Abstract
Background
Studies suggest that 10–15% of perinatal women experience depressive symptoms. Due to the risks, problems with detection, and barriers to treatment, effective universal preventive interventions are needed. The aim of this study was to assess the effectiveness of an automated internet intervention (‘Mamma Mia’) on perinatal depressive symptoms. Mamma Mia is tailored specifically to the perinatal phase and targets risk and protective factors for perinatal depressive symptoms.
Methods
A total of 1342 pregnant women were randomized to an intervention (‘Mamma Mia’) and control group. Data were collected at gestational week (gw) 21–25, gw37, 6 weeks after birth, and 3 and 6 months after birth. We investigated whether (1) the intervention group displayed lower levels of depressive symptoms compared with the control group, (2) the effect of Mamma Mia changed over time, (3) the effect on depressive symptoms was moderated by baseline depressive symptoms, previous depression, and parity, and (4) this moderation changed by time. Finally, we examined if the prevalence of mothers with possible depression [i.e. Edinburgh Postnatal Depression Scale (EPDS)-score ⩾10] differed between the intervention and control group.
Results
Participants in the Mamma Mia group displayed less depressive symptoms than participants in the control group during follow-up [F(1) = 7.03, p = 0.008]. There were indications that the effect of Mamma Mia was moderated by EPDS score at baseline. The prevalence of women with EPDS-score ⩾10 was lower in the Mamma Mia group at all follow-up measurements.
Conclusions
The study demonstrated the effects of the automated web-based universal intervention Mamma Mia on perinatal depressive symptoms.
Key words: Internet intervention, linear mixed effects models, perinatal depression, randomized controlled trial, universal prevention
Introduction
The perinatal period represents a time signified by much emotional turmoil for women, and studies suggest that 10–15% of women experience moderate to high levels of depressive symptoms during this period (O'Hara and Swain, 1996; Eberhard-Gran et al., 2004; Gavin et al., 2005). Factors associated with increased risk of perinatal depression include a history of depression, stressful life events, young age and other demographic variables (Robertson et al., 2004; Vesga-López et al., 2008; Lancaster et al., 2010; Silverman et al., 2017). A majority of studies emphasize the postpartum period as a particularly vulnerable time during which the woman is at increased risk for mental disorders (Munk-Olsen et al., 2006; Vesga-Lopez et al., 2008; O'Hara and McCabe, 2013). More recent studies, however, highlight how women seem to be at equal risk of developing depression during pregnancy (Melville et al., 2010), and approximately 50% of depressive episodes after birth, start during pregnancy (American Psychiatric Association, 2013). This underscores the importance of early intervention, as the chronicity of depressive symptoms is of particular concern for the consequences of depression (Sohr-Preston and Scaramella, 2006). Perinatal depression carries a high personal cost for both the woman and her family (Lovestone and Kumar, 1993; Lovejoy et al., 2000; Goodman et al., 2011) while all too often escaping detection and treatment (Bågedahl-Strindlund and Monsen Børjesson, 1998).
Although effective psychological treatments for perinatal depression exist (Stuart-Parrigon, and Stuart, 2014; Stephens, 2016), there are several complicating factors that impede women's ability to receive appropriate treatment, such as limited access to treatment (Payne and Myhr, 2010; WHO, 2015), difficulties in attending therapy during usual business hours, struggles with geographical location (Overland et al., 2007), transportation and childcare (Goodman, 2009). Additionally, many women are reluctant to seek treatment due to a perceived stigma (Beck, 2001; O'Mahen and Flynn, 2008; Goodman, 2009). Some women even fear that their children will be ‘taken away’ if health providers discover that they suffer from perinatal depression (Dennis and Chung-Lee, 2006). Furthermore, many new mothers are unfamiliar with what constitute depressive symptoms, and do not recognize that they suffered from perinatal depression until it was over. Consequently, many people suffer ‘in silence’. The many barriers associated with face-to-face treatments clearly underscore a need for innovative approaches to public health prevention and promotion.
Internet-delivered interventions represent an innovative approach that circumvents many of the obstacles encountered in the dissemination of face-to-face treatment (Kohn et al., 2004). Internet interventions allow anonymity, which reduces a sense of stigma and patients’ fear of seeking help (Dennis and Chung-Lee, 2006). In turn, such interventions can more readily reach the many people that suffer ‘in silence’. Indeed, many women with perinatal depression express an interest in internet interventions and report that they would use the internet to learn coping strategies for depressive symptoms (Maloni et al., 2013). Because of the scalability, internet-based interventions are likely cost-effective, which makes them especially advantageous for disorders characterized by a combination of high prevalence and low treatment-seeking rates.
Recent studies have demonstrated the acceptability and feasibility of internet interventions for perinatal depression (Danaher et al., 2012; Haga et al., 2013; Salonen, 2014; Logsdon et al., 2014). Findings from two recent systematic reviews on web-based interventions for perinatal mental health are encouraging and suggest they may be effective in reducing perinatal depression (Ashford et al., 2016; Lee et al., 2016). However, there is still an overall need for high-quality randomized controlled trials (RCTs) to assess the effects of web-based interventions on perinatal depression (Lee et al., 2016). Interestingly, the reviews address how remarkably few of the existing interventions are tailored specifically to pregnancy and the postpartum period, which is curious considering how perinatal women face changes, difficulties, and mental health issues that are quite specific to this period (Ashford et al., 2016). Interventions that target perinatal specific themes may increase perceived relevance and acceptability, which, in turn, may lower attrition rates (O'Mahen et al., 2012). The current intervention, Mamma Mia, was tailored specifically to the perinatal phase and targets risk and protective factors for perinatal depressive symptoms such as attachment, couple satisfaction, social support, and subjective well-being. It was developed to address the high prevalence rates of perinatal depressive symptoms and the need for preventive interventions. Our study aims to contribute to the understanding of the effectiveness of fully automated web-based preventive interventions for perinatal depression.
Aim of the study
The objective of the current study was to test the effectiveness of a universal preventive intervention for perinatal depressive symptoms (Mamma Mia) on depressive symptoms from pregnancy to 6 months after birth. Our main hypothesis was that the Mamma Mia group would display lower levels of depressive symptoms compared with the control group as measured by the Edinburgh Postnatal Depression Scale (EPDS) (Cox et al., 1987). Second, we investigated whether the effect of Mamma Mia changed over time. Third, we examined if the effect of Mamma Mia on depressive symptoms was moderated by baseline depressive symptoms, an indication of previously experienced depression, and parity, and whether this moderation changed over time. Finally, we examined if the prevalence of mothers with at least a probable minor depression (i.e. EPDS-score ⩾10) differed between the intervention and control group.
