Abstract
Auxin plays a key role in different plant growth and development processes, including flower opening and development. The perception and signaling of auxin depend on the cooperative action of various components, among which auxin/indole-3-acetic acid (Aux/IAA) proteins play an imperative role. In a recent study, the entire Aux/IAA gene family was identified and comprehensively analyzed in Hedychium coronarium, a scented species used as an ornamental plant for cut flowers. Phylogenetic analysis showed that the Aux/IAA gene family in H. coronarium is slightly contracted compared to Arabidopsis, with low levels of non-canonical proteins. Sequence analysis of promoters showed numerous cis-regulatory elements related to various phytohormones. HcIAA genes showed distinct expression patterns in different tissues and flower developmental stages, and some HcIAA genes showed significant responses to auxin and ethylene, indicating that Aux/IAAs may play an important role in linking hormone signaling pathways. Based on the expression profiles, HcIAA2, HcIAA4, HcIAA6 and HcIAA12, were selected as candidate genes and HcIAA2 and HcIAA4 were screened for further characterization. Downregulation of HcIAA2 and HcIAA4 by virus-induced gene silencing in H. coronarium flowers modified the total volatile compound content, suggesting that HcIAA2 and HcIAA4 play important roles in H. coronarium floral scent formation. The results presented here will provide insights into the putative roles of HcIAA genes and will assist the elucidation of their precise roles during floral scent formation.
Keywords: Hedychium coronarium, Aux/IAA, floral scent, HcIAA
1. Introduction
Auxin plays a substantial role in several aspects of plant growth and development, like cell division, apical dominance, vascular differentiation, lateral/adventitious root formation and fruit and flower development [1,2,3]. Aux/IAA genes constitute one of the three major classes of primary auxin-responsive genes, including SAUR (Small Auxin UP RNA) and GH3 (Gretchen Hagen 3) [4,5]. Aux/IAA proteins can act as transcriptional repressors through interactions with ARF (auxin response factor) proteins. Aux/IAAs inactivate ARFs, which can be either transcriptional repressors or activators of primary auxin-responsive genes [6,7]. Aux/IAAs are the primary responsive auxin genes, most of which are short-lived in the cytosol and nucleus [1,8,9]. Aux/IAA proteins are normally conserved with four domains known as domain I to domain IV, although proteins missing one or two domains were also included in this gene family [1]. Domain I consists of a leucine-rich repeat motif symbolized by “LxLxL” and acts as an active repression domain that can interact with the corepressor protein TOPLESS (TPL) [10,11]. Domain II is highly conserved with the degron sequence (GWPPV), leading towards Aux/IAA protein instability by ubiquitin degradation) [12,13,14,15]. Domain III and IV at the C-terminus intercede homo- and heterodimerization among Aux/IAA proteins and/or auxin ARF proteins [1,16,17]. Usually, auxin-responsive cis-elements (AuxREs) are present at the promoter regions of auxin-responsive genes that bind to ARFs to regulate the expression of auxin-mediated genes. To regulate auxin-responsive genes, Aux/IAA proteins do not bind directly to AuxREs; however, they interact with ARFs by controlling ARF activity [18]. The study of auxin regulation and activity is highly complex because of the extensive number of Aux/IAA and ARF family members, expression patterns, variations, and auxin-mediated transcriptional and post-transcriptional regulations.
Formerly, a large number of Aux/IAA genes were identified and characterized by mutant analysis, especially in Arabidopsis and tomato. In Arabidopsis, a functional mutation in IAA8 altered lateral branches, curled leaves, shortened primary inflorescence stems, decreased shoot apical dominance, induced the formation of abnormal flower organs (bent stigmas, short petal and stamen) and reduced the jasmonic acid level in the flowers. In tomato, compared with wild-type plants, SlIAA15 suppressed transgenic lines by showing a higher number of xylem cells. The monoterpene content, including β-phellandrene, α-terpinene, γ-element, α-humulene and, β-caryophyllene, in trichome exudates was reduced significantly in Sl-IAA15 downregulated leaves [19]. However, the silencing of the Sl-IAA27 gene showed multiple phenotypes related to vegetative and reproductive growth [20]. Furthermore, Sl-IAA27 silencing resulted in the downregulation of strigolactone biosynthesis by regulating the genes involved in strigolactone synthesis [21]. Overall, Aux/IAA plays a key role in monocots and dicots plants by affecting the development of flowers, roots and stems. It also affects some secondary metabolism, such as the biosynthesis of volatile compounds [22,23,24]. Aux/IAA gene family members have been identified in numerous plant species, including Arabidopsis [25], rice [3], maize [26], tomato [27], Vitis vinifera [28], Eucalyptus [29] and papaya [30]. However, the function of Aux/IAA family members in Hedychium coronarium is still unknown.
H. coronarium is a perennial herb frequently cultivated as a cut flower or garden plant in tropical and subtropical regions. The flower is famous for its fragrance and medicinal importance [31]. The blooming of flowers results in the emission of a blend of volatile compounds mainly consisting of monoterpenes (linalool, 1,8-cineole, (E)/(Z)-β-ocimene), sesquiterpenes (β-caryophyllene, α-farnesene) and some benzenoids [32,33,34,35]. Similar to color and shape, floral fragrance is also an important characteristic of any ornamental plant, refining its esthetic and economic value [36]. To the best of our knowledge, a genome-wide analysis of the Aux/IAA gene family in H. coronarium has not been performed, and the function of Aux/IAA genes in floral scent formation is still unknown. In the current study, we identified Aux/IAA family genes in H. coronarium genomes and analyzed their sequence characteristics, genomic structures, phylogeny and cis-regulatory elements. The spatiotemporal differential expression patterns of Aux/IAA in different tissues/organs and at different flower developmental stages were also studied. Additionally, we evaluated the roles of Aux/IAA members in floral scent formation through their response to various hormonal treatments. Moreover, we identified two nuclear-localized Aux/IAA genes (HcIAA2 and HcIAA4) that are involved in floral scent formation, as demonstrated by virus-induced gene silencing. Our findings will provide novel insights into the functions of Aux/IAA and will assist scientists in future studies on elucidating the precise biological functions of Aux/IAA genes in H. coronarium.
2. Results
2.1. Identification and Sequence Analysis of the Aux/IAA Gene Family in H. coronarium
According to a pBLAST search, a total of 35 candidate gene models were originally found. The annotation of these gene models was assessed by using H. coronarium transcriptome data. Falsely predicted Aux/IAA gene models were curated manually. A total of 27 H. coronarium Aux/IAA genes were identified and named HcIAA1–HcIAA27. Detailed information on these HcIAA genes, including gene names, sequence IDs, exon number, genome location, open reading frame (ORF) lengths, protein molecular weight (MW), length of the protein sequence and isoelectric point (pI), is listed in Table 1.
Table 1.
Description of the Aux/IAA gene family in Hedychium coronarium.
| Gene Name | Gene ID | ORF (bp) | Deduced Polypetide | Exon No. | Genome Location | ||
|---|---|---|---|---|---|---|---|
| Length (aa) | Mol. Wt (kda) | pI | |||||
| HcIAA1 | Hc118.198 | 552 | 183 | 20.698 | 9.13 | 5 | 1298266–1301736 |
| HcIAA2 | Hc909.36 | 735 | 244 | 26.869 | 8.65 | 6 | 311277–314281 |
| HcIAA3 | Hc563.22 | 501 | 166 | 19.177 | 4.84 | 4 | 170726–171832 |
| HcIAA4 | Hc215.30 | 855 | 284 | 31.179 | 9.04 | 6 | 354002–357876 |
| HcIAA5 | Hc1096.12 | 891 | 296 | 31.617 | 5.09 | 4 | 96290–98064 |
| HcIAA6 | Hc506.60 | 594 | 197 | 21.856 | 8.57 | 4 | 520699–522248 |
| HcIAA7 | Hc717.7 | 468 | 155 | 16.881 | 9.36 | 3 | 53508–54653 |
| HcIAA8 | Hc38.101 | 852 | 283 | 30.625 | 7.42 | 5 | 1966779–1969857 |
| HcIAA9 | Hc899.17 | 924 | 307 | 32.633 | 7.01 | 5 | 251006–254190 |
| HcIAA10 | Hc357.107 | 945 | 314 | 33.141 | 6.16 | 5 | 779004–783999 |
| HcIAA11 | Hc189.134 | 777 | 258 | 28.457 | 5.97 | 4 | 1076907–1077976 |
| HcIAA12 | Hc189.121 | 594 | 197 | 21.273 | 5.30 | 3 | 987719–988944 |
| HcIAA13 | Hc107.22 | 912 | 303 | 33.216 | 9.31 | 5 | 734366–735832 |
| HcIAA14 | Hc285.12 | 735 | 244 | 26.556 | 7.93 | 5 | 146079–148497 |
| HcIAA15 | Hc440.26 | 729 | 242 | 26.643 | 9.23 | 5 | 424147–425365 |
| HcIAA16 | Hc47.162 | 528 | 175 | 19.225 | 5.11 | 4 | 1790499–1791855 |
| HcIAA17 | Hc1004.1 | 537 | 178 | 20.500 | 4.66 | 3 | 64402–73225 |
| HcIAA18 | Hc412.59.2 | 933 | 310 | 34.252 | 5.91 | 6 | 489469–493695 |
| HcIAA19 | Hc269.7 | 1005 | 334 | 35.863 | 8.23 | 5 | 386761–389599 |
| HcIAA20 | Hc41.21 | 942 | 313 | 33.433 | 6.55 | 5 | 707930–711511 |
| HcIAA21 | Hc614.34 | 858 | 285 | 30.632 | 6.55 | 5 | 348817–352993 |
| HcIAA22 | Hc42.52 | 585 | 194 | 21.522 | 5.40 | 5 | 737421–740882 |
| HcIAA23 | Hc270.58 | 615 | 204 | 23.116 | 10.15 | 3 | 459106–460132 |
| HcIAA24 | Hc484.51.1 | 1059 | 352 | 39.190 | 6.29 | 7 | 480251–483666 |
| HcIAA25 | Hc566.42 | 546 | 181 | 19.907 | 5.45 | 4 | 628269–630501 |
| HcIAA26 | Hc641.57 | 762 | 253 | 27.553 | 7.14 | 5 | 340256–342758 |
| HcIAA27 | Hc326.19 | 522 | 173 | 19.566 | 6.78 | 4 | 329556–335070 |
The predicted HcIAA proteins vary in size from 155 (HcIAA7) to 352 amino acids (HcIAA24) with molecular masses ranging from 16 to 39 kDa (Table 1). The theoretical isoelectric points also differ greatly from 4.66 (HcIAA17) to 10.15 (HcIAA23), showing that they may have roles in diverse microenvironments. Pairwise analysis of HcIAA protein sequences revealed that the identity differs widely from 86.9% (between HcIAA9 and HcIAA10) to 14.4% (between HcIAA24 and HcIAA27) (Table S2). A similar large variation was reported in Arabidopsis [17], tomato [37] and Eucalyptus [29].
