Abstract
To explore the molecular mechanisms of BAY R3401, four types of novel photoaffinity probes bearing different secondary tags were synthesized. Their potency for glycogenolysis was evaluated in primary human liver HL-7702 cells and HepG2 cells. Probe 2d showed the best activity in primary human liver HL-7702 cells and HepG2 cells, with IC50 values of 4.45 μM and 28.49 μM, respectively. Likewise, probe 5d showed IC50 values of 6.46 μM in primary human liver HL-7702 cells and 15.29 μM in HepG2 cells, respectively. Photoaffinity labeling experiments were also performed and protein bands larger than 170 kDa were specifically tagged by probe 2d. The results suggest that the synthesized probe 2d might be a very promising tool for the isolation of the target proteins of BAY R3401.
Keywords: BAY R3401, molecular mechanism, type 2 diabetes, photoaffinity probe, glycogenolysis, photoaffinity labeling, target proteins
Academic Editor: Run Zhang, D. Amilan Jose, Hang Thu Ta and Mingqian Tan
1. Introduction
BAY R3401 is an orally bioavailable hypoglycemic agent for the treatment of type 2 diabetes, as reported by the Bayer Pharmaceutical Company [1]. This agent allows irreversible, nonselective suppression of hepatic glycogenolysis by inhibiting glycogen phosphorylase, which is the rate controlling enzyme of the glycogenolytic pathway [2]. The active metabolite, W1807, contributes significantly to its activity [3]. Nonetheless, much to the researchers’ surprise, BAY R3401 inactivated glycogen phosphorylase by 63%, but glucose output dropped by 83% in the perfused liver [4]. It is difficult to explain the effects based only on the reported mechanism. Therefore, the exact mode of action of BAY R3401 has not been established.
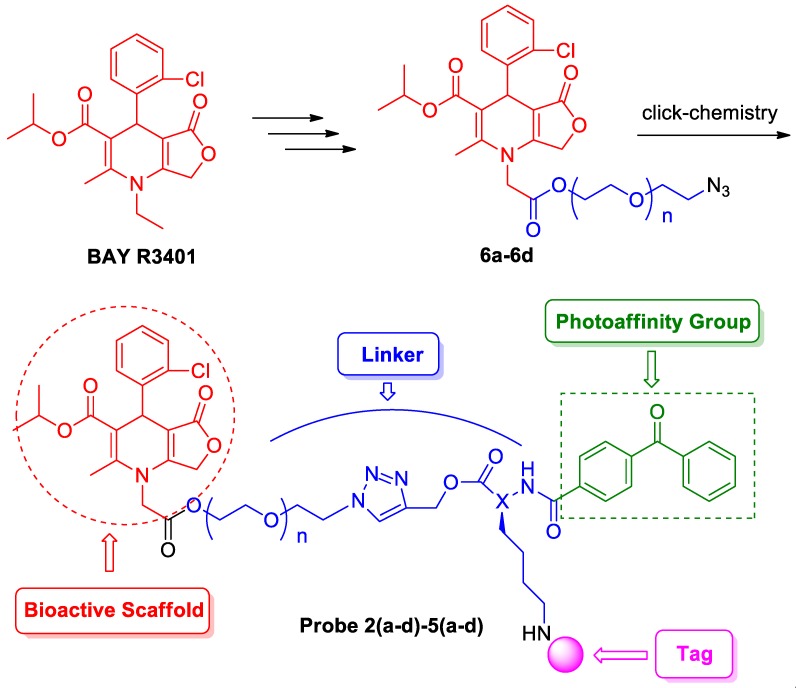
Photoaffinity labeling is one of the major methods to directly capture small-molecule binding proteins [5]. The conventional approach, however, usually relies on the synthesis of photoaffinity probes and the identification of photolabeled fragments in proteins [6]. In general, a typical photoaffinity probe contains three functional groups. A bioactive scaffold ferries the probe to the enzyme active site, a photoreactive group generates a covalent and irreversible linkage between the probe and its target macromolecule after UV irradiation, and a tag (such as biotin or fluorophore) detects and/or visualizes the modified target enzymes [7].
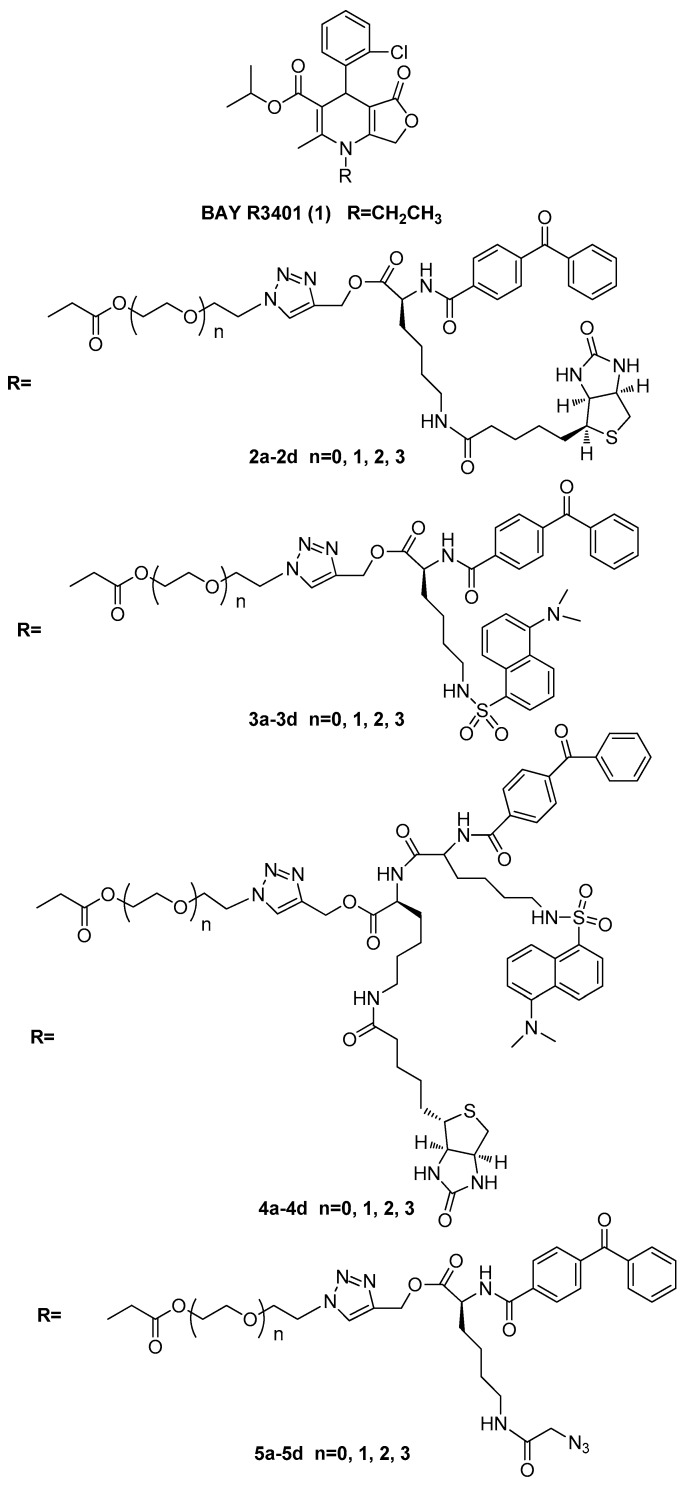
Herein, we report the synthesis and biological application of four types of photoaffinity probes based on BAY R3401, which contains both a benzophenone photophore for covalent labeling of target proteins and a secondary handle for the subsequent detection or manipulation of labeled proteins (Figure 1). Probes bearing different secondary tags were exploited, either by direct attachment of a dansyl fluorescent or a biotin tag for detection and enrichment. Moreover, we developed a dual-functional tag containing both a dansyl group and a biotin, which is suitable for both affinity purification and fluorescence applications. In order to avoid the sterically hindrance caused by the large tags, we designed another tag-free probe that employed an azide handle for downstream conjugation to the reporter tag via the click-chemistry reaction after proteome labeling. According to the previous primary structure-activity relationship of the dihydropyridine derivative, modification of the N1 position of BAY R3401 had little effect on its potency. However, the molecular size of the secondary tags is not very small compared to BAY R3401. Thus, it is not hard to speculate that hindrance might sterically occur due to interfere from the interaction between BAY R3401 and its target proteins when secondary tags are introduced to the N1 position of BAY R3401 directly. Therefore, appropriate linkages were designed to provide enough space between BAY R3401 and the secondary tags.
Figure 1.
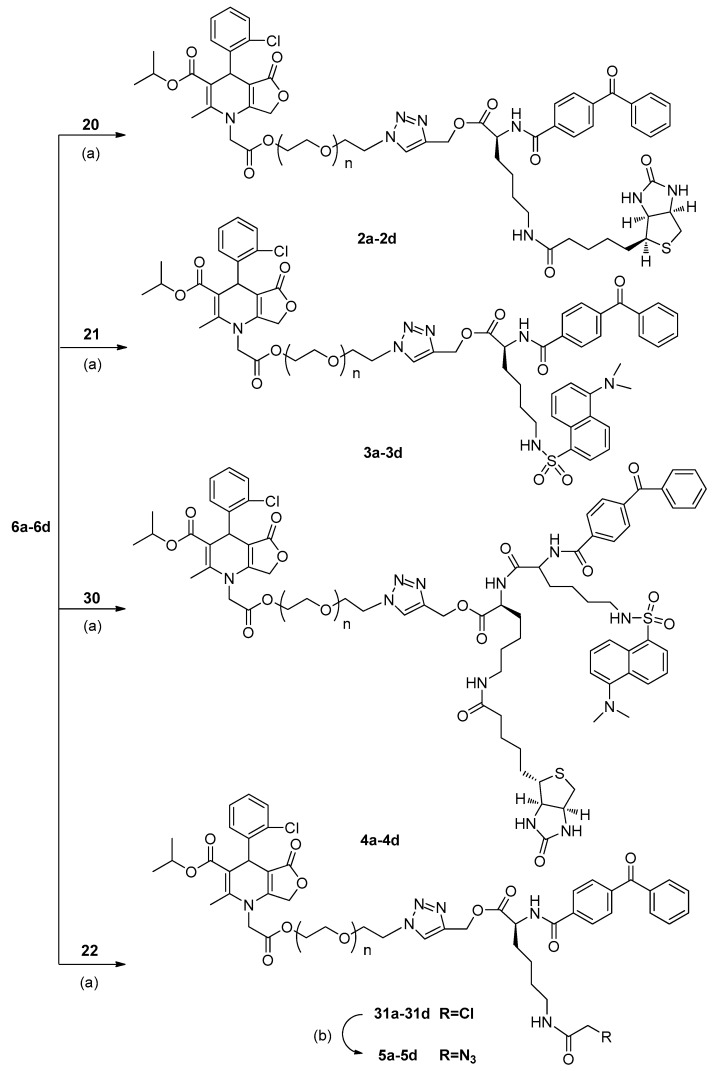
Structures of BAY R3401 (1), and synthetic photoaffinity probes possessing biotin (2a–2d), dansyl (3a–3d), a dual-functional tag (4a–4d), or azide (5a–5d).
2. Results and Discussion
2.1. Chemistry
The general synthetic strategy employing a click reaction for the described activity probes is outlined in Figure 2.
Figure 2.
General synthetic strategy for photoaffinity probes by click-chemistry reaction.
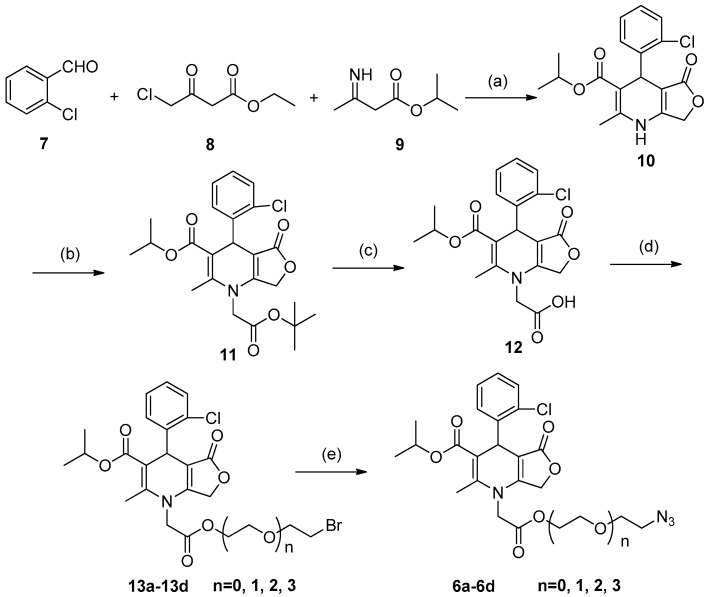
To realize the click chemistry between BAY R3401 and the photoaffinity label moiety, azide functions were first introduced into the position of the ethyl branches based on previous SAR studies (Scheme 1) [8]. Treatment of 2-chlorobenzaldehyde 7 and ethyl 4-chloroacetoacetate 8 with excess isopropyl 3-aminocrotonate 9 in a one-pot reaction at reflux overnight in isopropanol yielded the 1,4-dihydropyridine nucleus 10 with a 17% yield. The alkylation of 10 with tert-butyl chloroacetate produced 1-alkyl-1,4-dihydropyridine derivative 11 with a 40% yield. Deprotection of 11 with trifluoroacetic acid (TFA) produced carboxylic acid 12 (46%). Esterification of 12 with three different linker groups (such as 1,2-dibromoethane, 2,2’-Dibromodiethyl ether, and 1,2-Bis(2-bromoethoxy)ethane) yielded the corresponding bromides, 13a–13d (58–62%), which were converted to azides 6a–6d via a nucleophilic substitution reaction with sodium azide (NaN3) with satisfactory yields (59–90%).
Scheme 1.
Reagents and conditions: (a) isopropyl alcohol, reflux (17%); (b) (i) NaH, 0 °C to 80 °C; (ii) tert-butyl 2-chloroacetate, 80 °C (40% for 2 steps); (c) trifluoroacetic acid (TFA), CH2Cl2, 0 °C to r.t. (46%); (d) 1,2-dibromoethane or 2,2’-Dibromodiethyl ether or 1,2-Bis(2-bromoethoxy)ethane, K2CO3, anhydrous CH3CN, 0 °C to 80 °C (58–62%); (e) NaN3, DMF, r.t. (59–90%).
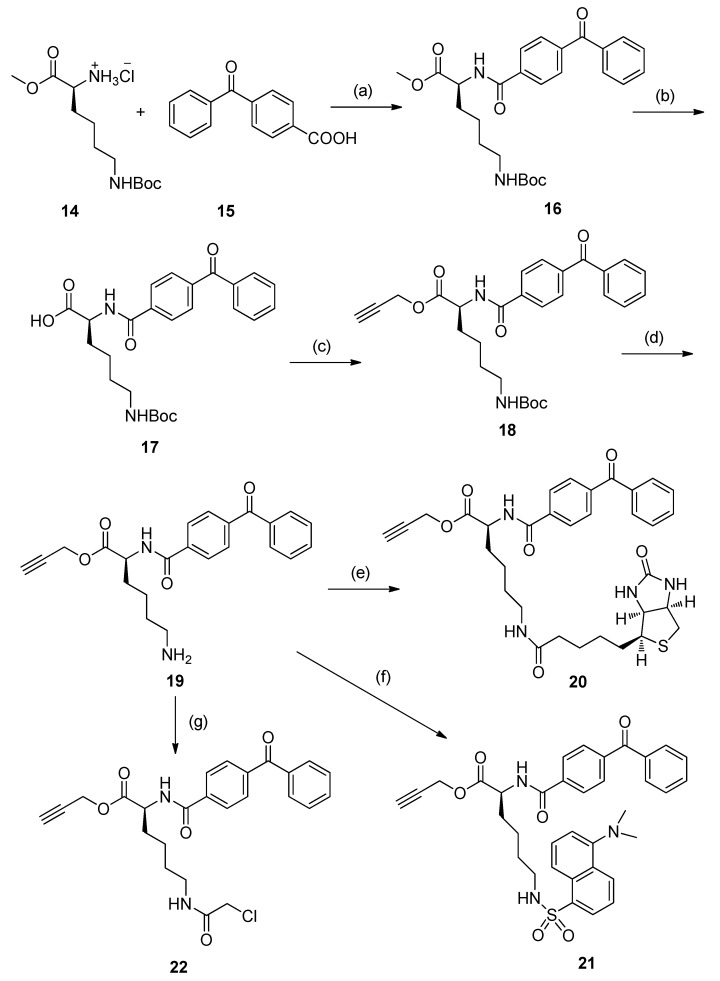
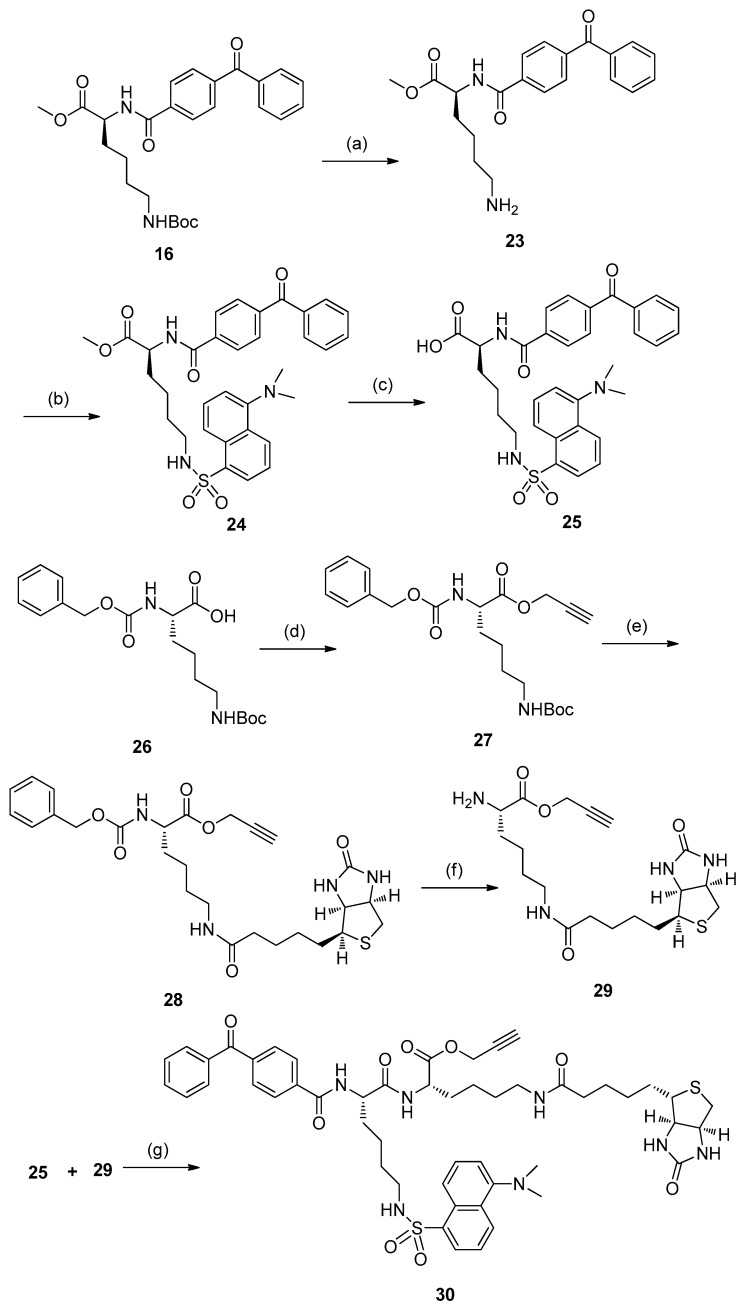
The key intermediates, 20–22, were prepared individually via procedures similar to those reported previously, with some modifications (Scheme 2) [9]. Treatment of Lys(Boc)-OMe with 4-benzoylbenzoic acid using EDCI/DMAP afforded compound 16, bearing a benzophenone photophore. Hydrolysis of 16 with aqueous NaOH yielded carboxylic acid 17, followed by an esterification with propargyl bromide to produce the alkyne 18. Subsequent deprotection of 18 using TFA gave amide 19, then coupling 19 with D-biotin in DMF in the presence of EDCI, HOBt, and DIPEA as condensing agents produced biotin conjugate 20 with a biotin tag. Treatment of amino 19 with dansyl chloride (DNS-Cl) gave the desired fluorescent derivative 21. Amidation of amino 19 with ClCH2COCl was carried out to produce chloroacetyl compound 22 for the next step of azide displacement.
