Abstract
Objectives
To determine the rate of a first recurrent venous thromboembolism (VTE) event after discontinuation of anticoagulant treatment in patients with a first episode of unprovoked VTE, and the cumulative incidence for recurrent VTE up to 10 years.
Design
Systematic review and meta-analysis.
Data sources
Medline, Embase, and the Cochrane Central Register of Controlled Trials (from inception to 15 March 2019).
Study selection
Randomised controlled trials and prospective cohort studies reporting symptomatic recurrent VTE after discontinuation of anticoagulant treatment in patients with a first unprovoked VTE event who had completed at least three months of treatment.
Data extraction and synthesis
Two investigators independently screened studies, extracted data, and appraised risk of bias. Data clarifications were sought from authors of eligible studies. Recurrent VTE events and person years of follow-up after discontinuation of anticoagulant treatment were used to calculate rates for individual studies, and data were pooled using random effects meta-analysis. Sex and site of initial VTE were investigated as potential sources of between study heterogeneity.
Results
18 studies involving 7515 patients were included in the analysis. The pooled rate of recurrent VTE per 100 person years after discontinuation of anticoagulant treatment was 10.3 events (95% confidence interval 8.6 to 12.1) in the first year, 6.3 (5.1 to 7.7) in the second year, 3.8 events/year (95% confidence interval 3.2 to 4.5) in years 3-5, and 3.1 events/year (1.7 to 4.9) in years 6-10. The cumulative incidence for recurrent VTE was 16% (95% confidence interval 13% to 19%) at 2 years, 25% (21% to 29%) at 5 years, and 36% (28% to 45%) at 10 years. The pooled rate of recurrent VTE per 100 person years in the first year was 11.9 events (9.6 to 14.4) for men and 8.9 events (6.8 to 11.3) for women, with a cumulative incidence for recurrent VTE of 41% (28% to 56%) and 29% (20% to 38%), respectively, at 10 years. Compared to patients with isolated pulmonary embolism, the rate of recurrent VTE was higher in patients with proximal deep vein thrombosis (rate ratio 1.4, 95% confidence interval 1.1 to 1.7) and in patients with pulmonary embolism plus deep vein thrombosis (1.5, 1.1 to 1.9). In patients with distal deep vein thrombosis, the pooled rate of recurrent VTE per 100 person years was 1.9 events (95% confidence interval 0.5 to 4.3) in the first year after anticoagulation had stopped. The case fatality rate for recurrent VTE was 4% (95% confidence interval 2% to 6%).
Conclusions
In patients with a first episode of unprovoked VTE who completed at least three months of anticoagulant treatment, the risk of recurrent VTE was 10% in the first year after treatment, 16% at two years, 25% at five years, and 36% at 10 years, with 4% of recurrent VTE events resulting in death. These estimates should inform clinical practice guidelines, enhance confidence in counselling patients of their prognosis, and help guide decision making about long term management of unprovoked VTE.
Systematic review registration
PROSPERO CRD42017056309.
Introduction
For patients with unprovoked venous thromboembolism (VTE), comprising deep vein thrombosis and pulmonary embolism, the optimal duration of anticoagulant treatment is uncertain. After three to six months of initial anticoagulation, extended treatment is highly effective at reducing the risk of recurrent VTE,1 but this clinical benefit is not maintained when anticoagulation is stopped.2 After discontinuation of anticoagulant treatment, patients with a first unprovoked VTE have a much higher risk of recurrence compared with patients with VTE provoked by a major transient risk factor.3 4 5 Consequently, anticoagulant treatment is discontinued after three to six months in patients with VTE due to a major transient provoking factor, whereas current guidelines suggest extended (ie, indefinite) anticoagulation in patients with unprovoked proximal deep vein thrombosis or pulmonary embolism who have a non-high bleeding risk.6 7 8 This is, however, a weak (grade 2B) recommendation, in large part as a result of uncertainty in estimates of the long term risk of major bleeding if treatment is continued, and, importantly, the long term risk of recurrent VTE if anticoagulation is discontinued. Thus deciding whether patients with a first episode of unprovoked VTE should receive indefinite anticoagulation or can stop treatment after the initial three to six months, remains an important challenge.
A previous individual patient data meta-analysis5 of 1732 patients with unprovoked VTE from six randomised trials, reported an overall risk of recurrent VTE of about 10% per year in the first two years after discontinuation of anticoagulation. That analysis did not assess the risk of recurrent VTE in men and women separately or in patients with isolated pulmonary embolism, and it only followed patients for 24 months. Furthermore, trials in that analysis were published before 2004, whereas since then additional prospective studies have reported on the risk for recurrent VTE after discontinuation of anticoagulant treatment in patients with a first episode of unprovoked VTE, with several of these studies having followed patients beyond 24 months and some up to 10 years.9 10 11 12 13 This offers an opportunity to obtain reliable estimates of the long term risk of recurrent VTE and to assess how the risk varies over time—knowledge that is crucial for deciding the need for indefinite anticoagulation, as well as defining the burden of illness in this common patient population.
Methods
We formed the Meta-Analysis of the long term Risk of recurrent Venous thromboEmboLism after stopping anticOagulation for acute Unprovoked venous thromboemboliSm (MARVELOUS) collaboration to undertake a systematic review and meta-analysis to determine the rate of a first recurrent VTE event in the first year, in the second year, in years 3-5, and in years 6-10 after discontinuation of anticoagulant treatment for a first episode of unprovoked VTE, as well as the cumulative incidence for recurrent VTE at 2, 5, and 10 years.
The study protocol was developed using guidance from the preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) statement,14 registered in PROSPERO and published.15 This systematic review and meta-analysis is reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.16
Search strategy and study selection
In conjunction with a medical librarian, we conducted a comprehensive systematic search (from inception to 15 March 2019) of Embase, Medline, and the Cochrane Central Register of Controlled Trials. For these searches, we combined terms related to VTE and anticoagulant treatment with those related to study design, without language restrictions (Appendix table 1 shows the systematic search strategy used for Embase). We supplemented the electronic searches by hand searching reference lists of relevant review articles, and without consideration of grey literature. Two authors (FK, AR) independently screened titles, abstracts, and full text publications, and a third author (MAR) resolved discrepancies.
Randomised controlled trials and prospective observational cohort studies were included if they were published in a peer reviewed journal; they enrolled patients with a first episode of objectively confirmed, symptomatic VTE that was either unprovoked or associated with minor transient risk factors, as defined according to the International Society on Thrombosis and Haemostasis guidance (see box 1)17; patients had completed at least three months of initial anticoagulation before stopping treatment, and the decision to stop anticoagulation was not influenced by stratification of the risk of VTE recurrence (eg, negative D dimer test result, clinical decision rules); patients were followed-up for at least nine months after discontinuation of anticoagulant treatment; and symptomatic recurrent VTE (as defined in individual studies) events were reported during follow-up after discontinuation of anticoagulant treatment. When more than one article included the same population of patients (ie, duplication), we included the publication with the longest follow-up.
Box 1. International Society on Thrombosis and Haemostasis definition of unprovoked venous thromboembolism.
VTE is defined as unprovoked if the following provoking risk factors are absent:
Persistent
Active cancer, defined as:
cancer that has not received potentially curative treatment, or
treatment is ongoing, or
evidence that treatment has not been curative
Major transient
Surgery with general anaesthesia for more than 30 minutes
Confined to bed (only “bathroom privileges”) for at least three days with an acute illness
Caesarean section
Minor transient
Surgery with general anaesthesia for less than 30 minutes
Admission to hospital for fewer than three days with an acute illness
Oestrogen treatment
Pregnancy or puerperium
Confined to bed out of hospital for at least three days with an acute illness
Leg injury associated with reduced mobility for at least three days
Data extraction and quality assessment
Using a standardised form, two authors (FK, AR) independently extracted data, with clarifications requested from the study’s authors when necessary. Data were extracted on study design; number of eligible patients; average age of patients; percentage of men; number of eligible patients with isolated distal deep vein thrombosis (ie, in deep veins of the calf), proximal deep vein thrombosis (ie, in the popliteal, femoral, or iliac veins), and pulmonary embolism (with or without deep vein thrombosis); and the definition of unprovoked VTE. For the calculation of event rates, we requested the following from the authors: aggregate data on the number of recurrent VTE events (any VTE, and subtypes deep vein thrombosis, pulmonary embolism, pulmonary embolism plus deep vein thrombosis, and fatal pulmonary embolism), and the total number of person years of follow-up during each of the specified intervals (to ensure appropriate censoring of deaths, patients lost to follow-up and those withdrawn from study). Patients who did not satisfy our eligibility criteria (eg, those with cancer or a second unprovoked VTE event) were not included in those aggregate data.
