Abstract
Hybrid male sterility (HMS) contributes to speciation by restricting gene flow between related taxa. Detailed cytological characterization of reproductive organs in hybrid males is important for identifying phenotypes that can help guide searches of speciation genes. To investigate possible cellular causes of HMS, we performed crosses between closely related species of the Anopheles gambiae complex: An. merus with An. gambiae or An. coluzzii. We demonstrate that HMS in African malaria mosquitoes involves two defects in the reciprocal crosses: a premeiotic arrest of germline stem cells in degenerate testes and a failure of the reductional meiotic division of primary spermatocytes in normal-like testes. The premeiotic arrest in degenerate testes of hybrids is accompanied by a strong suppression of meiotic and postmeiotic genes. Unlike pure species, sex chromosomes in normal-like testes of F1 hybrids are largely unpaired during meiotic prophase I and all chromosomes show various degrees of insufficient condensation. Instead of entering reductional division in meiosis I, primary spermatocytes prematurely undergo an equational mitotic division producing non-motile diploid sperm. Thus, our study identified cytogenetic errors in interspecies hybrids that arise during the early stages of postzygotic isolation.
Keywords: Anopheles, chromosome pairing, hybrid male sterility, meiosis, speciation, spermatogenesis
1. Introduction
When species diverge, hybrid offspring that are produced can suffer from reduced fitness. Hybrid male fertility is usually one of the first of these postzygotic phenotypes affected [1,2]. Therefore, the genetic factors, cellular basis and molecular mechanisms of hybrid male sterility (HMS) are of considerable interest, as they inform our understanding of both speciation and normal fertility function. Studies in animals commonly assess testis shape, size or weight, as well as sperm morphology, density or motility, to define male sterility phenotypes. However, the cellular basis of HMS is rarely investigated. Available cytological studies of spermatogenesis in sterile hybrids indicate that multiple mechanisms of functional sterility are possible. Sterile hybrids between Mus musculus domesticus and M. m. musculus have spermatogenic arrest in early meiosis I with disrupted homoeologous chromosome pairing and meiotic sex chromosome inactivation [3,4]. Studies of failed spermatogenesis in different Drosophila hybrids observed arrests at the premeiotic stage [5], reduced chromosome pairing, unequal chromosome segregation in meiosis [6,7], characteristic spermiogenic arrests [5], spermatid abnormalities [8], and problems in sperm bundling and motility [9,10]. Detailed analyses of cellular phenotypes in testes of various hybrid organisms could help to establish the order of origin of post-zygotic isolating barriers and to guide the identification of speciation genes.
The Anopheles gambiae complex consists of at least nine morphologically nearly indistinguishable sibling species of African malaria mosquitoes [11–13]. Genome-based estimations of the age of the An. gambiae complex vary from 1.85 [14] to as young as 0.526 Myr [15]. Genomic introgression is prevalent in autosomal regions of several species, indicating naturally occurring interspecies hybridization [14,15]. Experimental crosses of species from the An. gambiae complex often produce sterile F1 hybrid males, conforming to Haldane's rule of sterility or inviability of the heterogametic sex [16–20]. Early crossing experiments between members of the complex have found that HMS is associated with various degrees of testes atrophy and underdevelopment of sperm [18]. A large effect of the X chromosome on HMS has been demonstrated for crosses between An. gambiae and An. arabiensis [16,21]. However, an introgression of the Y chromosome from An. gambiae into the background of An. arabiensis has shown no apparent influence on male fertility, fitness or gene expression [17]. Because of the recent evolution and ease of hybridization, sibling species of the An. gambiae complex offer great opportunities to provide insights into the cellular basis and molecular mechanisms of HMS.
Here, we investigate possible cellular causes of male sterility in hybrids between sibling species of the An. gambiae complex. We asked the following three specific questions. (i) What cellular processes are involved in causing infertility in hybrid mosquito males? (ii) Are the defects leading to HMS premeiotic, meiotic or postmeiotic? (iii) What cytogenetic errors trigger spermatogenic breakdown? We demonstrate that HMS in malaria mosquitoes involves two cellular defects in reciprocal crosses: premeiotic arrest in germline stem cells and the failure of the reductional meiotic division in primary spermatocytes. Our data suggest that meiotic abnormality in hybrid males stems from the unpairing of the sex chromosomes and insufficient chromatin condensation. Thus, our study identifies cytogenetic errors in hybrids that arise during the early stages of postzygotic isolation.
2. Material and methods
(a). Mosquito strains and crossing experiments
Laboratory colonies of An. gambiae ZANU (MRA-594), An. coluzzii MOPTI (MRA-763), An. coluzzii MALI (MRA-860) and An. merus MAF (MRA-1156) were obtained from the Biodefense and Emerging Infections Research Resources Repository (BEI). To perform interspecies crosses, male and female pupae were separated to guarantee virginity of adult mosquitoes. After the emergence of adults, crossing experiments were performed by combining 30 females and 15 males in one cage. At least two blood meals were fed to females and at least three repeats of each cross were conducted. Male gonads were photographed using an Olympus phase contrast microscope BX41 and UC90 digital camera (Olympus, Tokyo, Japan). We observed the testes of 30 males and took pictures of the testes of five males from each cross. A movie of sperm motility for five hybrid males from each cross and five males of pure species was recorded using a UC90 digital camera.
