Abstract
The speed and dynamics of range expansions shape species distributions and community composition. Despite the critical impact of population growth rates for range expansion, they are neglected in existing empirical studies, which focus on the investigation of selected life-history traits. Here, we present an approach based on non-invasive genetic capture–mark–recapture data for the estimation of adult survival, fecundity and juvenile survival, which determine population growth. We demonstrate the reliability of our method with simulated data, and use it to investigate life-history changes associated with range expansion in 35 colonies of the bat species Rhinolophus hipposideros. Comparing the demographic parameters inferred for 19 of those colonies which belong to an expanding population with those inferred for the remaining 16 colonies from a non-expanding population reveals that range expansion is associated with higher net reproduction. Juvenile survival was the main driver of the observed reproduction increase in this long-lived bat species with low per capita annual reproductive output. The higher average growth rate in the expanding population was not associated with a trade-off between increased reproduction and survival, suggesting that the observed increase in reproduction stems from a higher resource acquisition in the expanding population. Environmental conditions in the novel habitat hence seem to have an important influence on range expansion dynamics, and warrant further investigation for the management of range expansion in both native and invasive species.
Keywords: capture–mark–recapture, life history, Rhinolophus hipposideros, range expansion, trade-off
1. Introduction
In the light of current global change, understanding range expansion mechanisms is an increasingly important challenge for ecology and conservation. A growing number of species are expanding their distribution range, both invasive species rapidly spreading into new areas and native species following their shifting climatic envelope [1,2]. Range expansion depends on the colonization and long-term settlement in new habitat patches and thus, on dispersal and reproduction [3]. Changes in life-history traits as a response to the environmental conditions encountered at the expansion front can promote range expansion if they lead to increased dispersal and reproduction [4]. The evolution of life-history traits such as increased dispersal by spatial sorting and higher reproduction due to relaxed density dependence [5–8] can considerably speed up spread [9] and play a crucial role for the long-term persistence of species following suitable climatic conditions in space [5–7].
While evolutionary theory predicts increased dispersal and reproduction rates in expanding populations, the reality is more complex: density dependence, resource limitation and resulting trade-offs, density-independent environmental fluctuations, as well as changes in interspecific interactions present potentially important and interacting components of selection shaping changes in life-history traits [5,8,10]. Empirical results are accordingly heterogeneous: range expansion studies document increases in reproduction or dispersal traits, but decreases in others, or no change at all (see [11] for an extensive review). In addition to inherent complexities of each study system, there is great variation between studies in the choice of the investigated trait(s), which inevitably influences the detection (or the absence thereof) of life-history changes [11].
The impact of range expansion is extremely challenging to quantify for all relevant life-history traits [8]. Ultimately, however, range expansion depends on dispersal and a positive local population growth rate [12,13] (i.e. reproduction plus immigration exceeding mortality plus emigration). Survival rates, therefore, play a crucial role for range expansion success [14]. Nevertheless, very few range expansion studies have investigated adult survival or related traits (e.g. [15–17]). Differences between edge and core populations in juvenile survival are, to our knowledge, not investigated at all, with empirical studies focusing rather on fecundity, even though net reproduction depends on both fecundity and juvenile survival. Neglecting adult and juvenile survival may bias predictions of range expansion dynamics because population growth is implicitly overestimated [15,18]. Changes in demographic parameters (adult and juvenile survival, fecundity) and resulting population dynamics (e.g. growth rates) therefore present a valuable source of information that allows a more realistic evaluation of range expansion speed and success.
Understanding and predicting critical ecological processes like range expansion requires the investigation of how demographic statistics vary among populations across spatial scales [19]. The deficiency of studies quantifying demographic parameters in the context of range expansion may stem from challenges associated with estimating adult and juvenile survival rates in wild populations. Here, we estimate those demographic parameters through capture–mark–recapture (CMR) data collected non-invasively over multiple years and multiple sites. Information from genetic parentage assignment is included to estimate fecundity. We finally combine the CMR dataset with population size estimates in an integrated population model (IPM) framework. IPMs are powerful modelling tools that allow to estimate population dynamic parameters by combining information obtained from different datasets, enhancing the reliability of estimations [20,21].
Previous empirical range expansion studies have mainly focused on species with fast life histories (i.e. the classical r-strategists) [11,22,23]. Climate change-induced range shift, however, also concerns species on the other extreme of the pace-of-life continuum [24]. For species with slow life histories (i.e. the classical K-strategists), it is less clear whether the predicted increase in reproduction can be achieved, because evolutionarily constraints can limit the number of offspring per reproduction event or age at maturity, for example [25]. We apply the developed CMR/IPM approach to Rhinolophus hipposideros (lesser horseshoe bat), a bat species for which range expansion is expected to play a central role in coping with climate change [26]. Even for bats of the temperate zone, Rhinolophus hipposideros has a slow life strategy [27] as it is relatively long-lived (maximum reported age: 29.4 years [28]) and uniparous [29,30].
We employ a comparative approach, contrasting estimated demographic parameters and resulting population dynamics of an expanding and a non-expanding Rhinolophus hipposideros population (equivalent to edge and core populations, respectively) to investigate if the predicted increase in reproduction in expanding populations can also be observed in this long-lived species where the individual number of offspring per reproduction event and the number of reproduction events per year are fixed. Relaxed density dependence is a potential driver which may lead to demographic responses in expanding populations, but directly testing density dependence is extremely challenging and requires long-term longitudinal data [31,32]. As such data are not available for our studied populations, we focus here on testing the hypothesis that reproduction is higher in an expanding population of a long-lived species. Furthermore, we elucidate which of fecundity or juvenile survival contributes most to this increase, and assess potential trade-offs between reproduction and survival.
2. Methods
(a). Data collection
(i). Study site and sampling protocol
We studied two Rhinolophus hipposideros populations. ‘Population’ here refers to a set of maternity colonies within a confined geographical region where individual movements between colonies are possible. The German population (Thuringia, 19 colonies sampled) is currently growing and expanding into previously occupied areas after a severe range contraction in the second half of the twentieth century [33], and hence referred to as the ‘expanding population’. The French population (Picardy, 16 colonies sampled), which has displayed a stable demographic trend and no expansion during the last decades (personal communication, Office National des Forêts, Compiègne, France), is termed the ‘non-expanding population’. The two are treated as distinct populations as they are demographically independent from each other.
In R. hipposideros, females aggregate in the so-called maternity colonies during spring and summer to give birth and raise their single offspring [29,30]. Males can be present in these maternity colonies to a varying extent [34], but we only consider females for the investigation of population dynamics.
