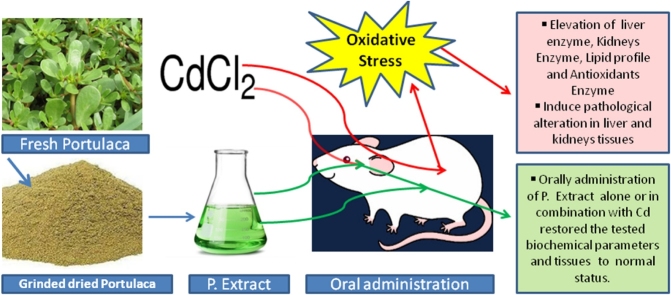
Graphical abstract
Keywords: Cadmium, Chemistry, Histopathology, Kidney, Liver, P. oleracea, Rat
Highlights
-
•
Cadmium (Cd) is a toxic heavy metal widely occurred around the world, particularly in soils, well water and contaminated food/feed.
-
•
Cadmium-induced harmful effects on liver/ kidneys function, lipid profile and antioxidant status in experimental rats.
-
•
Oral exposure to cadmium caused severe pathological alterations in renal and liver tissues in experimental rats.
-
•
Portulaca extract alone or in combination with Cd revered biochemical markers and tissues to the control status.
Abstract
The study was designed to clarify the hapato-nephroprotective effects of purslane ethanolic extract (PEE) against cadmium toxicity. Cadmium (Cd) is a toxic heavy metal. Cd occurs as environmental and food/ feed contamination causing public and animals health hazards. Liver and kidney are the main target organs for acute and chronic cadmium toxicity. Portulaca oleracea is rich in several vitamins, minerals, antioxidant components, and omega-3 fatty acids mainly α-linolenic acid and eicosapentaenoic acid. Results showed significant elevation of the liver and kidney functions, lipid profile and lipid peroxidation. In contrast to the antioxidants enzymatic were greatly decreased. The hepatic and renal tissues showed severe degeneration and necrosis accompanied by severe congestion and multifocal hemorrhages in Cd intoxicated rats. All parameters and tissues showed no changes in rates-treated with both Cd and purslane extract as compared with the control rats. The administration of PEE provided a significantly protection against Cd-induced hepato-nephrotoxicity.
1. Introduction
Food and feedstuff contamination with heavy-metals represent problems in human and animal health, therefore attracting worldwide attention [1]. Unfortunately, Cadmium (Cd) is a metal of commercial importance. It used in nickel-Cd batteries, plating, pigments and plastics manufacturer [2]. Cd is a harmful and broadly disseminated poisonous material that is greatly detected in soil, food, air, and water [3]. Sheep, goat and cattle flocks could be grazed in Cd-contaminated grasses, therefore, gaining access into the livestock body tissues [4]. Cd is capable of distribution through different organs rapidly and most of ingested Cd enters into the liver and kidneys [5]. When Cd is gaining access into renal cells the cellular production of reactive oxygen species (ROS) and apoptosis occurred. Cd accumulated in the kidneys leading to nephrotoxicity [6,7]. Cd was more pronounced in renal proximal convoluted tubules and clinically observed as glycosuria, proteinuria, and aminoaciduria [8]. The liver is the most target organ of Cd and Cd hepatotoxicity is the main reason for its acute lethality [9]. After absorption, cd is distributed in animal body through red blood cells or proteins. A major amount of Cd in RBCs joins with proteins of high molecular weight, whilst minor amount joins with hemoglobin so cd may cause anemia [10]. Cd is transferred to the liver and so caused damages and functional disturbance which confirmed histopathologically and biochemically. Nephrotoxicity is clarified via the structural damage of kidneys and the excretory functional changes [11,12]. Cd was recorded to induce toxicity in reproductive, endocrine, pancreas, immune and cardiovascular systems [13,14].
Medicinal Plants and formulated natural products have a wide important role in the elemination of many hepatorenal adverse effects that caused by espoure to enviromental and food contaminants [15,16,17]. Portulaca oleracea (P. oleracea) is a member of the Portulacaceae family commonly called purslane [18]. P. oleracea is richer in omega-3 fatty acids than other vegetables [19]. It contains vitamin A, vitamin C, carotenoids and some of vitamin B. Magnesium, calcium, potassium, and iron was detected. Also, two types of betalain alkaloid pigments which have antioxidant and antimutagenic effects [20]. P. oleracea have hepatoprotective effects against numerous toxins causing hepatotoxicity [21]. Some hepatotoxins induced liver fibrosis and increased all of the liver enzymes, total bilirubin and TNF-α in serum and malondialdehyde (MDA) in liver tissue. Also decreases the hepatic antioxidant activity. Conversely, by the administration of P. oleracea, all the disturbing biochemical parameters will return to normal [22]. P. oleracea have a promising role in the treatment of acute renal injury induced by nephrotoxins as cisplatin [23]. Application of purslane ethanolic extract treated the adverse changes in kidney functions, antioxidant, and peroxidation activity [18]. There is little information about the hepato-renal protection of Purslane (P. oleracea) against Cd toxicity. Therefore our aim in this experimental study is to clarify the hepato-renal protective effects of purslane against Cd toxicity.
2. Materials and methods
2.1. Preparation of Portulaca oleracea (purslane) ethanolic extract
Fresh P. oleracea plant was collected on July 2016 from a farm in Assiut governorate, Egypt. The leaves of P. oleracea were cleaned and dried at ambient temperature (25 °C). Then, 500 ml of (50% ethanol solution) was added to 50 g of powdered leaves and the mixture heated under reflux for 60 min. The decoction was filtered and frozen at −20 °C until used [24]. Finally, the preserved filtrate was thawed and then lyophilized. The crude yield of the lyophilized material was approximately 32% (w/w).
2.2. Phytochemical screening
Phytochemical analysis carried out to determine the presence of active chemical constituents in purslane extract such as tannins, terpenoids, alkaloids, saponins, and flavonoids. The presence of flavonoids and tannins were detected chemically according to Brain and Turner [25]. The detection of alkaloids carried out according to Finar [26]. Saponins were detected by the method of Parekh et al. [27]. Salkovski test indicates the presence of terpenoids performed using a small amount of extract solution according to Finar [26].