Method
Study design and recruitment
This was a two-armed RCT registered at http://www.controlled-trials.com (ISRCTN91808706). Participants were randomly assigned (ratio: 1:1) to one of two conditions: the intervention group (i.e. usual perinatal care and the web-based program Mamma Mia) or control group [i.e. usual perinatal care (Norwegian Directorate of Health, 2005, 2013)]. The overall goal with the well-baby clinic that offers perinatal care is to promote physical and mental health and prevent injuries and disease, and ensure a continuity of care for its users (Norwegian Directorate of Health, 2004). According to guidelines for prenatal care, all women are offered eight consultations throughout the pregnancy, and midwives are to identify women in need of extended care and offer additional consultations (Norwegian Directorate of Health, 2005). During the first 6 months following birth, the woman and her child should be offered six consultations. Although the child's development is of primary concern during these consultations, the psychological well-being of the mother is also addressed. Pregnant women across Norway were invited to take part in the study between December 2013 and February 2015. Participants were recruited at well-baby clinics across the country during routine prenatal care and via hospitals in eastern Norway during regular ultrasound [i.e. gestational week (gw)18–20]. Eligible participants had to be pregnant (up until gw25), at least 18 years of age, able to read and write Norwegian, have access to the internet and have an electronic mailing account.
The intervention – Mamma Mia
Participants in the intervention received Mamma Mia; a universal preventive intervention for perinatal depressive symptoms. It is a fully automated internet-based program available free of charge. The intervention comprises three phases. The first phase consists of 11 sessions beginning in the second trimester in gw 21–25 and ends in gw37. The second phase starts when the infant is 2–3-weeks-old, and lasts for 6 weeks, with three sessions per week. The final phase consists of 10 sessions over an 18-week period. In total, the intervention consists of 44 sessions over a period of 11.5 months. All sessions include themes specific to the perinatal period. Mamma Mia applies a tunneled design to guide the woman through the program in a step-by-step fashion in accordance with the psychological preparations of becoming a mother. The intervention is delivered by email and interactive websites, combining text, pictures, prerecorded audio files, and user input. Each session is designed to take about 10 min and must be completed before users can access the next session. This is done to ensure that relevant information has been reviewed and to create continuity and a narrative in the program (see Drozd et al., 2015 for a comprehensive description of Mamma Mia). A recent study demonstrates the feasibility and acceptability of Mamma Mia (Haga et al., 2013). For a demonstration of Mamma Mia, see: http://smarturl.it/psych_med.
Data collection and randomization
Data was gathered by means of internet-based surveys. First, participants reported on demographic information (incl. age, marital status, parity, education, and estimated due date). Based on the reported due date, it was calculated when participants were between gw21–25, and that was when they received their baseline questionnaire assessing depressive symptoms. This was done to ensure similarity in terms of when depressive symptoms were assessed. Upon completing the baseline assessment, an automated, unrestricted randomization procedure was carried out. Thus, each participant had an equal probability of being assigned to each of the two groups, resulting in 678 and 664 eligible participants being randomly assigned to the intervention and control group, respectively. Depressive symptoms were assessed twice during pregnancy; at baseline between gw21 and 25 and at gw37. After birth, depressive symptoms were assessed at 6 weeks, 3 and 6 months postpartum.
Depressive symptoms were measured by EPDS (Cox et al., 1987), which is a 10-item self-report instrument that assesses depressive symptoms during the last 7 days. It has been validated for use during pregnancy (Bunevicius et al., 2009; Bergink et al., 2011), and postpartum (Eberhard-Gran et al., 2001a). Items are rated on a 4-point scale from 0 to 3 to produce a summative score ranging from 0 to 30, with higher scores indicating elevated risk for perinatal depression. Cox et al. (1987) recommend a cut-off score of EPDS ⩾10 to detect possible depression. The present study used the validated Norwegian translation of the EPDS (Eberhard-Gran et al., 2001b). Continuous EPDS-scores were used in main analyses, as recommended for the EPDS in population research (Green, 2005), while a cut-off score of ⩾10 was used to assess group differences in prevalence rates of possible depression across time.
Statistical analysis
Baseline differences between groups were merely examined by descriptive information in line with recommendations in the CONSORT guidelines. Logistic regression analyses with group assignment as an independent variable were done at each measurement time to assess drop-out after baseline, unadjusted and adjusted for age, first language, parity, education, previous depression, and EPDS at baseline. A series of linear mixed effects (LME) models were estimated for the time development of EPDS-scores after randomization. Time was included as a categorical independent variable with four categories (gw37, 6 weeks, 3 and 6 months postpartum), and treatment assignment was included as a dichotomous independent variable (control or Mamma Mia). Mixed-effects models are commonly used for repeated measures data (Pinheiro and Bates, 2012) as they do not require balanced data and give valid results under the less restrictive missing at random assumption. LME models handle repeated measure data in a long format and are therefore especially advantageous in longitudinal studies with missing data, as all data available is used and no cases are deleted. All models included a random intercept. For fixed effects, the first model was by group and time only. The second model included a group by time interaction to test for differences in intervention effects during follow-up. The third model also included interactions of group by parity, previous depression and depressive symptoms at baseline, and the final fourth model included third-order interactions between the group, time, and these background characteristics. It should be noted that EPDS-scores at baseline were not included in the dependent variable because when randomization is successful, group differences at baseline are random.
In supplementary analyses of the prevalence of depression over time after randomization, we performed logistic regression analyses for repeated measurements by means of generalized estimating equations (GEE). The binary outcome was EPDS-scores <10 (coded as 0) and EPDS-scores ⩾10 (coded as 1). In addition, we performed separate logistic regressions at each time point, adjusted for a baseline from gw37 to 6 months postpartum. The significance level was set to 0.05, but in line with the recommendations of the American Statistical Association, this boundary was not used strictly (Wasserstein and Lazar, 2016). All tests were two-tailed. SPSS version 23 (IBM SPSS, Armonk, NY, USA) was used for descriptive analyses. The R (The R Foundation for Statistical Computing, Vienna, Austria) package nlme was employed for the mixed effects analyses and the GEE analyses employed the R package gee.
