Abstract
The most common cause of intrauterine growth restriction (IUGR) in the developed world is placental insufficiency, a concept often used synonymously with reduced utero-placental and umbilical blood flows. However, placental insufficiency and IUGR are associated with complex, coordinated and highly regulated changes in placental signaling and nutrient transport including inhibition of insulin and mTOR signaling and down-regulation of specific amino acid transporters, Na+/K+-ATPase, the Na+/H+-exchanger, folate and lactate transporters. In contrast, placental glucose transport capacity is unaltered and Ca2+-ATPase activity and the expression of proteins involved in placental lipid transport are increased in IUGR. These findings are not entirely consistent with the traditional view that the placenta is dysfunctional in IUGR, but rather suggest that the placenta adapts to reduce fetal growth in response to an inability of the mother to allocate resources to the fetus. This new model has implications for the understanding of the mechanisms underpinning IUGR and for the development of intervention strategies.
Keywords: syncytiotrophoblast, placental transport, fetal growth restriction, human, maternal-fetal exchange, fetal development
Introduction
Intrauterine growth restriction (IUGR) is often defined as the failure of the fetus to reach its genetic growth potential in utero and affects 3–7% of all newborns [1]. IUGR increases the risk of perinatal complications [2, 3], including stillbirth [4, 5], and predisposes the infant to the development of diabetes and cardiovascular disease in childhood and adult age [6–8]. However, the pathophysiology underlying IUGR remains poorly understood, no specific treatment is available and biomarkers for early detection are lacking [9, 10].
An incomplete trophoblast invasion resulting in poor spiral artery remodeling, which restricts the normal increase in utero-placental blood flow in mid-pregnancy is believed to be the most common etiology of IUGR in developed countries. In the literature it is often assumed that placental insufficiency, i.e. impaired delivery of nutrients and oxygen to the fetus, is directly caused by the reduced placental blood flow. However, the placental blood flow reduction per se is unlikely to adequately explain the impaired placental transfer in IUGR. Oxygen, which is highly lipophilic, diffuses rapidly across the placental barrier and placental oxygen transfer is therefore primarily limited by the rate of blood flow on the two sides of the barrier, and to some degree the barrier thickness. Thus, it is likely that the reduction in utero-placental and umbilical blood flows contribute to fetal hypoxia that develops in some IUGR fetuses. In contrast, transplacental transport of nutrients such as glucose and amino acids are less affected by changes in blood flow because the transport across the barrier is the primary limiting factor for the transfer of these molecules. Thus, placental insufficiency may not be just a matter of reduced blood flow or a small placenta.
Herein, we will briefly review experimental evidence suggesting that complex regulation of multiple placental signaling pathways and down-regulation of placental nutrient transporters directly contributes to the development of IUGR. Although the focus will be on changes in placental function in human IUGR, we will discuss selected animal models to address mechanistic links and to illustrate that very different causes of IUGR appear to be associated with strikingly similar changes in placental signaling and function. Moreover, we will speculate on the evolutionary origins of placental responses to changes in utero-placental blood flow as well as maternal nutrition and metabolism. Subsequently, we will present a model that can help explain a range of fetal growth patterns in response to variation in the ability of the maternal supply line to allocate resources for fetal growth. Finally, we will discuss novel avenues to prevent or improve outcomes in IUGR targeting placental function based on this new understanding.
The placental barrier in the human
In the term human placenta, there are only two largely continuous cell layers between the maternal blood in the intervillous space and the fetal blood in the capillaries of the terminal villi: the syncytiotrophoblast and the capillary endothelial cells (Fig 1). The syncytiotrophoblast is the transporting epithelium of the human placenta and is a true syncytium generated by fusion of underlying cytotrophoblast cells. Because the fetal-placental capillary endothelial cells are of the so called “continuous” type, and therefore allow a relatively unrestricted transfer of molecules such as glucose and amino acids through intercellular junctions [11], the syncytiotrophoblast represents the primary barrier for movement of most solutes from the maternal to the fetal circulations (Fig 1). Specifically, transfer across the two polarized plasma membranes of the syncytiotrophoblast, the apical or microvillous plasma membrane (MVM) directed to maternal blood in the intervillous space and the basal plasma membrane (BM) facing the fetal capillaries constitute the limiting step. Maternal-fetal exchange of most nutrients and ions occurs by mediated transfer involving transporter proteins expressed in the syncytiotrophoblast plasma membranes.
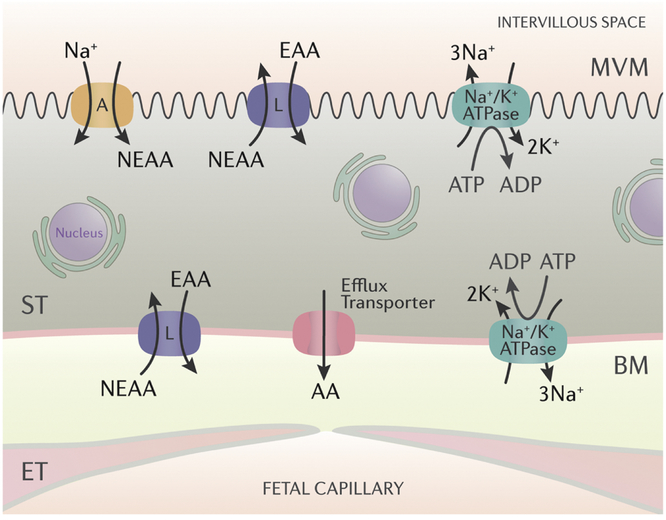
Fig 1. The placental barrier at term and a model for placental transport of neutral amino acids.
The syncytiotrophoblast cell layer (ST) and in particular its two polarized plasma membranes, the microvilllous (MVM) and basal plasma membranes (BM) constitute the primary barrier for the transfer of molecules such as glucose, amino acids and folate. The uptake of neutral amino acids from the maternal circulation across the MVM represents the active step of amino acid transport and is largely mediated by the System A and System L amino acid transporters. System A is predominantly expressed in the MVM and transports non-essential neutral amino acids (NEAA) against a concentration gradient energized by the inwardly directed Na+-gradient. System L is an exchanger, which uses the steep outwardly directed concentration gradient of some NEAAs to drive uptake of essential amino acids (EAA) against their concentration gradients. Amino acids are transferred across BM by facilitated diffusion driven by the outwardly directed concentration gradient mediated by System L and efflux transporters. N,nucleus; ET, endothelial cells of the fetal capillary.
The description of the human placental barrier above is a simplification. First, a number of other cell types are present in the barrier even at term, including cytotrophoblasts, macrophages (Hofbauer cells), and fibroblasts. Albeit not forming a continuous layer and therefore not directly limiting transplacental transfer, these cell types may influence transport across the barrier by their substrate metabolism. For example, it has been reported that cytotrophoblast cells contribute significantly to glycolysis and oxidative phosphorylation in the term placenta [12] and changes in cytotrophoblast metabolism could therefore impact transplacental transport of, for example, glucose. Second, for larger molecules including immunoglobulins and lipids, the endothelial cell barrier becomes limiting for transplacental transport and for these molecules mechanisms accounting for the transport across the endothelial cells must be considered in order to model transfer across the entire barrier [11, 13]. Third, in early pregnancy cytotrophoblast cells are highly abundant, forming a largely continuous cell layer between the syncytium and the fetal capillary [14] which is therefore likely to directly contribute to the barrier properties of the human placenta in first trimester.
Changes in placental signaling in IUGR
The syncytiotrophoblast serves not only as the transporting epithelium but also as the primary endocrine cell of the human placenta, integrating a multitude of maternal and fetal signals to balance maternal supply and fetal demand. It does so by altering placental growth and function and by secreting signaling molecules, which directly alter maternal physiology and fetal development. Although complex signaling pathways with extensive cross-talk regulate this functional diversity, the discussion of placental signaling in IUGR will be limited to a few signaling pathways that have been studied in some detail and that have been linked to specific placental functions. This data is summarized in Fig 2.
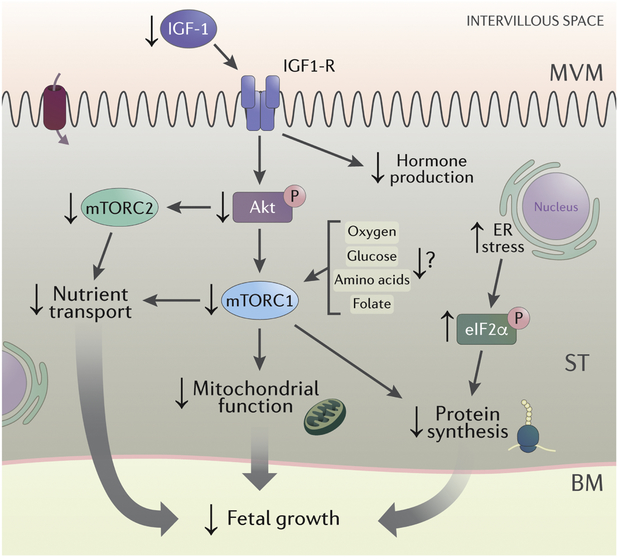
Fig 2. Changes in placental signaling in IUGR.
Placental insulin/IGF-1 and mTORC1 and mTORC2 signaling is inhibited and the ER stress pathway is activated in IUGR. For details, see text. Abbreviations: IGF-1: insulin-like growth factor I; IGF1-R: insulin-like growth factor 1 receptor; AKT: protein kinase B; mTORC1: mechanistic target of rapamycin complex 1; mTORC2: mechanistic target of rapamycin complex 2; eIF2α: eukaryotic initiation factor 2α.
Insulin/IGF signaling
The insulin-like growth factor (IGF) family consists of polypeptide ligands (insulin, IGF-1, and IGF-2), tyrosine kinase receptors (in particular the IGF1 receptor and insulin receptors) and IGF binding proteins (IGFBPs). Insulin [15–17] and IGF-1 [17–19] positively regulate the system A amino acid transporter in the trophoblast and the placenta. The insulin receptor (INSR) gene undergoes differential splicing generating two isoforms, INSR-A and INSR-B. Insulin binds to both INSR-A and ISNR-B with high affinity. IGF-1 predominantly interacts with the IGF-1 receptor and the biological effects of IGF-2 are mediated by binding to INSR-A [20]. IGFs, in particular IGF-1, are key regulators of fetal growth [21, 22] and placental and maternal IGFs promote placental growth and function [23–25], such as amino acid transport [26]. The bioavailability of IGFs is tightly regulated by IGF binding proteins and IGFBP-1 is believed to be the predominant IGF binding protein in the fetus and in pregnant women. Phosphorylation of IGFBP-1 at three serine residues (Ser101, 119 and 169) is known to markedly increase its affinity for binding IGF-1 [27], thus affecting the ability of IGF-1 to interact with the IGF-1 receptor, resulting in inhibition of IGF-1 function [28, 29]. In addition, phosphorylation makes IGFBP-1 more resistant to proteolysis [28, 30]. Functionally, phosphorylation increases the capacity of IGFBP-1 to inhibit IGF-1-stimulated cell proliferation, DNA synthesis, amino acid transport and apoptosis [31–33].
Insulin [34–38] and IGF-1 receptors [39] are abundantly expressed in the syncytiotrophoblast microvillous plasma membrane, and the actions of both insulin and IGF-1 in the maternal circulation and the maternal-fetal interface serve as powerful positive regulators of placental function and growth. Therefore, alterations in the maternal insulin, IGF-1 and/or IGFBP1 levels in pregnancies complicated by IUGR may directly contribute to the restricted fetal growth. Locally in the placental barrier, IGFBP-1 produced by the decidua inhibits trophoblast invasion by sequestering IGF-1 [40, 41]. Furthermore, the decidua constitutes the major source for maternal circulating IGFBP-1 in pregnancy [42, 43]. Serum IGF-1 concentrations are decreased in mothers delivering IUGR babies [44] and most [44–54], but not all [55–58], studies show that IUGR or low birth weight is associated with increased maternal serum IGFBP-1 levels. Moreover, placental IGFBP-1 protein expression has been reported to be increased in IUGR [59, 60]. We demonstrated that first trimester maternal plasma IGFBP-1 is hyperphosphorylated in women who later delivered IUGR infants [61]. This suggests that hyperphosphorylation of maternal IGFBP-1 may represent a novel early biomarker of IUGR with implications for early intervention and ultimately prevention of perinatal complications associated with this pregnancy complication.
In addition to decreased maternal circulating levels of IGF-1 in IUGR [44], decreased IGF-1 secretion from decidual explants [62] and lower placental expression of IGF-1 has been reported in human IUGR [63]. After ligand binding, the INSR and IGF1-R activate the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) and protein kinase B (AKT/PKB) pathways, which mediate many of the cellular effects of insulin/IGF-1. Overall, the activity of the placental insulin/IGF-1 signaling, as determined by the degree of phosphorylation of key kinases in the signaling pathway, is decreased in human IUGR [64–66] (Fig 2). The protein expression of INSR [67] and the gene [63] and protein expression [68] of IGF-1R have been reported to be decreased in the IUGR placenta. In contrast, other reports suggest that placental IGF-1R gene expression is increased in association with IUGR [69]. Moreover, Iniguez and co-workers reported that total protein expression of IGF-1R, IRS-1 and Akt was increased in the IUGR placenta [70], reminiscent to the increased total placental mTOR protein expression demonstrated in IUGR previously [71]. These changes may be compensatory to inhibition of basal insulin/IGF-1 activity in the IUGR placenta. However, other reports suggest that IGF-1R expression is decreased in the IUGR placenta [68]. Because the insulin/IGF-1 signaling regulates multiple trophoblast functions such as hormone synthesis including human placental lactogen, chorion gonadotrophin, and progesterone [72], nutrient transport [16, 73] and protein synthesis (mediated by mTOR signaling, see below), decreased placental insulin/IGF-1 signaling in IUGR is expected to inhibit these functions (Fig 2).
mTOR signaling
Mechanistic target of rapamycin (mTOR) is a serine/threonine kinase highly expressed in the syncytiotrophoblast [71]. mTOR regulates cellular metabolism, growth and proliferation [74–78]. mTOR is present in the cell in two complexes, mTOR complex (mTORC) 1 and 2, with the protein raptor associated to mTORC1 and the protein rictor constituting an integral part of mTORC2. mTORC1 promotes protein translation mediated by phosphorylation of S6K1 and 4EBP1 [74–79]. In contrast, activation of mTORC2 phosphorylates Akt, PKCα, and serum glucocorticoid-regulated kinase 1 (SGK1) and regulates the actin skeleton, cell-cycle progression, anabolism and cell survival [80–82]. Deptor is an endogenous inhibitor of both mTORC1 and 2 [83]. mTORC1 integrates a large number of metabolic signals, including hormones and growth factors, such as insulin, IGF-1 and EGF, cellular ATP levels, hypoxia, DNA damage, amino acids, glucose and fatty acids, to regulate cellular metabolism, growth and proliferation [84, 85]. In contrast to mTORC1, mTORC2 predominantly responds to insulin/PI3K signaling [84].
