Abstract
The results of studies that assessed the impact of metformin treatments on gestational diabetes mellitus (GDM) in patients with polycystic ovary syndrome (PCOS) are inconclusive. In addition, the impact of time and duration of metformin therapy for an optimum reduction of GDM has not been reported in these studies. This study aimed to summarize current knowledge regarding the effect of metformin-therapy before conception versus throughout pregnancy on the risk of GDM in women with PCOS. PubMed, Scopus, Google Scholar and ScienceDirect databases were searched to identify relevant studies. Both fixed and random effect models were used. Subgroup analyses were performed based on the on the study methodology. The association between the PCOS status and GDM was assessed using the univariate and multiple meta-regression analysis adjusted by the BMI and metformin therapy. Forty-eight of 1397 identified studies were included involving 5711 PCOS patients and 20,296 controls. Regardless of metformin therapy, the prevalence of GDM diagnosed in the second trimester among women with PCOS was significantly higher than healthy controls that was independent of obesity. Including all studies, the increased risk of GDM among women with PCOS, compared to healthy controls, disappeared after the adjustment of metformin-therapy (β = 0.08, 95% CI 0.04, 0.2; p = 0.624). By excluding observational studies as a source of bias, the prevalence of GDM among women with PCOS treated using metformin before conception till the end of pregnancy did not differ from treated just before conception (β = − 0.09, 95% CI − 0.2, 0.02; p = 0.092) or those without metformin therapy (β = − 0.05, 95% CI − 0.07, 0.04; p = 0.301). The results remained unchanged after the subgroup analysis based on the methodology of RCTs and non-RCTs studies. The main body of literature in the current meta-analysis was observational, which may be mixed with some sources of bias. Also, a lack of well-designed and high quality interventional studies means that the findings should be interpreted with cautious. In this respect, decisions regarding the continuation or discontinuation of metformin therapy in women with PCOS are somewhat arbitrary and can be made individually based on the patient’s condition given the presence or absence of other GDM risk factors. Additional well-designed RCTs still need for precise recommendation.
Electronic supplementary material
The online version of this article (10.1186/s13098-019-0453-7) contains supplementary material, which is available to authorized users.
Keywords: Gestational diabetes mellitus, Meta-analysis, Meta-regression, Metformin therapy, Polycystic ovary syndrome
Background
Polycystic ovary syndrome (PCOS) with a prevalence of 7–15% is one of the most common endocrinopathies among women in the reproductive age [1]. Hyperandrogenism and/or hyperandrogenemia, chronic oligo-ovulation and polycystic ovaries morphology are the main characteristics of this syndrome. The exact underlying pathogenic mechanisms of PCOS are not fully understood, but it is believed that insulin resistance (IR) with compensatory hyperinsulinemia is the cornerstone of its pathogenesis [2, 3].
It is well documented that non-pregnant women with PCOS face more metabolic and reproductive complications with an early or late term syndrome’s risks [4–6]. However, the effects of PCOS on pregnancy outcomes remain controversial. Normal pregnancy is characterized by the physiologic insulin resistance state, which is at its peak in the third trimester of pregnancy. Human placental lactogen, estradiol, progesterone and cortisol regulate the insulin status during pregnancy, which induce the diabetogenis state due to the facilitated diffusion and transfer of glucose to the fetus [7–9]. Pregnant women suffering from PCOS experience the additive preexisting state of insulin resistance, which may accompany adverse pregnancy outcomes [10]. Metformin as an insulin sensitizing agent have been wildly used for PCOS, but its effect on the prevention of GDM in PCOS is controversial.
According to available meta-analyses studies, women with PCOS have 2.8–4.3 higher risk of GDM compared to healthy controls [5, 10–13]. Mopreover, several studies were conducted to assess the impact of metformin treatments on GDM in patients with PCOS [14–19]. However, their results were inconclusive. For instance, Zheng et al. [19] in a meta-analysis study showed that the incidence of GDM was significantly lower among pregnant women with PCOS receiving metformin than those not received. Conversely, according to another meta-analysis, Zhuo et al. [17], metformin did not significantly reduced GDM in women with PCOS. These controversial results may be partly explained by the use of different eligibility criteria for the type of included studies (interventional versus observational) or selecting a non-homogenous control groups (PCOS not treated, or both not treated PCOS and non-PCOS ones) [14–17, 19], and not adjustment for most relevant confounders including age and body mass index. Moreover [18, 19], most of those meta-analyses did not assess the quality of included studies [14, 15, 18, 19] and none of them evaluated the risk of bias [14–19]. In addition, the impact of time and duration of metformin therapy for an optimum reduction of GDM has not been reported in these studies. Hence, we decided to conduct a meta-analysis to assess the effect of metformin-therapy before conception versus all throughout the pregnancy on the risk of gestational diabetes mellitus (GDM) in women with polycystic ovary syndrome (PCOS) after the adjustment for type of study (observational versus trials), age and BMI.
Methods
This systematic review and meta-analysis was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [20] with the following objectives:
Study of the prevalence of GDM among women with PCOS regardless of metformin therapy, compared to healthy controls;
Study of the effect of obesity on the prevalence of GDM among women with PCOS, compared to healthy controls;
Study of the prevalence of GDM among women with PCOS treated with metformin just before conception/before conception till the end of pregnancy, compared to healthy controls;
Study of the prevalence of GDM among women with PCOS treated with metformin before conception until the end of pregnancy, compared to women with PCOS treated with metformin just before conception.
Study of the prevalence of GDM among women with PCOS treated with metformin only before conception/before conception until the end of pregnancy, compared to untreated women with PCOS.
Search strategy, study selection and data extraction
A comprehensive literature search was performed in the PubMed (including Medline), Web of Science and Scopus databases for retrieving relevant randomized or non-randomized controlled trials (RCTs or NRS), cohort studies, cross sectional, and case–control studies published in English language up to August 2017. In addition, a manual search of the reference list of relevant studies was conducted to expand the search coverage.
The following MeSH terms keywords, alone or in combination, were used for the search process: “Polycystic Ovary Syndrome” OR “Polycystic Ovarian Syndrome” OR “polycystic ovary disease” OR “PCOS” OR “PCOD” OR “Stein Leventhal Syndrome” AND “insulin resistance” OR “gestational diabetes” OR “pregnancy complications” OR “obstetric complications” OR “adverse pregnancy outcome”.
The initial selection of articles was performed based on titles’ screening, followed by a second round of selection performed by one reviewer, who deleted duplicates and reviewed the abstracts of all remaining records. Any disagreement in the selection of abstracts was resolved through consensus or by a senior reviewer. The full text articles were evaluated.
Studies with subjects having diabetes or currently using antidiabetic drugs except metformin, reporting the prevalence of PCOS retrospectively in women with GDM and non-original studies were excluded. General characteristics of the studies including “authors, journal, publication year, design, recruitment source, ethnicity, sample size for cases and controls as well as group characteristics including diagnostic criteria of PCOS, screening time and strategy of GDM, age, body mass index (BMI), duration of metformin therapy, adjustment methods of confounders and prevalence of GDM were extracted.
Quality assessment
Quality of the studies was critically apprised in terms of methods and results. Two reviewers who were blind to the study’s author, journal and institution evaluated quality of the studies independently. Disagreements were resolved through consensus or by a senior reviewer.
The modified Consolidated Standards of Reporting Trials (CONSORT) was used as a validated quality assessment checklist for clinical trials [21]. Studies with a score ≥ 70% of the highest level of the CONSORT checklist score were considered as high quality, those with 40–70% of the score as moderate, and those with 20–40% of the score as low quality and with < 20% of the score as very low quality.
The quality of observational studies was also evaluated using the modification of the Newcastle–Ottawa Quality Assessment Scale for Nonrandomized Studies (NRS) [22], which assessed the quality of published nonrandomized studies in terms of selection, comparability and outcome. Studies with a score above 6 were considered high quality, 3–5 moderate and below than 3 low quality.
Risk of bias assessment
The risk of bias of NRS and other methodological studies was assessed using the ROBINS [23] and Cochrane Collaboration’s tool, respectively [24]. In this respect, the risk of bias based on the subgroups of low-, moderate-, critical- and unclear risk was assessed.
Statistical analysis
The STATA software package (version 12; STATA Inc., College Station, TX, USA) was used to conduct statistical analysis. Heterogeneity was evaluated using the Chi square test and P value > 0.05 was interpreted as homogeneity. Publication bias was assessed using the Begg’s test as a formalized statistical test for statistically estimating funnel plot asymmetry to find any possible publication bias. Accordingly, the random effect model without any correction were used for such analysis. The Meta-prop method was used for the pooled estimation of the prevalence of GDM. The Mantel–Haenszel method for meta-analysis was applied for the pooled estimation of age and BMI in various subgroups including women with PCOS (without metformin therapy, metformin therapy just before conception, and metformin therapy before conception until the end of pregnancy) and non-PCOS women. In addition, subgroup analysis was performed based on the study methodology. The association between the PCOS status and GDM was assessed using the univariate and multiple meta-regression analysis adjusted by the BMI and metformin therapy. The prevalence of GDM, PCOS status and weight given to each study was calculated using the fixed effect model based on the inverse of within-study variance, and was presented through the scatter bubble plots. P > 0.05 was set as statistically significant.
Results
Search and study selection
The search yielded 1397 potentially relevant articles. The flow chart indicating the selection process for the systematic review and meta-analysis was depicted as Additional file 1: Fig. S1. According to the inclusion criteria, 48 full-text articles were selected for the meta-analysis.
Study characteristics
Forty-eight studies published between 1998 and 2017 were included in the systematic review. Data on 5711 women with PCOS and 20,296 healthy controls was presented in Table 1. Overall, most studies were judged as having a low risk of bias for evaluated domains (Additional file 1: Figs. S2–S5). In addition, quality of the body of evidence in the current meta-analysis was classified as moderate. Twenty-three studies were identified as high quality [25–47] and other as moderate quality (Additional file 1: Tables S1–S3).
