Abstract
The insulin-like growth factor 2 (IGF2) mRNA binding protein IMP2 (IGF2BP2) is an oncogenic protein known to be overexpressed in different tumor types. Pancreatic cancer is a very lethal cancer that requires early diagnosis and new treatment options. The aim of our study was to investigate the role of IMP2 in the initiation and progression of pancreatic ductal adenocarcinoma (PDAC). IMP2 was significantly overexpressed in a human precursor (PanIN) lesions suggesting IMP2 as a marker for early stages of PDAC. In a PDAC cohort of matched normal and tumor samples IMP2 showed overexpression in tumor tissues compared with normal pancreatic tissue. Strict correlation analysis (threshold R2 > 0.75) revealed 22 genes highly positively and 9 genes highly negatively correlating with IMP2. Besides genes involved in the inhibition of apoptosis (Bcl-XL), especially factors involved in ubiquitination were strongly correlated with IMP2 expression: SMURF1 and FBXO45. Moreover, protein kinase C (PKC) signaling pathway was distinctly affected: DXS1179E encoding PKC iota, PKC substrate PLEK2, and inositol triphosphate receptor IP3R3 were positively correlated with IMP2 expression. Besides tumor initiation, IMP2 also seemed to have an impact on tumor progression. TGF-β treatment of Panc-1 pancreatic cancer cells to induce epithelial-mesenchymal transition (EMT) was accompanied by increased IMP2 expression. EMT is important for cancer cells to gain migratory and invasive potential, which is essential for metastasis. Concordantly, circulating tumor cells showed higher IMP2 levels as compared with normal tissue from tumor origin and with normal hematological cells. Accordingly, IMP2 protein levels correlated with poor survival. In conclusion, as IMP2 seems to promote tumor progression of PDAC, it might be an interesting diagnostic and prognostic marker as well as a novel target for the treatment of PDAC.
Keywords: p62, RBP
1. Introduction
Pancreatic adenocarcinoma is the seventh leading cause of cancer-related deaths worldwide [1]. Prognosis is poor and 5-year survival is only 9%. Most of the patients have advanced stage tumors at the time of diagnosis making tumor resection impossible. Insulin-like growth factor 2 (IGF2) mRNA binding proteins (IGF2BPs/IMPs) have been described to be oncogenic in several types of cancer including pancreatic cancer [2,3,4,5,6,7]. The IMP family member IMP3 has originally been identified and pancreatic cancer tissues [8] and studied in this cancer type in more detail compared to the other two IMPs [9,10,11,12]. In lung cancer IMP1 has been reported to increase Kras signaling [13], which is frequently altered in pancreatic cancer tissue. Recently, IMP2 has been reported to be the most abundant of the three members of the IMP family in most cancer types including pancreatic ductal adenocarcinoma (PDAC) [5]. However, beside gene expression in pancreatic cancer samples of the TCGA data set little is known about its role in pancreatic cancer progression and its prognostic relevance.
A well-known precursor of PDAC is Pancreatic Intraepithelial Neoplasia (PanIN). PanIN lesions progress from intraepithelial to invasive PDAC. Early detection of PanINs would help to interfere with PanIN progression to PDAC. IMP2 has been shown to promote carcinogenesis in the liver and to worsen chronic liver disease as a risk factor for liver cancer development [7,14].
This study shows for the first time that IMP2 expression is linked to progression and poor survival in pancreatic cancer.
