Abstract
Chronic periodontitis (ChP) is a prevalent inflammatory disease affecting 46% of the US population. ChP produces a profound local inflammatory response to dysbiotic oral microbiota that leads to destruction of alveolar bone and tooth loss. ChP is also associated with systemic illnesses, including cardiovascular diseases, malignancies, and adverse pregnancy outcomes. However, the mechanisms underlying these adverse health outcomes are poorly understood. In this prospective cohort study, we used a highly multiplex mass cytometry immunoassay to perform an in-depth analysis of the systemic consequences of ChP in patients before (n = 28) and after (n = 16) periodontal treatment. A high-dimensional analysis of intracellular signaling networks revealed immune system–wide dysfunctions differentiating patients with ChP from healthy controls. Notably, we observed exaggerated proinflammatory responses to Porphyromonas gingivalis–derived lipopolysaccharide in circulating neutrophils and monocytes from patients with ChP. Simultaneously, natural killer cell responses to inflammatory cytokines were attenuated. Importantly, the immune alterations associated with ChP were no longer detectable 3 wk after periodontal treatment. Our findings demarcate systemic and cell-specific immune dysfunctions in patients with ChP, which can be temporarily reversed by the local treatment of ChP. Future studies in larger cohorts are needed to test the boundaries of generalizability of our results.
Keywords: mass cytometry, CyTOF, innate immunity, human, oral systemic disease, cell signaling
Introduction
Chronic periodontitis (ChP), an inflammatory disease that affects 46% of US adults (Eke et al. 2015), is initiated by dysbiotic bacteria that activate the host’s local immune response, thereby destroying connective tissue and bone, forming periodontal pockets, and eventually causing tooth loss. Accumulating epidemiologic evidence also supports a strong association between ChP and systemic diseases, including cardiovascular disease, diabetes, Alzheimer’s disease, malignancies, and adverse pregnancy outcomes (Bassani et al. 2007; Conde-Agudelo et al. 2008; Nabet et al. 2010; Ajita et al. 2013; Morishita et al. 2013; Nguyen et al. 2015; Peng et al. 2017). Demonstrating a mechanistic link between ChP and adverse health outcomes is critical for implementation of effective strategies to prevent ChP and its negative health consequences.
The standard treatment of mechanical removal of subgingival biofilm and calculus has been shown to reduce clinical attachment loss in the short term (Smiley et al. 2015). However, without frequent maintenance, ChP relapses, and disease progression continues, even with antimicrobial adjuncts (Becker et al. 1984). Novel therapeutic approaches are critically needed to prevent systemic progression of ChP. As such, therapeutic strategies targeting peripheral inflammatory mechanisms implicated in ChP’s systemic manifestations will enhance treatment. To this end, basic principles can be gained from other fields, such as cardiovascular or cancer immunotherapies, which have demonstrated the importance of patient selection based on individual molecular and cellular characteristics (Hamilton et al. 2017; Ridker, Everett, et al. 2017; Ridker, MacFadyen, et al. 2017). To identify targetable immune mechanisms, local and systemic functional immune characteristics need to be mapped comprehensively across variations in ChP severity, as well as across individuals with similar disease stages.
Current periodontal research has primarily focused on local inflammatory mechanisms driving the destruction of alveolar bone (Abe et al. 2012; Hajishengallis 2014). In contrast, the systemic immunologic manifestations of ChP remain poorly understood. Seminal studies have provided important insight into the effect of ChP on circulating inflammatory (Ling et al. 2015) and proresolving (Van Dyke and Serhan 2003; Serhan 2014) mediators and the distribution of select immune cell subsets (Wilensky et al. 2015). However, technological limitations have precluded the simultaneous assessment of phenotypic and functional attributes across many immune cell subsets in patients with ChP. Therefore, analyses reflecting the complex behaviors of immune cells occurring in a multicellular in vivo environment are warranted to capture the systemic immunologic imprint of ChP.
The recent advent of mass cytometry, a high-dimensional flow cytometry platform that allows quantification of >50 proteomic parameters on a single-cell basis (Bendall et al. 2011; Spitzer and Nolan 2016), offers unparalleled capacity for single-cell profiling of immune mechanisms associated with disease pathogenesis (Bendall et al. 2011; Gaudillière et al. 2014; Spitzer and Nolan 2016; Aghaeepour, Kin, et al. 2017; Aghaeepour et al. 2018; Good et al. 2018). Here, we employed a high-dimensional mass cytometry immunoassay and a machine learning method developed for analysis of multicellular immune signaling networks (Aghaeepour, Ganio, et al. 2017) to characterize major innate and adaptive immune responses in peripheral blood from patients with and without ChP. The goals were to determine whether the peripheral immunologic state of patients with ChP differs from that of healthy individuals, to identify the cellular components of an immunologic signature of ChP, and to determine whether standard nonsurgical periodontal treatment modifies the peripheral immunologic signature of ChP.