Bat droppings, as a source of bat DNA, were collected from each of the 35 colonies during two successive sampling sessions per year. We sampled once before parturition when only adults were present in the colony and a second time when juveniles were weaned and flying, following the protocol described by Zarzoso-Lacoste et al. [34]. The pre-birth sampling session was performed in early to mid-June in France and around the end of June/in early July in Germany to match the respective parturition dates, which slightly differ between the geographical regions. The post-birth session accordingly took place in the beginning to middle of July in France, and in mid- to late August in Germany.
The number of adult and juvenile Rhinolophus hipposideros individuals in the sampled roosts was obtained by visual counts. Adults were counted during each sampling session, and juveniles were counted in mid-July, with a few exceptions where juvenile counts were not possible. More detailed information about visual counts is provided in the electronic supplementary material, file S1: Visual Counts. Sampling was performed from 2013 to 2016 in France, and from 2015 to 2017 in Germany. In the first sampling year (2013 in France, 2015 in Germany), the number of samples analysed per colony was twice the colony size (i.e. twice the maximum number of adults registered during visual counts in the respective year). For economic reasons, the number of samples analysed had to be reduced to the equivalent of the respective colony size for the pre-birth sampling session in Germany and for both sampling sessions in France in later sampling years.
To investigate whether our model (see ‘Integrated population model’) was able to reliably estimate demographic parameters despite slight differences between the sampling regimes and visual counts of juveniles in France and Germany, we simulated populations subjected to either sampling regime, whose demographic parameters were estimated in addition to those of the empirical dataset. More details are provided in the electronic supplementary material, file S2: Simulations.
(ii). Capture–recapture data and colony size estimation
Individual capture–recapture data were obtained by extracting DNA from bat droppings and genotyping them. The complete procedure is described by Zarzoso-Lacoste et al. [34]. The resulting CMR dataset encompassed 3480 individuals (distinct genotypes with identified alleles on at least seven of the nine loci) in the French colonies, 2437 of which were females, and 3826 individuals (2171 females) in the German dataset. Those datasets include both adults and juveniles. Only females were considered in the subsequent analyses, because males are not suitable to study demographic parameters in R. hipposideros due to their low roost fidelity and capture probability [34].
As females cannot be distinguished from males in visual counts and because the proportion of males present in maternity roosts can be highly variable between colonies [34], the number of adult females was estimated using a Bayesian estimator for single session CMR data [35,36]. This estimator was used for samples from the pre-birth session only, which is less prone to capture heterogeneity than the post-birth sampling session [34]. Given the low detection probability of juveniles [34] and the state uncertainty concerning samples from post-birth sessions (see below), we estimated the number of female juveniles by dividing the number of visually counted juveniles by two, because the average birth sex-ratio is balanced in R. hipposideros [29].
(b). Integrated population model
We developed an IPM for the estimation of demographic parameters in R. hipposideros from CMR data, parentage assignment analysis and abundance data. To assess its reliability, we progressively constructed three models: a multi-event model based on CMR data only (CMRo model), a multi-event model including information from parentage assignment (CMRpa model) and the IPM presented here, which includes a state–space model implementing information on the number of adult and juvenile females. We then ran all three models on the simulated datasets and assessed their respective reliability by computing the relative bias, the precision (standard error, s.e.) and the accuracy (mean squared error, MSE), following Abadi et al. [37]. We then applied the best-performing model to the empirical dataset (IPM, see Results and electronic supplementary material, table S1). Details of the CMRo and CMRpa models and the results of the performance test are presented in the electronic supplementary material.
The multi-events part of the IPM uses CMR and parentage assignment data. Maternity assignment was carried out independently for each year using the software Colony 2 with a full-pedigree likelihood method [38], with data processing as described by Zarzoso-Lacoste et al. [34]. All individuals initially sampled during the pre-birth session were considered as adults. All adults were given a single detection probability (pA). In the post-birth session, we distinguished mothers (i.e. adults that gave birth to a female juvenile), non-mothers (i.e. other adults) or juveniles. Only bats that were sampled for the first time in a post-birth session were considered as potential juveniles in the model and for parentage assignment.
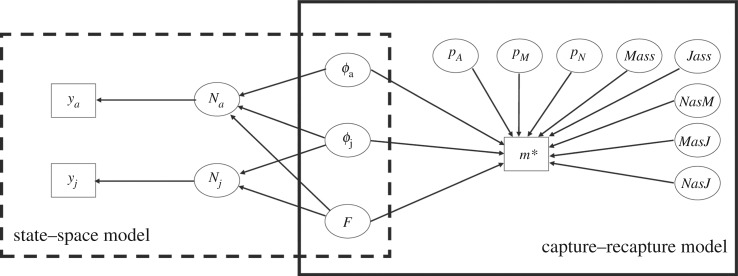
As the true number of juveniles among the individuals seen for the first time in a post-birth sampling session was unknown, and because parentage assignment may fail to detect all mother–juvenile pairs, we introduced state uncertainty [39] by estimating the proportions of mothers and juveniles that were correctly assigned (Mass and Jass) and the proportion of individuals wrongly assigned as mother or juvenile (NasM, MasJ and NasJ, figure 1). The detection probability of mothers (pM) was set to be higher than that of non-mothers (pN) due to their higher roost fidelity [34]. Juveniles can only be detected at one sampling event as juvenile, because they will already be considered as adult at the next sampling event (the year following their birth). Therefore, juvenile detection probability cannot be quantified per se.
Figure 1.
Acyclic graph illustrating the components of model likelihood in the IPM. ϕj = apparent juvenile survival, ϕa = adult survival, pA = adult detection probability, F = fecundity, m* = capture–recapture data including parentage assignment information, pM = mother detection probability, pN = non-mother detection probability, Mass = proportion of mothers correctly assigned, Jass = proportion of juveniles correctly assigned, NasM = proportion of non-mothers wrongly assigned as a mother, MasJ = proportion of mothers wrongly assigned as juvenile, NasJ = proportion of non-mothers wrongly assigned as a juvenile. ya = number of females estimated from single session CMR estimator, yj = number of juveniles estimated from visual counts, Na = adult colony size, Nj = juvenile colony size.
