Abstract
Gastrodia elata Blume (G. elata) is a valuable Traditional Chinese Medicine (TCM) with a wide range of clinical applications. G. elata polysaccharides, as one of the main active ingredients of G. elata, have interesting extraction, purification, qualitative analysis, quantitative analysis, derivatization, and pharmacological activity aspects, yet a review of G. elata polysaccharides has not yet been published. Based on this, this article summarizes the progress of G. elata polysaccharides in terms of the above aspects to provide a basis for their further research and development.
Keywords: Gastrodia elata Bl (G. elata), polysaccharides, phytochemistry, pharmacological action
1. Introduction
Gastrodia elata Blume (G. elata) is a precious traditional Chinese herbal medicine which was initially recorded in Shen Nong’s Herbal Classic about two thousand years ago. In the clinic, G. elata is used widely for the treatment of headaches, epilepsy, dizziness, rheumatism, neuralgia, cramps, high blood pressure, and other neurological diseases [1]. In the search for the active ingredients of G. elata, a series of small molecule compounds were found, including gastrodin, parishin, phenolic compounds, 4-hydroxybenzyl alcohol, and β-sitosterol [2,3,4,5,6]. Some of these small molecule compounds showed activity against headaches, high blood pressure [7], epilepsy, and other neurological diseases [2,5,6]. In 1981, Liu reported G. elata polysaccharides as another important active ingredient [8]. Since then, G. elata polysaccharides have received wide attention throughout the world, and a series of pharmacological activities were reported, such as anticancer [9], antivirus [10], antiosteoporosis [11], antioxidant [12], immunomodulatory [13], and neuroprotective effects [14], as well as a great effect on the cardiovascular system [15]. These results clearly indicate that G. elata polysaccharides play a key role in G. elata’s pharmacological activities.
Due to the importance of G. elata polysaccharides, it is necessary and urgent to summarize their actions. Up to now, studies on G. elata polysaccharides have focused on their extraction, purification, qualitative/quantitative analysis, derivatization, and pharmacological activity. However, no previous articles have synthetically summarized the research papers on G. elata polysaccharides. In this article, we summarize and review all the mentioned aspects of G. elata polysaccharides. The purpose of this article is to contribute to further and deeper research on the extraction, analysis technologies, quality control, bioactivities, and derivatization of G. elata polysaccharides.
2. Acquisition of G. elata Polysaccharides
2.1. Extraction of G. elata Polysaccharides
Phytomedicines contain fat-soluble ingredients which may interfere with the extraction of polysaccharides. Therefore, before the extraction of polysaccharides degreasing using organic solvents, including chloroform–ethanol [16,17], ether [17], petroleum ether [18,19], methanol [20], and different concentrations of ethanol [20,21] is required to remove some interfering components [22]. G. elata can be degreased with different concentrations of ethanol, such as 75% ethanol [23], 80% ethanol [24], 85% ethanol [25,26], and 95% ethanol [10,27]. Chen et al. obtained crude G. elata polysaccharide (RGP) in a yield of 6.11% after degreasing with 75% ethanol [23]. Lee et al. obtained 2.47 g of crude G. elata polysaccharide degreased with 80% ethanol [24]. Ming et al. found that the yield of G. elata polysaccharide (PGEB-3H) degreased with 85% ethanol was 0.797 g/kg [25]. Chen et al. found that the yield of crude G. elata polysaccharide (TM) degreased with 95% ethanol was 5.2% [27]. The relationship between the yield and degreasing concentration is vague and may be related to other extraction factors, but these results show that ethanol solution is a suitable solvent for the removal of fat-soluble ingredients from G. elata.
Water is widely used as the solvent to extract G. elata polysaccharides under different conditions. Most of the G. elata polysaccharides, e.g., glucan PGEB-3H [25,26], WGEW [10], WTMA [27], RGP-1a and RGP-1b [23], the acidic polysaccharides [24] and PGE [28], were extracted by traditional heating methods with different extraction times, extraction frequencies (for assisted methods), and extraction temperatures. Specific information can be found in Table 1. Qiu et al. obtained the alkali-soluble polysaccharide (AGEW) by extraction with 5% NaOH at 4 °C for 2 h [10]. Microwaves can penetrate the cell walls of Chinese medicines and interact with polar components to accelerate extraction [29]. Zhu et al. found the best method of extraction of G. elata polysaccharides, with a yield of 5.42%, to be microwave extraction with water (40×) at 120 °C for 3 h [30]. Ultrasound can enhance the conduction between plants and solvents [31,32] and destroy cell walls to improve the extractability of polysaccharides [33,34]. Zhang et al. indicated that the extraction rate of polysaccharides extracted with 45 volumes (v/w) of water at 66 °C for 34 min was 32.78% [35]. In addition the above methods, enzyme-assisted treatments, which can promote the release of polysaccharides, have also been applied to extract polysaccharides [36,37]. Tan et al. adopted the orthogonal method to determine the optimal process for enzymatic extraction of G. elata polysaccharides, and the amount of G. elata polysaccharides extracted was 46.63 mg/g under those conditions, involving an enzyme dosage of 8 mg/g [38]. Enzyme-assisted extractions are rarely used alone and are often combined with other extraction methods to increase the polysaccharide yield [39]. Wang et al. showed that the yield of G. elata polysaccharides extracted by hot water extraction was 22.380%, the yield of G. elata polysaccharides extracted by ultrasonic-assisted extraction was 33.089%, and the yield of G. elata polysaccharides enzymatically extracted was 50.315% [40]. At the same time, Wang et al. determined that the antioxidant activity of G. elata polysaccharides extracted by ultrasound in 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ferric reducing antioxidant power (FRAP) antioxidant evaluation systems was higher than after enzymatic extraction and hot water extraction, and in the 3-ethyl-benzothiazoline-6-sulfonic acid (ABTS) antioxidant evaluation system, the activity of G. elata polysaccharides obtained by enzymatic extraction was higher than by ultrasound auxiliary extraction and hot water extraction [40]. On the whole, ultrasonic-assisted extraction and enzymatic extraction may benefit the extraction of G. elata polysaccharides while maintaining high antioxidant activity.
Table 1.
The extraction methods of several polysaccharides from G. elata.
| Name [ref] | Defat | Extract | Yield |
|---|---|---|---|
| WGE [10] | 95% EtOH, 3 times | Boiling water, 4 times, 4 h | 5.1 g |
| AGE [10] | 95% EtOH, 3 times | 5% NaOH (2 h) at 4 °C, 2 times | 10 g |
| TM [27] | 95% EtOH for 7 days | Boiling water, four times, 4 h | 5.2% |
| PGEB-3H [26] | 85% EtOH (1000 mL × 3) at 70 °C for 4 h | Water (800 mL × 4) at r.t. for 3 h | - |
| GR-0 [24] | 10 volumes (v/w) of 80% EtOH at 60 °C, 2 times for 3 h | 2 L of boiling distilled H2O for 3 h | 2.47 g |
| RGP [23] | 75% EtOH, 3 times over 24 h | Water at 74 °C, 3 times for 66 min | 6.11% |
| GPs [13] | 3 volumes of absolute EtOH at 60 °C for 24 h | Water at 90 °C, 4 times at 4 h each | - |
| The crude polysaccharide [28] | - | 400 mL of distilled water for 2 h at 60 °C, 3 times | 10.12% |
| GEP [54] | - | Water at 90 °C for 4 h | - |
The extraction methods of G. elata polysaccharides are very traditional, and they have many drawbacks. First, a study showed that the yields of extraction by hot water are influenced by the extraction time, temperature, feed–liquid ratio, and other factors [41,42,43]. Long-term and high-temperature hot water extraction leads to degradation of the polysaccharides, which results in a decrease in the yield of G. elata polysaccharides. Second, although irradiation-assisted extraction can shorten the extraction time and improve the efficiency, microwaves [44,45] and ultrasound [46,47,48] can lead to the decomposition of G. elata polysaccharides. Third, the specificity and selectivity of enzymes are also influenced by temperature and pH. In recent years, some new technologies have been developed for polysaccharide extraction to improve the extraction efficiency, such as supercritical fluid extraction (SFE) [49], pressurized liquid extraction [50], and induced electric field [51]. SFE is an efficient, safe, and environmentally friendly method, which has been applied successfully to extract Pachyman from Poriacocos (Schw.) wolf [52]. Furthermore, a new method for extracting and isolating polysaccharides from Semen cassiae was established by microwave-assisted aqueous two-phase extraction [53]. The ethanol-soluble polysaccharides and water-soluble polysaccharides are separated simultaneously. These new extraction techniques could be used for the extraction of G. elata polysaccharides in order to obtain new polysaccharides with novel structures for research.
2.2. Purification of G. elata Polysaccharides
Aqueous extracts contain a lot of impurities, and protein removal is an important step in the purification process. Several methods are commonly used in deproteinization, such as the Sevag method, the trichlorotrifluoroethane method, and the trichloroacetic acid method. The merits of these methods are their compatibility with polysaccharides. Due to the fact the Sevag method is much milder than the others, G. elata aqueous extract is typically deproteinized using the Sevag method [23,41,54]. The enzyme method is another effective and alternative to the common methods to eliminate proteins. Zhu et al. found that the protein removal rate was 90.1% under an enzyme dosage of 2.0 U/mg for 5 h [55]. The use of proteolytic enzymes to remove proteins is an environmentally friendly method with a mild response. After the protein has been removed, small molecule impurities are removed by dialysis, and then ethanol is added to the dialysate for precipitation to obtain crude polysaccharides. After these steps, the crude polysaccharides are further separated to obtain pure polysaccharides.
Polysaccharides can be separated by column chromatography to obtain homogenous polysaccharides, for example, using ion-exchange chromatography and size exclusion chromatography. Typically, ion-exchange chromatography is used for the separation of neutral/acidic polysaccharides from negatively charged polysaccharides by gradient salt elution or pH changes [56]. The G. elata polysaccharides WGEW, AGEW, WTMA, and GPs are prepared by using DEAE-cellulose column chromatography to further purify the crude polysaccharides [10,13,27]. Size exclusion chromatography is usually used for separating polysaccharides according to differences in molecular weight or molecular size. For example, Zhu et al. separated the crude polysaccharides on a Sephadex G-200 column at a flow rate of 0.30 mL/min, and then collected and further purified them by ultra-filtration tubes to obtain G. elata polysaccharides (PGE) [28]. Moreover, it has been reported that polysaccharides, such as PGEB-3H [25,26], RGP-1a and RGP-1b, can be purified by ion-exchange chromatography combined with size exclusion chromatography [23]. The macroporous resin D101 purifies G. elata polysaccharides well and can increase the purity of G. elata polysaccharides to 65.7% under optimal purification conditions [57]. All of the above information on G. elata polysaccharides is listed in Table 2.
