Abstract
Hirschsprung disease (HSCR, OMIM 142623) is due to a failure of enteric precursor cells derived from neural crest (EPCs) to proliferate, migrate, survive or differentiate during Enteric Nervous System (ENS) formation. This is a complex process which requires a strict regulation that results in an ENS specific gene expression pattern. Alterations at this level lead to the onset of neurocristopathies such as HSCR. Gene expression is regulated by different mechanisms, such as DNA modifications (at the epigenetic level), transcriptional mechanisms (transcription factors, silencers, enhancers and repressors), postranscriptional mechanisms (3′UTR and ncRNA) and regulation of translation. All these mechanisms are finally implicated in cell signaling to determine the migration, proliferation, differentiation and survival processes for correct ENS development. In this review, we have performed an overview on the role of epigenetic mechanisms at transcriptional and posttranscriptional levels on these cellular events in neural crest cells (NCCs), ENS development, as well as in HSCR.
Keywords: enteric nervous system development, Hirschsprung disease, neural crest cells, epigenetic mechanisms
1. Introduction
Hirschsprung disease (HSCR, OMIM 142623) is a rare congenital disorder that occurs in approximately one per 5000 live births. It is characterized by the absence of enteric ganglia in the rectum and a variable continuous segment of the proximal intestine resulting in intestinal dysfunction. Based on the length of the aganglionic segment, the pathology is classified as short segment HSCR, long segment HSCR, and total colonic aganglionosis or total intestinal aganglionosis. Most often the disease appears as sporadic HSCR, although familial cases have also been reported. Although 70% of HSCR cases appear without any additional clinical manifestations (isolated HSCR), the remaining 30% of cases manifest with other disorders or congenital malformations (syndromic HSCR) [1].
HSCR results from a failure to fully colonize the gut by enteric precursor cells (EPCs) derived from neural crest cells (NCCs). Such incomplete gut colonization is due to alterations in EPCs proliferation, survival, migration and differentiation during enteric nervous system (ENS) development [2]. Various pathways have been described in relation with these cellular events. Among them, the main pathways described are RET/GFRα1/GDNF and EDNRB/EDN3/ECE1, as well as several transcription factors (PAX3, SOX10, ZFHX1B and PHOX2B) and some morphogens (netrins, semaphorins and SHH) [3]. Therefore, ENS formation is a complex process in which a large number of molecules that are tightly regulated by a specific gene expression pattern are implicated. In this sense, the epigenetic mechanisms among others are involved in the regulation of gene expression, being such regulatory processes an emerging research area in the field of ENS development and specifically in HSCR.
Epigenetic events are defined as “the structural adaptation of chromosomal regions so as to register, signal or perpetuate altered activity state” [4]. Mostly, modifications at this level are stable and are transmitted along generations, but the effect of the environmental agents has also been described [5]. Here, we emphasize the impact of alterations in epigenetic mechanisms such as methylation of DNA, post-translational modifications to histone proteins, polycomb repression, ATP dependent chromatin remodeling and non-coding RNA (ncRNA) [6,7,8,9].
In addition, in this review, we also highlight the above-mentioned regulatory mechanisms that may finally result in the onset of HSCR.
2. DNA Methylation
DNA methylation involves the insertion of a CH3 group at the C-5 position of the cytosine ring of DNA. Susceptibility regions of methylation (CpG islands) represent around 40% of all mammalian promoters. These regions are usually unmethylated when gene expression occurs [10]. DNA methylation is essential for many biological processes during mammal development [11,12]. This mechanism is carried out by the enzyme family known as DNA methyltransferases (DNMTs): DNMT1, DNMT3 (A and B). DNMT1 is the maintenance methyltransferase whereas both DNMT3s are de novo methyltransferases [13,14].
With respect to DNMT3A and its paralog DNMT3B, some evidence has shown their crucial role for normal mammal development, as well as their involvement in diseases [15,16,17,18]. Specifically, Dnmt3A homozygous knockout mice die some weeks after birth, and rostral neural tube defects and growth impairment have been observed for Dntm3B homozygous knockout embryos that finally leads to death, suggesting that both enzymes are essential during embryonic development [19]. Various studies have showed the potential involvement of both genes in NCC development. Specifically, in neural crest cells of chicken embryos, Dnmt3A downregulation leads to a reduced expression of genes which is directly implicated in neural crest specification (Snail, FoxD3, Sox10, Pax7 and Pax3) [20]. On the contrary, Dnmt3B is upregulated during chicken embryo neural crest formation [21]. Regarding studies in humans, DNMT3B mutations have been found in the immunodeficiency-centromeric instability-facial anomalies syndrome (OMIM#242860) [15,22]. In embryonic stem cells, DNMT3B knockdown leads to early neural crest differentiation as well as the upregulation of neural crest specifier genes [23]. The contribution of DNMT3B to the onset of HSCR was demonstrated because its downregulation in EPCs from HSCR patients versus controls correlated with a decrease of global DNA methylation levels. In addition, the synergistic effect of mutations in both DNMT3B and other HSCR–related genes on the severity of the phenotype in HSCR patients has been reported [24]. Such alterations resulted in an altered gene expression pattern [25] and an arrest of cell cycle of the EPCs through P53-P21 activity [26]. Therefore, all this evidence suggests the involvement of DNMT3B as a susceptibility gene for HSCR and demonstrates the crucial role of DNA methylation in ENS development and in the onset of HSCR.
Aberrant DNA methylation patterns affecting genes related to ENS development and HSCR have been described. The RET proto-oncogene encodes a receptor tyrosine kinase that plays crucial roles in ENS development. It is the main gene associated with HSCR, contains a 5′-CG-3′ rich region within its promoter, and the methylation levels of this region have been demonstrated to be related to its expression level in peripheral white blood cells from HSCR patients [27]. GFRA4 has been widely proposed as a susceptibility gene in the pathogenesis of HSCR [28]. It encodes for a RET co-receptor inducing neuronal survival and differentiation [29] through its interaction with members of the glial derived neurotrophic factors family [30] since RET-GDNF is one of the main pathways related to HSCR, as mentioned above. It has been described that the downregulation of GFRA4 in HSCR can be partly due to hypermethylation at its promoter region. Therefore, it has been proposed that DNA methylation contributes to the regulation of the neuroprotective role of GFRA4 on NCCs [31]. EDNRB (endothelin receptor type B) is another susceptibility gene for HSCR because the Endothelin 3-Endothelin Receptor B Signalling Pathway is crucial for the correct formation of enteric ganglia [32]. Tang et al. demonstrated that epigenetic inactivation of EDNRB might play a role in ENS development and in the onset of HSCR. Specifically, the upregulated expression level of EDNRB in HSCR tissue compared with controls correlated with a significantly lower ratio of its methylation level in these patients. [33]. Additionally, it has been described that methylation levels of the sonic hedgehog (SHH) promoter are significantly increased in patients with congenital anorectal malformations (ARM), which is correlated with lower levels of its expression [34]. This epigenetic modification on SHH may be responsible for abnormal ENS development, which is related to the onset of ARM. De Pontual et al. have identified an aberrant CpG dinucleotide methylation within the PHOX2B promoter in neuroblastoma, an embryonic tumor originating from NCCs. This outcome suggests that aberrant methylation patterns within PHOX2B might be also implicated in this pathology [35].