2.2. Multiple Sequence Alignment and Phylogenetic Analysis of HcIAA Genes
Alignment of the amino acid sequences of H. coronarium Aux/IAAs revealed that the typical four highly conserved domains (domains I, II, III and IV) were present in the majority of HcIAA proteins. A typical LxLxLx motif was present in domain I of the majority of HcIAA proteins, except HcIAA1 and HcIAA22. The consensus sequence (T/LELRLGLPG) in domain I was not well conserved in HcIAA3, HcIAA5 and HcIAA17 (Figure 1), and the conserved degron sequence VGWPP in domain II, which is important for degradation, was not found in HcIAA27. HcIAA12 was the only member that contained a truncated domain IV. In most of H. coronarium Aux/IAA proteins, two kinds of putative nuclear localization signals (NLS) were detected. The first NLS has a bipartite structure encompassing a conserved basic doublet, KR, between domains I and II and the next NLS is a basic residue-rich region situated in domain IV (Figure 1). Majority of the HcIAA proteins consist of both types of NLS and are hence most likely localized to the nucleus, consistent with their transcriptional activity. However, HcIAA12 and HcIAA24 lack the SV40-type NLS, whilst HcIAA11, HcIAA18, HcIAA19 and HcIAA27 lack the bipartite NLS. These putative NLSs suggest that HcIAAs are nuclear-located proteins (Figure 1).
Figure 1.
Multiple sequence alignment of the full-length HcIAA proteins obtained with Clustal W and manual correction. Domains I–IV of the H. coronarium IAA proteins are indicated with red lines. Color shading indicates identical and conserved amino acid residues. Nuclear localization signals (NLSs) are indicated by filled stars. The amino acid position is given to the right of each sequence. The yellow color box showed a typical LxLxLx motif present in domain I.
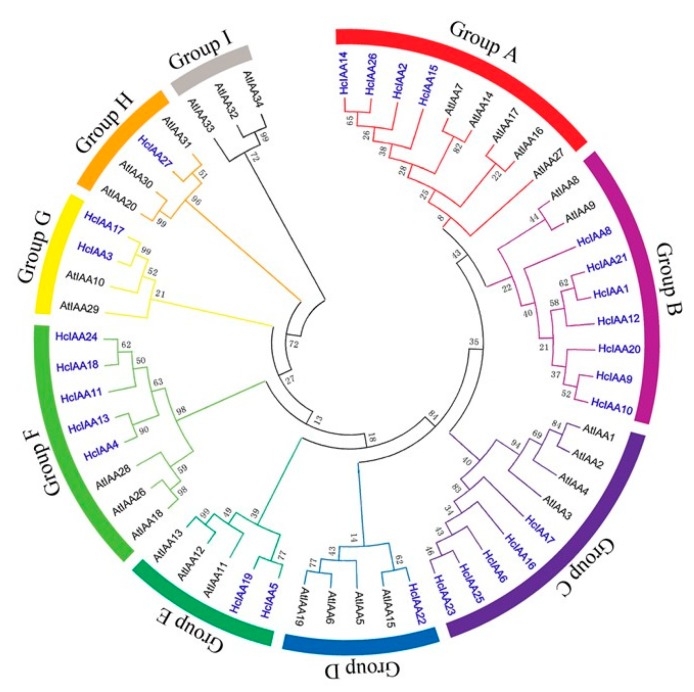
The Aux/IAA family in H. coronarium, with 27 members, is slightly contracted compared with the 31 in Oryza sativa, 31 in Zea mays and 29 members in Arabidopsis. Its size resembles to that of tomato and Eucalyptus, both of which contain 26 members [29,37].
To evaluate the relationship between H. coronarium and Arabidopsis Aux/IAAs, phylogenetic analysis was carried out by using the predicted full-length amino acid sequences of Aux/IAAs from H. coronarium and Arabidopsis. All Aux/IAA proteins were categorized into nine distinct groups named A-I (Figure 2). With reference to Arabidopsis, two groups (D and H) are contracted, and two groups (B and F) are expanded in H. coronarium. Group B contains seven genes in H. coronarium but only three members in Arabidopsis, while group F consists of five genes in H. coronarium and contains three members in Arabidopsis. The non-canonical group H, which lacks the conserved domain II, contains three members (AtIAA20, AtIAA30 and AtIAA31) in Arabidopsis but only one member in H. coronarium (HcIAA27; Figure 2). Group I, which also gathers non-canonical Aux/IAAs of Arabidopsis, is absent in H. coronarium. Overall, the non-canonical Aux/IAAs are overrepresented in Arabidopsis with six genes (AtIAA20, AtIAA30, AtIAA31, AtIAA32, AtIAA33 and AtIAA34), whereas only one was found in H. coronarium (HcIAA27).
Figure 2.
Phylogenetic analysis of H. coronarium and Arabidopsis Aux/IAA proteins. Full-length protein sequences were aligned by using the Clustal X 2.1 program. The phylogenetic tree was constructed by using MEGA 6 software and the neighbor-joining method with predicted Aux/IAA proteins. Bootstrap values are indicated at each node. Each Aux/IAA group (A–I) is indicated by a specific color. HcIAAs are noted in blue and bold.
2.3. Gene Structure and Motif Composition of HcIAA Genes
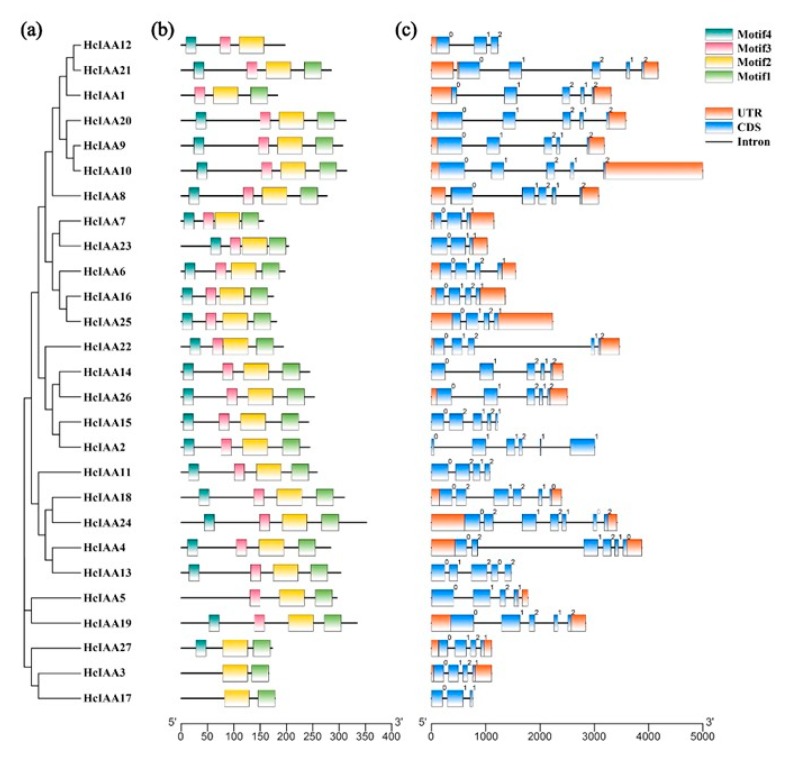
Schematic structures of HcIAA genes are shown in Figure 3. The MEME web server was used to analyze the domain distributions of HcIAA proteins. Four different conserved domains were mapped (Figure 3b). Most of the HcIAA proteins contain the four typical domains, while some of the IAA proteins have truncated domains, such as motif 4 (domain I), which is missing in HcIAA1, HcIAA3, HcIAA5 and HcIAA17; motif 3 (domain II), which is missing in HcIAA27, HcIAA3 and HcIAA17; and motif 1 (domain IV), which is missing in HcIAA12 (Figure 3b). The number of introns in all HcIAA genes was between two and six. The coding sequences of most (69%) of the HcIAA genes are disrupted by three or four introns, and the intron positions and phases are well conserved (Figure 3c). Variations were observed in some members involving mainly the loss of one intron (HcIAA7, HcIAA12, HcIAA17 and HcIAA23) and, in some cases, the gain of one or more additional intron (HcIAA2, HcIAA4, HcIAA18 and HcIAA24). The exon–intron organizations of all identified HcIAA genes were analyzed to gain more insight into the evolution of the Aux/IAA family in H. coronarium. Further analyses indicated that the distribution of introns and the intron phase coincided with the phylogenetic alignment of HcIAA genes (Figure 3a).
Figure 3.
Phylogenetic relationships, motif distribution analysis and gene structure in Aux/IAA genes from H. coronarium. (a) The phylogenetic tree was constructed based on the full-length sequences of H. coronarium Aux/IAA proteins using MEGA 6 software. (b) The motif distribution in H. coronarium IAA proteins. By using a MEME web server, motifs of Aux/IAA proteins were analyzed. Four motifs representing domains I, II, III and IV are mapped on all of the Aux/IAA proteins in different colors. (c) Exon–intron structure of H. coronarium Aux/IAA genes. Orange boxes indicate untranslated 5′- and 3′-regions; blue boxes indicate exons; black lines indicate introns. The number indicates the phases of corresponding introns.