2.3. Antioxidant activity
DPPH radical-scavenging activity percentage of the antioxidant activity evaluated by the method of Brand-Williams et al. [28] using DPPH (2, 2-diphenyl-1-picryl-hydrazyl-hydrate) for initiation of the free radicals. The resulting solution measured spectrophotometrically at 517 nm.
2.4. Experimental animals
Thirty-two Sprague-Dawley rats weighing 140–145 g were used. Rats housed in groups of 8 per cage with free access to standard laboratory chow and tap water. Rats housed in a temperature-controlled room (22 ± 2 °C and 40–60% RH) under 12 h light and dark cycles for 1 week prior to and during the experiment. The study conducted in accordance with ethical procedures and policies approved by the Animal Care and Use Committee of National Research Centre, Dokki, Egypt.
2.5. Kits
Alanine amino Transaminase (ALT), Aspartate amino Transaminase (AST), Alkaline Phosphatase, (ALP), Total Protein (TP) Urea, Creatinine, Triglyceride (TriG), Cholesterol (Cho.) kits from Spectrum Co. (Spain). Malondialdehyde (MDA), Catalase (CAT), Superoxide Dismutase (SOD) and Glutathione Peroxidase (GPx) kits from Bio-diagnostics Co. (Egypt).
2.6. Experimental design
Cadmium treatment and rat grouping: Cadmium chloride (CdCl2) dissolved in saline solution to induce chronic toxicity [29]. The dose of purslane extract was chosen as high dose to assure the protection against cadmium toxicity, while the dose 3.5 mg/kg of cadmium of body weight was chosen according to [30]. Rats randomly divided into four groups (n = 8) and treated regularly as single daily and intraperitoneally for 30 consecutive days by the following administrations:
First group: Rats were received normal saline and served as a negative control.
Second group: Rats were received an ethanolic extract of purslane at a dose of (2 g/kg bw).
Third group: Rats were received of CdCl2 at a dose of (3.5 mg/kg bw).
Fourth group: rats were received of CdCl2 at a dose of (3.5 mg/kg bw) and after 30 min the rats treated with ethanolic extract of purslane at a dose of (2 g/kg bw).
In 31st day, blood samples were collected via retro-orbital venous plexus and serum was separated [31] then rats sacrificed and liver and kidneys from rats were excised, weighed and rinsed in ice-cold 0.25 M sucrose solution and 10%w/v homogenate prepared in 0.05 M PBS (pH 7), centrifuged at 12,000×g / 60 min at 4 °C [32]. Supernatant collected and stored at−20 °C until monitored for oxidative stress parameters.
2.7. Determination of serum biochemical parameters
Serum Alanine-amino-Transaminase(ALT), Aspartate-amino-Transaminase(AST) [33] Alkaline Phosphatase (ALP) [34], Cholesterol (Cho) [35], Triglycerides (TriG) [36, Urea [37], Creatinine [38].
2.8. Protein estimation
Protein estimated in subcellular fractions using bovine serum albumin (BSA) as standard. The rapid and sensitive method was done for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding [39].
2.9. Measurement of Malondialdehyde (MDA) in liver homogenates
Malondialdehyde (MDA) used as a biomarker for lipid peroxidation. MDA reacts with thiobarbituric acid (TBA) as a thiobarbituric acid reactive substance (TBARS) to form a 1:2 MDA-TBA adduct, which absorbs at 532 nm. Thus, the quantity of TBARS is proportionate to the amount of MDA. The concentration of TBARS is determined and calculated using the MDA standard curve and was expressed as nmol/mg of protein [40].
2.10. Determination of cellular antioxidant status
Oxidative stress was measured by estimating the enzymatic and non-enzymatic antioxidants such as superoxide dismutase (SOD) [41], catalase [42], and glutathione peroxidase (GPx) [43] in liver tissue homogenate.
2.11. Statistical analysis
Data are presented as means ± SD and evaluated by one-way analysis of variance (ANOVA) implemented in SPSS Version 13.0 (SPSS Ltd., Surrey, UK). Individual comparisons assessed by Duncan’s multiple range test (DMRT). Differencesare statistically significant when p < 0.05.
2.12. Histopathological examination
Programmable post mortem examination was carried out for the four groups of rats. All macroscopical lesions were recorded. Tissue specimens from the liver, kidney were taken for histopathological processing. The collected tissue specimens were fixed in 10% neutral buffer formalin (NBF). Routine dehydration, embedding in paraffin wax and sectioning at 5 μm occurred. The tissue slides were stained with hematoxylin and eosin (H&E) for histopathological examination [44].
3. Results
All rats survived along the experimental period of 4 weeks till they were sacrificed in the 31st day. Daily observation over the experimental period showed detectable alterations in the healthy state of the cadmium treated rats (group 3).
3.1. Phytochemical constituents of Portulaca oleracea ethanolic extract
Table 1 illustrated the presence of alkaloids, flavonoids, steroids, saponins, and phenols and absence of tannins in ethanolic extracts of P. oleracea these findings in line with [45].
Table 1.
Phytochemical constituents of Portulaca oleracea ethanolic extract.
| Purslane Ethanolic extract | Antioxidants activity | Flavonoids | Tannins | Saponins | Terpenoids | Alkaloids |
|---|---|---|---|---|---|---|
| – | 69% | + | – | + | + | ++ |
(+) Presence of phytochemical constituents. (-) Absence of phytochemical constituents.
3.2. Changes in body, liver and kidney weights of the rats
Table 2 showed that the final body weight, body weight gain, liver, and kidneys weight showed significant difference among groups. Exposure to cd significantly (p < 0.05) decreased final body weight, body weight gain but increased in control and purslane treated groups. Inversely, liver and kidney weights significantly (p < 0.05) increased in cd treated group. Rats treated with Purslane extract plus cd showed a significant increase in final body weight, body weight gain, liver and kidney weights as compared to cd treated group. Rats treated with Purslane only exhibited no significant difference.
Table 2.