Scheme 2.
Reagents and conditions: (a) EDCI, NMM, DMAP, DMF, r.t. (87%); (b) 2 N NaOH, CH3OH, 0 °C to r.t.; (c) 3-Bromopropyne, K2CO3, DMF, r.t. (68%); (d) TFA, CH2Cl2, 0 °C to r.t.; (e) D-biotin, EDCI, HOBt, DIPEA, DMF (37%); (f) dansyl chloride (DNS-Cl), Et3N, CH2Cl2, r.t. (81%); (g) ClCH2COCl, Et3N, CH2Cl2, 0 °C to r.t. (86%).
The preparation of a dual-labeled moiety was accomplished as follows (Scheme 3). The synthesis cycle began with deprotection of 16 using TFA, producing amide 23. Then, amidation with DNS-Cl gave compound 24. Hydrolysis of 24 with aqueous LiOH afforded carboxylic acid 25 with an 87% yield. Treatment of N-Cbz-N’-Boc-L-lysine with propargyl bromide gave alkyne 27. Subsequent deprotection of 27 using TFA, followed by coupling with D-biotin in DMF in the presence of isobutyl chloroformate and Et3N as condensing agents, produced biotin conjugate 28. The N-Cbz group was hydrolyzed off in 30% HBr in acetic acid at room temperature to give the amide compound 29. Reaction of 29 with carboxylic acid derivative 25 in the presence of HATU and DIPEA produced the dual label moiety with a 43% yield.
Scheme 3.
Reagents and conditions: (a) TFA, CH2Cl2, 0 °C to r.t.; (b) DNS-Cl, Et3N, CH2Cl2, r.t. (67%); (c) 6 N LiOH, THF/H2O, 0 °C to r.t. (88%); (d) 3-Bromopropyne, K2CO3, DMF, r.t. (72%); (e) (i) TFA, CH2Cl2, 0 °C to r.t.; (ii) D-biotin, isobutyl chloroformate, Et3N, DMF (77%); (f) HBr/HOAC, 0 °C to r.t.; (g) HATU, DIPEA, DMF, 0 °C to r.t. (43%).
Click chemistry was handled as shown in Scheme 4. The corresponding azides and alkynes were dissolved in CH2Cl2-H2O, followed by the addition of catalytic sodium ascorbate and CuSO4·5H2O. Reaction of the above azides 6a–6d with alkynes 20, 21, and 30 gave, respectively, probes 2a–2d (21–25%), 3a–3d (13–27%), and 4a–4d (13–27%). In a similar way, the conversion of 6a–6d to the corresponding chloroacetyl compounds, 31a–31d, was followed by a reaction with NaN3 to give probes 5a–5d.
Scheme 4.
Reagents and conditions: (a) CuSO4·5H2O, sodium ascorbate, CH2Cl2-H2O, r.t.; (b) NaN3, DMF, r.t.
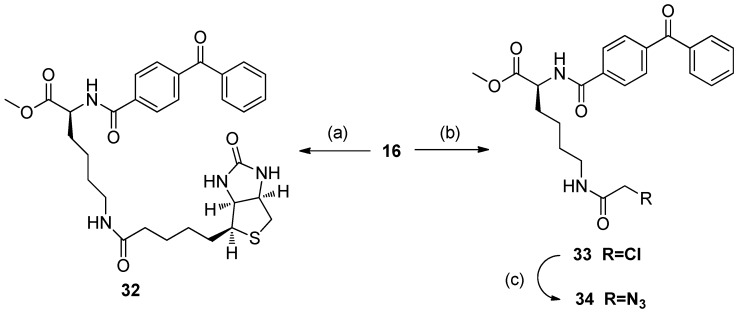
The synthesis of control compounds 32 and 34, which lack the bioactive ligand BAY R3401 and only contain the photoaffinity and tag groups, is shown in Scheme 5. The cycle was started with the deprotection step, as above, followed by amidation with D-biotin and ClCH2COCl to give the control compound 32, and intermediate haloester 33. Then, treatment of 33 with NaN3 produced the control compound 34 with a 77% yield.
Scheme 5.
(a) i) TFA, CH2Cl2, 0 °C to r.t.; ii) D-biotin, EDCI, HOBt, DIPEA, DMF (51%); (b) ClCH2COCl, Et3N, CH2Cl2, 0 °C to r.t. (68%); (c) NaN3, DMF, r.t. (77%).
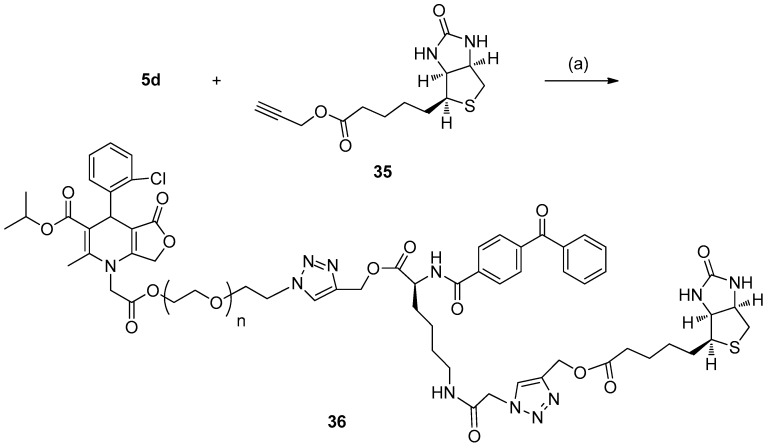
To confirm the efficacy of designed probes 5a–5d in combining the tag after protein labeling, the reaction of the tag-free probe 5d with the alkyne-coupled biotin derivative in a simple model system was also studied (Scheme 6). The biotin derivative 35 was prepared based on a previously reported procedure described in [10]. Treatment of probe 5d with alkyne-biotin 35 under typical click conditions (CuSO4·5H2O, with sodium ascorbate as the reducing agent) in CH2Cl2-H2O, the conjugate product 36 could be isolated with a 13% yield as a white solid. These results demonstrated that the azide handle of the tag-free probe can be subsequently conjugated with an alkyne-tag through biocompatible copper-catalyzed azide-alkyne cycloaddition.
Scheme 6.
(a) CuSO4·5H2O, sodium ascorbate, CH2Cl2-H2O, r.t. (13%).
2.2. Cell Assay and SAR Analysis.
It is obviously important that the synthesized probes retain potency in bioassays. To evaluate the effects of all probes, the glycogenolysis assays were established in vitro, with primary human liver HL-7702 cells and HepG2 cells, based on the published method [11]. A well-known chloroindole inhibitor of glycogenolysis, CP-91149, was used as a positive control in these experiments.
The IC50 values of the tested derivatives are listed in Table 1. Most of the newly synthesized probes maintained moderate inhibitory activity against glucagon-stimulated glycogenolysis in primary human liver HL-7702 cells and HepG2 cells. It is interesting to note that modification of dihydropyridine scaffold with bulky substituents resulted in a great increase in IC50 both in primary human liver HL-7702 cells and HepG2 cells (e.g., BAY R3401 vs. 2b–2d, 3b–3d, 5a, 5c, 5d, 6a). The results are consistent with the SAR analysis of W1807, the active metabolite in cells of BAY R3401, which revealed that the N1-substituent may productive van der Waals interactions between the substitutions and its target proteins [8]. With different linkers, SAR analysis in HepG2 cells shows that the distance between the BAY R3401 moiety and secondary tags’ moiety is important—a longer linker led to better inhibitory activity (e.g., 2a vs. 2d, 3a vs. 3d, and 5a vs. 5d). However, data analysis indicated no clear SAR for the distance in primary human liver HL-7702 cells. Within this series of compounds, probe 2d showed the best activity in primary human liver HL-7702 cells and HepG2 cells, with IC50 values of 4.45 μM and 28.49 μM, respectively. Likewise, probe 5d showed an IC50 value of 6.46 μM in primary human liver HL-7702 cells and 15.29 μM in HepG2 cells, respectively. Therefore, probe 2d and 5d may be very promising tools for isolation of the target proteins of BAY R3401.
Table 1.
Glycogenolysis inhibition assay for compounds 2(a–d)–6(a–d) in liver cells.
| Compound | IC50 a (μM, HL-7702 cells) | IC50 a (μM, HepG2 cells) | Compound | IC50 a (μM, HL-7702 cells) | IC50 a (μM, HepG2 cells) |
|---|---|---|---|---|---|
| 2a | 6.23 ± 2.86 | 53.12 ± 10.11 | 4d | 17.15 ± 4.97 | NI |
| 2b | 4.55 ± 1.57 | 37.69 ± 1.76 | 5a | 2.96 ± 0.57 | 45.94 ± 12.44 |
| 2c | 5.22 ± 1.17 | 38.00 ± 1.06 | 5b | 4.83 ± 1.48 | 59.89 ± 19.75 |
| 2d | 4.45 ± 0.50 | 28.49 ± 3.38 | 5c | 15.22 ± 3.29 | 18.73 ± 5.31 |
| 3a | 4.86 ± 1.22 | 106.16 ± 4.17 | 5d | 6.46 ± 3.60 | 15.29 ± 4.31 |
| 3b | 5.62 ± 1.67 | 44.75 ± 7.20 | 6a | 19.36 ± 2.00 | 40.02 ± 5.55 |
| 3c | 2.56 ± 0.59 | 42.32 ± 5.78 | 6b | 22.36 ± 1.97 | 54.38 ± 13.73 |
| 3d | 2.71 ± 0.54 | 33.71 ± 1.23 | 6c | 22.35 ± 24.36 | NI |
| 4a | 27.22 ± 4.94 | 49.09 ± 1.23 | 6d | 24.00 ± 12.44 | 40.05 ± 3.99 |
| 4b | 27.32 ± 9.27 | 96.71 ± 36.04 | BAY R3401 | 27.06 ± 9.63 | 52.83 ± 8.93 |
| 4c | 15.22 ± 3.29 | NIb | CP-91149 c | 2.53 ± 0.78 | 3.08 ± 1.16 |
a Each value represents the mean ± S.D. of three determinations. b NI means no inhibition. c CP-91149 was used as a positive control.
2.3. Application of Activity-Based Profiling to Target Discovery
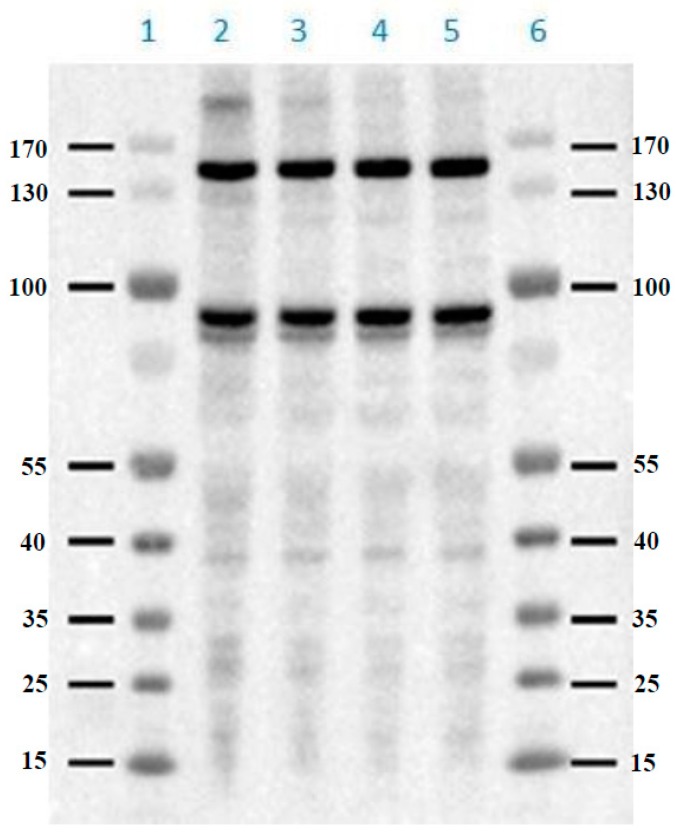
Based on the results of the potency in cell assay, probe 2d was selected for photolabeling studies to detect the binding proteins of BAY R3401 by PAGE and chemiluminescence [12]. The soluble proteomes prepared from HepG2 cells, were incubated with the 10 μM probe 2d, exposed to UV light for 30 min, followed by SDS-PAGE electrophoresis, and then transfer onto a PVDF membrane for detection with streptavidin-HRP [10]. Samples were prepared by incubating 2.0 mg/mL proteomes at different conditions: (Lane 1 and 6) marker; (Lane 2) with the 10 μM probe 2d and exposed to UV light for 30 min; (Lane 3) with the 10 μM probe 2d and BAY R3401, and then exposed to UV light for 30 min; (Lane 4) With the 10 μM control compound 32 and exposed to UV light for 30 min; and (Lane 5) with the 0 μM probe 2d and exposed to UV light for 30 min.
The results showed that a protein band larger than 170 kDa was seen in samples incubated with 2d. This labeling was specific since it was completed by BAY R3401, and no such labeling was seen when samples were incubated with control compound 32 rather than 2d (Figure 3). These data demonstrate that the synthesized probe 2d can efficiently and specifically identify the binding protein(s) of BAY R3401 and suggest that 2d will be suitable for isolating the binding protein(s) of BAY R3401 by avidin–agarose chromatography.
Figure 3.
Results of SDS-PAGE analysis of photoaffinity labeling of experiment by synthesized probe 2d.
Then, we used a more detailed proteomic analysis, with a mass spectrometric analysis method, to detect the protein band larger than 170 kDa. Several proteins have been identified, and some of the results are summarized in Table 2. We hypothesise that the PH-interacting protein, histone H4, hexokinase-2 and solute carrier family 2 might be the target proteins of BAY R3401 based on the protein probability scores in MaxQuant. We are now in the process of verifying these proteins. A comparison between the streptavidin blot analysis and Coomassie brilliant blue (CBB) staining was carried out. The detailed information of CBB is in Supplementary information. The results also showed that the streptavidin blot analysis was specific since the protein labeled by probe 2d is clearly shown after the streptavidin blot analysis while CBB staining had a strong background with the gel image under identical staining conditions.
Table 2.
Comparative analysis of the protein groups identified by LC-MS/MS.
| Description | Accession a | Score b | MW c [kDa] |
|---|---|---|---|
| Carbamoyl-phosphate synthase [ammonia] | P31327 | 703.64 | 164.83 |
| Myoferlin | Q9NZM1 | 491.05 | 234.56 |
| Coatomer subunit alpha | P53621 | 338.49 | 138.26 |
| Keratin, type II cytoskeletal 1 | P04264 | 332.99 | 66.0 |
| Vigilin | Q00341 | 325.29 | 141.37 |
| Sodium/potassium-transporting ATPase subunit alpha-1 | P05023 | 169.27 | 112.82 |
| Inositol 1,4,5-trisphosphate receptor type 3 | Q14573 | 146.90 | 303.91 |
| Epidermal growth factor receptor | P00533 | 110.92 | 134.19 |
| PH-interacting protein | Q8WWQ0 | 46.67 | 206.56 |
| Nuclear pore complex protein Nup153 | P49790 | 29.84 | 153.84 |
| Histone H4 | P62805 | 27.37 | 11.36 |
| Hexokinase-2 | E9PB90 | 26.18 | 98.91 |
| DNA (cytosine-5)-methyltransferase 1 | P26358 | 26.13 | 183.05 |
| U2 snRNP-associated SURP motif-containing protein | O15042 | 25.91 | 118.22 |
| Histone-lysine N-methyltransferase EHMT2 | A0A0G2JIS2 | 24.46 | 128.95 |
| Keratin, type II cytoskeletal 72 | Q14CN4 | 24.37 | 55.84 |
| 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase beta-3 | Q01970 | 22.73 | 138.71 |
| Solute carrier family 2, facilitated glucose transporter member 1 | P11166 | 20.46 | 54.05 |
| Inositol 1,4,5-trisphosphate receptor type 2 | Q14571 | 20.32 | 307.87 |
| Low-density lipoprotein receptor | P01130 | 18.50 | 95.31 |
| Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit alpha | O00443 | 16.86 | 190.56 |
| Inositol 1,4,5-trisphosphate receptor type 1 | Q14643 | 15.87 | 313.73 |
| Receptor tyrosine-protein kinase erbB-2 (Fragment) | J3KTI5 | 13.12 | 27.76 |
| Apolipoprotein B-100 | P04114 | 11.42 | 515.28 |
| Rho-associated protein kinase 2 | O75116 | 10.68 | 160.80 |
| Histone-lysine N-methyltransferase EHMT1 | A0A1B0GV09 | 10.33 | 138.17 |
a Protein accession number received from the Uniprot-Orytolagus cuniculus database. b Scores are based on the results of MaxQuant software (www.maxquant.org). The higher the score, the more consistent the mass spectrometry data are with data in the database. c The molecular mass data were recorded according to the LTQ-Orbitrap Fusion mass spectrometer.
3. Materials and Methods
3.1. Chemistry
NMR experiments were performed on a Bruker Avance III 400 MHz and a Bruker Fourier 300 MHz. The spectra are referenced internally according to the residual solvent signals of TMS (δ = 0.00 ppm). Positive or negative ion LCMS data were obtained at 303 K by a quadrupole Mass Spectrometer on Agilent LC/MSD 1200 Series using a 50 × 4.6 mm (5 μm) ODS column. Prep-HPLC experiments were performed by flash welchrom C18 column (150 × 20 mm) chromatography.
3.1.1. Isopropyl 2-methyl-4-(2-chlorophenyl)-5-oxo-1,4,5,7-tetrahydrofuro [3,4-b]pyridine-3-carboxylate (10)
A mixture of 9 (224.9 mL, 1.55 mol), ethyl 4-chloro-3-oxobutanoate (209.4 mL, 1.55 mol) and 2-chlorobenzaldehyde (174.4 mL, 1.55 mol) in isopropyl alcohol (1.5 L) was stirred at reflux temperature overnight. After cooling to r.t. overnight, the mixture was filtered. The residue was purified by recrystallized from isopropyl ether to give product 10 (90.0 g, 17%) as a yellow solid. HPLC analysis: 100.0%. M.p. 225–227 °C. 1H-NMR (400 MHz, d6-DMSO): 0.71 (d, J = 8.4 Hz, 3H), 1.12 (d, J = 8.4 Hz, 3H), 2.30 (s, 3H), 4.68–4.76 (m, 1H), 4.79 (s, 2H), 5.16 (s, 1H), 7.12–7.18 (m, 1H), 7.25–7.32 (m, 3H), 9.79 (s, 1H). 13C-NMR (100 MHz, d6-DMSO): 171.5, 166.3, 156.7, 146.6, 144.3, 132.4, 131.4, 129.2, 128.2, 127.7, 103.4, 100.7, 66.5, 65.5, 34.8, 22.0, 21.3, 19.2. ESI (MS) m/z: 348.1 (M + H)+. HRMS (ESI-TOF) calculated for C18H18ClNNaO4 (M + Na)+: 370.08166; found: 370.08298.
