Abstract
The introduction of immunotherapies has been a major development in the treatment of many advanced cancers, including hepatocellular carcinoma (HCC). We are entering a new era of systemic therapy for advanced HCC associated with an explosion of clinical trial activity. Data from phase I/II studies of checkpoint inhibitors in advanced HCC have been promising, with durable objective response rates of approximately 20% seen (in both first- and second-line settings) and acceptable safety profiles (including immune-mediated hepatitis). Phase III studies evaluating anti-programmed cell death protein 1 (anti-PD-1) and anti-programmed cell death ligand 1 (anti-PD-L1) antibodies compared with sorafenib are already underway. The potential synergistic effects of anti-PD-1/anti-PD-L1 when used in combination with agents against other checkpoint molecules, systemic therapies, as well as conventional surgical and locoregional therapies are also being explored in upcoming clinical trials. Aside from this, other strategies to harness the immune system, including chimeric antigen receptor-engineered T cells, natural killer cell therapies, and peptide vaccines directed against HCC antigens have entered phase I/II studies. Current limitations of immunotherapies and areas of future research include the accurate assessment and prediction of tumor response, overcoming the immunosuppressive effects of a hypoxic microenvironment, and the management of immune-related hepatitis in patients who already have limited liver reserve.
Keywords: combinational immunotherapy, hepatocellular carcinoma, immune cell-based therapy, immune checkpoints, immunotherapy
Introduction
Hepatocellular carcinoma (HCC) notoriously has a poor prognosis. It is currently the fifth most common malignancy and the second leading cause of cancer-related death worldwide.1,2 Current first-line systemic therapy, such as sorafenib, can only extend the overall survival of patients with advanced HCC by 3 months and is associated with significant adverse effects.3 Although HCC is an immunogenic cancer that expresses various tumor associated antigens (TAAs), immune therapies have not demonstrated meaningful efficacy against HCC for decades.4 Interest in immune therapies was recently reinvigorated by the success of a programmed cell death protein 1 (PD-1) blockade observed in the treatment of advanced melanoma first reported in 2010.5 Since then, several monoclonal antibodies directed against the immune inhibitory molecules PD-1, programmed cell death-ligand 1 (PD-L1), and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), have been trialed, with promising antitumor immune responses seen against many solid tumors from bench to bedside.
In the setting of HCC, clinical studies have shown that immune checkpoint inhibitor therapy can provide objective responses for a subset of patients with advanced HCC.6 To further improve the otherwise poor response rates in HCC patients, the combination of anti-PD-1/anti-PD-L1 with anti-CTLA-4 antibodies is being evaluated in phase I–III trials (NCT01658878, NCT03298451, NCT03298451). Evidence extrapolated from other cancers suggests that an anti-PD-1 antibody combined with targeted therapy or locoregional therapy may also be an effective treatment strategy for HCC. In this review, we summarize current knowledge and recent key developments in immunotherapies for the treatment of HCC, with a main focus on checkpoint inhibitors, while also highlighting cell-based and vaccine therapies. We also discuss current limitations in the application of immunotherapies, and offer perspectives on areas of future research.
Immunotherapy and HCC
The normal liver is exposed to antigens ranging from toxins and gut-derived microbial products. To prevent aberrant responses to continual pathogen exposure, and as a result of a variety of stromal cells and multiple immunoinhibitory molecules, the liver is a tolerogenic immune organ.7 Furthermore, HCC almost always occurs in the setting of chronic inflammation that contributes to the immunosuppressive milieu. The inhibition of antigen-specific immune surveillance in the chronic inflammatory state is mediated by changes in expression of inhibitory immune checkpoint molecules, alterations dendritic cell function, increases in number of regulatory T cells (Tregs), and release of immunosuppressive cytokines such as interleukin (IL)-10 and transforming growth factor-β (TGF-β).8 Chronic exposure to antigens also leads to the overexpression of inhibitory immune checkpoint molecules on T cells, which induces energy or cell exhaustion.9 HCCs also use this mechanism to evade the immune system and facilitate tumor progression.
There is evidence to suggest that enhancing and harnessing the immune reaction to HCC might be beneficial. Firstly, cases of spontaneous regression of HCC have been reported, and these cases were often related to systemic inflammatory responses.10 Secondly, the frequency of TAA-specific CD8+ T cell responses in the periphery and tumor tissue is higher in early-stage compared with late-stage HCC, and is associated with patient survival.11 Not only is the number of effector cells important, but their function (e.g. ability to secrete interferon-gamma) is also significant. Thirdly, earlier preclinical and clinical trials of immunotherapies such as infusions of IL-12, activated peripheral blood mononuclear cells, and/or dendritic cells, have suggested benefits in both early and advanced stage HCC.12–14 Here, we briefly describe some current immunotherapies, their mechanism of action and clinical experience.
Immune checkpoints
PD-1 and PD-L1/PD-L2
Immune checkpoints are T cell surface molecules that can inhibit or stimulate the immune system. Importantly, they are responsible for maintaining self-tolerance and preventing unwanted or exaggerated immune responses. PD-1 – an immune co-inhibitory receptor that is expressed mainly on T cells at the late activation stage in association with infection or an immune response – was first discovered by Tasuku Honjo as a T cell apoptosis inducer in 1992.15 PD-1 interacts with its ligands PD-L1 and PD-L2 and suppresses antigen-specific T cell activation. PD-L1 is universally expressed on normal peripheral tissues, most immune cells during the initiation of the immune response, and cancer cells. PD-L2 expression is selective and limited to antigen presenting cells (APCs) (which may explain why antibodies against PD-1 and PD-L1 are effective, while PD-L2 plays a limited role in anticancer immunity). In the HCC tumor microenvironment, PD-L1 is expressed mainly on Kupffer cells, other APCs, and tumor cells. During immune activation, tumor antigens on cancer cells are presented by APCs to T cells, and recognized by binding to T cell receptors. Activated T cells will then release perforin, granzymes, interferon-γ, and other cytokines to attack these cancer cells. HCCs may evade this attack by expressing low levels of costimulatory immune checkpoint molecules (CD80 and CD86) and increased levels of inhibitory immune checkpoint molecules (PD-L1).16 In particular, increased expression of PD-1 on CD8+ T cells and its ligand PD-L1 on cancer cells have been reported in patients with HCC, which results in inhibition of antitumor T cell responses (migration, proliferation, secretion of cytotoxic mediators) and immune tolerance to HCC.8,9 Their expression is significantly correlated with HCC stage and poor prognosis.17 High PD‑L1 expression on intratumoral and peripheral CD8+ T cells in HCC patients is also associated with disease progression and local recurrence after resection.18
Therapeutically, PD-1/PD-L1 blockade has been shown to suppress HCC tumor growth in preclinical models.19 Recently, a multicenter trial of nivolumab (a fully human IgG4 monoclonal antibody against PD-1) was performed in patients with advanced HCC.6 Nivolumab was evaluated in both the first-line (no prior systemic therapy) and second-line (progression despite prior systemic therapy, mainly sorafenib) settings. The combined phase I/II study included 48 patients in the dose-escalation phase (groups received between 0.1 mg/kg to 10 mg/kg of nivolumab every 2 weeks) and 214 patients in the dose-expansion phase (all received 3 mg/kg of nivolumab every 2 weeks). Patients in the dose-expansion group demonstrated objective response rates of 20% (including two patients with complete response), which was durable (median duration 9.9 months), and a favorable overall survival of 74% at 9 months.6 Similar results were seen in the dose-escalation group: 15% objective response rate and 17 months median duration of response. The durable benefits of nivolumab were observed irrespective of disease etiology or line of therapy. The median overall survival times were 28.6 months in sorafenib-naïve patients and 15 months in sorafenib-experienced patients.20 Most adverse events were mild and transient, with the commonest being rash (23%), pruritus (19%), and diarrhea (10%).6 Following this, nivolumab was promptly approved by the U.S. Food and Drug Administration (FDA) as second-line therapy for advanced HCC following failure of sorafenib in September 2017. Nivolumab is currently being evaluated in two phase III trials as first-line therapy (NCT02576509, CheckMate-459) compared with sorafenib and as adjuvant therapy (NCT03383458, CheckMate-9DX) in patients at high risk of recurrence after resection or ablation compared against placebo.
