Abstract
Sarcopenic obesity portends poor outcomes, yet it is under-recognized in practice. We collected baseline clinical data including data on body composition (total and segmental muscle mass and total body fat), grip strength, and 5-times sit-to-stand. We defined sarcopenia using cut-points for appendicular lean mass (ALM) and obesity using body-fat cut-points. A total of 599 clinic patients (78.5% female; mean age was 51.3 ± 14.2 years) had bioelectrical impedance analysis (BIA) data (83.8%). Mean body mass index (BMI) and waist circumference were 43.1 ± 8.9 kg/m2 and 132.3 ± 70.7 cm, respectively. All patients had elevated body fat. There were 284 (47.4%) individuals fulfilling criteria for ALM-defined sarcopenia. Sarcopenic obese persons had a lower BMI (38.2 ± 6.4 vs 47.6 ± 8.6; P < 0.001), fat-free mass (113.0 kg ± 16.1 vs 152.1 kg ± 29.4; P < 0.001), fat mass (48.4% ± 5.9 vs 49.5% ± 6.2; P = 0.03), and visceral adipose tissue (216.8 ± 106.3 vs 242.7 ± 133.6 cm3; P = 0.009) than those without sarcopenic obesity. Grip strength was lower in those with sarcopenic obesity (25.1 ± 8.0 vs 30.5 ± 11.3 kg; P < 0.001) and sit-to-stand times were longer (12.4 ± 4.4 vs 10.8 second ± 4.6; P = 0.03). Sarcopenic obesity was highly prevalent in a rural, tertiary care weight and wellness center.
Keywords: obesity, sarcopenic obesity, rural, patient-reported outcomes, muscle
Introduction
Current strategies for managing obesity in adults focus on lifestyle measures, including delivering intensive behavioral and nutritional therapy in clinical practices.1,2 A multidisciplinary treatment team is recommended by the 2014 American Heart Association guidelines that encourages weight loss of 5% which can lead to marked improvements in comorbidity.3 Most successful weight-loss programs struggle to meet such demands,4 particularly in rural areas,5 where staff availability and specialty are limited6 and reimbursement is poor.7
Sarcopenic obesity is an emerging syndrome that is observed in adults with obesity.8 Sarcopenia is broadly defined as the loss of muscle mass, strength, and/or function with aging.9,10 Yet, fat can infiltrate into muscle fibers, promoting insulin resistance and contributing to weakness and physical disability.8 Traditional lifestyle measures as outlined by current guidelines emphasize the importance of aerobic exercise but omit the contribution of obesity and weight loss to physiological muscle processes. A 1 kg weight loss not only leads to loss of fat but can lead to a loss of approximately .25 kg of muscle.11 There is promising evidence that resistance exercises can potentially treat sarcopenic obesity12,13 and is essential in the management of populations with obesity.
Anthropometric measures such as body mass index (BMI) or waist circumference (WC) are accepted as surrogate clinical measures for the assessment of both muscle and fat mass but are highly inaccurate and lack diagnostic accuracy.14,15 When available, body composition measures such as dual-energy x-ray absorptiometry (DXA) or bioelectrical impedance analysis (BIA) have augmented accurate assessment of muscle and fat mass within both the research and clinical settings.8,16-18 Using BIA in an office setting is a low-cost procedure, requires minimal training and oversight, can provide an accurate determination of the components of muscle and fat, and can overcome the inability to measure sarcopenic obesity in clinical practice.19 The ability to identify obese individuals with low fat-free or muscle mass has important clinical implications, in which it can guide providers to augment multimodality treatments with resistance training. While, the definition of sarcopenic obesity varies in the literature,20,21 the Foundation for the National Institutes of Health (FNIH) and other major societies do not specify diagnostic criteria for this entity. We defined sarcopenic obesity using cut-points of both FNIH10 and those from American Association of Clinical Endocrinologists22 for sarcopenia and body fat, respectively. As such, we sought to evaluate the prevalence of sarcopenic obesity, as defined by the FNIH cut-points for appendicular lean mass (ALM)10 and cut-points for body fat, in an academic, rural weight, and wellness center.
Methods
Study setting
The study setting was located at the Dartmouth-Hitchcock Weight and Wellness Center (WWC), an interdisciplinary clinic focusing on treatment of weight and weight-related comorbidities. Dartmouth-Hitchcock is a rural tertiary care medical center located in Lebanon, NH on the New Hampshire and Vermont border and is New Hampshire’s only academic medical center with a catchment population area of approximately 1.5 million persons. The study was reviewed and approved by the Committee for the Protection of Human Subjects at Dartmouth College. The STROBE statement for nutritional epidemiology is attached in the Supplementary materials (Supplemental Material).
Clinical program
The WWC clinic opened in January 2016 and offers multidisciplinary, comprehensive medical management. Patients require a referral from their primary-care clinician, allowing for ongoing coordination of care between primary and specialty teams. A patient-centered treatment plan was formulated at the initial consultation with a provider specializing in weight management. As part of a patient’s initial intake visit, a comprehensive survey instrument was completed in advance of their visit and a trained nurse completes objective anthropometric and functional measures (see below). All information was directly entered into the electronic medical record.
Study cohort
Between January 2016 and March 2018, 715 unique patients were evaluated by a clinician at the WWC, of whom 599 had a bioelectrical impedance analysis (BIA; Figure 1) at the intake visit. The remaining 116 patients did not have their measurements performed for the following reasons: a medical contraindication (n = 6, see below), lost to follow-up (n = 60), patient refusal (n = 5), machine malfunction (n = 12), or general staff unavailability (n = 38). As with any initiation of a clinical infrastructure, our measures changed slightly over time accounting for some of the missing functionality and questionnaire data in a subset of data. We excluded eight patients with missing comorbidities at the baseline visit for specific analyses using this covariate.
Figure 1.
