Abstract
Aims: Productivity losses related to premature cancer mortality have been assessed for most developed countries but results for Russia are limited to cross-sectional reports. The aim of this study was to quantify productivity costs due to cancer mortality in Russia between 2001 and 2015 and project this to 2030. Methods: Cancer mortality data (2001–2015) were acquired from the State Cancer Registry, whereas population data, labour force participation rates and annual earnings were retrieved from the Federal State Statistics Service. Cancer mortality was projected to 2030 and the human capital approach was applied to estimate productivity losses. Results: The total annual losses increased from US6.5b in 2001–2005 to US$8.1b in 2011–2015, corresponding to 0.24% of the annual gross domestic product. The value is expected to remain high in 2030 (US$7.5b, 0.14% of gross domestic product). Productivity losses per cancer death are predicted to grow faster in women (from US$18,622 to US$22,386) than in men (from US$25,064 to US$28,459). Total losses were found to be highest for breast cancer in women (US$0.6b, 20% of overall losses in women) and lung cancer in men (US$1.2b, 24%). The absolute predicted change of annual losses between 2011–2015 and 2026–2030 was greatest for cervix uteri (+US$214m) in women and for lip, oral and pharyngeal cancers in men (+US$182m). Conclusions: In Russia, productivity losses due to premature cancer mortality are substantial. Given the expected importance especially for potentially preventable cancers, steps to implement effective evidence-based national cancer control policies are urgently required.
Keywords: Cancer, premature mortality, productivity losses, Russia
Background
Cancer is the second leading cause of death in Russia [1]. While information on incidence reflects the causes of cancer, data for mortality capture combined information on relative success of primary and secondary prevention as well as on cancer management. Such data can be applied to illustrate societal burden, including economic impact. This latter can be separated into two components, namely direct healthcare expenses and indirect costs associated with lost contribution to the economy due to absence from work, known as lost productivity. Though often dismissed, lost productivity through premature cancer mortality of the working population may exceed treatment expenses and contribute substantially to the overall cost of cancer to societies [2].
Productivity losses related to premature cancer mortality have been assessed for most developed countries [3 –10]. However, large-scale assessment of productivity losses has never been conducted in Russia and given recent efforts of the Ministry of Healthcare to implement cancer control strategies, such information is very necessary to guide design of national cancer control plans [11].
Aims
The aim of this study was to quantify this aspect of cancer burden by estimating years of life lost (YLL) and thereby calculate productivity losses due to premature cancer mortality in Russia in 2001–2015 and project this up to 2030.
Methods
General approach
We measured the YLL and the societal burden of cancer in Russia by assessing the impact of premature cancer mortality on productivity losses. Overall, YLL were calculated for six calendar periods (2001–2005, 2006–2010, …, and 2026–2030) by sex and 18 age groups for the following cancer sites, classified using ICD-10 (the International Statistical Classification of Diseases and Related Health Problems): lip, oral cavity and pharynx (C00–14), oesophagus (C15), stomach (C16), colorectum (C18–21), liver (C22), pancreas (C25), larynx (C32), trachea and lung (C33–34), bone (C40), skin (melanoma) (C43), soft tissues (C46.1,3,7–9,47,49), female breast (C50), cervix uteri (C53), corpus uteri (C54), ovaries (C56), prostate (C61), kidney (C64), bladder (C67), brain and central nervous system (C70–72, CNS), hematopoietic and lymphoid malignancies (C81–96) and all cancers combined including non-melanoma skin cancers (C00–96). The ‘other’ category included both cancer sites not mentioned earlier and unspecified cancer deaths. This was calculated as the difference between the number of all cancer deaths combined and the number of deaths from cancer-specific sites mentioned above. To estimate productivity losses due to cancer-related premature mortality in Russia in the period between 2001 and 2015 and projected until 2030 we applied an incidence-based method using the human capital approach (HCA).
Cancer mortality and population data
Age- and sex-specific cancer mortality data between 2001 and 2015 were acquired from the State Cancer Registry (SCR) based in the Herzen Research Institute of Oncology in Moscow [12]. A system of obligatory registration and lifetime follow-up of cancer patients was established in the USSR in 1953. Mortality data are collected by civil registration services and aggregated by the Federal State Statistics Service (FSSS). Population data, based on population censuses of 1989, 2002, 2010 and average population projections until 2031 were retrieved from the FSSS [13]. Age-standardized rates (ASRs) of cancer incidence and mortality per 100,000 person-years were calculated using the modified world standard population proposed by Segi [14]. In order to estimate trends over time log-transformed ASRs were calculated. To predict cancer mortality rates, we applied the Norpred prediction tool with a linear trend [15]. We derived age-specific YLL for each 5-year period calculated as a function of cancer deaths and life expectancy. Cause-deleted life-tables by period were generated based on data from the Human Mortality Database and forecasts with a functional demographic model [16, 17]. Methods and limitation of the analysis for data from the SCR were previously described [18].
