Abstract
There are a variety of devices that quantify biological properties of cerebral tissue. Installing such device will cause a local insertion trauma, which will affect early measurements. Current literature proposes minimum one hour of observation before acquiring first measurements when using microdialysis. It is unknown whether this applies to other intracerebral devices. We therefore aimed to investigate time needed to reach steady state when using microdialysis and two intracerebral probes in a piglet model. Ten newborn piglets less than 24 hours of age were anaesthetized. Two probes (Codman and OxyLite/OxyFlo) and a microdialysis catheter (CMA Microdialysis) were installed 10 mm into the left hemisphere. Probes measured intracranial pressure, cerebral blood flow, and oxygen tension. The microdialysis catheter measured lactate, glucose, glycerol, and pyruvate. Measurements were acquired hourly for 20 hours. Lactate and glycerol peaked immediately after insertion and reached steady state after approximately four hours. Glucose, pyruvate, cerebral blood flow, and intracranial pressure reached steady state immediately. Oxygen tension reached steady state after 12 hours. With time, interindividual variability decreased for the majority of measurements. Consequently, time to stabilization after insertion depends on the choice of device and is crucial to obtain valid baseline values with high degree of precision.
Subject terms: Brain injuries, Sensors and probes
Introduction
To quantify biological properties of cerebral tissue, microdialysis and intracerebral probes are used1. In paediatric research, these devices are used in experimental animal studies to investigate the intracerebral consequence of perinatal hypoxia and ischemia and specific treatments2,3. In neurocritical care, microdialysis and intracerebral probes are used to detect secondary ischemia in patients with intracerebral bleeding, or to measure the influence of different interventions in animal experiments4,5. Accordingly, information on metabolism, intracranial pressure (ICP), cerebral blood flow (CBF), and oxygenation are essential for cerebral monitoring in both the clinical and experimental setting6. To map metabolism, microdialysis can be used with a standard panel of glucose, lactate, glycerol, glutamate, and pyruvate7. ICP can be measured invasively by a strain-gauge device8. Cerebral oxygenation and CBF can be measured directly through a probe inserted into the brain parenchyma3. However, when installing a microdialysis catheter or a probe, a local insertion trauma will occur which will affect early measures.
When using microdialysis in human muscle tissue, a minimum of two hours of observation was required for values to stabilize9,10. Sørensen et al. found a reduction in lactate, glycerol, and glutamate when comparing microdialysis measurements acquired at 20 minutes with those obtained at 40 minutes after insertion in muscle tissue11. Even longer time to stabilization have been reported when measures are carried out in cerebral tissue12,13. Mellergård et al. investigated the impact of microdialysis-catheter placement in patients with severe brain injury12. They found stable values of glycerol, lactate and pyruvate immediately after insertion; but substantial changes in glutamate and several cytokines and chemokines up to 48 hours after insertion12. Consensus statement from the 2014 International Microdialysis Forum states that during the first hour, microdialysis measurements should not be used due to interference from the insertion trauma7. There is a lack of data on the consequence of insertion trauma for other devices used in cerebral tissue. The aim of this newborn-piglet study was to determine whether one hour of observation after insertion of two intracerebral probes, and a microdialysis catheter was sufficient to reach steady state.
Results
All 10 piglets survived for the 20-hour observation period. One piglet had a malfunctioning microdialysis catheter and data was not obtained. ICP measurements were not recorded in two piglets due to probe failure. Vital signs and blood-gas values remained stable though the observation period (Table 1).
Table 1.
