Abstract
Background:
Venous thromboembolism (VTE) is an appreciable burden on healthcare. The protracted recumbency experienced by many spine patients juxtaposed with concerns for postoperative hemorrhage from early anticoagulation results in conflicting stances regarding chemoprophylaxis. Identifying risk factors associated with VTE is therefore instrumental in guiding management.
Objective:
To identify VTE risk factors in patients undergoing degenerative spine surgery
Methods
The Nationwide Readmission Database was queried for adults undergoing spine surgery for degenerative pathologies between 2010–2014. The 30-and 90-day VTE incidence was estimated from readmissions with new VTE diagnoses. A multivariate survey-adjusted logistic regression model was utilized to identify variables associated with VTE diagnoses on readmission.
Results
Of 838,507 degenerative spine cases queried, 3499 (0.42%) patients were readmitted with a VTE diagnosis within 30 days and 4321 patients (0.62%) were readmitted within 90 days. In multivariate analysis, steroids were independently associated with a higher likelihood of readmission with VTE at both 30 days (OR 1.58, p<0.001) and 90 days (OR 1.97, p<0.001). Significant associations were also identified with thoracolumbar surgery, length of stay and discharge to institutional care.
Conclusions
The incidence of readmission with VTE diagnoses in spine surgery is low. However, their devastating consequences underscore the need to identify those patients deemed high-risk. They include patients having thoracolumbar surgery, of advanced age, prolonged length of stay, corticosteroid use and disposition to institutional care (e.g. SNF, LTAC). Given the association between steroids and VTE, clinicians should be judicious about perioperative administration in spite of their obvious anti-inflammatory benefits.
Keywords: venous thromboembolism (VTE), pulmonary embolism (PE), deep vein thrombosis (DVT), spine surgery, nationwide database, readmission, adult cohort
Introduction
Venous thromboembolism (VTE) is comprised of proximal and distal deep vein thromboses (DVT) and, their more sinister corollary, pulmonary embolism (PE). DVTs are the source of as many as 90% of PEs1, with the latter carrying a mortality rate somewhere in the range of 30%2,3. Risk factors for VTE formation include restricted mobility from limb paresis or plegia, ventilator dependence, and poor baseline functional status, among others 4–8. While VTEs are common after major surgery 9,10, patients undergoing spine surgery often experience prolonged intraoperative and postoperative immobilization, which further predisposes them to thromboembolic complications11,12. Despite the appreciable VTE risk, concerns for symptomatic surgical site hemorrhage with adverse neurologic sequelae can hamper widespread and early chemoprophylaxis use13.
Previous investigations have identified several associations with VTE namely, neoplastic disease, infection, and advanced age 4,5,14–16. Other variables that have been implicated include increased procedure length and higher intraoperative blood loss 6,16–18 However, many studies are limited to single-institution cohorts and, if conducted using national databases, are largely restricted to VTE incidents diagnosed during the index admission. Because VTEs frequently develop in a delayed fashion, sufficient postoperative follow-up is warranted in order to accurately capture VTE events attributable to spine surgery. Another challenge is that VTE events are frequently missed because of different-hospital follow-up; in other words, patients sought treatment at facilities outside the original center where the index procedure was performed. Schairer et al found that as many as 40% of VTEs diagnosed after discharge presented to a hospital other than the original institution where surgery was performed19. The Nationwide Readmissions Database (NRD) affords longitudinal follow-up within a calendar year and circumvents the challenges of different-hospital follow-up, as it assigns anonymized linkage numbers that enable patients to be tracked throughout a state irrespective of the institution where care is being sought. We therefore endeavored to use the NRD to determine rates of hospital readmission with VTE diagnoses in patients undergoing elective spine surgery for degenerative pathologies.
There are no universally accepted guidelines pertaining to VTE prophylaxis in spine surgery. Given the delicate balance between VTE risks and hemorrhagic complications, identifying potentially modifiable risk factors is central to risk stratification during the perioperative window. In this study we evaluated a large heterogeneous cohort of spine surgery patients from the NRD to identify risk factors associated with postoperative VTE formation. The NRD is a pooled hospital admissions database featuring 20–27 states. De-identified patient data in this database permits researchers to track patient readmissions, thereby allowing for accurate characterization of hospital readmission with VTE after surgery. The goals of this investigation were: (1) to determine readmission rates with VTE diagnoses at 30-and 90-days after elective spine surgery for degenerative disease, (2) to characterize readmission trends and (3) to analyze variables that were predictive of increased VTE likelihood.
Methods
Data Source
We queried the 2010–2014 cohorts of the Nationwide Readmission Database (NRD) compiled by the Healthcare Cost and Utilization Project (HCUP). This database assigns de-identified linkage numbers to all patients admitted to an inpatient facility in the 20–27 participating states and captures approximately 50% of admissions within the United States. Patients are tracked within state lines via these linkage numbers over the course of a single calendar year. All coding within the database meets quality assurance standards for hospital discharge.
Study Population
Primary inclusion criteria were patients older than 18 years who underwent elective spine surgery for degenerative disease. Patients were extracted using the corresponding International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9CM) diagnosis and procedure codes detailed in Table 1. These codes have previously been employed in database studies of degenerative spine disease20. Index admission was defined as the initial hospitalization during which spine surgery was performed. Patients who died on index admission or who carried a diagnosis of acute or chronic VTE on index admission were excluded from our analysis. The remaining cohort was queried for 30-and 90-day hospital readmissions with a new diagnosis of acute VTE (451.1, 451.1x, 451.2, 451.81, 451.83, 451.9, 453.2, 453.3, 453.4x, 453.6, 453.8x, 453.9, 415.1, 415.1x). The NRD tracks patients over a single calendar year; hence, only patients discharged between January through November were included in the 30-day readmission analysis and only patients discharged between January through September were included in the 90-day readmission analysis in order to allow for sufficient follow-up.