All estimations were performed in open-source statistical software R (version 3.3.3, 2017-03-06)), using packages «nordpred» (accessed on 15 May 2016)[19] and «demography» (version 1.18) [20].
Economic data
The age- and sex-specific economic data were obtained from the FSSS: (a) labour-force participation rates (2001–2014); (b) averaged annual earnings (biennial, between 2002 and 2014); and (c) inflation rates (2001–2016). These data also included information on retirement and labour-force participation after retirement age (official retirement ages are 60 for men and 55 for women). Earnings were converted from Russian rubles to 2016 US dollars after adjustment for inflation based on yearly average currency exchange rates. We applied natural splines to interpolate employment rate integral and mean wages in 2001 and 2015, and also to project employment rates. For sensitivity analysis, wage growth was estimated based on two gross domestic product (GDP) scenarios: (a) GDP growth for 2016, 0.3%; 2018, 2.7%; 2022, 3.9%; 2024, 4.3%; 2027, 4.1%; 2030, 4%; and 2035, 3.8%, were used for base-case calculations, and (b) where GDP growth for 2016, 0.3%; 2018, 2.0%; 2022, 2.4%; 2024, 2.4%; 2027, 2.2%; 2030, 1.9%; and 2035, 1.7%, were used for sensitivity analysis.
Average age-specific wages weighted by age-specific labour force participation were then calculated. An annual discount rate of 2.5% was also applied for base-case calculations [21]. For sensitivity analysis, we additionally applied discount rates of 0% and 5%. GDP based on purchasing power parity in 2011 international US dollars was acquired from the World Bank Database along with a deflator in order to transform the GPD to 2016 US dollars. In the base-case calculations, any earnings from work force participation were discontinued at the age of 70 as currently reported by FSSS. However, in the sensitivity analysis we also explored additional scenarios: where earnings were discontinued at an earlier age (65 and 55) and where earnings were not discontinued but projected to allow workforce participation until the end of life.
Estimation of lost productivity
The HCA assumes that economic output of an individual equates to wage rate, so that premature death, by cutting short the working life, produces economic losses to society equal to the lost earnings. To calculate productivity losses, we used age- and period-specific death and economic data. All results were expressed in 2016 US dollars. First (2001–2005), most recent (2011–2015) and last projected (2026–2030) periods for the base-case scenario were reported in Table I.
Table I.
Annual productivity losses due to cancer mortality, productivity losses per death (US$) and % of gross domestic product (GDP) adjusted for purchasing power parity by cancer, sex and period in Russia.
| Cancer type (ICD-10) | Sex | Productivity losses 2016 US$m |
Productivity losses per death, 2016 US$ |
% of GDP |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2001–2005 | 2011–2015 | 2026–2030 | 2001–2005 | 2011–2015 | 2026–2030 | 2001–2005 | 2011–2015 | 2026–2030 | ||
| Lip, oral cavity, pharynx (C00–14) | Women | 27.0 | 50.5a | 68.0 | 13,688 | 25,731 | 35,013 | 0.0011 | 0.0015 | 0.0013 |
| Men | 222.5 | 322.8 | 405.3 | 26,251 | 38,871 | 49,033 | 0.0094 | 0.0094 | 0.0077 | |
| Oesophagus (C15) | Women | 11.8 | 21.3 | 28.0 | 7734 | 13,672 | 18,155 | 0.0005 | 0.0006 | 0.0005 |
| Men | 113.8 | 170.7 | 187.6 | 19,878 | 30,094 | 33,229 | 0.0048 | 0.005 | 0.0036 | |
| Stomach (C16) | Women | 239.7 | 237.9 | 161.6 | 19,265 | 18,961 | 12,791 | 0.0102 | 0.0069 | 0.0031 |
