Abstract
Detritus can fundamentally shape and sustain food webs, and shredders can facilitate its availability. Most of the biomass of the highly productive giant kelp, Macrocystis pyrifera, becomes detritus that is exported or falls to the seafloor as litter. We hypothesized that sea urchins process kelp litter through shredding, sloppy feeding and egestion, making kelp litter more available to benthic consumers. To test this, we conducted a mesocosm experiment in which an array of kelp forest benthic consumers were exposed to 13C- and 15N-labelled Macrocystis with or without the presence of sea urchins, Strongylocentrotus purpuratus. Our results showed that several detritivore species consumed significant amounts of kelp, but only when urchins were present. Although they are typically portrayed as antagonistic grazers in kelp forests, sea urchins can have a positive trophic role, capturing kelp litter before it is exported and making it available to a suite of benthic detritivores.
Keywords: marine food web, Strongylocentrotus purpuratus, Macrocystis, suspension feeders, detritivores, invertebrates
1. Introduction
Primary production is a fundamental driver of ecosystem complexity and function, with higher productivity linked to higher species diversity [1–3], secondary production [4,5], longer food chains [6,7] and more complex food webs [8]. Most primary production, however, is not consumed by herbivores but becomes detritus that may vary in its fate, residence time and lability [9,10]. Detrital resources can fundamentally shape and sustain food webs, increasing their stability [11], diversity and complexity [12]. Detrital inputs to food webs can be integrated over longer time scales than primary production alone, and accounting for sources, quantity and quality of detritus and their processing paths is needed to understand of the overall role of energy input in structuring communities [11].
Aquatic ecosystems are generally considered to host higher levels of herbivory [13] and to produce and accumulate less detritus than terrestrial systems [14], which can build up large deposits of macrophyte detritus such as wood and leaf litter [9]. Nevertheless, detritus can also be a significant resource in aquatic ecosystems in the form of senescent phytoplankton [15,16] and macrophytes like macroalgae and seagrass [17–19]. The role of macrophyte detritus in many aquatic systems, including marine ecosystems, is unclear, and in many cases, marine macrophyte detritus is apparently unavailable to local consumers because of its unpalatability [20,21] or short residence time due to export by water flow [14,22]. In terrestrial and freshwater ecosystems, the availability of such refractory detritus can be increased by a guild of consumers, called shredders, that process and to varying degrees consume leaves and other plant tissues, producing faeces and finer detrital particles that are more available for bacterial colonization and consumption by other organisms [23–25]. By contrast, such animal processing probably does not markedly improve the food quality of the phytoplankton detritus that dominates the oceans, explaining the apparent paucity of shredders in marine systems [26] (but see [27]). In shallow seas, where macrophyte detritus is abundant, however, marine shredders could play a key role in routing this detritus into coastal food webs.
As abundant herbivores and detritivores in many marine ecosystems [28,29], sea urchins are potential marine shredders. On coral reefs, urchins graze algae and favour slower-growing coral [30,31]. Urchins often regulate seagrass biomass, and can overgraze and limit the extent of seagrass beds [32–35]. On temperate rocky reefs, urchins can overgraze kelp, as well as other sessile organisms [36–38]. In this role, urchins have been considered a key instrument of trophic cascades: when urchin predators such as sea otters or fishes are scarce, their urchin prey can become overabundant and uninhibited in their movements, mowing down lush kelp forests and converting them to ‘urchin barrens' [39,40]. These shifts have been considered to affect rocky reef food webs in part by eliminating kelp and kelp detritus as food for grazers and suspension feeders [41–43].
However, sea urchins are common in most intact kelp forests as well, and are often abundant, thriving on the detritus produced by the kelp in the form of senescent blades and fronds that fall to the seafloor as litter [44–47]. In doing so, urchins act as archetypal shredders [48,49], converting the coarse particulate organic matter of kelp litter to fine fragments that are broken loose as they feed [50] and faecal pellets that may be high-quality food for detritivores [51,52].
We hypothesized that sea urchins act as shredders, channelling finer particulate kelp detritus into reef food webs. To test this, we assembled artificial communities of small kelp forest consumers, all putative detritivores, in mesocosms, and added isotopically labelled kelp blades with and without sea urchins present. We predicted that the urchins would make the kelp detritus available to the consumers, and that detritivore communities with urchins present would reflect this by incorporating more isotopically labelled kelp-derived carbon and nitrogen in their tissues.
2. Methods
(a). Study system and experimental design
The purple sea urchin Strongylocentrotus purpuratus is an important herbivore and detritivore in southern California giant kelp (Macrocystis pyrifera) forests that grow on shallow rocky reefs (approx. 3–20 m depth) off southern California, including Santa Barbara, where this study was done. To investigate the shredder activity of purple urchins, we assembled communities of common detritivores and suspension feeders from local reefs in mesocosms. Three species of brittle stars (Ophiopteris papillosa, Ophioplocus esmarki and Ophiothrix spiculata), one vermetid gastropod (Thylacodes squamigerus), two barnacles (Chthamalus sp. and Megabalanus californicus), one polychaete worm (Chaetopterus sp.) and three sea cucumbers (Cucumaria piperata, Pachythyone rubra and Cucumaria salma) were collected from the seafloor of local kelp forests at 5–15 m depth in the Santa Barbara Channel. Ten of each species were placed in each of six 50 l (51 × 38 × 27 cm) flow-through unfiltered seawater tanks, each containing two concrete bricks for hard substrate on top of 3 cm of sand. Because sea urchins are highly mobile, capable of bulldozing other occupants, we isolated them above the experimental communities on plastic mesh (1 cm) dividers that were secured horizontally across each mesocosm 16 cm above the bottom.
Ten individuals of each consumer species, along with 2–3 blades, totalling 47.3 g (±1.2 s.e.), of isotopically labelled kelp (see below) were placed on the floor of each tank, below the divider. On top of the mesh divider, we placed an additional 229.8 g (±6.1 s.e.) of enriched kelp blades, and in half of the tanks, 10 adult urchins (total mass 343.3 g ± 1.3 s.e.) per tank. The kelp below the mesh ensured that the detritivores had direct access to degrading kelp detritus regardless of the urchin treatment, similar to the situation in the kelp forest, allowing us to more clearly ascertain the degree to which the detritivores were dependent on urchins for kelp detritus assimilation. The experiment was run for 28 days (26 July–24 August 2016); above-mesh kelp was removed and replenished once after 13 days.
(b). Isotopic labelling of kelp
We labelled giant kelp with the stable isotopes 13C and 15N for the detritivore experiment. Six 1 m long kelp fronds were incubated for 3 days (23–26 July, 6–9 August 2016) in a closed 113 l open-top seawater tank in full natural sunlight that was kept cool by sitting in a much larger flow-through seawater tank. A 700 GPH submersible pump was used to maintain water circulation, and the kelp fronds were stirred three times per day to homogenize light exposure and help achieve more uniform isotope enrichment. NaH13CO3 and 15NH4Cl solution was added to each kelp tank at 66 µM 13C and 10 µM 15N final concentration on each of the first 2 days. On the 3rd enrichment day, no additional isotopes were added, but mixing and stirring continued.
(c). Isotope analysis
Enriched kelp and invertebrate consumers from the mesocosms were sampled and frozen at the beginning and end of the experiment. Tissues were dissected from all invertebrate species except barnacles, for which the whole body was extracted from the theca (electronic supplementary material, table S1). Dissected samples were dried at 60°C for a minimum of 48 h, ground into a fine powder with a mortar and pestle, acidified with a minimum of 190 µl 6% sulfurous acid or more until bubbles ceased forming to remove inorganic carbonates, and analysed for δ13C and δ15N using a Thermo Finnigan Delta-Plus Advantage isotope mass spectrometer coupled with a Costech EAS elemental analyser in the University of California Santa Barbara Marine Science Institute Analytical Laboratory. Instrument calibration was conducted using acetanilide reference standards run at the beginning of each set of 35 samples and tested every 5 samples within each set. Instrument precision, determined using replicate analyses of L-glutamic acid USGS40, was ±0.12 for 13C and ±0.06 for 15N. The abundances of 13C and 15N are expressed in standard δ notation and calculated as follows for element X:
where R = Xn/Xn−1, expressed as per mil (‰) relative to the PDB standard for carbon and atmospheric N2 for nitrogen.
