Abstract
Screening for hepatopulmonary syndrome (HPS) using pulse oximetry is recommended in liver transplant (LT) candidates since mortality is increased, independent of the severity of the oxygenation defect. LT exception points may be afforded to those with HPS and severe hypoxemia. We assessed the screening characteristics of pulse oximetry for HPS. The Pulmonary Vascular Complications of Liver Disease 2 study is a multicenter, prospective cohort study of adults undergoing their first LT evaluation. Patients underwent protocolized assessment of oxygen saturation by pulse oximetry (SpO2), arterial blood gas, spirometry, and contrast echocardiography (CE). HPS was defined as an alveolar-arterial gradient ≥ 15 mm Hg (≥ 20 mm Hg if age > 64 years), intrapulmonary vascular dilatation on CE, and absence of lung disease. The study sample included 363 patients. Of these, 75 (20.7%, 95% CI 16.6 – 25.2%) met criteria for HPS. The area under the receiver operating characteristic curve (AUROC or c-statistic) for SpO2 in discriminating HPS was 0.59 (95% CI 0.51 – 0.66). A SpO2 < 96%, recommended by practice guidelines as a threshold to require further testing, had low sensitivity (28%, 95% CI 18 – 28%). The c-statistic of SpO2 in discriminating HPS with a partial pressure of oxygen (PaO2) < 60 mm Hg (eligible for LT exception points) was 0.76 (95% CI 0.46 – 1.00). A SpO2 cutoff of < 96% had higher sensitivity for detecting HPS with PaO2 < 60 mm Hg (71%, 95% CI 38 – 100%), but was still inadequate.
Conclusion:
Pulse oximetry is not sufficiently sensitive to screen for HPS in LT candidates. Arterial blood gas and CE are required in LT candidates for diagnosis of HPS.
Keywords: cirrhosis, hepatopulmonary syndrome, hypoxemia, portal hypertension, pulse oximetry
Introduction
Hepatopulmonary syndrome (HPS) is a manifestation of chronic liver disease and/or portal hypertension and is characterized by intrapulmonary vascular dilatation and an increased alveolar-arterial gradient (A-a gradient).(1) Present in up to one-third of all patients without other etiologies of lung disease who are evaluated for liver transplant (LT), HPS represents the most common pulmonary vascular complication of liver disease, though its true prevalence may be underestimated.(2–4) As defined in this study, HPS is associated with at least a two-fold increase in mortality, as demonstrated in large prospective cohort studies of patients with advanced liver disease.(2, 4) Importantly, this increase in risk of mortality is independent of the degree of the oxygenation defect and severity of liver disease.(5–7) HPS of any severity is associated with an increased risk of death.
Although there are no effective medical therapies for HPS, LT is curative in the majority of cases.(5–8) In the US, HPS patients with the most profound oxygenation defects are prioritized for LT, translating to an increased LT rate and improved survival compared to those LT candidates without HPS.(7, 9) At the time of LT evaluation, assessment of oxygen saturation by pulse oximetry (SpO2) is recommended by practice guidelines to determine whether a full evaluation for HPS should be initiated.(10, 11) A SpO2 < 96% was reported in a single center study to have a sensitivity and specificity of 100% and 88%, respectively, to detect HPS patients with a partial pressure of oxygen in arterial blood (PaO2) < 60 mmHg, qualifying them for Model for End-Stage Liver Disease (MELD) exception points for LT in the US.(3, 9) Additionally, a SpO2 of ≤ 94% identified all patients with severe hypoxemia (PaO2 < 60 mmHg) and increased the specificity of the test to 93%. Given these data, its non-invasive nature, low cost, widespread availability, and lack of specialized training required for operation, pulse oximetry has become the screening test of choice for HPS.(10–12)