2.4. Analysis of Hormone-Related cis-Elements in the Promoter Regions of HcIAA Genes
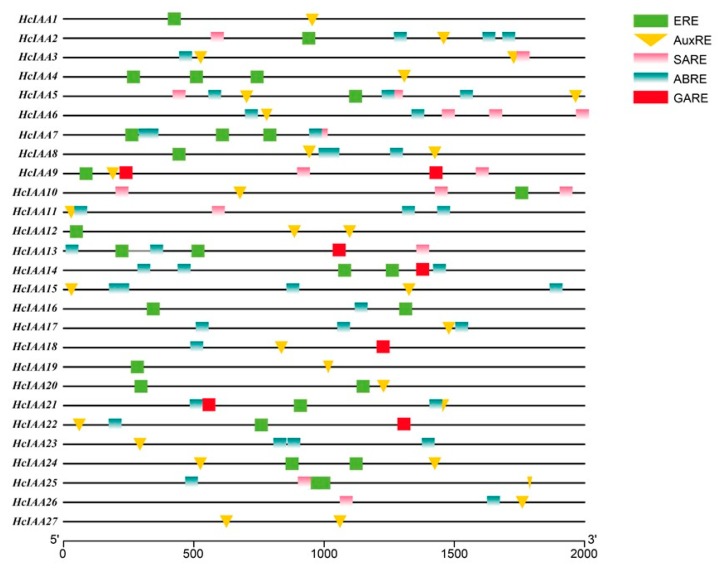
The 2000 bp upstream promoter regions were scanned to identify hormone-related cis-elements to gain insight into how the expression levels of HcIAA genes responded to hormonal stimuli. The results revealed that the majority (23 out of 27) of the HcIAA promoters contained AuxREs as either a degenerate (TGTCCC) or conserved (TGTCTC) motif. Interestingly, 18 out of the 27 HcIAA promoters contained conserved ethylene-response motifs (AWTTCAAA). Additionally, several kinds of hormone-related cis-elements were present in the promoter regions of HcIAA genes, such as HcIAA2, HcIAA9 and HcIAA21. One AuxRE, one SARE, one ERE and three ABREs were present in the promoter of HcIAA2; one AuxRE, two GAREs, two SAREs and one ERE were present in the promoter of HcIAA9 and one AuxRE, two ABREs, one ERE and one GARE were present in the promoter of HcIAA21 (Figure 4). Moreover, more than three ABREs were found in the promoters of HcIAA5 and HcIAA15. The existence of these cis-regulatory elements shows a potential regulation of the Aux/IAA genes not only by auxin but also by other hormones.
Figure 4.
Analysis of specific cis-elements in promoters. The 2000 bp promoter sequences of HcIAA genes were used to analyze specific hormone-related cis-elements, including AuxRE, SARE, GARE, ERE and ABRE, which are color-coded. The AuxRE element is indicated with an inverted triangle.
2.5. Expression Profiling of HcIAA Genes in Different Organs/Tissues
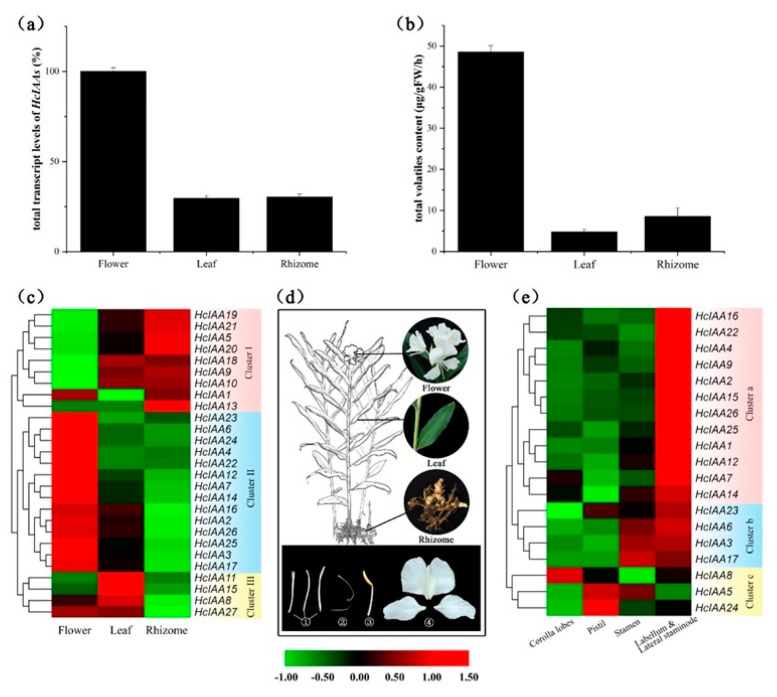
Total volatile contents and total transcript levels of the 27 Aux/IAAs were checked from three organs, including the flower, leaf and rhizome. In full-boom H. coronarium flowering plants, the mRNA accumulation of total HcIAAs reached its highest level, while low-level expression was observed in the leaves and rhizomes (Figure 5a). Similarly, the maximum amounts of volatiles were found in the flowers, while low amounts were detected in the leaves and rhizomes (Figure 5b). To gain insight into the spatial expression patterns of HcIAA genes, transcript levels were assessed by qRT-PCR in different plant tissues and organs. The relative transcript accumulation of all the HcIAA genes is presented in a heat map, and hierarchical clustering allowed us to group all the expression patterns into distinct clusters. Three clusters are shown in the heat map for different organ expression levels (Figure 5c). Many members of the three clusters were expressed in flowers, except HcIAA9, HcIAA10, HcIAA19, HcIAA20 and HcIAA21, which belong to cluster I. However, cluster I was preferentially expressed in vegetative organs (leaf and rhizome). All members in cluster II had higher expression levels in the flowers than in the vegetative organs. Members of cluster III were highly expressed in the leaves, but they diverged in their differential expressions in the rhizome when compared with cluster I. Notably, HcIAA13 was the only gene that was specifically expressed highly in the rhizome (Figure 5c). Interestingly, HcIAA4, HcIAA6, HcIAA22, HcIAA23 and HcIAA24 showed the highest specific expression in flowers. Furthermore, the flower was divided into four different parts, including mixed labellum and lateral staminode, corolla lobes, pistil and stamen (Figure 5d), and HcIAA genes that were expressed in flowers were selected for further analysis in detailed tissues (Figure 5e). A vast majority of members belonging to cluster a and cluster b showed the highest expression level in scented tissues (mixed labellum and lateral staminode), except HcIAA5, HcIAA8 and HcIAA24, which belong to cluster c. The members of cluster a were preferentially expressed in mixed labellum and lateral staminode compared to cluster b (Figure 5e).
Figure 5.
Expression profiles of HcIAA genes in various organs and tissues. (a) Total transcript levels of HcIAAs in flower, leaf and rhizome. (b) The total volatile contents in flower, leaf and rhizome. (c) Changes in the expression levels in three organs (flower, leaf and rhizome), which are schematically depicted above the displayed qRT-PCR data, are relative to RNA accumulation levels. Levels of downregulated expression (green) and upregulated expression (red) are shown on a log2 scale from the highest to the lowest expression for each HcIAA gene. (d) Pictorial view of different organs/tissues ((1) corolla lobes, (2) pistil, (3) stamen, (4) labellum and lateral staminode) of H. coronarium. (e) Changes in the expression levels in different parts of the flowers for 19 HcIAA genes, which are schematically depicted above the displayed qRT-PCR data, are relative to RNA accumulation levels. Levels of downregulated expression (green) or upregulated expression (red) are shown on a log2 scale from the highest to the lowest expression for each HcIAA gene.
2.6. Expression of HcIAA Genes at Different Flower Developmental Stages
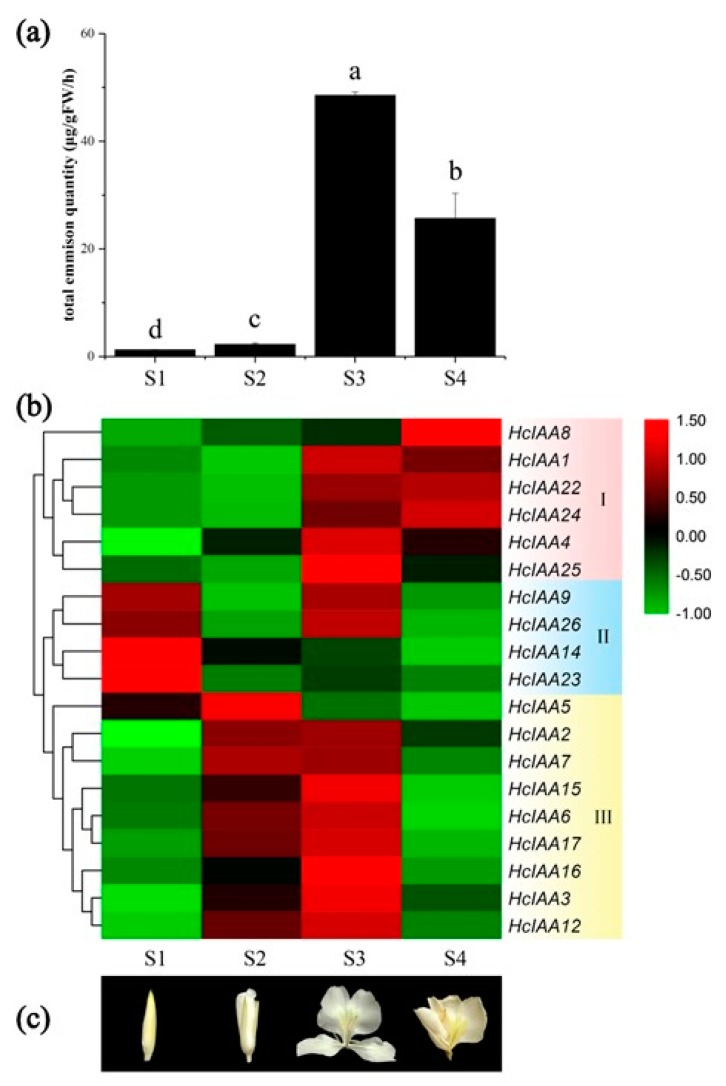
The formation of floral volatile compounds is closely associated with developmental processes in H. coronarium [29]. The total emission of flower volatile compounds was sampled by headspace collection and analyzed by gas chromatography–mass spectrometry at four stages (Figure 6a). The amount of emitted volatile compounds was low at the bud period (S1) and increased slightly at the initial flowering stage (S2), while the emissions continuously increased during the opening stage, reaching the highest level at the blooming period (S3) and declining thereafter at senescence (S4) (Figure 6a). To elucidate the functions of the HcIAA genes during the flower developmental period, their expression levels at four different developmental stages were categorized into three groups by heat map software. Several HcIAA genes (HcIAA1, HcIAA8, HcIAA22 and HcIAA24), which belong to Group I, showed a continuous increase in the expression level during the developmental process. However, some HcIAA genes, including HcIAA9, HcIAA14, HcIAA23 and HcIAA26, which belong to Group II, were gradually reduced (Figure 6b). Within Group I, HcIAA4 and HcIAA25 dramatically peaked at stage S3 and then declined significantly at stage S4. Members of both Groups I and III were most highly expressed at the full-bloom stage (S3), but Group I showed a dramatic increase from S2 to S3, whereas those of Group III maintained a high expression level from S2. The expression levels of most HcIAA genes were significantly changed during flower development, indicating their potential function during the process of flower development and floral scent emission.