Table 2.
The purification methods of several polysaccharides from G. elata.
| Name [ref] | Purify | Flow Rate | Eluting Solvent | Yield |
|---|---|---|---|---|
| WGEW [10] | DEAE-cellulose column (50 × 5 cm) | - | deionized water | 0.6 g from 5.1 g WGE |
| AGEW [10] | DEAE-cellulose column (50 × 5 cm) | - | deionized water | 1.8 g from 10.0 g AGE |
| WTMA [27] | DEAE-cellulose (50 cm × 5 cm, Cl− form) | - | 0.1M NaCl | 0.8 g from 6 g TM |
| PGEB-3H [26] | DEAE-cellulose A52 column (2.6 × 30 cm) | - | deionized water | 0.797 g/kg |
| Sephadex G-100 column (1.6 × 70 cm) | - | 0.1 M NaCl | ||
| The acidic polysaccharides [24] | DEAE-Sepharose CL-6B | - | 0, 0.05, 0.1, 0.2, 0.3, 0.4, and 0.5 M NaCl | 0.61 g from 2.47 g GR-0 |
| RGP-1a, PGP-1b [23] | DEAE-cellulose-52 column (2.6 cm × 80 cm) | 1 mL/min | deionized water | - |
| Sephadex G-100 column (1.8 cm × 100 cm) | 0.2 mL/min | deionized water | - | |
| GPs [13] | DEAE-52 cellulose column | 2.0 mL/min | distilled water and a gradient of 0→2 mol/L NaCl | - |
| PGE [28] | Sephadex G-200 column | 0.30 mL/min | distilled water | - |
Size exclusion chromatography has the obvious drawback that it can only be used for the analysis of relatively high molar mass samples and degrades ultra-high molar mass polymers, which makes it impossible to judge the exact molar mass of the sample [22]. Asymmetrical flow field-flow fractionation (AF4) is an effective fractionation technique that can solve this problem. Unlike chromatography, AF4 uses a separation channel without fillers, rather than a packed column. It can be applied to the characterization of colloids and macromolecules using low pressure and mild processing [58]. In addition, high-speed countercurrent chromatography (HSCCC) has been widely applied for the separation of natural products [59]. As a new technique, HSCCC has been applied successfully to separate and purify polysaccharides [60,61,62,63,64]. These methods could be used in the separation of G. elata polysaccharides to obtain higher purity G. elata polysaccharides for research.
3. Analysis of G. elata Polysaccharides
3.1. Qualitative Analysis of G. elata Polysaccharides
Analysis of the monosaccharide composition, ratio, and glycosidic linkages of polysaccharides is the most important step in the analysis of polysaccharides. The G. elata polysaccharides are degraded to monosaccharides by acid hydrolysis, and then these monosaccharides are separated and analyzed by various chromatographic techniques. The main advantages of thin layer chromatography (TLC) are its easy sample operation and the low cost required to detect the monosaccharide composition. Using TLC, Lee et al. found that the acidic polysaccharides obtained from G. elata included xylose, glucose, galacturonic acid, and glucuronic acid [24]. Gas chromatography (GC) is the most common method used to detect monosaccharide composition. Derivatization of the monosaccharides, for example, using methylation and acetylation, is required prior to the use of gas chromatography. Previous studies using GC analysis, indicated that G. elata polysaccharides are mainly composed of the monosaccharide glucose [10,13,25,27,28]. High performance liquid chromatography is an important method for the detection of polysaccharides. Using a refractive index detector, Chen et al. reported that G. elata RGP-1a is composed of fructose and glucose in a ratio of 1:10.68, and G. elata RGP-1b is only composed of glucose [24]. Using a carbohydrate column and a pulsed amperometric detector, Zhu et al. reported that the G. elata polysaccharide GEP is composed of glucose [54]. Through the use of ion chromatography to analyze the monosaccharide composition of G. elata polysaccharides, Li et al. reported that the G. elata polysaccharide is composed of rhamnose, galactose, glucose, xylose, and mannose [65].
Traditional acid hydrolysis will lead to a range of hydrolyzed monosaccharide ratios due to the poor selectivity of acid hydrolysis. To overcome this drawback, the use of specific glycosidases to digest polysaccharides has gradually replaced traditional acid hydrolysis methods. After digestion of the polysaccharides, the resulting monosaccharides can be separated by chromatographic techniques, such as high performance thin layer chromatography, high performance liquid chromatography, capillary electrophoresis tubes, PACE, and so on. Saccharides without ultraviolet absorption cannot be detected with an ultraviolet detector, unless the saccharide is derivatized. The evaporative light-scattering detector (ELSD) [66] and charged aerosol detector [67] can directly detect polysaccharides without derivatization. Matrix-assisted laser desorption/ionization can identify most polymers without derivatization and has been successfully used for the characterization of polysaccharides, such as seaweed polysaccharides [68]. Characterization and qualitative analysis are performed by the above methods [68,69,70]. Carbohydrate gel electrophoresis (PACE) has been applied to study the polysaccharides in Cordyceps [71,72,73] and Ganoderma [74]. Due to the individual preparation of the gel, the reproducibility of the glycan profile should be considered. High-efficiency thin-layer chromatography does not have this issue and can characterize ginseng polysaccharides [75]. These new techniques for separating monosacccharides after polysaccharide digestion could be applied for the analysis of G. elata polysaccharides to improve the accuracy of the analysis of G. elata polysaccharides.
Mass spectrometry is an efficient analysis method to obtain molecular information on polysaccharides. It is commonly used in the structural analysis of polysaccharides in conjunction with nuclear magnetic resonance (NMR) spectroscopy. NMR data (1D and 2D NMR) provide information on aspects of polysaccharide structure, such as the monosaccharide composition, sugar configuration, connection characteristics, and monosaccharide connection sequence. Some G. elata polysaccharides have a repeating structure with an α-(1→4) glucan and an α-(1→4) branch at O-6 according to NMR and GC-MS [10,25,27]. However, the structure of the polysaccharide PGE obtained from G. elata is a backbone of (1→4)-linked-d-Glcp and 1→3 and 1→4,6-branched glucopyranose [28]. Zhou et al. isolated the polysaccharide GEP II from G. elata, which was composed of glucose and mannose [76]. The main skeleton has 1→6 and 1→4 glycosidic bonds as well as 1→2 glycosidic bonds [76]. The monosaccharide composition, backbone, and molecular weight of polysaccharides derived from G. elata are summarized in Table 3.
Table 3.
The molecular weight, backbone, monosaccharide composition, and biological activities of polysaccharides derived from G. elata.
| Name [ref] | Molecular Weight (Da) | Monosaccharide Composition | Backbone | Biological Activities |
|---|---|---|---|---|
| WGEW [10] | 1.0 × 105 | Glucose | α-1,4-glucan and α-1,4,6-glucan | - |
| AGEW [10] | 2.8 × 105 | Glucose | α-1,4-glucan and α-1,4,6-glucan | - |
| WTMA [27] | 7.0 × 105 | Glucose | α-1,4-glucan and α-1,4,6-glucan | Anti-cancer |
| PGEB-3H [25] | 2.88 × 104 | Glucose | α-1,4-glucan and α-1,4,6-glucan | Cardiovascular system |
| The acidic polysaccharides [24] | - | Xylose, glucose, galacturonic acid, and glucuronic acid | - | Cardiovascular system |
| RGP-1a [23] | 1.925 × 104 | fructose: glucose = 1:10.68 | - | Immunological activity |
| RGP-1b [23] | 3.92 × 103 | Glucose | - | Immunological activity |
| GPs [13] | 2.71 × 105 | Glucose | - | Immunological activity |
| PGE [28] | 1.54 × 106 | Glucose | α-1,4-glucan, α-1,3-glucan and α-1,4,6-glucan | Cardiovascular system |
| GEP [54] | 875185 | Glucose | - | Antioxidant |
3.2. Quantitative Analysis of G. elata Polysaccharides
The extraction and purification of polysaccharides are key steps to obtain polysaccharides, which have a great influence on the polysaccharide composition and content. At present, the quantitative analysis methods for polysaccharides mainly include colorimetry, high performance liquid chromatography, and gas chromatography. The total content of G. elata polysaccharides is usually determined by colorimetric methods of different color systems, such as the phenol–sulfuric acid method and the anthrone–sulfuric acid method. Because of the differences between monosaccharides, when glucose is used as a reference, the monosaccharide composition has a great influence on the polysaccharide content [77]. The monosaccharide content of a polysaccharide can be quantified by GC and HPLC to calculate the number of monosaccharides released after hydrolysis of the polysaccharide. For example, using HPLC with an RI detector, Chen et al. found that G. elata RGP-1a is composed of fructose and glucose in a ratio of 1:10.68 [23]. High performance gel permeation chromatography (HPGPC) is ofter applied to determine the molecular weights of polysaccharides. A series of glucans with different molecular weights is used to establish the standard curve and determine the molecular weights of polysaccharides. All the molecular weights of G. elata polysaccharides, such as the polysaccharides WGEW, AGEW [10], WTMA [27], PGEB-3H [25], RGP-1a, RGP-1b [23], GPs [13], PGE [28], and GEP [54], are determined according to this method.
Application of both the colorimetric method and the HPGPC method for G. elata are too complicated, as they require an individual polysaccharide reference. Natural polysaccharides can be isolated by HPSEC, and then the molecular mass of each component can be determined by multi-angle laser light scattering. The polysaccharide or polysaccharide component can be quantified according to the response of the polysaccharide to the refractive index detector and the refractive index increment (dn/dc). The method without a reference substance is simple and accurate. The method has been used to quantitatively analyze the polysaccharides for species in the Panax genus, e.g., P. ginseng, P. notoginseng, and P. quinquefolius [78], as well as Lycium barbarum [79].
Previous studies suggest that several specific polysaccharides from G. elata with defined structures clearly consist only of glucose and have a main chain of 1–4 glycosidic bonds, for example, AGEW, WGEW, WTMA and PGEB-3H [10,25,27]. The structures of several polysaccharides are shown in Figure 1. Some polysaccharides from G. elata without a defined structure consist of glucose and several other monosaccharides. The similar structures of some G. elata polysaccharides and its monotonous monosaccharide compositions may be related to the lack of novelty of the research method of G. elata polysaccharides. Therefore, the development of a new research method for G. elata polysaccharides may result in a novel structure of G. elata polysaccharides, which, in turn, has promoted research on G. elata polysaccharides.
