Abstract
The Veronica genus, with more than 200 species, belongs to the Plantaginaceae family and is distributed over most of the Northern Hemisphere and in many parts of Southern Hemisphere. These plants are traditionally used in medicine for wound healing, in the treatment of rheumatism, and in different human diseases. This paper reviews the chemical composition of some valuable Veronica species, the possibilities Veronica extracts have in food preservation and as food ingredients, and their functional properties. Veronica species represent a valuable source of biological active secondary metabolites, including iridoid glycosides and phenolic compounds. In particular, due to presence of these phytochemicals, Veronica species exhibit a wide spectrum of biological activities, including antimicrobial and antioxidant. In fact, some studies suggest that some Veronica extracts can inhibit foodborne pathogens, such as Listeria monocytogenes, but only a few of them were performed in food systems. Moreover, anticancer, anti-inflammatory, and other bioactivities were reported in vitro and in vivo. The bioactivity of Veronica plants was demonstrated, but further studies in food systems and in humans are required.
Keywords: Veronica plants, speedwell, iridoids, phenolic compounds, natural preservatives
1. Introduction
The genus Veronica at present belongs to the family Plantaginaceae, while it was previously classified in the family Scrophulariaceae [1]. There are many suggestions (problems) related to the classification and rearrangement of this genus [2,3]. The family includes 120 plant genera with 7055 scientific plant names, of which 1614 are accepted species names [4]. The Plant List includes 1520 scientific plant names for the Veronica genus. Of these, 234 are accepted species names, 335 are synonyms, and 951 are unassessed. Thus, the total number of species belonging to the genus Veronica depends on synonym acceptance. These species are distributed over the Northern Hemisphere and into the Australasian region (Australia, New Zealand, New Guinea), with centers of diversity in western Asia and New Zealand [2]. Most of the species of Veronica occur in regions with a Mediterranean precipitation regime from the sea to high alpine elevations. Despite the importance in many habitats, aquatic plants of Veronica are mostly researched in modern biosystematic studies. The common member of the semi-aquatic plants in the Mediterranean region is Veronica sect. beccabunga [5]. Some other common species of Veronica genus are represented in Table 1.
Table 1.
List of some common Veronica species, their edibility, and medicinal uses [6].
| Latin Name | Common Name | Edibility | Medicinal Use |
|---|---|---|---|
| Veronica agrestis L. | Field speedwell, green field speedwell | Yes | Yes |
| Veronica americana Schwein. ex Benth. | American brooklime, American speedwell | Yes | Yes |
| Veronica anagallis-aquatica L. | Water speedwell | Yes | Yes |
| Veronica arvensis L. | Corn speedwell | No | Yes |
| Veronica beccabunga L. | Brooklime, European speedwell | Yes | Yes |
| Veronica catenata Pennell | Yes | No | |
| Veronica chamaedrys L. | Germander speedwell | Yes | Yes |
| Veronica hederifolia L. | Ivy-leaf speedwell | No | Yes |
| Veronica longifolia L. | Garden speedwell, long-leaf speedwell | Yes | No |
| Veronica officinalis L. | Common speedwell | Yes | Yes |
| Veronica peregrina L. | Necklace weed, neckweed, hairy purslane speedwell | No | Yes |
| Veronica polita Fr. | Gray field speedwell | Yes | Yes |
| Veronica scutellata L. | Marsh speedwell, skullcap speedwell | Yes | No |
| Veronica spuria L. | Bastard speedwell | Yes | No |
| Veronica undulata Wall. | Undulate speedwell | Yes | Yes |
| Veronica strum virginicum (L.) Farw. | Beaumont’s root, Culver’s root, Bowman’s root, Culver’s root, Black root | No | Yes |
Traditional medicine is a sum of great knowledge about health, disease prevention, treatment, and physical and mental illnesses. There are different types of traditional medicine such as ancient Iranian medicine, traditional Chinese medicine, Ayurveda, traditional African medicine, acupuncture, and so on [7]. In 2019, the World Health Organization published a global report on traditional and complementary medicine and stated that 88% of its member states, corresponding to 170 member states, reported that their population uses traditional medicines to treat illnesses [8]. The wide diverse distribution of Veronica plants, from aquatic to dry steppe habitats and from sea-level to high alpine regions [9], could be related to the wide range of traditional uses within these cultures (Table 1). As an example, Veronica peregrina L. is useful for treating hemorrhage, gastric ulcer, infections, and diseases related to macrophage-mediated inflammatory disorders, as illustrated in Korean traditional medicine [10]. Tea made from Veronica spicata L. is a well-known remedy in traditional medicine [11]. Veronica species have cytotoxic and anti-inflammatory activity. Veronica officinalis L. (common speedwell) is used for treating liver, eczema, ulceration, snake bites, wound healing, and skin lesions in Balkan traditional medicine [12]. Veronica species are used in traditional medicine for the treatment of rheumatism [13], hemoptysis, laryngopharyngitis, hernia [14], and lung and respiratory diseases (e.g., against cough or as an expectorant) [15]. They also have properties such as antiscorbutic and diuretic, as well as wound healing [16]. Three Veronica species, namely, V. officinalis, Veronica chamaedrys L., and Veronica herba DAC, are used in traditional Austrian herbal drugs [17]. V. officinalis is a popular medicinal plant, used as a commercial herbal product in many European countries [18]. There are around 79 Veronica species in Turkish flora, 26 of which are endemic. Different parts of Veronica species are used as a diuretic, for wound healing, and against rheumatic pains in Turkish folk medicine [19].
Thus, based on this knowledge, Veronica plants are potential sources of nutraceuticals and functional ingredients with a wide spectrum of bioactivities. In fact, Veronica species represent a valuable source of biological active compounds. Among others, the extracts of Veronica plants show antioxidant, antimicrobial, antifungal, anti-inflammatory, scolicidal, and anti-cancer activities, as well as inhibitory potential on acetylcholinesterase, tyrosinase, lipoxygenase, and xanthine oxidase [20]. Overall, these species might be considered good candidates for industrial or pharmacological applications. Therefore, this review covers information about the phytochemical composition of Veronica plants, mainly focused on phenolics and iridoids. Their biological properties are also detailed, as well as recent food applications.
2. Phytochemical Characterization of Veronica Plants
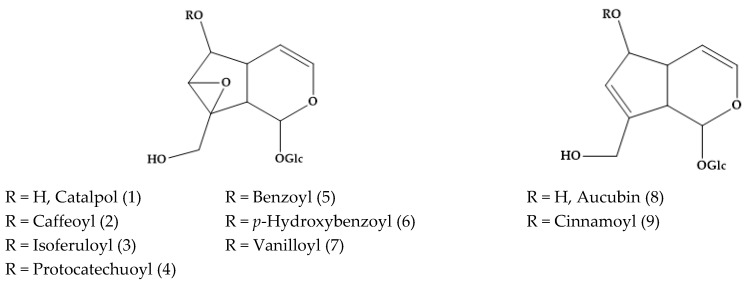
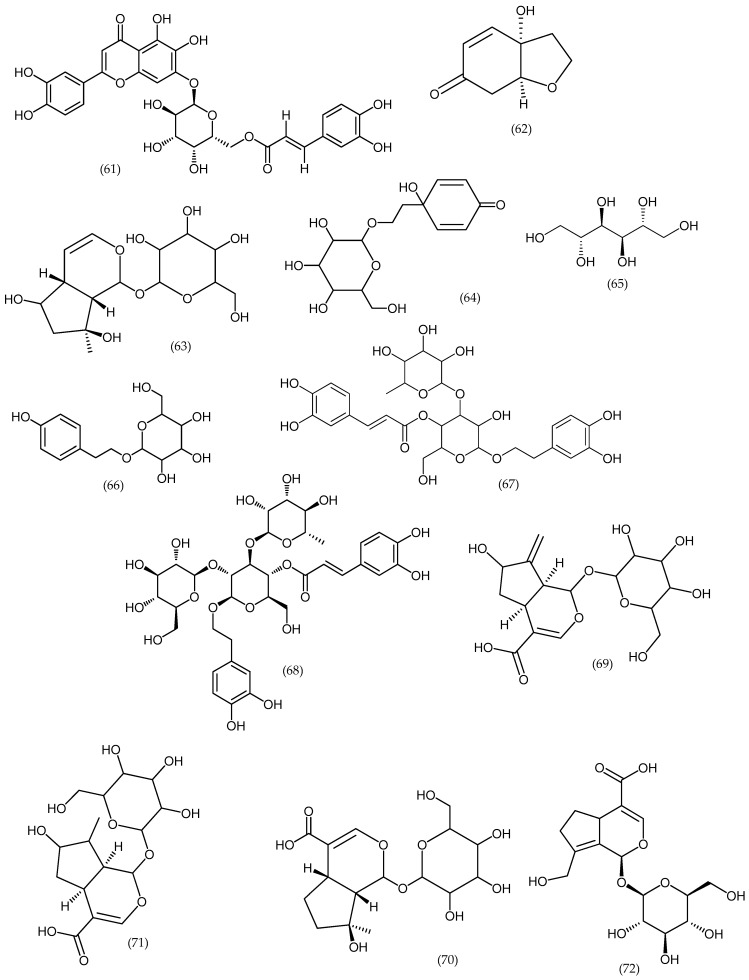
Several phytoconstituents isolated from the plant extracts were studied by means of mass spectrometry (MS) and spectroscopy techniques such as nuclear magnetic resonance (NMR). In the year 1973, Grayer-Barkmeijer and co-workers reported that catalpol (1) derivatives, i.e., caffeoyl-catalpol (2), isoferuloyl-catalpol (3), protocatechuoyl-catalpol (4), benzoyl-catalpol (5), p-hydroxybenzoyl catalpol (catalposide) (6), vanilloyl-catalpol (7), and cinnamoyl-aucubin (9), were isolated from several Veronica species, including sect. Paederota, Pseudolysimachia, Veronicastrum, Omphalospora, and Chamaedrys [21] (Figure 1). It depends on the species, e.g., neither of them was detected in Veronica virginica L. (sect. Paederota) and most of them were in Veronica persica Poir (Sect. Omphalospora). Verproside (6-O-protocatechuoylcatalpol) (4) was also isolated from V. officinalis [22]. In fact, Johansen and co-authors reported that 6-O-rhamnopyranosylcatalpol esters are chemical markers of Veronica sect. Hebe [23].
Figure 1.
Catalpol and aucubin derivatives described in several Veronica plants, including sect. Paederota, Pseudolysimachia, Veronicastrum, Omphalospora, and Chamaedrys.
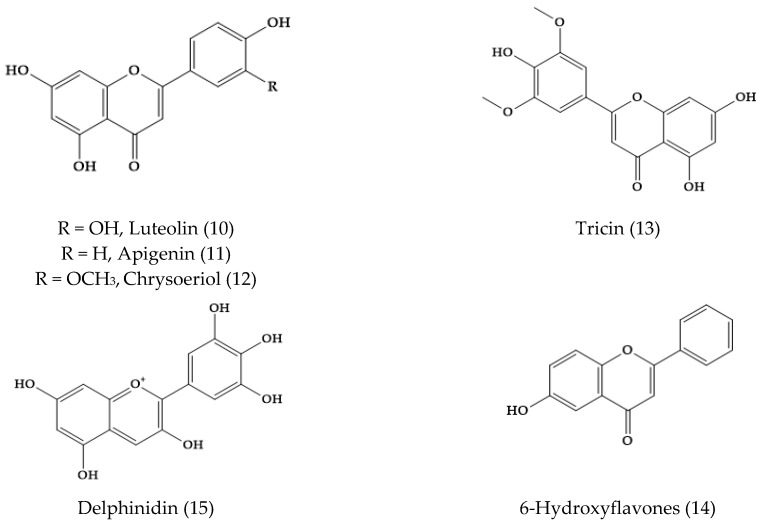
In Veronica, a larger variety of flavone aglycones was also found, e.g., luteolin (10), apigenin (11), chrysoeriol (12), tricin (13), and 6-hydroxyflavones (14) [24]. In fact, eight flavone aglycones were detected in 52 samples of 29 species of Veronica, and the most common ones were apigenin (11) and luteolin (10) (Figure 2) [25]. The observed exudate flavonoid aglycone profiles appeared to be characteristic for some related groups within Veronica genus, in consonance with the morphological, karyological, molecular, and other chemical data [25]. The presence of flavone glycosides was reported in several species, such as Veronica gentianoides Vahl., Veronica alpine L., and Veronica fruticans Jacq. The petals of the species of Veronica, like Veronica gentianoides Vahl, Veronica arvensis L., V. persica, Veronica filiformis Sm., Veronica hederifolia L., and V. chamaedrys, also showed the presence of the anthocyanidin delphinidin (15).
Figure 2.
Common flavonoid aglycones in several Veronica species.
Furthermore, the structure of other particular compounds in Veronica species is detailed in this section; in addition to the expected iridoid glucosides, Veronica species are sources of new phytochemicals.
2.1. Veronica filiformis
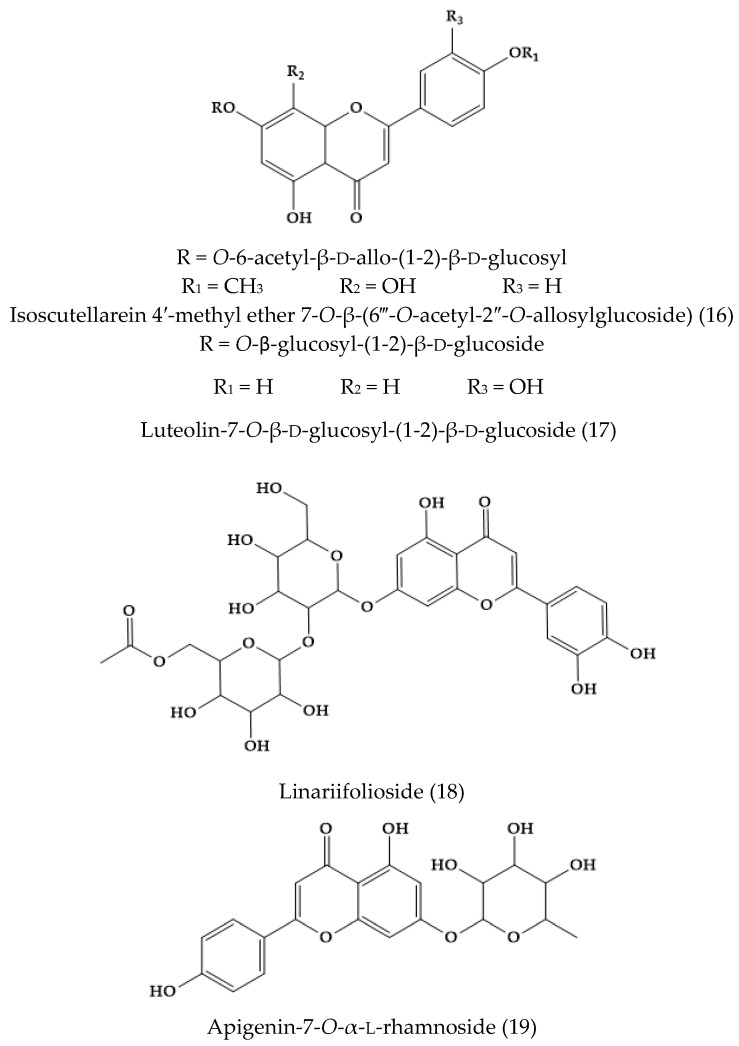
A non-common flavone glycoside was isolated from the whole plant of V. filiformis and identified by means of 13C NMR spectroscopy as isoscutellarein 4′-methyl ether 7-O-β-(6‴-O-acetyl-2″-O-allosylglucoside) (16) (Figure 3). This was the first report of 2-allosylglucose as a disaccharide unit of flavonoids [26].
Figure 3.
Flavone derivatives found in Veronica filiformis and Veronica linariifolia.