Materials and Methods
Study Design
This prospective study was conducted at Stanford University and the Bell Dental Center (San Leandro, CA) after approval by Stanford’s Institutional Review Board. Individuals aged >18 y with stage III-IV, grade B-C periodontitis (Tonetti et al. 2018) were eligible as patients. Exclusion criteria included immune-modulatory therapy, chemotherapy, radiation therapy, metastatic disease, active extraoral infection, antibiotics, metabolic disease, significant organ dysfunction, substance abuse, and smoking within the past 6 mo. Healthy adult individuals without ChP (clinical attachment loss <1 mm, no bleeding on probing) were eligible as controls. All participants signed written informed consent. Complete periodontal examination was performed (pocket depths at 6 sites per tooth with a Marquis color-coded probe, recession, bleeding on probing, mobility). Hard tissue examination and radiographs were also performed, with caries, periapical abscesses, and radiographic calculus recorded. This study conformed with STROBE guidelines for human observational studies.
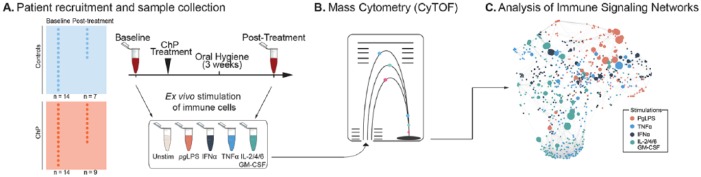
Sixteen participants were available for a return visit, including 7 controls and 9 patients with ChP. These patients underwent full-mouth disinfection treatment (Fang et al. 2016) consisting of scaling and root planing for patients with ChP or prophylactic cleaning for controls, followed by directed home oral hygiene, including 0.12% chlorhexidine mouth rinse twice daily for both study arms. A second blood draw for the treatment cohort was performed at 3 wk following this intervention (Fig. 1A), as well as qualitative periodontal examination and interview to determine compliance with the home hygiene regimen. In all cases, there was marked qualitative improvement of the periodontal condition and compliance with home hygiene.
Figure 1.
Experimental workflow and analytic approach. (A) Peripheral blood samples from 14 patients and 14 matched healthy controls were collected at baseline, with a subset of 16 patients (9 patients and 7 controls) undergoing standard nonsurgical treatment for chronic periodontitis (ChP) and an additional blood draw 3 wk after treatment. (B) Whole blood samples were either unstimulated or stimulated with receptor-specific ligands, including lipopolysaccharide isolated from Porphyromonas gingivalis (pgLPS), interferon α (IFN-α), tumor necrosis factor α (TNF-α), and a cytokine cocktail consisting of interleukin 2 (IL-2), IL-4, and IL-6, and granulocyte-macrophage colony-stimulating factor (GM-CSF). Samples were processed for mass cytometry (CyTOF) analysis. (C) A cell signaling elastic net analysis of the mass cytometry data set formed a correlation network of immune features highlighting coordinated intracellular signaling responses associated with ChP. The nodes are colored according to the stimulation condition as indicated.
Sample Collection and Mass Cytometry Analysis
Whole-blood samples collected at baseline (n = 28) and 3 wk after treatment (n = 16) were left unstimulated or stimulated with Porphyromonas gingivalis lipopolysaccharide (PgLPS), interferon α (IFNα), tumor necrosis factor α (TNFα), or a cytokine cocktail containing interleukins 2/4/6 (IL-2/4/6) and granulocyte-macrophage colony-stimulating factor (GM-CSF; Fig. 1A). Stimulation conditions were chosen to evaluate the integrity of intracellular signaling pathways activated downstream of key inflammatory mediators implicated in the pathogenesis of ChP (Appendix Methods). Samples were processed for mass cytometry with standardized protocols (Appendix Methods). The mass cytometry data were manually gated into 18 immune cell subsets representing major innate and adaptive compartments (Appendix Table 2).
Three categories of immune features were obtained:
Cell frequency: Cell counts were expressed as a percentage of leukocytes for neutrophils (Nφ’s) and mononuclear cells for other cell types.
Endogenous signaling immune features: Endogenous intracellular signaling activities—arcsinh transform of the signal for phospho (p)P38, pMAPKAPK2, prpS6, pNF-κB, pCREB, pERK1/2, pSTAT1, pSTAT3, pSTAT5, pSTAT6, and total IκB—reflective of canonical signaling pathways implicated in ChP were quantified with unstimulated samples.
Evoked intracellular signaling features: Signaling responses (arcsinh ratio of the intracellular signal intensity between the stimulated and unstimulated conditions) were analyzed in samples stimulated with PgLPS, IFNα, TNFα, or IL-2/4/6 with GM-CSF. The transformed ratios were z scored for further analysis.
Graphical Representation
Pairwise Spearman rank correlation analysis was performed on all immune features. Edges in the correlation network graphs represent a significant correlation (P < 5.0E-5) between 2 immune features. The layout was constructed with the t-SNE algorithm (Maaten and Hinton 2008).
Statistical Analysis
Statistical analysis of the mass cytometry data was performed with the cell signaling elastic net (csEN) method (Aghaeepour, Ganio, et al. 2017), a recent adaptation of the penalized linear regression elastic net (Zou and Hastie 2005), which considers previous biological knowledge of cell type– and receptor-specific signaling pathway activation. csEN models were optimized and trained via cross-validation, and a repeat hold-out approach was used to test for statistical significance. To aid the biological interpretation of the csEN model, individual model components were ranked according to the strength of the association with ChP (–log[P value], Wilcoxon rank sum test; details inAppendix Methods).
