Abstract
The population size and projected demographics of Vietnam’s 2 largest cities, Ho Chi Minh City (HCMC) and Hanoi, will change dramatically over the next decade. Demographic changes in an aging population coupled with income growth and changes in lifestyle will result in a very different distribution of common cancers in the future. The study aimed to project the number of cancer incidence in the 2 largest populated cities in Vietnam for the year 2025.
Cancer incidence data from 2004 to 2013 collected from population-based cancer registries in these 2 cities were provided by Vietnam National Cancer Institute. Incidence cases in 2013 and the previous decades average annual percent changes of age-standardized cancer incidence rates combined with expected population growth were modeled to project cancer incidence for each cancer site by gender to 2025.
A substantial double in cancer incidence from 2013 to 2025 resulted from a growing and aging population in HCMC and Hanoi. Lung, colorectum, breast, thyroid, and liver cancers, which represent 67% of the overall cancer burden, are projected to become the leading cancer diagnoses by 2025 regardless of genders. For men, the leading cancer sites in 2025 are predicted to be lung, colorectum, esophagus, liver, and pharynx cancer, and among women, they are expected to be breast, thyroid, colorectum, lung, and cervical cancer. We projected an epidemiological transition from infectious-associated cancers to a high burden of cancers that have mainly been attributed to lifestyle in both cities.
We predicted that with 16.9% growth in the overall population and dramatic aging with these 2 urban centers, the burdens of cancer incidence will increase sharply in both cities over the next decades. Data on projections of cancer incidence in both cities provide useful insights for directing appropriate policies and cancer control programs in Vietnam.
Keywords: cancer incidence, projections; average annual percent changes, age-standardized cancer incidence rates, demographic shifts
Introduction
Vietnam, a lower middle-income country, has undergone an epidemiological transition from infectious disease to a high burden of noncommunicable diseases (NCDs).1 Approximately 141 million new incidences and 478 900 death cases related to NCDs, accounted for 79% of total death cases, were estimated in 2017.2,3 Among NCDs, cancer is increasingly a public health problem as more and more people are being diagnosed with and dying from its related diseases.4
Based upon the GLOBOCAN 2018 database using data from HCMC Cancer Registry and mathematical modeling, the International Agency for Research on Cancer released that the age-standardized cancer incidence rates (ASIRs) per 100 000 people for all cancer sites in 2018 were 151.4 for both sexes, 186.7 for men, and 125.2 for women in Vietnam. The number of incident cancers was estimated to be 164 000 among a population slightly over 96 million.5 Liver, lung, stomach, colorectum, and nasopharynx cancers were estimated as the most common cancer diagnoses among men (with 21.5%, 18.4%, 12.3%, 8.4%, and 5.0% of new cases, respectively), which accounted for about 65.6% of new cases. Breast, colorectum, lung, stomach, and liver were the most common cancers among women (with 20.6%, 9.6%, 9.4%, 8.6%, and 7.8% of new cases, respectively), which accounted for 56% all newly diagnosed cases.5 In addition, cancer accounted for 19.9% of all deaths (increased by approximately 7% over 3 past decades) in Vietnam, placing this nation among those with the highest mortality rates worldwide.2,4 The cancer spectrum in Vietnam differs substantially from that seen in developed countries such as the United States and other Western countries.
Vietnam has shifted significantly in its age demographic structures with its population predicted to age considerably in the future.6 The proportion of adults who are 65 years or older is estimated to increase from 7.1% in 2014 to 18.1% (nearly triple) by 2049, whereas the proportion population between the ages 45 and 64 is projected to increase from 19.8% in 2014% to 26.0% in 2049.6 Demographic changes of an aging population coupled with income growth and changes in lifestyle will result in a different distribution of common cancers in the future. Projections of cancer incidence by taking into account demographic changes and historical cancer incidence trend could provide better understanding of the transition in the cancer spectrum. Currently, there are no available long-term national cancer projections for Vietnam.
There exists several notable statistic methods for projecting cancer burden with a variety in sophistication of applied model type from simple linear or log-linear regression of age-specific rates or counts against time to age–period–cohort (APC) modeling.7,8 It is difficult to find one method that is superior for every cancer site and every population cancer registry.9 Several sophisticated methods such as Bayesian APC models have not yet found the easy-to-use way into practice of epidemiologists and have been criticized to produce too wide prediction intervals.10 Among projection methods, projection based on demographic shifts and changes in ASIRs by cancer sites, which is one of the easy-to-use methods, were conducted in the United States11,12 and Germany.13 In the United States, Smith et al11 projected a marked increase in the number of cancer diagnoses in 2020 and 2030 due to demographic changes (ie, the boomer generation of adults over 65 years), whereas Rahib et al12 projected an unexpected increased burden of thyroid, liver, and pancreas cancers in the United States by 2030.12 In 2016, Quante et al projected a significant increase in cancer incidence for prostate, pancreas, and thyroid cancer, whereas breast and lung cancer will continuously increase in incidence over the next 2 decades in Germany with its higher rates of smoking.13 Quante et al also estimated the number of incident cancer cases in 2030, which was very similar to the cancer incidence projected by using a Bayesian statistical approach with a high computational burden.14
For planning and developing appropriate policies and implementation for cancer control programs in Vietnam, it is needed to quantify the future burden of cancer in this country as well as its urban centers. Using local data from the population-based cancer registries in the years 2004 to 2013, we estimated case numbers of common cancers in 2020 and 2025 in Ho Chi Minh City (HCMC) and Hanoi, which are the 2 largest population cities in the nation.