Figure 1.
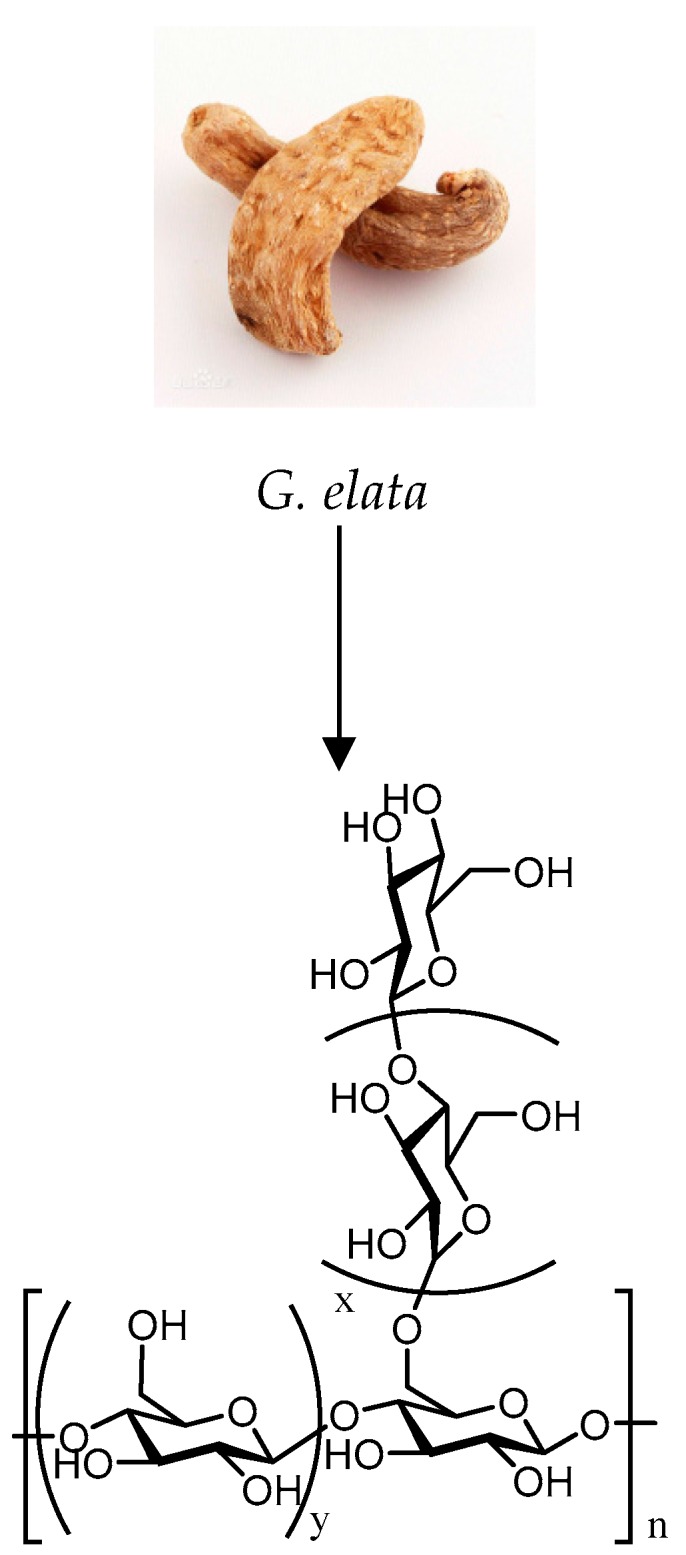
Chemical structure of part of the polysaccharides derived from G. elata: x + y = R; 1. AGEW: R = 14; 2. WTMA: R = 15; 3. WGEW: R = 16; and 4. PGEB-3H: R = 20 [5].
4. Modification of G. elata Polysaccharides
Polysaccharides from traditional Chinese herbal medicines have received a lot of attention due to their wide range of biological activities. The structure–activity relationships of polysaccharides are complex and hard to determine and are thus a big obstacle to the advancement of polysaccharide research. The pharmacological effects of polysaccharides depend on their characteristics, including molecular weight, solubility, viscosity and other physical or chemical properties [80,81,82,83]. The molecular derivatization of polysaccharides can change and enrich the structure and physico-chemical properties of polysaccharides. Physical, chemical, and biological pathways are three major methods of polysaccharide molecular derivatization [84]. In addition, through modification and derivatization of the chemical structure of polysaccharides, qualitative or quantitative analyses of polysaccharides have been performed [22]. Hence, more and more research has focused on the derivatization and modification of polysaccharides [85].
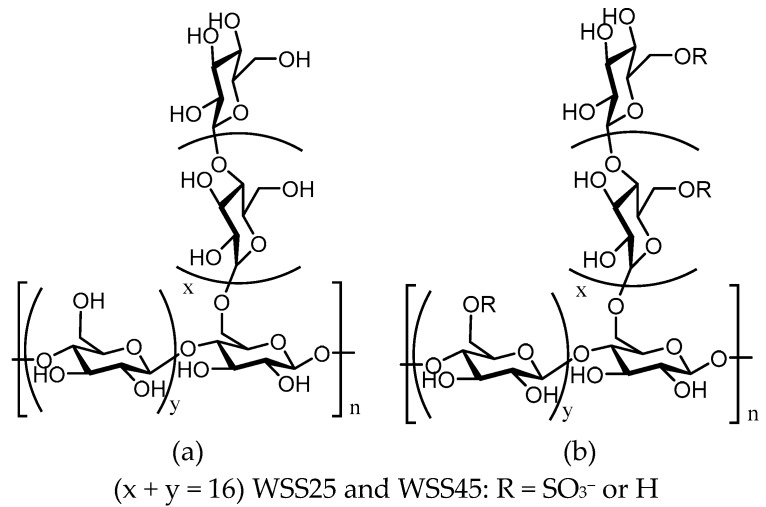
Up until now, several methods for the modification of polysaccharides have been reported, including sulfation [86], phosphorylation [87], carboxymethylation [88], selenization [89], acetylation [90], acid/alkali degradation [91], and others [92,93,94]. In 2007, Ding and their collaborators first isolated two polysaccharides from G. elata, WGEW and AGEW, and found that these two polysaccharides only consist of glucose [10]. Based on the reported structure–activity relationship between sulfated polysaccharides and anti-dengue virus bioactivities, they then sulfated WGEW and AGEW [10]. Using the chlorosulfonic acid-pyridine method, two sulfated derivatives of two polysaccharides—WSS25, WSS45, ASS25, and ASS45—were obtained at 25 and 45 °C, respectively. The basic information and structures of WGEW and AGEW and their sulfated derivatives is shown in Table 4 and Figure 2 [10]. The antiviral activity of WSS25, WSS45, and ASS45 was shown to be stronger than that of WGEW and AGEW. However, the selectivity index (CC50/EC50) of virus inhibition activities of WSS25 and ASS45 was lower than that of WSS45. WSS45 was shown to be a better dengue virus type 2 (DV2) inhibitor, with a selectivity index of more than 1000 [10]. Further study on the antiviral mechanism of WSS45 revealed that WSS45 does not directly kill the virus. WSS45 mainly interferes with the adsorption of DV2 by target cells, thereby strongly inhibiting DV2 infection. Furthermore, they found that the antiviral effect is related to the molecular weight of WSS45 and the degree of sulfation (DS) [10]. It has also been reported that the antivirus ability of sulfated polysaccharides increases as the degree of sulfation increases [10]. We can see that there is no correlation between the molecular weight of different sulfurized polysaccharides and their final efficacy strength and selectivity index for DV2. The structure–activity relationship between sulfated polysaccharides with different DS or molecular weights and their antiviral activities could be inferred.
Table 4.
Molecular weight, derivatization type, degree of substitution, and bioactivity of polysaccharides from G. elata and their derivatives.
| Name [ref] | Molecular Weight (Da) | Modification | DS 1 | Bioactivities 2 | ||
|---|---|---|---|---|---|---|
| a | b | c | ||||
| AGEW [10] | 1.0 × 105 | - | 0 | − | − | − |
| ASS25 [10] | 1.5 × 105 | sulfation | 0.579 | − | − | − |
| ASS45 [10] | 6.8 × 104 | 0.624 | + | − | − | |
| WGEW [10] | 2.8 × 105 | - | 0 | − | − | − |
| WSS25 [10,11,95,96,97] | 6.5 × 104 | sulfation | 0.206 | + | + | + |
| WSS45 [10] | 1.9 × 105 | 1.685 | + | − | − | |
| M1S [97] | 1.8 × 105 | 1.050 | − | + | − | |
| M2S [97] | 1.3 × 105 | 1.220 | − | + | − | |
| M3S [97] | 7.5 × 104 | 1.270 | − | + | − | |
| M4S [97] | 4.1 × 104 | 1.210 | − | + | − | |
| M5S [97] | 1.4 × 104 | 1.050 | − | − | − | |
| M6S [97] | 1.2 × 104 | 1.240 | − | − | − | |
| M7S [97] | 2.7 × 103 | 1.350 | − | − | − | |
| WGES1 [97] | 2.4 × 105 | 0.141 | − | + | − | |
| WGES2 [97] | 6.7 × 104 | 0.097 | − | + | − | |
| WGES3 [97] | 1.8 × 105 | 0.194 | − | + | − | |
| WGES4 [97] | 8.0 × 104 | 0.173 | − | + | − | |
| WGES5 [97] | 1.38 × 105 | 0.220 | − | + | − | |
| WGES6 [97] | 7.6 × 104 | 0.202 | − | + | − | |
| WGEA | 5.7 × 103 | aminopropylation | unknown | − | − | − |
| WGEC | 7.3 × 104 | carboxymethylation | − | − | − | |
| WGEP | 2.2 × 103 | phosphorylation | − | − | − | |
| WGEL | 6.0 × 105 | acetylation | − | − | − | |
1 DS is calculated as 162 × %W/(96-80 × %W); %W is the content of SO4 2−. 2 a. Anti-dengue virus; b. antiangiogenesis; c. antiosteoporosis.
Figure 2.
Structures of WGEW (a) and its sulfated derivatives WSS25 and WSS45 (b) [4].