2. Results and Discussion
2.1. IMP2 Is Overexpressed in Precursor Lesions, PDAC and Linked to Lower Rate of Survival
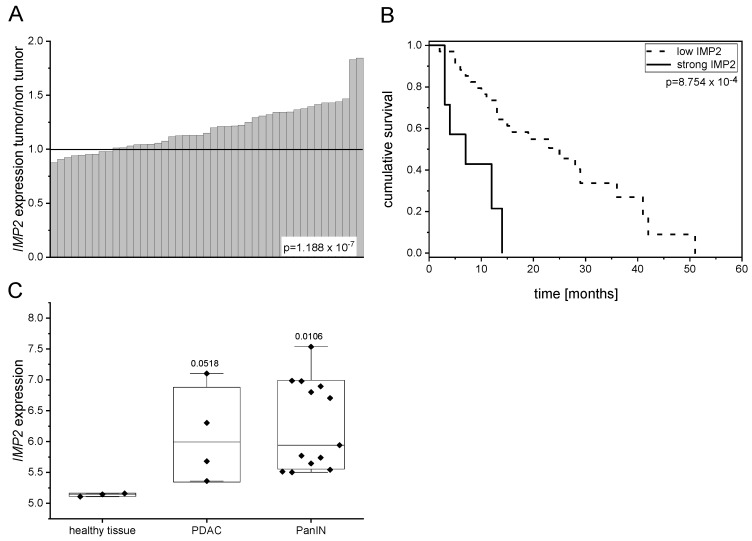
In order to study the expression of IMP2 in pancreatic cancer, publicly available datasets were investigated. Dai et al. recently showed that IMP2 is overexpressed in PDAC tissues of the publicly available TCGA cohort compared to normal tissues [5]. In concordance, we observed IMP2 overexpression in tumor tissues compared to normal tissues from a dataset containing matched normal and tumor samples (Figure 1A). Survival analysis revealed that high IMP2 expression is linked to lower survival rate (Figure 1B). Interestingly, IMP2 was overexpressed in PanIN lesions, which bear a high risk to develop pancreatic cancer (Figure 1C). In contrast to IMP2, IMP3 was shown to be highly specific for pancreatic tumor tissue and negative in premalignant tissues [15]. However, since biomarkers for early detection are needed to detect progression from PanIN towards PDAC, IMP2 might fulfill this need.
Figure 1.
IMP2 is overexpressed in PanINs and PDAC and leads to lower rate of survival. (A) Expression levels of IMP2 in human PDAC cohort as compared with matched normal pancreatic tissue (GEO ID: GSE28735; p = 1.188 × 10−7; n = 45); (B) Kaplan-Meier estimated cumulative survival of PDAC patients with strong or low IMP2 expression (GEO ID: GSE28735; p = 8.754 × 10−4; low IMP2 expression in tumor tissue < 6, n = 35; strong IMP2 expression > 6, n = 7); (C) IMP2 expression levels ±SEM in human PDAC and PanIN lesions (GEO ID: GSE43288).
Strict correlation analysis (threshold R2 > 0.75) revealed 22 genes highly positively and 9 genes highly negatively correlating with IMP2 (Table 1). Besides genes involved in the inhibition of apoptosis (Bcl-XL), especially factors involved in ubiquitination were strongly correlated with IMP2 expression: SMURF1 and FBXO45. Moreover, protein kinase C (PKC) signaling pathway was distinctly affected: DXS1179E encoding PKC iota, PKC substrate PLEK2, and inositol triphosphate receptor IP3R3. Negatively correlated genes are involved in apoptosis regulation and DNA repair (APO-J and CAF) as well as epigenetic regulation (AAM-B). Interestingly, IMP2 negatively correlated with KIAA0922, which antagonizes Wnt signaling, a pathway which has been described to be essential for pancreatic carcinogenesis [16,17].
Table 1.
Genes correlating with IMP2 expression. Table shows correlation coefficients for highly positively and negatively correlating genes (threshold R2 > 0.75 or R2 < −0.75, respectively).