3.1.2. Isopropyl 2-methyl-4-(2-chlorophenyl)-1-(2-tert-butoxy-2-oxoethyl)-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (11)
To a solution of 10 (62.60 g, 0.18 mol) in anhydrous DMF (1.5 L) was added a NaH (60% dispersion in mineral oil, 8.85 g, 0.22 mol) at 0 °C under a N2 atmosphere. The mixture was heated at 80 °C for 2 h and then tert-butyl 2-chloroacetate (36.10 g, 0.24 mol) was added slowly dropwise at r.t.. The mixture was stirred at 80 °C for 1 h. After cooling to r.t., the mixture was diluted with water (2.5 L) and extracted by EtOAc (1 L × 3). The combined organic phase was washed with brine (1 L × 2), dried over anhydrous Na2SO4, and evaporated. The residue was purified by being recrystallized with EtOAc (200 mL) to give product 11 (33.0 g, 40%) as a white solid. HPLC analysis: 90.3%. M.p. 174–176 °C. 1H-NMR (400 MHz, CDCl3): 0.85 (d, J = 8.4 Hz, 3H), 1.22 (d, J = 6.4 Hz, 3H), 1.55 (s, 9H), 2.42 (s, 3H), 4.07 (dd, J = 42.8, 18.4 Hz, 2H), 4.68 (s, 2H), 4.84–4.93 (m, 1H), 5.47 (s, 1H), 7.11 (td, J = 7.6, 1.6 Hz, 1H), 7.21 (td, J = 7.6, 1.2 Hz, 1H), 7.31 (dd, J = 8.0, 1.2 Hz, 1H), 7.42 (dd, J = 8.0, 1.6 Hz, 1H). 13C-NMR (100 MHz, CDCl3): 171.1, 166.9, 166.8, 156.4, 144.9, 142.2, 133.2, 131.1, 129.4, 127.9, 127.0, 109.0, 103.3, 84.0, 67.8, 65.0, 48.1, 35.0, 28.0, 21.7, 20.9, 15.1. ESI (MS) m/z: 462.0 (M + H)+. HRMS (ESI-TOF) calculated for C24H29ClNO6 (M + H)+: 462.16779; found: 462.16878.
3.1.3. 2-[2-Methyl-3-isopropoxycarbonyl-4-(2-chlorophenyl)-5-oxo-furo[3,4-b]pyridine-1(4H,5H,7H)-yl]acetic acid (12)
To a solution of 11 (10.0 g, 21.65 mmol) in DCM (100 mL) under a N2 atmosphere, TFA (15.0 mL, 0.20 mol) was added, dropwise, at 0 °C. The mixture was stirred at 0 °C for 2 h and then quenched with KHCO3 aq (3 N). The aqueous layer was acidified with acetic acid to pH = 2 and extracted by EtOAc (100 mL × 5). The combined organic phase was washed with brine (200 mL × 2), dried over anhydrous Na2SO4, and concentrated. The residue was purified by flash silica gel column chromatography [CH2Cl2-MeOH (100:1)] to obtain a white solid product 12 (4.0 g, 45%). HPLC analysis: 92.6%. M.p. 155–157 °C. 1H-NMR (400 MHz, d6-DMSO): 0.79 (d, J = 6.0 Hz, 3H), 1.14 (d, J = 6.4 Hz, 3H), 2.32 (s, 3H), 4.27 (q, J = 18.4 Hz, 2H), 4.71–4.78 (m, 1H), 4.87 (dd, J = 42.8, 16.4 Hz, 2H), 5.22 (s, 1H), 7.17 (td, J = 7.6, 1.6 Hz, 1H), 7.27 (td, J = 7.2, 0.8 Hz, 1H), 7.32 (dd, J = 7.6, 0.8 Hz, 1H), 7.49 (dd, J = 7.6, 0.8 Hz, 1H). 13C-NMR (100 MHz, d6-DMSO): 171.5, 170.9, 166.9, 159.1, 146.9, 143.7, 132.3, 131.6, 129.1, 128.4, 127.8, 107.4, 101.1, 67.2, 66.0, 48.6, 34.6, 21.9, 21.2, 15.3. ESI (MS) m/z: 405.9 (M + H)+. HRMS (ESI-TOF) calculated for C20H20ClNNaO6 (M + Na)+: 428.08714; found: 428.08765.
3.1.4. General Procedure for Synthesis of compounds 13a–13d
To a solution of 12 (12.20 g, 0.03 mol) in anhydrous acetonitrile (120 mL) was added K2CO3 (12.50 g, 0.09 mol) in groups, and then Bromo-PEG(n)-bromide (0.15 mol) was added to the reaction mixture slowly under an ice bath, and the temperature was raised to 80 °C for 1.5 h. After cooling to r.t., the mixture was diluted with water (150 mL) and extracted by EtOAc (100 mL × 2). The combined organic phase was washed with brine (100 mL × 2), dried over anhydrous Na2SO4, and evaporated. The residue was purified by flash column chromatography [petroleum ether-EtOAc (1:1)] to give the products 13a–13d.
Isopropyl 1-[2-(2-bromoethoxy)-2-oxoethyl]-4-(2-chlorophenyl)-2-methyl-5-oxo-1, 4, 5, 7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (13a). HPLC analysis: 96.8%. M.p. 154–156 °C. 1H-NMR (400 MHz, CDCl3): 0.85 (d, J = 6.4 Hz, 3H), 1.20 (d, J = 6.4 Hz, 3H), 2.41 (s, 3H), 3.58 (t, J = 6.0 Hz, 2H), 4.25 (dd, J = 57.6, 18.8 Hz, 2H), 4.57 (t, J = 5.6 Hz, 2H), 4.69 (s, 2H), 4.85–4.91 (m, 1H), 5.44 (s, 1H), 7.11 (td, J = 8.0, 1.6 Hz, 1H), 7.21 (t, J = 7.6, Hz, 1H), 7.30 (d, J = 7.6, 1H), 7.41 (dd, J = 7.6, 1.6 Hz, 1H). 13C-NMR (100 MHz, CDCl3): 171.0, 167.6, 166.7, 156.2, 144.4, 141.9, 133.3, 131.1, 129.4, 127.9, 127.0, 109.4, 103.6, 67.9, 65.3, 65.0, 47.2, 35.1, 28.3, 21.7, 20.9, 15.2. ESI (MS) m/z: 513.8 (M + H)+. HRMS (ESI-TOF) calculated for C22H24BrClNO6 (M + H)+: 512.047; found: 512.04884.
Isopropyl 1-{2-[2-(2-bromoethoxy)ethoxy]-2-oxoethyl}-4-(2-chlorophenyl)-2-methyl-5-oxo-1, 4, 5, 7-tetrahydrofuro[3, 4-b]pyridine-3-carboxylate (13b). HPLC analysis: 90.0%. M.p. 113–115 °C. 1H-NMR (400 MHz, CDCl3): 0.85 (d, J = 6.4 Hz, 3H), 1.21 (d, J = 6.4 Hz, 3H), 2.42 (s, 3H), 3.47 (t, J = 6.0 Hz, 2H), 3.78 (t, J = 4.8 Hz, 2H), 3.82 (t, J = 5.6 Hz, 2H), 4.24 (dd, J = 51.2, 18.8 Hz, 2H), 4.43–4.46 (m, 2H), 4.70 (s, 2H), 4.83–4.93 (m, 1H), 5.45 (s, 1H), 7.11 (td, J = 8.0, 1.6 Hz, 1H), 7.21 (td, J = 7.6, 1.2 Hz, 1H), 7.31 (dd, J = 7.6, 1.2 Hz, 1H), 7.41 (dd, J = 7.6, 1.6 Hz, 1H). 13C-NMR (100 MHz, CDCl3): 171.1, 167.9, 166.8, 156.4, 144.6, 142.0, 133.2, 131.1, 129.4, 127.9, 127.0, 109.3, 103.5, 71.0, 68.5, 67.9, 65.1, 64.8, 47.3, 35.0, 30.3, 21.7, 20.9, 15.2. ESI (MS) m/z: 557.9 (M + H)+. HRMS (ESI-TOF) calculated for C24H28BrClNO7 (M + H)+: 556.07322; found: 556.07444.
Isopropyl 1-(2-{2-[2-(2-bromoethoxy)ethoxy]ethoxy}-2-oxoethyl)-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (13c). HPLC analysis: 94.9%. M.p. 77–78 °C. 1H-NMR (400 MHz, CDCl3): 0.85 (d, J = 6.0 Hz, 3H), 1.21 (d, J = 6.4 Hz, 3H), 2.42 (s, 3H), 3.49 (t, J = 6.0 Hz, 2H), 3.67 (s, 4H), 3.77–3.82 (m, 4H), 4.24 (dd, J = 51.2, 18.8 Hz, 2H), 4.43–4.45 (m, 2H), 4.71 (s, 2H), 4.84–4.93 (m, 1H), 5.46 (s, 1H), 7.11 (td, J = 7.6, 1.6 Hz, 1H), 7.21 (td, J = 7.6, 0.8 Hz, 1H), 7.31 (dd, J = 8.0, 0.8 Hz, 1H), 7.41 (dd, J = 7.6, 1.6 Hz, 1H). 13C-NMR (100 MHz, CDCl3): 171.1, 167.9, 166.8, 156.4, 144.7, 142.0, 133.3, 131.1, 129.4, 127.9, 127.0, 109.3, 103.6, 71.2, 70.5, 68.8, 67.9, 65.1, 47.3, 35.1, 30.5, 21.7, 20.9, 15.2. ESI (MS) m/z: 602.0 (M + H)+. HRMS (ESI-TOF) calculated for C26H31BrClNNaO8 (M + Na)+: 622.08138; found: 622.08153.
Isopropyl 1-(14-bromo-2-oxo-3,6,9,12-tetraoxatetradecyl)-4- (2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (13d). HPLC analysis: 92.7%. 1H-NMR (400 MHz, CDCl3): 0.85 (d, J = 6.0 Hz, 3H), 1.21 (d, J = 6.4 Hz, 3H), 2.41 (s, 3H), 3.49 (t, J = 6.4Hz, 2H), 3.63–3.69 (m, 8H), 3.76 (t, J = 4.8 Hz, 2H), 3.81 (t, J = 6.4Hz, 2H), 4.24 (dd, J = 48.8, 18.4 Hz, 2H), 4.42–4.44 (m, 2H), 4.71 (s, 2H), 4.83–4.92 (m, 1H), 5.45 (s, 1H), 7.11 (td, J = 8.0, 1.6 Hz, 1H), 7.21 (td, J = 7.6, 1.2 Hz, 1H), 7.31 (dd, J = 7.6, 1.2 Hz, 1H), 7.41 (dd, J = 7.6, 1.6 Hz, 1H). 13C-NMR (100 MHz, CDCl3): 171.1, 167.9, 166.8, 156.5, 144.7, 142.1, 133.2, 131.1, 129.3, 127.9, 127.0, 109.2, 103.5, 71.1, 70.6, 70.5, 70.4, 68.7, 67.8, 65.1, 65.0, 47.3, 35.0, 30.5, 21.7, 20.9, 15.2. ESI (MS) m/z: 646.1 (M + H)+. HRMS (ESI-TOF) calculated for C28H35BrClNNaO9 (M + Na)+: 666.10759; found: 666.10903.
3.1.5. General Procedure for Synthesis of Compounds 6a–6d
A mixture of 13a–13d (1.0 equiv.) and NaN3 (1.5 equiv.) in DMF (20 mL) was stirred at r.t. for 26 h. The mixture was diluted with ice water (100 mL) and extracted by EtOAc (100 mL × 2). The combined organic phase was washed with brine (100 mL × 2), dried over anhydrous Na2SO4, and concentrated. The residue was purified by flash column chromatography [petroleum ether-EtOAc (2:1)] to give products 6a–6d as white solids.
1-[2-(2-Azidoethoxy)-2-oxoethyl]-4-(2-chlorophenyl)-2-methyl-5-oxo-1, 4, 5, 7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (6a). HPLC analysis: 94.2%. M.p. 142–144 °C. 1H-NMR (400 MHz, CDCl3): 0.86 (d, J = 6.0 Hz, 3H), 1.21 (d, J = 6.4 Hz, 3H), 2.42 (s, 3H), 3.59 (t, J = 4.8Hz, 2H), 4.25 (dd, J = 54.0, 18.4 Hz, 2H), 4.44 (t, J = 4.8 Hz, 2H), 4.69 (s, 2H), 4.84–4.93 (m, 1H), 5.46 (s, 1H), 7.12 (td, J = 7.6, 1.6 Hz, 1H), 7.22 (t, J = 7.6, 1H), 7.31 (d, J = 7.6, Hz, 1H), 7.42 (dd, J = 7.6, 1.2 Hz, 1H). 13C-NMR (100 MHz, CDCl3): 171.0, 167.8, 166.7, 156.2, 144.4, 141.9, 133.3, 131.1, 129.4, 127.9, 127.0, 109.5, 103.7, 67.9, 65.0, 64.6, 49.5, 47.1, 35.1, 21.7, 20.9, 15.2. ESI (MS) m/z: 474.9 (M + H)+. HRMS (ESI-TOF) calculated for C22H24ClN4O6 (M + H)+: 475.13789; found: 475.13970.
Isopropyl 1-{2-[2-(2-azidoethoxy)ethoxy]-2-oxoethyl}-4-(2-chlorophenyl)-2-methyl-5-oxo-1, 4, 5, 7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (6b). HPLC analysis: 96.8%. M.p. 118–120 °C. 1H-NMR (400 MHz, CDCl3): 0.84 (d, J = 6.4 Hz, 3H), 1.19 (d, J = 6.4 Hz, 3H), 2.39 (s, 3H), 3.37 (t, J = 4.8 Hz, 2H), 3.68 (t, J = 4.8 Hz, 2H), 3.75 (t, J = 4.4 Hz, 2H), 4.22 (dd, J = 57.2, 18.8 Hz, 2H), 4.42 (t, J = 7.6 Hz, 2H), 4.66 (s, 2H), 4.82–4.91 (m, 1H), 5.44 (s, 1H), 7.10 (td, J = 8.0, 1.6 Hz, 1H), 7.20 (t, J = 7.6 Hz, 1H), 7.29 (d, J = 7.2 Hz, 1H), 7.41 (dd, J = 7.6, 1.2 Hz, 1H). 13C-NMR (100 MHz, CDCl3): 171.2, 168.0, 166.8, 156.6, 144.7, 142.2, 133.2, 131.2, 129.3, 127.9, 127.1, 109.2, 103.4, 70.2, 68.6, 67.8, 65.1, 64.9, 50.5, 47.2, 35.0, 21.7, 20.9, 15.2. ESI (MS) m/z: 518.9 (M + H)+. HRMS (ESI-TOF) calculated for C24H28ClN4O7 (M + H)+: 519.1641; found: 519.16577.
Isopropyl 1-(2-{2-[2-(2- azidoethoxy)ethoxy]ethoxy}-2-oxoethyl)-4-(2-chlorophenyl)-2-methyl-5-oxo-1, 4, 5, 7-tetrahydrofuro[3, 4-b]pyridine-3-carboxylate (6c). HPLC analysis: 94.0%. M.p. 44–45 °C. 1H-NMR (400 MHz, CDCl3): 0.85 (d, J = 6.0 Hz, 3H), 1.21 (d, J = 6.4 Hz, 3H), 2.42 (s, 3H), 3.39 (t, J = 4.8Hz, 2H), 3.63–3.68 (m, 6H), 3.78 (t, J = 4.8Hz, 2H), 4.23 (dd, J = 51.6, 18.8 Hz, 2H), 4.43–4.45 (m, 2H), 4.70 (s, 2H), 4.83–4.93 (m, 1H), 5.46 (s, 1H), 7.11 (td, J = 7.6, 1.6 Hz, 1H), 7.21 (td, J = 7.6, 1.2 Hz, 1H), 7.31 (dd, J = 7.6, 1.2 Hz, 1H), 7.41 (dd, J = 7.6, 1.6 Hz, 1H). 13C-NMR (100 MHz, CDCl3): 171.1, 167.9, 166.8, 156.4, 144.7, 142.0, 133.3, 131.1, 129.4, 127.9, 127.0, 109.3, 103.5, 70.7, 70.6, 70.1, 68.8, 67.8, 65.1, 65.0, 50.6, 47.3, 35.1, 21.7, 20.9, 15.2. ESI (MS) m/z: 563.1 (M + H)+. HRMS (ESI-TOF) calculated for C26H31ClN4NaO8 (M + Na)+: 585.17226; found: 585.17324.
Isopropyl 1-(14-azido-2-oxo-3,6,9,12-tetraoxatetradecyl)-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (6d). HPLC analysis: 94.3%. M.p. 37–38 °C. 1H-NMR (400 MHz, CDCl3): 0.84 (d, J = 6.4 Hz, 3H), 1.20 (d, J = 6.4 Hz, 3H), 2.41 (s, 3H), 3.39 (t, J = 4.8 Hz, 2H), 3.65–3.68 (m, 10H), 3.75 (t, J = 4.4 Hz, 2H), 4.22 (dd, J = 50.4, 18.4 Hz, 2H), 4.41–4.43 (m, 2H), 4.70 (s, 2H), 4.84–4.90 (m, 1H), 5.45 (s, 1H), 7.11 (td, J = 7.6, 1.6 Hz, 1H), 7.21 (td, J = 7.6, 1.2 Hz, 1H), 7.30 (d, J = 8.0 Hz, 1H), 7.41 (dd, J = 7.6, 1.2 Hz, 1H). 13C-NMR (100 MHz, CDCl3): 171.1, 167.9, 166.8, 156.5, 144.7, 142.1, 133.2, 131.1, 129.3, 127.9, 127.0, 109.2, 103.5, 70.7, 70.6, 70.5, 70.4, 70.0, 68.7, 67.8, 65.1, 65.0, 50.7, 47.3, 35.0, 21.7, 20.9, 15.2. ESI (MS) m/z: 607.1 (M + H)+. HRMS (ESI-TOF) calculated for C28H35ClN4NaO9 (M + Na)+: 629.19848; found: 629.19933.
3.1.6. (S)-Methyl 2-(4-benzoylbenzamido)-6-(tert-butoxycarbonylamino)hexanoate (16)
Lys(Boc)-OMe·HCl (0.20 g, 0.67 mmol) was dissolved in 20 mL of DMF, to which 4-benzoylbenzoic acid (0.15 g, 0.67 mmol), N-[3-(dimethylamino)propyl]-N-ethylcarbodiimide hydrochloride (EDCI, 0.15 g,0.78 mmol), 4-(dimethylamino)pyridine (DMAP, 0.097 g, 0.79 mmol), and N-methylmorpholine (NMM, 0.26 mL, 2.36 mmol) were added. The reaction mixture was stirred at r.t. overnight and then poured into ice water (50 mL). The aqueous layer was extracted with EtOAc (50 mL × 2). The combined organic layer was washed with saturated brine (50 mL × 2), dried over anhydrous Na2SO4, filtered, and concentrated. The residue was purified by flash silica gel column chromatography [petroleum ether-EtOAc (4:1)] to give benzophenone-Lys(Boc)-OMe as a transparent solid (0.28 g, 89%). HPLC analysis: 98.6%. M.p. 66–67 °C. 1H-NMR (400 MHz, CDCl3): 1.40(s, 9H), 1.45–1.58 (m, 4H), 1.83–2.04 (m, 2H), 3.06–3.18 (m, 2H), 3.80 (s, 3H), 4.64 (br s, 1H), 4.79–4.84 (m, 1H), 6.97 (d, J = 5.6 Hz, 1H), 7.50 (t, J = 7.6 Hz, 2H), 7.62 (t, J = 7.2 Hz, 1H), 7.80 (d, J = 7.6 Hz, 2H), 7.85 (d, J = 8.0 Hz, 2H), 7.94 (d, J = 8.4 Hz, 2H). 13C-NMR (100 MHz, CDCl3): 195.9, 172.9, 166.4, 156.2, 140.3, 137.1, 137.0, 132.9, 130.1, 128.4, 127.2, 79.2, 52.64, 52.59, 40.0, 32.0, 29.7, 28.4, 22.5. ESI (MS) m/z: 491.2 (M + Na)+. HRMS (ESI-TOF) calculated for C26H32N2NaO6 (M + Na)+: 491.2158; found: 491.2164.