Several clinical trials of other anti-PD-1/anti-PD-L1 immunotherapies are currently underway. In separate phase II trials, pembrolizumab (another anti-PD-1 antibody) has demonstrated response rates of 17–33% and a median survival of 13–14 months in patients who were sorafenib-refractory or intolerant.21,22 In the larger multicenter Keynote-224 study, 104 patients with advanced HCC previously treated with sorafenib were given 200 mg pembrolizumab intravenously every 3 weeks for a median duration of 4.2 months.21 An objective response was seen in 17% of patients (one with complete response) while 44% had stable disease. Grade 3 or 4 treatment-related events were seen in a quarter of patients, with the most common being increased aspartate aminotransferase (7%), increased alanine aminotransferase (4%), and fatigue (4%). Based largely on this data, the FDA also approved pembrolizumab in November 2018 as second-line therapy for advanced HCC following failure of sorafenib.
There are currently two phase III trials of pembrolizumab as second-line therapy versus placebo in patients with advanced HCC (Keynote-240, NCT02702401 and Keynote-394, NCT03062358). Keynote-240 is a phase III, double-blind trial comparing pembrolizumab (200 mg fixed dose every 3 weeks for up to 35 cycles) plus best supportive care versus placebo plus best supportive care in patients with previously treated with systemic therapy (i.e. second-line therapy). The final analysis of the study, which enrolled 413 patients, was recently released.23 This showed a numerical improvement in overall survival [hazard ratio (HR) 0.78, 95% confidence interval (CI) 0.61–0.99, p = 0.023] and progression-free survival (HR, 0.78; 95% CI, 0.61–0.99; p = 0.021) in patients treated with pembrolizumab compared with placebo. However, these did not meet the prespecified cutoffs for statistical significance. Since superiority was not reached in either primary endpoint, the secondary endpoint of overall response rate was not formally tested. Adverse events were consistent with those observed in the phase II studies. A parallel phase III study (Keynote-394, NCT03062358) evaluating the same regimens and setting as Keynote-240 in Asian patients is currently recruiting.
Tislelizumab (anti-PD-1), camrelizumab (anti-PD-1), and durvalumab (anti-PD-L1) are currently all being, or about to be, evaluated in phase III trials (NCT03412773, NCT02989922) either as first-line or second-line monotherapy after demonstrating reasonable response rates in phase II trials (10–14% response rates).24–26
CTLA-4
CTLA-4 is a CD28 homolog expressed on activated T cells and Tregs. It inhibits T cell activation by outcompeting CD28 (which transmits immune stimulatory signals) for its ligand B7-1, and, in turn, delivering an inhibitory signal instead to the T cell.27–29 CTLA-4 can also regulate Treg activity and differentiation as well as disrupt the function of dendritic cells.30,31 The anti-CTLA-4 antibody was the first to be used for cancer treatment in 1996 by Allison and colleagues,32–34 who demonstrated on a mouse model that blockade of CTLA-4 with an inhibitory antibody could enhance effective immune responses against tumor cells. At the time of writing, tremelimumab is the only anti-CTLA-4 antibody that has been trialed as monotherapy or combination therapy in the advanced HCC setting. A small pilot clinical trial of 20 viremic patients with hepatitis C virus (HCV)-related HCC demonstrated not only antitumor activity (partial response rate of 17.6%) but also antiviral activity, with a significant drop in viral load seen.35 The treatment was, in general, well tolerated, and no corticosteroids were required for severe immune-mediated adverse events. The other major anti-CTLA-4 therapy, ipilimumab, is currently being assessed in combination with nivolumab (discussed below in section Combination strategies for immune therapy in HCC).
Other inhibitory checkpoints and costimulatory immune checkpoints
Aside from PD-1/PD-L1 and CTLA-4, other inhibitory receptors exist, including T cell immunoglobulin mucin 3 (TIM-3) and lymphocyte-activation gene 3 (LAG-3). In particular, TIM-3 has been shown to be involved in progression of HCC, and increased infiltration of TIM-3 positive cells in HCCs is associated with poorer prognosis.36,37 The expression of costimulatory molecules such as glucocorticoid-induced tumor necrosis factor receptor (GITR) and the inducible T cell costimulator is reported to contribute to the immunosuppressive microenvironment in HCC, which may be overcome by treatment with a specific ligand, for example, with a soluble GITR ligand (GITRL) for GITR.38 These may all present potential targets for future immune therapies in advanced HCC. Indeed, trials combining anti-PD-1/anti-PD-L1 therapy with agents targeting TIM-3 (NCT03099109) and LAG-3 (NCT03005782 and NCT01968109) are already underway.
Combination strategies for immune therapy in HCC
While the response rates of monotherapy with immune checkpoint inhibitors far exceed those seen with sorafenib, overall they are still low (<20%). Therefore, strategies to maximize patient response are constantly being explored. Aside from striving to better predict and choose patients who are likely to respond (discussed below in section Prediction of treatment response), it would seem logical to combine immune checkpoint inhibitors with other checkpoint inhibitors, small molecule kinase inhibitors, other systemic therapies, and locoregional therapies.
Since anti-PD-1/anti-PD-L1 and anti-CTLA-4 work on different targets, it is thought that their combination would have a synergistic effect. This was indeed seen in melanoma, where the combination of nivolumab and ipilimumab led to significantly improved progression-free survival (almost double that of nivolumab monotherapy and fourfold that of ipilimumab monotherapy) indicating that the two pathways are nonredundant.39 Unsurprisingly, there was also an increased frequency of grade 3 or 4 treatment-related adverse events (55%). This same combination is currently being evaluated in HCC as part of the CheckMate-040 trial (second-line therapy), and also as neoadjuvant therapy after resection (NCT01658878, NCT03222076, NCT03510871). Data already exists for another combination using durvalumab and tremelimumab, where a phase I/II trial showed a response rate of 20% without any unexpected safety signals.40 A phase III study of this combination in the first-line setting is currently recruiting (NCT03298451).
