Abstract
Quinoa (Chenopodium quinoa Willd.) was known as the “golden grain” by the native Andean people in South America, and has been a source of valuable food over thousands of years. It can produce a variety of secondary metabolites with broad spectra of bioactivities. At least 193 secondary metabolites from quinoa have been identified in the past 40 years. They mainly include phenolic acids, flavonoids, terpenoids, steroids, and nitrogen-containing compounds. These metabolites exhibit many physiological functions, such as insecticidal, molluscicidal and antimicrobial activities, as well as various kinds of biological activities such as antioxidant, cytotoxic, anti-diabetic and anti-inflammatory properties. This review focuses on our knowledge of the structures, biological activities and functions of quinoa secondary metabolites. Biosynthesis, development and utilization of the secondary metabolites especially from quinoa bran were prospected.
Keywords: quinoa (Chenopodium quinoa), secondary metabolites, biological activities, functions
1. Introduction
Quinoa (Chenopodium quinoa Willd.), a dicotyledonous plant belonging to Chenopodiaceae family, is one of the oldest native crops in the Andean region of South America, with approximately 7000 years of cultivation [1]. It has been considered as a pseudo-cereal because of the grain characteristics [2]. Consumption of seeds is the most common use of quinoa. Once the bran (also called hull or seed coats) containing saponins has been eliminated, the seeds can be consumed as entire grains or milled to flour for preparation of bread and pastry. The other parts such as leaves and stems were used as feed [2,3].
Quinoa has been recognized as a complete food due to a variety of vitamins, significant amounts of minerals, unsaturated fatty acids, dietary fiber, abounding proteins, and excellent balance of essential amino acids. The year 2013 was named “The Internaitonal Year of Quinoa” by the UN. Quinoa has been introduced and cultivated all over the world in the past ten years [3,4,5,6,7,8].
Quinoa possesses a large number of secondary metabolites, such as phenolic acids, flavonoids, terpenoids, steroids, and nitrogen-containing compounds. These metabolites play various physiological and ecological roles against harmful microorganisms, birds and insects. They also exhibit features beneficial to humans, including anti-diabetic [9], anticancer [10], cytotoxic [11], antimicrobial [12], anti-inflammatory [13], immunoregulatory [14] and adjuvant activities [15].
To our knowledge, there are many reviews on quinoa, most of them are focused on the nutritional, functional and antinutritional aspects [16,17,18], abiotic stress responses [19], biodiversity and sustainability [20], or only a specific topic of quinoa secondary metabolites and their biological activities such as steroids [21,22] and triterpenoid saponins [23], but no review covers almost all secondary metabolites and their biological activities. In this review, we summarize and discuss quinoa secondary metabolites on their structural diversity, biological activities or functions during the past 40 years.
2. Phenolic Acids and Their Biological Activities or Functions
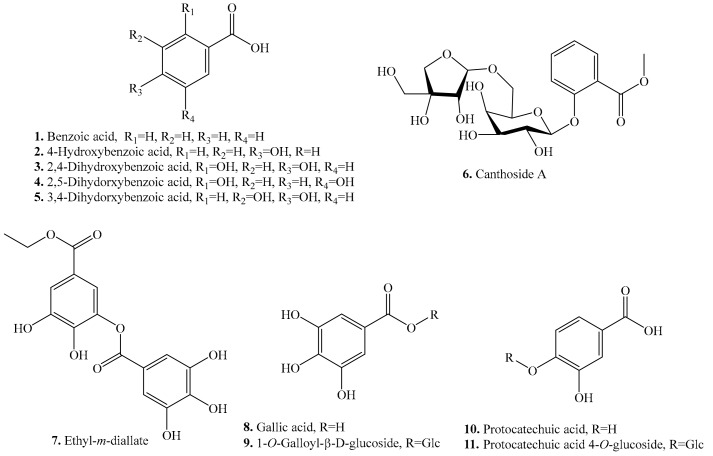
About 29 phenolic acid analogues have been identified in quinoa. According to their structural features, they can be classified as benzoic acid analogues (1–16) and cinnamic acid analogues (17–29). Benzoic acid (1) was derived from cinnamic acid (19) in planta in the biosynthetic pathway of phenolic acids [24]. Phenolic acid derivatives are present in either free or conjugated forms. The total of conjugated phenolic acids in quinoa were at comparable level as that of free ones, suggesting that conventional solvent extraction and chromatographic analysis of extractable phenolic acids might have significantly underestimated the total phenolic acid content in quinoa, as such methods only detect free phenolic acids [25].
Phenolic acids can be released by acid, alkaline, and enzymatic treatments from the conjugated forms. It was reported that at least 19 phenolic acids were released in the residue of quinoa which can enhance bioaccessibility [25]. Bound phenolic acid derivatives in conjugated forms were not affected by environmental stresses [26]. Higher content of phenolic acids showed stronger antioxidant and inhibitory activities of α-glucosidase and pancreatic lipase [25].
2.1. Benzoic Acid Analogues and Their Biological Activities or Functions
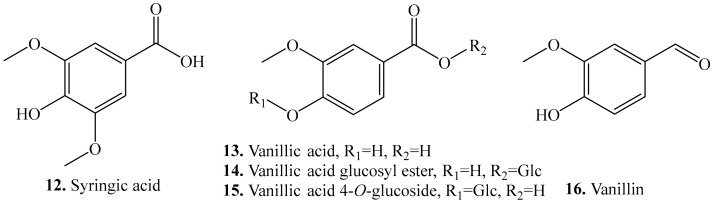
At least 16 benzoic acid analogues have been identified from quinoa. Their biological activities are listed in Table 1, and the structures are shown in Figure 1. Benzoic acid derivatives include benzoic acid (1), gallic acid (8), protocatechuic acid (10), syringic acid (12), vanillic acid (13), and their analogues. They are rich in the leaves and seeds of quinoa [25,27]. Though the benzoic acid analogues from quinoa have not been evaluated for their biological activities, these metabolites from other plant species have been reported to have antimicrobial [28,29], allelopathic [30], antioxidant [31], and antifeedant [32] activities (Table 1).
Table 1.
Benzoic acid analogues and their biological activities or functions.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Benzoic acid (1) | Leaves and flour | - | [27,33] |
| 4-Hydroxybenzoic acid = p-Hydroxybenzoic acid (2) | Seeds | - | [25] |
| Leaves and seeds | - | [27,34] | |
| Antimicrobial activity | [28] | ||
| Allelopathic effect | [30] | ||
| 2,4-Dihydroxybenzoic acid (3) | Seeds | - | [25,35] |
| 2,5-Dihydroxybenzoic acid (4) | Seeds | - | [35] |
| 3,4-Dihydroxybenzoic acid (5) | Seeds | - | [35] |
| Canthoside A (6) | Flour | - | [33] |
| Ethyl-m-digallate (7) | Flour | - | [33] |
| Antifeedant activity | [32] | ||
| Gallic acid (8) | Leaves, sprouts and seeds | - | [27,34] |
| Antioxidant activity | [31] | ||
| Antibacterial activity | [36] | ||
| 1-O-Galloyl-β-d-glucoside (9) | Seeds and flour | - | [33] |
| Protocatechuic acid (10) | Sprouts and seeds | - | [25,37] |
| Antioxidant activity | [31] | ||
| Anticancer activity | [38] | ||
| Antibacterial activity | [39] | ||
| Antiulcer activity | [40] | ||
| Antiageing activity | [41] | ||
| Anti-inflammatory, antiibrotic, antiatherosclerotic, hyperlipidemic, analgesic, hepatoprotective and nephroprotective activities | [42] | ||
| Antiviral activity | [43] | ||
| Protocatechuic acid 4-O-glucoside (11) | Flour | - | [33] |
| Antioxidant activity | [44] | ||
| Syringic acid (12) | Leaves and seeds | - | [25,26] |
| Allelopathic effect | [30] | ||
| Antioxidant activity | [31] | ||
| Antimicrobial activity | [45] | ||
| Hepatoprotective effect | [46] | ||
| Anti-inflammatory activity | [47] | ||
| Vanillic acid (13) | Leaves and seeds | - | [25,34] |
| Allelopathic effect | [30] | ||
| Hepatoprotective effect | [46] | ||
| Antioxidant and antimicrobial activities, and inhibitory activity on COX-I and COX-II | [48] | ||
| Vanillic acid glucosyl ester (14) | Seeds | - | [49] |
| Vanillic acid 4-O-glucoside (15) | Seeds | - | [35] |
| Vanillin (16) | Seeds and flour | - | [25,33,35] |
| Antioxidant activity | [50] | ||
| Antimicrobial activity | [51] | ||
| Antidepressant activity | [52] | ||
| Anti-angiogenic, anti-inflammatory and anti-nociceptive activities | [53] |
Figure 1.
Structures of the benzoic acid analogues isolated from quinoa.
2.2. Cinnamic Acid Analogues and Their Biological Activities or Functions
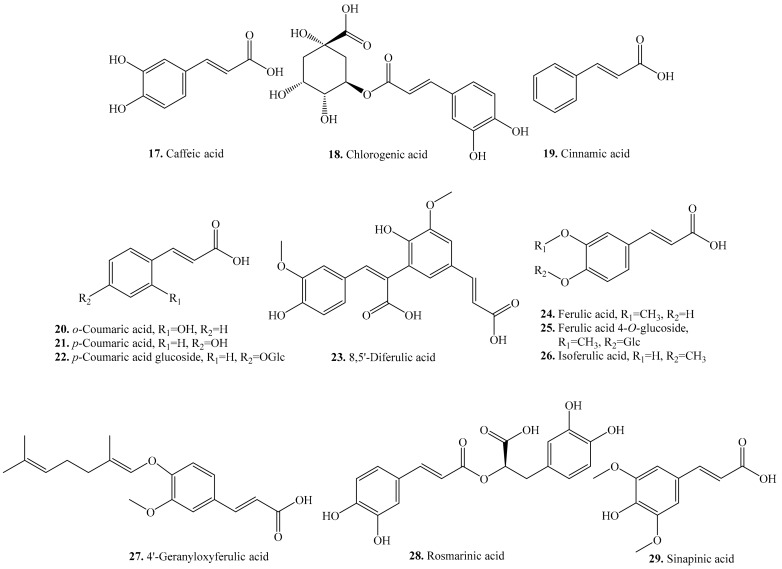
Thirteen cinnamic acid analogues have been identified from quinoa. Their biological activities are listed in Table 2, and the structures are shown in Figure 2. These cinnamic acid derivatives include caffeic acid (17), chlorogenic acid (18), cinnamic acid (19), coumaric acid (20/21), ferulic acid (24), rosmarinic acid (28), sinapinic acid (29), and their analogues. Ferulic acid (24) and its derivatives were the predominant phenolics in bound form to be present in quinoa seeds [17].
Table 2.
Cinnamic acid analogues and their biological activities or functions.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Caffeic acid (17) | Seeds | - | [25,34] |
| Antimicrobial activity | [29] | ||
| Allelopathic effect | [30] | ||
| Antioxidant activity | [31] | ||
| Anti-apoptotic activity | [55] | ||
| Inhibitory activity on xanthine oxidase | [57] | ||
| Chlorogenic acid (18) | Leaves and seeds | - | [25,26] |
| Antimicrobial activity | [29] | ||
| Antioxidant activity | [31] | ||
| Anti-diabetic activity | [56] | ||
| Hemolytic activity | [58] | ||
| Neuroprotective effects | [59] | ||
| Anti-obesity activity | [60] | ||
| Antihepatotoxic effect | [61] | ||
| Antibiofilm activity | [62] | ||
| Cinnamic acid (19) | Sprouts and seeds | - | [34] |
| o-Coumaric acid (20) | Leaves and seeds | - | [25,26] |
| Allelopathic effect | [30] | ||
| Antioxidant activity | [31] | ||
| p-Coumaric acid (21) | Leaves and seeds | - | [27,63] |
| Antilisterial activity | [64] | ||
| p-Coumaric acid glucoside (22) | Seeds | - | [35] |
| 8,5′-Diferulic acid (23) | Seeds | - | [25] |
| Ferulic acid (24) | Leaves, sprouts and seeds | - | [27,34,35] |
| Antimicrobial activity | [29] | ||
| Anti-apoptotic activity | [55] | ||
| Antioxidant activity | [65] | ||
| Cholesterol-lowering activity | [66] | ||
| Anti-thrombosis and anti-atherosclerosis effects | [67,68] | ||
| Anti-inflammatory activity | [69] | ||
| Anti-cancer activity | [70] | ||
| Ferulic acid 4-O-glucoside (25) | Flour | - | [33] |
| Isoferulic acid (26) | Seeds | - | [35] |
| Antioxidant activity | [71] | ||
| 4’-Geranyloxyferulic acid (27) | Seeds | - | [72] |
| Rosmarinic acid (28) | Seeds | - | [25] |
| Antimicrobial activity | [73] | ||
| Anti-inflammatory activity | [74] | ||
| Antioxidant activity | [75] | ||
| Antimutagenicity activity | [76] | ||
| Antiviral and anti-inflammatory effects | [77] | ||
| Sinapinic acid = trans-Sinapic acid (29) | Leaves | - | [27] |
| Seeds | - | [25] | |
| Antioxidant activity | [44] | ||
| Anxiolytic-like effects | [78] | ||
| Cerebral protective and cognition-improving effects | [79] |
Figure 2.
Structures of the cinnamic acid analogues isolated from quinoa.
Both ferulic acid (24) and sinapic acid (29) had more phytotoxic effects on cucumber seedling as compared to the other tested phenolic acids [54]. The phenolic acids from quinoa were also isolated from other plant species which showed a variety of biological activities such as antimicrobial [28], allelopathic [30], antioxidant [31], anti-apoptotic [55], anti-diabetic [56] activities that are mentioned in Table 2.
3. Flavonoids and Their Biological Activities or Functions
Flavonoids are based upon a fifteen-carbon skeleton consisting of two benzene rings linked via a heterocyclic pyrene ring [80]. They contain aglycones and their glycosides. The main flavonoid aglycones are kaempferol (35) and quercetin (46). Other aglycones in quinoa include acacetin (30), myricetin (45), daidzein (62), and genistein (63). According to the structural features, quinoa flavonoids can be classified as flavones (30–33), flavonols (34–54), flavanones (or dihydroflavones, 55–57), flavanols (58–60), and isoflavones (61–65). Flavonoids play important roles in plants against the feeding insects and herbivores [81]. Flavonoids also have deterrent effects with respect to feeding and physiological behavior against some soil herbivorous nematodes [82].
3.1. Flavones and Their Biological Activities or Functions
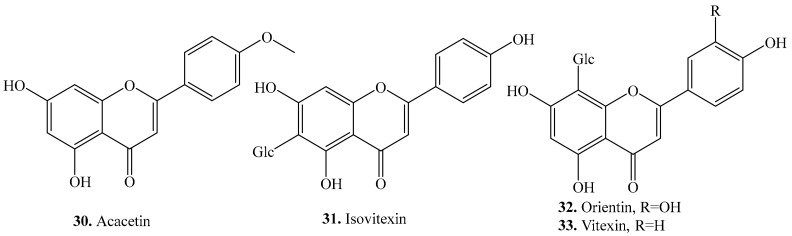
Four flavones, namely acacetin (30), isovitexin (31), orientin (32) and vitexin (33), have been identified from quinoa. Their biological activities are listed in Table 3, and the structures are shown in Figure 3. Flavones were significantly richer in sprouts than in other parts of quinoa. Quinoa sprouts grown in the darkness contained vitexin (33) and substantial amounts of isovitexin (31), whereas those grown in daylight only contained isovitexin (31). It is remarkable that no isovitexin (31) was present in quinoa seeds [34]. Acacetin (30), isovitexin (31), orientin (32) and vitexin (33) were also isolated from other plant species which showed various biological activities such as antioxidant [83], anti-inflammatory [84] activities, which are listed in Table 3.
Table 3.
Flavones and their biological activities or functions.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Acacetin (30) | Flour | - | [33] |
| Antioxidant activity | [83] | ||
| Spasmolytic and antinociceptive activities | [85] | ||
| Antiproliferative activity | [86] | ||
| Antiherpetic activity | [87] | ||
| Anticancer activity | [88] | ||
| Anti-inflammatory and antinociceptive activities | [89] | ||
| Hypouricemic effect | [90] | ||
| Isovitexin (31) | Sprouts | - | [34] |
| Anti-inflammatory and antioxidant activities | [84] | ||
| Anti-neoplastic effect | [91] | ||
| Anti-tumour activity | [92] | ||
| Neuroprotective effect | [93] | ||
| Anxiolytic property | [94] | ||
| Anti-Alzheimer‘s disease | [95] | ||
| Reduced postprandial blood glucose | [96] | ||
| Inhibitory effect on α-glucosidase | [97] | ||
| Inhibitory activity on rat lens aldose reductase | [98] | ||
| Orientin (32) | Seeds | - | [34] |
| Anticancer activity | [7] | ||
| Anti-inflammatory activity | [99] | ||
| Antioxidant activity | [100] | ||
| Antiapoptosis activity | [101] | ||
| Antithrombotic and antiplatelet activities | [102] | ||
| Antiproliferative activity | [103] | ||
| Vitexin (33) | Sprouts and seeds | - | [34] |
| Anti-carcinogenic effect | [91] | ||
| Anxiolytic property | [94] | ||
| Anti-Alzheimer’s disease property | [95] | ||
| Reduced postprandial blood glucose | [96] | ||
| Inhibitory effect on α-glucosidase | [97] | ||
| Induced apoptosis property | [104] | ||
| Agonist-induced regulation of vascular contractility | [105] | ||
| Antioxidant activity | [106] | ||
| Anti-inflammatory activity | [107] | ||
| Neuroprotective effect | [108] | ||
| Anti-depressant effect | [109] | ||
| Anti-convulsant effect | [110] | ||
| Antiepileptic effect | [111] | ||
| Anti-nociceptive effect | [112] | ||
| Anti-hypoxia/ischemia injury | [113] | ||
| Anti-ischemia/reperfusion injury | [114] | ||
| Anti-thyroid effect | [115] | ||
| Antimicrobial activity | [116] | ||
| Anti-viral effect | [117] |
Figure 3.
Structures of the flavones isolated from quinoa.
3.2. Flavonols and Their Biological Activities or Functions
About 21 flavonols have been identified in quinoa. Most of them are present in the seeds. Their biological activities are listed in Table 4, and their structures are shown in Figure 4.
Table 4.
Flavonols and their biological activities or functions.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
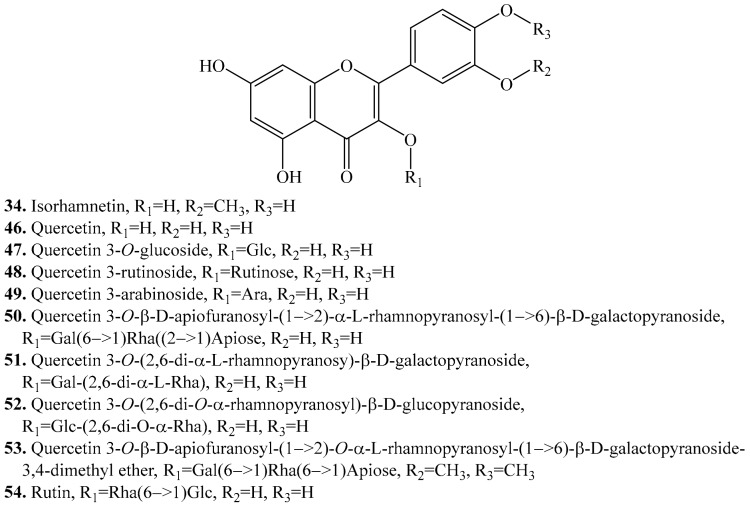
| Isorhamnetin (34) | Leaves | - | [27,125] |
| Chemopreventive activity | [126] | ||
| Antituberculosis activity | [127] | ||
| Antioxidant activity | [128] | ||
| Anti-tumor activity | [129,130] | ||
| Inhibitory activity on farnesyl protein transferase | [131] | ||
| Anti-inflammatory activity | [132] | ||
| Anticoagulant activity | [133] | ||
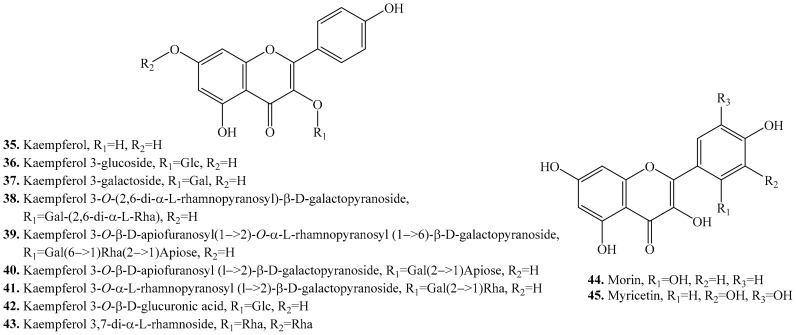
| Kaempferol (35) | Leaves and seeds | - | [25,26,35] |
| Antibacterial activity | [36] | ||
| Antioxidant activitiy | [134] | ||
| Inhibit UVB-induced COX-2 expression | [135] | ||
| Anti-inflammatory activitiy | [136] | ||
| Stimulate osteoblastic activity | [137] | ||
| Kaempferol 3-glucoside (36) | Seeds | - | [35] |
| Kaempferol 3-galactoside (37) | Seeds | - | [35] |
| Kaempferol 3-O-(2,6-di-α-l-rhamnopyranosyl)-β-d-galactopyranoside (38) | Seeds | Antioxidant activity | [49,120,138,139] |
| Kaempferol 3-O-β-d-apiofuranosyl-(1→2)-O-α-l-rhamnopyranosyl(1→6)-β-d-galactopyranoside (39) | Seeds | Antioxidant activity | [49,120,138] |
| Kaempferol 3-O-β-d-apiofuranosyl-(l→2)-β-d-galactopyranoside (40) | Seeds | Antioxidant activity | [120,138] |
| Kaempferol 3-O-α-l-rhamnopyranosyl-(1→2)-β-d-galactopyranoside (41) | Seeds | Antioxidant activity | [120] |
| Kaempferol 3-O-β-d-glucuronic acid (42) | Seeds | Antioxidant activity | [49] |
| Kaempferol 3,7-dirhamnoside (43) | Seeds | - | [35] |
| Morin (44) | Sprouts and seeds | - | [34] |
| Anti-biofilm activity | [140] | ||
| Anti-inflammatory activity | [141] | ||
| Antitumor activity | [142] | ||
| Inhibitory effect on the expression of α1 (I) collagen | [143] | ||
| Antioxidant activity | [144] | ||
| Anticancer activity | [145] | ||
| Inhibited the increase of ROS and reduced the apoptotic cell | [146] | ||
| Neuroprotective effect | [147] | ||
| Hepatoprotective activity | [148] | ||
| Myricetin (45) | Seeds | - | [63] |
| Antibacterial activity | [36] | ||
| Antioxidant and prooxidant activities | [149] | ||
| Anticancer activity | [150] | ||
| Anti-inflammatory activity | [151] | ||
| Analgesic activity | [152] | ||
| Quercetin (46) | Leaves and seeds | - | [27,35,125,139] |
| COX-I and COX-II inhibition activity | [48] | ||
| Stimulate osteoblastic activity | [137] | ||
| Antioxidant and prooxidant activities | [149] | ||
| Anti-inflammatory activity | [153] | ||
| Cytotoxic activity | [154] | ||
| Quercetin 3-O-glucoside (47) | Flour | - | [33] |
| Quercetin-3-rutinoside (48) | Seeds | - | [35] |
| Quercetin 3-arabinoside (49) | Seeds | - | [35] |
| Quercetin 3-O-β-d-apiofuranosyl-(1→2)-α-l-rhamnopyranosyl-(1→6)-β-d-galactopyranoside (50) | Seeds | Antioxidant activity | [120,139] |
| Quercetin 3-O-(2,6-di-α-l-rhamnopyranosy)-β-d-galactopyranoside (51) | Seeds | Antioxidant activity | [49,120] |
| Quercetin 3-O-(2,6-di-O-α-rhamnopyranosyl)-β-glucopyranoside (52) | Seeds | - | [139] |
| Quercetin 3-O-β-d-apiofuranosyl-(1→2)-O-α-l-rhamnopyranosyl-(1→6)-β-d-galactopyranoside-3,4-dimethyl ether (53) | Seeds | Antioxidant activity | [49] |
| Rutin (54) | Leaves, sprouts and seeds | - | [27,34] |
| Anti-diabetic activity | [56] | ||
| Antioxidant activity | [155] | ||
| Antiulcerogenic activity | [156] |
Figure 4.