Table 1.
Summary of studies assessing GDM prevalence in women PCOS and controls with or without metformin therapy
| Author, year | PCOS criteria | Time screening, Guideline | Group 1 characteristics (PCOS patient with metformin therapy in pregnancy) | Group 2 characteristics (PCOS patient without metformin therapy in pregnancy) | Group 3: characteristics (Non-PCOS pregnant group) | Metformin therapy in PCOS | Prevalence of GDM (%) |
|---|---|---|---|---|---|---|---|
| Abd El Hameed et al. (2011)1 | Rotterdam | Time: 8,24,36 w, guideline: NM° | N = 31, Age: 30.2 (3.8), BMI: 29.22 (2.3) | N = 26, Age: 28.1 (4.3), BMI: 28.3 (1.9) | – |
Group 1: before conception till the end of pregnancy Group 2: – |
2ed trimester Group 1: 3.2 Group 2: 23.08 |
| Begum et al. (2009)1 | Rotterdam | Time: NM guideline: NM | N = 29, Age: 28.1 (2.9), BMI: 28.2 (2.3) | N = 30, Age: 26.1 (3.6), BMI: 27.9 (2.4) | – |
Group 1: before conception till the end of pregnancy Group 2: before conception |
Group 1: 3.44 Group 2: 30 |
| Ashrafi et al. (2014)2 | Rotterdam | Time: 24–28 w, guideline: ADA | – | N = 234, Age: 29.6 (3.9), BMI: (26.1) |
Group 3a: non-PCOS, infertile N = 234, Age: 30.7 (4.7), BMI: 25.5 (4.2) Group 3b: non-PCOS, fertile N = 234, Age: 26.4 (5.5), BMI: 25.7 (3.8) |
Group 2: before conception Group 3: – Group 4: – |
Group 1: 44.4 Group 3a: 29.9 Group 3b: 7.3 |
| Ashrafi et al. (2017)2 | Rotterdam | Time: 24–28 w, guideline: ADA | – |
Group 2a: HA + AO + PCO N = 113, Age: 29.5 (3.8), BMI: 26.1 (3.1) Group 2b: AO + HA N = 5, Age: 28.6 (5.4), BMI: 27.7 (3.2) Group 2c: HA + PCO N = 74, Age: 29.90 (4.2), BMI: 25.94 (4.1) Group 2d: AO + PCO N = 16, Age: 28.3 (2.8), BMI: 25.9 (2.1) |
– |
Group 2a: no Group 2b: no Group 2c: no Group 2d: no |
Group 2a: 46 Group 2b: 100 Group 2c: 41.9 Group 2d: 43.8 |
| Bjercke et al. (2002)3 | NIH | TIME: NM, guideline: NM | – |
Group 2 a: without IR N = 29, Age: 31.5 (3.8), BMI: 25.2 (3.9) Group 2 b: with IR N = 23, Age: 31.1 (4.0), BMI: 27.7 (5.5) |
N = 355, Age: 32.7 (3.4), BMI: 21.9 (2.7) | – |
Group 2a: 7 Group 2b: 9 Group 3: 0.6 |
| D’Anna et al. (2012)3 | NM | Time: 24–28 w, guideline: NM | – |
N = 37, Age: 30.6 (4.2), BMI: 24.7 (3.9) |
– | Group 2: before conception | Group 2: 54 |
| De Fre`ne et al. (2014)3 | Rotterdam | Time: ~ 24 w, guideline: ADA | – |
Group 2 a: overweight N = 93, Age: 29 (4.2), BMI: 30.8 (27.7–33.5)a Group 2 b: Normal weight N = 107, Age: 28.4 (3.1), BMI: 20.9 (20–22.3)a |
– |
Group 2 a: NO Group 2 b: NO |
Group 2 a: 8.2 Group 2 b: 0 |
| De Leo et al. (2011)3 | AES | Time: NM, guideline: NM | N = 98, Age: 32 (6), BMI: 28.3 (2.1) | – | N = 110, Age: 33 (5), BMI: 26.6 (1.2) |
Group 1: before conception till 37 weeks’ gestation Group 2:– |
Group 1: 0 Group 3: 12.5 |
| deWilde et al. (2015)3 | Rotterdam | Time: 24–26 w, guideline: ADA | – | N = 72, Age: 29.6 [26.8–31.8]a, BMI: 24.4 [21.6–28.9]a | – | Group 2: before conception | Group 2: 31 |
| deWilde et al. (2014)3 | Rotterdam | Time: 24–26 w, guideline: NM | – | N = 189, Age: 29 [27–31]a, BMI: 24 [21–28]a | – | Group 2: before conception | Group 2: 22 |
| Dmitrovic et al. (2011)2 | NIH | Time: 6–10, 12–16, 24–28, 34–38 w, guideline: ADA | – | N = 17, Age: 29 (4), BMI: 32 (8) | N = 17, Age: 31 (5), BMI: 26 (7) |
Group 2: – Group 3: – |
Group 2: 47 Group 3: 12 |
| Elkholi et al. (2016)2 | Rotterdam | Time: 24–28 w, guideline: ADA | – |
Group 2a: metabolically obese N: 62, Age: 22.3 (1.2), BMI: 21.4 (1.5) Group 2b: metabolically healthy N: 47, Age: 21.1 (1.6), BMI: 21.6 (1.4) |
N: 35, Age: 20.4 (1.3), BMI: 21.3 (1.4) |
Group 2a: before conception Group 2b: before conception Group 3: No |
Group 2a: 9.8 Group 2b: 0 Group 3: 0 |
| Fougner et al. (2008)1 | Rotterdam | Time: 19, 32, 36 w, guideline: WHO | N = 18, Age: 28.9 (26.5–31.4)a, BMI: 32.1 (29.1–35.2)a | N: 22, Age: 28.3 (26.6–30.0)a, BMI: 29.3 (25.8–32.9)a | – |
Group 1: before conception till the end of pregnancy Group 2: before conception |
First trimester Group 1: 11.1 Group 2: 27.2 2ed trimester Group 1: 11.1 Group 2: 4.5 3rd trimester Group 1: 22.2 Group 2: 9 |
| Glueck et al. (2004)3 | Rotterdam | Time: 26–28 w, guideline: ADA | N = 90, Age: 33 (5), BMI: 33.8 (7.8) | – | N = 252, Age: 29 (6), BMI: 25.6 (5.9) |
Group 1: before conception till the end of pregnancy Group 3: – |
Group 1: 9.5 Group 3: 15.9 |
| Glueck et al. (2004)3 | Rotterdam | Time: 26–28 w, guideline: ADA | N = 39, Age: 30 (4), BMI: 34 (8.2) | – | – | Group 1: before conception till the end of pregnancy | Group 1: 7.6 |
| Glueck et al. (2002)3 | NIH | Time: 26–28 w, guideline: ADA | N = 33, Age: 34 (8), BMI: 33.9 | – | – | Group 1: before conception till the end of pregnancy | Group 1: 33 |
| Glueck et al. (2013)3 | Rotterdam | Time: NM, guideline: NM | N = 76, Age: 32 (5), BMI: 33.3 (7.4) | – | N = 156, Age: 30 (6), BMI: 26.9 (6.6) |
Group 1: before conception till the end of pregnancy Group 3:– |
Group 1: 10.5 Group 3: 14.7 |
| Glueck et al. (2008)3 | Rotterdam | Time: 26–28 w, guideline: ADA | N = 142, Age: 30 (5), BMI: 33.5 (7.9) | – | – | Group 1: before conception till the end of pregnancy | Group 1: 7 |
| Glueck et al. (2002)3 | Rotterdam | Time: 26–28 w, guideline: ADA | – | N = 68, Age: –, BMI: 33 (29–38.8)a | – | Group 2: before conception | Group 2: |
| Haakova et al. (2003)3 | Rotterdam | Time: second, third trimester, guideline: NM | – | N = 66, Age: 29.8 (4.9), BMI: 23.2 (3.8) | N = 66, Age: 29 (4.9), BMI: 23.2 (3.8) |
Group 2: – Group 3: – |
Group 2: 4.92 Group 3: 12.12 |
| Han et al. (2011)3 | Rotterdam | Time: 24 w, guideline: ADA | – |
Group 2a: Obese N = 64, Age: 31.6 (3.1), BMI: 27.46 (2.4) Group 2b: Non-obese N = 272, Age: 31.2 (2.7), BMI: 20.45 (2.0) |
Group 3a: Obese N = 117, Age: 32.2 (3.2), BMI: 27.5 (2) Group 3b: Non-obese N = 886, Age: 32.5 (2.8), BMI: 20.5 (1.9) |
Group 2a: no Group 2b: no Group 3a: no Group 3b: no |
Group 2a: 10.5 Group 2b: 1.1 Group 3a: 8.6 Group 3b: 1.8 |
| Hassanzahraeiet al. (2007)3 | NIH | Time: 24–28 w, guideline: NDDG | – | N = 47, Age: 27.8 (5.2), BMI: 25.1 (4.4) | N = 100, Age: 28 (4.9), BMI: 23.4 (3.3) |
Group 2: – Group 3: – |
Group 2: – Group 3: – |
| Joham et al. (2014)2 | NM | Time: NM, guideline: NM | – | N = 478, Age: 30.5 (1.4), BMI: 28 (7.2) | N = 8134, Age: 30.6 (1.5), BMI: 25.1 (5.6) |
Group 2: – Group 3: – |
Group 2: 11.2 Group 3: 3.8 |
| Khattab et al. (2011)3 | Rotterdam | Time: 5–12, 19, 32, 36 w, guideline: WHO | N = 31, Age: 30.2 (3.8), BMI: 29.22 (2.3) | N = 31, Age: 30.2 (3.8), BMI: 29.22 (2.3) | – |
Group 1: before conception till the end of pregnancy Group 2: before conception |
Group 1: 4 Group 2: 20 |
| Kollmann et al. (2015)3 |
1-NIH 2. Rotterdam (HA + PCO) 3. Rotterdam (OA + PCO) |
Time: 24–28 w, guideline: IADPSG | – |
Group 2a: NIH criteria N = 85, Age: 29 (26–32)a, BMI: 24.3 (21.4–29.2)a Group 2b: Rotterdam (HA + PCO) N = 14, Age: 31 (26–33) a, BMI: 25.5 (22.1–31.2)a Group 2c: Rotterdam (OA + PCO) N = 78, Age: 30 (27–33)a, BMI: 24.2 (20.5–29.7)a |
N = 708,Age: 30 (25–34)a, BMI: 22.5 (20.5–25.8)a |
Group 2a: – Group 2b: – Group 2c: – |
Group 2a: 18.8 Group 2b: 14.3 Group 2c: 26.9 Group 3: 2.5 |