Table 1.
International Classification of Diseases, 9th edition, diagnosis and procedures codes used to extract index cohort
| Region | Diagnosis code | Procedure code |
|---|---|---|
| Cervical | 721.0, 721.1, 722.0, 722.4, 722.71,723.0, 723.4, 722.81, 723.1, 723.7, 738.2 | 80.50, 80.51, 81.00, 81.01, 81.02, 81.03, 81.31, 81.32, 81.33, 81.62, 81.63, 03.09, 80.51 |
| Thoracolumbar | 722.10, 722.11, 722.51, 722.52, 722.72, 722.73, 724.2, 721.3, 721.4, 721.41, 721.42, 738.4 | 81.04, 81.05, 81.06, 81.07, 81.08, 81.34, 81.35, 81.36, 81.37, 81.38, 03.09, 80.51 |
Patient and Hospital Demographics
Association analyses were performed for various patient, procedure and hospital factors with the thrombotic events of interest. Variables identified as having an association with VTE in univariate analysis (p < 0.15) were subsequently factored into our multivariate model using two-level mixed effects modeling accounting for clustering. Hospital characteristics evaluated include teaching status and bed size (small, medium, or large). Demographic variables analyzed include gender and age categorized into the following cohorts: 18–44, 45–59, 60–74, ≥75. Insurance payer type and median household income by ZIP code were also considered. Clinical variables such as underlying comorbidities (Elixhauser index), length of stay and discharge disposition were analyzed. Additionally, clinical factors with a predisposition towards thrombosis were incorporated in our analysis: obesity16,21 (278.0, V85.3, V85.4), hypercoagulable state22 (289.81), steroid use8,23,24 (V58.65), SIRS criteria6,8,19,25 (995.9x, 785.52), prior chemotherapy26,27 (V58.11, V87.41), ventilator dependence8,23 (V46.1x), and chronic lung disease28 (CM_CHRNLUNG).
Statistical Analysis
The clinical outcome of interest in this study was readmission with VTE at 30-and 90-days following the index hospitalization. Readmissions were extracted from the NRD using standard HCUP methodology. In cases where multiple admissions were identified, only the first readmission was included. Variables with p <0.05 in univariate analyses were entered into the multivariate model. Multivariable analysis was performed using two-level mixed effects modeling accounting for clustering and reported using odds ratios (OR) with 95% confidence intervals (CI). Interactions between variables were tested to minimize the potential for confounding from collinearity. Statistical significance was defined as p < 0.05. All analysis was conducted with SAS 9.4 (Cary, NC).
Results
Patient and Hospital Baseline Characteristics
A total of 838,507 spine operations for degenerative disease were identified that met our inclusion criteria (Table 2). The majority were for thoracolumbar (61.4%, n = 515,077) followed by cervical pathology (38.6%, n = 323,430). The median length of stay on index admission was 2 days with a median cost of $64,736. The majority of patients fell in the 45–74 age cohort: 18–44 (19.7%), 45–59 (36.3%), 60–74 (33.5), and ≥75 (10.5%). Both genders were roughly equally represented in our cohort: males (48.9%, 410,331) and females (51.1%, n = 428,176). A significant proportion of patients had underlying comorbidities as identified by the Elixhauser index (67.3%, n = 564,607). Most patients had either Medicare (37.1%, n = 310,808) or private insurance (42.7%, n = 358,603) and were treated at hospitals with a large bed size (64.5%, n = 540,899).
Table 2.
Demographics of patients readmitted within 30-and 90-days of index hospitalization
| 30-day | 90-day | ||||
|---|---|---|---|---|---|
| Variable of Interest | No. readmitted |
% of readmitted |
No. readmitted |
% of readmitted |
|
|
Anatomical Location of Pathology |
Cervical | 945 | 27.01 | 1195 | 28.24 |
| Thoracolumbar | 2554 | 72.99 | 3036 | 71.76 | |
| Age | 18–44 | 434 | 12.4 | 472 | 11.16 |
| 45–59 | 934 | 26.69 | 1123 | 26.54 | |
| 60–74 | 1434 | 40.98 | 1732 | 40.94 | |
| >=75 | 697 | 19.92 | 904 | 21.37 | |
| Gender | Male | 1852 | 52.93 | 2235 | 52.82 |
| Female | 1647 | 47.07 | 1996 | 47.18 | |
|
Primary insurance |
Medicare | 1772 | 50.64 | 2252 | 53.23 |
| Medicaid | 202 | 5.77 | 247 | 5.84 | |
| Private insurance | 1176 | 33.61 | 1317 | 31.13 | |