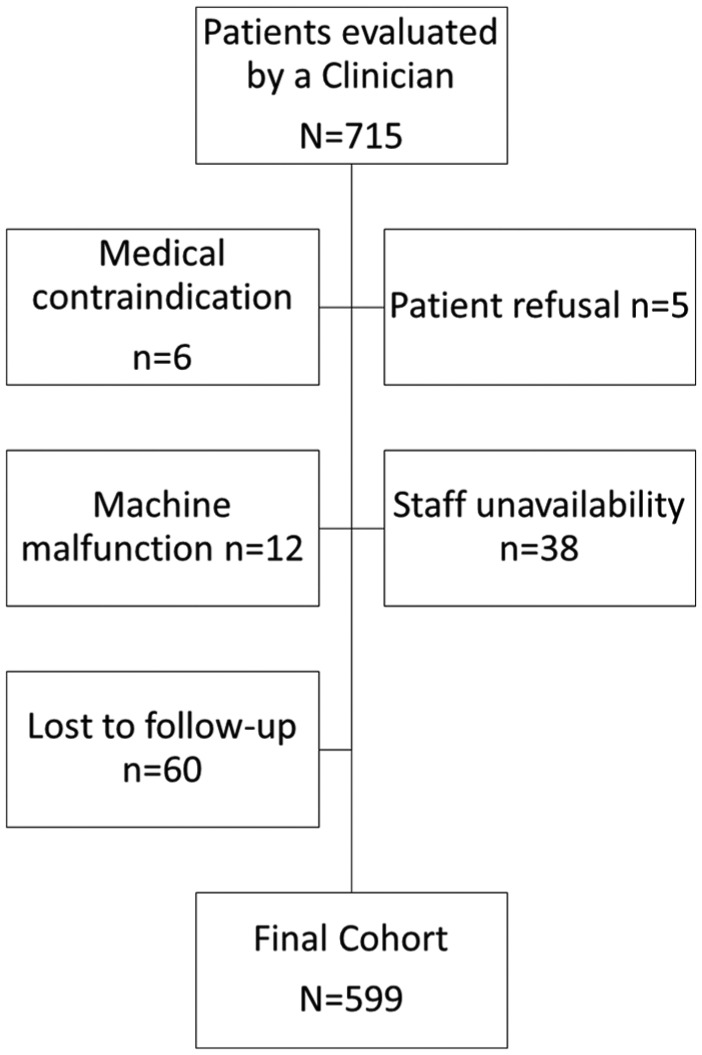
Participant flow and exclusion criteria of cohort.
Body composition
Body composition was assessed using the 8-point Seca m514 (Hamburg, Germany) bioelectrical impedance analyzer that has previously validated equations.23,24 The apparatus has the capabilities of determining segmental fat-free or muscle mass (appendicular and total body), overall fat mass, and visceral fat mass through multi-frequency analysis. Its capacity weight is 300 kg. All participants stood on the apparatus, barefoot, holding each side at the requisite contact points. The total duration of analysis was approximately 20 seconds. Height, date of birth, and self-reported physical activity levels (ranging on a scale from 0 to 5 [very inactive to very active]) were directly entered into the console by the nurse, who received formal training to perform these assessments. Contraindications to using a BIA included an uncontrolled cardiac arrhythmia, pregnancy status, an implantable device (pacemaker, automatic implantable cardiac defibrillator, TENS unit, infusion pumps, artificial hearts, or other portable electronic medical devices). Ascertainment of BIA did not account for a patient’s recent hydration status, alcohol or recent physical activity. Waist circumference was measured snuggly using a tape measure around the abdomen above the iliac crest, parallel to the floor at the end of exhalation.
Sarcopenic obesity definitions
Sarcopenia was defined using the definition of low ALM put forth by the 2014 Foundation of the National Institute of Health Sarcopenia Project.10 ALM was calculated as the sum of the BIA-measured lean mass of all four limbs (right arm, left arm, right leg, and right arm). Sex-specific cut-points of ALM of <19.75 kg in men and <15.02 kg in women defined sarcopenia. This group also stated the importance of a relationship between muscle mass and muscle strength, yet a causal, direct pathway does not exist. Elevated body fat was defined as values exceeding 25% and 35%, in men and women,15 respectively, and >88 cm and >102 cm for WC.25 Sarcopenic obesity was defined using the combination of ALM and body-fat percent cut-points.
Baseline characteristics
Data within the medical record was extracted for baseline demographic variables (age, sex, race/ethnicity, insurance, and smoking status), and comorbid conditions (anxiety, chronic obstructive pulmonary disease, depression, high cholesterol, osteoarthritis, sleep apnea, cerebrovascular disease, and non-alcoholic fatty liver disease) based on the International Classification of Disease 10 codes (http://www.who.int/classifications/icd/en/) from the problem list. Ethnicity was categorized as Hispanic versus non-Hispanic, and race was categorized as American Indian/Pacific Islander, Black or African-American, White, or other. Primary insurance status was classified as Medicare, Medicaid or charity care, commercial insurance, self-pay, or other. Smoking status is routinely assessed at all clinic visits and categorized as former smoker, current smoker, or non-smoker.
Functional measures
Grip Strength was assessed using a JAMAR dynamometer (Patterson Medical, Warrenville, IL). Patients are instructed to sit, flex their dominant arm at 90° on a level surface and squeeze the dynamometer which is adjusted to their hand size. Four measurements are obtained (two on each limb), and the mean (in kg) was entered into a designated field in the medical record. Low grip strength was classified as <26 kg in men and <16 kg in women.10 The five times sit-to-stand test is a measure of lower extremity strength.26 Patients sat in a chair with their arms crossed in a chair with back and were instructed to stand, unassisted with their arms crossed, extending their body fully upright and then sitting down completely, before rising again. This was done five times. Time (in seconds) was measured from the onset of the first rise to the onset of the fifth rise. The six-minute walk test was a measure of aerobic capacity performed in a 100-foot long corridor.27 This assessment was performed according to the American Thoracic Society guidelines by a nurse. Measurements of blood pressure, heart rate, and oxygen saturation before and after the evaluation. Distance in feet was recorded in the medical record and was converted to meters.