(d). MixSIAR modelling and isotope data analyses
The Bayesian mixing model MixSIAR (v.3.1.10) run in R [53] was used to estimate the proportion of detritivore's tissue comprised of enriched kelp at the conclusion of the mesocosm experiment. δ13C and δ15N values of (1) initial (pre-experiment) species-specific consumer tissue, and (2) enriched kelp tissue, were used as sources in the MixSIAR model. Trophic discrimination values were set to zero for the initial species' tissue values and to 0.5 δ13C (±1.2 s.d.) and 2.5‰ δ15N (±2.5 s.d.) for enriched kelp [54]. The Markov chain Monte Carlo parameter was set to very long run length, error structure in the model included residual and process error, and Bayesian priors were uninformed. Diagnostic Gelman–Rubin and Geweke tests were used to determine whether or not the model converged. The median and 95% confidence interval posterior distribution of estimated proportion kelp contributions to species tissues were compared to assess whether there were differences between consumer diets when urchins were present or absent. The final estimates of percentage kelp assimilation in the benthic consumers represent the amount of kelp assimilated within the constraints of the tissue turnover time of the consumer, which implies that they are underestimates given that the tissue turnover times for invertebrates are generally longer than the one-month length of the experiment [55–57].
(e). Urchin and kelp litter time series
To assess the potential magnitude and variability of urchin detrital processing, data from the Santa Barbara Channel Long Term Ecological Research (SBC LTER) program were used to estimate kelp litter availability and purple urchin excretion rates from 2008 to 2018 at three kelp forest sites: Arroyo Quemado (34° 28.127′ N, 120° 07.285′ W), Isla Vista (34° 24.155′ N, 119° 51.467′ W), Mohawk (34° 23.664′ N, 119° 43.800′ W) and Naples (34° 25.347′ N, 119° 57.181′ W)] [58,59]. Standing stock of kelp litter and purple urchin densities were measured quarterly on one fixed 40 × 2 m transect at each site. At each transect, quarterly monitoring included measurements of small (less than or equal to 2.5 cm test diameter) and large purple urchin densities. Additionally, all visible macroalgal and plant litter pieces were collected in mesh bags, brought back to the laboratory, identified to species, and weighed. Annual biomass estimates of purple urchins were calculated using the following species-specific test diameter (D) to wet mass (M) regression (R2 = 0.99) developed by Reed et al. [60]:
Seasonal production of urchin excreta (E) was estimated at sites commonly having excess Macrocystis litter using seasonal urchin density (d), annual urchin biomass at each site (M), published values of purple urchin ingestion rates (I) and gravimetric absorption (A) of Macrocystis [61,62]:
We used a Macrocystis ingestion rate of 0.12 (g g−1) d−1 (±0.01 s.e.) based on data from small urchins (20.7 g ± 1.7 s.e., n = 15) [62,63], which were in agreement with feeding rates at 16°C of larger urchins (79.5 g ± 4.9 s.e.) [61]. We converted wet mass (WM) ingested to dry mass (DM) and then carbon (C) using Macrocystis blade data from the Santa Barbara Coastal LTER (DM:WM = 0.095 ± 0.001 s.e., C : DM = 0.304 ± 0.001 s.e., n = 677). Gravimetric assimilation efficiency was assumed to be 79% (±2 s.e.) [61]. Error was propagated through the equation by adding the fractional uncertainties in quadrature. We compared these estimates to other organic carbon sources to assess the relative potential of the urchin faecal pathway.
3. Results
(a). Isotope values of sources
Kelp δ15N and δ13C enrichment was an order of magnitude or more above natural isotope abundances. After enrichment, kelp tissue δ13C averaged 116‰ (±7 s.e., range 62–216‰) and δ15N averaged 1821‰ (±106 s.e., range 1160–3364‰) (table 1). Initial (non-enriched) isotope values of consumer tissues were typical of benthic kelp forest invertebrates (reviewed by [43,64,65]), ranging from −19.4‰ to −13.7‰ δ13C and 1.5‰ to 12.2‰ δ15N, depending on species (table 1).
Table 1.
Species, sampled tissue type, mean (±s.e.) shell-free body mass (g wet weight), sample size (n) and mean (±s.e.) of δ13C and δ15N of source samples used in MixSIAR models.
| species | tissue type | body mass ± s.e. | n | δ13C ± s.e. | δ15N ± s.e. |
|---|---|---|---|---|---|
| Macrocystis pyrifera | lamina | n.a. | 32 | 116 ± 7 | 1821 ± 106 |
| Ophiopteris papillosa | dorsal disc | 1.2 ± 0.1 | 5 | −17.1 ± 0.3 | 3.7 ± 0.9 |
| Ophioplocus esmarki | dorsal disc | 2.5 ± 0.4 | 5 | −15.5 ± 0.6 | 11.5 ± 0.4 |
| Ophiothrix spiculata | dorsal disc | 0.4 ± 0.1 | 5 | −18.3 ± 0.2 | 7.0 ± 0.3 |
| Thylacodes squamigerus | foot | 1.8 ± 0.2 | 5 | −17.0 ± 0.2 | 10.5 ± 0.2 |
| Megabalanus californicus | whole | n.a. | 5 | −18.1 ± 0.2 | 10.6 ± 0.2 |
| Chthamalus sp. | whole | n.a. | 5 | −18.0 ± 0.2 | 10.9 ± 0.1 |
| Chaetopterus sp. | peristome | 3.2 ± 0.4 | 5 | −19.3 ± 0.1 | 9.0 ± 0.2 |
| Cucumaria piperata | body wall | 1.7 ± 0.9 | 5 | −17.0 ± 0.3 | 8.6 ± 0.4 |
| Pachythyone rubra | body wall | 0.3 ± 0.1 | 5 | −17.7 ± 0.3 | 7.3 ± 0.4 |
| Cucumaria salma | body wall | 8.3 ± 1.6 | 5 | −16.1 ± 0.3 | 10.4 ± 0.1 |
(b). Enhancement of kelp C and N use by reef consumers in the presence of urchins
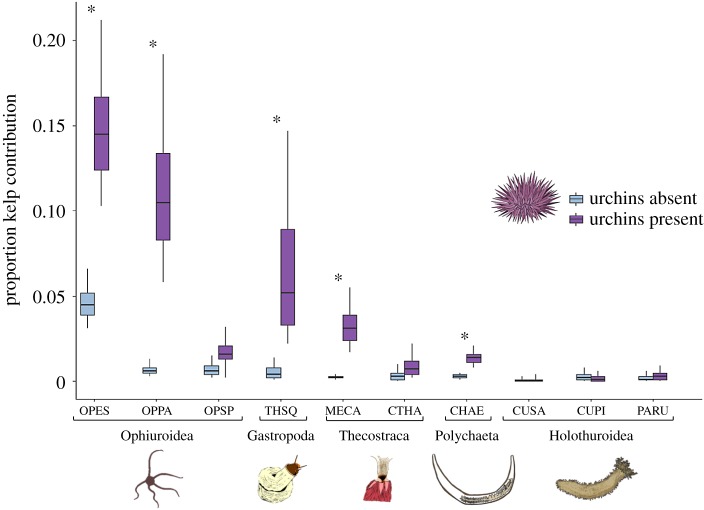
The presence of urchins significantly increased the proportional contribution of kelp to the diet in five of the 10 species (figure 1). The largest and most variable treatment effect was seen in the ophiuroids. Relative to the control, the presence of urchins increased the median proportion enriched kelp by 10.0% in Ophioplocus esmarki, 9.9% in Ophiopteris papillosa and 1.0% in Ophiothrix spiculata. The incorporation of labelled kelp by the vermetid gastropod Thylacodes squamigerus, the barnacle Megabalanus californicus and the polychaete Chaetopterus sp. also increased significantly in the presence of urchins compared with the control (by 4.8%, 2.9% and 1.1%, respectively). Across all species, median posterior estimates of percentage kelp in urchin present treatments averaged 3.7% (range: 0–14.5%). By contrast, the percentage of kelp contribution across species in the control was minimal, 0.7% with a maximum of 4.5% for Ophioplocus esmarki. Surprisingly, none of the holothurians assimilated significant levels of kelp C or N in either treatment (figure 1).