However, assessment of SpO2 by pulse oximetry can be relatively insensitive to changes in PaO2 and has been shown to be unreliable in certain settings and patient populations, including in smokers, children and cirrhotics.(13–15) Cirrhotics may have an abnormal oxygen dissociation curve on the basis of elevated levels of carbon monoxide and 2,3 diphosphoglycerate (DPG), affecting oxyhemoglobin.(16) Additionally, because of these alterations in the oxygen dissociation curve, there is increased dispersion of oxygen saturation for any given value of PaO2. Moreover, SpO2 by pulse oximetry may be normal in HPS patients who may have an increased A-a gradient but preserved PaO2 due to lower PaCO2 from hyperventilation, commonly seen in cirrhotics.(17) Lastly, elevated carboxyhemoglobin concentrations may lead to higher SpO2 measurements contributing to overestimation of arterial oxygenation.(18, 19)
An ideal screening test for HPS would, if “negative,” decrease the likelihood of a patient having HPS from what would be expected at baseline (pre-test probability) and increase the likelihood of having HPS if “positive.” Sensitivity (and negative predictive value) would be of greater priority for screening LT candidates for HPS, since it would be more detrimental to miss detecting the possible presence of HPS, which might increase the priority for LT allocation.(4, 9) To determine the screening characteristics of pulse oximetry for HPS, we prospectively examined patients undergoing the first LT evaluation at three academic centers in the US between February 2013 and December 2016. We aimed to determine the performance of pulse oximetry as a screening test for HPS.
Patients and Methods
Study Sample
The Pulmonary Vascular Complications of Liver Disease 2 (PVCLD2) study is a multicenter, prospective cohort study of adult patients with portal hypertension undergoing evaluation for LT or with portopulmonary hypertension.(20) The criteria for cohort assembly were the presence of portal hypertension with or without intrinsic liver disease, as determined by the site principal investigator, and presentation for LT evaluation. We excluded patients with active infection, recent gastrointestinal bleeding (<2 weeks from date of LT evaluation), or a history of prior liver or lung transplantation. The study sample for this analysis was drawn from 399 enrolled patients undergoing their first LT evaluation at the University of Pennsylvania, the Mayo Clinic, and the University of Texas at Houston between February 2013 and December 2016. We excluded those patients with portopulmonary hypertension, prescribed supplemental oxygen, or who failed to complete spirometry and/ or arterial blood gas (ABG) sampling, both of which were required by the research protocol and necessary for the diagnosis of HPS.
As established by the European Respiratory Society (ERS) Task Force Pulmonary-Hepatic Vascular Disorders Scientific Committee, HPS was defined as: 1) A-a gradient ≥ 15 mm Hg (or A-a gradient ≥ 20 mm Hg if age > 64 years); 2) late passage of contrast on contrast-enhanced echocardiography (CE) (defined below); and 3) absence of i) a significant obstructive ventilatory defect on spirometry, defined as forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) < 0.70 with FEV1 < 80% predicted, and/or ii) a significant restrictive ventilatory defect, defined as FVC <70% predicted; and 4) absence of intracardiac shunting (defined below).(1)
Two comparator groups without HPS were included in the analysis. The first group included all other patients undergoing their first LT evaluation who did not meet diagnostic criteria for HPS (n=288). The second group, a subset of the first, excluded patients with obstructive or restrictive ventilatory defects on spirometry (defined above) or those with intracardiac shunting (defined below) (n=127).
Study Procedures
All patients being seen for LT evaluation during the study period were screened for eligibility at each study site. Informed consent was obtained from eligible patients, who were then scheduled for research assessment, which included a history and physical examination, performance of anthropometrics, pulse oximetry, ABG sampling, spirometry, and CE. All study visits and study procedures were conducted in the outpatient setting. Patients were asked to avoid smoking before the research assessment.
