Abstract
Background
Despite the global distribution of the intestinal protozoan Dientamoeba fragilis, its clinical picture remains unclear. This results from underdiagnosis: microscopic screening methods either lack sensitivity (wet preparation) or fail to reveal Dientamoeba (formalin-fixed sample).
Aim
In a retrospective study setting, we characterised the clinical picture of dientamoebiasis and compared it with giardiasis. In addition, we evaluated an improved approach to formalin-fixed samples for suitability in Dientamoeba diagnostics.
Methods
This study comprised four parts: (i) a descriptive part scrutinising rates of Dientamoeba findings; (ii) a methodological part analysing an approach to detect Dientamoeba-like structures in formalin samples; (iii) a clinical part comparing demographics and symptoms between patients with dientamoebiasis (n = 352) and giardiasis (n = 272), and (iv) a therapeutic part (n = 89 patients) investigating correlation between faecal eradication and clinical improvement.
Results
The rate of Dientamoeba findings increased 20-fold after introducing criteria for Dientamoeba-like structures in formalin-fixed samples (88.9% sensitivity and 83.3% specificity). A further increase was seen after implementing faecal PCR. Compared with patients with giardiasis, the symptoms in the Dientamoeba group lasted longer and more often included abdominal pain, cramping, faecal urgency and loose rather than watery stools. Resolved symptoms correlated with successful faecal eradication (p < 0.001).
Conclusions
Previously underdiagnosed, Dientamoeba has become the most frequently recorded pathogenic enteroparasite in Finland. This presumably results from improved diagnostics with either PCR or detection of Dientamoeba-like structures in formalin-fixed samples, an approach applicable also in resource-poor settings. Symptoms of dientamoebiasis differ slightly from those of giardiasis; patients with distressing symptoms require treatment.
Keywords: Dientamoeba fragilis, intestinal, parasite, protozoan, Giardia, dientamoebiasis
Introduction
Despite the worldwide distribution of the enteroparasite Dientamoeba fragilis, its prevalence and clinical significance have remained obscure [1,2]. Spread by the faecal-oral route appears most likely [2]. Recently, a cyst stage was discovered [3,4] and its transmission – and association of the protozoan with symptomatic disease – was shown in a rat model [3,4]. Acquisition together with pinworms has also been suggested [1].
Historically, Dientamoeba has often remained undetected because of shortcomings of the stool parasite screening methods generally used: formalin-fixed samples were not considered applicable to detecting Dientamoeba and wet preparation has poor sensitivity [5]. Instead, diagnosis has required microscopy of specifically stained fresh stool samples (trichrome or modified iron-haematoxylin staining) [6-9] and clinicians familiar with the protozoan knowing how to search for it. Today, PCR methods have become available in many laboratories. The advantages of these methods are obvious, but results from studies using them vary considerably: prevalences between 0.2% and 71% have been reported [2], and some commercial assays have been shown to misidentify the animal protozoan Tritrichomonas foetus as Dientamoeba [10]. Nevertheless, now that the PCR methods have been widely adopted in the more affluent parts of the world, the reported prevalence of Dientamoeba is higher than when the traditional microscopical methods were used [2]. The main reasons for debating the pathogenicity of Dientamoeba are its high prevalence in some studies [2] and the large proportion of asymptomatic carriage ranging from 11% [11] to 39% [12].
The most frequently recorded symptoms include abdominal pain and diarrhoea or loose stools [13]; a chronic course lasting up to several years has been reported for 2% [14] to 32% [15] of the patients. Many studies have shown an association between clinical improvement and faecal eradication [2,11,16-19], while the results for children have been conflicting [20-22]. Greater virulence of specific Dientamoeba strains has been suggested to account for symptomatic disease. However, recent investigations suggest that there are only two major clonal lines of Dientamoeba worldwide, one much more common than the other, which rather points to host characteristics bringing about variation in the clinical picture [23].
In Finland, clinical experience of dientamoebiasis has been accrued since 2007, when our laboratory commenced informing clinicians of Dientamoeba-like structures detected in formalin samples, urging them to send in additional trichrome-stained samples. This led to an increase in the number of new findings. Later, in 2017, a multiplex PCR method for protozoan parasites (Dientamoeba fragilis, Giardia lamblia, Cryptosporidium parvum, Entamoeba histolytica) was implemented in clinical practice (Amplidiag Stool Parasites test, Mobidiag Ltd, Espoo, Finland) [24]. Spurred by the rise in Dientamoeba findings, we conducted a study depicting this development, presenting the formalin sample approach, analyses of demographics and clinical picture, and microbiological and clinical cure rates after antimicrobial therapy.
Methods
Study outline
We conducted a retrospective study of the diagnostics and clinical picture of dientamoebiasis. To this end, we collected data on microbiological results and clinical symptoms of patients with a positive finding in clinical faecal samples examined for Dientamoeba or Giardia in the period from January 2007 to March 2012 at Helsinki University Hospital Laboratory (HUSLAB).
The investigation comprised four parts: (i) a descriptive part relating the annual numbers of new Dientamoeba findings from 2007 to 2017; (ii) a methodological part presenting an approach to identify Dientamoeba in formalin-fixed samples and comparing the results to those from the same patients’ trichrome samples; (iii) a clinical part comparing the demographics and clinical picture between patients with dientamoebiasis and those with giardiasis; (iv) a therapeutic part analysing faecal eradication and clinical outcome after antiparasitic treatment.
The HUSLAB database was retrospectively searched for Dientamoeba and Giardia entries dated between January 2007 and March 2012.
The diagnosis of dientamoebiasis was based on positive faecal sample in microscopy after fixation with Ecofix (Meridian Bioscience, Inc., Cincinnati, United States) and modified trichrome staining [25]. In 2017, a multiplex PCR for intestinal protozoa was adopted in routine use and therefore, in the descriptive part of the study, also positive findings by PCR were covered.
Diagnosis of giardiasis was verified by microscopy of formalin-fixed faecal sample, by microscopy of Ecofix-fixed and trichrome-stained faecal sample or by positive antigen test (ProSpecT Giardia/Cryptosporidium Microplate Assay, Oxoid Ltd, Basingstoke, United Kingdom).