Furthermore, an important role of the methylation level of genes that encode for microRNA (miRNA) has also been described in HSCR. In this sense, miR-141, which belongs the tomiR-200 family that has been highly associated with different human pathologies [36], showed that hypermethylation of a CpG Island within its promoter correlated with its downregulation and the subsequent upregulation of its target genes (CD47 and CUL3) in colon tissues from HSCR patients compared with controls. Moreover, such upregulation of CD47 and CUL3 reduced proliferation and migration of 293T (sub-line of adenovirus-immortalized human embryonic kidney cells) and SH-SY5Y (subline of the neuroblastoma cell line SK-N-SH) cell lines. These results suggest that the methylation status of the promoter of the miR-141 gene might be a key factor in the pathogenesis of HSCR [37].
3. Histone Modifications
Histones are the main binding proteins associated with chromatin, and their association with the compacted DNA strand results in nucleosomes. Each nucleosome consists of four duplicated units of histones (H2A, H2B, H3 and H4), resulting in a structure formed by the combination of eight histones (nucleosome core) around which DNA rolls up with unstructured tails [38]. There are several posttranslational modifications described for the evolutionarily conserved histone tails (methylation, acetylation, deacetylation, phosphorylation, ubiquitination, and/or sumoylation) that regulate gene expression [39,40].
Specifically in eukaryotic cells, histone acetylation is established by two different enzymes, histone acetyltransferases (HATs) and histone deacetylases (HDACs) [41,42,43]. Histone acetylations closely associated with open chromatin are related to gene expression (i.e., H3K27ac) [44,45,46,47,48], whereas histone deacetylation is used to close chromatin, which conducts the repression of gene expression [49].
Various histone acetylation and methylation mechanisms have been associated with NCC development, although their implication in HSCR is still unknown. All this evidence suggests a potential role in the onset of this pathology that should be investigated.
In most cases, the histone acetylation corresponds to cis-regulatory regions in the neural crest. The HDAC repression complex promotes trunk crest cell specification [50] and regulates the migration of NCC [51,52,53]. In addition, HDACs take part in regulating downstream NCCs differentiation. In zebrafish, hdac1 and hdac4 are implicated in various developmental events and are expressed during neural crest cell differentiation [54,55,56]. Regarding human development, HDAC4 also relates to syndromes and other diseases derived from neural crest development [57,58]. For instance, brachydactyly mental retardation syndrome has been associated with haploinsufficiency of HDAC4, including craniofacial and skeletal abnormalities (OMIM#600430) [59]. HDAC3 and HDAC8 are related to the regulation of smooth muscle cell differentiation and cardiac outflow tract development in mice [60]. Specifically, HDAC8 epigenetically regulates skull morphogenesis in NCCs by inhibiting Lhx1 and Otx2 activity [61]. HDACs can maintain or induce the active gene state [62] together with HATs. In this sense, it has been shown that the binding of specific HDACs (HDAC1 and HDAC2) to promoters causes NCC differentiation to peripheral glia [63]. In zebrafish, hdac1 is required for eye development, the central nervous system and NCC populations [54,64,65,66,67]. Ignatius et al. showed the specific requirements for hdac1 function during the development of the neural crest in zebrafish and therefore in ENS formation [54].
Regarding histone methylation, JMJD2A mediates neural crest gene expression by modulating the epigenetic modification H3K9m3, that finally establishes the neural crest in the embryonic stage. JmjD2A knockdown resulted in a drastic loss of Snail2, FoxD3 and Sox10 expression. When H3K9m3 modification is present in the promoter regions of Sox10 and Snail2, their interaction with JMJD2A is unraveled by Chromatin immunoprecipitation (ChIP) assays [68]. Thus, JmjD2A is necessary to correct neural crest establishment during embryo development. Moreover, PHF8, can demethylate the H4K20me1 and H3K9me1 marks close to the start of the transcription site that turns active. Interestingly, this transcriptional regulator was previously associated with the regulation of neural crest development in diverse vertebrate models [69,70,71,72].
The only histone modulator factor that has been related to HSCR thus far is MECP2 (Methyl-CpG binding Protein 2). Its association with HDACs and histone methyltransferases (HTMs) forms stable repressor complexes for gene expression [73]. Zhou et al. identified a decrease in the expression levels of MECP2 in HSCR patients and, interestingly, the downregulation of this gene in SH-SY5Y caused a decline in cell proliferation. Nevertheless, in the methylation level of MECP2, there was no difference when analyzing both groups. Moreover, similar outcomes were found in miR-34b, which is a regulator of MECP2 expression. These results suggest that alteration in the expression level of MECP2 may be relevant in the etiology of HSCR through the regulation of histone modifications [74].
4. Polycomb Repressive Complex (PRC)
This complex is formed by a series of proteins that prevent the transcription of their target genes by catalyzing H3K27me3 epigenetic complex [75,76]. There are two classes of PRC, PRC1 and PRC2, which are implicated in embryonic development and differentiation of neural crest-derived craniofacial structures [77,78]. In this sense, the differentiation of the cranial neural crest in chondrocytes has been reported to be established by EZH2 (the enhancer of the zeste homolog 2), which is a subunit of PRC2, as well as Ring1b/Rnf2 (the single E3 ubiquitin ligase) in PRC1 [77,78,79].
Heterozygous mutant mice, with respect to the Aebp2 gene, which encodes for a component of PRC2 expressed in NCCs [80], show similar phenotypes to HSCR and Waardenburg syndrome patients. Both pathologies arise from defects in the development of NCCs [81,82,83]. Interestingly, these mutants showed an alteration in the neural crest gene expression levels, such as the lower expression of Sox10. This result is similar to the reduced SOX10 dosage frequently observed in Waardenburg syndrome type 4 [83]. Therefore, Aebp2 misregulation might be responsible for HSCR and the Waardenburg syndrome due to an aberrant epigenetic regulation of neural crest genes. In the same way, EED (Embryonic Ectoderm Development), one of the two core catalytic subunits of PRC2 has been described as a regulator of neural crest gene expression during NCC determination and migration [84]. In the HSCR context, there is a significant upregulation of EED in EPCs from HSCR patients with respect to controls [25].
5. ATP-Dependent Chromatin Remodeling
This epigenetic mechanism is mediated by protein complexes, such as CHD (chromodomain helicase DNA-binding), ISWI (imitation switch) and SWI/SNF (mating-type switch/sucrose nonfermenting), that change the structure of chromatin by ATP-hydrolysis. They promote regions with a lack of nucleosomes to facilitate transcription factor binding and binding of other regulatory proteins in these regions [85,86]. Specifically, CHD7 (Chromodomain Helicase DNA Binding Protein 7) together with PBAF (SWI/SNF) [87] induce neural crest specification in embryonic stem cells from humans [88]. Williams syndrome transcription factor is a subunit of WICH and WINAC, both being ATP-dependent chromatin remodeling complexes [89]. Such genes are transcription factor is related to William’s syndrome (OMIM#194050), a developmental disorder that shows alterations in neural crest-derived tissues [90,91]. In summary, all this evidence suggests a possible involvement of this mechanism in ENS formation and therefore in HSCR.