At the same time, the antiviral activity structure-activity relationship of sulfated polysaccharides is very complex. It is not enough to depend on only four sulfated polysaccharides to determine this structure–activity relationship. If the structure–activity relationship of sulfated polysaccharides from G. elata is to be studied further, more derivatization and pharmacological research on sulfated polysaccharides is essential.
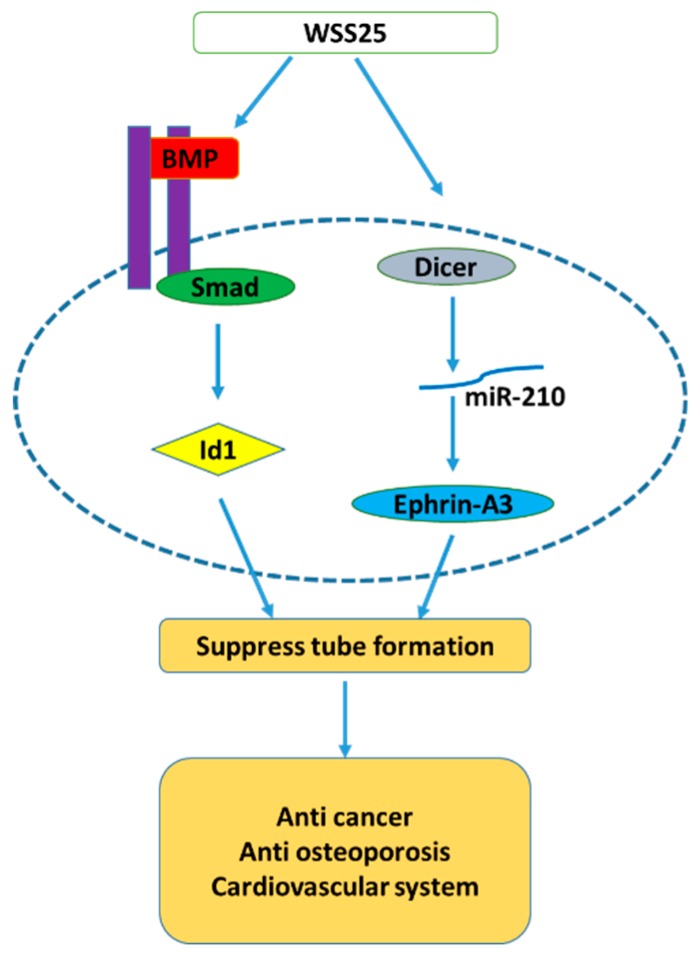
According to the structure–activity relationship between anti-angiogenic target proteins (Id1 and HS) and sulfated polysaccharides, WSS25 was confirmed to inhibit Id1 expression in HMEC-1 cells and block the BMP2/Smad/Id1 signaling pathway (Figure 3), thereby reducing tumor angiogenesis and inhibiting hepatic tumor cells [95]. Another molecular mechanism by which WSS25 inhibits angiogenesis is the inhibition of HMEC-1 cells by inhibiting dicer, a key enzyme in miRNA biosynthesis [96].
Figure 3.
The pathway of tube formation suppression by the sulfated polysaccharides WSS25 from G. elata.
Although the anti-angiogenic mechanism of WSS25 has been studied clearly, its structure–activity relationship is still complicated. Therefore, in order to further study the structure–activity relationship of the sulfated polysaccharides of WSS25 on its anti-angiogenesis effects [97], a dozen WSS25-based sulfated derivatives and several WGEW aminopropylation, carboxymethylation, phosphorylation, and acetylation derivatives were synthesized. Basic information about these derivatives is shown in Table 3. Only sulfated polysaccharides with a molecular weight higher than 41,000 Da were shown to have an anti-angiogenic effect. Because the longer sugar chain structure could interact with the target protein, polysaccharide derivatives containing other groups do not have this activity. The strength of anti-angiogenic activity depends on the DS of polysaccharide derivatives. Additionally, the derivatized polysaccharides with a DS value of 0.173 to 0.194 showed better activity. The glucose branch of the sulfated polysaccharide was found to contribute little to this activity.
It was also reported that WSS25 can interact with BMP-2 in hepatic cancer cells [95]. Meanwhile, BMP-2 plays an important regulatory role in osteoclast and osteoblast cells [98,99]. WSS25 can inhibit bone loss induced by ovariectomy in female mice. This result suggests that WSS25 might be an effective anti-osteoporosis drug for older women [11].
In summary, research on G. elata polysaccharide derivatives has mainly focused on the sulfated products WSS25 and WSS45. They have antiviral, antiangiogenic, and antiosteoporosis biological activity. Some regular patterns were discovered from research on the antiangiogenic structure-activity relationship of G. elata sulfate polysaccharides. Unfortunately, studies on other derivatives of G. elata polysaccharides, their related activities, and structure-activity relationships are rarely reported.
5. Pharmacological Activities and Functions of G. elata Polysaccharides
A lot of references point out that G. elata shows significant pharmacological activity [100,101]. The activities of G. elata polysaccharides are summarized in Table 5.
Table 5.
Pharmacological activities information about polysaccharides isolated from G. elata.
| Name [ref] | Activities | Cell Lines | Animals Model | Model of Action |
|---|---|---|---|---|
| WTMA [27] | anti-cancer | PANC-1, live LO2 cells | - | - |
| G. elata polysaccharides [9] | - | H22 tumor-bearing mice | increases caspase-3,8,9 levels and G0/G1 phase cell percentage, and decrease G2/M phase cell percentage | |
| G. elata polysaccharides [12] | anti-aging and antioxidation | - | aging mice | improves the activities of SOD and GSH-Px, inhibits MAO activity, and reduces the level of MDA to |
| G. elata polysaccharides [102] | - | aging mice | promote the recovery of cranial nerves, significantly improve the activity of enzymes related to oxidative metabolism in the body | |
| GEP [52] | - | aging mice | increases the activity of superoxide dismutase and glutathione peroxidase, as well as the serum and malondialdehyde levels | |
| RGP-1a, RGP-1b [23] | Immunomodulatory effects | RAW 264.7 cell macrophages | - | enhances NO production and phagocytic activity |
| G. elata polysaccharides [103] | - | Immunocompromised mice | increases serum IgA, IgG, and hemolysin, the spleen index, thymus index, and serum IgM levels | |
| GPs [13] | - | Kunming mice | augments serum IL-2, TNF-α, IFN-γ, IgG, IgA, and IgM levels, as well as the spleen and thymus indexes | |
| PGEB-3H [25] | - | mice | increases the Ach content in brain tissue | |
| PGB [104] | BDNF-positive cells and SCF-positive cells | Focal cerebral ischemia rats | up-regulates BDNF, Nestin, and SCF expression | |
| GEP [105] | neuroprotection cardiovascular system | PC12 cells | - | inhibits the endoplasmic reticulum stress-mediated pathway |
| PGB [104] | - | RHR rats | promotes the production of endogenous vasoactive substances such as nitric oxide and inhibits the release of endogenous vasoconstrictors such as plasma endothelin and angiotensin II | |
| PGEB-3H [25] | - | Hyperlipidemia rats | - | |
| Crude and acidic polysaccharides [106] | cardiovascular system | - | SD rats fed a high-fat diet | suppresses total cholesterol and LDL |
| Acidic polysaccharides [24] | - | SHR rats fed a high-fat diet | reduces total cholesterol, triglyceride, and LDL levels | |
| PGE [28] | - | - | - |
Research on the pharmacological activities of G. elata polysaccharides mentioned in this paper mainly involves two parts: animal experiments in vivo and cell experiments in vitro. While Liu et al. used intracortical injection as an administration method in their experiments [12], almost all animal experiments were performed by oral administration. However, none of the above studies on systemic activity mention any pharmacokinetic parameters and characteristics other than the mode of administration and the dosage of G. elata polysaccharides. The in vivo pharmacokinetic study of G. elata polysaccharides is still blank, and its molecular mechanism still needs further confirmation. The in vitro cell experiments mentioned mainly use a method in which the cells are co-incubated with the G. elata polysaccharides. Unfortunately, few studies have mentioned whether G. elata polysaccharides can enter cells or whether certain pathway affect cells.
5.1. Anti-Cancer Activities
Chen et al. reported that the growth of PANC-1 cells could be inhibited by WTMA, and the polysaccharides showed insignificant toxicity on PANC-1 cells but no inhibition effect on live LO2 cells [27]. Liu et al. found that G. elata polysaccharides have a significant anti-tumor effect on H22 tumor-bearing mice, which can reduce the tumor weight [9]. The high-dose G. elata polysaccharide inhibition rate was shown to reach 44.7%. A study showed that G. elata polysaccharide could increase the G0/G1 phase cell percentage and decrease the G2/M phase cell percentage. The mechanism of action might be related to cell cycle distribution, the suppression of cell proliferation, and the activation of the caspase system to induce tumor cell apoptosis [9].
5.2. Antioxidation Activities
G. elata polysaccharides show antioxidation activities [12]. Previous studies have revealed that G. elata polysaccharides can improve the activity of SOD in the brain and GSH-Px in the blood, and G. elata polysaccharides can inhibit MAO activity in the brain and reduce the level of MDA in the brain tissue of aging mice. Xie et al. reported that G. elata polysaccharides can improve the learning and memory ability of D-galactose–induced aging mice to improve the activity of enzymes related to oxidative metabolism in the body [102]. G. elata polysaccharides can also delay the aging of the human body related to free radicals and increase the activity of superoxide dismutase and glutathione peroxidase, as well as the serum malondialdehyde levels in aging mice through dose-dependent enhancement [52].
5.3. Immunological Activities
Chen et al. reported that RGP-1a and RGP-1b, isolated from G. elata, could enhance the NO production and phagocytic activity of RAW 264.7 macrophages in a dose-dependent manner [23]. Li et al. reported that for mice in a cyclophosphamide-induced immunocompromised state, the serum of IgA, IgG, and serum hemolysin were increased in the middle- and high-dose groups of G. elata polysaccharides (p < 0.01). The high-dose group increased the spleen index and thymus index, and the polysaccharide middle dose group significantly increased serum IgM levels (p < 0.05) [103]. The G. elata polysaccharide could alleviate the inhibitory effect of cyclophosphamide on humoral immune function in mice. Bao et al. showed that G. elata polysaccharides isolated from the dried rhizomes of G. elata augment serum IL-2, TNF-α, IFN-γ, IgG, IgA and IgM levels as well as the spleen and thymus indexes of Kunming mice with immunomodulatory activity in a dose-dependent manner following intragastric treatment [13].