Combination therapy has also been extended to include kinase inhibitors (both sorafenib and newer agents). Anti-PD-1 therapy has been paired with sorafenib in upcoming trials (NCT03211416, NCT01658878, and NCT02988440), although preclinical studies have not been supportive.41 Other kinase inhibitor combinations with anti-PD-1 include regorafenib (NCT03347292), lenvatinib (NCT03006926), cabozantinib (NCT03299946 and NCT01658878), and axitinib (NCT03289533). The results of these studies and others are eagerly awaited.
Preclinical data in metastatic renal cell carcinoma indicated that cotargeting vascular endothelial growth factor (VEGF) and PD-L1 might be effective due to blockade of VEGF enhancing APC and T cell trafficking.42 Indeed, there is rationale to support this combination in advanced cancers including HCC. Firstly, the VEGF overexpression that occurs in the setting of tumor hypoxia and angiogenesis can lead to an immunosuppressive microenvironment. Specifically, VEGF overexpression can inhibit dendritic cell maturation, increase intratumoral accumulation of Tregs and myeloid-derived suppressor cells, and decrease the infiltration and cytotoxic activity of CD8+ T cells.43 Secondly, blockade of the VEGF pathway with VEGF-specific antibodies or multikinase inhibitors has been shown to abrogate these immunosuppressive changes, which is due, in part, to normalization of tumor vasculature and alleviation of hypoxia.44 However, sustained or high doses of anti-VEGF therapy (outside the so called ‘normalization window’) may result in excessive regression of vasculature leading back to hypoxia and its immunosuppressive effects. Hence, the immunomodulatory effects of anti-VEGF therapies are complex, and whether they would work in synergism with checkpoint inhibitors is currently unclear (discussed further in section Overcoming tumor hypoxia). Nonetheless, the combination of an anti-VEGF agent and a checkpoint inhibitor has been interrogated in advanced HCC with a phase I trial of atezolizumab (anti-PD-L1 inhibitor) and bevacizumab (anti-VEGF antibody), which showed impressive and durable response rates of up to 34%.45 This combination is being further studied as a first-line option compared with sorafenib in a phase III trial (NCT03434379). A similar response rate was also seen when two other drugs [SHR-1210 (anti-PD-1 antibody) and apatinib (selective VEGFR2 inhibitor)] were used to target the PD-1 and VEGF pathways in a phase I trial, including 18 patients with advanced HCC.46
Synergism between immune checkpoint inhibitors and locoregional treatments including ablation, radiotherapy, and transarterial chemoembolization (TACE) is also being investigated. Tumors with low mutational burden and fewer neoantigens are typically less immunogenic, and have no/low response (or primary resistance) to checkpoint inhibitors. Locoregional therapies and radiotherapy induce inflammation, thermocoagulation and other DNA-disturbing activities that create conditions that stimulate the release of TAAs and neoantigens into the bloodstream.47 Human and mouse studies in advanced melanoma have shown that the addition anti-CTLA-4 antibody to radiotherapy had synergistic antitumor effects by increasing the diversity of the T cell receptor repertoire in intratumoral T cells and improving the CD8+ T cell to Treg ratio.48 The addition of anti-PD-L1 therapy improved responses further still. Therefore, it is anticipated that the combination of checkpoint inhibitors with locoregional therapies (especially when tumors are pretreated with locoregional therapy) would enhance sensitivity to checkpoint inhibitors. In a pilot study of 32 patients, tremelimumab was used in combination with radiofrequency ablation or TACE.49 A partial response was seen in up to one-quarter of patients. Patients who had a clinical benefit exhibited a dramatic increase in intratumoral CD8+ T cells on histology. An antiviral effect against HCV was also observed, akin to the tremelimumab monotherapy trial. Since then, other trials combining checkpoint inhibitors with locoregional therapies have surfaced, including: nivolumab with TACE (phase I, NCT03143270 and phase II, NCT03572582), pembrolizumab with TACE (phase I/II, NCT03397654), and nivolumab with yttrium-90 radioembolization (phase II, NCT03033446), among others.
The current clinical trials of immune checkpoint inhibitor monotherapies and combination therapies are listed in Table 1.
Table 1.
Current clinical trials of immune checkpoint inhibitors for HCC.
| Drug | ClinicalTrials.gov identifier | Phase | n | Line of therapy | Endpoint | Status |
|---|---|---|---|---|---|---|
| Nivolumab | ||||||
| Nivolumab | NCT01658878 | I/II | 42 | 1L/2L | DLT/MTD | Completed |
| Nivolumab | NCT01658878 | I/II | 214 | 1L/2L | ORR | Completed |
| Nivolumab | NCT01658878 | I/II | 200 | 1L | ORR | Completed |
| Nivolumab | NCT01658878 | I/II | 262 | 1L/2L | AEs | Completed |
| Nivolumab | NCT02576509 | III | 726 | 1L | TTP/OS | Recruiting |
| Nivolumab | NCT03383458 | III | 520 | Adjuvant | ||
| Pembrolizumab | ||||||
| Pembrolizumab | NCT02702414 | II | 100 | 2L | ORR | Completed |
| Pembrolizumab | NCT02702401 | III | 408 | 2L | PFS/OS | Recruiting |
| Pembrolizumab | NCT03062358 | III | 330 | 2L | OS | Recruiting |
| Pembrolizumab | NCT03211416 | I-II | 27 | 1L | ORR | Recruiting |
| Relatlimab | NCT01968109 | I-II | 168 | 2L | AEs/ORR | Recruiting |
| LY3321367/ LY3300054 |
NCT03099109 | I | 196 | 2L | DLT | Recruiting |
| BGB-A317 | NCT03412773 | III | 660 | 1L | OS | Recruiting |
| SHR-1210 | NCT02989922 | II | 220 | 2L | ORR | Completed |
| REGN3767 | NCT03005782 | I | 546 | 2L | ORR | Recruiting |
| Combinations with other immunotherapies | ||||||
| Nivolumab/ Ipilimumab | NCT01658878 | II | 620 | 2L | AEs | Completed |
| Nivolumab/ ipilimumab | NCT03222076 | II | 45 | Neoadjuvant | AEs | Recruiting |
| Nivolumab/ Ipilimumab | NCT03510871 | II | 40 | Neoadjuvant | ORR | Recruiting |
| Nivolumab/ Pexavec |
NCT03071094 | II | 30 | 2L | DLT/ORR | Recruiting |
| Durvalumab/ Tremelimumab |
NCT02519348 | II | 545 | 1L/2L | AEs | Recruiting |
| Durvalumab/ Tremelimumab | NCT03298451 | III | 1200 | 1L | OS | Recruiting |
| Relatlimab/ Nivolumab | NCT01968109 | I-II | 168 | 2L | AEs/ORR | Recruiting |
| REGN3767/ REGN2810 | NCT03005782 | I | 546 | 2L | ORR | Recruiting |
| LY3321367/ LY3300054 |
NCT03099109 | I | 196 | 2L | DLT | Recruiting |
| Atezolizumab/ bevacizumab |
NCT03434379 | III | 480 | 1L | OS/ PFS | Recruiting |
| Combinations with targeted agents | ||||||
| PDR001/ FGF401 |
NCT02325739 | II | 238 | 2L | DLT/TTP/ORR | Recruiting |
| PDR001/ INC280 |
NCT02795429 | II | 108 | 2L | DLT/ORR | Recruiting |
| Nivolumab/ Galunisertib | NCT02423343 | II | 75 | 2L | MTD | Completed |
| Regorafenib/ pembrolizumab | NCT03347292 | I | 40 | 1L | AEs/ DLT | Recruiting |
| Cabozantinib/ nivolumab | NCT03299946 | I | 15 | Neoadjuvant | AEs | Recruiting |
| Nivolumab/ CC-122 | NCT02859324 | I-II | 50 | 2L | AEs/ DLT/ ORR | Recruiting |
| PDR001/ Sorafenib |
NCT02988440 | II | 50 | 2L | AEs | Recruiting |
| Pembrolizumab/ Lenvatinib | NCT03006926 | I | 104 | 2L | AEs/ DLT | Recruiting |
| Combinations with locoregional therapies | ||||||
| Nivolumab/ TACE |
NCT03143270 | I | 14 | 2L | AEs | Recruiting |
| Nivolumab/ Y90 |
NCT03033446 | II | 40 | 2L | ORR | Recruiting |
| Nivolumab/ Y90 |
NCT02837029 | I | 35 | 2L | MTD | Recruiting |
| Pembrolizumab/ Y90 |
NCT03099564 | II | 30 | 2L | PFS | Recruiting |
AE, Adverse Event; DLT, dose-limiting toxicity; MTD, maximum tolerated dose; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; TTP, time to progression.