| Lesser et al. (1997)3 | NIH | Time: 20–28 w, guideline: NDDG | – | N = 24, Age: 29.8 (5.3), BMI: 28.4 (4.7) | N = 44, Age: 32 (4.6), BMI: 23.4 (2.79) |
Group 2: no Group 3: no |
Group 2: 16.7 Group 3: 6.7 |
| Mehrabian et al. (2013)3 | Rotterdam | Time: 24–28 w, guideline: ADA | – |
Group 2a: PCOS with GDM N = 50, Age: 34 (47.5), BMI: 28.9 (4.5) Group 2a: PCOS without GDM N = 130, Age: 33.3 (6.6), BMI: 25.9 (4.5) |
– | Group 2: no | Group 2 totally: 27.8 |
| Mikola et al. (2001)3 | NIH | Time: NM, guideline: NM | – | N = 99, Age: 30.4 (3.9), BMI: 25.6 (6.5) | N = 737, Age: 29.4 (4.8), BMI: 23 (4.6) |
Group 2: no Group 3: no |
Group 2: 20 Group 3: 9 |
| Mumm et al. (2015)3 | Rotterdam | Time: 14–20, 28–30 w guideline: NM | – | N = 157, Age: 29 (26–32)a, BMI: 25.9 (22.0–32.0)a | N = 995, Age: 29 (26–33)a, BMI: 23.2 (20.9–26.1)a |
Group 2: no Group 3: no |
Group 2: 6.4 Group 3: 13.8 |
| Naver et al. (2014)3 | Rotterdam | Time: NM, guidelines: national | – | N = 459, Age: 31.6, BMI: 22.9 | N = 5409, Age: 30.7, BMI: 23.4 |
Group 2: no Group 3: no |
Group 2: 2.4 Group 3: 1.1 |
| Nawaz et al. (2008)3 | Rotterdam | Time: 24–28 w, guideline: NM |
Group 1a: N = 40, Age: 28 (3.6), BMI: 29.6 (5.1) Group 1b: N = 20, Age: 29 (3.1), BMI: 30 (2.6) Group 1c: N = 45, Age: 27 (4.2), BMI: 29.3 (3.3) |
N = 32, Age: 30 (2.9), BMI: 31.2 (4.6) | – |
Group 1a: before conception till 4–16 weeks of gestation Group 1b: before conception till 32 weeks of gestation Group 1c: before conception till the end of pregnancy Group 2: no |
Group 1a: 37.5 Group 1b: 50 Group 1c: 28.8 Group 2: 40.6 |
| Ott et al. (2014)3 | Rotterdam | Time: second trimester, guideline: NM | – |
Group 2a: conceived with LOA + metformin N = 40, Age: 27.8 (4.9), BMI: 26.9 (5.0) Group 2b: conceived with CC + metformin N = 40, Age: 27.5 (4.5), BMI: 28.0 (6.0) Group 2c: conceived with metformin only N = 40, Age: 27.2 (4.6), BMI: (27.2 ± 5.6) |
– |
Group 2a: before conception Group 2b: before conception Group 2c: before conception |
Group 2a: 29.4 Group 2b: 31.3 Group 2c: 31.4 |
| Palomba et al. (2010)3 | Rotterdam | Time: NM, guideline: NM | – | N = 93, Age: 30 (20–33)a, BMI: 24.2 (18.1–29.1)a | N = 73, Age: 30 (19–34)a, BMI: 24 (17.8–29.4)a |
Group 2: no Group 3: no |
Group 2: 16.1 Group 3: 5.8 |
| Paradisi et al. (1998)3 | NIH | Time: NM, guideline: NM | – |
Grou2a: PCOS with GDM N = 5, Age: 32.2 (6.3), BMI: 28.3 (0.7) Group 2b: PCOS without GDM N = 8, Age: 28 (2.9), BMI: 28.3 (3.2) |
– | Group 2: no | Group 2: 38.4 |
| Radon et al. (1999)3 | ICD-9th revision | Time: 24–28 w, guideline: NM | – | N = 22, Age: 32.4 (4.1), BMI: 28.9 (8) | N = 66, Age: 31.1 (3.9), BMI: 28 (7.2) |
Group 2: no Group 3: no |
Group 2: 40.9 Group 3: 3 |
| Reyes-Muñoz et al. (2012)3 | Rotterdam | Time: 14–24 or 24–28 w, guideline: ADA | – | N = 52, Age: 29.1 (3.9), BMI: 27.5 (3.1) | N = 26, Age: 29 (3.8), BMI: 27.5 (3.3) |
Group 2: before conception Group 3: – |
Group 2: yes Group 3: no |
| Sterling et al. (2016)3 | Rotterdam criteria | Time: NM, guideline: NM | – | N = 71, Age: 33 (30–35)a, BMI: 22.7 (20.4–28.3)a | N = 323, Age: 35 (32–37)a, BMI: 22.6 (20.8–26.0)a |
Group 2: no Group 3: no |
Group 2: 15.5 Group 3: 5 |
| Turhan et al. (2003)3 | NIH | Time: 24–28 w, guideline: ADA | – | N = 38, Age: 27.6 (3.7), BMI: 31.5 (4.5) | N = 136, Age: 26.6 (4.7), BMI: 23.6 (4.3) |
Group 2: no Group 3: no |
Group 2: 2.6 Group 3: 8.1 |
| Vollenhoven et al. (2000)3 | NM | Time: 24–28 w, guideline: WHO | – | N = 60, Age: –, BMI: 27.1 (5.2) | N = 60, Age: –, BMI: 26.5 (4.9) |
Group 2: no Group 3: no |
Group 2: 22 Group 3: 17 |
| Vanky et al. (2004)1 | Rotterdam | Time: 19, 32, 36 w, guideline: WHO | N = 18, Age: 28.9 (4.8), BMI: 32.1 (6.1) | N = 22, Age: 28.3 (3.7), BMI: 29.3 (8) | – |
Group 1: before conception till the end of pregnancy Group 2: before conception |
First trimester Group 1: 16.6 Group 2: 27.2 2ed trimester Group 1: 11.1 Group 2: 4.5 3rd trimester Group 1: 22.2 Group 2: 9 |
| Vanky et al. (2010)1 | Rotterdam | Time: NM, guideline: NM |
N = 135, Age: 29.6 (4.4), BMI: 29.5 (7) |
N = 138, Age: 29.2 (4.4), BMI: 28.5 (7.2) | – |
Group 1: before conception till the end of pregnancy Group 2: before conception |
Group 1: 16.2 Group 2: 15.2 |
| Vanky et al. (2011)2 |
1. NIH 2. Rotterdam |
Time: 14, 28 w, guideline: WHO | – |
Group 2a: PCOS based on NIH criteria N = 164, Age: 29.1 (4.4), BMI: 29.5 (6.62) Group 2a: PCOS based on Rotterdam criteria N = 93, Age: 29.6 (4.4), BMI: 27.4 (6.4) |
– |
Group 2a: no group 2b: no |
First trimester Group 2a: 9 group 2b: 10 2ed trimester Group 2a: 11.1 Group 2b: 4.5 3rd trimester Group 1: 10 Group 2: 7 |
| Veltman-Verhulst et al. (2010)3 | Rotterdam | Time: 24–26 w, guideline: ADA | – |
Group 2a: PCOS with GDM N = 21, Age: 26.6 (3.5), BMI: 28.2 (5.8) Group 2a: PCOS without GDM N = 29, Age: 25.6 (3.0), BMI: 24.7 (5.7) |
– | Group 2: – | Group 2 totally: 42 |
| Wan et al. (2015)3 | Rotterdam | Time: NM, guideline: WHO | – | N = 25, Age: 31.4 (2), BMI: 22.8 (3.6) | N = 174, Age: 32.7 (3.1), BMI: 21.5 (2.6) |
Group 2: no Group 3: no |
Group 2: 29.2 Group 3: 29.8 |
| Wang et al. (2013) (120)3 | Rotterdam | Time: 24–28 w, guideline: ADA | – | N = 144, Age: 30.8 (3.9), BMI: 23 (2.6) | N = 594, Age: 29.1 (3.9), BMI: 20 (2.4) |
Group 2: no Group 3: no |
Group 2: 54.9 Group 3: 14.3 |
| Weerakiet et al. (2004)3 | NM | Time: 24–28 w, guideline: ADA | – | N = 47, Age: 31.6 (4), BMI: 24 (3) | N = 264, Age: 31.3 (3.8), BMI: 22.1 (3.6) |
Group 2: no Group 3: no |
Group 2: 22.2 Group 3: 18 |
| Xia et al. (2017)3 | Rotterdam | Time: 24–28 w, guideline: NM | – |
Group 2a: PCOS with GDM N = 31, Age: –, BMI: 24.0 (6.4) Group 2a: PCOS without GDM N = 63, Age: –, BMI: 23.2 (3.0) |
– | Group 2 totally: no | Group 2 totally: 32.9 |
| Zhang et al. (2016)2 | Rotterdam | Time: 24–28 w, guideline: ADA | – |
Group 2a: PCOS with GDM N = 45, Age: 28.87 (3.20), BMI: 24.30 (3.23) Group 2a: PCOS without GDM N = 223, Age: 28.06 (3.2), BMI: 23.2 (3.1) |
– | – | Group 2 totally: 16.7 |
N number, BMI body mass index, NM not mentioned, PCOS polycystic ovary syndrome, HA hyperandrogenism, AO anovulation, PCO polycystic ovary morphology, ADA American diabetes association, WHO World Health Organization
aMedian (25th–75th percentile)
1Experimental study
2Cross sectional study
3Prospective study
Forty-three studies had observational and five studies had interventional (four RCTs [48–51] and one NRS [52]) methods. Marking diversity was found in screening strategies for the diagnosis of GDM. Majority of the studies performed the GDM screening test in the second trimester of pregnancy; 6 reported the GDM prevalence during the first, second and third trimesters of pregnancy [27, 49, 50, 52–54]. Twelve studies implemented the two-step screening process with a 50-g Glucose Challenge test (GCT), following a 3-h, 100-g glucose tolerance test (OGTT) [7, 24, 25, 30, 32, 38, 39, 41, 55–58]; 29 studies applied the one-step screening process with a 3-h, 100-g OGTT [28, 47, 52, 59–61] or a 2-h OGTT with 75 g glucose [27, 29, 31, 33–37, 40, 42, 44–47, 49–51, 53, 54, 62–64] and 7 studies did not mention the GDM diagnostic criteria [26, 48, 65–69]. In addition, marking diversity was found in cutoff values of diagnostic criteria (Table 1).