Clinical data were collected from formal interviews with enrolled patients on the date of study procedures and from the medical record. Clinical laboratory results obtained closest to the date of the study visit were recorded. The Model for End-Stage Liver Disease, MELD, and MELD-Na, scores were calculated using the following formulas: MELD=10 * ((0.957 * ln(Creatinine)) + (0.378 * ln(Bilirubin)) + (1.12 * ln(INR))) + 6.43 and MELD-Na=MELD + (0.32 *(137 - Na)) - (0.033 * MELD * (137 - Na).(21, 22)
Pulse oximetry was performed using a standard professional grade oximeter (Datascope Accutorr Plus Vital Signs Monitor, Datascope Corp., Fairfield, NJ; Nonin Pulse Oximeter, Nonin Medical, Inc., Plymouth, MN; Welch Allyn MasimSET, Welch Allyn Medical Products, Skaneateles Falls, NY; or Quik Signs, Protocol Systems, Inc (now known as Welch Allen Protocol, Inc.), Berverton, OR) after the study participant maintained an upright posture for five minutes and then was repositioned supine for 5 minutes. ABG sampling was also performed on room air in a seated position after 10 minutes of rest. The samples were processed in a blood gas analyzer after a one-point calibration. The A-a gradient was calculated using the following formula: AaPO2=[(FiO2*[Patm - PH20])-(PaCO2 /R)]] - PaO2 where R was assumed to be 0.8 and Patm was the barometric pressure measured on the date (and in the city) of the study visit.(23)
Spirometry was performed according to American Thoracic Society (ATS) and European Respiratory Society (ERS) recommendations. A minimum of three efforts with no acceptability errors and at least 2 with repeatability per ATS-ERS standards (FVC within 150 mL of largest, FEV1 within 150 mL of largest, and peak flow within 15% of largest) were required.(24) Testing was continued until the above criteria were met, a total of 8 tests were performed, or the patient was unable to continue testing. Sex, age and race specific predicting equations were used to determine percent predicted based on spirometric reference values derived from the National Health and Nutrition Evaluation Survey III.(25)
CE was performed by the injection of agitated saline via a peripheral vein during transthoracic echocardiographic imaging. The apical four-chamber view was the preferred window for image acquisition, although the parasternal long axis, modified or para-apical four-chamber view, or subcostal views were utilized if the four-chamber view was suboptimal or unavailable. At least 10 continuous cardiac cycles were captured, beginning immediately prior to contrast injection to allow accurate assessment of cardiac cycles to determine delay from injection of agitated saline until visualization of contrast entering the left heart is observed. Identification of microbubbles in either the left atrium or left ventricle after ≥ 3 cardiac cycles was considered to indicate the presence of intrapulmonary vascular dilatation.(26) Patients with evidence of immediate (< 3 cycles) opacification of the left atrium or left ventricle were presumed to have an intra-cardiac shunt. A Doppler flow signal across the atrial septum was presumed to indicate a patent foramen ovale, considered an intra-cardiac right-to-left shunt. Post-Valsalva images were not utilized for study purposes. The Echocardiography Core Laboratory at the Mayo Clinic evaluated all CEs performed at individual study sites and echocardiographers interpreted the studies offline and were blinded to all clinical information.
Statistical Methods
Categorical variables were evaluated by frequencies and proportions, and continuous variables were summarized by means and standard deviations (SD) or medians and interquartile ranges (IQR), as appropriate. Categorical variables were compared using a χ2 test or Fisher’s exact test. Continuous variables were compared using a Student’s t-test or Wilcoxon rank sum test, as determined by the distribution of variable being analyzed.
The area under the receiver operating characteristic curve (AUROC or c-statistic) was used to assess the ability of SpO2 as assessed by pulse oximetry to discriminate patients with HPS from 1) all other patients being evaluated for LT and 2) patients without significant obstructive or restrictive lung disease and without intracardiac shunts being evaluated for LT. The sensitivities, specificities, and negative and positive predictive values (NPVs and PPVs) (with exact 95% confidence intervals, 95% CIs) were calculated for prior recommended SpO2 cutoffs and for the optimal SpO2 cut off as derived from the study data.(27) These analyses were repeated for the identification of HPS patients with severe hypoxemia, defined as a PaO2 of < 60 mm Hg, who are currently eligible for MELD exception points in the US.