Immune cell-based therapies
Chimeric antigen receptor-engineered T cell immunotherapy
T cells engineered with chimeric antigen receptors (CARs) gain the ability to recognize a defined antigen, which allows specific cells, including tumor cells, to be targeted. CAR-T cells recognize tumor cell surface antigens by using single-chain variable regions (ScFv) constructed from the variable heavy and light chains of a TAA-specific monoclonal antibody as the extracellular antigen recognition domain. This is connected by a spacer to the transmembrane and intracellular signaling domains for signal transduction and T cell activation. CAR-T based therapy has been successful in treating CD19-positive hematological malignancies, which paves the way for its application in solid tumors.50–52 In HCC, Glypican-3 (GPC3) has been most commonly used as the TAA for CAR-T therapy,53–55 with significant antitumor activity seen both in vitro and in vivo.54,55 Alpha-fetoprotein (AFP), which is commonly overexpressed in HCC (but also in healthy tissue), has also been used as a target, with evidence of potent antitumor response,56 although tumor-specificity remains a question.47 Interestingly, CAR-T cells specifically targeting hepatitis B virus (a major cause of HCC worldwide) and its envelope proteins have also been used to reduced viral replication in a mice model.57 The current limitations of CAR-T therapy include the shortage of appropriate TAAs, the inefficient homing of T cells into the tumor, the potential for on-target/off-tumor recognition of healthy tissue, and its unknown long-term safety.58,59 The combination of anti-CD19 CAR-T cells and PD-1 blockade showed synergistic effects in relapsed lymphoma patients and mouse models, which provides hope for this combination in HCC patients.60 There are currently at least 10 phase I/II clinical trials (almost all conducted in China) investigating the use of CAR-T cells in advanced HCC.61
Natural-killer-cell-based therapy
Natural killer (NK) cells (CD56-positive lymphocytes) are essential in the human innate immune system. They deliver cytotoxic granules, secrete effector cytokines, and engage death-inducing receptors to stimulate target cell apoptosis and mediate antibody-dependent cell-mediated cytotoxicity.62–66 NK cells make up 30–50% of the intrahepatic lymphocytes in human livers. Intrahepatic NK cells have unique phenotypic features and functional properties that demonstrate higher cytotoxic activity against tumor cells compared with circulating NK cells.67,68 During hepatocarcinogenesis, there is a reduction in both NK cell proportion as well as function in terms of cytokine (interferon-gamma) production and cytotoxic activity. Extensive preclinical studies have been performed using different strategies to (re)activate NK cells and harness their cytotoxic activity against tumor cells; such strategies include chemoimmunotherapy, adoptive transfer of NK cells or gene-modified NK cells, gene therapy, cytokine therapy, and therapy with a monoclonal antibody specific for NK inhibitory receptors.69 These studies have shown enhanced cytotoxicity of NK cells and increased anti-HCC effects in both in vivo and in vitro studies either when used alone or in combination with therapies such as sorafenib. There are currently 7 phase I/II clinical trials investigating NK cell-based immunotherapy in HCC patients with most employing adoptive transfer of autologous or allogeneic NK cells as their strategy.70
Peptide vaccines
Cancer peptide vaccines utilize TAAs to stimulate the adaptive immune system to induce the activation and proliferation of cytotoxic T cells that specifically recognize and kill cancer cells. Like in CAR-T cell immunotherapy, the most well-studied peptide vaccine in HCC is GPC3 because it is overexpressed in up to 80% of HCCs (including early-stage tumors) but not in normal tissues.71 Furthermore, its expression is associated with poorer prognosis.72
The initial phase I study of GPC3 peptide vaccine in 33 patients with advanced HCC showed that the vaccine was well tolerated and resulted in one patient with partial response (3%) and 19 with stable disease (58%) at 2 months.73 The GPC3 peptide vaccine induced a GPC3-specific cytotoxic T lymphocyte response in 90% of patients, which correlated with overall survival. The same authors also evaluated the use of GPC3 peptide vaccine in the adjuvant setting with a phase II study of 35 HCC patients who had undergone resection.74 While there were modest numerical reductions in recurrence rates at 1 year and 2 years after vaccination (28.6% versus 54.3% and 39.4% versus 54.5%, respectively), the recurrence rate at 1 year was significantly lower in those with GPC3-positive tumors who had received the vaccine (24% versus 48%). Preclinical studies have been performed with the aim of trying to increase responses to GPC3 peptide vaccines, including by direct intratumoral injection of the peptide, intravenous infusion of GPC3-coupled lymphocyte complex, and passive immunization with anti-GPC3 antibodies.75–77 However, since GPC3 is not a lethal gene to HCC cells, at best only partial responses have been documented. Hence, further studies are needed to explore the use of GPC3 peptide vaccines in combination with other therapies.
Current limitations and perspectives of immunotherapy
The enthusiasm with immune checkpoint blockade needs to be tempered by some limitations.