Body, liver and kidney weights of rats treated with P. oleracea ethanolic extract.
| Control | PEE (2 g/kg bw) | Cadmium (3.5 mg/kg bw) | PEE (2 g/kgbw) + Cadmium (3.5 mg/kg bw) |
||
|---|---|---|---|---|---|
| Body weight (g) | Initial | 143.6 ± 1.6 | 143.3 ± 2.2 | 144.5 ± 3.5 | 145.6 ± 1.6 |
| Final | 212.4 ± 2.3 | 219 ± 5.5 | 182.6 ± 4.5* | 196.3 ± 1.5 | |
| Weight gain (g) | 68.80 ± 3.4 | 75.7 ± 7.7 | 38.1 ± 7.9* | 50.7 ± 2.1* | |
| Liver weight (g) | 2.94 ± 0.15 | 2.81 ± 0.16 | 3.54 ± 0.12* | 2.81 ± 0.11 | |
| liver weight/ body weight X 100 | 1.38 ± 0.06 | 1.28 ± 0.09 | 1.94 ± 0.05* | 1.43 ± 0.06 | |
| kidney weight (g) | 1.70 ± 0.03 | 1.70 ± 0.03 | 1.90 ± 0.17* | 1.81 ± 0.14 | |
| Kidney weight/ body weight X 100 | 0.80 ± 0.03 | 0.80 ± 0.03 | 1.08 ± 0.8* | 0.90 ± 0.07 |
Each measurement was done at least in triplicate and the values are the means ± SD for eight rats in each group.
PEE = purslane ethanolic extract.
P < 0.05 significantly different from the normal control group.
3.3. Effect of cadmium and P. oleracea ethanolic extract on liver function of the rat
Table 3, after 30 concessive days treatment with cd, Purslane extract and their combination to rats. Cd-treated group developed hepatocellular damage that evident from significant elevation in serum activities of AST, ALT, ALP, TGi, and TP levels in comparison to control rats. The ethanolic extract of P. oleracea showed significant restoration of the altered biochemical parameters at a dose (2 mg/kg) when compared to Cd-treated group.
Table 3.
Effect of cadmium and P. oleracea ethanolic extract on liver function of the rat.
| ALT (IU/ml) | AST (IU/ml) | ALP (IU/ml) | TP (mg/dl) | |
|---|---|---|---|---|
| Control | 33.8 ± 0.4 | 37 ± 0.6 | 178.8 ± 3 | 6.2 ± 0.2 |
| PEE (2 g/kg bw) | 35.1 ± 1 | 39.1 ± 1.6 | 179.8 ± 1.8 | 6.3 ± 0.3 |
| Cadmium (3.5 mg/kg bw) | 85 ± 1.4* | 91.4 ± 2.9* | 216.8 ± 3* | 8.3 ± 0.3* |
| PEE (2 g/kg bw) + Cadmium (3.5 mg/kg bw) | 35.7 ± 2 | 40.7 ± 0.6 | 180.4 ± 0.9 | 6.5 ± 0.1 |
Each measurement was done at least in triplicate sample and Data is presented as mean ± SD (n = 8); PEE = purslane ethanolic extract; AST = aspartate transaminase; ALT = alanine transaminase; ALP = Alkaline Phosphatase; TP = Total Protein.
Significantly different from the normal control group at p < 0.05.
3.4. Effect of cadmium and P. oleracea on kidney function
Table 4 stated significant (p < 0.05) increase in serum urea and creatinine in Cd-treated group were noticed. Daily administration of P. oleracea efficiently maintained the normal serum urea and creatinine levels. The combination between P. oleracea and Cd caused significantly restored serum creatinine levels to normal as compared to Cd alone but serum urea had no significant difference from Cd group.
Table 4.
Effect of cadmium and P. oleracea ethanolic extract on kidney function.
| Creatinine (mg/dl) | Urea (mg/dl) | |
|---|---|---|
| Control | 0.60 ± 0.01 | 5.7 ± 0.07 |
| PEE (2 g/kg bw) | 0.61 ± 0.01 | 5.9 ± 0.3 |
| Cadmium (3.5 mg/kg bw) | 0.84 ± 0.02* | 7.8 ± 0.4* |
| PEE(2 g/kg bw) + Cadmium (3.5 mg/kg bw) | 0.64 ± 0.10 | 6.1 ± 0.9* |
Each measurement was done at least in triplicate samples and Data is presented as mean ± SD (n = 8).
PEE = purslane ethanolic extract.
Significantly different from the normal control group at p < 0.05.
3.5. Effect of cadmium and P. oleracea on serum triglycerides and cholesterol
Table 5 Summarized Significant elevation in triglyceride and cholesterol level in Cd-treated group was observed. Otherwise no significant difference (p < 0.05) in triglycerides and cholesterol levels of rats treated with purslane alone or purslane plus Cd when compared with the control group.
Table 5.
Effect of cadmium and P. oleracea ethanolic extract on serum triglycerides and Cholesterol.
| TriG (mg/dl) | Cholesterol (mg/dl) | |
|---|---|---|
| Control | 47.6 ± 0.7 | 177.3 ± 2.5 |
| PEE (2 g/kg bw) | 52.7 ± 2.2 | 180.2 ± 4.6 |
| Cadmium (3.5 mg/kg bw) | 177.2 ± 4.3* | 206.7 ± 2.1* |
| PEE(2 g/kg bw) + Cadmium (3.5 mg/kg bw) | 92.8 ± 4.8* | 179 ± 1.7 |
Each measurement was done at least in triplicate samples and Data is presented as mean ± SD (n = 8).
PEE = purslane ethanolic extract; TriG = Triglycerides.
Significantly different from the normal control group at p < 0.05.
3.6. Effect of cadmium and P. oleracea extract on antioxidant status and lipid peroxidation
Table 6 revealed that Cd- administration caused a significant reduction in antioxidant enzymes (SOD, CAT, Gpx) in Cd-treated group in comparison to control group. Inversely; Cd increases lipid peroxidation activity as measured as MDA products in serum. Rats received purslane extract alone showed significant enhancement in antioxidant capacities by elevation the levels of SOD, CAT, and GPx. Whereas rats received purslane plus Cd showed significant improvement in lipid peroxidation and antioxidant enzymes activity.
Table 6.