The same two authors independently assessed the risk of bias of studies. Since our objective was to establish pooled event rates after discontinuation of anticoagulant treatment, we evaluated all studies, including each arm of a randomised trial, as an independent observational cohort. As such, the risk of bias for each observational cohort was assessed using a modified version of the Newcastle-Ottawa scale score18 based on three selection criteria and three outcome criteria only (criteria assessing comparability were considered irrelevant in the context of this systematic review and meta-analysis). Given data clarifications obtained from the authors of all included studies, we judged the risk of bias assessment not only at the published study level but also based on authors’ clarifications. Following quality assessment standards of a previous meta-analysis,19 we considered studies in our meta-analysis that met four or more of these Newcastle-Ottawa scale criteria to be of higher quality. A third author (MAR) resolved discrepancies.
Data synthesis and analysis
For each study cohort we calculated the rates of recurrent VTE, expressed as number of events per 100 person years, from the number of first recurrent VTE events divided by the person years of follow-up obtained from the authors of original studies and categorised by four time intervals after discontinuation of anticoagulant treatment when a first recurrence of VTE occurred: year 1 (0-12 months), year 2 (12-24 months), years 3-5, and years 6-10. We used random effects meta-analyses to pool rates from each study cohort, with cohorts weighted according to their inverse variance.20 Given that we calculated rates of recurrent VTE in our analysis based on person time at risk accounting for deaths and other patient losses to follow-up, obtained from the authors of the included studies, during each of the studied intervals, we calculated the cumulative incidence for recurrent VTE at 2, 5, and 10 years after discontinuation of anticoagulation. To do this, we first determined the proportion of patients who did not experience recurrent VTE based on event rates during each of the intervals we studied. Then we determined the proportion of patients who did experience a recurrent VTE by multiplying the proportion of patients, in each year under consideration, who did not experience a recurrent VTE. For example, if the rate of recurrent VTE (per 100 person years) was 10 events in year 1, five events in year 2, and four events/year in years 3-5, then the proportion of patients who did not experience a recurrent VTE within five years=90.0% (year 1)×95.0% (year 2)×(96.0%)3 (years 3, 4, and 5)=75.6%, resulting in a cumulative incidence for recurrent VTE of 24.4% five years after discontinuation of anticoagulant treatment.
We determined the upper and lower limits of the 95% confidence interval for cumulative incidence by performing the calculations described on the upper and lower limits of the 95% confidence interval of the event rates, respectively. For example, if the rate of recurrent VTE (per 100 person years) was 10.0 events (95% confidence interval 9.0 to 11.0) in year 1 and 5.0 events (4.0 to 6.0) in year 2, resulting in a two year cumulative incidence of 14.5%, then the 95% confidence interval of the cumulative incidence was calculated as follows: using the lower bound 95% confidence intervals of 9.0 events (year 1) and 4.0 events (year 2), the proportion of patients who did not experience a recurrent VTE within two years=91.0% (year 1)×96.0% (year 2)=87.4%, resulting in a lower bound 95% confidence interval for the cumulative incidence of 12.6%. Using the upper bound 95% confidence interval of 11.0 events (year 1) and 6.0 events (year 2), the proportion of patients who did not experience a recurrent VTE within two years=89.0% (year 1)×94.0% (year 2)=83.7%, resulting in an upper bound 95% confidence interval for the cumulative incidence of 16.3%.
Lastly, to measure the clinical impact of disease recurrence after discontinuation of anticoagulant treatment, we determined the case fatality rate of recurrent VTE from the total number of fatal recurrent pulmonary embolism events divided by the total number of recurrent VTE events.
Heterogeneity between studies was assessed using the I2 statistic, with values of 75% or greater indicating substantial heterogeneity. All meta-analyses (with a requirement for at least three cohorts of patients) were performed using StatsDirect Version 3 (Cheshire, UK).21
We performed sensitivity and subgroup analyses to investigate several potential sources of between study heterogeneity: cohorts with event rates that were outliers, cohorts randomised to receive aspirin after completing initial anticoagulant treatment, cohorts derived from randomised trials versus prospective observational studies, sex, and site of initial VTE.
Patient and public involvement
Patients were not involved in the design or conduct of the study. Patient partners in the CanVECTOR network (www.canvector.ca) will be involved in dissemination or knowledge translation activities, or both.
Results
Search results
Of the 1034 records identified by the literature search, 604 remained after removal of duplicates. Of these, 512 were excluded after screening of titles and abstracts, leaving 92 articles for full text screening. A further 68 articles were excluded after full text screening because they were neither a randomised controlled trial nor prospective cohort study, did not include patients with unprovoked VTE, did not systematically stop anticoagulation, did not have patient follow-up lasting for a minimum of nine months after discontinuation of anticoagulation, did not report on recurrent VTE after anticoagulation, or included duplicate patients from other included studies. Thus, 24 studies were identified as eligible for inclusion in the meta-analysis (fig 1). We requested data clarifications for person years of follow-up and recurrent VTE events during follow-up from the authors of the 24 eligible studies. Data clarifications were obtained for 18 of the studies. The remaining six studies22 23 24 25 26 27 were excluded because the published manuscript did not provide the data required for our analysis.
Fig 1.
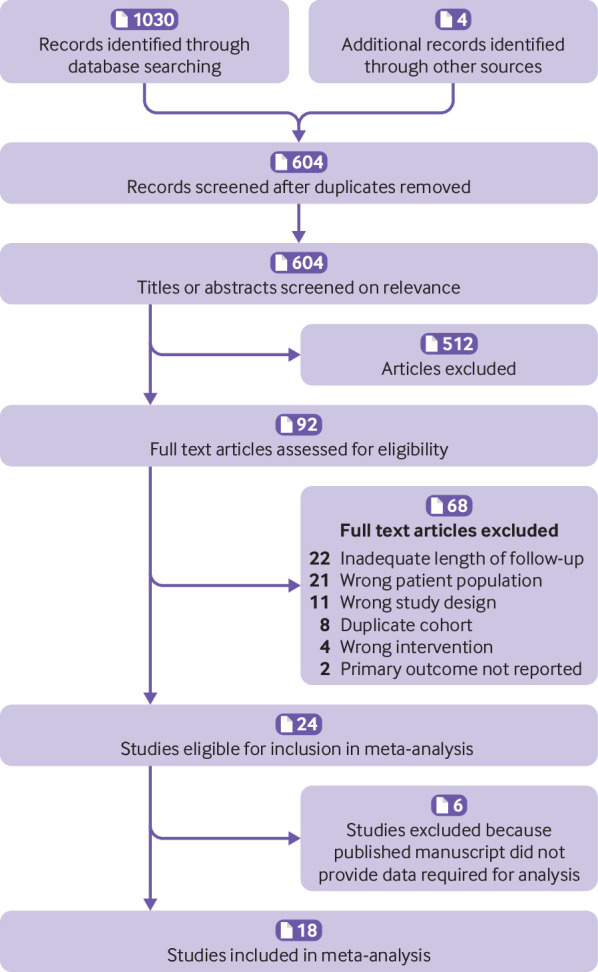
Flow diagram of study identification and selection
Characteristics of included studies
Eighteen studies2 9 10 11 12 13 28 29 30 31 32 33 34 35 36 37 38 39 with a total of 7515 patients were included in the analysis (fig 1). Four of the 18 studies were prospective observational cohort studies11 12 13 31 and 14 were randomised controlled trials2 9 28 29 30 32 33 34 35 36 37 38 39 (table 1). Fifteen studies2 10 11 12 13 28 29 30 31 32 33 34 36 38 39 met the criteria for the International Society on Thrombosis and Haemostasis definition of unprovoked VTE or VTE associated with minor transient risk factors (table 1). All 18 studies with 24 independent observational cohorts followed patients for one year after discontinuation of anticoagulant treatment. Thirteen studies2 9 10 11 12 13 28 31 32 33 35 36 39 (18 cohorts and 5078 patients) followed patients for two years, four studies10 11 12 13 (four cohorts and 2638 patients) followed patients for five years, three studies10 11 13 (three cohorts and 1975 patients) followed patients for 10 years after discontinuation of anticoagulation (table 1). All studies were of high quality according to the Newcastle-Ottawa scale (table 1). Appendix table 2 presents the components of the Newcastle-Ottawa scale score for all studies.