Results
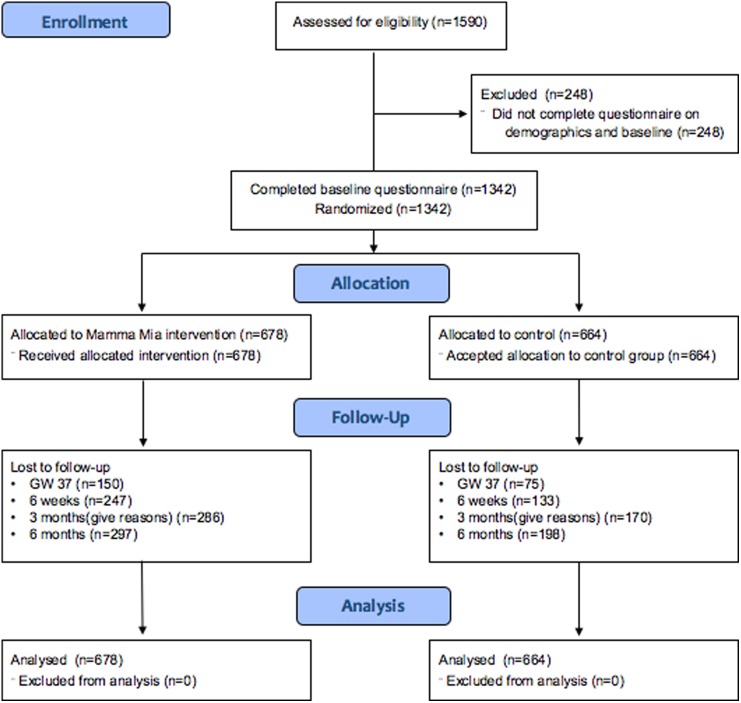
The flow of participants is illustrated in Fig. 1. In total, 678 participants were randomly assigned to the Mamma Mia group, and 644 were randomly assigned to the control group.
Fig. 1.
Participant flowchart.
The number of respondents in the total sample was 1342 at baseline, 1117 (83.2%) at gw37, and 962 (71.7%), 886 (66.0%), 847 (63.1%) at 6 weeks, 3 and 6 months postpartum, respectively.
Demographics are presented in Table 1. Mean maternal age was comparable with that of the average age for all births in Norway, which is 30.8 years (Statistics Norway, 2017b). In terms of education and parity, more than half of the mothers reported having higher education (i.e. ⩾4–5 years in college or university) and being primipara. This may indicate that the level of education was substantially higher than the 8.7% of women with higher education in the general population (Statistics Norway, 2017a), although the level of education among pregnant women in Norway is unknown. Using mothers’ first language as a proxy for ethnicity, the proportion of mothers with non-Scandinavian language was 7.7%, while the proportion of births given by mothers born outside of Norway was 27% in 2014 (The Norwegian Insitute of Public Health, 2015). The skewness in ethnicity may, however, be explained by the implicit inclusion criterion; the ability to read and understand Norwegian to participate in Mamma Mia.
Table 1.
Participant characteristics
| Characteristic | Mamma Mia (n = 678) | Control (n = 664) |
|---|---|---|
| Age (years) mean (s.d.) | 31.0 (4.6) | 31.1 (4.5) |
| Education n (%) | ||
| ⩽ high school | 100 (14.7) | 107 (16.1) |
| 1–3 years college or university | 189 (27.9) | 183 (27.6) |
| ⩾4–5 years college or university | 389 (57.4) | 374 (56.3) |
| First language n (%) | ||
| Scandinavian | 630 (92.9) | 609 (91.7) |
| Non-Scandinavian | 48 (7.1) | 55 (8.3) |
| No. of previous children n (%) | ||
| No previous children | 393 (58.0) | 382 (57.5) |
| ⩾1 children | 285 (42.0) | 282 (42.5) |
Table 2 shows levels of depressive symptoms in the Mamma Mia and control group over time. It also shows the percentage of participants who scored ⩾10 on the EPDS, which is an indication of possible depression. As can be seen, levels of depressive symptoms were highest at baseline in both groups, and the greatest reduction in symptoms occurred from baseline (gw21–25) to gw37. Noticeably, the proportion of women scoring above the cut-off at baseline was just over 23% in both groups. This is a higher proportion than what recent studies have found in Norway (Eberhard-Gran et al., 2002; Dørheim et al., 2009; Glavin et al., 2010; Haga et al., 2012, 2017), indicating that perhaps women with higher levels of depressive symptoms than the rest of the perinatal population have signed up for the study. This is not surprising considering that the participants were self-selected, and the study revolved around an intervention for perinatal depression.
Table 2.
Means, standard deviations, and number of women scoring above the cut-off for EPDS over time (N = 1 342)
| Mamma Mia (n = 678) | Control (n = 664) | |||||
|---|---|---|---|---|---|---|
| n (%) | EPDS (mean, s.d.) | EPDS ⩾10 (valid n, %) | n (%) | EPD (mean, s.d.) | EPDS ⩾10 (valid n, %) | |
| Baseline | 678 (100.0) | 6.5, 4.5 | 160 (23.6) | 664 (100.0) | 6.2, 4.4 | 156 (23.5) |
| gw37 | 528 (77.9) | 5.2, 4.0 | 72 (13.6) | 589 (88.7) | 5.8, 4.3 | 104 (17.7) |
| 6 weeks | 431 (63.6) | 5.2, 4.1 | 60 (13.9) | 531 (80.0) | 5.8, 4.2 | 94 (17.7) |
| 3 months | 392 (57.8) | 4.1, 4.0 | 37 (9.4) | 494 (74.4) | 4.5, 4.2 | 63 (12.8) |
| 6 months | 381 (56.2) | 4.0, 4.0 | 33 (8.7) | 466 (70.2) | 4.4, 4.3 | 55 (11.8) |
Drop-out and missing data
At all measurement times, drop-out was greater in the intervention group [odds ratio (OR) = 0.43–0.55, all ps <0.001 both in adjusted and unadjusted analysis]. There was little difference when comparing the adjusted and unadjusted analyses. For the whole sample, later dropout was predicted by a higher score on baseline EPDS (all ps ⩽0.047) and lower level of education (all ps ⩽0.004).