It was recently reported that mTORC1, but not mTORC2, is a positive regulator of oxidative phosphorylation mediated by influencing mitochondrial biogenesis [86]. Thus, by controlling syncytiotrophoblast ATP production, mTORC1 signaling indirectly regulates all energy-requiring processes including active transport and protein synthesis, In addition, using human placental villous explants and primary human trophoblast (PHT) cells, we have demonstrated that mTOR signaling directly regulates key trophoblast nutrient transporters [17, 71, 73, 87–89]. Specifically, inhibition of both mTORC1 and/or mTORC2 downregulates trophoblast System A and L amino acid transport activity by affecting the plasma membrane trafficking of specific System A (SNAT2, SLC38A2) and System L isoforms (LAT1, SLC7A5) [87]. Furthermore, it was demonstrated that Nedd4–2, an E3 ubiquitin ligase, is required for the regulation of plasma membrane trafficking of amino acid transporter isoforms by mTORC1, but not mTORC2 [88]. In contrast, mTORC2 regulates the plasma membrane trafficking of specific amino acid transporter isoforms activating the Rho GTPases Cdc42 and Rac1, which influence the actin skeleton (Rosario et al, unpublished observations). The powerful effects of mTOR signaling on nutrient transport are not limited to amino acids. For example, both mTORC1 and 2 are positive regulators of trophoblast folate uptake by modulating the cell surface expression of folate receptor-α (FR-α) and the reduced folate carrier (RFC) [89].
Using the phosphorylation of key downstream targets as functional readouts of mTOR signaling, we and others have reported that placental mTORC1 [90–94] and mTORC2 [92, 94] signaling activity is inhibited in human idiopathic IUGR as well in IUGR induced by placental malaria [94]. On the other hand, the total protein expression of mTOR has been reported to be up-regulated in the IUGR placenta in some studies [90, 95] but not all [91], possibly as a regulatory event in response to the inhibited activity.
ER stress signaling
Diverse signals may impair the ability of cells to properly execute folding and post-translational modifications of secretory and transmembrane proteins in the endoplasmic reticulum (ER) causing ER stress, which is associated with accumulation of misfolded proteins in the ER [96]. For survival, cells that undergo ER stress must rapidly restore protein-folding capacity to match protein-folding demand. In the presence of high levels of misfolded proteins in the ER, an intracellular signaling pathway called the unfolded protein response (UPR) initiates homeostatic mechanisms to prevent further damage but prolonged UPR activation can lead to cell death [96]. The UPR is comprised of three principal signaling pathways with involvement of PKR-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme 1 (IRE-1) and activating transcription factor 6 (ATF6). ER stress and activation of the UPR leads to increased phosphorylation of eukaryotic initiation factor 2α (eIF2α), resulting in inhibition of global translation but to increased translation of activating transcription factor (ATF) 4. ATF4 increases the expression of a small group of target genes involved in transport, metabolism, and oxidative stress [97]. IUGR has been reported to be associated with placental ER stress and protein synthesis inhibition [91, 93, 98]. The reduced protein synthesis in the IUGR placenta in response to inhibition of mTOR signaling and activation of the ER stress pathways has been proposed to contribute to a smaller placental size, which is characteristic of the IUGR phenotype. UPR activation may also indirectly regulate trophoblast nutrient transporters. First, ATF4 has been proposed to increase the transcription of the two amino acid transporter isoforms SNAT2 (SLC38A2) and CAT-1 (SLC7A1) [97]. Second, eIF2α phosphorylation results in inhibition of mTORC1 and mTORC2, which are positive regulators of several specific amino acid transporter isoforms, and is mediated in part by increased expression of REDD1 (regulated in development and DNA damage response 1) [99, 100].
PPARγ signaling
Peroxisome proliferator-activated receptor γ (PPARγ) is a ligand-activated transcription factor that regulates gene expression. PPARγ is involved in an array of biological processes including glucose homeostasis, adipocyte differentiation and inflammatory responses [101]. PPARγ forms a heterodimer with RXR and promotes fatty acid uptake and lipid storage, typically resulting in increased insulin sensitivity [101, 102]. PPARγ regulates trophoblast invasion and differentiation [103, 104] and controls placental vascular proliferation [105] and is therefore critical for placental development. PPARγ stimulates fatty acid uptake [106] and increases L-type amino acid and taurine transporter protein expression [107] in primary human trophoblast cells. The literature on placental PPARγ expression in IUGR is conflicting. Some investigators have reported an increased placental PPARγ expression in IUGR, which has been suggested to represent a protection against hypoxia and/or nutrient deficiency caused by placental insufficiency [108, 109]. However, Rodie and coworkers reported that PPARγ is unchanged [110], whereas other investigators observed a decreased placental PPARγ protein [107] and gene expression [111] in small-for-gestational age infants. Therefore, though the important regulatory role of PPARγ in placental development is clear, the relationship between alterations in placental PPARγ expression and the development of IUGR remains to be elucidated.
Placental mitochondrial abundance and function in IUGR
Increased mitochondrial activity and biogenesis across gestation sustains the metabolic activity of the placenta during normal pregnancy. Mitochondrial dysfunction and excess generation of reactive oxygen species can affect placental function, signaling and nutrient transport which are vital for normal fetal growth. Studies exploring placental mitochondrial DNA content, electron transport chain complex expression and function in IUGR have generated conflicting results. Specifically, placental mitochondrial DNA content has been reported to be increased [112, 113] unaltered [114] or decreased [115, 116] in IUGR. Moreover, using enzyme activity measurements Lefebvre and coworkers concluded that placental mitochondrial function is unchanged in IUGR [114], whereas Mando and collaborators studied cultured primary human cytotrophoblast cells using high resolution respirometry and reported increased trophoblast mitochondrial function in IUGR [112]. The reasons for the divergent findings in the different studies remains to be established but may be related to small sample sizes in some studies and variable definitions of IUGR. We recently reported that placental protein expression of electron transport chain complexes is decreased and positively correlated to mTOR signaling activity in IUGR [86]. These observations, together with the demonstration that mTORC1 is a positive regulator of trophoblast function [86], are consistent with the possibility that reduced placental mTOR activity in IUGR may impair mitochondrial respiration and contribute to placental insufficiency in this pregnancy complication.
Placental nutrient transport in IUGR
Nutrient availability is a key determinant of fetal growth, and changes in fetal nutrient supply not only results in abnormal fetal growth but programs the fetus for diseases later in life [8, 117–120]. Fetal nutrient availability is critically dependent on placental nutrient transport and metabolism and common pregnancy complications are associated with specific changes in placental nutrient transporter expression and activity [121–125]. Thus, various aspects of placental nutrient transport in IUGR have been studied in some detail, as reviewed in the literature [121–123, 126–135].
As discussed above, it is the syncytiotrophoblast cell layer and in particular its two polarized plasma membranes, the microvillous (MVM) and basal plasma membranes (BM), that constitute the actual barrier for the transfer of molecules such as glucose, amino acids, and folate. This provides the rationale for isolating these plasma membranes to study their transport characteristics in vitro. Reported changes in MVM or BM nutrient transporter activity in IUGR placentas are summarized in Fig 3.
Figure 3. Alterations in Placental Transport in IUGR.
Increased (red), decreased (blue) and unchanged (grey) transporter activity in microvillous (MVM) and basal (BM) syncytiotrophoblast plasma membranes isolated from IUGR placentas, as compared to gestational age matched AGA controls. For details, see text.
Glucose
Transplacental glucose transport is mediated by facilitated diffusion through specific glucose transporters (GLUTs). In late pregnancy GLUT 1 (SLC2A1) is believed to constitute the primary transporter mediating transfer of glucose across the placental barrier in the human [136] (Fig 3). GLUT1 expression has been estimated to be three-fold higher in the MVM compared to BM [136], reflecting the higher glucose transport activity in the MVM [136]. Given the 6–7-fold larger area of the microvillous surface of the syncytiotrophoblast compared to the basal plasma membrane at term it has been estimated that the total glucose transport capacity of the MVM may be 20-fold higher than BM [136]. Based on these findings it was suggested that transport across the BM constitutes the rate-limiting step of transplacental glucose transport [136]. In addition to GLUT1, additional glucose transporters have been shown to be expressed in different cell types of the human term placenta, however their functional significance remain to be fully established. For example, the insulin-sensitive GLUT4 is reported to be expressed in human term villous stromal cells [137], GLUT9 protein is expressed in both MVM and BM [138, 139] and GLUT3 is reported to be localized in the BM [140], though other investigators have found that GLUT3 protein expression in the term human placenta is restricted to fetal capillary endothelial cells [141]. In the first trimester, at least four different GLUT isoforms are expressed in the syncytiotrophoblast, primarily in the MVM: GLUT1, 3, 4 and 12 [142, 143] of which GLUT4 and 12 are sensitive to regulation by insulin.
MVM and BM GLUT1 protein and glucose transport activity are unaffected by IUGR [136, 144]. However, BM GLUT1 protein expression has been reported to be decreased in women residing at high altitude who give birth to smaller infants, albeit not meeting the clinical definition of IUGR [145]. Using immunohistochemistry, Janzen and co-workers found that GLUT3 protein expression is increased in the IUGR placenta, in particular in cytotrophoblasts [146]. Thus, fetal hypoglycemia in IUGR is unlikely to be due to changes in placental glucose transporter expression or activity. It is possible that an increased placental consumption of glucose in IUGR could explain a decreased transplacental glucose transfer, even with maintained GLUT transporter expression and activity. Some studies [147, 148], but not all [149], have provided evidence in support of this hypothesis.
Amino Acids
More than twenty-five amino acid transporters with distinct but overlapping substrate specificity are expressed in the syncytiotrophoblast [150, 151]. System A is a sodium-dependent transporter mediating the uptake of non-essential neutral amino acids into the cell [152]. All three known isoforms of System A, SNAT 1 (SLC38A1), SNAT 2 (SLC38A2), and SNAT 4 (SLC38A4) are expressed in the human placenta [153]. System A activity establishes the high intracellular concentration of amino acids like glycine, which is used to exchange for extracellular essential amino acids via system L. Thus, System A activity is critical for placental transport of both non-essential and essential amino acids (Fig 1). System L is a sodium-independent exchanger mediating cellular uptake of essential amino acids including leucine [154]. The System L amino acid transporter is a heterodimer, consisting of a light chain, typically LAT1 (SLC7A5) or LAT2 (SLC7A8), and a heavy chain, 4F2hc/CD98 (SLC3A2). System β or TAUT (SLC6A6) mediates cellular uptake of taurine, an essential amino acid in fetal life.
The activity of several placental amino acid transporters is reduced in IUGR pregnancies. The activity of system A has been consistently shown to be lower in MVM isolated from IUGR placentas [92, 94, 144, 155] due to a decreased MVM protein expression of SNAT 1 (SLC38A1) and SNAT 2 (SLC38A2) [92]. Moreover, MVM system A activity is related to the level of fetal compromise in IUGR. Term IUGR fetuses are less affected than preterm IUGR [144] and the most severe cases of IUGR, as defined by abnormal pulsatility index in the umbilical artery and abnormal fetal heart rate tracings, are associated with the largest decreases in MVM System A activity [156]. However, it is unclear whether the reduction in placental amino acid transport in women occurs prior to fetal growth restriction or as a consequence of reduced fetal needs. We have reported that down-regulation of key placental amino acid transport systems precedes the development of IUGR in rodents [157, 158] and non-human primates [159–161], supporting the concept that down-regulation of placental nutrient transport is a primary event, which directly contributes to IUGR, rather than a response to a changing fetal demand.
The MVM and/or BM activity of transporters of essential amino acids, including system β (taurine) and system L (lysine and leucine), is also reduced in IUGR [162, 163]. Importantly, these in vitro findings are in agreement with a study using stable isotope techniques in pregnant women demonstrating that placental transfer of the essential amino acids leucine and phenylalanine is reduced in IUGR term pregnancies [164]. The deceased activity of amino acid transporters in the placental barrier in association with IUGR is believed to result in reduced placental amino acid transport, which may account for the low plasma levels of certain amino acids in growth restricted fetuses [165, 166].
Lipids
Numerous proteins involved in fatty acid transfer are expressed in the placenta [134]. For fatty acids in maternal lipoproteins to be accessible for placental transport, triglycerides must be hydrolyzed into non-esterified fatty acids (NEFAs). This is accomplished by lipases associated with the MVM. The placenta expresses several triglyceride lipases [167], two of which function in the extracellular compartment: lipoprotein lipase (LPL) and endothelial lipase (EL). Upon entering the cell FAs are believed to be esterified with coenzyme A and transferred by cytosolic fatty acid binding proteins (FABPs) to either (1) mitochondria for oxidation, (2) endoplasmic reticulum for re-esterification and lipid droplet formation, or 3) the fetal-facing syncytiotrophoblast basal plasma membrane (BM) for transport to the fetal circulation. The membrane-bound fatty acid transport proteins (FATPs) are important for cellular uptake of long-chain fatty acids [168]. The mammalian placenta expresses mRNA for five of the six known FATP isoforms: FATP1–4 and FATP6 [169]. FATP1 and FATP4, as well as CD36/FAT, have been demonstrated in both the MVM and BM of human placenta [170–172]. BM FATP2 protein expression has been reported to be up-regulated in maternal obesity [173].
The activity of lipoprotein lipase (LPL), an enzyme responsible for hydrolysis of lipoproteins, is reduced in MVM and the gene expression of EL is down-regulated in IUGR placentas [174]. In contrast, placental LPL mRNA expression is increased in IUGR [175, 176], possibly representing a compensatory mechanism or reflecting that MVM LPL activity is not regulated on the transcriptional level [176]. IUGR is associated with a reduced placental expression of lipoprotein receptors, low-density lipoprotein (LDL) and scavenger receptor class B type-I (SRBI), the key receptors for cholesterol uptake from maternal LDL and/or HDL [176]. In contrast, MVM FATP6 and CD36 protein expression is increased and long chain polyunsaturated fatty acids (LCPUFA) are preferentially routed towards cellular storage in triglyceride in the IUGR placenta [177], possibly to protect against oxidative stress associated with cellular fatty acid accumulation. We speculate that these changes may be caused by impaired efflux of fatty acids across the fetal-facing syncytiotrophoblast basal plasma membrane in IUGR placenta. Collectively, these findings suggest that placental lipid handling is abnormal in the IUGR placenta. However, further studies are required to obtain a more complete understanding of placental lipid transport in normal pregnancy and in pregnancy complications, such as IUGR.