| Men | 518.5 | 522.8 | 362.4 | 31,149 | 30,640 | 21,094 | 0.022 | 0.0153 | 0.0069 | |
| Colorect al (C18–21) |
Women | 217.4 | 314.3 | 267.1 | 10,151 | 14,803 | 12,561 | 0.0092 | 0.0092 | 0.0051 |
| Men | 257.8 | 402.7 | 361.5 | 13,971 | 22,124 | 19,972 | 0.0109 | 0.0117 | 0.0069 | |
| Liver (C22) | Women | 53.2 | 70.1 | 73.9 | 12,798 | 17,047 | 18,029 | 0.0023 | 0.002 | 0.0014 |
| Men | 113.3 | 165 | 180.4 | 20,669 | 30,440 | 33,737 | 0.0048 | 0.0048 | 0.0034 | |
| Pancreas (C25) | Women | 71.9 | 122.9 | 115.9 | 8430 | 14,812 | 14,019 | 0.003 | 0.0036 | 0.0022 |
| Men | 196.3 | 281.2 | 267.2 | 21,884 | 31,879 | 30,722 | 0.0083 | 0.0082 | 0.0051 | |
| Larynx (C32) | Women | 4.8 | 7.9 | 13.1 | 15,885 | 26,697 | 44,887 | 0.0002 | 0.0002 | 0.0069 |
| Men | 132.0 | 134.2 | 109.6 | 35,684 | 35,110 | 28,759 | 0.0056 | 0.0039 | 0.0021 | |
| Lung (C33, 34) | Women | 115.8 | 174.4 | 177.0 | 12,273 | 18,589 | 18,938 | 0.0049 | 0.0051 | 0.0034 |
| Men | 952.3 | 1206.5 | 968.0 | 23,259 | 29,094 | 23,378 | 0.0403 | 0.0352 | 0.0184 | |
| Bone (C40, 41) | Women | 34.1 | 26.9 | 28.0 | 66,610 | 52,273 | 53,315 | 0.0014 | 0.0008 | 0.0005 |
| Men | 70.8 | 61.6 | 58.9 | 107,636 | 93,561 | 87,423 | 0.003 | 0.0018 | 0.0011 | |
| Melanoma of skin (C43) | Women | 47.8 | 62.9 | 60.2 | 25,092 | 33,306 | 31,854 | 0.002 | 0.0018 | 0.0011 |
| Men | 61.9 | 83.3 | 81.9 | 37,198 | 51,050 | 50,186 | 0.0026 | 0.0024 | 0.0016 | |
| Soft tissues (C46.1,3,7–9,47,49) | Women | 45.5 | 57.2 | 60.1 | 27,431 | 34,579 | 36,196 | 0.0019 | 0.0017 | 0.0011 |
| Men | 70.9 | 91.5 | 99.0 | 45,857 | 59,415 | 64,514 | 0.003 | 0.0027 | 0.0019 | |
| Breast (C50) | Women | 558.9 | 642.6 | 522.4 | 24,756 | 28,668 | 23,153 | 0.0237 | 0.0187 | 0.0099 |
| Cervix uteri (C53) | Women | 232.7 | 342.6 | 446.8 | 34,240 | 50,601 | 65,569 | 0.0099 | 0.01 | 0.0085 |
| Corpus uteri (C54) | Women | 99.0 | 145 | 137.9 | 14,426 | 21,358 | 20,272 | 0.0042 | 0.0042 | 0.0026 |
| Ovary (C56) | Women | 179.7 | 233.2 | 212.1 | 23,308 | 30,324 | 27,538 | 0.0076 | 0.0068 | 0.004 |
| Prostate (C61) | Men | 51.7 | 128.6 | 109.2 | 4332 | 12,151 | 10,589 | 0.0022 | 0.0038 | 0.0021 |
| Kidney (C64) | Women | 460 | 55.5 | 43.0 | 14,615 | 17,834 | 13,677 | 0.0019 | 0.0016 | 0.0008 |
| Men | 135.4 | 173.2 | 132.6 | 25,632 | 33,180 | 25,398 | 0.0057 | 0.0051 | 0.0025 | |
| Bladder (C67) | Women | 8.7 | 13.8 | 16.2 | 6150 | 9693 | 11,432 | 0.0004 | 0.0004 | 0.0003 |
| Men | 66.6 | 88.8 | 64.3 | 13,183 | 17,197 | 12,404 | 0.0028 | 0.0026 | 0.0012 | |
| Brain, central nervous system (C70–72) | Women | 140.0 | 165.7 | 186.3 | 37,720 | 45,747 | 52,049 | 0.0059 | 0.0048 | 0.0035 |
| Men | 217.2 | 264.8 | 296.0 | 58,855 | 73,167 | 82,157 | 0.0092 | 0.0077 | 0.0056 | |
| Hematopoietic, lymphoid (C81–96) | Women | 240.2 | 239 | 228.1 | 32,634 | 32,614 | 31,137 | 0.0102 | 0.007 | 0.0043 |
| Men | 389.1 | 412.7 | 420.0 | 52,064 | 55,265 | 56,467 | 0.0165 | 0.012 | 0.008 | |
| Othersb | Women | 183.3 | 232.1 | 239.0 | 14,194 | 18,148 | 18,722 | 0.0078 | 0.0068 | 0.0045 |
| Men | 341.3 | 426.3 | 408.7 | 28,867 | 36,447 | 35,031 | 0.0145 | 0.0124 | 0.0078 | |
| All cancer types combined (C00–96) | Women | 2,547.8 | 3,204.4 | 3,053.4 | 18,622 | 23,492 | 22,386 | 0.1079 | 0.0935 | 0.0581 |
| Men | 3,969.4 | 4,926.6 | 4,496.5 | 25,064 | 31,070 | 28,459 | 0.1681 | 0.1437 | 0.0855 | |
| Overall (sexes combined) | - | 6,517.2 | 8,131.0 | 7,549.9 | - | - | - | 0.276 | 0.2372 | 0.1436 |
Red cells represent increased losses compared with the previous period, green cells – reduced, grey – stable.