5.4. Neuroprotection Activities
The research results show that the polysaccharide PGEB-3H can improve the learning and memory ability of mice with scopolamine-induced memory disorders through increasing the Ach content in brain tissue [14]. G. elata polysaccharides (PGB) were shown to increase the number of BDNF-positive cells and SCF-positive cells and decrease the average gray value, suggesting that PGB has neuroprotective effects by up-regulating BDNF and SCF expression in brain tissues around ischemic lesions [104]. Electroacupuncture combined with PGB may improve the neurological function of rats with focal cerebral ischemia rats by increasing Nestin and brain-derived neurotrophic factor expression to accelerate the growth of neural stem cells in the basolateral amygdala [107]. The PC12 cells were protected from corticosterone-induced apoptosis and lactate dehydrogenase leakage, and intracellular reactive oxygen levels were reduced after treatment with 1000 μg/mL of polysaccharides from G. elata (GEP) before exposure to 200 μM of corticosterone by suppressing the endoplasmic reticulum stress-mediated pathway [105].
5.5. Cardiovascular System Activities
The G. elata polysaccharide PGB was shown to have a good antihypertensive effect on RHR rats by promoting the production of endogenous vasoactive substances, such as nitric oxide, and inhibiting the release of endogenous vasoconstrictors, such as plasma endothelin and angiotensin II [15]. By feeding PGEB-3H to rats with hyperlipidemia, Ming et al. showed that the polysaccharide PGEB-3H has potential lipid-lowering effects [25]. The crude and acidic polysaccharides of G. elata were shown to suppress the total cholesterol and LDL concentrations to decrease the atherosclerosis risk, and there were no significant differences in the serum triglyceride and HDL levels in SD rats fed a high-fat diet, indicating that the acidic polysaccharide of G. elata might significantly reduce the risk of cardiovascular disease (CVD) and atherosclerosis through suppressing the de novo synthesis of total cholesterol and LDL [106]. The acidic polysaccharides isolated from G. elata were shown to decrease blood pressure and improved serum lipid levels in SHR rats fed a high-fat diet by reducing the total cholesterol, triglyceride and LDL levels [24].
5.6. Other Functions
In addition to their good pharmaceutical potential, G. elata polysaccharides can also be applied in cosmetics and foods. G. elata polysaccharides show good functions related to appropriate viscosity, moisture absorption, and moisture retention due to their numerous hydroxyl groups [108]. G. elata polysaccharides also have good antioxidant and anti-aging activities [12,52]. A series of studies have applied G. elata polysaccharides to the preparation and development of moisturizers and skin creams [109,110,111]. Based on the anti-hypertension [15], anti-cancer [9,27], and anti-aging activities of water-soluble G. elata polysaccharides [12,52], Zhengjie and colleagues developed a G. elata polysaccharide-functional beverage [111].
6. Discussion
At present, the extraction of G. elata polysaccharides is based on traditional methods, including hot water extraction, ultrasonic extraction, microwave extraction, and so on. All of these methods use water as the extraction solvent and organic solvents for the removal of low-polarity small molecule compounds. The existing greener extraction methods, such as the microwave-assisted aqueous two-phase method [53], SFE extraction [52], and ionic liquids [112], could be applied to extract G. elata polysaccharides. Ionic liquids consist of organic cations and organic or inorganic anions, which exist in a liquid state at room temperature [112]. Ionic liquids have good solubility for bioactive polysaccharides, because of the stronger hydrogen bonds resulting from the interaction between polysaccharides and the large number of anions in the ionic liquid (such as AcO− and Cl−) [111]. For example, chitosan and β-d-glucan both exhibit good solubility in 1-ethyl-3-methylimidazolium acetate (EMIMAc) [113,114]. Therefore, we can infer that SFE and ionic liquids with melting points in the room temperature range and can be used as novel and green non-aqueous extraction solvents for the extraction of G. elata polysaccharides.
So far, the structures of only a few polysaccharides isolated from G. elata have been defined clearly, such as WTMA [27], WGEM [10], AGEM [10], and PGBE-3H [25]. Polysaccharides without a characterized specific structure do not have assured reproducibility, which is not conducive to further ensuring their clinical efficacy and drug stability. The structure–functional relationships of G. elata polysaccharides represent a blank field that needs further research to control the quality of polysaccharides. Wu et al. used the saccharide mapping method to control the quality of polysaccharides from Ganoderma spp. [74]. Saccharide mapping is a new quality control method for uncharacterized polysaccharides. It is based on a specific and mild digestion method, followed by chromatographic separation including HPTLC, HPSEC, CE, and PACE [21,70], which does not require knowledge of the clear structure of polysaccharides. However, saccharide mapping has not been put into use for the quality control of G. elata polysaccharides; it is necessary to develop a simple and reliable method for the quality control of G. elata polysaccharides to guarantee the quality of drugs developed using G. elata polysaccharides.
It is undeniable that G. elata polysaccharides have good biological activity in many aspects, including anticancer, antioxidation, antihypertensive, immune system modulation and nervous system regulation. Especially, the function of G. elata polysaccharides in neuroprotection and treatment of hypertension diseases has great potential to improve the health of middle-aged and elderly populations. However, most of the current studies on systemic pharmacological activity have neglected the mechanism of action of G. elata polysaccharides in animal experiments in vivo. The molecular mechanisms have also not been fully investigated in in vitro cell experiments. The field of G. elata polysaccharide pharmacokinetic studies is still blank.
In the derivatization of G. elata polysaccharides, research has mainly focused on sulfurized polysaccharides, resulting in a lack of information about other derivatives of G. elata polysaccharides. The anti-angiogenic biological activity of WSS25 and its structure–activity relationship have been well researched. However, the bioactivity and structure–activity relationship of other derivatized compounds of G. elata polysaccharides are still unknown. Based on the structure–activity relationships of other derivatized polysaccharides, there is potential to study the preparation, pharmacological activity, and structure–activity relationships of G. elata polysaccharides. However, the difficulty lies in the fact that the uniform composition and complex structure of G. elata polysaccharides causes great disturbance to the derivatization.
7. Conclusions
In summary, many new ideas and techniques have been developed for the study of polysaccharides in traditional Chinese medicine. However, the extraction, analysis, and derivatization of G. elata polysaccharides are still conducted using traditional methods. Consequently, G. elata polysaccharides have not been fully studied. There is still much room for deeper research. New extraction, analysis, and derivatization research methods should be applied to the study of G. elata polysaccharides to further discover its potential bioactivity, functions, and applications.
Acknowledgments
This investigation is part of National Key R & D Program of China.
Author Contributions
Writing—original draft preparation, H.Z., C.L., J.H. and H.L.; writing—review and editing, M.L.; supervision, W.W.
Funding
This research was funded by Special Fund for National Key R & D Program of China [2018YFC1707900, 2018YFC1707903, 2018YFC1707905].
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Liu Y., Huang G.L. The chemical composition, pharmacological effects, clinical applications and market analysis of Gastrodia elata. Pharm. Chem. J. 2017;51:211–215. doi: 10.1007/s11094-017-1584-5. [DOI] [Google Scholar]
- 2.Hsieh C.L., Lin J.J., Chiang S.Y., Su S.Y., Tang N.Y., Lin G.G., Lin I.H., Liu C.H., Hsiang C.Y., Chen J.C., et al. Gastrodia elata modulated activator protein 1 via c-Jun N-terminal kinase signaling pathway in kainic acid-induced epilepsy in rats. J. Ethnopharmacol. 2007;109:241–247. doi: 10.1016/j.jep.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Choi J.H., Lee D.U. A new citryl glycoside from Gastrodia elata and its inhibitory activity on GABA transaminase. Chem. Pharm. Bull. 2006;54:1720–1721. doi: 10.1248/cpb.54.1720. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y., Gong X.J., Zhou X., Kang Z.J. Relative bioavailability of gastrodin and parishin from extract and powder of Gastrodiae rhizoma in rat. J. Pharm. Biomed. Anal. 2014;100:309–315. doi: 10.1016/j.jpba.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Matias M., Silvestre S., Falcão A., Alves G. Gastrodia elata and epilepsy: Rationale and therapeutic potential. Phytomedicine. 2016;23:1511–1526. doi: 10.1016/j.phymed.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Shu C.H., Qiao N., Piye N., Ming W.H., Xiao S.S., Feng S., Sheng W., Opler M. Protective effects of Gastrodia elata on aluminium-chloride-induced learning impairments and alterations of amino acid neurotransmitter release in adult rats. Restor. Neurol. Neurosci. 2008;26:467–473. [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashi J., Sekine T., Deguchi S., Lin Q., Horie S., Tsuchiya S., Yano S., Watanabe K., Ikegami F. Phenolic compounds from Gastrodia rhizome and relaxant effects of related compounds on isolated smooth muscle preparation. Phytochemistry. 2002;59:513–519. doi: 10.1016/S0031-9422(02)00008-0. [DOI] [PubMed] [Google Scholar]