6. NcRNA
Several classes of ncRNA have emerged to play key roles in modulating many cellular processes, such as micro RNA (miRNAs), long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs). In particular, miRNAs are highly conserved RNAs (20–24 nucleotides) that inhibit gene expression by posttranscriptional mechanisms through complementary binding to the 3′-untranslated regions (3′-UTR) of target mRNA [92,93]. LncRNAs are defined as RNA transcripts longer than 200 bp which do not encode for proteins. They play an important regulatory role in gene expression through epigenetic mechanisms (chromatin remodeling, transcriptional and posttranscriptional processing) that finally will determine diverse cellular processes [94]. Finally, circRNAs form covalently closed continuous loop structures through specific splicing methods and work as transcription regulators [95] or as miRNA sponges [96].
Different research studies have related miRNAs, lncRNA and circRNA to HSCR [28,37,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121]. These associations have been based on either their differential expression in HSCR tissues, an aberrant expression of their target genes and, finally, alterations in migration, proliferation and/or apoptosis processes of NCCs during development (Table 1). Their potential role in cellular processes has been analyzed through in vitro approaches using various cell lines such as 293T and SH-SY5Y. Therefore, although much evidence suggests a relationship between ncRNA and HSCR, the role of these ncRNAs in the onset of the disease should be thoroughly clarified.
Table 1.
NcRNAs with a potential role into the pathogenesis of HSCR.
| Role on Cellular Processes | ncRNA/Reference | Expression in HSCR Tissue | Change |
|---|---|---|---|
| proliferation and migration | miR-141 [37] | downregulated | ↑CD47/CUL3 |
| miR-195 [104] | upregulated | ↓DIEXF | |
| miR-200a/141 [106] | downregulated | ↑PTEN | |
| miR-206 [102,112] | downregulated | ↑SDPR/FN1 | |
| miR-192/215 [121] | downregulated | ↑NID1 | |
| miR-218-1 [115] | upregulated | ↑SLIT2 ↓RET/PLAG1 | |
| miR-215 [103] | downregulated | ↓IARS2/↑SIGLEC-8 | |
| miR-369-3p [110] | upregulated | ↓SOX4 | |
| miR-483-3p [119] | downregulated | ↓IGF2 ↑FHL1 | |
| miR-214 [117] | upregulated | ↓PLAGL2 | |
| HOTTIP [118] | downregulated | ↓HOXA13 | |
| miR143HG [100] | upregulated | ↓miR-143/↑RBM24 | |
| AFAP1-AS [99] | downregulated | ↑miR-181a/↓RAP1B | |
| MEG3 [105] | downregulated | ↓miR-770-5p/↑SRGAP1 | |
| FAL1 [107] | downregulated | ↓AKT1 | |
| miR31HG [97] | downregulated | ↓miR-31/31* | |
| LOC100507600 [114] | downregulated | ↑miR128–1-3p/↓BMI1 | |
| cir-ZNF609 [111] | downregulated | ↑miR-150-5p/↓AKT3 | |
| circ-PRKCI [120] | downregulated | ↑miR-1324/↓PLCB1 | |
| cir-CCDC66 [116] | downregulated | ↑miR-488-3p/↓DCX | |
| proliferation and apoptosis | miR-483-5p [28] | upregulated | ↓GFRA4 |
| proliferation | miR-939 [98] | upregulated | ↓LRSAM1 |
| LOC101926975 [113] | downregulated | ↓FGF1 | |
| apoptosis | HN12 [101] | upregulated | - |
| Unknown | HA117 [108,109] | upregulated | ↓DPF3/FOXA1/DUSP6 |
↑: upregulation/↓: downregulation.
Finally, a relationship between 3′UTR RET variants and HSCR has been widely described. Fitze et al. characterized several RET polymorphisms in a group of HSCR patients and controls and found two variants located at the 3′UTR, c.3187+47T>C (rs2075912) and 3′UTR+124A>G) with a strong association with HSCR [122]. In contrast, Griseri et al. identified a “protective” RET haplotype characterized by the presence of an SNP, g.128496T>C (rs3026785) in the 3′UTR of RET [123]. Moreover, they suggested that the protective effect against HSCR of this allele might be due to lower mRNA degradation, which leads to an increase of gene transcripts and probably an increase in the amount of total RET protein. Similarly, Pan et al. screened the RET 3′UTR in the Chinese population and identified a combination of 7 SNPs that seems to act as protective haplotypes [124]. Implication in the miRNA-mediated regulation of gene expression of these RET polymorphisms still needs to be further elucidated.
7. Conclusions
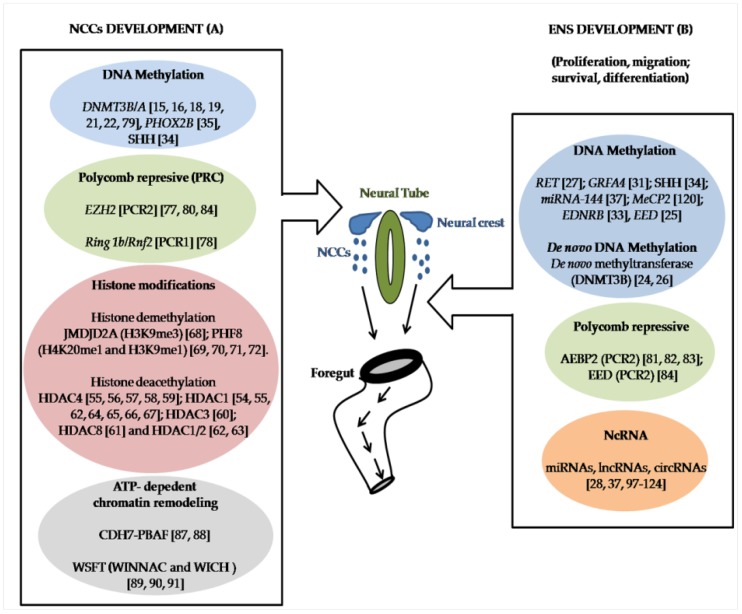
HSCR is a human congenital disorder due to an incorrect process in ENS formation attributed to an aberrant migration, proliferation, differentiation or survival of NCCs. Several epigenetic events have been related to ENS development and HSCR. Nevertheless, their potential role in the context of HSCR is just beginning to be defined. In this review, we have summarized the epigenetic mechanisms at transcriptional and posttranscriptional levels implicated in NCCs, ENS development and HSCR described so far (Figure 1). Nonetheless, additional studies are needed to improve the knowledge about the role of epigenetics in the pathogenesis of HSCR.
Figure 1.
Scheme of the epigenetic processes implicated in Neural Crest Cells (NCCs) and Enteric Nervous System (ENS) development in HSCR context. Epigenetic mechanisms identified in NCC development (A) and in ENS formation (B) with a possible role in the onset of the disease. Adapted from http://www.columbia.edu/itc/hs/medical/humandev/2006/HD10/ENS6.pdf.