Methods
Study Setting
The HCMC, located in the south of Vietnam, is the largest populated city and the largest commercial center within the country with a population of 7 165 200 permanent residents in 2009.15 After extending Hanoi’s land area in 2008, the capital of Vietnam has become the second largest populated city as well as the second largest economic hub of the nation which has a population of 6 448 800 inhabitants in 2009.15 The population of HCMC is projected to be 8 668 000 in 2020 and 9 018 300 in 2025, whereas population projections in 2020 and 2025 for Hanoi are projected to be 7 576 000 and 7 87 000. In 2025, the total of projected population of Hanoi and HCMC will be 16.9% of the population of Vietnam, 9.0% for HCMC, and 7.9% for Hanoi.6
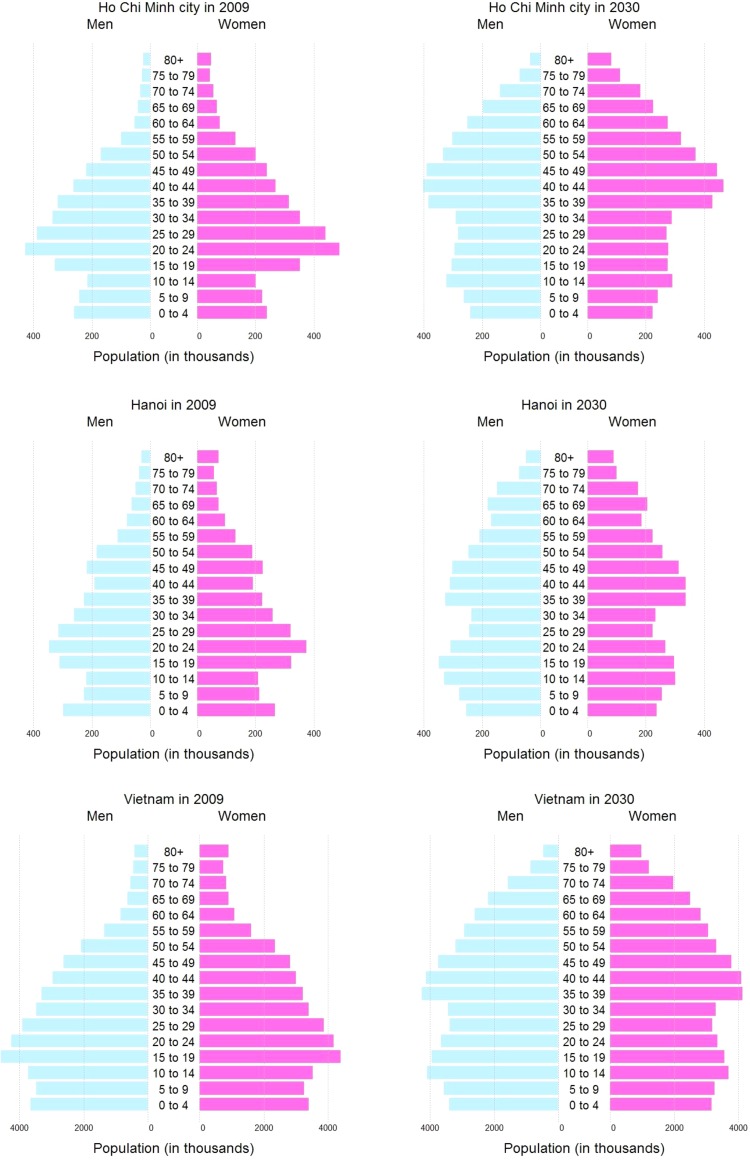
According to 4 conducted population and housing censuses from 1979 to 2009, the proportion of adults aged 65 years or older in HCMC is predicted to increase by 6.1% for men and 6.8% for women, whereas the proportion aged 65 years or older in Hanoi is also estimated to increase by 5.5% for men and 5.8% for women from 2009 to 2030. In the entire of Vietnam, the proportion of adults who are 65 years or older is estimated to increase 50% from 7.1% in 2014 to 11.5% in 2030, whereas the proportion population between the ages of 45 to 64 are predicted to increase from 19.8% in 2014 to 24.8% in 2030.6 (Figure 1).
Figure 1.
Age structure of the population in Ho Chi Minh City, Hanoi, and the entire of Vietnam in 2009 and 2030 (Data source: General Statistics Office).
Cancer Registry
Cancer registries for these 2 cities were established in 1987 in Hanoi and 1990 in HCMC. Details of history, objectives, and activities of these population-based cancer registries have been described previously.16-18 Briefly, the population-based cancer registries in HCMC and Hanoi are the first cancer registries in Vietnam with the highest overall practices and data quality in this nation covering 45 and 30 public and private hospitals, respectively.19 They have provided cancer incidence data to the World Health Organization/International Agency for Research on Cancer (IARC) publication series “Cancer Incidence in Five Continents” since 1991 in Hanoi and 1995 in HCMC. Case ascertainment by cancer registries in Hanoi and HCM primarily came from hospital inpatient and outpatient records as well as pathology diagnostic laboratories’ logs and reports. Registrars, who are routinely trained, visit hospital periodically from 2 to 6 months to review all medical records for verifying newly diagnosed cancer cases. They carry out a paper-based data collection with basic information recommended by IARC and use software CanReg5 for data entry, management, and quality control. Besides Hanoi and HCMC cancer registries, Vietnam has established cancer registries in 7 provinces including Thai Nguyen, Hai Phong, Thanh Hoa, Hue, Da Nang, Can Tho, and Kien Giang. The Vietnam National Cancer Institute (VNCI) plays an essential role to manage National Cancer Registry activities including training courses, data consolidation, and publications. Currently, only data from cancer registries are available, sufficient and well-validated for projecting cancer incidence in Vietnam, particularly in Hanoi and HCMC.
Outcome Ascertainments
Clinicians and oncologists at health settings classified cancer cases according to the International Classification of Diseases for Oncology version 3 (ICD-O-3) and converted to International Classification of Diseases, Tenth Edition (ICD-10) by using CanReg5.20 The ICD-O-3 classification of all cancer cases was rechecked by the registrars from the population-based cancer registries in HCMC and Hanoi. The cancer sites (individual sites or groups of sites) included in this study were (1) all cancer sites (C00-96) without nonmelatonin skin cancer (C44) (2) the 20 cancer sites in Hanoi and HCMC as follows: Oral cavity (C00-C08), pharynx (C9-C14), esophagus (C15), stomach (C16), colorectum (C18-C20), liver (C22), pancreas (C25), larynx (C32), lung and bronchus (lung; C33-34), breast (C50), cervix uteri (C53), uterine corpus (C54), ovary (C56), prostate (C61), kidney (C64), bladder (C67), brain, nervous system (C70-72), thyroid (C73), non-Hodgkin lymphoma (C82-85, C96), leukemia (C91-95), and others.
For the current analysis, we used data from the population-based cancer registries in HCMC and Hanoi provided by the VNCI throughout the years of 2004 to 2013. A total of 171 440 incident cancer cases were entered in these population-based cancer registries in the period. All cancer cases with missing information of gender, age, year of birth, and year of diagnosis were excluded (1379 cases). We further excluded from the study 30 770 cases diagnosed with nonmelatonin skin cancer and all in situ neoplasms coded by ICD-10 as D00 to D76. The final sample size for the analysis was 64 160 incident cancer cases of HCMC Cancer Registry and 75 131 incident cancer cases of Hanoi Cancer Registry.
Data Preparation
The estimation of 5-year age-specific population in the years 2004 to 2013 and 5-year age-specific population projections in the years 2014 to 2025 for HCMC and Hanoi were obtained from the General Statistics Office.6 Consistently, incident data for each site were summarized into 5-year age groups (0-4, 5-9,…, 70-74, 75+ years) by gender and city. The ASIRs and age-specific incidence rates were computed per 100 000 individuals for the years 2004 to 2013. The ASIRs were standardized using the World Standard Population.21 The standard errors of ASIRs also were calculated from the corresponding ASIRs and the number of cases in each site.
Data Analysis and Statistical Method
Cancer incidences for 2020 and 2025 were predicted with a methodology using a base year of 2013 and historic incidence trend data from a 10-year period (2004-2013) combined with expected population growth. Our projections are based on several assumptions including: (1) the trend pattern in cancer incidence remains consistent during the years 2014 to 2025 stratified by city; and (2) population projections for 2014 to 2025 are based on current developments and past trend as reported by the General Statistics Office.