Transport of solutes involved in acid-base balance
The fetus is dependent on the placenta for regulation of its acid-base balance, which involves the exchange of carbon dioxide, bicarbonate, protons, and lactate across the placental barrier. The sodium-proton exchanger (NHE) is the most important placental transporter involved in acid-base regulation. There are at least nine distinct human NHE genes (NHE1/SLC9A1-NHE9/SLC9A9) and this family of transporters is involved in a host of physiological processes in addition to removal of intracellular protons in exchange for sodium, including regulation of cell volume, proliferation and apoptosis [178]. NHEs are highly active in the MVM [179, 180], mediating the export of protons from the cytosol of the syncytium into maternal blood. Studies in villous fragments have confirmed that NHE is critical for maintenance of intracellular pH in the syncytiotrophoblast [181]. Pharmacological [182–184] and protein expression data [184, 185] suggest that NHE1 is the major isoform expressed in the MVM, however NHE2 and NHE3 are also present [184, 185]. Both the activity and expression of the Na+/H+ exchanger are reduced in IUGR [184, 185] and this finding could explain the development of acidosis in some of these infants due to decreased capacity to eliminate metabolically produced protons in the fetus to the maternal circulation.
Lactate is an important energy source in the fetal heart, brain and skeletal muscle. The placenta may be a source of lactate even when oxygen supplies are adequate [186]. However the placenta is also an important site for clearance of lactate produced in the fetus, especially in cases of fetal hypoxia [187]. Lactate is transported across cell membranes by members of the monocarboxylate transporter (MCT) family (SLC16), which mediates H+/lactate co-transport [188]. Both MVM [189, 190] and BM [190, 191] efficiently transport lactate in the presence of a proton gradient and the predominant MCT isoform in the MVM is MCT4, whereas MCT1 is highly expressed in the BM [190]. Decreased BM lactate transporter activity [192] may contribute to the increased fetal lactate levels associated with IUGR and impair the ability of the IUGR fetus to tolerate stress.
Sodium and calcium transport
A large number of Na+-dependent co-transporters have been shown to be active in MVM. These transporters utilize the inwardly directed sodium gradient across MVM to energize the uptake of, for example, amino acids, phosphate [193], biotin [194] and succinate [195] by co-transport or the efflux of molecules such as protons [179, 180] via exchange mechanisms. As in all other cells, low intracellular sodium concentrations in the syncytiotrophoblast are maintained by Na+K+-ATPase. However, in contrast to classical epithelia in which the sodium pump is expressed exclusively in the basolateral plasma membrane, Na+K+-ATPase is expressed and active in both BM [196, 197] and MVM [197] of the human syncytiotrophoblast. MVM Na+/K+ ATPase activity is decreased in IUGR [198], which may impair the function of all transporters dependent on the Na+ gradient for energy.
The fetus accumulates approximately 30g of calcium over nine months, predominantly in the third trimester when fetal bone mineralization occurs. As in other cells, the intracellular calcium concentration in the syncytiotrophoblast is four magnitudes lower than extracellular concentrations, which is critical for the role of intracellular calcium as a signal transducer. In principle, the transfer of calcium across the syncytiotrophoblast occurs in three distinct steps [reviewed in [199]] similar to calcium transport in the intestinal epithelium [reviewed in [200]]. The first step involves calcium entry into the cell across MVM. Subsequently, Ca2+ moves through the cytosol associated with calcium binding proteins and finally it is transported out of the cell mediated by Ca2+ ATPase. Interestingly, the BM Ca2+ ATPase is up-regulated in IUGR [201] possibly due to elevated fetal concentrations of PTHrp 38–94, a key regulator of the placental calcium pump [202]. In summary, the activity of syncytiotrophoblast Na+K+-ATPase and Ca2+ ATPase is altered in opposite directions, suggesting that functional changes in the IUGR placenta are due to homeostatic regulation rather than the result of injury.
Animals models of IUGR
The overall premise in the field is that a better understanding of the molecular and cellular mechanisms underpinning the development of IUGR will allow us to develop early biomarkers and novel intervention strategies to alleviate or prevent this serious pregnancy complication and its long-term adverse consequences. For example, prenatal identification of babies who are SGA significantly improves their perinatal outcomes as compared to diagnosis at birth [203]. Thus, identifying biomarkers for IUGR in early pregnancy could improve the clinical management of these patients by allowing early intervention. It is difficult, if not impossible, to perform mechanistic studies in women, and placental function can only be explored following delivery, typically in late gestation. These limitations can, at least in part, be circumvented by studies in carefully validated animal models, which have provided important insights into changes in placental function common to an array of different IUGR models in a range of species, changes in placental function across gestation and cause-and-effect links.
Changes in placental function in IUGR are remarkably similar in diverse animal models and are in general agreement with human IUGR due to placental insufficiency. For example, maternal calorie restriction from gestational day 30 to 165 (term 180–184) in the baboon was associated with an inhibition of placental insulin/IGF-1 and mTOR and signaling pathways, decreased expression of key placental amino acid and glucose transporter isoforms and lower fetal levels of essential amino acids and IUGR [161]. In pregnant rats subjected to protein restriction placental mTOR signaling is inhibited [158] and amino acid transport, as measured in vitro and in vivo, was reported to be decreased [157, 158, 204]. Maternal hyperthermia is one of the most common models of IUGR in the sheep. By increasing ambient temperature, maternal body temperature is elevated, which interferes with placental development, causing decreased placental oxygen diffusion and nutrient transport capacity [205]. Specifically, placental mTOR signaling is inhibited [206] and placental capacity to transport glucose [207], leucine [208], threonine [209] and ACP (a branched-chain amino acid analog) [210] is reduced in this IUGR model. In guinea-pigs, IUGR induced by uterine artery ligation in mid-pregnancy (GD 35) is associated with decreased placental capacity to transport amino acids, as measured in the unstressed chronically instrumented animal, in late pregnancy [211]. Similarly, uterine artery ligation in the rat resulted in a decreased in vivo transplacental glucose and amino acid transport and IUGR [212]. Furthermore, administration of corticosteroids to pregnant mice – a model of maternal stress - inhibits placental System A activity [213]. Chronic infusion of adiponectin in pregnant mice to mimic the high circulating adiponectin levels of very lean women, causes modest IUGR by inhibition of placental insulin and mTOR signaling and down regulation of placental nutrient transporters [214, 215]. Maternal folate deficiency inhibits placental mTOR signaling and placental amino acid transport and results in IUGR [216].
Importantly, animal IUGR models are not always associated with changes in placental function in agreement with this general model [217]. In particular, a series of studies in mice have provided evidence for compensatory increases in placental transport capacity in response to maternal under nutrition [218–220]. The possible reasons for the distinct placental responses to maternal under nutrition in mice as compared to the human, rat, guinea-pig and baboon have been discussed in some detail elsewhere [217]. Nevertheless, widely different causes of IUGR, such as maternal under nutrition, maternal stress, and reduced utero-placental blood flow following suboptimal trophoblast invasion, appear to result in strikingly similar changes in placental signaling and function, suggesting a common placental response to an inability of the maternal supply line to deliver nutrients and oxygen to the placenta.
In the absence of non-invasive approaches to study placental transport across gestation in women, gestational changes in placental signaling and function in normal and IUGR pregnancies can be explored in animal models. Studies in mice, baboons and rats strongly suggest that changes in placental nutrient transporters are a cause of, rather than a secondary consequence to, altered fetal growth [157–159, 161, 221]. Specifically, down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet [157] and in baboons fed a calorie-restricted diet [161, 222], suggesting that these placental transport changes are a cause, rather than a consequence, of IUGR.
Because of the available advanced gene tools, mouse models provide unique opportunities for rigorous cause-and-effect experiments. Traditional transgenesis using a short fragment of the human Cyp19 promoter, Cyp19I.1 as a trophoblast specific promoter [223–225] and lentivirus transfection of mouse blastocysts [226–228] have been used as approaches to achieve trophoblast-specific gene targeting. However, despite the promise of these approaches, they have not been entirely reproducible between labs and studies systematically targeting placental nutrient transporters have yet to be published.
Evolution and placental nutrient sensing
Imprinted genes are characterized by parental-specific monoallelic expression and many of the approximately ~100 imprinted genes currently known in humans are highly expressed in the placenta [229]. The “parental conflict theory” proposes that paternally expressed (i.e. maternally imprinted) genes in the placenta, such as IGF2, are designed to stimulate fetal growth. In contrast, maternally expressed (paternally imprinted) placental genes, including the IGF2 receptor (IGF2R), which functions as a IGF-2 clearance receptor, tend to limit nutrient supply to the embryo in order to preserve maternal reproductive resources [230]. Given the intimate relationship between placental nutrient transport and fetal growth, it is not surprising that some genes that encode transporter proteins (SLC38A4) or proteins that are involved in the regulation of nutrient transport (IGF2) are imprinted in the placenta. The expression of imprinted genes in the placenta may set the general trajectory for placental growth/function and fetal growth, whereas other regulatory mechanisms are believed to respond to changes in the ability of the maternal supply line to deliver oxygen and nutrients to the placenta.
The down-regulation of a multitude of placental nutrient transporters in IUGR in women and a range of animal models appears, at first glance, counterintuitive given the paradigm of the fetus as the ultimate parasite and the fact that classic physiology would predict a homeostatic up-regulation of placental nutrient transport in response to decreased fetal nutrient delivery. There are two fundamentally distinct and opposing, but not mutually exclusive, models for how placental function in general, and placental nutrient transporters in particular, are regulated by changes in the ability of the maternal circulation to supply oxygen and nutrients to the placenta. In the regulation by maternal supply model [123–125, 231, 232], the placenta responds to maternal nutritional cues, resulting in down-regulation of placental nutrient transporters in response to, for example, maternal under-nutrition, stress, high altitude hypoxia or restricted utero-placental blood flow (Fig 4). As a result, fetal nutrient availability is decreased and IUGR may develop. The regulation by maternal supply model therefore constitutes a mechanism by which fetal growth is matched to the ability of the maternal supply line to allocate resources to the fetus. In this model, changes in placental growth and nutrient transport directly contribute to, or cause, alterations in fetal growth. Maternal nutritional signaling molecules include maternal metabolic hormones such as insulin, cortisol, leptin, and adiponectin (Fig 4), which are known to be influenced by maternal nutrition and regulate placental transport. Other signals proposed to be sensed by the placenta are oxygen and nutrient levels. It has been suggested that placental mTOR signaling plays an important role in integrating all these signals and regulating fetal nutrient availability by modulating placental growth and nutrient transport [123–125, 231, 232].
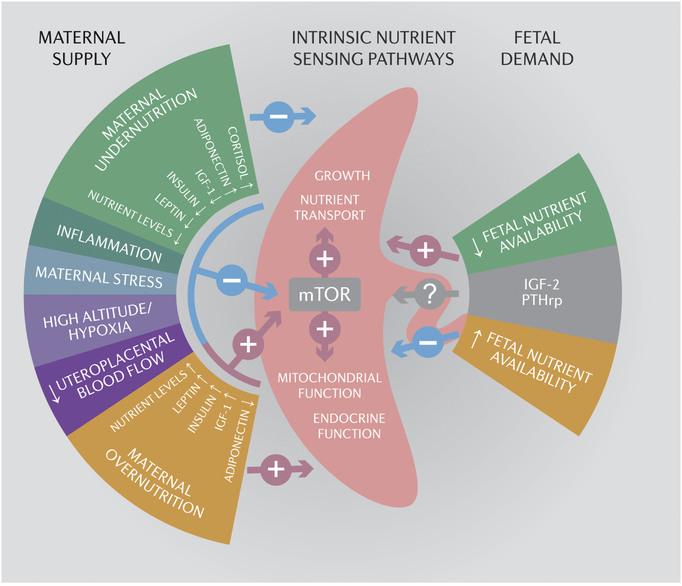
Fig 4. Placental nutrient sensing.
The placenta integrates signals conveying information on the ability of the maternal supply line to deliver nutrients and oxygen to the placenta (maternal supply) and on fetal demand through intrinsic nutrient sensors, such as mechanistic target of rapamycin (mTOR) signaling. These signals then regulate placental growth, mitochondrial respiration, nutrient transport and hormone secretion to balance fetal demand with the ability of the mother to support pregnancy. Thus, the placenta plays a critical role in modulating maternal-fetal resource allocation, thereby affecting fetal growth and the long-term health of the offspring. See text for detailed explanation. IGF-2, insulin-like growth factor 2; PTHrp, parathyroid hormone related peptide.
Predominantly based on elegant mouse studies, placental nutrient transport capacity has been proposed to be largely regulated by fetal demand signals [233–235]. According to this model, in response to limitations in fetal nutrient supply due to, for example, maternal under-nutrition or a lack of normal gestational increase in utero-placental blood flow, the fetus signals to the placenta to up-regulate placental growth and nutrient transport. This model represents a classical homeostatic mechanism by which the fetus compensates for changes in nutrient availability by regulating nutrient supply (i.e., placental transport) in the opposite direction.
As discussed above, most studies of placental transport functions in human IUGR show a down-regulation of key placental transporters for amino acids, lipids and ions (Fig 3), which is inconsistent with a homeostatic or fetal demand model for regulation of placenta transport. Indeed, there is a paucity of data demonstrating the presence of fetal demand signals in other species than mice. For example, when placental nutrient transport capacity and fetal growth were studied across the last third of pregnancy in rats exposed to protein restriction [157, 204] and baboons subjected to a 30% reduction in calorie intake [160, 161, 222] inhibition of placental mTOR signaling and down-regulation of placental nutrient transport occurred prior to slowing of fetal growth and without signs of compensatory up-regulation of placental function. However, fetal demand signals are difficult to identify unequivocally and it is possible that these signals exist in all species. Moreover, human studies of placental function in IUGR have been performed at term, or in a few cases using tissue obtained from preterm deliveries in third trimester [144, 174, 184]. It is therefore possible that compensatory changes consistent with fetal demand signals may be present earlier in pregnancy, as is evident in mouse models of IUGR. Finally, the distinct up-regulation of BM Ca2+-ATPase activity in IUGR placentas [236] may represent a compensatory activation of the placental calcium transport system stimulated by an increased fetal demand.