3.1.7. (S)-Prop-2-ynyl 2-(4-benzoylbenzamido)-6-(tert-butoxycarbonylamino)hexanoate (18)
To a solution of 16 (0.50 g, 1.07 mmol) in MeOH (10 mL), a NaOH aqueous (2 N, 10 mL) was added under an ice bath. The reaction mixture was stirred at r.t. for 3 h and then concentrated in vacuo. The residue was redissolved in H2O (20 mL), and then the aqueous was adjusted to pH = 2 with HCl aqueous (1 N), the suspension was extracted with EtOAc (20 mL × 3), and the combined organic layer was dried over anhydrous Na2SO4, filtered, and concentrated to give a white solid product (0.37 g, 76%), which was used directly in the next reaction without further purification. The above product 17 (10.0 g, 22.0 mmol) and K2CO3 (6.08 g, 44.0 mmol) in anhydrous DMF (120 mL) were slowly added to 3-Bromopropyne (2.85 mL, 33.04 mmol, 80% in toluene). The reaction mixture was stirred at r.t. overnight and then filtered. The filtrate was diluted with water (200 mL) and extracted with EtOAc (200 mL × 2). The combined organic layer was washed with 1 N HCl aqueous solution (200 mL), saturated NaHCO3 solution (200 mL), and saturated brine (200 mL), dried over anhydrous Na2SO4 and evaporated. The mixture was purified by column chromatography on silica gel [petroleum ether-EtOAc (4:1)] to give 18 (9.70 g, 90%) of a white solid. HPLC analysis: 98.6%. M.p. 79–81 °C. 1H-NMR (400 MHz, CDCl3): 1.40 (s, 8H), 1.53 (ddd, J = 26.4, 14.9, 7.2 Hz, 4H), 1.89 (dt, J = 13.2, 8.4 Hz, 1H), 1.96–2.10 (m, 1H), 2.53 (s, 1H),3.13 (s, 2H), 4.61 (s, 1H), 4.74 (dd, J = 15.4, 2.2 Hz, 1H), 4.85 (dt, J = 11.2, 4.0 Hz, 2H), 6.94 (d, J = 6.0 Hz, 1H), 7.50 (t, J = 7.6 Hz, 2H), 7.62 (t, J = 7.4 Hz, 1H), 7.80 (d, J = 7.6 Hz, 2H), 7.85 (d, J = 8.0 Hz, 2H), 7.94 (d, J = 8.2 Hz, 2H). 13C-NMR(100 MHz, CDCl3): 195.9, 171.7, 166.5, 156.2, 140.4, 137.1, 137.0, 132.9, 130.1, 128.5, 127.2, 79.2, 75.6, 52.9, 52.6, 39.9, 31.8, 29.7, 28.4, 22.4. ESI (MS) m/z: 515.2 (M + Na)+. HRMS (ESI-TOF) calculated for C28H32N2NaO6 (M + Na)+: 515.2158; found: 515.2165.
3.1.8. General Procedure for Synthesis of compounds 20 and 32
The crude product 19 or 23 (12.80 mmol) and D-Biotin (19.50 mmol) were dissolved in 200 mL of anhydrous DMF, to which EDCI (0.69 mmol), HOBt (0.69 mmol), and DIPEA (1.38 mmol) were added at 0 °C. The mixture was stirred at r.t. overnight under a N2 atmosphere and then diluted with water (10 mL) and extracted with EtOAc (10 mL × 3). The combined organic layer was washed with brine (10 mL × 2), dried over Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography over silica gel [CH2Cl2-MeOH (40:1)] to give target products 20 and 32 as white solids.
(S)-Prop-2-ynyl 2-(4-benzoylbenzamido)-6-(5-{(3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazole-4-yl}pentanamido)hexanoate (20). HPLC analysis: 95.4%. M.p. 130–132 °C. 1H-NMR (400 MHz, d6-DMSO): 1.30–1.59 (m, 10H), 1.83 (br s, 2H), 2.04 (t, J = 5.6 Hz, 2H), 2.57 (d, J = 12.4 Hz, 1H), 2.80–2.83 (m, 1H), 3.05 (br s, 3H), 3.63 (d, J = 36.0 Hz, 1H), 4.12 (br s, 1H), 4.30 (br s, 1H), 4.44–4.49 (m, 1H), 4.77 (br s, 2H), 6.35 (br s, 1H), 6.40 (br s, 1H), 7.55–7.64 (m, 2H), 7.72–7.84 (m, 5H), 8.06 (d, J = 7.2 Hz, 1H), 8.97 (d, J = 6.4 Hz, 1H). 13C-NMR (100 MHz, d6-DMSO): 195.4, 171.9, 171.4, 162.7, 139.4, 136.9, 136.6, 133.0, 129.7, 129.4, 128.7, 127.7, 78.2, 77.9, 61.0, 59.2, 55.4, 52.7, 52.2, 38.0, 35.2, 29.9, 28.7, 28.2, 28.0, 25.3, 23.1, 23.0. ESI (MS) m/z: 641.2 (M + H)+. HRMS (ESI-TOF) calculated for C33H38N4NaO6 (M + Na)+: 641.2410; found: 641.2418.
(S)-Methyl 2-(4-benzoylbenzamido)-6-(5-{(3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazole-4-yl}pentanamido)hexanoate (32). HPLC analysis: 90.2%. M.p. 148–150 °C. 1H-NMR (400 MHz, CDCl3): 1.34–1.62 (m, 10H), 1.92–1.95 (m, 2H), 2.16–2.18 (m, 2H), 2.69 (d, J = 12.4 Hz, 1H), 2.84–2.85 (m, 1H), 3.07–3.08 (m, 1H), 3.24 (br s, 2H), 3.77 (s, 3H), 4.22–4.29 (m, 1H), 4.44–4.50 (m, 1H), 4.73 (dd, J = 13.2, 8.0 Hz, 1H), 6.40 (br s, 1H), 6.65 (br s, 1H), 7.49 (t, J = 8.0 Hz, 2H), 7.59–7.66 (m, 2H), 7.80 (dd, J = 16.8, 7.2 Hz, 4H), 8.03 (d, J = 8.0 Hz, 2H). 13C-NMR (100 MHz, d6-DMSO): 195.4, 172.6, 171.9, 165.9, 162.7, 139.4, 137.0, 136.6, 133.0, 129.7, 129.4, 128.7, 127.7, 61.0, 59.1, 55.4, 52.8, 51.9, 38.0, 35.2, 33.5, 30.1, 28.7, 28.0, 25.3, 24.5, 23.1. ESI (MS) m/z: 595.3 (M + H)+. HRMS (ESI-TOF) calculated for C31H39N4O6S (M + H)+: 595.2590; found: 595.2598.
3.1.9. General Procedure for Synthesis of Compounds 21 and 24
To a solution of 16 or 18 (21.34 mmol) in anhydrous CH2Cl2 (50 mL), trifluoroacetic acid (TFA, 32 mL, 0.43 mol) was added. The reaction mixture was stirred at 0 °C for 2 h. Then, the reaction mixture was concentrated in vacuo. The residue was redissolved in EtOAc (20 mL) and washed with saturated NaHCO3 (20 mL), 1 N HCl aqueous (20 mL), and brine (20 mL), dried over Na2SO4, filtered, and concentrated to give the crude target product 19 or 23 as a white solid, which was used for the next reaction without further purification. To a mixture of the above crude product 19 or 23 (21.71 mmol) and Et3N (4.75 mL, 34.22 mmol) in anhydrous CH2Cl2/CH3OH (1:1, v/v, 200 mL) were slowly added dansyl chloride (9.23 g, 34.22 mmol) in groups under an ice bath. The reaction mixture was stirred at r.t. overnight. After the solvent was removed in vacuo, the residue was redissolved in CH2Cl2 (20 mL) and washed with brine (2 × 20 mL), dried over anhydrous Na2SO4, and evaporated. The mixture was purified by column chromatography on silica gel [CH2Cl2 = 100%] to give target the products 21 and 24 as pale yellow solids.
(S)-Prop-2-ynyl-6-dansylamide-2-(4-benzoylbenzamido)hexanoate (21). HPLC analysis: 100.0%. M.p. 76–78 °C. 1H-NMR (400 MHz, CDCl3): 1.43–1.56 (m, 4H), 1.73–1.82 (m, 1H), 1.85–1.92 (m, 1H), 2.52 (t, J = 6.4 Hz, 1H), 2.88 (s, 6H), 2.90–2.94 (m, 2H), 4.69–4.83 (m, 3H), 5.05 (br t, J = 6.0 Hz, 1H), 6.97 (d, J = 7.6 Hz, 1H), 7.16 (d, J = 7.2 Hz, 1H), 7.46–7.52 (m, 4H), 7.60 (tt, J = 7.6, 1.2 Hz, 1H), 7.77–7.83 (m, 4H), 7.94 (d, J = 8.4 Hz, 2H), 8.20 (dd, J = 7.2, 0.8 Hz, 1H), 8.28 (d, J = 8.4 Hz, 1H), 8.54 (d, J = 8.4 Hz, 1H). 13C-NMR (100 MHz, CDCl3): 195.9, 171.6, 166.6, 140.4, 137.0, 136.8, 134.8, 132.9, 130.4, 130.1, 129.9, 129.6, 129.5, 128.5, 128.4, 127.3, 123.2, 115.3, 77.0, 75.7, 52.9, 52.5, 45.4, 42.6, 31.5, 28.8, 22.0. ESI (MS) m/z: 648.2 (M + Na)+. HRMS (ESI-TOF) calculated for C35H35N3NaO6S (M + Na)+: 648.2144; found: 648.2152.
(S)-Methyl-6-dansylamide-2-(4-benzoylbenzamido)hexanoate (24). HPLC analysis: 98.1%. M.p. 69–71 °C. 1H-NMR (400 MHz, CDCl3): 1.39–1.55 (m, 4H), 1.70–1.79 (m, 1H), 1.82–1.90 (m, 1H), 2.88–2.92 (m, 8H), 3.76 (s, 3H), 4.71–4.76 (m, 1H), 5.13 (br t, J = 5.6 Hz, 1H), 6.99 (d, J = 7.6 Hz, 1H), 7.16 (d, J = 7.2 Hz, 1H), 7.47–7.52 (m, 4H), 7.61 (t, J = 7.6 Hz, 1H), 7.78 (d, J = 7.2 Hz, 2H), 7.83 (d, J = 8.0 Hz, 2H), 7.95 (d, J = 8.4 Hz, 2H), 8.21 (dd, J = 7.2, 0.8 Hz, 1H), 8.29 (d, J = 8.8 Hz, 1H), 8.53 (d, J = 8.4 Hz, 1H). 13C-NMR (100 MHz, CDCl3): 196.0, 172.8, 166.5, 140.3, 137.0, 136.9, 134.8, 132.9, 130.4, 130.1, 129.8, 129.6, 129.5, 128.5, 128.4, 127.3, 123.2, 115.3, 52.6, 52.5, 45.4, 42.6, 31.7, 28.9, 22.1. ESI (MS) m/z: 602.3 (M + H)+. HRMS (ESI-TOF) calculated for C33H35N3NaO6S (M + Na)+: 624.2144; found: 624.2152.
3.1.10. General Procedure for Synthesis of Compounds 22 and 33
To a solution of 16 or 18 (15.0 g, 30.45 mmol) in anhydrous CH2Cl2 (150 mL), trifluoroacetic acid (TFA, 46.0 mL, 0.62 mol) was added. The reaction mixture was stirred at 0 °C for 2 h. Then, the reaction mixture was concentrated in vacuo. The residue was redissolved in EtOAc (150 mL) and was washed with saturated NaHCO3 (200 mL × 2), 1 N HCl aqueous solution (200 mL × 2), and brine (200 mL × 2), and dried over Na2SO4, filtered, and concentrated to give the crude target product as a white solid, which was used for next reaction without further purification. To a mixture of the above crude product 19 or 23 (0.26 mmol) and Et3N (0.36 mmol) in anhydrous CH2Cl2/CH3OH (1:1, v/v, 20 mL), was slowly added acyl chloride (0.36 mmol) in groups under an ice bath. The reaction mixture was stirred at r.t. for another 12 h. After the solvent was removed in vacuo, the residue was redissolved in CH2Cl2 (20 mL) and washed with brine (2 × 20 mL), dried over anhydrous Na2SO4, and evaporated. The mixture was purified by column chromatography on silica gel [CH2Cl2 = 100%] to give target products 22 and 33 as pale yellow solids.
(S)-Prop-2-ynyl-2-(4-benzoylbenzamido)-6-(2-chloroacetamido)hexanoate (22). HPLC analysis: 99.5%. M.p. 123–124 °C. 1H-NMR (400 MHz, CDCl3): 1.47 (ddd, J = 29.2, 15.8, 6.8 Hz, 2H), 1.65 (tt, J = 13.6, 6.8 Hz, 2H), 1.87–1.98 (m, 1H), 2.05 (qd, J = 10.6, 5.4 Hz, 1H), 2.53 (t, J = 2.2 Hz, 1H), 3.26–3.43 (m, 2H), 3.94–4.06 (m, 2H), 4.75 (dd, J = 15.4, 2.4 Hz, 1H), 4.84 (ddd, J = 13.2, 5.4, 2.6 Hz, 2H), 6.67 (s, 1H), 6.98 (d, J = 7.4 Hz, 1H), 7.50 (t, J = 7.6 Hz, 2H), 7.62 (t, J = 7.4 Hz, 1H), 7.79 (d, J = 7.4 Hz, 2H), 7.85 (d, J = 8.2 Hz, 2H), 7.96(d, J = 8.2 Hz, 2H). 13C-NMR(100 MHz, CDCl3): 195.9, 171.6, 166.5, 166.3, 140.4, 137.0, 136.8, 132.9, 130.1, 128.5, 127.2, 77.0, 75.6, 53.0, 52.5, 42.6, 39.1, 31.5, 28.9, 22.3. ESI (MS) m/z: 491.1 (M + Na)+. HRMS (ESI-TOF) calculated for C25H25ClN2NaO5 (M + Na)+: 491.1350; found: 491.1356.
(S)-Methyl-2-(4-benzoylbenzamido)-6-(2-chloroacetamido)hexanoate (33). HPLC analysis: 99.6%. M.p. 129–130 °C. 1H-NMR (400 MHz, CDCl3): 1.39–1.67 (m, 4H), 1.84–2.06 (m, 2H),3.25–3.41 (m, 2H), 3.80 (s, 3H), 3.95–4.06 (m, 2H), 4.81 (td, J = 7.6, 5.0 Hz, 1H), 6.66 (s, 1H), 6.96 (d, J = 7.4 Hz, 1H), 7.50 (t, J = 7.6 Hz, 2H), 7.62 (t, J = 7.4 Hz, 1H), 7.80 (d, J = 7.2 Hz, 2H), 7.85 (d, J = 8.2 Hz, 2H), 7.96 (d, J = 8.2 Hz, 2H). 13C-NMR (100 MHz, CDCl3): 195.9, 172.8, 166.4, 166.2, 140.4, 137.0, 136.9, 132.9, 130.1, 128.5, 127.2, 52.6, 52.5, 42.6, 39.1, 31.8, 28.9, 22.4. ESI (MS) m/z: 443.1 (M − H)−. HRMS (ESI-TOF) calculated for C23H24ClN2O5 (M − H)−: 443.1374; found: 443.1380.
3.1.11. (S)-6-Dansylamide-2-(4-benzoylbenzamido)hexanoic acid (25)
To a solution of 24 (5.30 g, 8.81 mmol) in THF (50 mL), LiOH·H2O (6 N, 3.0 mL) was added under an ice bath. The reaction mixture was stirred at r.t. for 3 h and then concentrated in vacuo. The residue was redissolved in H2O (50 mL), and then the aqueous solution was adjusted to pH = 2 with 1 N HCl aqueous solution, and the suspension was extracted with EtOAc (50 mL × 3). The combined organic layer was dried over anhydrous Na2SO4, filtered, and concentrated. The residue was purified by flash silica gel column chromatography [CH2Cl2-MeOH (40:1)] to obtain the pale yellow solid product 25 (4.50 g, 87%). HPLC analysis: 99.5%. M.p. 83–85 °C. 1H-NMR (400 MHz, CDCl3): 1.40–1.51 (m, 4H), 1.84–2.06 (m, 2H), 2.90–2.97 (m, 8H), 4.75–4.81 (m, 2H), 6.85 (d, J = 6.8 Hz, 1H), 7.18 (d, J = 10.0 Hz, 1H), 7.52 (dd, J = 20.4, 10.0 Hz, 4H), 7.63 (t, J = 9.6 Hz, 1H), 7.82 (d, J = 9.6 Hz, 2H), 7.92 (dd, J = 32.8, 11.2 Hz, 4H), 8.26 (dd, J = 15.2, 10.4 Hz, 2H), 8.55 (d, J = 11.2 Hz, 1H). 13C-NMR (100 MHz, CDCl3): 196.0, 167.3, 151.6, 140.2, 136.9, 136.6, 134.9, 132.9, 130.3, 130.1, 129.9, 129.8, 129.6, 129.2, 128.4, 128.3, 127.5, 123.2, 119.0, 115.3, 45.4, 42.6, 31.1, 28.9, 22.3. ESI (MS) m/z: 588.3 (M + H)+. HRMS (ESI-TOF) calculated for C32H33N3NaO6S (M + Na)+: 610.19823; found: 610.20065.
3.1.12. Prop-2-ynylN-Cbz-N’-Boc-L-lysine (27)
To a stirred solution of 26 (3.81 g, 10.0 mmol) in anhydrous DMF (20 mL), K2CO3 (2.10 g, 15.19 mmol) was added at r.t.. After 0.5 h, 3-Bromopropyne (1.72 mL, 0.02 mol mmol, 80% in toluene) was slowly added dropwise. The reaction mixture was stirred at r.t. overnight and then filtered. The filtrate was diluted with water (40 mL) and extracted with EtOAc (40 mL × 3). The combined organic layer was washed with 1 N HCl aqueous (40 mL × 2), saturated NaHCO3 (40 mL × 2) and brine (40 mL × 2), dried over anhydrous Na2SO4, filtered, and evaporated. Purified by column chromatography on silica gel [petroleum ether-EtOAc (8:1)] to give product 27 (3.0 g, 72%) as a white solid. HPLC analysis: 96.5%. M.p. 119–121 °C. 1H-NMR (400 MHz, CDCl3): 1.37–1.48 (m, 13H), 1.70–1.91 (m, 2H), 2.51 (s, 1H), 2.98–3.16 (m, 2H), 4.37–4.42 (m, 1H), 4.62 (br s, 1H), 4.73 (ddd, J = 37.2, 15.6, 1.6 Hz, 2H), 5.10 (s, 2H), 5.49 (br d, J = 6.8 Hz, 1H), 7.29–7.36 (m, 5H). 13C-NMR (100 MHz, CDCl3): 171.7, 156.0, 155.9, 136.1, 128.4, 128.1, 128.0, 79.1, 75.4, 67.0, 53.6, 52.6, 39.8, 31.8, 29.5, 28.3, 22.1. ESI (MS) m/z: 419.2 (M + H)+. HRMS (ESI-TOF) calculated for C22H30N2NaO6 (M + Na)+: 441.19961; found: 441.20100.