Effect of cadmium and P. oleracea ethanolic extract on SOD, CAT, GPx and MDA.
| SOD (U/mgp) | CAT (U/mgp) | GPx (U/mgp) | MDA (nmol/mgp) | |
|---|---|---|---|---|
| Control | 8.31 ± 0.15 | 4.3 ± 0.01 | 6.43 ± 0.2 | 14.53 ± 0.2 |
| PEE (2 mg/kg bw) | 7.64 ± 0.04 | 4.1 ± 0.08 | 5.34 ± 0.2 | 14.31 ± 0.25 |
| Cadmium (3.5 mg/kg bw) | 5.55 ± 0.4* | 3.04 ± 0.15* | 2.44 ± 0.1* | 20.12 ± 0.4* |
| PEE (2 mg/kg bw) + Cadmium (3.5 mg/kg bw) | 7.50 ± 0.1 | 3.4 ± 0.25 | 3.86 ± 0.3 | 16.41 ± 1.06 |
Each measurement was done at least in triplicate and the values are the means ± SD for eight rats in each group;
PEE = purslane ethanolic extract; SOD = superoxide dismutase; CAT = catalase; GPx = glutathione Peroxidase MDA = Malondialdehyde.
P < 0.05 significantly different from the normal control group.
3.7. Histopathological findings
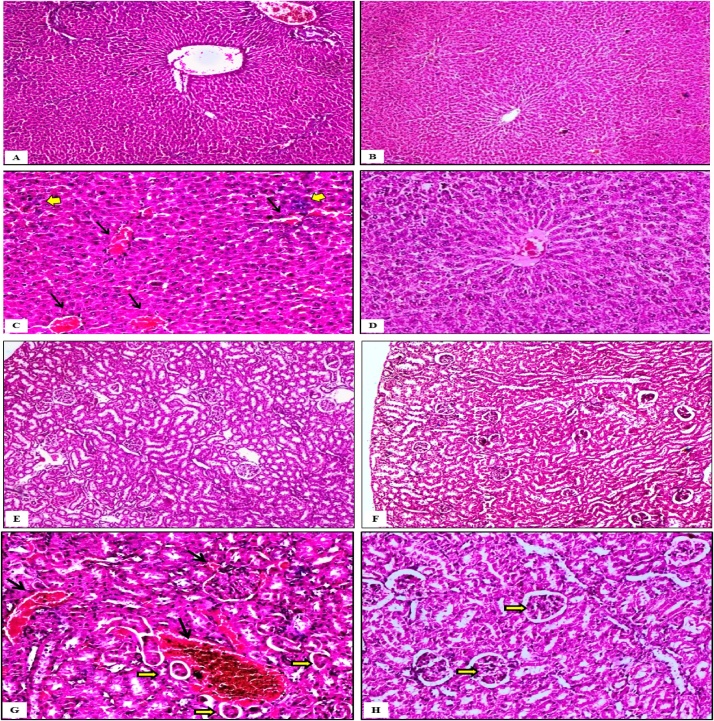
The histopathological findings in the four groups of rats in this experiment revealed that; Liver and kidney in the first group showed complete normal architectures and tissue details as in Fig. A&B. Group (2) which treated only with P. oleracea ethanolic extract, the hepatic and renal tissues do not exhibit any signs of toxicity either hemorrhages or tissue damages as in Fig. C&D. In contrast; Group (3) which treated with cadmium chloride (Cd) exhibited severe tissue toxicity mainly severe congestion in central veins of hepatic lobules accompanied by hemorrhages among hepatic cords. Moreover, mild multifocal infiltrations of inflammatory cells between the areas of hemorrhages were detected as in Fig. E. The renal tissue clarified severe congestion in the renal blood vessels accompanied with peri tubular and peri glomerular extravasation of RBCs. Moreover, large numbers of the proximal convoluted tubules in the peri vascular areas suffered from severe atrophy as in Fig G. Group (4) which firstly treated with Cd and then treated with P. oleracea ethanolic extract showed that; Liver exhibited recovery of the haemorrhagic and inflammatory condition and the hepatic tissue return to normal as denoted in Fig. F. The renal tissue became completely normal except presence of mild degeneration in the lining epithelium of the proximal convoluted tubules as in Fig H (Plate 1).
Plate 1.
Photomicrograph for experimental design done on female Sprague-Dawley rats classified into 4 groups and the route of administration is intraperitoneal injection: Group (1) control, Group (2) Rats treated with P. oleracea ethanolic extract (2 mg/kg bw), Group (3) rats treated with CdCl2 (3.5 mg/kg bw), Group (4) rats treated with (CdCl2 3.5 mg/kg bw + P. oleracea 2 mg/kg bw). All tissues pecimens were stained with H&E stain.
Liver: Fig A: Control group (1) showed completely normal tissue details in liver X 100. Fig B: group (2) liver exhibited normal tissue characters without histopathological changes X 100. Fig C: Liver of group (3) showed severe congestion in the central veins of hepatic lobules accompanied with hemorrhages among the hepatic cords (black arrows). Mild multifocal infiltrations of inflammatory cells among several hemorrhagic areas (yellow arrows) were noticed X 200. Fig D: Liver of the fourth group exhibited recovery of the hemorrhagic and inflammatory condition and the hepatic tissue return to normal X 200.
Kidney: Fig E: control group showed the normal renal tissue X 100. Fig F: group (2) kidney exhibited no histopathological changes than normal X 100. Fig G: Kidney of group (3) clarified severe congestion in most of the renal blood vessels associated with multifocal extravasation of blood among the proximal convoluted tubules and in the peri glomerular areas (black arrows). Also, severe atrophy in large numbers of the renal tubules in the peri vascular areas (yellow arrows) was observed X 200. Fig H: Kidney of group (4) the renal tissue became completely normal except presence of mild degeneration in the lining epithelium of proximal convoluted tubules (yellow arrows) X 200.
