Absolute pitch (AP) refers to the rare ability to effortlessly identify and/or produce the pitch of a sound without a reference pitch (Deutsch, 2013). Since AP has been proposed to constitute an ideal model for investigating the joint influences of genes and environment on the development of a highly specific ability and its neural underpinnings, it has intrigued researchers in music science, neuroscience, psychology, and genetics (Zatorre, 2003). Over the last 25 years, because of the advent of structural and functional neuroimaging, an increasing number of studies have begun to uncover the neural substrates of AP by comparing AP musicians with non-AP musicians. Nevertheless, our knowledge about the neural mechanisms underlying AP is still limited.
A recent Journal of Neuroscience article set forth to shed more light on the neural mechanisms that allow AP musicians to categorize tones as easily as most people name the colors of objects (McKetton et al., 2019). The study included 20 AP musicians, 20 non-AP musicians, and 20 non-musician control subjects. The three groups were well matched for demographic characteristics, and the two musician groups were additionally matched for their primary instruments, age of onset of musical training, and number of training hours per week. The authors used fMRI during acoustic stimulation with ascending and descending pure tone sweeps (“chirps”) to map neural responses in the primary auditory cortex (A1), the rostral part of auditory cortex (R), and the rostral-temporal part of auditory cortex (RT). All of these areas are part of Heschl's gyrus located on the supratemporal plane. Importantly, evidence from both humans and monkeys suggests that A1, R, and RT show tonotopic gradients, characterized by spatially organized neuronal populations, which are particularly sensitive to specific sound frequencies (i.e., center frequency). Moreover, neuronal populations in these regions show a characteristic tuning width determined by their frequency selectivity. In particular, populations with a broad tuning width respond to many frequencies, whereas populations with a sharper tuning width respond to fewer frequencies (Moerel et al., 2014). McKetton et al. (2019) computationally modeled the neural responses using population receptive field mapping (Dumoulin and Wandell, 2008) to create individual tonotopic maps of A1, R, and RT, determining both center frequencies and tuning sharpness of single voxels within these areas. In addition, they manually delineated these core areas of auditory cortex and calculated their respective volumes using morphometric techniques.
The McKetton et al. (2019) analyses revealed an interesting constellation of results: First, the authors observed overall larger bilateral Heschl's gyri in AP musicians compared with both non-AP musicians and controls, and these enlargements were found in A1, R, and RT. Second, and contrary to the authors' original hypothesis of sharper frequency tuning in AP musicians, the larger volumes were paralleled by broader frequency tuning in A1 and R in AP musicians compared with non-AP musicians. The authors concluded that the enlarged core areas form the anatomical basis for the engagement of a larger number of neuronal populations during sound processing in AP musicians (McKetton et al., 2019). However, an important question that remains largely unanswered is how these findings are related to previous studies demonstrating structural and functional alterations in AP and non-AP musicians.
Many of the previous studies on brain structure in AP and non-AP musicians found alterations in regions related to lower-level auditory perception. For example, Wengenroth et al. (2014) observed an increased volume of the right Heschl's gyrus in AP musicians compared with non-AP musicians. Moreover, earlier studies showed a substantially larger anteromedial area of bilateral Heschl's gyri, which corresponds approximately to core area A1, in non-AP musicians compared with controls (Schneider et al., 2002). Apart from primary auditory areas, the planum temporale, a secondary auditory region located immediately posterior to Heschl's gyrus, has been prominently linked to AP. In a seminal study, Schlaug et al. (1995) discovered a stronger leftward asymmetry of the planum temporale in AP compared with non-AP musicians. Subsequent studies have found that this asymmetry is mainly driven by a smaller right planum temporale in AP musicians (e.g., Keenan et al., 2001). Findings of a larger Heschl's gyrus and a smaller planum temporale in AP musicians can be easily reconciled with the notion that AP and non-AP musicians differ in their distribution of cortical volume rather than the absolute volume itself (Wengenroth et al., 2014). Here, McKetton et al. (2019) make an important contribution in explaining the perceptual function underlying enlarged Heschl's gyrus areas in AP musicians, namely, the broader frequency tuning of neuronal populations in response to acoustic stimulation.
Previous studies on brain function in AP and non-AP musicians found evidence for alterations in sensory and higher-order brain regions. Regarding the former, there is evidence for enhanced neural responses in AP in Heschl's gyrus and the planum temporale during acoustic stimulation (Wengenroth et al., 2014; Leipold et al., 2019) and altered connectivity patterns in Heschl's gyrus during rest (Brauchli et al., 2019). In addition, the planum polare, which is part of the auditory association cortex and located immediately anterior to Heschl's gyrus, has recently been implicated in AP (Kim and Knösche, 2017). On the other hand, there is also evidence for functional alterations in hierarchically higher-order regions, for instance, the superior temporal sulcus (Schulze et al., 2009), the inferior frontal gyrus (Zatorre et al., 1998; Wengenroth et al., 2014), and the DLPFC (Zatorre et al., 1998; but see Leipold et al., 2019).