Questionnaires
Participants completed several self-reported questionnaires using an electronic touchpad device or on their home computer in advance of the baseline clinic visit. The University of Rhode Island Change Assessment (URICA)28 is a scale based on the transtheoretical model of behavioral change29 consisting of 12 questions in four domains—pre-contemplation, contemplation, action, and maintenance. A final readiness score was calculated. A score <8 is suggestive of pre-contemplation, a score of 8-11.99 is considered a contemplation state, and a score >12 is classified as a preparation state for treatment. The Weight–Efficacy Lifestyle Short-Form (eight questions)30 assessed patient self-efficacy and the Patient Reported Outcome Measures Information System Global (10 items)31 measured physical function (six questions) and general health (physical and mental health).
Statistical analysis
Data from the BIA analyzer was aggregated with variables of interest from the electronic medical record extracted with the assistance of the Dartmouth-Hitchcock Analytics Institute into a composite data set. Descriptive statistics, means ± standard deviation, and counts (percentages) were calculated. Patients with obesity were categorized by lean mass (sarcopenia) status (low vs normal), and prevalence was further stratified by sex, race, and age category (18-40 years, 40-60 years, 60-70 years, and ⩾70 years). Comparisons between low and normal lean mass groups were conducted using unpaired t-tests for continuous variables and chi-square tests for categorical variables (or their non-parametric equivalences). All analyses were performed using STATA, version 15 (College Station, TX). A P value of less than 0.05 was considered statistically significant.
Results
Of the 715 patients, we included 599 in our analytic data set (Table 1). All patients were classified as having body-fat defined obesity. The prevalence of sarcopenic obesity using ALM-defined sarcopenia was 47.6% using the FNIH cut-point for ALM at their intial visit. Individuals with sarcopenic obesity were older (greater than 60 years old), more likely to be female, and were less likely to have osteoarthritis and sleep apnea.
Table 1.
Baseline characteristics of Weight and Wellness patients with obesity.
| Lean mass status |
P value | |||
|---|---|---|---|---|
| Overall |
Low |
Normal |
||
| N = 599 | N = 285 | N = 314 | ||
| Age, years | 51.3 ± 14.1 | 54.5 ± 13.3 | 48.5 ± 14.2 | <.001 |
| Age category | ||||
| 18-40 years | 143 (23.9) | 45 (15.8) | 98 (31.2) | <.001 |
| 40-60 years | 268 (44.7) | 129 (45.3) | 139 (44.3) | |
| 60-70 years | 131 (21.9) | 78 (27.4) | 53 (16.9) | |
| >70 years | 57 (9.5) | 33 (11.6) | 24 (7.6) | |
| Female sex | 470 (78.5) | 251 (88.1) | 219 (69.8) | <.001 |
| Hispanic race | 4 (0.7) | 1 (0.4) | 3 (1.0) | .30 |
| Ethnicity | .12 | |||
| American Indian | 2 (0.3) | 1 (0.4) | 1 (0.3) | |
| Black | 12 (2.0) | 2 (0.7) | 10 (3.2) | |
| White | 581 (97.0) | 279 (97.9) | 302 (96.2) | |
| Other | 4 (0.7) | 3 (1.1) | 1 (0.3) | |
| Insurance | .74 | |||
| Medicare | 133 (22.2) | 67 (23.2) | 66 (21.0) | |
| Medicaid/State assistance | 51 (8.5) | 23 (8.1) | 28 (8.9) | |
| Commercial/other | 415 (69.3) | 195 (68.7) | 220 (70.1) | |
| Smoking status | .48 | |||
| Current | 38 (6.3) | 15 (5.3) | 23 (7.3) | |
| Former | 179 (29.9) | 81 (28.4) | 98 (31.2) | |
| Never | 374 (62.4) | 184 (64.6) | 190 (60.5) | |
| Unknown | 8 (1.3) | 5 (1.8) | 3 (1.0) | |
| Comorbiditiesa | ||||
| Anxiety | 158 (26.4) | 73 (25.6) | 85 (27.1) | .62 |
| COPD | 7 (1.2) | 7 (2.5) | 0 | .005 |
| CAD | 40 (6.7) | 14 (4.9) | 26 (8.3) | .09 |
| Depression | 179 (29.9) | 84 (29.5) | 95 (30.3) | .76 |
| Diabetes | 102 (17.0) | 50 (17.5) | 52 (16.6) | .80 |
| High cholesterol | 178 (29.7) | 89 (31.2) | 89 (28.3) | .50 |
| Osteoarthritis | 115 (19.2) | 43 (15.1) | 72 (22.9) | .01 |
| Sleep apnea | 210 (35.1) | 85 (29.8) | 125 (39.8) | .007 |
| Cerebrovascular disease | 10 (1.7) | 7 (2.5) | 3 (1.0) | .16 |
| NAFLD | 33 (5.6) | 17 (6.0) | 16 (5.2) | .67 |
Values represented are means ± standard deviation, counts (column percents). All participants were classified as having body-fat defined obesity. Sarcopenia was defined using the FNIH-definition of ALM (<26 kg in men; <16 kg in women).
Abbreviations: ALM, appendicular lean mass; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; NAFLD, non-alcoholic fatty liver disease.
Eight participants did not have any medical problems documented in the problem list.
Table 2 highlights clinical and BIA-specific anthropometric and body composition measures. Individuals with ALM-defined sarcopenic obesity were lighter (100.1 kg ± 13.5 vs 137.8 kg ± 26.6; P < 0.001), had lower BMI (38.2 kg/m2 ± vs 47.6 kg/m2 ± 8.6; P < 0.001), and lower total body fat (48.7 kg ± 10.3 vs 68.6 ± 18.0 kg; P < 0.001) than individuals without sarcopenic obesity. Prevalence of sarcopenic obesity is depicted in Figure 2 (Appendix 1) in the overall sample of individuals BIA data, by sex, race, and age category.