| Self-pay | 42 | 1.2 | 54 | 1.28 | |
| No charge | 11 | 0.31 | 12 | 0.28 | |
| Other | 289 | 8.26 | 340 | 8.04 | |
| Hospital bed size | Small | 353 | 10.09 | 418 | 9.88 |
| Medium | 773 | 22.09 | 944 | 22.31 | |
| Large | 2373 | 67.82 | 2869 | 67.81 | |
| Teaching status | Teaching | 1983 | 56.67 | 2427 | 57.36 |
| Non-teaching | 1516 | 43.33 | 1804 | 42.64 | |
| Disposition | Routine | 1817 | 51.93 | 2102 | 49.68 |
| Short-term Hospital | 39 | 1.11 | 49 | 1.16 | |
| Transfer Other | 919 | 26.26 | 1245 | 29.43 | |
| Home Health Care | 720 | 20.58 | 831 | 19.64 | |
| Against Medical Advice | DS | DS | DS | DS | |
| Volume | Above 90th percentile | 1344 | 38.41 | 1621 | 38.31 |
| <= 90th percentile (652 / year) | 2155 | 61.59 | 2610 | 61.69 | |
|
Elixhauser comorbidity |
Yes | 2701 | 77.19 | 3323 | 78.54 |
| No | 798 | 22.81 | 908 | 21.46 | |
|
Medical complication |
Yes | 157 | 4.49 | 214 | 5.06 |
| No | 3342 | 95.51 | 4017 | 94.94 | |
|
Neurological complication |
Yes | 160 | 4.57 | 191 | 4.51 |
| No | 3339 | 95.43 | 4040 | 95.49 | |
| Obesity | Yes | 17 | 0.49 | 23 | 0.54 |
| No | 3482 | 99.51 | 4208 | 99.46 | |
|
Hypercoagulable state |
Yes | DS | DS | DS | DS |
| No | 3493 | 99.83 | 4225 | 99.86 | |
|
Index Length of
stay |
0–1days | 475 | 13.58 | 577 | 13.64 |
| 2days | 511 | 14.6 | 618 | 14.61 | |
| 3–4days | 1052 | 30.07 | 1245 | 29.43 | |
| >=5days | 1461 | 41.75 | 1791 | 42.33 | |
|
Median
household income for patient’s ZIP code, based on current year |
0–25percentile | 780 | 22.29 | 969 | 22.9 |
| 26–50percentile | 778 | 22.23 | 981 | 23.19 | |
| 51–75percentile | 944 | 26.98 | 1115 | 26.35 | |
| 76–100 percentile | 932 | 26.64 | 1090 | 25.76 | |
| Steroid use | Yes | 59 | 1.69 | 89 | 2.1 |
| No | 3440 | 98.31 | 4142 | 97.9 | |
| SIRS criteria | Yes | 42 | 1.2 | 59 | 1.39 |
| No | 3457 | 98.8 | 4172 | 98.61 | |
|
History of
chemotherapy |
Yes | 13 | 0.37 | 17 | 0.4 |
| No | 3486 | 99.63 | 4214 | 99.6 | |
|
Ventilator dependence |
Yes | DS | DS | DS | DS |
| No | 3497 | 99.94 | 4229 | 99.5 | |
|
Chronic lung disease |
Yes | 562 | 16.06 | 738 | 17.44 |
| No | 2937 | 83.94 | 3493 | 82.56 | |
DS = Data Suppressed according to HCUP/NRD regulations
Rates of hospital readmission with VTE and readmission demographics
3499 patients were readmitted with VTE 30-days after discharge from the index hospitalization with a corresponding incidence of 0.4%. Among those with VTE, 1487 (42.5%) patients were readmitted with DVT alone, 1253 (35.8%) with PE alone and 759 (21.7%) with both DVT and PE. The 30-day DVT and PE readmission rates were estimated to be 0.27% and 0.24%, respectively. The median time to readmission was 10 days with a median readmission cost of $34,000.
At 90-days, 4231 patients were readmitted with a diagnosis of VTE, corresponding to an incidence of 0.6%. There were 1932 (45.7%) patients with DVT alone, 1385 (32.7%) with PE alone and 914 (21.6%) with both. The 90-day DVT and PE readmission rates were 0.41% and 0.34%, respectively. The median time to 90-day readmission was 17 days with a median cost of $34, 266.
Factors associated with readmission with VTE within 30 and 90 days
Various clinical factors were independently associated with VTE development (Table 4, 5). Operations for thoracolumbar pathology carried a higher VTE risk relative to cervical procedures (OR 1.19–1.26, p < 0.001). Patients on corticosteroids had nearly twice the odds of developing a VTE at both 30-days (OR 1.58, p < 0.0005) and 90-days (1.97, p < 0.0001). Advanced age was also significantly associated with VTE readmission, with older patients (≥75) having nearly twice the odds compared to those 18–44 years (OR 1.63–1.76, p < 0.0001). In addition, increased length of stay on index admission correlated with higher VTE likelihood with stays ≥ 5 days having more than three-fold risk (OR 3.42–3.68, p <0.0001) compared to stays lasting 24 hours or less. Other factors with significant relationships with VTE include: male gender, presence of medical or neurological complications, and final disposition with home health resources or to institutional care (e.g. skilled nursing or long-term acute care facilities).
Table 4.