| Positive Correlation | Negative Correlation | ||
|---|---|---|---|
| Gene | Correlation Coefficient R2 | Gene | Correlation Coefficient R2 |
| ERO1-alpha | 0.867 | DMDL | −0.833 |
| CD318 | 0.830 | CAF | −0.814 |
| ARVD12 | 0.825 | SEPP1 | −0.801 |
| BEN | 0.818 | AAM-B | −0.796 |
| BCL-XL/S | 0.793 | ADAMTSL3 | −0.779 |
| IP3R3 | 0.787 | 8B | −0.774 |
| BM600-125KD | 0.783 | KIAA0922 | −0.765 |
| PLEK2 | 0.781 | SEB | −0.761 |
| TM9SF4 | 0.776 | GGTA1 | −0.760 |
| DYT17 | 0.774 | APO-J | −0.753 |
| TMCC1 | 0.772 | ADCL2 | −0.752 |
| DXS1179E | 0.770 | ||
| HSNOV1 | 0.764 | ||
| SDC4 | 0.762 | ||
| TFGA | 0.761 | ||
| SMURF1 | 0.761 | ||
| FAD104 | 0.760 | ||
| CT31 | 0.759 | ||
| FGD6 | 0.758 | ||
| FBXO45 | 0.750 | ||
2.2. IMP2 Is Involved in Metastasis
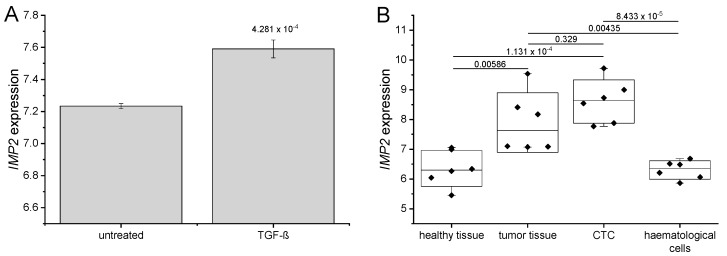
Epithelial-mesenchymal transition (EMT) is important for tumor cells to gain migratory and invasive potential. In glioblastoma, IMP2 promotes EMT and migration via the IGF2/PI3K/Akt pathway [18]. EMT can be induced in cell culture by treatment of cancer cells with TGF-β. In fact, TGF-β induced EMT was associated with increased IMP2 expression (Figure 2A).
Figure 2.
IMP2 is associated with metastasis (A) IMP2 expression in Panc-1 cells after EMT induction by treatment with 5 ng/mL TGF beta for 48 h (GEO ID: GSE23952, n = 3); (B) IMP2 expression in tumor tissue and CTC compared to healthy tissue and heamatological cells of the same donor as controls (GEO ID: GDS4329).
Metastases are a result of circulating tumor cells (CTC) that detach from the primary cancer and settle down in distant organs. In the publicly available dataset GDS4329 CTC, haematological cells, original tumour, and non-tumoural pancreatic control tissue were isolated from PDAC patients. CTC showed high IMP2 expression, significantly increased compared to healthy pancreatic tissue as well as to haematological cells (Figure 2B), suggesting a role for IMP2 in metastasis of pancreatic tumors. IMP2 protein expression is linked to the occurrence of metastasis in esophageal cancer [3]. IMP2 was further described to be involved in tumor growth and metastasis in non-small cell lung cancer (NSCLC) and to be targeted by the tumor suppressive microRNA miR-485-5p [19]. Png and colleagues reported that IMP2 is secreted from metastatic cells and recruits endothelial cells during metastasis [20] underlining the role of IMP2 in tumor progression.