The observed trends from 2004 to 2013 for each cancer site were assessed by using Joinpoint regression, which involves fitting a series joined line segments on a logarithmic scale to the trends in ASIRs with the years 2004 to 2013 as an independent variable. The models incorporated standard errors of ASIRs from 2004 to 2013 to select the best-fitting trend pattern in the period. The estimate slope was transformed back to represent an increasing or decreasing annual percentage in the ASIRs (ie, annual percent changes [AAPCs]). Specifically, the AAPC from 2004 to 2013 for the entire age range was calculated by summarizing the trend over the time period as weighted average of the AAPCs.22 Joinpoint regression program (version 4.6.0.0, April 16, 2018), a freely available software tool from National Cancer Institute, United States, calculated AAPC and respective 95% confidence interval (CI) for each site stratified by sex. A Monte Carlo permutation method was used for the tests of significance. The 95% CI of AAPCs indicate a significant difference from 0 at α of .05 level.
Projected Cancer Incidence
In this study, incident cases in 2013 and the AAPC from 2004 to 2013 combined with expected population projection were modeled to project cancer incidence in these 2 cities to 2020 and 2025. Our projection of cancer incidences was calculated for each cancer site (individual sites or groups of sites) by sex and city using the methods applied by Rahib et al12 for projections in the United States and Quante et al13 for projections in Germany. However, in contrast to Rahib and colleagues12, we still applied AAPCs of age-standardized incidence rates in the projections even though the AAPCs were not significantly different from 0. This approach is followed that of Quante et al in order to reduce bias.13
The following formulas were used to project incidences for 2020 and 2025.12
AAPCi is the AAPC of age-standardized incidence rates for each cancer site by sex. The n is the adjustment in years from the base year (2013), which was 7 for 2020 and 12 for 2025, respectively. Id is the projected incidence based on demographics for 2020 and 2025, which equals the sum of the age-specific incidence rate of year 2013 for each age group j multiplied by the corresponding estimated age-specific population in the modeled year, 2020 or 2025.13
Based on a consideration of the quality and coverage of data from Hanoi Population-based Cancer Registry in 2013, incidence cases in 2012 and the AAPC from 2004 to 2012 combined with expected population projection were modeled, to project cancer incidence in Hanoi to 2013, 2020, and 2025. The n is the adjustment in years from the base year (2012), which was 8 for 2020 and 13 for 2025, respectively.
Results
Table 1 demonstrated the projected cancer incidence for the 5 common cancer sites in HCMC and Hanoi regardless of gender. The number of cancer incidence for all sites is projected to increase from 28 506 in 2020 (HCMC: 11 001 cases, Hanoi: 17 505 cases) to 36 333 in 2025 (HCMC: 13 797 cases, Hanoi: 22 536 cases). Lung (8509 cases), colorectum (6767 cases), breast (3856 cases), thyroid (2971 cases), and liver (2284 cases) cancers are predicted to be the leading cancer sites in 2025, respectively account for 23.4%, 18.6%, 10.6%, 8.2%, and 6.3% of all projected cases (Table 1).
Table 1.
Projection of Cancer Incidence by 5 Common Cancer Sites in 2025 in Both Sexes Stratified by City.
| Cancer Sites | HCMC | Hanoi | Both Cities |
|---|---|---|---|
| # of Cases | # of Cases | # of Cases | |
| All (C00-C96 without C44) | |||
| 2013a | 7431 | 11 255 | 18 686 |
| 2020 | 11 001 | 17 505 | 28 506 |
| 2025 | 13 797 | 22 536 | 36 333 |
| Lung and bronchus (C33-34) | |||
| 2013a | 1078 | 2157 | 3235 |
| 2020 | 1926 | 3994 | 5920 |
| 2025 | 2736 | 5773 | 8509 |
| Colorectum (C18-20) | |||
| 2013a | 1007 | 1140 | 2147 |
| 2020 | 2233 | 2098 | 4331 |
| 2025 | 3741 | 3026 | 6767 |
| Breast (C50) | |||
| 2013a | 1040 | 1229 | 2269 |
| 2020 | 1676 | 1474 | 3150 |
| 2025 | 2245 | 1611 | 3856 |
| Thyroid (C73) | |||
| 2013a | 537 | 581 | 1118 |
| 2020 | 1028 | 992 | 2020 |
| 2025 | 1578 | 1393 | 2971 |
| Liver (C22) | |||
| 2013a | 574 | 759 | 1333 |
| 2020 | 681 | 1206 | 1887 |
| 2025 | 718 | 1566 | 2284 |
Abbreviation: HCMC, Ho Chi Minh City.
aThe number cancer incidence in 2013 was observed for HCMC and projected for Hanoi.
Tables 2 and 3 indicate the AAPC of ASIRs and projected cancer incidence based on changing demographics for the 10 common cancer sites among men and women, stratified by city. The ASIRs for all cancer (excluding non-melatonin skin cancer) are increasing for HCMC (men: 0.4% AAPC, women:1.0% AAPC) and for Hanoi (men: 3.0% AAPC, women: 1.9% AAPC). In HCMC, the number of all cancer incidence for all sites is projected to increase from 5057 in 2020 to 6465 in 2025 among men and from 5944 in 2020 to 7332 in 2025 among women. In Hanoi, projected incidence for all cancer sites are 10 114 male cases and 7391 female cases in 2020 and 13 502 male cases and 9034 female cases in 2025 (Table 2 and 3).
Table 2.
Projection of Cancer Cases by 10 Common Cancer Sites Among Men in 2025, Stratified by City.
| Cancer Sites | HCMC | Hanoi | Both Cities | ||
|---|---|---|---|---|---|
| APPCb | # of Cases | APPCb | # of Cases | # of Cases | |
| All (C00-C96 without C44) | |||||
| 2013a | 0.4 | 3349 | 3.0 | 6093 | 9442 |
| 2020 | 5057 | 10 114 | 15 171 | ||
| 2025 | 6465 | 13 502 | 19 967 | ||
| Lung and bronchus (C33-34) | |||||
| 2013a | 3.1 | 719 | 4.5 | 1544 | 2263 |
| 2020 | 1368 | 2873 | 4241 | ||
| 2025 | 2038 | 4160 | 6198 | ||
| Colorectum (C18-20) | |||||
| 2013a | 6.6 | 569 | 5.6 | 664 | 1233 |
| 2020 | 1345 | 1352 | 2697 | ||
| 2025 | 2386 | 2068 | 4454 | ||
| Esophagus (C15) | |||||
| 2013a | 0.6 | 106 | 6.1 | 616 | 722 |
| 2020 | 163 | 1245 | 1408 | ||
| 2025 | 207 | 1905 | 2112 | ||
| Liver (C22) | |||||
| 2013a | −3.6 | 430 | 2.4 | 612 | 1042 |
| 2020 | 511 | 976 | 1487 | ||
| 2025 | 538 | 1266 | 1804 | ||
| Pharynx (C9-14) | |||||
| 2013a | −1.4 | 220 | 4.3 | 467 | 687 |
| 2020 | 291 | 821 | 1112 | ||
| 2025 | 338 | 1141 | 1479 | ||
| Stomach (C16) | |||||
| 2013a | −4.8 | 197 | −0.5 | 732 | 929 |
| 2020 | 210 | 969 | 1179 | ||
| 2025 | 213 | 1093 | 1306 | ||
| Oral cavity (C0-8) | |||||
| 2013a | 1.2 | 152 | 3.3 | 207 | 359 |
| 2020 | 238 | 352 | 590 | ||
| 2025 | 305 | 467 | 772 | ||
| Bladder (C67) | |||||
| 2013a | 11.4 | 85 | −0.1 | 88 | 173 |
| 2020 | 263 | 116 | 379 | ||
| 2025 | 566 | 136 | 702 | ||
| Prostate (C61) | |||||
| 2013a | −0.7 | 98 | 4.3 | 129 | 227 |
| 2020 | 136 | 240 | 376 | ||
| 2025 | 173 | 370 | 543 | ||
| Thyroid (C73) | |||||
| 2013a | 4.3 | 91 | 5.4 | 118 | 209 |
| 2020 | 142 | 215 | 357 | ||
| 2025 | 189 | 308 | 497 | ||
Abbreviations: AAPC, average annual percent change; HCMC: Ho Chi Minh City.
aThe number of cancer incidence in 2013 was observed for HCMC and projected for Hanoi.
bThe bold AAPCs indicate a significant difference from 0 at α = .05 level.