Structures of the flavonols isolated from quinoa.
Both kaempferol (35) and quercetin (46) are two main flavonols. They are in the form of glycosides present in quinoa. Structure-activity relationship of their antioxidant activity showed that the ability to quench free hydroxyl radicals increased with the amount of hydroxyl groups in the ring B. For example, myricetin (45) was a stronger antioxidant than kaempferol (35) [118]. In addition, the compounds with 3’,4’-dihydroxy substituents in the ring B had much stronger antioxidative activities than those without ortho-dihydroxy substitution in the ring B [119]. Quercetin (46) was the strongest antioxidant among the flavonoids. Both isorhamnetin (34) and kaempferol (35) were the most abundant flavonoids in quinoa leaves, and it also contained large amounts of rutin (54) [27]. Four kaempferol 3-glycosides (38–41) exhibited moderate antioxidant activity while two quercetin 3-glycosides (50,51) showed strong antioxidant activity, suggesting that quinoa could represent an important source of free radical inhibitors [120].
Many flavonoids are characterized by antibacterial, antifungal and antiviral activities, not only against plant pathogens, but also against the pathogens for humans and animals (Table 4). Kaempferol (35) and its derivatives showed antibacterial activity against Gram-positive and Gram-negative bacteria, as well as against the fungus Candida glabrata [121,122].
About eight quercetin derivatives (46–53) have been identified in quinoa. Kaempferol (35), myricetin (45) and quercetin (46) acted as the deterrents against Radopholus similis and Meloidogyne incognita [82]. Quercetin-3-glucoside (47) and rutin (54) from Pinus banksiana inhibited the development of Lymantria dispar and increased its mortality [123]. Quercetin (46), quercetin 3-O-glucoside (47) and its six derivatives exhibited inhibitory activity on the shoot growth of Arabidopsis thaliana as well as on the spore germination of the fungus Neurospora crassa [124].
3.3. Flavanones and Their Biological Activities or Functions
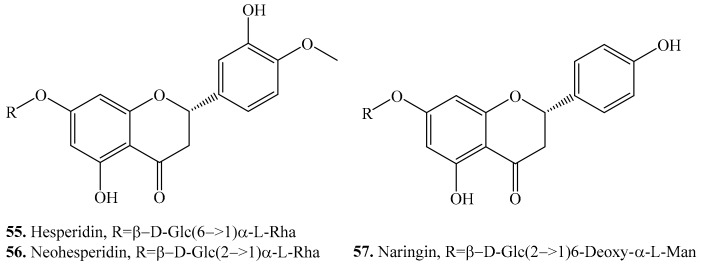
Three flavanones hesperidin (55), neohesperidin (56), and naringin (57) were identified in quinoa seeds (Table 5 and Figure 5). Both hesperidin (55) and neohesperidin (56) were found in the sprouts [34]. These flavanones isolated from other plant species were screened to show a variety of biological activities such as neuroprotective [147], antioxidant [157], anti-inflammatory [158] and antifungal [159] activities.
Table 5.
Flavanones and their biological activities.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Hesperidin (55) | Seeds | - | [34] |
| Neuroprotective effect | [147] | ||
| Antioxidant and cytotoxic activities | [157] | ||
| Anti-inflammatory activity | [158] | ||
| Antifungal activity | [159] | ||
| Anti-proliferative and apoptotic activities | [160] | ||
| Protects the liver against drug-induced injury | [161] | ||
| Cardioprotective activity | [162] | ||
| Neohesperidin (56) | Seeds | - | [34] |
| Neuroprotective effect | [147] | ||
| Antifungal activity | [159] | ||
| Antioxidant activity | [163] | ||
| Induces cell apoptosis | [164] | ||
| Naringin (57) | Seeds | - | [35] |
| Antifungal activity | [159] | ||
| Antioxidative activity | [165] | ||
| Anti-osteoporosis activity | [166] | ||
| Anti-inflammatory activity | [167] |
Figure 5.
Structures of the flavanones isolated from quinoa.
3.4. Flavanols and Their Biological Activities or Functions
Three flavanols namely catechin (58), epicatechin (59), and epigallocatechin (60) were found in quinoa seeds. Their biological activities are listed in Table 6, and their structures are shown in Figure 6. They generally showed antioxidant [149,168] and antimutagenic [169] activities.
Table 6.
Flavanols and their biological activities or functions.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Catechin (58) | Seeds | - | [25] |
| Antioxidant activity | [149] | ||
| Antimutagenic activity | [169] | ||
| Anti-metastatic activity | [170] | ||
| Antifungal activity | [171] | ||
| Apoptosis-inducing activity | [172] | ||
| Epicatechin (59) | Seeds | - | [35] |
| Antimutagenic activity | [169] | ||
| Antioxidant activity | [173] | ||
| Antiproliferative activity | [174] | ||
| Epigallocatechin (60) | Seeds | - | [33,35] |
| Antioxidant activity | [168] |
Figure 6.
Structures of the flavanols isolated from quinoa.
3.5. Isoflavones and Their Biological Activities or Functions
Five isoflavanones, i.e., biochanin (61), daidzein (62), genistein (63), prunetin (64), and puerarin (65) were found in quinoa (Table 7 and Figure 7). They showed antinematodal activities on Radopholus similis [82]. Isoflavones are recognized to be estrogenic compounds that are often associated with a reduced risk of cancers. The estrogenic activity can be enhanced after metabolization to more active compounds such as daidzein (62) and genistein (63) by gut microorganisms [175].
Table 7.
Isoflavones and their biological activities or functions.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Biochanin A (61) | Seeds | - | [35] |
| Daidzein (62) | Seeds | - | [176] |
| Antioxidant activity | [177] | ||
| Enhance adipocyte differentiation and PPARγ transcriptional activities | [178] | ||
| Affected human nonhormone-dependent cervical cancer cells | [179] | ||
| Modulate in vitro rat uterine contractile activity | [180] | ||
| Anti-hypoxia activity | [181] | ||
| Antithrombotic and antiallergic activities | [182] | ||
| Chemoprotective activity | [183] | ||
| Inhibits bone loss in ovariectomized mice | [184] | ||
| Antiproliferative activity | [185,186] | ||
| Genistein (63) | Seeds | - | [176] |
| Antiproliferative activity on human breat cancer cells | [185,186] | ||
| Modulate in vitro rat uterine contractile activity | [180] | ||
| Antioxidant activity | [187] | ||
| Inhibitory activity on tyrosine-specific protein kinases | [188] | ||
| Antitumor activity | [189] | ||
| Cytotoxic activity and anticancer activities | [190] | ||
| Antitumor and antiangiogenic activities | [191,192] | ||
| Antibacterial activity | [193] | ||
| Inhibition of cyclooxygenase-2 activity | [194] | ||
| Antiprostate cancer activity | [195] | ||
| Antileukemic activity | [196] | ||
| Induction of quinone reductase activity | [197] | ||
| Induces growth arrest and suppresses telomerase activities | [198] | ||
| Prunetin (64) | Seeds | - | [25] |
| Anti-inflammatory activity | [199] | ||
| Puerarin (65) | Seeds | - | [35] |
| Antithrombotic and antiallergic activities | [182] | ||
| Anti-apoptosis activity | [200] | ||
| Antioxidant activity | [201] | ||
| Antihyperglycemic effect | [202] |
Figure 7.
Structures of the the isoflavonoids isolated from quinoa.
4. Terpenoids and Their Biological Activities or Functions
The terpenoids in quinoa mainly include monoterpenoids and triterpenoids which are biosynthesized through the isoprenoid metabolic pathway. The monoterpenoids usually play functions as allelochemicals in quinoa. The triterpenoids are present in the seed coats (also called bran or hull), and have a characteristic bitter or astringent taste to protect it from birds and insects, and possess detergent properties [2]. The saponins are also of interest as valuable adjuvants and the first saponin-based vaccines have been introduced commercially [203].
4.1. Monoterpenoids and Their Biological Activities or Functions
Quinoa monoterpenoids and their biological activities are listed in Table 8. Their structures are shown in Figure 8. At least 15 monoterpenoids in the essential oils of quinoa from the East Mediterranean have been identified [204]. Penstebioside (74) was an iridoid glycoside isolated from the flour of quinoa [33]. γ-Terpinene (78) was also isolated from rice to show antibacterial activity on Xanthomonas oryzae pv. oryzae (Xoo) [205].
Table 8.
Monoterpenoids and their biological activities or functions.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| cis-Ascaridole (66) | Leaves | - | [204] |
| cis-Isoascaridole (67) | Leaves | - | [204] |
| Camphene (68) | Leaves | - | [204] |
| Camphor (69) | Leaves | - | [204] |
| trans-Carveol (70) | Leaves | - | [204] |
| p-Cymene (71) | Leaves | - | [204] |
| p-Mentha-1(7),8-diene (72) | Leaves | - | [204] |
| trans-p-Menth-2-en-1-ol (73) | Leaves | - | [204] |
| Penstebioside (74) | Flour | - | [33] |
| β-Pinene (75) | Leaves | - | [204] |
| Pinocarvone (76) | Leaves | - | [204] |
| α-Terpinene (77) | Leaves | - | [204] |
| γ-Terpinene (78) | Leaves | - | [204] |
| Antibacterial activity | [205] | ||
| Terpin-1-ol (79) | Leaves | - | [204] |
| α-Terpinyl acetate (80) | Leaves | - | [204] |
Figure 8.
Structures of the monoterpenoids isolated from quinoa.
4.2. Sesquiterpenoids and Their Biological Activities or Functions
Only one sesquiterpene namely caryophyllene (81) was identified in quinoa [204]. Its structure is shown in Figure 9.
Figure 9.
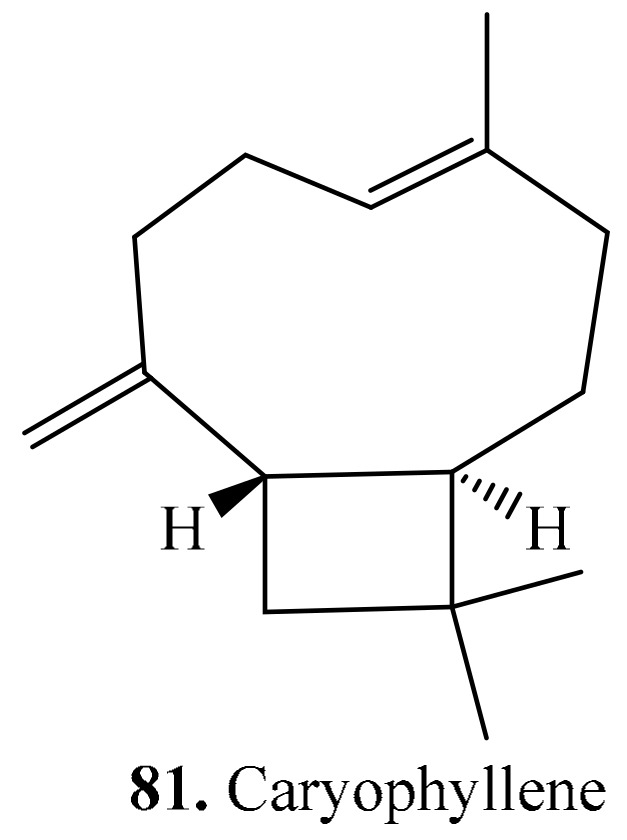
Structure of the sesquiterpenoid isolated from quinoa.
4.3. Triterpenoids and Their Biological Activities or Functions
Triterpenoids, including their aglycones (sapogenins) and glycosides (saponins), are mainly present in the bran to protect quinoa from pests and herbivores (i.e., birds and insects) and pathogenic microorganisms [206]. Quinoa saponins are characterized as the bitter metabolites. The quinoa could be classified into bitter and sweet varieties according to the triterpenoid saponin content, which is much lower in the sweet varieties and higher in the bitter ones [138,207].
The crude saponin fraction inhibited the growth of Candida albicans at 50 μg/mL [208]. The alkali-transformed saponin from quinoa bran showed inhibition against halitosis-related bacterium Fusobacterium nucleatum, with a minimum inhibitory concentration (MIC) of 31.3 μg/mL. It could be used as an antibacterial agent to treat halitosis [209]. When the fungal pathogen Botrytis cinerea was treated with the saponin extracts, mycelial growth and conidial germination were significantly inhibited [210].
When golden apple snails (Pomacea canaliculata, GAS) were treated with the crude saponin under laboratory conditions in 24 h at approximately 33 μg/mL, they were completely killed [211]. Similarly, when giant apple snails (Pomacea maculata) were treated with saponins above 7 μg/mL after 72 h, they were also 100% killed. Quinoa saponin could be a viable product to safely control P. maculata in rice fields [212]. Therefore, quinoa saponins could be developed into molluscicide. In addition, this molluscicide was found to be non-toxic to other non-target species such as goldfish (Carassius auratus) and tilapia (Oreochromis mossambicus), while providing adequate protection from Pomacea snails to newly sprouted rice seeds under laboratory conditions [211,213].
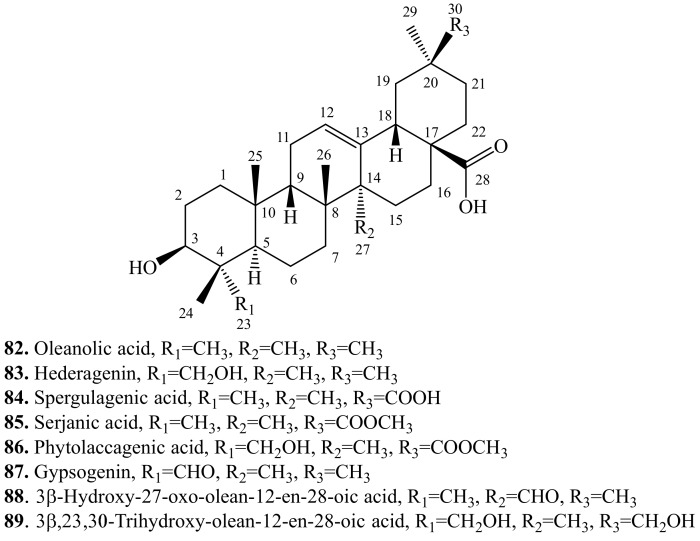
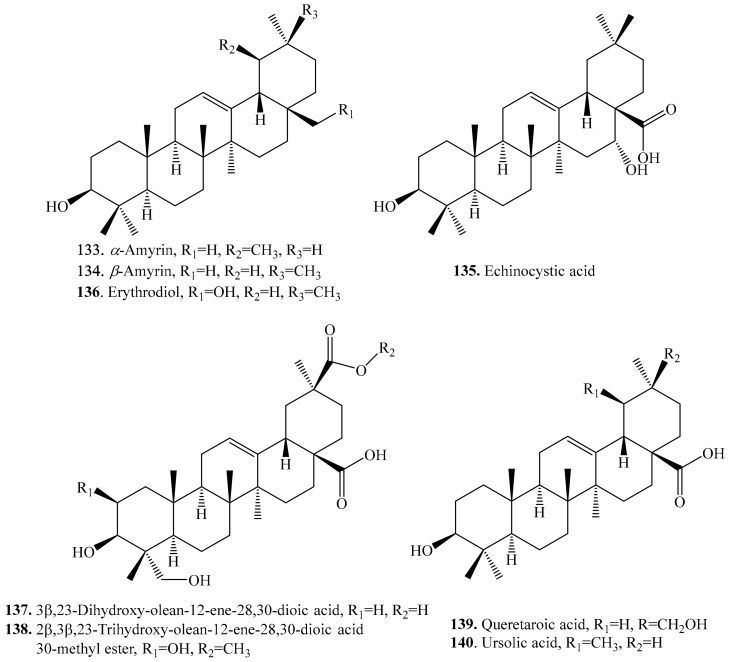
The quinoa triterpenoids contain either tetracycles or pentacycles in their core structures. Most of them are pentacyclic triterpenoids in the form of saponins. The saponins contain an aglycone (sapogenin) and one to three saccharide chains in their structures, and were classified according to the number of saccharide chains as mono-, di-, and tridesmosides. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) allowed a complete preassignment and identification of the major saponins and aglycones [214]. The main aglycones (Figure 10), which are oleanolic acid (82), hederagenin (83), spergulagenic acid (84), serjanic acid (85), phytolaccagenic acid (86), gypsogenin (or named 3β-hydroxy-23-oxo-olean-12-en-28-oic acid) (87), 3β-hydroxy-27-oxo-olean-12-en-28-oic acid (88), and 3β,23,30-trihydroxy-olean-12-en-28-oic acid (89), and their glycosides are shown in Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9 [23,215,216,217]. They have a five-ring skeleton, and are biosynthesized from β-amyrin in planta (134) [23]. Among them, oleanolic acid is the major aglycone [218]. Sugars, which were glucose (Glc), glucuronic acid (GlcA), galactose (Gal), arabinose (Ara), and xylose (Xyl), can be linked to the aglycone at C-3, C-23 or C-28 [214].
Figure 10.
Structures of the main triterpenoid aglycones in quinoa.
Table 9.
Oleanolic acid derivatives and their biological activities or functions.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Oleanolic acid (82) | Seeds and bran | - | [217,233] |
| Antimicrobial activity | [220,221] | ||
| Anti-HIV activity | [222] | ||
| Anti-inflammatory activity | [223] | ||
| Antioxidant activity | [225] | ||
| Antifertility activity | [226] | ||
| Antitumor activity | [227,228] | ||
| Inhibitory activities on serin protease and porcine pancreatic elastase | [232] | ||
| Methyl oleanate (90) | Bran | Anti-inflammatory activity | [234] |
| 3-O-α-l-Arabinopyranosyl-(1→3)-β-d-glucuronopyranosyl oleanolic acid 28-O-β-d-glucopyranosyl ester (91) | Seeds | - | [235,236] |
| 3-O-β-d-Glucopyranosyl oleanolic acid (92) | Seeds | - | [237] |
| Antidiabetogenic activity | [230] | ||
| Anti-inflammatory activity | [224] | ||
| Hemolytic activity | [231] | ||
| 3-O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl oleanolic acid 28-O-β-d-glucopyranosyl ester (93) | Flowers, fruits, seeds and bran | - | [11,235,236] |
| 3-O-β-d-Glucopyranosyl-(1→2)-β-d-glucopyranosyl-(1→3)-α-l-arabinopyranosyl oleanolic acid 28-O-β-d-glucopyranosyl ester (94) | Flowers, fruits, seeds and bran | - | [11,217,238] |
| 3-O-β-d-Glucuropyranosyl oleanolic acid (95) | Seeds | - | [208,238] |
| 3-O-β-d-Glucuronopyranosyl oleanolic acid 28-O-β-d-glucopyranosyl ester (96) | Flowers, fruits, seeds and bran | Hemolytic activity | [11,208,217,236] |
| 3-O-β-d-Xylopyranosy-(1→3)-β-d-glucuronopyranosyl oleanolic acid (97) | Seeds | - | [237] |
| 3-O-β-d-Xylopyranosy(1→3)-6-methyl-β-d-glucuronopyranosyl oleanolic acid (98) | Seeds | - | [237] |
| 3-O-β-d-Xylopyranosyl-(1→3)-β-d-glucuronopyranosyl oleanolic acid 28-O-β-d-glucopyranosyl ester (99) | Flowers, fruits, seeds and bran | - | [11,217,237] |
4.3.1. Oleanolic Acid Derivatives and Their Biological Activities or Functions
About 11 oleanolic acid analogues have been identified in quinoa. Their biological activities are listed in Table 9, and the structures are shown in Figure 11. The major sugars of the saccharide moieties are arabinose, glucose and galactose [219].
Figure 11.
Structures of the oleanolic acid and its glycosides isolated from quinoa.
Oleanolic acid and its glycosides are mainly present in the bran (seeds) of quinoa. They showed a variety of biological activities such as antimicrobial [220,221], anti-HIV [222], anti-inflammatory [223,224], antioxidant [225], antifertility [226], antitumor or anticancer [227,228,229], antidiabetogenic [230], anticomplement [231] properties. They also exhibited inhibitory activities on serin protease and porcine pancreatic elastase [232].
4.3.2. Hederagenin Derivatives and Their Biological Activities or functions
About 10 hederagenin analogues have been identified in quinoa. Their biological activities are listed in Table 10, and the structures are shown in Figure 12. Hederagenin (83) was the main aglycone of saponins from quinoa leaves [239]. Hederagenin glycosides existed in nature and possessed many biological activities such as molluscicidal [240], cytotoxic [241], antifungal [242], leishmanicidic [243], anti-inflammatory [244] activities, and they have been recently reported to show low cytotoxic properties for several human cancer cell lines with median effective concentration (EC50) >30 μM [245]. Hederagenin monodesmosides also showed strong haemolytic activity [208], hence the saponins have been considered as the serious antinutritional factors [246]. Hederagenin from the leaves of ivy (Hedera helix) induced apoptosis of LoVo cells through the mitochondrial apoptotic pathway, which indicated that hederagenin might be a promising therapeutic candidate for the prevention and treatment of human colon cancer [247].
Table 10.