Figure 6.
Heat map of the expression of HcIAA genes during different flower developmental stages. (a) The amount of floral volatiles emitted during developmental stages (S1, S2, S3 and S4). (b) The hierarchically clustered heat map was constructed with the relative expression qRT-PCR data for 19 HcIAA genes (indicated on the right) in four development stages (shown at the down). Levels of downregulated expression (green) or upregulated expression (red) are shown on a log2 scale from the highest to the lowest expression for each HcIAA gene. (c) Different flower developmental stages are shown as pictures.
2.7. Expression of HcIAA Genes in Response to Hormone Treatments
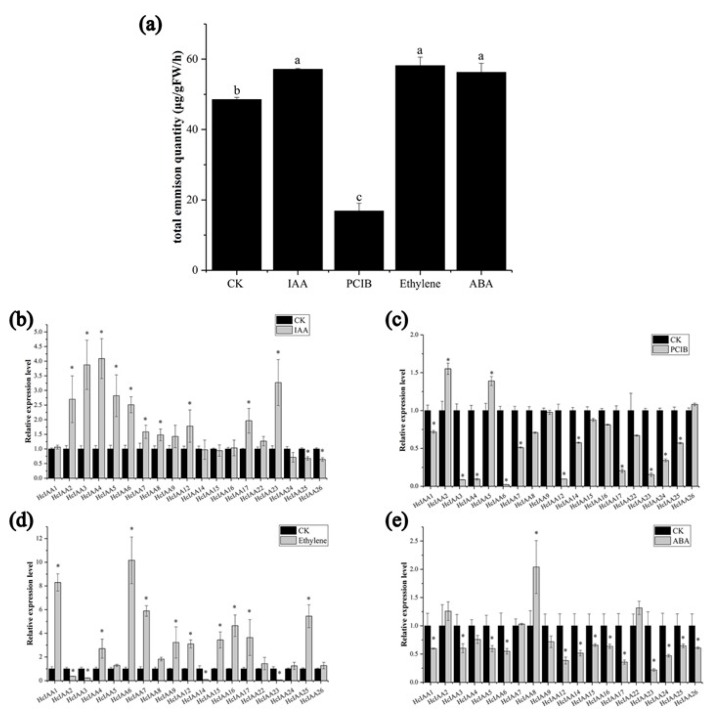
Auxin, ABA and ethylene are three major hormones involved in flower development and senescence [38,39]. PCIB, as an auxin signal inhibitor, also plays key roles in auxin signal transduction [40,41,42,43]. The total percentage of volatile compounds of H. coronarium flowers increased 16%, 20% and 21% under ABA, ethylene and IAA treatment, respectively, but decreased 52% with PCIB treatment (Figure 7a). The expression levels of HcIAA genes were verified by qRT-PCR under IAA, PCIB, ethylene and ABA treatments. The expression levels of HcIAA3, HcIAA4 and HcIAA23 were significantly upregulated by three-fold when treated with auxin, while HcIAA25 and HcIAA26 were reduced (Figure 7b). Under PCIB treatment, HcIAA3, HcIAA4, HcIAA6 and HcIAA12 were significantly reduced, while HcIAA2 and HcIAA5 were slightly upregulated (Figure 7c). Under ethylene treatment, HcIAA1, HcIAA6, HcIAA7, HcIAA16 and HcIAA25 were significantly upregulated, while HcIAA14 and HcIAA23 were downregulated (Figure 7d). The expression level of HcIAA8 was upregulated under ABA treatment, whereas most genes were downregulated (Figure 7e).
Figure 7.
Relative expression levels of HcIAA genes and emission of floral volatiles under different hormonal treatments. (a) The amount of floral volatiles during different treatment. The expression of HcIAA genes in response to (b) IAA, (c) PCIB, (d) ethylene and (e) ABA treatments was analyzed by qRT-PCR. The expression levels of HcIAA genes in control flowers were set to a value of 1. The expression levels of HcIAA genes in IAA (100 μM), PCIB (1.5 mM), ethylene (10 μL/L) and ABA (200 μM) treated flowers were compared to a mock treatment for relative mRNA levels. Error bars represent standard deviations from three biological replicates. Significant differences (* p < 0.05) between the hormone-treated samples and control are indicated by an asterisk.
2.8. Subcellular Localization of HcIAA Candidate Genes
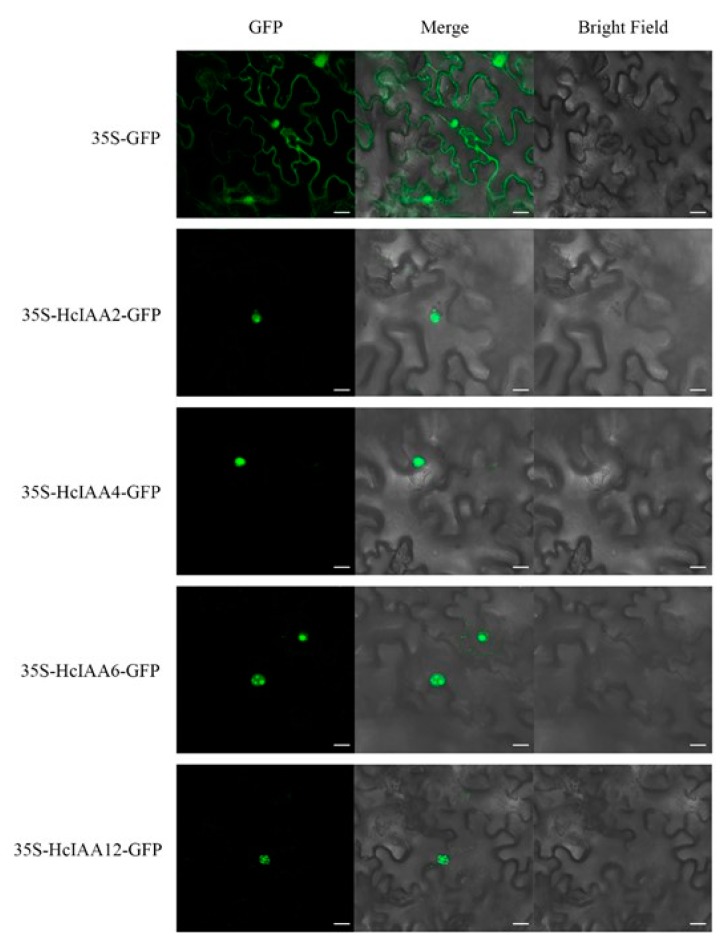
To identify the best candidate gene(s) potentially involved in floral scent formation, we defined several criteria for further functional characterization in plants: Transcript abundance in flower organs; high expression in the labellum and lateral staminode, which are the main parts that emit floral scent; responsiveness to IAA and PCIB, which significantly influence flower scent formation; and expression patterns during flower developmental stages that match the emission of volatile compounds. The combination of all these criteria is presented as a Venn diagram (Supplement Figure S1). HcIAA2, HcIAA4, HcIAA6 and HcIAA12 were identified as the most suitable candidates that matched the criteria. The results of the prediction suggested that the candidates were localized in the nucleus (Figure 1). To experimentally verify subcellular localization, the full-length sequences of HcIAA2, HcIAA4, HcIAA6 and HcIAA12 were fused to a GFP reporter gene and transferred to N. benthamiana leaves, which were subsequently analyzed for transient GFP expression by confocal laser scanning microscopy. The results revealed that the green fluorescence of HcIAA2-GFP, HcIAA4-GFP, HcIAA6-GFP and HcIAA12-GFP was all located in the nucleus (Figure 8), which clearly indicated that the candidate HcIAA proteins are targeted to the nucleus. Similarly, numerous Aux/IAA proteins from different plant species have been identified, which are targeted to the nucleus, from which EgrIAA4 is among one of them, which is located exclusively in the nucleus [44,45]. Thus, HcIAA2, HcIAA4, HcIAA6 and HcIAA12 proteins were located in the nucleus as predicted and were able to mediate an auxin response in vivo consistent with their transcriptional activity.
Figure 8.
Subcellular localization of HcIAA2, HcIAA4, HcIAA6 and HcIAA12 proteins. HcIAA2-GFP, HcIAA4-GFP, HcIAA6-GFP and HcIAA12-GFP fusion proteins were transiently expressed in N. benthamiana leaves, and their subcellular localization was examined by confocal laser scanning microscopy. The merged pictures of the green fluorescence channel (left panels) and the corresponding bright field (right panels) are shown (middle panels). The scale bar indicates 20 µm.
2.9. Silencing of HcIAA2 and HcIAA4 Altered the Flower Volatile Compound Amount
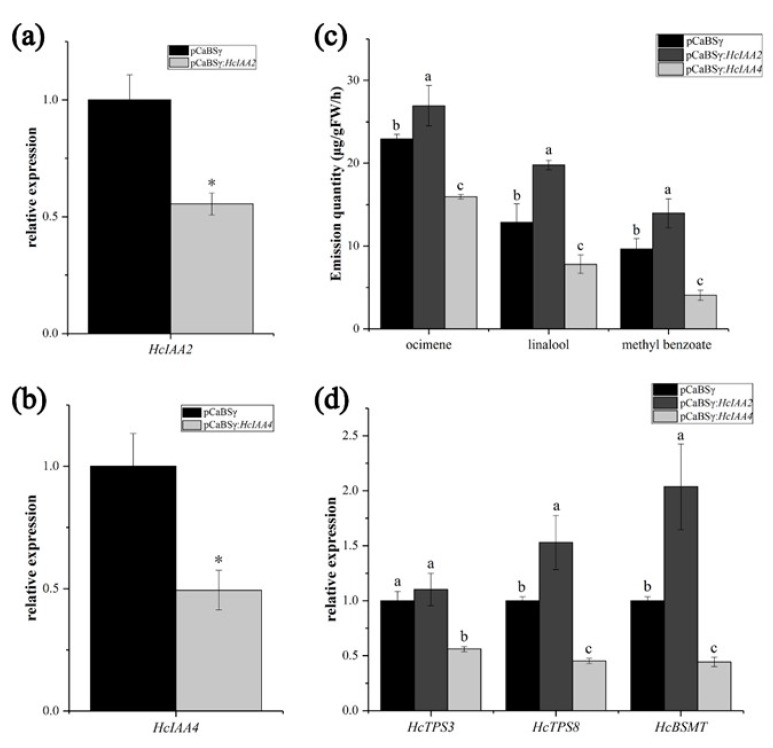
To investigate the potential function of HcIAA2 and HcIAA4 in floral volatile compound formation, we suppressed their expression levels by virus-induced gene silencing (VIGS). As shown in Figure 9, when HcIAA2 and HcIAA4 were silenced in flowers by VIGS, the expression levels of HcIAA2 and HcIAA4 were lower in the silenced flowers than in the pCaBS-γ control (Figure 9a,b). HcIAA2 silencing caused an increase in the amount of the main volatile compounds compared with the control. The contents of ocimene, linalool and methyl benzoate increased by approximately 17.5%, 54.6% and 44.5%, respectively. In contrast, the HcIAA4-silenced flowers showed lower volatile compound amounts than the pCaBSγ control. The contents of ocimene, linalool and methyl benzoate decreased to 30.5%, 39.1% and 58.6%, respectively (Figure 9c). In addition, the expression levels of the key volatile compound synthesis genes in H. coronarium, such as HcTPS3, HcTPS8 and HcBSMT, were analyzed [35]. In HcIAA2-silenced flowers, the expression levels of HcTPS8 and HcBSMT were significantly higher than those of the control; however, the expression levels were reduced in HcIAA4-silenced flowers (Figure 9d). The results indicated that HcIAA2 and HcIAA4 play an imperative role in floral volatile formation in H. coronarium.