Results
Single-Cell Profiling of Peripheral Immune Status in Patients with ChP
Thirty participants were enrolled in the study, including 15 with ChP receiving treatment and 15 sex- and age-matched healthy volunteers. One patient was excluded due to onset of treatment for autoimmune disease, and 1 control was excluded due to a hand infection. Demographics and comorbidities for all participants and the periodontal status of patients with ChP are listed in the Table. Nine patients and 7 controls were available to follow up 3 wk after scaling and root planing or prophylaxis as appropriate (Fig. 1A).
Table.
Demographic and Clinical Data.
| Patients with ChP (n = 14) | Healthy Controls (n = 14) | P Value | |
|---|---|---|---|
| Age, y, median (range) | 40.5 (29 to 61) | 36.5 (26 to 57) | 0.12a |
| Sex, n | >0.99b | ||
| Male | 6 | 6 | |
| Female | 8 | 8 | |
| Race/ethnicity, n (%) | 0.072b | ||
| African American | 2 (14) | 2 (14) | |
| Asian | 3 (21) | 6 (43) | |
| Caucasian | 0 (0) | 3 (21) | |
| Latino | 9 (64) | 3 (21) | |
| Body mass index, mean (range) | 28.9 (19 to 40) | 24.6 (19 to 31) | 0.07a |
| Comorbidities, n | |||
| Anemia | 1 | 0 | |
| Hypertension | 1 | 0 | |
| Morphea | 0 | 1 | |
| Thyroid disease | 1 | 0 | |
| Periodontal classification, n | |||
| Stage III | 10 | ||
| Stage IV | 4 | ||
| Deepest periodontal pocket, mm, mean (SD) | 7.60 (1.12) | ||
| Largest clinical attachment loss, mm, mean (SD) | 8.42 (1.73) | ||
| No. of pockets ≥5 mm, median (range) | 36 (7 to 84) | ||
| No. of teeth with furcation involvement, median (range) | 0 (0 to 2) | ||
| No. of sites with radiographic calculus, median (range) | 8 (2 to 27) |
ChP, chronic periodontitis.
T test.
Chi-square.
Analysis of Immune Signaling Networks Identifies a Peripheral Immune Signature of ChP
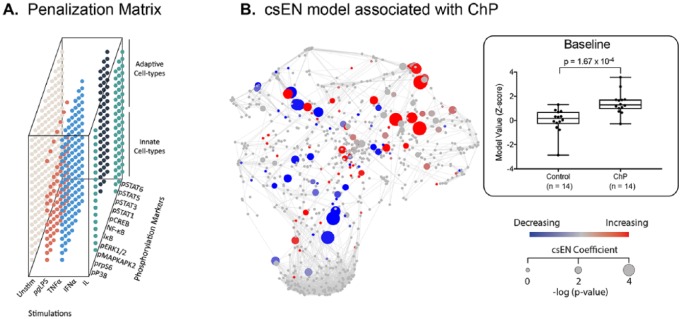
The high-dimensional mass cytometry data set formed a correlation network emphasizing the interconnectivity of measured immune features (Fig. 1B, C). The highly intercorrelated nature of the data justified the use of the csEN regularized regression algorithm (Aghaeepour, Ganio, et al. 2017). The csEN analysis identified a statistically stringent linear model that differentiated patients with ChP from controls according to their evoked signaling response to PgLPS, IFNα, TNFα, or IL-2/4/6 with GM-CSF stimulation (cross-validation P value = 1.673E-4; Fig. 2). Coefficients of the csEN model were visualized on the correlation network (Fig. 2B). Individual features selected by the model are listed in Appendix Table 3. Interestingly, no differences in immune cell frequencies were detected between patients with ChP and controls, suggesting that the functional attributes are more informative than the assessment of cell distribution in the immunologic profile of patients with ChP.
Figure 2.
A cell signaling–based elastic net (csEN) analysis identifies systemic immune signaling features associated with chronic periodontitis (ChP). (A) We adapted a previously implemented cell signaling–based penalization matrix for the csEN analysis that accounted for whether a cell type– and receptor-specific signaling response to each stimulation condition is supported by prior knowledge of signal transduction pathways. (B) (Left panel) The csEN identified immune signaling features that differentiate samples from patients with ChP and those from controls at baseline. Each node is colored by model coefficient. Red and blue indicate features elevated and decreased in samples from patients at baseline, respectively. Node size is proportional to the P value of the difference between patient and control samples (Wilcoxon rank sum test). (Right panel) For each of the 28 patients, a unique model value from the csEN is represented as a z score in the box plot showing that the model significantly differentiates the patients with ChP (n = 14) from the controls (n = 14). Values are presented as median, interquartile range, and range.
ChP Is Associated with Profound and Cell-Specific Peripheral Immune Dysfunctions
To further evaluate the biological implications of the results, features included in the csEN model were examined individually. Model components were ranked according to the strength of their association with ChP (–log[P value], Wilcoxon rank sum test). Examination of the model components that differed the most between patients with ChP and controls pointed at specific biological mechanisms differentially regulated in ChP, which provided the basis for the biological interpretation of the statistical model. Of the 37 csEN features that differed between patients with ChP and controls, the majority were signaling responses to the cytokine cocktail (40%) and PgLPS stimulations (35%). Innate immune cell signaling responses accounted for 76% of the features, while 34% were signaling responses in adaptive cells (Appendix Table 3).