Hederagenin derivatives and their biological activities or functions.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Hederagenin (83) | Seeds and bran | - | [215,216,217] |
| Inhibitory activity on serin protease, and porcine pancreatic elastase | [232] | ||
| Cytotoxic activity on P-388 mouse lymphoma, L-1210 mouse lymphomatic leukemia, HL-60 human promyelocytic leukemia and SNU-5 human stomach cancer cells | [248,249] | ||
| Haemolytic activity | [250] | ||
| Anti-inflammatory activity | [244] | ||
| Antidermatophytic activity | [251] | ||
| Antitrichomonas activity | [252] | ||
| Inducing apoptosis in human LoVo colon cells | [247] | ||
| 3-O-α-l-Arabinopyranosyl hederagenin (100) | Seeds | - | [208] |
| Molluscicidal activity | [140,253] | ||
| Cytotoxic activity on human carcinoma and melanoma cell lines DLD-1, PA1, A549, MCF7, PC3, and M4 | [241] | ||
| Antifungal activity | [242] | ||
| Leishmanicidic activity | [243] | ||
| Antidermatophytic activity | [251] | ||
| 3-O-α-l-Arabinopyranosyl hederagenin 28-O-β-d-glucopyranosyl ester (101) | Flowers, fruits, seeds and bran | - | [11,208,216,217] |
| Antidermatophytic activity | [251] | ||
| Anticomplementary activity | [254] | ||
| 3-O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl hederagenin (102) | Seeds and bran | - | [208,216,238] |
| Cytotoxic activity on A549, SK-OV-3, SK-MEL-2, XF498 and HCT15 | [255] | ||
| 3-O-β-d-Glucopyranosyl-(1→3)-β-d-galactopyranosyl hederagenin (103) | Bran | - | [216] |
| 3-O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranoside hederagenin 28-O-β-d-glucopyranosyl ester (104) | Flowers, fruits, seeds and bran | - | [11,208,216,217,236,238,256] |
| 3-O-β-d-Glucopyranosyl-(1→3)-β-d-galactopyranosyl hederagenin 28-O-β-d-glucopyranosyl ester (105) | Flowers, fruits, seeds and bran | - | [11,216,238] |
| 3-O-β-d-Glucopyranosyl-(1→4)-β-d-glucopyranosyl-(1→4)-β-d-glucopyranosyl hederagenin 28-O-β-d-glucopyranosyl ester (106) | Seeds | - | [256] |
| 3,23-Bis(O-β-d-glucopyranosyloxy) olean-12-en-28-oic acid 28-O-β-d-glucopyranosyl-(1→3)-α-l-arabinopyanosyl ester (107) | Seeds | - | [257] |
| 3-O-β-d-Glucuronopyranosyl hederagenin 28-O-β-glucopyranosyl ester (108) | Flowers, fruits, seeds and bran | - | [11,217,238] |
| 3-O-β-d-Xylopyranosyl-(1→3)-β-d-glucuronopyranosyl hederagenin 28-O-β-d-glucopyranosyl ester (109) | Bran | - | [217] |
Figure 12.
Structures of hederagenin and its glycosides isolated from quinoa.
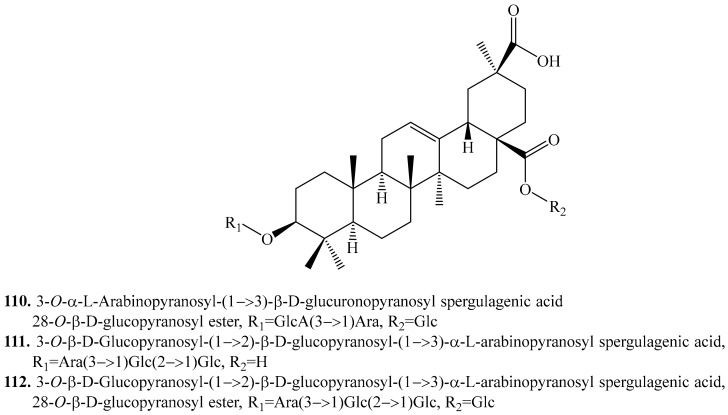
4.3.3. Spergulagenic Acid Derivatives and Their Biological Activities or Functions
Spergulagenic acid (84), a pentacyclic triterpene used in medicine, was found in diverse plant families [258]. Until now, three spergulagenic acid glycosides (Table 11) were identified in quinoa [217,256], though spergulagenic acid as the aglycone has not been isolated from quinoa. Their structures are shown in Figure 13.
Table 11.
Spergulagenic acid derivatives and their biological activities or functions.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| 3-O-α-l-Arabinopyranosyl-(1→3)-β-d-glucuronopyranosyl spergulagenic acid 28-O-β-d-glucopyranosyl ester (110) | Seeds | - | [256] |
| 3-O-β-d-Glucopyranosyl-(1→2)-β-d-glucopyranosyl-(1→3)-α-l-arabinopyranosyl spergulagenic acid (111) | Bran | - | [217] |
| 3-O-β-d-Glucopyranosyl-(1→2)-β-d-glucopyranosyl-(1→3)-α-l-arabinopyranosyl spergulagenic acid 28-O-β-d-glucopyranosyl ester (112) | Seeds | - | [256] |
Figure 13.
Structures of the spergulagenic acid glycosides isolated from quinoa.
4.3.4. Serjanic Acid Derivatives and Their Biological Activities or Functions
Serjanic acid (85) is the aglycone with only the bidesmosides to be found in quinoa [217]. About 5 serjanic acid analogues have been identified in quinoa (Table 12, Figure 14). Hemolysis tests showed that most monodesmoside saponins were active, and most bidesmoside saponins were inactive as the monodesmosides can reduce hydrophobic interactions with membrane lipids [208]. Similarly, both 3-O-α-l-arabinopyranosyl serjanic acid 28-O-β-d-glucopyranosyl ester (113) and 3-O-β-d-glucuronopyranosyl serjanic acid 28-O-β-d-glucopyranosyl ester (116) had weaker hemolytic activity (IC50 > 100 μg/mL) than their sapogenin (serjanic acid, IC50 = 50 μg/mL) [11].
Table 12.
Serjanic acid derivatives and their biological activities or functions.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Serjanic acid (85) | Flowers, fruits, seeds and bran | Cytotoxic activity on HeLa cell line | [11,217] |
| 3-O-α-l-Arabinopyranosyl serjanic acid 28-O-β-d-glucopyranosyl ester (113) | Flowers, fruits, seeds and bran | Cytotoxic activity on Hela cell line | [11] |
| 3-O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl serjanic acid 28-O-β-d-glucopyranosyl ester = 3-O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl-30-O-methyl spergulagenate 28-O-β-d-glucopyranosyl ester (114) | Flowers, fruits, seeds and bran | - | [11,236,238] |
| 3-O-β-d-Glucopyranosyl-(1→2)-β-d-glucopyranosyl-(1→3)-α-l-arabinopyranosyl serjanic acid 28-O-β-d-glucopyranosyl ester (?) = 3-O-β-d-Glucopyranosyl-(1→2)-β-d-glucopyranosyl-(1→3)-α-l-arabinopyranosyl-30-O-methyl spergulagenate 28-O-β-d-glucopyranosyl ester (115) | Flowers, fruits, seeds and bran | - | [11,217,238,256] |
| 3-O-β-d-Glucuronopyranosyl serjanic acid 28-O-β-d-glucopyranosyl ester (116) | Flowers, fruits, seeds and bran | Cytotoxic activity on HeLa cell line | [11] |
Figure 14.
Structures of serjanic acid and its glycosides isolated from quinoa.
4.3.5. Phytolaccagenic Acid Derivatives and Their Biological Activities or Functions
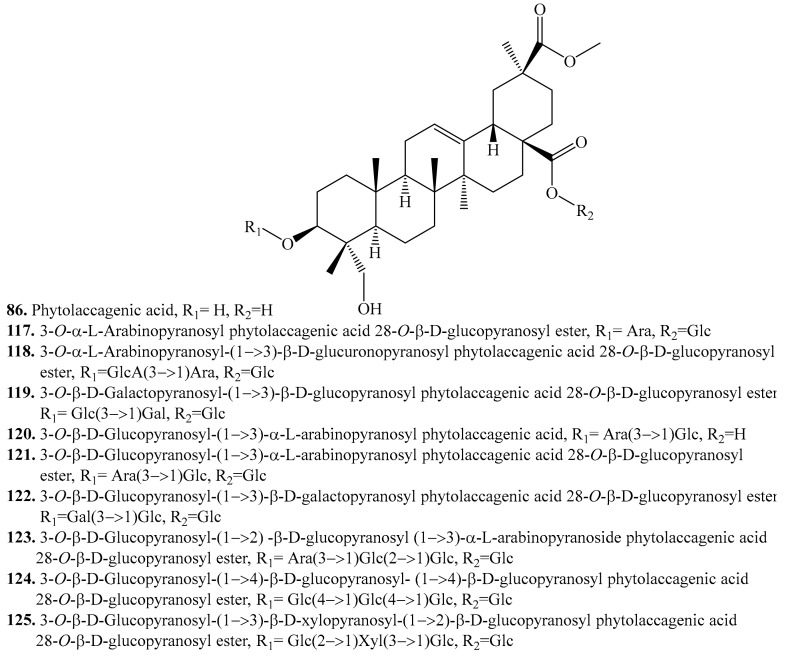
Phytolaccagenic acid (86) might be originated from serjanic acid (85) by subsequent oxidative enzymatic steps involving the formation of the corresponding alcohol substituted at C-23 in planta [11]. It is one of the main structures of quinoa sapogenins. About 10 phytolaccagenic acid analoques have been identified in quinoa. They are listed in Table 13, and the structures are shown in Figure 15.
Table 13.
Phytolaccagenic acid derivatives and their biological activities or functions.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Phytolaccagenic acid (86) | Bran | - | [216,217] |
| Bran | Anti-inflammatory activity | [234] | |
| 3-O-α-l-Arabinopyranosyl phytolaccagenic acid 28-O-β-d-glucopyranosyl ester (117) | Flowers, fruits, seeds and bran | - | [11,208,216,217,238] |
| 3-O-α-l-Arabinopyranosyl-(1→3)-β-d-glucuronopyranosyl phytolaccagenic acid 28-O-β-d-glucopyranosyl ester (118) | Seeds | - | [235,236,256] |
| 3-O-β-d-Galactopyranosyl-(1→3)-β-d-glucopyranosyl phytolaccagenic acid 28-O-β-d-glucopyranosyl ester (119) | Seeds | - | [238] |
| 3-O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl phytolaccagenic acid (120) | Seeds | Antifungal activity | [208,238] |
| 3-O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl phytolaccagenic acid 28-O-β-d-glucopyranosyl ester (121) | Flowers, fruits, seeds and bran | - | [11,208,216,217,235,236,238] |
| 3-O-β-d-Glucopyranosyl-(1→3)-β-d-galactopyranoside phytolaccagenic acid 28-O-β-d-glucopyranosyl ester (122) | Flowers, fruits, seeds and bran | - | [11,216,217,238] |
| 3-O-β-d-Glucopyranosyl(1→2)-β-d-glucopyranosyl-(1→3)-α-l-arabinopyranoside phytolaccagenic acid 28-O-β-d-glucopyranosyl ester (123) | Flowers, fruits, seeds and bran | - | [11,217,235,238] |
| 3-O-β-d-Glucopyranosyl-(1→4)-β-d-glucopyranosyl-(1→4)-β-d-glucopyranosyl phytolaccagenic acid 28-O-β-d-glucopyranosyl ester (124) | Flowers, fruits, seeds and bran | - | [11,256] |
| 3-O-β-d-Glucopyranosyl-(1→3)-β-d-xylopyranosyl-(1→2)-β-d-glucopyranosyl phytolaccagenic acid 28-O-β-d-glucopyranosyl ester (125) | Seeds | - | [235] |
Figure 15.
Structures of phytolacagenic acid and its glycosides isolated from quinoa.
Phytolaccagenic acid saponins are highly concentrated in the bran (seed coats), which are more exposed to water during germination compared to oleanolic acid saponins [259]. It was suggested that a short saccharide chain (1 or 2 glycosyl residues) requires the presence of an additional longer one to make the saponin water-soluble [260]. Phytolaccagenic acid was employed as the anti-inflammatory drug of oral administration [234].
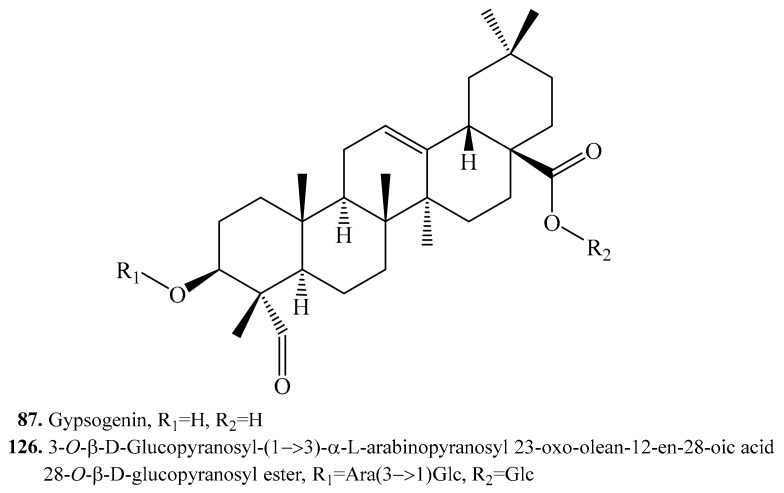
4.3.6. Gypsogenin Derivatives and Their Biological Activities or Functions
Gypsogenin (or named 3β-hydroxy-23-oxo-olean-12-en-28-oic acid) (87) and its glycoside 3-O-β-d-glucopyranosyl-(3)-α-l-arabinopyranosyl 23-oxo-olean-12-en-28-oic acid 28-O-β-d-gluco-pyranosyl ester (126) were isolated from quinoa (Table 14, Figure 16). They showed cytotoxic activity [11].
Table 14.
Gypsogenin derivatives and their biological activities or functions.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Gypsogenin = 3β-Hydroxy-23-oxo-olean-12-en-28-oic acid (87) | Flowers, fruits, seeds and bran | Cytotoxic activity on Hela cell line | [11] |
| 3-O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl 23-oxo-olean-12-en-28-oic acid 28-O-β-d-glucopyranosyl ester = 3β-[(O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl)oxy]-23-oxo-olean-12-en-28-oic acid 28-O-β-d-glucopyranosyl ester (126) | Flowers, fruits, seeds and bran | Cytotoxic activity on HeLa cell line | [11] |
Figure 16.
Structures of the gypsogenin derivatives isolated from quinoa.
4.3.7. 3β-Hydroxy-27-oxo-olean-12-en-28-oic Acid Derivatives and Their Biological Activities or Functions
3β-Hydroxy-27-oxo-olean-12-en-28-oic acid (88) and its glycoside 3-O-β-d-glucopyranosyl-(1→3)-α-l-arabinopyranosyl 27-oxo-olean-12-en-28-oic acid 28-O-β-d-glucopyranosyl ester (127) were isolated from quinoa (Table 15, Figure 17). 3β-Hydroxy-27-oxo-olean-12-en-28-oic acid (88) showed same cytotoxic effect as 3β-hydroxy-23-oxo-olean-12-en-28-oic acid (87) with an IC50 value of 25.4 μg/mL. This suggests that the CHO groups at C-23 or C-27 are correlated with the increased cytotoxicity [11].
Table 15.
3β-Hydroxy-27-oxo-olean-12-en-28-oic acid derivatives and their biological activities or functions.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| 3β-Hydroxy-27-oxo-olean-12-en-28-oic acid (88) | Flowers, fruits, seeds and bran | Cytotoxic activity on HeLa cell line | [11] |
| 3-O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl 27-oxo-olean-12-en-28-oic acid 28-O-β-d-glucopyranosyl ester = 3β-[(O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl)oxy]-27-oxo-olean-12-en-28-oic acid 28-O-β-d-glucopyranoside (127) | Flowers, fruits, seeds and bran | Cytotoxic activity on Hela cell line | [11] |
Figure 17.
Structures of the 3β-hydroxy-27-oxo-olean-12-en-28-oic acid triterpenoids isolated from quinoa.
4.3.8. 3β,23,30-Trihydroxy-olean-12-en-28-oic acid Triterpenoids and Their Biological Activities or Functions
3β,23,30-Trihydroxy-olean-12-en-28-oic acid (89) and 3-O-β-d-glucopyranosyl-(1→3)-α-l-arabinopyranosyl 3β,23,30-trihydroxy olean-12-en-28-oic acid 28-O-β-d-glucopyranosyl ester (128) have been isolated from quinoa (Table 16, Figure 18). Hederagenin (83) was considered as the substrate for the production of 3β,23,30-trihydroxyolean-12-en-28-oic acid (89), following a mechanism involving a stereochemically specific enzyme able to insert one hydroxyl group into the C-30 position of the triterpene skeleton [11].
Table 16.
3β,23,30-Trihydroxy-olean-12-en-28-oic acid triterpenoids and their biological activities or functions.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| 3β,23,30-Trihydroxy-olean-12-en-28-oic acid (89) | Flowers, fruits, seeds and bran | - | [11] |
| 3-O-β-d-Glucopyranosyl-(1→3)-α-l-arabinopyranosyl 3β,23,30-trihydroxyolean-12-en-28-oic acid 28-O-β-d-glucopyranosyl ester (128) | Flowers, fruits, seeds and bran | - | [11,214] |
Figure 18.
Structures of the 3β,23,30-trihydroxy-olean-12-en-28-oic acid triterpenoids isolated from quinoa.
4.3.9. Other Triterpenoids and Their Biological Activities or Functions
Other triterpenoids include tetracyclic and pentacyclic triterpenoids. Their biological activities are shown in Table 17, and the structures are shown in Figure 19 and Figure 20.
Table 17.
Other triterpenoids and their biological activities or functions.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Tetracyclic triterpenoids | |||
| Citrostadienol (129) | Seeds | - | [261] |
| Anticomplementary activity | [262] | ||
| Gramisterol (130) | Seeds | - | [261] |
| Anti-cancer activity on mouse leukemic cell line WEHI-3 | [8] | ||
| 24-Methylene-cycloartenol (131) | Seeds | - | [261] |
| Parkeol (132) | Seeds | - | [261] |
| Pentacyclic triterpenoids | |||
| α-Amyrin (133) | Seeds | - | [215] |
| Antibacterial activity | [263] | ||
| Antidiabetic effect | [264] | ||
| Antioxidant activity | [265] | ||
| Inhibitory activity against human oxidosqualene cyclase | [266] | ||
| β-Amyrin (134) | Seeds | - | [215] |
| Antibacterial activity | [263] | ||
| Antioxidant activity | [265] | ||
| Inhibitory activity against human oxidosqualene cyclase | [266] | ||
| Antifeedant and growth regulating activities | [267] | ||
| Insecticidal activity | [268] | ||
| Echinocystic acid (135) | Seeds | - | [215] |
| Erythrodiol (136) | Seeds | - | [215,261] |
| Antibacterial activity | [269] | ||
| Melanogenesis-inhibitory activity | [270] | ||
| Protecting the cardiovascular system | [271] | ||
| Antiproliferative and apoptotic activity | [272] | ||
| 3β,23-Dihydroxy-olean-12-ene-28,30-dioic acid (137) | Seeds | - | [208] |
| 2β,3β,23-Trihydroxy-olean-12-ene-28,30-dioic acid 30-methyl ester (138) | Bran | - | [216] |
| Queretaroic acid (139) | Seeds | - | [215] |
| Ursolic acid (140) | Seeds | - | [215] |
| Spasmolytic and antinociceptive activities | [85] | ||
| Cytotoxic activity | [270] | ||
| Anticancer activity | [273,274] |
Figure 19.
Structures of the tetracyclic triterpenoids isolated from quinoa.
Figure 20.
Structures of the other pentacyclic triterpenoids isolated from quinoa.
Four tetracyclic triterpenoids including two nortriterpenoids citrostadienol (129) and gramisterol (130) have been isolated from quinoa seeds [261]. Citrostadienol (129) showed anticomplementary activity [262], and gramisterol (130) showed anti-cancer activity [8].
Among the other pentacyclic triterpenoids, β-amyrin (133) was considered as the precursors of other triterpenoids in their biosynthetic pathways [23].
4.4. Meroterpenoids and Their Biological Activities or Functions
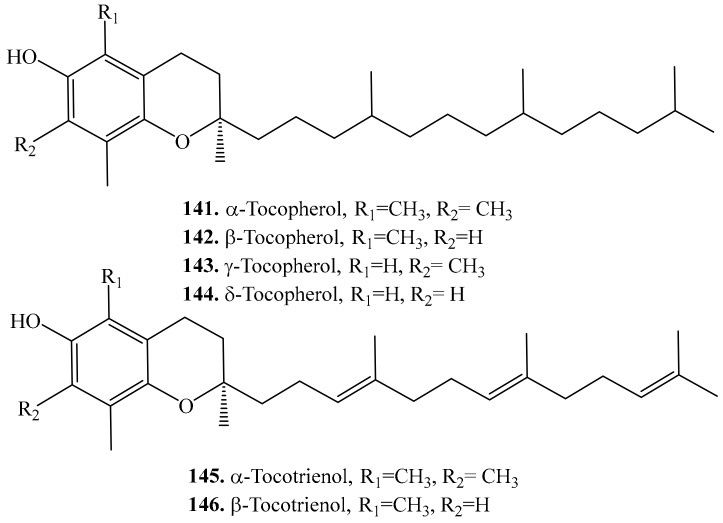
Mertoterpenoids are natural products of mixed biosynthetic origin which are partially derived from terpenoids. Meroterpenoids were also found in quinoa that include tocopherols (141–144) and tocotrienols (145,146). Their biological activities are listed in Table 18, and the structures are shown in Figure 21.
Table 18.
Meroterpenoids and their biological activities or functions.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| α-Tocopherol (141) | Seeds | - | [283] |
| Antioxidative, antihypercholesterolemic, anticancer, neuroprotective activities | [284] | ||
| β-Tocopherol (142) | Seeds | - | [37] |
| Antioxidative, antihypercholesterolemic, anticancer, neuroprotective activities | [284] | ||
| γ-Tocopherol (143) | Seeds | - | [283] |
| Antioxidative, antihypercholesterolemic, anticancer, neuroprotective activities | [284] | ||
| δ-Tocopherol (144) | Seeds | - | [37] |
| Antioxidative, antihypercholesterolemic, anticancer, neuroprotective activities | [284] | ||
| α-Tocotrienol (145) | Seeds | - | [275] |
| Antioxidant and anti-inflammatory activities | [282] | ||
| β-Tocotrienol (146) | Seeds | - | [275] |
| Antioxidant and anti-inflammatory activities | [282] |
Figure 21.
Structures of the meroterpenoids isolated from quinoa.
The total tocopherol content ranged from 37.49 to 59.82 μg/g [275]. All four tocopherol isoforms (α, β, γ, and δ) have been detected in quinoa seeds, with γ-tocopherol (143) to be the most abundant followed by α-tocopherol (141), β-tocopherol (142) and δ-tocopherol (144) was the least [276]. Tocopherols acted as strong antioxidants and had many essential physiological functions such as anticoagulant, essential regulator of metabolic processes including inflammation and cancer in humans [277,278]. Among 4 tocopherols homologues, α-tocopherol (141) was considered a stronger antioxidant, whereas γ-tocopherol (143) was a stronger anti-inflammatory agent [279,280]. γ-Tocopherol (143) was the main lipophilic tocopherol in quinoa [281].