Table 2.
Body composition and anthropometric measures in patients with obesity.
| Overall (n = 599) | Sarcopenia status |
P value | ||
|---|---|---|---|---|
| Present (n = 285) | Absent (n = 314) | |||
| Height, m | 1.66 ± 0.09 | 1.62 ± 0.08 | 1.70 ± 0.09 | <.001 |
| Weight, kg | 119.9 ± 28.5 | 100.1 ± 13.5 | 137.8 ± 26.6 | <.001 |
| Body mass index, kg/m2 | 43.1 ± 8.9 | 38.2 ± 6.4 | 47.6 ± 8.6 | <.001 |
| Waist Circumference, cm | 132.3 ± 70.7 | 127.9 ± 100.5 | 136.3 ± 18.7 | .15 |
| High WC (yes/no) | 594 (99.2) | 281 (98.6) | 313 (99.7) | .15 |
| Body fat, kg | 59.2 ± 39.2 | 48.7 ± 10.3 | 68.6 ± 18.0 | <.001 |
| Body fat, % | 49.0 ± 6.1 | 48.4 ± 5.9 | 49.5 ± 6.2 | .03 |
| High body fat, % | 599 (100) | 284 (100) | 314 (100) | 1.00 |
| Visceral adipose tissue, cm3 | 6.54 ± 4.47 | 4.50 ± 2.37 | 8.38 ± 5.08 | <.001 |
| Skeletal muscle mass, kg | 29.6 ± 8.0 | 24.4 ± 4.2 | 34.4 ± 7.6 | <.001 |
| ALM, kg | 16.7 ± 4.7 | 13.4 ± 2.2 | 19.8 ± 4.3 | <.001 |
| Right leg, kg | 15.0 ± 4.3 | 12.0 ± 1.85 | 17.8 ± 4.1 | <.001 |
| Left leg, kg | 14.9 ± 4.2 | 11.9 ± 1.82 | 17.6 ± 3.85 | <.001 |
| Left arm, kg | 3.4 ± 1.1 | 2.76 ± 0.68 | 3.97 ± 1.11 | <.001 |
| Right arm, kg | 3.54 ± 1.2 | 2.87 ± 0.71 | 4.15 ± 1.18 | <.001 |
| ALM | 0.39 ± 0.08 | 0.358 ± 0.068 | 0.421 ± 0.083 | <.001 |
All values presented are mean ± standard deviation, or counts (percent).
Abbreviations: ALM, appendicular lean mass; BMI, body mass index; WC, waist circumference.
Figure 2.
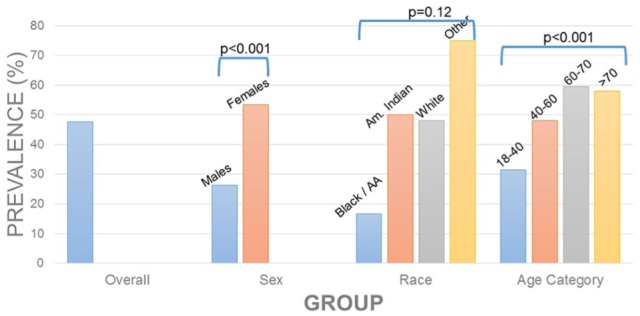
Prevalence of low lean mass in adults with obesity.
In our subset of patients who had functional measures (Table 3), grip strength was significantly lower (25.1 ± 8.0 vs 30.5 ± 11.3; P < 0.001) and sit-to-stand time was slower (12.4 ± 4.4 vs 10.8 ± 4.65 seconds, P < 0.001) in those with sarcopenic obesity. We did not observe differences in the 6 minute walk, readiness to change or self-efficacy scores. PROMIS physical function scoring and self-reported health was higher in those with sarcopenic obesity than those without. No differences in baseline characteristics were observed between those individuals with full functional measures and individuals with at least one missing measure (Appendix 2).
Table 3.
Functional and patient-reported outcome measures in patients with obesity.
| Overall | Low lean mass status |
P value | |||
|---|---|---|---|---|---|
| Low | Normal | ||||
| Strength measures | n | ||||
| Grip strength (kg) | 488 | 27.9 ± 10.3 | 25.1 ± 8.0 | 30.5 ± 11.3 | <.001 |
| Sit-to-stand (seconds) | 491 | 11.6 ± 4.6 | 12.4 ± 4.4 | 10.8 ± 4.65 | <.001 |
| 6-minute walk test, (m) | 495 | 426.8 ± 219.0 | 435.1 ± 106.7 | 418.5 ± 290.6 | .41 |
| Subjective measures | |||||
| URICA | |||||
| Readiness | 359 | 10.1 ± 2.0 | 10.3 ± 2.1 | 9.9 ± 1.9 | .11 |
| WEL-SF | 373 | 48.8 ± 17.3 | 50.5 ± 17.5 | 47.4 ± 17.0 | .08 |
| PROMIS | |||||
| Physical function | 359 | 43.4 ± 8.11 | 44.8 ± 8.0 | 42.2 ± 8.0 | .003 |
| Mental health | 373 | 44.1 ± 8.9 | 45.9 ± 8.7 | 42.5 ± 8.8 | <.001 |
| Physical health | 367 | 42.7 ± 7.7 | 44.0 ± 7.8 | 41.6 ± 7.4 | .003 |
All values presented are mean ± standard deviation, or counts (percent). A P value represents the differences between obese individuals with and without ALM-defined sarcopenia (<19.75 kg in men; <15.02 kg in women).