Vital signs and blood-gas values for 10 piglets during the 20-hour observation period presented as mean values with range.
| 1st hour | 6th hour | 12th hour | 18th hour | |
|---|---|---|---|---|
| Vital signs: | ||||
| Heart rate (min−1) | 139 (98–176) | 168 (130–230) | 164 (130–250) | 158 (126–231) |
| Mean arterial pressure (mmHg) | 46.2 (34.0–60.0) | 41.8 (39.0–46.0) | 39.9 (34.0–46.0) | 42.0 (35.5–47.0) |
| Rectal temperature (°C) | 38.0 (36.4–39.3) | 39.0 (38.3–39.7) | 38.9 (38.2–39.3) | 38.8 (38.2–39.4) |
| Blood-gas values: | ||||
| pH | 7.6 (7.4–7.7) | 7.6 (7.5–7.6) | 7.5 (7.4–7.6) | 7.6 (7.5–7.6) |
| pCO2 (kPa) | 4.1 (2.7–6.0) | 4.0 (3.3–4.7) | 4.3 (3.6–5.0) | 3.9 (3.3–4.4) |
| pO2 (kPa) | 10.8 (8.1–14.7) | 11.3 (7.9–19.2) | 10.4 (8.4–12.6) | 12.0 (9.1–17.6) |
| Lactate (mmol/L) | 2.1 (1.3–3.5) | 1.4 (1.1–1.9) | 1.3 (1.0–1.6) | 1.5 (1.1–1.8) |
| Glucose (mmol/L) | 7.4 (4.1–11.8) | 5.3 (4.5–6.3) | 6.4 (4.0–13.3) | 5.8 (4.3–7.6) |
| Na2+ (mmol/L) | 137.1 (135.0–140.0) | 135.3 (132.0–141.0) | 131.5 (123.0–141.0) | 128.0 (124.0–134.0) |
| K+ (mmol/l) | 3.5 (2.4–4.3) | 3.8 (2.8–4.5) | 4.0 (3.0–5.7) | 3.8 (2.8–4.1) |
Blood-gasses were sampled 1, 6, 12, and 18 hours (±1 hour) after insertion.
Microdialysis
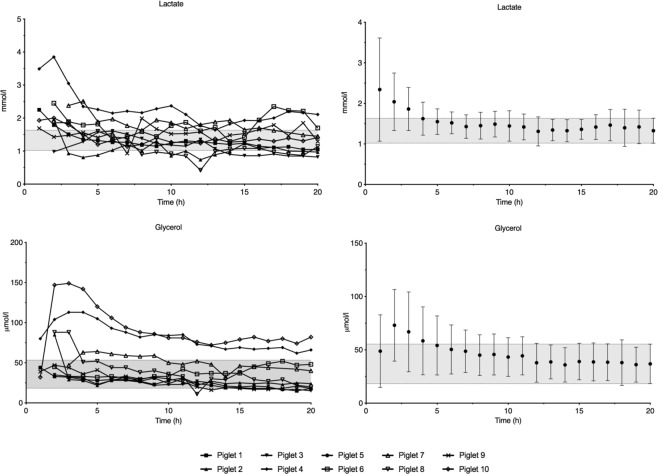
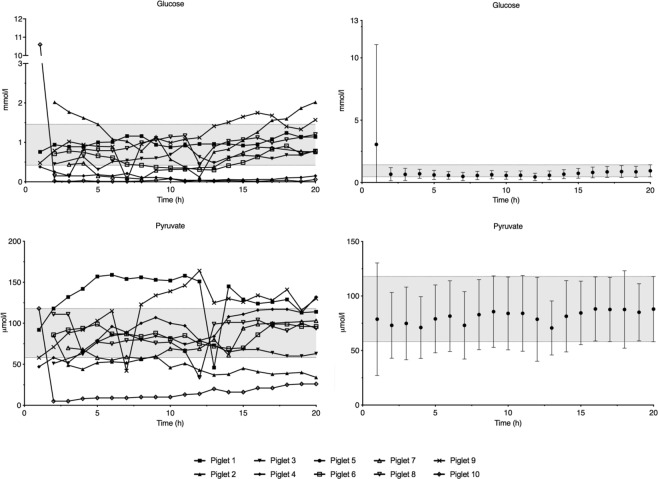
Lactate concentration peaked one hour after insertion and decreased to reach steady state at four hours (Fig. 1). Glycerol concentration increased to a peak at two hours and reached steady state after five hours of observation (Fig. 1). Except for one animal with an initially very high intracerebral glucose concentration, steady state was reached after one hour of observation for both glucose and pyruvate (Fig. 2). The interindividual variation of lactate and glycerol decreased during the first four and seven hours, respectively, while glucose and pyruvate remained constant throughout the observation period (Supplementary Data S1).