Twenty-seven studies did not use metformin in the women with PCOS [25–27, 29–35, 38, 40–47, 53, 61–64, 70, 71]; 13 studies used metformin therapy only before conception [25, 28, 37, 39, 48–51, 54, 55, 57, 59, 69]; 13 studies treated the women with PCOS using metformin before conception until the end of pregnancy [36, 48–52, 54, 58, 60, 65–68]; In one study, metformin therapy was used before conception until 4–16 weeks of gestations [36] and in one single study it was used before conception until the 32th week of gestation [36].
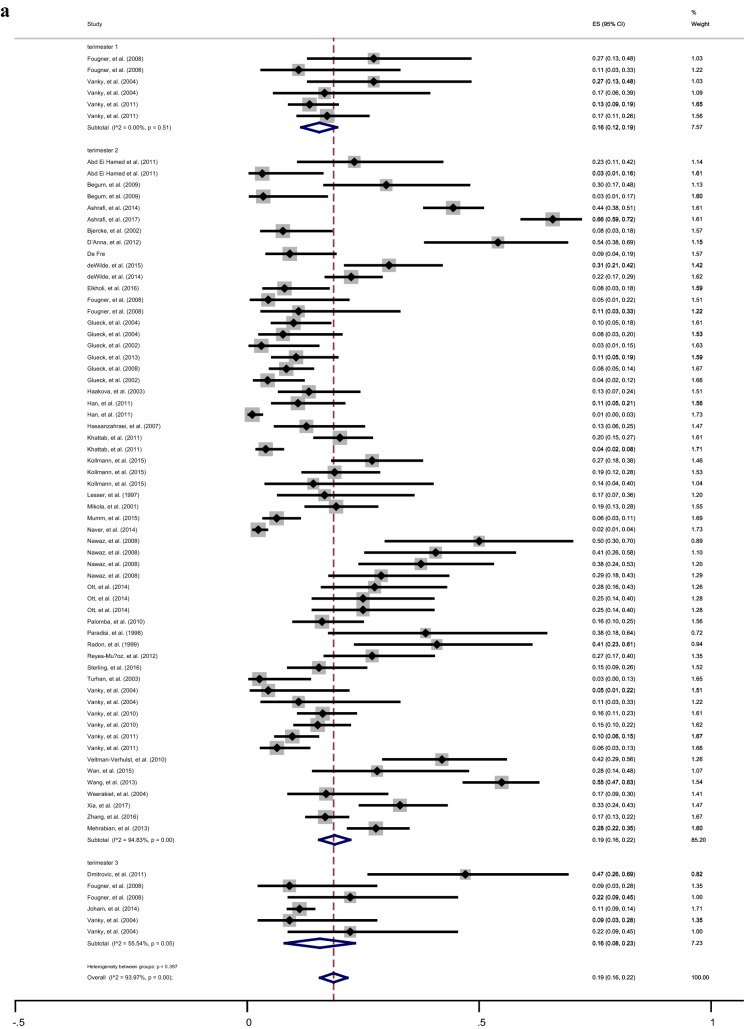
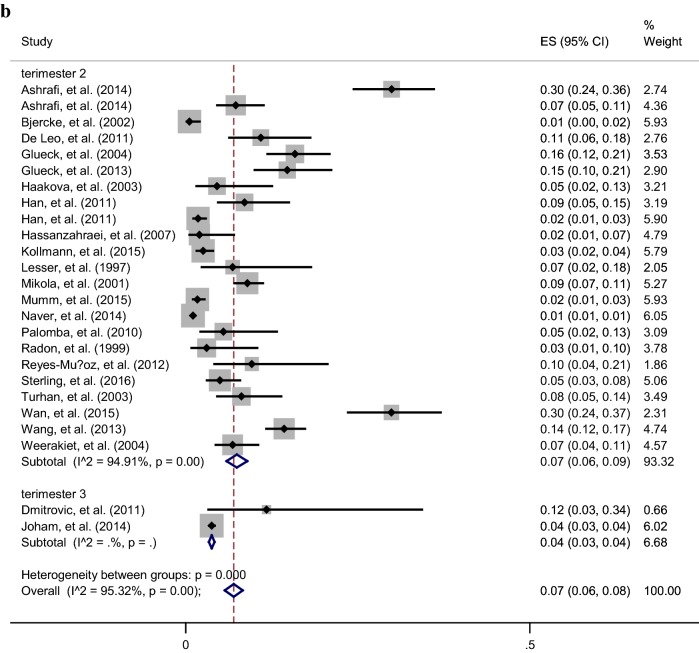
The overall pooled prevalence (95% CI) of GDM among different groups was presented in Table 2. According to the Chi square test and Begg’s test, a significant heterogeneity but no publication bias was found between the studies in various subgroups. Overall, the women with PCOS were younger [Random-pooled mean (95% CI) 29.4 (28.6, 30.3) vs. 30.6 (29.7, 30.9)] and had higher BMI [Random-pooled mean (95% CI): 28.0 (26.8, 29.3) vs. 24.4 (23.2, 25.6)] compared with healthy controls. The Random-pooled overall prevalence of GDM among women with PCOS and healthy controls in the second trimester of pregnancy were (Random-pooled overall p = 0.19, 95% CI 0.16–0.22) and (Random-pooled overall p = 0.07, 95% CI 0.06–0.09), respectively (Fig. 1a, b).
Table 2.
Results of heterogeneity and publication bias estimation and subgroup meta-analysis for various study population and metformin treatment among women with PCOS and without PCOS
| Sample size of participants | Chi square (df) | P value | Begg’s test | Pooled overall prevalence (95% CI) | |
|---|---|---|---|---|---|
| Gestational diabetes in first trimester of pregnancy | |||||
| PCOS | 337 | 4.25 (5) | 0.510 | 0.452 | 0.16 (0.12, 0.19) |
| Without metformin therapy | 257 | − (1) | – | 0.317 | 0.15 (0.10, 0.19) |
| Metformin therapy just before conception | 44 | − (1) | – | 1 | 0.27 (0.14, 0.40) |
| Metformin therapy before conception till end of pregnancy | 36 | − (1) | – | 0.317 | 0.13 (0.02, 0.25) |
| Non-PCOS | 0 | – | – | – | – |
| Gestational diabetes in second trimester of pregnancy | |||||
| PCOS | 5156 | 1123 (58) | 0.001 | 0.789 | 0.19 (0.16, 0.22) |
| Without metformin therapy | 3008 | 772 (28) | 0.001 | 0.341 | 0.20 (0.15, 0.25) |
| Metformin therapy just before conception | 1232 | 165 (15) | 0.001 | 0.786 | 0.23 (0.16, 0.30) |
| Metformin therapy before conception till end of pregnancy | 916 | 61 (13) | 0.001 | 0.555 | 0.11 (0.07, 0.16) |
| Non-PCOS | 12,059 | 433 (22) | 0.001 | 0.321 | 0.07 (0.06, 0.09) |
| Gestational diabetes in third trimester of pregnancy | |||||
| PCOS | 575 | 11 (5) | 0.001 | 0.573 | 0.16 (0.08, 0.23) |
| Without metformin therapy | 495 | − (1) | – | 1 | 0.12 (0.09, 0.15) |
| Metformin therapy just before conception | 44 | − (1) | – | 1 | 0.09 (0.01, 0.18) |
| Metformin therapy before conception till end of pregnancy | 36 | − (1) | – | 1 | 0.22 (0.09, 0.36) |
| Non-PCOS | 8151 | − (1) | – | 0.317 | 0.04 (0.03, 0.04) |
PCOS polycystic ovary syndrome
Fig. 1.