3.1.13. Prop-2-ynylN-Cbz-N’-(5-{(3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl}pentanoyl)-L-lysine (28)
To a solution of 27 (6.48 g, 15.50 mmol) in CH2Cl2 (20 mL), trifluoroacetic acid (TFA, 10 mL, 0.14 mol) was added, and the reaction mixture was stirred at 0 °C for 2 h. Then, the reaction mixture was concentrated in vacuo. The residue was redissolved in EtOAc (20 mL) and washed with saturated NaHCO3 (20 mL × 2), 1 M HCl aqueous (20 mL × 2), and brine (20 mL × 2), dried over Na2SO4, and concentrated to give crude Prop-2-ynyl N-Cbz-L-lysine as a colorless oil, which was used for next reaction without further purification. To a stirred solution of D-biotin (3.79 g, 15.50 mmol) and Et3N (2.57 mL, 18.60 mmol) in anhydrous DMF (20 mL), isobutyl chloroformate (2.12 g, 15.50 mmol) was slowly added at 0 °C under nitrogen. After 2 h, the reaction mixture was added successively to Prop-2-ynyl N-Cbz-L-lysine (5.50 g, 15.50 mmol) in anhydrous DMF (10 mL) and Et3N (3.10 g, 0.03 mol). The reaction mixture was stirred at r.t. overnight, and then poured into ice water (100 mL). The aqueous layer was extracted with EtOAc (50 mL × 3). The combined organic layer was washed with 1 N KHSO4 (50 mL × 2), 1 N NaHCO3 solution (50 mL × 2) and saturated brine (50 mL × 2), dried over anhydrous Na2SO4, filtered, and concentrated. The residue was purified by flash silica gel column chromatography [CH2Cl2-MeOH (8:1)] to give 28 as a white solid (6.50 g, 77%). HPLC analysis: 95.4%. M.p. 120–121 °C. 1H-NMR (400 MHz, CD3OD): 1.28–1.79 (m, 12H), 2.09 (t, J = 7.2 Hz, 2H), 2.59 (d, J = 12.8 Hz, 1H), 2.82 (dd, J = 12.8, 5.2 Hz, 1H), 2.86 (t, J = 2.4 Hz, 1H), 3.05–3.11 (m, 3H), 4.08–4.12 (m, 1H), 4.17–4.20 (m, 1H), 4.36–4.39 (m, 1H), 4.64 (ddd, J = 26.8, 15.6, 2.4 Hz, 2H), 5.00 (s, 2H), 7.18–7.26 (m, 5H), 7.85 (br t, J = 4.4 Hz, 1H). 13C-NMR (100 MHz, CD3OD): 176.0, 173.3, 166.1, 158.7, 138.2, 129.5, 129.0, 128.8, 78.5, 76.6, 67.7, 63.4, 61.6, 57.0, 55.4, 53.4, 41.0, 40.0, 36.8, 32.1, 29.9, 29.8, 29.5, 26.9, 24.2. ESI (MS) m/z: 545.3 (M + H)+. HRMS (ESI-TOF) calculated for C27H36N4NaO6S (M + Na)+: 567.22478; found: 567.22561.
3.1.14. (S)-Prop-2-ynyl-2-[(S)-6-dansylamide-2-(4-benzoylbenzamido)hexanamido] -6-(5-{(3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl}pentanamido)hexanoate (30)
Compound 28 (5.60 g, 10.28 mmol) was dissolved in a mixed solution of 30% HBr/HOAC (30.0 mL). The reaction mixture was stirred at r.t. for 1 h, and then concentrated in vacuo to give a colorless oil, which was almost a pure product and was used for the next reaction without further purification. The 29 (4.19 g, 10.20 mmol) was dissolved in anhydrous DMF (50 mL), to which O-(7-Azabenzotriazol-1-yl)-N,N,N’,N’-tetraMethyluronium hexafluorophosphate (HATU, 3.88 g, 10.21 mmol), DIPEA (5.05 mL, 30.56 mmol) was added at 0 °C. After the mixture was stirred for 5 min at r.t., 25 (6.00 g, 10.21 mmol) was added. The mixture was stirred at r.t. overnight. The mixture was diluted with water (100 mL) and extracted by EtOAc (100 mL × 2). The combined organic layer was washed with 1 N KHSO4 (100 mL × 2), saturated NaHCO3 solution (100 mL × 2), and saturated brine (100 mL × 2), dried over anhydrous Na2SO4, filtered, and concentrated. The residue was purified by preparative-reverse phase HPLC to give 30 (4.30 g, 43%) as a white solid. HPLC analysis: 95.1%. M.p. 105–108 °C. 1H-NMR (400 MHz, CDCl3): 1.43–1.88 (m, 18H), 2.05 (s, 2H), 2.49 (s, 1H), 2.71 (d, J = 12.8 Hz, 1H), 2.84 (s, 6H), 2.94 (s, 2H), 3.09 (s, 1H), 3.21 (s, 2H), 3.46 (s, 1H), 4.32 (s, 1H), 4.49–4.53 (m, 2H), 4.63–4.66 (m, 2H), 4.73–4.76 (m, 1H), 5.78 (s, 1H), 6.66–6.71 (m, 2H), 6.85 (s, 1H), 7.10 (d, J = 6.4 Hz, 1H), 7.40–7.49 (m, 4H),7.58–7.60 (m, 1H), 7.80 (dd, J = 17.6, 7.2 Hz, 4H), 7.91 (d, J = 5.2 Hz, 1H), 8.00 (d, J = 7.2 Hz, 2H), 8.18 (d, J = 6.0 Hz, 1H), 8.34 (d, J = 6.4 Hz, 2H), 8.48 (d, J = 8.0 Hz, 1H). 13C-NMR (100 MHz, CDCl3): 196.0, 173.7, 173.0, 171.6, 167.2, 164.3, 151.8, 140.3, 137.0, 136.8, 135.4, 132.9, 130.1, 129.9, 129.6, 129.1, 128.5, 128.1, 127.4, 123.2, 119.1, 115.1, 77.2, 75.6, 62.1, 60.2, 55.8, 54.2, 52.8, 52.3, 50.7, 45.4, 42.0, 40.6, 38.7, 35.3, 31.2, 30.7, 28.6, 28.5, 27.8, 25.3, 22.4, 22.2. ESI (MS) m/z: 980.0 (M + H)+. HRMS (ESI-TOF) calculated for C51H61N7NaO9S2 (M + Na)+: 1002.38644; found: 1002.38858.
3.1.15. General Procedure for Synthesis of compounds 2a–2d, 3a–3d, and 31a–31d
To a solution of 6 (0.32 mmol) and 20, 21, or 22 (0.32 mmol) in CH2Cl2 (1.5 mL) and H2O (1.5 mL), CuSO4·5H2O (0.39 mmol) and sodium ascorbate (0.51 mmol) were added. The resulting solution was stirred at r.t. overnight. The solvent was evaporated in vacuo, and then the residue was redissolved in EtOAc (10 mL), washed with water (10 mL × 3), and dried over anhydrous Na2SO4. The organic layer was evaporated, and the residue was purified by preparative-reverse phase HPLC to give target products 2a–2d, 3a–3d, and 31a–31d as white solids.
Isopropyl 1-(2-{2-[4-({[(S)-2-(4-benzoylbenzamido)-6-(5-{(3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl}pentanamido)hexanoyl]oxy}methyl)-1H-1,2,3-triazol-1-yl]ethoxy}-2-oxoethyl)-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (2a). HPLC analysis: 98.5%. M.p. 128–130 °C. 1H-NMR (400 MHz, d6-DMSO): 0.76 (d, J = 6.4 Hz, 3H), 1.11 (d, J = 6.4 Hz, 3H), 1.23–1.48 (m, 10H), 1.79–1.84 (m, 2H), 2.03 (t, J = 6.4 Hz, 2H), 2.23 (s, 3H), 2.55 (d, J = 12.8 Hz, 1H), 2.80 (dd, J = 12.0, 4.4 Hz, 1H), 3.00–3.08 (m, 3H), 4.08–4.11 (m, 1H), 4.26–4.30 (m, 1H), 4.38–4.50 (m, 3H), 4.59–4.63 (m, 2H), 4.72–4.92 (m, 5H), 5.18 (s, 3H), 6.34 (br s, 1H), 6.40(br s, 1H), 7.17 (t, J = 6.4 Hz, 1H), 7.26 (t, J = 7.2 Hz, 1H), 7.31 (d, J = 7.6 Hz, 1H), 7.35 (d, J = 7.6 Hz, 1H), 7.58 (t, J = 7.6 Hz, 2H), 7.71 (t, J = 7.2 Hz, 1H), 7.76 (d, J = 7.2 Hz, 3H), 7.81 (d, J = 8.4 Hz, 2H), 8.03 (d, J = 8.0 Hz, 2H), 8.22 (s, 1H), 8.94 (d, J = 7.2 Hz, 1H). 13C-NMR (100 MHz, d6-DMSO): 195.8, 172.4, 172.3, 171.3, 168.9, 166.7, 166.5, 163.2, 158.5, 146.1, 143.3, 142.3, 139.9, 137.4, 137.1, 133.5, 132.5, 131.3, 130.2, 129.9, 129.2, 129.1,128.5, 128.2, 127.8, 125.8, 107.9, 101.7, 67.4, 65.9,64.1, 61.5, 59.7, 58.2, 55.9, 53.4, 48.9, 47.6, 38.5, 35.7, 34.6, 30.4, 29.2, 28.7, 28.5, 25.8, 23.6, 21.8, 21.1, 15.2. ESI (MS) m/z: 1093.4 (M + H)+. HRMS (ESI-TOF) calculated for C55H61ClN8NaO12S (M + Na)+: 1115.3716; found 1115.3710.
Isopropyl 1-[2-(2-{2-[4-({[(S)-2-(4-benzoylbenzamido)-6-(5-{(3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl}pentanamido)hexanoyl]oxy}methyl)-1H-1,2,3-triazol-1-yl]ethoxy}ethoxy)-2-oxoethyl]-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (2b). HPLC analysis: 98.5%. M.p. 103–105 °C. 1H-NMR (400 MHz, d6-DMSO): 0.76 (d, J = 6.4 Hz, 3H), 1.10 (d, J = 6.4 Hz, 3H), 1.23–1.63 (m, 10H), 1.77–1.83 (m, 2H), 2.03 (t, J = 7.2 Hz, 2H), 2.28 (s, 3H), 2.55 (d, J = 10.4 Hz, 1H), 2.79 (dd, J = 15.2, 8.0 Hz, 1H), 3.00–3.08 (m, 3H), 3.66 (t, J = 5.2 Hz, 2H), 3.84 (t, J = 5.2 Hz, 2H), 4.08–4.11 (m, 1H), 4.29 (t, J = 4.0 Hz, 3H), 4.40–4.61 (m, 5H), 4.70–4.77 (m, 1H), 4.89 (dd, J = 36.4, 16.4 Hz, 2H), 5.20 (s, 1H), 5.21 (s, 2H), 6.36 (br s, 1H), 6.42 (br s, 1H), 7.17 (td, J = 7.6, 1.6 Hz, 1H), 7.27 (td, J = 7.2, 0.8 Hz, 1H), 7.32 (dd, J = 8.0, 1.2 Hz, 1H), 7.40 (d, J = 7.2 Hz, 1H), 7.58 (t, J = 8.0 Hz, 2H), 7.71 (tt, J = 7.2, 1.2 Hz, 1H), 7.76 (d, J = 7.2 Hz, 3H), 7.82 (d, J = 8.4 Hz, 2H), 8.03 (d, J = 8.4 Hz, 2H), 8.13 (s, 1H), 8.95 (d, J = 7.2 Hz, 1H). 13C-NMR (100 MHz, d6-DMSO): 195.3, 171.9, 171.8, 170.8, 168.7, 166.2, 166.1, 162.7, 158.2, 145.6, 142.8, 141.6, 139.4, 137.0, 136.6, 133.0, 132.0, 130.9, 129.7, 129.4, 128.7, 128.6, 128.0, 127.6, 127.3, 125.0, 107.5, 101.2, 68.5, 67.9, 66.9, 65.4, 64.4, 61.0, 59.2, 57.8, 55.4, 52.9, 49.3, 47.3, 38.0, 35.2, 34.2, 30.0, 28.7, 28.2, 28.0, 25.3, 23.1, 21.3, 20.6, 14.7. ESI (MS) m/z: 1137.4 (M + H)+. HRMS (ESI-TOF) calculated for C57H65ClN8NaO13S (M + Na)+: 1159.3978; found: 1159.3973.
Isopropyl 1-{2-[2-(2-{2-[4-({[(S)-2-(4-benzoylbenzamido)-6-(5-{(3aS, 4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl}pentanamido)hexanoyl]oxy}methyl)-1H-1,2,3-triazol-1-yl]ethoxy}ethoxy)ethoxy]-2-oxoethyl}-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (2c). HPLC analysis: 95.2%. M.p. 77–79 °C. 1H-NMR (400 MHz, d6-DMSO): 0.76 (d, J = 6.4 Hz, 3H), 1.11 (d, J = 6.4 Hz, 3H), 1.23–1.63 (m, 10H), 1.78–1.83 (m, 2H), 2.03 (t, J = 7.2 Hz, 2H), 2.30 (s, 3H), 2.55 (d, J = 12.4 Hz, 1H), 2.79 (dd, J = 12.4, 5.2 Hz, 1H), 2.98–3.08 (m, 3H), 3.49 (s, 4H), 3.61 (t, J = 4.8 Hz, 2H), 3.79 (t, J = 7.2 Hz, 2H), 4.08–4.11 (m, 1H), 4.27–4.30 (m, 3H), 4.41–4.61 (m, 5H), 4.71–4.77 (m, 1H), 4.89 (dd, J = 33.6, 16.4 Hz, 2H), 5.20 (s, 3H), 6.34 (br s, 1H), 6.40 (br s, 1H), 7.17 (td, J = 7.6, 1.6 Hz, 1H), 7.27 (t, J = 7.6 Hz, 1H), 7.32 (d, J = 7.6 Hz, 1H), 7.40 (d, J = 7.6 Hz, 1H), 7.58 (t, J = 7.6 Hz, 2H), 7.73 (t, J = 8.0 Hz, 1H), 7.76 (d, J = 7.2 Hz, 3H), 7.82 (d, J = 8.4 Hz, 2H), 8.03 (d, J = 8.0 Hz, 2H), 8.12 (s, 1H), 8.94 (d, J = 7.2 Hz, 1H). 13C-NMR (100 MHz, d6-DMSO): 195.4, 171.9, 171.9, 170.8, 168.7, 166.2, 166.0, 162.7, 158.2, 145.6, 142.8, 141.6, 139.4, 137.0, 136.6, 133.0, 132.0, 130.9, 129.7, 129.4, 128.7, 128.6, 128.0, 127.7, 127.3, 125.0, 107.5, 101.1, 69.5, 68.6, 68.1, 66.9, 65.4, 64.5, 61.0, 59.2, 57.8, 55.4, 52.9, 49.4, 47.4, 38.0, 35.2, 34.2, 29.9, 28.7, 28.2, 28.0, 25.3, 23.1, 21.3, 20.7, 14.7. ESI (MS) m/z: 1181.5 (M+H)+. HRMS (ESI-TOF) calculated for C59H69ClN8NaO14S (M + Na)+: 1203.4240; found: 1203.4235.
Isopropyl 1-{14-[4-({[(S)-2-(4-benzoylbenzamido)-6-(5-{(3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl}pentanamido)hexanoyl]oxy}methyl)-1H-1,2,3-triazol-1-yl]-2-oxo-3,6,9,12-tetraoxatetradecyl}-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (2d). HPLC analysis: 97.4%. M.p. 61–63 °C. 1H-NMR (400 MHz, d6-DMSO): 0.77 (d, J = 6.0 Hz, 3H), 1.11 (d, J = 6.0 Hz, 3H), 1.38–1.56 (m, 10H), 1.78–1.82 (m, 2H), 2.03 (t, J = 7.2 Hz, 2H), 2.30 (s, 3H), 2.55 (d, J = 9.2 Hz, 1H), 2.80 (dd, J = 12.4, 5.2 Hz, 1H), 2.99–3.08 (m, 3H), 3.44–3.52 (m, 8H), 3.64 (t, J = 4.0 Hz, 2H), 3.80 (t, J = 5.2 Hz, 2H), 4.08–4.11 (m, 1H), 4.27–4.32 (m, 3H), 4.40–4.61 (m, 5H), 4.71–4.77 (m, 1H), 4.85 (dd, J = 32.0, 15.2 Hz, 2H), 5.20 (s, 2H), 5.21 (s, 1H), 6.40 (br s, 2H), 7.17 (t, J = 8.4 Hz, 1H), 7.27 (t, J = 8.4 Hz, 1H), 7.32 (d, J = 8.0 Hz, 1H), 7.40 (d, J = 8.0 Hz, 1H), 7.58 (t, J = 7.6 Hz, 2H), 7.71 (t, J = 7.2 Hz, 1H), 7.76 (d, J = 7.2 Hz, 3H), 7.82 (d, J = 8.0 Hz, 2H), 8.03 (d, J = 8.0 Hz, 2H), 8.12 (s, 1H), 8.94 (d, J = 7.2 Hz, 1H). 13C-NMR (100 MHz, d6-DMSO): 195.4, 171.9, 171.8, 170.8, 168.7, 166.2, 166.0, 162.7, 158.2, 145.7, 142.8, 141.5, 139.4, 137.0, 136.6, 133.0, 132.0, 130.9, 129.7, 129.4, 128.7, 128.6, 128.0, 127.7, 127.3, 125.1, 107.4, 101.1, 69.7, 69.6, 69.5, 69.4, 68.6, 68.1, 66.9, 65.4, 64.5, 61.0, 59.2, 57.8, 55.4, 52.9, 49.4, 47.3, 38.0, 35.2, 34.2, 30.0, 28.7, 28.2, 28.0, 25.3, 23.1, 21.3, 20.6, 14.7. ESI (MS) m/z: 1225.5 (M + H)+. HRMS (ESI-TOF) calculated for C61H73ClN8NaO15S (M+Na)+: 1247.4502; found: 1247.4497.