Patients with a sample positive for Dientamoeba in trichrome staining were included in the Dientamoeba group and those with a positive sample in Giardia diagnostics comprised the Giardia group. To obtain a roughly equal number of patients in both groups, initially only the first five positive results per month were selected for the Giardia group. Later, in 2009, when Dientamoeba findings exceeded Giardia findings, all positive results were recorded in both groups.
Ethical statement
According to the Finnish Medical Research Act, review by an ethics committee is only required for research involving intervention. The study protocol was approved by the research boards of the Inflammation Center and the regional laboratory of HUSLAB, Helsinki University Hospital (HUH), and the Department of Social Services and Health Care, City of Helsinki.
Methodological part of the study
Formalin-fixed samples were prepared as follows: faecal samples (2–3 g) were fixed with 10 mL of 10% formalin, filtered through a cheese cloth to remove large debris and concentrated by the standard formalin-ethyl acetate method [26]. Approximately 25 μL of pellet was stained with an equal amount of Lugol’s iodine and examined using bright field microscopy by skilled laboratory personnel at 100× and 400× magnification for 3–5 minutes. In one positive sample, we generally identified several Dientamoeba-like structures.
The criteria for Dientamoeba-like structures in formalin-fixed samples were as follows: shape slightly flexible, structure round or oval, diameter 8–15 µm; surrounded by a thin cell membrane with no resemblance to Entamoeba cysts; coarseness of cytoplasm fine to medium; nuclei (if visible), when stained by iodine, dot-like. The structures looked jumbled because of vacuoles and multiple granules in the cytoplasm.
For photography, Lugol’s iodine solution was added (1:1) and mobility of sample was reduced by adding an equal amount of the fluid and acrylamide solution before polymerisation (40% acrylamide containing 0.5% ammonium persulfate and 0.25% TEMED). The samples were photographed using a 40× objective in a LEICA DM6000 microscope equipped with a LEICA DM2900 camera.
To determine the sensitivity and specificity of detecting Dientamoeba in formalin-fixed specimens, the results from them were compared with the same patients’ trichrome-stained samples. The samples of both kinds had either been taken at the same time or the trichrome sample shortly afterwards, on the laboratory’s recommendation prompted by findings in the formalin-fixed sample. For the sake of objectivity, we only included in the analyses formalin-fixed samples investigated at least 1 day earlier than the same patient’s trichrome samples (gold standard).
Clinical part of the study
Clinical data
Demographic data, results of faecal microbiological tests and clinical data were retrieved from the electronic patient charts of HUH and Helsinki City healthcare units. The demographic information comprised sex, age and chronic diseases. Symptoms were recorded as reported in patient charts by the clinicians. Data on treatment were collected only for the Dientamoeba group.
Additional microbiological data, when available, were collected on faecal pathogens (cultures for Salmonella spp., Yersinia spp., Campylobacter spp. and Shigella spp.; culture and toxin test for Clostridium difficile; enteric worms and ova (formalin-fixed samples); Cryptosporidium spp. (formalin-fixed modified Ziehl-Neelsen staining or antigen test ProSpecT Giardia/Cryptosporidium Microplate Assay, Oxoid Ltd, Basingstoke, United Kingdom); Entamoeba histolytica (Entamoeba Celisa Path Test Kit, CeLLabs Pty Ltd, Sydney, Australia); Enterobius vermicularis (microscopy of a perianal cotton swab)). Findings of apathogenic faecal microbes, including Blastocystis hominis, were recorded separately. Samples screened because of indications other than gastrointestinal symptoms were recorded separately.
Patients with findings positive for other pathogens were excluded. Lack of diagnostic samples for other pathogens did not lead to exclusion since, as opposed to Dientamoeba [13], bacterial and viral pathogens are not common as causes of prolonged intestinal complaints [27,28]. The number of patients with missing samples was recorded.
Treatment
Our analysis covered the first course of medication, and only patients with at least two control samples taken 2 weeks or more after completing the course were selected. Only data from patients treated with doxycycline, metronidazole, paromomycin or secnidazole – the alternatives recommended in the Finnish guidelines concerning treatment of dientamoebiasis – were included. Data on dosage and regimen duration were recorded when available, yet absence of such data did not result in exclusion because the recommended regimen is uniform in the Finnish guidelines.
Microbiological and clinical outcomes were recorded separately. Faecal clearance was evaluated by findings in trichrome-stained samples. Two or more negative control samples were classified as microbiological success, but even one positive control sample sufficed for interpretation as microbiological failure. Clinical outcome was judged by symptoms recorded in the patient charts before and after antiparasitic treatment. Clinical success was defined as complete or partial relief of symptoms. In our clinical experience, as for giardiasis [29], it is not uncommon for dientamoebiasis symptoms to be partly relieved, with full clinical recovery only occurring weeks after a successful faecal eradication.
Exclusion criteria
Patients were excluded from the methodological analysis if they had not provided either faecal sample, formalin-fixed or trichrome-stained, or if their trichrome samples had been investigated before their formalin samples. As for the clinical part, patients were excluded because of (i) a verified positive finding of some other intestinal pathogen(s), (ii) missing patient history, (iii) a previously diagnosed active chronic gastrointestinal disease (e.g. colitis ulcerosa) with or without exacerbation or (iv) samples taken as part of routine screening (e.g. recent immigrants). Patients were excluded from post-treatment analysis (i) if they had submitted less than two control samples or the specimens had been taken too early (during the first 2 weeks after completing the treatment), (ii) if they had received some drug other than doxycycline, metronidazole, paromomycin or secnidazole, (iii) if they had been asymptomatic before the treatment, or (iv) if their post-treatment clinical data was missing.
Statistical analysis
For medians, the range and interquartile range (IQR) were determined. Means with standard deviations and ranges were calculated. For continuous variables, appropriate tests were used (independent samples t-test or Mann–Whitney U-test). For categorical variable analyses, the chi-square test was applied. IBM SPSS Statistics (versions 21 to 24) software was used in statistical analyses.