We have proposed a model (placental nutrient sensing) in which the placenta integrates a multitude of maternal (regulation by maternal supply) and fetal nutritional cues (regulation by fetal demand) with information from intrinsic nutrient sensing signaling pathways to balance fetal demand with the ability of the mother to support the pregnancy by regulating maternal physiology, placental growth and nutrient transport (Figure 4). These regulatory loops may also function in the “reverse” direction in response to maternal ‘over-nutrition’, represented by maternal obesity and gestational diabetes. In support of this concept, placental capacity to transport amino acids [237, 238], glucose [138] [239] and fatty acids [167, 173, 174] may be increased in obese and/or diabetic women, particularly in cases of fetal overgrowth.
The link between changes in placental function and restricted fetal growth may be modulated by fetal sex because pregnancies with a male fetus are more likely to have a poor outcome [240–242], an association that is present also in IUGR or small-for-gestational age infants [243, 244]. The underlying mechanisms are elusive, however sexual dimorphism in placenta gene expression [245–247] and function [248] may play a role. Emerging evidence suggest that differences in polyamine metabolism underpin sex differences in placental function in normal pregnancies and in pregnancy complications such as IUGR [249].
We propose that placental nutrient sensing has evolved due to the evolutionary pressures of maternal under-nutrition or starvation, which likely occurred frequently across evolutionary time. Matching fetal growth to maternal resources in response to maternal under-nutrition will produce an offspring that is smaller in size but who, in most instances, will survive and reproduce. Reduced fetal growth may be a better alternative than the fetus extracting all the nutrients needed for normal growth from an already deprived mother, thereby potentially jeopardizing both maternal and fetal survival. It may be speculated that the relative importance of placental nutrient sensing and fetal demand signals for the regulation of placental function may differ between species and depend on the type, duration and severity of the nutritional perturbation. For example, it is plausible that regulation by fetal demand signals dominates when the nutritional challenge is moderate and brief whereas regulation by maternal supply may override fetal demand if the nutritional challenge is more severe or prolonged.
Avenues to target placental function to improve outcomes in IUGR
In terms of preventing the development of IUGR two interventions have received significant interest; aspirin and low molecular weight heparin. Low dose aspirin therapy has an array of actions that could offer benefits in preventing IUGR, including the well-established irreversible inhibition of the synthesis of platelet thromboxane A2, a powerful vasoconstrictor and prothrombotic agent, increased acetylation of endothelial nitric oxide synthase promoting nitric oxide release [250] and increased activity of heme oxygenase-1 which catabolizes heme leading to decreased oxidative stress and inflammation [251]. However, recent systematic meta-analyses [252, 253] suggest that aspirin only has a modest effect on decreasing the risk of a SGA infant in high-risk pregnancies. Low molecular weight heparin has been safely used in pregnancy on the indications thromboprophylaxis and venous thromboembolism and initial randomized clinical trials suggested that low molecular weight heparin may reduce the risk of developing preeclampsia and/or IUGR [254, 255]. However more recent meta-analyses suggest no clear benefit [256, 257].
A lack of normal increase in utero-placental blood flow across gestation is believed to underpin the development of IUGR, providing a strong premise for the efforts of the past 40 years to develop prevention and treatment strategies aiming at increasing utero-placental blood flow in IUGR [258, 259]. Unfortunately, systemic maternal administration of vasodilators has in many cases been reported to be associated with poor fetal outcomes. For example, maternal administration of prostacyclin, a potent vasodilator, in severe IUGR resulted in fetal demise [260] and a recent large trial of sildenafil, a phosphodiesterase type 5 inhibitor potentiating the action of nitric oxide, had to be halted after multiple fetal deaths [261]. There are several reasons for the lack of efficacy of systemically administered vasodilators in improving utero-placental blood flow and fetal outcomes in IUGR. One explanation is likely to be that for many vasodilators, the systemic circulation is more sensitive to the vasodilatory effects of the agent than the utero-placental circulation resulting in a decrease of placental perfusion due to lowered mean arterial blood pressure. Animal experiments [262] and pilot studies in women [263] have suggested that atrial natriuretic peptide may represent a vasodilator for which the opposite is true, i.e., the uteroplacental circulation is more sensitive for the vasodilatory effect of ANP. Collectively, there are currently no specific treatment of IUGR available but some innovative approaches targeting the uteroplacental vasculature, including maternal VEGF gene therapy to increase VEGF levels locally in the uterine artery [264, 265], are currently being developed.
As discussed in this review, placental insufficiency is much more than just a matter of reduced blood flow because IUGR is associated with complex, coordinated and highly regulated changes in placental signaling and nutrient transport capacity that directly contributes to poor outcomes. Thus, approaches targeting the trophoblast to improve placental function may improve fetal growth and alleviate adverse outcomes in IUGR. Albeit in very early stages of development, IGF-2 or the antioxidant MitoQ have been specifically targeted to trophoblast cells by using nanoparticles coated with tumor-homing peptide sequences (which is also recognized by the trophoblast) [266, 267]. Given that mTOR signaling regulates a wide range of key placental functions including amino acid and folate transport, mitochondrial biogenesis and protein synthesis and that IUGR is associated with inhibition of placental mTOR signaling, specific approaches to activate trophoblast mTOR signaling in IUGR hold promise for improving fetal growth and preventing poor outcomes in this important pregnancy complication.
In conclusion, the development of IUGR involves regulation of multiple placental signaling pathways and down-regulation of placental nutrient transporters, contributing to placental insufficiency and restricted fetal growth. Our proposed ‘placental nutrient sensing’ model provides a framework for considering the complex regulatory pathways by which the placenta balances maternal supply with fetal demand to optimize fetal growth. Further investigation into placental function and potential mechanistic pathways are needed to identify the time points at which intervention may help prevent the development of IUGR and to improve the clinical management of the already affected IUGR fetus.
Highlights.
IUGR involves complex regulation of multiple placental signaling pathways
An array of placental nutrient transporters are down-regulated in IUGR
These changes contribute to placental insufficiency and restricted fetal growth
Placental insufficiency is much more than just a matter of reduced blood flow
Altered placental function should be considered when IUGR therapies are developed
Acknowledgments
Supported by grants from NIH (HD089980, HD093950 HD065007, HD068370, and HD078376)
Abbreviations:
- GLUT1
glucose transporter 1
- A
system A
- L
system L
- β
system β
- NHE1
sodium/hydrogen exchanger 1
- LPL
lipoprotein lipase
- CD36
cluster of differentiation 36, fatty acid translocase
- FATP6
fatty acid transporter 6
- MCT1
monocarboxylate transporter 1
- Ala
alanine
- Ser
serine
- Gln
glutamine
- Gly
glycine
- Leu
leucine
- Tau
taurine
- TG
triglyceride
- FA
fatty acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Romo A, Carceller R, Tobajas J. Intrauterine growth retardation (IUGR): epidemiology and etiology. Pediatr Endocrinol Rev 2009; 6 Suppl 3:332–336. [PubMed] [Google Scholar]
- 2.Pallotto EK, Kilbride HW. Perinatal outcome and later implications of intrauterine growth restriction. Clin Obstet Gynecol 2006; 49:257–269. [DOI] [PubMed] [Google Scholar]
- 3.Dall’Asta A, Brunelli V, Prefumo F, Frusca T, Lees CC. Early onset fetal growth restriction. Matern Health Neonatol Perinatol 2017; 3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Froen JF, Gardosi JO, Thurmann A, Francis A, Stray-Pedersen B. Restricted fetal growth in sudden intrauterine unexplained death. Acta Obstet Gynecol Scand 2004; 83:801–807. [DOI] [PubMed] [Google Scholar]
- 5.Silver RM. Examining the link between placental pathology, growth restriction, and stillbirth. Best Pract Res Clin Obstet Gynaecol 2018; 49:89–102. [DOI] [PubMed] [Google Scholar]
- 6.Gluckman PD, Hanson MA. Living with the past: Evolution, development, and patterns of disease. Science 2004; 305:1733–1736. [DOI] [PubMed] [Google Scholar]
- 7.Barker DJP, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993; 341:938–941. [DOI] [PubMed] [Google Scholar]
- 8.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 2008; 359:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conde-Agudelo A, Papageorghiou AT, Kennedy SH, Villar J. Novel biomarkers for predicting intrauterine growth restriction: a systematic review and meta-analysis. BJOG 2013; 120:681–694. [DOI] [PubMed] [Google Scholar]
- 10.Ruchob R, Rutherford JN, Bell AF. A Systematic Review of Placental Biomarkers Predicting Small-for-Gestational-Age Neonates. Biol Res Nurs 2018; 20:272–283. [DOI] [PubMed] [Google Scholar]
- 11.Firth JA, Leach L. Not trophoblast alone: a review of the contribution of the fetal microvasculature to transplacental exchange. Placenta 1996; 17:89–96. [DOI] [PubMed] [Google Scholar]
- 12.Kolahi KS, Valent AM, Thornburg KL. Cytotrophoblast, Not Syncytiotrophoblast, Dominates Glycolysis and Oxidative Phosphorylation in Human Term Placenta. Sci Rep 2017; 7:42941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eaton BM, Leach L, Firth JA. Permeability of the fetal villous microvasculature in the isolated perfused term human placenta. J Physiol 1993; 463:141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones CJ, Fox H. Ultrastructure of the normal human placenta. Electron Microsc Rev 1991; 4:129–178. [DOI] [PubMed] [Google Scholar]
- 15.Karl PI, Alpy KL, Fisher SE. Amino acid transport by the cultured human placental trophoblast: effect of insulin on AIB transport. Am J Physiol 1992; 262:C834–C839. [DOI] [PubMed] [Google Scholar]
- 16.Jansson N, Greenwood SL, Johansson BR, Powell TL, Jansson T. Leptin stimulates the activity of the system A amino acid transporter in human placental villous fragments. J Clin Endocrinol Metab 2003; 88:1205–1211. [DOI] [PubMed] [Google Scholar]
- 17.Roos S, Kanai Y, Prasad PD, Powell TL, Jansson T. Regulation of placental amino acid transporter activity by mammalian target of rapamycin. Am J Physiol Cell 2009; 296:C142–C150. [DOI] [PubMed] [Google Scholar]
- 18.Fang J, Mao D, Smith CH, Fant ME. IGF regulation of neutral amino acid transport in the BeWo choriocarcinoma cell line (b30 clone): evidence for MAP kinase-dependent and MAP kinase-independent mechanisms. Growth Horm IGF Res 2006; 16:318–325. [DOI] [PubMed] [Google Scholar]
- 19.Karl PI. Insulin-like growth factor-1 stimulates amino acid uptake by the cultured human placental trophoblast. J Cell Physiol 1995; 165:83–88. [DOI] [PubMed] [Google Scholar]
- 20.Belfiore A, Malaguarnera R, Vella V, Lawrence MC, Sciacca L, Frasca F, Morrione A, Vigneri R. Insulin Receptor Isoforms in Physiology and Disease: An Updated View. Endocr Rev 2017; 38:379–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 1993; 75:73–82. [PubMed] [Google Scholar]
- 22.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 1993; 75:59–72. [PubMed] [Google Scholar]
- 23.Sferruzzi-Perri AN, Owens JA, Pringle KG, Roberts CT. The neglected role of insulin-like growth factors in the maternal circulation regulating fetal growth. J Physiol 2011; 589:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sferruzzi-Perri AN, Owens JA, Pringle KG, Robinson JS, Roberts CT. Maternal insulin-like growth factors I and II act via different pathways to promote fetal growth. Endocrinology 2006; 147:3344–3355. [DOI] [PubMed] [Google Scholar]
- 25.Sferruzzi-Perri AN, Owens JA, Standen P, Taylor RL, Heineman GK, Robinson JS, Roberts CT. Early treatment of the pregnant guinea pig with IGFs promotes placental transport and nutrition partitioning near term Am J Physiol Endocrinol Metab 2006; 292:E668–E676. [DOI] [PubMed] [Google Scholar]
- 26.Kniss DA, Shubert PJ, Zimmerman PD, Landon MB, Gabbe SG. Insulin growth factors: their regulation of glucose and amino acid transport in placental trophoblasts isolated from first-trimester chorionic villi. J Reprod Med 1994; 39:249–256. [PubMed] [Google Scholar]
- 27.Abu Shehab M, Iosef C, Wildgruber R, Sardana G, Gupta MB. Phosphorylation of IGFBP-1 at discrete sites elicits variable effects on IGF-I receptor autophosphorylation. Endocrinology 2013; 154:1130–1143. [DOI] [PubMed] [Google Scholar]
- 28.Gibson JM, Aplin JD, White A, Westwood M. Regulation of IGF bioavailability in pregnancy. Mol Hum Reprod 2001; 7:79–87. [DOI] [PubMed] [Google Scholar]
- 29.Jones JI, Busby WH Jr., Wright G, Smith CE, Kimack NM, Clemmons DR. Identification of the sites of phosphorylation in insulin-like growth factor binding protein-1. Regulation of its affinity by phosphorylation of serine 101. J Biol Chem 1993; 268:1125–1131. [PubMed] [Google Scholar]