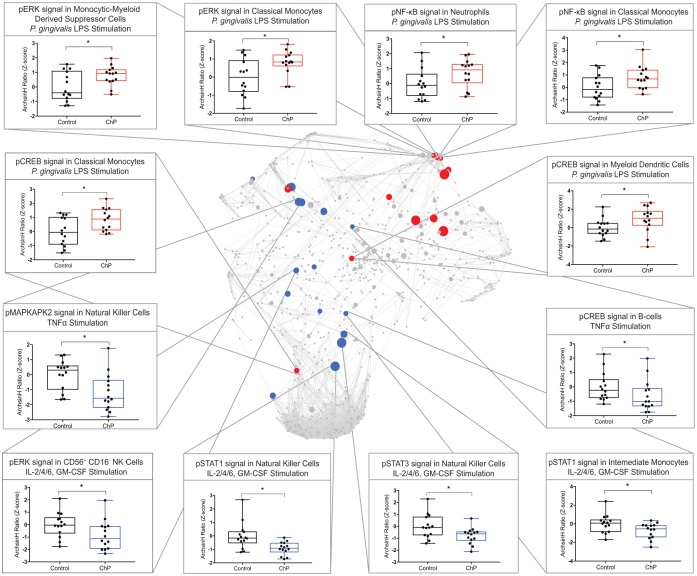
Seventeen model features were canonical elements of known signaling pathways activated in response to the specific stimulation (Appendix Table 4), while 20 features were noncanonical signaling responses. Six canonical csEN model features were signaling responses to PgLPS in various innate immune cell subsets and included pCREB, pNF-κB, and pERK signaling responses in classical (c)MCs, pNF-κB signaling response in Nφs, pCREB signaling response in myeloid dendritic cells, and pERK signaling response in monocytic myeloid-derived suppressor cells. These signaling responses to PgLPS were all increased in patients with ChP as compared with controls, suggesting that ChP is associated with heightened TLR-2/4 signaling in peripheral innate immune cells.
In contrast to PgLPS signaling responses, csEN model features representing signaling responses to TNFa, IFNa, or IL-2/4/6 and GM-CSF stimulations were suppressed in patients with ChP as compared with controls (Fig. 3). These csEN features included the pMAPKAPK2 signaling response to TNFa and the pSTAT1, pSTAT3, and pERK signaling response to IL-2/4/6 and GM-CSF in NK cells subsets; the pSTAT1 signaling response to IL-2/4/6 and GM-CSF in intermediate (int)MCs; and the pCREB signaling response to TNFa in B cells, representing broad immunosuppression.
Figure 3.
The cell signaling–based elastic net (csEN) model reveals systemic immune alterations associated with chronic periodontitis (ChP). (Center) Baseline immune network with the 17 canonical features selected by the csEN model, labeled on the correlation network, that differ between patients with ChP and controls. (Periphery) Box plots depicting 12 canonical csEN model components (arcsinh ratio of the stimulated samples as compared with unstimulated samples, z scored). Values are presented as median, interquartile range, and range. Additional model components are listed in Appendix Table 3. GM-CSF, granulocyte-macrophage colony-stimulating factor; LPS, lipopolysaccharide; TNFα, tumor necrosis factor α.
These results suggest that the capacity of specific immune cell subsets to respond to an inflammatory challenge is altered in peripheral blood samples from patients with ChP. The most prominent differences between the immune features of the 2 study arms were observed in innate immune cells and included heightened TLR-2/4 responses to PgLPS and depressed signaling responses to TNFa, IFNa, and IL-2/4/6 and GM-CSF. Features identified by the csEN model were not significantly associated with the sex (independent samples t test), race (1-way analysis of variance), body mass index, or age (Pearson’s correlation; Bonferroni-corrected a = 0.0014) of the patient.
To determine whether observed differences in immune responses between patients with ChP and controls resulted from the local dysbiosis of the oral cavity, rather than from variables that could not be captured in the study design, immune responses were again examined 3 wk after nonsurgical ChP treatment (n = 9) or prophylaxis (n = 7).