Adherence to the intervention
Adherence to the intervention refers to the completion of sessions. A total of 678 participants received the Mamma Mia intervention. Of these, 226 (33%) completed all 44 sessions. As many as 345 (51%) completed 36 or more sessions (>80% of the intervention), and merely 41 (6%) participants did not initiate using the program. The completion rate is high considering that Mamma Mia is a comprehensive, unguided, and fully automated prevention program that is delivered at a time that is already quite hectic.
Symptom reduction
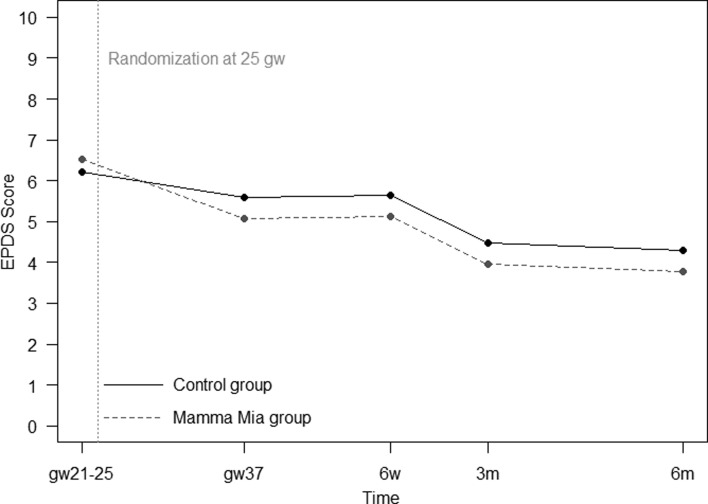
Our main hypothesis was that the level of depressive symptoms would differ between treatment and control group. The first model, by treatment assignment and time, showed a significant effect of Mamma Mia on depressive symptoms [F (1) = 7.03, p = 0.008] (see Fig. 2).
Fig. 2.
Mamma Mia and control group trajectories of depressive symptoms. Numbers at baseline are means, while numbers during follow-up are model-based estimates.
Effect on depressive symptoms over time
In the second model, there were no significant differences in the rate of change between the Mamma Mia and control group over time [F (3) = 1.02, p = 0.384 for the time by group interaction]. This means that the intervention group had lower levels of depressive symptoms compared with the control group, but both groups seemed to follow a similar trajectory in depressive symptoms. As seen in Table 3, participants in the Mamma Mia group had significantly lower symptoms than the control group at gw37 and 6 weeks postpartum. Group differences were not statistically significant at 3 and 6 months postpartum.
Table 3.
Contrasts between the Mamma Mia and control group at different time points (N = 1117)
| Model 2 (Conditional) | ||||
|---|---|---|---|---|
| Time | Contrast | Lower 95% bound | Upper 95% bound | p value |
| gw37 | −0.65 | −1.13 | −0.17 | 0.008 |
| 6 weeks | −0.56 | −1.07 | −0.05 | 0.031 |
| 3 months | −0.31 | −0.84 | 0.21 | 0.241 |
| 6 months | −0.25 | −0.78 | 0.28 | 0.358 |
Testing moderating variables – over time
In the third model, only EPDS-scores at baseline moderated the effect of group (p = 0.051). The group difference was somewhat more pronounced for higher values of baseline EPDS (data not shown). All other interactions were clearly non-significant (all ps ⩾0.317). In the final model, there was no indication of any third-order interactions (all ps ⩾0.133).
In sum, results from the mixed effects models indicate that participants in the Mamma Mia group scored lower on depressive symptoms than participants in the control group on EPDS during follow-up (gw37–6 months). The differences between the groups were significant at gw37 and 6 weeks. There were indications that the effect of Mamma Mia was moderated by EPDS score at baseline; indicating that a higher initial EPDS-score yielded a greater effect of Mamma Mia.
Prevalence of possible depression
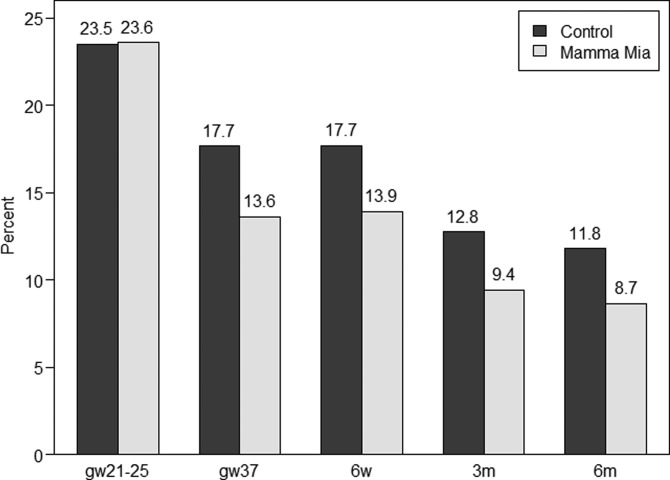
Finally, we examined if the prevalence of women with a possible minor depression (i.e. EPDS-score ⩾10) differed between the intervention and control group. Figure 3 below presents a histogram showing the percentage of women with EPDS-scores ⩾10 across time in the Mamma Mia and control group, respectively. These results indicate a 21.5–26.6% reduction in the prevalence of possible depression in the Mamma Mia group compared with the control group across time. The prevalence of women who scored ⩾10 on the EPDS was lower in the Mamma Mia group from gw37 and onwards (GEE adjusted for time and baseline, OR 0.75, 95% CI 0.58–0.96, p = 0.024). This difference was fairly stable over time (ORs between 0.71 and 0.78, see Table 4).
Fig. 3.
Percentages of women with EPDS-scores ⩾10 in the Mamma Mia and control group across time.
Table 4.
Results from separate logistic regression analyses comparing Mamma Mia to controls at each time point using EPDS ⩾10 as an outcome
| gw21–25 | gw37 | 6 weeks | 3 months | 6 months | |
|---|---|---|---|---|---|
| OR (95% CI) | 1.01 | 0.74 | 0.78 | 0.72 | 0.71 |
| (0.78–1.29) | (0.52–1.04) | (0.55–1.13) | (0.46–1.12) | (0.45–1.13) | |
| p value | 0.964 | 0.082 | 0.191 | 0.141 | 0.149 |
Estimates from gw37 to 6 months are adjusted for baseline EPDS.