Table 3.
Projection of Cancer Cases by 10 Common Cancer Sites Among Women in 2025, Stratified by City.
| Cancer Sites | HCMC | Hanoi | Both Cities | ||
|---|---|---|---|---|---|
| APPCb | # of Cases | APPCb | # of Cases | # of Cases | |
| All (C00-C96 without C44) | |||||
| 2013a | 1.0 | 4082 | 1.9 | 5162 | 9244 |
| 2020 | 5944 | 7391 | 13 335 | ||
| 2025 | 7332 | 9034 | 16 366 | ||
| Breast (C50) | |||||
| 2013a | 2.7 | 1021 | −0.8 | 1178 | 2199 |
| 2020 | 1652 | 1383 | 3035 | ||
| 2025 | 2217 | 1485 | 3702 | ||
| Thyroid (C73) | |||||
| 2013a | 7.7 | 446 | 5.5 | 463 | 909 |
| 2020 | 886 | 777 | 1663 | ||
| 2025 | 1389 | 1085 | 2474 | ||
| Colorectum (C18-20) | |||||
| 2013a | 4.9 | 438 | 2.8 | 476 | 914 |
| 2020 | 888 | 746 | 1634 | ||
| 2025 | 1355 | 958 | 2313 | ||
| Lung and bronchus (C33-34) | |||||
| 2013a | 0.9 | 359 | 5.0 | 613 | 972 |
| 2020 | 558 | 1121 | 1679 | ||
| 2025 | 698 | 1613 | 2311 | ||
| Cervical (C53) | |||||
| 2013a | −1.5 | 551 | 2.8 | 478 | 1029 |
| 2020 | 674 | 702 | 1376 | ||
| 2025 | 738 | 874 | 1612 | ||
| Ovary (C56) | |||||
| 2013a | 2.7 | 229 | 2.6 | 255 | 484 |
| 2020 | 372 | 365 | 737 | ||
| 2025 | 496 | 454 | 950 | ||
| Stomach (C16) | |||||
| 2013a | −5.7 | 135 | −2.7 | 377 | 512 |
| 2020 | 130 | 415 | 545 | ||
| 2025 | 117 | 410 | 527 | ||
| Liver (C22) | |||||
| 2013a | −3.4 | 144 | 2.9 | 147 | 291 |
| 2020 | 170 | 230 | 400 | ||
| 2025 | 180 | 300 | 480 | ||
| Corpus uteri (C54) | |||||
| 2013a | 3.2 | 136 | −1.8 | 119 | 255 |
| 2020 | 231 | 137 | 368 | ||
| 2025 | 323 | 136 | 459 | ||
| Pharynx (C9-14) | |||||
| 2013a | −1.9 | 63 | 0.5 | 142 | 205 |
| 2020 | 74 | 183 | 257 | ||
| 2025 | 79 | 210 | 289 | ||
Abbreviations: AAPC, average annual percent change; HCMC: Ho Chi Minh City.
aThe number of cancer incidence in 2013 was observed for HCMC and projected for Hanoi.
bThe bold AAPCs indicate a significant difference from 0 at α = .05 level.
Among men, the ASIRs are projected to increase for 5 of 10 common cancer sites in HCMC and almost all cancer sites in Hanoi. The ASIRs for colorectum (HCMC: 6.6% AAPC, Hanoi: 5.6% AAPC), lung (HCMC: 3.1% AAPC, Hanoi: 4.5% AAPC), and thyroid (HCMC: 4.3% AAPC and Hanoi: 5.4% AAPC) are considerably accelerating in both cities. We also observed a substantial increase in the ASIRs of esophagus (6.1% AAPC) in Hanoi and of bladder (11.4% AAPC) in HCMC, but a smaller decrease in the ASIRs of stomach and liver cancer in HCMC. The leading cancer sites in 2025 are predicted to be lung (6198 cases), colorectum (4454 cases), esophagus (2112 cases), liver (1804 cases) and pharynx (1479 cases) for men in both cities (Table 2).
Among women, the ASIRs are projected to increase for 6 of 10 common cancer sites in HCMC and 7 of 10 common cancer sites in Hanoi. In both cities, the ASIRs for thyroid (HCMC: 7.7% AAPC, Hanoi: 5.5% AAPC) and colorectal cancer (HCMC: 4.9% AAPC, Hanoi: 2.8% AAPC) are dramatically accelerating, particularly in HCMC. The AAPC of lung cancer in Hanoi and the AAPC of breast cancer in HCMC are respectively increasing by 5.0% and 2.7% in age-standardized incidence rate per year. We also predicted significant decreases in the ASIRs of liver (5.7% AAPC) and stomach (3.4% AAPC) cancer in HCMC. For women, the leading cancer sites is projected to be breast (3702 cases), thyroid (2474 cases), colorectum (2313 cases), lung (2311 cases), and cervical cancer (1612 cases) in 2025 (Table 3).
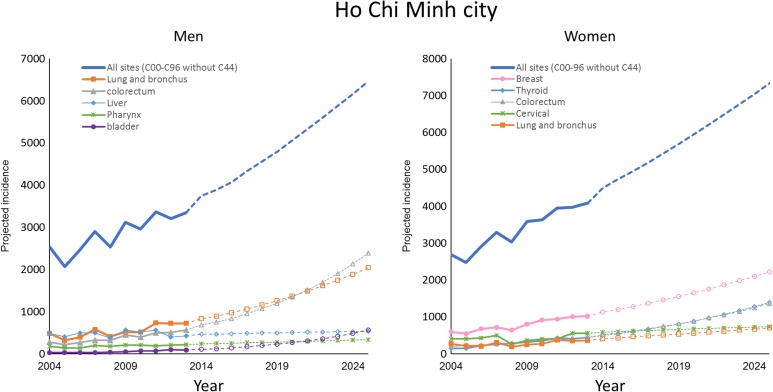
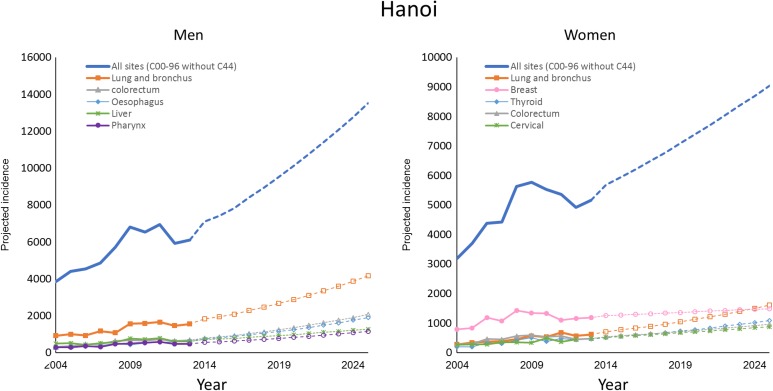
Figures 2 and 3 illustrate the observed and projected cancer incidence during 2004 to 2025 for all cancer sites and 5 most common cancer sites in 2025, stratified by gender and city. The number of cancer incidence for all sites is estimated to be nearly double from 2013 to 2025. The rank order of cancer sites was not homogenous between Hanoi and HCMC.