Both α-tocotrienol (145) and β-tocotrienol (146) were also identified in quinoa seeds [275]. They were the members of the vitamin E family to show antioxidant and anti-inflammatory properties [282].
5. Steroids and Their Biological Activities or Functions
Quinoa contains a lot of biologically active phytoecdysteroids, which have been implicated in plant defense from insects, and have displayed potential pharmacologic and metabolic properties in mammals. According to the carbon skeletons, quinoa steroids can be classified as C27-, C28- and C29-steroids.
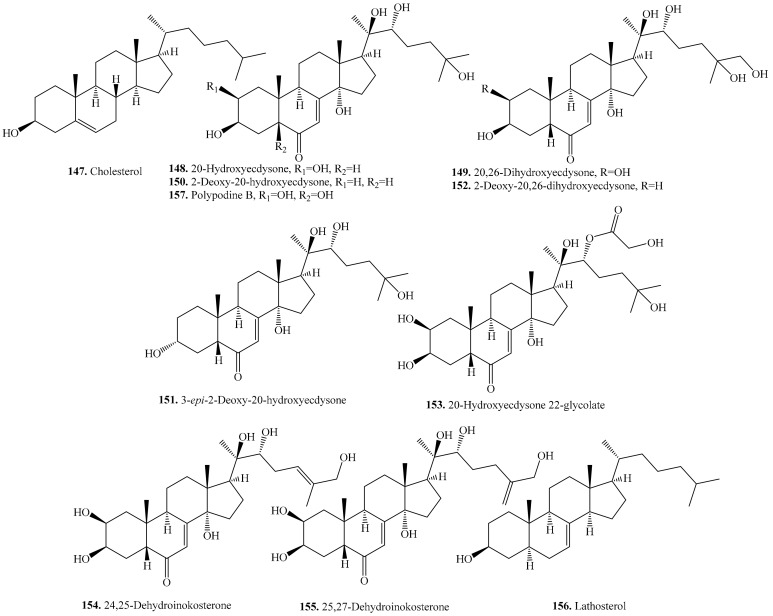
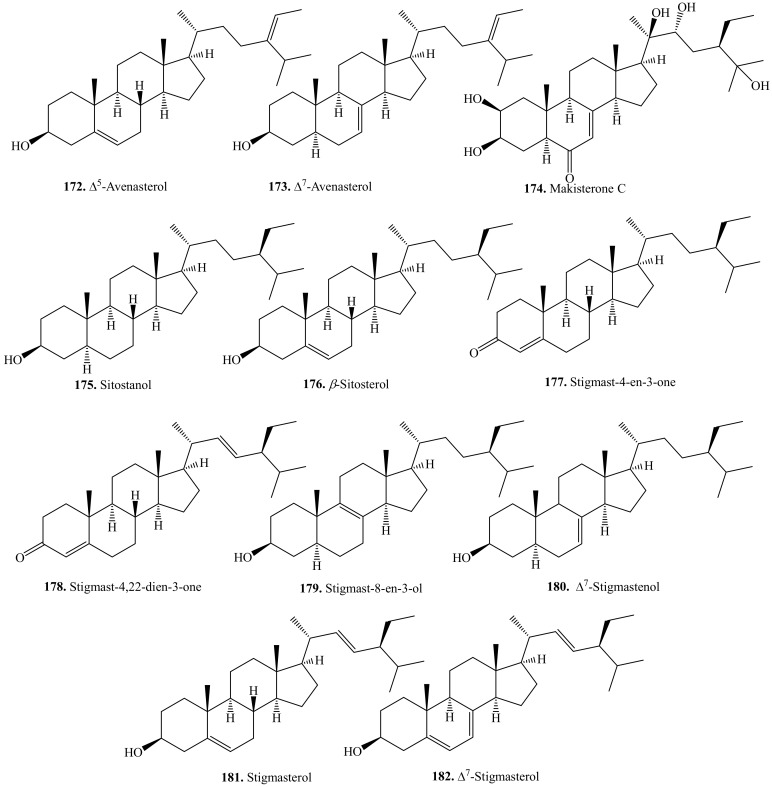
About 36 steroids have been identified in quinoa. Seven sterols were identified among the quinoa lipids, namely cholesterol (147), campesterol (160), Δ7-campesterol (161), Δ5-avenasterol (172), β-sitosterol (176), stigmasterol (181), and Δ7-stigmasterol (182) [285]. Eleven 4,4-desmethylsterols were assigned, with Δ7-avenasterol (173), β-sitosterol (176), and Δ7-stigmastenol (180) being the most abundant (8.7, 27.2, and 51.3% of total sterols, respectively) [261].
5.1. C27-Steroids and Their Biological Activities or Functions
Eleven C27-steroids were identified in quinoa seeds which are listed in Table 19. Their structures are shown in Figure 22. Among them, ecdysteroids are main steroids which are insect moulting hormones and protect plants against non-adapted insects and nematodes [21]. Ecdysteroids are mainly present in the bran, the major component is 20-hydroxyecdysone (148) possessing a 14α-hydroxy-7-en-6-one chromophore and A/B-cis ring fusion (5β-H) [21].
Table 19.
C27-Steroids and their biological activities or functions.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Cholesterol (147) | Seeds | - | [285,288] |
| 20-Hydroxyecdysone (148) | Seeds | Antioxidant activity | [289] |
| Inhibitory activity on collagenase | [287] | ||
| Insecticidal activity | [290] | ||
| 20,26-Dihydroxyecdysone (149) | Seeds | Antioxidant activity | [289] |
| Inhibitory activity on collagenase | [287] | ||
| 2-Deoxy-20-hydroxyecdysone (150) | Seeds | - | [22] |
| 3-epi-2-Deoxy-20-hydroxyecdysone (151) | Seeds | - | [22] |
| 2-Deoxy-20,26-dihydroxyecdysone (152) | Seeds | - | [22] |
| 20-Hydroxyecdysone 22-glycolate (153) | Seeds | Antioxidant activity and inhibitory activity on collagenase | [287] |
| 24,25-Dehydroinokosterone (154) | Seeds | - | [22] |
| 25,27-Dehydroinokosterone (155) | Seeds | - | [22] |
| Lathosterol (156) | Seeds | - | [261] |
| Polypodine B (157) | Seeds | - | [22] |
Figure 22.
Structures of the C27-steroids isolated from quinoa.
Eating quinoa seeds or quinoa-derived products provides significant amounts of ecdysteroids that may be beneficial to animal or human health [22]. Quinoa extract enriched in 20-hydroxyecdysone has an antiobesity activity in vivo and could be used as a nutritional supplement for the prevention and treatment of obesity and obesity-associated disorders. The findings indicated that the extract acted by reducing both fatty acid uptake and esterification in adipocyte [286]. It was found that 8 isolated ecdysteroids showed a stronger free-radical-scavenging activity, which was almost 3 to 8 times higher than that of the well-known antioxidant compound, BHA, and also possessed a strong ability to sequester ferrous ions. This observation supported that if the number of hydroxyl and methyl groups bearing the carbon skeleton of ecdysteroids is higher, the antioxidant activity becomes stronger. The ability of ecdysteroids to sequester ferrous ions is thought to be due to their carbonyl conjugated to a double bond attached to the C-7. Ecdysteroids are also able to inhibit skin collagenase, and could therefore also prevent skin ageing [287]. In addition, ecdysteroids have been reported to occur in Chenopodiaceae to show their possible chemotaxonomic and ecological implications [21].
5.2. C28-Steroids and Their Biological Activities or Functions
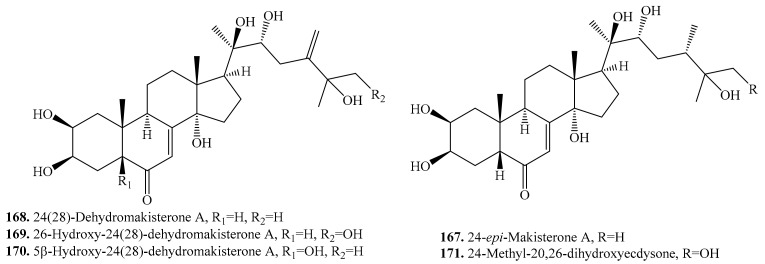
About 14 C28-steroids such as campesterol (160), makisterone A (166), and their derivatives have been identified from quinoa seeds [34]. Their biological activities are listed in Table 20, and the structures are shown in Figure 23. The main biological activities include antioxidant activity [289], antiangiogenic activity [291], and inhibitory activity on collagenase [287].
Table 20.
C28-Steroids and their biological activities or functions.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Brassicasterol (158) | Seeds | - | [292] |
| Campestanol (159) | Seeds | - | [261] |
| Campesterol = Δ5-Campesterol (160) | Seeds | - | [261,285,288] |
| Antiangiogenic activity | [291] | ||
| Δ7-Campesterol (161) | Seeds | - | [285,288] |
| Dacrysterone (162) | Seeds | - | [22] |
| Episterol (163) | Seeds | - | [261] |
| Ergost-7-en-3β-ol = Δ7-Ergostenol (164) | Seeds | - | [261] |
| Kancollosterone (165) | Seeds | - | [293] |
| Makisterone A (166) | Seeds | Antioxidant activity | [289] |
| Inhibitory activity on collagenase | [287] | ||
| 24-epi-Makisterone A (167) | Seeds | Antioxidant activity | [289] |
| Inhibitory activity on collagenase | [287] | ||
| 24(28)-Dehydromakisterone A (168) | Seeds | Antioxidant activity | [289] |
| Inhibitory activity on collagenase | [287] | ||
| 26-Hydroxy-24(28)-dehydromakisterone A (169) | Seeds | Antioxidant activity, inhibitory activity on collagenase | [287] |
| 5β-Hydroxy-24(28)-dehydromakisterone A (170) | Seeds | - | [22] |
| 24-Methyl-20,26-dihydroxyecdysone (171) | Seeds | - | [22] |
| Seeds | Antioxidant activity | [287] |
Figure 23.
Structures of the C28-steroids isolated from quinoa.
5.3. C29-Steroids and Their Biological Activities or Functions
The main C29-steroids in quinoa included avenasterol (172/173), sitosterol (176), stigmasterol (181), and their derivatives. They were all identified in the lipid extract of quinoa seeds [261]. Their biological activities are listed in Table 21, and the structures are shown in Figure 24.
Table 21.
C29-Steroids and their biological activities or functions.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
| Δ5-Avenasterol = Δ5,24(28)-Avenasterol (172) | Seeds | - | [261,285,288] |
| Δ7-Avenasterol = Δ7,24(28)-Avenasterol (173) | Seeds | - | [261] |
| Makisterone C (174) | Seeds | - | [22] |
| Sitostanol (175) | Seeds | - | [261] |
| β-Sitosterol (176) | Seeds | - | [285,288] |
| Insecticidal activity | [268] | ||
| Anti-inflammatory activity | [294] | ||
| Anti-oxidant activity | [295] | ||
| Antidiabetic activity | [296] | ||
| Inducing apoptosis | [302] | ||
| Hypocholesterolemic activity | [303,304] | ||
| Angiogenic effect | [305] | ||
| Genotoxicity effect | [306] | ||
| Anthelminthic and Anti-mutagenic activity | [307] | ||
| Immunomodulatory activity | [308] | ||
| Neuroprotection effect | [309] | ||
| Stigmast-4-en-3-one (177) | Seeds | [292] | |
| Stigmast-4,22-dien-3-one (178) | Seeds | [292] | |
| Stigmast-8-en-3-ol (179) | Seeds | - | [261] |
| Δ7-stigmastenol (180) | Seeds | - | [261] |
| Stigmasterol = Δ5-Stigmasterol (181) | Seeds | - | [261] |
| Anti-inflammatory activity | [297] | ||
| Anti-tumor activity | [298] | ||
| Antifungal activity | [299] | ||
| Anti-hypercholestrolemic activity | [300] | ||
| Cytotoxicity activity | [301] | ||
| Anti-osteoarthritic activity | [310] | ||
| Δ7-Stigmasterol (182) | Seeds | - | [285,288] |
| Seeds | - | [261] |
Figure 24.
Structures of the C29-steroids isolated from quinoa.
β-Sitosterol (176) has been reported to have a variety of biological activities such as anti-inflammatory [294], antioxidant [295], and antidiabetic [296] activities. Stigmasterol (181) also exhibited various biological activities such as anti-inflammatory [297], anti-tumor [298], antifungal [299], anti-hypercholestrolemic [300], and cytotoxicity [301] activities.
6. Nitrogen-Containing Metabolites and Their Biological Activities or Functions
About 12 nitrogen-containing metabolites have been identified in quinoa seeds. They belong to the derivatives of glycine and tyrosine. Their biological activities are listed in Table 22, and the structures are shown in Figure 25.
Table 22.
Nitrogen-containing metabolites and their biological activities or functions.
| Name | Quinoa Part Used for Isolation | Biological Activity or Function | Ref. |
|---|---|---|---|
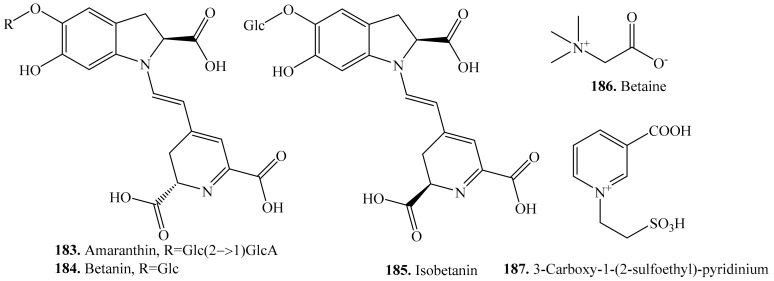
| Amaranthin (183) | Seeds | - | [316] |
| Betanin (184) | Seeds | - | [35] |
| Antioxidant activity | [317] | ||
| Isobetanin (185) | Seeds | - | [35] |
| Betaine (186) | Seeds | - | [35] |
| 3-Carboxy-1-(2-sulfoethyl)-pyridinium (187) | Seeds | - | [313] |
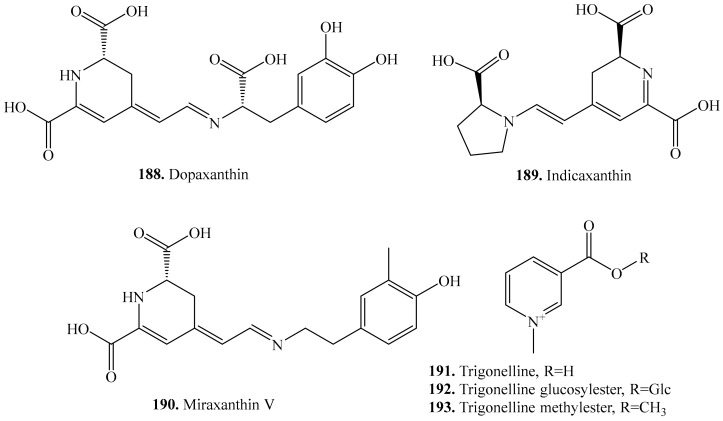
| Dopaxanthin (188) | Seeds | - | [316] |
| Antioxidant activity | [318] | ||
| Indicaxanthin (189) | Seeds | - | [316] |
| Miraxanthin V (190) | Seeds | - | [316] |
| Trigonelline (191) | Seeds | - | [313] |
| Anti-invasive activity | [319] | ||
| Hypoglycemic effect | [320] | ||
| Trigonelline glucosylester (192) | Seeds | - | [313] |
| Trigonelline methylester (193) | Seeds | - | [313] |
Figure 25.
Structures of the nitrogen-containing metabolites isolated from quinoa.
Betalains are tyrosine-derived red-violet and yellow pigments found in quinoa [311]. They are divided into two groups, betacyanins (red and purple) and betaxanthins (yellow and orange). Betacyanins are derivatives of betanidin, the conjugate of betalamic acid with cyclo-Dopa. Betacyanins, including amaranthin (183), betanin (184) and isobetanin (185), were confirmed in red and black quinoa seeds, instead of anthocyanins [35]. Betaxanthins are conjugates of betalamic acid with amino acids. Betaxanthins mainly include dopaxanthin (188), indicaxanthin (189), and miraxanthin V (190) in quinoa [312].
Betalains showed promising bioactive potential, such as high antioxidant and free radical scavenging activities [30]. Betacyanins and betaxanthins showed the highest antioxidant activity by comparing the white and black quinoa varieties [35]. These two varieties are characterized by a high content of dopaxanthin (188), whose dihydroxylated substructure demonstrated high antioxidant capacity [36].
Other nitrogen-containing metabolites in quinoa include betaine (186), trigonelline (191), and their derivatives. In mammals, betaine (186) acted as an osmolyte in the inner medulla of the kidney, preserving osmotic equilibrium, maintaining at the same time the tertiary structure of macromolecules [313]. Trigonelline (191) was considered to be an important multifunctional natural plant hormone with potential taxonomic value [314], and has been shown to stabilize enzyme activity in vitro [315].
7. Conclusions and Future Perspectives
This review focuses on the structures, isolation parts, biological activities or functions of quinoa secondary metabolites during the past 40 years. Flavonoids and phenolic acids were mostly derived from quinoa seeds. Steroids were mostly separated from quinoa bran. Triterpenoids were also mainly located in the bran. Their biological activities or functions have been reported but not comprehensive, and are needed to be systematically evaluated in the future.
The bitter taste associated with saponins (triterpenoids) greatly limits the use of quinoa as food [18]. Approximately 34% of quinoa saponins are present in the bran, indicating that dehulling could remove almost one half of the saponins. The seeds should be milled to remove the bran (seed coats) to make them edible [239]. Another method to remove saponins from the seeds is washing due to the high water solubility of saponins although this method can lead to the loss of some nutrients such as vitamins and minerals [18].
With the increased demand for quinoa, the problem that comes with it is that the bran is discarded as an industrial production waste. In order to increase the added value of quinoa, the bran (seed coat) should be fully exploited and utilized [321]. Quinoa saponins have shown their great potential applications. They can be used in the pharmaceutical industry as the saponins can induce changes in intestinal permeability which can be useful for the absorption of specific medicines and in hypocholesterolemia [15,322,323,324]. Quinoa saponins are also of interest as valuable adjuvants and the first saponin-based vaccines have been introduced commercially [203]. In addition, the saponins can be used as bitters, antibiotics to control pathogenic fungi and bacteria, or to protect crop against attack by birds and other pests [325]. Quinoa saponins have been successfully developed as a bioinsecticide in Bolivia [326]. They can also be used as emulsifiers and detergents due to surface active characteristics which saponins have [327]. Quinoa saponins might be developed into products like soaps, shampoos, and bitters in the future. As phenolic acids, flavonoids, and steroids are also abundant in the bran, they can be developed into antimicrobials, antioxidants, and insect moulting hormones, respectively [5,21]. It is worth mentioning that 20-hydroxyecdysone (148), mainly present in the bran, has potential for development as an insect moulting hormone [21]. After the above secondary metabolites are extracted from the bran, the remaining residues, which mainly contain cellulose, could be either used as feed, or femented into biofuels and biofertilizer.
Biosynthesis research on quinoa secondary metabolites has rarely been reported. Methyl jasmonate was reported to induce accumulation of saponins in quinoa leaves and induce the expression of saponin biosynthetic genes in quinoa [328]. Knowledge of the saponin biosynthesis and its regulation in quinoa may aid the further development of sweet cultivars. Genome sequencing of quinoa revealed a diversity of biosynthetic core genes of secondary metabolites [329], indicating the great potential of this plant to produce various secondary metabolites with biological activities or functions which merit further investigation.
Acknowledgments
This work was financed by the grant from the National Key R&D Program of China (2017YFD0201105).