Figure 1.
Median posterior distribution estimating proportion of invertebrate tissues composed of enriched kelp with presence or absence of Strongylocentrotus purpuratus purple urchins. Boxes represent 25–75% posterior distribution intervals and whiskers represent 2.5–97.5% posterior distribution intervals. Asterisks denote a significant difference (α = 0.05) between kelp assimilation posterior distributions across treatments and within each species. Ophiuroidea species include Ophiopteris papillosa (OPPA), Ophioplocus esmarki (OPES) and Ophiothrix spiculata (OPSP). The Gastropoda species is Thylacodes squamigerus (THSQ). The Thecostraca barnacle species are Chthamalus sp. (CTHA) and Megabalanus californicus (MECA). The Polychaeta species is Chaetopterus sp. (CHAE). Holothuroidea species include Cucumaria piperata (CUPI), Pachythyone rubra (PARU) and Cucumaria salma (CUSA). (Online version in colour.)
(c). Urchin and kelp litter dynamics in Santa Barbara channel kelp forests
Both urchin and kelp litter biomass were highly variable seasonally, annually and across different patch reefs. Prior to 2014, S. purpuratus were more abundant on SBC-LTER kelp reef transects while standing stock of kelp litter was low (electronic supplementary material, figure S1). From 2014 onwards, urchin abundances on SBC-LTER reefs have been lower, with larger amounts of kelp litter in fall and winter. Macrocystis litter availability never exceeded 80 g m−2 at any reef prior to late 2013. Litter availability from 2014 forward has been high, particularly in fall and winter, with a maximum of 300.6 g m−2 in August 2016 at Arroyo Quemado and mean ranges from 14.6 g m−2 (±4.8 s.e.) at Mohawk to 60.1 g m−2 (±22.2 s.e.) at Arroyo Quemado (electronic supplementary material, figure S1A). Urchin biomass did not track seasonal kelp litter pulses, but did vary on interannual time scales (electronic supplementary material, figure S1B). Assuming that urchins were the major cause of low apparent litter availability at reefs prior to 2014 and that the presence of any litter represented an excess rather than an estimate of supply, urchins were generally not food limited. Under this assumption, urchin faecal production rate averaged 0.11 gC m−2 d−1 (±0.02 s.e.) at Arroyo Quemado, 0.37 gC m−2 d−1 (±0.06 s.e.) at Mohawk and 0.51 gC m−2 d−1 (±0.07 s.e.) at Naples.
4. Discussion
Sea urchins are key grazers structuring kelp forest ecosystems, but their role in detrital processing and the consequences for benthic detritivore communities is virtually unknown. Our results show that as urchins process kelp during feeding they make this energy source more available to other common benthic detritivores. The presence of urchin shredders benefited a diverse array of detritivores including ophiuroids, barnacles, a vermetid gastropod and a polychaete (figure 1). Only one of the 10 detritivore species studied, the ophiuroid Ophioplocus esmarki, incorporated a significant amount of kelp detritus in the absence of urchins, and it was still approximately 3× less kelp carbon than in the presence of urchins. These results suggest that sea urchins can be a key trophic intermediate in kelp forests, potentially making large amounts of kelp litter available to communities of benthic fauna that are important foraging resources for fish and other mobile predators in the kelp forest [66–68].
While there are no data on the isotopic turnover times of our species, our MixSIAR results are likely underestimates of per cent kelp consumed in the experiment. The isotopic turnover time of invertebrates can be approximated as [57]
Mean shell-free body mass for the species in the mesocosms ranged from 0.3 to 8.3 g (table 1), corresponding to half-lives ranging from 20 to 42 days. Assuming that a species were eating a diet of 100% enriched kelp in a well-mixed system with first-order kinetics and high assimilation efficiency [69], the MixSIAR model outputs would estimate approximately 50% kelp contribution after the 30-day experiment. While the absolute magnitude of the kelp-urchin pathway cannot be assessed by these experiments, relative contributions between treatments are statistically meaningful and the body masses of species sampled are similar enough that we can qualitatively compare differences across species.
Our experiment revealed that some species may benefit more than others from urchin-processed kelp detritus, and feeding modes may explain these differences in kelp utilization. In our experiment, we found that species with more passive suspension feeding modes, especially those that were optimized for small particle capture (1–150 µm), did not use urchin-processed kelp. Urchin faeces are typically much larger (300 µm—greater than 1 mm) [70] than the small phytoplankton and detrital particles consumed by passive suspension feeders (reviewed in [71]). Moreover, although urchin faeces sink much slower than kelp litter [70], the faeces and kelp fragments were apparently unavailable to these passive suspension feeders, suggesting that they were not frequently resuspended. Observation of the tanks during the experiment confirmed this: visible detritus fragments and faeces accumulated on the bottom with apparently minimal suspension.
The suspension feeding species that consumed little or no kelp in either treatment were the holothurians, the brittle star Ophiothrix spiculata, the barnacle Cthamalus sp. and the tube-dwelling polychaete Chaetopterus sp. All three holothurian species in our experiment were members of the order Dendrochirotida, and have branching oral tentacles used primarily for passive suspension feeding on small phytoplankton [72,73], instead of the substrate-oriented, tube-shaped feeding tentacles characteristic of deposit-feeding holothurians [74]. Ophiothrix spiculata is also a passive suspension feeder, extending its spiny mucous-coated arms into the water column to trap small particles [75]. Megabalanus californicus assimilated more urchin-processed kelp compared with Cthamalus sp., and these barnacle species exhibit both active and passive suspension feeding using cirri that are optimized for capturing larger particles, such as zooplankton [76]. Megabalanus californicus is a much larger species with a more highly developed circulatory system, allowing it to maintain longer periods of active feeding through cirral motions [77], clear larger volumes of water per unit time [76,78], and potentially overcome the limited sinking time of the urchin faeces and kelp fragments. Chaetopterus sp. ate low, but significant levels of urchin-processed kelp. These polychaete worms are specialized for capturing small particles (greater than 1 µm) by drawing water through the small apertures on either end of their tube and sieving particles with an internal mucus net [79].
The brittle stars, Ophioplocus esmarki and Ophiopteris papillosa, and the vermetid gastropod consumed the highest levels of kelp, and probably acted as substrate-based detritivores rather than suspension feeders. Ophioplocus esmarki has smooth arms with no observed suspension feeding behaviour, while the coarsely spined Ophiopteris papillosa exhibited low levels of suspension feeding behaviour, and is probably one of the many ophiuroids with the ability to switch between detritivory and suspension feeding modes [80]. Vermetid gastropods, such as Thylacodes squamigerus, feed using long sticky mucus threads, which intermesh into a net that clings to the substrate to capture surface detritus and falling particles [81]. These sessile gastropods are essentially substrate-based detritivores, and well suited to capturing urchin-processed kelp.