- 8.Liu C.Y. The changes of the polysaccharide and its distribution in the development process of Gastrodia elate. Acta Botanic. Yunnan. 1981;3:375–380. [Google Scholar]
- 9.Liu X.H., Guo X.N., Zhan J.P., Xie Z.L., Wang J.M., Zhang Y.T., Chen Y.L., Li X.B. The effects of polysaccharide from Gastrodia elata B1 on cell cycle and caspase proteins activity in H22 tumor bearing mice. Chin. J. Gerontol. 2015;20:5681–5682. [Google Scholar]
- 10.Qiu H., Tang W., Tong X.K., Ding K., Zuo J.P. Structure elucidation and sulfated derivatives preparation of two α-d-glucans from Gastrodia elata. and their anti-dengue virus bioactivities. Carbohydr. Res. 2007;342:2230–2236. doi: 10.1016/j.carres.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Chen C., Qin Y., Fang J.P., Ni X.Y., Yao J., Wang H.Y., Ding K. WSS25, a sulfated polysaccharide, inhibits RANKL-induced mouse osteoclast formation by blocking SMAD/ID1 signaling. Acta Pharmacol. Sin. 2015;36:1053–1064. doi: 10.1038/aps.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J., Mori A. Antioxidant and free radical scavenging activities of Gastrodia elata Bl. and Uncaria rhynchophylla (Miq.) Jacks. Neuropharmacology. 1992;31:1287–1298. doi: 10.1016/0028-3908(92)90058-W. [DOI] [PubMed] [Google Scholar]
- 13.Bao Q.W., Qian L., Gong C., Shen X.Z. Immune-enhancing activity of polysaccharides from Gastrodia elata. J. Food. Process. Pres. 2017;41:e13016. doi: 10.1111/jfpp.13016. [DOI] [Google Scholar]
- 14.Ming J., Zeng K.F., Wu S.R., Fu A.L., Zhao G.H., Gui M.Y., Chen Z.D. Effect of soluble polysaccharide PGEB-3-H from Gastrodia elataume on scopolamine-induced learning and memory disorders in Mice. J. Food Sci. 2010;31:246–249. [Google Scholar]
- 15.Miao H.C., Shen Y.S. Antihypertensive effect of polysaccharides substracted from Gastrodia elataume. Chin. J. Hypertension. 2006;14:531–534. [Google Scholar]
- 16.Ju Y., Xue Y., Huang J., Zhai Q., Wang X.H. Antioxidant Chinese yam polysaccharides and its pro-proliferative effect on endometrial epithelial cells. Int. J. Biol. Macromol. 2014;66:81–85. doi: 10.1016/j.ijbiomac.2014.01.070. [DOI] [PubMed] [Google Scholar]
- 17.Lou X., Huang Y., Guo X., Zheng F., Jiang Z., Yang Y.P. Polysaccharides from Portulaca oleracea (purslane) supplementation lowers acute exercise induced oxidative stress in young rats. Afr. J. Pharm. Pharmacol. 2011;5:381–385. [Google Scholar]
- 18.Shu X., Liu X., Fu C., Liang Q. characterization and antitumor effect of the polysaccharides from star anise (Illicium verum Hook. f.) J. Med. Plants Res. 2010;4:2666–2673. [Google Scholar]
- 19.Wang L., Wang Z., Zhag J., Tang Z.S., Sun X.C., Song Z.X., Huang W.J., Liu L. Extraction and isolation of polysaccharide from Portulaca oleracea by traditional process combined with membrane separation technology and evaluation of its anti-oxidant activity. Chin. Trad. Herb. Drugs. 2016;47:1676–1681. [Google Scholar]
- 20.Zhao R., Gao X., Cai Y., Shao X., Jia G., Huang Y., Qin X., Wang J., Zheng X. Antitumor activity of Portulaca oleracea L. polysaccharides against cervical carcinoma in vitro and in vivo. Carbohydr. Polym. 2013;96:376–383. doi: 10.1016/j.carbpol.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Dong C.X., Hayashi K., Lee J.B., Hayashi T. Characterization of structures and antiviral effects of polysaccharides from Portulaca oleracea L. Chem. Pharm. Bull. 2010;58:507–510. doi: 10.1248/cpb.58.507. [DOI] [PubMed] [Google Scholar]
- 22.Zhao J., Deng Y., Li S.P. Advanced analysis of polysaccharides, novel functional components in food and medicine dual purposes Chinese herbs. Trends Anal. Chem. 2017;96:138–150. doi: 10.1016/j.trac.2017.06.006. [DOI] [Google Scholar]
- 23.Chen J.C., Tian S., Shu X.Y., Du H.T., Li N., Wang J.R. Extraction, Characterization and immunological activity of polysaccharides from Rhizoma gastrodiae. Int. J. Mol. Sci. 2016;17:1011. doi: 10.3390/ijms17071011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee O.H., Kim K.I., Han C.K., Kim Y.C., Hong H.D. Effects of acidic polysaccharides from Gastrodia Rhizome on systolic blood pressure and serum lipid concentrations in spontaneously hypertensive rats fed a high-fat diet. Int. J. Mol. Sci. 2012;13:698–709. doi: 10.3390/ijms13010698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ming J., Liu J., Wu S.R., Guo X.H., Chen Z.D., Zhao G.H. Structural characterization and hypolipidemic activity of a polysaccharide PGEB-3H from the fruiting bodies of Gastrodia elataume. Procedia. Eng. 2012;37:169–173. doi: 10.1016/j.proeng.2012.04.221. [DOI] [Google Scholar]
- 26.Zhao G.H., Kan J.Q., Li Z.X., Chen Z.D. Characterization and immunostimulatory activity of an (1→6)-α-d-glucan from the root of Ipomoea batatas. Int. Immunopharmacol. 2005;5:1436–1445. doi: 10.1016/j.intimp.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Chen X., Cao D.X., Zhou L., Jin H.Y., Dong Q., Yao J., Ding K. Structure of a polysaccharide from Gastrodia elata., and oligosaccharides prepared thereof with anti-pancreatic cancer cell growth activities. Carbohydr. Polym. 2011;86:1300–1305. doi: 10.1016/j.carbpol.2011.06.029. [DOI] [Google Scholar]
- 28.Zhu Z.Y., Chen C.J., Sun H.Q., Chen L.J. Structural characterisation and ACE-inhibitory activities of polysaccharide from Gastrodia elataume. Nat. Prod. Res. 2018;2:1–6. doi: 10.1080/14786419.2018.1434643. [DOI] [PubMed] [Google Scholar]
- 29.Chan C.H., Yusoff R., Ngoh G.C., Kung F.W.L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A. 2011;1218:6213–6225. doi: 10.1016/j.chroma.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 30.Zhu X.X., Luo X.G. Optimization of extraction parameters for polysaccharides of Gastrodia elata. Li Shizhen Med. Mater. Medica Res. 2007;18:906–907. [PubMed] [Google Scholar]
- 31.Vinatoru M., Toma M., Mason T.J. Ultrasonically assisted extraction of bioactive principles from plants and their constituents. Adv. Sonochem. 1999;5:209–248. [Google Scholar]
- 32.Glisic S.B., Ristic M., Skala D.U. The combined extraction of sage (Salvia officinalis L.): Ultrasound followed by supercritical CO2 extraction. Ultrason. Sonochem. 2011;18:318–326. doi: 10.1016/j.ultsonch.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Jia X.J., Ma L.S., Li P., Chen M.W., He C.W. Prospects of poriacocos polysaccharides: Isolation process, structural features and bioactivities. Trends Food. Sci. Technol. 2016;54:52–62. doi: 10.1016/j.tifs.2016.05.021. [DOI] [Google Scholar]
- 34.Fu L., Chen H., Dong P., Zhang X., Zhang M. Effects of ultrasonic treatment on the physicochemical properties and DPPH radical scavenging activity of polysaccharides from mushroom Inonotus obliquus. J. Food Sci. 2010;75:C322–C327. doi: 10.1111/j.1750-3841.2010.01590.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M.J., Xu H.D., An X.G. Research on ultrasonic wave extraction of elata. Polysaccharides. J. Northwest A & F Univ. (Natural Science Edition) 2007;35:91–95. [Google Scholar]
- 36.Li B.B., Smith B., Hossain M.M. Extraction of phenolics from citrus peels: II. enzyme-assisted extraction method. Sep. Purif. Technol. 2006;48:189–196. doi: 10.1016/j.seppur.2005.07.019. [DOI] [Google Scholar]
- 37.Pan L.H., Wang J., Ye X.Q., Zha X.Q., Luo J.P. Enzyme-assisted extraction of polysaccharides from Dendrobium chrysotoxum and its functional properties and immunomodulatory activity. LWT-Food Sci. Technol. 2015;60:1149–1154. doi: 10.1016/j.lwt.2014.10.004. [DOI] [Google Scholar]
- 38.Tan S., Zhu R.W., Zhang J., Li G.F., Li L., Zou T. Extraction process optimization of polysaccharide in Gastonia elate by enzymatic method. Food. Res. Dev. 2017;38:50–53. [Google Scholar]
- 39.Chen Y., Yao F.K., Ming K., Wang D.Y., Hu Y.L., Liu J.G. Polysaccharides from traditional Chinese medicines: Extraction, purification, modification, and biological activity. Molecules. 2016;21:1705. doi: 10.3390/molecules21121705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Q., Li D.D., Pan Y.Y., Wu W. Effect of different extraction methods on the extraction ratio and antioxidant activity of polysaccharides from Gastrodia elata. Food Mach. 2017;33:146–150. [Google Scholar]
- 41.Wang N., Zhang Y., Wang X., Huang X., Fei Y., Yu Y., Shou D. Antioxidant property of water-soluble polysaccharides from Poria cocos Wolf using different extraction methods. Int. J. Biol. Macromol. 2016;83:103–110. doi: 10.1016/j.ijbiomac.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 42.Govender S., Pillay V., Chetty D.J., Essack S.Y., Dangor C.M., Govender T. Optimisation and characterisation of bioadhesive controlled release tetracycline microspheres. Int. Pharm. J. 2005;306:24–40. doi: 10.1016/j.ijpharm.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 43.Yin G.H., Dang Y.L. Optimization of extraction technology of the Lycium barbarum polysaccharides by Box–Behnken statistical design. Carbohydr. Polym. 2008;74:603–610. doi: 10.1016/j.carbpol.2008.04.025. [DOI] [Google Scholar]