Discussion
The focus of this study was to evaluate the effectiveness of an internet-based program (‘Mamma Mia’) on depressive symptoms from pregnancy to 6 months postpartum. Results indicated that the reduction in mean depressive symptoms in the intervention group compared with the control group was small but significant. In fact, participants who received Mamma Mia displayed less depressive symptoms than participants in the control group on all measurements occasions after baseline. This difference was statistically significant at gw37 and 6 weeks postpartum. Although group differences were no longer statistically significant at 3 and 6 months postpartum, participants in the intervention group still had lower levels of depressive symptoms than the control group. As symptom severity is at its highest towards the end of pregnancy and early postpartum period (Cox et al., 1993; Eberhard-Gran et al., 2003; Gaynes et al., 2005; Munk-Olsen et al., 2006), and typically remits over time (Heron et al., 2004), it is not surprising that Mamma Mia was most effective during the third trimester and early postpartum period. Importantly, as the end of pregnancy (Eberhard-Gran et al., 2004) and first weeks after birth are particularly vulnerable time periods, it is reassuring that Mamma Mia is most effective at precisely those times.
Although Mamma Mia is a universal intervention, analyses were undertaken to assess whether Mamma Mia was more effective given certain background characteristics. Only EPDS-scores at baseline emerged as a moderator, indicating that a higher initial EPDS score yielded a greater effect of Mamma Mia. This finding is clinically meaningful as people with higher levels of distress have greater potential for improvement (Dennis, 2005).
The clinical implications of Mamma Mia for the individual and the population
Although the effect on the mean level of EPDS-scores was modest, is important to consider the clinical implications of Mamma Mia for the individual. Interesting research has investigated whether ‘a touch of depression matters’ (Brenner, 1985; Rose, 2008). What Brenner (1985) found was that the likelihood of needing health care support increased with every increase on a scale measuring depressive symptoms, regardless of whether the person was over or below the cut-off score. This finding implies that even the mildest subclinical degree of depression is problematic for the individual and associated with impaired functioning, suggesting that even a minor reduction in depressive symptoms over time will matter for a new mother and her baby. Because all levels of symptoms matter to the people involved, preventive medicine ought to target the whole spectrum of illness and health. What is more, ‘the mild symptom can be the father of the severe’ (Rose, 2008).
In a population, the mean level of symptoms is related to the prevalence of a disorder (Anderson et al., 1993; Whittington and Huppert, 1996). Universal interventions that can reduce mean symptoms will thus, in turn, reduce the prevalence of a disorder. Rose (2008) underscored this argument by using an analogy of how the ‘visible part of the iceberg (prevalence) is a function of its total mass (the population average)’ (Rose, 2008). In the current study, analyses supported this analogy by showing that a small mean sample reduction in depression was also accompanied by a reduced proportion of women with an EPDS-score ⩾10 in the Mamma Mia group. Indeed, EPDS-score of ⩾10 prevalence rates were reduced between 21.5 and 26.6% in the Mamma Mia group compared with the controls, across time. These are promising results indicating that Mamma Mia may prevent at least minor to moderate depression. Due to the substantial barriers to care once women become ill, Mamma Mia holds a considerable potential for clinical effectiveness.
Study limitations
Current drop-out rates fall within the typical range reported on internet-based interventions (Christensen et al., 2009), but is nevertheless a limitation in this study. The drop-out was greater in the intervention group than the control group and missing data analysis showed that participants with less formal education and those with higher EPDS-scores at baseline were more likely to drop out. Therefore, EPDS baseline score and educational level were used as covariates in the regression models as a deliberate effort to preclude systematic differences between drop-outs and completers. Nevertheless, both from a methodological and a prevention standpoint, it is unfortunate that participants with more depressive symptomatology were more likely to drop out. Participant drop-out may also have affected the power to detect differences, particularly at later measurement occasions because drop-out became more pronounced over time.
While we used continuous EPDS-scores in our main analyses, an EPDS-score ⩾10 was used as an indicator of possible depression. However, while the EPDS-scores are indicative of depressive symptomatology, scores do not confirm or refute the presence of depression (Cox and Holden, 2003). Cases were not confirmed with clinical assessment. The mean EPDS-scores in both groups at baseline, as well as the proportion of women with an EPDS-score ⩾10, were higher than found in recent Norwegian studies (Dørheim, 2009; Glavin et al., 2010; Haga et al., 2012; Haga et al., 2017). This is likely due to the self-selection of participants and that women with more depressive symptomatology may have been attracted to an intervention that targets depression. Thus, a limitation of the present study is the potential for sampling bias from not achieving a full inclusion of the intended population.
Finally, as in any effectiveness trial, a mix of good and poor responders will have been recruited, so the average therapeutic response may have been mitigated.
Future recommendations and studies
Research demonstrates how few of the existing interventions are tailored to the perinatal period, which is surprising as tailored interventions are likely perceived as more relevant and acceptable, and, in turn, associated with lower attrition rates. Moreover, perinatal care that is tailored to the woman's needs has been found to reduce the prevalence rate of perinatal depression (MacArthur et al., 2002). Mamma Mia is a comprehensive intervention that addresses a wide range of themes that preoccupy perinatal women, ranging from attachment, breastfeeding, regulatory capacity in infants to partner relationship, all of which comprise important health determinants for perinatal depression. A key challenge with such a comprehensive program, however, is the ‘black box’ phenomenon. That is, it is not clear which components are effective for whom and what minimum dosage of the intervention is required to achieve the effect. Moreover, the intervention might be too comprehensive for those most at risk. Future studies should attempt to link the use of intervention components and their effects and determine their dose-response relationships.
Although the current study took place in Norway, the content of Mamma Mia was largely developed based on international studies addressing risk and protective factors for perinatal depression. Moreover, the national clinical guidelines for perinatal care in Norway are in line with World Health Organization recommendations (2015) and practices are comparable with those in Britain (National Institute for Health and Excellence, 2014) and the other Scandinavian countries (Danish Health Authority, 2013; The Swedish Association of Midwives, 2016). Thus, there are reasons to assume that the current results are generalizable to other Western cultures. This should be addressed in future studies.