Author Contributions
Bibliographic research and original draft preparation, M.L.; manuscript discussion and corrections: P.H., Y.L. and W.W.; manuscript revision, D.L.; conception, design, supervision of the manuscript, L.Z. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Jacobsen S.E. The worldwide potential for quinoa (Chenopodium quinoa Willd.) Food Rev. Int. 2003;19:167–177. [Google Scholar]
- 2.Vega-Galvez A., Miranda M., Vergara J., Uribe E., Puente L., Martínez E.A. Nutrition facts and functional potential of quinoa (Chenopodium quinoa Willd.), an ancient Andean grain: A review. J. Sci. Food Agric. 2010;90:2541–2547. doi: 10.1002/jsfa.4158. [DOI] [PubMed] [Google Scholar]
- 3.Repo-Carrasco R., Espinoza C., Jacobsen S.E. Nutritional value and use of the Andean crops quinoa (Chenopodium quinoa) and kaniwa (Chenopodium pallidicaule) Food Rev. Int. 2003;19:179–189. [Google Scholar]
- 4.Ng S.C., Anderson A., Coker J., Ondrus M. Characterization of lipid oxidation products in quinoa (Chenopodium quinoa) Food Chem. 2007;101:185–192. [Google Scholar]
- 5.Abugoch L.E. Quinoa (Chenopodium quinoa Willd.): Composition, chemistry, nutritional and functional properties. Adv. Food Nutr. Res. 2009;58:1–31. doi: 10.1016/S1043-4526(09)58001-1. [DOI] [PubMed] [Google Scholar]
- 6.Jancurova M., Minarovicova L., Dandar A. Quinoa—A review. Czech J. Food Sci. 2009;27:71–79. [Google Scholar]
- 7.Kim S.J., Pham T.H., Bak Y., Ryu H.W., Oh S.R., Yoon D.Y. Orientin inhibits invasion by suppressing MMP-9 and IL-8 expression via the PKCα/ERK/AP-1/STAT3-mediated signaling pathways in TPA-treated MCF-7 breast cancer cells. Phytomedicine. 2018;50:35–42. doi: 10.1016/j.phymed.2018.09.172. [DOI] [PubMed] [Google Scholar]
- 8.Suttiarporn P., Chumpolsri W., Mahatheeranont S., Luangkamin S., Teepsawang S., Leardkamokkarn V. Structures of phytosterols and triterpenoids with potential anti-cancer activity in bran of black non-glutinous rice. Nutrients. 2015;7:1672–1687. doi: 10.3390/nu7031672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graf B.L., Poulev A., Kuhn P., Grace M.H., Lila M.A., Raskin I. Quinoa seeds leach phytoecdysteroids and other compounds with anti-diabetic properties. Food Chem. 2014;163:178–185. doi: 10.1016/j.foodchem.2014.04.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Y., Zhang J., Zou L., Fu C., Li P., Zhao G. Chemical characterization, antioxidant, immune-regulating and anticancer activities of a novel bioactive polysaccharide from Chenopodium quinoa seeds. Int. J. Biol. Macromol. 2017;99:622–629. doi: 10.1016/j.ijbiomac.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Kuljanabhagavad T., Thongphasuk P., Chamulitrat W., Wink M. Triterpene saponins from Chenopodium quinoa Willd. Phytochemistry. 2008;69:1919–1926. doi: 10.1016/j.phytochem.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Miranda M., Delatorre-Herrera J., Vega-Galvez A., Jorquera E., Quispe-Fuentes I., Martinez E.A. Antimicrobial potential and phytochemical content of six diverse sources of quinoa seeds (Chenopodium quinoa Willd. Agric. Sci. 2014;5:1015–1024. [Google Scholar]
- 13.Yao Y., Yang X., Shi Z., Ren G. Anti-inflammatory activity of saponins from quinoa (Chenopodium quinoa Willd.) seeds in lipopolysaccharide-stimulated RAW 264.7 macrophages cells. J. Food Sci. 2014;79:H1018–H1023. doi: 10.1111/1750-3841.12425. [DOI] [PubMed] [Google Scholar]
- 14.Yao Y., Shi Z., Ren G. Antioxidant and immunoregulatory activity of polysaccharides from quinoa (Chenopodium quinoa Willd.) Int. J. Mol. Sci. 2014;15:19307–19318. doi: 10.3390/ijms151019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estrada A., Li B., Laarveld B. Adjuvant action of Chenopodium quinoa saponins on the induction of antibody responses to intragastric and intranasal administered antigens in mice. Comp. Immunol. Microbiol. Infect. Dis. 1998;21:225–236. doi: 10.1016/s0147-9571(97)00030-1. [DOI] [PubMed] [Google Scholar]
- 16.Filho A.M.M., Pirozi M.R., Borge J.T.D.S., Sant’Ana H.M.P., Chaves J.B.P., Coimbra S.D.R. Quinoa: Nutritional, functional, and antinutritional aspects. Crit. Rev. Food Sci. Nutr. 2017;57:1618–1630. doi: 10.1080/10408398.2014.1001811. [DOI] [PubMed] [Google Scholar]
- 17.Tang Y., Tsao R. Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial effects: A review. Mol. Nutr. Food Res. 2017;61:1600767. doi: 10.1002/mnfr.201600767. [DOI] [PubMed] [Google Scholar]
- 18.Suarez-Estrella D., Torri L., Pagani M.A., Marti A. Quinoa bitterness: Causes and solutions for improving product acceptability. J. Sci. Food Agric. 2018;98:4033–4041. doi: 10.1002/jsfa.8980. [DOI] [PubMed] [Google Scholar]
- 19.Hinojosa L., Gonzalez J.A., Barrios-Masias F.H., Fuentes F., Murphy K.M. Quinoa abiotic stress responses: A review. Plants. 2018;7:106. doi: 10.3390/plants7040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz K.B., Biondi S., Oses R., Acuna-Rodriguez I.S., Antognoni F., Martinez-Mosquieira E.A., Coulidaly A., Canahua-Murillo A., Pinto M., Zurita-Silva A., et al. Quinoa biodiversity and sustainability for food security under climate change. A review. Agron. Sustain. Dev. 2014;34:349–359. [Google Scholar]
- 21.Dinan L. Phytoecdysteroids: Biological aspects. Phychemistry. 2001;57:325–353. doi: 10.1016/s0031-9422(01)00078-4. [DOI] [PubMed] [Google Scholar]
- 22.Kumpun S., Maria A., Crouzet S., Evrard-Todeschi N., Girault J.P., Lafont R. Ecdysteroids from Chenopodium quinoa Willd., an ancient Andean crop of high nutritional value. Food Chem. 2011;125:1226–1234. [Google Scholar]
- 23.Kuljanabhagavad T., Wink M. Biological activities and chemisty of saponins from Chenopodium quioa Willd. Phytochem. Rev. 2009;8:473–490. [Google Scholar]
- 24.Abd El-Mawla A.M.A., Beerhues L. Benzoic acid biosynthesis in cell cultures of Hypericum androsaemum. Planta. 2002;214:727–733. doi: 10.1007/s004250100657. [DOI] [PubMed] [Google Scholar]
- 25.Tang Y., Zhang B., Li X., Chen P.X., Zhang H., Tsao R. Bound phenolics of quinoa seeds released by acid, alkaline, and enzymatic treatments and their antioxidant and α-glucosidase and pancreatic lipase inhibitory effects. J. Agric. Food Chem. 2016;64:1712–1719. doi: 10.1021/acs.jafc.5b05761. [DOI] [PubMed] [Google Scholar]
- 26.Gómez-Caravaca A.M., Iafelice G., Lavini A., Pulvento C., Caboni M.F. Phenolic compounds and saponins in quinoa samples (Chenopodium quinoa Willd.) grown under different saline and nonsaline irrigation regimens. J. Agric. Food Chem. 2012;60:4620–4627. doi: 10.1021/jf3002125. [DOI] [PubMed] [Google Scholar]
- 27.Gawlik-Dziki U., Swieca M., Sułkowski M., Dziki D., Baraniak B., Czyz J. Antioxidant and anticancer activities of Chenopodium quinoa leaves extracts—In vitro study. Food Chem. Toxicol. 2013;57:154–160. doi: 10.1016/j.fct.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 28.Cho J.Y., Moon J.H., Seong K.Y., Park K.H. Antimicrobial activity of 4-hydroxybenzoic acid and trans 4-hydroxycinnamic acid isolated and identified from rice hull. Biosci. Biotech. Biochem. 1998;62:2273–2276. doi: 10.1271/bbb.62.2273. [DOI] [PubMed] [Google Scholar]
- 29.Tsou M.F., Hung C.F., Lu H.F., Wu L.T., Chang S.H., Chang H.L., Chen G.W., Chung J.G. Effects of caffeic acid, chlorogenic acid and ferulic acid on growth and arylamine N-acetyltransferase activity in Shigella sonnei (group D) Microbios. 2000;101:37–46. [PubMed] [Google Scholar]
- 30.Slimen I.B., Najar T., Abderrabba M. Chemical and antioxidant properties of betalains. J. Agric. Food Chem. 2017;65:675–689. doi: 10.1021/acs.jafc.6b04208. [DOI] [PubMed] [Google Scholar]
- 31.Ti H., Li Q., Zhang R., Zhang M., Deng Y., Wei Z., Chi J., Zhang Y. Free and bound phenolic profiles and antioxidant activity of milled fractions of different indica rice varieties cultivated in Southern China. Food Chem. 2014;159:166–174. doi: 10.1016/j.foodchem.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 32.Abou-Zaid M.M., Helson B.V., Nozzolillo C., Arnason J.T. Ethyl m-digallate from red maple, Acer rubrum L., as the major resistance factor to forest tent caterpillar, Malacosoma disstria Hbn. J. Chem. Ecol. 2001;27:2517–2527. doi: 10.1023/a:1013683600211. [DOI] [PubMed] [Google Scholar]
- 33.Gomez-Caravaca A.M., Segura-Carretero A., Fernandez-Gutierrez A., Caboni M.F. Simultaneous determination of phenolic compounds and saponins in quinoa (Chenopodium quinoa Willd) by a liquid chromatography-diode array detection-electrospray ionization-time-of-flight mass spectrometry methodology. J. Agric. Food Chem. 2011;59:10815–10825. doi: 10.1021/jf202224j. [DOI] [PubMed] [Google Scholar]
- 34.Pasko P., Sajewicz M., Gorinstein S., Zachwieja Z. Analysis of selected phenolic acids and flavonoids in Amaranthus cruentus and Chenopodium quinoa seeds and sprouts by HPLC. Acta Chromatogr. 2008;20:661–672. [Google Scholar]
- 35.Tang Y., Li X., Zhang B., Chen P.X., Liu R., Tsao R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015;166:380–388. doi: 10.1016/j.foodchem.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 36.Cai L., Wu C.D. Compounds from Syzygium aromaticum possessing growth inhibitory activity against oral pathogens. J. Nat. Prod. 1996;59:987–990. doi: 10.1021/np960451q. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez-Jubete L., Wijngaard H., Arendt E.K., Gallagher E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa, buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010;119:770–778. [Google Scholar]
- 38.Tanaka T., Tanaka T., Tanaka M. Potential cancer chemopreventive activity of protocatechuic acid. J. Exp. Clin. Med. 2011;3:27–33. [Google Scholar]
- 39.Liu K.S., Tsao S.M., Yin M.C. In vitro antibacterial activity of roselle calyx and protocatechuic acid. Phytother. Res. 2005;19:942–945. doi: 10.1002/ptr.1760. [DOI] [PubMed] [Google Scholar]
- 40.Kore K.J., Bramhakule P.P., Rachhadiya R.M., Shete R.V. Evaluation of antiulcer activity of protocatechuic acid ethyl ester in rats. Int. J. Pharm. Life Sci. 2011;2:909–915. [Google Scholar]
- 41.Shi G.F., An L.J., Jiang B., Guan S., Bao Y.M. Alpinia protocatechuic acid protects against oxidative damage in vitro and reduces oxidative stress in vivo. Neurosci. Lett. 2006;403:206–210. doi: 10.1016/j.neulet.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 42.Kakkar S., Bais S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014;2014:952943. doi: 10.1155/2014/952943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Z., Zhang Y., Ding X.R., Chen S.H., Yang J., Wang X.J., Wang S.Q. Protocatechuic aldehyde inhibits hepatitis B virus replication both in vitro and in vivo. Antivir. Res. 2007;74:59–64. doi: 10.1016/j.antiviral.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Galano A., Francisco-Márquez M., Alvarez-Idaboy J.R. Mechanism and kinetics studies on the antioxidant activity of sinapinic acid. Phys. Chem. Chem. Phys. 2011;13:11199–11205. doi: 10.1039/c1cp20722a. [DOI] [PubMed] [Google Scholar]
- 45.Chong K.P., Rossall S., Atong M. In vitro antimicrobial activity and fungitoxicity of syringic acid, caffeic acid and 4-hydroxybenzoic acid against Ganoderma boninense. J. Agric. Sci. 2009;1:15. [Google Scholar]
- 46.Itoh A., Isoda K., Kondoh M., Kawase M., Kobayashi M., Tamesada M., Yagi K. Hepatoprotective effect of syringic acid and vanillic acid on concanavalin a-induced liver injury. Biol. Pharm. Bull. 2009;32:1215–1219. doi: 10.1248/bpb.32.1215. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez M.A., Saenz M.T., Garcia M.D. Natural Products: Anti-inflammatory activity in rats and mice of phenolic acids isolated from Scrophularia frutescens. J. Pharm. Pharmacol. 1998;50:1183–1186. doi: 10.1111/j.2042-7158.1998.tb03332.x. [DOI] [PubMed] [Google Scholar]
- 48.El-Hawary S., Mohammed R., AbouZid S., Ali Z.Y., El-Gendy A.O., Elwekeel A. In-vitro cyclooxygenase inhibitory, antioxidant and antimicrobial activities of phytochemicals isolated from Crassula arborescens (Mill.) Willd. Int. J. Appl. Res. Nat. Prod. 2016;9:8–14. [Google Scholar]
- 49.Dini I., Tenore G.C., Dini A. Phenolic constituents of Kancolla seeds. Food Chem. 2004;84:163–168. [Google Scholar]
- 50.Tai A., Sawano T., Yazama F., Ito H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. BBA Gen. Subjects. 2011;1810:170–177. doi: 10.1016/j.bbagen.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Cava-Roda R.M., Taboada-Rodríguez A., Valverde-Franco M.T., Marín-Iniesta F. Antimicrobial activity of vanillin and mixtures with cinnamon and clove essential oils in controlling Listeria monocytogenes and Escherichia coli O157: H7 in milk. Food Bioprocess Technol. 2012;5:2120–2131. [Google Scholar]
- 52.Shoeb A., Chowta M., Pallempati G., Rai A., Singh A. Evaluation of antidepressant activity of vanillin in mice. Indian J. Pharmacol. 2013;45:141. doi: 10.4103/0253-7613.108292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lim E.J., Kang H.J., Jung H.J., Song Y.S., Lim C.J., Park E.H. Anti-angiogenic, anti-inflammatory and anti-nociceptive activities of vanillin in ICR mice. Biomol. Ther. 2008;16:132–136. [Google Scholar]
- 54.Gerig T.M., Blum U. Effects of mixtures of four phenolic acids on leaf area expansion of cucumber seedlings grown in Portsmouth B 1 soil materials. J. Chem. Ecol. 1991;17:29–40. doi: 10.1007/BF00994420. [DOI] [PubMed] [Google Scholar]
- 55.Khanduja K.L., Avti P.K., Kumar S., Mittal N., Sohi K.K., Pathak C.M. Anti-apoptotic activity of caffeic acid, ellagic acid and ferulic acid in normal human peripheral blood mononuclear cells: A Bcl-2 independent mechanism. BBA Gen. Subjects. 2006;1760:283–289. doi: 10.1016/j.bbagen.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 56.Hunyadi A., Martins A., Hsieh T.J., Seres A., Zupkó I. Chlorogenic acid and rutin play a major role in the in vivo anti-diabetic activity of Morus alba leaf extract on type II diabetic rats. PLoS ONE. 2012;7:e50619. doi: 10.1371/journal.pone.0050619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang W.S., Chang Y.H., Lu F.J., Chiang H.C. Inhibitory effects of phenolics on xanthine oxidase. Anticancer Res. 1994;14:501–506. [PubMed] [Google Scholar]
- 58.Ohnishi M., Morishita H., Iwahashi H., Toda S., Shirataki Y., Kimura M., Kido R. Inhibitory effects of chlorogenic acids on linoleic acid peroxidation and haemolysis. Phytochemistry. 1994;36:579–583. [Google Scholar]
- 59.Kwon S.H., Lee H.K., Kim J.A., Hong S.I., Kim H.C., Jo T.H., Jang C.G. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010;649:210–217. doi: 10.1016/j.ejphar.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 60.Cho A.S., Jeon S.M., Kim M.J., Yeo J., Seo K.I., Choi M.S., Lee M.K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010;48:937–943. doi: 10.1016/j.fct.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Kapil A., Koul I.B., Suri O.P. Antihepatotoxic effects of chlorogenic acid from Anthocephalus cadamba. Phytother. Res. 1995;9:189–193. [Google Scholar]
- 62.Karunanidhi A., Thomas R., Van Belkum A., Neela V. In vitro antibacterial and antibiofilm activities of chlorogenic acid against clinical isolates of Stenotrophomonas maltophilia including the trimethoprim/sulfamethoxazole resistant strain. BioMed. Res. Int. 2013;2013:392058. doi: 10.1155/2013/392058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Repo-Carrasco-Valencia R., Hellström J.K., Pihlava J.M., Mattila P.H. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kaniwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus) Food Chem. 2010;120:128–133. [Google Scholar]
- 64.Wen A., Delaquis P., Stanich K., Toivonen P. Antilisterial activity of selected phenolic acids. Food Microbiol. 2003;20:305–311. [Google Scholar]
- 65.Graf E. Antioxidant potential of ferulic acid. Free Radical Biol. Med. 1992;13:435–448. doi: 10.1016/0891-5849(92)90184-i. [DOI] [PubMed] [Google Scholar]
- 66.Kim H.K., Jeong T.S., Lee M.K., Park Y.B., Choi M.S. Lipid-lowering efficacy of hesperetin metabolites in high-cholesterol fed rats. Clin. Chim. Acta. 2003;327:129–137. doi: 10.1016/s0009-8981(02)00344-3. [DOI] [PubMed] [Google Scholar]
- 67.Ou S., Bao H., Lan Z. Advances on pharmacological study of ferulic acid and its derivatives. J. Chin. Med. Mater. 2001;24:220–221. [Google Scholar]
- 68.Ou S., Kwok K.C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004;84:1261–1269. [Google Scholar]
- 69.Sakai S., Kawamata H., Kogure T., Mantani N., Terasawa K., Umatake M., Ochiai H. Inhibitory effect of ferulic acid and isoferulic acid on the production of macrophage inflammatory protein-2 in response to respiratory syncytial virus infection in RAW264. 7 cells. Mediat. Inflamm. 1999;8:173–175. doi: 10.1080/09629359990513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mori H., Kawabata K., Yoshimi N., Tanaka T., Murakami T., Okada T., Murai H. Chemopreventive effects of ferulic acid on oral and rice germ on large bowel carcinogenesis. Anticancer Res. 1999;19:3775–3778. [PubMed] [Google Scholar]
- 71.Wang X., Li X., Chen D. Evaluation of antioxidant activity of isoferulic acid in vitro. Nat. Prod. Commun. 2011;6:1285–1288. [PubMed] [Google Scholar]
- 72.Fiorito S., Preziuso F., Epifano F., Scotti L., Bucciarelli T., Taddeo V.A., Genovese S. Novel biologically active principles from spinach, goji and quinoa. Food Chem. 2019;276:262–265. doi: 10.1016/j.foodchem.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 73.Bais H.P., Walker T.S., Schweizer H.P., Vivanco J.M. Root specific elicitation and antimicrobial activity of rosmarinic acid in hairy root cultures of Ocimum basilicum. Plant Physiol. Biochem. 2002;40:983–995. [Google Scholar]
- 74.Englberger W., Hadding U., Etschenberg E., Graf E., Leyck S., Winkelmann J., Parnham M.J. Rosmarinic acid: A new inhibitor of complement C3-convertase with anti-inflammatory activity. Int. J. Immunopharmacol. 1988;10:729–737. doi: 10.1016/0192-0561(88)90026-4. [DOI] [PubMed] [Google Scholar]
- 75.Cao H., Cheng W.X., Li C., Pan X.L., Xie X.G., Li T.H. DFT study on the antioxidant activity of rosmarinic acid. J. Mol. Struct. 2005;719:177–183. [Google Scholar]
- 76.Furtado M.A., de Almeida L.C.F., Furtado R.A., Cunha W.R., Tavares D.C. Antimutagenicity of rosmarinic acid in Swiss mice evaluated by the micronucleus assay. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2008;657:150–154. doi: 10.1016/j.mrgentox.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 77.Swarup V., Ghosh J., Ghosh S., Saxena A., Basu A. Antiviral and anti-inflammatory effects of rosmarinic acid in an experimental murine model of Japanese encephalitis. Antimicrob. Agents Chemother. 2007;51:3367–3370. doi: 10.1128/AAC.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoon B.H., Jung J.W., Lee J.J., Cho Y.W., Jang C.G., Jin C., Ryu J.H. Anxiolytic-like effects of sinapic acid in mice. Life Sci. 2007;81:234–240. doi: 10.1016/j.lfs.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 79.Karakida F., Ikeya Y., Tsunakawa M., Yamaguchi T., Ikarashi Y., Takeda S., Aburada M. Cerebral protective and cognition-improving effects of sinapic acid in rodents. Biol. Pharm. Bull. 2007;30:514–519. doi: 10.1248/bpb.30.514. [DOI] [PubMed] [Google Scholar]
- 80.Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013;2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harborne J.B., Williams C.A. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 82.Wuyts N., Swennen R., De Waele D. Effects of plant phenylpropanoid pathway products and selected terpenoids and alkaloids on the behaviour of the plant-parasitic nematodes Radopholus similis, Pratylenchus penetrans and Meloidogyne incognita. Nematology. 2006;8:89–101. [Google Scholar]
- 83.Kang G.-H., Chang E.-J., Choi S.-W. Antioxidative activity of phenolic compounds in roasted safflower (Carthamus tinctorius L.) seeds. J. Food Sci. Nutr. 1999;4:221–225. [Google Scholar]
- 84.Lv H., Yu Z., Zheng Y., Wang L., Qin X., Cheng G., Ci X. Isovitexin exerts anti-inflammatory and anti-oxidant activities on lipopolysaccharide-induced acute lung injury by inhibiting MAPK and NF-κB and activating HO-1/Nrf2 pathways. Int. J. Biol. Sci. 2016;12:72. doi: 10.7150/ijbs.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.González-Trujano M.E., Ventura-Martínez R., Chávez M., Díaz-Reval I., Pellicer F. Spasmolytic and antinociceptive activities of ursolic acid and acacetin identified in Agastache mexicana. Planta Med. 2012;78:793–796. doi: 10.1055/s-0031-1298416. [DOI] [PubMed] [Google Scholar]