Author Contributions
A.T.: major contributor to the writing of the manuscript. L.V.-B., B.L.-T.: writing and reviewing of the manuscript. R.M.F., G.A.: reviewing of the manuscript. S.B.: reviewing of the manuscript and funding acquisition. All authors read and approved the final manuscript.
Funding
This study was supported by Instituto de Salud Carlos III (ISCIII), Spanish Ministry of Economy and competitiveness, Spain and cofounded by European Union (ERDF/ESF, “Investing in your future”) (PI16/01422). CIBERER is an initiative of the ISCIII, Spanish Ministry of Economy and Competitiveness.
Conflicts of Interest
There is no Conflicts of Interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Chakravarti A., Lyonnet S. In: The Metabolic and Molecular Bases of Inherited Disease. 8th ed. Beaudet A.R., Scriver C.R., Sly W., Valle D., editors. McGraw-Hill; New York, NY, USA: 2001. [Google Scholar]
- 2.Amiel J., Sproat-Emison E., Garcia-Barcelo M., Lantieri F., Burzynski G., Borrego S., Pelet A., Arnold S., Miao X., Griseri P., et al. Hirschsprung disease, associated syndromes and genetics: A review. J. Med. Genet. 2008;45:1–14. doi: 10.1136/jmg.2007.053959. [DOI] [PubMed] [Google Scholar]
- 3.Lake J.I., Heuckeroth R.O. Enteric nervous system development: Migration, differentiation, and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;305:G1–G24. doi: 10.1152/ajpgi.00452.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 5.Calvanese V., Lara E., Fraga M.F. Epigenetic code and self-identity. Adv. Exp. Med. Biol. 2012;738:236–255. doi: 10.1007/978-1-4614-1680-7_14. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y., Xiao A. Epigenetic regulation in neural crest development. Birth Defects Res. Part AClin. Mol. Teratol. 2011;91:788–796. doi: 10.1002/bdra.20797. [DOI] [PubMed] [Google Scholar]
- 7.Fujita K., Ogawa R., Kawawaki S., Ito K. Roles of chromatin remodelers in maintenance mechanisms of multipotency of mouse trunk neural crest cells in the formation of neural crest-derived stem cells. Mech. Dev. 2014;133:126–145. doi: 10.1016/j.mod.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Sergi C.M., Caluseriu O., McColl H., Eisenstat D.D. Hirschsprung’s disease: Clinical dysmorphology, genes, micro-RNAs, and future perspectives. Pediatric Res. 2017;81:177–191. doi: 10.1038/pr.2016.202. [DOI] [PubMed] [Google Scholar]
- 9.Rogers J.M. Search for the missing lncs: Gene regulatory networks in neural crest development and long non-coding RNA biomarkers of Hirschsprung’s disease. Neurogastroenterol. Motil. 2016;28:161–166. doi: 10.1111/nmo.12776. [DOI] [PubMed] [Google Scholar]
- 10.Law J.A., Jacobsen S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010;11:204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 12.Ooi S.K., O’Donnell A.H., Bestor T.H. Mammalian cytosine methylation at a glance. J. Cell Sci. 2009;122:2787–2791. doi: 10.1242/jcs.015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu N., Strobl-Mazzulla P.H., Bronner M.E. Epigenetic regulation in neural crest development. Dev. Biol. 2014;396:159–168. doi: 10.1016/j.ydbio.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roellig D., Bronner M.E. The epigenetic modifier DNMT3A is necessary for proper otic placode formation. Dev. Biol. 2016;411:294–300. doi: 10.1016/j.ydbio.2016.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehrlich M., Sanchez C., Shao C., Nishiyama R., Kehrl J., Kuick R., Kubota T., Hanash S.M. ICF, an immunodeficiency syndrome: DNA methyltransferase 3B involvement, chromosome anomalies, and gene dysregulation. Autoimmunity. 2008;41:253–271. doi: 10.1080/08916930802024202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaenisch R., Bird A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003;33:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 17.Linhart H.G., Lin H., Yamada Y., Moran E., Steine E.J., Gokhale S., Lo G., Cantu E., Ehrich M., He T., et al. Dnmt3b promotes tumorigenesis in vivo by gene-specific de novo methylation and transcriptional silencing. Genes Dev. 2007;21:3110–3122. doi: 10.1101/gad.1594007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan X.J., Xu J., Gu Z.H., Pan C.M., Lu G., Shen Y., Shi J.Y., Zhu Y.M., Tang L., Zhang X.W., et al. Exome sequencing identifies somatic mutations of DNA methyltransferase gene DNMT3A in acute monocytic leukemia. Nat. Genet. 2011;43:309–315. doi: 10.1038/ng.788. [DOI] [PubMed] [Google Scholar]
- 19.Okano M., Bell D.W., Haber D.A., Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/S0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 20.Hu N., Strobl-Mazzulla P., Sauka-Spengler T., Bronner M.E. DNA methyltransferase3A as a molecular switch mediating the neural tube-to-neural crest fate transition. Genes Dev. 2012;26:2380–2385. doi: 10.1101/gad.198747.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams M.S., Gammill L.S., Bronner-Fraser M. Discovery of transcription factors and other candidate regulators of neural crest development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2008;237:1021–1033. doi: 10.1002/dvdy.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin B., Tao Q., Peng J., Soo H.M., Wu W., Ying J., Fields C.R., Delmas A.L., Liu X., Qiu J., et al. DNA methyltransferase 3B (DNMT3B) mutations in ICF syndrome lead to altered epigenetic modifications and aberrant expression of genes regulating development, neurogenesis and immune function. Hum. Mol. Genet. 2008;17:690–709. doi: 10.1093/hmg/ddm341. [DOI] [PubMed] [Google Scholar]
- 23.Lui V.C., Cheng W.W., Leon T.Y., Lau D.K., Garcia-Barcelo M.M., Miao X.P., Kam M.K., So M.T., Chen Y., Wall N.A., et al. Perturbation of hoxb5 signaling in vagal neural crests down-regulates ret leading to intestinal hypoganglionosis in mice. Gastroenterology. 2008;134:1104–1115. doi: 10.1053/j.gastro.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Torroglosa A., Enguix-Riego M.V., Fernandez R.M., Roman-Rodriguez F.J., Moya-Jimenez M.J., de Agustin J.C., Antinolo G., Borrego S. Involvement of DNMT3B in the pathogenesis of Hirschsprung disease and its possible role as a regulator of neurogenesis in the human enteric nervous system. Genet. Med. 2014;16:703–710. doi: 10.1038/gim.2014.17. [DOI] [PubMed] [Google Scholar]