Immune Signature Associated with ChP Resolves after ChP Treatment
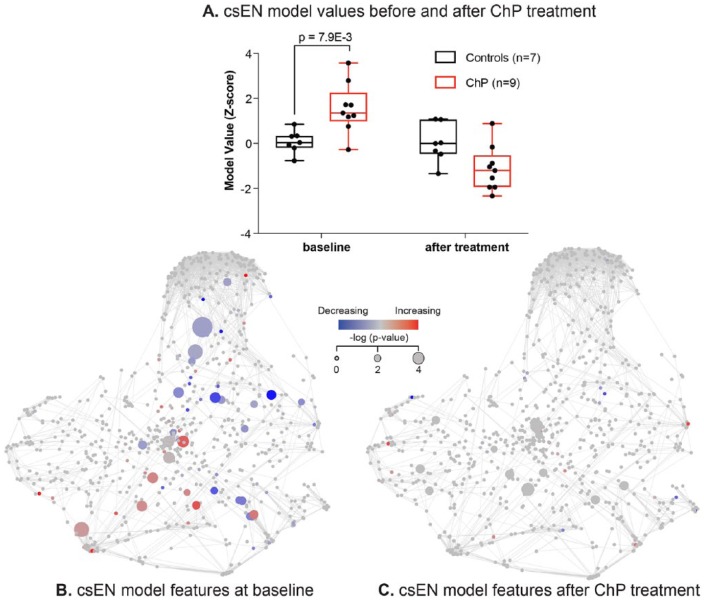
The csEN model trained on the immunologic data set derived from 28 patients at baseline was applied to the data set obtained from the analysis of blood samples collected from the 16 patients who underwent ChP treatment. This analysis provided a predicted value per study participant before and after ChP treatment. The data showed that the csEN predictions significantly separated the cohort of patients with ChP from the controls (P = 7.9E-3; Fig. 4A, B) at baseline but not after ChP treatment (Fig. 4C). These results suggest that the systemic immune signature of ChP identified in this study is driven, at least in part, by local pathologic processes that are sensitive to disinfection of the oral cavity.
Figure 4.
Chronic periodontitis (ChP) treatment reverses aspects of the systemic immune manifestations of ChP. (A) Box plot depicting the cell signaling–based elastic net (csEN) model values in samples from patients with ChP and controls in the treatment cohort, before and after ChP treatment. The csEN values are increased in samples from patients with ChP before treatment as compared with controls (9 patients, 7 controls, Wilcoxon rank sum test P = 7.9E-3) but not after treatment. Values are presented as median, interquartile range, and range. (B, C) csEN model component values overlaid on the immune signaling network for the patients who underwent scaling and root planing (n = 9) or prophylactic cleaning (n = 7). Nodes are colored by the median difference in signaling activity (arcsinh transform) between patients with ChP and controls. The size of the nodes varies according to the P value (Wilcoxon rank sum test, 1-tailed). (B) Before treatment. (C) After treatment.
Discussion
This study employed a multiplex mass cytometry assay to provide an in-depth survey of the systemic immunologic manifestations of ChP in patients, before and 3 wk after periodontal treatment. High-dimensional analysis of immune signaling networks identified a statistically stringent model of peripheral immune responses that accurately classified patients’ periodontal status. These findings demarcate a peripheral immune signature that specifies the systemic inflammatory state of patients with ChP.
The systemic consequences of ChP rely on the integration of numerous interconnected immunologic events. The ability to simultaneously quantify these immunologic events with single-cell resolution has been possible with only the recent development of high-parameter, single-cell technologies such as mass cytometry (Bendall et al. 2011). However, the large immunologic data set derived from the mass cytometry analysis of clinical samples presents several computational challenges (James et al. 2013). First, the number of measured immune features often greatly outnumbers the sample size, which leads to false-positive discoveries (Tibshirani 1996; Newell and Cheng 2016). Second, the nature of mass cytometry data is highly correlated, reflecting the coordinated interactions between immune cells (Zou and Hastie 2005). Both of these challenges are addressed with the csEN analysis, which was recently shown to outperform other common machine learning methods in the analysis of mass cytometry data sets (Aghaeepour, Ganio, et al. 2017).
The longitudinal profile of peripheral immune responses before and after ChP treatment revealed the remarkable plasticity of the peripheral immune system in patients with ChP. Globally, the integrated output of all measured immune responses captured by the csEN analysis strongly differentiated patients with ChP from controls at baseline. However, differences in csEN model values between controls and patients were markedly decreased 3 wk after ChP treatment. These results suggest that the immunologic differences observed before treatment are attributable to ChP rather than to unaccounted clinical or demographic variables. The findings also point to the ability of the peripheral immune system of patients with ChP to evolve from a dysfunctional to a relatively normal state after a 3-wk intervention targeting the local manifestations of ChP (Smiley et al. 2015). Interestingly, there was a trend toward a relative decrease in csEN model predictions for patients with ChP as compared with controls after treatment. This may reflect the fact that the patient’s immune system has not fully adjusted 3 wk after treatment. As such, the 3-wk posttreatment time point provides only a snapshot of the patient’s immune system adjustment after treatment.
In addition, high interpatient variability existed among immune responses. This variability may reflect patient-specific immunologic states underlying individual differences in periodontal pathogen communities or varying degrees of periodontitis. These findings are reminiscent of prior omics characterizations of systemic inflammatory states, such as aging and cancer, showing genetic, metabolic, and immune variability within a predefined clinical class that predicts adverse clinical outcomes or response to therapy (Gaudillière et al. 2014; Aghaeepour, Kin, et al. 2017; Good et al. 2018).