- 30.Dolcini L, Sala A, Campagnoli M, Labo S, Valli M, Visai L, Minchiotti L, Monaco HL, Galliano M. Identification of the amniotic fluid insulin-like growth factor binding protein-1 phosphorylation sites and propensity to proteolysis of the isoforms. FEBS J 2009; 276:6033–6046. [DOI] [PubMed] [Google Scholar]
- 31.Frost RA, Tseng L. Insulin-like growth factor-binding protein-1 is phosphorylated by cultured human endometrial stromal cells and multiple protein kinases in vitro. J Biol Chem 1991; 266:18082–18088. [PubMed] [Google Scholar]
- 32.Yu J, Iwashita M, Kudo Y, Takeda Y. Phosphorylated insulin-like growth factor (IGF)-binding protein-1 (IGFBP-1) inhibits while non-hosphorylated IGFBP-1 stimulates IGF-I-induced amino acid uptake in cultured trophoblast cells. Growth Hormone and IGF Research 1998; 8:65–70. [DOI] [PubMed] [Google Scholar]
- 33.Siddals KW, Westwood M, Gibson JM, White A. IGF-binding protein-1 inhibits IGF effects on adipocyte function: implications for insulin-like actions at the adipocyte. J Endocrinol 2002; 174:289–297. [DOI] [PubMed] [Google Scholar]
- 34.Whitsett JA, Lessard JL. Characteristics of the microvillus brush border of human placenta: insulin receptor localization in brush border membranes. Endocrinology 1978; 103:1458–1468. [DOI] [PubMed] [Google Scholar]
- 35.Nelson DM, Smith RM, Jarett L. Nonuniform distribution and grouping of insulin receptors on the surface of human placental syncytial trophoblast. Diabetes 1978; 27:530–538. [PubMed] [Google Scholar]
- 36.Whitsett JA, Johnson CL, Hawkins K. Differences in localization of insulin receptors and adenylate cyclase in the human placenta. Am J Obstet Gynecol 1979; 133:204–207. [DOI] [PubMed] [Google Scholar]
- 37.Tavare JM, Holmes CH. Differential expression of the receptors for epidermal growth factor and insulin in the developing human placenta. Cell Signal 1989; 1:55–64. [DOI] [PubMed] [Google Scholar]
- 38.Desoye G, Hartmann M, Blaschitz A, Dohr G, Hahn T, Kohnen G, Kaufmann P. Insulin receptors in syncytiotrophoblast and fetal endothelium of human placenta. Immunohistochemical evidence for developmental changes in distribution pattern. Histochemistry 1994; 101:277–285. [DOI] [PubMed] [Google Scholar]
- 39.Fang J, Furesz TC, Lurent RS, Smith CH, Fant M. Spatial polarization of insulin-like growth factor receptors on the human syncytiotrophoblast. Pediatr Res 1997; 41:258–265. [DOI] [PubMed] [Google Scholar]
- 40.Irwin JC, Giudice LC. Insulin-like growth factor binding protein-1 binds to placental cytotrophoblast alpha5beta1 integrin and inhibits cytotrophoblast invasion into decidualized endometrial stromal cultures. Growth Horm IGF Res 1998; 8:21–31. [DOI] [PubMed] [Google Scholar]
- 41.Lacey H, Haigh T, Westwood M, Aplin JD. Mesenchymally-derived insulin-like growth factor 1 provides a paracrine stimulus for trophoblast migration. BMC Dev Biol 2002; 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martina NA, Kim E, Chitkara U, Wathen NC, Chard T, Giudice LC. Gestational age-dependent expression of insulin-like growth factor-binding protein-1 (IGFBP-1) phosphoisoforms in human extraembryonic cavities, maternal serum, and decidua suggests decidua as the primary source of IGFBP-1 in these fluids during early pregnancy. J Clin Endocrinol Metab 1997; 82:1894–1898. [DOI] [PubMed] [Google Scholar]
- 43.Fang Q, Wang YX, Zhou Y. Insulin-like growth factor binding protein 1 and human embryonic development during 6 – 10 gestational weeks. Chin Med J (Engl) 2004; 117:488–491. [PubMed] [Google Scholar]
- 44.Holmes R, Montemagno R, Jones J, Preece M, Rodeck C, Soothill P. Fetal and maternal plasma insulin-like growth factors in pregnancies with appropriate or retarded fetal growth. Early Hum Dev 1997; 49:7–17. [DOI] [PubMed] [Google Scholar]
- 45.Hall K, Hansson U, Lundin G, Luthman M, Persson B, Povoa G, Stangenberg M, Ofverholm U. Serum levels of somatomedins and somatomedin-binding protein in pregnant women with type I or gestational diabetes and their infants. J Clin Endocrinol Metab 1986; 63:1300–1306. [DOI] [PubMed] [Google Scholar]
- 46.Hills FA, English J, Chard T. Circulating levels of IGF-I and IGF-binding protein-1 throughout pregnancy: relation to birthweight and maternal weight. J Endocrinol 1996; 148:303–309. [DOI] [PubMed] [Google Scholar]
- 47.Wang HS, Lim J, English J, Irvine L, Chard T. The concentration of insulin-like growth factor-I and insulin-like growth factor-binding protein-1 in human umbilical cord serum at delivery: relation to fetal weight. J Endocrinol 1991; 129:459–464. [DOI] [PubMed] [Google Scholar]
- 48.Boyne MS, Thame M, Bennett FI, Osmond C, Miell JP, Forrester TE. The relationship among circulating insulin-like growth factor (IGF)-I, IGF-binding proteins-1 and −2, and birth anthropometry: a prospective study. J Clin Endocrinol Metab 2003; 88:1687–1691. [DOI] [PubMed] [Google Scholar]
- 49.Gibson JM, Westwood M, Lauszus FF, Klebe JG, Flyvbjerg A, White A. Phosphorylated insulin-like growth factor binding protein 1 is increased in pregnant diabetic subjects. Diabetes 1999; 48:321–326. [DOI] [PubMed] [Google Scholar]
- 50.Langford K, Blum W, Nicolaides K, Jones J, McGregor A, Miell J. The pathophysiology of the insulin-like growth factor axis in fetal growth failure: a basis for programming by undernutrition? Eur J Clin Invest 1994; 24:851–856. [DOI] [PubMed] [Google Scholar]
- 51.Olausson H, Lof M, Brismar K, Forsum E, Sohlstrom A. Maternal serum concentrations of insulin-like growth factor (IGF)-I and IGF binding protein-1 before and during pregnancy in relation to maternal body weight and composition and infant birth weight. Br J Nutr 2010; 104:842–848. [DOI] [PubMed] [Google Scholar]
- 52.Baldwin S, Chung T, Rogers M, Chard T, Wang HS. Insulin-like growth factor binding protein-1, glucose tolerance and fetal growth in human pregnancy. J Endocrinol 1993; 136:319–325. [DOI] [PubMed] [Google Scholar]
- 53.Fowler D, Albaiges G, Lees C, Jones J, Nicolaides K, Miell J. The role of insulin-like growth factor binding protein-1 phosphoisoforms in pregnancies with impaired placental function identified by doppler ultrasound. Hum Reprod 1999; 14:2881–2885. [DOI] [PubMed] [Google Scholar]
- 54.Larsen T, Main K, Andersson AM, Juul A, Greisen G, Skakkebaeck NE. Growth hormone, insulin-like growth factor I and its binding proteins 1 and 3 in last trimester intrauterine growth retardation with increased pulsatility index in the umbilical artery. Clin Endocrinol 1996; 45:315–319. [DOI] [PubMed] [Google Scholar]
- 55.Bhatia S, Faessen G, Carland R, Balise RL, Gargosky SE, Druzin M, El-Sayed Y, Wilson DM, Giudice LC. A longitudinal analysis of maternal serum insulin-like growth factor I (IGF-I) and total and nonphosphorylated IGF-binding protein-1 in human pregnancies complicated by intrauterine growth restriction. J Clin Endocrinol Metab 2002; 87:1864–1870. [DOI] [PubMed] [Google Scholar]
- 56.Sifakis S, Akolekar R, Kappou D, Mantas N, Nicolaides KH. Maternal serum insulin-like growth factor (IGF-I) and binding proteins IGFBP-1 and IGFBP-3 at 11–13 weeks’ gestation in pregnancies delivering small for gestational age neonates. Eur J Obstet Gynecol Reprod Biol 2012; 161:30–33. [DOI] [PubMed] [Google Scholar]
- 57.Qiu C, Vadachkoria S, Meryman L, Frederick IO, Williams MA. Maternal plasma concentrations of IGF-1, IGFBP-1, and C-peptide in early pregnancy and subsequent risk of gestational diabetes mellitus. Am J Obstet Gynecol 2005; 193:1691–1697. [DOI] [PubMed] [Google Scholar]
- 58.Clapp JF 3rd, Schmidt F, Paranjape A, Lopez B. Maternal insulin-like growth factors levels (IGF) reflect placental mass and neonatal mass. Am J Obstet Gynecol 2004; 190:730–736. [DOI] [PubMed] [Google Scholar]
- 59.Reis FM, Florio P, Cobellis L, Luisi S, Severi FM, Bocchi C, Picciolini E, Centini G, Petraglia F. Human placenta as a source of neuroendocrine factors. Biol Neonate 2001; 79:150–156. [DOI] [PubMed] [Google Scholar]
- 60.Iwashita M, Sakai K, Kudo Y, Takeda Y. Phosphoisoforms of insulin-like growth factor binding protein-1 in appropriate-for-gestational-age and small-for-gestational-age fetuses. Growth Horm IGF Res 1998; 8:487–493. [DOI] [PubMed] [Google Scholar]
- 61.Gupta MB, Abu Shehab M, Nygard K, Biggar K, Singal SS, Santoro N, Powell TL, Jansson T. IUGR is associated with marked hyperphosphorylation of decidual and maternal plasma IGFBP-1. J Clin Endocrinol Metab 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heffner LJ, Bromley BS, Copeland KC. Secretion of prolactin and insulin-like growth factor I by decidual explant cultures from pregnancies complicated by intrauterine growth retardation. Am J Obstet Gynecol 1992; 167:1431–1436. [DOI] [PubMed] [Google Scholar]
- 63.Calvo MT, Romo A, Gutierrez JJ, Relano E, Barrio E, Ferrandez Longas A. Study of genetic expression of intrauterine growth factors IGF-I and EGFR in placental tissue from pregnancies with intrauterine growth retardation. J Pediatr Endocrinol Metab 2004; 17 Suppl 3:445–450. [PubMed] [Google Scholar]
- 64.Yung HW, Calabrese S, Hynx D, Hemmings BA, Cetin I, Charnock-Jones S, Burton GJ. Evidence of translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am J Pathol 2008; 173:311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laviola L, Perrini S, Belsanti G, Natalicchio A, Montrone C, Leonardini A, Vimercati A, Scioscia M, Selvaggi L, Giorgino R, Greco P, Giorgino F. Intrauterine Growth Restriction in Humans Is Associated with Abnormalities in Placental Insulin-Like Growth Factor Signaling. Endocrinology 2005; 146:1498–1505. [DOI] [PubMed] [Google Scholar]
- 66.Street ME, Viani I, Ziveri MA, Volta C, Smerieri A, Bernasconi S. Impairment of insulin receptor signal transduction in placentas of intra-uterine growth-restricted newborns and its relationship with fetal growth. Eur J Endocrinol 2011; 164:45–52. [DOI] [PubMed] [Google Scholar]
- 67.Potau N, Riudor E, Ballabriga A. Insulin receptors in human placenta in relation to fetal weight and gestational age. Pediatr Res 1981; 15:798–802. [PubMed] [Google Scholar]
- 68.Gurel D, Ozer E, Altunyurt S, Guclu S, Demir N. Expression of IGR-IR and VEGF and trophoblastic proliferative activity in placentas from pregnancies complicated by IUGR. Pathol Res Pract 2003; 199:803–809. [DOI] [PubMed] [Google Scholar]
- 69.Abu-Amero SN, Ali A, Bennett P, Vaughan JI, Moore GE. Expression of the insulin-like growth factors and their receptors in term placentas: A comparison between normal and IUGR births. Molecular Reproduction and Development 1998; 49:229–235. [DOI] [PubMed] [Google Scholar]
- 70.Iniguez G, Castro JJ, Garcia M, Kakarieka E, Johnson MC, Cassorla F, Mericq V. IGF-IR signal transduction protein content and its activation by IGF-I in human placentas: relationship with gestational age and birth weight. PLoS One 2014; 9:e102252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roos S, Jansson N, Palmberg I, Säljö K, Powell TL, Jansson T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growt. J Physiol 2007; 582:449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bowman CJ, Streck RD, Chapin RE. Maternal-placental insulin-like growth factor (IGF) signaling and its importance to normal embryo-fetal development. Birth Defects Res B Dev Reprod Toxicol 2010; 89:339–349. [DOI] [PubMed] [Google Scholar]
- 73.Roos S, Lagerlöf O, Wennergren M, Powell TL, Jansson T. Regulation of amino acid transporters by glucose and growth factors in cultured primary human trophoblast cells is mediated by mTOR signaling Am J Physiol Cell 2009; 297:C723–C731. [DOI] [PubMed] [Google Scholar]
- 74.Peng T, Golub TR, Sabatini DM. The Immunosuppressant Rapamycin Mimics a Starvation-Like Signal Distinct from Amino Acid and Glucose Deprivation. Mol. Cell. Biol 2002; 22:5575–5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tee AR, Blenis J. mTor, translational control and human disease Sem Cell Dev Biol 2005; 16:29–37. [DOI] [PubMed] [Google Scholar]
- 76.Martin DE, Hall MN. The expanding TOR network. Curr Opin Cell Biol 2005; 17:158–166. [DOI] [PubMed] [Google Scholar]
- 77.Hay N, Soneneberg N. Upstream and downstream of mTOR. Genes Dev 2004; 18:1926–1945. [DOI] [PubMed] [Google Scholar]
- 78.Jacinto E, Hall MN. TOR signalling in bugs, brain and brawn. Nature Rev Mol Cell Biol 2003; 4:117–126. [DOI] [PubMed] [Google Scholar]
- 79.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012; 149:274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005; 307:1098–1101. [DOI] [PubMed] [Google Scholar]
- 81.Garcia-Martinez JM, Alessi DR. mTOR complex-2 (mTORC2) controls hydrophobic motif phophorylation and activation of serum and glucocorticoid induced protein kinase-1 (SGK1). Biochem J 2008; 416:375–385. [DOI] [PubMed] [Google Scholar]
- 82.Facchinetti V, Ouyang W, Wei H, Soto N, Lazorchak A, Gould C, Lowry C, Newton AC, Mao Y, Miao RQ, Sessa WC, Qin J, et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J 2008; 27:1932–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl VM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frquently overexpressed in multiple myeloma cells and required for their survival. Cell 2009; 137:873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017; 169:361–371. [DOI] [PubMed] [Google Scholar]