Predictors of 30-day hospital readmissions with venous thromboembolic complications by multivariate analysis
| Variables of Interest | Odds Ratio | 95% Confidence Interval | p-value | ||
|---|---|---|---|---|---|
| Anatomic region | Cervical | 0.796 | 0.736 | 0.861 | <.0001 |
| Thoracolumbar | Reference | ||||
| Age | 45–59 | 1.078 | 0.96 | 1.211 | 0.2015 |
| 60–74 | 1.393 | 1.233 | 1.574 | <.0001 | |
| >=75 | 1.631 | 1.408 | 1.89 | <.0001 | |
| 18–44 | Reference | ||||
| Gender | Male | 1.336 | 1.249 | 1.43 | <.0001 |
| Female | Reference | ||||
|
Primary insurance |
Medicare | 0.905 | 0.77 | 1.063 | 0.2241 |
| Private insurance |
0.928 | 0.797 | 1.081 | 0.3402 | |
| Self-pay | 0.941 | 0.674 | 1.315 | 0.7224 | |
| No charge | 1.558 | 0.844 | 2.875 | 0.1563 | |
| Other | 0.758 | 0.632 | 0.91 | 0.0029 | |
| Medicaid | Reference | ||||
|
Discharge disposition |
Short-term Hospital |
2.174 | 1.565 | 3.019 | <.0001 |
| Transfer Other | 2.023 | 1.835 | 2.231 | <.0001 | |
| Home Health Care | 1.227 | 1.117 | 1.348 | <.0001 | |
| Against Medical Advice |
1.166 | 0.431 | 3.154 | 0.7626 | |
| Routine | Reference | ||||
| Medical | Yes | 1.243 | 1.052 | 1.467 | 0.0105 |
| complication | No | Reference | |||
|
Neurological complication |
Yes | 1.347 | 1.146 | 1.584 | 0.0003 |
| No | Reference | ||||
|
Index length of
stay |
2 days | 1.687 | 1.485 | 1.916 | <.0001 |
| 3–4 days | 2.173 | 1.933 | 2.443 | <.0001 | |
| >=5 days | 3.676 | 3.261 | 4.144 | <.0001 | |
| 0–1days | Reference | ||||
| Steroid use | Yes | 1.582 | 1.22 | 2.052 | 0.0005 |
| No | Reference | ||||
Table 5.
Predictors of 90-day hospital readmissions with venous thromboembolic complications by multivariate analysis
| Variables of Interest | Odds Ratio | 95% Confidence Interval | p-value | ||
|---|---|---|---|---|---|
|
Anatomic region |
Cervical | 0.838 | 0.78 | 0.899 | <.0001 |
| Thoracolumbar | Reference | ||||
| Age | 45–59 | 1.186 | 1.063 | 1.323 | 0.0022 |
| 60–74 | 1.479 | 1.318 | 1.66 | <.0001 | |
| >=75 | 1.758 | 1.534 | 2.015 | <.0001 | |
| 18–44 | Reference | ||||
| Gender | Male | 1.341 | 1.261 | 1.426 | <.0001 |
| Female | Reference | ||||
|
Primary insurance |
Medicare | 0.913 | 0.789 | 1.057 | 0.2227 |
| Private insurance | 0.86 | 0.748 | 0.988 | 0.0333 | |
| Self-pay | 1.001 | 0.744 | 1.347 | 0.9962 | |
| No charge | 1.413 | 0.783 | 2.549 | 0.2506 | |
| Other | 0.734 | 0.622 | 0.867 | 0.0003 | |
| Medicaid | Reference | ||||
|
Discharge disposition |
Short-term Hospital |
2.327 | 1.734 | 3.122 | <.0001 |
| Transfer Other | 2.386 | 2.185 | 2.605 | <.0001 | |
| Home Health Care | 1.239 | 1.135 | 1.353 | <.0001 | |
| Against Medical Advice | 1.017 | 0.377 | 2.739 | 0.9739 | |
| Routine | Reference | ||||
|
Medical complication |
Yes | 1.321 | 1.143 | 1.526 | 0.0002 |
| No | Reference | ||||
|
Neurological complication |
Yes | 1.296 | 1.117 | 1.504 | 0.0006 |
| No | Reference | ||||
|
Index Length of
stay |
2 days | 1.683 | 1.499 | 1.889 | <.0001 |
| 3–4 days | 2.041 | 1.834 | 2.272 | <.0001 | |
| >=5 days | 3.418 | 3.064 | 3.814 | <.0001 | |
| 0–1days | Reference | ||||
| Steroid use | Yes | 1.967 | 1.588 | 2.436 | <.0001 |
| No | Reference | ||||
Discussion
Unplanned hospital readmissions are a major source of patient morbidity and pose substantial burden to the healthcare system29. In 2011 alone, the estimated cost associated with readmissions exceeded US $41 billion30. Because readmissions are increasingly being recognized as a surrogate for quality of care delivered, hospitals now stand to face financial penalties from Medicare under the Hospital Readmissions Reduction Program (HRRP), which endeavors to curb excessive 30-day readmissions 31. VTEs are a documented cause of unplanned readmissions in post-surgical cohorts32. We therefore sought to evaluate the rates of hospital readmission with VTE in patients undergoing elective spine surgery for degenerative pathologies as well as identify risk factors predictive of thromboembolic complications.
VTE comprises deep vein thrombosis (DVT) and pulmonary embolism (PE). As DVT formation uniformly precedes PE development, and both are subject to the same predisposing factors, they were analyzed as a single entity in our multivariate models. Prior estimates of VTE incidence in the spine surgery literature range anywhere from 0.3 to 31% 11. This considerable variation is likely attributable to discrepancies in inclusion criteria,12,18,33–35 methods of VTE prophylaxis,18,36 and differences in screening criteria or modality for VTE detection 4,7,12,15,18,35,37 In our investigation, we determined 30-and 90-day incidences of hospital readmission with VTE to be 0.4% and 0.6%, respectively. On further breakdown, the corresponding DVT rates at 30-and 90-days were 0.27% and 0.41%, while the PE rates over the same period were 0.24% and 0.34%. These figures are lower than rates reported by Piper et al from a recent NSQIP database study5 in which their overall VTE incidence was 1.1% at 30-days, with DVT and PE rates of 0.8% and 0.4%, respectively. Reasons for this difference include but are not limited to: restriction of our cohort to degenerative spine pathologies alone versus a broader cohort in the Piper study, use of ICD-9 diagnosis and procedure codes for patient extraction in the NRD versus CPT codes in NSQIP, and other differences in study methodology.