Within giant kelp forests, kelp has significant physical engineering effects on community structure due to its size and the habitat it provides, but the extent to which kelp provides trophic support is poorly understood [43,82]. Results of our mesocosm experiments suggest that urchins could be a key link between giant kelp primary productivity and benthic secondary production. Urchins are most well-known as destructive grazers capable of denuding kelp from entire regions if unchecked by predators [39,83] (reviewed in [84]). While destructive grazing does occur as a response to algal drift shortages [46,85], urchins in thriving kelp forests have a minimal effect on attached kelp [86–89]. Instead, sea urchins in kelp forests feed primarily by capturing large pieces of detrital kelp litter sinking or transported by water currents [46,90,91], and produce large quantities of smaller faecal particles that sink and accumulate in crevices and depressions on the reef [52] where other benthic invertebrates are typically abundant [92]. In general, grazing on attached Macrocystis is thought to be low [93], with more than 90% of Macrocystis production ending up in the detrital pool [19]. Much of this detritus is exported to adjacent ecosystems, such as beaches [94] and deep sea canyons [95], and urchins thus retain litter within kelp forests that would have otherwise been lost to the kelp forest food web [90]. At moderate densities, therefore, urchins can facilitate kelp's trophic support of the ecosystem, rather than serving a primarily agonistic role in kelp forest function, as has previously been emphasized.
Urchin feeding not only captures and retains algal drift material [44,96] but may also make egested kelp-derived carbon more labile than fresh kelp [97]. Urchin digestive systems host a rich microbial community, including plant polysaccharide generalist bacteria [98], nitrogen fixers [99,100] and ciliates [101,102]. While assimilation efficiency of Macrocystis by S. purpuratus is around 70–90% [61,103], urchin faecal pellets are colonized heavily by microbes [101]. These gut microbes can increase the nutritional value of faeces by synthesizing valuable molecules such as essential amino acids [104], decrease C : N ratios and increase available energy content [51].
To assess the relative magnitude of urchin inputs, we can compare them with sources of primary production on area reefs. Mean estimated urchin faecal production rates ranged from 0.11 to 0.51 gC m−2 d−1, on the order of half of phytoplankton production in the Santa Barbara Channel (winter: 1.3 gC m−2 d−1 (±0.6 s.e.), spring: 3.1 gC m−2 d−1 (±1.5 s.e.)) [105], and comparable with near-shore microphytobenthos production that ranged from 0 to 0.27 gC m−2 d−1 [106,107]. Macroalgal production, which forms the base of urchin faecal production, is dominated by Macrocystis at 0.6–2.2 gC m−2 d−1 [108]. Other urchin food sources include foliose and turf algae, which had average productivity rates of 0.9 gC m−2 d−1 (±0.1 s.e.) and 0.4 gC m−2 d−1 (±0.1 s.e.), respectively [109]. These benthic algae are less preferred food sources for urchins compared with kelp [63], but they are consumed and can increase in abundance when Macrocystis biomass is low [110,111]. Given these food sources and our findings that an excess standing stock of Macrocystis litter was found at most time points in the three reefs we sampled, urchins are unlikely to be food limited most of the time (electronic supplemental material, figure S1) [90]. Therefore, urchin-processed kelp (including faecal pellets) is probably a readily available and nutritionally dense food source for benthic detritivores that rivals other resources in kelp forests.
Kelp forests support hundreds of ecologically and economically important species through physically altering reef environments [38,112,113], food provision [46] and species interactions [114,115]. This study demonstrates that sea urchins are not simply destructive herbivores in kelp forest ecosystems, and may play a more complex role in kelp forest trophic dynamics than previously appreciated. Understanding how kelp detritus and sea urchins may interact to support these ecosystems will give us a more complete view of kelp forest ecosystem function, and could also inform management. In particular, increasing urchin culling practices to manage and ‘restore' kelp forests [116–118] could be misguided if not carefully considered and managed.
Supplementary Material
Acknowledgements
We thank C. Pierre and C. Orsini for providing information on where to find and collect animals for the experiment, F. Puerzer and J. Stone-Farhat for assistance with sample processing, and G. Paradis for analytical laboratory training and assistance.
Data accessibility
Mesocosm isotope data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.38m3dc6 [119]. Santa Barbara Channel Long-Term Ecological Research data used in electronic supplementary material, figure S1 are available through the LTER data repository at https://portal.lternet.edu/nis/mapbrowse?packageid=knb-lter-sbc.119.2 and https://portal.lternet.edu/nis/mapbrowse?packageid=knb-lter-sbc.25.18
Authors' contributions
C.E.Y., R.J.M. and H.M.P. designed the study. C.E.Y. conducted the experiments, analysed the data and wrote the first draft of the manuscript, and all authors contributed to the final draft.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by the US. National Science Foundation's Long-Term Ecological Research programme under Division of Ocean Sciences grant nos 9982105 and 0620276, by NSF Bio-Ocean award 0962306 to H.M.P. and R.J.M., and by the National Aeronautics and Space Administration, Biodiversity and Ecological Forecasting Program (Grant NNX14AR62A), the Bureau of Ocean Energy Management, Environmental Studies Program (BOEM Agreement MC15AC00006) and the National Oceanic and Atmospheric Administration in support of the Santa Barbara Channel Marine Biodiversity Observation Network.
References
- 1.Chase JM, Leibold MA. 2002. Spatial scale dictates the productivity–biodiversity relationship. Nature 416, 427–430. ( 10.1038/416427a) [DOI] [PubMed] [Google Scholar]
- 2.Grace JB, et al. 2016. Integrative modelling reveals mechanisms linking productivity and plant species richness. Nature 529, 390–393. ( 10.1038/nature16524) [DOI] [PubMed] [Google Scholar]
- 3.Liang J, et al. 2016. Positive biodiversity–productivity relationship predominant in global forests. Science 354, aaf8957 ( 10.1126/science.aaf8957) [DOI] [PubMed] [Google Scholar]