Table 1.
Characteristics of studies included in meta-analysis
| Source (year) | Study design | No of patients (n=7515) | Men (%) | Age (range or SD) (years) | No and site of initial VTE | Definition of unprovoked VTE (minor transient risk factors included) | Follow-up (years)* | Independent adjudication of outcomes | Overall Newcastle-Ottawa scale score (out of 6) |
|---|---|---|---|---|---|---|---|---|---|
| LAFIT: Kearon et al28 | RCT | 83 | 53.0 | 58 (16) | 61 proximal DVT; 22 PE with or without DVT | International Society on Thrombosis and Haemostasis | 2 | Yes | 6 |
| WODIT-DVT: Agnelli et al29 | RCT | 133 | 61.2 | 67.7 (7.3) | 133 proximal DVT | International Society on Thrombosis and Haemostasis | 1 | Yes | 6 |
| DOTAVK: Pinede et al30: | RCT | 308 | 53 distal DVT; 145 proximal DVT; 18 PE; 92 PE plus DVT | International Society on Thrombosis and Haemostasis | Yes | 6 | |||
| Arm 1 | 161 | 47.6 | 58.2 (1.0) | 30 distal DVT; 79 proximal DVT; 8 PE; 44 PE plus DVT | 1 | ||||
| Arm 2 | 147 | 47.0 | 58.9 (0.9) | 23 distal DVT; 66 proximal DVT; 10 PE; 48 PE plus DVT | 1 | ||||
| Palareti et al31 | Cohort | 166 | 50.0 | 67 (12-91) | 4 distal DVT; 137 proximal DVT; 25 PE plus DVT | International Society on Thrombosis and Haemostasis | 2 | Yes | 6 |
| WODIT-PE: Agnelli et al32 | RCT | 181 | 72 PE; 109 PE plus DVT | International Society on Thrombosis and Haemostasis | Yes | 6 | |||
| Arm 1 | 91 | 41.6 | 61.0 (15.5) | 37 PE; 54 PE plus DVT | 2 | ||||
| Arm 2 | 90 | 39.4 | 62.9 (16.3) | 35 PE; 55 PE plus DVT | 2 | ||||
| PREVENT: Ridker et al9 | RCT | 160 | 52.9 | 67.7 (7.3) | 20 distal DVT with or without PE; 100 proximal DVT with or without PE; 40 unspecified VTE | Unprovoked VTE events were defined as those that did not occur within 90 days after surgery or trauma | 2 | Yes | 6 |
| DURAC I: Schulman et al10 | RCT | 272 | 61.4 | 60.6 (15.4) | 234 DVT with or without PE; 38 PE | International Society on Thrombosis and Haemostasis | 10 | Yes | 6 |
| Prandoni et al11 | Cohort | 864 | 45.2 | 66.0 (16-96) | 735 DVT with or without PE; 129 PE | International Society on Thrombosis and Haemostasis | 10 | Yes | 6 |
| AESOPUS: Prandoni et al33 | RCT | 151 | 57.6 | 69.0 (21-89) | 151 proximal DVT | International Society on Thrombosis and Haemostasis | 2 | Yes | 6 |
| EINSTEIN-Extension: Bauersachs et al34 | RCT | 465 | 58.5 | 57.6 (16.2) | 267 DVT; 144 PE; 46 PE plus DVT | International Society on Thrombosis and Haemostasis (oestrogen treatment; pregnancy and puerperium; leg trauma with transient impairment of mobility) | 1 | Yes | 6 |
| WARFASA: Becattini et al35 | RCT | 402 | 252 proximal DVT; 55 PE; 95 PE plus DVT | Yes | 6 | ||||
| Arm 1 | 197 | 61.9 | 62.1 (15.1) | 130 proximal DVT; 18 PE; 49 PE plus DVT | Unprovoked VTE events were defined as those that occurred in the absence of any known persistent or temporary risk factors for VTE | 2 | |||
| Arm 2 | 205 | 65.8 | 61.9 (15.3) | 122 proximal DVT; 37 PE; 46 PE plus DVT | 2 | ||||
| ASPIRE: Brighton et al36 | RCT | 822 | 468 proximal DVT; 231 PE; 114 PE plus DVT | International Society on Thrombosis and Haemostasis | Yes | 6 | |||
| Arm 1 | 411 | 54 | 54 (15.8) | 232 proximal DVT; 119 PE; 59 PE plus DVT | 2 | ||||
| Arm 2 | 411 | 55 | 55 (16) | 236 proximal DVT; 112 PE; 56 PE plus DVT | 2 | ||||
| RE-SONATE: Schulman et al37† | RCT | 651 | 42.4 | 56.1 (15.5) | Not available | Patients were initially treated for more than 10 months | 1 | Yes | 6 |
| PADIS-PE: Couturaud et al2 | RCT | 371 | 259 PE; 112 PE plus DVT | International Society on Thrombosis and Haemostasis (oestrogen treatment) | Yes | 6 | |||
| Arm 1 | 187 | 55.1 | 57.3 (17.4) | 131 PE; 56 PE plus DVT | 2 | ||||
| Arm 2 | 184 | 42.5 | 58.7 (16) | 128 PE; 56 PE plus DVT | 2 | ||||
| REVERSE I: Rodger et al11 | Cohort | 663 | 51.4 | 53.2 (18-95) | 346 proximal DVT; 194 PE; 123 PE plus DVT | International Society on Thrombosis and Haemostasis (oestrogen treatment) | 5 | Yes | 6 |
| AUREC: Kyrle et al12 | Cohort | 839 | 66.0 | 53 (14) | 154 distal DVT; 349 proximal DVT; 336 PE with or without DVT | International Society on Thrombosis and Haemostasis | 10 | Yes | 6 |
| EINSTEIN-Choice: Weitz et al38 | RCT | 880 | 56.7 | 58.4 (15.0) | 442 proximal DVT; 295 PE; 139 PE plus DVT | International Society on Thrombosis and Haemostasis (oestrogen treatment; pregnancy and puerperium; lower limb trauma with transient impairment of mobility) | 1 | Yes | 6 |
| PADIS-DVT: Couturaud et al39 | RCT | 104 | 104 proximal DVT | International Society on Thrombosis and Haemostasis (oestrogen treatment) | Yes | 6 | |||
| Arm 1 | 54 | 72.2 | 61.5 (14.5) | 2 | |||||
| Arm 2 | 50 | 62.0 | 59.0 (17.2) | 2 |
DVT=deep vein thrombosis; PE=pulmonary embolism; RCT=randomised controlled trial.
Duration of follow-up as applicable to intervals of 1, 2, 5, and 10 years after discontinuation of anticoagulation used in analysis.
Data corresponds to post-treatment follow-up in dabigatran arm. Data during 12 months of follow-up in placebo arm of trial were not accessible.
Recurrent VTE after anticoagulation
Table 2 presents the pooled person years of follow-up, number of events for recurrent VTE, deep vein thrombosis, pulmonary embolism, pulmonary embolism plus deep vein thrombosis, and fatal pulmonary embolism, as well as the corresponding rates and cumulative incidence for these outcomes. Appendix tables 3, 6, and 7 provide the results from individual study cohorts.
Table 2.