Statistical significance was declared for a 2-sided hypothesis test if the p-value was < 0.05. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and Stata version 14.2 (StataCorp, College Park, TX). The institutional review boards at all study sites approved the study protocol. All authors had access to the study data and reviewed and approved all statistical analyses as well as the resulting manuscript.
Results
From February 2013 through December 2016, 399 patients undergoing their first LT evaluation and without a diagnosis of portopulmonary hypertension were enrolled in PVCLD2 (Figure 1). Of these, 31 (7.8%) lacked spirometry, pulse oximetry and/or ABG data and 5 (1.3%) were using supplemental oxygen at the time of assessment, leaving 363 patients for the primary analysis. Of note, none of the patients on supplemental oxygen met diagnostic criteria for HPS. The median age of the cohort was 57 years (IQR 52 – 63 years) and 130 (35.8%) were women, consistent with the gender distribution of patients with chronic liver diseases undergoing LT evaluation.(28) Two hundred fifty-seven (70.8%) were non-Hispanic white, 31 (8.5%) were non-Hispanic black, 61 (16.8%) were Hispanic and 14 (3.9%) were of other races. The largest proportion of patients had hepatitis C infection as at least one of the underlying etiologies of their chronic liver disease (40.2%). The median calculated MELD score was 14 (IQR 10 – 16), MELD-Na 14 (IQR 10 – 18) and the median Child-Turcotte-Pugh Score was 6 (IQR 5 – 8) at the time of LT evaluation.
Figure 1:
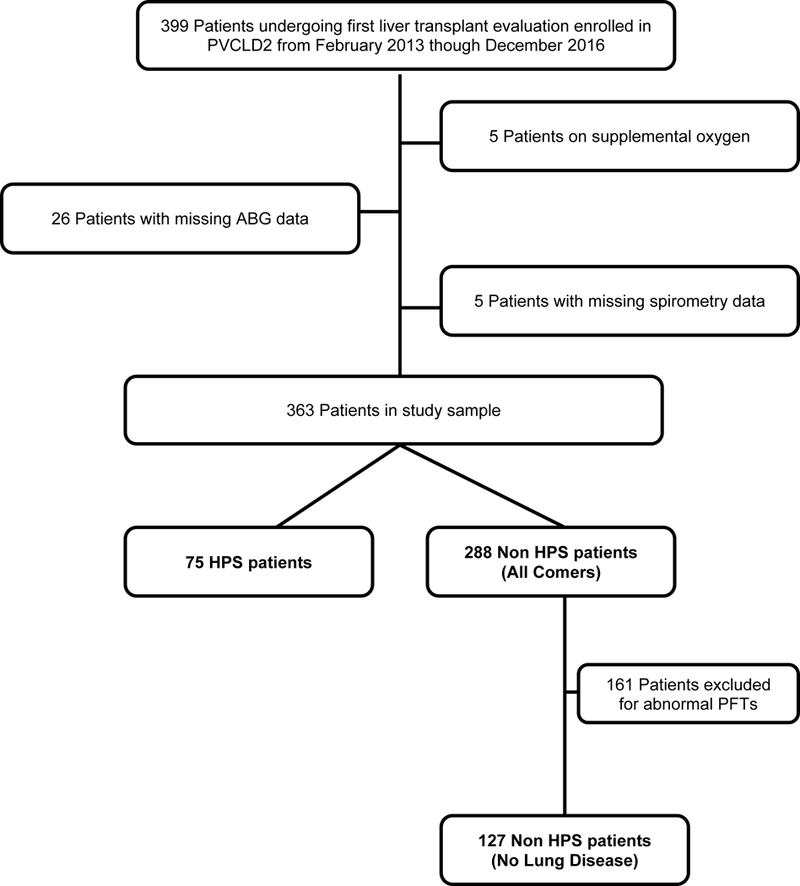
Selection of Study Sample
Of the 363 subjects in the study sample, 75 (20.7%, 95% CI 16.6 – 25.2%) patients had HPS (Table 1). Patients with HPS were more likely to be non-Hispanic white, but there were no differences with respect to age, etiology of liver disease or portal hypertension, liver disease manifestations, or the prevalence of hepatocellular carcinoma (HCC), as shown previously.(4) Patients with HPS had a trend towards having a higher calculated MELD and MELD-Na scores (with significantly higher total bilirubin and international normalized ratio (INR) when the constituents of the MELD score were considered as distinct variables) (Table 1), however the actual differences in the estimates were small and of questionable clinical significance. Patients with HPS had a higher Child-Turcotte-Pugh (CTP) score (p=0.01).