Functional alterations in higher-order regions are consistent with an influential theory proposing that AP relies on two cognitive processes, namely, long-term memory representations of pitches and their association with meaningful labels (e.g., C# or A) (Levitin, 1994; Levitin and Rogers, 2005). Findings of alterations in sensory brain regions, as in the study of McKetton et al. (2019), might point to a differential categorization of pitches into narrower categories at an earlier stage of perception (Zatorre, 2003). In light of the findings of McKetton et al. (2019), together with previous evidence of brain alterations in AP musicians, a question that arises is as follows: How does the broader frequency tuning in auditory core areas translate to stronger responses in secondary auditory regions and finally to the defining feature of AP, the labeling of pitches, which is presumably mediated by higher-order regions supporting cognitive functions?
In this context, the widely acknowledged dual-stream models of auditory processing might provide an interesting framework for future investigations (Fig. 1). Broadly speaking, these models differentiate a ventral stream, projecting from primary auditory areas along anterior (e.g., the planum polare) and middle temporal regions to the inferior frontal gyrus, and a dorsal stream, projecting from primary areas via the planum temporale to the DLPFC (Rauschecker and Scott, 2009). Currently, there is little doubt that the ventral stream is involved in processing spectrally complex sounds and facilitates auditory object recognition. The dorsal stream supports sensory-motor integration, articulation, and verbal memory functions (López-Barroso et al., 2013). Since AP can be understood as a particular form of auditory object recognition where sounds are perceived as discrete pitch categories (pitch categorization) and are mapped onto meaningful labels (pitch labeling), it is conceivable that the ventral stream plays a major role in AP (compare Kim and Knösche, 2017). This notion is compatible with human and animal studies demonstrating that auditory object recognition, including speech sounds, animal vocalizations, and environmental sounds, strongly depends upon the functional integrity of the ventral stream (Rauschecker and Scott, 2009). Indeed, the superior temporal sulcus, which is part of the ventral stream and crucial in sound categorization and recognition, has previously been linked to pitch categorization in AP musicians (Schulze et al., 2009). On the other hand, the dorsal stream might facilitate the access to verbal codes necessary for pitch labeling in AP musicians. Furthermore, AP-specific processing in the dorsal stream might also vary as a function of task demands, for instance, whether subjects are instructed to verbally or nonverbally respond, or not respond at all, during acoustic stimulation. Although these thoughts on the role of the two auditory streams in AP are largely speculative, integrating accounts of sensory and higher-order alterations will be crucial for future investigations into the neural substrates of perception and cognition in AP.
Figure 1.
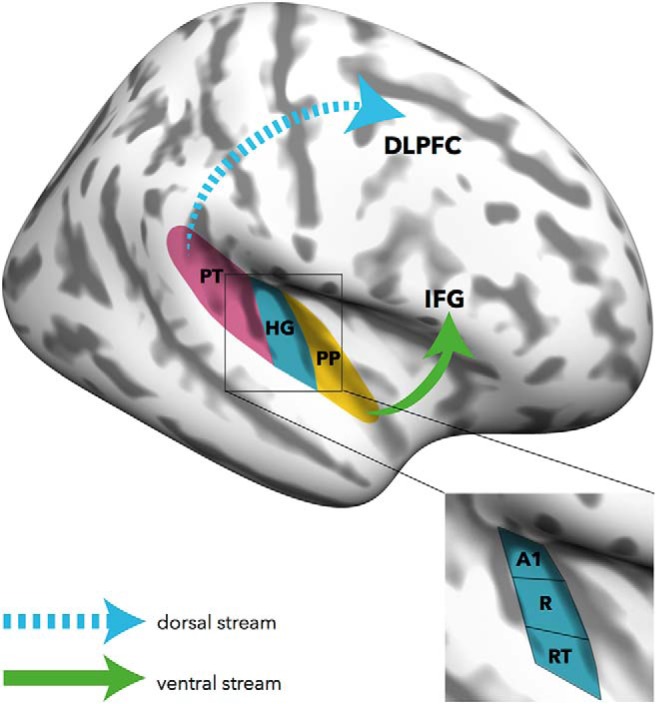
Ventral and dorsal streams in absolute pitch processing. Brain regions that have previously been associated with absolute pitch might be integrated in the context of dual-stream models of auditory processing. Apart from larger cortical volumes and broader frequency tuning in core areas of Heschl's gyrus (HG), absolute pitch-specific structural and functional alterations have been identified in the planum temporale (PT) and the planum polare (PP), but also in higher-order regions, such as the inferior frontal gyrus (IFG) and the DLPFC. The ventral stream might underlie pitch categorization in absolute pitch, whereas the dorsal stream might facilitate access to verbal codes necessary for pitch labeling. A1 = primary auditory cortex; R = rostral part of auditory cortex; RT = rostral-temporal part of auditory cortex.
In conclusion, the study of McKetton et al. (2019) advances our understanding of AP-specific functional and structural alterations in sensory brain regions associated with early stages of auditory perception. Future directions for AP research are the integration of findings concerning sensory regions with the frequently reported alterations in higher-order regions associated with cognition. The role of the two auditory streams in AP could provide a sensible framework for future investigations into this fascinating phenomenon.