In a 2009 meta-analysis examining VTE events after spine surgery, the authors found a pooled DVT rate of 2.1%11. While again much higher than our reported incidence, this study included variable follow-up as well as patients undergoing surgery for trauma and other cohorts with established propensity towards VTE development11. Additionally, the meta-analysis included studies with scheduled Doppler surveillance for DVT detection, which is not standard practice in clinical settings. This might have contributed to an artificial inflation of DVT incidence. We exclusively evaluated patients presenting for surgical management of degenerative spine disease. This essentially excludes patients undergoing surgery for trauma, neoplasm, infection and other risk factors implicated in VTE formation5,16,19. We also excluded VTE events during the index admission, which might have underestimated true VTE incidence. Our decision to do so was predicated on the goal of this investigation which was to identify predictors of hospital readmission with VTE after spine surgery. It should be noted that although there is a potential for understimating true VTE incidence in this manner, it also prevents overestimating of said events given that VTEs detected during the index stay could very well represent pre-existing events that were already present prior to admission, but that were only then detected on hospitalization.
Although the risk of VTE formation is low in patients undergoing spine surgery, it is not negligible and there is limited consensus regarding optimal prophylaxis13,18,36,38–40. Because of the risk of clinically significant surgical site hemorrhage requiring takeback to the operating room, many clinicians are wary to institute aggressive chemoprophylaxis13. Identification of high-risk VTE factors can therefore aid in tailoring medical management for high risk patients. Patients who underwent thoracolumbar procedures were identified as having higher likelihood for readmission with VTE than those who had cervical procedures. Previous studies on DVT development after spine surgery have reported a similar outcome7,18,35. The putative explanation for this observation is that prone intraoperative positioning exerts greater compression on the inguinal regions with resultant venous stasis in the lower extremities35. Additionally, and intuitively so, dissection of the much larger lower back muscles is likely to result in higher pain sequelae and lower likelihood of ambulation in the immediate hours—or even days—following surgery. In this regard, thoracolumbar procedures demonstrate longer periods of postoperative immobility and therefore higher VTE risk 7,18.
In multivariate analysis we found that corticosteroid use was independently associated with higher readmission rates with VTE diagnoses at both 30-and 90-days even after controlling for neurologic weakness as confounding variable. Corticosteroids are employed throughout spine surgery for their anti-inflammatory effects to reduce mass effect on the spinal cord and exiting nerves in cases of pathologic compression. The association between steroid use and thromboembolic phenomena is not new. In a 2013 case-control study from Denmark41, the authors analyzed 70,000 patients from the National Database of Reimbursed Prescriptions in which patients could be identified as being “former”, “recent” or “present” users of steroids. They found that not only did steroid use correlate with VTE events, but the temporality of use was also important: patients who were new to steroids (within 90 days) had the highest risk of VTE development. Other studies have also demonstrated higher VTE incidence in patients with Cushing’s disease, further implicating corticosteroids in thromboembolic complications42. The presumed explanation for this relationship is that steroids promote hypercoagulability via induction of factors VII, VIII, IX, von Willebrand’s factor, and thrombin43–45. The increased VTE risk conferred by steroids, coupled with their ubiquity, calls for more judicious administration to surgical patients and represents a modifiable risk factor that should be capitalized upon.
Consistent with other reports in the literature, we found that older age correlated with higher VTE rates 4,12,14,15,18,46, This is a well-established epidemiologic phenomenon, which has been partially attributed to the increased number of medical comorbidities in older patients. It follows then, that the presence of medical or neurological complications were also associated with higher VTE rates and indeed that was the case in our study as well. Patients with neurological deficits are more likely to experience extended periods of immobility resulting in venous stasis and subsequent VTE formation. Likewise, patients subjected to conditions that are not conducive to native levels of mobility—such as being hospitalized for prolonged periods—also demonstrate higher rates of VTE. In our analysis, increased length of stay was proportionate with higher odds of VTE formation, as patients hospitalized for 5 or more days had a three-to four-fold higher chance of readmission with VTE. These findings are in line with the available literature, including a 2016 NSQIP study by Sebastian et al in which spine patients hospitalized for longer than 6 days had a 4.07-fold higher risk of VTE development16.
Patients discharged with home health resources or to institutional care (e.g. skilled nursing facility, long-term acute care) exhibited higher likelihood of readmission with VTE. The risks conferred by institutional placement likely have to do with many of the same predisposing factors inherent to prolonged inpatient hospitalization. Regardless of resources, there are limitations to the level of individualized care that such facilities can provide in comparison to patients who are discharged home. Consequently, patients are inevitably at a lower functionality compared with their baseline. In a 2008 population-based study by Liebson et al there was a strong association between recent hospitalization and VTE development in nursing home residents47. Altogether, these data highlight institutional discharge as a high-risk feature for VTE in perioperative cohorts. Vigilance for thromboembolic complications through early mobilization, mechanical and chemical prophylaxis should thus be maintained when transitioning patient care from inpatient to outpatient nursing and rehabilitation facilities.