(b). Chromosome preparation and fluorescence in situ hybridization
Preparations of chromosomes, DNA probe labelling and fluorescent in situ hybridization (FISH) were performed as previously described [22,23]. Five DNA probes were used for FISH in this study (electronic supplementary material, table S1). Cytogenetic analyses were performed on at least 10 male individuals of each pure species and of each hybrid. Lengths of well-spread metaphase I chromosomes of each species and of each hybrid were measured using the ruler tool in Adobe Photoshop CS6 (Adobe Inc., San Jose, CA, USA; electronic supplementary material, table S2). For whole-mount FISH and analysis of chromosome pairing, testes from one-day-old adults of pure species and hybrids were dissected in 1 × PBS solution and fixed in 3.7% paraformaldehyde in 1 × PBS with 0.1% tween-20 (PBST) for 10 min at room temperature. After adding labelled DNA probes, testes were incubated at 75°C for 5 min (denaturation) and then at 37°C overnight (hybridization). Testes from six individuals of pure species and of hybrids were scanned with a Zeiss LSM 880 confocal laser scanning microscope (Carl Zeiss AG, Oberkochen, Germany) to analyse pairing and unpairing of the sex chromosomes. A total of 418 and 489 nuclei at the early stages of meiotic prophase I were analysed in pure species and hybrids, respectively (electronic supplementary material, table S3). The percentage of cells with pairing and no pairing of sex chromosomes was used to compare parental species and hybrids. Since the variances for both groups were equal (smax/smin < 2), a statistical two-sample pooled t-test was performed using the JMP 13 software (SAS Institute Inc., Cary, NC, USA). For more details, see the electronic supplementary material.
(c). RNA extraction and reverse transcription polymerase chain reaction
We dissected reproductive organs, which include both testes and male accessary glands (MAGs), from 30 males, only testes or only MAGs from 20 males, and ovaries from 30 females. These mosquitoes were 0–12-hour-old virgin adults of An. coluzzii MOPTI, An. merus MAF, and interspecies hybrids from crosses ♀An. coluzzii MOPTI × ♂An. merus and ♀An. merus × ♂An. coluzzii MOPTI. Total RNA was extracted using a Direct-Zol RNA MiniPrep Kit (Zymo Research, Irvine, CA, USA). Two-step RT-PCR was performed to analyse gene expression. cDNA for selected genes (electronic supplementary material, table S1) was generated in a 20 µl reaction containing 2 µl of 10 × RT buffer, 2 µl of 0.1 M dithiothreitol (DTT), 1 µl of 50 µM oligo(dT)20 primer, 1 µl of 10 mM dNTP mix solution, 1 µl of 25 mM MgCl2, 1 µl of RNaseOUT (40 U/µl−1), 1 µl of SuperScript III RT (200 U/µl−1), 2 µl of RNA and 6 µl of DEPC-treated water using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA, USA). Amplification products were visualized in a 2% agarose gel and photographed under the same parameters for each gene.
3. Results
(a). HMS phenotypes at the cellular level
To obtain interspecies hybrids, we performed reciprocal crosses between An. merus MAF and An. gambiae ZANU or An. coluzzii MOPTI and MALI. Backcrossing of F1 males to parental females resulted in induction of the laying of eggs that did not hatch, which confirmed mating of sterile F1 hybrid males since seminal fluids but not sperm are required to induce oviposition [24]. The observed sterility of hybrid males agrees with Haldane's rule [20] for the majority of interspecies crosses in the An. gambiae complex except for crosses between An. gambiae and An. coluzzii, which produce fertile hybrids of both sexes [16–19,25,26]. To investigate the developmental phenotypes involved in hybrid sterility, we dissected testes from adult males obtained from interspecies crosses and from pure species. Normal testes of pure species have a spindle-like shape (electronic supplementary material, figure S1A). We found obvious differences between testes morphology/size in one interspecies cross versus its reciprocal. Hybrid males from crosses between female An. merus and male An. gambiae or An. coluzzii display normal-like testes (electronic supplementary material, figure S1B). By contrast, F1 males from crosses between female An. gambiae or An. coluzzii and male An. merus show severely underdeveloped (degenerate) testes (electronic supplementary material, figure S1C). We then tested whether normal-like and degenerate testes of interspecies hybrids produce any sperm. Sperm reaches maturity within two days after emergence of Anopheles adult males. In squashed testes of 2–5-day-old adults of pure species, large amounts of mature spermatozoa with long tails can be seen. After we crushed the testes, spermatozoa with vibrant motility escaped from the ruptures (electronic supplementary material, figure S1A and movie S1). However, mature sperm or sperm motility could hardly be seen in squashed or crushed normal-like testes of 2–5-day-old adult hybrids from crosses when An. merus was the mother. Instead, we see fewer spermatids and mostly non-motile spermatozoa with large heads and often two short tails growing from opposite ends of the head (electronic supplementary material, figure S1B and movie S2). Staining with DAPI identified extended premeiotic and spermatogenic stages that cause delay of spermatogonia amplification, spermatocyte divisions and spermatid differentiation in the normal-like testes (electronic supplementary material, figure S2). Only undifferentiated round cells could be seen in degenerate testes of hybrids from reciprocal crosses when An. merus was the father (electronic supplementary material, figure S1C). Given the small size of the degenerate testes, these round cells may represent germline stem cells. Thus, neither normal-like nor underdeveloped testes of the interspecies hybrids produce mature motile spermatozoa.