Results
Rates of Dientamoeba findings
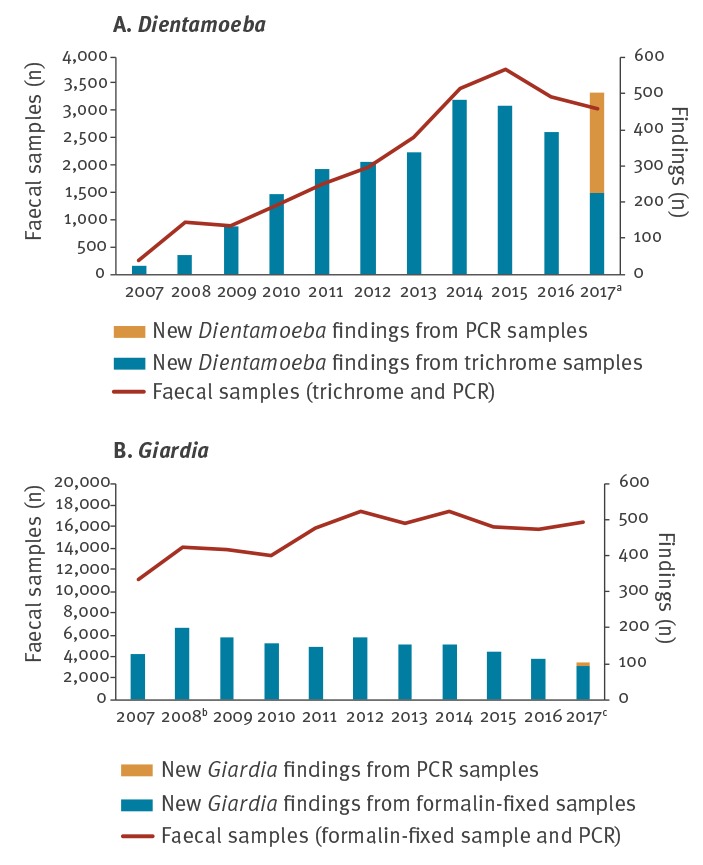
After adopting the formalin-fixed approach into detection of Dientamoeba in 2007, the number of positive trichrome samples multiplied. A steady 20-fold increase in new Dientamoeba findings was seen between 2007 and 2017 (Figure 1A), whereas the number of Giardia findings remained constant (Figure 1B). Multiplex PCR for Dientamoeba fragilis, Giardia lamblia, Cryptosporidium parvum and Entamoeba histolytica was implemented at the beginning of April 2017 and a 28% annual increase in new Dientamoeba findings was seen in 2017.
Figure 1.
Annual numbers of new Dientamoeba and Giardia findings and number of faecal samples investigated at HUSLAB, Helsinki, Finland 2007–2017 (n = 189,723 samples)
HUSLAB: Helsinki University Hospital laboratory.
a All specimens from 2007–16 were trichrome samples. In 2017, there were a total of 3,065 faecal specimens, consisting of 1,187 PCR and 1,878 trichrome samples.
b The peak in new Giardia findings in 2008 was due to a sewage epidemic in the town of Nokia, Finland [40].
c All specimens from 2007–16 were formalin samples. In 2017, there were a total of 16,422 faecal specimens, comprising 15,235 formalin and 1,187 PCR samples.
Subject groups
During the period from January 2007 to March 2012, we collected a total of 802 Dientamoeba and 849 Giardia findings from the HUSLAB database (see Methods about equal numbers in the patient groups). Detailed information of these entries was available for 44% (352/802) of the Dientamoeba and 32% (272/849) of the Giardia patients. Other pathogenic microbes were observed in 6% (20/352) of the Dientamoeba and 14% (39/272) of the Giardia entries, and apathogenic parasites in 69% (244/352) and 57% (155/272), respectively; a co-infection of Dientamoeba and Giardia was recorded for 11 patients (Supplementary Table S1).
After exclusions (Figure 2), the Dientamoeba group comprised 319 and the Giardia group 160 patients. Apathogenic parasites were identified in the specimens, respectively, of 61% (196/319) and 49% (78/160) of the patients scrutinised, and Blastocystis hominis proved the most common apathogen in both groups: 54% (172/319) and 43% (69/160), respectively. Table 1 and Supplementary Table S1 show the number of patients from whom the different faecal pathogens had been analysed and the proportions of positive findings in the microbiological tests among the two final subject groups.
Figure 2.
Study design for a retrospective investigation of patients with positive stool findings of Dientamoeba and Giardia, Helsinki Metropolitan Area, Finland, 2007–2012 (n = 624)
a Patients with both formalin and trichrome samples.
b Exclusion because of: findings of other concomitant pathogens (n = 20), initial diagnosis from immigration screening (n = 6), case history missing (n = 5) or active gastrointestinal disease (n = 2).
c Exclusion because of: findings of other concomitant pathogens (n = 39), initial diagnosis from immigration screening (n = 55), case history missing (n = 17) or active gastrointestinal disease (n = 1).
d Exclusion because of: no data available on treatment (n = 19), less than two faecal control samples collected or control samples taken too early (n = 172), antimicrobial drug other than doxycycline, metronidazole, paromomycin or secnidazole (n = 5), post-treatment clinical data missing (n = 14) or asymptomatic before treatment (n = 20).
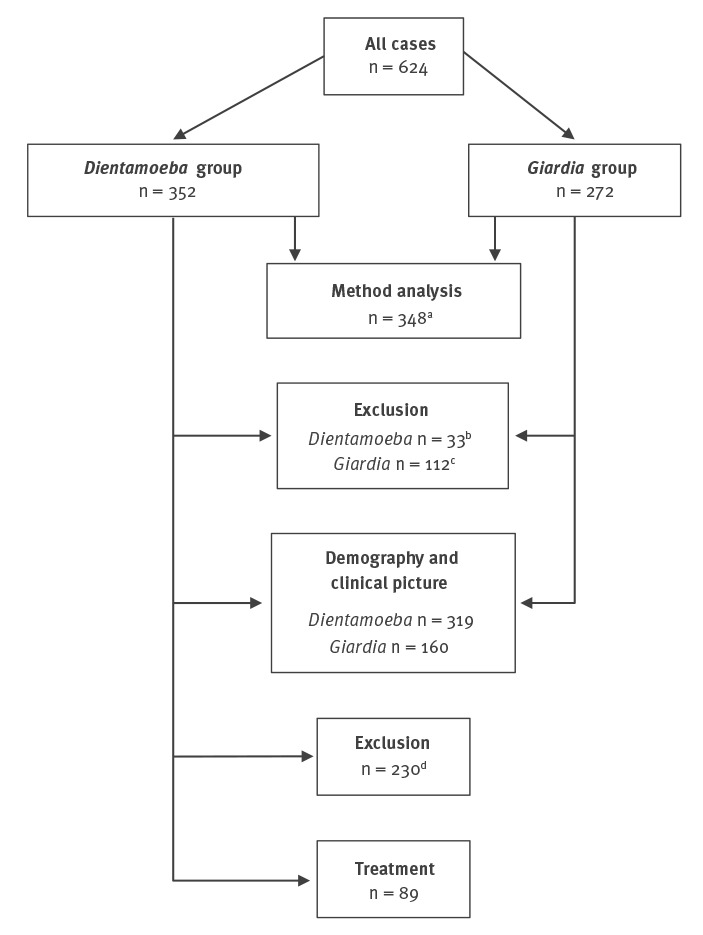
Table 1. Number of faecal samples and results of analyses among patients with dientamoebiasis and giardiasis, Helsinki Metropolitan Area, Finland, 2007–2012 (n = 479).