Our study is limited by the constraints imposed by pooled databases, such as clerical errors of omission or inaccurate transcription. While the NRD is the first nationwide database to specifically track hospital readmissions, patient linkage numbers are only valid for a single calendar year. Consequently, follow-up data on 30-and 90-day readmissions do not extend beyond November and September, respectively, resulting in the potential for under-reporting of readmission rates. Additionally, the NRD only allows patients to be tracked within state lines. As such, patients who seek care at hospitals outside the original state will not be captured in our analysis. Other potential limitations include the fact that certain demographic variables are omitted from NRD on account of patient confidentiality, which effectively limits any comprehensive analyses on socio-economic variables that potentially have a bearing on outcomes. Finally, the NRD does not provide details regarding VTE prophylaxis or other aspects of perioperative care. We are therefore constrained in our ability to draw correlations between events that were extracted and the corresponding clinical management that might have contributed to their occurrence.
Conclusions
Using the Nationwide Readmissions Database (NRD), we determined the VTE incidence in patients undergoing elective spine surgery for degenerative disease and identified factors that predicted thromboembolic complications. The 30-day VTE incidence was 0.4%, with corresponding DVT and PE rates of 0.27% and 0.24%, respectively. The 90-day VTE incidence was 0.6%, with corresponding DVT and PE rates of 0.41% and 0.34%. Various procedure and patient-related factors correlated with increased likelihood for readmission with VTE after spine surgery namely, thoracolumbar procedures, corticosteroid use and final disposition with home health resources or to institutional care. In addition, advanced age, presence of neurological or medical complications and increased hospital length of stay correlated with higher odds of VTE. The association between steroids and VTE, coupled with their ubiquity in spine surgery, underscores their importance as a potentially modifiable risk factor in VTE formation. Careful attention should be paid to balancing their anti-inflammatory benefits against their potential for venous thromboembolism.
Figure 1.
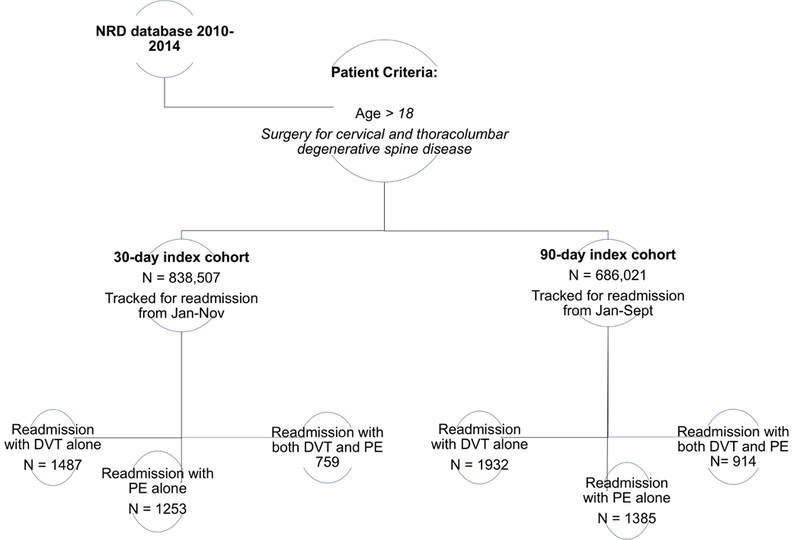
Schematic of Nationwide Readmission Database study design for charactering VTE readmission
Table 3.
Breakdown of venous thromboembolic complications by spine region
| 30 day readmission | 90 day readmission | |||
|---|---|---|---|---|
| Study Cohort | DVT (# events/rate) |
PE (# events/rate) |
DVT (# events/rate) |
PE (# events/rate) |
| Cervical | 574 (0.18) | 574 (0.18) | 777 (0.29) | 665 (0.25) |
| Thoracolumbar | 1672 (0.32) | 1438 (0.28) | 2069 (0.49) | 1634 (0.39) |
Abbreviations
- NRD
Nationwide Readmissions Database
- NSQIP
National Surgical Quality Improvement Project
- ICD-9CM
International Classification of Diseases, Ninth Edition, Clinical Modification
- VTE
venous thromboembolism
- DVT
deep vein thrombosis
- PE
pulmonary embolism
- SNF
skilled nursing facility
- LTAC
long-term acute care
Footnotes
Disclosure Statement: The authors have no conflicts of interest to disclose
Conflict of Interest: The authors have no conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Danish SF, Bumett MG, Stein SC. Prophylaxis for deep venous thrombosis in patients with craniotomies: a review. NeurosurgFocus. 2004;17(4):E2. [DOI] [PubMed] [Google Scholar]
- 2.Chibbaro S, Cebula H, Todeschi J, et al. Evolution of Prophylaxis Protocols for Venous Thromboembolism in Neurosurgery: Results from a Prospective Comparative Study on Low-Molecular-Weight Heparin, Elastic Stockings, and Intermittent Pneumatic Compression Devices. World Neurosurg. 2018;109:e510–e516. [DOI] [PubMed] [Google Scholar]
- 3.Simanek R, Vormittag R, Hassler M, et al. Venous thromboembolism and survival in patients with high-grade glioma. Neuro Oncol. 2007;9(2):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akeda K, Matsunaga H, Imanishi T, et al. Prevalence and countermeasures for venous thromboembolic diseases associated with spinal surgery: a follow-up study of an institutional protocol in 209 patients. Spine (Phila Pa 1976). 2014;39(10):791–797. [DOI] [PubMed] [Google Scholar]
- 5.Piper K, Algattas H, DeAndrea-Lazarus IA, et al. Risk factors associated with venous thromboembolism in patients undergoing spine surgery. J Neurosurg Spine. 2017;26(1):90–96. [DOI] [PubMed] [Google Scholar]
- 6.Sebastian AS, Currier BL, Clarke MJ, Larson D, Huddleston PM 3rd, Nassr A. Thromboembolic Disease after Cervical Spine Surgery: A Review of 5,405 Surgical Procedures and Matched Cohort Analysis. Global Spine J. 2016;6(5):465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshioka K, Murakami H, Demura S, Kato S, Tsuchiya H. Prevalence and risk factors for development of venous thromboembolism after degenerative spinal surgery. Spine (Phila Pa 1976). 2015;40(5):E301–306. [DOI] [PubMed] [Google Scholar]