Abbreviations: ALM, appendicular lean mass; PROMIS, Patient Reported Outcome Measurement Information Systems (higher score, 0-100 is suggestive of better health); URICA, University of Rhode Island Change Assessment (precontemplative <8; contemplation state 8-11.99; preparation state for treatment >12); WEL-SF, Weight Efficacy Short-Form (assessed weight self-efficacy).
Discussion
Prevalence of sarcopenic obesity defined using ALM and body-fat percent cut-points, approached 50% among adults at their intake visit upon referral for obesity management in a rural, academic weight treatment center. Our results suggest the importance of recognizing sarcopenic obesity, defined using low lean mass (LLM) and elevated body-fat mass, in adults, irrespective of age, as it may be associated with impaired objective physical function and likely is underdiagnosed in clinical practice.
Surprisingly, prevalence of sarcopenic obesity were higher than those reported in other literature estimates of cross-sectional data, including reports of participants younger than the age of 60 years as is the case in our center.20,32 In other studies, rates of sarcopenic obesity markedly increase with age,10,33-35 which was not consistently observed in our sample. There are several potential explanations for these findings. First, cross-sectional, population-based studies are often representative of community-dwelling adults and may not be representative of individuals seeking obesity management within a health-care setting. Second, sarcopenic obesity is strongly associated with functional impairments and disability, rising with age.36,37 Presumably, individuals with a significant disability may be less likely to seek care for obesity leading to the lower rates in those aged >70 years. Third, younger individuals at risk or with physical impairments may seek care due to the reduced co-morbidity burden as compared to older adults. The baseline comorbid characteristics are reflective of such. Last, there continues to be a bias against managing obesity in an older adult population38 despite emerging evidence suggesting its importance.39
Our objective, patient-reported outcome measures provide exploratory, pragmatic information regarding the baseline characteristics of patients with sarcopenic obesity presenting in a real-world setting. The relationship between fat-free (muscle mass) and muscle strength is not causal nor linear,10,33,34 yet our results demonstrate parallel relationships between muscle and strength. Our results demonstrate significant differences in grip strength and in sit-to-stand strength. Such robust measures and trends of upper and lower extremity strength parallel findings observed in epidemiological studies.10,33-35 The lack of differences in 6-minute walk test suggests that sarcopenic obesity may have a lesser impact on cardiopulmonary and aerobic fitness as opposed to its biologic effect of muscle although larger sample sizes could be helpful in clarifying this association. An alternative explanation was this population had higher baseline performance as compared to other interventional studies.40 Readiness-to-change, and indicator of willingness to engage in therapy, was no different in these populations suggesting a potential referral bias in those with sarcopenic obesity. In addition, the significantly higher baseline scores on the PROMIS scales in those with sarcopenic obesity is intriguing. While purely speculative, we hypothesize that the individuals with sarcopenic obesity seeking weight loss perceive themselves as healthier and likely believe they may be able to improve their health. These findings may be indicators of health-status and could be considered, irrespective of sarcopenia status, of engagement in such a program. Sarcopenic obesity is generally associated with reduced quality of life41 and thus future work is needed to clarify and ascertain with the differences in PROMIS are in fact due to sarcopenia or other factors.
Our study has many strengths. These findings are the first to our knowledge evaluating sarcopenic obesity in the context of a natural experiment of obesity using clinic-based data from an electronic medical record. Given that data were collected as part of routine care, complete case ascertainment was excellent (>85%). Our overall functional and patient-reported outcome measure completion rate was lower (55.5%); however, our sensitivity analysis comparing the baseline characteristics of individuals with and without such measures found no significant differences. This approach suggests that our analytical sample is likely representative of the true population referred for treatment.
There are some limitations to our analysis in addition to the missing information. We are reliant on administrative data in the electronic medical record for our analyses, whose use has been challenged by others.42,43 We also may not have sufficiently captured medical problems. Nursing staff conducts much of the objective information, and while trained, we have no method in a natural environment to validate their technique and hence they may be subject to measurement bias. Our clinic may not be representative of the entire population and is subject to referral bias seen in academic centers. While our population may have external validity among rural, caucasian referral populations that can provide initial formative estimates on its prevalence within rural settings, we caution generalization to other populations. While BIA has been proven to be helpful in the ascertainment of body composition, it has considerable limitations itself and reduced diagnostic accuracy as compared to DXA17,18 and indeed may have lower accuracy in adults with obesity.44,45 While BIA may over- or under-estimate fat or lean mass, it may be well within acceptable ranges considering the poor sensitive of BMI. Furthermore, there are no practical or pragmatic means of performing DXA in all patients outside of a research setting due to regulatory and reimbursement issues ultimately prompting the application of DXA-derived cutoffs to BIA. Such an approach has been previously suggested in guidelines. FNIH also uses ALMBMI as a cut-point for sarcopenia that normalizes muscle mass for BMI. However, our previous work suggests that ALM may, in fact, be a better predictor of adverse metabolic and inflammatory outcomes.46 Integrating an ALMBMI cut-point for sarcopenia while using percent body fat for obesity may lead to potential confounding and collinearity.
These preliminary findings have considerable implications. Adults of all ages are at risk for sarcopenic obesity and clinicians failing to recognize and identify sarcopenia in individuals with obesity could potentially be detrimental to this referral population. Unopposed weight loss in obese individuals with sarcopenia can further propagate its development, promoting not only fat loss but furthering muscle and bone loss.12,40 This loss of muscle loss places individuals at additional risk of adverse outcomes, including cardiovascular disease,47 disability48 and early mortality.49 Weight-loss programs should include resistance-based exercise programs,40,50 vitamin D supplementation,51 and potential augmentation of protein intake in efforts to mitigate sarcopenia.52
Conclusions
Sarcopenic obesity is common in a tertiary care, academic rural obesity clinic, suggesting its importance in its recognition. Our results highlight the importance of identifying sarcopenia using the definition of LLM in adults with obesity, irrespective of age as their objective physical function may be impaired.