Other cancer types includes all other cancers not specified above.
Ethical approval
This study utilized publicly available secondary aggregate data, and thus did not require, according to the current legislature, ethical approval.
Results
Trends in age-standardized mortality rates per 100,000 and YLL from cancer
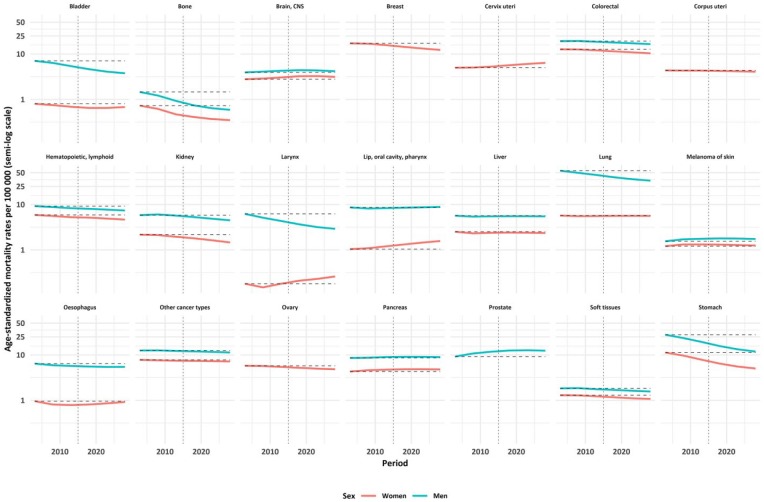
Mortality for most cancer types was going down during the study period (Figure 1; Supplementary material Table S1). There was an upward trend between 2001 and 2015 for melanoma, pancreas, brain and CNS cancer mortality in both men and women, for lip, oral and pharynx, larynx and cervix uteri cancer mortality in women, and for prostate cancer mortality in men. Overall YLL increased for most cancer sites, except larynx in men, and stomach and bone in both men and women. The overall annual YLL due to premature cancer mortality in men increased from 11 million years in 2001–2005 to 12 million years in 2011–2015. It was predicted to further increase to 14 million years in 2026–2030.
Figure 1.
Age-standardized mortality rates per 100,000 (presented on a semi-log scale) according to cancer types and sex between 2001 and 2030 in Russia (dash–dot line separates recent and future predicted rates).
Taking into account both relative and absolute changes in the overall YLL between 2001 and 2015, cervix uteri, pancreas and colorectal cancer showed the largest increases. Lip, oral and pharynx cancer showed the largest relative change, while cervix uteri cancer exhibited the largest absolute growth in women. Prostate cancer showed the highest absolute and relative increase in YLL in men, with major increases also in lip, oral and pharynx, colorectal and pancreas cancer in men in both relative and absolute terms (Supplementary material Figure S1).
Overall productivity losses and losses per one death
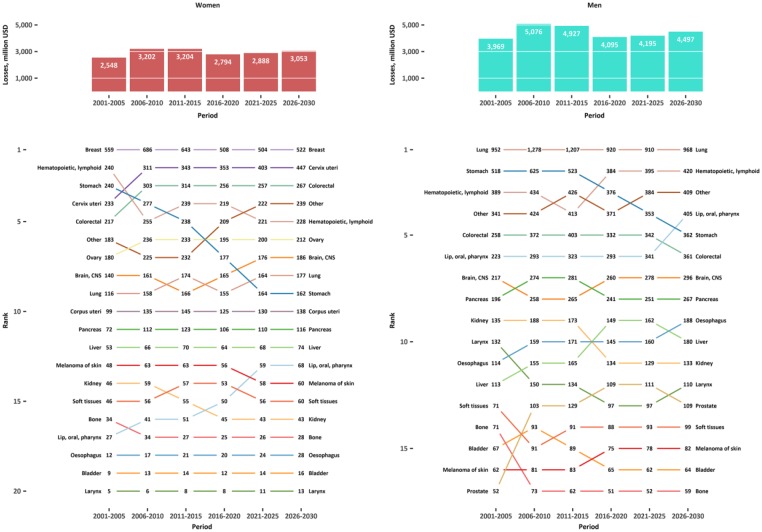
Annual productivity losses due to cancer mortality in Russia for the reported cancer types are presented in Table I. The annual overall productivity losses due to cancer mortality were reaching a peak of US$8.3b in 2006–2010 (US$3.2b in women and US$5.1b in men) (Figure 2).