- 86.Hsu Y.L., Kuo P.L., Liu C.F., Lin C.C. Acacetin-induced cell cycle arrest and apoptosis in human non-small cell lung cancer A549 cells. Cancer Lett. 2004;212:53–60. doi: 10.1016/j.canlet.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 87.Hayashi K., Hayashi T., Arisawa M., Morita N. Antiviral agents of plant origin. Antiherpetic activity of acacetin. Antivir. Chem. Chemoth. 1993;4:49–53. [Google Scholar]
- 88.Liu L.Z., Jing Y., Jiang L.L., Jiang X.E., Jiang Y., Rojanasakul Y., Jiang B.H. Acacetin inhibits VEGF expression, tumor angiogenesis and growth through AKT/HIF-1α pathway. Biochem. Bioph. Res. Commun. 2011;413:299–305. doi: 10.1016/j.bbrc.2011.08.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carballo-Villalobos A.I., Gonzalez-Trujano M.E., Lopez-Munoz F.J. Evidence of mechanism of action of anti-inflammatory/antinociceptive activities of acacetin. Eur. J. Pain. 2014;18:396–405. doi: 10.1002/j.1532-2149.2013.00378.x. [DOI] [PubMed] [Google Scholar]
- 90.Nguyen M.T.T., Awale S., Tezuka Y., Shi L., Zaidi S.F.H., Ueda J.Y., Kadota S. Hypouricemic effects of acacetin and 4,5-O-dicaffeoylquinic acid methyl ester on serum uric acid levels in potassium oxonate-pretreated rats. Biol. Pharm. Bull. 2005;28:2231–2234. doi: 10.1248/bpb.28.2231. [DOI] [PubMed] [Google Scholar]
- 91.Shochet G.E., Drucker L., Pasmanik-Chor M., Pomeranz M., Fishman A., Matalon S.T., Lishner M. First trimester human placental factors induce breast cancer cell autophagy. Breast Cancer Res. Treat. 2015;149:645–654. doi: 10.1007/s10549-015-3266-x. [DOI] [PubMed] [Google Scholar]
- 92.Lee C.Y., Chien Y.S., Chiu T.H., Huang W.W., Lu C.C., Chiang J.H., Yang J.S. Apoptosis triggered by vitexin in U937 human leukemia cells via a mitochondrial signaling pathway. Oncol. Rep. 2012;28:1883–1888. doi: 10.3892/or.2012.2000. [DOI] [PubMed] [Google Scholar]
- 93.De Oliveira D.R., Zamberlam C.R., Gaiardo R.B., Rêgo G.M., Cerutti J.M., Cavalheiro A.J., Cerutti S.M. Flavones from Erythrina falcata are modulators of fear memory. BMC Complement. Altern. Med. 2014;14:288. doi: 10.1186/1472-6882-14-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Soulimani R., Younos C., Jarmouni S., Bousta D., Misslin R., Mortier F. Behavioural effects of Passiflora incarnata L. and its indole alkaloid and flavonoid derivatives and maltol in the mouse. J. Ethnopharmacol. 1997;57:11–20. doi: 10.1016/s0378-8741(97)00042-1. [DOI] [PubMed] [Google Scholar]
- 95.Choi J.S., Islam M.N., Ali M.Y., Kim E.J., Kim Y.M., Jung H.A. Effects of C-glycosylation on anti-diabetic, anti-Alzheimer’s disease and anti-inflammatory potential of apigenin. Food Chem. Toxicol. 2014;64:27–33. doi: 10.1016/j.fct.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 96.Peng X., Zheng Z., Cheng K.W., Shan F., Ren G.X., Chen F., Wang M. Inhibitory effect of mung bean extract and its constituents vitexin and isovitexin on the formation of advanced glycation endproducts. Food Chem. 2008;106:475–481. [Google Scholar]
- 97.Shibano M., Kakutani K., Taniguchi M., Yasuda M., Baba K. Antioxidant constituents in the dayflower (Commelina communis L.) and their α-glucosidase-inhibitory activity. J. Nat. Med. 2008;62:349. doi: 10.1007/s11418-008-0244-1. [DOI] [PubMed] [Google Scholar]
- 98.Li H.M., Hwang S.H., Kang B.G., Hong J.S., Lim S.S. Inhibitory effects of Colocasia esculenta (L.) Schott constituents on aldose reductase. Molecules. 2014;19:13212–13224. doi: 10.3390/molecules190913212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoo H., Ku S.-K., Lee T., Bae J.-S. Orientin inhibits HMGB1-induced inflammatory responses in HUVECs and in murine polymicrobial sepsis. Inflammation. 2014;37:1705–1717. doi: 10.1007/s10753-014-9899-9. [DOI] [PubMed] [Google Scholar]
- 100.An F., Yang G., Tian J., Wang S. Antioxidant effects of the orientin and vitexin in Trollius chinensis Bunge in D-galactose-aged mice. Neural Regen. Res. 2012;7:2565. doi: 10.3969/j.issn.1673-5374.2012.33.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li F., Zong J., Zhang H., Zhang P., Xu L., Liang K., Qian W. Orientin reduces myocardial infarction size via eNOS/NO signaling and thus mitigates adverse cardiac remodeling. Front. Pharm. 2017;8:926. doi: 10.3389/fphar.2017.00926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee W., Bae J.S. Antithrombotic and antiplatelet activities of orientin in vitro and in vivo. J. Funct. Foods. 2015;17:388–398. [Google Scholar]
- 103.Thangaraj K., Natesan K., Palani M., Vaiyapuri M. Orientin, a flavanoid, mitigates 1,2-dimethylhydrazine-induced colorectal lesions in Wistar rats fed a high-fat diet. Toxicol. Rep. 2018;5:977–987. doi: 10.1016/j.toxrep.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen J., Zhong J., Liu Y., Huang Y., Luo F., Zhou Y., Wang J. Purified vitexin compound 1, a new neolignan isolated compound, promotes PUMA-dependent apoptosis in colorectal cancer. Cancer Med. 2018;7:6158–6169. doi: 10.1002/cam4.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Je H.G., Hong S.M., Je H.D., Sohn U.D., Choi Y.S., Seo S.Y., Park E.S. The inhibitory effect of vitexin on the agonist-induced regulation of vascular contractility. J. Pharm. Sci. 2014;69:224–228. [PubMed] [Google Scholar]
- 106.Praveena R., Sadasivam K., Kumaresan R., Deepha V., Sivakumar R. Experimental and DFT studies on the antioxidant activity of a C-glycoside from Rhynchosia capitata. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2013;103:442–452. doi: 10.1016/j.saa.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 107.Borghi S.M., Carvalho T.T., Staurengo-Ferrari L., Hohmann M.S., Pinge-Filho P., Casagrande R., Verri W.A., Jr. Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines. J. Nat. Prod. 2013;76:1141–1149. doi: 10.1021/np400222v. [DOI] [PubMed] [Google Scholar]
- 108.Abbasi E., Nassiri-Asl M., Sheikhi M., Shafiee M. Effects of vitexin on scopolamine-induced memory impairment in rats. Chin. J. Physiol. 2013;56:184–189. doi: 10.4077/CJP.2013.BAB123. [DOI] [PubMed] [Google Scholar]
- 109.Can O.D., Ozkay U.D., Ucel U.I. Anti-depressant-like effect of vitexin in BALB/c mice and evidence for the involvement of monoaminergic mechanisms. Eur. J. Pharm. 2013;699:250–257. doi: 10.1016/j.ejphar.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 110.Abbasi E., Nassiri-Asl M., Shafeei M., Sheikhi M. Neuroprotective effects of vitexin, a flavonoid, on pentylenetetrazole-induced seizure in rats. Chem. Biol. Drug Des. 2012;80:274–278. doi: 10.1111/j.1747-0285.2012.01400.x. [DOI] [PubMed] [Google Scholar]
- 111.Aseervatham G.S.B., Suryakala U., Sundaram S., Bose P.C., Sivasudha T. Expression pattern of NMDA receptors reveals antiepileptic potential of apigenin 8-C-glucoside and chlorogenic acid in pilocarpine induced epileptic mice. Biomed. Pharm. 2016;82:54–64. doi: 10.1016/j.biopha.2016.04.066. [DOI] [PubMed] [Google Scholar]
- 112.Ozkay U.D., Can O.D. Anti-nociceptive effect of vitexin mediated by the opioid system in mice. Pharm. Biochem. Behav. 2013;109:23–30. doi: 10.1016/j.pbb.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 113.Min J.-W., Hu J.-J., He M., Sanchez R.M., Huang W.-X., Liu Y.-Q., Bsoul N.B., Han S., Yin J., Liu W.-H., et al. Vitexin reduces hypoxia–ischemia neonatal brain injury by the inhibition of HIF-1alpha in a rat pup model. Neuropharmacology. 2015;99:38–50. doi: 10.1016/j.neuropharm.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 114.Wang Y., Zhen Y., Wu X., Jiang Q., Li X., Chen Z., Dong L. Vitexin protects brain against ischemia/reperfusion injury via modulating mitogen-activated protein kinase and apoptosis signaling in mice. Phytomedicine. 2015;22:379–384. doi: 10.1016/j.phymed.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 115.Brahmbhatt S., Brahmbhatt R.M., Boyages S.C. Thyroid ultrasound is the best prevalence indicator for assessment of iodine deficiency disorders: A study in rural/tribal schoolchildren from Gujarat (Western India) Eur. J. Endocrinol. 2000;143:37–46. doi: 10.1530/eje.0.1430037. [DOI] [PubMed] [Google Scholar]
- 116.Basile A., Giordano S., Lopez-Saez J.A., Cobianchi R.C. Antibacterial activity of pure flavonoids isolated from mosses. Phytochemistry. 1999;52:1479–1482. doi: 10.1016/s0031-9422(99)00286-1. [DOI] [PubMed] [Google Scholar]
- 117.Knipping K., Garssen J., van’t Land B. An evaluation of the inhibitory effects against rotavirus infection of edible plant extracts. Virol. J. 2012;9:137. doi: 10.1186/1743-422X-9-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arora A., Nair M.G., Strasburg G.M. Structure-activity relationships for antioxidant activities of a series of flavonoids in a liposomal system. Free Radical Biol. Med. 1998;24:1355–1363. doi: 10.1016/s0891-5849(97)00458-9. [DOI] [PubMed] [Google Scholar]
- 119.Rice-evans C.A., Miller N.J., Bolwell P.G., Bramley P.M., Pridham J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radical Res. 1995;22:375–383. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 120.Zhu N., Sheng S., Li D., LaVoie E.J., Karwe M.V., Rosen R.T., HO C.T. Antioxidative flavonoid glycosides from quinoa seeds (Chenopodium quinoa Willd) J. Food Lipids. 2001;8:37–44. [Google Scholar]
- 121.Bloor S.J. An antimicrobial kaempferol-diacyl-rhamnoside from Pentachondra pumila. Phytochemistry. 1995;38:1033–1035. doi: 10.1016/0031-9422(94)00661-c. [DOI] [PubMed] [Google Scholar]
- 122.Martini N.D., Katerere D.R.P., Eloff J.N. Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae) J. Ethnopharmacol. 2004;93:207–212. doi: 10.1016/j.jep.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 123.Treutter D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 2005;7:581–591. doi: 10.1055/s-2005-873009. [DOI] [PubMed] [Google Scholar]
- 124.Parvez M.M., Tomita-Yokotani K., Fujii Y., Konishi T., Iwashina T. Effects of quercetin and its seven derivatives on the growth of Arabidopsis thaliana and Neurospora crassa. Biochem. Syst. Ecol. 2004;32:631–635. [Google Scholar]
- 125.Bahrman N., Jay M., Gorenflot R. Contribution to the chemosystematic knowledge of some species of the genus Chenopodium, L. Lett. Bot. 1985;2:107–113. [Google Scholar]
- 126.Saud S.M., Young M.R., Jones-Hall Y.L., Ileva L., Evbuomwan M.O., Wise J., Bobe G. Chemopreventive activity of plant flavonoid isorhamnetin in colorectal cancer is mediated by oncogenic Src and β-catenin. Cancer Res. 2013;73:5473–5484. doi: 10.1158/0008-5472.CAN-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jnawali H.N., Jeon D., Jeong M.-C., Lee E., Jin B., Ryoo S., Yoo J., Jung I.D., Lee S.J., Park Y.M., et al. Antituberculosis activity of a naturally occurring flavonoid, isorhamnetin. J. Nat. Prod. 2016;79:961–969. doi: 10.1021/acs.jnatprod.5b01033. [DOI] [PubMed] [Google Scholar]
- 128.Torres R., Faini F., Modak B., Urbina F., Labbé C., Guerrero J. Antioxidant activity of coumarins and flavonols from the resinous exudate of Haplopappus multifolius. Phytochemistry. 2006;67:984–987. doi: 10.1016/j.phytochem.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 129.Teng B.S., Lu Y.H., Wang Z.T., Tao X.Y., Wei D.Z. In vitro anti-tumor activity of isorhamnetin isolated from Hippophae rhamnoides L. against BEL-7402 cells. Pharm. Res. 2006;54:186–194. doi: 10.1016/j.phrs.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 130.Li C., Yang D., Zhao Y., Qiu Y., Cao X., Yu Y., Yin X. Inhibitory effects of isorhamnetin on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-2/9. Nutr. Cancer. 2015;67:1191–1200. doi: 10.1080/01635581.2015.1073763. [DOI] [PubMed] [Google Scholar]
- 131.Oh H.M., Kwon B.M., Baek N.I., Kim S.H., Chung I.S., Park M.H., Kim D.K. Inhibitory activity of isorhamnetin from Persicaria thunbergii on farnesyl protein transferase. Arch. Pharm. Res. 2005;28:169–171. doi: 10.1007/BF02977709. [DOI] [PubMed] [Google Scholar]
- 132.Chirumbolo S. Anti-inflammatory action of isorhamnetin. Inflammation. 2014;37:1200. doi: 10.1007/s10753-014-9846-9. [DOI] [PubMed] [Google Scholar]
- 133.Ku S.K., Kim T.H., Bae J.S. Anticoagulant activities of persicarin and isorhamnetin. Vasc. Pharm. 2013;58:272–279. doi: 10.1016/j.vph.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 134.Kim S.K., Kim H.J., Choi S.E., Park K.H., Choi H.K., Lee M.W. Anti-oxidative and inhibitory activities on nitric oxide (NO) and prostaglandin E 2 (COX-2) production of flavonoids from seeds of Prunus tomentosa Thunberg. Arch. Pharm. Res. 2008;3:424. doi: 10.1007/s12272-001-1174-9. [DOI] [PubMed] [Google Scholar]
- 135.Lee K.M., Lee K.W., Jung S.K., Lee E.J., Heo Y.S., Bode A.M., Dong Z. Kaempferol inhibits UVB-induced COX-2 expression by suppressing Src kinase activity. Biochem. Pharm. 2010;80:2042–2049. doi: 10.1016/j.bcp.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rho H.S., Ghimeray A.K., Yoo D.S., Ahn S.M., Kwon S.S., Lee K.H., Cho J.Y. Kaempferol and kaempferol rhamnosides with depigmenting and anti-inflammatory properties. Molecules. 2011;16:3338–3344. doi: 10.3390/molecules16043338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Prouillet C., Mazière J.C., Mazière C., Wattel A., Brazier M., Kamel S. Stimulatory effect of naturally occurring flavonols quercetin and kaempferol on alkaline phosphatase activity in MG-63 human osteoblasts through ERK and estrogen receptor pathway. Biochem. Pharm. 2004;67:1307–1313. doi: 10.1016/j.bcp.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 138.De Simone F., Dini A., Pizza C., Saturnino P., Schettino O. Two flavonol glycosides from Chenopodium quinoa. Phytochemistry. 1990;29:3690–3692. doi: 10.1016/0031-9422(90)85310-c. [DOI] [PubMed] [Google Scholar]
- 139.Hirose Y., Fujita T., Ishii T., Ueno N. Antioxidative properties and flavonoid composition of Chenopodium quinoa seeds cultivated in Japan. Food Chem. 2010;119:1300–1306. [Google Scholar]
- 140.Chemmugil P., Lakshmi P.T.V., Annamalai A. Exploring Morin as an anti-quorum sensing agent (anti-QSA) against resistant strains of Staphylococcus aureus. Microb. Pathog. 2019;127:304–315. doi: 10.1016/j.micpath.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 141.Qu Y., Wang C., Liu N., Gao C., Liu F. Exhibits anti-inflammatory effects on IL-1β-stimulated human osteoarthritis chondrocytes by activating the Nrf2 signaling pathway. Cell. Physiol. Biochem. 2018;51:1830–1838. doi: 10.1159/000495684. [DOI] [PubMed] [Google Scholar]
- 142.Ji Y., Jia L., Zhang Y., Xing Y., Wu X., Zhao B., Qiao X. Antitumor activity of the plant extract morin in tongue squamous cell carcinoma cells. Oncol. Rep. 2018;40:3024–3032. doi: 10.3892/or.2018.6650. [DOI] [PubMed] [Google Scholar]
- 143.Yuan W., Ahmad S., Najar A. Morin, a plant derived flavonoid, modulates the expression of peroxisome proliferator-activated receptor-γ coactivator-1α mediated by AMPK pathway in hepatic stellate cells. Am. J. Transl. Res. 2017;9:5662. [PMC free article] [PubMed] [Google Scholar]
- 144.Chen X., Deng Z., Zhang C., Zheng S., Pan Y., Wang H., Li H. Is antioxidant activity of flavonoids mainly through the hydrogen-atom transfer mechanism? Food Res. Int. 2019 doi: 10.1016/j.foodres.2018.11.018. [DOI] [Google Scholar]
- 145.Sithara T., Arun K.B., Syama H.P., Reshmitha T.R., Nisha P. Morin Inhibits proliferation of SW480 colorectal cancer cells by inducing apoptosis mediated by reactive oxygen species formation and uncoupling of Warburg effect. Front. Pharmacol. 2017;8:640. doi: 10.3389/fphar.2017.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang N., Zhang J., Qin M., Yi W., Yu S., Chen Y., Zhang R. Amelioration of streptozotocin-induced pancreatic β cell damage by morin: Involvement of the AMPK-FOXO3-catalase signaling pathway. Int. J. Mol. Med. 2018;41:1409–1418. doi: 10.3892/ijmm.2017.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Carmona V., Martín-Aragon S., Goldberg J., Schubert D., Bermejo-Bescós P. Several targets involved in Alzheimer’s disease amyloidogenesis are affected by morin and isoquercitrin. Nutr. Neurosci. 2019 doi: 10.1080/1028415x.2018.1534793. [DOI] [PubMed] [Google Scholar]
- 148.Bhakuni G.S., Bedi O., Bariwal J., Kumar P. Hepatoprotective activity of morin and its semi-synthetic derivatives against alcohol induced hepatotoxicity in rats. Indian J. Physiol. Pharmacol. 2017;61:175–190. [Google Scholar]
- 149.Fukumoto L.R., Mazza G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000;48:3597–3604. doi: 10.1021/jf000220w. [DOI] [PubMed] [Google Scholar]
- 150.Lu J., Papp L.V., Fang J., Rodriguez-Nieto S., Zhivotovsky B., Holmgren A. Inhibition of mammalian thioredoxin reductase by some flavonoids: Implications for myricetin and quercetin anticancer activity. Cancer Res. 2006;66:4410–4418. doi: 10.1158/0008-5472.CAN-05-3310. [DOI] [PubMed] [Google Scholar]
- 151.Wang S.J., Tong Y., Lu S., Yang R., Liao X., Xu Y.F., Li X. Anti-inflammatory activity of myricetin isolated from Myrica rubra Sieb. et Zucc. leaves. Planta Med. 2010;76:1492–1496. doi: 10.1055/s-0030-1249780. [DOI] [PubMed] [Google Scholar]
- 152.Tong Y., Zhou X.M., Wang S.J., Yang Y., Cao Y.L. Analgesic activity of myricetin isolated from Myrica rubra Sieb. et Zucc. leaves. Arch. Pharm. Res. 2009;32:527–533. doi: 10.1007/s12272-009-1408-6. [DOI] [PubMed] [Google Scholar]
- 153.Comalada M., Ballester I., Bailon E., Sierra S., Xaus J., Galvez J., Zarzuelo A. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: Analysis of the structure–activity relationship. Biochem. Pharmacol. 2006;72:1010–1021. doi: 10.1016/j.bcp.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 154.Silva T.B., Costa C.O., Galvão A.F., Bomfim L.M., Rodrigues A.C., Mota M.C., Dantas A.A., Santos T.R., Soares M.B., Bezerra D.P. Cytotoxic potential of selected medicinal plants in northeast Brazil. BMC Complement. Altern. Med. 2016;16:199. doi: 10.1186/s12906-016-1166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ng T.B., Liu F., Wang Z.T. Antioxidative activity of natural products from plants. Life Sci. 2000;66:709–723. doi: 10.1016/s0024-3205(99)00642-6. [DOI] [PubMed] [Google Scholar]
- 156.Shimoyama A.T., Santin J.R., Machado I.D., de Silva A.M.D.O., De Melo I.L.P., Mancini-Filho J., Farsky S.H. Antiulcerogenic activity of chlorogenic acid in different models of gastric ulcer. N-S. Arch. Pharmacol. 2013;386:5–14. doi: 10.1007/s00210-012-0807-2. [DOI] [PubMed] [Google Scholar]
- 157.Al-Ashaal H.A., El-Sheltawy S.T. Antioxidant capacity of hesperidin from citrus peel using electron spin resonance and cytotoxic activity against human carcinoma cell lines. Pharm. Biol. 2011;49:276–282. doi: 10.3109/13880209.2010.509734. [DOI] [PubMed] [Google Scholar]
- 158.Parhiz H., Roohbakhsh A., Soltani F., Rezaee R., Iranshahi M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015;29:323–331. doi: 10.1002/ptr.5256. [DOI] [PubMed] [Google Scholar]
- 159.Salas M.P., Céliz G., Geronazzo H., Daz M., Resnik S.L. Antifungal activity of natural and enzymatically-modified flavonoids isolated from citrus species. Food Chem. 2011;124:1411–1415. [Google Scholar]
- 160.Cincin Z.B., Unlu M., Kiran B., Bireller E.S., Baran Y., Cakmakoglu B. Anti-proliferative, apoptotic and signal transduction effects of hesperidin in non-small cell lung cancer cells. Cell. Oncol. 2015;38:195–3204. doi: 10.1007/s13402-015-0222-z. [DOI] [PubMed] [Google Scholar]
- 161.Mahmoud A.M. Hesperidin protects against cyclophosphamide-induced hepatotoxicity by upregulation of PPARγ and abrogation of oxidative stress and inflammation. Can. J. Physiol. Pharm. 2014;92:717–724. doi: 10.1139/cjpp-2014-0204. [DOI] [PubMed] [Google Scholar]
- 162.Agrawal Y.O., Sharma P.K., Shrivastava B., Ojha S., Upadhya H.M., Arya D.S., Goyal S.N. Hesperidin produces cardioprotective activity via PPAR-γ pathway in ischemic heart disease model in diabetic rats. PLoS ONE. 2014;9:e111212. doi: 10.1371/journal.pone.0111212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee J.H., Lee S.H., Kim Y.S., Jeong C.S. Protective effects of neohesperidin and poncirin isolated from the fruits of Poncirus trifoliata on potential gastric disease. Phytother. Res. 2009;23:1748–1753. doi: 10.1002/ptr.2840. [DOI] [PubMed] [Google Scholar]