Figure 9.
Silencing of HcIAA2 and HcIAA4 alters the amount of the main volatile compounds in flowers. (a, b) Expression of the HcIAA2 and HcIAA4 genes in barley stripe mosaic virus (BSMV)-HcIAA2/4-silenced and control flowers were analyzed by qRT-PCR. (c) GC–MS analysis of main volatile compounds of H. coronarium flower. (d) Expression analysis of key volatile biosynthesis genes in BSMV-HcIAA2/4-silenced vs. control flowers by qRT-PCR. The results are the means of three biological replicates with standard deviations. Asterisks indicate statistically significant differences (Student’s t-test, * p < 0.05).
3. Discussion
Auxins have been shown to play a very important role in plant growth and development [43,45,46]. Two protein families, Aux/IAA and ARF, are known to mediate the auxin signaling molecule pathway [25]. With the development of genome sequencing, the Aux/IAA gene family has been reported in more than 30 plant species, including 29 genes from Arabidopsis thaliana, 17 from Medicago truncatula, 27 from Cucumis sativus, 26 from Solanum lycopersicum, 26 from V. vinifera, 35 from Populus trichocarpa and 26 from Citrus [47]. Especially in Arabidopsis, the functions of Aux/IAA genes are quite well understood [17]. However, in H. coronarium, there is very little information available on Aux/IAA genes. The characterization and expression pattern analysis of HcIAA genes unravel the mechanisms behind auxin involvement in flower development and the floral scent formation of H. coronarium. In total, 27 Aux/IAA genes were identified in H. coronarium, which was slightly less than that of model plants, such as Arabidopsis (29) and rice (31). However, the H. coronarium Aux/IAA gene family contains a lower number of genes compared with Arabidopsis, and two groups are considerably expanded. Groups B and F contain seven and five Aux/IAA genes in H. coronarium, respectively, but only three members were present in both groups of Arabidopsis. Group expansion has also been found in other higher plants, such as S. lycopersicum and P. trichocarpa [37,48]. Particularly, group H, comprising three non-canonical members (AtIAA20, AtIAA30 and AtIAA31) in Arabidopsis that lack the conserved domain II, which is important for protein degradation, is also represented in H. coronarium with one member (HcIAA27). Aux/IAA proteins classified into the same groups may have similar functions in events common to both monocot and dicot plants [49]. The results of phylogenetic analyses of H. coronarium Aux/IAA proteins will pave the way for their functional analysis.
The promoter analysis revealed several well-identified hormone response elements, including the well-conserved AuxREs, present in the promoter regions of the majority of HcIAA genes (Figure 4). Different cis-elements in the promoters of HcIAA genes partly show that auxin signaling transduction can interact with other metabolic pathways. Furthermore, our results revealed that ethylene responsive elements were enriched in most promoters of HcIAA genes (18 out of 27), suggesting that auxin and ethylene play key roles through cross-talk via HcIAAs in H. coronarium flowers; this result is similar to that found in tomato, in which the same pattern was reported, and 16 out of the 25 Sl-IAA promoters contained ethylene-response motifs [37]. This result indicated that Aux/IAA genes might also be regulated by ethylene in H. coronarium flowers.
The expression profiles of HcIAA genes in different tissues and organs disclosed that some Aux/IAAs have preferential expression patterns. Though with structural similarity, their expression patterns showed tissue or organ specificity, suggesting the functional diversity of gene families in different biological processes. For example, HcIAA2 and HcIAA15 have 79.6% identity, but the expression patterns were not the same. HcIAA2 showed much higher mRNA accumulation in flowers, whereas HcIAA15 was preferentially expressed in the leaves (Figure 5c). From the 27 HcIAAs, seven members had high expression levels in the vegetative organs; however, 14 Aux/IAAs showed higher expression levels in the flowers. HcIAA15 and HcIAA11 showed higher transcript accumulation in the leaves, indicating their functional role in leaf development. Some HcIAA genes have temporal and spatial expression patterns, such as HcIAA2, HcIAA4, HcIAA16 and HcIAA22, which were specifically expressed in the labellum and lateral staminode, the main tissues for the emission of volatile compounds, indicating their important roles in floral scent formation. In H. coronarium, the flower volatile compounds start to release during the flower bud stage (S1), then increase at the half-open stage and peak at the full-bloom stage, decreasing in the senescence stage. Interestingly, the expression pattern of Group III HcIAAs showed stage-specific expression patterns during the flower developmental stages that were similar to the amount of volatile compounds during the flower developmental stages. This result indicated that Group III members are potentially involved in floral scent formation. Tissue-specific and developmental stage-specific expression manners of Aux/IAA genes have been demonstrated in many species, such as chickpea, soybean, maize and cotton [26,50,51]. In the tomato flower development process, from the flower bud to fully open stages, a high mRNA gradient level of SlIAA9 was established and played a key role in regulating the initiation of fruit set [49]. In chickpea, during flower development, the Aux/IAA genes, such as CaIAA4, CaIAA7, CaIAA8, CaIAA10 and CaIAA13, also revealed higher transcript accumulation during the full-bloom stage and then declined at the senescence stage, suggesting their possible role in flower development. For soybean, GmIAA6, GmIAA31 and GmIAA33 exhibited higher transcript levels in floral buds, suggesting their putative role in the development of flower buds [51]. In cotton, the expression levels of GhAuxs showed different patterns during fiber initiation and development stages, contributing to the development of fiber [49]. The stage-specific differential expression suggests the diverse and overlapping functions of these proteins during plant growth and development. Overall, the tissue-preferential and stage-specific expressions exhibited by several Aux/IAA genes in H. coronarium flowers suggest their involvement in the biology of specific tissues and flower scent formation.
In agreement with previous reports, our data showed that the transcript levels of many HcIAA genes were regulated by auxin treatment (Figure 7b). Among these genes, HcIAA3, HcIAA4, HcIAA5 and HcIAA23 showed higher upregulation under auxin treatment. A similar study has already been identified in Arabidopsis, tomato, rice and papaya [8,17,30,37]. Arabidopsis Aux/IAA gene family members have also been shown to respond to exogenous IAA in a highly differential fashion with respect to time and dose [52]. In tomato, the transcript levels of 17 out of 19 Sl-IAA genes were upregulated by auxin treatment in seedlings, while Sl-IAA2, Sl-IAA3, Sl-IAA17 and Sl-IAA19 were significantly induced [37]. In rice, the transcript levels of the majority of OsIAA genes were upregulated by auxin treatment, and the effect was more pronounced on OsIAA9, OsIAA14, OsIAA19, OsIAA20 and OsIAA24 [53,54]. Interestingly, the expression levels of HcIAA3, HcIAA4, HcIAA6, HcIAA7 and HcIAA12 were dramatically reduced by treatment with the auxin signal inhibitor PCIB. The number of volatile compounds was reduced significantly under the PCIB treatment of H. coronarium flowers (Figure 7a), showing the importance of auxin signal transduction in floral scent formation. These results indicated that these genes may play key roles in floral scent formation through their expression levels or accumulated proteins in H. coronarium flowers. Additionally, the data showed that the content of volatiles in the flowers and the expression levels of some HcIAA genes were induced by ethylene treatment (Figure 7a,d). Aux/IAA gene responsiveness to ethylene was first described in late immature green tomato fruit [55]. Recent studies provide a comprehensive analysis of the ethylene regulation of Aux/IAA genes, revealing that ethylene clearly induced the expression of some genes. In tomato, the transcript accumulation level of Sl-IAA29 was most strongly upregulated by ethylene, while Sl-IAA2, Sl-IAA11, Sl-IAA17 and Sl-IAA19 were reduced in etiolated seedlings [38]. In papaya, the expression levels of CpIAA3, CpIAA15a, CpIAA15b, CpIAA19, CpIAA27 and CpIAA32 were significantly upregulated under 1-aminocyclopropane-1-carboxylic acid (ACC) treatment, an ethylene precursor [30]. In H. coronarium, the expression levels of some members were upregulated by auxin and ethylene at the same time, such as HcIAA4 and HcIAA6, which were highly upregulated by both auxin and ethylene (Figure 7b,d). Similarly, Sl-IAA3 transcript accumulation was positively regulated by auxin and ethylene in tomato seedlings [55]. The effect of auxin and ethylene treatment on the transcript levels of HcIAA genes reflects their role in auxin and ethylene cross talk in signal transduction. The potential role of the ethylene-regulated Aux/IAA genes in mediating the cross-talk between ethylene and auxin remains to be further studied, particularly during flower volatile formation during flower development.
Based on our data, HcIAA2, HcIAA4, HcIAA6 and HcIAA12 were considered to be suitable candidate genes to regulate flower volatile formation in H. coronarium, while HcIAA2 and HcIAA4 were chosen for functional characterization by VIGS. HcIAA2 is the ortholog of the Arabidopsis gene AtIAA7. The function of AtIAA7 has been studied through gain-of-function experiments. The AtIAA7 mutation axr2-1 conferred late flowering under short daylight in Arabidopsis [56]. The silencing of HcIAA2 in H. coronarium flowers did not change the flowering process, but the total volatile contents increased. Interestingly, the total volatile content was decreased in silenced flowers (HcIAA4), which was in contrast to HcIAA2. Similarly, in suppressed Sl-IAA15 tomato transgenic plants, the content of some monoterpenes in leaf trichome exudates was significantly reduced [19]. The difference in the contents of the volatiles after the silencing of HcIAA2 and HcIAA4 may be because they interact with different proteins, such as ARFs, that have an active or repressive function in the auxin signal response. These results, along with the expression patterns of HcIAA2 and HcIAA4, strongly suggest that HcIAA2 and HcIAA4 play important roles in flower scent formation in H. coronarium.