- 4.McNaughton SJ, Oesterheld M, Frank DA, Williams KJ. 1991. Primary and secondary production in terrestrial ecosystems. In Comparative analyses of ecosystems (eds Cole J, Lovett G, Findlay S), pp. 120–139. New York, NY: Springer. [Google Scholar]
- 5.Winder M, Carstensen J, Galloway AWE, Jakobsen HH, Cloern JE. 2017. The land–sea interface: a source of high-quality phytoplankton to support secondary production. Limnol. Oceanogr. 62, S258–S271. ( 10.1002/lno.10650) [DOI] [Google Scholar]
- 6.Wallace JB, Eggert SL, Meyer JL, Webster JR. 1997. Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science 277, 102–104. ( 10.1126/science.277.5322.102) [DOI] [Google Scholar]
- 7.Young HS, Mccauley DJ, Dunbar RB, Hutson MS, Ter-kuile M, Dirzo R. 2013. The roles of productivity and ecosystem size in determining food chain length in tropical terrestrial ecosystems. Ecology 94, 692–701. ( 10.1890/12-0729.1) [DOI] [PubMed] [Google Scholar]
- 8.Polis GA, Strong DR. 1996. Food web complexity and community dynamics. Am. Nat. 147, 813–846. ( 10.1086/285880) [DOI] [Google Scholar]
- 9.Cebrian J, Lartigue J. 2004. Patterns of herbivory and decomposition in aquatic and terrestrial ecosystems. Ecol. Monogr. 74, 237–259. ( 10.1890/03-4019) [DOI] [Google Scholar]
- 10.Ward CL, McCann KS, Rooney N. 2015. HSS revisited: multi-channel processes mediate trophic control across a productivity gradient. Ecol. Lett. 18, 1190–1197. ( 10.1111/ele.12498) [DOI] [PubMed] [Google Scholar]
- 11.Moore JC, et al. 2004. Detritus, trophic dynamics and biodiversity. Ecol. Lett. 7, 584–600. ( 10.1111/j.1461-0248.2004.00606.x) [DOI] [Google Scholar]
- 12.Hairston NG Jr, Hairston NG Sr. 1993. Cause-effect relationships in energy flow, trophic structure, and interspecific interactions. Am. Nat. 142, 379–411. ( 10.1086/285546) [DOI] [Google Scholar]
- 13.Cyr H, Pace ML. 1993. Magnitude and patterns of herbivory in aquatic and terrestrial ecosystems. Nature 361, 148–150. ( 10.1038/361148a0) [DOI] [Google Scholar]
- 14.Cebrian J. 1999. Patterns in the fate of production in plant communities. Am. Nat. 154, 449–468. ( 10.1086/303244) [DOI] [PubMed] [Google Scholar]
- 15.Goedkoop W, Gullberg KR, Johnson RK, Ahlgren I. 1997. Microbial response of a freshwater benthic community to a simulated diatom sedimentation event: interactive effects of benthic fauna. Microb. Ecol. 34, 131–143. ( 10.1007/s002489900043) [DOI] [PubMed] [Google Scholar]
- 16.Josefson A, Forbes T, Rosenberg R. 2002. Fate of phytodetritus in marine sediments: functional importance of macrofaunal community. Mar. Ecol. Prog. Ser. 230, 71–85. ( 10.3354/meps230071) [DOI] [Google Scholar]
- 17.Newman RM. 1991. Herbivory and detritivory on freshwater macrophytes by invertebrates: a review. J. North Am. Benthol. Soc. 10, 89–114. ( 10.2307/1467571) [DOI] [Google Scholar]
- 18.Lepoint G, Cox A-S, Dauby P, Poulicek M, Gobert S. 2006. Food sources of two detritivore amphipods associated with the seagrass Posidonia oceanica leaf litter. Mar. Biol. Res. 2, 355–365. ( 10.1080/17451000600962797) [DOI] [Google Scholar]
- 19.Krumhansl KA, Scheibling RE. 2012. Production and fate of kelp detritus. Mar. Ecol. Prog. Ser. 467, 281–302. ( 10.3354/meps09940) [DOI] [Google Scholar]
- 20.Newell R. 1963. The role of detritus in the nutrition of two marine deposit feeders, the prosobranch Hydrobia ulva and the bivalve Macoma balthica. Proc. Zool. Soc. Lond. 144, 25–45. ( 10.1111/j.1469-7998.1965.tb05164.x) [DOI] [Google Scholar]
- 21.Norderhaug KM, Fredriksen S, Nygaard K. 2003. Trophic importance of Laminaria hyperborea to kelp forest consumers and the importance of bacterial degradation to food quality. Mar. Ecol. Prog. Ser. 255, 135–144. ( 10.3354/meps255135) [DOI] [Google Scholar]
- 22.Duarte CM, Cebrián J. 1996. The fate of marine autotrophic production. Limnol. Oceanogr. 41, 1758–1766. ( 10.4319/lo.1996.41.8.1758) [DOI] [Google Scholar]
- 23.Vannote RL, Minshall WG, Cummins KW, Sedell JR, Cushing CE. 1980. The river continuum concept. Can. J. Fish. Aquat. Sci. 37, 130–137. ( 10.1139/f80-017) [DOI] [Google Scholar]
- 24.Gessner MO, Swan CM, Dang CK, McKie BG, Bardgett RD, Wall DH, Hättenschwiler S. 2010. Diversity meets decomposition. Trends Ecol. Evol. 25, 372–380. ( 10.1016/j.tree.2010.01.010) [DOI] [PubMed] [Google Scholar]
- 25.Wallace JB, Eggert SL, Meyer JL, Webster JR, Sobczak WV. 2015. Stream invertebrate productivity linked to forest subsidies: 37 stream-years of reference and experimental data. Ecology 96, 1213–1228. ( 10.1890/14-1589.1.sm) [DOI] [PubMed] [Google Scholar]
- 26.Plante CJ, Jumars PA, Baross JA. 1990. Digestive associations between marine detritivores and bacteria. Annu. Rev. Ecol. Syst. 21, 93–127. ( 10.1146/annurev.es.21.110190.000521) [DOI] [Google Scholar]
- 27.Iversen MH, Poulsen LK. 2007. Coprorhexy, coprophagy, and coprochaly in the copepods Calanus helgolandicus, Pseudocalanus elongatus, and Oithona similis. Mar. Ecol. Prog. Ser. 350, 79–89. ( 10.3354/meps07095) [DOI] [Google Scholar]
- 28.Harrold C, Pearse J. 1987. The ecological role of echinoderms in kelp forests. Echinoderm Stud. 2, 137–233. [Google Scholar]
- 29.Pearse JS. 2006. Ecological role of purple sea urchins. Science 314, 940–941. ( 10.1126/science.1131888) [DOI] [PubMed] [Google Scholar]
- 30.Ogden JC, Lobel PS. 1978. The role of herbivorous fishes and urchins in coral reef communities. Environ. Biol. Fishes 3, 49–63. ( 10.1007/bf00006308) [DOI] [Google Scholar]
- 31.Edmunds PJ, Carpenter RC. 2001. Recovery of Diadema antillarum reduces macroalgal cover and increases abundance of juvenile corals on a Caribbean reef. Proc. Natl Acad. Sci. USA 98, 5067–5071. ( 10.1073/pnas.071524598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valentine J, Heck K. 1991. The role of sea urchin grazing in regulating subtropical seagrass meadows: evidence from field manipulations in the northern Gulf of Mexico. J. Exp. Mar. Bio. Ecol. 154, 215–230. ( 10.1016/0022-0981(91)90165-S) [DOI] [Google Scholar]
- 33.Klumpp DW, Salita-Espinosa JT, Fortes MD. 1993. Feeding ecology and trophic role of sea urchins in a tropical seagrass community. Aquat. Bot. 45, 205–229. ( 10.1016/0304-3770(93)90022-O) [DOI] [Google Scholar]
- 34.Rose CD, et al. 1999. Overgrazing of a large seagrass bed by the sea urchin Lytechinus variegatus in Outer Florida Bay. Mar. Ecol. Prog. Ser. 190, 211–222. ( 10.3354/meps190211) [DOI] [Google Scholar]
- 35.Eklöf JS, de la Torre-Castro M, Gullström M, Uku J, Muthiga N, Lyimo T, Bandeira SO.. 2008. Sea urchin overgrazing of seagrasses: a review of current knowledge on causes, consequences, and management. Estuar. Coast. Shelf Sci. 79, 569–580. ( 10.1016/j.ecss.2008.05.005) [DOI] [Google Scholar]