Figure 2.
Observed and projected cancer incidence for all cancer sites and 5 common cancer sites in 2025, stratified by gender in Ho Chi Minh City.
Figure 3.
Observed and projected cancer incidence for all cancer sites and 5 common cancer sites in 2025, stratified by gender in Hanoi (the number of cancer cases during 2004-2009 was estimated for a combination of old Hanoi and Ha Tay province).
In HCMC, colorectal cancer is expected to surpass lung cancer to the most common cancer by 2025, whereas bladder is predicted to be the top 5 leading cancer and surpass liver, and pharynx to rank as the third most common cancer by 2025 among men. For women, the changes in the rank order over time is due to the AAPC increase for thyroid and colorectum cancer. These two cancers are expected to surpass cervix uteri and lung cancer to rank as the second and third most frequent malignancy by 2025 (Figure 2).
In Hanoi, lung cancer is expected to remain the most common cancer for men and continuously increase in incidence over the next decades. Colorectum and esophagus cancer are predicted to surpass liver and pharynx cancer to rank as the second and third leading cancer diagnoses among men by 2025. Lung cancer is expected to surpass breast cancer to become the most common cancer among women by 2025. Thyroid, colorectum, and cervical cancer are projected to remain the 5 most frequent malignancies through 2025 (Figure 3).
Discussion
The study projected that the burdens of cancer incidence is projected to increase sharply in both cities over the next decades in HCMC and Hanoi. The number of cases is projected to double for all cancer sites from 2013 to 2025 and to substantially increase for most types of cancers, which is driven by the aging population and the increasing populations within the 2 cities. Lung, colorectum, breast and thyroid, and liver cancer represent approximately 67% of the overall cancer burden. They are projected to become the leading cancer diagnoses in 2020 and consistently remain the most frequent malignancies through 2025.
In our model, lung cancer, a smoking-related cancer, remains the most common cancer diagnosis among men and the fourth leading cancer among women in both cities. Thanks to the efforts in national tobacco control program, the prevalence of current smoking in Vietnamese men dramatically reduced from 72.8% in 1997 to 56.5% in 2008 (52.7% in HCMC and 51.3% in Hanoi) and 45.3% in 2015.23-25 The prevalence of smoking in women was noted as very small (∼1.4%), but over 70% of adults reported exposing to secondhand smoking in national surveys.25 The impact of reduction in smoking among men over the past 20 years has been largely not yet reflected in the incidence data used for our projection. In the coming decades in both cities, it is projected that the number of lung cancer and other smoking related cancers (esophagus, bladder and, pharynx cancer) is predicted to continuously increase among men and women due to the impact of historical smoking patterns and the secondhand smoke exposures. Because of the absence of smoking information in our projection, the increasing trend in smoking-related cancers is projected in a larger extent without a gradual damping through the impact of reduction in smoking. Beside tobacco, alcohol consumption in Vietnamese adults might be attributable to cancer trends. According to the Global Status Report on Alcohol 2004, total alcohol consumption per Vietnamese adult showed an increase of approximately 2.5 times from 1993 to 2000.26 In addition, a study on mental health in 2000 reported that 5.5% Vietnamese adults were affected by alcohol abuse.27 High consumption of alcohol is associated with an increased risk of lung, larynx, esophagus, bladder, pancreas and kidney cancer.8 However, there is a limitation in evidence and prevention programs in Vietnam regarding alcohol consumption and alcohol-related health problems, particularly cancer diseases. Therefore, the government, scientists, and activists must continue efforts into maintain and broaden tobacco control policies, as well as implementing broad educational programs raising awareness on the health consequences of alcohol consumption and smoking, particularly secondhand smoking.25
Lifestyle factors including excess weight and physical inactivity play a major role in etiology of cancers such as colorectum, breast, thyroid, uterus, esophagus, kidney, and pancreas cancer.8 Rapid socioeconomic growth, changing lifestyles, and decreasing physical activity among adults contributed to rise in the prevalence of diabetes (1.5% in 1990s to 4.1% in 2015) and overweight and obesity (from 2.3% in 1993 to 14.5% in 2015) in Vietnamese adults aged 25 to 64 years, which indirectly reflects an increase in the incidence data of colorectal cancer.24,28-30 This type of cancer will be predicted to surpass lung cancer to become the most common cancer in HCMC by 2025. Therefore, there is a need to raise awareness of people aged 50 to 70 in community for regular checkups to detect and remove adenomas before they become cancerous, and this should be considered as the first solution to prevent the cancer disease.31 The number cases of breast cancer, the most frequently diagnosed cancer among women, has been on the rise over the past 2 decades presumably related to changes in dietary and reproductive behaviors and aging populations.32 Part of the observed increase may reflect the implementation of large-scale localized, provincial, and regional screening programs on adult women who were residing in both cities.33 Between 2008 and 2016, approximately 166 000 women were screened for breast and cervical cancer by at least 3 screening programs with domination of Hanoi and HCMC,33,34 which lead to a transient elevation in incidence due to the detection of a prevalent pool of undiagnosed cancers during the model’s baseline period. The significantly increasing number of thyroid cancer cases among women was observed in both cities, which is similar to the trends observed in developed countries.8,12 The contribution of advanced diagnostic techniques into the detection of small, subclinical thyroid carcinomas has led to an increased diagnosis, particularly in women, and may result in the significantly increasing thyroid cancer incidence.35 Applying ultrasonography, computed tomography scan, magnetic resonance imaging, and fine needle aspiration biopsy for the diagnosis of thyroid cancer is very popular in Hanoi and HCMC in the past decade. However, the increasing trend in thyroid cancer incidence also could be partially attributed to overdiagnosis.35 In general, maintain a healthy weight and being physically active may be an opportunity for preventing and reducing the risk of cancers associated with lifestyle in Vietnamese adults.8
Vietnam is a highly endemic country of hepatitis B virus (HBV), which was estimated to be around 8.4 million in 2005.36 The prevalence of hepatitis B surface antigen in Vietnam was as high as 8% or over and was various by different region.37 The HBV infection prevalence in HCMC and Hanoi was from 10% to 14% and as high as 18.8% to 19% in some rural areas.37 The HBV-related liver cirrhosis and hepatocellular carcinoma incidence were estimated to increase to 58 650 and 25 000 in 2025.36 The burden of HBV- and hepatitis C virus (HCV)-related liver diseases is attributable to the inadequate use of neonatal HBV vaccination.38 However, the ASIRs of liver cancer in our projection are projected to decrease in the next decade among men and women in HCMC, but not in Hanoi. The decrease in the ASIRs of liver cancer in HCMC may be the result of the high rate of late presentation for cancer screen and treatment as well as cancer registry replying on hospitalization data and missing cancers diagnosed and treated outside the hospital.