Figure 1.
Average lactate and glycerol concentrations in 9 piglets during the 20-hour observation period. Data are means with 95% confidence interval. The 95% confidence interval of the measurement at 20 hours is marked with a grey field.
Figure 2.
Average glucose and pyruvate concentrations in 9 piglets during the 20-hour observation period. Data are means with 95% confidence interval. The 95% confidence interval of the measurement at 20 hours is marked with a grey field.
Probes
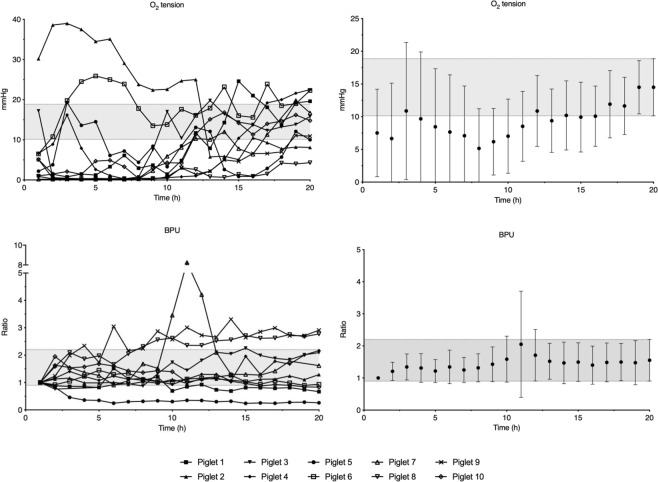
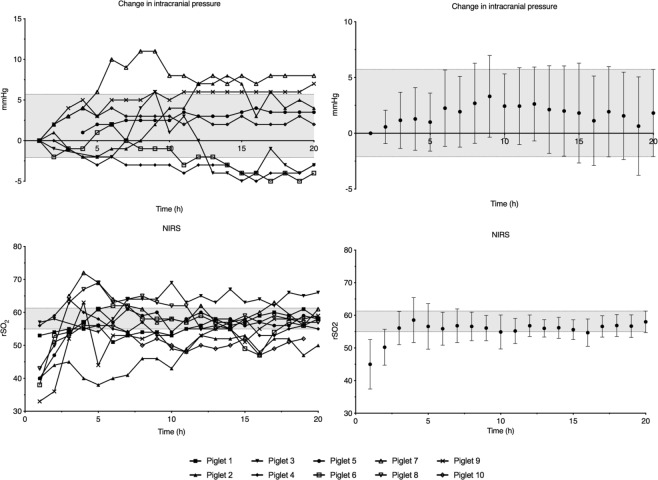
Mean oxygen tension increased through the whole observation period despite normal blood-gas values, although steady state was reached after 12 hours (Fig. 3). Some animals required 10–15 hours of observation before stable oxygen-tension values could be detected (Fig. 3). Interindividual variation of oxygen tension decreased until 10 hours of observation (Supplementary Data S1). Change in CBF and ICP reached steady state in the 2nd hour (Figs 3 and 4). After insertion of the probes, animals presented with a minor change in ICP and CBF, thus resulting in an increased interindividual variation for both measures (Supplementary Data S1). After approximately 10 hours of observation, ICP stabilized as presented in the raw data of Fig. 4.
Figure 3.
Average measurements of O2 tension and cerebral blood flow in 10 piglets during the 20-hour observation period. Data are means with 95% confidence interval. The 95% confidence interval of the measurement at 20 hours is marked with a grey field. BPU; blood perfusion units.
Figure 4.