Risk of recurrent venous thromboembolism (VTE) after discontinuation of anticoagulation in patients with a first unprovoked VTE event
| Interval after anticoagulation | Person years of follow-up | Recurrent events | Event rate per 100 person years* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VTE | DVT | PE | PE+DVT | Fatal PE | VTE | DVT | PE | PE+DVT | Fatal PE | |||
| 1st year | 6678.0 | 644 | 350 | 194 | 20 | 28 | 10.3 (8.6 to 12.1); 81, <0.001 | 6.2 (4.8 to 7.7); 79, <0.001 | 3.3 (2.4 to 4.2); 68, <0.001 | 0.3 (0.1 to 0.5); 44, 0.008 | 0.4 (0.2 to 0.7); 57, <0.001 | |
| 2nd year | 3906.0 | 262 | 151 | 82 | 7 | 12 | 6.3 (5.1 to 7.7); 56, 0.002 | 3.7 (2.8 to 4.7); 55, 0.003 | 2.0 (1.4 to 2.6); 36, 0.07 | 0.2 (0.1 to 0.4); 0, 0.63 | 0.3 (0.2 to 0.6); 10, 0.34 | |
| 2 year cumulative incidence, % (95% CI) | 16.0 (13.3 to 18.8) | 9.7 (7.5 to 12.0) | 5.2 (3.7 to 6.7) | 0.5 (0.2 to 0.9) | 0.7 (0.4 to 1.3) | |||||||
| Years 3-5 | 4772.0 | 182 | 116 | 54 | 5 | 6 | 3.8 (3.2 to 4.5); 24, 0.27 | 2.5 (2.0 to 2.9); 0, 0.59 | 1.0 (0.4 to 1.8); 83, <0.001 | 0.1 (0.0 to 0.3); 71, 0.02 | 0.1 (0.0 to 0.3); 53, 0.09 | |
| 5 year cumulative incidence, % (95% CI) | 25.2 (21.3 to 29.3) | 16.3 (12.9 to 19.5) | 8.0 (4.0 to 11.6) | 0.8 (0.2 to 1.8) | 1.0 (0.4 to 2.2) | |||||||
| Years 6-10 | 3023.4 | 99 | 67 | 27 | 0 | 3 | 3.1 (1.7 to 4.9); 84, <0.001 | 2.2 (1.0 to 3.8); 86, <0.001 | 0.7 (0.2 to 1.6); 79, 0.009 | 0.0 (0.0 to 0.1); 0, 1.00 | 0.1 (0.0 to 0.3); 0, 0.37 | |
| 10 year cumulative incidence, % (95% CI) | 36.1 (27.8 to 45.0) | 25.1 (17.2 to 33.7) | 11.2 (5.9 to 18.4) | 0.8 (0.2 to 2.3) | 1.5 (0.4 to 3.6) | |||||||
DVT=deep vein thrombosis; PE=pulmonary embolism.
Data are event rate (95% CI); I2 (%), P value unless stated otherwise. P value is for heterogeneity.
In the first year after discontinuation of anticoagulation, the pooled rate of recurrent VTE per 100 person years was 10.3 events (95% confidence interval 8.6 to 12.1; I2=81%). The rate of recurrent VTE events per 100 person years for deep vein thrombosis was 6.2 (95% confidence interval 4.8 to 7.7; I2=79%), for pulmonary embolism was 3.3 (2.4 to 4.2; I2=68%), and for pulmonary embolism plus deep vein thrombosis was 0.3 (0.1 to 0.5; I2=44%) (table 2).
In the second year after discontinuation of treatment, the pooled rate of recurrent VTE events per 100 person years was 6.3 (95% confidence interval 5.1 to 7.7; I2=56%). The rate of recurrent VTE events per 100 person years for deep vein thrombosis was 3.7 (2.8 to 4.7; I2=55%), for pulmonary embolism was 2.0 (1.4 to 2.6; I2=36%), and for pulmonary embolism plus deep vein thrombosis was 0.2 (0.1 to 0.4; I2=0%) (table 2).
In years 3-5 after discontinuation of treatment, the pooled rate of recurrent VTE per 100 person years was 3.8 events/year (95% confidence interval 3.2 to 4.5; I2=24%). The recurrent VTE events annually per 100 person years for deep vein thrombosis was 2.5 (95% confidence interval 2.0 to 2.9; I2=0%), for pulmonary embolism was 1.0 (0.4 to 1.8; I2=83%), and for pulmonary embolism plus deep vein thrombosis was 0.1 (0.0 to 0.3; I2=71%) (table 2).
During years 6-10 after discontinuation of treatment, the pooled rate of recurrent VTE per 100 person years was 3.1 events/year (95% confidence interval 1.7 to 4.9; I2=84%). The recurrent VTE events annually per 100 person years for deep vein thrombosis was 2.2 (95% confidence interval 1.0 to 3.8; I2=86%), for pulmonary embolism was 0.7 (0.2 to 1.6; I2=79%), and 0.0 for pulmonary embolism plus deep vein thrombosis was 0.0 (0.0 to 0.1; I2=0%) (table 2).
The cumulative incidence for recurrent VTE was 16.0% (95% confidence interval 13.3% to 18.8%) at 2 years, 25.2% (21.3% to 29.3%) at 5 years, and 36.1% (27.8% to 45.0%) at 10 years after discontinuation of anticoagulant treatment. The corresponding values for recurrent deep vein thrombosis were 9.7% (95% confidence interval 7.5% to 12.0%), 16.3% (12.9% to 19.5%), and 25.1% (17.2% to 33.7%), for recurrent pulmonary embolism were 5.2% (3.7% to 6.7%), 8.0% (4.0% to 11.6%), and 11.2% (5.9% to 18.4%), and for recurrent pulmonary embolism plus deep vein thrombosis were 0.5% (0.2% to 0.9%), 0.8% (0.2% to 1.8%), and 0.8% (0.2% to 2.3%) after discontinuation of anticoagulation (table 2).
Fatal recurrent pulmonary embolism and case fatality rate of recurrent VTE
After discontinuation of anticoagulation, the pooled rate of fatal recurrent pulmonary embolism events per 100 person years was 0.4 (95% confidence interval 0.2 to 0.7; I2=57%) in the first year, 0.3 (0.2 to 0.6; I2=10%) in the second year, and 0.1 (95% confidence interval 0.0 to 0.3) in both years 3-5 (I2=53%) and years 6-10 (I2=0%) (table 2). The cumulative incidence for fatal recurrent pulmonary embolism was 0.7% (95% confidence interval 0.4% to 1.3%) at 2 years, 1.0% (0.4% to 2.2%) at 5 years, and 1.5% (0.4% to 3.6%) at 10 years (table 2).
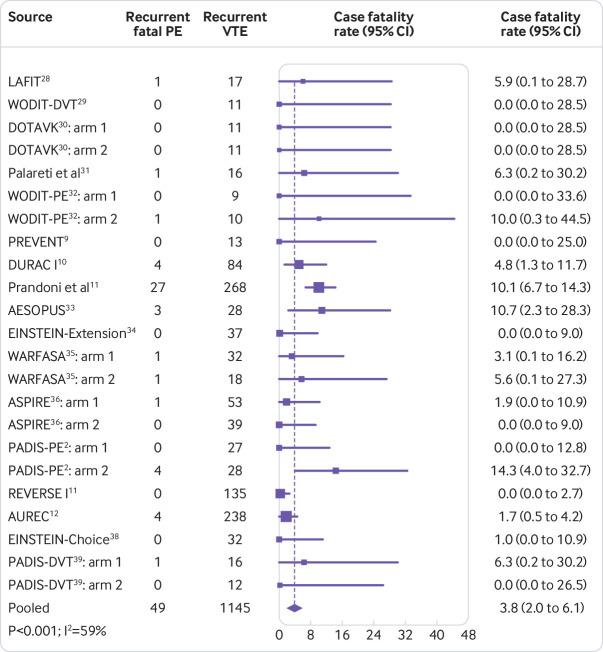
Based on 17 studies involving 6864 patients with information available on both fatal recurrent pulmonary embolism (n=49) and recurrent VTE (n=1145), the pooled case fatality rate of recurrent VTE after discontinuation of anticoagulation was 3.8% (95% confidence interval 2.0% to 6.1%; I2=59%) (fig 2), which remained constant over time (Appendix table 5).
Fig 2.