Floral scent formation is a complex process that is influenced by photoperiod, temperature and phytohormones [57,58,59,60]. Of these key elements, many studies have indicated that hormone signal transduction plays a pivotal role in floral scent formation [61,62,63,64]. Treatment with exogenous IAA and its signal inhibitor, PCIB, significantly influenced the amount of floral volatiles in H. coronarium (Figure 7a). Although HcIAA genes act as the primary auxin-responsive genes, their mode of action in floral scents needs to be investigated further. In this study, we identified the Aux/IAA family genes, analyzed the gene expression profiles, and selected several genes that were important candidates for further functional characterization. By VIGS, we identified that HcIAA2 and HcIAA4 are functionally involved in floral volatile formation. Overall, the information reported here for HcIAA genes could assist further investigations related to their functions in floral scent formation and elucidate the complicated auxin signaling transduction cascade.
4. Materials and Methods
4.1. Plant Material, Growth Conditions and Hormone Treatment
H. coronarium was grown in the growth chamber in South China Agricultural University Guangzhou, China under natural light conditions at 26 ± 2 °C with 13 h light and 11 h dark cycles. Plant samples were instantaneously frozen in liquid nitrogen after collected from the growth chamber and stored at –80 °C. For the analysis of tissue-specific expression patterns, three different samples were used: Full-bloom flowers, mature green leaves and healthy rhizomes of two-year-old H. coronarium plants (Figure 5d). The flower was divided into four parts, i.e., mixed labellum and lateral staminode, corolla lobes, pistil and stamen (Figure 5d). The flower developmental process was divided into four stages: Bud stage (S1), initial flowering stage (S2), full-bloom flower (S3) and senescence (S4; Figure 6b).
The flowers used for hormone treatment were purchased from the H. coronarium cut flower market. After brought back to laboratory, the flowers were immediately cultured in Murashige and Skoog (MS) liquid medium. The cut flowers had a similar flower developmental process as natural flowers (mentioned above). For IAA, 2-(4-chlorophenoxy)-isobutyric acid (PCIB) and abscisic acid (ABA) treatment, the flowers were chosen at the developmental stage between S1 and S2. The flower stems were shortly cut into 40 cm and then placed in sterilized water containing 100 μM IAA, 1.5 mM PCIB and 200 μM ABA for 12 h in a chamber with 14 h daylight and 10 h dark at 25 °C. For ethylene treatment, flowers were incubated with 10 μL/L of ethylene for 12 h in a sealed bottle. The volatile compound analysis was carried out at the full-bloom stage of treated flowers, which were subsequently frozen in liquid nitrogen and stored at –80 °C. Nicotiana benthamiana, for subcellular localization experiments, were grown in a growth room at 25 °C with a 12 h daylight and 12 h dark period.
4.2. RNA Isolation, cDNA Synthesis and Quantitative Real-Time PCR (qRT-PCR)
Total RNA from different organs/tissues and flower developmental stages was extracted using a HiPure plant RNA mini kit (Magen, Guangzhou, China) according to the manufacturer’s suggestions. In total RNA, genomic DNA contamination was removed by DNase I. The qRT-PCR analysis was executed in an ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Waltham, MA, USA) using iTaq™ Universal SYBR Green Supermix (BIO-RAD, Hercules, CA, USA) following the manufacturer’s protocols. PCR was performed in a total volume of 20 μL comprising cDNA, iTaq™ Universal SYBR® Green Supermix and forward and reverse primer. The temperature conditions were as follows: Initial temperature 95 °C for one min followed by 40 cycles of 95 °C for 15 s and 55 °C for 30 s, concluding at 72 °C for 30 s. The relative expression level of genes was calculated according to the formula 2−ΔΔCt method [65]. A similar procedure was carried out with different treatments, and the sequence-specific primers used for qRT-PCR are listed in Table S1. A heat map was constructed to visualize the qRT-PCR data by the Dual System Plotter software.
4.3. Genome-Wide Identification of HcIAA Genes
Based on the transcriptome data [29] and genomic data (data unpublished) of H. coronarium, which was obtained from the Beijing Novogene Bioinformatics Technology Corporation (China), HcIAA genes were identified using 29 Arabidopsis Aux/IAA protein sequences. These Arabidopsis Aux/IAA protein sequences were used to search for related proteins predicted in the H. coronarium genomic data (data unpublished), and 35 potential Aux/IAA proteins were identified. Afterward, the Pfam database Aux/IAA domain (PF02309) and NCBI conserved domain database web servers were used to observe the conserved domains. The incorrectly predicted and redundant sequences were manually discarded. A total of 27 Aux/IAA genes were finally identified in the H. coronarium genome. The obtained sequences were identified as unique genes for comprehensive analysis. The basic physical and chemical parameters of the H. coronarium Aux/IAA genes were calculated by the online ProtParam tool. Sequence information of H. coronarium Aux/IAA genes is given in the Table S3.
4.4. Phylogenetic Tree Construction, Gene Structure and Motif Prediction
Multiple sequence alignment of full-length sequences of HcIAA was performed using Clustal X 2.1. A phylogenetic tree was constructed with the aligned AtIAA and HcIAA protein sequences using MEGA6 [66] and choosing the neighbor-joining method. The predictions of the four classical domains (I, II, III and IV) in HcIAA proteins were performed with the DNAMAN software. The DNA and cDNA sequences corresponding to each predicted gene were obtained from the H. coronarium genome, and the exon–intron organization of the HcIAA genes was analyzed by the Gene Structure Display Server (GSDS). To identify conserved motifs in HcIAA proteins, the Multiple Expectation Maximization for Motif Elicitation (MEME) online program was used for protein sequence analysis. The optimized parameters were designed as follows: The incidences of a single motif 0 or 1 per sequence, motif width ranges from 10 to 60 amino acids, the maximum number of motifs to find four and other parameters defaulted.
4.5. Analysis of Hormone-Related cis-Elements
To investigate cis-elements in the promoter sequences of H. coronarium Aux/IAA genes, sequences 2000 bp upstream of the initiation codon were selected from the H. coronarium genome database. Numerous hormone-related cis-elements were analyzed, including auxin-responsive element (AuxRE), ethylene-responsive element (ERE), ABA-responsive element (ABRE), SA-responsive element (SARE) and gibberellin-responsive element (GARE). Moreover, to identify the putative cis-regulatory elements along the promoter sequences of each HcIAA family gene, the PLACE website was used [67].
4.6. Subcellular Localization of HcIAA Genes
The open reading frame (ORFs) of HcIAA2, HcIAA4, HcIAA6 and HcIAA12 were fused into the vector pEAQ-HT-GFP [68] using the Age I enzyme at the restriction site. The ClonExpress® II one step cloning kit (Vazyme, China) was used to construct the vectors. Sequencing confirmed that no errors had been introduced. The primers used in this experiment are listed in Table S1. The combined plasmids were introduced into Agrobacterium tumefaciens (strain EHA105). The cultures were grown in Luria–Bertani (LB) medium with antibiotics and shaken overnight to stationary phase. The following day, pellets were collected by centrifugation at 2000 g and resuspended in MMA (10 mM MgCl2, 100 µM acetosyringone, 10 mM MES (2-[N-morpholino] ethane sulfonic acid) with pH 5.8 to an OD600 of 0.6 for 2-3 h incubation at room temperature. The suspensions were infiltrated into N. benthamiana leaves. The infected tissues were visualized 48 h after infiltration by a Leica TCS SP2 AOBS Spectral Confocal Scanner mounted on a Leica DM RXA2 upright fluorescence microscope with 409 × 0.75 numerical aperture objectives, and images were further processed using Adobe Photoshop.
4.7. Headspace Analysis of Floral Volatiles
For volatile analysis, the whole flower was enclosed in a 500 mL glass bottle supplemented with an internal standard. After 30 min, a PDMS (polydimethylsiloxane) fiber was inserted into to adsorb volatiles for 30 min followed by injection into a gas chromatography–mass spectrometry (GC–MS) system (Agilent) for volatile analysis as described previously [35].
4.8. Virus-Induced Gene Silencing (VIGS)
The barley stripe mosaic virus (BSMV) system was selected and successfully applied in monocots for virus-induced gene silencing [69,70,71]. pCaBS-α, pCaBS-β and pCaBSγ are essential components of the BSMV system and they ensure the high-efficiency infectivity and transformation of the virus in the cell [71]. The Agro/LIC BSMV-VIGS vectors used in this experiment were provided by Dr. Dawei Li (State Key Laboratory of Agro-Biotechnology, China Agricultural University, Beijing, People’s Republic of China). The empty vector pCaBSγ was linearized with Apa I to insert the fragments. At the 3′ end of HcIAA2 and HcIAA4, 280 bp fragments were amplified by PCR from H. coronarium cDNAs, especially to silence HcIAA2 and HcIAA4. The fragments were then inserted into the pCaBSγ empty vector to generate the pCaBSγ:HcIAA2 and pCaBSγ:HcIAA4 constructs. The primers used for amplifying HcIAA2 and HcIAA4 are listed in Table S1. The pCaBS-α [71], pCaBS-β [71], pCaBSγ [71], pCaBSγ:HcIAA2 and pCaBSγ:HcIAA4 vectors were transformed into A. tumefaciens strain EHA105. The transformed A. tumefaciens lines were cultured in LB medium supplemented with 50 µg/mL kanamycin and 25 µg/mL rifampicin. The cultures were harvested by centrifugation at 5000 rpm for 10 min and resuspended in infiltration buffer (10 mM MgCl2, 0.1 mM acetosyringone, 10 mM MES, pH 5.6) to a final OD600 of approximately 1.0. Mixtures of cultures containing an equal ratio (v/v/v) of pCaBS-α, pCaBS-β and pCaBSγ, or pCaBS-α, pCaBS-β and pCaBSγ:HcIAA2, or pCaBS-α, pCaBS-β and pCaBSγ:HcIAA4. The culture mixtures were placed at room temperature in the dark for 3 to 5 h before vacuum infiltration into the flowers. For VIGS, the flowers were collected at the S1 stage. Vacuum infiltration was carried out by immersing the flowers in the bacterial suspension. After the release of the vacuum, the flowers were washed in deionized water, placed into an MS medium liquid culture, and then maintained with a 12/12 h day/night cycle at 16 °C for five days. The total volatile compounds were collected and analyzed at the full-bloom stage by GC–MS as described above. The experiment was replicated three times.