- 85.Roux PP, Topisirovic I. Signaling Pathways Involved in the Regulation of mRNA Translation. Mol Cell Biol 2018; 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosario FJ, Gupta MB, Myatt L, Powell TL, Glenn JP, Cox LA, Jansson T. Mechanistic Target of Rapamycin Complex 1 is a Positive Regulator of Genes Encoding Electron Transport Chain Proteins and Oxidative Phosphorylation, which is inhibited in Placentas of Growth Restricted Fetuses Sci Rep 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rosario FJ, Kanai Y, Powell TL, Jansson T. Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells. J Physiol 2013; 591:609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rosario FJ, Dimasuay KG, Kanai Y, Powell TL, Jansson T. Regulation of Amino Acid Transporter Trafficking by mTORC1 in Primary Human Trophoblast cells is Mediated by the Ubiquitin Ligase Nedd4–2. Clin Sci (Lond) 2016; 130:499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rosario FJ, Powell TL, Jansson T. Mechanistic target of rapamycin (mTOR) regulates trophoblast folate uptake by modulating the cell surface expression of FR-alpha and the RFC. Sci Rep 2016; 6:31705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roos S, Jansson N, Palmberg I, Saljo K, Powell TL, Jansson T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J Physiol 2007; 582:449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yung HW, Calabrese S, Hynx D, Hemmings BA, Cetin I, Charnock-Jones DS, Burton GJ. Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am J Pathol 2008; 173:451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen YY, Rosario FJ, Shehab MA, Powell TL, Gupta MB, Jansson T. Increased ubiquitination and reduced plasma membrane trafficking of placental amino acid transporter SNAT-2 in human IUGR. Clin Sci (Lond) 2015; 129:1131–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hung TH, Hsieh TT, Wu CP, Li MJ, Yeh YL, Chen SF. Mammalian target of rapamycin signaling is a mechanistic link between increased endoplasmic reticulum stress and autophagy in the placentas of pregnancies complicated by growth restriction. Placenta 2017; 60:9–20. [DOI] [PubMed] [Google Scholar]
- 94.Dimasuay KG, Aitken EH, Rosario F, Njie M, Glazier J, Rogerson SJ, Fowkes FJ, Beeson JG, Powell T, Jansson T, Boeuf P. Inhibition of placental mTOR signaling provides a link between placental malaria and reduced birthweight. BMC Med 2017; 15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aiko Y, Askew DJ, Aramaki S, Myoga M, Tomonaga C, Hachisuga T, Suga R, Kawamoto T, Tsuji M, Shibata E. Differential levels of amino acid transporters System L and ASCT2, and the mTOR protein in placenta of preeclampsia and IUGR. BMC Pregnancy Childbirth 2014; 14:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oakes SA, Papa FR. The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol 2015; 10:173–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab 2009; 20:436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yung HW, Cox M, Tissot van Patot M, Burton GJ. Evidence of endoplasmic reticulum stress and protein synthesis inhibition in the placenta of non-native women at high altitude. FASEB J 2012; 26:1970–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jin HO, Seo SK, Woo SH, Kim ES, Lee HC, Yoo DH, Choe TB, Hong SI, Kim JI, Park IC. SP600125 negatively regulates the mammalian target of rapamycin via ATF4-induced Redd1 expression. FEBS Lett 2009; 583:123–127. [DOI] [PubMed] [Google Scholar]
- 100.Appenzeller-Herzog C, Hall MN. Bidirectional crosstalk between endoplasmic reticulum stress and mTOR signaling. Trends Cell Biol 2012; 22:274–282. [DOI] [PubMed] [Google Scholar]
- 101.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell 2005; 123:993–999. [DOI] [PubMed] [Google Scholar]
- 102.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O’Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 1999; 402:880–883. [DOI] [PubMed] [Google Scholar]
- 103.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell 1999; 4:585–595. [DOI] [PubMed] [Google Scholar]
- 104.Schaiff WT, Carlson MG, Smith SD, Levy R, Nelson DM, Sadovsky Y. Peroxisome proliferator-activated receptor-gamma modulates differentiation of human trophoblast in a ligand-specific manner. J Clin Endocrinol Metab 2000; 85:3874–3881. [DOI] [PubMed] [Google Scholar]
- 105.Nadra K, Quignodon L, Sardella C, Joye E, Mucciolo A, Chrast R, Desvergne B. PPARgamma in placental angiogenesis. Endocrinology 2010; 151:4969–4981. [DOI] [PubMed] [Google Scholar]
- 106.Schaiff WT, Bildirici I, Cheong M, Chern PL, Nelson DM, Sadovsky Y. Peroxisome proliferator-activated receptor-gamma and retinoid X receptor signaling regulate fatty acid uptake by primary human placental trophoblasts. J Clin Endocrinol Metab 2005; 90:4267–4275. [DOI] [PubMed] [Google Scholar]
- 107.Chen Z, He P, Ding X, Huang Y, Gu H, Ni X. PPARgamma stimulates expression of L-type amino acid and taurine transporters in human placentas: the evidence of PPARgamma regulating fetal growth. Sci Rep 2015; 5:12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Biron-Shental T, Schaiff WT, Ratajczak CK, Bildirici I, Nelson DM, Sadovsky Y. Hypoxia regulates the expression of fatty-acid binding proteins in primary term human trophoblasts. Am J Obstet Gynecol 2007; 197:516.e511–516.e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Holdsworth-Carson SJ, Lim R, Mitton A, Whitehead C, Rice GE, Permezel M, Lappas M. Peroxisome proliferator-activated receptors are altered in pathologies of the human placenta: gestational diabetes mellitus, intrauterine growth restriction and preeclampsia. Placenta 2010; 31:222–229. [DOI] [PubMed] [Google Scholar]
- 110.Rodie VA, Young A, Jordan F, Sattar N, Greer IA, Freeman DJ. Human placental peroxisome proliferator-activated receptor delta and gamma expression in healthy pregnancy and in preeclampsia and intrauterine growth restriction. J Soc Gynecol Investig 2005; 12:320–329. [DOI] [PubMed] [Google Scholar]
- 111.Diaz M, Bassols J, Lopez-Bermejo A, Gomez-Roig MD, de Zegher F, Ibanez L. Placental expression of peroxisome proliferator-activated receptor gamma (PPARgamma): relation to placental and fetal growth. J Clin Endocrinol Metab 2012; 97:E1468–1472. [DOI] [PubMed] [Google Scholar]
- 112.Mando C, De Palma C, Stampalija T, Anelli GM, Figus M, Novielli C, Parisi F, Clementi E, Ferrazzi E, Cetin I. Placental mitochondrial content and function in intrauterine growth restriction and preeclampsia. Am J Physiol Endocrinol Metab 2014; 306:E404–413. [DOI] [PubMed] [Google Scholar]
- 113.Lattuada D, Colleoni F, Martinelli A, Garretto A, Magni R, Radaelli T, Cetin I. Higher mitochondrial DNA content in human IUGR placenta. Placenta 2008; 29:1029–1033. [DOI] [PubMed] [Google Scholar]
- 114.Lefebvre T, Roche O, Seegers V, Cherif M, Khiati S, Gueguen N, Desquiret-Dumas V, Geffroy G, Blanchet O, Reynier P, Legendre G, Lenaers G, et al. Study of mitochondrial function in placental insufficiency. Placenta 2018; 67:1–7. [DOI] [PubMed] [Google Scholar]
- 115.Diaz M, Aragones G, Sanchez-Infantes D, Bassols J, Perez-Cruz M, de Zegher F, Lopez-Bermejo A, Ibanez L. Mitochondrial DNA in placenta: associations with fetal growth and superoxide dismutase activity. Horm Res Paediatr 2014; 82:303–309. [DOI] [PubMed] [Google Scholar]
- 116.Poidatz D, Dos Santos E, Duval F, Moindjie H, Serazin V, Vialard F, De Mazancourt P, Dieudonne MN. Involvement of estrogen-related receptor-gamma and mitochondrial content in intrauterine growth restriction and preeclampsia. Fertil Steril 2015; 104:483–490. [DOI] [PubMed] [Google Scholar]
- 117.Gluckman PD, Hanson MA, Pinal C. The developmental origins of adult disease. Mat Child Nutr 2005; 1:130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab 2004; 15:183–187. [DOI] [PubMed] [Google Scholar]
- 119.Boney CM, Verma A, Tucker R, Vohr BS. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes. Pediatrics 2005; 115:e290–e296. [DOI] [PubMed] [Google Scholar]
- 120.Barker DJ, Thornburg KL. Placental programming of chronic diseases, cancer and lifespan: a review. Placenta 2013; 34:841–845. [DOI] [PubMed] [Google Scholar]
- 121.Sibley C, D´Souza S, Glazier J, Greenwood S. Mechanisms of solute transfer across the human placenta: effects of intrauterine growth restriction. Fetal Maternal Medicine Rev 1998; 10:197–206. [Google Scholar]
- 122.Sibley CP, Turner MA, Cetin I, Ayuk P, Boyd CAR, Souza SW, Glazier JD, Greenwood SL, Jansson T, Powell T. Placental Phenotypes of Intrauterine Growth. Pediatr Res 2005; 58:827–832. [DOI] [PubMed] [Google Scholar]
- 123.Jansson T, Powell TL. IFPA 2005 Award in Placentology Lecture. Human Placental Transport in Altered Fetal Growth: Does the Placenta Function as a Nutrient Sensor? - A Review. Placenta 2006; 27 SupplA S91–97. [DOI] [PubMed] [Google Scholar]
- 124.Diaz P, Powell TL, Jansson T. The role of placental nutrient sensing in maternal-fetal resource allocation. Biol Reprod 2014; 91:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jansson T, Powell TL. Role of placental nutrient sensing in developmental programming. Clin Obstet Gynecol 2013; 56:591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Desforges M, Sibley CP. Placental nutrient supply and fetal growth. Int J Dev Biol 2010; 54:377–390. [DOI] [PubMed] [Google Scholar]
- 127.Jansson T, Myatt L, Powell TL. The role of trophoblast nutrient and ion transporters in the development of pregnancy complications and adult disease. Curr Vasc Pharmacol 2009; 7:521–533. [DOI] [PubMed] [Google Scholar]
- 128.Gaccioli F, Lager S. Placental Nutrient Transport and Intrauterine Growth Restriction. Front Physiol 2016; 7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Winterhager E, Gellhaus A. Transplacental Nutrient Transport Mechanisms of Intrauterine Growth Restriction in Rodent Models and Humans. Front Physiol 2017; 8:951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cetin I, Alvino G. Intrauterine growth restriction: implications for placental metabolism and transport. A review. Placenta 2009; 30 Suppl A:S77–82. [DOI] [PubMed] [Google Scholar]
- 131.Brett KE, Ferraro ZM, Yockell-Lelievre J, Gruslin A, Adamo KB. Maternal-fetal nutrient transport in pregnancy pathologies: the role of the placenta. Int J Mol Sci 2014; 15:16153–16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Regnault TRH, Friedman JE, Wilkening RB, Anthony RV, Hay WW Jr., Fetoplacental transport and utilization of amino acids in IUGR- A review. Placenta 2005; 26 Suppl A:S52–S62. [DOI] [PubMed] [Google Scholar]
- 133.Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol 2018; 218:S745–S761. [DOI] [PubMed] [Google Scholar]
- 134.Lager S, Powell TL. Regulation of nutrient transport across the placenta. J Pregnancy 2012; 2012:179827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Avagliano L, Garo C, Marconi AM. Placental amino acids transport in intrauterine growth restriction. J Pregnancy 2012; 2012:972562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jansson T, Wennergren M, Illsley NP. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab 1993; 77:1554–1562. [DOI] [PubMed] [Google Scholar]
- 137.Xing AY, Challier JC, Lepercq J, Cauzac M, Charron MJ, Girard J, Hauguel-de Mouzon S. Unexpected expression of glucose transporter 4 in villous stromal cells of human placenta. J Clin Endocrinol Metab 1998; 83:4097–4101. [DOI] [PubMed] [Google Scholar]
- 138.Acosta O, Ramirez VI, Lager S, Gaccioli F, Dudley DJ, Powell TL, Jansson T. Increased glucose and placental GLUT-1 in large infants of obese nondiabetic mothers. Am J Obstet Gynecol 2015; 212:227 e221–227. [DOI] [PubMed] [Google Scholar]
- 139.Bibee KP, Illsley NP, Moley KH. Asymmetric syncytial expression of GLUT9 splice variants in human term placenta and alterations in diabetic pregnancies. Reprod Sci 2011; 18:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brown K, Heller DS, Zamudio S, Illsley NP. Glucose transporter 3 (GLUT3) protein expression in human placenta across gestation. Placenta 2011; 32:1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hauguel-de Mouzon S, Challier JC, Kacemi A, Cauzac M, Malek A, Girard J. The GLUT3 glucose transporter isoform is differentially expressed within human placental types. J Clin Endocrinol Metab 1997; 82:2689–2694. [DOI] [PubMed] [Google Scholar]
- 142.Ericsson A, Hamark B, Powell TL, Jansson T. Glucose transporter isoform 4 is expressed in the syncytiotrophoblast of first trimester human placenta. Hum. Reprod 2005; 20:521–530. [DOI] [PubMed] [Google Scholar]
- 143.Gude NM, Stevenson JL, Rogers S, Best JD, Kalionis B, Huisman MA, Erwich JJ, Timmer A, King RG. GLUT12 expression in human placenta in first trimester and term. Placenta 2003; 24:566–570. [DOI] [PubMed] [Google Scholar]
- 144.Jansson T, Ylvén K, Wennergren M, Powell TL. Glucose transport and system A activity in syncytiotrophoblast microvillous and basal membranes in intrauterine growth restriction. Placenta 2002; 23:392–399. [DOI] [PubMed] [Google Scholar]
- 145.Zamudio S, Baumann MU, Illsley NP. Effects of chronic hypoxia in vivo on the expression of human placental glucose transporters. Placenta 2006; 27:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Janzen C, Lei MY, Cho J, Sullivan P, Shin BC, Devaskar SU. Placental glucose transporter 3 (GLUT3) is up-regulated in human pregnancies complicated by late-onset intrauterine growth restriction. Placenta 2013; 34:1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Challis DE, Pfarrer CD, Ritchie KJW, Koren G, Adamson SL. Glucose metabolism is elevated and vascular resistance and maternofetal transfer is normal in perfused placental cotyledons from severely growth-restricted fetuses. Pediatr Res 2000; 47:309–315. [DOI] [PubMed] [Google Scholar]
- 148.Lee MH, Jeon YJ, Lee SM, Park MH, Jung SC, Kim YJ. Placental gene expression is related to glucose metabolism and fetal cord blood levels of insulin and insulin-like growth factors in intrauterine growth restriction. Early Hum Dev 2010; 86:45–50. [DOI] [PubMed] [Google Scholar]
- 149.Magnusson AL, Powell TL, Wennergren M, Jansson T. Glucose metabolism in the human preterm and term placenta of IUGR fetuses. Placenta 2002; 25:337–346. [DOI] [PubMed] [Google Scholar]