Forest plot of prevalence of GDM among women with PCOS (a) and healthy controls (b) in the first, second and third trimesters of pregnancy
Results of meta-regression analysis
The results of univariate, and multiple weighted, linear meta-regression analysis were presented in Table 3. Unadjusted meta-regression revealed that regardless of metformin therapy, the prevalence of GDM diagnosed in second trimester among women with PCOS was 9% higher than healthy controls (β = 0.09, 95% CI 0.04, 0.16; p = 0.002) (Table 3 model 1 and Additional file 1: Fig. S2); those higher rate remained significant after adjustment of age, BMI, study design, PCOS criteria, GDM definition and quality assessment (Table 3, Models 2–8). In all studies (observational and trials), the increased risk of GDM among women with PCOS, compared to healthy controls, disappeared after the adjustment of metformin-therapy (β = 0.08, 95% CI 0.04, 0.2; p = 0.624); meta-regression analyses demonstrating that the prevalence of GDM among the women with PCOS treated before conception was statistically higher than the healthy controls (β = 0.13, 95% CI 0.06, 0.2; p = 0.001). Nevertheless, this prevalence among women with PCOS all throughout the pregnancy were as the same as the healthy controls (β = 0.037, 95% CI − 0.03, 0.1; p = 0.276) (Table 4).
Table 3.
Meta-regression results for univariate and multiple (adjusted effect) models assessing the effect of PCOS on gestational diabetes in different trimester of pregnancy
| Trimester 1 (n = 6) | Trimester 2 (n = 82) | Trimester 3 (n = 8) | ||||
|---|---|---|---|---|---|---|
| β (95% CI for β) | P value | β (95% CI for β) | P value | β (95% CI for β) | P value | |
| Unadjusted model 1 | ||||||
| Effect PCOS | –a | 0.097 (0.04, 0.16) | 0.002 | 0.09 (− 0.08, 0.26) | 0.234 | |
| Adjusted Models | ||||||
| Model 2 | ||||||
| Effect of BMI | 0.016 (− 0.05, 0.09) | 0.522 | − 0.007 (− 0.02, 0.002) | 0.127 | 0.03 (− 0.03, 0.09) | 0.359 |
| Effect of PCOS | –a | 0.10 (0.05, 0.2) | 0.001 | − 0.02 (− 0.4, 0.3) | 0.887 | |
| Model 3 | ||||||
| Effect of BMI | 0.017 (− 0.056, 0.09) | 0.522 | − 0.005 (− 0.02, 0.004) | 0.242 | 0.03 (− 0.06, 0.1) | 0.413 |
| Effect of PCOS | –a | 0.10 (0.04, 0.2) | 0.002 | − 0.02 (− 0.5, 0.5) | 0.887 | |
| Effect of age | − 0.04 (− 0.29, 0.21) | 0.650 | − 0.003 (− 0.02, 0.01) | 0.621 | 0.01 (− 0.2, 0.1) | 0.413 |
| Model 4 | ||||||
| Effect of BMI | 0.022 (− 0.11, 0.16) | 0.548 | − 0.005 (− 0.02, 0.006) | 0.344 | 0.03 (− 0.09, 0.2) | 0.450 |
| Effect of PCOS | –a | 0.08 (0.04, 0.2) | 0.624 | − 0.04 (− 0.7, 0.6) | 0.852 | |
| Effect of age | − 0.072 (− 0.65, 0.50) | 0.647 | − 0.003 (− 0.02, 0.01) | 0.624 | − 0.02 (− 0.4, 0.3) | 0.862 |
| Effect of metformin therapy | − 0.023 (− 0.38, 0.33) | 0.803 | − 0.0001 (− 0.05, 0.05) | 0.997 | − 0.05 (− 0.4, 0.3) | 0.680 |
| Model 5 | ||||||
| Effect of BMI | 0.005 (− 0.1, 0.1) | 0.854 | − 0.004 (− 0.01, 0.01) | 0.425 | 0.03 (− 0.03, 0.08) | 0.253 |
| Effect of PCOS | –a | 0.10 (0.05, 0.2) | 0.001 | − 0.01 (− 0.2, 2) | 0.840 | |
| Effect of age | 0.04 (− 0.4, 0.5) | 0.740 | − 0.001 (− 0.01, 0.01) | 0.771 | − 0.1 (− 0.3, 0.06) | 0.134 |
| Effect of study design | − 0.04 (− 0.2, 0.1) | 0.396 | 0.05 (0.02, 0.08) | 0.004 | 0.1 (− 0.02, 0.2) | 0.073 |
| Model 6 | ||||||
| Effect of BMI | 0.04 (− 0.1, 0.2) | 0.334 | − 0.005 (− 0.02, 0.004) | 0.259 | 0.02 (− 0.07, 0.1) | 0.403 |
| Effect of PCOS | –a | 0.10 (0.02, 0.2) | 0.011 | –a | ||
| Effect of age | − 0.1 (− 0.6, 0.3) | 0.380 | − 0.004 (− 0.02, 0.01) | 0.519 | − 0.10 (− 0.1, 0.2) | 0.761 |
| Effect of PCOS definition | 0.2 (− 0.5, 0.8) | 0.396 | − 0.04 (− 0.1, 0.03) | 0.267 | 0.4 (− 0.29, 0.99) | 0.144 |
| Model 7 | ||||||
| Effect of BMI | 0.02 (− 0.05, 0.09) | 0.522 | − 0.005 (− 0.02, 0.004) | 0.256 | 0.02 (− 0.1, 0.2) | 0.649 |
| Effect of PCOS | –a | 0.10 (0.05, 0.2) | 0.001 | 0.02 (− 0.9, 0.9) | 0.953 | |
| Effect of age | − 0.04 (− 0.3, 0.2) | 0.634 | − 0.002 (− 0.02, 0.01) | 0.711 | 0.03 (− 0.3, 0.4) | 0.792 |
| Effect of quality assessment | –a | 0.02 (− 0.05, 0.08) | 0.612 | − 0.06 (− 0.7, 0.6) | 0.775 | |
| Model 8 | ||||||
| Effect of BMI | − 0.005 (− 0.12, 0.111) | 0.880 | − 0.006 (− 0.02, 0.004) | 0.236 | 0.06 (− 0.1, 0.2) | 0.306 |
| Effect of PCOS | –a | 0.1 (0.04, 0.2) | 0.002 | − 0.2 (− 0.9, 0.6) | 0.565 | |
| Effect of age | − 0.06 (− 0.4, 0.3) | 0.513 | − 0.003 (− 0.02, 0.01) | 0.680 | − 0.02 (− 0.3, 0.2) | 0.857 |
| Effect of GDM definition | 0.2 (− 0.4, 0.7) | 0.352 | 0.01 (− 0.04, 0.06) | 0.608 | − 0.1 (− 0.5, 0.3) | 0.459 |
Italic values indicate statistically significant results (p < 0.05)
aInsufficient data for analysis
Model 1: Univariate models assessing the effect of PCOS on Prevalence of GDM (Crude model)
Model 2: Multiple meta-regresion analysis effect of PCOS on Prevalence of GDM adjusted by BMI
Model 3: Multiple meta-regresion analysis effect of PCOS on Prevalence of GDM adjusted by BMI and age
Model 4: Multiple meta-regresion analysis effect of PCOS on Prevalence of GDM adjusted by BMI, age and metformin therapy
Model 5: Multiple meta-regresion analysis effect of PCOS on Prevalence of GDM adjusted by BMI, age and study design
Model 6: Multiple meta-regresion analysis effect of PCOS on Prevalence of GDM adjusted by BMI, age and PCOS definition
Model 7: Multiple meta-regresion analysis effect of PCOS on Prevalence of GDM adjusted by BMI, age and quality assessment
Model 8: Multiple meta-regresion analysis effect of PCOS on Prevalence of GDM adjusted by BMI, age and GDM definition
Table 4.
Meta-regression results for effect of different metformin therapy strategy among subgroup of POCS women and healthy controls and study methodology
| Regression coefficient (95% CI) | P value | |
|---|---|---|
| Comparison between PCOS and healthy controls | ||
| Women with PCOS, No treated with metformin vs. healthy controls | ||
| Non-RCTs~ | 0.10 (0.02, 0.17) | 0.006 |
| RCTs | –* | – |
| Women with PCOS, treated with metformin only before conception vs. healthy controls | ||
| Non-RCTs | 0.14 (0.07, 0.2) | 0.000 |
| RCTs | –* | – |
| Women with PCOS, treated with metformin before conception till the end of pregnancy vs. healthy controls | ||
| Non-RCTs | 0.035 (− 0.03, 0.1) | 0.324 |
| RCTs | –* | –* |
| Comparison between PCOS population | ||
| Women with PCOS, treated with metformin only before conception vs. without metformin therapy | ||
| Non-RCTs | 0.08 (− 0.03, 0.2) | 0.390 |
| RCTs | –* | – |
| Women with PCOS, treated with metformin before conception till the end of pregnancy vs. without metformin therapy | ||
| Non-RCTs | − 0.05 (− 0.07, 0.04) | 0.602 |
| RCTs | –* | – |
| Women with PCOS, treated with metformin before conception till the end of pregnancy vs. only before conception | ||
| Non-RCTs | − 0.11 (− 0.24, 0.02) | 0.097 |
| RCTs | − 0.03 (− 0.25, 0.20) | 0.757 |
* Insufficient data for analysis
~ Randomized clinical trial
Meta-regression analyses among women with PCOS treated with metformin before conception versus all throughout the pregnancy and non-users were presented in Table 4. It shown that by excluding observational studies, the prevalence of GDM among women with PCOS treated using metformin before conception until the end of pregnancy did not differ from those treated just before conception (β = − 0.09, 95% CI −0.2, 0.02; p = 0.092) or those without metformin therapy (β = − 0.05, 95% CI: − 0.07, 0.04; p = 0.301). In addition, the results remained unchanged after the subgroup analysis based methodology of RCTs and non-RCTs studies.
Discussion
It is well documented that the prevalence of GDM among women with PCOS is higher that healthy controls. In addition, the debate whether metformin therapy can change the risk of developing GDM among women with PCOS is continued. Additionally, in term of prevention of GDM in PCOS women, the effect of metformin that can be used before conception versus all throughout the pregnancy has not been compared yet. Previous meta-analyses are controversial and inconclusive mostly due to different study designs, non-homogenous control groups and un-adjustment for possible confounding factors of age and BMI.