| Faecal samples | Dientamoeba (n = 319) | Giardia (n = 160) | ||
|---|---|---|---|---|
| n | % of patients | n | % of patients | |
| Formalin-fixed samplea | ||||
| Patients with sample | 289 | 91 | 159 | 99 |
| Dientamoeba-positive | 251 | 79 | 0 | 0 |
| Giardia-positive | 0 | 0 | 156 | 98 |
| Trichrome sample | ||||
| Patients with sample | 319 | 100 | 20 | 13 |
| Dientamoeba-positive | 319 | 100 | 0 | 0 |
| Giardia-positive | 0 | 0 | 18 | 11 |
| Giardia/Cryptosporidium antigen testb | ||||
| Patients with sample | 54 | 17 | 37 | 23 |
| Giardia-positive | 0 | 0 | 25 | 16 |
| Cryptosporidium-positive | 0 | 0 | 0 | 0 |
| Enterobiasis cotton swab | ||||
| Patients with sample | 32 | 10 | 13 | 8 |
| Enterobiasis-positive | 6 | 2 | 8 | 5 |
| Faecal bacterial culture | ||||
| Patients with sample | 166 | 52 | 111 | 69 |
| Sample positive for pathogensc | 0 | 0 | 0 | 0 |
| Clostridium samplesd | ||||
| Patients with sample | 68 | 21 | 27 | 17 |
| Clostridium-positive | 0 | 0 | 0 | 0 |
| Entamoeba histolytica samplee | ||||
| Patients with sample | 40 | 13 | 10 | 6 |
| E. histolytica-positive | 0 | 0 | 0 | 0 |
a Dientamoeba positivity is indicated by Dientamoeba-like structures observed in microscopy of formalin sample.
b Formalin-fixed modified Ziehl-Nielsen staining or antigen test (ProSpecT Giardia/Cryptosporidium Microplate Assay, Oxoid Ltd, Basingstoke, United Kingdom).
c Culture for Salmonella spp., Yersinia spp., Shigella spp. and Campylobacter spp.
d Culture and toxin test.
e Entamoeba histolytica (Entamoeba Celisa Path Test Kit, CeLLabs Pty Ltd, Sydney, Australia).
The demographics were similar for both groups (Table 2): 49% (157/319) of the Dientamoeba patients and 54% (86/160) of the Giardia patients were female; the age medians were 29 years (IQR: 8–47; range: 1–82 and 31 years (IQR: 20–45; range: 1–76), respectively. The two groups had similar age distributions. An underlying chronic disease was reported for 35% (113/319) of the Dientamoeba patients and 32% (51/160) of the Giardia patients.
Table 2. Demographics of patients with dientamoebiasis and giardiasis, Helsinki Metropolitan Area, Finland, 2007–2012.
| Characteristics | Dientamoeba (n = 319) | Giardia (n = 160) | p value |
|---|---|---|---|
| Sex (n) | |||
| Male | 162 | 74 | 0.349a
|
| Female | 157 | 86 | |
| Age (years) | |||
| Median | 29 | 31 | 0.266b
|
| IQR | 8–47 | 20–45 | |
| Range | 1–81 | 1–76 | |
| Age groups (n) | |||
| 0–6 | 64 | 17 | |
| 7–15 | 66 | 17 | |
| 16–29 | 32 | 41 | |
| 30–49 | 85 | 58 | |
| 50–69 | 61 | 20 | |
| ≥ 70 | 11 | 7 | |
| Chronic diseases (n) | 113 | 51 | 0.476a |
IQR: interquartile range.
a Chi-square test.
b Mann–Whitney U-test.
Dientamoeba-like structures in formalin-fixed samples: specificity and sensitivity
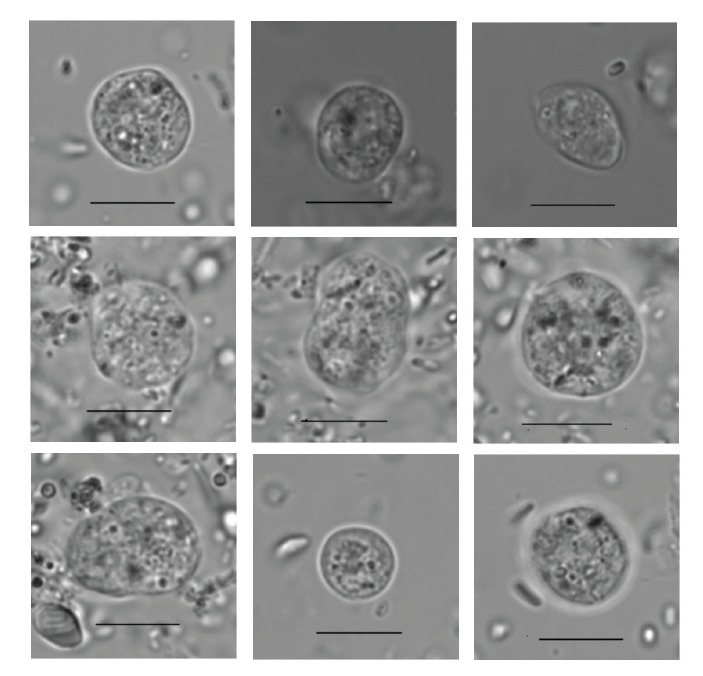
Figure 3 shows nine representative formalin-fixed samples with Dientamoeba-like structures from 8.5 to 17 μm in diameter, round or oval in shape, and with a thin cellular wall. Inside them there were variable numbers (two to six) of darker staining areas with a diameter of ca 0.5 to 1.0 μm. These intracellular structures had a surrounding halo; their identity has not been confirmed, but some of them might be nuclei of the organism. For the formalin sample to be interpreted as positive for Dientamoeba-like structures, size and inner structures were the main criteria.