Isopropyl 1-(2-{2-[4-({[(S)-6-dansylamide-2-(4-benzoylbenzamido)hexanoyl]oxy}methyl)-1H-1,2,3-triazol-1-yl]ethoxy}-2-oxoethyl)-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (3a). HPLC analysis: 91.8%. M.p. 119–121 °C. 1H-NMR (400 MHz, d6-DMSO): 0.75 (d, J = 6.4 Hz, 3H), 1.10 (d, J = 6.4 Hz, 3H), 1.22–1.34 (m, 4H), 1.63–1.69 (m, 2H), 2.22 (s, 3H), 2.73–2.79 (m, 2H), 2.82 (s, 6H), 4.29–4.54 (m, 3H), 4.59–4.61 (m, 2H), 4.70–4.92 (m, 5H), 5.15 (d, J = 3.6 Hz, 2H), 5.18 (s, 1H), 7.16 (tt, J = 8.0, 1.6 Hz, 1H), 7.25 (t, J = 6.8 Hz, 2H), 7.33 (dd, J = 8.0, 14.8 Hz, 2H), 7.55–7.62 (m, 4H), 7.71 (t, J = 7.2 Hz, 1H), 7.76 (d, J = 8.0 Hz, 2H), 7.80 (d, J = 8.4 Hz, 2H), 7.89 (t, J = 7.6 Hz, 1H), 7.99 (d, J = 8.0 Hz, 2H), 8.08 (d, J = 8.0 Hz, 1H), 8.20 (s, 1H), 8.29 (d, J = 8.8 Hz, 1H), 8.44 (d, J = 8.0 Hz, 1H), 8.87 (d, J = 7.2 Hz, 1H). 13C-NMR (100 MHz, d6-DMSO): 195.3, 171.7, 170.7, 168.4, 166.1, 166.0, 158.0, 150.7, 145.5, 142.7, 141.7, 139.3, 136.9, 136.6, 136.1, 133.0, 132.0, 130.8, 129.6, 129.4, 129.1, 129.0, 128.8, 128.7, 128.6, 128.1, 128.0, 127.7, 127.6, 127.2, 125.2, 123.6, 119.4, 115.3, 107.4, 101.1, 66.8, 65.3, 63.5, 57.6, 52.7, 48.4, 47.0, 45.0, 42.1, 34.1, 29.7, 28.7, 22.7, 21.3, 20.6, 14.6. ESI (MS) m/z: 1100.5 (M + H)+. HRMS (ESI-TOF) calculated for C57H59ClN7O12S (M + H)+: 1100.3631; found: 1100.3625.
Isopropyl 1-[2-(2-{2-[4-({[(S)-6-dansylamide-2-(4-benzoylbenzamido)hexanoyl]oxy}methyl)-1H-1,2,3-triazol-1-yl]ethoxy}ethoxy)-2-oxoethyl]-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (3b). HPLC analysis: 96.6%. M.p. 124–126 °C. 1H-NMR (400 MHz, d6-DMSO): 0.76 (d, J = 6.0 Hz, 3H), 1.10 (d, J = 6.4 Hz, 3H), 1.23–1.32 (m, 4H), 1.62–1.68 (m, 2H), 2.27 (s, 3H), 2.73–2.78 (m, 2H), 2.82 (s, 6H), 3.65 (t, J = 5.2 Hz, 2H), 3.83 (t, J = 5.2 Hz, 2H), 4.26–4.32 (m, 3H), 4.46–4.59 (m, 4H), 4.70–4.76 (m, 1H), 4.88 (dd, J = 36.4, 16.8 Hz, 2H), 5.18 (s, 2H), 5.20 (s, 1H), 7.16 (td, J = 7.6, 1.2 Hz, 1H), 7.23–7.27 (m, 2H), 7.31 (d, J = 8.0 Hz, 1H), 7.40 (d, J = 8.0 Hz, 1H), 7.54–7.62 (m, 4H), 7.71 (t, J = 7.2 Hz, 1H), 7.76 (d, J = 7.2 Hz, 2H), 7.80 (d, J = 8.4 Hz, 2H), 7.88 (t, J = 6.0 Hz, 1H), 7.99 (d, J = 8.0 Hz, 2H), 8.08 (d, J = 11.2 Hz, 1H), 8.10 (s, 1H), 8.29 (d, J = 9.2 Hz, 1H), 8.44 (d, J = 8.4 Hz, 1H), 8.86 (d, J = 6.8 Hz, 1H). 13C-NMR (100 MHz, d6-DMSO): 195.4, 171.8, 170.8, 168.7, 166.2, 166.0, 158.2, 150.9, 145.6, 142.8, 141.6, 139.4, 136.9, 136.6, 136.2, 133.0, 132.0, 130.9, 129.7, 129.4, 129.2, 129.1, 128.9, 128.7, 128.6, 128.2, 128.0, 127.7, 127.6, 127.3, 125.0, 123.6, 119.4, 115.2, 107.5, 101.1, 68.5, 67.8, 66.9, 65.4, 64.4, 57.7, 52.8, 49.3, 47.3, 45.1, 42.2, 34.2, 29.8, 28.8, 22.7, 21.3, 20.6, 14.7. ESI (MS) m/z: 1144.5 (M + H)+. HRMS (ESI-TOF) calculated for C59H63ClN7O13S (M + H)+: 1144.3893; found: 1144.3888.
Isopropyl 1-{2-[2-(2-{2-[4-({[(S)-6-dansylamide-2-(4-benzoylbenzamido)hexanoyl]oxy}methyl)-1H-1,2,3-triazol-1-yl]ethoxy}ethoxy)ethoxy]-2-oxoethyl}-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (3c). HPLC analysis: 97.8%. M.p. 96–98 °C. 1H-NMR (400 MHz, d6-DMSO): 0.76 (d, J = 7.2 Hz, 3H), 1.10 (d, J = 6.4 Hz, 3H), 1.23–1.37 (m, 4H), 1.62–1.67 (m, 2H), 2.29 (s, 3H), 2.73–2.78 (m, 2H), 2.82 (s, 6H), 3.48 (s, 4H), 3.59 (t, J = 5.6 Hz, 2H), 3.78 (t, J = 5.2 Hz, 2H), 4.27–4.33 (m, 3H), 4.48–4.61 (m, 4H), 4.70–4.76 (m, 1H), 4.89 (dd, J = 34.0, 16.4 Hz, 2H), 5.17 (s, 2H), 5.20 (s, 1H), 7.16 (td, J = 7.6, 1.2 Hz, 1H), 7.23–7.28 (m, 2H), 7.31 (d, J = 7.6 Hz, 1H), 7.40 (d, J = 8.0 Hz, 1H), 7.54–7.62 (m, 4H), 7.71 (t, J = 7.6 Hz, 1H), 7.76 (d, J = 8.0 Hz, 2H), 7.80 (d, J = 8.4 Hz, 2H), 7.88 (t, J = 4.8 Hz, 1H), 7.99 (d, J = 8.4 Hz, 2H), 8.08 (d, J = 7.2 Hz, 1H), 8.10 (s, 1H), 8.29 (d, J = 8.8 Hz, 1H), 8.44 (d, J = 9.2 Hz, 1H), 8.86 (d, J = 7.6 Hz, 1H). 13C-NMR (100 MHz, d6-DMSO): 195.4, 171.8, 170.8, 168.7, 166.2, 166.0, 158.2, 150.6, 145.6, 142.8, 141.5, 139.4, 136.9, 136.6, 136.2, 133.0, 132.0, 130.9, 129.7, 129.4, 129.1, 129.0, 128.9, 128.7, 128.6, 128.2, 128.0, 127.7, 127.6, 127.3, 125.0, 123.7, 119.5, 115.3, 107.5, 101.1, 69.5, 68.6, 68.1, 66.9, 65.4, 64.5, 57.7, 52.8, 49.4, 47.3, 45.1, 42.2, 34.2, 29.8, 28.8, 22.7, 21.3, 20.6, 14.7. ESI (MS) m/z: 1188.5 (M + H)+. HRMS (ESI-TOF) calculated for C61H67ClN7O14S (M + H)+: 1188.4155; found: 1188.4150.
Isopropyl 1-{14-[4-({[(S)-6-dansylamide-2-(4-benzoylbenzamido)hexanoyl]oxy}methyl)-1H-1,2,3-triazol-1-yl]-2-oxo-3,6,9,12-tetraoxatetradecyl}-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (3d). HPLC analysis: 96.9%. M.p. 84–86 °C. 1H-NMR (400 MHz, d6-DMSO): 0.76 (d, J = 6.0 Hz, 3H), 1.10 (d, J = 6.0 Hz, 3H), 1.23–1.37 (m, 4H), 1.61–1.67 (m, 2H), 2.29 (s, 3H), 2.72–2.78 (m, 2H), 2.82 (s, 6H), 3.42–3.51 (m, 8H), 3.63 (t, J = 6.0 Hz, 2H), 3.79 (t, J = 5.2 Hz, 2H), 4.28–4.33 (m, 3H), 4.49–4.61 (m, 4H), 4.70–4.77 (m, 1H), 4.89 (dd, J = 32.8, 16.4 Hz, 2H), 5.17 (s, 2H), 5.21 (s, 1H), 7.16 (td, J = 9.2, 1.6 Hz, 1H), 7.25 (t, J = 7.6 Hz, 2H), 7.30 (t, J = 8.0 Hz, 1H), 7.39 (d, J = 7.6 Hz, 1H), 7.54–7.62 (m, 4H), 7.71 (t, J = 7.6 Hz, 1H), 7.76 (d, J = 6.8 Hz, 2H), 7.80 (d, J = 8.4 Hz, 2H), 7.88 (t, J = 5.2 Hz, 1H), 7.99 (d, J = 8.0 Hz, 2H), 8.07 (d, J = 6.8 Hz, 1H), 8.10 (s, 1H), 8.29 (d, J = 8.4 Hz, 1H), 8.44 (d, J = 8.8 Hz, 1H), 8.86 (d, J = 6.8 Hz, 1H). 13C-NMR (100 MHz, d6-DMSO): 195.4, 171.8, 170.8, 168.7, 166.2, 166.0, 158.2, 150.8, 145.6, 142.8, 141.5, 139.4, 136.9, 136.6, 136.2, 133.0, 132.0, 130.9, 129.7, 129.4, 129.1, 129.0, 128.9, 128.7, 128.6, 128.2, 128.0, 127.7, 127.6, 127.3, 125.0, 123.6, 119.4, 115.3, 107.4, 101.1, 69.7, 69.6, 69.5, 69.4, 68.6, 68.1, 66.9, 65.4, 64.5, 57.7, 52.8, 49.4, 47.3, 45.1, 42.2, 34.2, 29.8, 28.8, 22.7, 21.3, 20.7, 14.7. ESI (MS) m/z: 1232.5 (M + H)+. HRMS (ESI-TOF) calculated for C63H70ClN7NaO15S (M + Na)+: 1254.4237; found: 1254.4231.
Isopropyl 1-(2-{2-[4-({[(S)-6-(2-chloroacetamido)-2-(4-benzoylbenzamido)hexanoyl]oxy}methyl)-1H-1,2,3-triazol-1-yl]ethoxy}-2-oxoethyl)-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (31a). HPLC analysis: 90.9%. M.p. 89–91 °C. 1H-NMR (400 MHz, CDCl3): 0.81 (d, J = 6.4 Hz, 3H), 1.16 (dd, J = 6.0, 1.6 Hz, 3H), 1.38–1.60 (m, 4H), 1.82–1.99 (m, 2H), 2.32 (s, 3H), 3.24–3.33 (m, 2H), 3.93–4.02 (m, 2H), 4.11–4.29 (m, 2H), 4.61–4.69 (m, 7H), 4.80–4.87 (m, 1H), 5.29–5.34 (m, 2H), 5.38 (s, 1H), 6.80–6.83 (m, 1H), 7.06 (tt, J = 7.6, 1.6 Hz, 1H), 7.15 (t, J = 6.4 Hz, 1H), 7.24–7.34 (m, 3H), 7.50 (t, J = 7.6 Hz, 2H), 7.62 (t, J = 7.6 Hz, 1H), 7.77–7.83 (m, 5H), 7.95 (dd, J = 8.4, 2.8 Hz, 2H). 13C-NMR (100 MHz, CDCl3): 196.0, 172.1, 171.4, 167.8, 166.8, 166.7, 166.6, 156.7, 144.4, 141.9, 140.4, 137.0, 136.8, 133.3, 133.0, 131.1, 130.1, 130.0, 129.4, 128.5, 128.0, 127.3, 127.0, 124.4, 109.4, 103.3, 68.0, 65.2, 63.6, 58.4, 53.0, 48.8, 47.1, 42.7, 38.9, 35.1, 30.8, 28.8, 22.5, 21.6, 20.9, 15.2. ESI (MS) m/z: 945.1 (M + H)+. HRMS (ESI-TOF) calculated for C47H49Cl2N6O11 (M + H)+: 943.28364; found: 943.2932.
Isopropyl 1-[2-(2-{2-[4-({[(S)-6-(2-chloroacetamido)-2-(4-benzoylbenzamido)hexanoyl]oxy}methyl)-1H-1,2,3-triazol-1-yl]ethoxy}ethoxy)-2-oxoethyl]-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (31b). HPLC analysis: 93.5%. M.p. 64–66 °C. 1H-NMR (400 MHz, CDCl3): 0.81 (d, J = 6.0 Hz, 3H), 1.18 (d, J = 6.4 Hz, 3H), 1.41–1.67 (m, 4H), 1.88–1.97 (m, 2H), 2.38 (s, 3H), 3.25–3.32 (m, 2H), 3.68 (t, J = 4.0 Hz, 2H), 3.88 (t, J = 5.2 Hz, 2H), 3.94–4.03 (m,2H), 4.20–4.37 (m, 4H), 4.51 (t, J = 5.2 Hz, 2H), 4.72–4.76 (m,3H), 4.83–4.88 (m, 1H), 5.32 (s, 2H), 5.42 (s, 1H), 6.72–6.78 (m, 1H), 7.06–7.18 (m, 3H), 7.25–7.29 (m, 1H), 7.37 (dt, J = 7.6, 1.2 Hz, 1H), 7.50 (t, J = 7.6 Hz, 2H), 7.62 (t, J = 7.6 Hz, 1H), 7.74 (s, 1H), 7.82 (dd, J = 17.2, 8.8 Hz, 4H), 7.95 (t, J = 8.4 Hz, 2H). 13C-NMR (100 MHz, CDCl3): 195.0,171.1, 170.5, 167.2, 165.8, 165.7, 165.6, 156.2, 143.7,141.2, 139.3, 135.9, 135.8, 132.2, 132.0, 130.1, 129.1, 129.0, 128.2, 127.5, 126.9, 126.3, 126.1, 108.3, 102.1, 68.1, 67.5, 66.9,64.4, 63.7, 57.5, 52.0, 49.1, 46.4, 41.7, 38.0, 33.9, 30.0, 27.7, 21.5, 20.6, 19.9, 14.2. ESI (MS) m/z: 987.1 (M + H)+. HRMS (ESI-TOF) calculated for C49H52Cl2N6NaO12 (M + Na)+: 1009.29125; found: 1009.29266.
Isopropyl 1-{2-[2-(2-{2-[4-({[(S)-6-(2-chloroacetamido)-2-(4-benzoylbenzamido)hexanoyl]oxy}methyl)-1H-1,2,3-triazol-1-yl]ethoxy}ethoxy)ethoxy]-2-oxoethyl}-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (31c). HPLC analysis: 91.1%. M.p. 51–52 °C. 1H-NMR (400 MHz, CDCl3): 0.82 (d, J = 6.4 Hz, 3H), 1.18 (d, J = 6.0 Hz, 3H), 1.41–1.59 (m, 4H), 1.83–1.99 (m, 2H), 2.39 (s, 3H), 3.21–3.34 (m, 2H), 3.55 (s, 4H), 3.65(t, J = 4.8 Hz, 2H), 3.84 (t, J = 4.8 Hz, 2H), 3.95–4.04 (m, 2H), 4.19–4.32 (m, 2H), 4.37–4.39 (m, 2H), 4.51 (t, J = 4.8 Hz, 2H), 4.73 (s, 2H), 4.74–4.78 (m, 1H), 4.82–4.88 (m, 1H), 5.32 (s, 2H), 5.42 (s, 1H), 6.78 (t, J = 7.2 Hz, 1H), 7.06–7.16 (m, 3H), 7.25–7.28 (m, 1H), 7.38 (dd, J = 7.6, 1.6 Hz, 1H), 7.50 (t, J = 8.0 Hz, 2H), 7.62 (t, J = 7.2 Hz, 1H), 7.79 (s, 1H), 7.83 (t, J = 8.0 Hz, 4H), 7.95 (t, J = 8.8 Hz, 2H). 13C-NMR (100 MHz, CDCl3): 195.9, 172.1, 171.3, 168.0, 166.8, 166.6, 166.4, 156.9, 144.7, 142.1, 140.4, 137.0, 136.9, 133.2, 132.9, 131.1, 130.1, 130.0, 129.3, 128.5, 127.9, 127.3, 127.0, 125.0, 109.3, 103.3, 70.5, 70.4, 69.3, 68.8, 67.9, 65.4, 64.9, 58.6, 52.8, 50.3, 47.5, 42.7, 39.1, 34.9, 31.3, 28.7, 22.5, 21.6, 20.9, 15.2. ESI (MS) m/z: 1031.1 (M + H)+. HRMS (ESI-TOF) calculated for C51H56Cl2N6NaO13 (M + Na)+: 1053.31746; found: 1053.31881.
Isopropyl 1-{14-[4-({[(S)-6-(2-chloroacetamido)-2-(4-benzoylbenzamido)hexanoyl]oxy}methyl)-1H-1,2,3-triazol-1-yl]-2-oxo-3,6,9,12-tetraoxatetradecyl}-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (31d). HPLC analysis: 90.6%. M.p. 51–52 °C. 1H-NMR (400 MHz, CDCl3): 0.82(d, J = 6.0 Hz, 3H), 1.18(d, J = 6.4 Hz, 3H), 1.42–1.58(m, 4H), 1.82–2.01 (m, 2H), 2.39(s, 3H), 3.24–3.33(m, 2H), 3.54–3.61 (m, 8H), 3.71 (t, J = 4.4 Hz, 2H), 3.86(t, J = 4.8 Hz, 2H), 3.95–4.04 (m, 2H), 4.19 (d, J = 18.4 Hz, 1H), 4.31 (dd, J = 14.4, 7.6 Hz, 1H), 4.39–4.41 (m, 2H), 4.53 (t, J = 4.8 Hz, 2H), 4.71 (s, 2H), 4.74–4.79(m, 1H), 4.82–4.88(m, 1H), 5.27–5.35(m, 2H), 5.42(s, 1H), 6.78 (t, J = 5.6 Hz, 1H), 7.07–7.10 (m, 2H), 7.15–7.19 (m, 1H), 7.26–7.28 (m, 1H), 7.39(d, J = 8.0 Hz, 1H), 7.50 (t, J = 8.0 Hz, 2H), 7.62(t, J = 7.2 Hz, 1H), 7.80 (d, J = 7.2 Hz, 2H), 7.82–7.85 (m, 3H), 7.94–7.97 (m, 2H). 13C-NMR (100 MHz, CDCl3): 195.0,171.1, 170.4, 167.1, 165.8, 165.6, 165.5, 156.0, 143.8,141.2, 141.1, 139.3, 135.98, 135.96, 132.2, 131.9, 130.1, 129.1, 129.0, 128.3, 127.5, 126.9, 126.3, 126.1, 124.1, 108.2, 102.2, 69.4, 68.3, 67.7, 66.8, 64.3, 63.9, 57.5, 51.8, 49.3, 46.4, 41.7, 38.1, 34.0, 30.2, 27.7, 21.5, 20.6, 19.9, 14.2. ESI (MS) m/z: 1075.1 (M + H)+. HRMS (ESI-TOF) calculated for C53H60Cl2N6NaO14 (M + Na)+: 1097.34368; found: 1097.34555.
3.1.16. General Procedure for Synthesis of compounds 4a–4d
To a solution of 6a–6d (0.32 mmol) and 30 (0.31 g, 0.32 mmol) in CH2Cl2 (1.5 mL) and H2O (1.5 mL), CuSO4·5H2O (97.37 mg, 0.39 mmol) and sodium ascorbate (0.10 g, 0.51 mmol) were added. The resulting solution was stirred at r.t. overnight. The solvent was evaporated in vacuo, and then the residue was redissolved in EtOAc (10 mL), washed by water (10 mL × 2), and dried over anhydrous Na2SO4. The combined organic layer was evaporated, and the residue was purified by preparative-reverse phase HPLC to give the target products of 4a–4d as pale yellow solids.