- 8.Algattas H, Kimmell KT, Vates GE, Jahromi BS. Analysis of Venous Thromboembolism Risk in Patients Undergoing Craniotomy. World Neurosurg. 2015;84(5): 1372–1379. [DOI] [PubMed] [Google Scholar]
- 9.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458–464. [DOI] [PubMed] [Google Scholar]
- 10.Anderson FA Jr, Wheeler HB, Goldberg RJ, Hosmer DW, Forcier A . The prevalence of risk factors for venous thromboembolism among hospital patients. Arch Intern Med. 1992;152(8): 1660–1664. [PubMed] [Google Scholar]
- 11.Glotzbecker MP, Bono CM, Wood KB, Harris MB. Thromboembolic disease in spinal surgery: a systematic review. Spine (Phila Pa 1976). 2009;34(3):291–303. [DOI] [PubMed] [Google Scholar]
- 12.Zacharia BE, Kahn S, Bander ED, et al. Incidence and risk factors for preoperative deep venous thrombosis in 314 consecutive patients undergoing surgery for spinal metastasis. J Neurosurg Spine. 2017;27(2): 189–197. [DOI] [PubMed] [Google Scholar]
- 13.Sansone JM, del Rio AM, Anderson PA. The prevalence of and specific risk factors for venous thromboembolic disease following elective spine surgery. J Bone Joint Surg Am. 2010;92(2):304–313. [DOI] [PubMed] [Google Scholar]
- 14.Cloney MB, Hopkins B, Dhillon ES, Dahdaleh NS. The timing of venous thromboembolic events after spine surgery: a single-center experience with 6869 consecutive patients. J Neurosurg Spine. 2018;28(1):88–95. [DOI] [PubMed] [Google Scholar]
- 15.Kim DY, Kobayashi L, Chang D, Fortlage D, Coimbra R. Early pharmacological venous thromboembolism prophylaxis is safe after operative fixation of traumatic spine fractures. Spine (Phila Pa 1976). 2015;40(5):299–304. [DOI] [PubMed] [Google Scholar]
- 16.Sebastian AS, Currier BL, Kakar S, et al. Risk Factors for Venous Thromboembolism following Thoracolumbar Surgery: Analysis of 43,777 Patients from the American College of Surgeons National Surgical Quality Improvement Program 2005 to 2012. Global Spine J. 2016;6(8):738–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang TY, Sakamoto JT, Nayar G, et al. Independent Predictors of 30-Day Perioperative Deep VeinThrombosis in 1346 Consecutive Patients After Spine Surgery. World Neurosurg. 2015;84(6): 1605–1612. [DOI] [PubMed] [Google Scholar]
- 18.Yoshioka K, Murakami H, Demura S, et al. Comparative study of the prevalence of venous thromboembolism after elective spinal surgery. Orthopedics. 2013;36(2):e223–228. [DOI] [PubMed] [Google Scholar]
- 19.Schairer WW, Pedtke AC, Hu SS. Venous Thromboembolism After Spine Surgery. Spine (Phila Pa 1976). 2014. [DOI] [PubMed] [Google Scholar]
- 20.Chitale R, Campbell PG, Yadla S, Whitmore RG, Maltenfort MG, Ratliff JK. International classification of disease clinical modification 9 modeling of a patient comorbidity score predicts incidence of perioperative complications in a nationwide inpatient sample assessment of complications in spine surgery. JSpinal Disord Tech. 2015;28(4): 126–133. [DOI] [PubMed] [Google Scholar]
- 21.Hoefnagel D, Kwee LE, van Putten EH, Kros JM, Dirven CM, Dammers R. The incidence of postoperative thromboembolic complications following surgical resection of intracranial meningioma. A retrospective study of a large single center patient cohort. Clin Neurol Neurosurg. 2014;123:150–154. [DOI] [PubMed] [Google Scholar]
- 22.Kshettry VR, Rosenbaum BP, Seicean A, Kelly ML, Schiltz NK, Weil RJ. Incidence and risk factors associated with in-hospital venous thromboembolism after aneurysmal subarachnoid hemorrhage. J Clin Neurosci. 2014;21(2):282–286. [DOI] [PubMed] [Google Scholar]
- 23.Rolston JD, Han SJ, Bloch O, Parsa AT. What clinical factors predict the incidence of deep venous thrombosis and pulmonary embolism in neurosurgical patients? J Neurosurg. 2014; 121(4):908–918. [DOI] [PubMed] [Google Scholar]
- 24.Senders JT, Goldhaber NH, Cote DJ, et al. Venous thromboembolism and intracranial hemorrhage after craniotomy for primary malignant brain tumors: a National Surgical Quality Improvement Program analysis. JNeurooncol. 2018;136(1): 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Liu YH, Mao Q. Retractorless surgery for third ventricle tumor resection through the transcallosal approach. Clin Neurol Neurosurg. 2017;155:58–62. [DOI] [PubMed] [Google Scholar]
- 26.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110(10):2339–2346. [DOI] [PubMed] [Google Scholar]
- 27.Marras LC, Geerts WH, Perry JR. The risk of venous thromboembolism is increased throughout the course of malignant glioma: an evidence-based review. Cancer. 2000;89(3):640–646. [DOI] [PubMed] [Google Scholar]
- 28.Goldhaber SZ. Risk factors for venous thromboembolism. J Am Coll Cardiol. 2010;56(1): 1–7. [DOI] [PubMed] [Google Scholar]
- 29.Eskander RN, Chang J, Ziogas A, Anton-Culver H, Bristow RE. Evaluation of 30-day hospital readmission after surgery for advanced-stage ovarian cancer in a medicare population. J Clin Oncol. 2014;32(36):4113–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hines AL BM, Jiang HJ, Steiner CA Conditions With the Largest Number of Adult Hospital Readmissions by Payer, 2011 HCUP Statistical Brief #172. April 2014. Rockville, MD: Agency for Healthcare Research and Quality;2014. [PubMed] [Google Scholar]
- 31.Patient Protection and Affordable Care Act. In. 42 U.S.C.2010.