State transition from the post-birth session to the pre-birth session allowed us to estimate local adult and juvenile survival rates (ϕa and ϕj, respectively), i.e. the probability to survive to the next year and to remain in the same colony. Survival greatly differs between adult and juvenile bats [40–42] and was therefore estimated separately for the two categories. The transition from pre-birth to post-birth states allows the estimation of fecundity (F). Fecundity is here defined as the proportion of females giving birth to a juvenile (only female juveniles considered). As the annual number of offspring per female in R. hipposideros is either zero or one, this is equivalent to the number of female juveniles per adult female in the colony per year. More details about this part of the model can be found in the electronic supplementary materials (CMRpa model), and the likelihood of this model is LCMRpa = (m|ϕa, ϕj, F, pA, pM, pN, Mass, Jass, NasM, MasJ, NasJ).
In addition to the individual CMR data, the IPM uses colony size estimates of adult females (Bayesian estimator for single session CMR) and juvenile females (visual counts). Population dynamic parameters were linked to colony size in a state–space model with the following Poisson distributions to account for demographic stochasticity:
and
As colony sizes can be challenging to estimate from visual counts, and because different surveyors performed the visual counts in our study, we considered counting error independently for each sampling session and colony in the model, assuming a greater observation error when the colony is larger [43]. Estimated colony size (yx,t, where x represents either adults (a) or juveniles (j) and t corresponds to the year) was assumed to be related to actual colony size (Nx,t) with a Poisson distribution as . The likelihood of the state–space model is LSS = (y|N, ϕa, ϕj, F), which makes the joint likelihood used in the IPM (figure 1): LIPM = (y, m|N, ϕa, ϕj, F, pA, pM, pN, Mass, Jass, NasM, MasJ, NasJ). A JAGS script of this model is provided in the electronic supplementary material.
We did not consider emigration or immigration in our simulations and models, assuming no dispersal between colonies, because it would result in more than 60 additional states which need to be considered in the multi-event models, requiring immense computational power which was not at our disposal. Furthermore, R. hipposideros females are reported to be highly philopatric [44–46], which was confirmed in our study: only 0.37% of females of the non-expanding population were detected in different colonies (i.e. had dispersed) during the 4 years of sampling (less than 0.1% detected dispersers per year), and only 0.57% in the expanding population during 3 years of sampling (less than 0.2% per year). We also assumed in the models that demographic parameters were constant over years to focus on differences between colonies rather than on temporal variations of these parameters.
(c). Model computation
We calculated the posterior distribution of the demographic parameters (ϕa, ϕj and F) for all three models with Markov chain Monte Carlo computations implemented in the program JAGS [47]. JAGS was executed from R version 3.3.3 [48] with the package jagsUI [49]. We ran 1 200 000 iterations and discarded the first 1 100 000 iterations as a burn-in, with a thinning interval of two. Convergence of the models was checked with the potential scale reduction factor [50].
(d). Statistical analyses
All statistical analyses were performed in R version 3.3.3 [48]. We first tested for differences in demographic parameters (adult and juvenile survival, fecundity, net reproduction [fecundity × juvenile survival], and colony growth rate [adult survival + net reproduction]) between the expanding and the non-expanding population. For this purpose, we used linear models with the demographic parameter of interest as a response variable and the population of origin (expanding or non-expanding) as an explanatory variable. We used a weighted least-square model to take into consideration the uncertainty in parameter estimates (standard error) provided by the IPM (electronic supplementary material, table S2). To test the significance of the influence of the population of origin, we performed an analysis of variance (ANOVA) on the respective model. We repeated these analyses with jackknifing colonies (one at a time) to assess if outliers were influencing the overall outcome. ANOVA p-values were re-calculated for all jackknifed datasets.
Correlations between demographic parameters among colonies were assessed for both populations with a Spearman correlation test. We additionally used a Bartlett test to examine if the variance between colonies in the estimated demographic parameters differed between the expanding and the non-expanding population.
3. Results
For the estimation of adult survival and fecundity from simulated data, the IPM provided accurate (relative bias < 0.02) estimates of high precision, irrespectively of the sampling regime (s.e. < 0.03; MSE < 0.001; electronic supplementary material, table S1 and figure S7). Juvenile survival was systematically overestimated (relative bias < 0.12) and also less precise (s.e. < 0.13), resulting in an overall higher MSE of up to 0.017 (electronic supplementary material, table S1 and figure S7). The overestimation of juvenile survival was consistent between the two simulated datasets representing the two populations of the empirical dataset (electronic supplementary material, table S1 and figure S7). Compared with the simpler models using only part of the information, the performance of the IPM was clearly superior (electronic supplementary material, table S1 and figure S7).
For the empirical datasets, the resulting estimates provided by the IPM revealed that all demographic parameters were significantly increased in the expanding compared to the non-expanding population (table 1). The largest difference was observed in juvenile survival, followed by fecundity (table 1). Average net reproduction and average growth rate of the expanding population concordantly exceeded those of the non-expanding one (table 1).
Table 1.
Demographic parameters (fecundity, adult and juvenile survival) estimated via the IPM for an expanding and a non-expanding population of R. hipposideros and corresponding standard errors. Average values of the calculated net reproduction rates (fecundity × juvenile survival) and growth rates (adult survival + net reproduction) for both populations. An ANOVA on weighted least-square models was used to test for significant differences in these parameters between populations. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
| parameter | expanding | non-expanding | significance |
|---|---|---|---|
| fecundity | 0.42 ± 0.03 | 0.33 ± 0.05 | ** |
| adult survival | 0.80 ± 0.02 | 0.74 ± 0.02 | * |
| juvenile survival | 0.56 ± 0.02 | 0.35 ± 0.03 | *** |
| net reproduction rate | 0.23 ± 0.02 | 0.11 ± 0.02 | *** |
| growth rate | 1.03 ± 0.02 | 0.85 ± 0.02 | *** |
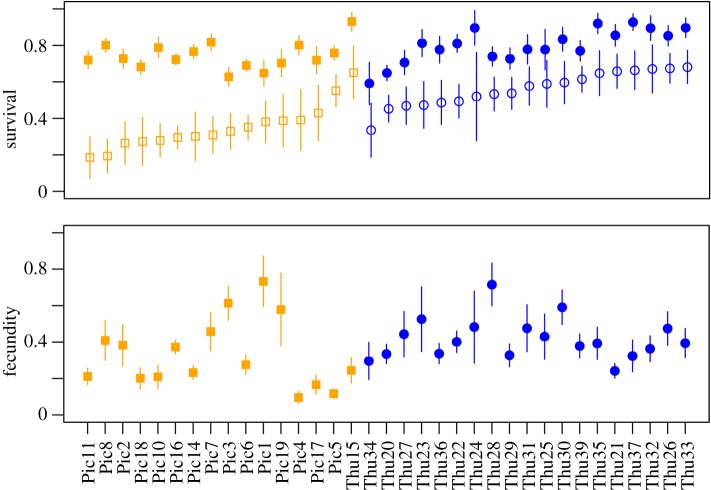
Adult survival differed only marginally between the two populations (figure 2 and table 1). When jackknifing, the difference became non-significant when removing from the dataset any of the three colonies with the highest adult survival from the expanding population (Thu33, Thu35 and Thu37) or one specific colony with relatively low adult survival from the non-expanding population (Pic6; figure 2). Jackknifing did not change the results for any of the other investigated demographic parameters.