We also predicted stomach cancer will not be in the top 5 leading cancer diagnoses in the next decade due to a decreasing AAPC for the malignancies that was observed among men and women in both cities. The decrease in stomach incidence rates may be largely attributable to the increased recognition and treatment of the Gram-negative bacterium Helicobacter pylori infection,39 which reported high rates in Hanoi and HCMC.40 A meta-analysis of prospective studies found that the increased risk of stomach cancer was associated with high salt intake, which may cause damage to gastric mucosa and help H pylori colonization. In addition, dietary nitrate (salted foods), which may result in endogenous N-nitrosation, can increase the risk stomach cancer.41 Reported changes in diet such as high fruit and vegetable consumption and reduced salt intake and dietary nitrate in Vietnam community may be contributed to the downward trend of stomach cancer.24 Reducing population salt intake has been globally identified as an important and cost-effective measure for improving population health outcomes and NCD prevention.24
Cervical cancer is expected to be in the top 5 common cancer among women in both cities, although a decreasing AAPC for the malignancies was observed in HCMC. Nearly 97% cases of invasive cervical cancer among Vietnamese women were due to the infection with human papillomavirus (HPV).42 The prevalence of HPV infection among women in Hanoi and HCMC was 6.13% and 8.27% with a domination of HPV type 16, 18 and 58, which could be prevented effectively by vaccination.43,44 Nevertheless, HPV vaccine is still costly, whereas individual must pay out-of-pocket for the vaccine. That leads to a significant obstacle for Vietnamese people to access the service of HPV vaccine. Therefore, expanding health insurance coverage for HPV vaccine and its package of benefits for screening services with Papanicolaou test for women should be considered in order to prevent or early detect and treat precancerous lesions. It seems that the cancer prevention programs in Vietnam neglected men who are at risk for HPV-associated oral cavity, pharynx, head and neck, and genital cancers. To date, few large-scale screening programs for cancer have been implemented in Vietnam due to the lack of appropriate diagnostic equipment, difficulties of follow-up regularly in community, and financial burden.9
Bladder cancer is not a common cancer in the 2004 to 2013 time period but is surprisingly projected to rank the third most common cancer by 2025 among men in HCMC. Besides smoking, which is a major risk factor for bladder cancer, epidemiological studies have released the association between occupational exposures and this cancer.45 In a case–control study, approximately 6.5% of bladder cancer incidence was attributed to motor vehicle driver, textile dyer, motor transport, and aromatic amines.46 Two pooled analyses of epidemiology studies were conducted in developed countries in North America and Europe and found a significant association between tap water consumption (contains chloroform and other trihalomethanes) and the risk of bladder cancer.14 Thus, the trend of this disease should be continuously observed before making a firm conclusion and recommendation.
Oblivious gender-related differences in the projected number incidence case are seen in both Hanoi and HCMC. Cancer is predicted to be more common in males than in females except for breast cancer, thyroid cancer, and the other female cancers. In addition, our projection showed differences in the predicted number of cases between HCMC and Hanoi. These differences by gender as well as geographical region are influenced in part by variation in cancer risk factors including lifestyle factors, occupational and environmental contaminants, and genetic susceptibility. For example, the number of stomach, esophagus, and pharynx caner is surprisingly predominant in Hanoi rather than HCMC. A study reported the infection of H pylori strains carrying vacAm1, which was strongly associated with an increase in prevalence of peptic ulcer in Hanoi, might contribute to the explanation for the difference in the number of cancer cases as well as ASIRs of these types of cancer between Hanoi and HCMC.40 The geographic variation in cancer incidence may also be explained by the availability of screening and diagnosed services, while the different rates of participation for cancer screening and treatment might be attributable to gender-related differences. Finally, the variation in cancer registry practices between HCMC and Hanoi could also explain some of geographical differences in cancer distribution.
During 2013 to 2025, the rank order of stomach cancer among men and cervical cancer among women is expected to be remarkably changed. Our projection showed an epidemiological transition from infectious-associated cancers such as stomach and cervical cancer to a high burden of cancers that have mainly been attributed to a “western lifestyle” in HCMC and Hanoi.47 To curbing the epidemic of cancer in the nation and in particularly these cities, developing appropriate policies and specific cancer control programs integrating in current tobacco control and NCD prevention programs for Vietnamese people is essential. The cancer control strategies should highlight the important role of nutrition and dietary, physical activity, overweight, and obesity in relation to cancer prevention as well as the need for continuing efforts to stop smoking, reduce alcohol intake, improve uptake of cancer screening, and increase use of HPV vaccination. The cancer control strategies also should leverage many resources to meet health-care requirements and reduce the burden of cancer in Vietnam. Last, additional etiological research is needed to better understand risk factors and guide prevention efforts.
Globally, the most frequently applied method for projecting cancer incidence is the APC model, which accounts for the effects of age, year of diagnosis (period), and year of birth (cohort). The Nordpred method is one of the most widely used approach for cancer projection worldwide, especially in the Nordic countries and the United Kingdom.48-50 The method has been shown to give more realistic predictions, especially for long-term projection,51 but requires a long-term cancer registries data (ie, a minimum of 15 years of data). Due to the shorter time series of the available incidence data in our study (<15 years of data), the methodical approach developed by Rahib et al was an alternative to Nordpred method for HCMC and Hanoi, which has shifted significantly in their age demographic structures with their population predicted to age considerably in the future. This method is an easy-to-use mathematical modeling method based on demographic shifts and changes in ASIRs by cancer sites, which were applied for projecting cancer incidence in the United States and Germany.12,13 Similar to an APC model, the methodical approach developed by Rahib et al does not include assumption about changes in risk factors or screening activity. In our projection, we followed the approach modified by Quante and colleagues that the value of nonsignificant AAPCs were kept and used for projections in order to reduce bias. Considering nonsignificant AAPCs as zero, it means the projected new cases were only based on demographic changes, but not on the changes in the incidence rates. This might result in a probable underestimate of the number of cases for those cancers with larger nonsignificant positive AAPCs (eg, lung, thyroid, and corpus uteri cancer in HCMC as well as prostate, thyroid, and pharynx cancer in Hanoi with AAPCs >3.0%). All AAPCs used in our projection were for the whole period from 2004 to 2013 in HCMC and 2004 to 2012 in Hanoi, although some significant Joinpoint were identified in both HCMC and Hanoi’s data (Supplemental Tables 1-4).