Average measurements of change in intracranial pressure for 8 piglets and NIRS for 10 piglets during the 20-hour observation period. Data are means with 95% confidence interval. The 95% confidence interval of the measurement at 20 hours is marked with a grey field. NIRS; near infrared spectroscopy.
Near Infrared Spectroscopy (NIRS)
NIRS values increased and researched steady state after three hours of observation (Fig. 4). Interindividual variation decreased during the first 11 hours of observation (Supplementary Data S1).
Discussion
We showed that sufficient observation time to reach steady state is essential to acquire valid baseline measurements when using intracerebral probes and microdialysis. Time needed to reach steady state was longer than expected for the majority of measurements and varied depending on the device and the specific metabolite measured. We also showed that adequate observation time is important to get homologous baseline values, as interindividual variability decreased for the majority of measures during the first hours of observation.
Benveniste et al. showed that the recovery from a microdialysis catheter will initially be increased after insertion, thus potentially producing falsely increased values during the first 30–60 minutes14. They also showed that the insertion trauma will cause an acute leak over the blood-brain barrier during the first 10 minutes after insertion, which will affect early measures further14. These acute changes are consistent with our results. Further, microdialysis recovery will be affected by other factors related to the technical details (e.g., membrane length and flow rate)7. A flow rate of 0.3 μL/min is commonly used7. To ensure sufficient sample volume, we chose a flow rate of 0.5 μL/min. This needs to be acknowledged in study-to-study comparison of absolute values, but should not affect overall trend.
Glycerol is considered a marker of phosolipid degeneration and cell wall destruction4. We found a delay with glycerol concentration peak two hours after the insertion trauma followed by a decrease towards steady-state values. A similar pattern was seen with regards to lactate (Fig. 1). Lactate production may be a consequence of mitochondrial dysfunction, and lactate concentrations were increased shortly after insertion and then decreased to reach steady state15. The insertion trauma thus appeared to cause cell lysis and metabolic compromise with release of metabolites, which may have influenced early microdialysis measures. O2 tension increased during our observation period and required the longest time to reach steady state of all measurements (Fig. 3). O’Hara et al., used a similar device, and reported an average of 52 minutes of observation before acquiring stable values16. They found haemorrhage in the insertion canal of the probe on post-mortem examination, which together with tissue compression, temperature changes, and location of the probe may have affected early readings16. They retracted the probe 0.5 mm after insertion to relieve pressure from the tip; a procedure we did not perform. We placed the piglet in a supine position after placement of the probe, which may have resulted in additional tissue compression in our study. We also found blood and microscopic bone fragments inside the probe canal on post-mortem examination and we believe that this may be a likely cause of the prolonged time to steady state seen in our animals. Compared to glycerol and lactate concentration, the insertion trauma may have caused low readings right after insertion due to bleeding and potential blockage of the tip of the device – rather than producing metabolites picked up by the device resulting in early elevated readings. These findings depict two different mechanisms on how the insertion trauma may interfere with early readings.
Cerebral oximetry by NIRS is known to have a wide intraindividual variability and may therefore best be used as a trend monitor17. Surprisingly, NIRS measurements increased for all animals during the first hours of observation and reach steady state at 3 hours (Fig. 4). Placed on the contralateral side of the head, NIRS measures should not be directly affected by the insertion of the probes or microdialysis catheter. Mechanical damage caused by the insertion of a device into cerebral tissue will trigger spreading depressions, i.e., short depolarizations with neuronal swelling, ion flux, change in CBF, increased glucose consumption, and increased oxygen demand18. Most of the harmful effects of spreading depressions resolve within minutes after the insertion trauma19. However, oxygen demand may be increased for up to two hours after recovery20. This is in line with our findings as spreading depressions may alter oxygen demand on the contralateral side due to their migrating nature, and proposes the possibility that inserting a device into cerebral tissue might also affect early reading from extracerebral devices such as NIRS20.