Figure 3.
Identification of Dientamoeba fragilis from formalin-fixed faecal samples, Helsinki Metropolitan Area, Finland, March 2015 (n = 8)
Nine panels showing representative Dientamoeba-like structures in formalin samples from eight patients with typical D. fragilis trophozoites in trichrome-stained faecal smears. The line indicating the scale in each panel corresponds to 10 µm. The largest Dientamoeba-like structures in the picture are seen in the middle panel of the second row and the left panel of the bottom row (13 × 17 and 12 × 17 µm).
A total of 363 patients (320 from the Dientamoeba and 43 from the Giardia group) had provided both formalin-fixed and trichrome-stained specimens. The trichrome sample had been examined before the formalin sample for 4% (15/363) of patients; these were excluded from further analyses. Judged by results of trichrome staining, 77% (268/348) of the formalin-fixed samples proved true Dientamoeba positives, 2% false positives (7/348), 11% false negatives (38/348), and 10% (35/348) true negatives. A sensitivity of 89% (95% confidence interval (CI): 85.2–92.1) and specificity of 83% (95% CI: 68.6–93.3) were recorded for formalin-fixed specimens in detecting Dientamoeba.
Symptoms
A total of 85% Dientamoeba and 88% Giardia patients had reported symptoms, most commonly diarrhoea, prolonged diarrhoea (lasting over 2 weeks), abdominal pain and flatulence, and abdominal swelling or discomfort (Table 3). The indications for stool sample screening among asymptomatic patients are given in Table 3. Dientamoeba patients differed from those with Giardia in reporting more frequently loose stools, abdominal pain, constipation and faecal urgency. Symptoms had persisted considerably longer in the Dientamoeba group before a correct diagnosis had been made.
Table 3. Clinical symptoms of patients with dientamoebiasis and giardiasis, Helsinki Metropolitan Area, Finland, 2007–2012 (n = 479).
| Symptoms | Dientamoeba (n = 319) | Giardia (n = 160) | p value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Symptomatic | 270 | 85 | 140 | 88 | NS |
| Diarrhoea (all) | 202 | 64 | 116 | 72 | 0.034 |
| Watery stools | 41 | 13 | 54 | 34 | < 0.001 |
| Loose stools | 145 | 46 | 55 | 34 | 0.023 |
| Both watery and loose stools | 16 | 5 | 7 | 4 | NS |
| Continuous diarrhoea ≥ 2 weeks | 160 | 50 | 100 | 63 | 0.011 |
| Abdominal pain and cramps | 175 | 55 | 72 | 45 | 0.043 |
| Flatus, abdominal swelling and discomfort | 104 | 33 | 72 | 45 | NS |
| Nausea | 40 | 13 | 46 | 29 | < 0.001 |
| Weight loss | 37 | 12 | 17 | 11 | NS |
| Vomiting | 30 | 9 | 19 | 12 | NS |
| Fatigue | 27 | 9 | 12 | 8 | NS |
| Fever | 27 | 9 | 24 | 15 | 0.025 |
| Constipation | 23 | 7 | 4 | 3 | 0.036 |
| Anal pruritus | 23 | 7 | 6 | 4 | NS |
| Faecal urgency | 14 | 4 | 0 | 0 | 0.026 |
| Bloody stools | 11 | 3 | 2 | 1 | NSa |
| Abnormally smelly stools | 8 | 3 | 2 | 1 | NSa |
| Heartburn | 2 | 1 | 2 | 1 | NSa |
| Duration of symptoms before diagnosis (days, median)b | 180 (IQR: 345; range: 2–3,650) | 45 (IQR: 106; range: 1–1,800) | < 0.001c | ||
IQR: interquartile range; NS: not significant.
a 2-sided Fisher’s Exact test.
b Data missing for 162 patients in the Dientamoeba group and for 97 patients in the Giardia group.
c Mann–Whitney U-test.
As for asymptomatic cases: 23 asymptomatic Dientamoeba and four asymptomatic Giardia cases were found when asymptomatic family members of symptomatic carriers were screened for intestinal parasites. Faecal samples of 18 Dientamoeba and 12 Giardia asymptomatic cases were examined for unknown reasons. Six Dientamoeba and four Giardia asymptomatic cases were detected when investigating the subjects’ peripheral venous eosinophilia. Two asymptomatic Dientamoeba cases were found in routine examination of faeces from faecal material donors.
Within the Dientamoeba group, abdominal pain and cramping had been reported more frequently among the patients younger than 18 years than among the adults (64% vs 49%, p = 0.006, chi-square test); flatulence, abdominal swelling and discomfort had been more commonly listed by the adults (20% vs 42%, p < 0.001, chi-square test).
Clinical success and parasite eradication
Of the 319 patients with Dientamoeba, 28% (89/319) were included in the therapeutic part of the study (Figure 2). Data on dosage and regimen are shown in Supplementary Table S2. The median time until the first control sample was 36 days (IQR: 29–62; range: 16–298) and until the second, it was 76 days (IQR: 40–132; range: 16–378) after completing treatment.
Of all Dientamoeba patients treated, clinical success had been reported by the physician for 66% (59/89) and eradication of the parasite from faeces was observed for 53% (47/89). Partial resolution of symptoms was seen among 25 of 59 patients with clinical success. Clinical success was more frequent among patients with successful eradication: 39 of 59 vs eight of 30, with p < 0.001 (chi-square test). The same result was seen in a subgroup analysis of adults (≥ 18 years: n = 63; p < 0.001, chi-square test), whereas in the subgroup of those under 18 years, clinical success did not correlate with eradication of parasites from stool (n = 26; p = 0.683, Fisher’s exact test). When comparing treatment success between the subgroups, faecal clearance was more common among adults than those under 18 years (59% vs 38%), yet the difference was not significant (p = 0.104, chi-square test). Clinical success was not found to differ between the two age groups (68% vs 62%; p = 0.624, chi-square test).