- 25.Villalba-Benito L., Torroglosa A., Fernandez R.M., Ruiz-Ferrer M., Moya-Jimenez M.J., Antinolo G., Borrego S. Overexpression of DNMT3b target genes during Enteric Nervous System development contribute to the onset of Hirschsprung disease. Sci. Rep. 2017;7:6221. doi: 10.1038/s41598-017-06539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torroglosa A., Villalba-Benito L., Fernandez R.M., Moya-Jimenez M.J., Antinolo G., Borrego S. Dnmt3b knock-down in enteric precursors reveals a possible mechanism by which this de novo methyltransferase is involved in the enteric nervous system development and the onset of Hirschsprung disease. Oncotarget. 2017;8:106443–106453. doi: 10.18632/oncotarget.22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munnes M., Patrone G., Schmitz B., Romeo G., Doerfler W. A 5′-CG-3′-rich region in the promoter of the transcriptionally frequently silenced RET protooncogene lacks methylated cytidine residues. Oncogene. 1998;17:2573–2583. doi: 10.1038/sj.onc.1202165. [DOI] [PubMed] [Google Scholar]
- 28.Wang G., Guo F., Wang H., Liu W., Zhang L., Cui M., Wu X. Downregulation of microRNA-483-5p Promotes Cell Proliferation and Invasion by Targeting GFRA4 in Hirschsprung’s Disease. DNA Cell Biol. 2017;36:930–937. doi: 10.1089/dna.2017.3821. [DOI] [PubMed] [Google Scholar]
- 29.Yang J., Runeberg-Roos P., Leppanen V.M., Saarma M. The mouse soluble GFRalpha4 receptor activates RET independently of its ligand persephin. Oncogene. 2007;26:3892–3898. doi: 10.1038/sj.onc.1210161. [DOI] [PubMed] [Google Scholar]
- 30.Jing S., Wen D., Yu Y., Holst P.L., Luo Y., Fang M., Tamir R., Antonio L., Hu Z., Cupples R., et al. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 1996;85:1113–1124. doi: 10.1016/S0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- 31.Wang G., Zhang L., Wang H., Cui M., Liu W., Liu Y., Wu X. Demethylation of GFRA4 Promotes Cell Proliferation and Invasion in Hirschsprung Disease. DNA Cell Biol. 2018;37:316–324. doi: 10.1089/dna.2017.3928. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez-Mejias A., Fernandez R.M., Lopez-Alonso M., Antinolo G., Borrego S. New roles of EDNRB and EDN3 in the pathogenesis of Hirschsprung disease. Genet. Med. Off. J. Am. Coll. Med. Genet. 2010;12:39–43. doi: 10.1097/GIM.0b013e3181c371b0. [DOI] [PubMed] [Google Scholar]
- 33.Tang W., Li B., Tang J., Liu K., Qin J., Wu W., Geng Q., Zhang J., Chen H., Xu X., et al. Methylation analysis of EDNRB in human colon tissues of Hirschsprung’s disease. Pediatric Surg. Int. 2013;29:683–688. doi: 10.1007/s00383-013-3308-6. [DOI] [PubMed] [Google Scholar]
- 34.Huang Y., Zhang P., Zheng S., Dong R. Hypermethylation of SHH in the pathogenesis of congenital anorectal malformations. J. Pediatric Surg. 2014;49:1400–1404. doi: 10.1016/j.jpedsurg.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 35.de Pontual L., Trochet D., Bourdeaut F., Thomas S., Etchevers H., Chompret A., Minard V., Valteau D., Brugieres L., Munnich A., et al. Methylation-associated PHOX2B gene silencing is a rare event in human neuroblastoma. Eur. J. Cancer. 2007;43:2366–2372. doi: 10.1016/j.ejca.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 36.Du Y., Xu Y., Ding L., Yao H., Yu H., Zhou T., Si J. Down-regulation of miR-141 in gastric cancer and its involvement in cell growth. J. Gastroenterol. 2009;44:556–561. doi: 10.1007/s00535-009-0037-7. [DOI] [PubMed] [Google Scholar]
- 37.Tang W., Qin J., Tang J., Zhang H., Zhou Z., Li B., Geng Q., Wu W., Xia Y., Xu X. Aberrant reduction of MiR-141 increased CD47/CUL3 in Hirschsprung’s disease. Cell. Physiol. Biochem. 2013;32:1655–1667. doi: 10.1159/000356601. [DOI] [PubMed] [Google Scholar]
- 38.Gibney E.R., Nolan C.M. Epigenetics and gene expression. Heredity. 2010;105:4–13. doi: 10.1038/hdy.2010.54. [DOI] [PubMed] [Google Scholar]
- 39.Berger S.L. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 40.Kouzarides T. SnapShot: Histone-modifying enzymes. Cell. 2007;128:802. doi: 10.1016/j.cell.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 41.de Ruijter A.J., van Gennip A.H., Caron H.N., Kemp S., van Kuilenburg A.B. Histone deacetylases (HDACs): Characterization of the classical HDAC family. Biochem. J. 2003;370:737–749. doi: 10.1042/bj20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marks P.A., Miller T., Richon V.M. Histone deacetylases. Curr. Opin. Pharmacol. 2003;3:344–351. doi: 10.1016/S1471-4892(03)00084-5. [DOI] [PubMed] [Google Scholar]
- 43.Roth S.Y., Denu J.M., Allis C.D. Histone acetyltransferases. Annu. Rev. Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- 44.Bonn S., Zinzen R.P., Girardot C., Gustafson E.H., Perez-Gonzalez A., Delhomme N., Ghavi-Helm Y., Wilczynski B., Riddell A., Furlong E.E. Tissue-specific analysis of chromatin state identifies temporal signatures of enhancer activity during embryonic development. Nat. Genet. 2012;44:148–156. doi: 10.1038/ng.1064. [DOI] [PubMed] [Google Scholar]
- 45.Cotney J., Leng J., Oh S., DeMare L.E., Reilly S.K., Gerstein M.B., Noonan J.P. Chromatin state signatures associated with tissue-specific gene expression and enhancer activity in the embryonic limb. Genome Res. 2012;22:1069–1080. doi: 10.1101/gr.129817.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Creyghton M.P., Cheng A.W., Welstead G.G., Kooistra T., Carey B.W., Steine E.J., Hanna J., Lodato M.A., Frampton G.M., Sharp P.A., et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. USA. 2010;107:21931–21936. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heintzman N.D., Hon G.C., Hawkins R.D., Kheradpour P., Stark A., Harp L.F., Ye Z., Lee L.K., Stuart R.K., Ching C.W., et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459:108–112. doi: 10.1038/nature07829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rada-Iglesias A., Bajpai R., Swigut T., Brugmann S.A., Flynn R.A., Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470:279–283. doi: 10.1038/nature09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahringer J. NuRD and SIN3 histone deacetylase complexes in development. Trends Genet. 2000;16:351–356. doi: 10.1016/S0168-9525(00)02066-7. [DOI] [PubMed] [Google Scholar]
- 50.Murko C., Lagger S., Steiner M., Seiser C., Schoefer C., Pusch O. Histone deacetylase inhibitor Trichostatin A induces neural tube defects and promotes neural crest specification in the chicken neural tube. Differ. Res. Biol. Divers. 2013;85:55–66. doi: 10.1016/j.diff.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 51.Hatta K., Takagi S., Fujisawa H., Takeichi M. Spatial and temporal expression pattern of N-cadherin cell adhesion molecules correlated with morphogenetic processes of chicken embryos. Dev. Biol. 1987;120:215–227. doi: 10.1016/0012-1606(87)90119-9. [DOI] [PubMed] [Google Scholar]