The most informative features of the ChP immune signature included increased elements of the TLR-2/4 signaling responses to PgLPS, 1 of 4 major virulence factors of Pg (Johansson and Dahlen 2018), in multiple innate immune cell types. These results resonate with previous reports showing an exacerbation of Nϕ activation in local gingival tissues and in peripheral blood from patients with ChP (Hajishengallis 2015). For example, Ling et al. (2015) observed an increase in proinflammatory Pg-related cytokines IL-8 and IL-1β from peripheral Nϕs in patients with ChP as compared with controls. Furthermore, in a bovine study, TLR-2/4 gene expression was upregulated in periodontal tissues of animals with periodontitis (Borsanelli et al. 2018). The increased signaling responses to PgLPS suggests a priming effect of ChP on peripheral innate immune cells, perhaps through upregulation of the TLR-2/4 receptors. Whether the priming of peripheral innate immune cells to subsequent PgLPS exposure results from a chronic exposure to circulating periodontal pathogens or indirectly from the chronic release of proinflammatory cytokines, such as TNFα and IL-1β, from periodontal tissue remains to be determined.
Other findings are unexpected. For example, in contrast to enhanced innate responses to PgLPS, a dampening of signaling responses to GM-CSF and proinflammatory cytokines TNFα and IL-2/4/6 was observed in NK cells, intMCs, and B cells. Interestingly, the decreased pMAPKAPK2 seen in the CD56loCD16+ NK cell subset was related to the severity of ChP, with patients with stage IV ChP having a greater degree of suppression than those with stage III ChP (Appendix Table 5). Previous studies of NK cells have predominantly focused on local mechanisms and suggest that NK cell activity promotes periodontal destruction. In mice, periodontal pathogens can activate NK cells to secrete IFNγ and TNFa, leading to fibroblast and osteoclast stimulation and subsequent degradation of extracellular matrix and alveolar bone (Wilensky et al. 2015). These studies also point to the expansion of NK cells in the local periodontal tissues (Górska et al. 2003). Our findings of decreased peripheral NK cell function in patients with ChP suggest that circulating NK cell responses may not reflect local NK cell function. In contrast, the relative NK cell anergy observed in the peripheral blood of patients with ChP may be implicated in the pathogenesis of systemic illnesses that are linked to periodontitis, such as systemic infections (endocarditis, pneumonia) and malignancies (Lorini et al. 1994).
This study has certain limitations. The small sample size of patients recruited from a single clinic may not represent the entire population or the entire spectrum of the disease, limiting the generalizability of the results. Larger studies from multiple sites will be required for validation of these findings. As all comorbidities and potential confounders cannot be eliminated—such as clinical or demographic variables potentially associated with ChP that were not captured in the study design, including nutrition, socioeconomic status, or undiagnosed illness—a study linking local pathology from gingival tissue and the peripheral immune response will be critical to better understand systemic dysfunction. In addition, as the periodontal status tends to relapse without frequent oral maintenance therapy, we would expect the immune signature of ChP to recur over a period of several weeks to months, which is not captured in the present study, with only a 3-wk follow-up. This expected relapse of the peripheral immune dysfunction justifies the development of adjunct therapies that complement standard periodontal treatment. While the mass cytometry assay allows measurement of >40 parameters per cell, the list of phenotypic markers and signaling responses tested is not exhaustive, nor is the stimulation panel. Similarly, the analysis was performed on manually gated populations to allow application of the csEN method. As such, we cannot exclude that additional features would have been detected with an unbiased clustering approach. However, the approach provides the analytic framework to expand the immunoassay to allow assessment of all immune cell dysfunctions associated with ChP and provide the basis for future multiomics research (Ghaemi et al. 2018) on ChP.
The data-driven development of cell- and pathway-specific immune therapies represents a paradigm shift in the treatment of ChP and the prevention of associated systemic illnesses (Tonetti et al. 2011). As an essential step toward this goal, our study revealed systemic immune cell dysfunctions associated with ChP that were reversible after treatment, highlighting a peripheral immune signature of ChP. These findings and analytic approach provide the framework for future efforts toward testing the boundaries of generalizability of our results and identifying modifiable targets for the development of novel therapies to prevent the systemic consequences of ChP.
Author Contributions
D.K. Gaudilliere, contributed to conception, design, and data analysis, drafted the manuscript; A. Culos, K. Djebali, A.S. Tsai, A. Maghaireh, contributed to data analysis, drafted the manuscript; E.A. Ganio, X. Han, B. Choisy, Q. Baca, J.F. Einhaus, J.J. Hedou, B. Bertrand, K. Ando, R. Fallahzadeh, M.S. Ghaemi, R. Okada, N. Stanley, A. Tanada, T. Alpagot, J.A. Helms, contributed to data analysis, critically revised the manuscript; W.M. Choi, M. Tingle, M.S. Angst, contributed to conception and design, critically revised the manuscript; N. Aghaeepour, contributed to conception, design, data analysis, critically revised the manuscript; B. Gaudilliere, contributed to conception, design, data analysis, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034519857714 for Systemic Immunologic Consequences of Chronic Periodontitis by D.K. Gaudilliere, A. Culos, K. Djebali, A.S. Tsai, E.A. Ganio, W.M. Choi, X. Han, A. Maghaireh, B. Choisy, Q. Baca, J.F. Einhaus, J.J. Hedou, B. Bertrand, K. Ando, R. Fallahzadeh, M.S. Ghaemi, R. Okada, N. Stanley, A. Tanada, M. Tingle, T. Alpagot, J.A. Helms, M.S. Angst, N. Aghaeepour and B. Gaudilliere in Journal of Dental Research
Acknowledgments
We acknowledge Robert Tibshirani, PhD, for his expertise and advice with our statistical models. We thank the dentists at the Bell Dental Center, San Leandro, California (Dr. Andrew Doan, Dr. Ina Kim, Dr. Richard Lai, Dr. Bao Chau Nguyen, Dr. Chris Park, and Dr. Ngan Tran), for dedication and efforts in patient recruitment and treatment.