- 44.Chen R.Z., Jin C.G., Tong Z.G., Lu J., Tan L., Tian L., Chang Q.Q. Optimization extraction, characterization and antioxidant activities of pectic polysaccharide from tangerine peels. Carbohydr. Polym. 2016;136:187–197. doi: 10.1016/j.carbpol.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 45.Fishman M.L., Chau H.K., Cooke P.H., Yadav M.P., Hotchkiss A.T. Physico-chemical characterization of alkaline soluble polysaccharides from sugar beet pulp. Food Hydrocolloid. 2009;23:1554–1562. doi: 10.1016/j.foodhyd.2008.10.015. [DOI] [Google Scholar]
- 46.Zhou C.S., Ma H.L. Ultrasonic degradation of polysaccharide from a red algae (Porphyrayezoensis) J. Agric. Food Chem. 2006;54:2223–2228. doi: 10.1021/jf052763h. [DOI] [PubMed] [Google Scholar]
- 47.Ebringerova A., Hromadkova Z. The effect of ultrasound on the structure and properties of the water-soluble corn hull heteroxylan. Ultrason. Sonochem. 1997;4:305–309. doi: 10.1016/S1350-4177(97)00037-0. [DOI] [PubMed] [Google Scholar]
- 48.Liu H., Bao J.G., Du Y.M., Zhou X., Kennedy J.F. Effect of ultrasonic treatment on the biochemphysical properties of chitosan. Carbohydr. Polym. 2006;64:553–559. doi: 10.1016/j.carbpol.2005.11.007. [DOI] [Google Scholar]
- 49.Reverchon E., Marco I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluid. 2006;38:146–166. doi: 10.1016/j.supflu.2006.03.020. [DOI] [Google Scholar]
- 50.Saravana P.S., Cho Y.J., Park Y.B., Woo H.C., Chun B.S. Structural, antioxidant, and emulsifying activities of fucoidan from Saccharina japonica using pressurized liquid extraction. Carbohydr. Polym. 2016;153:518–525. doi: 10.1016/j.carbpol.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 51.Yang N., Zhang N., Jin Y., Jin Z., Xu X. Development of a fluidic system for efficient extraction of mulberry leaves polysaccharide using induced electric fields. Sep. Purif. Technol. 2017;172:318–325. doi: 10.1016/j.seppur.2016.08.025. [DOI] [Google Scholar]
- 52.Glisic S., Ivanovic J., Ristic M., Skala D. Extraction of sage (Salvia officinalis L.) by supercritical CO2: Kinetic data, chemical composition and selectivity of diterpenes. J. Supercrit. Fluid. 2010;52:62–70. doi: 10.1016/j.supflu.2009.11.009. [DOI] [Google Scholar]
- 53.Chen Z., Zhang W., Tang X., Fan H., Xie X., Wan Q., Wu X., Tang J.Z. Extraction and characterization of polysaccharides from Semen Cassiae by microwaveassisted aqueous two-phase extraction coupled with spectroscopy and HPLC. Carbohydr. Polym. 2016;144:263–270. doi: 10.1016/j.carbpol.2016.02.063. [DOI] [PubMed] [Google Scholar]
- 54.Chen L., Zhang Y.P., Jin L.X. Preparation, characterization and anti-ageing activity of Gastrodia elataume polysaccharide. Acta Aliment. 2018;47:210–219. doi: 10.1556/066.2018.47.2.10. [DOI] [Google Scholar]
- 55.Zhu X.X., Zhang Y. Purification of Gastrodia elata polysaccharides. Chin. J. Ethnomed. Ethnopharm. 2010:102–103. [Google Scholar]
- 56.Xu S.Y., Huang X.S., Cheong K.L. Recent advances in marine algae Polysaccharides: Isolation, structure, and activities. Mar. Drugs. 2017;15:388. doi: 10.3390/md15120388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen C., Li X.X., Wei W., Liu X., Tian H.L., Feng Z.B., Hu H.Z., Zheng H.X. Macroporous adsorption resin for the purification of polysaccharides from Gastrodia elata. J. Sichuan Univ. (Natural Science Edition) 2018;55:1109–1115. [Google Scholar]
- 58.Runyon J.R., Ulmius M., Nilsson L. A perspective on the characterization of colloids and macromolecules using asymmetrical flow field-flow fractionation. Colloids. Surf. A. 2014;442:25–33. doi: 10.1016/j.colsurfa.2013.04.010. [DOI] [Google Scholar]
- 59.Song H., Lin J.H., Zhu X., Chen Q. Developments in high-speed countercurrent chromatography and its applications in the separation of terpenoids and saponins. J. Sep. Sci. 2016;39:1574–1591. doi: 10.1002/jssc.201501199. [DOI] [PubMed] [Google Scholar]
- 60.Zhou X.Y., Zhang J., Xu R.P., Ma X., Zhang Z.Q. Aqueous biphasic system based on low-molecular-weight polyethylene glycol for one-step separation of crude polysaccharides from Pericarpium granati using high-speed countercurrent chromatography. J. Chromatogr. A. 2014;1362:129–134. doi: 10.1016/j.chroma.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 61.Yang W., Wang Y., Li X., Yu P. Purification and structural characterization of Chinese yam polysaccharide and its activities. Carbohydr. Polym. 2015;117:1021–1027. doi: 10.1016/j.carbpol.2014.09.082. [DOI] [PubMed] [Google Scholar]
- 62.Li W.L., Wu T. Rapid separation of polysaccharides using a novel spiral coil column by high-speed countercurrent chromatography. J. Sep. Sci. 2016;39:1404–1410. doi: 10.1002/jssc.201501402. [DOI] [PubMed] [Google Scholar]
- 63.Yu P., Sun H.S. Purification of a fucoidan from kelp polysaccharide and its inhibitory kinetics for tyrosinase. Carbohydr. Polym. 2014;99:278–283. doi: 10.1016/j.carbpol.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 64.Xu Z., Li X., Feng S.L., Liu J., Zhou L.J., Yuan M., Ding C.B. Characteristics and bioactivities of different molecular weight polysaccharides from camellia seed cake. Int. J. Biol. Macromol. 2016;91:1025–1032. doi: 10.1016/j.ijbiomac.2016.06.067. [DOI] [PubMed] [Google Scholar]
- 65.Li C., Wang J.R., Ji X.H., Lu X.L. Isolation of Gastrodia elata. polysaccharides and analysis of its composition of monosaccharide. Chin. Agric. Sci. Bull. 2008;24:89–92. [Google Scholar]
- 66.Karlsson G., Winge S., Sandberg H. Separation of monosaccharides by hydrophilic interaction chromatography with evaporative light scattering detection. J. Chromatogr. A. 2005;1092:246–249. doi: 10.1016/j.chroma.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 67.Márquez-Sillero I., Cárdenas S., Valcárcel M. Comparison of two evaporative universal detectors for the determination of sugars in food samples by liquid chromatography. Microchem. J. 2013;110:629–635. doi: 10.1016/j.microc.2013.07.008. [DOI] [Google Scholar]
- 68.Menshova R.V., Anastyuk S.D., Ermakova S.P., Shevchenko N.M., Isakov V.I., Zvyaginstseva T.N. Structure and anticancer activity in vitro of sulfated galactofucan from brown alga Alaria angusta. Carbohydr. Polym. 2015;132:118–125. doi: 10.1016/j.carbpol.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 69.Hu D.J., Cheong K.L., Zhao J., Li S.P. Chromatography in characterization of polysaccharides from medicinal plants and fungi. J. Sep. Sci. 2013;36:1–19. doi: 10.1002/jssc.201200874. [DOI] [PubMed] [Google Scholar]
- 70.Guan J., Li S.P. Discrimination of polysaccharides from traditional Chinese medicines using saccharide mapping-enzymatic digestion followed by chromatographic analysis. J. Pharm. Biomed. Anal. 2010;51:590–598. doi: 10.1016/j.jpba.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 71.Wu D.T., Meng L.Z., Wang L.Y., Lv G.P., Cheong K.L., Hu D.J., Guan J., Zhao J., Li S.P. Chain conformation and immunomodulatory activity of a hyperbranched polysaccharide from Cordyceps sinensis. Carbohydr. Polym. 2014;110:405–414. doi: 10.1016/j.carbpol.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 72.Wu D.T., Xie J., Wang L.Y., Ju Y.J., Lv G.P., Leong F., Zhao J., Li S.P. Characterization of bioactive polysaccharides from Cordyceps militaris produced in China using saccharide mapping. J. Funct. Foods. 2014;9:315–323. doi: 10.1016/j.jff.2014.05.005. [DOI] [Google Scholar]
- 73.Wu D.T., Cheong K.L., Wang L.Y., Lv G.P., Ju Y.J., Feng K., Zhao J., Li S.P. Characterization and discrimination of polysaccharides from different species of Cordyceps using saccharide mapping based on PACE and HPTLC. Carbohydr. Polym. 2014;103:100–109. doi: 10.1016/j.carbpol.2013.12.034. [DOI] [PubMed] [Google Scholar]
- 74.Wu D.T., Xie J., Hu D.J., Zhao J., Li S.P. Characterization of polysaccharides from Ganoderma spp. using saccharide mapping. Carbohydr. Polym. 2013;97:398–405. doi: 10.1016/j.carbpol.2013.04.101. [DOI] [PubMed] [Google Scholar]
- 75.Cheong K.L., Wu D.T., Hu D.J., Zhao J., Cao K.Y., Qiao C.F., Han B.X., Li S.P. Comparison and characterization of the glycome of Panax species by high performance thin-layer chromatography. J. Planar Chromat. Modern TLC. 2014;27:449–453. doi: 10.1556/JPC.27.2014.6.8. [DOI] [Google Scholar]
- 76.Zhou B.H., Yang L., Yuan Y., Shen H., Feng Q., Guo Z.L., Liu G. Isolation and structure identification of an acidic and heteropolysaccharide from Gastrodia elataume. Chin. J. Hosp. Pharm. 2009;29:2002–2006. [Google Scholar]
- 77.Cheong K.L., Wu D.T., Zhao J., Li S.P. A rapid and accurate method for the quantitative estimation of natural polysaccharides and their fractions using high performance size exclusion chromatography coupled with multi-angle laser light scattering and refractive index detector. J. Chromatogr. A. 2015;1400:98–106. doi: 10.1016/j.chroma.2015.04.054. [DOI] [PubMed] [Google Scholar]
- 78.Cheong K.L., Wu D.T., Deng Y., Leong F., Zhao J., Zhang W.J., Li S.P. Qualitation and quantification of specific polysaccharides from Panax species using GC- MS, saccharide mapping and HPSEC-RID-MALLS. Carbohydr. Polym. 2016;153:47–54. doi: 10.1016/j.carbpol.2016.07.077. [DOI] [PubMed] [Google Scholar]