We installed the devices into healthy cerebral tissue. In neurocritical care, microdialysis is often installed during operation for various pathologies (e.g., traumatic brain injury or sub arachnoid haemorrhage)21. When used in perilesional tissue compared to healthy tissue in patients with traumatic brain injury, microdialysis measurements showed altered baseline values and a different reaction to change in other physiological parameters such as intracranial pressure and oxygenation22. Although device size, shape, and stiffness may influence the severity of the insertion trauma, little is known on whether the number of devices placed in close proximity might influence the mearuements23. To ensure sampling of the same anatomical location, we used an inter-device distance of 3 mm. Thus, the insertion trauma from one device might have influenced the tissue surrounding the neighbouring device.
Researchers are ethically obliged to utilize as few animals as possible, according to the “reduction” in the 3R-principple, as originally described by Russell and Burch in 195924. In this study, we show that sufficient observation time before interventions will result in a decreased interindividual variation and thus fewer animals needed to obtain the same power when comparing two or more groups (Supplementary Data S1).
The insertion trauma is most likely affected by the skill and dexterity of the researcher placing the device. This might be limited by the use of a stereotactic frame, which was not used in the current study. Another weakness is related to the precision of the device placement. We aimed to standardize device placement through measurements made before hand on autopsy in pilot animals. The final location was only verified in a few animals by macroscopic examination post-mortem. To reduce variation, all devices were placed by the same researcher (TCKA) according to standardized measurements made beforehand. A strength of this study is the long observation time. This allowed for repeated measurements, which for some animals were needed to get the stabile baseline data with little inter-individual variation. Our findings are likely generalizable to the use of intracerebral devices in other animal species or humans. Steady state was determined by means of 95% confidence intervals after 20 hours observation, as we expected the impact of the insertion trauma to be minimal at this time. This simple strategy of determining steady state, combined with graphing of raw data along with the presentation of averaged data, was chosen to allow for critical revision of the temporal changes by others.
Accordingly, one hour of observation after insertion was insufficient to acquire stable measurements in our setup. The observation time depends on the choice of device and the specific metabolite measured. The early measurements will not only be affected by the insertion trauma but also by other factors, such as the state of the cerebral tissue, recovery of microdialysis, type and number of devices. This study underlines the importance of appropriate observation time after insertion when using intracerebral devices. We demonstrate that appropriate observation time is crucial to retrieve information with a minimum of interindividual variation, which in turn will reduce the number of animals needed to detect an effect of a given intervention.
Methods
We used a neonatal piglet model, previously described by our group25. The project was conducted according to the guidelines given by the Danish Animal Experimentation Inspectorate and was approved by this institution (2016–15–0201–01146). The study comprised 10 newborn Danish landrace piglets, 12–24 hours old, with a mean body weight of 1.7 (1.4–2.0) kg and mean haemoglobin of 5.0 (4.3–6.8) mmol/L. The project is reported in accordance with the ARRIVE guidelines26.
Anaesthesia and monitoring
The piglets were anesthetized by inhalation of 2–4% sevoflurane. Intravenous access was acquired through an ear vein and a bolus of propofol 10 mg/kg, fentanyl 30 μg/kg, and rocuronium 1 mg/kg was administered. The animals were intubated ventilated with a target end-tidal CO2 (ECO2) of 4.5–5.5 kPa. After intubation, sedation and analgesia was maintained by continuous infusion of propofol 4–12 mg/kg/h and fentanyl 10–20 μg/kg/h. For monitoring purposes, blood sampling, and fluid and glucose administration, umbilical venous and arterial catheters were placed. Electrolyte and glucose levels were kept within the normal range by infusion with 0.72% NaCl (5 mmol/l K and 10% glucose) at 5–10 ml/kg/h. Mean arterial blood pressure (MAP), heart rate, oxygen saturation (SatO2%), fraction of inspired oxygen (FiO2), ECO2, and temperature were monitored and transferred to a computer by dedicated software (Datex Ohmeda S/5 Collect, Finland). Core temperature was measured by a rectal thermometer. All animals were treated with subcutaneous benzylpenicillin 15,000 IE/kg once every 12 hours. Plasma electrolytes, glucose, and blood-gas values was regularly measured during the 20-hour observation period and fluids, electrolytes, and ventilator settings was adjusted accordingly. We aimed to maintain a target MAP above 40 mmHg. Mean blood pressures below the target MAP were treated by first reducing anaesthesia and analgesia infusion. If hypotension persisted the animal was treated with noradrenaline infusion 0.25–1.5 μg/kg/min27.