Recent research shows that internet-based programs in combination with guidance from health personnel for treating perinatal depression may produce effects equivalent to those of face-to-face interventions (Cuijpers et al., 2010). Therefore, guidelines for the clinical use of Mamma Mia (i.e. with added guidance) and implementation in well-baby clinics have been developed (Drozd et al., 2017), designed to fit with existing national guidelines for pre- and postnatal care for well-baby clinics (Norwegian Directorate of Health, 2005, 2013). With a few case-finding questions health personnel could identify women at risk of depression who should be offered the guided program, while the unguided version of Mamma Mia, as tested in this study, may continue to be disseminated as universal prevention. A future study should, however, investigate whether adding guidance by well-baby personnel enhances the effects Mamma Mia. However, until then, conditions are in place for primary care to make the unguided internet-based program available as universal prevention to perinatal women.
Conclusion
Due to the prevalence, substantial risks, problems with detection of perinatal depression, as well as the substantial barriers to care once women become ill, an effective universal intervention holds substantial potential for clinical effectiveness. The present study demonstrated promising effects of Mamma Mia, and it is readily available for perinatal women at no cost. Primary health services are in a position to relay information about preventive programs for perinatal depression, and Mamma Mia has the potential to aid health care professionals in preventing depressive symptoms in the perinatal population.
Ethics
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The participants were regularly screened for depressive symptoms, and those scoring above a given threshold were provided feedback about where they could receive support and help (beyond what the program could offer). Power analyses were conducted so as not to include (and burden) any more participants than necessary. The trial was approved by the Regional Ethics Committee, Norway, South East (project number: 2012/1716).
Acknowledgements
First and foremost, we would like to thank all the women who took part in this comprehensive study at the same time as they were having a baby. We would also like to thank the hospitals and well-baby clinics who helped immensely with recruitment. Mamma Mia was developed in Norway and is owned by The Norwegian Women's Public Health Association (N.K.S.) who offers the program free of charge to women (see https://tinyurl.com/MammaMia-NKS). Mamma Mia is currently only offered in Norwegian language. The program was developed by Changetech AS in collaboration with the Department for Infant Mental Health at RBUP (Regional Centre for Child and Adolescent Mental Health, Eastern and Southern Norway). The project was supported by funding provided by the Research Council of Norway (#213737).
Conflict of interest
Mamma Mia was developed by Changetech in collaboration with the Regional Centre for Child and Adolescent Mental Health (RBUP) for The Norwegian Women's Public Health Association (N.K.S.).
References
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders, 5th Edn. Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Anderson J, Huppert F and Rose G (1993) Normality, deviance and minor psychiatric morbidity in the community. A population-based approach to general health questionnaire data in the health and lifestyle survey. Psychological Medicine 23, 475–485. [DOI] [PubMed] [Google Scholar]
- Ashford MT, Olander EK and Ayers S (2016) Computer- or Web-based interventions for perinatal mental health: a systematic review. Journal of Affective Disorders 197, 134–146. [DOI] [PubMed] [Google Scholar]
- Bågedahl-Strindlund M and Monsen Børjesson K (1998) Ostnatal depression: a hidden illness. Acta Psychiatrica Scandinavia 98, 272–275. [DOI] [PubMed] [Google Scholar]
- Beck CT (2001) Predictors of postpartum depression: an update. Nursing Research 50, 275–285. [DOI] [PubMed] [Google Scholar]
- Bergink V, Kooistra L, Lambregtse-van den Berg MP, Wijnen H, Bunevicius R, van Baar A and Pop V (2011) Validation of the Edinburgh Depression Scale during pregnancy. Journal of Psychosomatic Research 70, 385–389. [DOI] [PubMed] [Google Scholar]
- Brenner B (1985) Continuity between the presence and absence of the depressive syndrome. Paper presented at the 113th Annual Meeting of the American Public Health Association, Washington, DC.
- Bunevicius A, Kusminskas L, Pop VJ, Pedersen CA and Bunevicius R (2009) Screening for antenatal depression with the Edinburgh Depression Scale. Journal of Psychosomatic Obstetrics & Gynecology 30, 238–243. [DOI] [PubMed] [Google Scholar]
- Christensen H, Griffiths KM and Farrer L (2009) Adherence in internet interventions for anxiety and depression: systematic review. Journal of Medical Internet Research 11, e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JL and Holden J (2003) Perinatal Mental Health: A Guide to the Edinburgh Postnatal Depression Scale (EPDS). London: Gaskell. [Google Scholar]
- Cox JL, Holden JM and Sagovsky R (1987) Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. The British Journal of Psychiatry 150, 782–786. [DOI] [PubMed] [Google Scholar]
- Cox JL, Murray D, Chapman G, Cox JL, Murray D and Chapman G (1993) A controlled study of the onset, duration and prevalence of postnatal depression A controlled study of the onset, duration prevalence of postnatal depression. The British Journal of Psychiatry 163, 27–31. doi: 10.1192/bjp.163.1.27. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Donker T, van Straten A, Li J and Andersson G (2010) Is guided self-help as effective as face-to-face psychotherapy for depression and anxiety disorders? A systematic review and meta-analysis of comparative outcome studies. Psychological Medicine 40, 1943–1957. [DOI] [PubMed] [Google Scholar]
- Danaher BG, Milgrom J, Seeley JR, Stuart S, Schembri C, Tyler MS, Ericksen J, Lester W, Gemmill AW and Lewinsohn P (2012) Web-based intervention for postpartum depression: formative research and design of the MomMoodBooster program. JMIR Research Protocols 1, e18. [Medline: 23612274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danish Health Authority (2013) Available at https://www.sst.dk/da/sundhed-og-livsstil/graviditet-og-foedsel/anbefalinger-for-svangreomsorgen
- Dennis CL (2005) Psychosocial and psychological interventions for prevention of postnatal depression: systematic review. British Medical Journal 331, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis CL and Chung-Lee L (2006) Postpartum depression help-seeking barriers and maternal treatment preferences: a qualitative systematic review. Birth (Berkeley, Calif ) 33, 323–331. [Medline: 17150072. [DOI] [PubMed] [Google Scholar]
- Dørheim SK, Bondevik GT, Eberhard-Gran M and Bjorvatn B (2009) Sleep and depression in postpartum women: a population-based study. Sleep 32, 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozd F, Haga SM, Brendryen H and Slinning K (2015) An Internet-based intervention (Mamma Mia) for postpartum depression: mapping the development from theory to practice. JMIR Research Protocols 4, e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozd F, Haga SM and Slinning K (2017) From science to practice: implementation and clinical guidelines for an internet intervention for postpartum depression In Langrial SU (ed.), Web-Based Behavioral Therapies for Mental Disorders. Hershey, PA: IGI Global, pp. 79–110. 10.4018/978-1-5225-3241-5.ch004 [DOI] [Google Scholar]
- Eberhard-Gran M, Eskild A, Tambs K, Opjordsmoen S and Samuelsen SO (2001a) Review of validation studies of the Edinburgh postnatal depression scale. Acta Psychiatrica Scandinavica 104, 243–249. [DOI] [PubMed] [Google Scholar]
- Eberhard-Gran M, Eskild A, Tambs K, Schei B and Opjordsmoen S (2001b) The Edinburgh Postnatal Depression Scale: validation in a Norwegian community sample. Nordic Journal of Psychiatry 55, 113–117. [DOI] [PubMed] [Google Scholar]
- Eberhard-Gran M, Eskild A, Tams K and Samuelson SO (2002) Depression in post-partum women: prevalence and risk factors. Acta Psychiatrica Scandinavica 106, 426–433. [DOI] [PubMed] [Google Scholar]
- Eberhard-Gran M, Tambs K, Opjordsmoen S, Skrondal A and Eskild A (2003) A comparison of anxiety and depressive symptomatology in postpartum and nonpostpartum mothers. Social Psychiatry and Psychiatric Epidemiology 38, 551–556. [DOI] [PubMed] [Google Scholar]
- Eberhard-Gran M, Tambs K, Opjordsmoen S, Skrondal A and Eskild A (2004) Depression during pregnancy and after delivery: a repeated measurement study. Journal of Psychosomatic Obstetrics & Gynecology 25, 15–21. [DOI] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G and Swinson T (2005) Perinatal depression: a systematic review of prevalence and incidence. Obstetrics & Gynecology, 106(5 Pt 1): 1071–1083. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Brody S and Miller WC (2005) Perinatal Depression: Prevalence, Screening Accuracy, and Screening Outcomes. Evidence Report/Technology Assessment No. 119. (Prepared by the RTI University of North Carolina Evidence-based Practice Center, under Contract No. 290-02-0016.) AHRQ Publication No. 05-E006-2. Rockville, MD: Agency for Healthcare Research and Quality. February 2005.
- Glavin K, Smith L, Sorum R and Ellefsen B (2010) Redesigned community postpartum care to prevent and treat postpartum depression in women – a one-year follow-up study. Journal of Clinical Nursing 9, 3051–3062. [DOI] [PubMed] [Google Scholar]
- Goodman JH (2009) Women's attitudes, preferences, and perceived barriers to treatment for perinatal depression. Birth (Berkeley, Calif ) 36, 60–69. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM and Heyward D (2011) Maternal Depression and Child Psychopathology: A Meta-Analytic Review. Clinical Child and Family Psychology Review 14(1), 1–C27. [DOI] [PubMed] [Google Scholar]
- Green JM (2005) What is the EPDS measuring and how should we use it in research? In Henshaw C and Elliott S (eds), Screening for Perinatal Depression. London: Jessica Kingsley Publishers, pp. 141–147. [Google Scholar]
- Haga SM, Ulleberg P, Slinning K, Kraft P, Steen TB and Staff A (2012) A longitudinal study of postpartum depressive symptoms: multilevel growth curve analyses of emotion regulation strategies, breastfeeding self-efficacy, and social support. Archives of Women's Mental Health 15, 175–184. [DOI] [PubMed] [Google Scholar]
- Haga SM, Drozd F, Brendryen H and Slinning K (2013) Mamma Mia: a feasibility study of a web-based intervention to reduce the risk of postpartum depression and enhance subjective well-being. JMIR Research Protocols 2, e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga SM, Lisøy C, Drozd F, Valla L and Slinning K (2017) A population-based study of the relationship between perinatal depressive symptoms and breastfeeding: a cross-lagged panel study. Archives of Women's Mental Health 21, 235–242. [DOI] [PubMed] [Google Scholar]
- Heron J, O'Connor TG, Evans J, Golding J and Glover V (2004) The course of anxiety and depression through pregnancy and the postpartum in a community sample. Journal of Affective Disorders 80, 65–73. [DOI] [PubMed] [Google Scholar]
- Kohn R, Saxena S, Levav I and Saraceno B (2004) The treatment gap in mental health care. Bulletin of the World Health Organization 82, 858–866. [PMC free article] [PubMed] [Google Scholar]
- Lancaster CA, Gold KJ, Flynn HA, Yoo H, Marcus SM and Davis MM (2010) Risk factors for depressive symptoms during pregnancy: a systematic review. American Journal of Obstetrics and Gynecology 202, 5–14.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EW, Denison FC, Hor K and Reynolds RM (2016) Web-based interventions for prevention and treatment of perinatal mood disorders: a systematic review. BMC Pregnancy and Childbirth 6, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon MC, Bennett G, Crutzen R, Martin L, Eckert D, Robertson A, Myers J, Tomasulo R, Gregg J, Barone M, et al. (2014) Preferred health resources and use of social media to obtain health and depression information by adolescent mothers. Journal of Child and Adolescent Psychiatric Nursing 27, 63–168. [DOI] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O'Hare E and Neuman G (2000) Maternal depression and parenting behavior: a meta-analytic review. Clinical Psychology Review 20, 561–592. [DOI] [PubMed] [Google Scholar]
- Lovestone S and Kumar R (1993) Postnatal psychiatric illness: the impact on partners. British Journal Psychiatry 163, 210–216. [DOI] [PubMed] [Google Scholar]
- MacArthur C, Winter HR, Bick DE, Knowles H, Lilford R, Henderson C, Lancashire RJ, Braunholtz DA and Gee H (2002) Effects of redesigned community postnatal care on womens’ health 4 months after birth: a cluster randomized controlled trial. The Lancet 359, 378–385. [DOI] [PubMed] [Google Scholar]
