting to determine whether Col2a1-Cre specifically targets chondrocyte-like NP cells. In addition, future studies are warranted to determine when notochordal cells or their descendants become Col2a1-positive cells.
In conclusion, our study revealed that all these Cre lines direct recombination in IVD tissues at the postnatal growing and adult stages, but with different cell type specificity and/or targeting efficiency. Thus, these tools can be utilized to dissect cell type-specific gene functions in IVD homeostasis and degeneration.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Dr. Ting Chen (Beijing Institute of Biological Sciences, Beijing, China) and Dr. Xiaochun Bai (Southern Medical University, Guangzhou, China) for providing us Shh-CreERT2 and Osx-Cre mice, respectively. This study was supported by the National Key R&D Program of China (2016YFC1100203) and the National Natural Science Foundation of China (81772294).
Funding information
National Key R&D Program of China, Grant/Award Number: 2016YFC1100203; National Natural Science Foundation of China, Grant/Award Number: 81772294
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- Bedore J, Quesnel K, Quinonez D, Séguin CA, & Leask A (2016). Targeting the annulus fibrosus of the intervertebral disc: Col1a2-Cre (ER)T mice show specific activity of Cre recombinase in the outer annulus fibrosus. Journal of Cell Communication and Signaling, 10, 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedore J, Sha W, McCann MR, Liu S, Leask A, & Séguin CA (2013). Impaired intervertebral disc development and premature disc degeneration in mice with notochord-specific deletion of CCN2. Arthritis and Rheumatism, 65(10), 2634–2644. [DOI] [PubMed] [Google Scholar]
- Chen J, & Long F (2013). β-Catenin promotes bone formation and suppresses bone resorption in postnatal growing mice. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research, 28(5), 1160–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, & Long F (2018). mTOR signaling in skeletal development and disease. Bone Research, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Li S, Xie W, Wang B, & Chen D (2014b). Col2CreER(T2), a mouse model for a chondrocyte-specific and inducible gene deletion. European Cells & Materials, 28, 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Lichtler AC, Sheu TJ, Xie C, Zhang X, O’Keefe RJ, & Chen D (2007). Generation of a transgenic mouse model with chondrocyte-specific and tamoxifen-inducible expression of Cre recombinase. Genesis, 45(1), 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Shi Y, Regan J, Karuppaiah K, Ornitz DM, & Long F (2014a). Osx-Cre targets multiple cell types besides osteoblast lineage in postnatal mice. PLoS One, 9(1), e85161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KS, & Harfe BD (2011). Hedgehog signaling is required for formation of the notochord sheath and patterning of nuclei pulposi within the intervertebral discs. Proceedings of the National Academy of Sciences of the United States of America, 108(23), 9484–9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KS, Cohn MJ, & Harfe BD (2008). Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: Implications for disk degeneration and chordoma formation. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 237(12), 3953–3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, & Tabin CJ (2004). Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell, 118(4), 517–528. [DOI] [PubMed] [Google Scholar]
- Hayashi S, & McMahon AP (2002). Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: A tool for temporally regulated gene activation/inactivation in the mouse. Developmental Biology, 244(2), 305–318. [DOI] [PubMed] [Google Scholar]
- Henry SP, Jang CW, Deng JM, Zhang Z, Behringer RR, & de Crombrugghe B (2009). Generation of aggrecan-CreERT2 knockin mice for inducible Cre activity in adult cartilage. Genesis, 47(12), 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Shen J, Wang B, Wang M, Shu B, & Chen D (2011). TGF-β signaling plays an essential role in the growth and maintenance of intervertebral disc tissue. FEBS Letters, 585(8), 1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone DP, Genoud S. é, Atanasoski S, Grausenburger R, Berger P, Metzger D, … Suter U (2003). Tamoxifen-inducible glia-specific Cre mice for somatic mutagenesis in oligodendrocytes and Schwann cells. Molecular and Cellular Neuroscience, 22(4), 430–440. [DOI] [PubMed] [Google Scholar]
- Lim J, Burclaff J, He G, Mills JC, & Long F (2017). Unintended targeting of Dmp1-Cre reveals a critical role for Bmpr1a signaling in the gastrointestinal mesenchyme of adult mice. Bone Research, 5, 16049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Strecker S, Wang L, Kronenberg MS, Wang W, Rowe DW, & Maye P (2013). Osterix-cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PLoS One, 8(8), e71318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long F (2011). Building strong bones: Molecular regulation of the osteoblast lineage. Nature Reviews Molecular Cell Biology, 13(1), 27–38. [DOI] [PubMed] [Google Scholar]
- Long F, & Ornitz DM (2013). Development of the endochondral skeleton. Cold Spring Harbor Perspectives in Biology, 5(1), a008334–a008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long F, Zhang XM, Karp S, Yang Y, & McMahon AP (2001). Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development, 128(24), 5099–5108. [DOI] [PubMed] [Google Scholar]
- McCann MR, Tamplin OJ, Rossant J, & Seguin CA (2012). Tracing notochord-derived cells using a Noto-cre mouse: Implications for intervertebral disc development. Disease Models & Mechanisms, 5(1), 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi T, Pinho S, Ahmed J, Kunisaki Y, Hanoun M, Mendelson A, … Frenette PS (2014). Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Developmental Cell, 29(3), 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, & Luo L (2007). A global double-fluorescent Cre reporter mouse. Genesis, 45(9), 593–605. [DOI] [PubMed] [Google Scholar]
- Nagao M, Cheong CW, & Olsen BR (2016). Col2-Cre and tamoxifen-inducible Col2-CreER target different cell populations in the knee joint. Osteoarthritis and Cartilage, 24(1), 188–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponticos M, Abraham D, Alexakis C, Lu QL, Black C, Partridge T, & Bou-Gharios G (2004). Col1a2 enhancer regulates collagen activity during development and in adult tissue repair. Matrix Biology: Journal of the International Society for Matrix Biology, 22(8), 619–628. [DOI] [PubMed] [Google Scholar]
- Risbud MV, & Shapiro IM (2014). Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nature Reviews Rheumatology, 10(1), 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda SJ, & McMahon AP (2006). Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development, 133(16), 3231–3244. [DOI] [PubMed] [Google Scholar]
- Wang M, Tang D, Shu B, Wang B, Jin H, Hao S, … Chen D (2012). Conditional activation of β-catenin signaling in mice leads to severe defects in intervertebral disc tissue. Arthritis and Rheumatism, 64(8), 2611–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xiong C, Kudelko M, Li Y, Wang C, Wong YL, … Chan D (2018). Early onset of disc degeneration in SM/J mice is associated with changes in ion transport systems and fibrotic events. International Society for Matrix biology: Journal of the Matrix Biology, 70, 123–139. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Liu C, Ni L, Liu Z, Mirando AJ, Lin J, … Chen J (2018). Cell type-specific effects of Notch signaling activation on intervertebral discs: Implications for intervertebral disc degeneration. Journal of Cellular Physiology, 233(7), 5431–5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