In an attempt to answer this important question, this meta-analysis was conducted using different approaches. Comparison of the prevalence of GDM among PCOS patients versus healthy controls showed that the prevalence of GDM regardless of metformin therapy was significantly higher in women with PCOS, and the increased risk disappeared after metformin therapy during pregnancy. However, as a source of bias, all included studies were observational that might be influenced by the various biases that influence interpretation of results. In the second approach, comparison of PCOS patients, either without or with metformin therapy in various times revealed that before and during pregnancy it could not decrease the prevalence of GDM in metformin treated women with POCS compared to those with PCOS who did not received metformin or were treated only before conception. The results of subgroup analysis based on RCTs and non-RCTs confirmed such findings. However, these results of clinical trials should also be interpreted with caution mainly due to the small number of trials, moderate quality mainly due to the randomization and blindness.
This meta-analysis confirmed earlier findings regarding the higher risk of GDM among the women with PCOS [10–13, 72, 73]. Some mechanisms have been suggested to explain the established predisposition of women with PCOS for developing GDM. It has been demonstrated that profound IR in PCOS due to peripheral target tissue resistance, decreased hepatic clearance, beta-cell dysfunction and increased pancreatic sensitivity [74, 75] is exacerbated through innate IR during pregnancy mainly by the secretion of some insulin-desensitizing placental adipokines and hormones including tumor necrosis factor (TNF)-α, growth hormone, cortisol and human placental lactogen [63, 76]. However, Metformin as an insulin sensitizer is widely used by infertile women with PCOS, which could have reduced ovarian androgens, luteinizing hormone and sex hormone binding globulins. In addition, it is helpful to improve hyperandrogenemia and insulin sensitivity via inhibiting hepatic glucose production, increasing peripheral glucose uptake and utilization, and decreasing insulin levels. Metformin recently has been considered a potentially effective agent during pregnancy to prevent GDM.
There are six meta-analyses on the effect of metformin on the occurrence of GDM in women with PCOS [14–19].
Three of them reported that metformin therapy throughout pregnancy decreased the risk of GDM in pregnant PCOS women [16, 18, 19]. However, they were subject of potential bias as their major limitations were different eligibility criteria for the type of included studies (interventional vs. observational) [18, 19] or selecting of a non-homogenous control groups (PCOS not treated, or both not treated PCOS and non-PCOS ones) [16, 18, 19].
Other three meta-analyses concluded that metformin did not significantly affect GDM among women with PCOS [14, 15, 17]. While these studies were performed a subgroup analysis of RCTs as the most stringent method of determining whether a cause-effect relation existed between the intervention and outcome, they missed some eligible studies [17], including epi-analysis [77], which re-evaluated the results of two former RCTs [50, 51] leading to duplication of previous data [17]. Also, they used the heterogeneous population as controls [15, 17] and misclassification of included studies [48] in subgroup analysis [14] was reported.
Moreover, the quality assessment and risk of bias evaluation did not perform in most those meta-analyses, and the effect of potential confounder of age and BMI did not assessed in most previous meta-analyses.
According to the PRISMA guidelines, the current meta-analysis has standard criteria and presents reliable results. The main strength of this meta-analysis was the large number of eligible studies reviewed in this study, and also the adjustment for potential confounders, which made it possible to present the real feature of this syndrome. In addition, using the homogenous controls (PCOS not treated, or both not treated PCOS and non-PCOS ones) helped us to control the source of heterogeneity. Moreover, the impact of time and duration of metformin therapy for optimum reduction of GDM were evaluated. In addition, most studies included an estimated moderate or high quality with the low risk of bias that helped us provide high quality evidence, sensitivity analysis based on risk of bias showed no difference as well.
Nevertheless, it should be noted that, despite this meta-analysis, it seems the evidence about the metformin therapy among women with PCOS who had risk factor for GDM e.g. advanced maternal age [78], previous macrosomia [79], maternal obesity [80], maternal impaired glucose tolerance [81], ethnicity [82] and family history of diabetes [83], is insufficient and treatment should be prescribed individually for each patient.
However, as the limitations of the present study, there was inadequate evidence, and lack of large scale well-designed RCTs to establish the influence of metformin therapy in the various trimesters of pregnancy on the prevalence of GDM. While the onset of metformin therapy before conception was exactly specified, the duration of metformin treatment before pregnancy was unclear in some included studies and we could not adjust it as a potential confounding factor in this meta-analysis. Moreover, most studies were performed in infertility treatment center, may limit the validity of the results.
Conclusion
The main body of literature in the current meta-analysis was observational, which may be mixed with some sources of bias. Also, a lack of well-designed and high quality interventional studies means that the findings should be interpreted with cautious. In this respect, decisions regarding the continuation or discontinuation of metformin therapy in women with PCOS are somewhat arbitrary and can be made individually based on the patient’s condition given the presence or absence of other GDM risk factors. Additional well-designed RCTs still need for precise recommendation.
Additional file
Additional file 1. Additional figures and tables.
Acknowledgements
The authors thank Mrs. Marzieh Atashkar, the staff of the Research Institute for Endocrine Sciences Library, for assistance with the literature search. Also, authors wish to acknowledge Ms. Niloofar Shiva for critical editing of English grammar and syntax of the manuscript.
Abbreviations
- GDM
gestational diabetes mellitus
- PCOS
polycystic ovary syndrome
- BMI
body mass index
- RCT
randomized clinical trial
- IR
insulin resistance
- PRISMA
preferred reporting items for systematic reviews and meta-analyses
- NRS
nonrandomized Studies
Authors’ contributions
RBY was involved in study design, search in databases, study selection, data analysis, manuscript drafting, and submitting manuscript. SBG and FRT were involved in study design, data analysis, manuscript drafting and critical discussion. MA contributed in study selection, data analysis, and critical discussion. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request
Ethics approval and consent to participate
This study was approved by the ethics committee of the Research Institute for Endocrine Sciences and a written informed consent was obtained from all subjects before initiation of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tehrani FR, Simbar M, Tohidi M, Hosseinpanah F, Azizi F. The prevalence of polycystic ovary syndrome in a community sample of Iranian population: Iranian PCOS prevalence study. Reprod Biol Endocrinol. 2011;9:39. doi: 10.1186/1477-7827-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behboudi-Gandevani S, Ramezani Tehrani F, Rostami Dovom M, Farahmand M, Bahri Khomami M, Noroozzadeh M, et al. Insulin resistance in obesity and polycystic ovary syndrome: systematic review and meta-analysis of observational studies. Gynecol Endocrinol. 2016;32:343–353. doi: 10.3109/09513590.2015.1117069. [DOI] [PubMed] [Google Scholar]
- 3.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 4.Dennett CC, Simon J. The role of polycystic ovary syndrome in reproductive and metabolic health: overview and approaches for treatment. Diabetes Spectr. 2015;28:116–120. doi: 10.2337/diaspect.28.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palomba S, Santagni S, Falbo A, La Sala GB. Complications and challenges associated with polycystic ovary syndrome: current perspectives. Int J Womens Health. 2015;7:745–763. doi: 10.2147/IJWH.S70314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41. doi: 10.1186/1741-7015-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newbern D, Freemark M. Placental hormones and the control of maternal metabolism and fetal growth. Curr Opin Endocrinol Diabetes Obes. 2011;18:409–416. doi: 10.1097/MED.0b013e32834c800d. [DOI] [PubMed] [Google Scholar]
- 8.Sonagra AD, Biradar SM, Dattatreya K, Murthy DSJ. Normal pregnancy—a state of insulin resistance. J Clin Diagn Res. 2014;8:CC01–3. doi: 10.7860/JCDR/2014/10068.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005;26:19–39. [PMC free article] [PubMed] [Google Scholar]
- 10.Qin JZ, Pang LH, Li MJ, Fan XJ, Huang RD, Chen HY. Obstetric complications in women with polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol. 2013;11:56. doi: 10.1186/1477-7827-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:673–683. doi: 10.1093/humupd/dml036. [DOI] [PubMed] [Google Scholar]
- 12.Kjerulff LE, Sanchez-Ramos L, Duffy D. Pregnancy outcomes in women with polycystic ovary syndrome: a metaanalysis. Am J Obstet Gynecol. 2011;204(558):e1–e6. doi: 10.1016/j.ajog.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Toulis KA, Goulis DG, Kolibianakis EM, Venetis CA, Tarlatzis BC, Papadimas I. Risk of gestational diabetes mellitus in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Fertil Steril. 2009;92:667–677. doi: 10.1016/j.fertnstert.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 14.Feng L, Lin XF, Wan ZH, Hu D, Du YK. Efficacy of metformin on pregnancy complications in women with polycystic ovary syndrome: a meta-analysis. Gynecol Endocrinol. 2015;31:833–839. doi: 10.3109/09513590.2015.1041906. [DOI] [PubMed] [Google Scholar]
- 15.Tan X, Li S, Chang Y, Fang C, Liu H, Zhang X, Wang Y. Effect of metformin treatment during pregnancy on women with PCOS: a systematic review and meta-analysis. Clin Invest Med. 2016;39:E120–E131. doi: 10.25011/cim.v39i4.27091. [DOI] [PubMed] [Google Scholar]
- 16.Zeng XL, Zhang YF, Tian Q, Xue Y, An RF. Effects of metformin on pregnancy outcomes in women with polycystic ovary syndrome: a meta-analysis. Medicine. 2016;95:e4526. doi: 10.1097/MD.0000000000004526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhuo Z, Wang A, Yu H. Effect of metformin intervention during pregnancy on the gestational diabetes mellitus in women with polycystic ovary syndrome: a systematic review and meta-analysis. J Diabetes Res. 2014;2014:381231. doi: 10.1155/2014/381231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J, Liu X, Zhang W. The effect of metformin therapy for preventing gestational diabetes mellitus in women with polycystic ovary syndrome: a meta-analysis. Exp Clin Endocrinol Diabetes. 2018;1:1. doi: 10.1055/a-0603-3394. [DOI] [PubMed] [Google Scholar]
- 19.Zheng J, Shan PF, Gu W. The efficacy of metformin in pregnant women with polycystic ovary syndrome: a meta-analysis of clinical trials. J Endocrinol Invest. 2013;36:797–802. doi: 10.3275/8932. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells G, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed 19 Oct 2009.