- 164.Xu F., Zang J., Chen D., Zhang T., Zhan H., Lu M., Zhuge H. Neohesperidin induces cellular apoptosis in human breast denocarcinoma MDA-MB-231 cells via activating the Bcl-2/Bax-mediated signaling pathway. Nat. Prod. Commun. 2012;7:1475–1478. [PubMed] [Google Scholar]
- 165.Jeon S.M., Bok S.H., Jang M.K., Lee M.K., Nam K.T., Park Y.B., Choi M.S. Antioxidative activity of naringin and lovastatin in high cholesterol-fed rabbits. Life Sci. 2001;69:2855–2866. doi: 10.1016/s0024-3205(01)01363-7. [DOI] [PubMed] [Google Scholar]
- 166.Wei M., Yang Z., Li P., Zhang Y., Sse W.C. Anti-osteoporosis activity of naringin in the retinoic acid-induced osteoporosis model. Am. J. Chin. Med. 2007;35:663–667. doi: 10.1142/S0192415X07005156. [DOI] [PubMed] [Google Scholar]
- 167.Amaro M.I., Rocha J., Vila-Real H., Eduardo-Figueira M., Mota-Filipe H., Sepodes B., Ribeiro M.H. Anti-inflammatory activity of naringin and the biosynthesised naringenin by naringinase immobilized in microstructured materials in a model of DSS-induced colitis in mice. Food Res. Int. 2009;42:1010–1017. [Google Scholar]
- 168.Huang S.W., Frankel E.N. Antioxidant activity of tea catechins in different lipid systems. J. Agric. Food Chem. 1997;45:3033–3038. [Google Scholar]
- 169.Geetha T., Garg A., Chopra K., Kaur I.P. Delineation of antimutagenic activity of catechin, epicatechin and green tea extract. Mutat. Res.-Fund. Mol. Mech. Mutagen. 2004;556:65–74. doi: 10.1016/j.mrfmmm.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 170.Menon L.G., Kuttan R., Kuttan G. Anti-metastatic activity of curcumin and catechin. Cancer Lett. 1999;141:159–165. doi: 10.1016/s0304-3835(99)00098-1. [DOI] [PubMed] [Google Scholar]
- 171.Hirasawa M., Takada K. Multiple effects of green tea catechin on the antifungal activity of antimycotics against Candida albicans. J. Antimicro. Chemoth. 2004;53:225–229. doi: 10.1093/jac/dkh046. [DOI] [PubMed] [Google Scholar]
- 172.Saeki K., Hayakawa S., Isemura M., Miyase T. Importance of a pyrogallol-type structure in catechin compounds for apoptosis-inducing activity. Phytochemistry. 2000;53:391–394. doi: 10.1016/s0031-9422(99)00513-0. [DOI] [PubMed] [Google Scholar]
- 173.Iacopini P., Baldi M., Storchi P., Sebastiani L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008;21:589–598. [Google Scholar]
- 174.Kinjo J., Nagao T., Tanaka T., Nonaka G.I., Okawa M., Nohara T., Okabe H. Activity-guided fractionation of green tea extract with antiproliferative activity against human stomach cancer cells. Biol. Pharm. Bull. 2002;2:1238–1240. doi: 10.1248/bpb.25.1238. [DOI] [PubMed] [Google Scholar]
- 175.Han Z., Wang G., Yao W., Zhu W. Isoflavonic phytoestrogens-new prebiotics for farm animals: A review on research in China. Curr. Issues Intest. Microbiol. 2006;7:53–60. [PubMed] [Google Scholar]
- 176.Lutz M., Martínez A., Martínez E.A. Daidzein and genistein contents in seeds of quinoa (Chenopodium quinoa Willd.) from local ecotypes grown in arid Chile. Ind. Crop. Prod. 2013;49:117–121. [Google Scholar]
- 177.Foti P., Erba D., Riso P., Spadafranca A., Criscuoli F., Testolin G. Comparison between daidzein and genistein antioxidant activity in primary and cancer lymphocytes. Arch. Biochem. Biophys. 2005;433:421–427. doi: 10.1016/j.abb.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 178.Cho K.W., Lee O.H., Banz W.J., Moustaid-Moussa N., Shay N.F., Kim Y.C. Daidzein and the daidzein metabolite, equol, enhance adipocyte differentiation and PPARγ transcriptional activity. J. Nutr. Biochem. 2010;21:841–847. doi: 10.1016/j.jnutbio.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 179.Guo J.M., Kang G.Z., Xiao B.X., Liu D.H., Zhang S. Effect of daidzein on cell growth, cell cycle, and telomerase activity of human cervical cancer in vitro. Int. J. Gynecol. Cancer. 2004;14:882–888. doi: 10.1111/j.1048-891X.2004.14525.x. [DOI] [PubMed] [Google Scholar]
- 180.Picherit C., Dalle M., Neliat G., Lebecque P., Davicco M.J., Barlet J.P., Coxam V. Genistein and daidzein modulate in vitro rat uterine contractile activity. J. Steroid Biochem. Mol. Biol. 2000;75:201–208. doi: 10.1016/s0960-0760(00)00179-5. [DOI] [PubMed] [Google Scholar]
- 181.Zeng J., Huang Z., Qiu F., Yao X., Ye H. The anti-hypoxia activity of daidzein. Chin. J. Mod. Appl. Pharm. 2004;21:454–456. [Google Scholar]
- 182.Choo M.K., Park E.K., Yoon H.K., Kim D.H. Antithrombotic and antiallergic activities of daidzein, a metabolite of puerarin and daidzin produced by human intestinal microflora. Biol. Pharm. Bull. 2002;25:1328–1332. doi: 10.1248/bpb.25.1328. [DOI] [PubMed] [Google Scholar]
- 183.Lepri S.R., Luiz R.C., Zanelatto L.C., Da Silva P.B.G., Sartori D., Ribeiro L.R., Mantovani M.S. Chemoprotective activity of the isoflavones, genistein and daidzein on mutagenicity induced by direct and indirect mutagens in cultured HTC cells. Cytotechnology. 2013;65:213–222. doi: 10.1007/s10616-012-9476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fujioka M., Uehara M., Wu J., Adlercreutz H., Suzuki K., Kanazawa K., Ishimi Y. Equol, a metabolite of daidzein, inhibits bone loss in ovariectomized mice. J. Nutr. 2004;134:2623–2627. doi: 10.1093/jn/134.10.2623. [DOI] [PubMed] [Google Scholar]
- 185.Choi E.J., Kim G.H. Antiproliferative activity of daidzein and genistein may be related to ERα/c-erbB-2 expression in human breast cancer cells. Mol. Med. Rep. 2013;7:781–784. doi: 10.3892/mmr.2013.1283. [DOI] [PubMed] [Google Scholar]
- 186.Finking G., Wohlfrom M., Lenz C., Wolkenhauer M., Eberle C., Hanke H. The phytoestrogens genistein and daidzein, and 17 beta-estradiol inhibit development of neointima in aortas from male and female rabbits in vitro after injury. Coron. Artery Dis. 1999;10:607–615. doi: 10.1097/00019501-199912000-00010. [DOI] [PubMed] [Google Scholar]
- 187.Record I.R., Dreosti I.E., McInerney J.K. The antioxidant activity of genistein in vitro. J. Nutr. Biochem. 1995;6:481–485. [Google Scholar]
- 188.Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S.I., Itoh N., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 189.Banerjee S., Zhang Y., Ali S., Bhuiyan M., Wang Z., Chiao P.J., Sarkar F.H. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res. 2005;65:9064–9072. doi: 10.1158/0008-5472.CAN-05-1330. [DOI] [PubMed] [Google Scholar]
- 190.Uckun F.M., Narla R.K., Jun X., Zeren T., Venkatachalam T., Waddick K.G., Rostostev A., Myers D.E. Cytotoxic activity of epidermal growth factor-genistein against breast cancer cells. Clin. Cancer Res. 1998;4:901–912. [PubMed] [Google Scholar]
- 191.Büchler P., Reber H.A., Büchler M.W., Friess H., Lavey R.S., Hines O.J. Antiangiogenic activity of genistein in pancreatic carcinoma cells is mediated by the inhibition of hypoxia-inducible factor-1 and the down-regulation of VEGF gene expression. Cancer. 2004;10:201–210. doi: 10.1002/cncr.11873. [DOI] [PubMed] [Google Scholar]
- 192.Farina H.G., Pomies M., Alonso D.F., Gomez D.E. Antitumor and antiangiogenic activity of soy isoflavone genistein in mouse models of melanoma and breast cancer. Oncol. Rep. 2006;16:885–891. [PubMed] [Google Scholar]
- 193.Hong H., Landauer M.R., Foriska M.A., Ledney G.D. Antibacterial activity of the soy isoflavone genistein. J. Basic Microbiol. 2006;46:329–335. doi: 10.1002/jobm.200510073. [DOI] [PubMed] [Google Scholar]
- 194.Ye F., Wu J., Dunn T., Yi J., Tong X., Zhang D. Inhibition of cyclooxygenase-2 activity in head and neck cancer cells by genistein. Cancer Lett. 2004;211:39–46. doi: 10.1016/j.canlet.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 195.Aditya N.P., Shim M., Lee I., Lee Y., Im M.H., Ko S. Curcumin and genistein coloaded nanostructured lipid carriers: In vitro digestion and antiprostate cancer activity. J. Agric. Food Chem. 2013;61:1878–1883. doi: 10.1021/jf305143k. [DOI] [PubMed] [Google Scholar]
- 196.Raynal N.J.M., Momparler L., Charbonneau M., Momparler R.L. Antileukemic activity of genistein, a major isoflavone present in soy products. J. Nat. Prod. 2007;71:3–7. doi: 10.1021/np070230s. [DOI] [PubMed] [Google Scholar]
- 197.Kim J.S., Nam Y.J., Kwon T.W. Induction of quinone reductase activity by genistein, soybean isoflavone. Food Sci. Biotechnol. 1996;5:70–75. [Google Scholar]
- 198.Khaw A.K., Yong J.W.Y., Kalthur G., Hande M.P. Genistein induces growth arrest and suppresses telomerase activity in brain tumor cells. Gene. Chromosome Canc. 2012;51:961–974. doi: 10.1002/gcc.21979. [DOI] [PubMed] [Google Scholar]
- 199.Yang G., Ham I., Choi H.Y. Anti-inflammatory effect of prunetin via the suppression of NF-κB pathway. Food Chem. Toxicol. 2013;58:124–132. doi: 10.1016/j.fct.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 200.Xu X., Zhang S., Zhang L., Yan W., Zheng X. The Neuroprotection of puerarin against cerebral ischemia is associated with the prevention of apoptosis in rats. Planta Med. 2005;71:585–591. doi: 10.1055/s-2005-871261. [DOI] [PubMed] [Google Scholar]
- 201.Cherdshewasart W., Sutjit W. Correlation of antioxidant activity and major isoflavonoid contents of the phytoestrogen-rich Pueraria mirifica and Pueraria lobata tubers. Phytomedicine. 2008;15:38–43. doi: 10.1016/j.phymed.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 202.Hsu F.L., Liu I.M., Kuo D.H., Chen W.C., Su H.C., Cheng J.T. Antihyperglycemic effect of puerarin in streptozotocin-induced diabetic rats. J. Nat. Prod. 2003;66:788–792. doi: 10.1021/np0203887. [DOI] [PubMed] [Google Scholar]
- 203.Sun H.X., Xie Y., Ye Y.P. Advances in saponin-based adjuvants. Vaccine. 2009;27:1787–1796. doi: 10.1016/j.vaccine.2009.01.091. [DOI] [PubMed] [Google Scholar]
- 204.Dembitsky V., Shkrob I., Hanus L.O. Ascaridole and related peroxides from the genus Chenopodium. Biomed. Pap. Med. Fac. Palacky. Olomouc. Czech. Repub. 2008;152:209–215. doi: 10.5507/bp.2008.032. [DOI] [PubMed] [Google Scholar]
- 205.Yoshitomi K., Taniguchi S., Tanaka K., Uji Y., Akimitsu K., Gomi K. Rice terpene synthase 24 (PsTPS24) encodes a jamonate-responsive monoterpene synthase that produces an antibacterial γ-terpinene against rice pathogen. J. Plant Physiol. 2016;191:120–126. doi: 10.1016/j.jplph.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 206.Ruiz K.B., Khakimov B., Engelsen S.B., Bak S., Biondi S., Jacobsen S.-E. Quinoa seed coats as an expanding and sustainable source of bioactive compounds: An investigation of genotypic diversity in saponin profiles. Ind. Crop. Prod. 2017;104:156–163. [Google Scholar]
- 207.Mastebroek H.D., Limburg H., Gilles T., Marvin H.J.P. Occurrence of sapogenins in leaves and seeds of quinoa (Chenopodium quinoa Willd) J. Sci. Food Agric. 2000;80:152–156. [Google Scholar]
- 208.Woldemichael G.M., Wink M. Identification and biological activities of triterpenoid saponins from Chenopodium quinoa. J. Agric. Food Chem. 2001;49:2327–2332. doi: 10.1021/jf0013499. [DOI] [PubMed] [Google Scholar]
- 209.Sun X., Yang X., Xue P., Zhang Z., Ren G. Improved antibacterial effects of alkali-transformed saponin from quinoa husks against halitosis-related bacteria. BMC Complem. Altern. Med. 2019;19:46. doi: 10.1186/s12906-019-2455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Stuardo M., San Martín R. Antifungal properties of quinoa (Chenopodium quinoa Willd) alkali treated saponins against Botrytis cinerea. Ind. Crop. Prod. 2008;27:296–302. [Google Scholar]
- 211.San Martín R., Ndjoko K., Hostettmann K. Novel molluscicide against Pomacea canaliculata based on quinoa (Chenopodium quinoa) saponins. Crop Prot. 2008;27:310–319. [Google Scholar]
- 212.Castillo-Ruiz M., Cañon-Jones H., Schlotterbeck T., Lopez M.A., Tomas A., San Martin R. Safety and efficacy of quinoa (Chenopodium quinoa) saponins derived molluscicide to control of Pomacea maculata in rice fields in the Ebro Delta, Spain. Crop Prot. 2018;111:42–49. [Google Scholar]
- 213.Joshi R.C., San Martin R., Saez-Navarrete C., Alarcon J., Sainz J., Antolin M.M., Martin R., Sebastian L.S. Efficacy of quinoa (Chenopodium quinoa) saponins against golden apple snail (Pomacea canaliculata) in the Philippines under laboratory conditions. Crop Prot. 2008;27:553–557. [Google Scholar]
- 214.Madl T., Sterk H., Mittelbach M., Rechberger G.N. Tandem mass spectrometric analysis of a complex triterpene saponin mixture of Chenopodium quinoa. J. Am. Soc. Mass. Spectr. 2006;17:795–806. doi: 10.1016/j.jasms.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 215.Burnouf-Radosevich M., Delfel N.E., England R. Gas chromatography-mass spectrometry of oleanane-and ursane-type triterpenes–application to Chenopodium quinoa triterpenes. Phytochemistry. 1985;24:2063–2066. [Google Scholar]
- 216.Mizui F., Kasai R., Ohtani K., Tanaka O. Saponins from brans of quinoa, Chenopodium quinoa Willd. I. Chem. Pharm. Bull. 1988;36:1415–1418. [Google Scholar]
- 217.Mizui F., Kasai R., Ohtani K., Tanaka O. Saponins from brans of quinoa, Chenopodium quinoa Willd. II. Chem. Pharm. Bull. 1990;38:375–377. [Google Scholar]
- 218.Burnouf-Radosevich M., Delfel N.E. High-performance liquid chromatography of oleanane-type triterpenes. J. Chromatogr. A. 1984;292:403–409. [Google Scholar]
- 219.Quispe-Fuentes I., Vega-Galvez A., Miranda M., Lemus-Mondaca R., Lozano M., Ah-Hen K. A kinetic approach to saponin extraction during washing of quinoa (Chenopodium quinoa Willd.) seeds. J. Food Process Eng. 2013;36:202–210. [Google Scholar]
- 220.Horiuchi K., Shiota S., Hatano T., Yoshida T., Kuroda T., Tsuchiya T. Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci (VRE) Biol. Pharm. Bull. 2007;30:1147–1149. doi: 10.1248/bpb.30.1147. [DOI] [PubMed] [Google Scholar]
- 221.Wolska K.I., Grudniak A.M., Fiecek B., Kraczkiewicz-Dowjat A., Kurek A. Antibacterial activity of oleanolic and ursolic acids and their derivatives. Cent. Eur. J. Biol. 2010;5:543–553. [Google Scholar]
- 222.Kashiwada Y., Wang H.K., Nagao T., Kitanaka S., Yasuda I., Fujioka T., Ikeshiro Y. Anti-AIDS agents. 30. Anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids. J. Nat. Prod. 1998;61:1090–1095. doi: 10.1021/np9800710. [DOI] [PubMed] [Google Scholar]
- 223.Singh G.B., Singh S., Bani S., Gupta B.D., Banerjee S.K. Anti–inflammatory activity of oleanolic acid in rats and mice. J. Pharm. Pharmacol. 1992;44:456–458. doi: 10.1111/j.2042-7158.1992.tb03646.x. [DOI] [PubMed] [Google Scholar]
- 224.Ghosh D., Thejomoorthy P. Anti-inflammatory and analgesic activities of oleanolic acid 3-/3-glucoside (RDG-1) from Randia dumetorum (Rubiaceae) Indian J. Pharmacol. 1983;15:331. [Google Scholar]
- 225.Wang X., Ye X.L., Liu R., Chen H.L., Bai H., Liang X., Hai C.X. Antioxidant activities of oleanolic acid in vitro: Possible role of Nrf2 and MAP kinases. Chem.-Biol. Interact. 2010;184:328–337. doi: 10.1016/j.cbi.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 226.Rajasekaran M., Bapna J.S., Lakshmanan S., Nair A.R., Veliath A.J., Panchanadam M. Antifertility effect in male rats of oleanolic acid, a triterpene from Eugenia jambolana flowers. J. Ethnopharmacol. 1988;24:115–121. doi: 10.1016/0378-8741(88)90142-0. [DOI] [PubMed] [Google Scholar]
- 227.Dzubak P., Hajduch M., Vydra D., Hustova A., Kvasnica M., Biedermann D., Sarek J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006;23:394–411. doi: 10.1039/b515312n. [DOI] [PubMed] [Google Scholar]
- 228.Petronelli A., Pannitteri G., Testa U. Triterpenoids as new promising anticancer drugs. Anti-Cancer Drug. 2009;20:880–892. doi: 10.1097/CAD.0b013e328330fd90. [DOI] [PubMed] [Google Scholar]
- 229.Zhu Y.Y., Huang H.Y., Wu Y.L. Anticancer and apoptotic activities of oleanolic acid are mediated through cell cycle arrest and disruption of mitochondrial membrane potential in HepG2 human hepatocellular carcinoma cells. Mol. Med. Rep. 2015;12:5012–5018. doi: 10.3892/mmr.2015.4033. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 230.Yoshikawa M., Matsuda H. Antidiabetogenic activity of oleanolic acid glycosides from medicinal foodstuffs. BioFactors. 2000;13:231–237. doi: 10.1002/biof.5520130136. [DOI] [PubMed] [Google Scholar]
- 231.Park S.H., Oh S.R., Jung K.Y., Ahn K.S., Kim J.G., Lee J.J., Lee H.K. Anticomplement activities of oleanolic acid monodesmosides and bisdesmosides isolated from Tiarella polyphylla. Arch. Pharm. Res. 1999;22:428–431. doi: 10.1007/BF02979071. [DOI] [PubMed] [Google Scholar]
- 232.Facino R.M., Carini M., Stefani R., Aldini G., Saibene L. Anti–elastase and anti–hyaluronidase activities of saponins and sapogenins from Hedera helix, Aesculus hippocastanum, and Ruscus aculeatus: Factors contributing to their efficacy in the treatment of venous insufficiency. Archiv. Der. Pharm. 1995;328:720–724. doi: 10.1002/ardp.19953281006. [DOI] [PubMed] [Google Scholar]
- 233.Ruiz W.A., Farfan J.A. Determination of oleanolic acid in quinoa by gas-liquid chromatography (Chenopodium quinoa, Willd cv Kcancolla) Bol. Soc. Quim. Peru. 1979;45:266–276. [Google Scholar]
- 234.Lozano M., Gonzales E., Flores Y., Almanza G.R. Effect in acute inflammation of sapogenin extract and isolated sapogenins from quinoa waste (Chenopodium quinoa Willd) Rev. Boliv. Quim. 2013;30:115–121. [Google Scholar]
- 235.Dini I., Schettino O., Simioli T., Dini A. Studies on the constituents of Chenopodium quinoa seeds: Isolation and characterization of new triterpene saponins. J. Agric. Food Chem. 2001;49:741–746. doi: 10.1021/jf000971y. [DOI] [PubMed] [Google Scholar]
- 236.Dini I., Tenore G.C., Dini A. Oleanane saponins in “Kancolla”, a sweet variety of Chenopodium quinoa. J. Nat. Prod. 2002;65:1023–1026. doi: 10.1021/np010625q. [DOI] [PubMed] [Google Scholar]
- 237.Ma W.W., Heinstein P.F., Mclaughlin J.L. Additional toxic, bitter saponins from the seeds of Chenopodium quinoa. J. Nat. Prod. 1989;52:1132–1135. doi: 10.1021/np50065a035. [DOI] [PubMed] [Google Scholar]
- 238.Zhu N., Sheng S., Sang S., Jhoo J.-W., Bai N., Karwe M.V., Rosen R.T., Ho C.-T. Triterpene saponins from debittered quinoa (Chenopodium quinoa) seeds. J. Agric. Food Chem. 2002;50:865–867. doi: 10.1021/jf011002l. [DOI] [PubMed] [Google Scholar]
- 239.Chauhan G.S., Eskin N.A.M., Tkachuk R. Nutrients and antinutrients in quinoa seed. Cereal Chem. 1992;69:85–88. [Google Scholar]
- 240.Hostettmann K. Saponins with molluscicidal activity from Hedera helix L. Helv. Chim. Acta. 1980;63:606–609. [Google Scholar]
- 241.Barthomeuf C., Debiton E., Mshvildadze V., Kemertelidze E., Balansard G. In vitro activity of hederacolchisid A1 compared with other saponins from Hedera colchica against proliferation of human carcinoma and melanoma cells. Planta Med. 2002;68:672–675. doi: 10.1055/s-2002-33807. [DOI] [PubMed] [Google Scholar]
- 242.Favel A., Steinmetz M.D., Regli P., Vidal-Ollivier E., Elias R., Balansard G. In vitro antifungal activity of triterpenoid saponins. Planta Med. 1994;60:50–53. doi: 10.1055/s-2006-959407. [DOI] [PubMed] [Google Scholar]
- 243.Majester-Savornin B., Elias R., Diaz-Lanza A.M., Balansard G., Gasquet M., Delmas F. Saponins of the ivy plant, Hedera helix, and their leishmanicidic activity. Planta Med. 1991;57:260–262. doi: 10.1055/s-2006-960086. [DOI] [PubMed] [Google Scholar]
- 244.Lee S.J., Shin E.J., Son K.H., Chang H.W., Kang S.S., Kim H.P. Anti-inflammatory activity of the major constituents of Lonicera japonica. Arch. Pharm. Res. 1995;18:133. [Google Scholar]
- 245.Rodríguez-Hernández D., Demuner A.J., Barbosa L.C., Csuk R., Heller L. Hederagenin as a triterpene template for the development of new antitumor compounds. Eur. J. Med. Chem. 2015;105:57–62. doi: 10.1016/j.ejmech.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 246.Khalil A.H., El-Adawy T.A. Isolation, identification and toxicity of saponin from different legumes. Food Chem. 1994;50:197–201. [Google Scholar]
- 247.Liu B.-X.-Z., Zhou J.-Y., Li Y., Zou X., Wu J., Gu J.-F., Yuan J.-R., Zhao B.-J., Feng L., Jia X.-B., et al. Hederagenin from the leaves of ivy (Hedera helix L.) induces apoptosis in human LoVo colon cells through the mitochondrial pathway. BMC Complement. Altern. Med. 2014;14:412. doi: 10.1186/1472-6882-14-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lee K.-T., Sohn I.-C., Park H.-J., Kim D.-W., Jung G.-O., Park K.-Y. Essential moiety for antimutagenic and cytotoxic activity of hederagenin monodesmosides and bisdesmosides isolated from the stem bark of Kalopanax pictus. Planta Med. 2000;66:329–332. doi: 10.1055/s-2000-8539. [DOI] [PubMed] [Google Scholar]