- Maloni JA, Przeworski A and Damato EG (2013) Web recruitment and internet use and preferences reported by women with postpartum depression after pregnancy complications. Archives of Psychiatric Nursing 27, 90–95. [DOI] [PubMed] [Google Scholar]
- Melville JL, Gavin A, Guo Y, Fan M-Y and Katon WJ (2010) Depressive disorders during pregnancy: prevalence and risk factors in a large urban sample. Obstetrics and Gynecology 116, 1064–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk-Olsen T, Laursen TM, Pedersen CB, Mors O and Mortensen PB (2006) New parents and mental disorders: a population-based register study. The Journal of the American Medical Association 296, 2582–2589. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Excellence (2014) Antenatal and postnatal mental health: clinical management and service guidance. Clinical guideline [CG192]. Retrieved from: https://www.nice.org.uk/guidance/cg192. [PubMed]
- Norwegian Directorate of Health. (2004) Kommunenes helsefremmende og forebyggende arbeid i helsestasjons- og skolehelsetjenesten: Veileder til forskrift av 3. april 2003 nr. 450 [Municipalities health promotion and prevention in well-baby clinics and schools: Guidelines to regulations April 3, no. 450]. Oslo: Norwegian Directorate of Health. [Google Scholar]
- Norwegian Directorate of Health (2005) Retningslinjer for svangerskapsomsorgen [Guidelines for prenatal care]. Oslo: Norwegian Directorate of Health. [Google Scholar]
- Norwegian Directorate of Health (2013) Nytt liv og trygg barseltid for familien. Retningslinjer for barselomsorgen [A new life and a safe puerperium for the family. Guidelines for postnatal care]. Oslo: Norwegian Directorate of Health. [Google Scholar]
- O'Hara MW and McCabe JE (2013) Postpartum depression: current status and future directions. Annual Review of Clinical Psychology 9, 379–407. [DOI] [PubMed] [Google Scholar]
- O'Hara MW and Swain AM (1996) Rates and risk of postpartum depression: a meta analysis. International Review of Psychiatry 8, 37–54. [Google Scholar]
- O'Mahen HA and Flynn HA (2008) Preferences and perceived barriers to treatment for depression during the perinatal period. Journal of Women's Health (Larchmont) 17, 1301–1309. [DOI] [PubMed] [Google Scholar]
- O'Mahen HA, Fedock G, Henshaw E, Himle J, Forman J and Flynn HA (2012) Modifying CBT for perinatal depression: what do women want? A qualitative study. Cognitive and Behavioral Practice 19, 359–371. [Google Scholar]
- Overland S, Glozier N, Krokstad S and Mykletun A (2007) Undertreatment before the award of a disability pension for mental illness: the HUNT study. Psychiatric Services 58, 1479–1482. [DOI] [PubMed] [Google Scholar]
- Payne KA and Myhr G (2010) Increasing access to Cognitive-Behavioural Therapy (CBT) for the treatment of mental illness in Canada: a research framework and call for action. Healthcare Policy 5, e173–e185. [PMC free article] [PubMed] [Google Scholar]
- Pinheiro JC and Bates DM (2012) Approximations to the log-likelihood function in the nonlinear mixed-effects model. Journal of Computational and Graphical Statistics 4, 12–35. [Google Scholar]
- Robertson E, Grace S, Wallington T and Stewart DE (2004) Antenatal risk factors for postpartum depression: a synthesis of recent literature. General Hospital Psychiatry 26, 289–295. [DOI] [PubMed] [Google Scholar]
- Rose G (2008) Rose's Strategy of Preventive Medicine. Oxford University Press, Oxford. [Google Scholar]
- Salonen AH, Pridham KF, Brown RL and Kaunonen M (2014) Impact of an internet-based intervention on Finnish mothers’ perceptions of parenting satisfaction, infant centrality and depressive symptoms during the postpartum year. Midwifery 30, 112–122. [DOI] [PubMed] [Google Scholar]
- Silverman ME, Reichenberg A, Savitz DA, Cnattingius S, Lichtenstein P, Hultman CM, et al. (2017) The risk factors for postpartum depression: a population-based study. Depression and Anxiety 34, 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohr-Preston SL and Scaramella LV (2006) Implications of timing of maternal depressive symptoms for early cognitive and language development. Clinical Child and Family Psychology Review 9, 65–83. [DOI] [PubMed] [Google Scholar]
- Statistics Norway (2017a) Population level of education Available at https://www.ssb.no/utniv/ (Accessed 6 July 2017).
- Statistics Norway (2017b) Births, 2016 Available at https://www.ssb.no/fodte (Accessed 6 July 2017).
- Stephens S, Ford E, Paudyal P and Smith H (2016) Effectiveness of psychological interventions for postnatal depression in primary care: a meta-analysis. Annals of Family Medicine 14, 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart-Parrigon K and Stuart S (2014) Perinatal depression: an update and overview. Current Psychiatry Reports 16, 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Norwegian Insitute of Public Health (2015) 9 av 10 barn fødes etter fullgåtte svangerskap [9 out of 10 children are born after full-term pregnancies] Available at https://www.fhi.no/nyheter/2015/9-av-10-barn-fodes-etter-fullgatte-/ (Accessed 6 July 2017).
- The Swedish Association of Midwives (2016) Mödrahälsovård, Sexuell och Reproduktiv Hälsa SFOG. Interessegruppen för mödrahälsovård. Samordningsbarnmorskorna inom SBF i samarbete med Mödrahälsovårdspsykologernasförening [Maternity care, sexual and reproductive health, Swedish Society of Obstetrics and Gynecology. The interest group for Coordination midwives within the Swedish Association of Midwives in collaboration with Maternity care Psychologists Association]. 2008/2016 Report nr. 76 Retrieved from http://libris.kb.se/bib/11279400
- Vesga-López O, Blanco C, Keyes K, et al. (2008) Psychiatric disorders in pregnant and postpartum women in the United States. Archives of General Psychiatry 65, 805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserstein RL and Lazar NA (2016) The ASA's statement on p values: context, process, and purpose. The American Statistician 70, 129–133. Available at http://amstat.tandfonline.com/doi/abs/10.1080/00031305.2016.1154108#.Vt2XIOaE2MN. [Google Scholar]
- Whittington JE and Huppert FA (1996) Changes in the prevalence of psychiatric disorder in a community are related to changes in the mean level of psychiatric symptoms. Psychological Medicine 26, 1253–1260. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2015) Pregnancy, childbirth, postpartum and newborn care: a guide for essential practice – 3rd ed. Available at http://www.who.int/maternal_child_adolescent/documents/imca-essential-practice-guide/en/ [PubMed]