Figure 2.
Overall observed and predicted average annual productivity losses due to premature cancer mortality in Russia in 2001–2030 and ranking according to cancer sites and sex.
Productivity losses per cancer death were higher in men (Table I). Yet, productivity losses per cancer death in women are predicted to grow faster than in men, from UD$18,622 in 2001–2005 to US$22,386 in 2026–2030. Productivity losses per cancer death were highest for bone, brain and CNS, cervix uteri, soft tissues cancer hematopoietic and lymphoid malignancies and melanoma of the skin (Supplementary material Figure S2).
In total, the estimated productivity losses decreased from 0.28% of GDP in 2001–2005 to 0.24% in 2011–2015 and are predicted to further decrease to 0.14% in 2026–2030. While this decline was seen for most cancer sites increases were found for lip, oral and pharyngeal, pancreatic, lung, cervix uteri in women; prostate in men; and oesophagus cancer in men and women.
Productivity losses by cancer type
Annual overall productivity losses were highest for breast cancer in women (US$0.6b or 20% of all losses in 2011–2015) and lung cancer in men ($1.2b or 24% in 2011–2015), which remain the highest-ranking cancers in the next two decades of the study period (Figure 2).
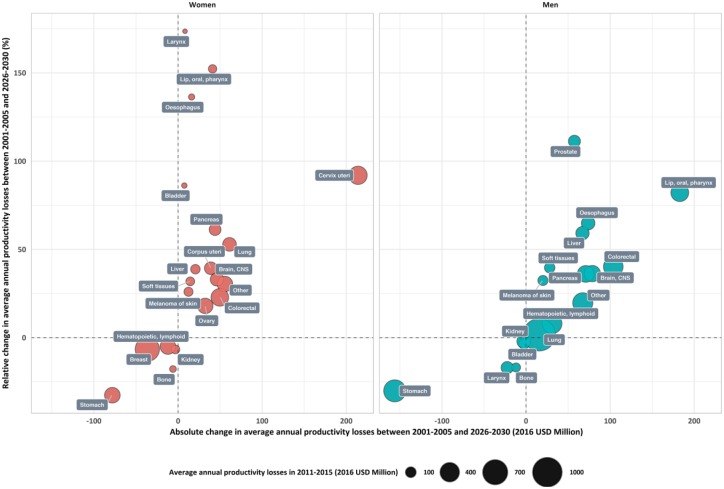
Relative change in productivity losses between 2011–2015 and 2026–2030 in women was highest for the larynx (174%), lip, oral and pharynx (152%), oesophagus (136%) and cervix uteri cancer (111%). During the same period, the absolute predicted change was highest for cervix uteri (+ US$214m) and lung cancer (+ US$61m) in women (Figure 3). In men, the relative change was highest for prostate cancer (111%), lip, oral and pharyngeal cancer (82%), oesophagus (64%), and liver cancer (69%), while the largest absolute increase was noted for lip, oral and pharyngeal (+ US$182m) and colorectal cancer (+ US$103m) (Figure 3).
Figure 3.
Change in in annual productivity losses due to premature cancer mortality in Russia between 2001–2005 and 2026–2030 according to cancer type and sex.
Sensitivity analyses
Changing the discount rate affected the overall amount of annual productivity losses but did not affect the relative distribution of losses by cancer types (Supplementary material Figures S3 and S4). Cutting the earnings at a certain age decreased the amount of productivity losses. In the scenario, where earlier age at earnings was discontinued, more productivity losses were assigned to cancer types affecting younger age groups (e.g. cervix uteri) and less productivity losses to those with deaths at older age groups (e.g. prostate) (Supplementary material Figures S5–S7). However, the major findings reported for the base-case scenario remained. The maximum overall annual productivity losses for 2011–2015 were reached without discontinuation of earnings with 0% discount and the base-case GDP growth scenario – US$4.03b in women and US$6.04b in men – while the lowest figures were obtained with discontinuation at the age 55 with a 5% discount and the second GDP growth scenario – US$1.52b in women and US$2.11b in men (Supplementary material Figures S9–S10).
Discussion
To our knowledge, this is the first analysis of trends and prediction of cancer mortality related productivity costs in Russia. A large overall cost of cancer death in Russia was found amounting to US$8.1b or 0.24% of GDP in 2011–2015. It is expected to remain high in 2030 (US$7.5b or 0.14% of GDP). The result also provides a wide perspective of major contributors to these losses, the greatest cause being lung cancer (US$1.2b or 24% of the total loss) in men and breast cancer (US$0.6b or 20%) in women in 2011–2015. Quantitative assessment of the rising losses linked to cancers known to be related to human papilloma virus (HPV) infection, revealed a figure for cervical cancer in women of US$233m in 2001, which is expected to almost double to US$447m in 2030. This study provides an economic appraisal for the Russian government to set priorities in cancer control activities, including primary prevention (e.g. tobacco control or HPV control) and population-based cancer screening (cervical, breast and colorectal).