- 52.Nakagawa S., Takeichi M. Neural crest cell-cell adhesion controlled by sequential and subpopulation-specific expression of novel cadherins. Development. 1995;121:1321–1332. doi: 10.1242/dev.121.5.1321. [DOI] [PubMed] [Google Scholar]
- 53.Taneyhill L.A., Coles E.G., Bronner-Fraser M. Snail2 directly represses cadherin6B during epithelial-to-mesenchymal transitions of the neural crest. Development. 2007;134:1481–1490. doi: 10.1242/dev.02834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ignatius M.S., Unal Eroglu A., Malireddy S., Gallagher G., Nambiar R.M., Henion P.D. Distinct functional and temporal requirements for zebrafish Hdac1 during neural crest-derived craniofacial and peripheral neuron development. PloS ONE. 2013;8:e63218. doi: 10.1371/journal.pone.0063218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ignatius M.S., Moose H.E., El-Hodiri H.M., Henion P.D. colgate/hdac1 Repression of foxd3 expression is required to permit mitfa-dependent melanogenesis. Dev. Biol. 2008;313:568–583. doi: 10.1016/j.ydbio.2007.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeLaurier A., Nakamura Y., Braasch I., Khanna V., Kato H., Wakitani S., Postlethwait J.H., Kimmel C.B. Histone deacetylase-4 is required during early cranial neural crest development for generation of the zebrafish palatal skeleton. BMC Dev. Biol. 2012;12:16. doi: 10.1186/1471-213X-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park J.W., Cai J., McIntosh I., Jabs E.W., Fallin M.D., Ingersoll R., Hetmanski J.B., Vekemans M., Attie-Bitach T., Lovett M., et al. High throughput SNP and expression analyses of candidate genes for non-syndromic oral clefts. J. Med. Genet. 2006;43:598–608. doi: 10.1136/jmg.2005.040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alsdorf R., Wyszynski D.F. Teratogenicity of sodium valproate. Expert Opin. Drug Saf. 2005;4:345–353. doi: 10.1517/14740338.4.2.345. [DOI] [PubMed] [Google Scholar]
- 59.Williams S.R., Aldred M.A., Der Kaloustian V.M., Halal F., Gowans G., McLeod D.R., Zondag S., Toriello H.V., Magenis R.E., Elsea S.H. Haploinsufficiency of HDAC4 causes brachydactyly mental retardation syndrome, with brachydactyly type E, developmental delays, and behavioral problems. Am. J. Hum. Genet. 2010;87:219–228. doi: 10.1016/j.ajhg.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh N., Trivedi C.M., Lu M., Mullican S.E., Lazar M.A., Epstein J.A. Histone deacetylase 3 regulates smooth muscle differentiation in neural crest cells and development of the cardiac outflow tract. Circ. Res. 2011;109:1240–1249. doi: 10.1161/CIRCRESAHA.111.255067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haberland M., Mokalled M.H., Montgomery R.L., Olson E.N. Epigenetic control of skull morphogenesis by histone deacetylase 8. Genes Dev. 2009;23:1625–1630. doi: 10.1101/gad.1809209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Z., Zang C., Cui K., Schones D.E., Barski A., Peng W., Zhao K. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell. 2009;138:1019–1031. doi: 10.1016/j.cell.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jacob C., Lotscher P., Engler S., Baggiolini A., Varum Tavares S., Brugger V., John N., Buchmann-Moller S., Snider P.L., Conway S.J., et al. HDAC1 and HDAC2 control the specification of neural crest cells into peripheral glia. J. Neurosci. 2014;34:6112–6122. doi: 10.1523/JNEUROSCI.5212-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cunliffe V.T. Histone deacetylase 1 is required to repress Notch target gene expression during zebrafish neurogenesis and to maintain the production of motoneurones in response to hedgehog signalling. Development. 2004;131:2983–2995. doi: 10.1242/dev.01166. [DOI] [PubMed] [Google Scholar]
- 65.Nambiar R.M., Henion P.D. Sequential antagonism of early and late Wnt-signaling by zebrafish colgate promotes dorsal and anterior fates. Dev. Biol. 2004;267:165–180. doi: 10.1016/j.ydbio.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 66.Stadler J.A., Shkumatava A., Norton W.H., Rau M.J., Geisler R., Fischer S., Neumann C.J. Histone deacetylase 1 is required for cell cycle exit and differentiation in the zebrafish retina. Dev. Dyn. 2005;233:883–889. doi: 10.1002/dvdy.20427. [DOI] [PubMed] [Google Scholar]
- 67.Yamaguchi M., Tonou-Fujimori N., Komori A., Maeda R., Nojima Y., Li H., Okamoto H., Masai I. Histone deacetylase 1 regulates retinal neurogenesis in zebrafish by suppressing Wnt and Notch signaling pathways. Development. 2005;132:3027–3043. doi: 10.1242/dev.01881. [DOI] [PubMed] [Google Scholar]
- 68.Strobl-Mazzulla P.H., Sauka-Spengler T., Bronner-Fraser M. Histone demethylase JmjD2A regulates neural crest specification. Dev. Cell. 2010;19:460–468. doi: 10.1016/j.devcel.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maxson R., Ishii M. The Bmp pathway in skull vault development. Front. Oral Biol. 2008;12:197–208. doi: 10.1159/0000115042. [DOI] [PubMed] [Google Scholar]
- 70.Monsoro-Burq A.H., Wang E., Harland R. Msx1 and Pax3 cooperate to mediate FGF8 and WNT signals during Xenopus neural crest induction. Dev. Cell. 2005;8:167–178. doi: 10.1016/j.devcel.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 71.Phillips B.T., Kwon H.J., Melton C., Houghtaling P., Fritz A., Riley B.B. Zebrafish msxB, msxC and msxE function together to refine the neural-nonneural border and regulate cranial placodes and neural crest development. Dev. Biol. 2006;294:376–390. doi: 10.1016/j.ydbio.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 72.Takahashi K., Nuckolls G.H., Takahashi I., Nonaka K., Nagata M., Ikura T., Slavkin H.C., Shum L. Msx2 is a repressor of chondrogenic differentiation in migratory cranial neural crest cells. Dev. Dyn. 2001;222:252–262. doi: 10.1002/dvdy.1185. [DOI] [PubMed] [Google Scholar]
- 73.McGinty J.F. The many faces of MeCP2. Neuropsychopharmacology. 2012;37:313–314. doi: 10.1038/npp.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou Z., Qin J., Tang J., Li B., Geng Q., Jiang W., Wu W., Rehan V., Tang W., Xu X., et al. Down-regulation of MeCP2 in Hirschsprung’s disease. J. Pediatric Surg. 2013;48:2099–2105. doi: 10.1016/j.jpedsurg.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 75.Mikkelsen T.S., Ku M., Jaffe D.B., Issac B., Lieberman E., Giannoukos G., Alvarez P., Brockman W., Kim T.K., Koche R.P., et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schuettengruber B., Chourrout D., Vervoort M., Leblanc B., Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–745. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 77.Schwarz D., Varum S., Zemke M., Scholer A., Baggiolini A., Draganova K., Koseki H., Schubeler D., Sommer L. Ezh2 is required for neural crest-derived cartilage and bone formation. Development. 2014;141:867–877. doi: 10.1242/dev.094342. [DOI] [PubMed] [Google Scholar]