Despite a long history of cancer registries, the available incidence data were from population-based cancer registries in both cities that were reported about challenges in terms of quality of data due to a shortage of human and financial resources and a heterogeneous quality of primary health information,18 which result in an underrepresentation of cancer incidence cases. In order to improve the data quality in Hanoi and HCMC as well as generally in this nation, a comprehensive assessment of data quality including completeness, timeliness, validity, and comparability in all established cancer registries should be the first step for the development of national standard cancer registry procedure. Cancer registration should be legally mandated in Vietnam’s urban centers such as Hanoi, HCMC, Hai Phong, and Da Nang. All local hospitals are required to notify these Cancer Registries of all newly diagnosed cancer cases. Besides basic information recommended by IARC, further collected information on risk factors and behaviors such as smoking habits, alcohol consumption, obesity, HBV/HCV infection, family history of cancer, screening activities, and treatments would be useful to project cancer burden and evaluate their effect on the trends of cancer incidence and mortality in the future.
This study is the first in which data from the population-based cancer registries were used to project case numbers and proportionate distribution of common cancers to 2020 and 2025 in in Hanoi and HCMC. All previous studies focused on evaluating the temporal trends of cancer incidence in Hanoi, HCMC, and combination of both cities in a short period of time. The strength of our projection is that it was based on a well-validated population-based cancer registries data from a 10-year period with the best overall practices and data quality in the nation covering 45 and 30 hospitals, respectively.19 Moreover, the population estimation in the years 2004 to 2013 and population projections in the years 2014 to 2025 for both cities were obtained from the General Statistics Office, which is reliable and accurate.6
Several limitations in our study should be considered when interpreting and explaining for the projections in HCMC and Hanoi. Using local data from the population-based cancer registries in the years 2004 to 2013, cancer incidence projections for 2020 and 2025 inherently carry some uncertainty as they depend on an assumption about the continuity of past trend. Our projection assumed that the current trend pattern in cancer incidence remains consistent into the future without assumption about changes in cancer causes, the exposure to risk factors, and the effects of screening in cancer incidence, thus may lead to theoretically reducing the accuracy of the calculated cancer burdens. We assumed that the trend of cancer incidence as well as ASIRs did not change considerably before and after extending Hanoi’s land area because of the similarity in age structure population between old Hanoi and the surrounding area. However, our projections might be useful in evaluating the effects of preventive intervention. If observed incidence and ASIRs in the future differ from those projected, this suggests that the risk or prevention influences changes in incidence and ASIRs. In future, examination and validation of different modeling strategies and methods using long-term cancer registries data to select the most appropriate method for Vietnam’s setting would be worthwhile. Finally, projecting changes in cancer spectrum in these 2 largest population cities may not be generalizable to this nation.
In conclusion, a double burden of cancer incidence is projected in both cities over the next decades, as a result of the aging population and the increasing population size. We also projected an epidemiological transition from infectious-associated cancers to a high burden of cancers that have mainly been attributed to rapid socioeconomic growth, changes in lifestyle, and decreasing physical activity among adults in these 2 cities.
Supplemental Material
Supplemental_tables_(2) for Projecting Cancer Incidence for 2025 in the 2 Largest Populated Cities in Vietnam by Sang Minh Nguyen, Stephen Deppen, Giang Huong Nguyen, Dung Xuan Pham, Tung Duc Bui and Thuan Van Tran in Cancer Control
Acknowledgments
The authors would like to thank the research staff members from Hanoi Cancer Registry and Ho Chi Minh Cancer Registry, without whom this study would not have been possible. The authors also are grateful to Dr Huong Tran (Hanoi Medical University and Vietnam National Cancer Institute) and Dr Xiao-Ou Shu (Vanderbilt University) for inspiring and advising for the manuscript.
Authors’ Note: SMN and TVT designed the study, with advice and inputs from SD, DXP, TDB, and GHN. Data were managed and analyzed by SMN and TDB with advice from SD and TVT. SMN, SD, and GHN drafted the manuscript, with extensive feedback from other coauthors. This research was not a study conducted on animal and human and was exempt from the institutional review board’s review.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: SMN was supported by a VECD Global Health Fellowship, funded by the National Cancer Institute (NCI) and the Fogarty International Center (FIC) of the NIH (D43 TW009337). The views expressed are solely those of the authors and do not necessary represent the views of the NIH.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Ministry of Health. Health Statistics Yearbook 2010. Hanoi, Vietnam: Ministry of Health; 2011. [Google Scholar]
- 2. Institute for Health Metrics and Evaluation (IHME). GBD Compare data visualization. 2018. http://vizhub.healthdata.org/gbd-compare. Accessed October 17, 2018.
- 3. World Health Organization. UN Interagency Task Force On NCDs Joint Country Mission to Viet Nam. World Health Organization; 2016. https://www.who.int/ncds/un-task-force/vietnam-mission-september-2016/en/. Accessed October 17, 2018. [Google Scholar]
- 4. World Health Organization. Raised Blood Pressure: Situation and Trends. Geneva: World Health Organization; 2012. [Google Scholar]
- 5. International Agency for Research on Cancer (IARC). Fact Sheets by Population. 2018. http://gco.iarc.fr/today/fact-sheets-populations. Accessed October 17, 2018.
- 6. General Statistics Office, United Nations Population Fund. Vietnam Population Projection, 2014-2049. Hanoi, Vietnam: Vietnam News Agency Publishing House; 2016. [Google Scholar]
- 7. Bray F, Moller B. Predicting the future burden of cancer. Nat Rev Cancer. 2006;6(1):63–74. [DOI] [PubMed] [Google Scholar]
- 8. Xie L, Semenciw R, Mery L. Cancer incidence in Canada: trends and projections (1983-2032). Health Promot Chronic Dis Prev Can. 2015;35(suppl 1):2–186. [PubMed] [Google Scholar]
- 9. Zhu L, Pickle LW, Ghosh K, et al. Predicting US- and state-level cancer counts for the current calendar year: part II: evaluation of spatiotemporal projection methods for incidence. Cancer. 2012;118(4):1100–1109. [DOI] [PubMed] [Google Scholar]
- 10. Clements MS, Armstrong BK, Moolgavkar SH. Lung cancer rate predictions using generalized additive models. Biostatistics. 2005;6(4):576–589. [DOI] [PubMed] [Google Scholar]
- 11. Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27(17):2758–2765. [DOI] [PubMed] [Google Scholar]
- 12. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. [DOI] [PubMed] [Google Scholar]
- 13. Quante AS, Ming C, Rottmann M, et al. Projections of cancer incidence and cancer-related deaths in Germany by 2020 and 2030. Cancer Med. 2016;5(9):2649–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Villanueva CM, Fernandez F, Malats N, Grimalt JO, Kogevinas M. Meta-analysis of studies on individual consumption of chlorinated drinking water and bladder cancer. J Epidemiol Community Health. 2003;57(3):166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The 2009 Viet Nam population and housing census: completed results. 2009. http://www.gso.gov.vn/default.aspx?tabid=512&idmid=5&ItemID=10798. Accessed January 11, 2011.
- 16. Anh PT, Parkin DM, Hanh NT, Duc NB. Cancer in the population of Hanoi, Vietnam, 1988-1990. Br J Cancer. 1993;68(6):1236–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nguyen QM, Nguyen HC, Parkin DM. Cancer incidence in Ho Chi Minh City, Viet Nam, 1995-1996. Int J Cancer. 1998;76(4):472–479. [DOI] [PubMed] [Google Scholar]
- 18. Anh PT, Duc NB. The situation with cancer control in Vietnam. Jpn J Clin Oncol. 2002;32(Suppl):S92–97. [DOI] [PubMed] [Google Scholar]
- 19. Mery L, Torode J, Pearlman P. Global initiative for cancer registry development partners task force visit to Vietnam. 2017. https://www.cancer.gov/about-nci/organization/cgh/blog/2016/cancer-registration-vietnam. Accessed October 17, 2018.