2.3. IMP2 Protein Is Overexpressed in PDAC Tissue Compared to Healthy Tissue and Associated with Lower Rate of One-Year Survival
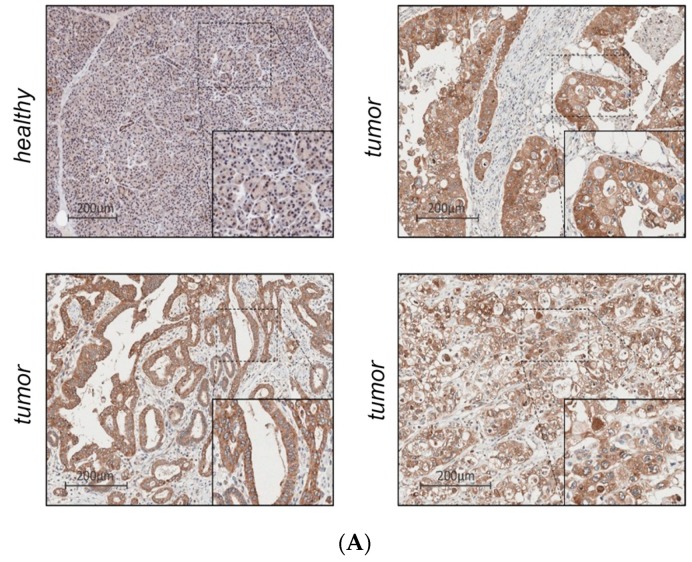
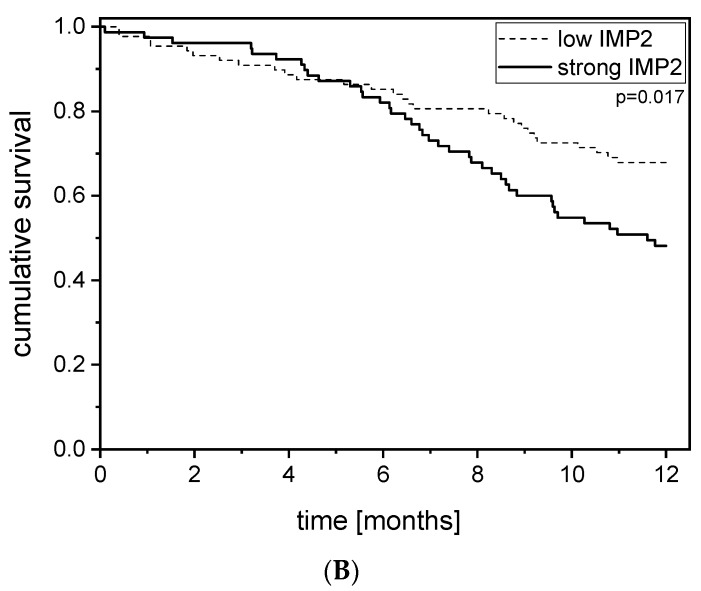
Since increased protein levels are crucial for the usage of IMP2 as a biomarker, tissue microarrays of a PDAC sample collection from 210 PDAC patients in total were analyzed by immunohistochemistry. IMP2 was significantly overexpressed in tumor tissue (p = 0.26 × 10−4; Figure 3A). In healthy tissues IMP2 immunoreactivity was found in 91% of samples. (score 0: 9%; score 1: 55%; score 2: 27%; score 3: 9%). All tumor tissues (n = 204) were positive for IMP2: score 1: 7.4%; score 2: 40%; score 2/3: 2.9%; score 3: 49.5%). Kaplan-Meier analysis showed no effect of IMP2 staining intensity on overall survival, but strong IMP2 expression (score3) was linked to lower rate of one-year survival (Figure 3B). This is in accordance to findings in several other malignancies, in which a subgroup of tumors with highest IMP2 expression is linked to short survival [2,3,6,21,22,23].
Figure 3.
IMP2 protein is linked to poor one-year survival. (A) Tissue microarrays of PDAC sample collection from n = 210 PDAC patients (tumor tissue: n = 210 healthy tissue: n = 11) were analyzed by immunohistochemistry. IMP2 was significantly overexpressed in tumor tissue (p = 0.26 × 10−4). In healthy tissues IMP2 immunoreactivity was found in 91% of samples (score 0: 9%; score 1: 55%; score 2: 27%; score 3: 9%). All tumor tissues (n = 204) were positive for IMP2 expression: score 1: 7.4%; score 2: 40%; score 2/3: 2.9%; score 3: 49.5%); (B) Kaplan-Meier analysis of one-year survival of patients with strong IMP2 staining (score 3) versus low IMP2 staining in pancreatic tumor tissues.
In conclusion, IMP2 is frequently overexpressed in PDAC and significantly associated with poor prognosis. IMP2 seems to promote tumor progression of PDAC. Thus, it might be an interesting prognostic marker as well as a novel target for the treatment of PDAC.
3. Materials and Methods
3.1. Analysis of Human Gene Omnibus (GEO) Datasets
Preprocessed and normalized data from the RNA microarray GEO datasets GSE28735 [24,25], GSE43288 [26], and GDS4329 [27] were analyzed. In GSE28735 differential gene expression was analyzed between PDAC and non-tumor tissues (n = 45 each). Pearson correlation was applied to detect possible co-expressions between genes of interest and other genes in the dataset (threshold: R2 ≥ 0.75 or ≤−0.75, respectively).