- 23.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashrafi M, Sheikhan F, Arabipoor A, Hosseini R, Nourbakhsh F, Zolfaghari Z. Gestational diabetes mellitus risk factors in women with polycystic ovary syndrome (PCOS) Eur J Obstet Gynecol Reprod Biol. 2014;181:195–199. doi: 10.1016/j.ejogrb.2014.07.043. [DOI] [PubMed] [Google Scholar]
- 26.Joham AE, Ranasinha S, Zoungas S, Moran L, Teede HJ. Gestational diabetes and type 2 diabetes in reproductive-aged women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99:E447–E452. doi: 10.1210/jc.2013-2007. [DOI] [PubMed] [Google Scholar]
- 27.Vanky E, Stridsklev S, Skogøy K, Kleggetveit O, Hjelle S, Brandis PV, et al. PCOS–what matters in early pregnancy?—data from a cross-sectional, multicenter study. Acta Obstet Gynecol Scand. 2011;90:398–404. doi: 10.1111/j.1600-0412.2010.01064.x. [DOI] [PubMed] [Google Scholar]
- 28.de Wilde MA, Veltman-Verhulst SM, Goverde AJ, Lambalk CB, Laven JS, Franx A, et al. Preconception predictors of gestational diabetes: a multicentre prospective cohort study on the predominant complication of pregnancy in polycystic ovary syndrome. Hum Reprod. 2014;29:1327–1336. doi: 10.1093/humrep/deu077. [DOI] [PubMed] [Google Scholar]
- 29.Haakova L, Cibula D, Rezabek K, Hill M, Fanta M, Zivny J. Pregnancy outcome in women with PCOS and in controls matched by age and weight. Hum Reprod. 2003;18:1438–1441. doi: 10.1093/humrep/deg289. [DOI] [PubMed] [Google Scholar]
- 30.Hassanzahraei R, Janighorban M. Complications and outcome of pregnancy in infertile PCOS patients. Iran J Nurs Midw Res. 2007;12:101–105. [Google Scholar]
- 31.Kollmann M, Klaritsch P, Martins WP, Guenther F, Schneider V, Herzog SA, et al. Maternal and neonatal outcomes in pregnant women with PCOS: comparison of different diagnostic definitions. Hum Reprod. 2015;30:2396–2403. doi: 10.1093/humrep/dev187. [DOI] [PubMed] [Google Scholar]
- 32.Lesser KB, Garcia FA. Association between polycystic ovary syndrome and glucose intolerance during pregnancy. J Matern Fetal Med. 1997;6:303–307. doi: 10.1002/(SICI)1520-6661(199709/10)6:5<303::AID-MFM14>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 33.Mikola M, Hiilesmaa V, Halttunen M, Suhonen L, Tiitinen A. Obstetric outcome in women with polycystic ovarian syndrome. Hum Reprod. 2001;16:226–229. doi: 10.1093/humrep/16.2.226. [DOI] [PubMed] [Google Scholar]
- 34.Mumm H, Jensen DM, Sørensen JA, Andersen LL, Ravn P, Andersen M, et al. Hyperandrogenism and phenotypes of polycystic ovary syndrome are not associated with differences in obstetric outcomes. Acta Obstet Gynecol Scand. 2015;94:204–211. doi: 10.1111/aogs.12545. [DOI] [PubMed] [Google Scholar]
- 35.Naver KV, Grinsted J, Larsen SO, Hedley PL, Jørgensen FS, Christiansen M, et al. Increased risk of preterm delivery and pre-eclampsia in women with polycystic ovary syndrome and hyperandrogenaemia. BJOG. 2014;121:575–581. doi: 10.1111/1471-0528.12558. [DOI] [PubMed] [Google Scholar]
- 36.Nawaz FH, Khalid R, Naru T, Rizvi J. Does continuous use of metformin throughout pregnancy improve pregnancy outcomes in women with polycystic ovarian syndrome? J Obstet Gynaecol Res. 2008;34:832–837. doi: 10.1111/j.1447-0756.2008.00856.x. [DOI] [PubMed] [Google Scholar]
- 37.Ott J, Kurz C, Nouri K, Wirth S, Vytiska-Binstorfer E, Huber JC, et al. Pregnancy outcome in women with polycystic ovary syndrome comparing the effects of laparoscopic ovarian drilling and clomiphene citrate stimulation in women pre-treated with metformin: a retrospective study. Reprod Biol Endocrinol. 2010;8:45. doi: 10.1186/1477-7827-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radon PA, McMahon MJ, Meyer WR. Impaired glucose tolerance in pregnant women with polycystic ovary syndrome. Obstet Gynecol. 1999;94:194–197. doi: 10.1016/s0029-7844(99)00252-5. [DOI] [PubMed] [Google Scholar]
- 39.Reyes-Muñoz E, Castellanos-Barroso G, Ramírez-Eugenio BY, Ortega-González C, Parra A, Castillo-Mora A, et al. The risk of gestational diabetes mellitus among Mexican women with a history of infertility and polycystic ovary syndrome. Fertil Steril. 2012;97:1467–1471. doi: 10.1016/j.fertnstert.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 40.Sterling L, Liu J, Okun N, Sakhuja A, Sierra S, Greenblatt E. Pregnancy outcomes in women with polycystic ovary syndrome undergoing in vitro fertilization. Fertil Steril. 2016;105(791–797):e2. doi: 10.1016/j.fertnstert.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Turhan NO, Seçkin NC, Aybar F, Inegöl I. Assessment of glucose tolerance and pregnancy outcome of polycystic ovary patients. Int J Gynaecol Obstet. 2003;81:163–168. doi: 10.1016/S0020-7292(03)00003-1. [DOI] [PubMed] [Google Scholar]
- 42.Vollenhoven B, Clark S, Kovacs G, Burger H, Healy D. Prevalence of gestational diabetes mellitus in polycystic ovarian syndrome (PCOS) patients pregnant after ovulation induction with gonadotrophins. Aust N Z J Obstet Gynaecol. 2000;40:54–58. doi: 10.1111/j.1479-828X.2000.tb03167.x. [DOI] [PubMed] [Google Scholar]
- 43.Veltman-Verhulst SM, van Haeften TW, Eijkemans MJ, de Valk HW, Fauser BC, Goverde AJ. Sex hormone-binding globulin concentrations before conception as a predictor for gestational diabetes in women with polycystic ovary syndrome. Hum Reprod. 2010;25:3123–3128. doi: 10.1093/humrep/deq272. [DOI] [PubMed] [Google Scholar]
- 44.Wan HL, Hui PW, Li HW, Ng EH. Obstetric outcomes in women with polycystic ovary syndrome and isolated polycystic ovaries undergoing in vitro fertilization: a retrospective cohort analysis. J Matern Fetal Neonatal Med. 2015;28:475–478. doi: 10.3109/14767058.2014.921673. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Zhao X, Zhao H, Ding H, Tan J, Chen J, et al. Risks for gestational diabetes mellitus and pregnancy-induced hypertension are increased in polycystic ovary syndrome. Biomed Res Int. 2013;2013:182582. doi: 10.1155/2013/182582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weerakiet S, Srisombut C, Rojanasakul A, Panburana P, Thakkinstian A, Herabutya Y. Prevalence of gestational diabetes mellitus and pregnancy outcomes in Asian women with polycystic ovary syndrome. Gynecol Endocrinol. 2004;19:134–140. doi: 10.1080/09513590400007242. [DOI] [PubMed] [Google Scholar]
- 47.Xia H, Zhang R, Sun X, Wang L, Zhang W. Valuable predictors of gestational diabetes mellitus in infertile Chinese women with polycystic ovary syndrome: a prospective cohort study. Gynecol Endocrinol. 2017;33:448–451. doi: 10.1080/09513590.2017.1290074. [DOI] [PubMed] [Google Scholar]
- 48.Begum MR, Khanam NN, Quadir E, Ferdous J, Begum MS, Khan F, et al. Prevention of gestational diabetes mellitus by continuing metformin therapy throughout pregnancy in women with polycystic ovary syndrome. J Obstet Gynaecol Res. 2009;35:282–286. doi: 10.1111/j.1447-0756.2008.00876.x. [DOI] [PubMed] [Google Scholar]
- 49.Fougner KJ, Vanky E, Carlsen SM. Metformin has no major effects on glucose homeostasis in pregnant women with PCOS: results of a randomized double-blind study. Scand J Clin Lab Invest. 2008;68:771–776. doi: 10.1080/00365510802254620. [DOI] [PubMed] [Google Scholar]
- 50.Vanky E, Salvesen KA, Heimstad R, Fougner KJ, Romundstad P, Carlsen SM. Metformin reduces pregnancy complications without affecting androgen levels in pregnant polycystic ovary syndrome women: results of a randomized study. Hum Reprod. 2004;19:1734–1740. doi: 10.1093/humrep/deh347. [DOI] [PubMed] [Google Scholar]
- 51.Vanky E, Stridsklev S, Heimstad R, Romundstad P, Skogøy K, Kleggetveit O, et al. Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: a randomized, controlled multicenter study. J Clin Endocrinol Metab. 2010;95:E448–E455. doi: 10.1210/jc.2010-0853. [DOI] [PubMed] [Google Scholar]
- 52.El Hameed A, Shreif HE, Mowafy HE. The role of continuing metformin therapy during pregnancy in the reduction of gestational diabetes and improving pregnancy outcomes in women with polycystic ovary syndrome. Middle East Fertil Soc J. 2011;16:204–208. doi: 10.1016/j.mefs.2011.04.002. [DOI] [Google Scholar]