Isopropyl 1-(2-{2-[4-({[(2-[(S)-6-dansylamide-2-(4-benzoylbenzamido)hexanamido] -6-(5-{(3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl}pentanamido) hexanoyl]oxy}methyl)-1H-1,2,3-triazol-1-yl]ethoxy}-2-oxoethyl)-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (4a). HPLC analysis: 96.6%. M.p. 93–95 °C. 1H-NMR (400 MHz, d6-DMSO): 0.76 (d, J = 6.4 Hz, 3H), 1.11 (d, J = 6.0 Hz, 3H), 1.28–1.61 (m, 18H), 2.03 (t, J = 7.6 Hz, 2H), 2.24 (s, 3H), 2.55 (d, J = 12.4 Hz, 1H), 2.74–2.80 (m, 3H), 2.82 (s, 6H), 2.95–2.99 (m, 2H), 3.04–3.09 (m, 1H), 4.07–4.12 (m, 1H), 4.18–4.23 (m, 1H), 4.28 (t, J = 6.4 Hz, 1H), 4.40–4.62 (m, 5H), 4.71–4.77 (m, 3H), 4.85 (dd, J = 40.0, 16.0 Hz, 2H), 5.12 (s, 2H), 5.19 (s, 1H), 6.40 (br s, 2H), 7.17 (t, J = 9.2 Hz, 1H), 7.24–7.36 (m, 4H), 7.55–7.63 (m, 4H), 7.69–7.80 (m, 6H), 7.90 (t, J = 6.0 Hz, 1H,), 8.00 (d, J = 8.0 Hz, 2H), 8.09 (d, J = 7.2 Hz, 1H), 8.19 (s, 1H), 8.31 (t, J = 7.6 Hz, 2H), 8.44 (d, J = 8.8 Hz, 1H), 8.55 (d, J = 7.6 Hz, 1H). 13C-NMR (100 MHz, d6-DMSO): 195.4, 172.0, 171.9, 171.7, 170.7, 168.4, 166.2, 165.7, 162.7, 158.1, 150.6, 145.5, 142.7, 141.8, 139.2, 137.4, 136.7, 136.2, 132.9, 132.0, 130.8, 129.7, 129.3, 129.1, 128.9, 128.7, 128.6, 128.1, 128.0, 127.7, 127.3, 125.1, 123.7, 119.6, 115.4, 107.4, 101.2, 66.9, 65.3, 63.6, 61.0, 59.2, 57.6, 55.4, 53.3, 51.9, 48.5, 47.1, 45.1, 42.4, 38.1, 35.2, 34.2, 31.0, 30.3, 29.1, 28.7, 28.2, 28.0, 25.3, 22.8, 22.7, 21.3, 20.6, 14.7. ESI (MS) m/z: 1454.5 (M + H)+. HRMS (ESI-TOF) calculated for C73H84ClN11NaO15S2 (M + Na)+: 1476.5176; found: 1476.5171.
Isopropyl 1-[2-(2-{2-[4-({[(2-[(S)-6-dansylamide-2-(4-benzoylbenzamido)hexanamido] -6-(5-{(3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl}pentanamido) hexanoyl]oxy}methyl)-1H-1,2,3-triazol-1-yl]ethoxy}ethoxy)-2-oxoethyl]-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (4b). HPLC analysis: 98.2%. M.p. 133–135 °C. 1H-NMR (400 MHz, d6-DMSO): 0.76 (d, J = 6.0 Hz, 3H), 1.10 (d, J = 6.4 Hz, 3H), 1.28–1.61 (m, 18H), 2.03 (t, J = 7.6 Hz, 2H), 2.28 (s, 3H), 2.55 (d, J = 12.4 Hz, 1H), 2.75–2.80 (m, 3H), 2.82 (s, 6H), 2.95–3.00 (m, 2H), 3.04–3.09(m, 1H), 3.66 (t, J = 4.4 Hz, 2H), 3.84 (t, J = 5.2 Hz, 2H), 4.08–4.11 (m, 1H), 4.18–4.23 (m, 1H), 4.26–4.30 (m, 3H), 4.38–4.60 (m, 5H), 4.71–4.77 (m, 1H), 4.89 (dd, J = 34.4, 16.0 Hz, 2H), 5.14 (d, J = 3.2 Hz, 2H), 5.21 (s, 1H), 6.40 (br s,2H), 7.17 (td, J = 8.0, 0.8 Hz, 1H), 7.24–7.28 (m, 2H), 7.32 (d, J = 8.0 Hz, 1H), 7.40 (d, J = 7.6 Hz, 1H), 7.55–7.63 (m, 4H), 7.69–7.80 (m, 6H), 7.90 (t, J = 5.6 Hz, 1H), 8.01 (d, J = 8.0 Hz, 2H), 8.09 (d, J = 6.0 Hz, 2H), 8.31 (t, J = 6.4 Hz, 2H), 8.44 (d, J = 8.4 Hz, 1H), 8.55 (d, J = 7.6 Hz, 1H). 13C-NMR (100 MHz, d6-DMSO): 195.4, 172.0, 171.9, 171.8, 170.8, 168.7, 166.2, 165.7, 162.7, 158.2, 150.6, 145.6, 142.8, 141.5, 139.2, 137.4, 136.7, 136.2, 133.0, 132.0, 130.9, 129.7, 129.3, 129.1, 128.8, 128.7, 128.6, 128.1, 128.0, 127.7, 127.6, 127.3, 124.9, 123.7, 119.6, 115.3, 107.5, 101.2, 68.5, 67.9, 66.9, 65.4, 64.4, 61.0, 59.2, 57.6, 55.4, 53.2, 51.9, 49.3, 47.3, 45.1, 42.4, 38.1, 35.2, 34.2, 31.0, 30.3, 29.1, 28.7, 28.2, 27.9, 25.3, 22.8, 22.7, 21.3, 20.6, 14.7. ESI (MS) m/z: 1499.0 (M + H)+. HRMS (ESI-TOF) calculated for C75H88ClN11NaO16S2 (M + Na)+: 1520.5438; found: 1520.5433.
Isopropyl 1-{2-[2-(2-{2-[4-({[(2-[(S)-6-dansylamide-2-(4-benzoylbenzamido)hexanamido] -6-(5-{(3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl}pentanamido) hexanoyl]oxy}methyl)-1H-1,2,3-triazol-1-yl]ethoxy}ethoxy)ethoxy]-2-oxoethyl}-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (4c). HPLC analysis: 98.9%. M.p. 119–121 °C. 1H-NMR (400 MHz, d6-DMSO): 0.76 (d, J = 6.0 Hz, 3H), 1.10 (d, J = 6.0 Hz, 3H), 1.28–1.61 (m, 18H), 2.03 (t, J = 7.6 Hz, 2H), 2.30 (s, 3H), 2.55 (d, J = 12.4 Hz, 1H), 2.77–2.80 (m, 3H), 2.82 (s, 6H), 2.95–3.00 (m, 2H), 3.04–3.09 (m, 1H), 3.49 (s, 4H), 3.61 (t, J = 4.4 Hz, 2H), 3.78 (t, J = 4.8 Hz, 2H), 4.08–4.11 (m, 1H), 4.18–4.24 (m, 1H), 4.26–4.31 (m, 3H), 4.38–4.61 (m, 5H), 4.71–4.77 (m, 1H), 4.89 (dd, J = 32.4, 16.4 Hz, 2H), 5.14 (d, J = 3.6 Hz, 2H), 5.21 (s, 1H), 6.40 (br s,2H), 7.17 (t, J = 7.6 Hz, 1H), 7.24–7.28 (m, 2H), 7.32 (d, J = 8.0 Hz, 1H), 7.40 (d, J = 7.2 Hz, 1H), 7.55–7.63 (m, 4H), 7.69–7.80 (m, 6H), 7.89 (t, J = 6.0 Hz, 1H), 8.01 (d, J = 8.0 Hz, 2H), 8.09 (d, J = 4.8 Hz, 2H), 8.31 (t, J = 7.6 Hz, 2H), 8.44 (d, J = 8.8 Hz, 1H), 8.55 (d, J = 8.0 Hz, 1H). 13C-NMR (100 MHz, d6-DMSO): 195.4, 172.0, 171.9, 171.8, 170.8, 168.7, 166.2, 165.7, 162.7, 158.2, 150.5, 145.6, 142.8, 141.5, 139.2, 137.4, 136.7, 136.2, 133.0, 132.0, 130.9, 129.7, 129.3, 129.1, 128.8, 128.7, 128.6, 128.1, 128.0, 127.7, 127.6, 127.3, 124.9, 123.7, 119.6, 115.4, 107.5, 101.1, 69.5, 68.6, 68.1, 66.9, 65.4, 64.5, 61.0, 59.2, 57.7, 55.4, 53.2, 51.9, 49.4, 47.3, 45.1, 42.4, 38.1, 35.2, 34.2, 31.0, 30.3, 29.1, 28.7, 28.2, 28.0, 25.3, 22.8, 22.7, 21.3, 20.7, 14.7. ESI (MS) m/z: 1544.0 (M + H)+. HRMS (ESI-TOF) calculated for C77H92ClN11NaO17S2 (M + Na)+: 1564.5700; found: 1564.5695.
Isopropyl 1-{14-[4-({[(2-[(S)-6-dansylamide-2-(4-benzoylbenzamido)hexanamido] -6-(5-{(3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl}pentanamido) hexanoyl]oxy}methyl)-1H-1,2,3-triazol-1-yl]-2-oxo-3,6,9,12-tetraoxatetradecyl}-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (4d). HPLC analysis: 99.0%. M.p. 158–160 °C. 1H-NMR (400 MHz, d6-DMSO): 0.77 (d, J = 6.4 Hz, 3H), 1.11 (d, J = 6.4 Hz, 3H), 1.23–1.61 (m, 18H), 2.03 (t, J = 6.8 Hz, 2H), 2.30 (s, 3H), 2.55 (d, J = 12.8 Hz, 1H), 2.77–2.80 (m, 3H), 2.82 (s, 6H), 2.95–3.00 (m, 2H), 3.04–3.09 (m, 1H), 3.44–3.50 (m, 8H), 3.64 (t, J = 4.4 Hz, 2H), 3.80 (t, J = 4.8 Hz, 2H), 4.08–4.11 (m, 1H), 4.19–4.24 (m, 1H), 4.26–4.32 (m, 3H), 4.40–4.61 (m, 5H), 4.71–4.77 (m, 1H), 4.89 (dd, J = 32.4,16.4 Hz, 2H), 5.14 (d, J = 4.8 Hz, 2H), 5.21 (s, 1H), 6.39 (br s,2H), 7.16 (t, J = 7.2 Hz, 1H), 7.25 (t, J = 7.6 Hz, 2H), 7.31 (t, J = 8.0 Hz, 1H), 7.40 (d, J = 7.6 Hz, 1H), 7.54–7.62 (m, 4H), 7.71 (t, J = 6.0 Hz, 2H), 7.77 (dd, J = 15.2, 8.0 Hz, 4H), 7.88 (t, J = 6.0 Hz, 1H), 8.01 (d, J = 8.4 Hz, 2H), 8.09 (d, J = 5.2 Hz, 2H), 8.30 (d, J = 8.8 Hz, 2H), 8.44 (d, J = 8.4 Hz, 1H), 8.54 (d, J = 7.6 Hz, 1H).13C-NMR (100 MHz, d6-DMSO): 195.4, 172.0, 171.9, 171.8, 170.8, 168.7, 166.2, 165.7, 162.7, 158.2, 150.8, 145.6, 142.8, 141.5, 139.2, 137.4, 136.7, 136.2, 133.0, 132.0, 130.9, 129.7, 129.3, 129.1, 129.0,128.9, 128.7, 128.6, 128.1, 128.0, 127.7, 127.6, 127.3, 125.0,123.6, 119.4, 115.2, 107.5, 101.1, 69.7, 69.6, 69.59, 69.50, 68.6, 68.1, 66.9, 65.4, 64.5, 61.0, 59.2, 57.6, 55.4, 53.2, 51.9, 49.4, 47.3, 45.1, 42.4, 38.1, 35.2, 34.2, 31.0, 30.3, 29.1, 28.7, 28.2, 28.0, 25.3, 22.8, 22.7, 21.3, 20.7, 14.7. ESI (MS) m/z: 1586.0 (M + H)+. HRMS (ESI-TOF) calculated for C79H96ClN11NaO18S2 (M + Na)+: 1608.5962; found: 1608.5957.
3.1.17. General Procedure for Synthesis of compounds 5a-5d
A mixture of 31a–31d (0.37 mmol) and NaN3 (0.56 mmol) in DMF (8.0 mL) was stirred at r.t. overnight. The mixture was diluted with ice water (100 mL) and extracted by EtOAc (100 mL × 2). The combined organic phase was washed with brine (100 mL × 2), dried over anhydrous Na2SO4, and concentrated. The residue was purified by preparative-reverse phase HPLC to give 5a–5d as pale yellow solids.
Isopropyl 1-(2-{2-[4-({[(S)-6-(2-azidoacetamido)-2-(4-benzoylbenzamido)hexanoyl]oxy}methyl)-1H-1,2,3-triazol-1-yl]ethoxy}-2-oxoethyl)-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (5a). HPLC analysis: 91.4%. M.p. 81–83 °C. 1H-NMR (400 MHz, CDCl3): 0.81 (d, J = 6.4 Hz, 3H), 1.17 (dd, J = 6.4, 2.4 Hz, 3H), 1.36–1.59 (m, 4H), 1.83–1.98 (m, 2H), 2.33 (s, 3H), 3.23–3.32 (m, 2H), 3.85–3.95 (m, 2H), 4.12–4.31 (m, 2H), 4.61–4.69 (m, 7H), 4.81–4.87 (m, 1H), 5.30–5.35 (m, 2H), 5.39 (s, 1H), 6.56–6.60 (m, 1H), 7.07 (tt, J = 8.0, 1.2 Hz, 1H), 7.14–7.22 (m, 2H), 7.25–7.27 (m, 1H), 7.32 (d, J = 8.0 Hz, 1H), 7.50 (t, J = 8.0 Hz, 2H), 7.62 (tt, J = 7.2, 1.2 Hz, 1H), 7.74 (d, J = 2.8 Hz, 1H), 7.78–7.85 (m, 4H), 7.96 (dd, J = 8.4, 3.2 Hz, 2H). 13C-NMR (100 MHz, CDCl3): 195.1,171.2, 170.7, 166.9, 166.5, 166.0, 165.8, 156.3, 143.6, 141.9, 141.2, 139.3, 135.9, 132.1, 132.0, 130.1, 129.1, 128.9, 128.2, 127.5, 127.0, 126.4, 126.1, 123.6, 108.3, 102.0, 67.0, 64.4, 62.7, 57.3, 52.1, 51.4, 47.9, 46.2, 37.7, 33.9, 29.8, 27.7, 21.7, 20.6, 19.9, 14.2. ESI (MS) m/z: 950.1 (M + H)+. HRMS (ESI-TOF) calculated for C47H48ClN9NaO11 (M + Na)+: 972.3060; found: 972.3054.
Isopropyl 1-[2-(2-{2-[4-({[(S)-6-(2- azidoacetamido)-2-(4-benzoylbenzamido)hexanoyl]oxy}methyl)-1H-1,2,3-triazol-1-yl]ethoxy}ethoxy)-2-oxoethyl]-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (5b). HPLC analysis: 94.1%. M.p. 67–68 °C. 1H-NMR (400 MHz, CDCl3): 0.81 (d, J = 6.4 Hz, 3H), 1.16 (d, J = 6.4 Hz, 3H), 1.34–1.56 (m, 4H), 1.90–1.99 (m, 2H), 2.37 (s, 3H), 3.22–3.29 (m, 2H), 3.68 (t, J = 2.8 Hz, 2H), 3.85–3.91 (m, 4H), 4.20–4.38 (m, 4H), 4.50 (t, J = 4.8 Hz, 2H), 4.68–4.76 (m, 3H), 4.81–4.88 (m, 1H), 5.31 (s, 2H), 5.41 (s, 1H), 6.62–6.66 (m, 1H), 7.04–7.10 (m, 1H), 7.13–7.22 (m, 2H), 7.25–7.28 (m, 1H), 7.38 (d, J = 7.6 Hz, 1H), 7.50 (t, J = 7.6 Hz, 2H), 7.62 (t, J = 7.6 Hz, 1H), 7.74 (s, 1H), 7.81 (dd, J = 15.6, 7.6 Hz, 4H), 7.95 (t, J = 8.4 Hz, 2H). 13C-NMR (100 MHz, CDCl3): 195.0,171.2, 170.6, 167.2, 166.4, 165.8, 165.7, 156.3, 143.7,141.3, 141.2, 139.3, 135.9, 135.8,132.1, 132.0, 130.1, 129.1, 128.9, 128.2, 127.5, 127.0, 126.3, 126.1, 123.9, 108.3, 102.0, 68.1, 67.5, 66.9, 64.5, 63.7, 57.4, 52.0, 51.4, 49.1, 46.4, 37.6, 33.9, 30.0, 27.7, 21.6, 20.6, 19.9, 14.2. ESI (MS) m/z: 994.1 (M + H)+. HRMS (ESI-TOF) calculated for C49H52ClN9NaO12 (M + Na)+: 1016.3322; found: 1016.3316.
Isopropyl 1-{2-[2-(2-{2-[4-({[(S)-6-(2- azidoacetamido)-2-(4-benzoylbenzamido)hexanoyl]oxy}methyl)-1H-1,2,3-triazol-1-yl]ethoxy}ethoxy)ethoxy]-2-oxoethyl}-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (5c). HPLC analysis: 93.2%. M.p. 73–74 °C. 1H-NMR (400 MHz, CDCl3): 0.82 (d, J = 6.0 Hz, 3H), 1.18 (d, J = 6.0 Hz, 3H), 1.38–1.57 (m, 4H), 1.89–2.03 (m, 2H), 2.39(s, 3H), 3.20–3.29(m, 2H), 3.55 (s, 4H), 3.66 (t, J = 4.4 Hz, 2H), 3.84 (t, J = 5.2 Hz, 2H), 3.90 (d, J = 4.0 Hz, 2H), 4.20–4.39 (m,4H), 4.51 (t, J = 4.4 Hz, 2H), 4.69–4.77(m, 3H), 4.82–4.88(m, 1H), 5.31(s, 2H), 5.41(s, 1H), 6.65–6.71 (m, 1H), 7.04–7.10 (m, 1H), 7.13–7.18 (m, 1H), 7.22–7.27 (m, 2H), 7.38 (d, J = 7.6 Hz, 1H), 7.50 (t, J = 7.6 Hz, 2H), 7.62 (t, J = 7.6 Hz, 1H), 7.79 (d, J = 8.4 Hz, 2H), 7.83 (t, J = 3.6 Hz, 3H), 7.95 (t, J = 8.4 Hz, 2H). 13C-NMR (100 MHz, CDCl3): 195.0, 171.1, 170.6, 167.1, 166.3, 165.8, 165.7, 156.2, 143.8, 141.2, 141.1, 139.3, 135.9, 132.1, 132.0, 130.1, 129.1, 129.0, 128.3, 127.5, 126.9, 126.3, 126.1, 124.0, 108.2, 102.1, 69.4, 69.3, 68.2, 67.7, 66.9, 64.4, 63.9, 57.5, 51.9, 51.4, 49.2, 46.4, 37.7, 33.9, 30.2, 27.7, 21.5, 20.6, 19.9, 14.2. ESI (MS) m/z: 1038.1 (M + H)+. HRMS (ESI-TOF) calculated for C51H56ClN9NaO13 (M + Na)+: 1060.3584; found: 1060.3578.