Intracerebral measurements
Using a cannula (diameter of 2.1 mm for the first and 1.5 mm for the second and third hole) three holes of 10 mm depth where made through the scalp. The most rostral hole was made 5 mm posterior to a straight line between the eyes and 10 mm laterally of the midline. The two additional holes were made 3 mm apart, the first being 3 mm posterior to the first hole. All three holes were made on the left side of the midline, targeting the left parasagittal cortex. In the most rostral hole a probe measuring ICP was installed (Codman, Sweden). The ICP probe was calibrated to zero in room air before insertion. A probe measuring CBF by laser-doppler technique (presented as blood perfusion units [BPU]), temperature, and oxygen tension was installed in the second hole (OxyLite/OxyFlo, Oxford Optronics, UK). In the third hole, a CMA 20 Elite microdialysis catheter (4 mm membrane length) with central nervous system, sterile isotonic perfusion fluid with a flowrate 0.5 μl/min, was installed and connected to a CMA 402 syringe pump (CMA Microdialysis, Kista, Sweden). The microdialysate was analysed for lactate, pyruvate, glucose, and glycerol on a CMA 600 microdialysis analyser. The probes and the microdialysis catheter were then secured to the scalp. To serve as a non-invasive cerebral measurement, a near infrared spectroscopy (NIRS) neonatal somasensor (Medtronic, MN, USA) was placed on the parietal region on the right side of the scalp. The piglets were then put in a supine position.
Experimental protocol
After placement of the intracerebral probes and microdialysis catheter, animals were allowed to rest for 30–60 minutes. The first measurements were then acquired (defined as time point “1”). Then animals were observed for 20 hours. Measurements were acquired once every hour. At the end of the experiment, the animals were euthanized through a lethal injection with pentobarbital 400 mg/ml, 0.4 ml/kg.
Statistics
From the Oxford-Optronics probe, measurements were registered for 5–10 minutes once every hour and a mean value during that time was noted as a value for that hour. Data from the ICP probe are presented as change in mmHg from insertion. When calculating change in ICP and CBF (BPU), measurements from the 1st hour were used as the reference. Due to the exploratory nature of this study, we applied the Resource Equation Method to determine sample size28. Despite a small sample size and risk of loss of animals throughout the study, we estimated that 10 animals was sufficient to complete the aim of this study. We expected that all three devices would have reached baseline at 20 hours of observation. Therefore, the mean with 95% confidence intervals (CI) for that time point were used as a reference when determining at what time point steady state was reached. All intracerebral measurements are presented as mean with 95% CI as well as raw data for each of the measures. 95% CI at 20 hours are marked with a grey field in each graph. Demographic data are presented as mean with range. Within-population variations are presented as standard deviations in Supplementary Data S1.
Supplementary information
Acknowledgements
This study was funded by The Lundbeck Foundation (grant nr: R20 8-2015-3647), the foundation of Marie Dorthea and Holger From, Haderslevs, and Aarhus University. The authors would like to acknowledge the staff at the animal facilities at Institute of Clinical Medicine at Aarhus University.
Author Contributions
T.C.K.A., K.J.K., V.E.H., M.P. and T.B.H. designed the study. T.C.K.A., M.V.P., N.B. and C.O. undertook the experiments. T.C.K.A. performed statistical analysis and drafted the manuscript. All authors have critically reviewed the drafted manuscript. All authors have approved the final manuscript and agree to be accountable for all aspects of the work.