Supplemental Material
Supplemental material, Supplemental_Table_1 for Prevalence of Sarcopenia Obesity in Patients Treated at a Rural, Multidisciplinary Weight and Wellness Center by John A Batsis, Diane Gilbert-Diamond, Auden C McClure, Aaron Weintraub, Diane Sette, John N Mecchella, Sivan Rotenberg, Summer B Cook and Richard I Rothstein in Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders
Acknowledgments
The authors thank the staff of the Dartmouth Weight and Wellness Center including Tara Efstathiou, Laurie Gelb, Eugene Soboleski, and Jennifer Snide from the Dartmouth-Hitchcock Analytics Institute.
Appendix
Appendix 1.
Prevalence of sarcopenic obesity in a weight center.
| Sarcopenia |
P value | ||
|---|---|---|---|
| Present | Absent | ||
| Overall | 285 (47.6) | 314 (52.4) | |
| Sex | |||
| Male | 34 (26.4) | 95 (73.6) | <.001 |
| Female | 251 (53.4) | 219 (46.7) | |
| Race | |||
| American Indian/Alaskan | 1 (50) | 1 (50) | .12 |
| Black | 2 (16.7) | 10 (83.3) | |
| White | 279 (48.0) | 302 (52.0) | |
| Other | 3 (75.0) | 1 (25.0) | |
| Age category | <.001 | ||
| 18-40 years | 45 (31.5) | 98 (68.5) | |
| 40-60 years | 129 (48.1) | 139 (51.9) | |
| 60-70 years | 78 (59.5) | 53 (40.5) | |
| >70 years | 33 (58.0) | 24 (42.1) | |
All values presented are counts (percent).
Appendix 2.
Comparison of individuals without versus with patient-reported measures.
| Characteristics | Without measures |
With measures |
P value |
|---|---|---|---|
| N = 267 | N = 332 | ||
| Age, year | 51.4 ± 13.6 | 51.3 ± 14.5 | .92 |
| Female (%) | 208 (77.9) | 262 (78.9) | .76 |
| Race (%) | |||
| Black | 6 (2.3) | 6 (1.8) | |
| White | 257 (96.3) | 324 (97.6) | .08 |
| Other | 4 (1.5) | 4 (0.6) | |
| Ethnicity (%) | |||
| Hispanic/Latino | 3 (1.1) | 1 (0.3) | .37 |
| Insurance (%) | |||
| Medicare | 47 (17.6) | 86 (25.9) | |
| Medicaid/assistance | 21 (7.9) | 30 (9.0) | .03 |
| Commercial | 199 (74.5) | 216 (65.1) | |
| Smoking (%) | |||
| Current | 16 (6.0) | 22 (6.6) | |
| Former | 79 (29.6) | 100 (30.1) | .69 |
| Never | 170 (63.7) | 204 (61.5) | |
| Comorbidities | |||
| Anxiety | 70 (26.8) | 70 (21.2) | .11 |
| Chronic obstructive pulmonary disease | 2 (0.8) | 5 (1.5) | .40 |
| Coronary artery disease | 16 (6.1) | 24 (7.3) | .58 |
| Diabetes | 44 (16.9) | 58 (17.6) | .82 |
| Depression | 71 (27.2) | 108 (32.7) | .15 |
| High cholesterol | 80 (30.7) | 98 (29.7) | .79 |
| Osteoarthritis | 52 (19.9) | 63 (19.1) | .81 |
| Sleep apnea | 91 (34.9) | 119 (36.1) | .76 |
| Cardiovascular disease | 5 (1.9) | 5 (1.5) | .71 |
| Non-alcoholic fatty liver disease | 10 (3.8) | 23 (7.0) | .99 |
| Body composition measures | |||
| Weight | 118.5 ± 26.1 | 120.9 ± 30.4 | .31 |
| Waist circumference | 135.1 ± 91.1 | 130.1 ± 48.4 | .39 |
| High waist circumference | 265 (99.3) | 329 (99.1) | .84 |
| Sarcopenia status (%) | 127 (47.6) | 158 (47.6) | .99 |
| Body mass index, kg/m2 | 42.7 ± 8.2 | 43.4 ± 9.5 | .35 |
| Appendicular lean mass, kg | 16.6 ± 4.5 | 16.8 ± 4.8 | .63 |
| Fat mass, kg | 58.1 ± 16.3 | 60.0 ± 19.0 | .21 |
| Body fat, % | 48.7 ± 6.2 | 49.1 ± 5.9 | .45 |
| Visceral adipose tissue, cm | 6.26 ± 3.97 | 6.76 ± 4.82 | .78 |
| Skeletal muscle mass | 29.6 ± 7.8 | 29.7 ± 8.2 | .80 |
Excluded individuals consisted of study subjects with at least one functional measure (grip strength, sit-to-stand, 6 minute walk, URICA, WEL-SF, PROMIS) missing in their data. All values presented are mean ± standard deviation, or counts (percent). A P value represents the differences between individuals with and without ALM-defined sarcopenia (<19.75 kg in men; <15.02 kg in women).