4.9. Statistical Analysis
Statistical analysis was performed using the SPSS 19.0 program (SPSS Inc. Chicago, IL, USA). Comparisons between two groups were executed by using a Student’s t-test at a significance level of 0.05. All data are presented as the mean, SD, and p < 0.05 was considered statistically significant. The expression analyses were performed between three to four biological replicates.
4.10. Data Availability
All data generated or analyzed during this study are included in the main text or supplement of this published article.
Acknowledgments
We thank Xiaozhou Liu and Yanyan Zeng (College of Forestry and Landscape Architecture) for their assistance in GC-MS data.
Abbreviations
| Aux/IAA | Auxin/indole-3-acetic acid |
| SAUR | Small Auxin UP RNA |
| GH3 | Gretchen Hagen 3 |
| ARF | Auxin response factor |
| AuxREs | Auxin-responsive cis-elements |
| MS | Murashige and Skoog |
| ABA | Abscisic acid |
| PCIB | 2-(4-chlorophenoxy)-isobutyric acid |
| IAA | Indole-3-Acetic Acid |
| GSDS | Gene Structure Display Server |
| MEME | Multiple Expectation Maximization for Motif Elicitation |
| AuxRE | Auxin-responsive Element |
| ERE | Ethylene-responsive element |
| GARE | Gibberellin-responsive element |
| SARE | SA-responsive element |
| ABRE | ABA-responsive element |
| ORFs | Open reading frame |
| LB | Luria-Bertani |
| PDMS | Polydimethylsiloxane |
| GC-MS | Gas chromatography-mass spectrometry |
| VIGS | Virus-induced gene silencing |
| BSMV | Barley stripe mosaic virus |
| MW | Molecular weight |
| pI | Isoelectric point |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| GFP | Green fluorescence protein |
Supplementary Materials
The supplementary materials are available online at https://www.mdpi.com/1422-0067/20/13/3235/s1.
Author Contributions
Conceptualization, Y.F.; Data curation, Y.K. and F.A.; Formal analysis, F.A., Y.Z. and X.L.; Funding acquisition, R.Y. and Y.F.; Investigation, Y.K. and Y.Yu; Methodology, Y.K., Y.Yue and X.L.; Project administration, Y.F.; Resources, R.Y.; Software, F.A., Y.Z. and Y.Yue; Supervision, Y.F.; Validation, R.Y., X.L. and Y.Yu; Visualization, R.Y. and Y.Yu; Writing—original draft, Y.K.; Writing—review & editing, F.A. and Y.F.
Funding
This work was supported in part by the National Natural Science Foundation of China to Y.F. (Grant no. 31770738 and 31370694) and supported by Natural Science Foundation of China to R.Y. (Grant no. 31870690), People’s livelihood science and technology projects of Guangzhou (Grant no. 201903010054), Key research project of the ministry of science and technology of China.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Hagen G., Guilfoyle T. Auxin-responsive gene expression: Genes, promoters and regulatory factors. Plant Mol. Biol. 2002;49:373–385. doi: 10.1023/A:1015207114117. [DOI] [PubMed] [Google Scholar]
- 2.Friml J., Vieten A., Sauer M., Weijers D., Scwartz H., Hamann T., Offringa R., Jürgens G. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- 3.Jain M., Kaur N., Garg R., Thakur J.K., Tyagi A.K., Khurana J.P. Structure and expression analysis of early auxin-responsive Aux/IAA gene family in rice (Oryza sativa) Funct. Integr. Genom. 2006;6:47–55. doi: 10.1007/s10142-005-0005-0. [DOI] [PubMed] [Google Scholar]
- 4.Theologis A., Huynh T.V., Davis R.W. Rapid induction of specific mRNAs by auxin in pea epicotyl tissue. J. Mol. Biol. 1985;183:53–68. doi: 10.1016/0022-2836(85)90280-3. [DOI] [PubMed] [Google Scholar]
- 5.Oeller P.W., Keller J.A., Parks J.E., Silbert J.E., Theologis A. Structural characterization of the early indoleacetic acid-inducible genes, PS-IAA4/5 and PS-IAA6, of pea (Pisum sativum L.) J. Mol. Biol. 1993;233:789–798. doi: 10.1006/jmbi.1993.1555. [DOI] [PubMed] [Google Scholar]
- 6.Dharmasiri S., Estelle M. The role of regulated protein degradation in auxin response. Plant Mol. Biol. 2002;49:401–408. doi: 10.1023/A:1015203013208. [DOI] [PubMed] [Google Scholar]
- 7.Tiwari S.B., Wang X.J., Hagen G., Guilfoyle T.J. Aux/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell. 2001;13:2809–2822. doi: 10.1105/tpc.010289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abel S., Oeller P.W., Theologis A. Early auxin-induced genes encode short-lived nuclear proteins. Proc. Natl. Acad. Sci. USA. 1994;91:326–330. doi: 10.1073/pnas.91.1.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arase F., Nishitani H., Egusa M., Nishimoto N., Sakurai S., Sakamoto N., Kaminaka H. IAA8 involved in lateral root formation interacts with the TIR1 auxin receptor and ARF transcription factors in Arabidopsis. PLoS ONE. 2012;7:e43414. doi: 10.1371/journal.pone.0043414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiwari S.B. Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell. 2004;16:533–543. doi: 10.1105/tpc.017384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szemenyei H., Hannon M., Long J.A. TOPLESS Mediates Auxin-Dependent Transcriptional Repression During Arabidopsis Embryogenesis. Science. 2008;319:1384–1386. doi: 10.1126/science.1151461. [DOI] [PubMed] [Google Scholar]
- 12.Dharmasiri N., Dharmasiri S., Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- 13.Kepinski S., Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;435:446–451. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- 14.Tan X., Calderon-Villalobos L.I.A., Sharon M., Zheng C., Robinson C.V., Estelle M., Zheng N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007;446:640–645. doi: 10.1038/nature05731. [DOI] [PubMed] [Google Scholar]
- 15.Leyser O. Auxin Signaling. Plant Physiol. 2018;176:465–479. doi: 10.1104/pp.17.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Remington D.L. Contrasting modes of diversification in the Aux/IAA and ARF gene families. Plant Physiol. 2004;135:1738–1752. doi: 10.1104/pp.104.039669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Overvoorde P.J. Functional genomic analysis of the Auxin/indole-3-acetic acid gene family members in Arabidopsis thaliana. Plant Cell. 2005;17:3282–3300. doi: 10.1105/tpc.105.036723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiwari S.B. The Roles of Auxin Response Factor Domains in Auxin-Responsive Transcription. Plant Cell. 2003;15:533–543. doi: 10.1105/tpc.008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng W., Yan F., Liu M., Wang X., Li Z. Down-regulation of SlIAA15 in tomato altered stem xylem development and production of volatile compounds in leaf exudates. Plant Signal. Behav. 2014;7:911–913. doi: 10.4161/psb.20723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassa C., Mila I., Bouzayen M., Audran-Delalande C. Phenotypes associated with down-regulation of Sl-IAA27 support functional diversity among AUX/IAA family members in tomato. Plant Cell Physiol. 2012;53:1583–1595. doi: 10.1093/pcp/pcs101. [DOI] [PubMed] [Google Scholar]
- 21.Guillotin B., Etemadi M., Audran C., Bouzayen M., Bécard G., Combier J. Sl-IAA27 regulates strigolactone biosynthesis and mycorrhization in tomato (var. Micro Tom) New Phytol. 2016;213:1124–1132. doi: 10.1111/nph.14246. [DOI] [PubMed] [Google Scholar]
- 22.Woodward A.W., Bartel B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu B.Q., Xu X.Q., Wu Y.W., Duan C.Q., Pan Q.H. Isolation and characterization of two hydroperoxide lyase genes from grape berries: HPL isogenes in Vitis vinifera grapes. Mol. Biol. Rep. 2012;39:7443–7455. doi: 10.1007/s11033-012-1577-0. [DOI] [PubMed] [Google Scholar]
- 24.Kitomi Y., Inahashi H., Takehisa H., Sato Y., Inukai Y. OsIAA13-mediated auxin signaling is involved in lateral root initiation in rice. Plant Sci. 2012;190:116–122. doi: 10.1016/j.plantsci.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Liscum E., Reed J.W. Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol. Biol. 2002;49:387–400. doi: 10.1023/A:1015255030047. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y., Deng D., Bian Y., Lv Y., Xie Q. Genome-wide analysis of primary auxin-responsive Aux/IAA gene family in maize (Zea mays. L.) Mol. Biol. Rep. 2010;37:3991–4001. doi: 10.1007/s11033-010-0058-6. [DOI] [PubMed] [Google Scholar]
- 27.Wu J., Peng Z., Liu S.Y., He Y.J., Cheng L., Kong F., Wang J., Lu G. Genome-wide analysis of Aux/IAA gene family in Solanaceae species using tomato as a model. Mol. Genet. Genom. 2012;287:295–311. doi: 10.1007/s00438-012-0675-y. [DOI] [PubMed] [Google Scholar]
- 28.Çakir B., Kiliçkaya O., Olcay A. Genome-wide analysis of Aux/IAA genes in Vitis vinifera: Cloning and expression profiling of a grape Aux/IAA gene in response to phytohormone and abiotic stresses. Acta Physiol. Plant. 2013;35:365–377. doi: 10.1007/s11738-012-1079-7. [DOI] [Google Scholar]
- 29.Yue Y., Yu R., Fan Y. Transcriptome profiling provides new insights into the formation of floral scent in Hedychium coronarium. BMC Genom. 2015;16:470. doi: 10.1186/s12864-015-1653-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu K., Yuan C., Feng S., Zhong S., Li H., Zhong J., Shen C., Liu J. Genome-wide analysis and characterization of Aux/IAA family genes related to fruit ripening in papaya (Carica papaya L.) BMC Genom. 2017;18:351. doi: 10.1186/s12864-017-3722-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Z.Y., Raven H.P. Flora of China. Volume 24. Missouri Botanical Garden Press; Saint Louis, MO, USA: 2000. pp. 370–377. [Google Scholar]
- 32.Baez D., Pino J.A., Morales D. Floral scent composition in Hedychium coronarium J. Koenig analyzed by SPME. J. Essent. Oil Res. 2011;23:64–67. doi: 10.1080/10412905.2011.9700460. [DOI] [Google Scholar]