Discussion
This investigation yielded four noteworthy findings: (i) Dientamoeba fragilis can be detected in formalin samples with high sensitivity and specificity; (ii) the clinical picture of dientamoebiasis differs in several aspects from that of giardiasis; (iii) faecal clearance of the parasite is associated with alleviation of symptoms especially among the adult population; (iv) in 2017, Dientamoeba was the most common pathogenic parasitological finding in the Helsinki Metropolitan Area.
Formalin fixation approach in diagnostics
While microscopy of wet preparations enables detection of Dientamoeba with very low sensitivity [5], formalin-fixed samples have not allowed detection at all. In countries like Finland, where classical formalin-fixed samples have been used as the sole approach (until 2017) to screen for stool parasites, the practise has inevitably led to underdiagnostics of Dientamoeba. This is clearly evidenced by the 20-fold upsurge of new Dientamoeba findings over the 10-year-period after adopting in 2007 our novel approach to identify Dientamoeba-like structures in formalin-fixed samples. Indeed, the number of Dientamoeba findings exceeded Giardia already before the availability of the PCR methods in 2017. With a specificity of 83.3% and sensitivity of 88.9%, identification of Dientamoeba-like structures has proved a viable screening method even if – like all other parasitological microscopy – it requires a trained technician. Nevertheless, as a major advantage over PCR methodology in global settings, by identifying Dientamoeba in formalin-fixed samples, diagnostics can also be considerably improved in indigent regions where this is the sole approach available.
Demographics
Concurring with large epidemiological surveys from Denmark [30], the two largest age cohorts in the Dientamoeba group appeared to be daycare and school children, and 30- to 49-year-olds. This finding accords with the conception of Dientamoeba infecting especially daycare-aged children and those caring for them [31,32]. This was the first investigation to compare chronic illnesses between patients with dientamoebiasis and those with giardiasis; no significant differences were found.
Symptoms
As in previous studies [13,15], the Dientamoeba group was characterised by a prolonged course of disease (median 180 days). Low awareness of the disease among practitioners and diagnostic difficulties presumably accounted at least partly for such late diagnosis.
The most common symptoms in the Dientamoeba group were loose stools, abdominal pain and flatus or abdominal discomfort, all in accordance with earlier studies [2,13]. Our data confirmed previously reported differences in clinical presentations between dientamoebiasis and giardiasis [16]: stomach ache and loose stools were more frequent among Dientamoeba patients, while those with Giardia more often reported severe illness, watery diarrhoea and even fever. In contrast to a previous investigation [9], faecal urgency was more frequent in the Dientamoeba than the Giardia group: this may reflect colonic mucosal irritation induced by Dientamoeba [2], whereas Giardia is generally known to cause disease in the jejunum.
Treatment success
We evaluated the association between faecal clearance and clinical success. Our data showed a distinct correlation between the two (p < 0.001), consistent with several previous investigations [2,11,16-19]. However, this finding appears to contradict four recent paediatric studies reporting no association between eradication and symptom relief [20,21] or between Dientamoeba carriage and symptoms [33,34]. Indeed, scrutinising our data separately for subgroups of children and adults, a difference was revealed: parasitological clearance and clinical outcome were not found to be closely connected (p = 0.683) among children, while a significant correlation was observed (p < 0.001) for adults. This may simply reflect adults’ better skills in describing their symptoms.
The possibility that some of the treatment failures actually were reinfections cannot be ruled out. Transmission within families, especially in households with small children, appears common [35]. Data on family members were not collected, since they are rarely found in patient charts. Variation in failure rates has been shown between various regimens [36], demonstrating that a substantial part of treatment failures are not reinfections.
Doubts about the pathogenicity of D. fragilis are presumably related to diagnostic challenges and high proportion of asymptomatic carriers, especially among children [33,34]. In studies with no correlation between clinical and microbiological outcomes, the protozoan has been identified by PCR [20,21], the sensitive method enabling detection of minuscule amounts of microbes [37,38]. No data exist on positive correlation between asymptomatic individuals’ PCR findings and high CT values in PCR. In fact, we found no studies exploring the CT values among symptomatic adult patients without irritable bowel syndrome (IBS); studies applying IBS criteria are not valid for dientamoebiasis, since they only cover patients with a certain selection of symptoms (Rome III criteria). No correlation has been found between dientamoebiasis and IBS-type symptoms in previous investigations [39].
Limitations
The principal limitations reside in the retrospective study setting and the diagnostics: Firstly, other pathogens were not conclusively excluded from all patients. Viral enteropathogens and diarrhoeagenic Escherichia coli, for example, were not tested at HUSLAB during the study period. However, these pathogens typically cause acute watery diarrhoea [28,29], not the clinical picture characteristic of dientamoebiasis that we described. Secondly, in cases where a trichrome sample had not been taken, the exclusion of dientamoebiasis in the Giardia group relied on formalin-fixed samples. This should not be a crucial point: presuming a specificity of 83.3% for the formalin-fixed sample to identify Dientamoeba would give in the Giardia group 23 false-negative results at most. With 23 false-negative Dientamoeba patients, the co-infection rate of dientamoebiasis and giardiasis would be 9% (34/375) (Supplementary Table S1). Thirdly, the symptoms could only be reported according to what the clinician had recorded in the patient charts; this limitation applies to the Dientamoeba and Giardia groups alike. Finally, our study design did not allow estimating the proportion of asymptomatic subjects in the Dientamoeba group, which can be assumed to be high.
Conclusions
We present an increase in the number of Dientamoeba findings presumably stemming from implementation of a simple and inexpensive novel diagnostic approach to screen dientamoebiasis from formalin-fixed samples. This approach may prove valuable also – and especially – in low-income countries, where microscopy often remains the only diagnostic laboratory tool available. Already before the implementation of PCR methods, our approach revealed Dientamoeba to be much more common in the Helsinki Metropolitan Area than previously thought. In fact, the rate exceeds that of Giardia. As abdominal complaints are among the most common reasons for seeking medical care, active efforts are warranted to increase clinicians’ awareness about this pathogen as a cause of prolonged stomach disorders.
Acknowledgements
Jukka Ollgren, MSc is acknowledged for expert help in the statistical analyses.