Isopropyl 1-{14-[4-({[(S)-6-(2- azidoacetamido)-2-(4-benzoylbenzamido)hexanoyl]oxy}methyl)-1H-1,2,3-triazol-1-yl]-2-oxo-3,6,9,12-tetraoxatetradecyl}-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate(5d). HPLC analysis: 90.7%. M.p. 66–68 °C. 1H-NMR (400 MHz, CDCl3): 0.82 (d, J = 6.4 Hz, 3H), 1.18 (d, J = 6.0 Hz, 3H), 1.38–1.56 (m, 4H), 1.83–1.93 (m, 2H), 2.39 (s, 3H), 3.23–3.29 (m, 2H), 3.54–3.61 (m, 8H), 3.71 (t, J = 4.4 Hz, 2H), 3.85(t, J = 4.8 Hz, 2H), 3.90 (d, J = 4.8 Hz, 2H), 4.27 (dd, J = 51.6, 18.8 Hz, 2H), 4.38–4.41 (m, 2H), 4.53 (t, J = 5.2 Hz, 2H), 4.72 (s, 2H), 4.74–4.77 (m, 1H), 4.82–4.88 (m, 1H), 5.26–5.35(m, 2H), 5.41 (s, 1H), 6.64–6.69 (m, 1H), 7.05–7.10 (m, 1H), 7.15–7.19 (m, 2H), 7.25–7.27 (m, 1H), 7.39 (dd, J = 7.6, 1.6 Hz, 1H), 7.50 (t, J = 7.6 Hz, 2H), 7.62 (t, J = 7.2 Hz, 1H), 7.78–7.85 (m, 5H), 7.94–7.97 (m, 2H). 13C-NMR (100 MHz, CDCl3): 195.0, 171.1, 170.5, 167.2, 166.3, 165.8, 165.7, 156.1, 143.8, 141.3, 141.1, 139.3, 136.0, 132.1, 132.0, 130.1, 129.1, 129.0, 128.2, 127.5, 126.9, 126.3, 126.1, 124.1, 108.2, 102.1, 69.45, 69.43, 69.41, 68.2, 67.7, 66.8, 64.4, 63.9, 57.4, 51.9, 51.4, 49.3, 46.4, 37.7, 33.9, 30.2, 27.7, 21.5, 20.6, 19.9, 14.2. ESI (MS) m/z: 1082.1 (M+H)+. HRMS (ESI-TOF) calculated for C53H60ClN9NaO14 (M + Na)+: 1104.3846; found: 1104.3840.
3.1.18. (S)-Methyl-6-(2-azidoacetamido)-2-(4-benzoylbenzamido)hexanoate (34)
A mixture of 33 (0.11 g, 0.25 mmol) and NaN3 (0.25 g, 0.38 mmol) in DMF (10.0 mL) was stirred at 75 °C for 2 h. The mixture was diluted with ice water (20 mL) and extracted by EtOAc (20 mL × 2). The combined organic phase was washed with brine (20 mL × 2), dried over anhydrous Na2SO4, and concentrated. The residue was purified by flash silica gel column chromatography [petroleum ether-EtOAc (4:1)] to obtain 34 (83.0 mg, 61%) as a white solid product. HPLC analysis: 96.0%. M.p. 121–122 °C. 1H-NMR (400 MHz, CDCl3): 1.36–1.54 (m, 2H), 1.62 (tt, J = 14.1, 7.0 Hz, 2H), 1.88 (ddd, J = 22.8, 11.4, 7.4 Hz, 1H), 1.96–2.07 (m, 1H), 3.24–3.39 (m, 2H), 3.80 (s, 3H), 3.88–4.00 (m, 2H), 4.81 (dd, J = 12.2, 7.2 Hz, 1H), 6.44 (s, 1H), 6.97 (s, 1H), 7.50 (t, J = 7.6 Hz, 2H), 7.62 (t, J = 7.4 Hz, 1H), 7.80 (d, J = 7.6 Hz, 2H), 7.85 (d, J = 7.6 Hz, 2H), 7.96 (d, J = 8.2 Hz, 2H). 13C-NMR (100 MHz, CDCl3): 195.9, 172.8, 166.9, 166.4, 140.4, 137.0, 136.9, 132.9, 130.1, 128.5, 127.2, 52.7, 52.5, 38.7, 31.8, 28.9, 22.4. ESI (MS) m/z: 450.2 (M − H)−. HRMS (ESI-TOF) calculated for C23H24N5O5 (M−H)−: 450.1777; found: 450.1783.
3.1.19. Isopropyl 1-(14-{4-[({(S)-2-(4-benzoylbenzamido)-6-[2-(4-{[(5-{(3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl}pentanoyl)oxy]methyl}-1H-1,2,3-triazol-1-yl)acetamido]hexanoyl}oxy)methyl]-1H-1,2,3-triazol-1-yl}-2-oxo-3,6,9,12-tetraoxatetradecyl)-4-(2-chlorophenyl)-2-methyl-5-oxo-1,4,5,7-tetrahydrofuro[3,4-b]pyridine-3-carboxylate (36)
To a solution of 5d (0.39 g, 0.36 mmol) and 35 (0.12 g, 0.43 mmol) in CH2Cl2 (4 mL) and H2O (4 mL), CuSO4·5H2O (0.10 mg, 0.40 mmol) and sodium ascorbate (0.10 g, 0.51 mmol) were added. The resulting solution was stirred at r.t. overnight. The solvent was evaporated in vacuo, and then the residue was redissolved in EtOAc (10 mL), washed by water (10 mL × 2), and dried over anhydrous Na2SO4. The organic layer was evaporated, and the residue was purified by preparative-reverse phase HPLC to give 36 (66.0 mg, 13%) as a pale yellow solid. HPLC analysis: 93.6%. M.p. 71–73 °C. 1H-NMR (400 MHz, d6-DMSO): 0.76 (d, J = 6.0 Hz, 3H), 1.11 (d, J = 6.0 Hz, 3H), 1.29–1.56 (m, 10H), 1.79–1.84 (m, 2H), 2.30–2.33 (m, 5H), 2.57 (d, J = 12.4 Hz, 1H), 2.81 (dd, J = 12.4, 5.2 Hz, 1H), 3.06–3.10 (m, 3H), 3.44–3.52 (m, 8H), 3.64 (t, J = 4.4 Hz, 2H), 3.80 (t, J = 5.2 Hz, 2H), 4.10–4.13 (m, 1H), 4.28–4.32 (m, 3H), 4.43–4.61 (m, 5H), 4.71–4.77 (m, 1H), 4.89 (dd, J = 32.0, 16.8 Hz, 2H), 5.06 (s, 2H), 5.12 (s, 2H), 5.21 (s, 3H), 6.34 (br s, 1H), 6.40 (br s, 1H), 7.17 (td, J = 7.6, 1.6 Hz, 1H), 7.27 (td, J = 7.6, 1.2 Hz, 1H), 7.31 (dd, J = 8.0, 1.2 Hz, 1H), 7.40 (dd, J = 7.6, 1.6 Hz, 1H), 7.58 (t, J = 7.6 Hz, 2H), 7.71 (t, J = 7.6 Hz, 1H), 7.76 (d, J = 7.2 Hz, 2H), 7.82 (d, J = 8.0 Hz, 2H), 8.03 (d, J = 8.4 Hz, 2H), 8.07 (s, 1H), 8.12 (s, 1H), 8.32 (t, J = 5.6 Hz, 1H), 8.95 (d, J = 7.2 Hz, 1H). 13C-NMR (100 MHz, CDCl3): 195.1, 172.4, 171.6, 170.7, 167.3, 165.9, 165.8, 164.6, 156.3, 143.9, 141.9, 141.3, 141.1, 139.2, 136.0, 135.9, 132.1, 131.9, 130.1, 129.1, 129.0, 128.2, 127.5, 126.9, 126.5, 126.1, 124.6, 124.1, 108.1, 102.0, 69.4, 69.3, 68.2, 67.7, 66.8, 64.5, 63.9, 60.7, 59.2, 57.4, 56.4, 54.5, 52.2, 51.6, 49.3, 46.4, 39.5, 38.0, 33.9, 32.7, 29.9, 27.3, 27.1, 27.0, 23.6, 21.7, 20.7, 19.9, 14.2. ESI (MS) m/z: 1364.1 (M + H)+. HRMS (ESI-TOF) calculated for C66H78ClN11NaO17S (M + Na)+: 1386.4884; found: 1386.4879.
3.2. Glycogenolysis Inhibition in Liver HL-7702 Cells and HepG2 Cells
The inhibition of hepatic glycogenolysis was monitored by the measurement of liver glycogen using a microplate reader (BIO-RAD, Bio-Rad Laboratories, Hercules, CA, USA), which was done quantitatively by the anthrone reagent (Sigma, Saint Louis, MO, USA) colorimetric method based on the published method [11]. Primary human liver HL-7702 cells or HepG2 cells (Sigma) were treated with the test compound or DMSO solvent (final concentration, 0.10%), followed by 60 min incubation with 0.3 nM glucagon (GGN). Assays were terminated by centrifugation, and cells were digested with 30% KOH followed by glycogen determination. The IC50 values were estimated by fitting the inhibition data to a dose-dependent curve using a logistic derivative equation.
3.3. Photoaffinity Labeling Experiment
(1) According to our previous report [10], a complete Dulbecco’s Modified Eagle’s Medium (DMEM) (100 U/mL penicillin, 100 mg/mL streptomycin, 12% fetal bovine serum, pH 7.4) was used to culture the HepG2 cells in standard incubator conditions (37 °C, 5% CO2) until about 80% confluence. HepG2 cells were harvested by trypsinization and washed twice with phosphate buffered saline (PBS). HepG2 cells were lysed in a lysis buffer (7 M urea, 2 M thiourea, 4% (w/v) CHAPS, 40 mM Tris, 65 mM DTT, 2% (v/v) IPG Buffer pH 3–10, 1% (v/v) Protease Inhibitor cocktail), and then centrifuged using a Soniprep instrument with 100 W, 5 × 10 s pulses and short pauses in between; these cells were kept on ice for 1 h. The resulting lysate was centrifuged at 100,000× g at 4 °C for 1 h to remove cell debris. Finally, the precipitate was resuspended in 5 volumes of 50 mM Tris·HCl buffer (pH 7.4) and stored at −80 °C. The protein concentration of the suspension was measured according to the Bradfor method. (2) The soluble proteomes prepared from HepG2 cells were diluted to 2.0 mg/mL with 50 mM Tris·HCl buffer (pH 7.4). The labeling reation was initiated by incubating proteomes with the probe (dissolved in DMSO) at 4 °C for 8 h, and then exposing them to UV 365 nm (220 v, 6 W, 365 nm) at a distance of 3 cm. The reaction mixture was centrifuged at 48,000× g for 10 min, and then the supernatant was removed, and the precipitate was resuspended in a lysis buffer (urea 480 mg/L, chaps 40 mg/L, Tris-base 4.8 mg/L, DTT 10 mg/L, Ampholate 50 mL/L, and bromophenol blue 0.002%) at 4 °C for 1 h and centrifuged at 18,000× g for 2 h. The supernatant was dialyzed against 50 mM Tris·HCl buffer (pH 7.4). To detect the proteins photo-cross-linked by test probe, the homogenate photolabeled with the probe was subjected to SDS-PAGE electrophoresis and then transferred onto a polyvinylidene fluoride membrane. The membrane was blocked with 1% BSA in PBS with 0.05% Tween 20 at room temperature for 1h, washed using PBS with 0.05% Tween 20 for 5 min two times, and then incubated with a streptavidin-HRP polymer conjugate (Sigma, Cat. S 2438) diluted 1:10,000 in PBS with 0.05% Tween 20 at room temperature for 1 h. After six washes for 5 min each using PBS with 0.05% Tween 20, the membrane was treated with Enhanced Chemiluminescence (ECL, Amersham, Boston, MA, USA) detection reagents. Biotinylated proteins were visualized by exposure to X-ray and imaging development.
3.4. Protein Digestion and Mass Spectrometry
(1) The 170 kDa band was excised from the blot and processed for internal sequence analysis as described [13]. Briefly, in situ digestion was done using 0.01 μg/μL trypsin (in 100 μL of 25 mM NH4HCO3) for 30 min at 4 °C, until the trypsin was completely absorbed by the gel particles. Then, the resulting mixture was added to 100 μL of 25 mM NH4HCO3, the supernatant was collected by centrifugation and transfered in another Eppendorf tube. The extraction buffer (5% TFA, 95% ddH2O) was added into the pellets for 1 h, and then treated with an extraction buffer (2.5% TFA, 50% ACN, 47.5% ddH2O) for 1 h and stored at −20 °C. (2) Mass Spectrometry analysis was carried out using the Tanon-6100 Chemiluminescent Imaging system (Tanon Science and technology Co., Ltd., Shanghai, China). The band densities were calculated with Quantity One software 4.6.2 (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The excised gel was digested with trypsin and subjected to LC-MS/MS. The data were recorded on an LTQ-Orbitrap Fusion mass spectrometer (ThermoFisher Scientific Inc., Waltham, MA, USA) coupled to an Easy-nLC 1000 LC System (ThermoFisher Scientific Inc., Waltham, MA, USA). Label-free MS analysis was performed by Thermo Q-Exactive mass spectrometry, and the MS raw data were processed using MaxQuant software with the Uniprot-Orytolagus cuniculus database.
4. Conclusions
In summary, this work described the design and synthesis of novel photoaffinity probes of dihydropyridine derivatives, BAY R3401, and evaluated their potency in an inhibition assay of glycogenolysis. Probe 2d exhibited the best inhibitory activity, with an IC50 value of 4.45 μM and 28.49 μM in primary human liver HL-7702 cells and HepG2 cells, respectively. Photoaffinity labeling experiments were also performed, and protein bands larger than 170 kDa were tagged by probe 2d in crude proteomes. These data suggest that the synthesized probe 2d might be used to label, identify, and purify the potential target enzymes of BAY R3401. Mass spectrometric analysis was used to detect protein bands larger than 170 kDa, and we hypothesise that the PH-interacting proteins, histone H4, hexokinase-2, and solute carrier family 2 might be the target proteins of BAY R3401 based on the results. We are now in the process of verifying these proteins.
Supplementary Materials
Supplementary materials associated with this article are available online. Contents: Data of Coomassie brilliant blue (CBB) and NMR spectra of the prepared compounds.
Author Contributions
Conceived and designed the study: L.Z. and Z.Y. Performed the experiments: L.Z., Z.Y., Y.W., C.S., and G.M. Analyzed the data: Z.Y. and Y.W. Contributed reagents/materials/analysis tools: L.Z., Z.Y., Y.W., C.S., and G.M. Wrote the manuscript: L.Z. and Z.Y.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81473101 and 81001401), the Natural Science Foundation of Hebei Province (No. H2017406049), and Key Program of Education Department of Hebei Province (No. ZD2018080).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Goldmann S., Ahr H., Puls W., Bischoff H., Petzinna D., Schlossmann K., Ben-der J. Dihydropyridine compounds and their use in reducing blood sugar. 4,786,641. U.S. Patent. 1988 Nov 22;
- 2.Kurukulasuriya R., Link J.T., Madar D.J., Pei Z., Richards S.J., Rohde J.J., Souers A.J., Szczepankiewicz B.G. Potential drug targets and progress towards pharmacologic inhibition of hepatic glucose production. Curr. Med. Chem. 2003;10:123–153. doi: 10.2174/0929867033368556. [DOI] [PubMed] [Google Scholar]
- 3.Kato A., Nasu N., Takebayashi K., Adachi I., Minami Y., Sanae F., Asano N., Watson A.A., Nash R.J. Structure-activity relationships of flavonoids as potential inhibitors of glycogen phosphorylase. J. Agric. Food Chem. 2008;56:4469–4473. doi: 10.1021/jf800569s. [DOI] [PubMed] [Google Scholar]
- 4.Bergans N., Stalmans W., Goldmann S., Vanstapel F. Molecular mode of inhibition of glycogenolysis in rat liver by the dihydropyridine derivative, BAY R3401: Inhibition and inactivation of glycogen phosphorylase by an activated metabolite. Diabetes. 2000;49:1419–1426. doi: 10.2337/diabetes.49.9.1419. [DOI] [PubMed] [Google Scholar]
- 5.Sakurai K., Hiraizumi M., Isogai N., Komatsu R., Shibatal T., Ohta Y. Synthesis of a fluorescent photoaffinity probe of OSW-1 by site-selective acylation of an inactive congener and biological evaluation. Chem. Commun. 2017;53:517–520. doi: 10.1039/C6CC08955K. [DOI] [PubMed] [Google Scholar]
- 6.Pan S., Zhang H., Wang C., Yao S.C., Yao S.Q. Target identification of natural products and bioactive compounds using affinity-based probes. Nat. Prod. Rep. 2016;33:612–620. doi: 10.1039/C5NP00101C. [DOI] [PubMed] [Google Scholar]
- 7.Smith E., Collins I. Photoaffinity labeling in target - and binding-site identification. Future Med. Chem. 2015;7:159–183. doi: 10.4155/fmc.14.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogawa A.K., Willoughby C.A., Bergeron R., Ellsworth K.P., Geissler W.M., Myers R.W., Yao J., Harris G., Chapman K.T. Glucose-lowering in a db/db mouse model by dihydropyridine diacid glycogen phosphorylase inhibitors. Bioorg. Med. Chem. Lett. 2003;13:3405–3408. doi: 10.1016/S0960-894X(03)00798-4. [DOI] [PubMed] [Google Scholar]
- 9.Rowland M.M., Bostic H.E., Gong D., Speers A.E., Lucas N., Cho W., Cravatt B.F., Best M.D. Phosphatidylinositol 3,4,5-trisphosphate activity probes for the labeling and proteomic characterization of protein binding partners. Biochemistry. 2011;50:11143–11161. doi: 10.1021/bi201636s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L., Zhang Y., Dong J., Liu J., Zhang L., Sun H. Design and synthesis of novel photoaffinity probes for study of the target proteins of oleanolic acid. Bioorg. Med. Chem. Lett. 2012;22:1036–1039. doi: 10.1016/j.bmcl.2011.11.123. [DOI] [PubMed] [Google Scholar]
- 11.Martin W.H., Hoover D.J., Armento S.J., Stock I.A., McPherson R.K., Danley D.E., Stevenson R.W., Barrett E.J., Treadway J.L. Discovery of a human liver glycogen phosphorylase inhibitor that lowers blood glucose in vivo. Proc. Natl. Acad. Sci. 1998;95:1776–1781. doi: 10.1073/pnas.95.4.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olivo H.F., Perez-Hernandez N., Liu D., Iruthayanathan M., O’Leary B., Homan L.L., Dillon J.S. Synthesis and application of a photoaffinity analog of dehydroepiandrosterone (DHEA) Bioorg. Med. Chem. Lett. 2010;20:1153–1155. doi: 10.1016/j.bmcl.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erdjument-Bromage H., Lui M., Lacomis L., Grewal A., Annan R.S., McNulty D.E., Carr S.A., Tempst P. Examination of micro-tip reversed-phase liquid chromatographic extraction of peptide pools for mass spectrometric analysis. J. Chromatogr. A. 1998;826:167–181. doi: 10.1016/S0021-9673(98)00705-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.