Our findings can be compared with reports from other countries, which give similar relative estimates when costs are compared to GDP, taking into account differences in mortality and population size. In Europe, productivity losses due to cancer mortality in 2008 were 0.36% of total GDP ranging between 0.15 and 0.67% by country [22]. In the USA, productivity losses were higher: about 1.11% of GDP based on the 2000 estimates [3]. Cancer mortality related productivity costs in BRICS countries in 2008 were estimated as 0.21% GDP for Brazil, 0.25% for Russia, 0.34% GDP for China, 0.36% GDP for India and 0.49% for South Africa. Our results were consistent with these findings. [10].
We estimated an annual loss of US$8.1b due to premature deaths from cancer in Russia in 2011–2015. Extrapolating this estimate to total costs of cancer in Russia without high-quality data for direct medical costs related to cancers remains challenging. In a recent systematic review the proportion of cancer mortality related productivity losses was reported to be over 50% of the total cost [7]. However, in a large European study this proportion ranged from 24 to 54% for 27 countries [22, 23]. With some uncertainty, overall cancer costs in Russia can be estimated to be at least US$15b but probably more than US$20b per year based on extrapolation from our findings.
Productivity losses by cancer type
In general, we have observed a decline in the mortality from cancers in Russia with ASRs dropping between 2001 and 2015 from 94 to 87 per 100,000 in women (7%) and from 190 to 167 per 100,000 in men (12%), which reflected a decrease in the burden of cancer to the GDP from 0.27 to 0.24%. Some of this decrease can be related to the decline in smoking-related cancers in Russia as much of the productivity losses, particularly among men, are driven by smoking-related cancers such as lung cancer (24% of the total productivity loss in 2011–2015). The Russian Federation has been a Party to the WHO Framework Convention on Tobacco Control since 2008 and Federal Law on Health Protection from Exposure to Environmental Tobacco Smoke and the Consequences of Tobacco Consumption was adopted in 2013 – actions that clearly will contribute to further mortality decline. Yet, the smoking prevalence remains high among men in Russia (daily smoking for 51% of men in 2015) [24]. Unlike many reports in other European countries, costs associated with women lung cancer deaths were moderate, ranking eighth of all costs in women probably related to a relatively low daily smoking prevalence (i.e. 15% in 2015) [24]. A slight increase in rates of lung cancer has been observed in women and continued increase in lung-cancer rates reported in other countries caution for a similar rise in Russia. Considering these factors, additional smoking cessation counselling might be implemented as part of screening programmes [25].
On the other hand, we saw a substantial increase in the contribution of HPV-related cancers (cervix uteri, oral and pharynx) to future productivity losses in Russia. At the moment, HPV vaccination is not available as a nationwide programme and cervical cancer screening remains opportunistic for a select proportion of the population [26]. Rising productivity losses from oral and pharynx cancers in both sexes adds additional motivation to put forward population-based HPV control activities at the national level.
We also saw a rapid rise in productivity losses from oesophagus, liver and pancreas cancer mortality, all traditionally related to smoking and alcohol consumption, that can also be partly explained by the increasing obesity prevalence in Russia and other lifestyle risk factors [27]. In addition, growing incidence and substantial losses from colorectal cancer mortality as well as a large contribution of breast cancer to the economic cost underline the need to assess the major risk factors for these cancers and also the effectiveness of early detection and management in order to adapt existing national control policies.
A few limitations of the study should be noted. Quality of the data used in the analysis may affect the result, and as such the data input for this study needs to be considered when interpreting reported results. The ‘other cancer types’ category in our analysis consisted of two groups: the first including unspecified tumors (C76–C80) with around 4% out of the total reported deaths. The second group is other specific cancer types (around 3%), for which data were not available for the whole study period. The HCA is only one of several approaches to estimate societal burden of cancer. The friction cost approach (FCA) is an alternative. As such, losses based on the HCA calculations are considerably higher than calculated by FCA [28]. Yet, the HCA has become widely used and the methodology has become a standard to estimate productivity losses for calculating indirect non-medical costs due to mortality [23, 29]. This analysis includes only paid productivity losses, so other indirect and direct losses, like unpaid work such as caring for children or sick relatives, and volunteering work, as well as other payments, are not included. Additionally, our method does not consider productivity losses that occur due to illness and disability, and hence loss of income, related to cancer. Estimating individual losses is an optimal approach but it is rarely feasible and renders such results incomparable to other studies due to differences in data collection and availability [30]. Furthermore, projected losses need to be carefully interpreted due to uncertainty in the economic, population and cancer predictions.