- 32.Sherrod BA, Johnston JM, Rocque BG. Risk factors for unplanned readmission within 30 days after pediatric neurosurgery: a nationwide analysis of 9799 procedures from the American College of Surgeons National Surgical Quality Improvement Program. J Neurosurg Pediatr. 2016;18(3):350–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De la Garza Ramos R, Goodwin CR, Abu-Bonsrah N, et al. Patient and operative factors associated with complications following adolescent idiopathic scoliosis surgery: an analysis of 36,335 patients from the Nationwide Inpatient Sample. J Neurosurg Pediatr. 2016;25(6):730–736. [DOI] [PubMed] [Google Scholar]
- 34.Jain A, Hassanzadeh H, Puvanesarajah V, et al. Incidence of perioperative medical complications and mortality among elderly patients undergoing surgery for spinal deformity: analysis of 3519 patients. J Neurosurg Spine. 2017;27(5):534–539. [DOI] [PubMed] [Google Scholar]
- 35.Oda T, Fuji T, Kato Y, Fujita S, Kanemitsu N. Deep venous thrombosis after posterior spinal surgery. Spine (Phila Pa 1976). 2000;25(22):2962–2967. [DOI] [PubMed] [Google Scholar]
- 36.McClendon J Jr, Smith TR, O’Shaughnessy BA, Sugrue PA, Thompson SE, Koski TR. Time to Event Analysis for the Development of Venous Thromboembolism After Spinal Fusion >/= 5 Levels. World Neurosurg 2015;84(3):826–833. [DOI] [PubMed] [Google Scholar]
- 37.Moayer A, Mohebali N, Razmkon A. Incidence of Deep Vein Thrombosis in Patients Undergoing Degenerative Spine Surgery onProphylactic Dalteparin; A Single Center Report. Bull Emerg Trauma. 2016;4(1):38–42. [PMC free article] [PubMed] [Google Scholar]
- 38.Cox JB, Weaver KJ, Neal DW, Jacob RP, Hoh DJ. Decreased incidence of venous thromboembolism after spine surgery with early multimodal prophylaxis: Clinical article. J Neurosurg Spine. 2014;21(4):677–684. [DOI] [PubMed] [Google Scholar]
- 39.Dhillon ES, Khanna R, Cloney M, et al. Timing and risks of chemoprophylaxis after spinal surgery: a single-center experience with 6869 consecutive patients. J Neurosurg Spine. 2017;27(6):681–693. [DOI] [PubMed] [Google Scholar]
- 40.Khan NR, Patel PG, Sharpe JP, Lee SL, Sorenson J. Chemical venous thromboembolism prophylaxis in neurosurgical patients: an updated systematic review and meta-analysis. J Neurosurg. 2017:1–10. [DOI] [PubMed] [Google Scholar]
- 41.Johannesdottir SA, Horvath-Puho E, Dekkers OM, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med. 2013;173(9):743–752. [DOI] [PubMed] [Google Scholar]
- 42.Squizzato A, Gerdes VE, Ageno W, Buller HR. The coagulation system in endocrine disorders: a narrative review. Intern Emerg Med. 2007;2(2):76–83. [DOI] [PubMed] [Google Scholar]
- 43.Van Zaane B, Nur E, Squizzato A, et al. Hypercoagulable state in Cushing’s syndrome: a systematic review. J Clin Endocrinol Metab. 2009;94(8):2743–2750. [DOI] [PubMed] [Google Scholar]
- 44.van Zaane B, Nur E, Squizzato A, et al. Systematic review on the effect of glucocorticoid use on procoagulant, anti-coagulant and fibrinolytic factors. J Thromb Haemost. 2010;8(11):2483–2493. [DOI] [PubMed] [Google Scholar]
- 45.van Zaane B, Stuijver DJ, Squizzato A, Gerdes VE. Arterial and venous thrombosis in endocrine diseases. Semin Thromb Hemost. 2013;39(5):489–495. [DOI] [PubMed] [Google Scholar]
- 46.Wei J, Li W, Pei Y, Shen Y, Li J. Clinical analysis of preoperative risk factors for the incidence of deep venous thromboembolism in patients undergoing posterior lumbar interbody fusion. J Orthop SurgRes. 2016; 11(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liebson CL, Petterson TM, Bailey KR, Melton LJ 3rd, Heit JA. Risk factors for venous thromboembolism in nursing home residents. Mayo Clin Proc. 2008;83(2): 151–157. [PubMed] [Google Scholar]