Funding: J-PP: Finnish Medical Society Duodecim; AK: Paulo Foundation, SSAC Foundation, Finnish Governmental subsidy for Health Science Research. The sponsors did not contribute to the study design, the collection of data, analyses or interpretation of results.
Supplementary Data
Conflict of interest: None declared.
Authors’ contributions: Study concept and design: TM, HS, TSJ, AK; acquisition of materials: TM, ET, A-MK, LP; collection of data: J-PP; drafting of the first version of the manuscript: J-PP, AK; critical revision of the manuscript for important intellectual content: TM, HS, TSJ; statistical analysis: J-PP; obtained funding: J-PP, AK; technical support: ET, A-MK, LP; study supervision: TM, HS, TSJ, AK; approved final manuscript: all authors.
References
- 1. Clark CG, Röser D, Stensvold CR. Transmission of Dientamoeba fragilis: pinworm or cysts? Trends Parasitol. 2014;30(3):136-40. 10.1016/j.pt.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 2. Stark D, Barratt J, Chan D, Ellis JT. Dientamoeba fragilis, the neglected trichomonad of the human bowel. Clin Microbiol Rev. 2016;29(3):553-80. 10.1128/CMR.00076-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Munasinghe VS, Vella NG, Ellis JT, Windsor PA, Stark D. Cyst formation and faecal-oral transmission of Dientamoeba fragilis--the missing link in the life cycle of an emerging pathogen. Int J Parasitol. 2013;43(11):879-83. 10.1016/j.ijpara.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 4. Stark D, Garcia LS, Barratt JL, Phillips O, Roberts T, Marriott D, et al. Description of Dientamoeba fragilis cyst and precystic forms from human samples. J Clin Microbiol. 2014;52(7):2680-3. 10.1128/JCM.00813-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Gool T, Weijts R, Lommerse E, Mank TG. Triple Faeces Test: an effective tool for detection of intestinal parasites in routine clinical practice. Eur J Clin Microbiol Infect Dis. 2003;22(5):284-90. [DOI] [PubMed] [Google Scholar]
- 6. Stark D, Beebe N, Marriott D, Ellis J, Harkness J. Detection of Dientamoeba fragilis in fresh stool specimens using PCR. Int J Parasitol. 2005;35(1):57-62. 10.1016/j.ijpara.2004.09.003 [DOI] [PubMed] [Google Scholar]
- 7. Verweij JJ, Mulder B, Poell B, van Middelkoop D, Brienen EA, van Lieshout L. Real-time PCR for the detection of Dientamoeba fragilis in fecal samples. Mol Cell Probes. 2007;21(5-6):400-4. 10.1016/j.mcp.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 8. Stensvold CR, Nielsen HV. Comparison of microscopy and PCR for detection of intestinal parasites in Danish patients supports an incentive for molecular screening platforms. J Clin Microbiol. 2012;50(2):540-1. 10.1128/JCM.06012-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ken Dror S, Pavlotzky E, Barak M. Evaluation of the NanoCHIP Gastrointestinal Panel (GIP) test for simultaneous detection of parasitic and bacterial enteric pathogens in fecal specimens. PLoS One. 2016;11(7):e0159440. 10.1371/journal.pone.0159440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chan D, Barratt J, Roberts T, Phillips O, Šlapeta J, Ryan U, et al. Detection of Dientamoeba fragilis in animal faeces using species specific real time PCR assay. Vet Parasitol. 2016;227:42-7. 10.1016/j.vetpar.2016.07.025 [DOI] [PubMed] [Google Scholar]
- 11. Stark D, Barratt J, Roberts T, Marriott D, Harkness J, Ellis J. A review of the clinical presentation of dientamoebiasis. Am J Trop Med Hyg. 2010;82(4):614-9. 10.4269/ajtmh.2010.09-0478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holtman GA, Kranenberg JJ, Blanker MH, Ott A, Lisman-van Leeuwen Y, Berger MY. Dientamoeba fragilis colonization is not associated with gastrointestinal symptoms in children at primary care level. Fam Pract. 2017;34(1):25-9. 10.1093/fampra/cmw111 [DOI] [PubMed] [Google Scholar]
- 13. Barratt JL, Harkness J, Marriott D, Ellis JT, Stark D. A review of Dientamoeba fragilis carriage in humans: several reasons why this organism should be considered in the diagnosis of gastrointestinal illness. Gut Microbes. 2011;2(1):3-12. 10.4161/gmic.2.1.14755 [DOI] [PubMed] [Google Scholar]
- 14. Banik GR, Barratt JL, Marriott D, Harkness J, Ellis JT, Stark D. A case-controlled study of Dientamoeba fragilis infections in children. Parasitology. 2011;138(7):819-23. 10.1017/S0031182011000448 [DOI] [PubMed] [Google Scholar]
- 15. Stark D, Beebe N, Marriott D, Ellis J, Harkness J. Prospective study of the prevalence, genotyping, and clinical relevance of Dientamoeba fragilis infections in an Australian population. J Clin Microbiol. 2005;43(6):2718-23. 10.1128/JCM.43.6.2718-2723.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vandenberg O, Peek R, Souayah H, Dediste A, Buset M, Scheen R, et al. Clinical and microbiological features of dientamoebiasis in patients suspected of suffering from a parasitic gastrointestinal illness: a comparison of Dientamoeba fragilis and Giardia lamblia infections. Int J Infect Dis. 2006;10(3):255-61. 10.1016/j.ijid.2005.05.011 [DOI] [PubMed] [Google Scholar]
- 17. Kurt O, Girginkardeşler N, Balcioğlu IC, Ozbilgin A, Ok UZ. A comparison of metronidazole and single-dose ornidazole for the treatment of dientamoebiasis. Clin Microbiol Infect. 2008;14(6):601-4. 10.1111/j.1469-0691.2008.02002.x [DOI] [PubMed] [Google Scholar]
- 18. Vandenberg O, Souayah H, Mouchet F, Dediste A, van Gool T. Treatment of Dientamoeba fragilis infection with paromomycin. Pediatr Infect Dis J. 2007;26(1):88-90. 10.1097/01.inf.0000247139.89191.91 [DOI] [PubMed] [Google Scholar]