The primary strength of our study is that we provide comprehensive analysis of the trends and changes in productivity losses over time while most similar studies report cross-sectional findings. That allowed us to capture how the changing cancer burden is affecting economic losses in Russia. We used combined economic and epidemiological data available for the whole period of the study. In the absence of the reliable individual-level data, results of our and similar studies must be used to approximate the overall cancer costs in Russia, but future research would benefit from having more detailed data to estimate the economic burden of cancer in Russia.
Conclusions
Productivity losses due to premature cancer mortality in Russia are substantial and amount to US$8b per year. The losses are expected to drop from 0.28% of GDP in 2001 to 0.14% in 2030 mostly due to a decline in the mortality from cancers in Russia. While the losses are highest for breast cancer in women and lung cancer in men, the relative growth in productivity losses was highest for HPV-related cancer mortality.
Supplemental Material
Supplemental material, Supplementary_material_Barchuk2 for Productivity losses associated with premature mortality due to cancer in Russia: A population-wide study covering 2001–2030 by Anton Barchuk, Alexander Bespalov, Heini Huhtala, Tuvshinjargal Chimed, Alexey Belyaev, Malcolm Moore, Ahti Anttila, Anssi Auvinen, Alison Pearce and Isabelle Soerjomataram in Scandinavian Journal of Public Health
Footnotes
Conflict of interest: None declared.
Funding: This work was funded by the Faculty of Social Sciences of the University of Tampere and the Finnish Cancer Registry grants for participants of the International Doctoral Programme in Epidemiology.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Anton Barchuk  https://orcid.org/0000-0002-4629-3326
https://orcid.org/0000-0002-4629-3326
References
- [1]. Kaprin AD, Starinskiy V, Petrova G. Zlokachestvennye novoobrazovaniya v Rossii v 2014 godu (zabolevaemost‘ i smertnost’) (Malignancies in Russia in 2014 (incidence and mortality)). Moscow: P. Herzen Research Institute of Oncology, nmicr.ru/upload/doc/2017/2016_zno_2014.pdf (2016). [Google Scholar]
- [2]. Krol M, Brouwer W, Rutten F. Productivity costs in economic evaluations: past, present, future. Pharmacoeconomics 2013; 31: 537–549. [DOI] [PubMed] [Google Scholar]
- [3]. Bradley CJ, Yabroff KR, Dahman B, et al. Productivity costs of cancer mortality in the United States: 2000–2020. J Natl Cancer Inst 2008; 100: 1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Ortiz-Ortiz KJ, Pérez-Irizarry J, Marín-Centeno H, et al. Productivity loss in Puerto Rico’s labor market due to cancer mortality. P R Health Sci J 2010; 29: 241–249. [PubMed] [Google Scholar]
- [5]. Tingstedt B, Andersson E, Flink A, et al. Pancreatic cancer, healthcare cost, and loss of productivity: a register-based approach. World J Surg 2011; 35: 2298–2305. [DOI] [PubMed] [Google Scholar]
- [6]. Binazzi A, Scarselli A, Marinaccio A. The burden of mortality with costs in productivity loss from occupational cancer in Italy. Am J Ind Med 2013; 56: 1272–1279. [DOI] [PubMed] [Google Scholar]
- [7]. Gol-Montserrat J, del Burgo MLM, Quecedo L, et al. Analysis of productivity costs in cancer: a systematic review. Grhta 2017;4:1–10. [Google Scholar]
- [8]. Cher BP, Chen C, Yoong J. Prevalence-based, disease-specific estimate of the social cost of smoking in Singapore. BMJ Open 2017; 7: e014377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Łyszczarz B, Nojszewska E. Productivity losses and public finance burden attributable to breast cancer in Poland, 2010–2014. BMC Cancer 2017; 17: 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Pearce A, Sharp L, Hanly P, et al. Productivity losses due to premature mortality from cancer in Brazil, Russia, India, China, and South Africa (BRICS): a population-based comparison. Cancer Epidemiol 2018; 53: 27–34. [DOI] [PubMed] [Google Scholar]
- [11]. The Ministry of Healthcare of the Russian Federation. Prikaz Minzdrava RF ot 03 fevralja 2015 g. N36an ’Ob utverzhdenii poryadka provedeniya dispanserizacii ohtdelennyh grupp vzroslogo naseleniya’. (The order of the Ministry of Healthcare of the Russian Federation #36an from 03 February 2015 ’On implementing the order of dispanserization of certain groups of adult population’.)gnicpm.ru/UserFiles/disp_prikaz_N36an.pdf. (2015).