Assessment of treatment response
The assessment of treatment response is not straightforward. Traditionally, response in solid organ cancers has been assessed solely by reduction in tumor size using the World Health Organization criteria, and, more recently, the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines. However, in HCC, there is a poor correlation between tumor shrinkage and antitumor activity (e.g. the induction of intratumoral necrotic areas) provided by locoregional and targeted therapies.78 Subsequently, the RECIST criteria were modified (mRECIST) such that the evaluation of response now measures the diameters of viable (arterially enhancing) target lesions.79 mRECIST has neither been validated prospectively nor evaluated for immunotherapies. Furthermore, it has since emerged that a distinct radiologic response pattern known as pseudoprogression occurs in <10% of patients treated with immunotherapy where deep and durable tumor responses are noted after an initial progression.80 This is thought to be due to the initial recruitment of activated T cells to the tumor site before they have any antitumor activity, causing the tumor to swell and artificially increase in size. This has led to the development of immune-specific response criteria, such as immune-related RECIST (irRECIST) guidelines and immunotherapy RECIST (iRECIST), which requires confirmation of progression with repeat imaging 4–8 weeks after the first documented response. Currently, RECIST v1.1 is used to assess response in clinical trials of immunotherapy every 6–9 weeks, while the use of mRECIST, irRECIST, and iRECIST has been inconsistent (NCT02576509).6,21,22
Prediction of treatment response
Clearly a response is not seen in all patients. The cause of nonresponse (excluding pseudoprogression) and treatment resistance is complex and involves mutations affecting the immunogenicity of cancer itself, defective cytokine signaling, and upregulation of alternative escape immune checkpoint pathways (e.g. CTLA-4, TIM-3, LAG-3) by tumor cells.81 Immunological profiling of HCC has revealed a novel molecular class of tumors (in approximately 25% of patients) with an enriched inflammatory response characterized by the overexpression of immune-related genes and high expression of PD-1 and PD-L1, which may predict response to checkpoint inhibitor immunotherapy.82 However, responsiveness is not necessarily correlated with PD-1/PD-L1 expression levels. The CheckMate-040 trial reported that baseline tumor cell PD-L1 status did not have any apparent effect on response rates.6 A similar subset of patients with high lymphocyte infiltration (in 22% of all HCC patients) has been described in a study by The Cancer Genome Atlas (TCGA) consortium, which performed multiplatform integrative molecular subtyping on 196 HCCs.83 The latter study also showed that tumors carrying the Catenin Beta 1 (CTNNB1) mutation were associated with a lack of immune infiltrate (so called cold tumors). Accordingly, in a recent first report of prospective genotyping of advanced HCC by next generation sequencing, Wnt/CTNNB1 mutations were associated with primary resistance to immune checkpoint inhibitors.84 The 10/27 (37%) patients exhibiting Wnt/CTNNB1 mutations all had progressive disease as their best response, and their median survival was 9.1 months compared with 15.2 months in those without mutations. Further validation of this clinical signal is required.
Currently, there are otherwise few biomarkers to predict response to immunotherapy in advanced HCC. Identification of biomarkers based on experience with immunotherapy in other solid organ cancers provide direction for further investigation. Tumor mutational burden, defined as the total number of mutations (nonsynonymous single nucleotide variants) present in a tumor specimen, has been shown to be an independent predictor of response across multiple cancers, especially melanoma and nonsmall cell lung cancer.85 Indeed, a strong positive correlation exists between tumor mutational burden and objective responses to PD-1 inhibition across cancers overall as shown in a recent meta-analysis.86 The density of tumor-infiltrating lymphocytes (particularly cytotoxic T cells and PD-1+ T cells) is another candidate biomarker that has repeatedly been demonstrated to predict response to checkpoint inhibitor therapy in melanoma patients.87,88
There is increasing evidence to suggest the gut microbiome is a key player in liver inflammation and hepatocarcinogenesis.89 A recent study by Routy and colleagues demonstrated that primary resistance to checkpoint inhibitors could be attributed to abnormal gut microbiome composition (antibiotic-related dysbiosis) in patients with nonsmall cell lung cancers, renal cell carcinomas, and urothelial carcinomas.90 In particular, an increased abundance of Akkermansia muciniphila was found in patients with objective responses and longer progression-free survival (>3 months). Promisingly, the efficacy of PD-1 blockade could be restored by fecal microbiota transplantation of stool samples from responders or oral supplementation of A. muciniphila in murine models. Furthermore, not only can gut microbiome signatures predict response to checkpoint inhibitors, they are also associated with the development of immune-related adverse events (IRAEs) such as enterocolitis.91 Currently, the influence of gut microbiota on response to immune checkpoint inhibitors has not yet been validated in HCC, and these studies are urgently needed.
Aside from nonresponders, reports of hyperprogressors after the use of immunotherapy (especially anti-PD-1/anti-PD-L1 monotherapy) in advanced cancers (including HCC) have recently emerged. Definitions of hyperprogressors have included: time to treatment failure of <2 months after initiation of treatment, >50% increase in tumor burden compared with preimmunotherapy imaging, and increase in tumor growth rate by >50–100%.92–94 As distinct from pseudoprogressors (who also have initial progressive disease), hyperprogressors do not undergo a subsequent delayed tumor response. The rate of hyperprogression in advanced HCC after immunotherapy has been reported to be 8%,94 which is similar to that of other cancers.92 Currently, little is known about the characteristics of these hyperprogressors or the underlying mechanism driving their disease. However, they appear to have an association with mouse double minute (MDM) 2 and 4 amplification (odds ratio 10.8) or epidermal growth factor receptor (EGFR) aberrations (odds ratio 8.4) suggesting that genomic testing may need to be considered prior to starting checkpoint inhibitor therapy in the future.93
Therefore, predicting responders and nonresponders (including hyperprogressors) to immune therapies remains a challenge and is an area of active research. Further studies that define signatures associated with tumor responses in tumor genomics, the tumor microenvironment, and the gut microbiome during checkpoint blockade are needed to help clinicians to personalize therapeutic strategy and design effective combinations in the future.
Overcoming tumor hypoxia
HCC is a hypervascular tumor with structurally and functionally abnormal blood vessels that negatively impact on the delivery of systemic therapy and infiltration of effector immune cells into the tumor.95 Furthermore, tumor hypoxia also creates an immunosuppressive microenvironment by recruiting Tregs and reprogramming of resident macrophages to a tumor-promoting M2-polarization. In particular, there is an upregulation of immune checkpoint molecules (e.g. PD-L1) by immune, stromal, and tumor cells.44,96 Preclinical studies have shown that normalization of vessels and the microenvironment can improve the efficacy of immunotherapies, including checkpoint inhibitors.97 Furthermore, synergism between checkpoint inhibition and tumor vessel normalization (where checkpoint blockade can in turn enhance vessel normalization via a positive feedback loop mediated by CD4+ T cells) has also been reported using experimental cancer models.98 Hence the use of immunotherapies in conjunction with vascular normalization strategies should be explored in the future.