- 20. World Health Organization. International Statistical Classification of Diseases and Related Health Problems. 10th Revision ed Geneva, Switzerland: World Health Organization; 1994. [Google Scholar]
- 21. Surveillance e, and End Results Program (SEER). World (WHO 2000-2025) Standard. 2013. https://seer.cancer.gov/stdpopulations/world.who.html. Accessed February 24, 2019.
- 22. Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat Med. 2009;28(29):3670–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jenkins CN, Dai PX, Ngoc DH, et al. Tobacco use in Vietnam. Prevalence, predictors, and the role of the transnational tobacco corporations. JAMA. 1997;277(21):1726–1731. [DOI] [PubMed] [Google Scholar]
- 24. Ministry of Health—General Department of Preventive Medicine. National Survey of Risk Factors for Non-Communicable Disease (Steps) Vietnam 2015. Hanoi, Vietnam: Ministry of Health—General Department of Preventive Medicine; 2016. [Google Scholar]
- 25. Minh HV, Ngan TT, Mai VQ, et al. Tobacco control policies in Vietnam: review on MPOWER implementation progress and challenges. Asian Pac J Cancer Prev. 2016;17(S1):1–9. [DOI] [PubMed] [Google Scholar]
- 26. World Health Organization. Global Status Report On Alcohol 2004. Geneva, Switzerland: Department of Mental Health and Substance Abuse; 2004. [Google Scholar]
- 27. Vietnam Ministry of Health, Health Partnership Group. Joined Annual Health Review: Strengthening Prevention and Control of Non-Communicable Disease. Hanoi, Vietnam: Vietnam Ministry of Health; 2014. [Google Scholar]
- 28. Vietnam Ministry of Health. Fact Sheet: Vietnam Stepwise Approach To Surveillance Adults Aged 25-64 Years Old Hanoi. Hanoi, Vietnam: Vietnam Ministry of Health; 2010. [Google Scholar]
- 29. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. [DOI] [PubMed] [Google Scholar]
- 30. Nguyen TT, Hoang MV. Non-communicable diseases, food and nutrition in Vietnam from 1975 to 2015: the burden and national response. Asia Pac J Clin Nutr. 2018;27(1):19–28. [DOI] [PubMed] [Google Scholar]
- 31. Nguyen LH, Laohasiriwong W, Stewart JF, Wright P, Nguyen YTB, Coyte PC. Cost-effectiveness analysis of a screening program for breast cancer in Vietnam. Value Health Reg Issues. 2013;2(1):21–28. [DOI] [PubMed] [Google Scholar]
- 32. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. [DOI] [PubMed] [Google Scholar]
- 33. Jenkins C, Minh LN, Anh TT, et al. Breast cancer services in Vietnam: a scoping review. Glob Health Action. 2018;11(1):1435344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thuan TV, Anh PT, DV T, Huong TTT. Cancer control in Vietnam. Where are we? Cancer Control. 2016. http://www.cancercontrol.info/cc2016/cancer-control-in-vietnam-where-we-are/. Accessed October 17, 2018. [Google Scholar]
- 35. Kent WD, Hall SF, Isotalo PA, Houlden RL, George RL, Groome PA. Increased incidence of differentiated thyroid carcinoma and detection of subclinical disease. CMAJ. 2007;177(11):1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nguyen VT, Law MG, Dore GJ. An enormous hepatitis B virus-related liver disease burden projected in Vietnam by 2025. Liver Int. 2008;28(4):525–531. [DOI] [PubMed] [Google Scholar]
- 37. Gish RG, Bui TD, Nguyen CT, et al. Liver disease in Viet Nam: screening, surveillance, management and education: a 5-year plan and call to action. J Gastroenterol Hepatol. 2012;27(2):238–247. [DOI] [PubMed] [Google Scholar]
- 38. Huy Do S. Epidemiology of hepatitis B and C virus infections and liver cancer in Vietnam. Euroasian J Hepatogastroenterol. 2015;5(1):49–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. IARC Working Group on the Evaluation of Carcinogenic Risk to Humans. Schistosomes, Liver Flukes And Helicobacter Pylori. Lyon, France: International Agency for Research on Cancer; 1994. [Google Scholar]
- 40. Nguyen TL, Uchida T, Tsukamoto Y, et al. Helicobacter pylori infection and gastroduodenal diseases in Vietnam: a cross-sectional, hospital-based study. BMC Gastroenterol. 2010;10:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nagini S. Carcinoma of the stomach: a review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol. 2012;4(7):156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Quek SC, Lim BK, Domingo E, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical intraepithelial neoplasia across 5 countries in Asia. Int J Gynecol Cancer. 2013;23(1):148–156. [DOI] [PubMed] [Google Scholar]
- 43. Vu LT, Le HT. Cervical human papilloma virus infection among the general female population in Vietnam: a situation analysis. Asian Pac J Cancer Prev. 2011;12(2):561–566. [PubMed] [Google Scholar]
- 44. Vu LT, Bui D. Prevalence of cervical human papilloma virus infection among married women in Vietnam, 2011. Asian Pac J Cancer Prev. 2012;13(1):37–40. [DOI] [PubMed] [Google Scholar]
- 45. Letasiova S, Medve’ova A, Sovcikova A, et al. Bladder cancer, a review of the environmental risk factors. Environ Health. 2012;11(suppl 1):S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Siemiatycki J, Dewar R, Nadon L, Gerin M. Occupational risk factors for bladder cancer: results from a case-control study in Montreal, Quebec, Canada. Am J Epidemiol. 1994;140(12):1061–1080. [DOI] [PubMed] [Google Scholar]
- 47. Fitzmaurice C. Epidemiological transition of cancer: a systematic analysis from the global burden of disease study 2016. J Global Oncol. 2018;4(suppl 2):80s–80s. [Google Scholar]
- 48. Moller B, Fekjaer H, Hakulinen T, et al. Prediction of cancer incidence in the Nordic countries up to the year 2020. Eur J Cancer Prev. 2002;11(suppl 1):S1–S96. [PubMed] [Google Scholar]
- 49. Mistry M, Parkin DM, Ahmad AS, Sasieni P. Cancer incidence in the United Kingdom: projections to the year 2030. Br J Cancer. 2011;105(11):1795–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Smittenaar CR, Petersen KA, Stewart K, Moitt N. Cancer incidence and mortality projections in the UK until 2035. Br J Cancer. 2016;115(9):1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moller B, Fekjaer H, Hakulinen T, et al. Prediction of cancer incidence in the Nordic countries: empirical comparison of different approaches. Stat Med. 2003;22(17):2751–2766. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental_tables_(2) for Projecting Cancer Incidence for 2025 in the 2 Largest Populated Cities in Vietnam by Sang Minh Nguyen, Stephen Deppen, Giang Huong Nguyen, Dung Xuan Pham, Tung Duc Bui and Thuan Van Tran in Cancer Control