- 78.van der Velden Y.U., Wang L., Querol Cano L., Haramis A.P. The polycomb group protein ring1b/rnf2 is specifically required for craniofacial development. Plos ONE. 2013;8:e73997. doi: 10.1371/journal.pone.0073997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martins-Taylor K., Schroeder D.I., LaSalle J.M., Lalande M., Xu R.H. Role of DNMT3B in the regulation of early neural and neural crest specifiers. Epigenetics. 2012;7:71–82. doi: 10.4161/epi.7.1.18750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim H., Kang K., Kim J. AEBP2 as a potential targeting protein for Polycomb Repression Complex PRC2. Nucleic Acids Res. 2009;37:2940–2950. doi: 10.1093/nar/gkp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahola J.A., Koivusalo A., Sairanen H., Jokinen E., Rintala R.J., Pakarinen M.P. Increased incidence of Hirschsprung’s disease in patients with hypoplastic left heart syndrome--a common neural crest-derived etiology? J. Pediatric Surg. 2009;44:1396–1400. doi: 10.1016/j.jpedsurg.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 82.Inoue K., Shilo K., Boerkoel C.F., Crowe C., Sawady J., Lupski J.R., Agamanolis D.P. Congenital hypomyelinating neuropathy, central dysmyelination, and Waardenburg-Hirschsprung disease: Phenotypes linked by SOX10 mutation. Ann. Neurol. 2002;52:836–842. doi: 10.1002/ana.10404. [DOI] [PubMed] [Google Scholar]
- 83.Kim H., Kang K., Ekram M.B., Roh T.Y., Kim J. Aebp2 as an epigenetic regulator for neural crest cells. Plos ONE. 2011;6:e25174. doi: 10.1371/journal.pone.0025174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tien C.L., Jones A., Wang H., Gerigk M., Nozell S., Chang C. Snail2/Slug cooperates with Polycomb repressive complex 2 (PRC2) to regulate neural crest development. Development. 2015;142:722–731. doi: 10.1242/dev.111997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kwon C.S., Wagner D. Unwinding chromatin for development and growth: A few genes at a time. Trends Genet. 2007;23:403–412. doi: 10.1016/j.tig.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 86.Wu J.I., Lessard J., Crabtree G.R. Understanding the words of chromatin regulation. Cell. 2009;136:200–206. doi: 10.1016/j.cell.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muchardt C., Yaniv M. When the SWI/SNF complex remodels...the cell cycle. Oncogene. 2001;20:3067–3075. doi: 10.1038/sj.onc.1204331. [DOI] [PubMed] [Google Scholar]
- 88.Bajpai R., Chen D.A., Rada-Iglesias A., Zhang J., Xiong Y., Helms J., Chang C.P., Zhao Y., Swigut T., Wysocka J. CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature. 2010;463:958–962. doi: 10.1038/nature08733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barnett C., Krebs J.E. WSTF does it all: A multifunctional protein in transcription, repair, and replication. Biochem. Cell Biol. = Biochim. Et Biol. Cell. 2011;89:12–23. doi: 10.1139/O10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barnett C., Yazgan O., Kuo H.C., Malakar S., Thomas T., Fitzgerald A., Harbour W., Henry J.J., Krebs J.E. Williams Syndrome Transcription Factor is critical for neural crest cell function in Xenopus laevis. Mech. Dev. 2012;129:324–338. doi: 10.1016/j.mod.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoshimura K., Kitagawa H., Fujiki R., Tanabe M., Takezawa S., Takada I., Yamaoka I., Yonezawa M., Kondo T., Furutani Y., et al. Retraction for Yoshimura et al., Distinct function of 2 chromatin remodeling complexes that share a common subunit, Williams syndrome transcription factor (WSTF) Proc. Natl. Acad. Sci. USA. 2014;111:2398. doi: 10.1073/pnas.1323397111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hausser J., Zavolan M. Identification and consequences of miRNA-target interactions--beyond repression of gene expression. Nat. Rev. Genet. 2014;15:599–612. doi: 10.1038/nrg3765. [DOI] [PubMed] [Google Scholar]
- 93.Pillai R.S. MicroRNA function: Multiple mechanisms for a tiny RNA? RNA. 2005;11:1753–1761. doi: 10.1261/rna.2248605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cao J. The functional role of long non-coding RNAs and epigenetics. Biol. Proced. Online. 2014;16:11. doi: 10.1186/1480-9222-16-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Z., Huang C., Bao C., Chen L., Lin M., Wang X., Zhong G., Yu B., Hu W., Dai L., et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015;22:256–264. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 96.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 97.Cai P., Li H., Huo W., Zhu H., Xu C., Zang R., Lv W., Xia Y., Tang W. Aberrant expression of LncRNA-MIR31HG regulates cell migration and proliferation by affecting miR-31 and miR-31* in Hirschsprung’s disease. J. Cell. Biochem. 2018;119:8195–8203. doi: 10.1002/jcb.26830. [DOI] [PubMed] [Google Scholar]
- 98.Chen G., Du C., Shen Z., Peng L., Xie H., Zang R., Li H., Xia Y., Tang W. MicroRNA-939 inhibits cell proliferation via targeting LRSAM1 in Hirschsprung’s disease. Aging. 2017;9:2471–2479. doi: 10.18632/aging.101331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen G., Peng L., Zhu Z., Du C., Shen Z., Zang R., Su Y., Xia Y., Tang W. LncRNA AFAP1-AS Functions as a Competing Endogenous RNA to Regulate RAP1B Expression by sponging miR-181a in the HSCR. Int. J. Med. Sci. 2017;14:1022–1030. doi: 10.7150/ijms.18392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Du C., Shen Z., Zang R., Xie H., Li H., Chen P., Hang B., Xu X., Tang W., Xia Y. Negative feedback circuitry between MIR143HG and RBM24 in Hirschsprung disease. Biochim. Et Biophys. Acta. 2016;1862:2127–2136. doi: 10.1016/j.bbadis.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 101.Du C., Xie H., Zang R., Shen Z., Li H., Chen P., Xu X., Xia Y., Tang W. Apoptotic neuron-secreted HN12 inhibits cell apoptosis in Hirschsprung’s disease. Int. J. Nanomed. 2016;11:5871–5881. doi: 10.2147/IJN.S114838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Budi N.Y.P., Kalim A.S., Santiko W., Musthofa F.D., Iskandar K., Makhmudi A. Aberrant expressions of miRNA-206 target, FN1, in multifactorial Hirschsprung disease. Orphanet J. Rare Dis. 2019;14:5. doi: 10.1186/s13023-018-0973-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lei H., Li H., Xie H., Du C., Xia Y., Tang W. Role of MiR-215 in Hirschsprung’s Disease Pathogenesis by Targeting SIGLEC-8. Cell. Physiol. Biochem. 2016;40:1646–1655. doi: 10.1159/000453214. [DOI] [PubMed] [Google Scholar]