3.2. Tissue Microarray and Immunohistochemistry
Formalin-fixed, paraffin-embedded pancreatic tissue samples and the corresponding clinical data were provided by the Biobank Graz under the permission of the ethics commission (Ethikkommission Medizinische Universität Graz, 12/2013, EK number 25-259 ex 12/13). A total of 200 patients (operated between 1991 and 2005) with a median age of 64 (range 31–81) years were retrospectively evaluated. The series included 184 ductal, 5 glandular, 3 intraductal papillary mucinous neoplasms (IPMN), and 2 endocrine tumors. For 25 patients survival data were missing. Immunohistochemical stainings against IMP2 were performed as previously described [23] using the Dako Envision AEC Kit (#K4009, Dako, Germany) for antibody detection according to the manufacturer’s instructions. TMAs contained three tissue spots per tumor. Stainings were evaluated for cytoplasmatic intensity by two independent, blinded investigators. Intensity was scored using the following scoring system: score 0 = no staining, score 1 = weak staining, score 2 = moderate staining, score 3 = strong staining. If the replicates of the same tumor differed in staining intensity median score was used for further analysis.
3.3. Statistical Analysis
Data analysis and statistics of experimental data were performed using either R software or OriginPro software (Origin 2019; OriginLabs). Differential expression analysis was based on the Kolmogorov–Smirnov test. Pearson correlation was applied to detect correlations between genes of interest. All tests are two-sided, and differences were considered statistically significant when p-values were less than 0.05. Data are shown as mean values ± SD (if not indicated differently), or as individual values and boxplots ± interquartile range with median. Depending on normal distribution, which was tested by the Shapiro-Wilk method, statistical differences were estimated by independent two-sample t-test or ANOVA (for multiple groups) combined with Tukey post hoc test, or Kruskal-Wallis-ANOVA respectively.
Abbreviations
| IGF2 | Insulin-like growth factor 2 |
| IGF2BP2/IMP2 | IGF2 mRNA binding protein IMP2 |
| NSCLC | Non-small cell lung cancer |
| PDAC | Pancreatic ductal adenocarcinoma |
| CTC | Circulating tumor cells |
Author Contributions
Conceptualization, S.M.K. and J.H.; software, A.B.; formal analysis, C.D., A.B., P.P., J.H., and S.M.K.; investigation, C.D., A.B., P.P., J.H., and S.M.K.; writing—original draft preparation, C.D., J.H., S.M.K.; writing—review and editing, A.H., P.P., F.K.; visualization, C.D., A.B., S.M.K.; supervision, S.M.K. and J.H.; project administration, S.M.K.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Rawla P., Sunkara T., Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019;10:10–27. doi: 10.14740/wjon1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barghash A., Helms V., Kessler S.M. Overexpression of IGF2 mRNA-Binding Protein 2 (IMP2/p62) as a feature of basal-like breast cancer correlates with short survival. Scand. J. Immunol. 2015;82:142–143. doi: 10.1111/sji.12307. [DOI] [PubMed] [Google Scholar]
- 3.Barghash A.G.-S.N., Helms V., Haybaeck J., Kessler S.M. Elevated expression of the IGF2 mRNA binding protein 2 (IGF2BP2/IMP2) is linked to short survival and metastasis in esophageal adenocarcinoma. Oncotarget. 2016;7:49743–49750. doi: 10.18632/oncotarget.10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell J.L., Wächter K., Mühleck B., Pazaitis N., Köhn M., Lederer M., Hüttelmaier S. Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs): Post-transcriptional drivers of cancer progression? Cell. Mol. Life Sci. 2013;70:2657–2675. doi: 10.1007/s00018-012-1186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai N., Ji F., Wright J., Minichiello L., Sadreyev R., Avruch J. IGF2 mRNA binding protein-2 is a tumor promoter that drives cancer proliferation through its client mRNAs IGF2 and HMGA1. Elife. 2017;6:e27155. doi: 10.7554/eLife.27155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janiszewska M., Suvà M.L., Riggi N., Houtkooper R.H., Auwerx J., Clément-Schatlo V., Radovanovic I., Rheinbay E., Provero P., Stamenkovic I. Imp2 controls oxidative phosphorylation and is crucial for preservin glioblastoma cancer stem cells. Genes Dev. 2012;26:1926–1944. doi: 10.1101/gad.188292.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kessler S.M., Laggai S., Barghash A., Schultheiss C.S., Lederer E., Artl M., Helms V., Haybaeck J., Kiemer A.K. IMP2/p62 induces genomic instability and an aggressive hepatocellular carcinoma phenotype. Cell Death Dis. 2015;6:e1894. doi: 10.1038/cddis.2015.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müeller-Pillasch F., Lacher U., Wallrapp C., Micha A., Zimmerhackl F., Hameister H., Varga G., Friess H., Büchler M., Beger H.G., et al. Cloning of a gene highly overexpressed in cancer coding for a novel KH-domain containing protein. Oncogene. 1997;14:2729. doi: 10.1038/sj.onc.1201110. [DOI] [PubMed] [Google Scholar]
- 9.Morimatsu K., Aishima S., Yamamoto H., Hayashi A., Nakata K., Oda Y., Shindo K., Fujino M., Tanaka M., Oda Y. Insulin-like growth factor II messenger RNA–binding protein-3 is a valuable diagnostic and prognostic marker of intraductal papillary mucinous neoplasm. Hum. Pathol. 2013;44:1714–1721. doi: 10.1016/j.humpath.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Schaeffer D.F., Owen D.R., Lim H.J., Buczkowski A.K., Chung S.W., Scudamore C.H., Huntsman D.G., Ng S.S.W., Owen D.A. Insulin-like growth factor 2 mRNA binding protein 3 (IGF2BP3) overexpression in pancreatic ductal adenocarcinoma correlates with poor survival. BMC Cancer. 2010;10:59. doi: 10.1186/1471-2407-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wachter D.L., Schlabrakowski A., Hoegel J., Kristiansen G., Hartmann A., Riener M.O. Diagnostic value of immunohistochemical IMP3 expression in core needle biopsies of pancreatic ductal adenocarcinoma. Am. J. Surg. Pathol. 2011;35:873–877. doi: 10.1097/PAS.0b013e3182189223. [DOI] [PubMed] [Google Scholar]
- 12.Zhao H., Mandich D., Cartun R.W., Ligato S. Expression of K homology domain Containing protein Overexpressed in cancer in pancreatic FNA for diagnosing adenocarcinoma of pancreas. Diagn. Cytopathol. 2007;35:700–704. doi: 10.1002/dc.20739. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld Y.B.-Z., Krumbein M., Yeffet A., Schiffmann N., Mishalian I., Pikarsky E., Oberman F., Fridlender Z., Yisraeli J.K. VICKZ1 enhances tumor progression and metastasis in lung adenocarcinomas in mice. Oncogene. 2019 doi: 10.1038/s41388-019-0715-8. [DOI] [PubMed] [Google Scholar]
- 14.Simon Y., Kessler S.M., Bohle R.M., Haybaeck J., Kiemer A.K. The insulin-like growth factor 2 (IGF2) mRNA-binding protein p62/IGF2BP2-2 as a promoter of NAFLD and HCC? Gut. 2014;63:861–863. doi: 10.1136/gutjnl-2013-305736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yantiss R.K., Woda B.A., Fanger G.R., Kalos M., Whalen G.F., Tada H., Andersen D.K., Rock K.L., Dresser K. KOC (K homology domain containing protein overexpressed in cancer): A novel molecular marker that distinguishes between benign and malignant lesions of the pancreas. Am. J. Surg. Pathol. 2005;29:188–195. doi: 10.1097/01.pas.0000149688.98333.54. [DOI] [PubMed] [Google Scholar]