- [12]. Petrova G, Kaprin AD, Gretsova O, et al. Zlokachestvennye novoobrazovaniya v Rossii (obzor statisticheskoi informacii za 1993–2013 gg.) (Malignancies in Russia. Statistical information review for the period 1993–2013.) Moscow: P. Hertzen Research Institute of Oncology, 2015. [Google Scholar]
- [13]. Federal State Statistics Service. Demografia: Federalnaya sluzhba gosudarstvennoi statistiki. (Demography: Federal State Statistics Service.), www.gks.ru/wps/wcm/connect/rosstat_main/rosstat/ru/statistics/population/demography/.
- [14]. Doll R, Cook P. Summarizing indices for comparison of cancer incidence data. Int J Cancer 1967; 2: 269–279. [DOI] [PubMed] [Google Scholar]
- [15]. Møller B, Fekjær H, Hakulinen T, et al. Prediction of cancer incidence in the Nordic countries: empirical comparison of different approaches. Stat Med 2003; 22: 2751–2766. [DOI] [PubMed] [Google Scholar]
- [16]. University of California, Berkeley USA, Max Planck Institute for Demographic Research Germany. Human Mortality Database, www.mortality.org
- [17]. Hyndman RJ, Shahid Ullah M. Robust forecasting of mortality and fertility rates: A functional data approach. Comput Statist Data Anal 2007; 51: 4942–4956. [Google Scholar]
- [18]. Barchuk A, Bespalov A, Huhtala H, et al. Breast and cervical cancer incidence and mortality trends in Russia 1980–2013. Cancer Epidemiol 2018; 55: 73–80. [DOI] [PubMed] [Google Scholar]
- [19]. Fekjær H, Møller B. Nordpred R package. kreftregisteret.no, www.kreftregisteret.no/en/Research/Projects/Nordpred/Nordpred-software/
- [20]. Hyndman RJ, Booth H, Tickle L, et al. Demography: Forecasting mortality, fertility, migration and population data. R package version 2011; 1–09. [Google Scholar]
- [21]. Ghauri K. Report on analysis of economic losses due to iron and folic acid deficiencies in Kazakhstan. The Global Alliance for Improved Nutrition (GAIN), www.gainhealth.org/wp-content/uploads/2017/06/Analysis-of-Economic-Losses-in-Kazakhstan.pdf (2017). [Google Scholar]
- [22]. Luengo-Fernandez R, Leal J, Gray A, et al. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol 2013; 14: 1165–1174. [DOI] [PubMed] [Google Scholar]
- [23]. Östensson E, Silfverschiöld M, Greiff L, et al. The economic burden of human papillomavirus-related precancers and cancers in Sweden. PLoS ONE 2017; 12: e0179520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Bilano V, Gilmour S, Moffiet T, et al. Global trends and projections for tobacco use, 1990–2025: an analysis of smoking indicators from the WHO Comprehensive Information Systems for Tobacco Control. The Lancet 2015; 385: 966–976. [DOI] [PubMed] [Google Scholar]
- [25]. Gorini G, Carreras G, Giordano L, et al. The Pap smear screening as an occasion for smoking cessation and physical activity counselling: effectiveness of the SPRINT randomized controlled trial. BMC Public Health 2012; 12: 805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Basu P, Ponti A, Anttila A, et al. Status of implementation and organization of cancer screening in The European Union Member States-Summary results from the second European screening report. Int J Cancer 2018; 142: 44–56. [DOI] [PubMed] [Google Scholar]
- [27]. Rtveladze K, Marsh T, Webber L, et al. Obesity trends in Russia. The impact on health and healthcare costs 2012. Health 2012; 4: 1471–1484. [Google Scholar]
- [28]. Menzin J, Marton JP, Menzin JA, et al. Lost productivity due to premature mortality in developed and emerging countries: an application to smoking cessation. BMC Med Res Methodol 2012; 12: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Bolin K, Lindgren B. Smoking, healthcare cost, and loss of productivity in Sweden 2001. Scand J Public Health 2016; 35: 187–196. [DOI] [PubMed] [Google Scholar]
- [30]. Martikainen JA, Kautiainen H, Rantalaiho V, et al. Longterm work productivity costs due to absenteeism and permanent work disability in patients with early rheumatoid arthritis: A nationwide register study of 7831 Patients. J Rheumatol 2016; 43: 2101–2105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_material_Barchuk2 for Productivity losses associated with premature mortality due to cancer in Russia: A population-wide study covering 2001–2030 by Anton Barchuk, Alexander Bespalov, Heini Huhtala, Tuvshinjargal Chimed, Alexey Belyaev, Malcolm Moore, Ahti Anttila, Anssi Auvinen, Alison Pearce and Isabelle Soerjomataram in Scandinavian Journal of Public Health