Figure 2.
Survival rates of adults (filled symbols) and juveniles (empty symbols) and fecundity inferred with the IPM (sorted by juvenile survival estimates in ascending order within each population). Squares, non-expanding population; circles, expanding population. Lines represent the standard error. (Online version in colour.)
The variance of fecundity was significantly higher in the non-expanding population (0.034) than in the expanding one (0.012; p = 0.04; figure 2). Adult and juvenile survival were positively correlated in the expanding population (Spearman's rS = 0.74; p < 0.005), but not in the non-expanding one. No significant correlation was detected between other life-history traits in either population.
4. Discussion
(a). IPM performance and demographic estimates
The general superiority of the IPM compared with simpler models indicates that including population size estimates of adults (via CMR) and juveniles (via visual counts) greatly improves the estimation of demographic parameters, stressing the importance of monitoring data. Overall, the demographic parameters estimated by the IPM are in concordance with values of adult survival, fecundity and juvenile survival reported for temperate zone bat species [40–42,46,51], even though testing the performance of the IPM with simulated data indicates a positive bias in estimated juvenile survival. Other studies dealing with adult survival in Rhinolophus hipposideros reported lower survival rates than those estimated here [52,53]. However, the authors of these previous studies concede that survival rates may have been lowered due to the invasiveness of banding, the marking method used in their studies. The fecundity values estimated in our study were in the range of values previously reported for R. hipposideros [54,55].
The overestimation of juvenile survival in the simulated dataset may stem from adult individuals first sampled in a post-birth sampling session and thus considered as potential juveniles. Juvenile survival can be overestimated in our study system when adults sampled late (i.e. detected for the first time in the second sampling session) are wrongly considered as a juvenile and assigned to a mother, because their mother or another closely related female is present in the colony. As average survival is higher in adults than in juveniles, such cases would result in an overestimation of juvenile survival. Another explanation might be a particularly high mortality of juveniles in the first weeks after birth [33] (i.e. before the post-birth sampling session). Juveniles who are thus never sampled would artificially decrease the estimated fecundity because their mother would appear to be a non-mother. Moreover, they would increase the estimated juvenile survival as juveniles which die before being sampled are not considered in the estimation of juvenile survival.
It is important to note that a downward bias in the survival rates estimated for the empirical dataset may potentially be introduced because emigration and immigration are currently not implemented in our model. Therefore, emigrating bats are considered dead, whereas immigrating ones are registered as previously unsampled individuals. Overall, we expect the resulting bias to be negligible for population dynamics, because of the high philopatry of R. hipposideros females as discussed in the Methods section. While being rare, proportionally more dispersal events were detected in the expanding population (details are provided in the last paragraph of the ‘Integrated population model’ section), so survival rates are more prone to be underestimated in the expanding population. Our results are thus conservative, since the adult survival rate and average growth rate of the expanding population could exceed those of the non-expanding one to an even higher degree.
(b). Higher reproduction in the expanding population
The higher average net reproduction and average growth rate observed in the expanding population (figure 2 and table 1) are in concordance with the population dynamics expected under range expansion. Notably, R. hipposideros females cannot bear more than one juvenile per year, and there is very limited variation in the age at first parturition [29]. Investigating demographic parameters demonstrates that proportionally more birth events, and hence higher reproduction rates, can be detected in the expanding population. A major part of the observed increase in reproduction however originates from higher juvenile survival. In previous empirical studies, range expansion induced changes in reproduction were mainly associated with altered clutch or litter size and age at sexual maturity [11,22,56–58]. Our findings demonstrate that net reproduction rate can increase during range expansion also in a species where the annual number of offspring per female cannot be increased above one and where the minimum age at sexual maturity can be reduced only marginally [27,59]. The great contribution of juvenile survival to the observed increase in net reproduction suggests that the latter may be achieved via different mechanisms in long-lived, uniparous species and in short-lived, multiparous species, with juvenile survival potentially playing a more important role in species of limited annual reproductive output.
(c). Searching for trade-offs
No trade-off with increased reproduction was detected in the expanding population, as it had a higher average growth rate and an adult survival rate which at least equalled, or even exceeded that of the non-expanding population. The theory of life-history evolution under range expansion postulates that increased reproduction and dispersal entail negative fitness consequences for other traits, but empirical studies frequently fail to detect the expected trade-offs [11]. This can be the case if the affected trait is not investigated, for example, or if it is not under strong selection pressure and the resulting fitness loss is low [11]. Trade-offs diminishing competitive ability present a typical case: they are negligible during colonization due to low population density, but selection pressure on this trait increases with rising population density in the post-colonization phase, mediating the attenuation of dispersal and/or reproduction [5,60].
Importantly, trade-offs can also be masked when variation in resource acquisition dominates over the variation in resource allocation [61]. Studies on female performance (albeit not in a range expansion context) often find no detectable trade-offs with reproduction, but strong variation between individuals, which is attributed to individual differences in resource acquisition [62,63]. Our study suggests that this phenomenon can also occur at a higher level (here, at the colony level): the expanding population displays a greater average growth rate, suggesting higher resource acquisition. This is consistent with the detected positive correlation between adult and juvenile survival for the expanding population. While a negative correlation between traits indicates a trade-off, a positive correlation is generally indicative of a strong influence of resource acquisition [61]. Conclusively, the higher average juvenile survival and fecundity observed in the expanding population could be a manifestation of individuals acquiring more resources there.
(d). Differing selection pressures for fecundity and adult survival
Variation in resource acquisition within the non-expanding population (in addition to variation in resource acquisition between populations) might further explain the high variance in fecundity observed in this population. High variance in fecundity, and thus reproduction, but not in adult survival under suboptimal conditions (i.e. populations at or close to carrying capacity), is in concordance with the life-history strategy of R. hipposideros. In long-lived, iteroparous species, lifetime reproductive success is critically determined by lifespan [64] and resource limitation or environmental variation manifest in lowered reproduction or juvenile survival rather than adult survival [61,62,65]. In bats, longevity is particularly important for lifetime reproduction success, because their reproductive potential diminishes only marginally with age [51].