(b). The progress of meiosis in males of pure species
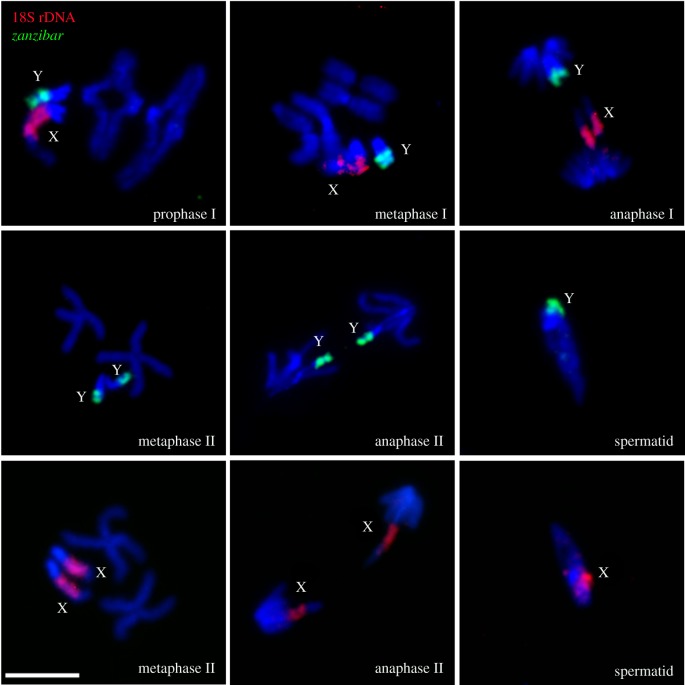
Here, we provide the first description of chromosome behaviour during meiosis of pure Anopheles species. The chromosome complement of Anopheles males consists of three chromosome pairs: two autosomes (2 and 3), and X and Y sex chromosomes. With the help of sex-chromosome-specific fluorescent probes (electronic supplementary material, table S1), we followed the progress of meiosis in An. gambiae, An. coluzzii and An. merus. Figure 1 shows normal activities of meiotic chromosomes in the testes of An. gambiae ZANU. In primary spermatocytes, all homologous autosomes and sex chromosomes pair and display chiasmata in diplotene/diakinesis of prophase I, they align with each other at the cell equator in metaphase I, and then move from each other in anaphase I. In secondary spermatocytes, sister chromatids of each chromosome align with each other at the cell equator in metaphase II and go to opposite poles of the cell during anaphase II. Meiotic divisions produce spermatids that contain a haploid set of autosomes and either a Y or X chromosome. An. coluzzii and An. merus males show similar behaviours of chromosomes during meiosis (electronic supplementary material, figures S3 and S4).
Figure 1.
Chromosome behaviour during meiosis in testes of An. gambiae. X chromosomes are labelled with 18S rDNA (red); Y chromosomes are labelled with retrotransposon zanzibar (green). Chromosomes are counterstained with DAPI (blue). Scale bar 5 µm.
(c). Meiotic and premeiotic failures in F1 males of interspecies hybrids
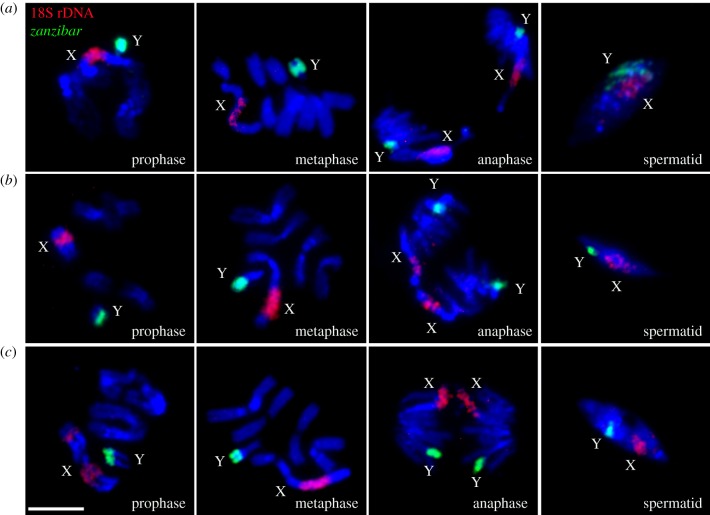
To determine possible cytogenetic mechanisms of HMS, we analysed chromosome behaviour in normal-like testes of hybrids from the ♀An. merus × ♂An. coluzzii/An. gambiae crosses (figure 2). In primary spermatocytes, homoeologous autosomes pair and form chiasmata in prophase I as in pure species. Unlike pure species, X and Y chromosomes do not pair or display chiasmata in diplotene/diakinesis of prophase I in these hybrids. We found this pattern consistent in all analysed hybrid males. Metaphase chromosomes in hybrids are visibly longer than at the same stage in pure species, indicating insufficient chromatin condensation. Besides, homoeologous chromosomes in hybrids do not segregate during anaphase. Instead, sister chromatids move to opposite poles of the dividing cell. Because reductional division does not occur in hybrid males, both X and Y chromatids move to the same pole during anaphase. As a result, haploid secondary spermatocytes are not present in these males. Our FISH analysis demonstrated that spermatids in testes of pure species normally contain either an X or Y chromosome. By contrast, we found both X and Y chromosomes present in spermatids of the hybrids (electronic supplementary material, figure S5). Moreover, the abnormal spermatids are larger in size due to insufficient chromatin condensation and the double chromosome content. Thus, we discovered that chromosomes in normal-like testes of hybrid males start with meiotic behaviour in prophase and then prematurely switch to mitotic behaviour in anaphase, thus skipping the reductional division. The equational division of primary spermatocytes results in dysfunctional diploid sperm in hybrids when An. merus is the mother. Degenerate testes of F1s from the reciprocal ♀An. coluzzii/gambiae × ♂An. merus crosses have only undifferentiated round germline stem cells. To visualize sex chromosomes, we performed whole-mount FISH with labelled 18S rDNA (Cy3) and satellite AgY53B (Cy5) to mark the sex chromosomes of the hybrid (electronic supplementary material, figure S6). Only interphase sex chromosomes in the nuclei of germline stem cells are detected indicating that meiosis does not start in the underdeveloped testes of F1 hybrids if An. merus is the father. Thus, premeiotic arrest in degenerate testes is the reason for the lack of spermatids in these hybrids.
Figure 2.
Chromosome behaviour during meiosis in testes of interspecies hybrids. (a) ♀An. merus × ♂An. coluzzii MALI. (b) ♀An. merus × ♂An. coluzzii MOPTI. (c) ♀An. merus × ♂An. gambiae ZANU. X chromosomes are labelled with 18S rDNA (red) and Y chromosomes are labelled with retrotransposon zanzibar (green). Chromosomes are counterstained with DAPI (blue). Scale bar 5 µm.
(d). Gene expression in pure species and interspecies hybrids
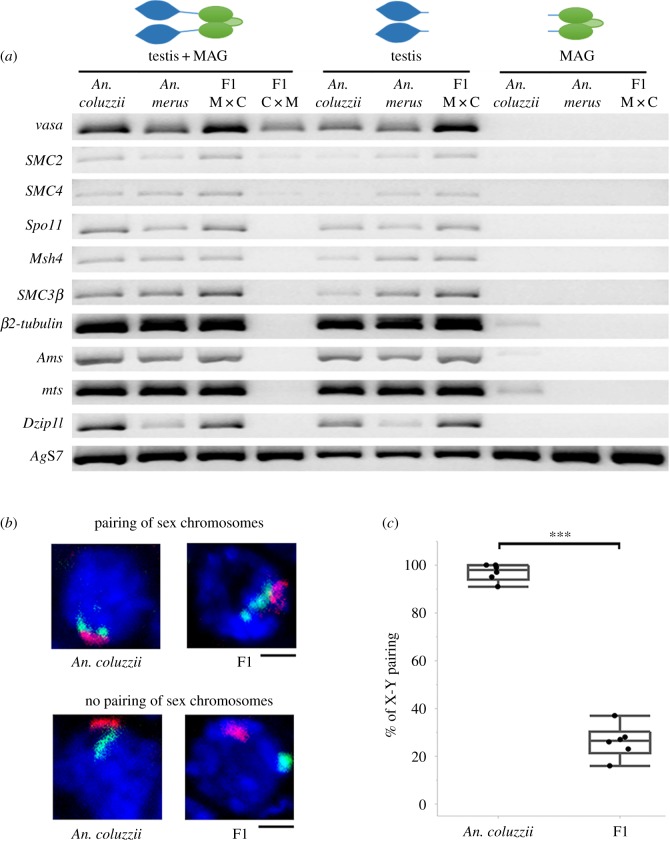
To test if the aforementioned premeiotic or meiotic failures are associated with gene misexpression, we analysed the transcript profile of the premeiotic and meiotic vasa [27], SMC2 and SMC4 genes [28,29], as well as the meiotic and postmeiotic Spo11, Msh4, SMC3 [28,29], β2-tubulin [30,31], Ams, mts and Dzip1l [32] genes in F1 hybrid males (electronic supplementary material, table S1). The RT-PCR results show that all these genes express in the reproductive tissues of An. coluzzii, An. merus and F1 hybrids from the ♀An. merus × ♂An. coluzzii cross. A slight but noticeable increase was observed for the vasa, SMC2, SMC4 and SMC3β (paralog of SMC3) transcripts in normal-like testes of these hybrids compared with testes of any of the parental species (figure 3a). This expression profile was consistent between two independent RT-PCR experiments that involved testes combined with MAGs and testes without MAGs. A separate experiment using MAGs only shows no vasa, SMC2, SMC4 or SMC3β transcripts in this tissue. Although vasa, SMC2 and SMC4 express in degenerate gonads of ♀An. coluzzii MOPTI × ♂An. merus F1 hybrids, transcripts of all tested meiotic and postmeiotic genes are virtually absent in these hybrids (figure 3a) supporting our observation that meiosis does not occur in degenerate testes (electronic supplementary material, figure S6). Expression of vasa indicated the development of germline stem cells even in degenerate testes of these interspecies hybrids. To test whether gene misexpression occurs in fertile female hybrids, we performed RT-PCR for the same genes using ovaries of pure species and hybrid females (electronic supplementary material, figure S7). Our results show that, unlike males, all analysed genes have similar transcript profiles in females of pure species and hybrids from the reciprocal crosses.
Figure 3.
Molecular and chromosomal abnormalities in interspecies hybrids. (a) Gene expression in male reproductive organs of An. coluzzii MOPTI, An. merus, F1 hybrids of ♀An. merus × ♂An. coluzzii MOPTI (F1 M × C) and F1 hybrids of ♀An. coluzzii MOPTI × ♂An. merus (F1C × M) analysed by RT-PCR. AgS7—an endogenous control gene. (b) Confocal images of primary spermatocytes with FISH showing pairing and unpairing of X (red) and Y (green) chromosomes in An. coluzzii MOPTI males and F1 hybrid males from the ♀An. merus × ♂An. coluzzii MOPTI cross. (c) Percentage of the X and Y chromosome pairing in primary spermatocytes of six An. coluzzii MOPTI males and six F1 hybrid males from the ♀An. merus × ♂An. coluzzii MOPTI cross. Statistical significance was assessed with a two-sample pooled t-test (p < 0.001).
(e). Chromosomal abnormalities in interspecies hybrids
Here, we performed quantitative analyses of chromatin condensation and X-Y chromosome pairing in pure species and their hybrids. To determine the extent of chromatin condensation in normal-like testes of interspecies hybrids compared with pure species, we measured the lengths of metaphase chromosomes (electronic supplementary material, table S2). The results of statistical analysis with a two-sample pooled t-test show that chromosomes in F1 hybrids are typically longer than chromosomes in An. coluzzii, An. gambiae or An. merus (electronic supplementary material, figure S8). For example, chromosomes of the An. merus origin in a hybrid background always show significant (p < 0.001) elongation by at least 1.3 times compared to pure An. merus. The X chromosome of An. merus suffered the most serious undercondensation in hybrids, exceeding the length of the X chromosome in the pure species background by 1.6–1.9-fold.
In our cytogenetic study of interspecies hybrids from the crosses ♀An. merus × ♂An. coluzzii/An. gambiae, the X and Y chromosomes do not show pairing or chiasmata in diplotene/diakinesis of prophase I (figure 2). We hypothesized that sex chromosome pairing is affected in early prophase I when individual chromosomes cannot be distinguished by direct visualization. To analyse the X-Y chromosome pairing at the pachytene stage of prophase I, we performed a whole-mount FISH and examined spatial positions of the X- and Y-specific fluorescent signals in confocal optical sections of nuclei in testes of pure species and their hybrids (electronic supplementary material, figure S9). We recorded the number of nuclei with X and Y fluorescent signals colocalized versus X and Y fluorescent signals located separately (figure 3b; electronic supplementary material, table S3). The results of a statistical analysis with a two-sample pooled t-test demonstrate that X and Y chromosomes pair in more than 90% of primary spermatocytes in An. coluzzii, while they pair in less than 30% of primary spermatocytes in F1 hybrids of ♀An. merus × ♂An. coluzzii (figure 3c).
4. Discussion
In this study, we performed the first detailed cytological analysis of spermatogenesis in pure species and hybrids of mosquitoes. We demonstrate that premeiotic and meiotic defects are involved in HMS in reciprocal crosses (electronic supplementary material, figure S10). This observation is at odds with the commonly accepted view that hybrid males suffer postmeiotic sterility problems more often than premeiotic or meiotic sterility problems [1,33]. Although many postmeiotic defects seen in Drosophila hybrids are indeed related to problems in sperm bundling and motility [5,9,33], some sperm abnormalities may stem from meiotic failures. For example, our data show that sperm abnormalities such as non-motility, two-tailed heads and chromatin decompaction in sperm heads can result from impaired meiosis I (figure 2). Additional studies of interspecies hybrids of various organisms should determine whether the first meiotic division commonly fails when fertility is affected.
Our cytogenetic investigation of meiosis in Anopheles species demonstrates that the X and Y chromosomes pair with each other throughout prophase I (figures 1 and 3b,c; electronic supplementary material, figures S3 and S4). Unlike pure species, meiotic prophase I in F1 mosquito hybrids shows cytogenetic anomalies—low percentages of X-Y chromosome pairing and insufficient chromatin condensation (figure 3b,c; electronic supplementary material, figure S8). The interplay between these two phenotypes may result in failing a reductional meiotic division and prematurely proceeding to an equational mitotic division (figure 4). All chromosomes must achieve synapsis in pachynema, complete DNA repair, and disassemble their synaptonemal complexes in diplonema as homologous chromosomes prepare to segregate [34]. The presence of chiasmata and tension exerted across homologues ensures that cells undergo reductional segregation [35]. Asynapsis of chromosomes almost invariably triggers pachytene checkpoint and meiotic breakdown [36].
Figure 4.
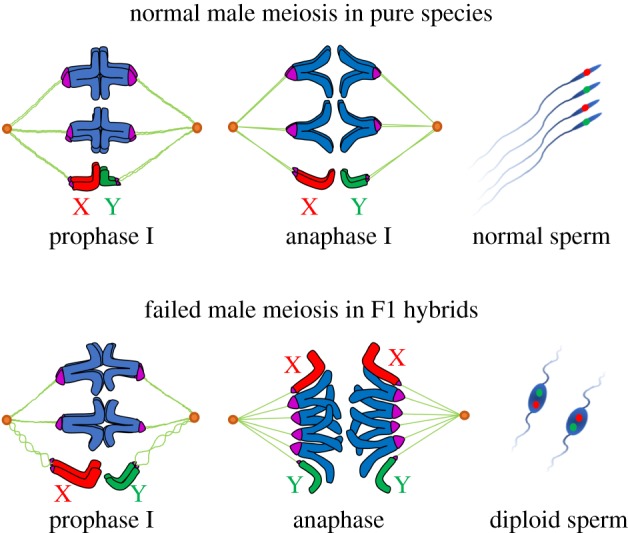
Scheme of male meiosis in pure species and interspecies hybrids of the An. gambiae complex. Unlike pure species, X and Y chromosomes in meiotic prophase I of hybrids are largely unpaired. The absence of chiasmata between the sex chromosomes and insufficient chromatin condensation reduce tension exerted across the homoeologous chromosomes, triggering premature sister chromatid segregation. As a result, instead of a reductional division, primary spermatocytes in interspecies hybrids undergo an equational mitotic division leading to diploid non-motile sperm.
Haldane's rule predicts that meiosis would be aberrant in males but may be normal in females [20]. This could be due to differences in the mechanisms of X-Y and X-X pairing. For example, in D. melanogaster, X and Y chromosomes pair through specific pairing sites located in heterochromatin [37], which could be relatively quickly altered during evolution. By contrast, the X-X and autosome-autosome pairing capacity is widely distributed along the chromosome arm [38] and is probably more robust to evolutionary changes. A study of introgression of the Y chromosome from An. gambiae into An. arabiensis suggests that the Y chromosome does not cause sterility [17]. This runs counter to our findings that X-Y unpairing underlies sterility in male hybrids. Identification and analysis of X-Y pairing sites in species of the An. gambiae complex may shed light on this problem.
Reciprocal crosses in malaria mosquitoes and other organisms usually produce hybrids with sterility phenotypes of different degrees of severity [6,18,39]. This observation indicates that malfunction of multiple stages of spermatogenesis can be involved in postzygotic isolation. At the early stages of speciation, premeiotic, meiotic and postmeiotic genes still express in hybrids and meiotic errors are characterized by decreased pairing between sex chromosomes, insufficient chromatin condensation and skipping reductional division. These phenotypes are seen in sterile hybrids from the ♀An. merus × ♂An. coluzzii/An. gambiae crosses (figures 2–4). Higher expression levels of the vasa, SMC2 and SMC4 genes (figure 3a) are consistent with our observation of extended premeiotic and meiotic stages in these hybrids (electronic supplementary material, figure S2). It is possible that the observed overexpression of SMC3β in the hybrid males may contribute to abandoning homologous chromosome segregation in favour of sister-chromatid cohesion, recombination and premature segregation. This is because the SMC1 and SMC3 proteins function together with other proteins to control cell cycle transitions by participating in sister-chromatid cohesion, recombination and faithful segregation [29,40,41]. To support crossover formation between homologous chromosomes and their subsequent segregation in meiosis, repair from the more readily available homologous sequences on the sister chromatid must be suppressed [34].
As species continue to diverge, meiotic errors become more prominent and new hybrid phenotypes appear as has been seen, for example, in sterile male hybrids from the ♀D. pseudoobscura × ♂D. persimilis cross [6,7]. At this stage of postzygotic isolation, meiosis is manifested by the unpairing of most of the chromosomes and by malfunction of the abnormally elongated spindle, resulting in spermatids with unbalanced chromosome content in sterile male hybrids [6,7]. In mouse hybrids, spermatogenic arrest occurs in early meiosis I when homoeologous chromosomes fail to pair and meiotic sex chromosome inactivation is disrupted, resulting in increased apoptosis of spermatocytes [3,4,42]. Disruption of synapsis between heterospecific chromosomes in prophase I has been proposed as a recurrently evolving trigger for the meiotic arrest of interspecific F1 hybrids [3,43]. Finally, spermatogenic abnormalities in hybrids can happen before meiosis starts. A spermatogenic arrest at the premeiotic stage is characterized by the repression of meiotic and postmeiotic genes and by the lack of spermatocytes in degenerate testes of hybrid males. These phenotypes have been observed in sterile hybrids from the ♀D. mauritiana × ♂D. sechellia cross [5] and in sterile hybrids from the ♀An. coluzzii/An. gambiae × ♂An. merus crosses (our study).
Charles Darwin, in the chapter on ‘Hybridism' in Origin of species, rightly argued that hybrid sterility ‘is not a specially endowed quality, but is incidental on other acquired differences’ [44]. Identification of the germline-specific cellular and molecular differences acquired during the early stages of postzygotic isolation between species is crucial to explaining both speciation and normal fertility function. Our study suggests that meiotic abnormalities in hybrid males stem from the unpairing of the sex chromosomes and insufficient chromatin condensation. Cytological analyses of spermatogenesis in interspecies hybrids from diverse groups of organisms may highlight general patterns and mechanisms in the origin and evolution of postzygotic isolation. Recently evolved members of the An. gambiae complex represent an excellent new system for studying the genetic basis and molecular mechanisms of species incompatibilities at the early stages of postzygotic reproductive isolation.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
The following mosquito strains were obtained through BEI Resources, NIAID, NIH: An. coluzzii, Strain Mali-NIH, Eggs, MRA-860 contributed by Nora J. Besansky; An. coluzzii, Strain MOPTI, Eggs, MRA-763 contributed by Gregory C. Lanzaro; An. gambiae, Strain ZANU, MRA-594 contributed by Hilary Ranson and Frank H. Collins; An. merus, Strain MAF, MRA-1156 contributed by Maureen Coetzee. We thank Kristin Rose and Janet Webster for editing the text.
Data accessibility
This article has no additional data.
Authors' contributions
I.V.S. conceived the study. J.L. conducted mosquito maintenance and crosses. J.L. and I.V.S. performed mosquito dissection and sperm motility assays. J.L. conducted FISH, microscopy and RT-PCR. J.L. and I.V.S. did data analysis. I.V.S. led the writing of the manuscript with critical contributions from J.L. All authors read and approved the final manuscript.
Competing interests
We declare we have no competing interests.
References
- 1.Powell JR. 1997. Progress and prospects in evolutionary biology. New York, NY: Oxford University Press. [Google Scholar]
- 2.Turissini DA, McGirr JA, Patel SS, David JR, Matute DR. 2018. The rate of evolution of postmating-prezygotic reproductive isolation in Drosophila. Mol. Biol. Evol. 35, 312–334. ( 10.1093/molbev/msx271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharyya T, Gregorova S, Mihola O, Anger M, Sebestova J, Denny P, Simecek P, Forejt J. 2013. Mechanistic basis of infertility of mouse intersubspecific hybrids. Proc. Natl Acad. Sci. USA 110, E468–E477. ( 10.1073/pnas.1219126110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forejt J, Ivanyi P. 1974. Genetic studies on male sterility of hybrids between laboratory and wild mice (Mus musculus L.). Genet. Res. 24, 189–206. ( 10.1017/S0016672300015214) [DOI] [PubMed] [Google Scholar]
- 5.Kulathinal R, Singh RS. 1998. Cytological characterization of premeiotic versus postmeiotic defects producing hybrid male sterility among sibling species of the Drosophila melanogaster complex. Evolution 52, 1067–1079. ( 10.1111/j.1558-5646.1998.tb01834.x) [DOI] [PubMed] [Google Scholar]
- 6.Dobzhansky T. 1934. Studies on hybrid sterility. I. Spermatogenesis in pure and hybrid Drosophila pseudoobscura. Zeitschr. Zelljorsch. U. Mikrosk. Anat. 21, 169–223. ( 10.1007/BF00374056) [DOI] [Google Scholar]
- 7.Dobzhansky T. 1933. On the sterility of the interracial hybrids in Drosophila pseudoobscura. Proc. Natl Acad. Sci. USA 19, 397–403. ( 10.1073/pnas.19.4.397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardy RW, Lougheed A, Markow TA. 2011. Reproductive tract and spermatid abnormalities of hybrid males from reciprocal crosses between Drosophila mojavensis and D. arizonae. Fly (Austin) 5, 76–80. ( 10.4161/fly.5.2.15571) [DOI] [PubMed] [Google Scholar]
- 9.Coyne JA. 1984. Genetic-basis of male-sterility in hybrids between 2 closely related species of Drosophila. Proc. Natl Acad. Sci. USA 81, 4444–4447. ( 10.1073/pnas.81.14.4444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masly JP, Jones CD, Noor MA, Locke J, Orr HA. 2006. Gene transposition as a cause of hybrid sterility in Drosophila. Science 313, 1448–1450. ( 10.1126/science.1128721) [DOI] [PubMed] [Google Scholar]
- 11.Coluzzi M, Sabatini A, della Torre A, Di Deco MA, Petrarca V. 2002. A polytene chromosome analysis of the Anopheles gambiae species complex. Science 298, 1415–1418. ( 10.1126/science.1077769) [DOI] [PubMed] [Google Scholar]
- 12.Coetzee M, Hunt RH, Wilkerson R, Della Torre A, Coulibaly MB, Besansky NJ. 2013. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa 3619, 246–274. ( 10.11646/zootaxa.3619.3.2) [DOI] [PubMed] [Google Scholar]
- 13.Barron MG, et al. 2018. A new species in the Anopheles gambiae complex reveals new evolutionary relationships between vector and non-vector species. bioRxiv ( 10.1101/460667) [DOI] [Google Scholar]
- 14.Fontaine MC, et al. 2015. Extensive introgression in a malaria vector species complex revealed by phylogenomics. Science 347, 1258524 ( 10.1126/science.1258524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thawornwattana Y, Dalquen D, Yang Z. 2018. Coalescent analysis of phylogenomic data confidently resolves the species relationships in the Anopheles gambiae species complex. Mol. Biol. Evol. 35, 2512–2527. ( 10.1093/molbev/msy158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slotman M, Della Torre A, Powell JR. 2004. The genetics of inviability and male sterility in hybrids between Anopheles gambiae and An. arabiensis. Genetics 167, 275–287. ( 10.1534/genetics.167.1.275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernardini F, et al. 2017. Cross-species Y chromosome function between malaria vectors of the Anopheles gambiae species complex. Genetics 207, 729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson G, Paterson HE, Coluzzi M, Mason GF, Micks DW. 1967. The Anopheles gambiae complex. In Genetics of insect vectors of diesease (eds Wright JW, Pal R), pp. 211–249. New York, NY: Elsevier Publishing Company. [Google Scholar]
- 19.Presgraves DC, Orr HA. 1998. Haldane's rule in taxa lacking a hemizygous X. Science 282, 952–954. ( 10.1126/science.282.5390.952) [DOI] [PubMed] [Google Scholar]
- 20.Haldane JBS. 1922. Sex ratio and unisexual sterility in hybrid animals. J. Genet. 12, 101–109. ( 10.1007/BF02983075) [DOI] [Google Scholar]
- 21.Curtis CF. 1982. The mechanism of hybrid male sterility from crosses in the Anopheles gambiae and Glossina morsitans complexes. In Recent developments in the genetics of disease vectors (eds Steiner WM, Tabachnick WJ, Rai KS, Narang S), pp. 290–312. Champaign, IL: Stipes Publishing Company. [Google Scholar]
- 22.Timoshevskiy VA, Sharma A, Sharakhov IV, Sharakhova MV. 2012. Fluorescent in situ hybridization on mitotic chromosomes of mosquitoes. J. Vis. Exp. 67, e4215 ( 10.3791/4215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharakhova MV, George P, Timoshevskiy V, Sharma A, Peery A, Sharakhov IV. 2015. Mosquitoes (Diptera). In Protocols for cytogenetic mapping of arthropod genomes (ed. Sharakhov IV.), pp. 93–170. Boca Raton, FL: CRC Press. [Google Scholar]
- 24.Thailayil J, Magnusson K, Godfray HC, Crisanti A, Catteruccia F. 2011. Spermless males elicit large-scale female responses to mating in the malaria mosquito Anopheles gambiae. Proc. Natl Acad. Sci. USA 108, 13 677–13 681. ( 10.1073/pnas.1104738108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diabate A, Dabire RK, Millogo N, Lehmann T. 2007. Evaluating the effect of postmating isolation between molecular forms of Anopheles gambiae (Diptera: Culicidae). J. Med. Entomol. 44, 60–64. ( 10.1603/0022-2585(2007)44[60:ETEOPI]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 26.Aboagye-Antwi F, et al. 2015. Experimental swap of Anopheles gambiae's assortative mating preferences demonstrates key role of X-chromosome divergence island in incipient sympatric speciation. PLoS Genet. 11, e1005141 ( 10.1371/journal.pgen.1005141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papathanos PA, Windbichler N, Menichelli M, Burt A, Crisanti A. 2009. The vasa regulatory region mediates germline expression and maternal transmission of proteins in the malaria mosquito Anopheles gambiae: a versatile tool for genetic control strategies. BMC Mol. Biol. 10, 65 ( 10.1186/1471-2199-10-65) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malik SB, Pightling AW, Stefaniak LM, Schurko AM, Logsdon JM Jr. 2007. An expanded inventory of conserved meiotic genes provides evidence for sex in Trichomonas vaginalis. PLoS ONE 3, e2879 ( 10.1371/journal.pone.0002879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Losada A, Hirano T. 2005. Dynamic molecular linkers of the genome: the first decade of SMC proteins. Genes Dev. 19, 1269–1287. ( 10.1101/gad.1320505) [DOI] [PubMed] [Google Scholar]
- 30.Catteruccia F, Crisanti A, Wimmer EA. 2009. Transgenic technologies to induce sterility. Malar. J. 8, S7 ( 10.1186/1475-2875-8-S2-S7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kemphues KJ, Kaufman TC, Raff RA, Raff EC. 1982. The testis-specific beta-tubulin subunit in Drosophila melanogaster has multiple functions in spermatogenesis. Cell 31, 655–670. ( 10.1016/0092-8674(82)90321-X) [DOI] [PubMed] [Google Scholar]
- 32.Krzywinska E, Krzywinski J. 2009. Analysis of expression in the Anopheles gambiae developing testes reveals rapidly evolving lineage-specific genes in mosquitoes. BMC Genomics 10, 300 ( 10.1186/1471-2164-10-300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coyne JA, Orr HA. 2004. Speciation. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 34.Subramanian VV, Hochwagen A. 2014. The meiotic checkpoint network: step-by-step through meiotic prophase. Cold Spring Harb. Perspect. Biol. 6, a016675 ( 10.1101/cshperspect.a016675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyazaki S, Kim J, Sakuno T, Watanabe Y. 2017. Hierarchical regulation of centromeric cohesion protection by Meikin and Shugoshin during meiosis I. Cold Spring Harb. Symp. Quant. Biol. 82, 259–266. ( 10.1101/sqb.2017.82.033811) [DOI] [PubMed] [Google Scholar]
- 36.Bolcun-Filas E, Schimenti JC. 2012. Genetics of meiosis and recombination in mice. Int. Rev. Cell Mol. Biol. 298, 179–227. ( 10.1016/B978-0-12-394309-5.00005-5) [DOI] [PubMed] [Google Scholar]
- 37.McKee BD, Yan R, Tsai JH. 2012. Meiosis in male Drosophila. Spermatogenesis 2, 167–184. ( 10.4161/spmg.21800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKee BD. 2009. Homolog pairing and segregation in Drosophila meiosis. Genome Dyn. 5, 56–68. ( 10.1159/000166619) [DOI] [PubMed] [Google Scholar]
- 39.Good JM, Handel MA, Nachman MW. 2008. Asymmetry and polymorphism of hybrid male sterility during the early stages of speciation in house mice. Evolution 62, 50–65. ( 10.1111/j.1558-5646.2007.00257.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Revenkova E, Eijpe M, Heyting C, Hodges CA, Hunt PA, Liebe B, Scherthan H, Jessberger R. 2004. Cohesin SMC1 beta is required for meiotic chromosome dynamics, sister chromatid cohesion and DNA recombination. Nat. Cell Biol. 6, 555–562. ( 10.1038/ncb1135) [DOI] [PubMed] [Google Scholar]
- 41.Biswas U, Stevense M, Jessberger R. 2018. SMC1α substitutes for many meiotic functions of SMC1β but cannot protect telomeres from damage. Curr. Biol. 28, 249–261e244. ( 10.1016/j.cub.2017.12.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwahn DJ, Wang RJ, White MA, Payseur BA. 2018. Genetic dissection of hybrid male sterility across stages of spermatogenesis. Genetics 210, 1453–1465. ( 10.1534/genetics.118.301658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhattacharyya T, Reifova R, Gregorova S, Simecek P, Gergelits V, Mistrik M, Martincova I, Pialek J, Forejt J. 2014. X chromosome control of meiotic chromosome synapsis in mouse inter-subspecific hybrids. PLoS Genet. 10, e1004088 ( 10.1371/journal.pgen.1004088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darwin C. 1859. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life, p. 261 London, UK: John Murray. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.