4. Discussion
Environmental contamination by Cd is a worldwide problem. Cd is a highly toxic heavy metal and its toxicity occurred by ingestion and inhalation [46]. Metabolism and accumulation of Cd occurred in liver and kidney so they are exposed to Cd toxicity [47,48]. In this study ethanolic extract of P. oleracea experimented for overcoming the Cd toxicity in liver and kidneys of rats. The mean body weight of Cd-treated group significantly decreased with a significant increase in relative liver weight, which comes in agreement with the findings of El-demerdash et al. [49]. Rahman et al. [50] reported that; long-time exposure to Cd is directly proportional to increase the risk of diabetes mellitus which expounds the weight loss in rats. Also, exposure to heavy metals deteriorates the glucocorticoid system which in turn associated with weight gain/loss [51]. The rats treated with P. oleracea alone showed no significant effect in liver functions. Significant restoration of hepatic enzymes was observed, so purslane extract contributing hepato-protection against Cd toxicity. The protective effect of P. oleracea may be due to its constituents from the active components as Flavonoids, polyphenols, and Alkaloids. Polyphenols have been reported to possess a membrane stabilizing activity by inhibiting the generation of ROS induced by Cd and maintain the cell membrane structural integrity [52]. Our findings exhibited elevation in liver enzymes in Cd-treated group. These findings come in the same line with Toppo et al. [53] who revealed that; Cd toxicity causing hepatic cell damage and its enzymes AST, ALP and ALT released into circulation, therefore the level of these enzymes in blood elevated than normal [54]. The functional nephrotoxicity in the study was indexed through urea and creatinine which was elevated in Cd-treated rats as matched to control rats. Marked improvement was detected in renal function that supports the protective effect of purslane against Cd nephrotoxicity. Our results agreed with Liang et al. [55] who reported that Purslane extract reduced urea and creatinine levels. The data of the present study pointed out that; there was marked an increase in cholesterol, triglycerides levels, this might be due to Cd-induced oxidative stress leading to high level of H2O2 which in turn causing impairment in lipid metabolism and lipid peroxidation which is correlated with Cd toxicity [56]. More to the point, oxidative stress may induce a significant increase in serum cholesterol and triglycerides concentration was observed in comparison to control rats as a result of cadmium toxicity. This rise in serum cholesterol level could be due to the changes in the gene expression of some hepatic enzymes such as hydroxyl-methyl-glutaryl Co A reductase (HMG-CoA) due to cadmium [57]. HMG CoA reductase is the rate-limiting enzyme in cholesterol biosynthesis. Cadmium might have stimulated the activity of this enzyme [58]. Decreased activity of cytochrome P450 enzymes could also be the reason for increased cholesterol concentration [59]. High serum cholesterol concentration may also be due to hepatic dysfunction [57,60]. Co-treatment with P. oleracea showed effective prevention in lipid peroxidation. Our findings are agreed with the records of Sharma [61]. The anti-oxidant activity of P. oleracea was the main cause of the reduction in lipid oxidation. Other studies added that; the poly-saccharide contents of P. oleracea possess great antioxidant ability by scavenging the free radicals like nitric oxide, hydroxyl and superoxide radicals [62]. The pretreatment of Magnesium at 40 mg Mg/kg b.w. against Cd-treatment was efficient in restoring renal and testis, GSH levels towards the control group, but had no effect on hepatic GS after the in mice intoxicated with cadmium. Also, the enhanced dietary Mg intake during Cd exposure can have at least partly beneficial effect on Cd-induced alterations in homeostasis of zinc, copper, and magnesium [63]. In the same concern [64], showed that Cd exposure Caused to diminished activities of SOD, CAT, GR, and GPx and a concomitant increase in LPx and GST activities. Administration of Ca + Zn and Vit-E with Cd significantly reversed Cd-induced alterations in oxidative stress marker enzymes. However, Vit-E showed more inhibitory activity against Cd than did Ca + Zn, and it protected against Cd-induced nephrotoxicity.
Moreover, the P. oleracea Co-treated rats with Cd led to a significant decrease of oxidative stress markers when compared with Cd-treated rats might be imputed to the antioxidant effects of polyphenols components present in purslane extract which protect cells from free radicals. In the same concern, CAT prevents oxidative hazards from H2O2 by catalyzing the formation of H2O and O2 [65]. Glutathione is a non-enzymatic antioxidant that quenches ROS as lipid peroxides [66]. Measuring of enzymatic and non-enzymatic antioxidant activities in our study emphasizes the protection of purslane on Cd-induced oxidative stress. Cd decreased the activities of SOD, CAT, and GPx in liver homogenates of rats were also reported by Chen et al. [52]
The main histopathological findings occur in Cd-treated group is; generalized congestion in blood vessels and hemorrhagic inflammation in both kidney and liver. These findings could be clarified as; recent researches highlight the relationship between vascular diseases and Cd toxicity where the endothelial lining of blood vessels is impaired via Cd toxicity [67]. There is a close relationship between Cd hepatotoxicity and inflammation; after Cd intoxication the neutrophilic infiltration and Kupffer cell activation were occurred and released several inflammatory mediators as ROS, nitric oxide which activated the expression of adhesion molecules as vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (IMAC-1) in liver, these mediators are responsible for adherence and activation of circulating inflammatory cells leading to inflammation and liver damage [8]. Our findings on renal tissue are; severe congestion of renal blood vessels and extravasation of RBCs among glomeruli and proximal convoluted tubules. Degeneration and desquamation in the lining epithelium of proximal convoluted tubules observed, some other tubules showed atrophy. These finding could be explained as; Cd-induced nephrotoxicity is mediated by the formation of Cd metallothionein complex (Cd-Mt), which is constituted in liver and released to bloodstream. Cd-Mt was filtrated by renal glomeruli and taken up by the proximal convoluted tubular cells. This complex leads to tissue damage and gradual loss of the organ’s function [68].
In the present study, liver and kidney in Cd and P. oleracea co-treated group exhibited recovery of their inflammatory and hemorrhagic conditions and returning of these tissues into normal. These finding could be related to the varieties of bioactivities of P. oleracea as; the antibacterial, analgesic and anti-inflammatory effects of P. oleracea [69], in addition to the free radical scavenging activity of P. oleracea due to its content from the high level of total flavonoids [70]. Other literature also revealed that; P. oleracea has an antioxidative influence in the cardiovascular system of mice by elevating the SOD activity [20]. Our findings in the present study come in accordance with Karimi et al. [23] they reported that; P. oleracea possesses the obvious nephroprotective capability and has a promising effect in the treatment of acute renal damage induced by nephrotoxins. Purslane is considered as a very good source for omega-3 fatty acid which has a great impact in development, growth, prevention of several cardiovascular diseases, and keeping the healthiness of immune system [71].