A strong selection pressure on adult survival in our study species is also supported by the comparison of this parameter between the expanding and the non-expanding population. In contrast to the reproduction parameters, adult survival was only marginally higher in the expanding population, and the difference became non-significant when removing any of the three colonies with the highest adult survival rate from the dataset of the expanding population. This finding indicates that the observed significant difference in adult survival between the two populations is strongly influenced by a few single colonies in our dataset. Moreover, weighting by the standard error may result in a very strong influence of values close to the parameter interval limits, and the variance of estimates will be lower for colonies with higher adult survival because they are closer to the maximum value of one [65]. A marginal or even non-significant increase in adult survival despite assumed higher resource acquisition in the expanding population is therefore in concordance with the strong selection pressure on adult survival in long-lived, iteroparous species. For them, adult survival will already be close to the possible maximum, with little potential for further increase.
(e). Potential mechanisms behind demographic differences
The detected support for a strong influence of resource acquisition and the role played by juvenile survival and fecundity are consistent with the expanding population's higher average reproduction rate being a demographic response to relaxed density dependence [66]. Relaxed density dependence at the expansion edge represents exactly the conditions under which most theoretical [5,67] and experimental [68,69] models of range expansions are built, but for which empirical examples are lacking. Such conditions favour increased dispersal and reproduction [5,8], and can lead to highly variable invasion speed [67–69].
Though density dependence per se is notoriously difficult to disentangle from density-independent processes in driving demographic rates [31,32], both kinds of factors are likely to come into play in most empirical situations [70] (see [71] for an example). Density-independent factors include climatic conditions and the availability and quality of resources such as roosts and foraging areas. Weather conditions during spring transition, which may differ between the two populations, have been demonstrated to be highly relevant for reproduction success in R. hipposideros [72] and two other bat species [73,74]. The availability of high-quality roosts may also be better for the expanding population due to an extensive conservation programme for roost amelioration and protection which was implemented there a decade ago [44].
5. Conclusion
Taken together, the present study highlights that range expansion is associated with increased reproduction in R. hipposideros, a long-lived, uniparous species, and that this increase can be achieved by a combination of proportionally more females giving birth and higher juvenile survival. The latter had a particularly pronounced impact, indicating that in species where individual annual reproductive output cannot be increased, juvenile survival rather than the number of offspring may be the critical factor to achieve a higher annual reproduction rate.
Our results further suggest that the observed differences between the expanding and the non-expanding populations are due to resource acquisition varying between populations, but also between colonies of the non-expanding population. The fact that we did not detect a trade-off between survival and increased reproduction, as well as the positive correlation between adult and juvenile survival in the expanding population, indicate that these differences in resource acquisition dominate over resource allocation effects in shaping the observed population dynamics. The characteristics of the novel habitat being colonized may thus have a strong impact on resource acquisition, potentially influencing range expansion dynamics [5,9,13,67].
More studies are needed to identify key environmental factors determining net per capita resource availability, which could be used in species distribution models to assess the overall suitability of the area which is expected to be colonized during the range expansion process. Combining such species distribution models with the investigation of changes in population dynamics and variation in life-history traits could provide a tool to predict range expansion speed and success more accurately, benefitting the development of according management strategies [19,68,75]. Overall, range expansion success may be difficult to predict due to a potentially larger than expected influence of stochastic events [76]. Studies investigating variation in demographic parameters and the resulting population dynamics across sufficiently large numbers of demes and spatial scales are crucial to understand complex ecological phenomena such as range shifts and thus, for the development of monitoring programmes and conservation strategies [19].
Supplementary Material
Acknowledgements
We thank the Interessengemeinschaft Fledermausschutz und -forschung Thüringen IFT e.V. and the Office National des Forêts of Picardie and the Conservatoire d'espaces naturels of Picardie, and their staff and volunteer members who provided logistic support in the field. We thank Ben Phillips and two anonymous reviewers for comments that substantially improved the quality of the paper.
Ethics
No permit was required to access roosts in France. Sampling in Germany was approved by local authorities (permit number Jena AV09_AGO7_17).
Data accessibility
The capture history, multilocus genotypes and mother–daughter pairs detected are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.66bp77v [77]. All other data are in electronic supplementary material.
Authors' contributions
E.J.P. and S.J.P. designed the study; G.K., E.J.P., P.L.G. and S.J.P. supervised the project; G.K., E.J.P. and S.J.P. acquired funding; L.L. and M.B. did fieldwork with input from W.S.; A.-L.B., L.L. and P.-L.J. carried out the laboratory analyses; L.L. and P.-L.J. performed data analyses; P.-L.J. and P.L.G. developed the models and simulated data; L.L., E.J.P., P.L.G., P.-L.J. and S.J.P. interpreted the results; L.L. and P.-L.J. wrote the original manuscript. All authors critically discussed and edited the manuscript and approved its final version.
Competing interests
We declare we have no competing interests.
Funding
L.L. was funded by a PhD position in the framework of the RTG 2010 Research Training Program (Deutsche Forschungsgemeinschaft, German Science Foundation DFG; http://www.dfg.de; grant awarded to S.-J.P. and G.K.). P.-L.J. and all the work conducted on the French population benefited from funding by the Office National des Forêts (ONF, granted to E.J.P.). Funding by the PROCOPE/DAAD (project no. 57211773) and PROCOPE/PHC (project no. 35454SB) programmes (awarded to S.-J.P. and E.J.P.) and by the DFG (RESPONSE exchange grant, awarded to P.-L.J.) allowed us to collaborate and jointly work on the project.