- 79.Wu D.T., Lam S.C., Cheong K.L., Wei F., Lin P.C., Long Z.R., Lv X.J., Zhao J., Ma S.C., Li S.P. Simultaneous determination of molecular weights and contents of water-soluble polysaccharides and their fractions from Lycium barbarum collected in China. J. Pharm. Biomed. Anal. 2016;129:210–218. doi: 10.1016/j.jpba.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 80.Lu X., Mo X., Guo H., Zhang Y. Sulfation modification and anticoagulant activity of the polysaccharides obtained from persimmon (Diospyros kaki L.) fruits. Int. J. Biol. Macromol. 2012;51:1189–1195. doi: 10.1016/j.ijbiomac.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 81.Li S.Q., Shah N.P. Antioxidant and antibacterial activities of sulphated polysaccharides from Pleurotus eryngii and streptococcus thermophilus ASCC 1275. Food Chem. 2014;165:262–270. doi: 10.1016/j.foodchem.2014.05.110. [DOI] [PubMed] [Google Scholar]
- 82.Hattori K., Yoshida T., Nakashima H., Premanathan M., Aragaki R., Mimura T., Kaneko Y., Yamamoto N., Uryu T. Synthesis of sulfonated amino-polysaccharides having anti-HIV and blood anticoagulant activities. Carbohydr. Res. 1998;312:1–8. doi: 10.1016/S0008-6215(98)00198-0. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y., Peng Y., Wei X., Yang Z., Xiao J., Jin Z. Sulfation of tea polysaccharides: Synthesis, characterization and hypoglycemic activity. Int. J. Biol. Macromol. 2010;46:270–274. doi: 10.1016/j.ijbiomac.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 84.Li S., Xiong Q., Lai X., Li X., Wan M., Zhang J., Yan Y., Cao M., Lu L., Guan J., et al. Molecular modification of polysaccharides and resulting bioactivities. Compr. Rev. Food. Sci. F. 2016;15:237–250. doi: 10.1111/1541-4337.12161. [DOI] [PubMed] [Google Scholar]
- 85.Wu Y., Ye M., Du Z., Jing L., Surahio M., Yang L. Carboxymethylation of an exopolysaccharide from Lachnum and effect of its derivatives on experimental chronic renal failure. Carbohydr. Polym. 2014;114:190–195. doi: 10.1016/j.carbpol.2014.07.075. [DOI] [PubMed] [Google Scholar]
- 86.Chen T., Li B., Li Y., Zhao C., Shen J., Zhang H. Catalytic synthesis and antitumor activities of sulfated polysaccharide from Gynostemma pentaphyllum Makino. Carbohydr. Polym. 2011;83:554–560. doi: 10.1016/j.carbpol.2010.08.024. [DOI] [Google Scholar]
- 87.Liu X., Wan Z., Shi L., Lu X. Preparation and antiherpetic activities of chemically modified polysaccharides from Polygonatum cyrtonema Hua. Carbohydr. Polym. 2011;83:737–742. doi: 10.1016/j.carbpol.2010.08.044. [DOI] [Google Scholar]
- 88.Lee C.M., Jeong H.J., Kim D.W., Lee K.Y. Alginate/carboxymethyl scleroglucan hydrogels for controlled release of protein drugs. Macromol. Res. 2008;16:429–433. doi: 10.1007/BF03218541. [DOI] [Google Scholar]
- 89.Qin T., Chen J., Wang D., Hu Y., Wang M., Zhang J., Nguyen T.L., Liu C., Liu X. Optimization of selenylation conditions for Chinese angelica polysaccharide based on immune-enhancing activity. Carbohydr. Polym. 2013;92:645–650. doi: 10.1016/j.carbpol.2012.08.097. [DOI] [PubMed] [Google Scholar]
- 90.Du X., Zhang J., Lv Z., Ye L., Yang Y., Tang Q. Chemical modification of an acidic polysaccharide (TAPA1) from Tremella aurantialba and potential biological activities. Food. Chem. 2014;143:336–340. doi: 10.1016/j.foodchem.2013.07.137. [DOI] [PubMed] [Google Scholar]
- 91.Hou Y., Wang J., Jin W., Zhang H., Zhang Q. Degradation of Laminaria japonica fucoidan by hydrogen peroxide and antioxidant activities of the degradation products of different molecular weights. Carbohydr. Polym. 2012;87:153–159. doi: 10.1016/j.carbpol.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 92.Han B., Gao Y., Wang Y., Wang L., Shang Z., Wang S., Pei J. Protective effect of a polysaccharide from rhizoma Atractylodis Macrocephalae on acute liver injury in mice. Int. J. Biol. Macromol. 2016;87:85–91. doi: 10.1016/j.ijbiomac.2016.01.086. [DOI] [PubMed] [Google Scholar]
- 93.Tao Y., Xu W. Microwave-assisted solubilization and solution properties of hyperbranched polysaccharide. Carbohydr. Res. 2008;343:3071–3078. doi: 10.1016/j.carres.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 94.Zhong K., Zhang Q., Tong L., Liu L., Zhou X., Zhou S. Molecular weight degradation and rheological properties of schizophyllan under ultrasonic treatment. Ultrason. Sonochem. 2015;23:75–80. doi: 10.1016/j.ultsonch.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 95.Qiu H., Yang B., Pei Z., Zhang Z., Ding K. WSS25 Inhibits growth of xenografted hepatocellular cancer cells in nude mice by disrupting angiogenesis via blocking bone morphogenetic protein (BMP)/Smad/Id1 Signaling. J. Biol. Chem. 2010;285:32638–32646. doi: 10.1074/jbc.M110.105544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xiao F., Qiu H., Zhou L., Shen X., Yang L., Ding K. WSS25 inhibits dicer, downregulating microRNA-210, which targets ephrin-A3, to suppress human microvascular endothelial cell (HMEC-1) tube formation. Glycobiology. 2013;23:524–535. doi: 10.1093/glycob/cwt004. [DOI] [PubMed] [Google Scholar]
- 97.Chen X., Xiao F., Wang Y., Fang J., Ding K. Structure–activity relationship study of WSS25 derivatives with anti-angiogenesis effects. Glycoconj. J. 2012;29:389–398. doi: 10.1007/s10719-012-9424-z. [DOI] [PubMed] [Google Scholar]
- 98.Itoh K., Udagawa N., Katagiri T., Iemura S., Ueno N., Yasuda H., Higashio K., Quinn J.M., Gillespie M.T., Martin T.J., et al. Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factor-kappaB ligand. Endocrinology. 2001;142:3656–3662. doi: 10.1210/endo.142.8.8300. [DOI] [PubMed] [Google Scholar]
- 99.Pham L., Beyer K., Jensen E.D., Rodriguez J.S., Davydova J., Yamamoto M., Petryk A., Gopalakrishnan R., Mansky K.C. Bone morphogenetic protein 2 signaling in osteoclasts is negatively regulated by the BMP antagonist, twisted gastrulation. J. Cell. Biochem. 2011;112:793–803. doi: 10.1002/jcb.23003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu B., Gao J.M., Li F., Gong Q.H., Shi J.S. Gastrodin attenuates bilateral common carotid artery occlusion-induced cognitive deficits via regulating Aβ-related proteins and reducing autophagy and apoptosis in rats. Front. Pharmacol. 2018;9:405. doi: 10.3389/fphar.2018.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shi A.H., Xiang J.M., He F.Y., Zhu Y.P., Zhu G.B., Lin Y.H., Zhou N.N. The phenolic components of Gastrodia elata improve prognosis in rats after cerebral ischemia/reperfusion by enhancing the endogenous antioxidant mechanisms. Oxid. Med. Cell. Longev. 2018;2018:7642158. doi: 10.1155/2018/7642158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xie X.Y., Chao Y.M., Du Z., Zhang Y. Effects of polysaccharides from Gastrodia elata on anti-aging of ageing Mice. Pharm. J. Chin. PLA. 2010;26:206–209. [Google Scholar]
- 103.Li X.B., Zhan J.P., Zhang Y.T., Xie Z.L., Zhu Y.Q., Chen Y.L., Cheng K., Wang J.M., Li R.Q. Effect of polysaccharide from Gastrodia elata on humoral immune function in immunosuppressed mice induced by cyclophosphamide. Chin. J. Gerontol. 2016;36:1027–1028. [Google Scholar]
- 104.Li H.B., Wu F., Miao H.C., Xiong K.R. Effects of polysaccharide of Gastrodia elataume and electro-acupuncture on expressions of brain-derived neurotrophic factor and stem cell factor protein in caudate putamen of focal cerebral ischemia rats. Med. Sci. Monit. Basic. Res. 2016;22:175–180. doi: 10.12659/MSMBR.901524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou B.H., Tan J., Zhang C., Wu Y. Neuroprotective effect of polysaccharides from Gastrodia elataume against corticosterone-induced apoptosis in PC12 cells via inhibition of the endoplasmic reticulum stress-mediated pathway. Mol. Med. Rep. 2018;17:1182–1190. doi: 10.3892/mmr.2017.7948. [DOI] [PubMed] [Google Scholar]
- 106.Kim K.J., Lee O.H., Han C.K., Kim Y.C., Hong H.D. Acidic Polysaccharide extracts from Gastrodia rhizomes suppress the atherosclerosis risk index through inhibition of the serum cholesterol composition in sprague dawley rats fed a high-fat diet. Int. J. Mol. Sci. 2012;13:1620–1631. doi: 10.3390/ijms13021620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang R., Zhao J., Wu F., Miao H.C., Ding J., Xiong K.R. Effect of electroacupuncture plus polysaccharide of Gastrodia elataume on expression of nestin and brain derived neurotrophic factor in the basolateral amygdala of focal cerebral ischemia rats. Zhen Ci Yan Jiu. 2016;41:230–234. [PubMed] [Google Scholar]
- 108.Zan L.X., Wang Y., Hu L.L., Xu H., Zhao H., Yang P.J., Jiang J.L., Chen C. Application of Gastrodia elata polysaccharide to the skin cream. J. Shaanxi Univ. Tech. (Natural Science Edition) 2016;32:53–64. [Google Scholar]
- 109.Wang Y., Zan L.X., Hu L.L., Xu H., Yang P.J., Zhao H., Chen C. Formulation study of O/W creams cosmetics Tianma crude extract. Asia-Pac. Tradit. Med. 2016;12:21–23. [Google Scholar]
- 110.Du S.J., Chen D.L. Extraction of polysaccharides from Tianma stem and preparation of moisturizer. Yunnan Chem. Tech. 2018;45:102–104. [Google Scholar]
- 111.Zheng J., Sun M.M., Hu A.J., Hu X.H., Ren Y.Y., Yu S.Y. Extraction of Polysaccharide from Gastrodia elata Blume and preparation of its drinks. Food Res. Dev. 2018;39:123–128. [Google Scholar]
- 112.Seddon K.R. Ionic liquids for clean technology. J. Chem. Tech. Biotechnol. 1997;68:351–356. doi: 10.1002/(SICI)1097-4660(199704)68:4<351::AID-JCTB613>3.0.CO;2-4. [DOI] [Google Scholar]
- 113.Xie H.B., Zhang S.B., Li S.H. Chitin and chitosan dissolved in ionic liquids as reversible sorbents of CO2. Green Chem. 2006;8:630–633. doi: 10.1039/b517297g. [DOI] [Google Scholar]
- 114.Liu H.Z., Li Y.A., Gao J., Shi A., Liu A., Hu H., Putri N., Yu H., Fan W., Wang Q. Effects of microfluidization with ionic liquids on the solubilization and structure of β-d-glucan. Int. J. Biol. Macromol. 2015;84:394–401. doi: 10.1016/j.ijbiomac.2015.12.014. [DOI] [PubMed] [Google Scholar]