Case fatality rate of recurrent venous thromboembolism (VTE) after discontinuation of anticoagulant treatment in patients with a first unprovoked VTE event. P value is for heterogeneity
Recurrent VTE according to sex
Among men with a first unprovoked VTE, the pooled rate of recurrent VTE per 100 person years after discontinuation of anticoagulation was 11.9 events (95% confidence interval 9.6 to 14.4; I2=76%) in the first year, 7.3 events (5.3 to 9.5; I2=63%) in the second year, 4.4 events/year (95% confidence interval 3.2 to 5.7; I2=60%) in years 3-5, and 3.8 events/year (1.6 to 6.9; I2=89%) in years 6-10 (table 3). The cumulative incidence for recurrent VTE in men was 18.3% (95% confidence interval 14.4% to 22.5%) at 2 years, 28.6% (22.3% to 35.0%) at 5 years, and 41.2% (28.4% to 55.6%) at 10 years (table 3).
Table 3.
Risk of recurrent venous thromboembolism (VTE) after discontinuation of anticoagulation in patients with first unprovoked VTE event according to sex
| Interval after anticoagulation | Person years of follow-up | Recurrent VTE | Event rate per 100 person years* | |||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |||
| 1st year | 3273.8 | 2528.1 | 377 | 205 | 11.9 (9.6 to 14.4); 76, <0.001 | 8.9 (6.8 to 11.3); 72, <0.001 | ||
| 2nd year | 2026.8 | 1738.1 | 160 | 97 | 7.3 (5.3 to 9.5); 63, <0.001 | 5.2 (3.6 to 7.0); 57, 0.003 | ||
| 2 year cumulative incidence, % (95% CI) | 18.3 (14.4 to 22.5) | 13.6 (10.1 to 17.5) | ||||||
| Years 3-5 | 2880.6 | 1891.7 | 125 | 57 | 4.4 (3.2 to 5.7); 60, 0.06 | 3.0 (1.6 to 4.7); 74, 0.01 | ||
| 5 year cumulative incidence, % (95% CI) | 28.6 (22.3 to 35.0) | 21.2 (14.4 to 28.6) | ||||||
| Years 6-10 | 1820.6 | 1202.4 | 76 | 23 | 3.8 (1.6 to 6.9); 89, <0.001 | 2.0 (1.3 to 2.9); 0, 1.02 | ||
| 10 year cumulative incidence, % (95% CI) | 41.2 (28.4 to 55.6) | 28.8 (19.8 to 38.4) | ||||||
Data are event rate (95% CI); I2 (%), P value unless stated otherwise. P value is for heterogeneity.
Among women with a first unprovoked VTE, the pooled rate of recurrent VTE per 100 person years after discontinuation of anticoagulation was 8.9 events (95% confidence interval 6.8 to 11.3; I2=72%) in the first year, 5.2 events (3.6 to 7.0; I2=57%) in the second year, 3.0 events/year (95% confidence interval 1.6 to 4.7; I2=74%) in years 3-5, and 2.0 events/year (1.3 to 2.9; I2=0%) in years 6-10 (table 3). The cumulative incidence for recurrent VTE in women was 13.6% (95% confidence interval 10.1% to17.5%) at 2 years, 21.2% (14.4% to 28.6%) at 5 years, and 28.8% (19.8% to 38.4%) at 10 years (table 3).
Overall, men had 1.4 times the rate of recurrent VTE compared with women (rate ratio 1.4, 95% confidence interval 1.3 to 1.6, P<0.001) (see table 5).
Recurrent VTE according to site of initial VTE
In patients with a first unprovoked distal deep vein thrombosis, the pooled rate of recurrent VTE in the first year after discontinuation of anticoagulation was 1.9 events per 100 person years (95% confidence interval 0.5 to 4.3; I2=0%) (table 4).
Table 4.
Risk of recurrent venous thromboembolism (VTE) after discontinuation of anticoagulation in patients with a first unprovoked VTE event according to site of initial event
| Interval after anticoagulation | Site of initial VTE | |||
|---|---|---|---|---|
| Distal DVT | Proximal DVT | Isolated PE | PE+DVT | |
| 1st year | ||||
| Total person years of follow-up | 198.0 | 2387.4 | 1200.5 | 638.9 |
| Total recurrent VTE events | 3 | 233 | 86 | 66 |
| Event rate per 100 person years (95% CI); I2 (%), P value* | 1.9 (0.5 to 4.3); 0, 0.56 | 10.6 (8.1 to 13.3); 73, <0.001 | 7.7 (5.6 to 10.2); 49, 0.02 | 10.2 (6.7 to 14.2); 59, 0.005 |
| 2nd year | ||||
| Total person years of follow-up | NA | 1417.1 | 763.5 | 347.9 |
| Total recurrent VTE events | NA | 89 | 36 | 25 |
| Event rate per 100 person years (95% CI); I2 (%), P value* | NA | 6.5 (5.2 to 7.8); 0, 0.55 | 4.5 (2.6 to 6.8); 45, 0.07 | 7.6 (4.7 to 11.2); 23, 0.24 |
| 2 year cumulative incidence, % (95% CI) | NA | 16.4 (12.9 to 20.1) | 11.9 (8.1 to 16.3) | 17.0 (11.1 to 23.8) |
DVT=deep vein thrombosis; PE=pulmonary embolism; NA=not available.
P is for heterogeneity.
Among patients with a first unprovoked proximal deep vein thrombosis, the pooled rate of recurrent VTE in the first year after discontinuation of anticoagulation was10.6 events per 100 person years (8.1 to 13.3; I2=73%). In the second year after treatment had stopped, the rate was 6.5 events per 100 person years (5.2 to 7.8; I2=0%) (table 4). The cumulative two year incidence for recurrent VTE was 16.4% (95% confidence interval 12.9% to 20.1%) (table 4).
In patients with a first unprovoked isolated pulmonary embolism, the pooled rate of recurrent VTE in the first year after discontinuation of anticoagulation was 7.7 events per 100 person years (95% confidence interval 5.6 to 10.2; I2=49%). In the second year after treatment had stopped, the rate was 4.5 events per 100 person years (2.6 to 6.8; I2=45%) (table 4). The cumulative two year incidence for recurrent VTE was 11.9% (8.1% to 16.3%) (table 4).
Among those with pulmonary embolism plus deep vein thrombosis as the initial unprovoked VTE, the pooled rate of recurrent VTE in the first year after discontinuation of anticoagulation was 10.2 events per 100 person years (6.7 to 14.2; I2=59%). In the second year after treatment had stopped, the rate was 7.6 events per 100 person years (4.7 to 11.2; I2=23%) (table 4). The cumulative two year incidence for recurrent VTE was 17.0% (95% confidence interval 11.1% to 23.8%) (table 4).
Overall, patients with distal deep vein thrombosis had a lower rate of recurrent VTE compared to patients with proximal deep vein thrombosis (rate ratio 0.2, 95% confidence interval 0.04 to 0.5, P<0.001), isolated pulmonary embolism (0.2, 0.05 to 0.7, P=0.009), as well as pulmonary embolism plus deep vein thrombosis (0.2, 0.03 to 0.5, P<0.001). Patients with proximal deep vein thrombosis had 1.4 times the rate of recurrent VTE of patients with isolated pulmonary embolism (1.4, 1.1 to 1.7, P=0.004), and 0.9 times the rate of recurrent VTE of patients with pulmonary embolism plus deep vein thrombosis (0.9, 0.7 to 1.2, P=0.47). Patients with pulmonary embolism plus deep vein thrombosis had 1.5 times the rate of recurrent VTE of patients with isolated pulmonary embolism (1.5, 1.1 to 1.9, P=0.005) (table 5).
Table 5.