- 16.Sano M., Driscoll D.R., DeJesus-Monge W.E., Quattrochi B., Appleman V.A., Ou J., Zhu L.J., Yoshida N., Yamazaki S., Takayama T., et al. Activation of WNT/β-Catenin Signaling Enhances Pancreatic Cancer Development and the Malignant Potential Via Up-regulation of Cyr61. Neoplasia. 2016;18:785–794. doi: 10.1016/j.neo.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y., Morris J.P., Yan W., Schofield H.K., Gurney A., Simeone D.M., Millar S.E., Hoey T., Hebrok M., Pasca di Magliano M. Canonical Wnt Signaling Is Required for Pancreatic Carcinogenesis. Cancer Res. 2013;73:4909–4922. doi: 10.1158/0008-5472.CAN-12-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mu Q., Wang L., Yu F., Gao H., Lei T., Li P., Liu P., Zheng X., Hu X., Chen Y., et al. Imp2 regulates GBM progression by activating IGF2/PI3K/Akt pathway. Cancer Biol. Ther. 2015;16:623–633. doi: 10.1080/15384047.2015.1019185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang R.-S., Zheng Y.-L., Li C., Ding C., Xu C., Zhao J. MicroRNA-485-5p suppresses growth and metastasis in non-small cell lung cancer cells by targeting IGF2BP2. Life Sci. 2018;199:104–111. doi: 10.1016/j.lfs.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Png K.J., Halberg N., Yoshida M., Tavazoie S.F. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature. 2011;481:190. doi: 10.1038/nature10661. [DOI] [PubMed] [Google Scholar]
- 21.Kessler S.M., Pokorny J., Zimmer V., Laggai S., Lammert F., Bohle R.M., Kiemer A.K. IGF2 mRNA binding protein p62/IMP2-2 in hepatocellular carcinoma: Antiapoptotic action is independent of IGF2/PI3K signaling. Am. J. Physiol Gastrointest Liver Physiol. 2013;304:G328–G336. doi: 10.1152/ajpgi.00005.2012. [DOI] [PubMed] [Google Scholar]
- 22.He X., Li W., Liang X., Zhu X., Zhang L., Huang Y., Yu T., Li S., Chen Z. IGF2BP2 Overexpression Indicates Poor Survival in Patients with Acute Myelocytic Leukemia. Cell. Physiol. Biochem. 2018;51:1945–1956. doi: 10.1159/000495719. [DOI] [PubMed] [Google Scholar]
- 23.Kessler S.M., Lederer E., Laggai S., Golob-Schwarzl N., Hosseini K., Petzold J., Schweiger C., Reihs R., Keil M., Hoffmann J., et al. IMP2/IGF2BP2 expression, but not IMP1 and IMP3, predicts poor outcome in patients and high tumor growth rate in xenograft models of gallbladder cancer. Oncotarget. 2017;8:89736–89745. doi: 10.18632/oncotarget.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang G., He P., Tan H., Budhu A., Gaedcke J., Ghadimi B.M., Ried T., Yfantis H.G., Lee D.H., Maitra A., et al. Integration of metabolomics and transcriptomics revealed a fatty acid network exerting growth inhibitory effects in human pancreatic cancer. Clin. Cancer Res. 2013;19:4983–4993. doi: 10.1158/1078-0432.CCR-13-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang G., Schetter A., He P., Funamizu N., Gaedcke J., Ghadimi B.M., Ried T., Hassan R., Yfantis H.G., Lee D.H., et al. DPEP1 inhibits tumor cell invasiveness, enhances chemosensitivity and predicts clinical outcome in pancreatic ductal adenocarcinoma. PLoS ONE. 2012;7:e31507. doi: 10.1371/journal.pone.0031507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crnogorac-Jurcevic T., Chelala C., Barry S., Harada T., Bhakta V., Lattimore S., Jurcevic S., Bronner M., Lemoine N.R., Brentnall T.A. Molecular analysis of precursor lesions in familial pancreatic cancer. PLoS ONE. 2013;8:e54830. doi: 10.1371/journal.pone.0054830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sergeant G., van Eijsden R., Roskams T., Van Duppen V., Topal B. Pancreatic cancer circulating tumour cells express a cell motility gene signature that predicts survival after surgery. BMC Cancer. 2012;12:527. doi: 10.1186/1471-2407-12-527. [DOI] [PMC free article] [PubMed] [Google Scholar]