Immune-related adverse events
An expected side effect of boosting the body’s immune response to cancer is autoimmune disease. Although a wide range of IRAEs have been reported (Table 2),99 immune-related hepatotoxicity deserves particular mention. In clinical trials of anti-PD-1/anti-PD-L1 therapies, elevations in liver enzymes occurred in the minority, and were typically mild (15–20% overall, <10% grade 3 or 4).6,21 Immune-mediated hepatitis requiring systemic glucocorticoids occurred in only 5% of treated patients. However, patients with HCCs have relatively limited liver reserve compared with other cancer patients. Because the majority (80–90%) of HCCs occur on a background of liver cirrhosis,100 a flare of checkpoint inhibitor-induced hepatitis can potentially result in decompensation of cirrhosis, and even liver failure. In the CheckMate-040 dose-escalation and -expansion trial, all patients had Child-Pugh A or B7 cirrhosis6; however, a recent cohort study of 49 Child-Pugh B7-8 patients in the CheckMate-040 study demonstrated similar safety profiles without higher rates of discontinuation compared with Child-Pugh A patients from the dose-escalation and -expansion trial (24.5% grade 3 or 4 drug-related adverse events versus 22.5%, respectively).101 Clearly, careful patient selection is still critical. Another consideration is that the treatment of IRAEs typically involves dose reduction with the use of immunosuppression including corticosteroids and immunomodulators such as mycophenolate or azathioprine), which may be deleterious in terms of promoting cancer progression.102 Finally, up to 43% of patients with unresectable HCC die due to complications of liver disease (rather than cancer progression); hence, the management of underlying cirrhosis should not be neglected.103
Table 2.
Immune-related adverse events associated with immunotherapy.
| Dermatological |
|---|
| Rash |
| Vitiligo |
| Uveitis |
| Gastrointestinal |
| Enterocolitis |
| Hepatitis |
| Pancreatitis |
| Mucositis |
| Endocrine |
| Thyroiditis |
| Hypophysitis |
| Adrenal insufficiency |
| Autoimmune diabetes |
| Neurological |
| Encephalitis |
| Aseptic meningitis |
| Neuropathy |
| Other |
| Nephritis |
| Pneumonitis |
| Myocarditis |
| Arthralgia |
| Vasculitis |
| Thrombocytopenia |
| Anemia |
Conclusion
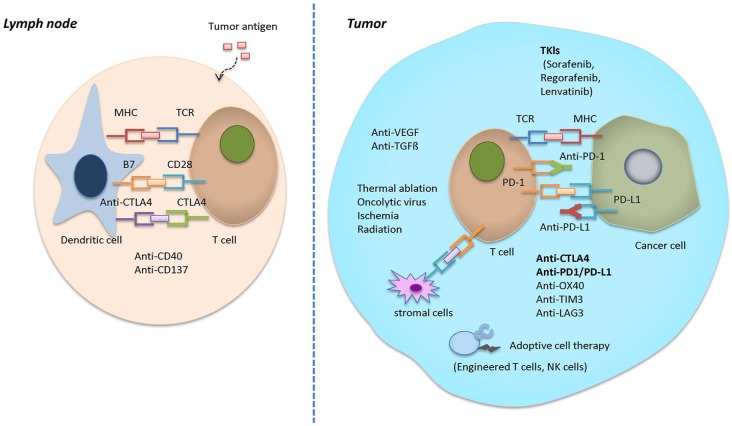
We are now striding into a new era of anticancer treatment for HCC, where immune checkpoint inhibitor-based strategies will soon become a cornerstone, both as monotherapy and in combination with other checkpoint inhibitors and kinase inhibitors, as well as conventional surgical and locoregional therapies (Figure 1). We continue to follow the rapid advances in the therapeutic use of immune checkpoint inhibitors with great interest.
Figure 1.
Potential synergistic mechanisms in combinational therapies of immune checkpoint inhibitors and other tyrosine kinase inhibitors.
Acknowledgments
Authors Weiqi Xu and Ken Liu contributed equally.
Footnotes
Funding: This work was supported by the National Natural Science Foundation (Grant No: 81772596).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Jiansong Ji  https://orcid.org/0000-0003-4727-2794
https://orcid.org/0000-0003-4727-2794
Contributor Information
Weiqi Xu, Department of Hepatic Surgery and Department of Oncology, Fudan University Shanghai Cancer Center, Shanghai Medical College, China.
Ken Liu, AW Morrow Gastroenterology and Liver Centre, Royal Prince Alfred Hospital, Sydney, NSW, Australia, Sydney Medical School, The University of Sydney, Australia; and Liver Injury and Cancer Program, The Centenary Institute, Sydney, Australia.
Minjiang Chen, Key Laboratory of Imaging Diagnosis and Minimally Invasive Intervention Research and Department of Radiology, The Fifth Affiliated Hospital of Wenzhou Medical University; Affiliated Lishui Hospital of Zhejiang University; and The Central Hospital of Zhejiang Lishui, China.
Jin-Yu Sun, Department of General Surgery, The First Affiliated Hospital of Nanjing Medical University, China, and Sparkfire Scientific Research Group, Nanjing Medical University, China.
Geoffrey W McCaughan, AW Morrow Gastroenterology and Liver Centre, Royal Prince Alfred Hospital, Sydney, NSW, Australia, Sydney Medical School, The University of Sydney, Australia; and Liver Injury and Cancer Program, The Centenary Institute, Sydney, Australia.
Xiao-Jie Lu, Department of General Surgery, The First Affiliated Hospital of Nanjing Medical University, 210029 China.
Jiansong Ji, Department of Radiology and Key Laboratory of Imaging Diagnosis and Minimally Invasive Intervention Research, The Fifth Affiliated Hospital of Wenzhou Medical University; Affiliated Lishui Hospital of Zhejiang University; and The Central Hospital of Zhejiang Lishui, China.
References
- 1. Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 2016; 150: 835–853. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–E386. [DOI] [PubMed] [Google Scholar]
- 3. Llovet JM, Villanueva A, Lachenmayer A, et al. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol 2015; 12: 408–424. [DOI] [PubMed] [Google Scholar]
- 4. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2015; 12: 681–700. [DOI] [PubMed] [Google Scholar]
- 5. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 389: 2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pardee AD, Butterfield LH. Immunotherapy of hepatocellular carcinoma: unique challenges and clinical opportunities. Oncoimmunology 2012; 1: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Makarova-Rusher OV, Medina-Echeverz J, Duffy AG, et al. The yin and yang of evasion and immune activation in HCC. J Hepatol 2015; 62: 1420–1429. [DOI] [PubMed] [Google Scholar]
- 9. Hato T, Goyal L, Greten TF, et al. Immune checkpoint blockade in hepatocellular carcinoma: current progress and future directions. Hepatology 2014; 60: 1776–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huz JI, Melis M, Sarpel U. Spontaneous regression of hepatocellular carcinoma is most often associated with tumour hypoxia or a systemic inflammatory response. HPB 2012; 14: 500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flecken T, Schmidt N, Hild S, et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology 2014; 59: 1415–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takayama T, Sekine T, Makuuchi M, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet 2000; 356: 802–807. [DOI] [PubMed] [Google Scholar]
- 13. Lee WC, Wang HC, Hung CF, et al. Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J Immunother 2005; 28: 496–504. [DOI] [PubMed] [Google Scholar]
- 14. Kayashima H, Toshima T, Okano S, et al. Intratumoral neoadjuvant immunotherapy using IL-12 and dendritic cells is an effective strategy to control recurrence of murine hepatocellular carcinoma in immunosuppressed mice. J Immunol 2010; 185: 698–708. [DOI] [PubMed] [Google Scholar]
- 15. Honjo T. Seppuku and autoimmunity. Science 1992; 258: 591–592. [DOI] [PubMed] [Google Scholar]
- 16. Tatsumi T, Takehara T, Katayama K, et al. Expression of costimulatory molecules B7–1 (CD80) and B7–2 (CD86) on humany hepatocellular carcinoma. Hepatology 1997; 25: 1108–1114 [DOI] [PubMed] [Google Scholar]
- 17. Wu K, Kryczek I, Chen L, et al. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res 2009; 69: 8067–8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi F, Shi M, Zeng Z, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer 2011; 128: 887–889. [DOI] [PubMed] [Google Scholar]
- 19. Kuang DM, Zhao Q, Peng C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med 2009; 206: 1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sangro B, Melero I, Yau T, et al. Nivolumab in sorafenib-naive and -experienced patients with advanced hepatocellular carcinoma (HCC): survival, hepatic safety, and biomarker assessments in CheckMate-040. Hepatology 2017; 66(Suppl. 1): abstract #141. [Google Scholar]
- 21. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018; 19: 940–952. [DOI] [PubMed] [Google Scholar]
- 22. Fuen LG, Li YY, Wangpaichitr M, et al. Phase II study of pembrolizumab in advanced, unresectable hepatocellular carcinoma. J Clin Oncol 2018; 36(15 Suppl.): 4086. [Google Scholar]
- 23. Merck Sharp & Dohme Corp. Merck provides update on KEYNOTE-240, a phase 3 study of KEYTRUDA® (pembrolizumab) in previously treated patients with advanced hepatocellular carcinoma. Merck Newsroom. https://www.mrknewsroom.com/news-release/oncology/merck-provides-update-keynote-240-phase-3-study-keytruda-pembrolizumab-previou. (accessed 4 May 2019).
- 24. Qin S, Finn RS, Kudo M, et al. A phase 3, randomized, open-label, multicenter study to compare the efficacy and safety of tislelizumab, an anti-PD-1 antibody, versus sorafenib as first-line treatment in patients with advanced hepatocellular carcinoma. J Clin Oncol 2018; 36 (15 Suppl.): TPS3110. [Google Scholar]
- 25. Qin SK, Ren ZG, Meng ZQ, et al. A randomized multicentered phase II study to evaluate SHR-1210 (PD-1 antibody) in subjects with advanced hepatocellular carcinoma (HCC) who failed or intolerable to prior systemic treatment. Ann Oncol 2018; 29 (Suppl.8). [Google Scholar]
- 26. Wainberg ZA, Segal NH, Jaeger D, et al. Safety and clinical activity of durvalumab monotherapy in patients with hepatocellular carcinoma (HCC). J Clin Oncol 2017; 35 (15 Suppl): 4071. [Google Scholar]
- 27. Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008; 322: 271–275. [DOI] [PubMed] [Google Scholar]
- 28. Ramagopal UA, Liu W, Garrett-Thomson SC, et al. Structural basis for cancer immunotherapy by the first-in-class checkpoint inhibitor ipilimumab. Proc Natl Acad Sci U S A 2017; 114: E4223–E4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med 2000; 192: 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Han Y, Chen Z, Yang Y, et al. Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology 2014; 59: 567–579. [DOI] [PubMed] [Google Scholar]
- 31. Duggleby R, Danby RD, Madrigal JA, et al. Clinical grade regulatory CD4(+) T cells (Tregs): moving toward cellular-based immunomodulatory therapies. Front Immunol 2018; 9: 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med 1996; 183: 2533–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krummel MF, Sullivan TJ, Allison JP. Superantigen responses and co-stimulation: CD28 and CTLA-4 have opposing effects on T cell expansion in vitro and in vivo. Int Imunol 1996; 8: 519–523. [DOI] [PubMed] [Google Scholar]
- 34. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996; 271: 1734–1736. [DOI] [PubMed] [Google Scholar]
- 35. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013; 59: 81–88. [DOI] [PubMed] [Google Scholar]
- 36. Yan W, Liu X, Ma H, et al. Tim-3 fosters HCC development by enhancing TGF-beta-mediated alternative activation of macrophages. Gut 2015; 64: 1593–1604. [DOI] [PubMed] [Google Scholar]
- 37. Li Z, Li N, Li F, et al. Immune checkpoint proteins PD-1 and TIM-3 are both highly expressed in liver tissues and correlate with their gene polymorphisms in patients with HBV-related hepatocellular carcinoma. Medicine (Baltimore) 2016; 95: e5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pedroza-Gonzalez A, Kwekkeboom J, Sprengers D. T-cell suppression mediated by regulatory T cells infiltrating hepatic tumors can be overcome by GITRL treatment. Oncoimmunology 2013; 2: e22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): phase I safety and efficacy analyses. J Clin Oncol 2017; 35 (15 Suppl): 4073. [Google Scholar]
- 41. Chen Y, Ramjiawan RR, Reiberger T, et al. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology 2015; 61: 1591–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wallin JJ, Bendell JC, Funke R, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun 2016; 7: 12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hato T, Zhu AX, Duda DG. Rationally combining anti-VEGF therapy with checkpoint inhibitors in hepatocellular carcinoma. Immunotherapy 2016; 8: 299–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jain RK. Angiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell 2014; 26: 605–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pishvaian MJ, Lee MS, Ryoo B, et al. Updated safety and clinical activity results from a Phase Ib study of atezolizumab + bevacizumab in hepatocellular carcinoma (HCC). Ann Oncol 2018; 29(Suppl. 8). [Google Scholar]
- 46. Xu J, Kim TW, Shen L, et al. Results of a randomized, double-blind, placebo-controlled, phase III trial of trifluridine/tipiracil (TAS-102) monotherapy in Asian patients with previously treated metastatic colorectal cancer: the TERRA study. J Clin Oncol 2018; 36: 350–358. [DOI] [PubMed] [Google Scholar]
- 47. Greten TF, Sangro B. Targets for immunotherapy of liver cancer. J Hepatol 2018; 68: 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Twyman-Saint VC, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015; 520: 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Duffy AG, Ulahannan SV, Makorova-Rusher O, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol 2017; 66: 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013; 368: 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brentjens RJ, Riviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011; 118: 4817–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012; 119: 2709–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dargel C, Bassani-Sternberg M, Hasreiter J, et al. T cells engineered to express a T-cell receptor specific for glypican-3 to recognize and kill hepatoma cells in vitro and in mice. Gastroenterology 2015; 149: 1042–1052. [DOI] [PubMed] [Google Scholar]
- 54. Gao H, Li K, Tu H, et al. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res 2014; 20: 6418–6428. [DOI] [PubMed] [Google Scholar]
- 55. Li W, Guo L, Rathi P, et al. Redirecting T cells to glypican-3 with 4–1BB zeta chimeric antigen receptors results in Th1 polarization and potent antitumor activity. Hum Gene Ther 2017; 28: 437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu H, Xu Y, Xiang J, et al. Targeting alpha-fetoprotein (AFP)-MHC complex with CAR T-cell therapy for liver cancer. Clin Cancer Res 2017; 23: 478–488. [DOI] [PubMed] [Google Scholar]
- 57. Krebs K, Bottinger N, Huang LR, et al. T cells expressing a chimeric antigen receptor that binds hepatitis B virus envelope proteins control virus replication in mice. Gastroenterology 2013; 145: 456–465. [DOI] [PubMed] [Google Scholar]
- 58. Kakarla S, Gottschalk S. CAR T cells for solid tumors: armed and ready to go? Cancer J 2014; 20: 151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ma W, Wu L, Zhou F, et al. T cell-associated immunotherapy for hepatocellular carcinoma. Cell Physiol Biochem 2017; 41: 609–622. [DOI] [PubMed] [Google Scholar]
- 60. Morales-Kastresana A, Labiano S, Quetglas JI, et al. Better performance of CARs deprived of the PD-1 brake. Clin Cancer Res 2013; 19: 5546–5548. [DOI] [PubMed] [Google Scholar]
- 61. Chen Y, E CY, Gong ZW, et al. Chimeric antigen receptor-engineered T-cell therapy for liver cancer. Hepatobiliary Pancreat Dis Int 2018; 17: 301–309. [DOI] [PubMed] [Google Scholar]
- 62. Vivier E, Tomasello E, Baratin M, et al. Functions of natural killer cells. Nat Immunol 2008; 9: 503–510. [DOI] [PubMed] [Google Scholar]
- 63. Herberman RB, Reynolds CW, Ortaldo JR. Mechanism of cytotoxicity by natural killer (NK) cells. Annu Rev Immunol 1986; 4: 651–680. [DOI] [PubMed] [Google Scholar]
- 64. Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol 2008; 8: 713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. de Saint Basile G, Menasche G, Fischer A. Molecular mechanisms of biogenesis and exocytosis of cytotoxic granules. Nat Rev Immunol 2010; 10: 568–579. [DOI] [PubMed] [Google Scholar]
- 66. Wang W, Erbe AK, Hank JA, et al. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol 2015; 6: 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Takeda K, Hayakawa Y, Smyth MJ, et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med 2001; 7: 94–100. [DOI] [PubMed] [Google Scholar]
- 68. Shi FD, Ljunggren HG, La Cava A, et al. Organ-specific features of natural killer cells. Nat Rev Immunol 2011; 11: 658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sun C, Sun HY, Xiao WH, et al. Natural killer cell dysfunction in hepatocellular carcinoma and NK cell-based immunotherapy. Acta Pharmacol Sin 2015; 36: 1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hosseinzadeh F, Verdi J, Ai J, et al. Combinational immune-cell therapy of natural killer cells and sorafenib for advanced hepatocellular carcinoma: a review. Cancer Cell Int 2018; 18: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Capurro M, Wanless IR, Sherman M, et al. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003; 125: 89–97. [DOI] [PubMed] [Google Scholar]
- 72. Shirakawa H, Suzuki H, Shimomura M, et al. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci 2009; 100: 1403–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sawada Y, Yoshikawa T, Nobuoka D, et al. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res 2012; 18: 3686–3696. [DOI] [PubMed] [Google Scholar]
- 74. Sawada Y, Yoshikawa T, Ofuji K, et al. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology 2016; 5: e1129483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nobuoka D, Yoshikawa T, Takahashi M, et al. Intratumoral peptide injection enhances tumor cell antigenicity recognized by cytotoxic T lymphocytes: a potential option for improvement in antigen-specific cancer immunotherapy. Cancer Immunol Immunother 2013; 62: 639–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wu Q, Le Trinh T, Zuo C, et al. A novel vaccine targeting glypican-3 as a treatment for hepatocellular carcinoma. Mol Ther 2017; 25: 2299–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhu AX, Gold PJ, El-Khoueiry AB, et al. First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin Cancer Res 2013; 19: 920–928. [DOI] [PubMed] [Google Scholar]
- 78. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010; 30: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008; 100: 698–711. [DOI] [PubMed] [Google Scholar]
- 80. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017; 18: e143–e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Park YJ, Kuen DS, Chung Y. Future prospects of immune checkpoint blockade in cancer: from response prediction to overcoming resistance. Exp Mol Med 2018; 50: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sia D, Jiao Y, Martinez-Quetglas I, et al. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology 2017; 153: 812–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell 2017; 169: 1327–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Harding JJ, Nandakumar S, Armenia J, et al. Prospective genotyping of hepatocellar carcinoma: clinical implications of next generation sequencing for matching patients to targeted and immune therapies. Clin Cancer Res 2019; 25: 2116–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Goodman AM, Kato S, Bazhenova L, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 2017; 16: 2598–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med 2017; 377: 2500–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vilain RE, Menzies AM, Wilmott KS, et al. Dynamic changes in PD-L1 expression and immune infiltrates early during treatment predict response to PD-1 blockade in melanoma. Clin Cancer Res 2017; 23: 5024–5033. [DOI] [PubMed] [Google Scholar]
- 88. Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515: 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yu LX, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nature Rev Gastroenterol Hepatol 2017; 14: 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018; 359: 91–97. [DOI] [PubMed] [Google Scholar]
- 91. Soularue E, Lepage P, Colombel JF, et al. Enterocolitis due to immune checkpoint inhibitors: a systematic review. Gut 2018; 67: 2056–2067. [DOI] [PubMed] [Google Scholar]
- 92. Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 2017; 23: 1920–1928. [DOI] [PubMed] [Google Scholar]
- 93. Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 2017; 23: 4242–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Scheiner B, Kirstein MM, Hucke F, et al. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment Pharmacol Ther 2019; 49: 1323–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liu K, Zhang X, Xu W, et al. Targeting the vasculature in hepatocellular carcinoma treatment: Starving versus normalizing blood supply. Clin Transl Gastroenterol 2017; 8: e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pinter M, Jain RK. Targeting the renin-angiotensin system to improve cancer treatment: implications for immunotherapy. Sci Transl Med 2017; 9: pii: eaan5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhao Y, Ting KK, Li J, et al. Targeting vascular endothelial-cadherin in tumor-associated blood vessels promotes T-cell-mediated immunotherapy. Cancer Res 2017; 77: 4434–4447. [DOI] [PubMed] [Google Scholar]
- 98. Tian L, Goldstein A, Wang H, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 2017; 544: 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018; 378: 158–168. [DOI] [PubMed] [Google Scholar]
- 100. Giannini EG, Risso D, Testa R, et al. Prevalence and prognostic significance of the presence of esophageal varices in patients with hepatocellular carcinoma. Clin Gastroenterol Hepatol 2006; 4: 1378–1384. [DOI] [PubMed] [Google Scholar]
- 101. Kudo M, Matilla AM, Santoro A, et al. Nivolumab in patients with Child-Pugh B advanced hepatocellular carcinoma (aHCC) in the Checkmate-040 study. Hepatology 2018; 68: S1. [DOI] [PubMed] [Google Scholar]
- 102. Cheng R, Cooper A, Kench J, et al. Ipilimumab-induced toxicities and the gastroenterologist. J Gastroenterol Hepatol 2015; 30: 657–666. [DOI] [PubMed] [Google Scholar]
- 103. Couto OF, Dvorchik I, Carr BI. Causes of death in patients with unresectable hepatocellular carcinoma. Dig Dis Sci 2007; 52: 3285–3289. [DOI] [PubMed] [Google Scholar]