5. Conclusion
It could be concluded that administration of cadmium chloride at (3.5 mg/kg bw) for 30 days caused significant (p < 0.05) elevation in liver and kidney function levels, in addition to elevation in levels of cholesterol and triglyceride. Whereas the daily administration of P. oleracea ethanolic extract in combination with Cd restored levels of liver function and lipid markers to normalcy. Moreover, it significantly restored creatinine to a normal level but the urea level showed no significant difference from the Cd-treated group. As regard to the antioxidant system, Cd- administration caused a significant (p < 0.05) decline in of the antioxidant enzymes activities associated with elevating MDA level in serum. While P. oleracea extract showed enhancement in antioxidant capacities through increasing activities of antioxidant enzymes associated with reducing the MDA level significantly (p < 0.05). Furthermore, Cd-administration exhibited severe alterations histopathological detected in the liver and renal tissues. The administration of P. oleracea extract ameliorated liver and kidneys tissues through facilitating recovery of the hemorrhagic and inflammatory conditions.
References
- 1.Ye X., Qian H., Xu P., Zhu L., Longnecker M.P., Fu H. Nephrotoxicity, neurotoxicity, and mercury exposure among children with and without dental amalgam fillings. Int. J. Hyg. Environ. Health. 2009;212:378–386. doi: 10.1016/j.ijheh.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang W., Zhi J., Cui Y., Zhang F., Habyarimana A., Cambier C., Gustin P. Potentiated interaction between ineffective doses of budesonide and formoterol to control the inhaled cadmium-induced up-regulation of metalloproteinases and acute pulmonary inflammation in rats. PLoS One. 2014;9(10):e109–136. doi: 10.1371/journal.pone.0109136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buha A., Antonijević B., Bulat Z., Jaćević V., Milovanović V., Matović V. The impact of prolonged cadmium exposure and co-exposure with polychlorinated biphenyls on thyroid function in rats. Toxicol. Lett. 2013;221(2):83–90. doi: 10.1016/j.toxlet.2013.06.216. [DOI] [PubMed] [Google Scholar]
- 4.Ihedioha J., Okoye C. Cadmium and lead levels in muscle and edible offal of cow reared in Nigeria. Bull. Environ. Contam. Toxicol. 2012;88(3):422–427. doi: 10.1007/s00128-011-0509-3. [DOI] [PubMed] [Google Scholar]
- 5.Monsefi M., Fereydouni B. The effects of cadmium pollution on female rat reproductive system. J. Infertil. Reprod. Biol. 2013;1(1):1–6. [Google Scholar]
- 6.Rana M.N., Tangpong J., Rahman M.M. Toxicodynamics of lead, cadmium, mercury and arsenic-induced kidney toxicity and treatment strategy: a mini review. Toxicol. Rep. 2018;5:704–713. doi: 10.1016/j.toxrep.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gobe G., Crane D. Mitochondria, reactive oxygen species and cadmium toxicity in the kidney. Toxicol. Lett. 2010;198(1):49–55. doi: 10.1016/j.toxlet.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim M.A., Almaeen A.H., El Moneim M.A., Tammam H.G., Khalifa A.M., Nasibe M.N. Cadmium-induced hematological, renal, and hepatic toxicity: the amelioration by spirulina platensis. Saudi J. Forensic Med. Sci. 2018;1:5–13. [Google Scholar]
- 9.Klaassen C.D., Liu J., Diwan B.A. Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharm. 2009;238(3):215–220. doi: 10.1016/j.taap.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andjelkovic M., Djordjevic A.B., Antonijevic E., Antonijevic B., Stanic M., Stevuljevic J.K., Kalimanovska V.S., Jovanovic M., Boricic N., Wallace D., Bulat Z. Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int. J. Environ. Res. Public Health. 2019;16:274–295. doi: 10.3390/ijerph16020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yildirim S., Celikezen F.C., Oto G., Sengul E., Bulduk M., Tasdemir M., Ali C.D. An investigation of protective effects of lithium borate on blood and histopathological parameters in acute cadmium-induced rats. Biol. Trace Elem. Res. 2018;182:287–294. doi: 10.1007/s12011-017-1089-9. [DOI] [PubMed] [Google Scholar]
- 12.Ezedom T., Asagba S.O. Effect of a controlled food-chain mediated exposure to cadmium and arsenic on oxidative enzymes in the tissues of rats. Toxicol. Rep. 2016;3:708–715. doi: 10.1016/j.toxrep.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mezynska M., Brzóska M.M. Environmental exposure to cadmium—a risk for health of the general population in industrialized countries and preventive strategies. Environ. Sci. Pollut. Res. 2018;25(4):3211–3232. doi: 10.1007/s11356-017-0827-z. [DOI] [PubMed] [Google Scholar]
- 14.Prabu S.M., Muthumani M., Shagirtha K. Quercetin potentially attenuates cadmium induced oxidative stress mediated cardiotoxicity and dyslipidemia in rats. Eur. Rev. Med. Pharmacol. Sci. 2013;17(5):582–595. [PubMed] [Google Scholar]
- 15.Darwesh O.M., Sultan Y.Y., Seif M.M., Marrez D.A. Bio evaluation of crustacean and fungal napplying as food ingredient. Toxicol. Rep. 2018;5:348–356. doi: 10.1016/j.toxrep.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohamed S.S., El-Sedeek L.E., Seif M.M., Amer M.M., Mohamed S.R., Abdel-Aziz M.H.M. Protective efect of cymbopogon schoenanthus extract against formalin hazard in rats. Res. J. Med. Plants. 2017:8–13. [Google Scholar]
- 17.Seif M.M., Farid O.A.H., Aboulthana W.M.A. Evaluation of the protective effect of Acacia (senegal extract against di -(2ethyehexyel phthalat) induced hepato and neurotoxicity in rats. ARRb. 2017 [Google Scholar]
- 18.Azuka O.I., Mary A., Abu O.L. A review on Portulaca oleracea (Purslane) plant – its nature and biomedical benefits. Int. J. Biomed. Res. 2014;5(2):70–80. [Google Scholar]