- 249.Park H.J., Kwon S.H., Lee J.H., Lee K.H., Miyamoto K.I., Lee K.T. Kalopanaxsaponin A is a basic saponin structure for the anti-tumor activity of hederagenin monodesmosides. Planta Med. 2001;67:118–121. doi: 10.1055/s-2001-11516. [DOI] [PubMed] [Google Scholar]
- 250.Voutquenne L., Lavaud C., Massiot G., Men-Olivier L.L. Structure-activity relationships of haemolytic saponins. Pharm. Biol. 2002;40:253–262. [Google Scholar]
- 251.Houghton P., Patel N., Jurzysta M., Biely Z., Cheung C. Antidermatophyte activity of medicago extracts and contained saponins and their structure–activity relationships. Phytother. Res. 2006;20:1061–1066. doi: 10.1002/ptr.1995. [DOI] [PubMed] [Google Scholar]
- 252.He W., Van Puyvelde L., Maes L., Bosselaers J., De Kimpe N. Antitrichomonas in vitro activity of Cussonia holstii Engl. Nat. Prod. Res. 2003;17:127–133. doi: 10.1080/1478641031000103713. [DOI] [PubMed] [Google Scholar]
- 253.Gopalsamy N., Gueho J., Julien H.R., Owadally A.W., Hostettmann K. Molluscicidal saponins of Polyscias dichroostachya. Phytochemistry. 1990;29:793–795. [Google Scholar]
- 254.Oh S.R., Jung K.Y., Son K.H., Park S.H., Ahn K.S., Lee H.K. In vitro anticomplementary activity of hederagenin saponins isolated from roots of Dipsacus asper. Arch. Pharm. Res. 1999;22:317–319. doi: 10.1007/BF02976371. [DOI] [PubMed] [Google Scholar]
- 255.Jung H.-J., Lee C.O., Lee K.-T., Choi J., Park H.-J. Structure–activity relationship of oleanane disaccharides isolated from Akebia quinata versus cytotoxicity against cancer cells and NO inhibition. Biol. Pharm. Bull. 2004;27:744–747. doi: 10.1248/bpb.27.744. [DOI] [PubMed] [Google Scholar]
- 256.Dini I., Tenore G.C., Schettino O., Dini A. New oleanane saponins in Chenopodium quinoa. J. Agric. Food Chem. 2001;49:3976–3981. doi: 10.1021/jf010361d. [DOI] [PubMed] [Google Scholar]
- 257.Meyer B.N., Heinstein P.F., Burnoufradosevich M., Delfel N.E., Mclaughlin J.L. Bioactivity-directed isolation and characterization of quinoside A: One of the toxic/bitter principles of quinoa seeds (Chenopodium quinoa Willd.) J. Agric. Food Chem. 1990;38:205–208. [Google Scholar]
- 258.Montoya G., Gutierrez G., D’vries R., Ellena J., Panay A.J. Spergulagenic acid A: Isolation and single crystal structure elucidation. J. Mol. Struct. 2018;1173:937–941. [Google Scholar]
- 259.Lazo-Vélez M.A., Guajardo-Flores D., Mata-Ramírez D., Gutiérrez-Uribe J.A., Serna-Saldivar S.O. Characterization and quantitation of triterpenoid saponins in raw and sprouted Chenopodium berlandieri spp. (Huauzontle) grains subjected to germination with or without selenium stress conditions. J. Food Sci. 2016;81:C19–C26. doi: 10.1111/1750-3841.13174. [DOI] [PubMed] [Google Scholar]
- 260.Vincken J.P., Heng L., de Groot A., Gruppen H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry. 2007;68:275–297. doi: 10.1016/j.phytochem.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 261.Fanali C., Beccaria M., Salivo S., Tranchida P., Tripodo G., Farnetti S., Dugo L., Dugo P., Mondello L. Non-polar lipids characterization of quinoa (Chenopodium quinoa) seed by comprehensive two-dimensional gas chromatography with flame ionization/mass spectrometry detection and non-aqueous reversed-phase liquid chromatography with atmospheric pressure chemical ionization mass spectrometry detection. J. Sep. Sci. 2015;38:3151–3160. doi: 10.1002/jssc.201500466. [DOI] [PubMed] [Google Scholar]
- 262.Lee I.S., Oh S.R., Jung K.Y., Kim D.S., Kim J.H., Lee H.K. Anticomplementary activity and complete 13C NMR assignment of citrostadienol from Schizandra chinensis. Int. J. Pharmacogn. 1997;35:358–363. [Google Scholar]
- 263.Saeed M.A., Sabir A.W. Antibacterial activity of Caesalpinia bonducella seeds. Fitoterapia. 2001;72:807–809. doi: 10.1016/s0367-326x(01)00292-1. [DOI] [PubMed] [Google Scholar]
- 264.Giacoman-Martínez A., Alarcón-Aguilar F.J., Zamilpa A., Hidalgo-Figueroa S., Navarrete-Vazquez G., Garcia-Macedo R., Almanza-Perez J.C. Triterpenoids from Hibiscus sabdariffa L. with PPAR δ/γ dual agonist action: In vivo, in vitro and in silico studies. Planta Med. 2019;85:412–423. doi: 10.1055/a-0824-1316. [DOI] [PubMed] [Google Scholar]
- 265.Cardoso B.K., de Oliveira H.L.M., Melo U.Z., Fernandez C.M.M., Campo C.F.D.A.A., Goncalves J.E., Gazim Z.C. Antioxidant activity of α- and β-amyrin isolated from Myrcianthes pungens leaves. Nat. Prod. Res. 2019;33 doi: 10.1080/14786419.2018.1525715. [DOI] [PubMed] [Google Scholar]
- 266.Chen D., Xu F., Zhang P., Deng J., Sun H., Wen X., Liu J. Practical synthesis of α-amyrin, β-amyrin, and lupeol: The potential natural inhibitors of human oxidosqualene cyclase. Arch. Pharm. 2017;350:1700178. doi: 10.1002/ardp.201700178. [DOI] [PubMed] [Google Scholar]
- 267.Kannan S., Vijayakumar B., Sureshkumar C., Mohankumar R., Narasimhan S. Insect antifeedant and growth regulating activities of β-amyrin from Sarcostemma acidum. Asian J. Chem. 2013;25:1167–1168. [Google Scholar]
- 268.Mhalla D., Ben Farhat-Touzri D., Tounsi S., Trigui M. Combinational effect of Rumex tingitanus (Polygonaceae) hexane extract and Bacillus thuringiensis δ-endotoxin against Spodoptera littoralis (Lepidoptera: Noctuidae) BioMed. Res. Int. 2018;2018:3895834. doi: 10.1155/2018/3895834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kemboi D. Phytochemistry and antimicrobial activity of extracts from medicinal plant Olea africana and Olea europea. Int. J. Biochem. Res. Rev. 2016;12:25863. [Google Scholar]
- 270.Zhang J., Yamada S., Ogihara E., Kurita M., Banno N., Qu W., Akihisa T. Biological activities of triterpenoids and phenolic compounds from Myrica cerifera bark. Chem. Biodivers. 2016;13:1601–1609. doi: 10.1002/cbdv.201600247. [DOI] [PubMed] [Google Scholar]
- 271.Ntchapda F., Talla E., Sakava P., Tanzi F., Fohouo F.N.T., Tanyi J.M., Dimo T. Nitric oxide-dependent vasodilation and Ca2+ signalling induced by erythrodiol in rat aorta. Asian Pac. J. Trop. Dis. 2015;5:S214–S223. [Google Scholar]
- 272.Juan M.E., Wenzel U., Daniel H., Planas J.M. Erythrodiol, a natural triterpenoid from olives, has antiproliferative and apoptotic activity in HT-29 human adenocarcinoma cells. Mol. Nutr. Food. Res. 2008;52:595–599. doi: 10.1002/mnfr.200700300. [DOI] [PubMed] [Google Scholar]
- 273.Zheng Q., Li P., Jin F., Yao C., Zhang G., Zang T., Ai X. Ursolic acid induces ER stress response to activate ASK1-JNK signaling and induce apoptosis in human bladder cancer T24 cells. Cell. Signal. 2013;25:206–213. doi: 10.1016/j.cellsig.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 274.Wang C.M., Tsai S.J., Jhan Y.L., Yeh K.L., Chou C.H. Anti-proliferative activity of triterpenoids and sterols isolated from Alstonia scholaris against non-small-cell lung carcinoma cells. Molecules. 2017;22:2119. doi: 10.3390/molecules22122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Tang Y., Li X., Chen P.X., Zhang B., Hernandez M., Zhang H., Marcone M.F., Liu R., Tsao R. Characterisation of fatty acid, carotenoid, tocopherol/tocotrienol compositions and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015;174:502–508. doi: 10.1016/j.foodchem.2014.11.040. [DOI] [PubMed] [Google Scholar]
- 276.Alvarez-Jubete L., Holse M., Hansen A., Arendt E.K., Gallagher E. Impact of baking on vitamin E content of pseudocereals amaranth, quinoa, and buckwheat. Cereal Chem. 2009;86:511–515. [Google Scholar]
- 277.Ju J., Picinich S.C., Yang Z., Zhao Y., Suh N., Kong A.N., Yang C.S. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis. 2009;31:533–542. doi: 10.1093/carcin/bgp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Gil-Chávez G.J., Villa J.A., Ayala-Zavala J.F., Heredia J.B., Sepulveda D., Yahia E.M., González-Aguilar G.A. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An overview. Compr. Rev. Food Sci. Food Saf. 2013;12:5–23. [Google Scholar]
- 279.Sen C.K., Khanna S., Roy S. Tocotrienols in health and disease: The other half of the natural vitamin E family. Mol. Asp. Med. 2007;28:692–728. doi: 10.1016/j.mam.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Zingg J.-M. Vitamin E: An overview of major research directions. Mol. Asp. Med. 2007;28:400–422. doi: 10.1016/j.mam.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 281.Pereira E., Encina-Zelada C., Barros L., Gonzales-Barron U., Cadavez V., Ferreira I.C. Chemical and nutritional characterization of Chenopodium quinoa Willd (quinoa) grains: A good alternative to nutritious food. Food Chem. 2019;280:110–114. doi: 10.1016/j.foodchem.2018.12.068. [DOI] [PubMed] [Google Scholar]
- 282.Ahsan H., Ahad A., Iqbal J., Siddiqui W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. 2014;11:52. doi: 10.1186/1743-7075-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kozioł M.J. Chemical composition and nutritional evaluation of quinoa (Chenopodium quinoa Willd.) J. Food Compos. Anal. 1992;5:35–68. [Google Scholar]
- 284.Sookwong P., Murata K., Nakagawa K., Shibata A., Kimura T., Yamaguchi M., Kojima Y., Miyazawa T. Cross-fertilization for enhancing tocotrienol biosynthesis in rice plants and QTL analysis of their F2 progenies. J. Agric. Food Chem. 2009;57:4620–4625. doi: 10.1021/jf900394t. [DOI] [PubMed] [Google Scholar]
- 285.Ahamed N.T., Singhal R.S., Kulkarni P.R., Pal M. A lesser-known grain, Chenopodium quinoa: Review of the chemical composition of its edible parts. Food Nutr. Bull. 1998;19:61–70. [Google Scholar]
- 286.Foucault A.S., Mathé V., Lafont R., Even P., Dioh W., Veillet S., Tomé D., Huneau J.F., Hermier D., Quignard-Boulangé A. Quinoa extract enriched in 20-hydroxyecdysone protects mice from diet-induced obesity and modulates adipokines expression. Obesity. 2012;20:270–277. doi: 10.1038/oby.2011.257. [DOI] [PubMed] [Google Scholar]
- 287.Nsimba R.Y., Kikuzaki H., Konishi Y. Ecdysteroids act as inhibitors of calf skin collagenase and oxidative stress. J. Biochem. Mol. Toxic. 2008;22:240–250. doi: 10.1002/jbt.20234. [DOI] [PubMed] [Google Scholar]
- 288.Dini A., Rastrelli L., Saturnino P., Schettino O. A compositional study of Chenopodium quinoa seeds. Nahrung. 1992;36:400–404. [Google Scholar]
- 289.Zhu N., Kikuzaki H., Vastano B.C., Nakatani N., Karwe M.V., Rosen R.T., Ho C.T. Ecdysteroids of quinoa seeds (Chenopodium quinoa Willd.) J. Agric. Food Chem. 2001;49:2576–2578. doi: 10.1021/jf0014462. [DOI] [PubMed] [Google Scholar]
- 290.Xu D., Ali S., Huang Z. Insecticidal activity influence of 20-hydroxyecdysone on the pathogenicity of Isaria fumosorosea against Plutella xylostella. Biol. Control. 2011;56:239–244. [Google Scholar]
- 291.Choi J.M., Lee E.O., Lee H.J., Kim K.H., Ahn K.S., Shim B.S., Kim N.I., Song M.C., Baek N.I., Kim S.H. Identification of campesterol from Chrysanthemum coronarium L. and its antiangiogenic activities. Phytother. Res. 2007;21:954–959. doi: 10.1002/ptr.2189. [DOI] [PubMed] [Google Scholar]
- 292.Villacrés E., Pástor G., Quelal M.B., Zambrano I., Morales S.H. Effect of processing on the content of fatty acids, tocopherols and sterols in the oils of quinoa (Chenopodium quinoa Willd), lupine (Lupinus mutabilis Sweet), amaranth (Amaranthus caudatus L.) and sangorache (Amaranthus quitensis L.) Glob. Adv. Res. J. Food Sci. Technol. 2013;2:44–53. [Google Scholar]
- 293.Dini I., Tenore G.C., Dini A. Nutritional and antinutritional composition of Kancolla seeds: An interesting and underexploited andine food plant. Food Chem. 2005;92:125–132. [Google Scholar]
- 294.Prieto J.M., Recio M.C., Giner R.M. Anti-inflammatory activity of β-sitosterol in a model of oxazoloneinduced contact-delayed-type hypersensitivity. Bol. Lat. Am. Caribb. Bull. Med. Plants. 2006;5:57–62. [Google Scholar]
- 295.Vivancos M., Moreno J.J. β-Sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radical Bio. Med. 2005;39:91–97. doi: 10.1016/j.freeradbiomed.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 296.Radika M.K., Viswanathan P., Anuradha C.V. Nitric oxide mediates the insulin sensitizing effects of β-sitosterol in high fat diet-fed rats. Nitric Oxide Biol. Chem. 2013;32:43–53. doi: 10.1016/j.niox.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 297.Garcia M.D., Saenz M.T., Gomez M.A., Fernandez M.A. Topical antiinflammatory activity of phytosterols isolated from Eryngium foetidum on chronic and acute inflammation models. Phytother. Res. 1999;13:78–80. doi: 10.1002/(SICI)1099-1573(199902)13:1<78::AID-PTR384>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 298.Ghosh T., Maity T.K., Singh J. Evaluation of antitumor activity of stigmasterol, a constituent isolated from Bacopa monnieri Linn aerial parts against Ehrlich Ascites Carcinoma in mice. Orient. Pharm. Exp. Med. 2011;11:41–49. [Google Scholar]
- 299.Mbambo B., Odhav B., Mohanlall V. Antifungal activity of stigmasterol, sitosterol and ergosterol from Bulbine natalensis Baker. (Asphodelaceae) J. Med. Plants Res. 2012;6:5135–5141. [Google Scholar]
- 300.Batta A.K., Xu G., Honda A., Miyazaki T., Salen G. Stigmasterol reduces plasma cholesterol levels and inhibits hepatic synthesis and intestinal absorption in the rat. Metabolism. 2006;55:292–299. doi: 10.1016/j.metabol.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 301.Huang J.G., Zhou L.J., Xu H.H., Li W.O. Insecticidal and cytotoxic activities of extracts of Cacalia tangutica and its two active ingredients against Musca domestica and Aedes albopictus. J. Econ. Entomol. 2009;102:1444–1447. doi: 10.1603/029.102.0407. [DOI] [PubMed] [Google Scholar]
- 302.Chai J.W., Kuppusamy U.R., Kanthimathi M.S. Beta-sitosterol induces apoptosis in MCF-7 cells. Malay. J. Biochem. Mol. Biol. 2008;16:28–30. [Google Scholar]
- 303.Saeidnia S., Manayi A., Gohari A.R., Abdollahi M. The story of beta-sitosterol-a review. Eur. J. Med. Plants. 2014;4:590. [Google Scholar]
- 304.Sugano M., Morioka H., Ikeda I. A comparison of hypocholesterolemic activity of β-sitosterol and β-sitostanol in rats. J. Nutr. 1977;107:2011–2019. doi: 10.1093/jn/107.11.2011. [DOI] [PubMed] [Google Scholar]
- 305.Moon E.J., Lee Y.M., Lee O.H., Lee M.J., Lee S.K., Chung M.H., Kim K.W. A ncovel angiogenic factor derived from Aloe vera gel: β-sitosterol, a plant sterol. Angiogenesis. 1999;3:117–123. doi: 10.1023/a:1009058232389. [DOI] [PubMed] [Google Scholar]
- 306.Paniagua-Pérez R., Madrigal-Bujaidar E., Reyes-Cadena S., Molina-Jasso D., Gallaga J.P., Silva-Miranda A., Chamorro G. Genotoxic and cytotoxic studies of beta-sitosterol and pteropodine in mouse. Biomed. Res. Int. 2005;2005:242–247. doi: 10.1155/JBB.2005.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Villasenor I.M., Angelada J., Canlas A.P., Echegoyen D. Bioactivity studies on β-sitosterol and its glucoside. Phytother. Res. 2002;16:417–421. doi: 10.1002/ptr.910. [DOI] [PubMed] [Google Scholar]
- 308.Bouic P.J.D., Etsebeth S., Liebenberg R.W., Albrecht C.F., Pegel K., Van Jaarsveld P.P. Beta-sitosterol and beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: Implications for their use as an immunomodulatory vitamin combination. Int. J. Immunopharmacol. 1996;18:693–700. doi: 10.1016/s0192-0561(97)85551-8. [DOI] [PubMed] [Google Scholar]
- 309.Shi C., Wu F., Zhu X., Xu J. Incorporation of β-sitosterol into the membrane increases resistance to oxidative stress and lipid peroxidation via estrogen receptor-mediated PI3K/GSK3β signaling. BBA Gen. Subj. 2013;1830:2538–2544. doi: 10.1016/j.bbagen.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 310.Gabay O., Sanchez C., Salvat C., Chevy F., Breton M., Nourissat G., Wolf C., Jacques C., Berenbaum F. Stigmasterol: A phytosterol with potential anti-osteoarthritic properties. Osteoarthr. Cartil. 2010;18:106–116. doi: 10.1016/j.joca.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 311.Imamura T., Takagi H., Miyazato A., Ohki S., Mizukoshi H., Mori M. Isolation and characterization of the betalain biosynthesis gene involved in hypocotyl pigmentation of the allotetraploid Chenopodium quinoa. Biochem. Bioph. Res. Commun. 2018;496:280–286. doi: 10.1016/j.bbrc.2018.01.041. [DOI] [PubMed] [Google Scholar]
- 312.Kobayashi N., Schmidt J., Wray V., Schliemann W. Formation and occurrence of dopamine-derived betacyanins. Phytochemistry. 2001;56:429–436. doi: 10.1016/s0031-9422(00)00383-6. [DOI] [PubMed] [Google Scholar]
- 313.Dini I., Tenore G.C., Trimarco E., Dini A. Two novel betaine derivatives from Kancolla seeds (Chenopodiaceae) Food Chem. 2006;98:209–213. [Google Scholar]
- 314.Tramontano W.A., Jouve D. Trigonelline accumulation in salt-stressed legumes and the role of other osmoregulators as cell cycle control agents. Phytochemistry. 1997;44:1037–1040. [Google Scholar]
- 315.Jones G.P., Paleg L.G. In vitro thermal and salt stability of pyruvate kinase are increased by proline analogues and trigonelline. Funct. Plant. Biol. 1991;18:279–286. [Google Scholar]
- 316.Escribano J., Cabanes J., Jimenez-Atienzar M., Ibañez-Tremolada M., Gomez-Pando L.R., García-Carmona F., Gandía-Herrero F. Characterization of betalains, saponins and antioxidant power in differently colored quinoa (Chenopodium quinoa) varieties. Food Chem. 2017;234:285–294. doi: 10.1016/j.foodchem.2017.04.187. [DOI] [PubMed] [Google Scholar]
- 317.Esatbeyoglu T., Wagner A.E., Motafakkerazad R., Nakajima Y., Matsugo S., Rimbach G. Free radical scavenging and antioxidant activity of betanin: Electron spin resonance spectroscopy studies and studies in cultured cells. Food Chem. Toxicol. 2014;73:119–126. doi: 10.1016/j.fct.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 318.Cai Y., Sun M., Corke H. Antioxidant activity of betalains from plants of the Amaranthaceae. J. Agric. Food Chem. 2003;51:2288–2294. doi: 10.1021/jf030045u. [DOI] [PubMed] [Google Scholar]
- 319.Hirakawa N., Okauchi R., Miura Y., Yagasaki K. Anti-invasive activity of niacin and trigonelline against cancer cells. Biosci. Biotech. Biochem. 2005;69:653–658. doi: 10.1271/bbb.69.653. [DOI] [PubMed] [Google Scholar]
- 320.Shah S.N., Bodhankar S.L., Bhonde R., Mohan V. Hypoglycemic activity of the combination of active ingredients isolated from Trigonella foenumgraecum in alloxan induced diabetic mice. Pharmacologyonline. 2006;1:65–82. [Google Scholar]
- 321.Letelier M.E., Rodríguez-Rojas C., Sánchez-Jofré S., Aracena-Parks P. Surfactant and antioxidant properties of an extract from Chenopodium quinoa Willd seed coats. J. Cereal Sci. 2011;53:239–243. [Google Scholar]
- 322.Johnson I.T., Gee J.M., Price K., Curl C., Fenwick G.R. Influence of saponins on gut permeability and active nutrient transport in vitro. J. Nutr. 1986;116:2270–2277. doi: 10.1093/jn/116.11.2270. [DOI] [PubMed] [Google Scholar]
- 323.Gee J.M., Price K.R., Ridout C.L., Wortley G.M., Hurrell R.F., Johnson I.T. Saponins of quinoa (Chenopodium quinoa): Effects of processing on their abundance in quinoa products and their biological effects on intestinal mucosal tissue. J. Sci. Food Agric. 1993;63:201–209. [Google Scholar]
- 324.Sharma V., Chandra S., Dwivedi P., Parturkar M. Quinoa (Chenopodium quinoa Willd.): A nutritional healthy grain. Int. J. Adv. Res. 2015;3:725–736. [Google Scholar]
- 325.Risi J.C. The Chenopodium grains of the Andes: Inca crops for modern agriculture. Adv. Appl. Biol. 1984;10:145–216. [Google Scholar]
- 326.Jiang X., Hansen H.C.B., Strobel B.W., Cedergreen N. What is the aquatic toxicity of saponin-rich plant extracts used as biopesticides? Environ. Pollut. 2018;236:416–424. doi: 10.1016/j.envpol.2018.01.058. [DOI] [PubMed] [Google Scholar]
- 327.Juneja V.K., Dwivedi H.P., Yan X. Novel natural food antimicrobials. Annu. Rev. Food Sci. Technol. 2012;3:381–403. doi: 10.1146/annurev-food-022811-101241. [DOI] [PubMed] [Google Scholar]
- 328.Fiallos-Jurado J., Pollier J., Moses T., Arendt P., Barriga-Medina N., Morillo E., Arahana V., de Lourdes Torres M., Goossens A., Leon-Reyes A. Saponin determination, expression analysis and functional characterization of saponin biosynthetic genes in Chenopodium quinoa leaves. Plant Sci. 2016;250:188–197. doi: 10.1016/j.plantsci.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 329.Jarvis D.E., Ho Y.S., Lightfoot D.J., Schmockel S.M., Li B., Borm T.J.A., Ohyanagi H., Mineta K., Michell C.T., Saber N., et al. The genome of Chenopodium quinoa. Nature. 2017;542:307–312. doi: 10.1038/nature21370. [DOI] [PubMed] [Google Scholar]