- 36.Watanabe J, Harrold C. 1991. Destructive grazing by sea urchins Strongylocentrotus spp. in a central California kelp forest: potential roles of recruitment, depth, and predation. Mar. Ecol. Prog. Ser. Oldend. 71, 125–141. ( 10.3354/meps071125) [DOI] [Google Scholar]
- 37.Ling SD, et al. 2018. Global regime shift dynamics of catastrophic sea urchin overgrazing. Phil. Trans. R. Soc. B 370, 20130269 ( 10.1098/rstb.2013.0269) [DOI] [Google Scholar]
- 38.Miller RJ, Lafferty KD, Lamy T, Kui L, Rassweiler A, Reed DC. 2018. Giant kelp, Macrocystis pyrifera, increases faunal diversity through physical engineering. Proc. R. Soc. B 285, 20172571 ( 10.1098/rspb.2017.2571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Estes J, Palmisano J. 1974. Sea otters: their role in structuring nearshore communities. Science 185, 1058–1060. ( 10.1126/science.185.4156.1058) [DOI] [PubMed] [Google Scholar]
- 40.Shears N, Babcock R. 2003. Continuing trophic cascade effects after 25 years of no-take marine reserve protection. Mar. Ecol. Prog. Ser. 246, 1–16. ( 10.3354/meps246001) [DOI] [Google Scholar]
- 41.Duggins DO, Simenstad CA, Estes JA. 1989. Magnification of secondary production by kelp detritus in coastal marine ecosystems. Science 245, 170–173. ( 10.1126/science.245.4914.170) [DOI] [PubMed] [Google Scholar]
- 42.Kaehler S, Pakhomov E, Kalin R, Davis S. 2006. Trophic importance of kelp-derived suspended particulate matter in a through-flow subAntarctic system. Mar. Ecol. Prog. Ser. 316, 17–22. ( 10.3354/meps316017) [DOI] [Google Scholar]
- 43.Miller RRJ, Page HHM. 2012. Kelp as a trophic resource for marine suspension feeders: a review of isotope-based evidence. Mar. Biol. 159, 1391–1402. ( 10.1007/s00227-012-1929-2) [DOI] [Google Scholar]
- 44.Mattison J, Trent J, Shanks A, Akin T, Pearse J. 1977. Movement and feeding activity of red sea urchins (Strongylocentrotus franciscanus) adjacent to a kelp forest. Mar. Biol. 39, 25–30. ( 10.1007/BF00395589) [DOI] [Google Scholar]
- 45.Vadas R. 1977. Preferential feeding: an optimization strategy in sea urchins. Ecol. Monogr. 47, 337–371. ( 10.2307/1942173) [DOI] [Google Scholar]
- 46.Harrold C, Reed D. 1985. Food availability, sea urchin grazing, and kelp forest community structure. Ecology 66, 1160–1169. ( 10.2307/1939168) [DOI] [Google Scholar]
- 47.Foster M, Schiel D. 1988. Kelp communities and sea otters: keystone species or just another brick in the wall? In The community ecology of sea otters (eds VanBlaricom GR, Estes JA), pp. 92–115. Berlin, Germany: Springer. [Google Scholar]
- 48.Cummins KW, Klug MJ. 1979. Feeding ecology of stream invertebrates. Annu. Rev. Ecol. Syst. 10, 147–172. ( 10.1146/annurev.es.10.110179.001051) [DOI] [Google Scholar]
- 49.Cummins KW, Wilzbach MA, Gates DM, Perry JB, Taliaferro WB. 1989. Shredders and riparian vegetation. Bioscience 39, 24–30. ( 10.2307/1310804) [DOI] [Google Scholar]
- 50.Koehl MAR, Wainwright SA. 1977. Mechanical adaptations of a giant kelp. Limnol. Oceanogr. 22, 1067–1071. ( 10.4319/lo.1977.22.6.1067) [DOI] [Google Scholar]
- 51.Sauchyn L, Scheibling R. 2009. Degradation of sea urchin feces in a rocky subtidal ecosystem: implications for nutrient cycling and energy flow. Aquat. Biol. 6, 99–108. ( 10.3354/ab00171) [DOI] [Google Scholar]
- 52.Sauchyn L, Lauzon-Guay J, Scheibling R. 2011. Sea urchin fecal production and accumulation in a rocky subtidal ecosystem. Aquat. Biol. 13, 215–223. ( 10.3354/ab00359) [DOI] [Google Scholar]
- 53.Stock BC, Semmens BX.. 2016. MixSIAR GUI User Manual. Version 3.1. [DOI] [PubMed]
- 54.Vander Zanden MJ, Rasmussen JB. 2001. Variation in δ 15 N and δ 13 C trophic fractionation: implications for aquatic food web studies. Limnol. Oceanogr. 46, 2061–2066. ( 10.4319/lo.2001.46.8.2061) [DOI] [Google Scholar]
- 55.Mcintyre PB, Flecker AS. 2006. Rapid turnover of tissue nitrogen of primary consumers in tropical freshwaters. Ecophysiology 148, 12–21. ( 10.1007/s00442-005-0354-3) [DOI] [PubMed] [Google Scholar]
- 56.Dubois S, Jean-Louis B, Bertrand B, Lefebvre S. 2007. Isotope trophic-step fractionation of suspension-feeding species: implications for food partitioning in coastal ecosystems. J. Exp. Mar. Biol. Ecol. 351, 121–128. ( 10.1016/j.jembe.2007.06.020) [DOI] [Google Scholar]
- 57.Vander Zanden MJ, Clayton MK, Moody EK, Solomon CT, Weidel BC. 2015. Stable isotope turnover and half-life in animal tissues: a literature synthesis. PLoS ONE 10, e0116182 ( 10.1371/journal.pone.0116182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reed DC. 2017. Data from: SBC LTER: Reef: long-term experiment: kelp removal: detritus biomass Environ. Data Initiat. ( 10.6073/pasta/490d9479fe3dfffe42140650246b870a) [DOI]
- 59.Reed DC. 2019. Data from: SBC LTER: Reef: long-term experiment: biomass for kelp forest species, ongoing since 2008 Environ. Data Initiat. ( 10.6073/pasta/7c69f5230905e0de9970c803a5ac44c9) [DOI]
- 60.Reed DC, Nelson JC, Harrer SL, Miller RJ. 2016. Estimating biomass of benthic kelp forest invertebrates from body size and percent cover data. Mar. Biol. 163, 101 ( 10.1007/s00227-016-2879-x) [DOI] [Google Scholar]
- 61.Azad AK, Pearce CM, McKinley RS. 2011. Effects of diet and temperature on ingestion, absorption, assimilation, gonad yield, and gonad quality of the purple sea urchin (Strongylocentrotus purpuratus). Aquaculture 317, 187–196. ( 10.1016/j.aquaculture.2011.03.019) [DOI] [Google Scholar]
- 62.Foster MC, Byrnes JEK, Reed DC. 2014. Data from: SBC LTER: Effect of algal diet on consumption, growth, and gonad weight of the purple sea urchin (Strongylocentrotus pupuratus) Environ. Data Initiat. ( 10.6073/pasta/511fb37b21e90bb924b3ac691789c568) [DOI] [PMC free article] [PubMed]
- 63.Foster MC, Byrnes JEK, Reed DC. 2015. Effects of five southern California macroalgal diets on consumption, growth, and gonad weight, in the purple sea urchin Strongylocentrotus purpuratus. PeerJ 3, e719 ( 10.7717/peerj.719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Page HM, Reed D, Brzezinski M, Melack J, Dugan J. 2008. Assessing the importance of land and marine sources of organic matter to kelp forest food webs. Mar. Ecol. Prog. Ser. 360, 47–62. ( 10.3354/meps07382) [DOI] [Google Scholar]
- 65.Page HM, et al. 2013. Stable isotopes reveal trophic relationships and diet of consumers in temperate kelp forest and coral reed ecosystems. Oceanography 26, 180–189. ( 10.5670/oceanog.2013.61) [DOI] [Google Scholar]
- 66.Larson RJ. 1972. The food habits of four kelp-bed rockfishes (Scorpaenidae, Sebastes) off Santa Barbara, California, Santa Barbara, CA: University of California. [Google Scholar]
- 67.Schmitt RJ, Holbrook SJ. 1984. Ontogeny of prey selection by black surfperch Embiotoca jacksoni (Pisces: Embiotocidae): the roles of fish morphology, foraging behavior, and patch selection. Mar. Ecol. Prog. Ser. 18, 225–239. ( 10.3354/meps018225) [DOI] [Google Scholar]
- 68.Castañeda-Fernández-de-Lara V, Serviere-Zaragoza E, Hernández-Vázquez S, Butler MJ IV. 2005. Feeding ecology of juvenile spiny lobster, Panulirus interruptus, on the Pacific coast of Baja California Sur, Mexico. New Zeal . J. Mar. Freshw. Res. 39, 425–435. ( 10.1080/00288330.2005.9517322) [DOI] [Google Scholar]
- 69.Del Rio MC, Wolf N, Carleton SA, Gannes LZ. 2009. Isotopic ecology ten years after a call for more laboratory experiments. Biol. Rev. 84, 91–111. ( 10.1111/j.1469-185X.2008.00064.x) [DOI] [PubMed] [Google Scholar]
- 70.Wernberg T, Filbee-Dexter K. 2018. Grazers extend blue carbon transfer by slowing sinking speeds of kelp detritus. Sci. Rep. 8, 17180 ( 10.1038/s41598-018-34721-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shimeta J, Jumars P. 1991. Physical mechanisms and rates of particle capture by suspension feeders. Ocean. Mar. Biol. Annu. Rev 29, 191–257. [Google Scholar]
- 72.Mckenzie JD. 1987. The ultrastructure of the tentacles of eleven species of dendrochirote holothurians studied with special reference to the surface coats and papillae. Cell Tissue Res. 248, 187–199. ( 10.1007/bf01239980) [DOI] [Google Scholar]
- 73.Hamel J, Mercier A. 1998. Diet and feeding behaviour of the sea cucumber Cucumaria frondosa in the St. Lawrence estuary, eastern Canada. Can. J. Zool. 76, 1194–1198. ( 10.1139/z98-040) [DOI] [Google Scholar]
- 74.Roberts A, Gebruk AV, Levin V, Manship B. 2000. Feeding and digestive strategies in deposit-feeding holothurians. Oceanogr. Mar. Biol. An Annu. Rev. 38, 257–310. ( 10.1017/CBO9781107415324.004) [DOI] [Google Scholar]
- 75.Austin WC. 1966. Feeding mechanisms, digestive tracts and circulatory systems in ophiuroids: Ophiothrix spiculata Le Conte, 1851 and Ophiura luetkeni (Lyman, 1860). Stanford, CA: Stanford University Press. [Google Scholar]
- 76.Riisgård HU, Larsen PS. 2010. Particle capture mechanisms in suspension-feeding invertebrates. Mar. Ecol. Prog. Ser. 418, 255–293. ( 10.3354/meps08755) [DOI] [Google Scholar]
- 77.Southward AJ. 1987. Barnacle biology. Rotterdam, The Netherlands: CRC Press. [Google Scholar]
- 78.Graf G. 1999. Do benthic animals control the particle exchange between bioturbated sediments and benthic trubidity zones? In Biogeochemical cycling and sediment ecology, vol. 59 (eds Gray JS, Ambrose W Jr, Szaniawska A.), pp. 153–160. Dordrecht, The Netherlands: Springer Science+Business Media. [Google Scholar]
- 79.Jørgensen C, Kørboe T, Møhlenberg F, Riisgård H. 1984. Ciliary and mucus-net filter feeding, with special reference to fluid mechanical characteristics. Mar. Ecol. Prog. Ser. 15, 283–292. ( 10.3354/meps015283) [DOI] [Google Scholar]
- 80.Pentreath RJ. 2010. Feeding mechanisms and the functional morphology of podia and spines in some New Zealand ophiuroids (Echinodermata). J. Zool. 161, 395–429. ( 10.1111/j.1469-7998.1970.tb04520.x) [DOI] [Google Scholar]
- 81.Kappner I, Al-Moghrabi SM, Richter C. 2000. Mucus-net feeding by the vermetid gastropod Dendropoma maxima in coral reefs. Mar. Ecol. Prog. Ser. 204, 309–313. ( 10.3354/meps204309) [DOI] [Google Scholar]
- 82.Koenigs C, Miller R, Page H. 2015. Top predators rely on carbon derived from giant kelp Macrocystis pyrifera. Mar. Ecol. Prog. Ser. 537, 1–8. ( 10.3354/meps11467) [DOI] [Google Scholar]
- 83.Breen PA, Mann KH. 1976. Changing lobster abundance and the destruction of kelp beds by sea urchins. Mar. Biol. 34, 137–142. ( 10.1007/BF00390755) [DOI] [Google Scholar]
- 84.Graham MH, Vasquez JA, Buschmann AH. 2007. Global ecology of the giant kelp Macrocystis: from ecotypes to ecosystems. Ocean. Mar. Biol. Annu. Rev. 45, 39–88. [Google Scholar]
- 85.Dean T, Schroeter S, Dixon J. 1984. Effects of grazing by two species of sea urchins (Strongylocentrotus franciscanus and Lytechinus anamesus) on recruitment and survival of two species of kelp (Macrocystis pyrifera and Pterygophora californica). Mar. Biol. 78, 301–313. ( 10.1007/BF00393016) [DOI] [Google Scholar]
- 86.Vanderklift M, Kendrick G. 2005. Contrasting influence of sea urchins on attached and drift macroalgae. Mar. Ecol. Prog. Ser. 299, 101–110. ( 10.3354/meps299101) [DOI] [Google Scholar]
- 87.Lowry M, Pearse J. 1973. Abalones and sea urchins in an area inhabited by sea otters. Mar. Biol. 23, 213–219. ( 10.1007/BF00389487) [DOI] [Google Scholar]
- 88.Foster M. 1975. Algal succession in a Macrocystis pyrifera forest. Mar. Biol. 32, 313–329. ( 10.1007/BF00388989) [DOI] [Google Scholar]
- 89.Cowen R, Agegian C, Foster M. 1982. The maintenance of community structure in a central California giant kelp forest. J. Exp. Mar. Biol. 64, 189–201. ( 10.1016/0022-0981(82)90152-6) [DOI] [Google Scholar]
- 90.Gerard V. 1976. Some aspects of material dynamics and energy flow in a kelp forest in Monterey Bay, California. Santa Cruz, CA: University of California. [Google Scholar]
- 91.Donohoe J, Lowe A, Dethier M. 2013. Capture efficiency of various species and sizes of drift macrophytes by red urchins, Strongylocentrotus franciscanus. Seattle, WA: University of Washington. [Google Scholar]
- 92.Lowe AT, Whippo R, Galloway AWE, Britton-Simmons KH, Dethier MN. 2015. Sedentary urchins influence benthic community composition below the macroalgal zone. Mar. Ecol. 36, 129–140. ( 10.1111/maec.12124) [DOI] [Google Scholar]
- 93.Mann K. 2000. Ecology of coastal waters, with implications for management, vol. 2 Oxford, UK: Blackwell Science. [Google Scholar]
- 94.Dugan JE, Hubbard DM, McCrary MD, Pierson MO. 2003. The response of macrofauna communities and shorebirds to macrophyte wrack subsidies on exposed sandy beaches of southern California. Estuar. Coast. Shelf Sci. 58, 25–40. ( 10.1016/S0272-7714(03)00045-3) [DOI] [Google Scholar]
- 95.Britton-Simmons KH, Rhoades AL, Pacunski RE, Galloway AWE, Lowe AT, Sosik EA, Dethier MN, Duggins DO. 2012. Habitat and bathymetry influence the landscape-scale distribution and abundance of drift macrophytes and associated invertebrates. Limnol. Oceanogr. 57, 176–184. ( 10.4319/lo.2012.57.1.0176) [DOI] [Google Scholar]
- 96.Roberts SN. 2012. A comparison of laboratory algal feeding rates with in situ capture of drift algae by the red urchin (Strongylocentrotus franciscanus). Boulder, CO: University of Colorado Boulder. [Google Scholar]
- 97.Dethier MN, Hoins G, Kobelt J, Lowe AT, Gallowat AWE, Schram JB, Raymore M, Duggins DO. 2019. Feces as food: the nutritional value of urchin feces and implications for benthic food webs. Ecology 514, 95–102. ( 10.1016/j.jembe.2019.03.016) [DOI] [Google Scholar]
- 98.De Ridder CH, Foret T.. 2001. Non-parasitic symbioses between echinoderms and bacteria. In Echinoderm studies (eds Jangoux M, Lawrence JM), pp. 111–169. Rotterdam, The Netherlands: Balkema Publ. [Google Scholar]
- 99.Guerinot ML, Fong W, Patriquin DG. 1977. Nitrogen fixation (acetylene reduction) associated with sea urchins (Strongylocentrotus droebrachiensis) feeding on seaweeds and eelgrass. J. Fish. Res. Board Can. 34, 416–420. ( 10.1139/f77-067) [DOI] [Google Scholar]
- 100.Guerinot ML, Patriquin DG. 1981. The association of N2-fixing bacteria with sea urchins. Mar. Biol. 62, 197–207. ( 10.1007/BF00388183) [DOI] [Google Scholar]
- 101.Lasker R, Giese A. 1954. Nutrition of the sea urchin, Strongylocentrotus purpuratus. Biol. Bull. 106, 328–340. ( 10.2307/1538767) [DOI] [Google Scholar]
- 102.Berger J. 1964. The morphology, systematics, and biology of the entocommensal ciliates of echinoids. Urbana, IL: University of Illinois at Urbana-Champaign. [Google Scholar]
- 103.Boolootian RA, Lasker R. 1964. Digestion of brown algae and the distribution of nutrients in the purple sea urchin Strongylocentrotus pupuratus. Comp. Biochem. Physiol. 11, 273–289. ( 10.1016/0010-406X(64)90109-4) [DOI] [PubMed] [Google Scholar]
- 104.Fong W, Mann KH. 1980. Role of gut flora in the transfer of amino acids through a marine food chain. Can. J. Fish. Aquat. Sci. 37, 88–96. ( 10.1139/f80-009) [DOI] [Google Scholar]
- 105.Brzezinski MA, Washburn L. 2011. Phytoplankton primary productivity in the Santa Barbara Channel: effects of wind-driven upwelling and mesoscale eddies. J. Geophys. Res. 116, C12013 ( 10.1029/2011JC007397) [DOI] [Google Scholar]
- 106.Montani S, Magni P, Abe N. 2003. Seasonal and interannual patterns of intertidal microphytobenthos in combination with laboratory and areal production estimates. Mar. Ecol. Prog. Ser. 249, 79–91. ( 10.3354/meps249079) [DOI] [Google Scholar]
- 107.Longphuirt SN, Clavier J, Grall J, Chauvaud L, Le Loc'h F, Le Berre I, Flye-Sainte-Marie J, Richard J, Leynaert A.. 2007. Primary production and spatial distribution of subtidal microphytobenthos in a temperate coastal system, the Bay of Brest, France. Estuar. Coast. Shelf Sci. 74, 367–380. ( 10.1016/J.ECSS.2007.04.025) [DOI] [Google Scholar]
- 108.Reed DC, Rassweiler A, Arkema KK. 2008. Biomass rather than growth rate determines variation in net primary productivity by giant kelp. Ecology 89, 2493–2505. ( 10.1890/07-1106.1) [DOI] [PubMed] [Google Scholar]
- 109.Miller RJ, Reed DC, Brzezinski MA. 2009. Community structure and productivity of subtidal turf and foliose algal assemblages. Mar. Ecol. Prog. Ser. 388, 1–11. ( 10.3354/meps08131) [DOI] [Google Scholar]
- 110.Miller RJ, Reed DC, Brzezinski MA. 2011. Partitioning of primary production among giant kelp (Macrocystis pyrifera), understory macroalgae, and phytoplankton on a temperate reef. Limnol. Oceanogr. 56, 119–132. ( 10.4319/lo.2011.56.1.0119) [DOI] [Google Scholar]
- 111.Castorani MCN, Reed DC, Miller RJ. 2018. Loss of foundation species: disturbance frequency outweighs severity in structuring kelp forest communities. Ecology 99, 2442–2454. ( 10.1002/ecy.2485) [DOI] [PubMed] [Google Scholar]
- 112.Gaylord B, et al. 2007. Spatial patterns of flow and their modification within and around a giant kelp forest. Limnol. Oceanogr. 52, 1838–1852. ( 10.4319/lo.2007.52.5.1838) [DOI] [Google Scholar]
- 113.Arkema K, Reed D, Schroeter S. 2009. Direct and indirect effects of giant kelp determine benthic community structure and dynamics. Ecology 90, 3126–3137. ( 10.1890/08-1213.1) [DOI] [PubMed] [Google Scholar]
- 114.Byrnes J, Stachowicz JJ, Hultgren KM, Randall Hughes A, Olyarnik SV, Thornber CS. 2006. Predator diversity strengthens trophic cascades in kelp forests by modifying herbivore behaviour. Ecol. Lett. 9, 61–71. ( 10.1111/j.1461-0248.2005.00842.x) [DOI] [PubMed] [Google Scholar]
- 115.Estes JA, Burdin A, Doak DF. 2016. Sea otters, kelp forests, and the extinction of Steller's sea cow. Proc. Natl Acad. Sci. USA 113, 880–885. ( 10.1073/pnas.1502552112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Filbee-Dexter K, Scheibling RE. 2014. Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Mar. Ecol. Prog. Ser. 495, 1–25. ( 10.3354/meps10573) [DOI] [Google Scholar]
- 117.Tracey SR, Baulch T, Hartmann K, Ling SD, Lucieer V, Marzloff MP, Mundy C. 2015. Systematic culling controls a climate driven, habitat modifying invader. Biol. Invasions 17, 1885–1896. ( 10.1007/s10530-015-0845-z) [DOI] [Google Scholar]
- 118.Sanderson JC, Ling SD, Dominguez JG, Johnson CR. 2016. Limited effectiveness of divers to mitigate ‘barrens’ formation by culling sea urchins while fishing for abalone. Mar. Freshw. Res. 67, 84 ( 10.1071/MF14255) [DOI] [Google Scholar]
- 119.Yorke CE, Page HM, Miller RJ. 2016. Data from: Sea urchins mediate the availability of kelp detritus to benthic consumers. Dryad Digital Repository ( 10.5061/dryad.38m3dc6). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Reed DC. 2017. Data from: SBC LTER: Reef: long-term experiment: kelp removal: detritus biomass Environ. Data Initiat. ( 10.6073/pasta/490d9479fe3dfffe42140650246b870a) [DOI]
- Reed DC. 2019. Data from: SBC LTER: Reef: long-term experiment: biomass for kelp forest species, ongoing since 2008 Environ. Data Initiat. ( 10.6073/pasta/7c69f5230905e0de9970c803a5ac44c9) [DOI]
- Foster MC, Byrnes JEK, Reed DC. 2014. Data from: SBC LTER: Effect of algal diet on consumption, growth, and gonad weight of the purple sea urchin (Strongylocentrotus pupuratus) Environ. Data Initiat. ( 10.6073/pasta/511fb37b21e90bb924b3ac691789c568) [DOI] [PMC free article] [PubMed]
- Yorke CE, Page HM, Miller RJ. 2016. Data from: Sea urchins mediate the availability of kelp detritus to benthic consumers. Dryad Digital Repository ( 10.5061/dryad.38m3dc6). [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Mesocosm isotope data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.38m3dc6 [119]. Santa Barbara Channel Long-Term Ecological Research data used in electronic supplementary material, figure S1 are available through the LTER data repository at https://portal.lternet.edu/nis/mapbrowse?packageid=knb-lter-sbc.119.2 and https://portal.lternet.edu/nis/mapbrowse?packageid=knb-lter-sbc.25.18