- 19.Simopoulos-Artemis P. Omega-3 fatty acids and antioxidants in edible wild plants. Biol. Res. 2004;37:263–277. doi: 10.4067/s0716-97602004000200013. [DOI] [PubMed] [Google Scholar]
- 20.Caballero-Salazar S., Riverón-Negrete L., Ordáz-Téllez M.G., Abdullaev F., Espinosa Aguirre J.J. Evaluation of the antimutagenic activity of different vegetable extracts using an in vitro screening test. Proc. West. Pharmacol. Soc. 2002;45:101–103. (PMID: 12434546). [PubMed] [Google Scholar]
- 21.Anusha M., Venkateswarlu M., Prabhakaran V., Taj S.S., Kumari P.B., Ranganayakulu D. Hepatoprotective activity of aqueous extract of Portulaca oleracea in combination with lycopene in rats. Indian J. Pharmacol. 2011;43(5):563–567. doi: 10.4103/0253-7613.84973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali S.I., Said M.M., Hassan E.K. Prophylactic and curative effects of purslane on bile duct ligation-induced hepatic fibrosis in albino rats. Ann. Hepatol. 2011;10(3):340–346. (PMID: 21677337) [PubMed] [Google Scholar]
- 23.Karimi G., Khoei A., Omidi A., Kalantari M., Babaei J., Taghiabadi E., Razavi B.M. Protective effect of aqueous and ethanolic extracts of Portulaca oleracea against cisplatin induced nephrotoxicity. Iran. J. Basic Med. Sci. 2010;13(2):31–35. [Google Scholar]
- 24.Eidi A., Mortazavi P., Moghadam J.Z., Mardani P.M. Hepatoprotective effects of Portulaca oleracea extract against CCl4-induced damage in rats. Pharm. Biol. 2015;53(7):1042–1051. doi: 10.3109/13880209.2014.957783. [DOI] [PubMed] [Google Scholar]
- 25.Brain K.R., Turner T.D. 1st ed. Wright science technical; 1975. The Practical Evaluation of Phytopharmaceuticals; p. p. 144.https://trove.nla.gov.au/version/12773771 [Google Scholar]
- 26.Finar I.L. 5th ed. vol. 2. Pearson Education; Delhi: 2003. Organic chemistry; pp. 769–771. (Stereochemistry and the Chemistry of Natural Products). [Google Scholar]
- 27.Parekh J., Karathia N., Chanda S. Evaluation of antibacterial activity and phytochemical analysis of Bauhinia variegata L. bark. Afr. J. Biomed. Res. 2006;9:53–56. [Google Scholar]
- 28.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. Lebenson Wiss. Technol. 1995;28:25–30. [Google Scholar]
- 29.Tzirogiannis K.N., Panoutsopoulos G.I., Demonakou M.D., Hereti R.I., Alexandropoulou K.N., Basayannis A.C., Mykoniatis M.G. Time-course of cadmium-induced acute hepatotoxicity in the rat liver: the role of apoptosis. Arch. Toxicol. 2003;77:694–701. doi: 10.1007/s00204-003-0499-y. [DOI] [PubMed] [Google Scholar]
- 30.Amin A., Hamza A.A., Daoud S., Hamza W. Spirulina protects against cadmium-induced hepatotoxicity in rats. Am. J. Pharmacol. Toxicol. 2006;1(2):21–25. [Google Scholar]
- 31.Cocchetto D.M., Bjornsson T.D. Methods for vascular access and collection of body fluids from laboratory rat. J. Pharm. Sci. 1983;72(5):465–492. doi: 10.1002/jps.2600720503. (PMID: 6345750) [DOI] [PubMed] [Google Scholar]
- 32.Oyedemi S.O., Bradley G., Afolayan A.J. In-vitro and -vivo antioxidant activities of aqueous extract of Strychnos henningsii Gilg. Afr. J. Pharm. Pharmacol. 2010;4:70–78. [Google Scholar]
- 33.Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 34.Kind P.R., King E.G. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J. Clin. Pathol. 1954;7(4):322–326. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allain C.C., Poon L.S., Chan C.S., Richmond W.F.P.C., Fu P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974;20(4):470–475. [PubMed] [Google Scholar]
- 36.Fassati P., Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982;28(10):2077–2080. (PMID: 6812986) [PubMed] [Google Scholar]
- 37.Fawcett J.K., Scott J.E. A rapid and precise method for the determination of urea. J. clin. Path. 1960;13(2):156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartels H., Böhmer M., Heierli C. Serum creatinine determination without protein precipitation. Clin. Chim. Acta. 1972;37:193–197. doi: 10.1016/0009-8981(72)90432-9. (PMID: 5022083) [DOI] [PubMed] [Google Scholar]
- 39.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 40.Mihara M., Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978;86(1):271–278. doi: 10.1016/0003-2697(78)90342-1. (PMID: 655387) [DOI] [PubMed] [Google Scholar]
- 41.Marklund A., Marklund G. Involvement of the superoxide anion radical in the auto-oxidation of pyrogallol and convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. (PMID: 4215654) [DOI] [PubMed] [Google Scholar]
- 42.Takahara S., Hamilton H.B., Neel J.V., Kobara T.Y., Ogura Y., Nishimura E.T. Hypo-acatalasemia: a new genetic carrier state. J. Clin. Invest. 1960;39(4):610–619. doi: 10.1172/JCI104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.G., Hoekstra W.G. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588–590. doi: 10.1126/science.179.4073.588. (PMID: 4686466) [DOI] [PubMed] [Google Scholar]
- 44.Suvarna K., Layton C., Bancroft J. 7th ed. Churchill Livingstone; New York: 2012. Theory and Practice of Histological Techniques. P1-645. [Google Scholar]
- Al-Moghazy M., Ammar M.S., Sief M.M., Mohamed S.R. Evaluation the antimicrobial activity of Artemisia and Portulaca plant extracts in beef burger. Food Sci. Nutr. 2017;1(1) [Google Scholar]
- 46.Satarug S., Garrett S., Sens M.A., Sens D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010;118(2):182–190. doi: 10.1289/ehp.0901234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar N., Kumari V., Ram C., Bharath Kumar B.S., Verma S. Impact of oral cadmium intoxication on levels of diff ;erent essential trace elements and oxidative stress measures in mice: a response to dose. Environ. Sci. Pollut. Res. Int. 2017;25(6):5401–5411. doi: 10.1007/s11356-017-0868-3. [DOI] [PubMed] [Google Scholar]