- 33.Fan Y.P., Yu R.C., Huang Y., Chen Y.F. Studies on the essential constituent of Hedychium flavum and H. coronarium. Acta Horti. Sinica. 2003;30:475. [Google Scholar]
- 34.Fan Y.P., Wang X.R., Yu R.C., Yang P. Analysis on the aroma components in several species of Hedychium. Acta Horti. Sinica. 2007;34:231–234. [Google Scholar]
- 35.Yue Y., Yu R., Fan Y. Characterization of two monoterpene synthases involved in floral scent formation in Hedychium coronarium. Planta. 2014;240:745–762. doi: 10.1007/s00425-014-2127-x. [DOI] [PubMed] [Google Scholar]
- 36.Pichersky E., Dudareva N. Scent engineering: Toward the goal of controlling how flowers smell. Trends Biotechnol. 2007;25:105–110. doi: 10.1016/j.tibtech.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Audran-Delalande C., Bassa C., Mila I., Regad F., Zouine M., Bouzayen M. Genome-Wide identification, functional analysis and expression profiling of the Aux/IAA gene family in tomato. Plant Cell Physiol. 2012;53:659–672. doi: 10.1093/pcp/pcs022. [DOI] [PubMed] [Google Scholar]
- 38.Wils C.R., Kaufmann K. Gene-regulatory networks controlling inflorescence and flower development in Arabidopsis thaliana. Biochim. Biophys. Acta Gene Regul. Mech. 2017;1860:95–105. doi: 10.1016/j.bbagrm.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Zubo Y.O., Yamburenko M.V., Kusnetsov V.V., Börner T. Methyl jasmonate, gibberellic acid, and auxin affect transcription and transcript accumulation of chloroplast genes in barley. J. Plant Physiol. 2011;168:1335–1344. doi: 10.1016/j.jplph.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Agarwal P.K., Agarwal P., Custers J.B.M., Liu C., Bhojwani S.S. PCIB an anti-auxin enhances microspore embryogenesis in microspore culture of Brassica juncea. Plant Cell Tissue Organ Cult. 2006;86:201–210. doi: 10.1007/s11240-006-9108-0. [DOI] [Google Scholar]
- 41.Fransson P. Studies on the interaction of antiauxin and native auxin in wheat roots. Physiol. Plantarum. 1958;11:644–654. doi: 10.1111/j.1399-3054.1958.tb08260.x. [DOI] [Google Scholar]
- 42.Heupel T., Stange L. The auxin antagonist p-chlorophenoxyisobutyric acid abolishes polar distribution of DNA synthesizing cells within the meristem of Riella helicophylla. J. Plant Physiol. 1995;146:757–759. doi: 10.1016/S0176-1617(11)81946-2. [DOI] [Google Scholar]
- 43.Van-Doorn W.G., Dole I., Çelikel F.G., Harkema H. Opening of Iris flowers is regulated by endogenous auxins. J. Plant Physiol. 2013;170:161–164. doi: 10.1016/j.jplph.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 44.Yu H., Soler M., San Clemente H., Mila I., Paiva J.A.P., Myburg A.A., Bouzayen M., Grima-Pettenati J., Cassan-Wang H. Comprehensive genome-wide analysis of the Aux/IAA gene family in Eucalyptus: Evidence for the role of EgrIAA4 in wood formation. Plant Cell Physiol. 2015;56:700–714. doi: 10.1093/pcp/pcu215. [DOI] [PubMed] [Google Scholar]
- 45.Tyurin A.A., Kabardaeva K.V., Berestovoy M.A., Sidorchuk Y.V., Fomenkov A.A., Nosov A.V., Goldenkova-Pavlova I.V. Simple and reliable system for transient gene expression for the characteristic signal sequences and the estimation of the localization of target protein in plant cell. Russ. J. Plant Physiol. 2017;64:672–679. doi: 10.1134/S1021443717040173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bohn-Courseau I. Auxin: A major regulator of organogenesis. Comptes Rendus Biologies. 2010;333:290–296. doi: 10.1016/j.crvi.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Gaudinová A., Malbeck J., Dobrev P., Kubelková D., Špak J., Vanková R. Cytokinin, auxin, and abscisic acid dynamics during flower development in white and red currants infected with Black currant reversion virus. Physiol. Mol. Plant Pathol. 2008;73:119–125. doi: 10.1016/j.pmpp.2009.03.004. [DOI] [Google Scholar]
- 48.Luo J., Zhou J., Zhang J. Aux/IAA Gene Family in Plants: Molecular Structure, Regulation, and Function. Int. J. Mol. Sci. 2018;19:259. doi: 10.3390/ijms19010259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalluri U.C., Difazio S.P., Brunner A.M., Tuskan G.A. Genome-wide analysis of Aux/IAA and ARF gene families in (Populus trichocarpa) BMC Plant Biol. 2007;7:59. doi: 10.1186/1471-2229-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J., Yan D., Yuan T., Gao X., Lu Y. A gain-of-function mutation in IAA8 alters Arabidopsis floral organ development by change of jasmonic acid level. Plant Mol. Biol. 2013;82:71–83. doi: 10.1007/s11103-013-0039-y. [DOI] [PubMed] [Google Scholar]
- 51.Han X., Xu X., Fang D.D., Zhang T., Guo W. Cloning and expression analysis of novel Aux/IAA family genes in Gossypium hirsutum. Gene. 2012;503:83–91. doi: 10.1016/j.gene.2012.03.069. [DOI] [PubMed] [Google Scholar]
- 52.Singh V.K., Jain M. Genome-wide survey and comprehensive expression profiling of Aux/IAA gene family in chickpea and soybean. Front. Plant Sci. 2015;6:918. doi: 10.3389/fpls.2015.00918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reed J.W. Roles and activities of Aux/IAA proteins in Arabidopsis. Trends Plant Sci. 2001;6:420–425. doi: 10.1016/S1360-1385(01)02042-8. [DOI] [PubMed] [Google Scholar]
- 54.Jain M., Kaur N., Tyagi A.K., Khurana J.P. The auxin-responsive GH3 gene family in rice (Oryza sativa) Funct. Integr. Genom. 2006;6:36–46. doi: 10.1007/s10142-005-0142-5. [DOI] [PubMed] [Google Scholar]
- 55.Meir S., Philosoph-Hadas S., Sundaresan S., Selvaraj K.S.V., Burd S., Ophir R., Kochanek B., Reid M.S., Jiang C.Z., Lers A. Microarray analysis of the abscission-related transcriptome in the tomato flower abscission zone in response to auxin depletion. Plant Physiol. 2010;154:1929–1956. doi: 10.1104/pp.110.160697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chaabouni S., Jones B., Delalande C., Wang H., Li Z., Mila I., Frasse P., Latché A., Pech J., Bouzayen M. Sl-IAA3, a tomato Aux/IAA at the crossroads of auxin and ethylene signaling involved in differential growth. J. Exp. Bot. 2009;60:1349–1362. doi: 10.1093/jxb/erp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mai Y.X., Wang L., Yang H.Q. A gain-of-function mutation in IAA7/AXR2 confers late flowering under short-day light in Arabidopsis. J. Integr. Plant Biol. 2011;53:480–492. doi: 10.1111/j.1744-7909.2011.01050.x. [DOI] [PubMed] [Google Scholar]
- 58.Abbas F., Ke Y., Yu R., Yue Y., Amanullah S., Jahangir M.M., Fan Y. Volatile terpenoids: Multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta. 2017;246:803–816. doi: 10.1007/s00425-017-2749-x. [DOI] [PubMed] [Google Scholar]
- 59.Abbas F., Ke Y., Yu R., Fan Y. Functional characterization and expression analysis of two terpene synthases involved in floral scent formation in Lilium ‘Siberia’. Planta. 2019;249:71–93. doi: 10.1007/s00425-018-3006-7. [DOI] [PubMed] [Google Scholar]
- 60.Dudareva N., Negre F., Nagegowda D.A., Orlova I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006;25:417–440. doi: 10.1080/07352680600899973. [DOI] [Google Scholar]
- 61.Dudareva N., Klempien A., Muhlemann J.K., Kaplan I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013;198:16–32. doi: 10.1111/nph.12145. [DOI] [PubMed] [Google Scholar]
- 62.Kwangjin K., Mijung K., Heungdeug K., Song J.S., Eunha Y., Junggun C. The effect of flower scent and essential oils on reduction of concentration of cortisol, a stress hormone. Korean J. Hortic. Sci. 2006;30:1198–1209. [Google Scholar]
- 63.Ramya M., Kwon O.K., An H.R., Park P.M., Baek Y.S., Park P.H. Floral scent: Regulation and role of MYB transcription factors. Phytochem. Lett. 2017;19:114–120. doi: 10.1016/j.phytol.2016.12.015. [DOI] [Google Scholar]
- 64.Schmelz E.A., Engelberth J., Alborn H.T., Donnell P., Sammons M., Toshima H., Tumlinson J.H. Simultaneous analysis of phytohormones, phytotoxins, and volatile organic compounds in plants. Proc. Natl. Acad. Sci. USA. 2003;100:10552–10557. doi: 10.1073/pnas.1633615100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Livakm K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 66.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2013;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Higo K., Ugawa I.M., Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999;27:297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sainsbury F., Thuenemann E.C., Lomonossoff G.P. pEAQ: Versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotechnol. J. 2009;7:682–693. doi: 10.1111/j.1467-7652.2009.00434.x. [DOI] [PubMed] [Google Scholar]
- 69.Mahadevan C., Jaleel A., Deb L., Thomas G., Sakuntala M. Development of an efficient virus induced gene silencing strategy in the non-model wild ginger Zingiber zerumbet and investigation of associated proteome changes. PLoS ONE. 2014;10:e124518. doi: 10.1371/journal.pone.0124518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Renner T., Bragg J., Driscoll H.E., Cho J., Jackson A.O., Specht C.D. Virus-Induced Gene Silencing in the Culinary Ginger (Zingiber officinale): An effective mechanism for down-regulating gene expression in tropical monocots. Mol. Plant. 2009;2:1084–1094. doi: 10.1093/mp/ssp033. [DOI] [PubMed] [Google Scholar]
- 71.Yuan C., Li C., Yan L., Jackson A.O., Liu Z., Han C., Yu J., Li D. A high throughput barley stripe mosaic virus vector for virus induced gene silencing in monocots and dicots. PLoS ONE. 2011;6:e26468. doi: 10.1371/journal.pone.0026468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.