- 150.Jansson T Amino acid transporters in the human placenta. Pediatr Res 2001; 49:141–147. [DOI] [PubMed] [Google Scholar]
- 151.Cleal JK, Lewis RM. The mechanisms and regulation of placental amino acid transport to the human foetus. J Neuroendocrinol 2008; 20:419–426. [DOI] [PubMed] [Google Scholar]
- 152.Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Arch 2004; 447:784–795. [DOI] [PubMed] [Google Scholar]
- 153.Desforges M, Lacey HA, Glazier JD, Greenwood SL, Mynett KJ, Speake PF, Sibley CP. The SNAT4 isoform of the system A amino acid transporter is expressed in human placenta. Am J Physiol Cell Physiol 2006; 290:C305–C312. [DOI] [PubMed] [Google Scholar]
- 154.Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch 2003. [DOI] [PubMed] [Google Scholar]
- 155.Mahendran D, Donnai P, Glazier JD, D’Souza SW, Boyd RDH, Sibley CP. Amino acid (System A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr Res 1993; 34:661–665. [DOI] [PubMed] [Google Scholar]
- 156.Glazier JD, Cetin I, Perugino G, Ronzoni S, Grey AM, Mahendran D, Marconi AM, Pardi G, Sibley CP. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res 1997; 42:514–519. [DOI] [PubMed] [Google Scholar]
- 157.Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol 2006; 576:935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rosario FJ, Jansson N, Kanai Y, Prasad PD, Powell TL, Jansson T. Maternal protein restriction in the rat inhibits placental insulin, mTOR, and STAT3 signaling and down-regulates placental amino acid transporters. Endocrinology 2011; 152:1119–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pantham P, Rosario FJ, Weintraub S, Nathanielsz PW, Powell TL, Li C, Jansson T. Down-regulation of Placental Transport of Amino Acids Precedes the Development of Intrauterine Growth Restriction in Maternal Nutrient Restricted Baboons Biol Reprod 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pantham P, Rosario FJ, Nijland M, Cheung A, Nathanielsz PW, Powell TL, Galan HL, Li C, Jansson T. Reduced placental amino acid transport in response to maternal nutrient restriction in the baboon. Am J Physiol Regul Integr Comp Physiol 2015; 309:R740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kavitha JV, Rosario FJ, Nijland MJ, McDonald TJ, Wu G, Kanai Y, Powell TL, Nathanielsz PW, Jansson T. Down-regulation of placental mTOR, insulin/IGF-I signaling, and nutrient transporters in response to maternal nutrient restriction in the baboon. Faseb J 2014; 28:1294–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Norberg S, Powell TL, Jansson T. Intrauterine growth restriction is associated with a reduced activity of placental taurine transporters. Pediatr Res 1998; 44:233–238. [DOI] [PubMed] [Google Scholar]
- 163.Jansson T, Scholtbach V, Powell TL. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr Res 1998; 44:532–537. [DOI] [PubMed] [Google Scholar]
- 164.Paolini CL, Marconi AM, Ronzoni S, Di Noio M, Fennessey PV, Pardi G, Battaglia FC. Placental transport of leucine, phenylalanine, glycine, and proline in intrauterine growth-restricted pregnancies. J Clin Endocrinol Metab 2001; 86:5427–5432. [DOI] [PubMed] [Google Scholar]
- 165.Economides DL, Nicolaides KH, Gahl WA, Bernadini I, Evans MI. Plasma amino acids in appropriate- and small-for-gestational-age fetuses. Am J Obstet Gynecol 1989; 161:1219–1227. [DOI] [PubMed] [Google Scholar]
- 166.Cetin I, Marconi AM, Bozzetti P, Sereni LP, Corbetta C, Pardi G, Battaglia FC. Umbilical amino acid concentrations in appropriate and small for gestational age infants: a biochemical difference present in utero. Am J Obstet Gynecol 1988; 158:120–126. [DOI] [PubMed] [Google Scholar]
- 167.Lindegaard ML, Damm P, Mathiesen ER, Nielsen LB. Placental triglyceride accumulation in maternal type 1 diabetes is associated with increased lipase gene expression. J Lipid Res 2006; 47:2581–2588. [DOI] [PubMed] [Google Scholar]
- 168.Kazantzis M, Stahl A. Fatty acid transport proteins, implications in physiology and disease. Biochim Biophys Acta 2012; 1821:852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mishima T, Miner JH, Morizane M, Stahl A, Sadovsky Y. The expression and function of fatty acid transport protein-2 and −4 in the murine placenta. PLoS One 2011; 6:e25865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dube E, Gravel A, Martin C, Desparois G, Moussa I, Ethier-Chiasson M, Forest JC, Giguere Y, Masse A, Lafond J. Modulation of Fatty Acid Transport and Metabolism by Obesity in the Human Full-Term Placenta. Biol Reprod 2012. [DOI] [PubMed] [Google Scholar]
- 171.Campell FM, Gordon MJ, Dutta-Roy AK. Placental membrane fatty acid-binding protein preferentially binds arachidonic and docosahexaenoic acids. Life Sci 1998; 63:235–240. [DOI] [PubMed] [Google Scholar]
- 172.Duttaroy AK. Transport of fatty acids across the human placenta: a review. Prog Lipid Res 2009; 48:52–61. [DOI] [PubMed] [Google Scholar]
- 173.Lager S, Ramirez VI, Gaccioli F, Jang B, Jansson T, Powell TL. Protein expression of fatty acid transporter 2 is polarized to the trophoblast basal plasma membrane and increased in placentas from overweight/obese women. Placenta 2016; 40:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Magnusson AL, Waterman IJ, Wennergren M, Jansson T, Powell TL. Triglyceride hydrolase activities and expression of fatty acid binding proteins in human placenta in pregnancies complicated by IUGR and diabetes. J Clin Endocrinol Metab 2004; 89:4607–4614. [DOI] [PubMed] [Google Scholar]
- 175.Gauster M, Hiden U, Blaschitz A, Frank S, Lang U, Alvino G, Cetin I, Desoye G, Wadsack C. Dysregulation of placental endothelial lipase and lipoprotein lipase in intrauterine growth-restricted pregnancies. J Clin Endocrinol Metab 2007; 92:2256–2263. [DOI] [PubMed] [Google Scholar]
- 176.Wadsack C, Tabano S, Maier A, Hiden U, Alvino G, Cozzi V, Hüttinger M, Schneider WJ, Lang U, Cetin I, Desoye G. Intrauterine growth restriction is associated with alterations in placental lipoprotein receptors and maternal lipoprotien composition Am J Physiol 2007; 292:E476–E484. [DOI] [PubMed] [Google Scholar]
- 177.Chassen SS, Ferchaud-Roucher V, Gupta MB, Jansson T, Powell TL. Alterations in placental long chain polyunsaturated fatty acid metabolism in human intrauterine growth restriction. Clin Sci (Lond) 2018; 132:595–607. [DOI] [PubMed] [Google Scholar]
- 178.Kemp G, Young H, Fliegel L. Structure and function of the human Na+/H+ exchanger isoform 1. Channels (Austin) 2008; 2:329–336. [DOI] [PubMed] [Google Scholar]
- 179.Balkovetz DF, Leibach FH, Mahesh VB, Devoe LD, Cragoe EJ Jr., Ganapathy V. Na+-H+ exchanger of human placental brush-boder membrane:identification and characterization. Am J Physiol 1986; 251:C852–860. [DOI] [PubMed] [Google Scholar]
- 180.Sibley CP, Glazier JD, Greenwood SL, Lacey H, Mynett K, Speake P, Jansson T, Johansson M, Powell TL. Regulation of placental transfer: The Na+/H+ exchanger. Placenta 2002; 23 SupplA:S39–S46. [DOI] [PubMed] [Google Scholar]
- 181.Powell TL, Illsley NP. A novel technique for studying cellular function in human placenta: Gestational changes in intracellular pH regulation. Placenta 1996; 17:661–668. [DOI] [PubMed] [Google Scholar]
- 182.Kulanthaivel P, Leibach FH, Mahesh VB, Cragoe EJ Jr., Ganapathy V. The Na(+)-H+ exchanger of the placental brush-border membrane is pharmacologically distinct from that of the renal brush-border membrane. J Biol Chem 1990; 265:1249–1252. [PubMed] [Google Scholar]
- 183.Kulanthaivel P, Furesz TC, Moe AJ, Smith CH, Mahesh VB, Leibach FH, Ganapathy V. Human placental syncytiotrophoblast expresses two pharmacologically distinguishable types of Na(+)-H+ exchangers, NHE-1 in the maternal-facing (brush border) membrane and NHE-2 in the fetal-facing (basal) membrane. Biochem J 1992; 284 (Pt 1):33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hughes JL, Doughty IM, Glazier JD, Powell TL, Jansson T, D’Souza SW, Sibley CP. Activity and expression of the Na+/H+ exchanger in the microvillous plasma membrane of the syncytiotrophoblast in relation to gestation and small for gestational age birth. Pediatr Res 2000; 48:652–659. [DOI] [PubMed] [Google Scholar]
- 185.Johansson M, Jansson T, Glazier JD, Powell TL. Activity and expression of the Na+/H+ exchanger is reduced in syncytiotrophoblast microvillous plasma membranes isolated from preterm intrauterine growth restriction pregnancies. J Clin Endocrinol Metab 2002; 87:5686–5694. [DOI] [PubMed] [Google Scholar]
- 186.Burd LI, Jones MDJ, Simmons MA, Makowski EL, Meschia G, Battaglia FC. Placental production and foetal utilisation of lactate and pyruvate. Nature 1975; 254:710–711. [DOI] [PubMed] [Google Scholar]
- 187.Hooper SB, Walker DW, Harding R. Oxygen, glucose, and lactate uptake by fetus and placenta during prolonged hypoxemia. Am J Physiol 1995; 268:R303–R309. [DOI] [PubMed] [Google Scholar]
- 188.Halestrap AP. The monocarboxylate transporter family--Structure and functional characterization. IUBMB Life 2012; 64:1–9. [DOI] [PubMed] [Google Scholar]
- 189.Balkovetz DF, Leibach FH, Mahesh VB, Ganapathy V. A proton gradient is the driving force for uphill transport of lactate in human placental brush border membrane vesicles. J Biol Chem 1988; 263:13823–13830. [PubMed] [Google Scholar]
- 190.Settle P, Mynett KJ, Speake PF, Champion E, Doughty IM, Sibley CP, D’Souza SW, Glazier JD. Polarized lactate transporter activity and expression in the syncytiotrophoblast of the term human placenta. Plcenta 2004; 25:496–504. [DOI] [PubMed] [Google Scholar]
- 191.Inuyama M, Ushigome F, Emoto A, Koyabu N, Satoh S, Tsukimori K, Nakano H, Ohtani H, Sawada Y. Characteristics of L-lactic acid transport in basal membrane vesicles of human placental syncytiotrophoblast. Am J Physiol Cell Physiol 2002; 283:C822–830. [DOI] [PubMed] [Google Scholar]
- 192.Settle P, Sibley CP, Doughty IM, Johnston T, Glazier JD, Powell TL, Jansson T, D’Souza SW. Placental lactate transporter activity and expression in intrauterine growth restriction. J Soc Gynecol Investig 2006; 13:357–363. [DOI] [PubMed] [Google Scholar]
- 193.Brunette MG, Allard S. Phosphate uptake by syncytial brush border membranes of human placenta. Pediatr Res 1985; 19:1179–1182. [DOI] [PubMed] [Google Scholar]
- 194.Karl PI, Fisher SE. Biotin transport in microvillous membrane vesicles, cultured trophoblasts, and isolated perfused human placenta. Am J Physiol 1992; 262:C302–308. [DOI] [PubMed] [Google Scholar]
- 195.Ganapathy V, Ganapathy ME, Tiruppathi C, Miyamoto Y, Mahesh VB, Leibach FH. Sodium-gradient-driven, high-affinity, uphill transport of succinate in human placental brush-border membrane vesicles. Biochem J 1988; 249:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Whitsett J, Wallick E. (3H)-ouabain binding and Na+/K+ ATPase activity in human placenta. Am J Physiol 1980; 238:E38–E45. [DOI] [PubMed] [Google Scholar]
- 197.Johansson M, Jansson T, Powell TL. Na+/K+-ATPase is distributed to microvillous and basal membrane of the syncytiotrophoblast in the human placenta. Am J Physiol 2000; 279:R287–R294. [DOI] [PubMed] [Google Scholar]
- 198.Johansson M, Karlsson L, Wennergren M, Jansson T, Powell TL. Activity and protein expression of Na+K+ ATPase are reduced in microvillous syncytiotrophoblast plasma membranes isolated from pregnancies complicated by intrauterine growth restriction. J Clin Endocrinol Metab 2003; 88:2831–2837. [DOI] [PubMed] [Google Scholar]
- 199.Belkacemi L, Bedard I, Simoneau L, Lafond J. Calcium channels, transporters and exchangers in placenta: a review. Cell Calcium 2005; 37:1–8. [DOI] [PubMed] [Google Scholar]
- 200.Khanal RC, Nemere I. Regulation of intestinal calcium transport. Annu Rev Nutr 2008; 28:179–196. [DOI] [PubMed] [Google Scholar]
- 201.Strid H, Bucht E, Jansson T, Wennergren M, Powell TL. ATP-dependent Ca2+ transport across basal membrane of human syncytiotrophoblast in pregnancies complicated by intrauterine growth restriction or diabetes. Placenta 2003; 24:445–452. [DOI] [PubMed] [Google Scholar]
- 202.Strid H, Care A, Jansson T, Powell T. Parathyroid hormone-related peptide (38–94) amide stimulates ATP-dependent calcium transport in the basal plasma membrane of the human syncytiotrophoblast. J Endocrinol 2002; 175:517–524. [DOI] [PubMed] [Google Scholar]
- 203.Lindqvist PG, Molin J. Does antenatal identification of small-for-gestational age fetuses significantly improve their outcome? Ultrasound Obstet Gynecol 2005; 25:258–264. [DOI] [PubMed] [Google Scholar]
- 204.Malandro MS, Beveridge MJ, Kihlberg MS, Novak DA. Effect of low-protein diet-induced intrauterine growth retardation on rat placental amino acid transport. Am J Physiol 1996; 271:C295–C303. [DOI] [PubMed] [Google Scholar]
- 205.Barry JS, Rozance PJ, Anthony RV. An animal model of placental insufficiency-induced intrauterine growth restriction. Semin Perinatol 2008; 32:225–230. [DOI] [PubMed] [Google Scholar]
- 206.Arroyo JA, Brown LD, Galan HL. Placental mammalian target of rapamycin and related signaling pathways in an ovine model of intrauterine growth restriction. Am J Obstet Gynecol 2009; 201:616e611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Thureen PJ, Trembler KA, Meschia G, Makowski EL, Wilkening RB. Placental glucose transport in heat-induced fetal growth retardation. Am J Physiol 1992; 263:R578–585. [DOI] [PubMed] [Google Scholar]
- 208.Ross JC, Fennessey PV, Wilkening RB, Battaglia FC, Meschia G. Placental transport and fetal utilization of leucine in a model of fetal growth retardation. Am J Physiol 1996; 270:E491–E503. [DOI] [PubMed] [Google Scholar]
- 209.Anderson AH, Fennessey PV, Meschia G, Wilkening RB, Battaglia FC. Placental transport of threonine and its utilization in the normal and growth-restricted fetus. Am J Physiol 1997; 272:E892–900. [DOI] [PubMed] [Google Scholar]