Data Availability
The datasets generated and analysed during the current study are available from the corresponding author on request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-47052-4.
References
- 1.Hillered L, Persson L. Microdialysis for neurochemical monitoring of the human brain. Scand. Cardiovasc. J. 2003;37:13–17. doi: 10.1080/14017430310006974. [DOI] [PubMed] [Google Scholar]
- 2.Frøen JF, Amerio G, Stray-Pedersen B, Saugstad OD. Detrimental effects of nicotine and endotoxin in the newborn piglet brain during severe hypoxemia. Biol. Neonate. 2002;82:188–196. doi: 10.1159/000063610. [DOI] [PubMed] [Google Scholar]
- 3.Lyng K, Munkeby BH, Saugstad OD, Stray-Pedersen B, Frøen JF. Effect of interleukin-10 on newborn piglet brain following hypoxia-ischemia and endotoxin-induced inflammation. Biol. Neonate. 2005;87:207–216. doi: 10.1159/000083131. [DOI] [PubMed] [Google Scholar]
- 4.Hillered L, Valtysson J, Enblad P, Persson L. Interstitial glycerol as a marker for membrane phospholipid degradation in the acutely injured human brain. J. Neurol. Neurosurg. Psychiatry. 1998;64:486–491. doi: 10.1136/jnnp.64.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enblad P, et al. Middle cerebral artery occlusion and reperfusion in primates monitored by microdialysis and sequential positron emission tomography. Stroke. 2001;32:1574–1580. doi: 10.1161/01.STR.32.7.1574. [DOI] [PubMed] [Google Scholar]
- 6.Nordström CH, Koskinen LO, Olivecrona M. Aspects on the physiological and biochemical foundations of neurocritical care. Front. Neurol. 2017;8:274. doi: 10.3389/fneur.2017.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutchinson PJ, et al. Consensus statement from the 2014 International Microdialysis Forum. Intensive Care Med. 2015;41:1517–1528. doi: 10.1007/s00134-015-3930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawoos U, McCarron RM, Auker CR, Chavko M. Advances in intracranial pressure monitoring and its significance in managing traumatic brain injury. International Journal of Molecular Sciences. 2015;16:28979–28997. doi: 10.3390/ijms161226146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turkina, M. V., Ghafouri, N., Gerdle, B. & Ghafouri, B. Evaluation of dynamic changes in interstitial fluid proteome following microdialysis probe insertion trauma in trapezius muscle of healthy women. Sci. Rep. 7 (2017). [DOI] [PMC free article] [PubMed]
- 10.Flodgren GM, et al. Glutamate and prostaglandin E2in the trapezius muscle of female subjects with chronic muscle pain and controls determined by microdialysis. Eur. J. Pain. 2005;9:511–515. doi: 10.1016/j.ejpain.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Sørensen, L. B. et al. Investigation of biomarkers alterations after an acute tissue trauma in human trapezius muscle, using microdialysis. Sci. Rep. 8 (2018). [DOI] [PMC free article] [PubMed]
- 12.Mellergard P, Aneman O, Sjogren F, Pettersson P, Hillman J. Changes in extracellular concentrations of some cytokines, chemokines, and neurotrophic factors after insertion of intracerebral microdialysis catheters in neurosurgical patients. Neurosurgery. 2008;62:151–158. doi: 10.1227/01.NEU.0000311072.33615.3A. [DOI] [PubMed] [Google Scholar]
- 13.Bouras, T. I. et al. Neuro-inflammatory sequelae of minimal trauma in the non-traumatized human brain. A microdialysis study. J. Neurotrauma, 10.1089/neu.2011.1790 (2011). [DOI] [PubMed]
- 14.Benveniste H. Brain Microdialysis. J. Neurochem. 1989;52:1667–1679. doi: 10.1111/j.1471-4159.1989.tb07243.x. [DOI] [PubMed] [Google Scholar]