- 104.Lei H., Tang J., Li H., Zhang H., Lu C., Chen H., Li W., Xia Y., Tang W. MiR-195 affects cell migration and cell proliferation by down-regulating DIEXF in Hirschsprung’s disease. BMC Gastroenterol. 2014;14:123. doi: 10.1186/1471-230X-14-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li H., Li B., Zhu D., Xie H., Du C., Xia Y., Tang W. Downregulation of lncRNA MEG3 and miR-770-5p inhibit cell migration and proliferation in Hirschsprung’s disease. Oncotarget. 2017;8:69722–69730. doi: 10.18632/oncotarget.19207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li H., Tang J., Lei H., Cai P., Zhu H., Li B., Xu X., Xia Y., Tang W. Decreased MiR-200a/141 suppress cell migration and proliferation by targeting PTEN in Hirschsprung’s disease. Cell. Physiol. Biochem. 2014;34:543–553. doi: 10.1159/000363021. [DOI] [PubMed] [Google Scholar]
- 107.Li Y., Zhou L., Lu C., Shen Q., Su Y., Zhi Z., Wu F., Zhang H., Wen Z., Chen G., et al. Long non-coding RNA FAL1 functions as a ceRNA to antagonize the effect of miR-637 on the down-regulation of AKT1 in Hirschsprung’s disease. Cell Prolif. 2018;51:e12489. doi: 10.1111/cpr.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu H., Luo Y., Li S., Wang S., Wang N., Jin X. Expression profiles of HA117 and its neighboring gene DPF3 in different colon segments of Hirschsprung’s disease. Int. J. Clin. Exp. Pathol. 2014;7:3966–3974. [PMC free article] [PubMed] [Google Scholar]
- 109.Luo Y., Li S., Teng Y., Wang N., Li L., Liu H., Jin X. Differential expression of FOXA1, DUSP6, and HA117 in colon segments of Hirschsprung’s disease. Int. J. Clin. Exp. Pathol. 2015;8:3979–3986. [PMC free article] [PubMed] [Google Scholar]
- 110.Pan W., Yu H., Zheng B., Gao Y., Li P., Huang Q., Xie C., Ge X. Upregulation of MiR-369-3p suppresses cell migration and proliferation by targeting SOX4 in Hirschsprung’s disease. J. Pediatric Surg. 2017;52:1363–1370. doi: 10.1016/j.jpedsurg.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 111.Peng L., Chen G., Zhu Z., Shen Z., Du C., Zang R., Su Y., Xie H., Li H., Xu X., et al. Circular RNA ZNF609 functions as a competitive endogenous RNA to regulate AKT3 expression by sponging miR-150-5p in Hirschsprung’s disease. Oncotarget. 2017;8:808–818. doi: 10.18632/oncotarget.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sharan A., Zhu H., Xie H., Li H., Tang J., Tang W., Zhang H., Xia Y. Down-regulation of miR-206 is associated with Hirschsprung disease and suppresses cell migration and proliferation in cell models. Sci. Rep. 2015;5:9302. doi: 10.1038/srep09302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shen Z., Peng L., Zhu Z., Xie H., Zang R., Du C., Chen G., Li H., Xia Y., Tang W. Downregulated Expression of Long Non-Coding RNA LOC101926975 Impairs both Cell Proliferation and Cell Cycle and Its Clinical Implication in Hirschsprung Disease Patients. Int. J. Med. Sci. 2016;13:292–297. doi: 10.7150/ijms.14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Su Y., Wen Z., Shen Q., Zhang H., Peng L., Chen G., Zhu Z., Du C., Xie H., Li H., et al. Long non-coding RNA LOC100507600 functions as a competitive endogenous RNA to regulate BMI1 expression by sponging miR128-1-3p in Hirschsprung’s disease. Cell Cycle. 2018;17:459–467. doi: 10.1080/15384101.2017.1403688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tang W., Tang J., He J., Zhou Z., Qin Y., Qin J., Li B., Xu X., Geng Q., Jiang W., et al. SLIT2/ROBO1-miR-218-1-RET/PLAG1: A new disease pathway involved in Hirschsprung’s disease. J. Cell. Mol. Med. 2015;19:1197–1207. doi: 10.1111/jcmm.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wen Z., Shen Q., Zhang H., Su Y., Zhu Z., Chen G., Peng L., Li H., Du C., Xie H., et al. Circular RNA CCDC66 targets DCX to regulate cell proliferation and migration by sponging miR-488-3p in Hirschsprung’s disease. J. Cell. Physiol. 2018 doi: 10.1002/jcp.27733. [DOI] [PubMed] [Google Scholar]
- 117.Wu L., Yuan W., Chen J., Zhou Z., Shu Y., Ji J., Liu Z., Tang Q., Zhang X., Shu X. Increased miR-214 expression suppresses cell migration and proliferation in Hirschsprung disease by interacting with PLAGL2. Pediatric Res. 2019 doi: 10.1038/s41390-019-0324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xie H., Zhu D., Xu C., Zhu H., Chen P., Li H., Liu X., Xia Y., Tang W. Long none coding RNA HOTTIP/HOXA13 act as synergistic role by decreasing cell migration and proliferation in Hirschsprung disease. Biochem. Biophys. Res. Commun. 2015;463:569–574. doi: 10.1016/j.bbrc.2015.05.096. [DOI] [PubMed] [Google Scholar]
- 119.Zhi Z., Zhu H., Lv X., Lu C., Li Y., Wu F., Zhou L., Li H., Tang W. IGF2-derived miR-483-3p associated with Hirschsprung’s disease by targeting FHL1. J. Cell. Mol. Med. 2018;22:4913–4921. doi: 10.1111/jcmm.13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou L., Li Y., Jiang W., Zhang H., Wen Z., Su Y., Wu F., Zhi Z., Shen Q., Li H., et al. Down-regulation of circ-PRKCI inhibits cell migration and proliferation in Hirschsprung disease by suppressing the expression of miR-1324 target PLCB1. Cell Cycle. 2018;17:1092–1101. doi: 10.1080/15384101.2018.1480210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhu D., Xie H., Li H., Cai P., Zhu H., Xu C., Chen P., Sharan A., Xia Y., Tang W. Nidogen-1 is a common target of microRNAs MiR-192/215 in the pathogenesis of Hirschsprung’s disease. J. Neurochem. 2015;134:39–46. doi: 10.1111/jnc.13118. [DOI] [PubMed] [Google Scholar]
- 122.Fitze G., Schierz M., Kuhlisch E., Schreiber M., Ziegler A., Roesner D., Schackert H.K. Novel intronic polymorphisms in the RET proto-oncogene and their association with Hirschsprung disease. Hum. Mutat. 2003;22:177. doi: 10.1002/humu.9161. [DOI] [PubMed] [Google Scholar]
- 123.Griseri P., Lantieri F., Puppo F., Bachetti T., Di Duca M., Ravazzolo R., Ceccherini I. A common variant located in the 3′UTR of the RET gene is associated with protection from Hirschsprung disease. Hum. Mutat. 2007;28:168–176. doi: 10.1002/humu.20397. [DOI] [PubMed] [Google Scholar]
- 124.Pan Z.W., Luo C.F., Liu Z.J., Li J.C. RET 3′UTR polymorphisms and its protective role in Hirschsprung disease in southeastern Chinese. J. Pediatric Surg. 2012;47:1699–1705. doi: 10.1016/j.jpedsurg.2012.03.057. [DOI] [PubMed] [Google Scholar]