- 210.de Vrijer B, Regnault TR, Wilkening RB, Meschia G, Battaglia FC. Placental uptake and transport of ACP, a neutral nonmetabolizable amino acid, in an ovine model of fetal growth restriction. Am J Physiol 2004; 287:E1114–E1124. [DOI] [PubMed] [Google Scholar]
- 211.Jansson T, Persson E. Placental transfer of glucose and aminoacids in intrauterine growth retardation: Studies with substrate analogs in the awake guinea pig. Pediatr Res 1990; 28:203–208. [DOI] [PubMed] [Google Scholar]
- 212.Nitzan M, Orloff S, Schulman JD. Placental transfer of analogs of glucose and amino acids in experimental intrauterine growth retardation. Pediatr Res 1979; 13:100–103. [DOI] [PubMed] [Google Scholar]
- 213.Audette MC, Challis JR, Jones RL, Sibley CP, Matthews SG. Antenatal dexamethasone treatment in midgestation reduces system A-mediated transport in the late-gestation murine placenta. Endocrinology 2011; 152:3561–3570. [DOI] [PubMed] [Google Scholar]
- 214.Rosario FJ, Schumacher MA, Jiang J, Kanai Y, Powell TL, Jansson T. Chronic maternal infusion of full-length adiponectin in pregnant mice down-regulates placental amino acid transporter activity and expression and decreases fetal growth. J Physiol 2012; 590:1495–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jones HN, Jansson T, Powell TL. Full length adiponectin attenuates insulin signaling and inhibits insulin-stimulated amino acid transport in human primary trophoblast cells. Diabetes 2010; 59:1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Rosario FJ, Nathanielsz PW, Powell TL, Jansson T. Maternal folate deficiency causes inhibition of mTOR signaling, down-regulation of placental amino acid transporters and fetal growth restriction in mice. Sci Rep 2017; 7:3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gaccioli F, Lager S, Powell TL, Jansson T. Placental transport in response to altered maternal nutrition. J DOHaD 2013; 4:101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sferruzzi-Perri AN, Vaughan OR, Coan PM, Suciu MC, Darbyshire R, Constancia M, Burton GJ, Fowden AL. Placental-specific Igf2 deficiency alters developmental adaptations to undernutrition in mice. Endocrinology 2011; 152:3202–3212. [DOI] [PubMed] [Google Scholar]
- 219.Coan PM, Vaughan OR, Sekita Y, Finn SL, Burton GJ, Constancia M, Fowden AL. Adaptations in placental phenotype support fetal growth during undernutrition of pregnant mice. J Physiol 2010; 588:527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Coan PM, Vaughan OR, McCarthy J, Mactier C, Burton GJ, Constancia M, Fowden AL. Dietary composition programmes placental phenotype in mice. J Physiol 2011; 589:3659–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Aye IL, Rosario FJ, Powell TL, Jansson T. Adiponectin supplementation in pregnant mice prevents the adverse effects of maternal obesity on placental function and fetal growth. Proc Natl Acad Sci U S A 2015; 112:12858–12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Pantham P, Rosario FJ, Weintraub ST, Nathanielsz PW, Powell TL, Li C, Jansson T. Down-Regulation of Placental Transport of Amino Acids Precedes the Development of Intrauterine Growth Restriction in Maternal Nutrient Restricted Baboons. Biol Reprod 2016; 95:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wenzel PL, Leone G. Expression of Cre recombinase in early diploid trophoblast cells of the mouse placenta. Genesis 2007; 45:129–134. [DOI] [PubMed] [Google Scholar]
- 224.Howerton CL, Bale TL. Targeted placental deletion of OGT recapitulates the prenatal stress phenotype including hypothalamic mitochondrial dysfunction. Proc Natl Acad Sci U S A 2014; 111:9639–9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, Blakely RD, Deneris ES, Levitt P. A transient placental source of serotonin for the fetal forebrain. Nature 2011; 472:347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Okada Y, Ueshin Y, Isotani A, Saito-Fujito T, Nakashima H, Kimura K, Mizoguchi A, Ohhora M, Ogata M, Oshima RG, Okabe M, Ikawa M. Complementation of placental defects and embryonic lethality by trophoblast-specific lentiviral gene transfer. Nat Biotechnol 2007; 25:233–237. [DOI] [PubMed] [Google Scholar]
- 227.Georgiades P, Cox B, Gertsenstein M, Chawengsaksophak K, Rossant J. Trophoblast-specific gene manipulation using lentivirus-based vectors. Biotechniques 2007; 42:317–325. [DOI] [PubMed] [Google Scholar]
- 228.Kumasawa K, Ikawa M, Kidoya H, Hasuwa H, Saito-Fujita T, Morioka Y, Takakura N, Kimura T, Okabe M. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci U S A 2011; 108:1451–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Reik W, Constancia M, Fowden A, Anderson N, Dean W, Ferguson-Smith A, Tycko B, Sibley C. Regulation of supply and demand for maternal nutrients in mammals by imprinted genes. J Physiol (Lond) 2003; 547:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Coan PM, Burton GJ, Ferguson-Smith AC. Imprinted genes in the placenta--a review. Placenta 2005; 26 Suppl A:S10–20. [DOI] [PubMed] [Google Scholar]
- 231.Roos S, Powell TL, Jansson T. Placental mTOR links maternal nutrient availability to fetal growth. Biochem Soc Trans 2009; 37:295–298. [DOI] [PubMed] [Google Scholar]
- 232.Jansson T, Aye IL, Goberdhan DC. The emerging role of mTORC1 signaling in placental nutrient-sensing. Placenta 2012; 33 Suppl 2:e23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sibley CP, Brownbill P, Dilworth M, Glazier JD. Review: Adaptation in placental nutrient supply to meet fetal growth demand: implications for programming. Placenta 2010; 31 Suppl:S70–74. [DOI] [PubMed] [Google Scholar]
- 234.Constancia M, Angiolini E, Sandovici I, Smith P, Kelsey G, Dean W, Ferguson-Smith A, Sibley CP, Reik W, Fowden A. Adaptation of nutrient supply to fetal demand in the mouse involves interaction between the Igf2 gene and placental transporter systems. Proc Natl Acad Sci 2005; 102:19219–19224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Angiolini E, Coan PM, Sandovici I, Iwajomo OH, Peck G, Burton GJ, Sibley CP, Reik W, Fowden AL, Constancia M. Developmental adaptations to increased fetal nutrient demand in mouse genetic models of Igf2-mediated overgrowth. Faseb J 2011; 25:1737–1745. [DOI] [PubMed] [Google Scholar]
- 236.Strid H, Bucht E, Jansson T, Wennergren M, Powell T. ATP-dependent Ca2+ transport across basal membrane of human syncytiotrophoblast in pregnancies complicated by diabetes or intrauterine growth restriction. Placenta 2003; 24:445–452. [DOI] [PubMed] [Google Scholar]
- 237.Jansson T, Ekstrand Y, Björn C, Wennergren M, Powell TL. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes 2002; 51:2214–2219. [DOI] [PubMed] [Google Scholar]
- 238.Jansson N, Rosario FJ, Gaccioli F, Lager S, Jones HN, Roos S, Jansson T, Powell TL. Activation of Placental mTOR Signaling and Amino Acid Transporters in Obese Women Giving Birth to Large Babies. J Clin Endocrinol Metab 2013; 98:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Jansson T, Wennergren M, Powell TL. Placental glucose transport and GLUT 1 expression in insulin dependent diabetes. Am J Obstet Gynecol 1999; 180:163–168. [DOI] [PubMed] [Google Scholar]
- 240.Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJ. Boys live dangerously in the womb. Am J Hum Biol 2010; 22:330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ingemarsson I Gender aspects of preterm birth. BJOG 2003; 110 Suppl 20:34–38. [DOI] [PubMed] [Google Scholar]
- 242.Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gend Med 2007; 4:19–30. [DOI] [PubMed] [Google Scholar]
- 243.Eskes M, Waelput AJM, Scherjon SA, Bergman KA, Abu-Hanna A, Ravelli ACJ. Small for gestational age and perinatal mortality at term: An audit in a Dutch national cohort study. Eur J Obstet Gynecol Reprod Biol 2017; 215:62–67. [DOI] [PubMed] [Google Scholar]
- 244.Simchen MJ, Weisz B, Zilberberg E, Morag I, Weissmann-Brenner A, Sivan E, Dulitzki M. Male disadvantage for neonatal complications of term infants, especially in small-for gestational age neonates. J Matern Fetal Neonatal Med 2014; 27:839–843. [DOI] [PubMed] [Google Scholar]
- 245.Cox LA, Li C, Glenn JP, Lange K, Spradling KD, Nathanielsz PW, Jansson T. Expression of the placental transcriptome in maternal nutrient reduction in baboons is dependent on fetal sex. J Nutr 2013; 143:1698–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sedlmeier EM, Brunner S, Much D, Pagel P, Ulbrich SE, Meyer HH, Amann-Gassner U, Hauner H, Bader BL. Human placental transcriptome shows sexually dimorphic gene expression and responsiveness to maternal dietary n-3 long-chain polyunsaturated fatty acid intervention during pregnancy. BMC Genomics 2014; 15:941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Gonzalez TL, Sun T, Koeppel AF, Lee B, Wang ET, Farber CR, Rich SS, Sundheimer LW, Buttle RA, Chen YI, Rotter JI, Turner SD, et al. Sex differences in the late first trimester human placenta transcriptome. Biol Sex Differ 2018; 9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kalisch-Smith JI, Simmons DG, Dickinson H, Moritz KM. Review: Sexual dimorphism in the formation, function and adaptation of the placenta. Placenta 2017; 54:10–16. [DOI] [PubMed] [Google Scholar]
- 249.Gong S, Sovio U, Aye IL, Gaccioli F, Dopierala J, Johnson MD, Wood AM, Cook E, Jenkins BJ, Koulman A, Casero RA Jr., Constancia M, et al. Placental polyamine metabolism differs by fetal sex, fetal growth restriction, and preeclampsia. JCI Insight 2018; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Taubert D, Berkels R, Grosser N, Schroder H, Grundemann D, Schomig E. Aspirin induces nitric oxide release from vascular endothelium: a novel mechanism of action. Br J Pharmacol 2004; 143:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Grosser N, Abate A, Oberle S, Vreman HJ, Dennery PA, Becker JC, Pohle T, Seidman DS, Schroder H. Heme oxygenase-1 induction may explain the antioxidant profile of aspirin. Biochem Biophys Res Commun 2003; 308:956–960. [DOI] [PubMed] [Google Scholar]
- 252.Meher A, Randhir K, Mehendale S, Wagh G, Joshi S. Maternal Fatty Acids and Their Association with Birth Outcome: A Prospective Study. PLoS One 2016; 11:e0147359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol 2017; 216:110–120 e116. [DOI] [PubMed] [Google Scholar]
- 254.de Vries JI, van Pampus MG, Hague WM, Bezemer PD, Joosten JH, Investigators F. Low-molecular-weight heparin added to aspirin in the prevention of recurrent early-onset preeclampsia in women with inheritable thrombophilia: the FRUIT-RCT. J Thromb Haemost 2012; 10:64–72. [DOI] [PubMed] [Google Scholar]
- 255.Rey E, Garneau P, David M, Gauthier R, Leduc L, Michon N, Morin F, Demers C, Kahn SR, Magee LA, Rodger M. Dalteparin for the prevention of recurrence of placental-mediated complications of pregnancy in women without thrombophilia: a pilot randomized controlled trial. J Thromb Haemost 2009; 7:58–64. [DOI] [PubMed] [Google Scholar]
- 256.Rodger MA, Gris JC, de Vries JIP, Martinelli I, Rey E, Schleussner E, Middeldorp S, Kaaja R, Langlois NJ, Ramsay T, Mallick R, Bates SM, et al. Low-molecular-weight heparin and recurrent placenta-mediated pregnancy complications: a meta-analysis of individual patient data from randomised controlled trials. Lancet 2016; 388:2629–2641. [DOI] [PubMed] [Google Scholar]
- 257.Mastrolia SA, Novack L, Thachil J, Rabinovich A, Pikovsky O, Klaitman V, Loverro G, Erez O. LMWH in the prevention of preeclampsia and fetal growth restriction in women without thrombophilia. A systematic review and meta-analysis. Thromb Haemost 2016; 116:868–878. [DOI] [PubMed] [Google Scholar]
- 258.Groom KM, David AL. The role of aspirin, heparin, and other interventions in the prevention and treatment of fetal growth restriction. Am J Obstet Gynecol 2018; 218:S829–S840. [DOI] [PubMed] [Google Scholar]
- 259.Nawathe A, David AL. Prophylaxis and treatment of foetal growth restriction. Best Pract Res Clin Obstet Gynaecol 2018; 49:66–78. [DOI] [PubMed] [Google Scholar]
- 260.Steel SA, Pearce JM. Specific therapy in severe fetal intrauterine growth retardation: failure of prostacyclin. J R Soc Med 1988; 81:214–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hawkes N Trial of Viagra for fetal growth restriction is halted after baby deaths. BMJ 2018; 362:k3247. [DOI] [PubMed] [Google Scholar]
- 262.Jansson T Low dose infusion of atrial natriuretic peptide in the conscious guinea pig increases blood flow to the placenta of growth retarded fetuses. Am J Obstet Gynecol 1992; 166:213–218. [DOI] [PubMed] [Google Scholar]
- 263.Grünewald C, Nisell H, Jansson T, Kublickas M, Thornström S, Nylund L. Possible improvement in uteroplacental blood flow during atrial natriuretic peptide infusion in preeclampsia. Obstet Gynecol 1994; 84:235–239. [PubMed] [Google Scholar]
- 264.Carr DJ, Wallace JM, Aitken RP, Milne JS, Mehta V, Martin JF, Zachary IC, Peebles DM, David AL. Uteroplacental adenovirus vascular endothelial growth factor gene therapy increases fetal growth velocity in growth-restricted sheep pregnancies. Hum Gene Ther 2014; 25:375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Carr DJ, Wallace JM, Aitken RP, Milne JS, Martin JF, Zachary IC, Peebles DM, David AL. Peri- and Postnatal Effects of Prenatal Adenoviral VEGF Gene Therapy in Growth-Restricted Sheep. Biol Reprod 2016; 94:142. [DOI] [PubMed] [Google Scholar]
- 266.Harris LK. Could peptide-decorated nanoparticles provide an improved approach for treating pregnancy complications? Nanomedicine (Lond) 2016; 11:2235–2238. [DOI] [PubMed] [Google Scholar]
- 267.Beards F, Jones LE, Charnock J, Forbes K, Harris LK. Placental Homing Peptide-microRNA Inhibitor Conjugates for Targeted Enhancement of Intrinsic Placental Growth Signaling. Theranostics 2017; 7:2940–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]