Footnotes
A supplemental appendix to this article is available online.
This study was supported by the National Institutes of Health / National Institute of Dental and Craniofacial Research (R21 DE027728-01). Additional funding was provided by the Department of Anesthesiology, Perioperative and Pain Medicine at Stanford University. B.G. was supported by the National Institutes of Health (1K23GM111657-03). N.A. was supported by an Ann Schreiber Mentored Investigator Award from the Ovarian Cancer Research Fund (292495), a Canadian Institute of Health Research Postdoctoral Fellowship (321510), and an International Society for Advancement of Cytometry Scholarship.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Data and Materials Availability: Raw data, processed data, and source code for reproduction of the results are publicly available at https://flowrepository.org/id/FR-FCM-ZYT6.
ORCID iDs: R. Okada  https://orcid.org/0000-0002-4361-0146
https://orcid.org/0000-0002-4361-0146
J.A. Helms  https://orcid.org/0000-0002-0463-396X
https://orcid.org/0000-0002-0463-396X
References
- Abe T, Hosur KB, Hajishengallis E, Reis ES, Ricklin D, Lambris JD, Hajishengallis G. 2012. Local complement-targeted intervention in periodontitis: proof-of-concept using a C5a receptor (CD88) antagonist. J Immunol. 189(11):5442–5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghaeepour N, Ganio EA, McIlwain D, Tsai AS, Tingle M, Van Gassen S, Gaudilliere DK, Baca Q, McNeil L, Okada R, et al. 2017. An immune clock of human pregnancy. Sci Immunol. 2(15):eaan2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghaeepour N, Kin C, Ganio EA, Jensen KP, Gaudilliere DK, Tingle M, Tsai A, Lancero HL, Choisy B, McNeil LS, et al. 2017. Deep immune profiling of an arginine-enriched nutritional intervention in patients undergoing surgery. J Immunol. 199(6):2171–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghaeepour N, Simonds EF, Knapp DJHF, Bruggner RV, Sachs K, Culos A, Gherardini PF, Samusik N, Fragiadakis GK, Bendall SC, et al. 2018. Gatefinder: projection-based gating strategy optimization for flow and mass cytometry. Bioinformatics. 34(23):4131–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajita M, Karan P, Vivek G, S MA, Anuj M. 2013. Periodontal disease and type 1 diabetes mellitus: associations with glycemic control and complications: an indian perspective. Diabetes Metab Syndr. 7(2):61–63. [DOI] [PubMed] [Google Scholar]
- Bassani DG, Olinto MT, Kreiger N. 2007. Periodontal disease and perinatal outcomes: a case-control study. J Clin Periodontol. 34(1):31–39. [DOI] [PubMed] [Google Scholar]
- Becker W, Becker BE, Berg LE. 1984. Periodontal treatment without maintenance: a retrospective study in 44 patients. J Periodontol. 55(9):505–509. [DOI] [PubMed] [Google Scholar]
- Bendall SC, Simonds EF, Qiu P, Amir E-aD, Krutzik PO, Finck R, Bruggner RV, Melamed R, Trejo A, Ornatsky OI, et al. 2011. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 332(6030):687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsanelli AC, Lappin DF, Viora L, King G, Bennett D, Dutra IS, Riggio MP. 2018. Evaluation of tissue levels of toll-like receptors and cytokine mrnas associated with bovine periodontitis and oral health. Res Vet Sci. 118:439–443. [DOI] [PubMed] [Google Scholar]
- Conde-Agudelo A, Villar J, Lindheimer M. 2008. Maternal infection and risk of preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 198(1):7–22. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, Taylor GW, Page RC, Beck JD, Genco RJ. 2015. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. 86(5):611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Han M, Li QL, Cao CY, Xia R, Zhang ZH. 2016. Comparison of full-mouth disinfection and quadrant-wise scaling in the treatment of adult chronic periodontitis: a systematic review and meta-analysis. J Periodont Res. 51(4):417–430. [DOI] [PubMed] [Google Scholar]
- Gaudillière B, Fragiadakis GK, Bruggner RV, Nicolau M, Finck R, Tingle M, Silva J, Ganio EA, Yeh CG, Maloney WJ, et al. 2014. Clinical recovery from surgery correlates with single-cell immune signatures. Sci Transl Med. 6(255):255ra131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaemi MS, DiGiulio DB, Contrepois K, Callahan B, Ngo TTM, Lee-McMullen B, Lehallier B, Robaczewska A, McIlwain D, Rosenberg-Hasson Y, et al. 2018. Multiomics modeling of the immunome, transcriptome, microbiome, proteome, and metabolome adaptations during human pregnancy. Bioinformatics. 35(1):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good Z, Sarno J, Jager A, Samusik N, Aghaeepour N, Simonds EF, White L, Lacayo NJ, Fantl WJ, Fazio G, et al. 2018. Single-cell developmental classification of B cell precursor acute lymphoblastic leukemia at diagnosis reveals predictors of relapse. Nat Med. 24(4):474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Górska R, Gregorek H, Kowalski J, Laskus-Perendyk A, Syczewska M, Madaliński K. 2003. Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J Clin Periodontol. 30(12):1046–1052. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G. 2014. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 35(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G. 2015. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 15(1):30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JA, Hasturk H, Kantarci A, Serhan CN, Van Dyke T. 2017. Atherosclerosis, periodontal disease, and treatment with resolvins. Curr Atheroscler Rep. 19(12):57. [DOI] [PubMed] [Google Scholar]