- 19. Girginkardeşler N, Coşkun S, Cüneyt Balcioğlu I, Ertan P, Ok UZ. Dientamoeba fragilis, a neglected cause of diarrhea, successfully treated with secnidazole. Clin Microbiol Infect. 2003;9(2):110-3. 10.1046/j.1469-0691.2003.00504.x [DOI] [PubMed] [Google Scholar]
- 20. Röser D, Simonsen J, Stensvold CR, Olsen KE, Bytzer P, Nielsen HV, et al. Metronidazole therapy for treating dientamoebiasis in children is not associated with better clinical outcomes: a randomized, double-blinded and placebo-controlled clinical trial. Clin Infect Dis. 2014;58(12):1692-9. 10.1093/cid/ciu188 [DOI] [PubMed] [Google Scholar]
- 21. de Jong MJ, Korterink JJ, Benninga MA, Hilbink M, Widdershoven J, Deckers-Kocken JM. Dientamoeba fragilis and chronic abdominal pain in children: a case-control study. Arch Dis Child. 2014;99(12):1109-13. 10.1136/archdischild-2014-305942 [DOI] [PubMed] [Google Scholar]
- 22. Ögren J, Dienus O, Löfgren S, Einemo IM, Iveroth P, Matussek A. Dientamoeba fragilis prevalence coincides with gastrointestinal symptoms in children less than 11 years old in Sweden. Eur J Clin Microbiol Infect Dis. 2015;34(10):1995-8. 10.1007/s10096-015-2442-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cacciò SM. Molecular epidemiology of Dientamoeba fragilis. Acta Trop. 2018;184:73-7. 10.1016/j.actatropica.2017.06.029 [DOI] [PubMed] [Google Scholar]
- 24. Pelkonen T, Dos Santos MD, Roine I, Dos Anjos E, Freitas C, Peltola H, et al. Potential diarrheal pathogens common also in healthy children in Angola. Pediatr Infect Dis J. 2018;37(5):424-8. 10.1097/INF.0000000000001781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garcia LS, Shimizu RY. Evaluation of intestinal protozoan morphology in human fecal specimens preserved in EcoFix: comparison of Wheatley’s trichrome stain and EcoStain. J Clin Microbiol. 1998;36(7):1974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia LS, Bruckner DA. Diagnostic Medical Parasitology. 5th ed. Washington, DC: American Society of Microbiology; 2007. [Google Scholar]
- 27. DuPont HL. Persistent diarrhea: a clinical review. JAMA. 2016;315(24):2712-23. 10.1001/jama.2016.7833 [DOI] [PubMed] [Google Scholar]
- 28. DuPont HL. Clinical practice. Bacterial diarrhea. N Engl J Med. 2009;361(16):1560-9. 10.1056/NEJMcp0904162 [DOI] [PubMed] [Google Scholar]
- 29. Escobedo AA, Hanevik K, Almirall P, Cimerman S, Alfonso M. Management of chronic Giardia infection. Expert Rev Anti Infect Ther. 2014;12(9):1143-57. 10.1586/14787210.2014.942283 [DOI] [PubMed] [Google Scholar]
- 30. Röser D, Simonsen J, Nielsen HV, Stensvold CR, Mølbak K. Dientamoeba fragilis in Denmark: epidemiological experience derived from four years of routine real-time PCR. Eur J Clin Microbiol Infect Dis. 2013;32(10):1303-10. 10.1007/s10096-013-1880-2 [DOI] [PubMed] [Google Scholar]
- 31. Ögren J, Dienus O, Löfgren S, Iveroth P, Matussek A. Dientamoeba fragilis DNA detection in Enterobius vermicularis eggs. Pathog Dis. 2013;69(2):157-8. 10.1111/2049-632X.12071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Röser D, Nejsum P, Carlsgart AJ, Nielsen HV, Stensvold CR. DNA of Dientamoeba fragilis detected within surface-sterilized eggs of Enterobius vermicularis. Exp Parasitol. 2013;133(1):57-61. 10.1016/j.exppara.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 33. Jokelainen P, Hebbelstrup Jensen B, Andreassen BU, Petersen AM, Röser D, Krogfelt KA, et al. Dientamoeba fragilis, a commensal in children in Danish day care centers. J Clin Microbiol. 2017;55(6):1707-13. 10.1128/JCM.00037-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heusinkveld M, Mughini-Gras L, Pijnacker R, Vennema H, Scholts R, van Huisstede-Vlaanderen KW, et al. Potential causative agents of acute gastroenteritis in households with preschool children: prevalence, risk factors, clinical relevance and household transmission. Eur J Clin Microbiol Infect Dis. 2016;35(10):1691-700. 10.1007/s10096-016-2714-9 [DOI] [PubMed] [Google Scholar]
- 35. Menéndez Fernández-Miranda C, Fernández-Suarez J, Rodríguez-Pérez M, Menéndez Fernández-Miranda P, Vázquez F, Boga Ribeiro JA, et al. Prevalence of D. fragilis infection in the household contacts of a group of infected patients. Enferm Infecc Microbiol Clin. 2018;36(7):423-7. 10.1016/j.eimc.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 36. van Gestel RS, Kusters JG, Monkelbaan JF. A clinical guideline on Dientamoeba fragilis infections. Parasitology. 2018;1-9. 10.1017/S0031182018001385 [DOI] [PubMed] [Google Scholar]
- 37. Gough R, Ellis J, Stark D. Comparison and Recommendations for the use of Dientamoeba fragilis Real-Time PCR assays. J Clin Microbiol. 2019;57(5):e01466-18. 10.1128/JCM.01466-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lääveri T, Antikainen J, Pakkanen SH, Kirveskari J, Kantele A. Prospective study of pathogens in asymptomatic travellers and those with diarrhoea: aetiological agents revisited. Clin Microbiol Infect. 2016;22(6):535-41. 10.1016/j.cmi.2016.02.011 [DOI] [PubMed] [Google Scholar]
- 39. Brands MR, Van de Vijver E, Haisma SM, Heida A, van Rheenen PF. No association between abdominal pain and Dientamoeba in Dutch and Belgian children. Arch Dis Child. 2019;104(7):686-9. 10.1136/archdischild-2018-316383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Laine J, Huovinen E, Virtanen MJ, Snellman M, Lumio J, Ruutu P, et al. An extensive gastroenteritis outbreak after drinking-water contamination by sewage effluent, Finland. Epidemiol Infect. 2011;139(7):1105-13. 10.1017/S0950268810002141 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