- 15.Bouzat P, Oddo M. Lactate and the injured brain: Friend or foe? Current Opinion in Critical Care. 2014;20:133–140. doi: 10.1097/MCC.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 16.O’Hara JA, et al. Simultaneous measurement of rat brain cortex PtO2 using EPR oximetry and a fluorescence fiber-optic sensor during normoxia and hyperoxia. Physiol. Meas. 2005;26:203–213. doi: 10.1088/0967-3334/26/3/006. [DOI] [PubMed] [Google Scholar]
- 17.Thavasothy M, Broadhead M, Elwell C, Peters M, Smith M. A comparison of cerebral oxygenation as measured by the NIRO 300 and the INVOS 5100 near-infrared spectrophotometers. Anaesthesia. 2002;57:999–1006. doi: 10.1046/j.1365-2044.2002.02826.x. [DOI] [PubMed] [Google Scholar]
- 18.Somjen GG. Mechanisms of Spreading Depression and Hypoxic Spreading Depression-Like Depolarization. Physiol. Rev. 2001;81:1065–1096. doi: 10.1152/physrev.2001.81.3.1065. [DOI] [PubMed] [Google Scholar]
- 19.Ayata C, Lauritzen M. Spreading Depression, Spreading Depolarizations, and the Cerebral Vasculature. Physiol. Rev. 2015;95:953–993. doi: 10.1152/physrev.00027.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piilgaard H, Lauritzen M. Persistent increase in oxygen consumption and impaired neurovascular coupling after spreading depression in rat neocortex. J. Cereb. Blood Flow Metab. 2009;29:1517–1527. doi: 10.1038/jcbfm.2009.73. [DOI] [PubMed] [Google Scholar]
- 21.Hillered Lars, Vespa Paul M., Hovda David A. Translational Neurochemical Research in Acute Human Brain Injury: The Current Status and Potential Future for Cerebral Microdialysis. Journal of Neurotrauma. 2005;22(1):3–41. doi: 10.1089/neu.2005.22.3. [DOI] [PubMed] [Google Scholar]
- 22.Timofeev Ivan, Czosnyka Marek, Carpenter Keri L.H., Nortje Jurgens, Kirkpatrick Peter J., Al-Rawi Pippa G., Menon David K., Pickard John D., Gupta Arun K., Hutchinson Peter J. Interaction between Brain Chemistry and Physiology after Traumatic Brain Injury: Impact of Autoregulation and Microdialysis Catheter Location. Journal of Neurotrauma. 2011;28(6):849–860. doi: 10.1089/neu.2010.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozai Takashi D. Y., Jaquins-Gerstl Andrea S., Vazquez Alberto L., Michael Adrian C., Cui X. Tracy. Brain Tissue Responses to Neural Implants Impact Signal Sensitivity and Intervention Strategies. ACS Chemical Neuroscience. 2015;6(1):48–67. doi: 10.1021/cn500256e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell, W. M. S. & Burch, R. L. The Principles of Humane Experimental Technique. In The Principles of Humane Experimental Technique10.1017/CBO9781107415324.004 (1959).
- 25.Kyng, K. J. et al. A Piglet Model of Neonatal Hypoxic-Ischemic Encephalopathy. J. Vis. Exp. e52454 10.3791/52454 (2015). [DOI] [PMC free article] [PubMed]
- 26.Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Editorial: Animal research: Reporting in vivo experiments-The ARRIVE Guidelines. J. Cereb. Blood Flow Metab. 2011;31:991–993. doi: 10.1038/jcbfm.2010.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nachar RA, et al. Dose-dependent hemodynamic and metabolic effects of vasoactive medications in normotensive, anesthetized neonatal piglets. Pediatr. Res. 2011;70:473–479. doi: 10.1203/PDR.0b013e31822e178e. [DOI] [PubMed] [Google Scholar]
- 28.Festing MFW, Altman DG. Guidelines for the Design and Statistical Analysis of Experiments Using Laboratory Animals. ILAR J. 2002;43:244–258. doi: 10.1093/ilar.43.4.244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on request.