Abbreviations: ALM, appendicular lean mass; PROMIS, Patient Reported Outcome Measurement Information Systems; URICA, University of Rhode Island Change Assessment; WEL-SF, Weight Efficacy Short-Form.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Batsis receives funding from the National Institute on Aging of the National Institutes of Health (NIH) under award number K23AG051681 and from the Friends of the Norris Cotton Cancer Center at Dartmouth and National Cancer Institute Cancer Center Support Grant 5P30 CA023108-37 Developmental Funds. Dr Batsis has also received honoraria from the Royal College of Physicians of Ireland, Endocrine Society, and Dinse, Knapp, McAndrew LLC, legal firm. Support was also provided by the Department of Medicine, Geisel School of Medicine, Dartmouth Health Promotion and Disease Prevention Research Center supported by Cooperative Agreement Number U48DP005018 from the Centers for Disease Control and Prevention. The Dartmouth Clinical and Translational Science Institute, under award number UL1TR001086 from the National Center for Advancing Translational Sciences (NCATS) of the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the official position of the Centers for Disease Control and Prevention.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ Note: Work to be presented at The Obesity Society 2018 Annual Meeting, Nashville, TN. Committee for the Protection of Human Subjects #:30801
Author Contributions: JAB Designed and implementated the research, to the analysis of the results, critically revised the manuscript, an approved the final version,DGD Designed and implementated the research, contributed to the analysis of the results, critically revised the manuscript, and approved the final version, ACM Designed and implementated the research, contributed to the analysis of the results, critically revised the manuscript, and approved the final version, AW Implementated the research, contributed to the analysis of the results, critically revised the manuscript, and approved the final version, DS Contributed to the analysis of the results, critically revised the manuscript, and approved the final version, JNM Implementated the research, contributed to the analysis of the results, critically revised the manuscript, and approvedthe final version, SR Implementated the research, contributed to the analysis of the results, critically revised the manuscript, and approvedthe final version, SBC Implementated the research, contributed to the analysis of the results, critically revised the manuscript, and approvedthe final version, RIR Implementated the research, contributed to the analysis of the results, critically revised the manuscript, and approvedthe final version.
ORCID iDs: John A. Batsis  https://orcid.org/0000-0002-0845-4416
https://orcid.org/0000-0002-0845-4416
Aaron Weintraub  https://orcid.org/0000-0002-0139-5898
https://orcid.org/0000-0002-0139-5898
Supplemental material: Supplemental material for this article is available online.
References
- 1. McTigue KM, Harris R, Hemphill B, et al. Screening and interventions for obesity in adults: summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;139:933–949. doi: 10.7326/0003-4819-139-11-200312020-00013. [DOI] [PubMed] [Google Scholar]
- 2. Moyer VA, U.S. Preventive Services Task Force. Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:373–378. doi: 10.7326/0003-4819-157-5-201209040-00475. [DOI] [PubMed] [Google Scholar]
- 3. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults. Circulation. 2014;129:S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DesRoches CM, Buerhaus P, Dittus RS, Donelan K. Primary care workforce shortages and career recommendations from practicing clinicians. Acad Med. 2015;90:671–677. doi: 10.1097/ACM.0000000000000591. [DOI] [PubMed] [Google Scholar]
- 5. MacDowell M, Glasser M, Fitts M, Nielsen K, Hunsaker M. A national view of rural health workforce issues in the USA. Rural Remote Health. 2010;10: 1531. [PMC free article] [PubMed] [Google Scholar]
- 6. Mitchell NS, Prochazka AV, Glasgow RE. Time to RE-AIM: why community weight loss programs should be included in academic obesity research. Prev Chronic Dis. 2016;13:E37. doi: 10.5888/pcd13.150436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Batsis JA, Huyck KL, Bartels SJ. Challenges with the Medicare obesity benefit: practical concerns & proposed solutions. J Gen Intern Med. 2015;30:118–122. doi: 10.1007/s11606-014-3031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Batsis JA, Villareal DT. Sarcopenic obesity in older adults: definition, etiology, epidemiology, treatment and future directions. Nat Rev Endocrinol. 2018. September;14(9):513–537. doi: 10.1038/s41574-018-0062-9. Review [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2018;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heymsfield SB, Gonzalez MC, Shen W, Redman L, Thomas D. Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes Rev. 2014;15:310–321. doi: 10.1111/obr.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weiss EP, Jordan RC, Frese EM, Albert SG, Villareal DT. Effects of weight loss on lean mass, strength, bone, and aerobic capacity. Med Sci Sports Exerc. 2017;49:206–217. doi: 10.1249/MSS.0000000000001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Batsis JA, Mackenzie TA, Bartels SJ, Sahakyan KR, Somers VK, Lopez-Jimenez F. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999–2004. Int J Obes. 2016;40:761–767. doi: 10.1038/ijo.2015.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes. 2008;32:959–966. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis-part I: review of principles and methods. Clin Nutr. 2004;23:1226–1243. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 18. Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004;23:1430–1453. doi: 10.1016/j.clnu.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 19. Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:177–180. [PMC free article] [PubMed] [Google Scholar]
- 20. Batsis JA, Barre LK, Mackenzie TA, Pratt SI, Lopez-Jimenez F, Bartels SJ. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: dual-energy X-ray absorptiometry data from the National Health and Nutrition Examination Survey 1999-2004. J Am Geriatr Soc. 2013;61:974–980. doi: 10.1111/jgs.12260. [DOI] [PubMed] [Google Scholar]
- 21. Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14:513–537. doi: 10.1038/s41574-018-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garvey WT, Mechanick JI, Brett EM, et al. American association of clinical endocrinologists and American college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22:1–203. doi: 10.4158/EP161365.GL. [DOI] [PubMed] [Google Scholar]