2.2. Veronica linariifolia Pall. ex Link
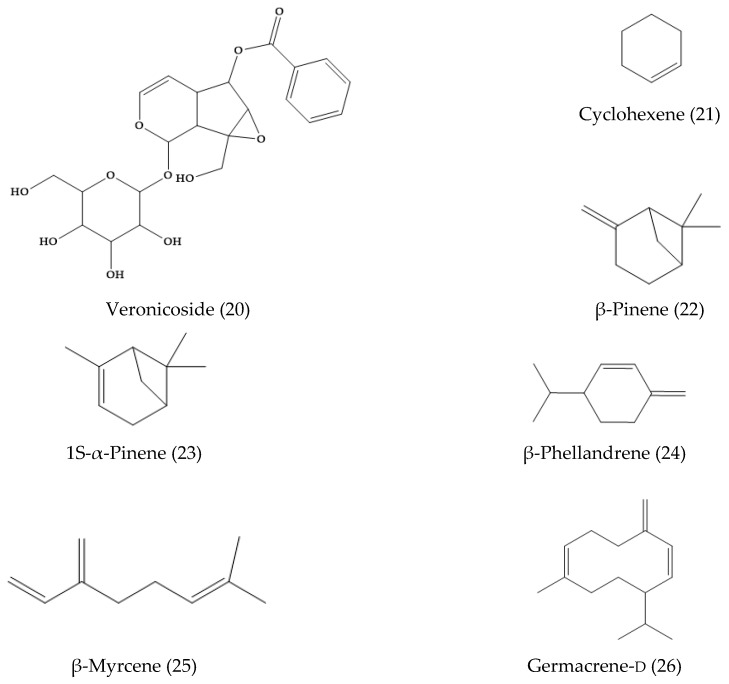
A new flavonoid glycoside, linariifolioside (18) (Figure 3), was isolated from the alcohol extract of the dried whole herb. In addition, four known compounds, luteolin-7-O-β-d-glucosyl-(1-2)-β-d-glucoside (17), apigenin-7-O-α-l-rhamnoside (19), luteolin (10), and apigenin (11) were isolated from the same fraction and identified [27]. Another work showed the presence of 3′,4′,5,6,7-pentahydroxyflavone-7-O-β-d-glucosyl-(1″→2′)-β-d-glucoside, 4′,5,7-trihydroxy-3′,6-dimethoxyflavone-7-O-β-d-glucoside, apigenin-7-O-β-d-glucuronide methyl ester, apigenin-7-O-β-d-glucuronide ethyl ester, apigenin-7-O-β-d-glucuronide buthyl ester, apigenin (11), luteolin (10), vanillic acid, p-hydroxybenzoic acid, protocatechuic acid, protocatechuic acid ethyl ester, isoerulic acid, catechol, and emodin [28]. Alternatively, the essential oil was extracted by steam distillation approaches. The main compounds isolated were cyclohexene (21), β-pinene (22), 1S-α-pinene (23), β-phellandrene (24), β-myrcene (25), and germacrene-d (26) (Figure 4) [29].
Figure 4.
Phytochemicals from Veronica linariifolia essential oil.
2.3. Veronica fushii
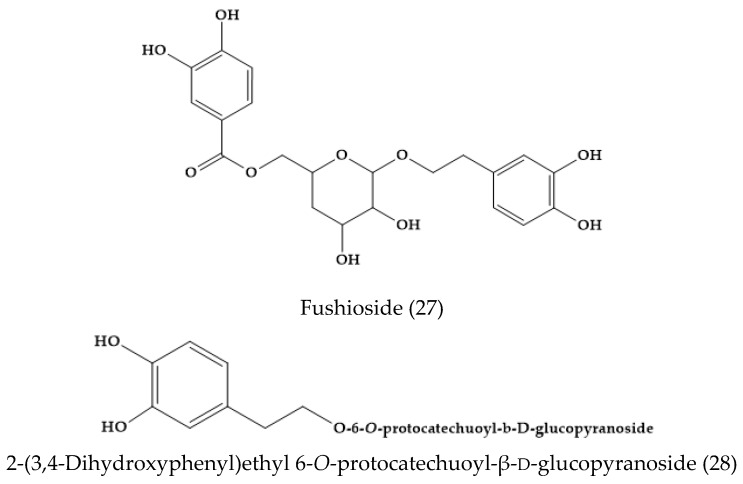
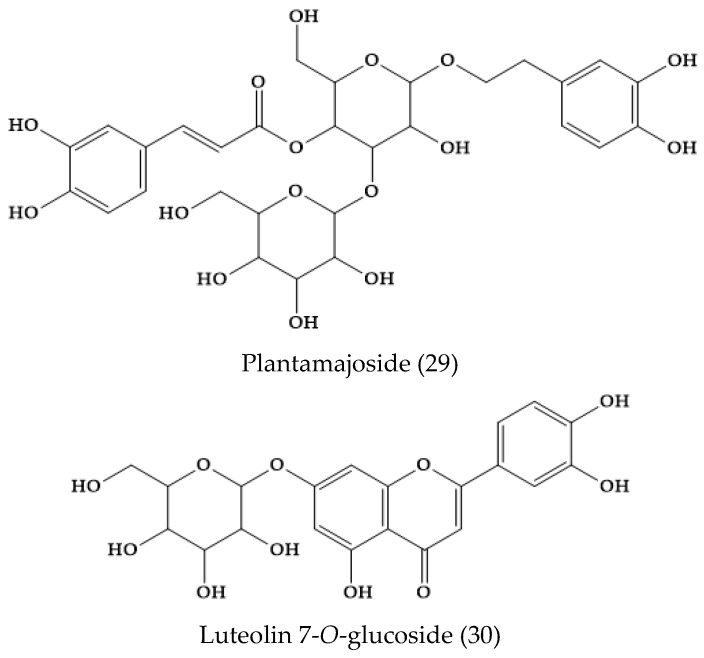
From the methanolic extract of the aerial parts of V. fuhsii, Ozipek and co-workers isolated and reported fushioside (27) and 2-(3,4-dihydroxyphenyl)ethyl 6-O-protocatechuoyl-β-d-glucopyranoside (28), along with a known phenylethanoid glycoside, plantamajoside (29), and a flavone glucoside, luteolin 7-O-glucoside (30) (Figure 5) [30]. Note that this species is endemic of Middle Anatolia [30], and it is not included in the plant list.
Figure 5.
Phytoconstituents in Veronica fushii.
2.4. Veronica cymbalaria Bodard
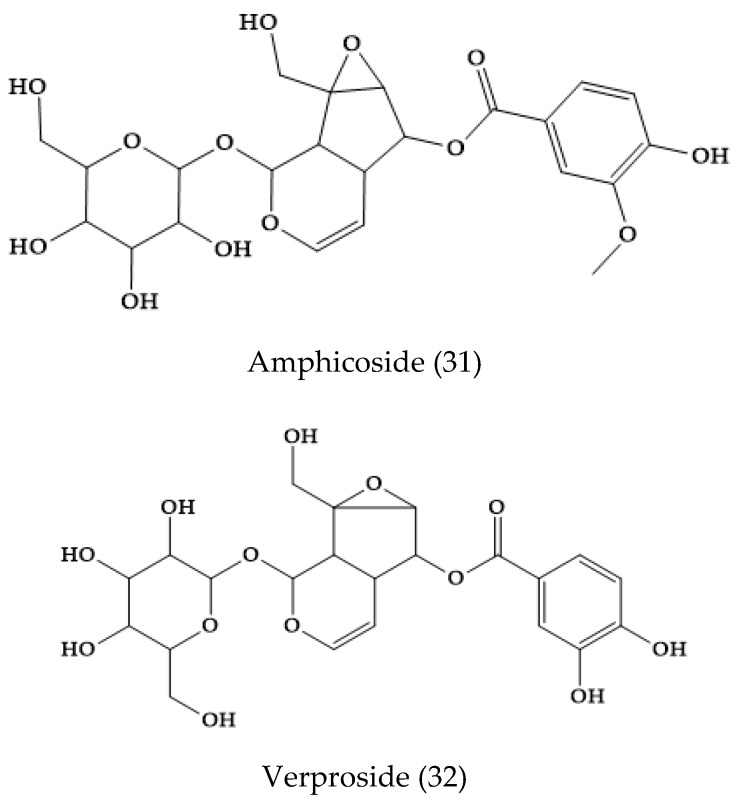
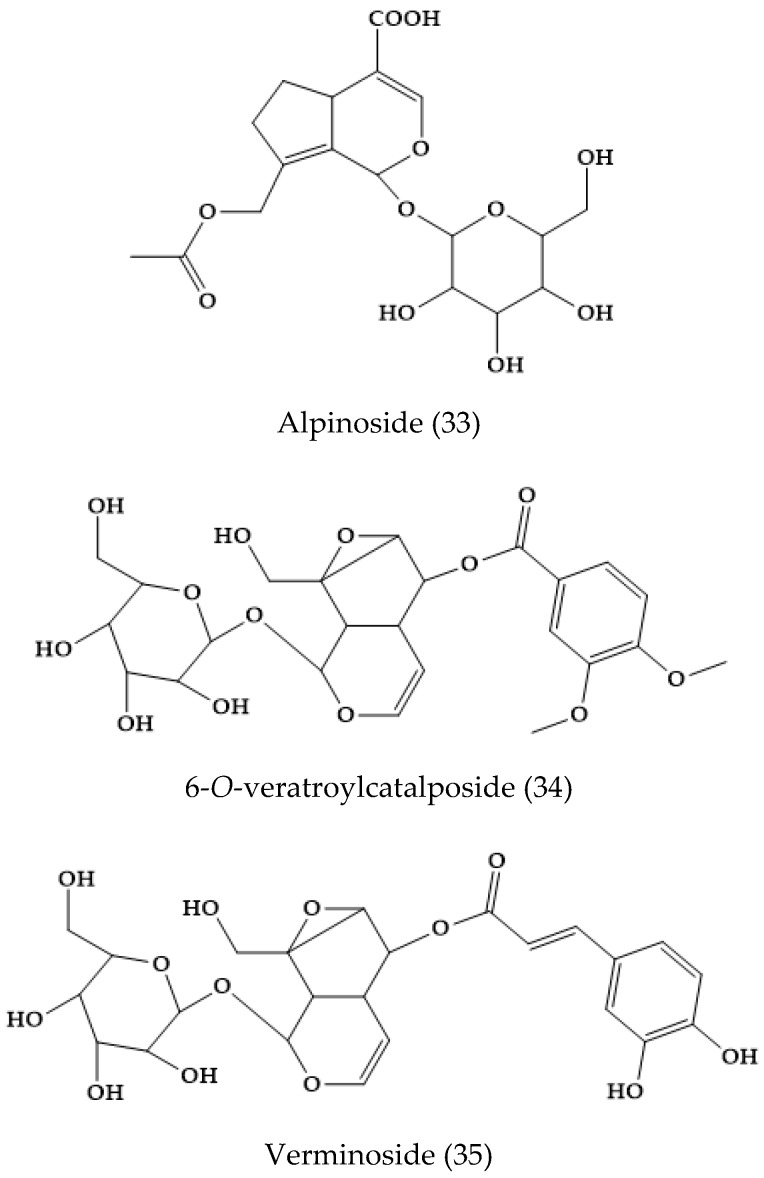
The main iridoids and iridoid-phenolic constituents of extracts from this plant were catalpol (1), amphicoside (31), and verproside (32), together with alpinoside (33), aucubin (8), 6-O-veratroylcatalpol (34), and verminoside (35) (Figure 6). The iridoid alpinoside with a 8,9-double bond was found for the first time in genus Veronica [31]. In V. cymbalaria, other authors found the iridoid glucosides aucubin (8), catalpol (1), veronicoside (20), verproside (32), amphycoside, verminoside (35), catalposide (6), 6-O-veratroylcatalposide (34), and 6-O-isovanilloylcatalpol [32].
Figure 6.
Phytoconstituents from Veronica cymbalaria.
2.5. Veronica anagallis-aquatica L.
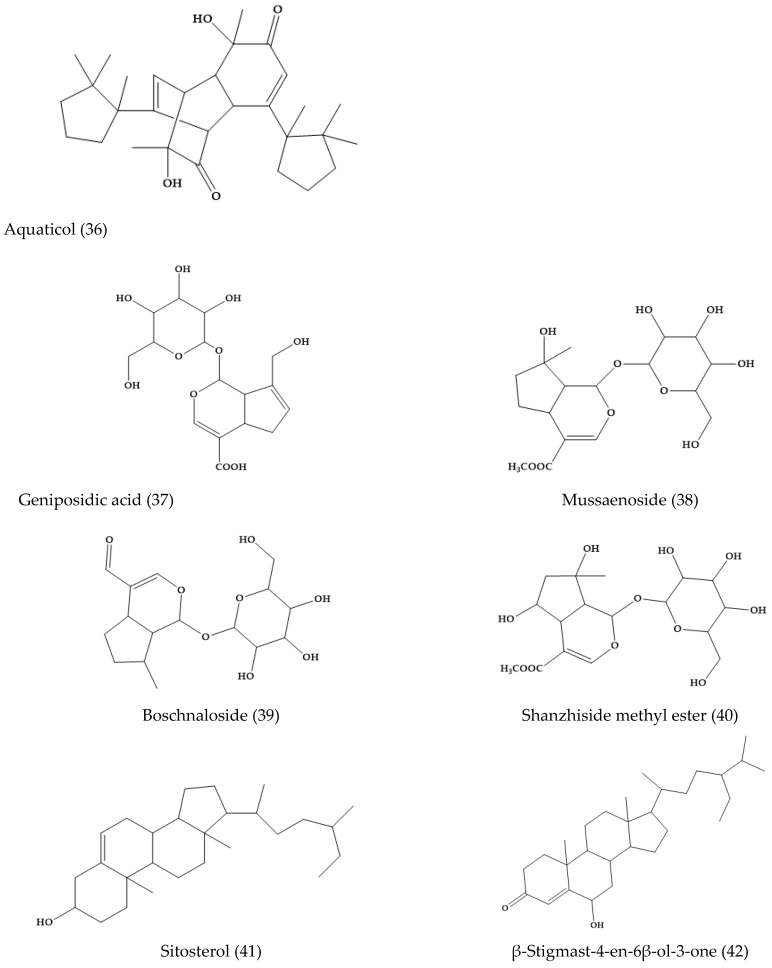
Aquaticol (36), an unusual bis-sesquiterpene, was isolated from the methanol extracts of V. anagallis-aquatica. The other 11 well-known compounds are aucubin (8), geniposidic acid (37), mussaenoside (38), catalposide (6), verproside (32), amphicoside (31), catalpol (1), boschnaloside (39), shanzhiside methyl ester (40), sitosterol (41), and β-stigmast-4-en-6β-ol-3-one (42) (Figure 7) [33].
Figure 7.
Selected phytochemicals from Veronica anagallis-aquatica and others.
2.6. Veronica persica
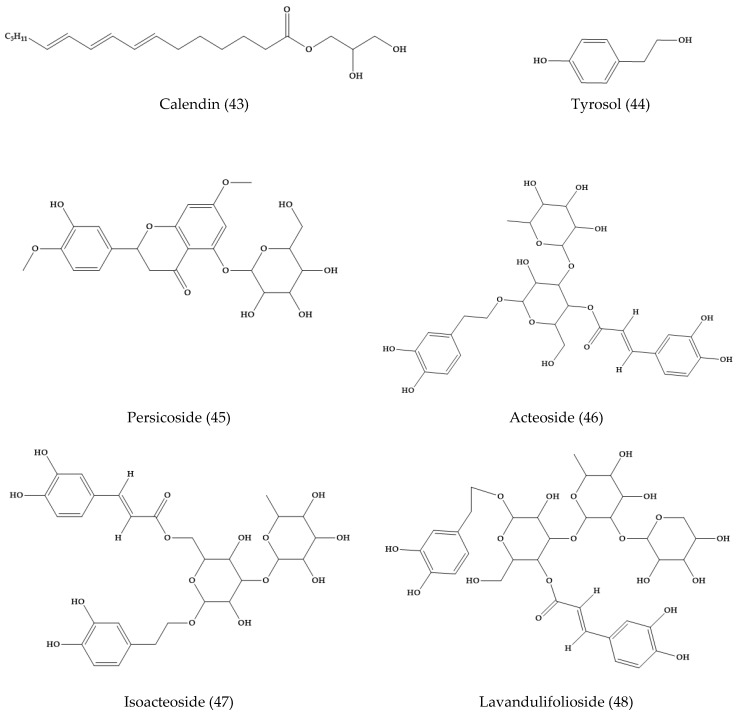
V. persica, “common field speedwell” or “Persian speedwell”, is a neophytic weed originally from southwest Asia and widely distributed in the temperate regions. In dichloromethane extracts of this plant, calendin (43), tyrosol (44), and two benzoic acid derivatives were isolated [34] (Figure 8). Moreover, in the aerial parts of V. persica, a new phenylethanoid glycoside, persicoside (45), and three known phenylethanoid glycosides, acteoside (46), isoacteoside (47), and lavandulifolioside (48) (Figure 8), were isolated and the structure was confirmed by spectroscopic techniques.
Figure 8.
Phytoconstituents from Veronica persica.
In addition to phenylethanoid glycosides, hexitol, dulcitol, and seven known iridoid glucosides, aucubin (8), veronicoside (20), amphicoside (31), 6-O-veratroyl-catalpol (34), catalposide (6), verproside (32), and verminoside (35) were isolated from this plant species [35].
2.7. Veronica longifolia L. and Veronica liwanensis K. Koch
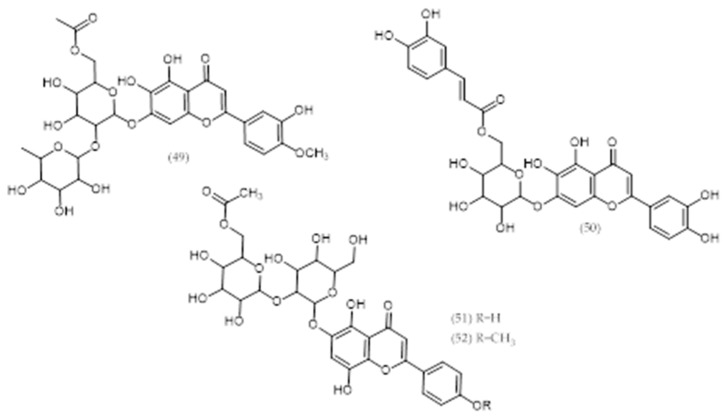
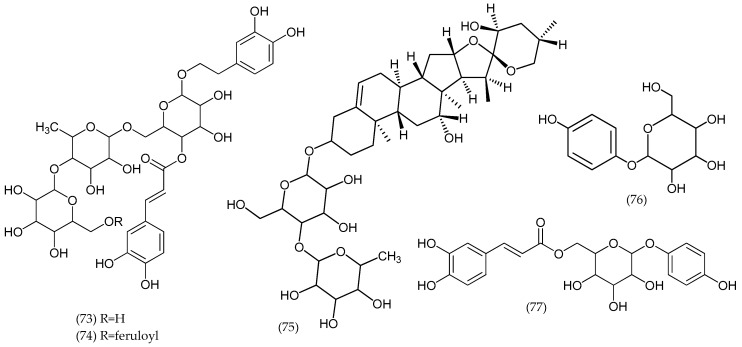
Two new acylated 5,6,7,3′,4′-pentahydroxyflavone (6-hydroxyluteolin) glycosides and two unusual allose-containing acylated 5,7,8,4′-tetrahydroxyflavone (isoscutellarein) glycosides were isolated from V. longifolia and V. liwanensis and characterized by NMR spectroscopy as 6-hydroxyluteolin 4′-methyl ether 7-O-α-rhamnopyranosyl(1‴→2″)[6″-O-acetyl-β-glucopyranoside] (49) and 6-hydroxyluteolin 7-O-(6″-O-(E)-caffeoyl)-β–glucopyranoside (50), respectively (see Table 2 and Figure 9) [36]. Moreover, three chlorinated iridoid glucosides, asystasioside E (92) and its 6-O-esters, named longifoliosides A and B (93, 94), were also detected [37].
Table 2.
Phytoconstituents obtained from ethanol extracts of some Veronica species (based on Reference [3]).
| Species | Extract | Compounds |
|---|---|---|
| Veronica argute-serrata | Ethanol | Mannitol (65), catalpol (1), aucubin (8), gardoside (69), ajugol (63), mussaenosidic acid (70), epiloganica acid (71), arborescosidic acid (72), verbascoside-like compounds, acetyl-flavone glycoside |
| Veronica arvensis L. | Ethanol | Mannitol (65), cornoside (64), ajugol (63), salidroside (66), verbascoside-like compounds |
| Veronica biloba schreb. ex L. | Ethanol | Catalpol (1), aucubin (8), ajugol (63), epiloganic acid (71), alpinoside (33) |
| Veronica campylopoda Boiss. | Ethanol | Mannitol (65), catalpol (1), aucubin (8), ajugol (63), verminoside (35), acetyl-flavone glycoside |
| Veronica chamaedryoides Engl. | Ethanol | Verbascoside-like compounds; some iridoid |
| Veronica dillenii Crantz | Ethanol | Verbascoside (67) and cornoside (64) |
| Veronica longifolia L. | Ethanol | Mannitol (65), catalpol (1), aucubin (8), verposide, catalposide (6), verminoside (35), catalpol ester, flavones |
| Veronica magna M.A.Fisch. | Ethanol | Verbascoside-like compounds |
| Veronica micans (M.A.Fisch.) Landolt | Ethanol | Verbascoside (67) and cornoside |
| Veronica micrantha Hoffmanns. & Link | Ethanol | Mannitol (65), aucubin (8), verpectoside B (68), triterpene glycosides |
| Veronica orbelica | Ethanol | Verbascoside-like compounds |
| Veronica vindobonensis (M.A.Fisch.) M.A.Fisch. | Ethanol | Verbascoside (67) and cornoside (64) |
Figure 9.
Phytoconstituents from Veronica longifolia, Veronica liwanensis, and Veronica orientalis.
2.8. Veronica orientalis Mill.
Isoscutellarein 7-O-(6‴-O-acetyl)-β-allopyranosyl(1‴→2″)-β-glucopyranoside (51) and its 4′-methyl ether (52) were obtained from V. orientalis species (Figure 9). The former was also found in Veronica intercedens Bornm. [36], although it is still an unresolved name [4].
2.9. Veronica thymoides P. H. Davis
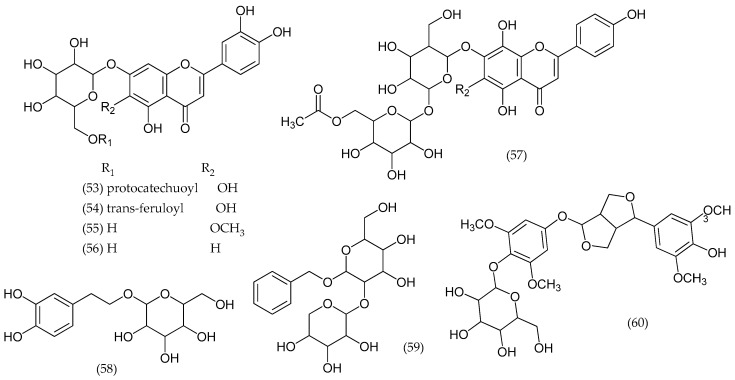
From V. thymoides subsp. pseudocinerea, a new acylated flavone glucoside, 3′-hydroxyscutellarein 7-O-(6″-O-protocatechuoyl)-β-glucopyranoside (53), and a new phenol glucoside, 3,5-dihydroxyphenethyl alcohol 3-O-β-glucopyranoside were isolated, along with seven flavone, phenol, and lignan glycosides: 3′-hydroxyscutellarein-7-O-(6″-O-trans-feruloyl)-β-glucopyranoside (54), 3′-hydroxy, 6-O-methylscutellarein 7-O-β-glucopyranoside (55), luteolin 7-O-β-glucopyranoside (56), isoscutellarein 7-O-(6‴-O-acetyl)-β-allopyranosyl (1‴→2″)-β-glucopyranoside (57), 3,4-dihydroxyphenethyl alcohol 8-O-β-glucopyranoside (58), benzyl alcohol 7-O-β-xylopyranosyl (1″→2′)-β-glucopyranoside (59), and (+)-syringaresinol 4′-O-β-glucopyranoside (60) (Figure 10) [38].
Figure 10.
Phytoconstituents from Veronica thymoides.
2.10. Veronica arvensis
The water extracts of this plant revealed the presence of cornoside aglycone and rengyolone (62). The iridoid glucoside, ajugol (63), and the phenylethanoid glucoside, cornoside (64) (Figure 11), were isolated from species of Veronica for the first time [39]. Other compounds are described in Table 2.
Figure 11.
Phytoconstituents reported in Veronica arvensis and other species (Table 2).
2.11. Veronica turrilliana Stoj. & Stef.
From V. turrilliana aerial parts, two phenylethanoid glycosides, turrilliosides A and B, and a steroidal saponin, turrillianoside, were isolated and their structures elucidated as β-(3,4-dihydroxyphenyl) ethyl-4-O-E-caffeoyl-O-[β-glucopyranosyl-(1→4)-α-rhamnopyranosyl -(1→6)]-β-glucopyranoside (73), β-(3,4-dihydroxyphenyl)ethyl-4-O-E-caffeoyl-[6-O-E-feruloyl -β-glucopyranosyl-(1→4)-α-rhamnopyranosyl-(1→6)]-β-glucopyranoside (74), and (23S,25S)-12β,23- dihydroxyspirost-5-en-3β-yl O-α-rhamnopyranosyl-(1→4)-β-glucopyranoside (75) (Figure 12), respectively. Other glucosides were reported, namely, catalpol (1), catalposide (6), verproside (32), amphicoside (31), isovanilloylcatalpol, aucubin (8), arbutin (76), and 6-O-E-caffeoylarbutin (77) [40].
Figure 12.
Phytoconstituents from Veronica turrilliana.
2.12. Veronica cuneifolia D. Don
Column chromatography of iridoid fractions of V. cuneifoia subsp. cuneifolia methanol extract resulted in the isolation of verproside (32), verminoside (35), amphicoside (31), veronicoside (20), catalposide (6), and catalpol (1). The comparison of the iridoid fractions of V. cuneifolia subsp. cuneifolia and V. cymbalaria using an HPLC diode array detector (DAD) system with 40% MeOH showed that iridoid fractions of V. cymbalaria contained veratroylcatalpol (34), isovanilloylcatalpol, and aucubin (8) in addition to the compounds found in iridoid fractions [19]. Additionally, seven iridoid glucosides, aucubin (8), catalpol (1), veronicoside (20), verproside (32), amphycoside, verminoside (28), and catalposide (6), were identified [32].
2.13. Veronica derwentiana Andrews and Veronica catarractae G. Forst.
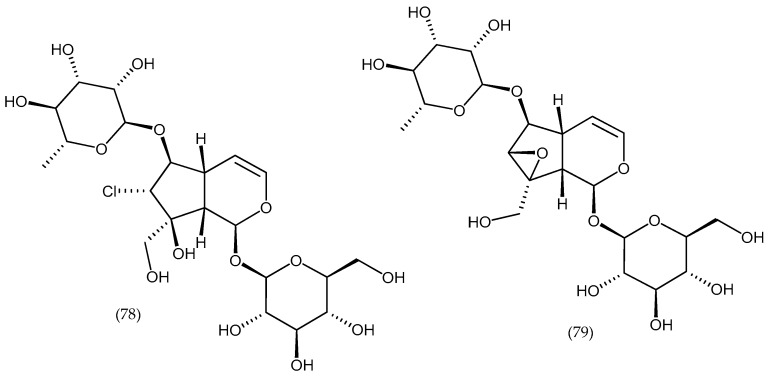
Three unusual substituted benzoyl esters of aucubin were obtained from V. derwentiana and a chlorinated iridoid glycoside (catarractoside) (78) (Figure 13) from V. catarractae, in addition to other iridoids common to the genus. The chemical profile of V. perfoliata is similar to that of Northern Hemisphere species of Veronica because of the presence of characteristic 6-O-catalpol esters. The profile of V. derwentiana is unique, since 6-O-esters of aucubin rather than of catalpol dominate; however, the acyl groups are the same as those present in catalpol esters found in some other Veronica sections. V. catarractae also contains one of the catalpol esters characteristic of Veronica, in addition to three 6-O-rhamnopyranosyl substituted iridoid glycosides, one of which is 6-O-rhamnopyranosylcatalpol (79) (Figure 13) [41].
Figure 13.
Structures of isolated compounds catarractoside and 6-O-rhamnopyranosylcatalpol (species Veronica derwentiana and Veronica catarractae).
2.14. Veronica sibirica L.
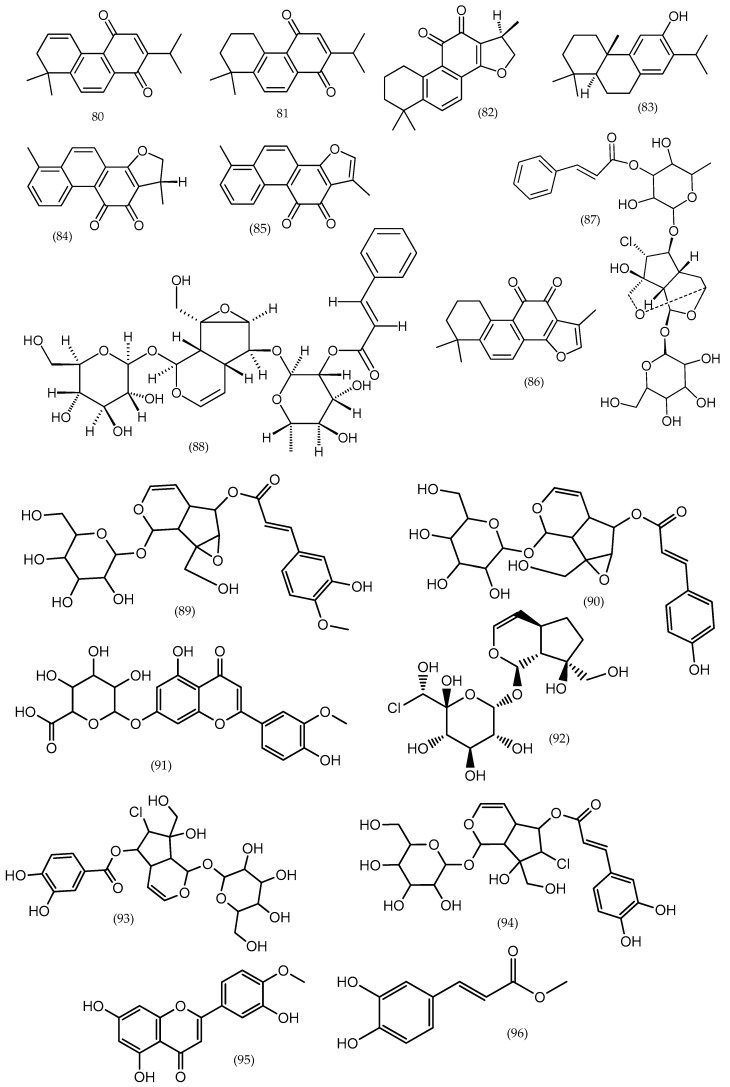
Eight compounds were isolated from V. sibirica L. and identified as 1,2-dehydrocryptotanshinone, sibiriquinone A (80), sibiriquinone B (81), cryptotanshinone (82), ferruginol (83), dihydrotanshinone I (84), tanshinone I (85), and tanshinone IIA (86) [42]. A new iridoid glycoside, versibirioside (87), and a known iridoid glycoside, verbaspinoside (88) (Figure 14), were also reported from the whole plant. In addition, versibirioside was isolated from the whole plant of V. sibirica [43].
Figure 14.
Several phytoconstituents in Veronica sibirica and Veronica peregrina.
2.15. Veronica Peregrina L.
Eight iridoid glycosides and four phenolic compounds were isolated from the ethyl acetate extract of V. peregrine. The compounds were identified as protocatechuic acid, luteolin (10), veronicoside (20), minecoside (89), specioside (90), amphicoside (31), catalposide (6), 6-O-cis-p-coumaroyl catalpol, p-hydroxybenzoic acid methyl ester, verproside (32), verminoside (35), and chrysoeriol 7-glucuronide (91) by spectroscopic analysis (Figure 14) [12]. In the methanolic extract, the presence of chrysoeriol (12), diosmetin (95), 4-hydroxybenzoic acid, apigenin (11), caffeic acid methylester (96), and protocatechuic acid was reported [44]. The phenolic acid 4-hydroxybenzoic acid was also found by other authors [45].
2.16. Veronica montana L., Veronica polita Fr., and Veronica spuria L.
The phenolic compounds of V. montana, V. polita, and V. spuria showed that flavones were the major compounds (V. montana: seven phenolic acids, five flavones, four phenylethanoids, and one isoflavone; V. polita: 10 flavones, five phenolic acids, two phenylethanoids, one flavonol, and one isoflavone; V. spuria: 10 phenolic acids, five flavones, two flavonols, two phenylethanoids, and one isoflavone). V. spuria possessed the highest contents in all groups of phenolic compounds, except flavones [46].
2.17. Veronica spicata
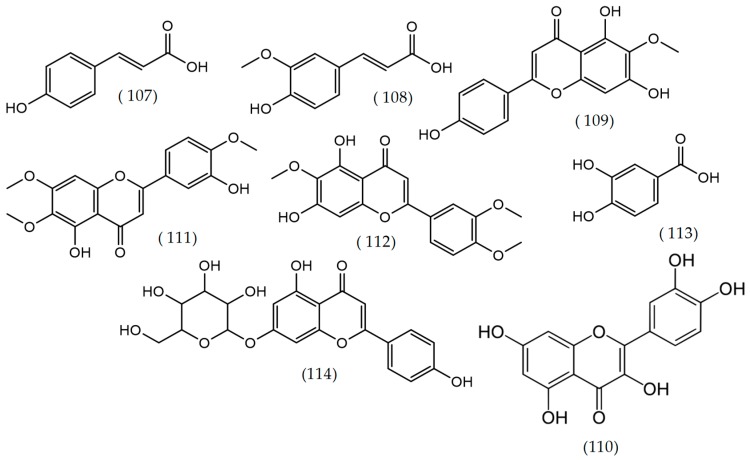
Six 6-hydroxyluteolin glycosides acylated with phenolic acids were elucidated in this species, three of which were new compounds and called spicoside (61) derivatives. A flavonoid survey of seven more species belonging to subgenus Pseudolysimachium and eight species of V. subgenus Pentasepalae showed that all the Pseudolysimachium species and four of the Pentasepalae species produced 6-hydroxyflavone glycosides, whereas the remaining four Pentasepalae species contained acetylated 8-hydroxyflavone glycosides [39]. Moreover, from the species, 10 phenolic compounds were characterized: chrysin (98), rutin (99), and quercitrin (100), as well as cichoric (101), ferulic (102), protocatechuic (103), syringic (104), rosmarinic (105), and tannic acids (106) (Figure 15) [11].
Figure 15.
Other phytoconstituents in Veronica spicata.
2.18. Veronica officinalis
The main phenolic compounds characterized in V. officinalis L. were p-coumaric acid (107), ferulic acid (108), luteolin (10), apigenin (11), quercitrin (100), hispidulin (109), quercetin (110) (Figure 16), and the sterol β-sitosterol (41). Some of these compounds (ferulic acid, coumaric acid, apigenin, and luteolin) were also found in V. teucrium L. and V. orchidea Crantz. Aglycones hispidulin, eupatorin (111), and eupatilin (112) (Figure 16) were also detected for the first time in the Veronica genus, mainly after hydrolysis, suggesting the presence of glycosylated forms. Eupatilin was found only in V. orchidea extracts. This chemical composition showed intravarietal differences [47].
Figure 16.
Structures of compounds reported in several Veronica species: Veronica officinalis, Veronica ciliata, and Veronica rosea.
2.19. Veronica ciliata Fisch.
Five main compounds, including two iridoid glycosides (catalposide (6), verproside (32)) and three phenolic compounds (luteolin (10), 4-hydroxy benzoic acid, and 3,4-dihydroxy benzoic acid (113)) (Figure 16), were isolated from the crude extract of V. ciliata by high-speed countercurrent chromatography [48].
2.20. Veronica rosea Desf.
The phytochemical study of butanolic extract of aerial parts of V. rosea led to the isolation and identification of four phytoconstituents: apigenin-7-O-β-glucopyranoside (114) (Figure 16), isoscutellarein-7-O-β-d-glucopyranoside, isoscutellarein7-O-[(6′’’-O-acetyl-β-d-allopyranosyl-(1→2)]-β-glucopyranoside, and mannitol (65) [49].
2.21. Others Phytochemicals and Species
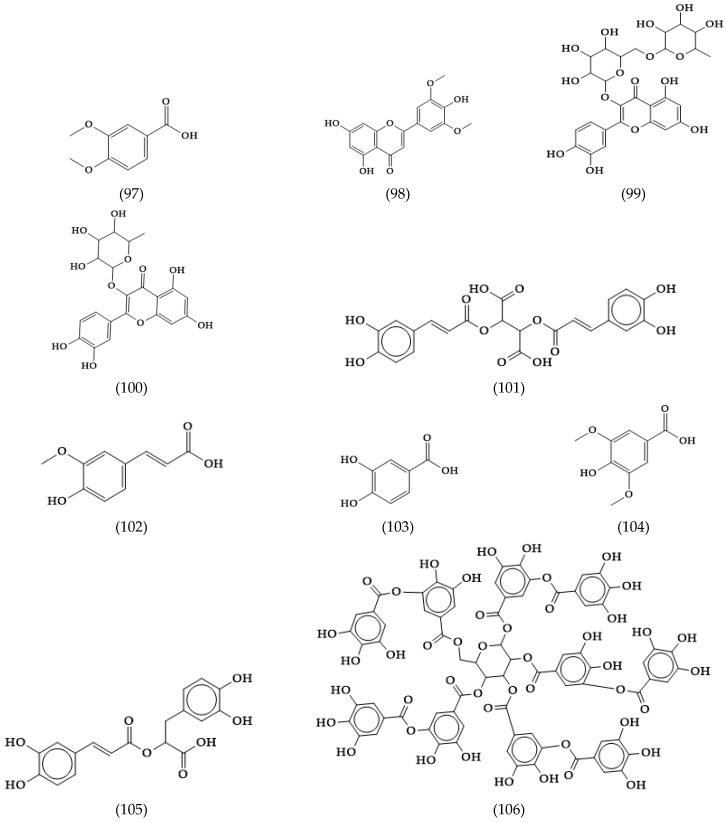
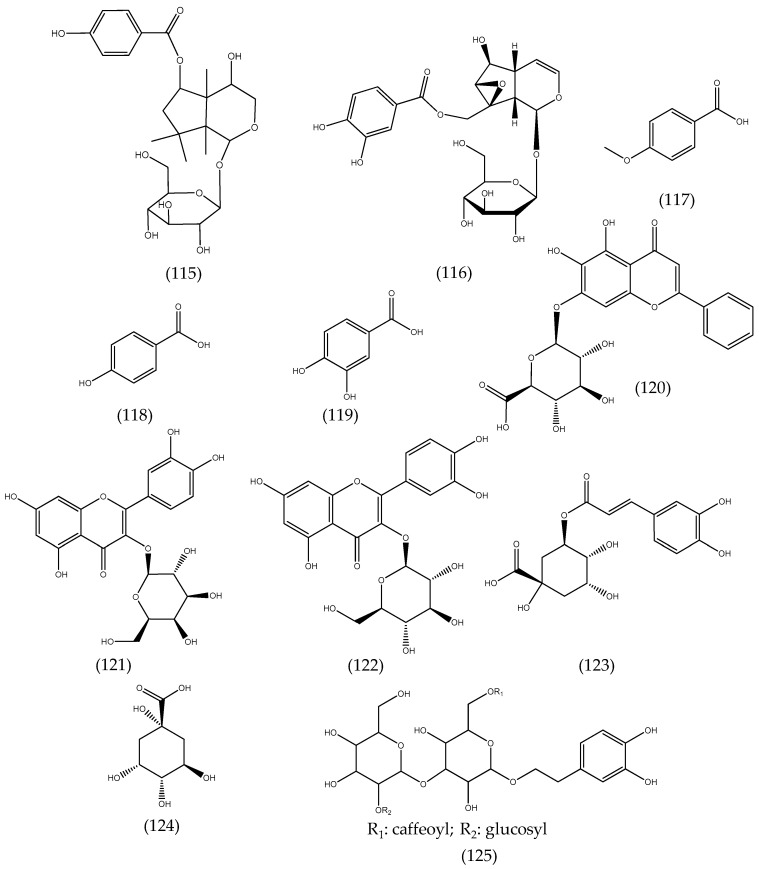
Veronica americana Schwein. ex Benth. methanolic extracts revealed new iridoids identified as 4β-hydroxy-6-O-(p-hydroxybenzoyl)-tetrahydrolinaride (115) and 10-O-protocatechuyl-catalpol (116), along with four known aromatic compounds, veratric acid (97), p-methoxybenzoic acid (117), p-hydroxybenzoic acid (118), and protocatechuic acid (119) (Figure 17) [50]. Kroll-Møller and co-workers isolated iridoid glucosides from Veronica hookeri (Buchanan) Garn.-Jones and Veronica pinguifolia Hook. f. from New Zealand [51]. Thirty-three water-soluble compounds were isolated from Veronica pulvinaris (Hook. f.) Cheeseman and Veronica thomsonii (Buchanan) Cheeseman. Most of the isolated compounds were esters of phenylethanoid and iridoid glycosides [52]. A chemosystematic investigation of the water-soluble compounds in Veronica cheesemanii and Veronica hookeriana Walp. showed that both species contained mannitol (65), in considerable amounts, and some iridoids such as aucubin (8), catalpol (1), and their esters [53].
Figure 17.
Structures of compounds reported in several Veronica species: Veronica americana, Veronica jacquinii, Veronica teucrium, and Veronica urticifolia.
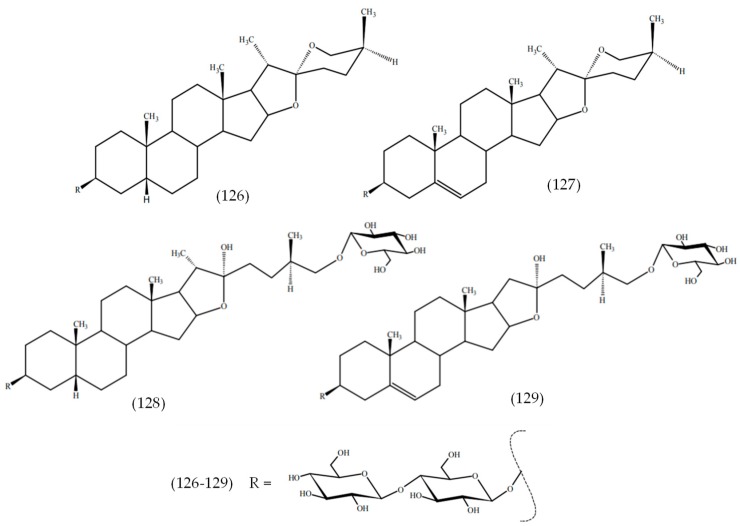
The phenolic compounds baicalin (120), hyperoside (121), isoquercetin (122), and chlorogenic acid (123), as well as quinic acid (124), were the main components in aqueous-acetone extracts of Veronica jacquinii Baumg., Veronica teucrium L., and Veronica urticifolia Jacq. (Figure 17) [13]. A new phenylethanoid triglycoside, chionoside J (125), was isolated from Veronica beccabunga L. (brooklime) [54], while two new spirostane glycosides, chamaedrosides C (126) and C1 (127), two new furostane glycosides, chamaedrosides E (128) and E1 (129), and two new furospirostane glycosides were found in V. chamaedrys (Figure 18) [55]. Compounds in other species are detailed in Table 2, i.e., Veronica argute-serrata, Veronica biloba schreb. ex L., Veronica campylopoda Boiss., Veronica chamaedryoides Engl., Veronica dillenii Crantz, Veronica magna M.A.Fisch., Veronica micans (M.A.Fisch.) Landolt, Veronica micrantha Hoffmanns. & Link, Veronica orbelica, and Veronica vindobonensis (M.A.Fisch.) M.A.Fisch.
Figure 18.
Structures of compounds reported in Veronica beccabunga L.
3. Antimicrobial Activities of Veronica Plants
Many plants were used since ancient times to treat infections caused by bacteria that are now resistant to antibiotics [56]. There are several traditional uses of the genus Veronica that could be related to antimicrobial properties. For example, these species are used as expectorants, restoratives, tonics, and for the treatment of influenza and other respiratory diseases in traditional Chinese medicine [57]. In Romanian medicine, aerial parts of Veronica plants are known for their wound-healing properties and are also used for the treatment of cough and catarrh [47]. Despite their widespread usage in folk medicine, there is a lack of information about the antimicrobial activity. Only a few studies confirmed that certain Veronica species showed noticeable bioactivity such as antibacterial, antifungal, and antiviral activity.
3.1. Antibacterial Activity
Although Veronica species are traditionally used for their antibacterial effect, there are only a few studies that showed its strong antibacterial effect. The antibacterial activity against Gram-positive and Gram-negative species depends on the extract type (solvent used, part extracted, species, etc.). As an example, the antimicrobial properties of aerial parts of V. spicata extracts with ethyl-acetate, methanol, or water using the diffusion method and microdilution method were examined. The bacterial strains used in study were found to be susceptible toward methanol and ethyl-acetate extracts, with minimum inhibitory concentration (MIC) values between 1.25 and 5 mg/mL using the microdilution method, while aqueous extracts were inactive. The extracts prepared from leaves showed larger zones of inhibition compared to flowers and steam extracts of V. spicata, indicating stronger antimicrobial activity [11,58]. In another work and using the microdilution method, Živković et al. (2014) investigated the antibacterial effect of the V. urticifolia methanol extract against the Gram-negative bacteria Escherichia coli, Enterococcus faecalis, and Pseudomonas aeruginosa, and Gram-positive bacteria Staphylococcus aureus, L. monocytogenes, and Bacillus cereus [59]. After the measurement of the minimum bactericidal concentration (MBC) and MIC values, it was found that the most sensitive germ was Staphylococcus aureus. The antistaphylococcal effect is due to a main phenolic compound acteoside, which could inhibit the incorporation of leucine and disturb protein synthesis. The same antistaphylococcal effects were found in other studies [20]. Other chemical compounds could be also responsible for this antibacterial activity, e.g., β-sitosterol, campesterol, stigmasterol, hispidulin, and flavonoids. These results are important for human health because S. aureus is a pathogen germ difficult to treat with the development of antibiotic resistance. For this reason, natural alternative therapies to solve this problem are useful. Other studies showed the antibacterial activity of methanol, ethanol, or aqueous extracts from Veronica urticifolia Jacq., Veronica orchidea Crantz, V. persica, and Veronica montana L. against Gram-positive and Gram-negative bacteria [20,47,57,59]. The summary of the antimicrobial activity of different Veronica species is represented in Table 3.
Table 3.
Summary of the antimicrobial activity of different Veronica species. MIC—minimum inhibitory concentration; MBC—minimum bactericidal concentration.
| Species | Plant Part | Extract | Effect | Reference |
|---|---|---|---|---|
| Veronica spicata L. | Flowers and stem | Methanol and ethyl-acetate extracts | MIC values were between 1.25 and 5 mg/mL against Staphylococcus aureus, Microccocus flavus, Listeria monocytogenes, Enterobacter cloacae, Escherichia coli, Bacillus cereus, and Pseudomonas aeruginosa | [11,58] |
| Veronica urticifolia Jacq. | The aerial parts | Methanol extract | The most sensitive germ was Staphylococcus aureus (MIC and MBC = 7.5 mg/mL) | [59] |
| Veronica lycica E. Lehm. | The aerial parts | Methanol extract | The antimicrobial activity was determined against E. coli, S. aureus, Klebsiella pneumoniae, P. aeruginosa, Proteus vulgaris, B. cereus, Mycobacterium smegmatis, L. monocytogenes, Micrococcus luteus, Candida albicans, Rhodotorula rubra, and Kluyveromyces fragilis. The weak antimicrobial effect was observed against the tested microorganisms | [60] |
| Veronica anagallis-aquatica L. | The aerial parts | Methanol extract | The extracts were tested against five bacterial and two yeast strains. They showed significant inhibition compared to the positive control (gentamicin) | [61] |
| Veronica officinalis L., Veronica teucrium L., Veronica orchidea Crantz | The aerial parts | 70% ethanol extract | Two anaerobic bacterial strains were used: Peptostreptococcus anaerobius and Fusobacterium nucleatum. V. teucrium and V. orchidea presented a higher activity (MIC = 31.25 mg/mL and MBC = 62.5 mg/mL) than V. officinalis (MIC and MBC of 62.5 mg/mL), with the most sensitive strain being Peptostreptococcus anaerobius | [62] |
| V. officinalis, V. teucrium, V. orchidea | The aerial parts | 70% ethanol extract | Eight bacterial strains were used: Staphylococcus aureus, Bacillus cereus, Listeria monocytogenes, Listeria ivanovii, Pseudomonas aeruginosa, Enterococcus faecalis, Salmonella typhimurium, and Escherichia coli. The most sensitive strains were Staphylococcus aureus, Listeria monocytogenes, and Listeria ivanovii with MIC values between 3.9 and 15.62 mg/mL | [47] |
| Veronica persica Poir | The aerial parts | 70% methanol extract | V. persica extract demonstrated an antifungal effect against Candida albicans and Aspergillus niger at a concentration 300 μg/mL of extract | [20] |
As commented before, not all results were positive. Dulger and Ugurlu (2005) evaluated the antimicrobial activity of the methanol extracts obtained from endemic Plantaginaceae members from Turkey, including Veronica lycica E. Lehm. The antimicrobial activity was determined in E. coli (American Type Culture Collection (ATCC) 11230), S. aureus (ATCC 6538P), Klebsiella pneumoniae (UC57), P. aeruginosa (ATCC 27853), Proteus vulgaris (ATCC 8427), B. cereus (ATCC 7064), Mycobacterium smegmatis (Czech Collection of Microorganisms (CCM) 2067), L. monocytogenes (ATCC 15313), Micrococcus luteus (CCM 169), Candida albicans (ATCC 10231), Rhodotorula rubra (German Collection of Microorganisms (DSM) 70403), and Kluyveromyces fragilis (ATCC 8608) using the disc diffusion method. In this case, V. lycica had weak antimicrobial effect against the tested microorganisms [60]. V. anagallis-aquatica was tested using the agar well diffusion assay against five bacterial and two yeast strains. None of the extracts of this Veronica species showed significant inhibition comparing to the positive control (gentamicin) [61].
In some cases, the antimicrobial activity of the isolated compounds was determined. In a study conducted by Mocan and collaborators, V. officinalis, V. teucrium, and V. orchidea were selected from Romanian natural flora and investigated for their antioxidant and antimicrobial effects against anaerobic bacterial strains with emphasis on the isolated compounds, caffeic and chlorogenic acids. V. teucrium and V. orchidea presented a higher activity (MIC = 31.25 mg/mL and MBC = 62.5 mg/mL) than V. officinalis (MIC and MBC of 62.5 mg/mL), with the most sensitive strain being Peptostreptococcus anaerobius. All analyzed species contained both caffeic and chlorogenic acids, where the richest source of caffeic acid was V. officinalis and the highest amount of chlorogenic acid was found in V. teucrium [62].
3.2. Antifungal, Antiviral, and Antiparasitic Activity
Other studies demonstrated the antifungal effect of Veronica species [11,20,47]. Mocan and co-workers investigated the antifungal properties of V. persica against Aspergillus niger and Penicillium hirsutum using the Kirby–Bauer diffusimetric method. The antifungal effect was higher for A. niger than P. hirsutum and it is due to phenolic compounds [47]. Using the same method, antifungal effects of V. persica extract against Candida albicans and A. niger were demonstrated in another study [20]. The results showed that the highest antifungal effect for both fungal pathogens was obtained at a concentration 300 μg/mL of extract. Dunkic et al. demonstrated in another study the antifungal effect of methanol V. spicata extract at MIC values ranging from 1.25 mg/mL to 5 mg/mL. In addition, the methanol extract prepared only from V. spicata’s leaves also had an effect against the dermatophyte Microsporum gypseum [11]. As a result of these positive data, Veronica plants can be considered good natural therapeutic alternatives for mild fungal infections.
A new biological property, i.e., antiviral activity (against herpes simplex viruses HSV1 and HSV2), of V. persica was demonstrated in a recent research conducted by Sharifi-Rad et al. (2018) [63]. In this study, the ethanol extract of V. persica was tested on Vero cells infected with both types of viruses. The stronger antiviral activity was found in the 80% methanol fraction of V. persica extract during and after infection of the cells with viruses, thus suggesting the interference of the extract with the intracellular entry of the virus and a possible inhibition of the viral intracellular replication of endogenous herpetic viruses. HSV1 was much more sensitive to the action of the methanolic fraction compared to HSV2. In addition, the 80% MeOH fraction of V. persica extract administered to the cells at the same time with aciclovir (antiviral drug) showed a synergistic effect in reducing plaque formation by herpetic viruses [63]. This antiviral action indicates the usefulness of the V. persica extract in combination with the antiviral medication (such as aciclovir) for decreasing the severity of symptomatic episodes of oral herpetic infection, which relapses when the immune system is weak. Moreover, in a recent investigation conducted by these authors, in vitro and in vivo susceptibility of Leishmania major to V. persica extract was evaluated. Antileishmanial activity of plant extract was investigated on cultured L. major promastigotes and in mice. In vitro tests showed a high and dose-dependent inhibitory activity of plant extract, which was able to reduce the survival time of promastigotes in a concentration-dependent manner; for example, the survival time of promastigotes decreased to 10% at 750 μg/mL after 72 h of exposure time. There was a significant influence of V. persica extracts in vivo on accelerating the healing process, as well as reducing the overall disease burden, in an animal model by inducing nitric oxide production in macrophage cells [64].
4. Antioxidant Activities of Veronica Plants
4.1. In Vitro Studies
Medicinal plants, including Veronica species, are excellent sources of phytochemicals with potent antioxidant activities. Extracts from several species were tested in vitro through several methods such as DPPH (2,2-diphenyl-1-picrylhydrazyl) free-radical scavenging, the phosphomolybdate method [56,65,66,67,68], hydrogen peroxide scavenging and bleomycin-dependent deoxyribonucleic acid (DNA) damage test [69], oxygen radical absorbance capacity (ORAC) assay [12], ferric-reducing antioxidant power test [70], and ABTS (2,2-azinobis 3- ethylbenzothiazoline-6-sulfonate) radical scavenging ability [56].
Mocan and co-workers reported that ethanolic extracts of V. officinalis, V. teucrium, and V. orchidea, which contain phenolic acids and flavonoids, showed potent antioxidant activity [47]. The Trolox equivalent (TE) antioxidant capacity (TEAC) assay indicated that V. officinalis (157.99 ± 6.58 mg TE/g dry weight (d.w.)) and V. orchidea (155.41 ± 1.58 mg TE/g d.w.) exhibited similar antioxidant capacities, but higher than that of V. teucrium (96.67 ± 0.26 mg TE/g d.w.). This was also suggested using antioxidant activity measured with electron paramagnetic resonance (EPR) spectroscopy using Fremy’s salt. In another work, 14 Veronica species were tested for their radical scavenging activity against DPPH, superoxide (SO), and nitric oxide (NO) radicals. V. chamaedrys was the most active against SO radical (half maximal inhibitory concentration (IC50) 113.40 µg/mL) and V. officinalis against DPPH (IC50 40.93 µg/mL) and NO radicals (IC50 570.33 µg/mL) [68]. In another work, the antioxidant potential of various extracts obtained from aerial flowering parts of three Veronica species, V. teucrium, V. jacquinii, and V. urticifola, was evaluated in vitro by DPPH free-radical scavenging activity (IC50 values 12.58 to 66.34 µg/mL) and ferric-reducing antioxidant power assays (0.97 to 4.85 mmol Fe2+/g) [70]. V. spicata extracts obtained by different solvents (water, methanol, and ethyl-acetate) also demonstrated a radical scavenging effect [11], especially methanol ones. Although butylated hydroxytoluene and butylhydroxyanisole are generally more active [11,70], the former is a source of natural ingredients.
Antioxidant activities of Veronica species could be attributed to their content of iridoids and phenolic acids [65]. Moreover, acylated flavonoids and phenol glycosides from V. thymoides subsp. pseudocinerea exhibited potent radical scavenging activity against DPPH radicals [38]. The two phenylethanoid glycosides, turrilliosides A and B, isolated from Veronica turrilliana Stoj. & Stef. were found to be potent DPPH radical scavengers, approximately 1.6-fold better than the flavonoid quercetin [40]. In another work, the antioxidant capacity of V. persica phenolic-rich extracts was correlated with their total phenol content [56].
4.2. In Vivo Studies
The antioxidant activity of three Veronica species, V. teucrium, V. jacquinii, and V. urticifola, was examined in vivo in rats, and their effect on several hepatic antioxidant systems was tested, i.e., on the activity of glutathione peroxidase, glutathione reductase (GR), peroxidase (Px), catalase (CAT), and xanthine oxidase, as well as glutathione (GSH) content and level of thiobarbituric acid reactive substances (TBARS). Treatment with Veronica extracts (methanolic, aqueous-acetone, and water extracts) (100 mg/kg) inhibited CCl4-induced liver injury by decreasing TBARS level, increasing GSH content, and bringing the activities of antioxidative enzymes CAT, Px, and GR to control levels. The study suggested that the extracts analyzed could protect the liver cells from CCl4-induced liver damage by their antioxidative effect on hepatocytes [70]. Veronica ciliata Fisch. is also a considerable candidate for protecting liver injuries due to its antioxidant and anti-apoptosis properties [71].
The antioxidant activity of other Veronica extracts was associated with other bioactivities. As an example, Lu and co-authors reported that isolated compounds from V. ciliata showed anti-hepatocarcinoma activities against HepG2 liver hepatocellular carcinoma cells, which could be associated with its antioxidant activity [48]. Lee and co-workers evaluated the antioxidant activity, cytotoxicity, and collagen synthesis activity in vitro in order to test the anti-wrinkle effect of a formulated cream containing V. officinalis extract. Antioxidant evaluation was performed in fibroblast cells. The ethanolic extract showed good antioxidant activity against DPPH free radicals (103.50 µg/mL) and also exhibited a significant effect on collagen synthesis activity without cytotoxicity. In a placebo-controlled trial on women, the treatment with the formulated cream (Scoti-Speedwell) for 56 days significantly resulted in anti-wrinkle activity [72].
5. Anticancer Activities of Veronica Species
Plant-derived metabolites are beneficial sources of new anti-cancer drugs with reduced cytotoxicity and increased activity. More than 60% of today’s drugs with proven anticancer properties originated from plants [73]. Extracts obtained from the aerial parts of various Veronica species are globally used as folk medicine for the therapy of cancer [16]. Despite this fact, only a few amounts of Veronica species were investigated for their cytotoxic and anticancer activities (as an example, see Table 4). With the exception of one in vivo confirmation, all of the investigations were performed in vitro against various cancer cell lines. Also, there is a lack of mechanistic studies for compounds isolated from Veronica species.
Table 4.
Some bioactive effects of Veronica plants and potential active compounds.
| Type of Studies | Primary Outcomes | Active Compounds | Veronica spp. | References | |
|---|---|---|---|---|---|
| In vitro | Human neuroblastoma cell line SH-SY5Y | Neuroprotective against H2O2 induced cytotoxicity | Iridoid glucosides acteoside, and aucubin (only in V. urticifolia) |
Veronica urticifolia Jacq. Veronica teucrium L. Veronica jacquinii Baumg. |
[79] |
| Human endothelial cells EA.hy 926 | Angiogenic | Phenylpropanoids and flavonoids |
V. jacquinii
V. teucrium V. urticifolia |
||
| Human lung epithelial cells A549 | Anti-inflammatory in lung diseases (anti-asthmatic) | Iridoid glycosides (verminoside, verproside) | Veronicaofficinalis L. | [15] | |
| Human cancer cell lines HF-6 (colon), PC-3 (prostate) human normal MRC-5 cells (fetal lung fibroblast) | Cytotoxic | Iridoids | Veronica americana Schwein. ex Benth. | [50] | |
| SK-N-SH human neuroblastoma cell line, BEL-7402 human hepatoma cell line | Cytotoxic | Diterpenes | Veronica sibirica L. | [42] | |
| In vivo | Phenyl-p-benzoquinone writhing test and carrageenan induced hind paw edema model in mice | Antinociceptive and anti-inflammatory | Iridoid glucosides, catalposide and verproside | Veronica anagallis-aquatica L. | [16] |
| Rats′ paw edema induced by dextran | Anti-inflammatory | Phenolic compounds and iridoids | Veronica persica Poir | [65] | |
| Clinical | Study design: randomized, placebo controlled for 58 days | Anti-wrinkles, antiaging of skin | Verbascoside | V. officinalis | [72] |
5.1. In Vitro Studies
Methanolic and water extracts of several Veronica species were tested against cancer cells in vitro. Harput and collaborators studied the cytotoxic activity of five Veronica species: V. cymbalaria, V. hederifolia, Veronica pectinata L., V. persica, and V. polita. Their methanolic extracts showed dose-dependent cytotoxicity against KB (human epidermoid carcinoma) and B16 (mouse melanoma) cells. Additional fractionation of investigated extracts pointed out that the active compounds occurred in the chloroform fraction, but not the water one. Moreover, KB cells were more sensitive to the CHCl3-soluble parts of the extracts compared to B16 cells. Except for V. cymbalaria, Veronica species exhibited similar activity against KB cells, while V. persica, V. polita, and V. pectinata demonstrated potent activity against B16 cells [74]. Saracoglu et al. (2011) evaluated the cytotoxicity of aqueous extracts (10–800 µL) of V. cuneifolia subsp. cuneifolia and V. cymbalaria using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay against various cell lines, Hep-2 (human epidermoid carcinoma), RD (human rhabdomyosarcoma), and L-20B (transgenic murine L-cells), as well as in non-cancerous Vero cells (African green monkey kidney cells). Both samples showed cytotoxic activity against used cell lines, but V. cuneifolia subsp. cuneifolia showed a stronger effect with IC50 values ranging from 250.4 (for RD line) to 410.9 (for Vero cell line) µg/mL [75]. Moreover, a previous pharmacological investigation on edible V. americana (Raf.) Schwein species (American speedwell) showed that the methanolic extract of the aerial parts exhibited cytotoxic activity against colon (HF-6) and prostate (PC-3) human cancer cell lines with IC50 values of 0.169 and 1.460 µg/mL, respectively [76].
Other studies focused on the elucidation of the active compounds. As an example, the authors of Reference [50] conducted bioassay-guided fractionation of V. americana methanolic extract, employing the cytotoxic activity against two previously mentioned cancer cell lines in order to determine active components. Compounds in this extract that demonstrated activity higher than camptothecin (used as the positive control) were 4β-hydroxy-6-O-(p-hydroxybenzoyl)-tetrahydrolinaride and 10-O-protocatechuyl catalpol. Their activity was higher against HF-6 cells with IC50 values of 0.031 and 0.066 µM for americanoside and 10-O-protocatechuyl-catalpol, respectively. Also, according to the calculated selectivity index (SI), both compounds showed more selective cytotoxicity against applied cancer cell lines than against human normal MRC-5 (fetal lung fibroblast) cells. Yin and co-workers investigated anti-hepatocarcinoma activity of 95% ethanol extract of V. ciliata aerial parts and its fractions, using human hepatocellular carcinoma cells HepG2 and the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) test. They demonstrated that active compounds were concentrated in the ethyl acetate fraction of the extract [77]. Iridoid compounds veronicoside, catalposide, amphicoside, and verminoside strongly inhibited HepG2 cell proliferation in a dose-dependent manner. Their inhibition rates (with the exception of veronicoside) were significantly higher compared to that of 5-fluorouracil used as a positive control, i.e., IC50 values ranged from 15.54 to 28.32 µg/mL, while the 5-fluorouracil IC50 value was 29.62 µg/mL. In the later study conducted by the same group of authors [48], the anti-hepatocarcinoma activities of five other compounds isolated from V. ciliata were also determined in vitro against the HepG2 cell line using the Cell Counting Kit-8 (CCK-8) method. The results showed that the proliferation of HepG2 cells was notably inhibited by these five compounds in a dose-dependent manner, and their activities decreased in the following order: luteolin > verproside > catalposide > 3,4-dihydroxy benzoic acid > 4-hydroxybenzoic acid, with IC50 values ranging from 102.36 to 444.76 µg/mL. The authors hypothesized that the exhibited activity of isolated compounds could be associated, at least in part, with their antioxidant activity. According to them, the higher cytotoxic potential of luteolin compared to analyzed iridoid glucosides and phenolic acids could be attributed to the higher number of phenolic groups in the molecule.
In a recent study, the cytotoxic properties of eight compounds isolated from V. sibirica were tested in vitro through the MTT assay against two cell lines: human neuroblastoma (SK-N-SH) and human hepatocellular carcinoma (BEL-7402). Compounds sibiriquinone A, cryptotanshinone, ferruginol, dihydrotanshinone I, tanshinone I, and tanshinone IIA showed beneficial inhibitory effects on the SK-N-SH cell growth, while compounds sibiriquinone A, cryptotanshinone, dihydrotanshinone I, and tanshinone IIA inhibited growth of the BEL-7402 cell line [42]. Moreover, the cytotoxic activity of iridoid compounds characteristic for Veronica species (aucubin, catalpol, and catalpol derivatives) was also determined against previously mentioned cell lines Hep-2, RD, and L-20B. Among tested compounds, verminoside showed very strong activity with IC50 values of 128 µM, 70 µM, and 103 µM, respectively. The activities of amphicoside and veronicoside were lower, while verminoside, verproside, and 6-O-veratroylcatalposide showed cytostatic activity [78]. Moreover, the in vitro antitumor activity of diterpenes, chemical compounds from V. sibirica, was also shown using the SK-N-SH human neuroblastoma cell line and BEL-7402 human hepatoma cell line [42].
5.2. In Vivo Studies
Tumors and different types of cancers are difficult to treat, but classical conventional chemotherapy can be combined with cytotoxic natural remedies. Živković and co-workers evaluated the antitumor activity of V. urticifolia methanolic extract in vivo in animals with Ehrlich ascites carcinoma (EAC) [59]. It is an aggressive, fast-growing carcinoma initially described as a spontaneous murine mammary adenocarcinoma [80]. The antitumor properties of V. urticifolia methanolic extract were estimated via determination of the tumor cell count, ascites volume, and cell viability, and the results were compared with those achieved for positive control N-acetyl-l-cysteine. Pretreatment (2 mg/kg body weight) for seven days before EAC implantation showed a statistically significant decrease in tumor cell viability, while ascites volume and tumor cell count were reduced to some extent, but not statistically significant.
There are too few data about the in vivo antitumor effects of compounds isolated from Veronica species. Acteoside (phenylpropanoid compound dominant in V. urticifolia extract) applied intraperitoneally (i.p.) in a concentration of 50 mg/kg in C57BL/6 mice as pretreatment for 13 days before implantation of melanoma cells induced a statistically significant increase in survival rate in animals [81]. According to the aforementioned in vitro studies, other candidates for the development of effective anticancer therapeutic agents could be verminoside, 4β-hydroxy-6-O-(p-hydroxybenzoyl)-tetrahydrolinaride, and 10-O-protocatechuyl catalpol.
6. Anti-Inflammatory Activity
6.1. In Vitro Studies
The anti-inflammatory properties of several Veronica species were evaluated in vitro and in vivo (Table 4). In particular, the anti-inflammatory effect of Veronica peregrina L. was demonstrated in a recent study [10]. Different concentrations of V. peregrina methanolic extracts were incubated with C57BL/6 mouse peritoneal macrophages and the release of pro-inflammatory mediators, cyclooxygenase-2 (COX2) and nitric oxide (NO), mediated by lipopolysaccharides was assessed; these were reduced dependent on the concentration of the extract. The mechanism of anti-inflammatory action was also highlighted: the inhibition of inducible nitric oxide synthase (iNOS) enzyme and consecutive decrease of NO production [10]. This study revealed the usefulness of V. peregrina extract as synergistic natural therapy in inflammatory diseases mediated by activated mast cells, such as arthritis, obesity, and atherosclerosis [82]. A similar study conducted by Harput et al. demonstrated the same biological anti-inflammatory activity of a methanol extract of five Veronica species which inhibited NO release from activated macrophages [74].
In another study, aqueous-acetone extracts of V. teucrium, V. jacquinii, and V. urticifola were tested for this effect on calcium ionophore-stimulated platelets, which release pro-inflammatory enzymes 12-lipoxygenase (12-LOX) and cyclooxygenase-1 (COX-1) with tromboxane B2 (TXB2) and prostaglandin E2 (PGE2). COX1 and its metabolite PGE2 were inhibited by V. urticifolia extract, while the 12-LOX enzyme was blocked only by the V. jacquinii extract (IC50 = 1.072 mg/mL). This anti-inflammatory activity is correlated with the presence of the main chemical compounds genistein, baicalin, isoquercetin, and hyperoside, but the mechanism of action is not yet completely known [13]. The results of these studies are important for human health, because natural remedies can successfully complete anti-inflammatory medical treatments.
Extracts of V. chamaedrys and V. officinalis also revealed in vitro anti-inflammatory activity on peroxisome proliferator-activated receptors (PPARs) and on the pro-inflammatory mediators, interleukin-8 (IL-8) and E-selectin [17]. Among the active constituents, V. officinalis extract rich in iridoid glycosides (verproside and verminoside) inhibited pro-inflammatory mediators via the nuclear factor kappa B (NF-κB) signaling pathway in a human lung cell line [15].
In another study, the anti-inflammatory action of V. officinalis extract in allergic inflammatory lung diseases was evaluated using A549 human lung epithelial cells. The results showed that the V. officinalis extract (40 to 160 μg/mL) inhibited in a dose-dependent manner the release of the main pro-inflammatory mediators IL-6 and IL-8, prostaglandin E2, and eotaxin. The data of the study did not clarify the mode of action of V. officinalis extract, but it is supposed that there is a link between the most abundant and important active compounds, iridoid glycosides (verminoside and verproside), and the anti-inflammatory effect [15]. These positive molecular results are in contrast to other studies that showed that the oral bioavailability of verproside is very low [83]. Nonetheless, more studies are required to elucidate the metabolites formed and their bioactivity. Moreover, an alternative way to administer V. officinalis extracts could be the pulmonary route using a spray for inhalation with the direct release of active substances to the lung epithelium [83].
6.2. In Vivo Studies
Inflammatory bowel diseases (IBD) (Crohn′s disease and ulcerative colitis) are a heterogeneous group of diseases with incomplete elucidate pathogenesis defined by inflammation, persistence, or recurrence in the gastrointestinal tract, different in terms of extension of lesions, symptoms, prognosis, and treatment [84]. The triggers of these diseases are complex and mediated by cytokines (interleukins, IL) [85] and signal transducer activator of transcription 3 (STAT3) [86,87], pro-inflammatory enzymes such as cyclooxygenase-2 (COX2) [88], and non-specific pro-inflammatory mediators, e.g., thromboxanes, prostaglandins, leukotrienes, oxygen free radicals, and NO [89]. Understanding these mechanisms led to research of new therapies, including natural alternatives. Thus, Akanda and co-workers investigated the anti-inflammatory property of V. polita species on experimental colitis induced in mice using dextran sulfate sodium (DSS) [90]. Forty mice divided into four groups were included in the study: control, mice treated with DSS, mice treated with DSS plus V. polita extract (200 mg/kg), and the last group received DSS associated with dexamethasone. The results of the study were surprising because, for the mice treated with V. polita associated with dexamethasone compared to those treated with DSS alone, the following data were obtained: pro-inflammatory cytokines (IL-1β, IL-6, tumor necrosis factor alpha (TNF-α)) were not activated and NO production in the intestine was reduced. In addition, COX-2, NF-κB, and the Janus kinase 2 (JAK2)/STAT3 signaling pathway were blocked in mice co-treated with DSS and V. polita, similarly to the group of mice co-treated with DSS and dexamethasone [90]. This can be attributed to the high content of total phenols and flavonoids of this species [46].
Kupeli et al. conducted a study concerning the antinociceptive and anti-inflammatory effect of the methanol and aqueous extracts of the species V. anagallis-aquatica, using a phenyl-p-benzoquinone (PBQ) writhing test and carrageenan-induced hind paw edema model in mice. Only the methanolic extract (500 mg/kg) showed significant antinociceptive and anti-inflammatory effects, which could be related to the presence of iridoid glucosides, catalposide and verproside. In addition, no toxic and adverse irritant gastric effects were revealed [16].
A new pharmaceutical formulation consisting of a microemulsion of V. persica was tested for anti-inflammatory effects on rat paw edema induced by two different substances—kaolin, stimulating pro-inflammatory cytokines, and dextran, increasing the release of histamine and consequently inducing vascular permeability. Inflammation induced by dextran was totally reduced following the co-administration of V. persica microemulsion compared to the kaolin inflammation. This effect was correlated with its high content of polyphenols and iridoids [65].
7. Other Properties
The neuroprotective and angiogenic effects of the methanolic and aqueous-acetone extracts of three Veronica species, V. teucrium, V. jacquinii, and V. urticifolia, were determined on human SH-SY5Y cell line neuroblastoma. The neurotoxicity was induced on the cells by oxidative and nitrosative stress using H2O2 and nitroprusside sodium, respectively. The six extracts showed a moderate neuroprotective effect, and this effect was higher for the cytotoxicity induced by oxidative stress and using V. urticifolia extract. Moreover, these authors showed that Veronica extracts, especially the V. jacquinni methanol extract and V. teucrium aqueous-acetone extract, showed antiangiogenic properties by preventing the formation of tubular structures in a Matrigel assay. Although this effect was probably due to the content of acteoside and aucubine, the mechanisms of action are unclear [79].
8. Food Applications of Veronica Plants and Other Uses
The enzymatic activity in foodstuffs result in some chemical reactions leading to food spoilage, thus making foods inedible or decreasing their qualities [91,92]. In addition to being an important health issue, which may sometimes lead to death, it may also yield great economic losses [93]. The World Health Organization reported that unsafe food resulted in illnesses in approximately two billion people throughout the world, and some of the cases were actually fatal [94]. There are some other methods that could prevent food spoilage, such as thermal processing; however, this technique might also result in a decrease in the nutritional value and quality of the food while reducing vegetative microorganisms. Therefore, to prevent food-related diseases and even death, a preservative should be used and this preservative has to be appropriate for the health of consumers and should not yield toxic materials [95]. Food manufacturers also search for compounds that could meet the expectations of healthy food trends, and this is also an important issue since synthetic preservatives are known to yield unwanted health results [96]. Veronica species have widespread traditional use throughout the world and, in addition to this usage, stems and leaves of some of the species are consumed as food in certain regions. Moreover, according to the Food Additive Status List by the Food and Drug Administration (FDA), Veronica species are listed as substances in conjunction with flavors that can be used in alcoholic beverages only [97]. However, since some members of this genus are also consumed by humans and used in traditional medicine, we can consider that usage of this plant is relatively safe [57].
Thus, the use of Veronica species in food preservation could be plausible with no adverse effects since some of them were demonstrated to possess antimicrobial effects (see Section 4). Although not directed on their use in food matrices, these studies also proved that the species extracts possess antibacterial effects and, thus, this genus has a potential to be used in food preservation [11,47,98,99,100]. In this sense, the antimicrobial effect of Veronica plants extracts against foodborne pathogenic and food contaminant bacteria was demonstrated by several authors, but with modest activity. As an example, the results of the experiment conducted by Mocan et al. (2015) on the antibacterial activity of V. officinalis, V. teucrium, and V. orchidea ethanolic extracts, tested using the microdilution assay, revealed that the most sensitive bacterial strains to V. officinalis were Listeria monocytogenes (ATCC 19114) and Listeria ivanovii (ATCC 19119), with the same values of minimum inhibitory (MIC) and minimum bactericidal concentration (MBC) of 7.81 mg/mL. In the case of V. teucrium antibacterial activity, the strains of Staphylococcus aureus (ATCC 49444), Bacillus cereus (ATCC 11778), and Enterococcus faecalis (ATCC 29212) showed equal values for MIC and MBC (7.81 mg/mL), being the most sensitive. The ethanolic extract of V. orchidea inhibited L. monocytogenes and L. ivanovii with MIC = 3.9 mg/mL and MBC = 7.81 mg/mL. Regarding the V. officinalis extract, Bacillus cereus, Pseudomonas aeruginosa (ATCC 27853), and Escherichia coli (ATCC 25922) were the most resistant species with MIC and MBC values higher than 15.62 mg/mL. According to Mocan et al. (2015), the activity of these extracts against Gram-positive bacteria like Listeria species and S. aureus could be related to their high β-sitosterol, campesterol, and stigmasterol content. Phenolic constituents such as apigenin, luteolin, and their glycosides could also contribute [11,20,47]. Another work also suggested that V. montana L. water extract and its main phenolic compound, protocatechuic acid, showed the highest antibacterial effect against S. aureus (ATCC 6538) (MIC and MBC, 7.5 mg/mL) but a poor effect against B. cereus (human isolate) (MIC = 22.5 mg/mL; MBC 45 mg/mL) [57].
The incorporation of these species in a food system was investigated recently. In this sense, the water extract of V. montana exerted antimicrobial effects against six pathogenic bacteria, but it was more effective against L. monocytogenes (MIC, 7.5 mg/L; MBC, 15.0 mg/mL). Its major compound, protocatechuic acid (15.7 mg/g), also showed antibacterial properties (MIC, 0.75 mg/L; MBC, 1.0 mg/mL) and it was evaluated after its incorporation in cream cheese, using this bacterium as a cheese contaminant. This compound preserved cream cheese by inhibiting the growth of L. monocytogenes at room temperature and in the refrigerator after three days of inoculation, without compromising the sensory properties. The compound was shown to alter the permeability of the bacterial cytoplasmic membrane, and this makes this plant species and its major component promising antibacterial food preservatives [57].
The antioxidant properties (see Section 5) can be also exploited to preserve food quality and reduce rancidity and off-flavors, but these studies were not carried out on food systems. In this context, further studies are required to establish their real applicability as natural preservatives instead of synthetic ones, including dosages, advantages, and disadvantages.
In another context, some wild edible plants are attracting attention as novel food ingredients for gourmet in agro-tourism, for example, for salads and refreshing candy products. Ricola® (Laufen, Switzerland) sweets are made with V. officinalis in conjunction with other medicinal plants, used to refresh the mouth and throat. Blanco-Salas et al. (2019) suggested that V. anagallis-aquatica, among other wild plants in “Sierra Grande de Hornachos” (Spain), already entered the high Spanish hotel industry and small selected market niches, due to its sensory and nutritional characteristics [101]. As commented before, this is a natural source of iridoids and other phytochemicals.
9. Conclusions
More than 100 phytochemicals were identified in Veronica plants, which mainly belong to iridoid glycosides and phenolic compounds, particularly flavones and terpenoids. Veronica plants are described in traditional medicine for the treatment of many diseases, especially related to inflammatory disorders. In addition, they represent importance in cosmetic and food industries. The review of the literature highlights that Veronica plants have good antioxidant, anti-inflammatory, antimicrobial, and anticancer abilities, which are mainly related to the presence of iridoid glucosides and phenolic constituents. The antioxidant properties of Veronica species were determined through different in vitro and in vivo studies. In addition, Veronica plants showed interesting antimicrobial effects, and most studies focused on its effects against both Gram-positive and Gram-negative bacteria. This also included foodborne pathogens, such as L. monocytogenes. The anti-inflammatory studies agreed with the use of Veronica remedies for anti-inflammatory medical treatments. Many authors also hypothesized that the cytotoxic activity of Veronica plants is associated with their antioxidant and anti-inflammatory effects. However, studies proving the therapeutic effects of the Veronica genus in humans are scarce, with the exception of V. officinalis as an anti-wrinkle agent. Only few studies were conducted in vivo, while the molecular mechanisms of the pharmacotherapeutic effects remain still unknown. When looking at the food applications of Veronica plants, promissory reports showed that its incorporation into several food matrices, such as dairy products, results in an improvement of the shelf-life, through exerting antimicrobial and antioxidant effects. Thus, these plants may be conceived as upcoming and effective natural food preservatives.
Acknowledgments
M.d.M.C. is grateful for funding from the “Acción 6 del Plan de Apoyo a la Investigación de la Universidad de Jaén, 2017–2019”. N. Martins would like to thank the Portuguese Foundation for Science and Technology (FCT-Portugal) for the Strategic project ref. UID/BIM/04293/2013 and “NORTE2020 - Northern Regional Operational Program” (NORTE-01-0145-FEDER-000012).
Author Contributions
All authors contributed to the manuscript. Conceptualization, B.S. and J.S.-R.; validation, investigation, resources, data reviewing, and writing, all authors; review and editing, J.S.-R., M.d.M.C., F.S., N.M., and W.C.C. All authors read and approved the final manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Footnotes
Sample Availability: Samples of compounds are not available from authors.
References
- 1.Olmstead R.G. Whatever happened to scrophulariaceae. Fremontia. 2002;30:13–22. [Google Scholar]
- 2.Albach D.C., Martınez-Ortega M.M., Fischer M.A., Chase M.W. A new classification of the tribe veroniceae-problems and a possible solution. Taxon. 2004;53:429–452. doi: 10.2307/4135620. [DOI] [Google Scholar]
- 3.Jensen S.R., Albach D.C., Ohno T., Grayer R.J. Veronica: Iridoids and cornoside as chemosystematic markers. Biochem. Syst. Ecol. 2005;33:1031–1047. doi: 10.1016/j.bse.2005.03.001. [DOI] [Google Scholar]
- 4.The Plant List. Version 1.1. [(accessed on 1 January 2019)]; Available online: http://www.theplantlist.org/
- 5.Ellmouni F.Y., Karam M.A., Ali R.M., Albach D.C. Molecular and morphometric analysis of Veronica l. Section Beccabunga (hill) dumor. Aquat. Bot. 2017;136:95–111. doi: 10.1016/j.aquabot.2016.09.010. [DOI] [Google Scholar]
- 6.Plants for a Future. [(accessed on 1 January 2019)]; Available online: https://pfaf.org/user/DatabaseSearhResult.aspx.
- 7.Wangkheirakpam S. Traditional and folk medicine as a target for drug discovery. In: Mandal S.C., Mandal V., Konishi T., editors. Natural Products and Drug Discovery: An Integrated Approach. Elsevier; Amsterdam, The Netherlands: 2018. pp. 29–56. [Google Scholar]
- 8.World Health Organization . WHO Global Report on Traditional and Complementary Medicine 2019. World Health Organization; Geneva, Switzerland: 2019. pp. 10–80. [Google Scholar]
- 9.Vural C., Özcan S., Akbulut M. New combination in Veronica (Scrophulariaceae s.l.) based on morphological characters and the seed storage protein polymorphism. J. Syst. Evol. 2009;47:168–172. doi: 10.1111/j.1759-6831.2009.00016.x. [DOI] [Google Scholar]
- 10.Jeon H. Anti-inflammatory activity of Veronica peregrina. Nat. Prod. Sci. 2012;18:141–146. [Google Scholar]
- 11.Dunkic V., Kosalec I., Kosir I.J., Potocnik T., Cerenak A., Koncic M.Z., Vitali D., Muller I.D., Kopricanec M., Bezic N., et al. Antioxidant and antimicrobial properties of Veronica spicata L. (plantaginaceae) Curr. Drug Targets. 2015;16:1660–1670. doi: 10.2174/1389450116666150531161820. [DOI] [PubMed] [Google Scholar]
- 12.Kwak J.H., Kim H.J., Lee K.H., Kang S.C., Zee O.P. Antioxidative iridoid glycosides and phenolic compounds from Veronica peregrina. Arch. Pharm. Res. 2009;32:207–213. doi: 10.1007/s12272-009-1137-x. [DOI] [PubMed] [Google Scholar]
- 13.Beara I., Zivkovic J., Lesjak M., Ristic J., Savikin K., Maksimovic Z., Jankovic T. Phenolic profile and anti-inflammatory activity of three veronica species. Ind. Crop. Prod. 2015;63:276–280. doi: 10.1016/j.indcrop.2014.09.034. [DOI] [Google Scholar]
- 14.Su B., Zhu Q., Jia Z. Aquaticol, a novel bis-sesquiterpene from Veronica anagallis-aquatica. Tetrahedron Lett. 1999;40:357–358. doi: 10.1016/S0040-4039(98)02303-X. [DOI] [Google Scholar]
- 15.Grundemann C., Garcia-Kaufer M., Sauer B., Stangenberg E., Konczol M., Merfort I., Zehl M., Huber R. Traditionally used Veronica officinalis inhibits proinflammatory mediators via the nf-kb signalling pathway in a human lung cell line. J. Ethnopharmacol. 2013;145:118–126. doi: 10.1016/j.jep.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 16.Kupeli E., Harput U.S., Varel M., Yesilada E., Saracoglu I. Bioassay-guided isolation of iridoid glucosides with antinociceptive and anti-inflammatory activities from Veronica anagallis-aquatica L. J. Ethnopharmacol. 2005;102:170–176. doi: 10.1016/j.jep.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 17.Vogl S., Picker P., Mihaly-Bison J., Fakhrudin N., Atanasov A.G., Heiss E.H., Wawrosch C., Reznicek G., Dirsch V.M., Saukel J., et al. Ethnopharmacological in vitro studies on austria’s folk medicine-an unexplored lore in vitro anti-inflammatory activities of 71 austrian traditional herbal drugs. J. Ethnopharmacol. 2013;149:750–771. doi: 10.1016/j.jep.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raclariu A.C., Mocan A., Popa M.O., Vlase L., Ichim M.C., Crisan G., Brysting A., Boer H. Veronica officinalis product authentication using DNA metabarcoding and hplc-ms reveals widespread adulteration with Veronica chamaedrys. Front. Pharmacol. 2017;8:378. doi: 10.3389/fphar.2017.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harput-Hudaverdi U.S., Oztunca F.H., Saracoglu I. Comparative phytochemical and biological studies on Veronica cuneifolia subsp. Cuneifolia and v. Cymbalaria. Planta Med. 2008;74:PC88. [Google Scholar]
- 20.Sharifi-Rad J., Tayeboon G.S., Niknam F., Sharifi-Rad M., Mohajeri M., Salehi B., Iriti M., Sharifi-Rad M. Veronica persica poir. Extract–antibacterial, antifungal and scolicidal activities, and inhibitory potential on acetylcholinesterase, tyrosinase, lipoxygenase and xanthine oxidase. Cell. Mol. Biol. 2018;64:50–56. doi: 10.14715/cmb/2018.64.8.8. [DOI] [PubMed] [Google Scholar]
- 21.Grayer-Barkmeijer R.J. A chemosystematic study of Veronica: Iridoid glucosides. Biochem. Syst. Ecol. 1973;1:101–110. doi: 10.1016/0305-1978(73)90023-9. [DOI] [Google Scholar]
- 22.Afifi-Yazar F.U., Sticher O. Verproside, a new iridoid glucoside from Veronica officinalis L. (scrophulariaceae) Helivetica Chim. Acta. 1980;63:1905–1907. doi: 10.1002/hlca.19800630716. [DOI] [Google Scholar]
- 23.Johansen M., Larsen T.S., Mattebjerg M.A., Gotfredsen C.H., Jensen S.R. Chemical markers in Veronica sect. Hebe. Biochem. Syst. Ecol. 2007;35:614–620. doi: 10.1016/j.bse.2007.04.010. [DOI] [Google Scholar]
- 24.Grayer-Barkmeijer R.J. Flavonoids in Parahebe and Veronica: A chemosystematic study. Biochem. Syst. Ecol. 1978;6:131–137. doi: 10.1016/0305-1978(78)90039-X. [DOI] [Google Scholar]
- 25.Nikolova M.T., Taskova R.M., Peev D.R. Exudate flavonoid aglycones of Veronica: Ecological and systematic implications. Biochem. Syst. Ecol. 2005;33:1258–1268. doi: 10.1016/j.bse.2005.07.006. [DOI] [Google Scholar]
- 26.Chari V.M., Grayer-Barkmeijer R.J., Harborne J.B., Österdahl B.G. An acylated allose-containing 8-hydroxyflavone glycoside from Veronica filiformis. Phytochemistry. 1981;20:1977–1979. doi: 10.1016/0031-9422(81)84048-4. [DOI] [Google Scholar]
- 27.Ma C.Y., Zhu K.X., Yang D.M., Yang J.S., Yu D.Q. Studies on chemical constituents of Veronica linariifolia pall. Ex link. Sub. Dilatata (nakai et kitagawa) hong. Yao Xue Xue Bao = Acta Pharm. Sin. 1991;26:203–208. [PubMed] [Google Scholar]
- 28.Hong J.L. Phenolic Constituents of Veronica linariifolia. Chin. J. Nat. Med. 2008;6:126–129. doi: 10.3724/SP.J.1009.2008.00126. [DOI] [Google Scholar]
- 29.Li F. Analysis of chemical constituents of essential oil in Veronica linariifolia by gas chromatography-mass spectrometry. Chin. J. Anal. Chem. 2002;30:822–825. [Google Scholar]
- 30.Ozipek M., Saracoglu I., Kojima K., Ogihara Y., Calis I. Fuhsioside, a new phenylethanoid glucoside from Veronica fuhsii. Chem. Pharm. Bull. 1999;47:561–562. doi: 10.1248/cpb.47.561. [DOI] [Google Scholar]
- 31.Taskova R., Handjieva N., Evstatieva L., Popov S. Iridoid glucosides from Plantago cornuti, Plantago major and Veronica cymbalaria. Phytochemistry. 1999;52:1443–1445. doi: 10.1016/S0031-9422(99)00182-X. [DOI] [Google Scholar]
- 32.Handan Öztunca F., Saracoglu I., Harput U. Comparative hplc determination of iridoid contents in Veronica cuneifolia subsp. Cuneifolia and V. cymbalaria. Turk. J. Pharm. Sci. 2011;8:63–70. doi: 10.3109/13880209.2011.575790. [DOI] [PubMed] [Google Scholar]
- 33.Su B.N., Yang L., Gao K., Jia Z.J. Aquaticol, a bis-sesquiterpene and iridoid glucosides from Veronica anagallis-aquatica. Planta Med. 2000;66:281–283. doi: 10.1055/s-2000-8564. [DOI] [PubMed] [Google Scholar]
- 34.Sarker S.D., Bright C., Bartholomew B., Watson A.A., Nash R.J. Calendin, tyrosol and two benzoic acid derivatives from Veronica persica (scrophulariaceae) Biochem. Syst. Ecol. 2000;28:799–801. doi: 10.1016/S0305-1978(99)00122-2. [DOI] [PubMed] [Google Scholar]
- 35.Harput U.S., Saracoglu I., Inoue M., Ogihara Y. Phenylethanoid and iridoid glycosides from Veronica persica. Chem. Pharm. Bull. 2002;50:869–871. doi: 10.1248/cpb.50.869. [DOI] [PubMed] [Google Scholar]
- 36.Albach D.C., Grayer R.J., Jensen S.R., Ozgokce F., Veitch N.C. Acylated flavone glycosides from Veronica. Phytochemistry. 2003;64:1295–1301. doi: 10.1016/j.phytochem.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Jensen S.R., Gotfredsen C.H., Harput U.S., Saracoglu I. Chlorinated iridoid glucosides from Veronica longifolia and their antioxidant activity. J. Nat. Prod. 2010;73:1593–1596. doi: 10.1021/np100366k. [DOI] [PubMed] [Google Scholar]
- 38.Saracoglu I., Varel M., Harput U.S., Nagatsu A. Acylated flavonoids and phenol glycosides from Veronica thymoides subsp. Pseudocinerea. Phytochemistry. 2004;65:2379–2385. doi: 10.1016/j.phytochem.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 39.Albach D.C., Grayer R.J., Kite G.C., Jensen S.R. Veronica: Acylated flavone glycosides as chemosystematic markers. Biochem. Syst. Ecol. 2005;33:1167–1177. doi: 10.1016/j.bse.2005.01.010. [DOI] [Google Scholar]
- 40.Kostadinova E.P., Alipieva K.I., Kokubun T., Taskova R.M., Handjieva N.V. Phenylethanoids, iridoids and a spirostanol saponin from Veronica turrilliana. Phytochemistry. 2007;68:1321–1326. doi: 10.1016/j.phytochem.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 41.Jensen S., Gotfredsen C., Grayer R. Unusual iridoid glycosides in Veronica sects. Hebe and labiatoides. Biochem. Syst. Ecol. 2008;36:207–215. doi: 10.1016/j.bse.2007.09.011. [DOI] [Google Scholar]
- 42.Teng J., Li H.Q., Yao Z., Zhang Y.W., Zhang F.G., Duan H.Q. Anticancer activity of diterpenes from Veronica sibirica in vitro. Chin. Tradit. Herb. Drugs. 2008;39:967–970. [Google Scholar]
- 43.Teng J., Zhang F.G., Zhang Y.W., Takaishi Y., Duan H.Q. A new iridoid glycoside from Veronica sibirica. Chin. Chem. Lett. 2008;19:450–452. doi: 10.1016/j.cclet.2008.01.035. [DOI] [Google Scholar]
- 44.Ahn D., Lee S.I., Yang J.H., Cho C.H., Hwang Y.H., Park J.H., Kim D.K. Superoxide radical scavengers from the whole plant of Veronica peregrina. Nat. Prod. Sci. 2011;17:142–146. [Google Scholar]
- 45.Kim D.K., Jeon H., Cha D.S. 4-hydroxybenzoic acid-mediated lifespan extension in Caenorhabditis elegans. J. Funct. Foods. 2014;7:630–640. doi: 10.1016/j.jff.2013.12.022. [DOI] [Google Scholar]
- 46.Barreira J.C.M., Dias M.I., Živković J., Stojković D., Soković M., Santos-Buelga C., Ferreira I.C.F.R. Phenolic profiling of Veronica spp. Grown in mountain, urban and sandy soil environments. Food Chem. 2014;163:275–283. doi: 10.1016/j.foodchem.2014.04.117. [DOI] [PubMed] [Google Scholar]
- 47.Mocan A., Vodnar D.C., Vlase L., Crisan O., Gheldiu A.M., Crisan G. Phytochemical characterization of Veronica officinalis l., v. Teucrium l. And v. Orchidea crantz from romania and their antioxidant and antimicrobial properties. Int. J. Mol. Sci. 2015;16:21109–21127. doi: 10.3390/ijms160921109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu Q., Sun Y., Shu Y., Tan S., Yin L., Guo Y., Tang L. Hsccc separation of the two iridoid glycosides and three phenolic compounds from Veronica ciliata and their in vitro antioxidant and anti-hepatocarcinoma activities. Molecules. 2016;21:1234. doi: 10.3390/molecules21091234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ouache R., Harkat H., Pale P., Oulmi K. Phytochemical compounds and anti-corrosion activity of Veronica rosea. Nat. Prod. Res. 2018;16:1–5. doi: 10.1080/14786419.2018.1474464. [DOI] [PubMed] [Google Scholar]
- 50.Moreno-Escobar J.A., Alvarez L., Rodríguez-López V., Bahena S.M. Cytotoxic glucosydic iridoids from Veronica americana. Phytochem. Lett. 2013;6:610–613. doi: 10.1016/j.phytol.2013.07.017. [DOI] [Google Scholar]
- 51.Kroll-Møller P., Pedersen K., Gousiadou C., Kokubun T., Albach D., Taskova R., Garnock-Jones P.J., Gotfredsen C.H., Jensen S.R. Iridoid glucosides in the genus Veronica (plantaginaceae) from new zealand. Phytochemistry. 2017;140:174–180. doi: 10.1016/j.phytochem.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 52.Taskova R.M., Kokubun T., Ryan K.G., Garnock-Jones P.J., Jensen S.R. Phenylethanoid and iridoid glycosides in the new zealand snow hebes (Veronica, plantaginaceae) Chem. Pharm. Bull. 2010;58:703–711. doi: 10.1248/cpb.58.703. [DOI] [PubMed] [Google Scholar]
- 53.Maggi A., Taskova R., Gotfredsen C.H., Bianco A., Jensen S.R. Chemical markers in Veronica sect. Hebe. Iiiq. Biochem. Syst. Ecol. 2009;37:731–736. doi: 10.1016/j.bse.2009.11.001. [DOI] [Google Scholar]
- 54.Jensen S.R., Opitz S.E.W., Gotfredsen C.H. A new phenylethanoid triglycoside in Veronica beccabunga L. Biochem. Syst. Ecol. 2011;39:193–197. doi: 10.1016/j.bse.2011.02.008. [DOI] [Google Scholar]
- 55.Marchenko A., Kintya P., Wyrzykiewicz B., Gorincioi E. Steroidal glycosides from Veronica chamaedrys l. Part i. The structures of chamaedrosides c, c1, c2, e, e1 and e2. Nat. Prod. Commun. 2012;7:565–568. doi: 10.1177/1934578X1200700504. [DOI] [PubMed] [Google Scholar]
- 56.Sharifi-Rad M., Tayeboon G.S., Sharifi-Rad J., Iriti M., Varoni E.M., Razazi S. Inhibitory activity on type 2 diabetes and hypertension key-enzymes, and antioxidant capacity of Veronica persica phenolic-rich extracts. Cell. Mol. Biol. 2016;62:80–85. [PubMed] [Google Scholar]
- 57.Stojkovic D.S., Zivkovic J., Sokovic M., Glamoclija J., Ferreira I.C.F.R., Jankovic T., Maksimovic Z. Antibacterial activity of Veronica montana L. Extract and of protocatechuic acid incorporated in a food system. Food Chem. Toxicol. 2013;55:209–213. doi: 10.1016/j.fct.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Dunki V., Kosalec I., Kosir J.I., Potonik T., Gerenak A., Koncik M.Z., Vitali D., Müller I.D., Koprianec M., Bezi N., et al. Antioxidant and antimicrobial properties of Veronica spicata L. (plantaginaceae) Curr. Drug Targets. 2014;15:1–12. doi: 10.2174/1389450116666150531161820. [DOI] [PubMed] [Google Scholar]
- 59.Zivkovic J., Barreira J.C.M., Stojkovic D., Cebovic T., Santos-Buelga C., Maksimovic Z., Ferreira I.C.F.R. Phenolic profile, antibacterial, antimutagenic and antitumour evaluation of Veronica urticifolia jacq. J. Funct. Foods. 2014;9:192–201. doi: 10.1016/j.jff.2014.04.024. [DOI] [Google Scholar]
- 60.Dulger B., Ugurlu E. Evaluation of antimicrobial activity of some endemic scrophulariaceae members from turkey. Pharm. Biol. 2005;43:275–279. doi: 10.1080/13880200590928870. [DOI] [Google Scholar]
- 61.Ginovyan M.M., Trchounian A.H. Screening of some plant materials used in armenian traditional medicine for their antimicrobial activity. Chem. Biol. 2017;51:44–53. doi: 10.1186/s12906-017-1573-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mocan A., Vlase L., Arsene A.L., Vodnar D., Bischin C., Silaghi-Dumitrescu R., Crişan G. Hplc/ms analysis of caffeic and chlorogenic acids from three romanian Veronica species and their antioxidant and antimicrobial properties. Farmacia. 2015;63:890–896. [Google Scholar]
- 63.Sharifi-Rad J., Iriti M., Setzer W.N., Sharifi-Rad M., Roointan A., Salehi B. Antiviral activity of Veronica persica poir. On herpes virus infection. Cell. Mol. Biol. 2018;64:11–17. doi: 10.14715/cmb/2018.64.8.2. [DOI] [PubMed] [Google Scholar]
- 64.Sharifi-Rad J., Roointan A., Setzer W.N., Sharifi-Rad M., Iriti M., Salehi B. Susceptibility of Leishmania major to Veronica persica poir. Extracts-in vitro and in vivo assays. Cell. Mol. Biol. 2018;64:44–49. doi: 10.14715/cmb/2018.64.8.7. [DOI] [PubMed] [Google Scholar]
- 65.Fierascu R.C., Georgiev M.I., Fierascu I., Ungureanu C., Avramescu S.M., Ortan A., Georgescu M.I., Sutan A.N., Zanfirescu A., Dinu-Pirvu C.E., et al. Mitodepressive, antioxidant, antifungal and anti-inflammatory effects of wild-growing romanian native Arctium lappa L. (Asteraceae) and Veronica persica poiret (plantaginaceae) Food Chem. Toxicol. 2018;111:44–52. doi: 10.1016/j.fct.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 66.Saracoglu I., Harput U.S., Inoue M., Ogihara Y. New phenylethanoid glycosides from Veronica pectinata var. Glandulosa and their free radical scavenging activities. Chem. Pharm. Bull. 2002;50:665–668. doi: 10.1248/cpb.50.665. [DOI] [PubMed] [Google Scholar]
- 67.Valyova M., Hadjimitova V., Stoyanov S., Ganeva Y., Petrov T., Petrov I. Free radicals scavenging activity of extracts from bulgarian Veronica officinalis L. and GC-MS analysis of ethanol extract. Int. J. Aesthet. Antiaging Med. 2009;2:1–5. [Google Scholar]
- 68.Harput U.S., Genc Y., Khan N., Saracoglu I. Radical scavenging effects of different Veronica species. Rec. Nat. Prod. 2011;5:100–107. [Google Scholar]
- 69.Sharifi-Rad J., Sharifi-Rad M., Salehi B., Iriti M., Roointan A., Mnayer D., Afshari A. In vitro and in vivo assessment of free radical scavenging and antioxidant activities of Veronica persica poir. Cell. Mol. Biol. 2018;64:57–64. doi: 10.14715/cmb/2018.64.8.9. [DOI] [PubMed] [Google Scholar]
- 70.Zivkovic J., Cebovic T., Maksimovic Z. In vivo and in vitro antioxidant effects of three Veronica species. Cent. Eur. J. Biol. 2012;7:559–568. [Google Scholar]
- 71.Sun Y., Lu Q., He L., Shu Y., Zhang S., Tan S., Tang L. Active fragment of Veronica ciliata fisch. Attenuates t-bhp-induced oxidative stress injury in hepg2 cells through antioxidant and antiapoptosis activities. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/4727151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee H., Ghimeray A., Yim J., Chang M. Antioxidant, collagen synthesis activity in vitro and clinical test on anti-wrinkle activity of formulated cream containing Veronica officinalis extract. J. Cosmet. Dermatol. Sci. Appl. 2015;5:45–51. [Google Scholar]
- 73.Shin S.A., Moon S.Y., Kim W.Y., Paek S.M., Park H.H., Lee C.S. Structure-based classification and anti-cancer effects of plant metabolites. Int. J. Mol. Sci. 2018;19:2651. doi: 10.3390/ijms19092651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harput U.S., Iclal S., Makoto I., Yukio O. Anti-inflammatory and cytotoxic activities of five Veronica species. Biol. Pharm. Bull. 2002;25:483–486. doi: 10.1248/bpb.25.483. [DOI] [PubMed] [Google Scholar]
- 75.Saracoglu I., Oztunca F.H., Nagatsu A., Harput U.S. Iridoid content and biological activities of Veronica cuneifolia subsp. Cuneifolia and v. Cymbalaria. Pharm. Biol. 2011;49:1150–1157. doi: 10.3109/13880209.2011.575790. [DOI] [PubMed] [Google Scholar]
- 76.Moreno-Escobar J.A., Bazaldúa S., Villarreal M.L., Bonilla-Barbosa J.R., Mendoza S., Rodríguez-López V. Cytotoxic and antioxidant activities of selected lamiales species from mexico. Pharm. Biol. 2011;49:1243–1248. doi: 10.3109/13880209.2011.589454. [DOI] [PubMed] [Google Scholar]
- 77.Yin L., Qiuxia L., Shancai T., Lisheng D., Yiran G., Fang C., Lin T. Bioactivity-guided isolation of antioxidant and anti-hepatocarcinoma constituents from Veronica ciliata. Chem. Cent. J. 2016;10:27. doi: 10.1186/s13065-016-0172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saracoglu I., Harput U.S. In vitro cytotoxic activity and structure activity relationships of iridoid glucosides derived from veronica species: Bioactivities of iridoid glucosides derived from Veronica species. Phytother. Res. 2012;26:148–152. doi: 10.1002/ptr.3546. [DOI] [PubMed] [Google Scholar]
- 79.Ignjatović D., Živković J., Tovilović G., Šavikin K., Tomić M., Maksimović Z., Janković T. Evaluation of angiogenic and neuroprotective potential of different extracts from three Veronica species. Front. Life Sci. 2015;8:107–116. doi: 10.1080/21553769.2014.998297. [DOI] [Google Scholar]
- 80.Gomes N.M., Rezende C.M., Fontes S.P., Hovell A.M.C., Landgraf R.G., Matheus M.E., Pinto A.C., Fernandes P.D. Antineoplasic activity of Copaifera multijuga oil and fractions against ascitic and solid ehrlich tumor. J. Ethnopharmacol. 2008;119:179–184. doi: 10.1016/j.jep.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 81.Ohno T., Inoue M., Ogihara Y., Saracoglu I. Antimetastatic activity of acteoside, a phenylethanoid glycoside. Biol. Pharm. Bull. 2002;25:666–668. doi: 10.1248/bpb.25.666. [DOI] [PubMed] [Google Scholar]
- 82.Rang N.Y., Sungmo J., Seung H.S. Metabolic features of macrophages innflammatory diseases and cancer. Cancer Lett. 2018;413:46–58. doi: 10.1016/j.canlet.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 83.Park E.J., Lee H.S., Oh S.R., Lee H.K., Lee H.S. Pharmacokinetics of verproside after intravenous and oral administration in rats. Arch. Pharm. Res. 2009;32:559–564. doi: 10.1007/s12272-009-1412-x. [DOI] [PubMed] [Google Scholar]
- 84.Baumgart D.C., Carding S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 85.Huang Y., Chen Z. Inflammatory bowel disease related innate immunity and adaptive immunity. Am. J. Transl. Res. 2016;8:2490–2497. [PMC free article] [PubMed] [Google Scholar]
- 86.Boland B.S., Sandborn W.J., Chang J.T. Update on janus kinase antagonists in inflammatory bowel disease. Gastroenterol. Clin. N. Am. 2014;43:603–617. doi: 10.1016/j.gtc.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coskun M., Salem M., Pedersen J., Nielsen O.H. Involvement of jak/stat signaling in the pathogenesis of inflammatory bowel disease. Pharmacol. Res. 2013;76:1–8. doi: 10.1016/j.phrs.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 88.Pearl D.S., Shah K., Whittaker M.A., Nitch-Smith H., Brown J.F., Shute J.K., Trebble T.M. Cytokine mucosal expression in ulcerative colitis, the relationship between cytokine release and disease activity. J. Crohn’s Colitis. 2013;7:481–489. doi: 10.1016/j.crohns.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 89.Soufli I., Toumi R., Rafa H., Touil-Boukoffa C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J. Gastrointest. Pharmacol. 2016;7:353–360. doi: 10.4292/wjgpt.v7.i3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Akanda M.R., Nam H.W., Wishun T., Islam A., Choo B.K., Park B.Y. Regulation of jak2/stat3 and nf-κb signal transduction pathways; Veronica polita alleviates dextran sulfate sodium-induced murine colitis. Biomed. Pharmacother. 2018;100:296–303. doi: 10.1016/j.biopha.2018.01.168. [DOI] [PubMed] [Google Scholar]
- 91.Albarracin H.W., Alfonso A.C., Sanchez B. Application of essential oils as a preservative to improve the shelf life of Nile tilapia. Vitae Rev. Fac. Quim. Farm. 2012;19:34–40. [Google Scholar]
- 92.Zahran S.E., El Shehedi M.A. Efficacy of antimicrobial activity of some herbs on improving quality of some chicken products. Anim. Health Res. J. 2017;5:181–194. [Google Scholar]
- 93.Fernandes D.B.R.V., Guimaraes C.I., Ferreira R.C.L., Botrel A.D., Borges V.S., De Souza U.A. Microencapsulated rosemary (Rosmarinus officinalis) essential oil as a biopreservative in minas frescal cheese. J. Food Process. Preserv. 2017;41:e12759. doi: 10.1111/jfpp.12759. [DOI] [Google Scholar]
- 94.Hintz T., Matthews K.K., Di R. The use of plant antimicrobial compound for food preservation. Biomed. Res. Int. 2015 doi: 10.1155/2015/246264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tzima K., Makris D., Nikiforidis C.V., Mourtzinos I. Potential use of rosemary, propolis and thyme as natural food preservatives. J. Nutr. Health. 2015;1:6. [Google Scholar]
- 96.Antolak H., Kregiel D. Food preservatives from plants, food additives, desiree nedra karunaratne and geethi pamunuwa. IntechOpen. 2017 doi: 10.5772/intechopen.70090. [DOI] [Google Scholar]
- 97.Food and Drug Administration (FDA) Food Additive Status List. [(accessed on 27 November 2018)]; Available online: https://www.fda.gov/food/food-additives-petitions/food-additive-status-list.
- 98.Çelik E., Çelik Y.G., Meysun A. Essential oil composition and antibacterial activity of some plant species. J. Appl. Biol. Sci. 2010;4:45–48. [Google Scholar]
- 99.Kovaleva A.M., Osmachko A.P., Kashpur N.V., Grudko I.V. The antibacterial activity of complexes of Veronica longifolia herb. Ukr. Biopharm. J. 2016;1:58–62. doi: 10.24959/ubphj.16.12. [DOI] [Google Scholar]
- 100.Doğan A., Otlu S., Çelebi Ö., Aksu Kılıçle P., Gülmez Sağlam A., Doğan A.N.C., Mutlu N. An investigation of antibacterial effects of steroids. Turk. J. Vet. Anim. Sci. 2017;41:302–305. doi: 10.3906/vet-1510-24. [DOI] [Google Scholar]
- 101.Blanco-Salas J., Gutiérrez-García L., Labrador-Moreno J., Ruiz-Téllez T. Wild plants potentially used in human food in the protected area “Sierra Grande de Hornachos” of Extremadura (Spain) Sustainability. 2019;11:456. doi: 10.3390/su11020456. [DOI] [Google Scholar]