Comparison of rate of recurrent venous thromboembolism (VTE) after discontinuation of anticoagulation in subgroups of patients with a first unprovoked VTE event
| Patient subgroups | Recurrent VTE rate ratio (95% CI) | P value |
|---|---|---|
| Men versus women | 1.4 (1.3 to 1.6) | <0.001 |
| Distal DVT versus proximal DVT | 0.2 (0.04 to 0.5) | <0.001 |
| Distal DVT versus isolated PE | 0.2 (0.05 to 0.7) | 0.009 |
| Distal DVT versus PE+DVT | 0.2 (0.03 to 0.5) | <0.001 |
| Proximal DVT versus isolated PE | 1.4 (1.1 to 1.7) | 0.004 |
| Proximal DVT versus PE+DVT | 0.9 (0.7 to 1.2) | 0.47 |
| PE+DVT versus isolated PE | 1.5 (1.1 to 1.9) | 0.005 |
DVT=deep vein thrombosis; PE=pulmonary embolism.
Sensitivity analyses
Estimates of the rate of recurrent VTE events were not different in the overall and subgroup analyses excluding outliers or cohorts among included trials with participants randomised to receive aspirin after completing initial anticoagulant treatment, as well as in cohorts derived from either randomised trials or prospective observational studies (Appendix tables 3, 4, 6, and 7).
Discussion
In this meta-analysis of 7515 patients with a first unprovoked venous thromboembolism (VTE) event who had completed at least three months of anticoagulant treatment, we found that the long term risk for recurrent VTE was substantial. The risk reached 10.3% in the first year after discontinuation of treatment, with a cumulative incidence of 16% at 2 years, 25% at 5 years, and 36% at 10 years.
Our observed rate of recurrent VTE in the year after discontinuation of anticoagulation is consistent with that of 9.8 events per 100 person years (95% confidence interval 8.7 to 11.2) reported in a previous individual patient data meta-analysis,5 but our study provides precise estimates for the risk of symptomatic recurrent VTE up to 10 years. Thus our findings extend the knowledge for the prognosis of unprovoked VTE. Our results indicate that after diagnosis of a first unprovoked VTE, 36% of patients will experience a recurrent VTE within 10 years after discontinuation of anticoagulant treatment, underscoring that unprovoked VTE is a chronic disease imposing a substantial long term burden.
To measure the clinical impact of VTE recurrence in this patient population, we determined the case fatality rate of recurrent VTE after discontinuation of anticoagulant treatment. Our analysis showed that after treatment for a first unprovoked VTE, 3.8% (95% confidence interval 2.0% to 6.1%) of recurrent VTE events are fatal. This case fatality rate is similar to the reported 3.6% (1.9% to 5.7%) in a previous study of patients with VTE.40
Clinicians, patients, and policymakers currently lack clear guidance on decision making about duration of anticoagulation for unprovoked VTE. To date, no randomised trial has compared the risk after 3-6 months of initial treatment with that of continuing treatment indefinitely. Furthermore, no trial that has compared durations of anticoagulant treatment for VTE has been powered to detect differences in mortality. Consequently, we must rely on indirect evidence to project absolute long term rates of recurrent VTE and major bleeding, and combine these rates with surrogate measure of mortality (ie, case fatality rate) to balance the risks and benefits of anticoagulant treatment.
Our results provide clinicians, patients, and policymakers with rigorous benchmarks as well as a management framework in which to consider the long term risks and consequences of recurrent VTE if anticoagulation is stopped. When weighed against current best estimates for risks and consequences of major bleeding if anticoagulation is continued, our results could be used to decide whether to consider indefinite anticoagulation for unprovoked VTE. For example, in a typical patient with a first episode of unprovoked VTE with a risk for recurrent VTE of 36% at 10 years, combined with a case fatality rate for recurrent VTE of 4% as determined by our analysis, the risk of death from a first recurrent VTE after discontinuation of anticoagulant treatment would be about 1.44% by 10 years. Indeed, our pooled results showed a cumulative risk of recurrent fatal VTE of 1.5% at 10 years after discontinuation of treatment. For the same patient, the annual risk for major bleeding if treatment is continued is estimated at 1.2%,1 translating to a 10 year risk for major bleeding of 12%. When combined with the case fatality rate for major bleeding of 11%,40 the risk of death from major bleeding if anticoagulation is continued would be about 1.32% at 10 years. Hence, over a 10 year horizon, patients with a first unprovoked VTE might be expected to derive a small net long term mortality benefit from continuing anticoagulation, consistent with current guidelines that suggest considering indefinite anticoagulation in patients with unprovoked proximal deep vein thrombosis or pulmonary embolism who are not at high risk for bleeding.6 7 8 Nevertheless, our estimation of the net mortality benefit is limited owing to the uncertainty in estimates of the long term risk of major bleeding and case fatality rate of major bleeding during extended anticoagulation in patients with a first unprovoked VTE event, which should be the focus of future research.
Because the overall reduction in mortality with indefinite anticoagulation is small, other factors that affect the risk of recurrence (eg, sex, site of initial VTE) and the risk of bleeding, as well as patient preferences, could influence decisions about whether to continue or stop treatment. We found that the cumulative risk of recurrent VTE at 10 years after discontinuation of anticoagulation was 41% in men and 29% in women with a first unprovoked VTE event. Estimating the long term mortality risks over a 10 year horizon shows that a net long term mortality benefit from continuing anticoagulation might be expected in men (1.64% risk of fatal recurrent VTE versus 1.32% risk of fatal major bleeding) but not in women (1.16% risk of fatal recurrent VTE versus 1.32% risk of fatal major bleeding). Consequently, our findings affirm the importance of considering a patient’s sex in deciding the optimal duration of treatment, suggesting that there might be a stronger argument for indefinite anticoagulation in men with a first unprovoked VTE than in women. However, given the closely balanced risks of mortality from recurrent VTE and major bleeding, as well as the lack of precise sex specific estimates of the risks for major bleeding during extended anticoagulation in this patient population, the need for an individualised, patient centred approach in the long term management of unprovoked VTE is emphasised.
Certainly, risk stratification approaches enable individualised management of unprovoked VTE. Although there appears to be no clear subgroup of men who can be identified as having low risk of recurrent VTE41 42 the prospectively validated HERDOO2 (Hyperpigmentation, Edema, or Redness in either leg; D-dimer level ≥250 μg/L; Obesity with body mass index ≥30; or Older age, ≥65 years) clinical decision rule43 allows about 50% of women with a first unprovoked VTE to be classified as having a low risk of recurrent VTE (3% in the year after discontinuing treatment), with a long term risk of recurrent VTE of less than 10% at eight years after discontinuing anticoagulation.11 On the other hand, as suggested by current guidelines,6 7 it is unlikely that patients with a major bleeding risk that exceeds 3% a year would ever experience a net long term mortality benefit from indefinite anticoagulation. Currently, however, there are no validated prediction tools to identify subgroups of patients with VTE at high risk of major bleeding.
Additional findings from our study are noteworthy. Firstly, our results show that it is unlikely that patients with a first unprovoked distal deep vein thrombosis will benefit from indefinite anticoagulation given the low rate of recurrent VTE (1.9%, 95% confidence interval 0.5% to 4.3%) in the year after discontinuation of treatment. Secondly, a cohort study44 suggested that patients with a first unprovoked pulmonary embolism have a higher rate of recurrent VTE after discontinuation of anticoagulation is discontinued than patients with a first unprovoked proximal deep vein thrombosis, whereas another cohort study suggested the opposite.45 Our meta-analysis shows that the rate of recurrent VTE after discontinuation of anticoagulation in patients with a first unprovoked proximal deep vein thrombosis is 1.4-fold higher than in patients with a first unprovoked isolated pulmonary embolism and is comparable to the rate of recurrent VTE in patients with pulmonary embolism plus deep vein thrombosis. Thirdly, our study establishes that the absolute risk of recurrent VTE in patients with a first unprovoked VTE varies considerably over time—it is highest in the first year after treatment, reaching 10.3% (95% confidence interval 8.6% to 12.1%), declines in the second year after treatment to 6.3% (5.1% to 7.7%), and then significantly drops to an average of 3.1% to 3.8% (95% confidence interval 1.7% to 4.9%) per year in the subsequent eight years. Given current guideline recommendations on treatment duration for unprovoked VTE, this finding might help clinicians counsel patients who have already stopped treatment and want advice about resuming anticoagulation. Our results suggest that patients who have not experienced a recurrence within two years of discontinuing anticoagulant treatment are unlikely to experience a net long term mortality benefit from restarting anticoagulation.
Strengths and limitations of this study
This study has several strengths. Firstly, despite a heterogeneous population of patients with VTE in studies included in our analysis, we were able to obtain and pool data from a large number of patients specifically with a first episode of unprovoked VTE who were prospectively followed for recurrent VTE after stopping anticoagulant treatment. Secondly, with the help of data clarifications from authors of included studies, we were able to capture accurately the time varying risk for recurrent VTE by standardising the varying durations of follow-up across patient cohorts, as well as compare the rates of recurrent VTE in six relevant subgroups of patients with unprovoked VTE.
Limitations of our study include the finding of moderate to high statistical heterogeneity in the primary analyses. However, the extent of heterogeneity as measured by the I2 statistic tends to be larger for meta-analyses of proportions.46 We were unable to fully explain between study heterogeneity through subgroup and sensitivity analyses, which could potentially be explored better with a meta-analysis of individual patient data. Secondly, owing to the lack of individual patient data, we could not account for death from causes other than pulmonary embolism as a competing event for recurrent VTE, adjust for other potentially confounding variables such as patient’s age, and explore the potential effect of an age-sex interaction on the differences in the risk of recurrent VTE observed between men and women. Lastly, we did not assess the risk of major bleeding during extended anticoagulant treatment, as well as other long term consequences of recurrent VTE that should also be considered in weighing the long term risk and benefits of anticoagulation, including the risk for post-thrombotic syndrome, chronic thromboembolic pulmonary hypertension, and quality of life.
Conclusion
In patients with a first episode of unprovoked VTE who have completed at least three months of anticoagulant treatment, the risk of recurrent VTE after discontinuing anticoagulation reached 10% in the first year, 16% at 2 years, 25% at 5 years, and 36% at 10 years, with 4% of recurrent VTE events resulting in death. These findings provide rigorous benchmarks of the long term risks and consequences of recurrent VTE that should inform clinical practice guidelines, enhance confidence in counselling patients of their prognosis, and help guide decision making about long term management of unprovoked VTE.
What is already known on this topic
Anticoagulant treatment is highly effective at reducing the risk of recurrent venous thromboembolism (VTE) after a first episode of unprovoked VTE, but this clinical benefit is not maintained once anticoagulation is discontinued
Deciding whether patients with a first unprovoked VTE should stop or continue anticoagulation indefinitely requires balancing the long term risks of recurrent VTE if anticoagulation is stopped and major bleeding if treatment is continued
The long term risk of recurrent VTE after discontinuing anticoagulation in patients with first unprovoked VTE is uncertain
What this study adds
In this meta-analysis of 18 studies involving 7515 patients with a first unprovoked VTE, the risk of recurrent VTE after discontinuing anticoagulation was 10% in the first year, 16% at two years, 25% at five years, and 36% at 10 years, with 4% of recurrent events resulting in death
These findings provide patients, clinicians, and policymakers with reliable estimates for the long term risks and consequences of recurrent VTE to help guide decision making about long term management of unprovoked VTE
Web extra.
Extra material supplied by authors
Supplementary information: additional tables 1-7
Contributors: FK and MAR conceived the study. FK, AR, BH, GAW, and MAR developed the design and methodology of the study. All authors were involved in the acquisition, analysis, or interpretation of data. FK and MAR drafted the manuscript and all authors critically revised the manuscript for important intellectual content. All authors gave final approval of the version to be published. FK and MAR are guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: MC, CK, SS, JIW, and MAR are investigators of the CanVECTOR Network; the Network receives grant funding from the Canadian Institutes of Health Research (CDT-142654). FK was supported by the Canada Graduate Scholarship from the Canadian Institutes of Health Research (CIHR), the CIHR Drug Safety and Effectiveness Cross-Disciplinary Training award, the Queen Elizabeth II Graduate Scholarship in Science and Technology, and is supported by the CIHR Fredrick Banting and Charles Best Doctoral Research Award. CK is supported by the Jack Hirsh Fellowship in Thromboembolism, McMaster University. MAR is supported by a Heart and Stroke Foundation Career Investigator Award and a University of Ottawa, Faculty of Medicine Tier 1 Clinical Research Chair. The funding organisations did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf and declare: MC has received grants from Leo Pharma, Bristol-Myers Squibb, Bayer, Octapharma, personal fees from Sanofi Aventis, Pfizer, Boehringer Ingelheim, Leo Pharma, Bayer Pfizer, Servier, and been on the advisory board for Leo Pharma and Sanofi Aventis, outside the submitted work; CK has received grants from Bayer, outside the submitted work. JIW has received personal fees from Bayer, Boehringer Ingelheim, Bristol Myers Squibb Daiichi-Sankyo, Ionis Pharmaceuticals, Janssen, Merck, Pfizer, and Portola, outside the submitted work; SS has received grants from Boehringer Ingelheim and Octapharma, personal fees from Boehringer Ingelheim, Bayer, Daiichi Sankyo, Octapharma, Sanofi, Alnylam, and Bristol-Myers-Squibb, outside the submitted work; FC has received grants from Pfizer, and personal fees from Bayer, BMS, Aztra Zeneca, leopharma, outside the submitted work; CB has received personal fees from Bayer HealthCare, Daiichi Sankyo, Bristol Myers Squibb, and Servier, outside the submitted work; GA has received personal fees from Bristol-Myers-Squibb, Bayer Healthcare, Boehringer Ingelheim, and Daiichi Sankyo, outside the submitted work; AWAL reports being an employee of Bayer HealthCare; MHP has received personal fees from Pfizer and Daiichi Sankyo, outside the submitted work; BH reports past research from Cornerstone Research Group for methodologic advice related to the conduct of systematic reviews and meta-analysis, outside the submitted work; GP has received personal fees from Alfasigma, Pfizer, BMS, Roche, and Werfen, outside the submitted work. There are no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: No additional data available.
Transparency: The lead author (FK) and senior author (MAR) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
References
- 1. Castellucci LA, Cameron C, Le Gal G, et al. Efficacy and safety outcomes of oral anticoagulants and antiplatelet drugs in the secondary prevention of venous thromboembolism: systematic review and network meta-analysis. BMJ 2013;347:f5133. 10.1136/bmj.f5133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Couturaud F, Sanchez O, Pernod G, et al. PADIS-PE Investigators Six months vs extended oral anticoagulation after a first episode of pulmonary embolism: The PADIS-PE randomized clinical trial. JAMA 2015;314:31-40. 10.1001/jama.2015.7046 [DOI] [PubMed] [Google Scholar]
- 3. Ost D, Tepper J, Mihara H, Lander O, Heinzer R, Fein A. Duration of anticoagulation following venous thromboembolism: a meta-analysis. JAMA 2005;294:706-15. 10.1001/jama.294.6.706 [DOI] [PubMed] [Google Scholar]
- 4. Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med 2010;170:1710-6. 10.1001/archinternmed.2010.367 [DOI] [PubMed] [Google Scholar]
- 5. Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011;342:d3036. 10.1136/bmj.d3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016;149:315-52. 10.1016/j.chest.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 7. Konstantinides SV, Torbicki A, Agnelli G, et al. Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:3033-69, 3069a-3069k. 10.1093/eurheartj/ehu283 [DOI] [PubMed] [Google Scholar]
- 8. Mazzolai L, Aboyans V, Ageno W, et al. Diagnosis and management of acute deep vein thrombosis: a joint consensus document from the European society of cardiology working groups of aorta and peripheral circulation and pulmonary circulation and right ventricular function. Eur Heart J 2018;39:4208-18. 10.1093/eurheartj/ehx003. [DOI] [PubMed] [Google Scholar]
- 9. Ridker PM, Goldhaber SZ, Danielson E, et al. PREVENT Investigators Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003;348:1425-34. 10.1056/NEJMoa035029 [DOI] [PubMed] [Google Scholar]
- 10. Schulman S, Lindmarker P, Holmström M, et al. Post-thrombotic syndrome, recurrence, and death 10 years after the first episode of venous thromboembolism treated with warfarin for 6 weeks or 6 months. J Thromb Haemost 2006;4:734-42. 10.1111/j.1538-7836.2006.01795.x [DOI] [PubMed] [Google Scholar]
- 11. Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007;92:199-205. 10.3324/haematol.10516 [DOI] [PubMed] [Google Scholar]
- 12. Rodger MA, Scarvelis D, Kahn SR, et al. Long-term risk of venous thrombosis after stopping anticoagulants for a first unprovoked event: A multi-national cohort. Thromb Res 2016;143:152-8. 10.1016/j.thromres.2016.03.028 [DOI] [PubMed] [Google Scholar]
- 13. Kyrle PA, Kammer M, Eischer L, et al. The long-term recurrence risk of patients with unprovoked venous thromboembolism: an observational cohort study. J Thromb Haemost 2016;14:2402-9. 10.1111/jth.13524 [DOI] [PubMed] [Google Scholar]
- 14. Shamseer L, Moher D, Clarke M, et al. PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 15. Khan F, Rahman A, Carrier M, et al. MARVELOUS Collaborators Long-term risk of recurrence after discontinuing anticoagulants for a first unprovoked venous thromboembolism: protocol for a systematic review and meta-analysis. BMJ Open 2017;7:16950. 10.1136/bmjopen-2017-016950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9, W64. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 17. Kearon C, Ageno W, Cannegieter SC, Cosmi B, Geersing GJ, Kyrle PA, Subcommittees on Control of Anticoagulation, and Predictive and Diagnostic Variables in Thrombotic Disease Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost 2016;14:1480-3. 10.1111/jth.13336 [DOI] [PubMed] [Google Scholar]
- 18.Wells GA, Shea B, O’Connnell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf. Accessed December, 2017.