- 23. Bosy-Westphal A, Jensen B, Braun W, Pourhassan M, Gallagher D, Muller MJ. Quantification of whole-body and segmental skeletal muscle mass using phase-sensitive 8-electrode medical bioelectrical impedance devices. Eur J Clin Nutr. 2017;71:1061–1067. doi: 10.1038/ejcn.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bosy-Westphal A, Schautz B, Later W, Kehayias JJ, Gallagher D, Muller MJ. What makes a BIA equation unique? Validity of eight-electrode multifrequency BIA to estimate body composition in a healthy adult population. Eur J Clin Nutr. 2013;67:S14–S21. doi: 10.1038/ejcn.2012.160. [DOI] [PubMed] [Google Scholar]
- 25. Batsis JA, Nieto-Martinez RE, Lopez-Jimenez F. Metabolic syndrome: from global epidemiology to individualized medicine. Clin Pharmacol Ther. 2007;82:509–524. doi: 10.1038/sj.clpt.6100355. [DOI] [PubMed] [Google Scholar]
- 26. Buatois S, Miljkovic D, Manckoundia P, et al. Five times sit to stand test is a predictor of recurrent falls in healthy community-living subjects aged 65 and older. J Am Geriatr Soc. 2008;56:1575–1577. doi: 10.1111/j.1532-5415.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- 27. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 28. DiClemente CC, Schlundt D, Gemmell L. Readiness and stages of change in addiction treatment. Am J Addict. 2004;13:103–119. doi: 10.1080/10550490490435777. [DOI] [PubMed] [Google Scholar]
- 29. Romain AJ, Bernard P, Hokayem M, Gernigon C, Avignon A. Measuring the processes of change from the transtheoretical model for physical activity and exercise in overweight and obese adults. Am J Health Promot. 2016;30:272–278. doi: 10.1177/0890117116633829. [DOI] [PubMed] [Google Scholar]
- 30. Ames GE, Heckman MG, Grothe KB, Clark MM. Eating self-efficacy: development of a short-form WEL. Eat Behav. 2012;13:375–378. doi: 10.1016/j.eatbeh.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 31. Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Batsis JA, Mackenzie TA, Emeny RT, Lopez-Jimenez F, Bartels SJ. Low lean mass with and without obesity, and mortality: results from the 1999-2004 national health and nutrition examination survey. J Gerontol A Biol Sci Med Sci. 2017;72:1445–1451. doi: 10.1093/gerona/glx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alley DE, Shardell MD, Peters KW, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:559–566. doi: 10.1093/gerona/glu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cawthon PM, Peters KW, Shardell MD, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:567–575. doi: 10.1093/gerona/glu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dam TT, Peters KW, Fragala M, et al. An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci. 2014;69:584–590. doi: 10.1093/gerona/glu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Batsis JA, Mackenzie TA, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity, and functional impairments in older adults: National Health and Nutrition Examination Surveys 1999-2004. Nutr Res. 2015;35:1031–1039. doi: 10.1016/j.nutres.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12:1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 38. Batsis JA, Zagaria AB. Addressing obesity in aging patients. Med Clin North Am. 2018;102:65–85. doi: 10.1016/j.mcna.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Batsis JA, Gill LE, Masutani RK, et al. Weight loss interventions in older adults with obesity: a systematic review of randomized controlled trials since 2005. J Am Geriatr Soc. 2017;65:257–268. doi: 10.1111/jgs.14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Villareal DT, Aguirre L, Gurney AB, et al. Aerobic or resistance exercise, or both in dieting obese older adults. N Engl J Med. 2017;376:1943–1955. doi: 10.1056/NEJMoa1616338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rizzoli R, Reginster JY, Arnal JF, et al. Quality of life in sarcopenia and frailty. Calcif Tissue Int. 2013;93:101–120. doi: 10.1007/s00223-013-9758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Iezzoni LI, Foley SM, Daley J, Hughes J, Fisher ES, Heeren T. Comorbidities, complications, and coding bias: does the number of diagnosis codes matter in predicting in-hospital mortality? JAMA. 1992;267:2197–2203. [DOI] [PubMed] [Google Scholar]
- 43. Mears SC, Bawa M, Pietryak P, et al. Coding of diagnoses, comorbidities, and complications of total hip arthroplasty. Clin Orthop Relat Res. 2002;402:164–170. doi: 10.1097/00003086-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 44. Bosaeus M, Karlsson T, Holmang A, Ellegard L. Accuracy of quantitative magnetic resonance and eight-electrode bioelectrical impedance analysis in normal weight and obese women. Clin Nutr. 2014;33:471–477. doi: 10.1016/j.clnu.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 45. Wang ZH, Yang ZP, Wang XJ, Dong YH, Ma J. Comparative analysis of the multi-frequency bio-impedance and dual-energy X-ray absorptiometry on body composition in obese subjects. Biomed Environ Sci. 2018;31:72–75. doi: 10.3967/bes2018.008. [DOI] [PubMed] [Google Scholar]
- 46. Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68:1001–1007. doi: 10.1038/ejcn.2014.117. [DOI] [PubMed] [Google Scholar]
- 47. Hamer M, O’Donovan G. Sarcopenic obesity, weight loss, and mortality: the English Longitudinal Study of Ageing. Am J Clin Nutr. 2017;106:125–129. doi: 10.3945/ajcn.117.152488. [DOI] [PubMed] [Google Scholar]
- 48. Hirani V, Blyth F, Naganathan V, et al. Sarcopenia is associated with incident disability, institutionalization, and mortality in community-dwelling older men: the concord health and ageing in men project. J Am Med Dir Assoc. 2015;16:607–613. doi: 10.1016/j.jamda.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 49. Tian S, Xu Y. Association of sarcopenic obesity with the risk of all-cause mortality: a meta-analysis of prospective cohort studies. Geriatr Gerontol Int. 2016;16:155–166. doi: 10.1111/ggi.12579. [DOI] [PubMed] [Google Scholar]
- 50. Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 51. Villareal DT, Apovian CM, Kushner RF, et al. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82:923–934. [DOI] [PubMed] [Google Scholar]
- 52. Porter Starr KN, Pieper CF, Orenduff MC, et al. Improved function with enhanced protein intake per meal: a pilot study of weight reduction in frail, obese older adults. J Gerontol A Biol Sci Med Sci. 2016;71:1369–1375. doi: 10.1093/gerona/glv210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Table_1 for Prevalence of Sarcopenia Obesity in Patients Treated at a Rural, Multidisciplinary Weight and Wellness Center by John A Batsis, Diane Gilbert-Diamond, Auden C McClure, Aaron Weintraub, Diane Sette, John N Mecchella, Sivan Rotenberg, Summer B Cook and Richard I Rothstein in Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders


