Abstract
Rationale:
With increasing number of complex medical patients with renal transplant who get pregnant, clinicians need to be aware of abdominal compartment syndrome which may masquerade as acute renal allograft injury in pregnancy.
Presenting concerns of the patient:
A 34-year-old nulliparous Caucasian female with end-stage renal disease (ESRD) due to type 1 diabetes mellitus who received a simultaneous pancreas-kidney transplant (SPK) in 2006 and then after rejection of renal allograft another, kidney-only allograft from a donation after circulatory death became pregnant in May 2013 with dichorionic, diamniotic twins without reproductive technology, and during pregnancy, she developed two episodes of acute injury to the renal allograft.
Diagnoses:
End-stage renal disease secondary to type I diabetes, acute renal allograft injury, tacrolimus toxicity, abdominal pain.
Interventions (including prevention and lifestyle):
She received intravenous hydration, medications contributing to renal failure were held, and pain and nauseas were controlled appropriately. Abdominal compartment syndrome was managed by maintaining intravascular pressure and optimizing regional and systemic vascular perfusion by appropriate fluid balance, evacuating intraluminal contents by decompressing gastrointestinal system, and improving abdominal wall compliance by using appropriate analgesics, sedation, and patient positioning.
Outcomes:
With advancing pregnancy, the patient developed progressive abdominal pain, nausea, leg edema, and rising creatinine that were not responsive to ongoing therapies and required delivery via Cesarean section at 31 weeks of gestational age.
Lessons learned:
In the era of increasing number of pregnant renal transplant patients with multiple medical issues, we need organized approach to diagnosis of acute renal allograft injury in pregnancy and we need to consider abdominal compartment syndrome as one of the causes.
Keywords: AKI (acute kidney injury), pregnancy, renal transplant, pancreatic transplant, abdominal compartment syndrome
Abrégé
Justification:
Le nombre croissant de grossesses chez les greffées d’un rein aux prises avec des problèmes de santé complexes oblige les cliniciens à connaître le syndrome du compartiment abdominal; un trouble qui, pendant la grossesse, peut contribuer à une insuffisance rénale aiguë du greffon.
Présentation du cas:
Une femme nullipare de 34 ans d’origine caucasienne et atteinte d’insuffisance rénale terminale (IRT) consécutive à un diabète de type 1. La patiente avait subi une première greffe simultanée rein-pancréas en 2006 puis, pour cause de rejet, une deuxième transplantation d’un rein seulement, lequel provenait d’un donneur décédé d’un problème circulatoire. La patiente est tombée enceinte de jumeaux diachroniques et diamniotiques en mai 2013 sans procréation assistée. La grossesse a été ponctuée de deux épisodes d’insuffisance rénale aiguë du greffon.
Diagnostic:
IRT consécutive à un diabète de type 1, insuffisance rénale aiguë du greffon, toxicité du tacrolimus, douleurs abdominales.
Interventions (prévention et habitudes de vie):
La patiente a été réhydratée par intraveineuse, les douleurs abdominales et les nausées ont été soulagées, et les médicaments contribuant à l’insuffisance rénale ont été temporairement cessés. Le syndrome du compartiment abdominal a été traité en maintenant la pression intravasculaire et en optimisant la perfusion vasculaire locale et systémique par un équilibre hydrique approprié, en évacuant le contenu intraluminal par décompression du système gastro-intestinal, et en améliorant la compliance de la paroi abdominale par l’administration d’analgésiques, par la sédation et par le positionnement de la patiente.
Issue:
Avec la progression de la grossesse, les symptômes de douleurs abdominales, nausées, œdème aux membres inférieurs et augmentation de la créatinine ayant cessé de répondre aux traitements, la patiente a dû accoucher par césarienne à 31 semaines.
Enseignements tirés:
Le nombre croissant de femmes enceintes greffées d’un rein et atteintes de problèmes de santé complexes plaide pour une approche concertée dans le diagnostic de l’insuffisance aiguë du greffon pendant la grossesse. Le syndrome du compartiment abdominal doit être envisagé comme l’une des causes de l’insuffisance rénale aiguë en grossesse.
What was known before
There is a scarcity of literature that addresses the epidemiology of acute kidney injury (AKI) in pregnancy after renal transplantation. This is made further challenging by the potential for multiple gestations in the current era of assistive reproductive technology. It is known that in the nontransplant population, multiple gestational pregnancies are associated with a higher risk of hyperemesis gravidarum, pre-eclampsia, and urinary obstruction—all of which increase the risk of AKI.1
What this adds
In the setting of the pregnant renal allograft recipient, approaching AKI via gestational age, the proper use of urinalysis, calcineurin inhibitor (CNI) level, presence of donor-specific antibody (DSA), and pre-eclampsia laboratory investigations allows for efficient and accurate narrowing of differential diagnoses. Intra-abdominal hypertension should be considered in pregnant patients presenting with AKI, increasing abdominal pain and severe leg edema. Bladder pressure should be measured in a standardized way to rule out intra-abdominal hypertension and management based on gestational age and maternal and fetal status. Our table allows the attending team to make this an efficient approach.
Case Presentation
A 34-year-old nulliparous Caucasian female with end-stage renal disease (ESRD) due to type 1 diabetes mellitus received a simultaneous pancreas-kidney transplant (SPK) in 2006 after having been treated with hemodialysis for 2 years. Post-transplantation, she experienced early severe acute T-cell-mediated rejection. In 2007, she developed unprovoked deep vein thrombosis with pulmonary embolism and progressive peripheral vascular disease leading to unilateral below-knee amputation. The kidney allograft failed in 2008 as a late consequence of the aforementioned rejection, at which point she resumed hemodialysis. She was re-transplanted in 2011 with a kidney-only allograft from donation after circulatory death. In 2012, she was treated for osteomyelitis of the foot and she underwent an abdominal incisional hernia repair and abdominal skin advancement flaps. By year-end 2012, both her pancreas and kidney grafts remained well functioning with serum creatinine of 80 μmol/L (estimated glomerular filtration rate [eGFR] 77 mL/min/1.73 m²), no proteinuria, no hypertension, and a hemoglobin A1C of 5.5%. Her maintenance immunosuppression included extended release tacrolimus achieving target trough concentration between 6 and 8 μg/L, mycophenolate sodium 540 mg twice daily, and prednisone 5 mg daily.
In early 2013, the patient expressed a desire to become pregnant, and after appropriate counseling, she was switched from mycophenolate sodium to azathioprine 125 mg daily in anticipation of pregnancy. In May of 2013, she became pregnant without reproductive assistance with dichorionic diamniotic twins. Due to her history of venous thromboembolism events, low-molecular-weight heparin, enoxaparin 40 mg, subcutaneously per day was initiated. She was also started on aspirin 81 mg by mouth daily to lower the risk of preeclampsia. The pregnancy was initially unremarkable with excellent renal allograft function with a creatinine nadir of 67 μmol/L (eGFR 94 mL/min/1.73 m²) at 20 weeks gestation. She had no proteinuria. Home blood pressure measurements throughout the first 2 trimesters were 90 to 118/65 to 85 mmHg. Her tacrolimus levels were closely monitored and she required progressively increasing doses throughout pregnancy to maintain trough concentrations between 5 and 7 μg/L. At 28 weeks gestation, she presented with constant lower abdominal pain and decreased appetite. She was hospitalized for further investigations. There was no history of fever, headache, seizure, visual changes, chest pain, or shortness of breath. She was mildly nauseated, but reported no vomiting, diarrhea, or constipation. She denied decreased urine output, graft tenderness, edema, or vaginal bleeding. On presentation, her blood pressure was 111/72 mmHg with a heart rate of 101 beats per minute. She had a normal precordial and pulmonary exam. Her abdominal exam was unremarkable and specifically she demonstrated no tenderness over the kidney allograft or right upper quadrant. Her uterus was palpable with a fundal height appropriate for her gestational age with twin pregnancy.
Initial laboratory investigations on day 1 revealed a serum creatinine of 95 μmol/L, bland urinalysis, and a protein-to-creatinine ratio of 19.04 mg/mmol. Hemoglobin was 101 g/L; white blood cells and platelet counts were 6.9 × 109 L−1 and 209 × 109 L−1, respectively. Her coagulation profile was normal. Glucose, lactate dehydrogenase (LDH), haptoglobin, and hepatic transaminases were normal. A peripheral blood smear revealed anemia with mild macrocytosis but no schistocytes to suggest hemolysis. Fetal ultrasound revealed normal biophysical profiles for both fetuses. Her urine culture was negative.
She was treated with dimenhydrinate for nausea. She received regular diet and otherwise her medications remained unchanged till day 4 of hospitalization. Abdominal pain did not improve. Serum creatinine was remeasured on day 4 of hospitalization and was found to have risen up to 215 μmol/L (eGFR 24 mL/min/1.73m²). Nephrology was consulted at that point. She had a blood pressure of 93/50 mmHg and a heart rate of 105 beats per minute. She had no leg edema. Repeat cell count, glucose, LDH, transaminases, haptoglobin, and peripheral blood smear remained within normal limits. Urinalysis was negative for microscopic hematuria and a protein-to-creatinine ratio was 18.34 mg/mmol and her serum calcium concentration was also elevated at 2.76 mmol/L. An urgent Doppler ultrasound of the renal allograft revealed normal transplant renal artery and cortical blood flows with no evidence of hydronephrosis. Chest and abdominal X-rays were also normal. Magnetic resonance imaging of the abdomen did not reveal any complications with the pancreatic allograft, bile ducts, liver, or bowels; the uterus and 2 fetuses appeared normal as well.
We were facing medically complex pregnant renal transplant recipient with acute rise in creatinine and broad differential diagnosis.
Approach to the Differential Diagnosis
Our most likely diagnoses included the following:
Prerenal insult secondary to volume depletion, hypercalcemia, and high tacrolimus concentrations;
Sepsis from chorioamnionitis;
Acute tubular necrosis (ATN);
Atypical preeclampsia (absence of hypertension);
Rejection;
Ureteric obstruction from enlarged uterus.
The typical approach to AKI in pregnancy involves traditional division of pre-renal, post-renal, and renal aetiologies and involves pregnancy-specific complications (Table 1). In a transplant patient, approach needs to include transplant-specific complications (Table 2). There is virtually no literature regarding approach to AKI in a pregnant renal transplant patient. Moreover, the Risk, Injury, Failure, Loss, and End-Stage Renal Disease (RIFLE) criteria and other AKI diagnostic criteria derived from RIFLE are not very helpful in this context.
Table 1.
Possible Etiologies of Acute Kidney Injury in a Pregnant Patient With a Native Kidney Based on Traditional Pre-Renal, Renal, and Post-Renal Approach.
| Causes of AKI in pregnancy |
|---|
| Pre-renal • Hyperemesis gravidarum • Hemorrhage • Heart failure |
| Intra-renal • Acute tubular necrosis • Acute cortical necrosis • Acute fatty liver of pregnancy • Preeclampsia/HELLP • Thrombotic thrombocytopenic purpura/atypical hemolytic uremic syndrome • Pyelonephritis • Amniotic fluid embolus • Lupus nephritis • Acute interstitial nephritis |
| Post-renal • Hydronephrosis due to uterine compression • Injury to ureters or bladder during C-section • Ureteral obstruction from stones or tumor • Obstruction at bladder outlet |
Source. Reprinted with permission from Jim and Garovic.2
Note. AKI = acute kidney injury; HELLP = hemolysis, elevated liver function tests, low platelet count.
Table 2.
Causes of Acute Kidney Injury in Kidney Transplant Recipients.
| AKI susceptibility factors | • Solitary kidney • Calcineurin inhibitors • Iodine contrast-enhanced studies • Nephrotoxic antibiotics |
| Immunologic | • Acute cellular rejection • Acute antibody-mediated rejection • Mixed rejection |
| Recurrence of native kidney disease | • C3 glomerulonephritis • Atypical hemolytic uremic syndrome • Primary focal segmental glomerulosclerosis • IgA glomerulonephritis • Primary hyperoxaluria • Other (membranous nephropathy, pauci-immune glomerulonephritis, anti-GBM disease, lupus nephritis, diabetic nephropathy) |
| Medication induced | • Calcineurin inhibitors • Intravenous immunoglobulin • Nephrotoxic antibiotics |
| Infections | • BK virus nephropathy • Urinary tract infections/pyelonephritis • Other (CMV, EBV, histoplasma infections) |
| Urinary tract obstruction | • Ureteral stricture • Bladder outlet obstruction • Neurogenic bladder • Ureteral stent • Lymphocele • Seroma • Hematoma • Urinoma |
| Hematologic | • Renal artery thrombosis • Renal vein thrombosis • Kidney allograft thrombosis |
| Malignancy | • Post-transplant lymphoproliferative disorder • Nephrotoxic chemotherapy |
Source. Reprinted with permission from AbuJawdeh and Govil.3
Note. AKI = acute kidney injury; IgA = immunoglobulin A; GBM = glomerular basement membrane; CMV = cytomegalovirus; EBV = Epstein-Barr virus.
The workup can become quite cumbersome and inefficient as the likelihood of particular diagnosis of AKI during gestation changes with time. Therefore, we propose a combination of traditional approach with trimester-based approach that makes diagnostic workup more efficient and could serve as a general guide to approach AKI in pregnant kidney transplant recipient (Table 3). We will analyze this approach with the current case.
Table 3.
Approach to Acute Kidney Injury in a Pregnant Patient With Renal Transplant Based on Traditional Division of Pre-Renal, Renal and Post-Renal Etiologies Combined With Gestational Age.
| Diagnosis | 1-12 weeks gestation | 13-20 weeks gestation | >20 weeks gestation | |
|---|---|---|---|---|
| Pre-renal | Hyperemesis gravidarum | + | + | − |
| Nausea/vomiting/diarrhea/low fluid intake due to any cause | + | + | + | |
| Septic abortion | + | + | − | |
| Early pregnancy hemorrhage due to miscarriage, ectopic pregnancy, placental abruption | + | + | − | |
| Intra-abdominal sepsis | + | + | + | |
| Viral infections (influenza, CMV induced diarrhea) or bacterial infections | + | + | + | |
| Calcineurin inhibitor toxicity | + | + | + | |
| Vasoconstriction due to medications, cocaine, hypercalcemia | + | + | + | |
| Late pregnancy hemorrhage due to placental abruption, placenta previa, uterine rupture, vasa previa | − | − | + | |
| Chorioamnionitis | − | − | + | |
| Amniotic fluid/pulmonary embolism | −/+ | −/+ | +/+ | |
| Heart failure from peripartum cardiomyopathy | − | − | + | |
| Renal | ATN | + | + | + |
| Interstitial nephritis | + | + | + | |
| Recurrent or de novo glomerular disease | + | + | + | |
| Vasculitis | + | + | + | |
| Rejection: T-cell mediated or antibody mediated | + | + | + | |
| Infection: BK virus or pyelonephritis | + | + | + | |
| Vascular compromise | ||||
| Transplant artery stenosis | + | + | + | |
| Abdominal compartment syndrome | − | +/− | + | |
| Uterine compression of allograft vessels | − | + | + | |
| Pregnancy-specific thrombotic microangiopathy | ||||
| Pre-eclampsia, HELLP syndrome | − | Very rare | + | |
| Acute fatty liver of pregnancy | − | − | + | |
| Disseminated intravascular coagulation | + | + | + | |
| Thrombotic thrombocytopenic purpura (TTP), typical/atypical hemolytic uremic syndrome (HUS/aHUS), broad differential should include | + | + | + | |
| • Systemic lupus erythematosus | + | + | + | |
| • Cryoglobulinemia | + | + | + | |
| • Antiphospholipid antibody syndrome | + | + | + | |
| Allograft-specific thrombotic microangiopathy | ||||
| Drug-induced (CNIs), cytomegalovirus infection, antibody-mediated rejection | + | + | + | |
| Post-renal | Obstruction: | |||
| Nephrolithiasis | + | + | + | |
| Uterine compression of allograft ureter | − | + | + | |
| Allograft ureteric stenosis | + | + | + | |
| Neurogenic bladder | + | + | + |
Once the possible etiologies based on Table 3 are generated, further analysis of clinical history, physical exam, blood tests, urinalysis, proteinuria, and diagnostic imaging will aid in narrowing the differential diagnosis.
We have to consider physiologic adaptations of pregnancy that are well described4,5 and include higher GFR and lower creatinine concentrations typically 35 to 70 μmol/L, urinary stasis in dilated ureters,6 and lower urea concentrations7 which also occur in allograft.8,9
Furthermore, it is important to establish a pregestation and early pregnancy baseline allograft function including urinalysis and proteinuria. There are no current recommendations for frequency of bloodwork monitoring. In our practice, we obtain blood work weekly to every 2 weeks in a stable patient and it includes urinalysis, protein to creatinine ratio, electrolytes, creatinine, and CNI drug levels. This may change if complications develop. Pregnancy results in lower whole blood tacrolimus concentrations, but there is no significant change in unbound concentrations if no adjustment in dosage is made,10 which makes monitoring and adjusting dosages more difficult. Also, many patients may have had their immunosuppressive regimen changed recently in anticipation of pregnancy, so monitoring for possible rejection is quite important.11
Our patient presented with AKI at 28 weeks gestational age, which falls into the third time period in our approach (Table 3). Sepsis, pyelonephritis or chorioamnionitis were unlikely with blood pressure unchanged from her baseline, absence of fever and negative urine and blood cultures. It was felt that she was moderately volume contracted based on her low oral intake, lower range of her baseline blood pressure, and absence of leg edema.
She did not have signs or symptoms of heart failure or pulmonary embolism and chest X-ray was normal. Fetal and placental ultrasounds ruled out late pregnancy hemorrhage.12 Her kidney allograft ultrasound and Doppler did not reveal any renal stones, hydronephrosis, vascular compromise, or neurogenic bladder.
In our patient, protein-to-creatinine ratio was slightly above normal. Investigations for complete blood count, haptoglobin, liver enzymes, partial thromboplastin time (PTT), and international normalized ratio (INR) were normal and peripheral blood smear did not show any schistocytes. This made pre-eclampsia; hemolysis, elevated liver function tests, low platelet count (HELLP) syndrome; de novo glomerulonephritis; acute fatty liver of pregnancy; disseminated intravascular coagulation; and any form of microangiopathic hemolytic anemia unlikely. However, pre-eclampsia may present in atypical way, and therefore, patient had daily noninvasive fetal monitoring and frequent ultrasounds which did not show any fetal distress.
Her plasma cytomegalovirus polymerase chain reaction (CMV PCR) study and urine for PCR for polyomavirus (BK) were negative. The Luminex single-antigen bead study was negative for donor-specific antibodies. At that point, we felt that her high tacrolimus trough concentration and hypercalcemia along with relative hypotension and tachycardia suggested that her AKI was likely secondary to vasoconstriction and volume contraction. While our approach helped to guide us to our working diagnosis, it is not possible to definitively differentiate rejection or CNI toxicity without an allograft biopsy.
Nonetheless, based on the working diagnosis, her tacrolimus and calcitriol were held, and she was given intravenous normal saline for volume resuscitation between day 4 and day 8 of hospitalization. Despite these interventions, her urine output decreased to 115 mL per day and her creatinine peaked at 380 μmol/L (eGFR 12.6 mL/min/1.73m²) with urea of 17.2 mmol/L on day 8 of hospitalization. Calcium level normalized but tacrolimus trough concentration remained elevated at 13 μg/L. She was becoming subjectively short of breath on exertion, although her oxygen saturation remained normal on room air, and objectively edematous. Intravenous fluid was stopped. Repeated fetal monitoring did not reveal any fetal distress.
How Would You Confirm the Diagnosis of Tacrolimus Toxicity?
In summary, we were confronted with acutely deteriorating renal allograft function and oliguria in a patient at 29 weeks gestation with a twin pregnancy. The lack of response to intravenous fluid and the persistently elevated tacrolimus level suggested severe AKI from tacrolimus toxicity, in conjunction with initial hypercalcemia-induced vasoconstriction and volume contraction. Her urea level was now 17.2 mmol/L, which may compromise fetal survival.13 A definitive diagnosis was urgently needed to confirm this clinical diagnosis, to rule out allograft rejection, and to prognosticate the recovery time which would help us to make decision regarding dialysis. Therefore, after discussion among the patient, nephrologist, obstetrician, and radiologist, a renal allograft biopsy was performed.
The safety of native kidney biopsy has been well established. A recent study shows that the rate of complications in renal allograft biopsy is also low with only 1.11% cases of hematomas, 2.23% of gross hematuria, 0.37% of hydronephrosis, and 0.74% of hemoglobin decline.14 Studies examining the rates of complications in renal transplant biopsy versus kidney biopsies have conflicting results, with some studies showing lower rates of complications in renal allografts and others showing higher rates of complications.15 The limited literature regarding renal biopsy in pregnancy indicates 7% vs 1% risk of bleeding in pregnancy vs postpartum period.16 There is no literature regarding renal transplant biopsy risks specifically in pregnancy.
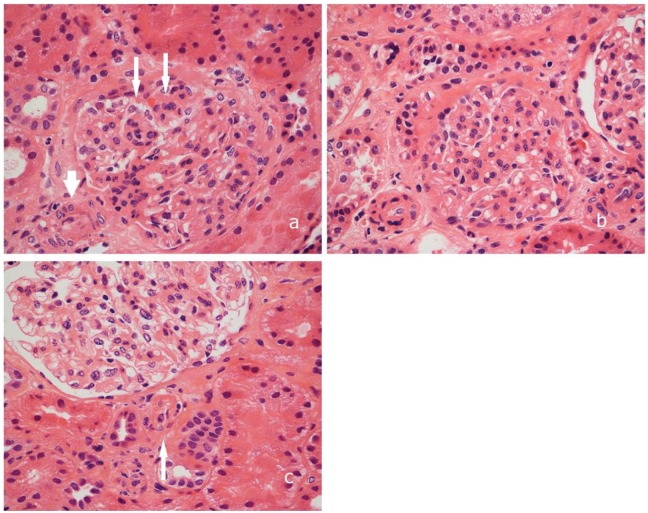
Our patient’s renal biopsy (Figure 1) revealed mild tubular injury without evidence of tubulitis, glomerulitis, or peritubular capillaritis (Banff Score: G0 CG0 I0 CI0 T0 CT0 V0 CV0 AH0 MM1 PTC0). Immunofluorescence for c4d was negative. The small arteries were within normal limits; however, the arterioles demonstrated endothelial swelling and proliferation to the extent of luminal obliteration. Many arterioles showed hyperplastic changes within their walls, an obliterative thrombus was noted within one of the arterioles, suggestive of thrombotic microangiopathy. With this histology, we were able to confidently rule out rejection.
Figure 1.
Pictures showing findings on the renal biopsy. (A) arteriolar intimal swelling (short arrow) accompanied by segmental endocapillary hypercellularity and endothelial swelling (long arrows). (B) Hyperplastic arteriolar thickening consistent with injury. (C) glomerular arteriole containing an organizing thrombus (arrow). All hematoxylin-eosin, original magnification (200×).
The main differential diagnosis for the localized thrombotic microangiopathy17 in this case was narrowed down to tacrolimus toxicity (Tables 1 and 2). This helped us to make a decision to wait with renal replacement therapy as long as there were no acute maternal indications or fetal distress. Within 24 hours after renal biopsy, her tacrolimus trough concentration decreased to 8.8 μg/L. Her urine output improved and her creatinine gradually normalized to her baseline of 86 μmol/L (eGFR 75.9 mL/min/1.73 m²) over the next 5 days. The improvement of renal allograft function with conservative management (holding and then restarting tacrolimus at a lower dose) provided further support for the diagnosis of tacrolimus-induced localized thrombotic microangiopathy.
How Would You Further Diagnose Her Abdominal Pain?
However, we still were uncertain why, in the first place, she had abdominal pain and low oral intake which led to fluid depletion and subsequently elevated tacrolimus and calcium levels. She required now opioids for pain management.
Over the next 2 weeks, her abdominal pain continued to worsen and she developed severe lower limb edema, and a second episode of AKI with a creatinine increasing again to 175 μmol/L (eGFR 32 mL/min/1.73 m²) associated with decreased urine output. A repeat analysis of AKI was done using Table 3. Repeat urinalysis, laboratory investigations, and a renal allograft ultrasound, including fetal ultrasonography, were within normal limits. The tacrolimus trough concentrations were maintained between 4 and 6 μg/L.
In summary, we had a patient with increasing abdominal pain, increasing leg edema which was not responding to diuretics, decreasing urine output, and worsening creatinine with otherwise nonrevealing blood work. This was pointing toward increased intra-abdominal pressure. After review of literature, we performed bladder pressure measurement in a standardized way.18,19 It was 29 mmHg (normal 10-15 mmHg at physiological, near-term pregnancy), indicating increased intra-abdominal pressure, and with rising creatinine suggestive of abdominal compartment syndrome.
Management of Abdominal Compartment Syndrome in Pregnancy
On the same day, the patient underwent emergent Caesarian section at 32 weeks gestational age. After delivery, her renal function normalized within 48 hours and the abdominal pain resolved immediately. The twin newborns required brief neonatal intensive care monitoring, though sustained no overt complications.
Discussion
Since the completion of the first successful kidney transplant in 1950, surgical techniques, immunosuppression, and outcomes have improved significantly. More women of childbearing age are undergoing kidney transplantation and there are an increasing number of pregnancies among kidney transplant recipients.20-24 This creates a new subset of pregnant patients who require unique considerations if confronted with renal failure.25-29
There is a scarcity of literature that addresses the epidemiology of AKI in pregnancy after renal transplantation. This is made further challenging by the potential for multiple gestations in the current era of assistive reproductive technology. It is known that in the nontransplant population, multiple gestational pregnancies are associated with a higher risk of hyperemesis gravidarum, pre-eclampsia and urinary obstruction—all of which increase the risk of AKI.1
Several sources have presented a differential diagnosis of AKI in pregnancy as well as AKI of the kidney allograft,12,1-3 but there is no widely accepted approach to AKI specific to pregnant renal transplant recipients. When approaching AKI in this select population, it is helpful to be aware of pregnancy-specific complications as well as those that are unique to the renal allograft (Table 3).
As illustrated by the presented case, where 2 separate episodes of AKI occurred, the approach allowed for very efficient and appropriate investigations to be carried out. We present this approach as a general guidance to investigate AKI in pregnant kidney transplant recipients. It is not meant to be comprehensive nor does it offer management options.
There is a handful of cases of multiple gestational pregnancies after renal allograft transplantation,20-24 and there is a growing number of singleton pregnancies in patients who have had an SPK transplant.30,31 To our knowledge, this is the first documented case of successful twin pregnancy after SPK transplantation, and this case was particularly complicated in that the patient had 2 apparently unrelated episodes of AKI: thrombotic microangiopathy associated with tacrolimus toxicity, followed by abdominal compartment syndrome.
After further consideration of the case, it became more obvious that increasing intra-abdominal pressure was the cause of abdominal pain and low fluid intake which in turn led to hypovolemia, increased calcium and tacrolimus levels, and the initial episode of AKI. While we addressed the latter, her gestation was advancing and intra-abdominal pressure continued to rise leading to organ dysfunction manifesting as the second episode of AKI, this time caused by organ hypoperfusion.
Pregnancy is typically associated with chronically elevated intra-abdominal pressure (IAP) in the range of 10 to 15 mmHg. Physiologic adaptation usually prevents decompensation, even with pressures consistent with intra-abdominal hypertension (IAH).32-35
Our suspicion of evolving abdominal compartment syndrome was ultimately a diagnosis of exclusion, having ruled out all other etiologies of AKI, and with clear evidence of elevated IAP and end organ injury. This patient had multiple risk factors including twin pregnancy and previous intra-abdominal surgeries (ie, SPK transplantation, hernia repair with mesh, abdominal skin advancement flaps). The ability of the abdominal wall and skin to stretch through pregnancy was restricted in this patient. The severe abdominal pain without obvious cause and relatively normal fetal and uterine physiology also pointed toward abdominal compartment syndrome.
Literature on IAH and abdominal compartment syndrome is limited in the setting of pregnancy.18 Normal IAP varies between sub-atmospheric up to 6.5 mmHg in uncomplicated hospitalized patients.36 IAH is defined as IAP > 12 mmHg and can culminate as abdominal compartment syndrome if the pressure exceeds 20 mmHg with evidence of organ failure, such as AKI or bowel ischemia.32,37 Additional risk factors include twin pregnancy and previous intra-abdominal surgeries.
The treatment of intra-abdominal hypertension-abdominal compartment syndrome should balance the risk to the mother and fetus depending on gestational age and with the desire to achieve more advanced gestational age in premature pregnancy.
The uterus is the ideal environment that enables the fetus to grow and develop the complex body systems essential for extrauterine survival. Prematurely delivered neonates have mortality rates largely determined by gestational age at birth: 100% at 22 weeks, 43% at 28 weeks, 5% to 10% at 28 to 31 weeks, and 1% to 2% at 32 to 34 weeks.38,39 Neonates born between 28 and 34 weeks of gestational age are at high risk of complications including acute respiratory distress syndrome, requirement for assisted ventilation, bronchodysplasia, and intraventricular hemorrhage.40 Usually, the longer one can prolong intrauterine gestation, the better the neonatal outcome. A multidisciplinary team of nephrology, obstetrics, and maternal-fetal medicine, along with the patient, should come to consensus for AKI management on a case-by-case basis.
In case of intra-abdominal hypertension and premature pregnancy, expectant management if possible consist of maintaining intravascular pressure and optimizing regional and systemic vascular perfusion by appropriate fluid balance, evacuating intraluminal contents by decompressing gastrointestinal system, and improving abdominal wall compliance by using appropriate analgesics, sedation and patient positioning.41,42 However, worsening renal function, liver dysfunction, bowel ischemia, maternal instability or signs of fetal discomfort are indications for delivery.
In conclusion, in the setting of the pregnant renal allograft recipient, approaching AKI via gestational age, the proper use of urinalysis, CNI level, the presence of DSA, and pre-eclampsia laboratory investigations allows for efficient and accurate narrowing of differential diagnoses. Decisions such as renal allograft biopsy or need for renal replacement therapy should be carefully weighed against the gestational age and the likely outcome of the fetus.
Intra-abdominal hypertension should be considered in pregnant patients presenting with AKI, increasing abdominal pain and severe leg edema. Bladder pressure should be measured in a standardized way and management based on gestational age and maternal and fetal status.
Footnotes
Ethics Approval and Consent to Participate: No ethics was required as our article reviewed existing literature. Patient provided consent for the case report.
Consent for Publication: MM and KW reviewed the literature and are the primary authors. RP provided supervision and clinical expertise. All authors have consented for publication.
Availability of Data and Materials: All data is provided in the article and supplementary tables. Primary data can be obtained directly from the original articles.
Authors’ Note: This article has been peer reviewed. Patient consent was obtained.
Author Contributions: Magdalena Michalska and Kevin Wen contributed to the design of the manuscript. All of the authors contributed to the drafting and revision of the manuscript and to the collection of data and approved the final version submitted for publication.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Magdalena Michalska  https://orcid.org/0000-0002-2784-2959
https://orcid.org/0000-0002-2784-2959
References
- 1. Chung PH, Abramowicz JS, Edgar DM, Sherer DM. Acute maternal obstructive renal failure in a twin gestation despite normal physiological pregnancy-induced urinary tract dilation. Am J Perinatol. 1994;11:242-244. doi: 10.1055/s-2008-1040755. [DOI] [PubMed] [Google Scholar]
- 2. Jim B, Garovic V. Acute kidney injury in pregnancy. Semin Nephrol. 2017;37(4):378-385. doi: 10.1016/j.semnephrol.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. AbuJawdeh BG, Govil A. Acute kidney injury in transplant setting: differential diagnosis and impact on health and health care. Adv Chronic Kidney Dis. 2017;24(4):228-232. doi: 10.1053/j.ackd.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 4. Conrad K, Davison JM. The renal circulation in normal pregnancy and preeclampsia: is there a place for relaxin? Am J Physiol Renal Physiol. 2014;306:1121-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davison JM, Dunlop W. Renal hemodynamics and tubular function normal human pregnancy. Kidney Int. 1980;18(2):152-161. [DOI] [PubMed] [Google Scholar]
- 6. Côté AM, Firoz T, Mattman A, Lam EM, von Dadelszen P, Magee LA. The 24-hour urine collection: gold standard or historical practice? Am J Obstet Gynecol. 2008;199(6):625.e1-625.e6. [DOI] [PubMed] [Google Scholar]
- 7. Lind T, Godfrey KA, Otun H, Philips PR. Changes in serum uric acid concentrations during normal pregnancy. Br J Obstet Gynaecol. 1984;91(2):128-132. [DOI] [PubMed] [Google Scholar]
- 8. Davison JM. The effect of pregnancy on kidney function in renal allograft recipients. Kidney Int. 1985;27:74-79. [DOI] [PubMed] [Google Scholar]
- 9. Cardonick E, Moritz M, Armenti V. Pregnancy in patients with organ transplantation: a review. Obstet Gynecol Surv. 2004;59(3):214-221. [DOI] [PubMed] [Google Scholar]
- 10. Zheng S, Easterling TR, Umans JG, et al. Pharmacokinetics of tacrolimus during pregnancy. Ther Drug Monit. 2012;34:660-970. doi: 10.1097/FTD.0b013e3182708edf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fuchs KP, Wu D, Ebcioglu Z. Pregnancy in renal transplant recipients. Semin Perinatol. 2007;31:339-347. [DOI] [PubMed] [Google Scholar]
- 12. Nwoko R, Plecas D, Garovic VD. Acute kidney injury in the pregnant patient. Clin Nephrol. 2012;78:478-486. doi: 10.5414/cn107323. [DOI] [PubMed] [Google Scholar]
- 13. Asamiya Y, Otsubo S, Matsuda Y, et al. The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int. 2009;75(11):1217-1222. [DOI] [PubMed] [Google Scholar]
- 14. Tsai SF, Chen CH, Shu K-H, et al. Current safety of renal allograft biopsy with indication in adult recipients. Medicine (Baltimore). 2016;95(6):e2816. doi: 10.1097/MD.0000000000002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prasanna A, Weerakkody RM, Wijewickrama ES, Cassim MRN, Wijeyarathne M. Salvage of bleeding renal allograft following biopsy, with suture technique; a case report. J Med Case Rep. 2016;10:82. doi: 10.1186/s13256-016-0870-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Piccoli GB, Diadola G, Attini R, et al. Kidney biopsy in pregnancy: evidence for counselling? a systematic narrative review. BJOG. 2013;120: 412-427. [DOI] [PubMed] [Google Scholar]
- 17. Iwami D, Harada H, Hotta K, et al. A case of pregnancy-induced thrombotic thrombocytopenic purpura with a kidney allograft recipient. Clin Transplant. 2010;24(suppl 22):66-69. doi:10.1111/j.1399-0012.2010.01272.x. [DOI] [PubMed] [Google Scholar]
- 18. Chun R, Baghirzada L, Tiruta C, Kirkpatrick AW. Measurement of intra-abdominal pressure in term pregnancy: a pilot study. Int J Obstet Anesth. 2012. doi: 10.1016/j.ijoa.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 19. DeKeulenaer BL, DeWaele JJ, Powell B, Malbrain ML. What is normal intra-abdominal pressure and how is it affected by positioning, body mass and positive end-expiratory pressure. Intensive Care Med. 2009;35:969-976. doi:10.1007/s00134-009-1445-0. [DOI] [PubMed] [Google Scholar]
- 20. Gizzo S, Noventa M, Saccardi C, et al. Twin pregnancy after kidney transplantation: what’s on? a case report and review of literature. J Matern Fetal Neonatal Med. 2014;27:17:1816-1819. [DOI] [PubMed] [Google Scholar]
- 21. Furman B, Wiznitzer A, Hackmon R, Gohar J, Mazor M. Multiple pregnancies in women after renal transplantation. Eur J Obstet Gynecol Reprod Biol. 1999;84:107-110. [DOI] [PubMed] [Google Scholar]
- 22. Nicovani V, Poblete H, Carrera M, Perez L. Successful multiple pregnancy (triplets) in a kidney transplant recipient: a case report. Transplant Proc. 2009;41:2688-2690. [DOI] [PubMed] [Google Scholar]
- 23. delMarColon M, Hibbard JU. Obstetric considerations in the management of pregnancy in kidney transplant recipients. Adv Chronic Kidney Dis. 2007;14:168-177. doi: 10.1053/j.ackd.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 24. Farr A, Bader Y, Husslein PW, Gyori G, Muhlbacher F, Margreiter M. Ultra-high-risk pregnancies in women after renal transplantation. Eur J Obstet Gynecol Reprod Biol. 2014;180:72-76. doi: 10.1016/j.ejogrb.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 25. First MR, Combs CA, Weislttel P, Miodovnik M. Lack of effect of pregnancy on renal allograft survival or function. Transplantation. 1992;59:249-287. [PubMed] [Google Scholar]
- 26. Rizzoni G, Ehrich JHH, Broyer M, et al. Successful pregnancies in women on renal replacement therapy: report from the EDTA Registry. Nephrol Dial Transplant. 1992;7:279-287. doi:10.1093/oxfordjournals.ndt.a092129. [DOI] [PubMed] [Google Scholar]
- 27. Sturgiss SN, Davison JM. Effect of pregnancy on long term function of renal allografts. Am J Kidney Dis. 1992;19:167-172. [DOI] [PubMed] [Google Scholar]
- 28. Hou S. Pregnancy in renal transplant recipients. Adv Chronic Kidney Dis. 2013;20:253-259. [DOI] [PubMed] [Google Scholar]
- 29. Salmela KT, Kullonen LEJ, Holmberg C, Gronhagen-Riska C. Impaired renal function after pregnancy in renal transplant recipients. Transplantation. 1993;56:1372-1375. [DOI] [PubMed] [Google Scholar]
- 30. Koyama S, Tomimatsu T. The first case report in Japan. J Obstet Gynaecol Res. 2011;37:1711-1716. [DOI] [PubMed] [Google Scholar]
- 31. Smyth A, Gaffney G, Hickey D, Lappin D, Reddan D, Dunne F. Case report successful pregnancy after simultaneous pancreas-kidney transplantation. Case Rep Obstet Gynecol. 2011:983592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malbrain ML, Cheatham ML, Kirkpatrick A, et al. Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. I. Definitions. Intensive Care Med. 2006;32:1722-1732. [DOI] [PubMed] [Google Scholar]
- 33. Sugerman HJ. Effects of increased intra-abdominal pressure in severe obesity. Surg Clin North Am. 2001;81:1063-1075. [DOI] [PubMed] [Google Scholar]
- 34. Malbrain ML, DeKeulenaer BL, Oda J, et al. Intra-abdominal hypertension and abdominal compartment syndrome in burns, obesity, pregnancy, and general medicine. Anaesthesiol Intensive Ther. 2015;47:228-240. doi: 10.5603/AIT.a2015.0021. [DOI] [PubMed] [Google Scholar]
- 35. Chun R, Kirkpatrick AW. Intra-abdominal pressure, intra-abdominal hypertension, and pregnancy: a review. Ann Intensive Care. 2012(2 suppl 1):S5. doi: 10.1186/2110-5820-2-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, De Waele J, Ivatury R. Abdominal compartment syndrome: it’s time to pay attention! Intensive Care Med. 2006;32:1912-1914. [DOI] [PubMed] [Google Scholar]
- 37. Cheatham ML, Malbrain ML, Kirkpatrick A, et al. Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. II. Recommendations. Intensive Care Med. 2007;33:951-962. [DOI] [PubMed] [Google Scholar]
- 38. Moore TA, Berger AM, Wilson ME. A new way of thinking about complications of prematurity. Biol Res Nurs. 2014;16:72-82. doi: 10.1177/1099800412461563. [DOI] [PubMed] [Google Scholar]
- 39. Stoll BJ, Hansen NI. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443-456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spinillo A, Capuzzo E, Stronati M, Iasci A, Ometto A, Solerte L. Early neonatal complications after elective preterm delivery in hypertensive pregnancy. J Perinat Med. 1995;23:175-181. [DOI] [PubMed] [Google Scholar]
- 41. Hunt L, Frost S, Hillman K, Newton PJ, Davidson PM. Management of intra-abdominal hypertension and abdominal compartment syndrome: a review. J Trauma Manag Outcomes. 2014;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheatham M. Nonoperative management of intraabdominal hypertension and abdominal compartment syndrome. World J Surg. 2009;33:1116-1122. [DOI] [PubMed] [Google Scholar]