- 19. Douketis J, Tosetto A, Marcucci M, et al. Risk of recurrence after venous thromboembolism in men and women: patient level meta-analysis. BMJ 2011;342:d813. 10.1136/bmj.d813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 21.StatsDirect. Cheshire, UK. www.statsdirect.com
- 22. Baglin T, Luddington R, Brown K, Baglin C. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet 2003;362:523-6. 10.1016/S0140-6736(03)14111-6 [DOI] [PubMed] [Google Scholar]
- 23. Schulman S, Wåhlander K, Lundström T, Clason SB, Eriksson H, THRIVE III Investigators Secondary prevention of venous thromboembolism with the oral direct thrombin inhibitor ximelagatran. N Engl J Med 2003;349:1713-21. 10.1056/NEJMoa030104 [DOI] [PubMed] [Google Scholar]
- 24. Campbell IA, Bentley DP, Prescott RJ, Routledge PA, Shetty HG, Williamson IJ. Anticoagulation for three versus six months in patients with deep vein thrombosis or pulmonary embolism, or both: randomised trial. BMJ 2007;334:674-7. 10.1136/bmj.39098.583356.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andresen MS, Sandven I, Brunborg C, et al. Mortality and recurrence after treatment of VTE: long term follow-up of patients with good life-expectancy. Thromb Res 2011;127:540-6. 10.1016/j.thromres.2011.02.017 [DOI] [PubMed] [Google Scholar]
- 26. Agnelli G, Buller HR, Cohen A, et al. AMPLIFY-EXT Investigators Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013;368:699-708. 10.1056/NEJMoa1207541 [DOI] [PubMed] [Google Scholar]
- 27. Franco Moreno AI, García Navarro MJ, Ortiz Sánchez J, et al. A risk score for prediction of recurrence in patients with unprovoked venous thromboembolism (DAMOVES). Eur J Intern Med 2016;29:59-64. 10.1016/j.ejim.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 28. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999;340:901-7. 10.1056/NEJM199903253401201 [DOI] [PubMed] [Google Scholar]
- 29. Agnelli G, Prandoni P, Santamaria MG, et al. Warfarin Optimal Duration Italian Trial Investigators Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. N Engl J Med 2001;345:165-9. 10.1056/NEJM200107193450302 [DOI] [PubMed] [Google Scholar]
- 30. Pinede L, Ninet J, Duhaut P, et al. Investigators of the “Durée Optimale du Traitement AntiVitamines K” (DOTAVK) Study Comparison of 3 and 6 months of oral anticoagulant therapy after a first episode of proximal deep vein thrombosis or pulmonary embolism and comparison of 6 and 12 weeks of therapy after isolated calf deep vein thrombosis. Circulation 2001;103:2453-60. 10.1161/01.CIR.103.20.2453 [DOI] [PubMed] [Google Scholar]
- 31. Palareti G, Legnani C, Cosmi B, Guazzaloca G, Pancani C, Coccheri S. Risk of venous thromboembolism recurrence: high negative predictive value of D-dimer performed after oral anticoagulation is stopped. Thromb Haemost 2002;87:7-12. 10.1055/s-0037-1612936 [DOI] [PubMed] [Google Scholar]
- 32. Agnelli G, Prandoni P, Becattini C, et al. Warfarin Optimal Duration Italian Trial Investigators Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med 2003;139:19-25. 10.7326/0003-4819-139-1-200307010-00008 [DOI] [PubMed] [Google Scholar]
- 33. Prandoni P, Prins MH, Lensing AW, et al. AESOPUS Investigators Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med 2009;150:577-85. 10.7326/0003-4819-150-9-200905050-00003 [DOI] [PubMed] [Google Scholar]
- 34. Bauersachs R, Berkowitz SD, Brenner B, et al. EINSTEIN Investigators Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499-510. 10.1056/NEJMoa1007903 [DOI] [PubMed] [Google Scholar]
- 35. Becattini C, Agnelli G, Schenone A, et al. WARFASA Investigators Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012;366:1959-67. 10.1056/NEJMoa1114238 [DOI] [PubMed] [Google Scholar]
- 36. Brighton TA, Eikelboom JW, Mann K, et al. ASPIRE Investigators Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012;367:1979-87. 10.1056/NEJMoa1210384 [DOI] [PubMed] [Google Scholar]
- 37. Schulman S, Kearon C, Kakkar AK, et al. RE-MEDY Trial Investigators. RE-SONATE Trial Investigators Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013;368:709-18. 10.1056/NEJMoa1113697 [DOI] [PubMed] [Google Scholar]
- 38. Weitz JI, Lensing AWA, Prins MH, et al. EINSTEIN CHOICE Investigators Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med 2017;376:1211-22. 10.1056/NEJMoa1700518 [DOI] [PubMed] [Google Scholar]
- 39. Couturaud F, Pernod G, Presles E, et al. Six months versus two years of oral anticoagulation after a first episode of unprovoked deep-vein thrombosis. The PADIS-DVT randomized clinical trial. Haematologica 2019;314:31-40. 10.3324/haematol.2018.210971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carrier M, Le Gal G, Wells PS, Rodger MA. Systematic review: case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med 2010;152:578-89. 10.7326/0003-4819-152-9-201005040-00008 [DOI] [PubMed] [Google Scholar]
- 41. Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008;179:417-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kearon C, Spencer FA, O’Keeffe D, et al. D-dimer Optimal Duration Study Investigators D-dimer testing to select patients with a first unprovoked venous thromboembolism who can stop anticoagulant therapy: a cohort study. Ann Intern Med 2015;162:27-34. 10.7326/M14-1275 [DOI] [PubMed] [Google Scholar]
- 43. Rodger MA, Le Gal G, Anderson DR, et al. REVERSE II Study Investigators Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ 2017;356:j1065. 10.1136/bmj.j1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eichinger S, Weltermann A, Minar E, et al. Symptomatic pulmonary embolism and the risk of recurrent venous thromboembolism. Arch Intern Med 2004;164:92-6. 10.1001/archinte.164.1.92 [DOI] [PubMed] [Google Scholar]
- 45. Kovacs MJ, Kahn SR, Wells PS, et al. Patients with a first symptomatic unprovoked deep vein thrombosis are at higher risk of recurrent venous thromboembolism than patients with a first unprovoked pulmonary embolism. J Thromb Haemost 2010;8:1926-32. 10.1111/j.1538-7836.2010.03958.x [DOI] [PubMed] [Google Scholar]
- 46. Mills EJ, Jansen JP, Kanters S. Heterogeneity in meta-analysis of FDG-PET studies to diagnose lung cancer. JAMA 2015;313:419. 10.1001/jama.2014.16482 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: additional tables 1-7