References
- 1.Travis JMJ, Dytham C. 2002. Dispersal evolution during invasions. Evol. Ecol. Res. 4, 1119–1129. [Google Scholar]
- 2.Lenoir J, Svenning JC. 2014. Climate-related range shifts—a global multidimensional synthesis and new research directions. Ecography 38, 15–28. ( 10.1111/ecog.00967) [DOI] [Google Scholar]
- 3.Chaine AS, Clobert J. 2012. Dispersal. In Behavioral responses to a changing world (eds Candolin U, Wong BBM), pp. 64–79. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Hassel K, Pedersen B, Söderström L. 2005. Changes in life-history traits in an expanding moss species: phenotypic plasticity or genetic differentiation? A reciprocal transplantation experiment with Pogonatum dentatum. Ecography 28, 71–80. ( 10.1111/j.0906-7590.2005.03910.x) [DOI] [Google Scholar]
- 5.Burton OJ, Phillips BL, Travis JMJ. 2010. Trade-offs and the evolution of life-histories during range expansion. Ecol. Lett. 13, 1210–1220. ( 10.1111/j.1461-0248.2010.01505.x) [DOI] [PubMed] [Google Scholar]
- 6.Kubisch A, Holt RD, Poethke H, Fronhofer EA. 2014. Where am I and why? Synthesizing range biology and the eco-evolutionary dynamics of dispersal. Oikos 123, 5–22. ( 10.1111/j.1600-0706.2013.00706.x) [DOI] [Google Scholar]
- 7.Colautti RI, Alexander JM, Dlugosch KM, Keller SR, Sultan SE. 2017. Invasions and extinctions through the looking glass of evolutionary ecology. Phil. Trans. R. Soc. B 372, 20160031 ( 10.1098/rstb.2016.0031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips BL, Brown GP, Shine R. 2010. Life-history evolution in range-shifting populations. Ecology 91, 1617–1627. ( 10.1890/09-0910.1) [DOI] [PubMed] [Google Scholar]
- 9.Phillips BL, Brown GP, Shine R. 2010. Evolutionarily accelerated invasions: the rate of dispersal evolves upwards during the range advance of cane toads. J. Evol. Biol. 23, 2595–2601. ( 10.1111/j.1420-9101.2010.02118.x) [DOI] [PubMed] [Google Scholar]
- 10.Reznick D, Bryant MJ, Bashey F. 2002. r- and K-selection revisited: the role of population regulation in life-history evolultion. Ecology 83, 1509–1520. ( 10.1890/0012-9658(2002)083[1509:RAKSRT]2.0.CO;2) [DOI] [Google Scholar]
- 11.Chuang A, Peterson CR. 2016. Expanding population edges: theories, traits, and trade-offs. Glob. Change Biol. 22, 494–512. ( 10.1111/gcb.13107) [DOI] [PubMed] [Google Scholar]
- 12.Phillips BL. 2009. The evolution of growth rates on an expanding range edge. Biol. Lett. 5, 802 ( 10.1098/rsbl.2009.0367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iles DT, Salguero-Gómez R, Adler PB, Koons DN. 2015. Linking transient dynamics and life history to biological invasion success. J. Ecol. 104, 399–408. ( 10.1111/1365-2745.12516) [DOI] [Google Scholar]
- 14.Capellini I, Baker J, Allen WL, Street SE, Venditti C. 2015. The role of life history traits in mammalian invasion success. Ecol. Lett. 18, 1099–1107. ( 10.1111/ele.12493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanski I, Saastamoinen N, Ovaskainen O. 2005. Dispersal-related life-history trade-offs in a butterfly metapopulation. J. Anim. Ecol. 75, 91–100. ( 10.1111/j.1365-2656.2005.01024.x) [DOI] [PubMed] [Google Scholar]
- 16.Amundsen P-A, Salonen E, Niva T, Gjelland KØ, Præbel K, Sandlund OT, Knudsen R, Bøhn T. 2012. Invader population speeds up life history during colonization. Biol. Invasions 14, 1501–1513. ( 10.1007/s10530-012-0175-3) [DOI] [Google Scholar]
- 17.Kilkenny FF, Galloway LF. 2012. Adaptive divergence at the margin of an invaded range. Evolution 67, 722–731. ( 10.1111/j.1558-5646.2012.01829.x) [DOI] [PubMed] [Google Scholar]
- 18.Clark JS, Lewis M, Horvath L. 2001. Invasion by extremes: population spread with variation in dispersal and reproduction. Am. Nat. 157, 537–554. ( 10.1086/319934) [DOI] [PubMed] [Google Scholar]
- 19.Gurevitch J, Fox GA, Fowler NL, Graham CH. 2016. Landscape demography: population change and its drivers across spatial scales. Q. Rev. Biol. 91, 459–485. ( 10.1086/689560) [DOI] [PubMed] [Google Scholar]
- 20.Schaub M, Gimenez O, Sierro A, Arlettaz R. 2007. Use of integrated modeling to enhance estimates of population dynamics obtained from limited data. Conserv. Biol. 21, 945–955. ( 10.1111/j.1523-1739.2007.00743.x) [DOI] [PubMed] [Google Scholar]
- 21.Schaub M, Abadi F. 2011. Integrated population models: a novel analysis framework for deeper insights into population dynamics. J. Ornithol. 152, 227–237. ( 10.1007/s10336-010-0632-7) [DOI] [Google Scholar]
- 22.Jaspers C, Marty L, Kiørboe T. 2017. Selection for life-history traits to maximize population growth in an invasive marine species. Glob. Change Biol. 24, 1164–1174. ( 10.1111/gcb.13955) [DOI] [PubMed] [Google Scholar]
- 23.Reim E, Blesinger S, Förster L, Fischer K. 2018. Successful despite poor flight performance: range expansion is associated with enhanced exploratory behaviour and fast development. J. Evol. Biol. 31, 1165–1179. ( 10.1111/jeb.13294) [DOI] [PubMed] [Google Scholar]
- 24.Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio P-O. 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil. Trans. R. Soc. B 365, 4051–4063. ( 10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pianka ER. 1972. r and K selection or b and d selection? Am. Nat. 106, 581–588. ( 10.1086/282798) [DOI] [Google Scholar]
- 26.Rebelo H, Tarroso P, Jones G. 2010. Predicted impact of climate change on European bats in relation to their biogeographic patterns. Glob. Change Biol. 16, 561–576. ( 10.1111/j.1365-2486.2009.02021.x) [DOI] [Google Scholar]
- 27.Gaisler J. 1989. The r-K selection model and life-history strategies in bats. In European bat research 1987 (eds Hanák V, Horáček I, Gaisler J), pp. 117–124. Prague, Czech Republic: Charles University Press. [Google Scholar]
- 28.Gaisler J, Hanák V, Hanzal V, Jarsky V. 2003. Results of bat banding in the Czech and Slovak Republics, 1948–2000. Vespertilio 7, 3–61. [Google Scholar]
- 29.Gaisler J. 1966. Reproduction in the lesser horseshoe bat (Rhinolophus hipposideros hipposideros Bechstein, 1800). Bijdr. Tot Dierkd. 36, 45–64. ( 10.1163/26660644-03601003) [DOI] [Google Scholar]
- 30.Gaisler J. 1965. The female sexual cycle and reproduction in the lesser horseshoe bat (Rhinolophus hipposideros hipposideros Bechstein, 1800). Vest. Cls. Spol. Zool. 29, 336–352. [Google Scholar]
- 31.Hassell MP, Latto J, May RM. 1989. Seeing the wood for the trees: detecting density dependence from existing life-table studies. J. Anim. Ecol. 58, 883–892. ( 10.2307/5130) [DOI] [Google Scholar]
- 32.Lebreton J-D, Gimenez O. 2013. Detecting and estimating density dependence in wildlife populations. J. Wildl. Manag. 77, 12–23. ( 10.1002/jwmg.425) [DOI] [Google Scholar]
- 33.Tress J, Biedermann M, Geiger H, Prüger J, Schorcht W, Tress C, Welsch K-P. 2012. Fledermäuse in Thüringen, 2nd edn Jena, Germany: Naturschutzreport 27. [Google Scholar]
- 34.Zarzoso-Lacoste D, Jan P, Lehnen L, Girard T, Besnard A, Puechmaille SJ, Petit EJ. 2018. Combining noninvasive genetics and a new mammalian sex-linked marker provides new tools to investigate population size, structure and individual behaviour: an application to bats. Mol. Ecol. Resour. 18, 217–228. ( 10.1111/1755-0998.12727) [DOI] [PubMed] [Google Scholar]
- 35.Petit E, Valière N. 2006. Estimating population size with noninvasive capture-mark-recapture data. Conserv. Biol. 20, 1062–1073. ( 10.1111/j.1523-1739.2006.00417.x) [DOI] [PubMed] [Google Scholar]
- 36.Puechmaille SJ, Petit EJ. 2007. Empirical evaluation of non-invasive capture–mark–recapture estimation of population size based on a single sampling session. J. Appl. Ecol. 44, 843–852. ( 10.1111/j.1365-2664.2007.01321.x) [DOI] [Google Scholar]
- 37.Abadi F, Botha A, Altwegg R. 2013. Revisiting the effect of capture heterogeneity on survival estimates in capture-mark-recapture studies: does it matter? PLoS ONE 8, e62636 ( 10.1371/journal.pone.0062636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones OR, Wang J. 2010. COLONY: a program for parentage and sibship inference from multilocus genotype data. Mol. Ecol. Resour. 10, 551–555. ( 10.1111/j.1755-0998.2009.02787.x) [DOI] [PubMed] [Google Scholar]
- 39.Pradel R, Choquet R, Lima MA, Merritt J, Crespin L. 2010. Estimating population growth rate from capture—recapture data in presence of capture heterogeneity. J. Agric. Biol. Environ. Stat. 15, 248–258. ( 10.1007/s13253-009-0008-8) [DOI] [Google Scholar]
- 40.Rossiter SJ, Jones G, Ransome RD, Barratt EM. 2001. Outbreeding increases offspring survival in wild greater horseshoe bats (Rhinolophus ferrumequinum). Proc. R. Soc. Lond. B 268, 1055–1061. ( 10.1098/rspb.2001.1612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sendor T, Simon M. 2003. Population dynamics of the pipistrelle bat: effects of sex, age and winter weather on seasonal survival. J. Anim. Ecol. 72, 308–320. ( 10.1046/j.1365-2656.2003.00702.x) [DOI] [Google Scholar]
- 42.Humphrey SR, Oli MK. 2015. Population dynamics and site fidelity of the cave bat, Myotis velifer, in Oklahoma. J. Mammal. 96, 946–956. ( 10.1093/jmammal/gyv095) [DOI] [Google Scholar]
- 43.Kéry M, Schaub M. 2011. Bayesian population analysis using WinBUGS: a hierarchical perspective. New York, NY: Academic Press. [Google Scholar]
- 44.Biedermann M, Karst I, Schorcht W.. 2012. Kleine Hufeisennase Rhinolophus hipposideros. In Fledermäuse in thüringen (eds Tress J, Biedermann M, Geiger H, Prüger J, Schorcht W, Tress C, Welsch K-P), pp. 245–266. Jena, Germany: Naturschutzreport 27. [Google Scholar]
- 45.Dietz C, Nill D, Von Helversen O. 2016. Handbuch der fledermäuse: Europa und nordwestafrika, 2nd edn Stuttgart, Germany: Kosmos. [Google Scholar]
- 46.Dool SE, Puechmaille SJ, Kelleher C, McAney K, Teeling EC. 2016. The effects of human-mediated habitat fragmentation on a sedentary woodland-associated species (Rhinolophus hipposideros) at its range margin. Acta Chiropt. 18, 377–393. ( 10.3161/15081109ACC2016.18.2.006) [DOI] [Google Scholar]
- 47.Plummer M. 2003. JAGS: a program for analysis of Bayesian graphical models using Gibbs sampling. In Proc. of the 3rd Int. workshop on distributed statistical computing See www.r-project.org/conferences/DSC-2003/Proceedings/Plummer.pdf. [Google Scholar]
- 48.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://cran.r-project.org. [Google Scholar]
- 49.Kellner K. 2017. jagsUI: a wrapper around ‘rjags’ to streamline ‘JAGS’ analyses. See https://CRAN.R-project.org/package=jagsUI.
- 50.Brooks SP, Gelman A. 1998. General methods for monitoring convergence of iterative simulations. J. Comput. Graph. Stat. 7, 434–455. ( 10.2307/1390675) [DOI] [Google Scholar]
- 51.Fleischer T, Gampe J, Scheuerlein A, Kerth G. 2017. Rare catastrophic events drive population dynamics in a bat species with negligible senescence. Sci. Rep. 7, 7370 ( 10.1038/s41598-017-06392-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Heerdt PF, Sluiter JW, Bezem JJ. 1960. Population statistics of five species of the bat genus Myotis and one of the genus Rhinolophus hibernating in the caves of S. Limburg. Arch. Nèerlandaises Zool. 13, 511–539. ( 10.1163/036551660X00170) [DOI] [Google Scholar]
- 53.Kepka O. 1960. Die Ergebnisse der Fledermausberingung in Steiermark vom Jahre 1949 bis 1960. Bonn. Zool. Beitr. 11, 54–76. [Google Scholar]
- 54.Schofield HW. 1996. The ecology and conservation of Rhinolophus hipposideros, the lesser horseshoe bat. PhD thesis, University of Aberdeen. [Google Scholar]
- 55.Petit E, Le Texier E, Farcy O. 2014. Suivi démographique de quatre espèces patrimoniales en Bretagne : analyse statistique de 11 années de comptage. Symbioses 32, 63–67. [Google Scholar]
- 56.Courant J, Secondi J, Bereiziat V, Herrel A. 2017. Resources allocated to reproduction decrease at the range edge of an expanding population of an invasive amphibian. Biol. J. Linn. Soc. 122, 157–165. ( 10.1093/biolinnean/blx048) [DOI] [Google Scholar]
- 57.Lustenhouwer N, Moran EV, Levine JM. 2017. Trait correlations equalize spread velocity across plant life histories. Glob. Ecol. Biogeogr. 26, 1398–1407. ( 10.1111/geb.12662) [DOI] [Google Scholar]
- 58.Masson L, Masson G, Beisel JN, Gutowsky LFG, Fox MG. 2018. Consistent life history shifts along invasion routes? An examination of round goby populations invading on two continents. Divers. Distrib. 24, 841–852. ( 10.1111/ddi.12726) [DOI] [Google Scholar]
- 59.Racey PA, Entwistle AC. 2000. Life-history and reproductive strategies of bats. In Reproductive biology of bats (eds Crichton EG, Krutzsch PH), pp. 363–414. London, UK: Academic Press. [Google Scholar]
- 60.Perkins TA, Boettiger C, Phillips BL. 2016. After the games are over: life-history trade-offs drive dispersal attenuation following range expansion. Ecol. Evol. 6, 6425–6434. ( 10.1002/ece3.2314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Noordwijk AJ, de Jong G. 1986. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 128, 137–142. ( 10.1086/284547) [DOI] [Google Scholar]
- 62.Hamel S, Gaillard J, Yoccoz NG, Loison A, Bonenfant C, Descamps S. 2010. Fitness costs of reproduction depend on life speed: empirical evidence from mammalian populations. Ecol. Lett. 13, 915–935. ( 10.1111/j.1461-0248.2010.01478.x) [DOI] [PubMed] [Google Scholar]
- 63.Gimenez O, Gaillard J-M. 2017. Estimating individual fitness in the wild using capture–recapture data. Popul. Ecol. 60, 101–109. ( 10.1007/s10144-017-0598-x) [DOI] [Google Scholar]
- 64.Clutton-Brock TH. 1988. Reproductive success: studies of individual variation in contrasting breeding systems. Chicago, IL: University of Chicago Press. [Google Scholar]
- 65.Gaillard J-M, Yoccoz NG. 2003. Temporal variation in survival of mammals: a case of environmental canalization? Ecology 84, 3294–3306. ( 10.1890/02-0409) [DOI] [Google Scholar]
- 66.Eberhardt LL. 2002. A paradigm for population analysis of long-lived vertebtrates. Ecology 83, 2841–2854. ( 10.1890/0012-9658(2002)083[2841:APFPAO]2.0.CO;2) [DOI] [Google Scholar]
- 67.Phillips BL. 2015. Evolutionary processes make invasion speed difficult to predict. Biol. Invasions 17, 1949–1960. ( 10.1007/s10530-015-0849-8) [DOI] [Google Scholar]
- 68.Ochocki BM, Miller TEX. 2017. Rapid evolution of dispersal ability makes biological invasions faster and more variable. Nat. Commun. 8, 14315 ( 10.1038/ncomms14315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weiss-Lehman C, Hufbauer RA, Melbourne BA. 2017. Rapid trait evolution drives increased speed and variance in experimental range expansions. Nat. Commun. 8, 14303 ( 10.1038/ncomms14303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turchin P. 1999. Population regulation: a synthetic view. Oikos 84, 153–159. ( 10.2307/3546876) [DOI] [Google Scholar]
- 71.Coulson T, Catchpole EA, Albon SD, Morgan BJT, Pemberton JM, Clutton-Brock TH, Crawley MJ, Grenfell BT. 2001. Age, sex, density, winter weather, and population crashes in Soay sheep. Science 292, 1528–1531. ( 10.1126/science.292.5521.1528) [DOI] [PubMed] [Google Scholar]
- 72.Jan P-L, Farcy O, Boireau J, Le Texier E, Baudoin A, Le Gouar P, Puechmaille SJ, Petit EJ. 2017. Which temporal resolution to consider when investigating the impact of climatic data on population dynamics? The case of the lesser horseshoe bat (Rhinolophus hipposideros). Oecologia 184, 749–761. ( 10.1007/s00442-017-3901-9) [DOI] [PubMed] [Google Scholar]
- 73.Lučan RK, Weiser M, Hanák V. 2013. Contrasting effects of climate change on the timing of reproduction and reproductive success of a temperate insectivorous bat. J. Zool. 290, 151–159. ( 10.1111/jzo.12021) [DOI] [Google Scholar]
- 74.Grindal SD, Collard TS, Brigham RM, Barclay RMR. 2001. The influence of precipitation on reproduction by Myotis bats in British Columbia. Am. Midl. Nat. 128, 339–344. ( 10.2307/2426468) [DOI] [Google Scholar]
- 75.Angert AL, Crozier LG, Rissler LJ, Gilman SE, Tewksbury JJ, Chunco AJ. 2011. Do species' traits predict recent shifts at expanding range edges? Ecol. Lett. 14, 677–689. ( 10.1111/j.1461-0248.2011.01620.x) [DOI] [PubMed] [Google Scholar]
- 76.Anderson SC, Branch TA, Cooper AB, Dulvy NK. 2017. Black-swan events in animal populations. Proc. Natl Acad. Sci. USA 114, 3252–3257. ( 10.1073/pnas.1611525114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jan P-L, Lehnen L, Besnard A-L, Kerth G, Biedermann M, Schorcht W, Petit EJ, Le Gouar P, Puechmaille SJ. 2019. Data from: Range expansion is associated with increased survival and fecundity in a long-lived bat species Dryad Digital Repository. ( 10.5061/dryad.66bp77v) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Jan P-L, Lehnen L, Besnard A-L, Kerth G, Biedermann M, Schorcht W, Petit EJ, Le Gouar P, Puechmaille SJ. 2019. Data from: Range expansion is associated with increased survival and fecundity in a long-lived bat species Dryad Digital Repository. ( 10.5061/dryad.66bp77v) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The capture history, multilocus genotypes and mother–daughter pairs detected are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.66bp77v [77]. All other data are in electronic supplementary material.