- 48.Matović V., Buha A., Ðukić-Ćosić D., Bulat Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015;78:130–140. doi: 10.1016/j.fct.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 49.El-demerdash F.M., Yousef I.M., Radwan M.E. Ameliorating effect of curcumin on sodium arsenite-induced oxidative damage and lipid peroxidation in different rat organs. Food Chem. Toxicol. 2009;47(1):249–254. doi: 10.1016/j.fct.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 50.Rahman M., Tondel M., Ahmad S.A. Diabetes mellitus associated with arsenic exposure in Bangladesh. Am. J. Epidemiol. 1998;148(2):198–203. doi: 10.1093/oxfordjournals.aje.a009624. [DOI] [PubMed] [Google Scholar]
- 51.Kaltreider R.C., Davis A.M., Lariviere J.P., Hamilton J.W. Arsenic alters the function of the glucocorticoid receptor as a transcription factor. Environ. Health Perspect. 2001;109(3):245–251. doi: 10.1289/ehp.01109245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y., Hu Y., Liu S., H Zheng, Wu X., Huang Z. Whole-body aerosol exposure of cadmium chloride (CdCl2) and tetrabromobisphenol A (TBBPA) induced hepatic changes in CD-1 male mice. J. Hazard. Mater. 2016;318:109–116. doi: 10.1016/j.jhazmat.2016.06.054. [DOI] [PubMed] [Google Scholar]
- 53.Toppo R., Roy B.K., Gora R.H., Baxla S.L., Kumar P. Hepatoprotective activity of Moringa oleifera against cadmium toxicity in rats. Vet. World. 2015;8(4):537–540. doi: 10.14202/vetworld.2015.537-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liju V.B., Jeena K., Kuttan R. Acute and subchronic toxicity as well as mutagenic evaluation of essential oil from turmeric (Curcuma longa L) Food Chem. Toxicol. 2013;53:52–61. doi: 10.1016/j.fct.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 55.Liang Y., Lei L., Nilsson J., Li H., Nordberg M., Bernard A., Nordberg G.F., Bergdahl I.A., Jin T. Renal function after reduction in cadmium exposure: an 8-year follow-up of residents in cadmium-polluted areas. Environ. Health Perspect. 2012;120:223–228. doi: 10.1289/ehp.1103699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bernhoft R.A. Cadmium toxicity and treatment. Sci. World J. 2013;2013 doi: 10.1155/2013/394652. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kojima M., Masui T., Nemoto K., Degawa M. Lead nitrate-induced development of hypercholesterolemia in rats: sterol-independent gene regulation of hepatic enzymes responsible for cholesterol homeostasis. Toxicol. Lett. 2004;154(1-2):35–44. doi: 10.1016/j.toxlet.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 58.Twisk J., Gillian-Daniel D.L., Tebon A., Wang L., Barrett P.H.R., Attie A.D. The role of the LDL receptor in apolipoprotein B secretion. J. Clin. Invest. 2000;105(4):521–532. doi: 10.1172/JCI8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Springer; Boston, MA: 2001. Mechanisms of Metal Toxicity and Carcinogenesis; pp. 41–47. [Google Scholar]
- 60.Kantola T., Kivistö K.T., Neuvonen P.J. Grapefruit juice greatly increases serum concentrations of lovastatin and lovastatin acid. Clin. Pharmacol. Ther. 1998;63(4):397–402. doi: 10.1016/S0009-9236(98)90034-0. [DOI] [PubMed] [Google Scholar]
- 61.Sharma R. In vivo delivery of Tinospora cordifolia root extract preventing radiation-induced dystrophies in mice ovaries. Evid. Based Complement. Altern. Med. 2015 doi: 10.1155/2015/346427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fan X.J., Liu S.Z., Li H.H., He J., Feng J.T., Zhang X., Yan H. Effects of Portulaca oleracea L. extract on lipid oxidation and color of pork meat during refrigerated storage. Meat Sci. 2019;147:82–90. doi: 10.1016/j.meatsci.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 63.Djukić-Ćosić D., Ninković M., Maličević Z., Matović V., Soldatović D. Effect of magnesium pretreatment on reduced glutathione levels in tissues of mice exposed to acute and subacute cadmium intoxication: a time course study. Magnes. Res. 2007;20(3):177–186. [PubMed] [Google Scholar]
- 64.Adi P.J., Burra S.P., Vataparti A.R., Matcha B. Calcium, zinc and vitamin E ameliorate cadmium-induced renal oxidative damage in albino Wistar rats. Toxicol. Rep. 2016;3:591–597. doi: 10.1016/j.toxrep.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rajeshkumar N.V., Kuttan R. Modulation of carcinogenic response and antioxidant enzymes of rats administered with 1,2-dimethylhydrazine by Picroliv. Cancer Lett. 2003;191(2) doi: 10.1016/s0304-3835(02)00203-3. (PMID: 12618326) [DOI] [PubMed] [Google Scholar]
- 66.Padma V.V., Baskaran R., Divya S., Priya L.B., Saranya S. Modulatory effect of Tinospora cordifolia extract on Cd-induced oxidative stress in Wistar rats. Integr. Med. Res. 2016;5(1):48–55. doi: 10.1016/j.imr.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jomova K., Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283(2-3):65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 68.El-Refaiy A.I., Eissa F.I. Histopathology and cytotoxicity as biomarkers in treated rats with cadmium and some therapeutic agents. Saudi J. Biol. Sci. 2013;20(3):265–280. doi: 10.1016/j.sjbs.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uddin K., Juraimi A.S., Ali E., Ismail M.R. Evaluation of antioxidant properties and mineral composition of purslane (Portulaca oleracea L.) at different growth stages. Int. J. Mol. Sci. 2012;13:10257–10267. doi: 10.3390/ijms130810257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dini I., Tenore G.C., Dini A. Effect of industrial and domestic processing on antioxidant properties of pumpkin pulp. LWT - Food Sci. Technol. 2013;53(1):382–385. [Google Scholar]
- 71.Uddin M.D.K., Juraimi A.S., Hossain M.D.S., Nahar Most. A.U., Ali M.D.E., Rahman M.M. Purslane weed (Portulaca oleracea): a prospective plant source of nutrition, Omega-3 fatty acid, and antioxidant attributes. Sci. World J. 2014;951019:6. doi: 10.1155/2014/951019. [DOI] [PMC free article] [PubMed] [Google Scholar]