- 53.Dmitrovic R, Katcher HI, Kunselman AR, Legro RS. Continuous glucose monitoring during pregnancy in women with polycystic ovary syndrome. Obstet Gynecol. 2011;118:878–885. doi: 10.1097/AOG.0b013e31822c887f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khattab S, Mohsen IA, Aboul Foutouh I, Ashmawi HS, Mohsen MN, van Wely M, et al. Can metformin reduce the incidence of gestational diabetes mellitus in pregnant women with polycystic ovary syndrome? Prospective cohort study. Gynecol Endocrinol. 2011;27:789–793. doi: 10.3109/09513590.2010.540600. [DOI] [PubMed] [Google Scholar]
- 55.Elkholi DGE, Nagy HM. The endocrine-metabolic disorders and adverse pregnancy outcomes in metabolically obese normal weight women with polycystic ovary syndrome. Womens Health Gynecol. 2016;2:68. [Google Scholar]
- 56.Ashrafi M, Sheikhan F, Arabipoor A, Rouhana N, Hosseini R, Zolfaghari Z. Gestational diabetes mellitus and metabolic disorder among the different phenotypes of polycystic ovary syndrome. Oman Med J. 2017;32:214–220. doi: 10.5001/omj.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.D’Anna R, Di Benedetto V, Rizzo P, Raffone E, Interdonato ML, Corrado F, et al. Myo-inositol may prevent gestational diabetes in PCOS women. Gynecol Endocrinol. 2012;28:440–442. doi: 10.3109/09513590.2011.633665. [DOI] [PubMed] [Google Scholar]
- 58.Glueck CJ, Pranikoff J, Aregawi D, Wang P. Prevention of gestational diabetes by metformin plus diet in patients with polycystic ovary syndrome. Fertil Steril. 2008;89:625–634. doi: 10.1016/j.fertnstert.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 59.de Wilde MA, Goverde AJ, Veltman-Verhulst SM, Eijkemans MJ, Franx A, Fauser BC, et al. Insulin action in women with polycystic ovary syndrome and its relation to gestational diabetes. Hum Reprod. 2015;30:1447–1453. doi: 10.1093/humrep/dev072. [DOI] [PubMed] [Google Scholar]
- 60.Glueck CJ, Wang P, Kobayashi S, Phillips H, Sieve-Smith L. Metformin therapy throughout pregnancy reduces the development of gestational diabetes in women with polycystic ovary syndrome. Fertil Steril. 2002;77:520–525. doi: 10.1016/S0015-0282(01)03202-2. [DOI] [PubMed] [Google Scholar]
- 61.Palomba S, Falbo A, Russo T, Tolino A, Orio F, Zullo F. Pregnancy in women with polycystic ovary syndrome: the effect of different phenotypes and features on obstetric and neonatal outcomes. Fertil Steril. 2010;94:1805–1811. doi: 10.1016/j.fertnstert.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 62.De Frène V, Vansteelandt S, T’Sjoen G, Gerris J, Somers S, Vercruysse L, et al. A retrospective study of the pregnancy, delivery and neonatal outcome in overweight versus normal weight women with polycystic ovary syndrome. Hum Reprod. 2014;29:2333–2338. doi: 10.1093/humrep/deu154. [DOI] [PubMed] [Google Scholar]
- 63.Bjercke S, Dale PO, Tanbo T, Storeng R, Ertzeid G, Abyholm T. Impact of insulin resistance on pregnancy complications and outcome in women with polycystic ovary syndrome. Gynecol Obstet Invest. 2002;54:94–98. doi: 10.1159/000067719. [DOI] [PubMed] [Google Scholar]
- 64.Zhang YJ, Jin H, Qin ZL, Ma JL, Zhao H, Zhang L, et al. Predictors of gestational diabetes mellitus in chinese women with polycystic ovary syndrome: a cross-sectional study. Gynecol Obstet Invest. 2016;81:220–224. doi: 10.1159/000440618. [DOI] [PubMed] [Google Scholar]
- 65.De Leo V, Musacchio MC, Piomboni P, Di Sabatino A, Morgante G. The administration of metformin during pregnancy reduces polycystic ovary syndrome related gestational complications. Eur J Obstet Gynecol Reprod Biol. 2011;157:63–66. doi: 10.1016/j.ejogrb.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 66.Glueck CJ, Bornovali S, Pranikoff J, Goldenberg N, Dharashivkar S, Wang P. Metformin, pre-eclampsia, and pregnancy outcomes in women with polycystic ovary syndrome. Diabet Med. 2004;21:829–836. doi: 10.1111/j.1464-5491.2004.01251.x. [DOI] [PubMed] [Google Scholar]
- 67.Glueck CJ, Goldenberg N, Wang P, Loftspring M, Sherman A. Metformin during pregnancy reduces insulin, insulin resistance, insulin secretion, weight, testosterone and development of gestational diabetes: prospective longitudinal assessment of women with polycystic ovary syndrome from preconception throughout pregnancy. Hum Reprod. 2004;19:510–521. doi: 10.1093/humrep/deh109. [DOI] [PubMed] [Google Scholar]
- 68.Glueck CJ, Goldenberg N, Pranikoff J, Khan Z, Padda J, Wang P. Effects of metformin-diet intervention before and throughout pregnancy on obstetric and neonatal outcomes in patients with polycystic ovary syndrome. Curr Med Res Opin. 2013;29:55–62. doi: 10.1185/03007995.2012.755121. [DOI] [PubMed] [Google Scholar]
- 69.Glueck CJ, Wang P, Goldenberg N, Sieve-Smith L. Pregnancy outcomes among women with polycystic ovary syndrome treated with metformin. Hum Reprod. 2002;17:2858–2864. doi: 10.1093/humrep/17.11.2858. [DOI] [PubMed] [Google Scholar]
- 70.Mehrabian F, Rezae M. Sex hormone binding globulin measurement before conception as a predictor of gestational diabetes in women with polycystic ovarian syndrome. J Res Med Sci. 2013;18:637–640. [PMC free article] [PubMed] [Google Scholar]
- 71.Paradisi G, Fulghesu AM, Ferrazzani S, Moretti S, Proto C, Soranna L, et al. Endocrino-metabolic features in women with polycystic ovary syndrome during pregnancy. Hum Reprod. 1998;13:542–546. doi: 10.1093/humrep/13.3.542. [DOI] [PubMed] [Google Scholar]
- 72.Boomsma CM, Fauser BC, Macklon NS. Pregnancy complications in women with polycystic ovary syndrome. Semin Reprod Med. 2008;26:72–84. doi: 10.1055/s-2007-992927. [DOI] [PubMed] [Google Scholar]
- 73.Yu HF, Chen HS, Rao DP, Gong J. Association between polycystic ovary syndrome and the risk of pregnancy complications: a PRISMA-compliant systematic review and meta-analysis. Medicine. 2016;95:e4863. doi: 10.1097/MD.0000000000004863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Malin SK, Kirwan JP, Sia CL, González F. Pancreatic β-cell dysfunction in polycystic ovary syndrome: role of hyperglycemia-induced nuclear factor-κB activation and systemic inflammation. Am J Physiol Endocrinol Metab. 2015;308:E770–E777. doi: 10.1152/ajpendo.00510.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Galluzzo A, Amato MC, Giordano C. Insulin resistance and polycystic ovary syndrome. Nutr Metab Cardiovasc Dis. 2008;18:511–518. doi: 10.1016/j.numecd.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 76.Harlev A, Wiznitzer A. New insights on glucose pathophysiology in gestational diabetes and insulin resistance. Curr Diab Rep. 2010;10:242–247. doi: 10.1007/s11892-010-0113-7. [DOI] [PubMed] [Google Scholar]
- 77.Vanky E, Zegher DE, Díaz M, Ibáñez L, Carlsen SM. On the potential of metformin to prevent preterm delivery in women with polycystic ovary syndrome—an epi-analysis. Acta Obstet Gynecol Scand. 2012;91:1460–1464. doi: 10.1111/aogs.12015. [DOI] [PubMed] [Google Scholar]
- 78.Kuo CH, Chen SC, Fang CT, et al. Screening gestational diabetes mellitus: the role of maternal age. PLoS ONE. 2017;12:e0173049. doi: 10.1371/journal.pone.0173049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Magenheim R, Tabák A, Lengyel Z, Tóth K, Lévárdi F. Is previous macrosomia a risk factor for gestational diabetes in the era of general screening? BJOG. 2007;114:512–513. doi: 10.1111/j.1471-0528.2006.01260.x. [DOI] [PubMed] [Google Scholar]
- 80.Miao M, Dai M, Zhang Y, Sun F, Guo X, Sun G. Influence of maternal overweight, obesity and gestational weight gain on the perinatal outcomes in women with gestational diabetes mellitus. Sci Rep. 2017;7:305. doi: 10.1038/s41598-017-00441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wei J, Gao J, Cheng J. Gestational diabetes mellitus and impaired glucose tolerance pregnant women. Pak J Med Sci. 2014;30:1203–1208. doi: 10.12669/pjms.306.5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hedderson MM, Darbinian JA, Ferrara A. Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatr Perinat Epidemiol. 2010;24:441–448. doi: 10.1111/j.1365-3016.2010.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moosazadeh M, Asemi Z, Lankarani KB, Tabrizi R, Maharlouei N, Naghibzadeh-Tahami A, et al. Family history of diabetes and the risk of gestational diabetes mellitus in Iran: a systematic review and meta-analysis. Diabetes Metab Syndr. 2017;11:S99–S104. doi: 10.1016/j.dsx.2016.12.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Additional figures and tables.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request