- James G, Witten D, Hastie T, Tibshirani R. 2013. An introduction to statistical learning: with applications in R. New York (NY): Springer. [Google Scholar]
- Johansson A, Dahlen G. 2018. Bacterial virulence factors that contribute to periodontal pathogenesis. In: Bostanci N, Belibasakis G, editors. Pathogenesis of periodontal diseases. Cham (Switzerland): Springer; p. 31–49. [Google Scholar]
- Ling MR, Chapple IL, Matthews JB. 2015. Peripheral blood neutrophil cytokine hyper-reactivity in chronic periodontitis. Innate Immun. 21(7):714–725. [DOI] [PubMed] [Google Scholar]
- Lorini R, Moretta A, Valtorta A, d’Annunzio G, Cortona L, Vitali L, Bozzola M, Severi F. 1994. Cytotoxic activity in children with insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 23(1):37–42. [DOI] [PubMed] [Google Scholar]
- Maaten L, Hinton G. 2008. Visualizing data using t-SNE. J Mach Learn Res. 9:2579–2605. [Google Scholar]
- Morishita M, Ariyoshi W, Okinaga T, Usui M, Nakashima K, Nishihara T. 2013. A. actinomycetemcomitans LPS enhances foam cell formation induced by LDL. J Dent Res. 92(3):241–246. [DOI] [PubMed] [Google Scholar]
- Nabet C, Lelong N, Colombier ML, Sixou M, Musset AM, Goffinet F, Kaminski M, Epipap G. 2010. Maternal periodontitis and the causes of preterm birth: the case-control Epipap study. J Clin Periodontol. 37(1):37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell EW, Cheng Y. 2016. Mass cytometry: blessed with the curse of dimensionality. Nat Immunol. 17(8):890–895. [DOI] [PubMed] [Google Scholar]
- Nguyen CM, Kim JWM, Quan VH, Nguyen BH, Tran SD. 2015. Periodontal associations in cardiovascular diseases: the latest evidence and understanding. J Oral Biol Craniofac Res. 5(3):203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng CH, Yang YS, Chan KC, Kornelius E, Chiou JY, Huang CN. 2017. Periodontal treatment and the risks of cardiovascular disease in patients with type 2 diabetes: a retrospective cohort study. Intern Med. 56(9):1015–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. 2017. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 377(12):1119–1131. [DOI] [PubMed] [Google Scholar]
- Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, Group CT. 2017. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 390(10105):1833–1842. [DOI] [PubMed] [Google Scholar]
- Serhan CN. 2014. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 510(7503):92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley CJ, Tracy SL, Abt E, Michalowicz BS, John MT, Gunsolley J, Cobb CM, Rossmann J, Harrel SK, Forrest JL, et al. 2015. Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc. 146(7):508–524.e505. [DOI] [PubMed] [Google Scholar]
- Spitzer MH, Nolan GP. 2016. Mass cytometry: single cells, many features. Cell. 165(4):780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R. 1996. Regression shrinkage and selection via the lasso. J Royal Stat Soc Series B. 58:267–288. [Google Scholar]
- Tonetti MS, Chapple IL; Working Group 3 of Seventh European Workshop on Periodontology. 2011. Biological approaches to the development of novel periodontal therapies—consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 38 Suppl 11:114–118. [DOI] [PubMed] [Google Scholar]
- Tonetti MS, Greenwell H, Kornman KS. 2018. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Clin Periodontol. 45 Suppl 20:S149–S161. [DOI] [PubMed] [Google Scholar]
- Van Dyke TE, Serhan CN. 2003. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 82(2):82–90. [DOI] [PubMed] [Google Scholar]
- Wilensky A, Chaushu S, Shapira L. 2015. The role of natural killer cells in periodontitis. Periodontol 2000. 69(1):128–141. [DOI] [PubMed] [Google Scholar]
- Zou H, Hastie T. 2005. Regularization and variable selection via the elastic net. J Royal Stat Soc Series B. 67(2):301–320. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034519857714 for Systemic Immunologic Consequences of Chronic Periodontitis by D.K. Gaudilliere, A. Culos, K. Djebali, A.S. Tsai, E.A. Ganio, W.M. Choi, X. Han, A. Maghaireh, B. Choisy, Q. Baca, J.F. Einhaus, J.J. Hedou, B. Bertrand, K. Ando, R. Fallahzadeh, M.S. Ghaemi, R. Okada, N. Stanley, A. Tanada, M. Tingle, T. Alpagot, J.A. Helms, M.S. Angst, N. Aghaeepour and B. Gaudilliere in Journal of Dental Research