Footnotes
Editor's Note: These short reviews of recent JNeurosci articles, written exclusively by students or postdoctoral fellows, summarize the important findings of the paper and provide additional insight and commentary. If the authors of the highlighted article have written a response to the Journal Club, the response can be found by viewing the Journal Club at www.jneurosci.org. For more information on the format, review process, and purpose of Journal Club articles, please see http://www.jneurosci.org/content/jneurosci-journal-club.
S.L. and M.G. were supported by Swiss National Science Foundation Grant 320030_163149 (to Lutz Jäncke). We thank Professor Lutz Jäncke for support; Chantal Oderbolz for critical reading of the manuscript; and Isabel Hotz for assistance in creating the figure.
The authors declare no competing financial interests.
References
- Brauchli C, Leipold S, Jäncke L (2019) Univariate and multivariate analyses of functional networks in absolute pitch. Neuroimage 189:241–247. 10.1016/j.neuroimage.2019.01.021 [DOI] [PubMed] [Google Scholar]
- Deutsch D. (2013) Absolute pitch. In: The psychology of music, pp 141–182. Amsterdam: Elsevier. [Google Scholar]
- Dumoulin SO, Wandell BA (2008) Population receptive field estimates in human visual cortex. Neuroimage 39:647–660. 10.1016/j.neuroimage.2007.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan JP, Thangaraj V, Halpern AR, Schlaug G (2001) Absolute pitch and planum temporale. Neuroimage 14:1402–1408. 10.1006/nimg.2001.0925 [DOI] [PubMed] [Google Scholar]
- Kim SG, Knösche TR (2017) Resting state functional connectivity of the ventral auditory pathway in musicians with absolute pitch. Hum Brain Mapp 38:3899–3916. 10.1002/hbm.23637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipold S, Brauchli C, Greber M, Jäncke L (2019) Absolute and relative pitch processing in the human brain: neural and behavioral evidence. Brain Struct Funct. Advance online publication. Retrieved April 9, 2019. doi: 10.1007/s00429-019-01872-2. 10.1007/s00429-019-01872-2 [DOI] [PubMed] [Google Scholar]
- Levitin DJ. (1994) Absolute memory for musical pitch: evidence from the production of learned melodies. Percept Psychophys 56:414–423. 10.3758/BF03206733 [DOI] [PubMed] [Google Scholar]
- Levitin DJ, Rogers SE (2005) Absolute pitch: perception, coding, and controversies. Trends Cogn Sci 9:26–33. 10.1016/j.tics.2004.11.007 [DOI] [PubMed] [Google Scholar]
- López-Barroso D, Catani M, Ripollés P, Dell'Acqua F, Rodríguez-Fornells A, de Diego-Balaguer R (2013) Word learning is mediated by the left arcuate fasciculus. Proc Natl Acad Sci U S A 110:13168–13173. 10.1073/pnas.1301696110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKetton L, DeSimone K, Schneider KA (2019) Larger auditory cortical area and broader frequency tuning underlie absolute pitch. J Neurosci 39:2930–2937. 10.1523/JNEUROSCI.1532-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerel M, De Martino F, Formisano E (2014) An anatomical and functional topography of human auditory cortical areas. Front Neurosci 8:225. 10.3389/fnins.2014.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Scott SK (2009) Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat Neurosci 12:718–724. 10.1038/nn.2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Jäncke L, Huang Y, Steinmetz H (1995) In vivo evidence of structural brain asymmetry in musicians. Science 267:699–701. 10.1126/science.7839149 [DOI] [PubMed] [Google Scholar]
- Schneider P, Scherg M, Dosch HG, Specht HJ, Gutschalk A, Rupp A (2002) Morphology of Heschl's gyrus reflects enhanced activation in the auditory cortex of musicians. Nat Neurosci 5:688–694. 10.1038/nn871 [DOI] [PubMed] [Google Scholar]
- Schulze K, Gaab N, Schlaug G (2009) Perceiving pitch absolutely: comparing absolute and relative pitch possessors in a pitch memory task. BMC Neurosci 10:106. 10.1186/1471-2202-10-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengenroth M, Blatow M, Heinecke A, Reinhardt J, Stippich C, Hofmann E, Schneider P (2014) Increased volume and function of right auditory cortex as a marker for absolute pitch. Cereb Cortex 24:1127–1137. 10.1093/cercor/bhs391 [DOI] [PubMed] [Google Scholar]
- Zatorre RJ. (2003) Absolute pitch: a model for understanding the influence of genes and development on neural and cognitive function. Nat Neurosci 6:692–695. 10.1038/nn1085 [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Perry DW, Beckett CA, Westbury CF, Evans AC (1998) Functional anatomy of musical processing in listeners with absolute pitch and relative pitch. Proc Natl Acad Sci U S A 95:3172–3177. 10.1073/pnas.95.6.3172 [DOI] [PMC free article] [PubMed] [Google Scholar]