Table 1.
Baseline Characteristics of Patients with HPS Compared to those without HPS (All Comers)
| HPS (N=75) |
No HPS (N=288) |
PValue |
|
|---|---|---|---|
| Age, Median (IQR), years | 56 (52, 61) | 58 (52, 63) | 0.09 |
| Sex, N (%) | 0.16 | ||
| Female | 32 (42.7) | 98 (34.0) | |
| Male | 43 (57.3) | 190 (66.0) | |
| Race/Ethnicity, N (%) | 0.02 | ||
| Non-Hispanic White | 61 (81.3) | 196 (68.1) | |
| Black | 2 (2.7) | 29 (10.1) | |
| Hispanic | 12 (16.0) | 49 (17.0) | |
| Other | 0 (0.0) | 14 (4.8) | |
| Body Mass Index (BMI), Mean (SD) | 31.1 (7.0) | 30.6 (7.0) | 0.57 |
| Liver Disease Etiology, N (%)* | |||
| Alcohol | 31 (41.3) | 112 (38.9) | 0.70 |
| Autoimmune Hepatitis | 3 (4.0) | 11 (3.8) | 1.00 |
| Cryptogenic | 3 (4.0) | 16 (5.6) | 0.77 |
| Hepatitis C | 34 (45.3) | 112 (39.9) | 0.31 |
| Non-alcoholic Fatty Liver Disease | 8 (10.7) | 44 (15.3) | 0.31 |
| Primary Biliary Cholangitis | 8 (10.7) | 14 (4.9) | 0.06 |
| Primary Sclerosing Cholangitis | 4 (5.3) | 11 (3.8) | 0.52 |
| HCC, N (%) | 20 (26.7) | 86 (33.3) | 0.27 |
| Liver Disease Manifestations, N (%) | |||
| Ascites | 57 (76.0) | 201 (69.8) | 0.29 |
| Gastrointestinal bleeding (Variceal) | 27 (36.0) | 89 (30.9) | 0.40 |
| Hepatic Encephalopathy | 47 (62.7) | 166 (57.6) | 0.43 |
| Hepatic Hydrothorax | 9 (12.0) | 35 (12.2) | 0.97 |
| Liver Disease Severity, Median (IQR) | |||
| MELD Score, (N=358) | 14 (12, 17) | 13 (10, 16) | 0.08 |
| MELD-Na Score, (N=357) | 15 (12, 19) | 14 (10, 18) | 0.06 |
| CTP Score, (N=363) | 7 (6, 8) | 6 (5, 7) | 0.01 |
| Laboratory Studies, Median (IQR) | |||
| Albumin, g/dL (N=361) | 3.1 (2.5, 3.4) | 3.1 (2.8, 3.6) | 0.06 |
| Bilirubin, Total, mg/dL (N=362) | 2.5 (1.6, 3.7) | 1.7 (1.0, 3.2) | 0.002 |
| Creatinine, mg/dL (N=362) | 1.0 (1.0, 1.1) | 1.0 (1.0, 1.2) | 0.05 |
| INR (N=359) | 1.4 (1.2, 1.6) | 1.3 (1.2, 1.5) | 0.06 |
| Sodium, mEq/L (N=361) | 138 (134, 141) | 139 (135, 141) | 0.56 |
| Signs and Symptoms, N (%) | |||
| Clubbing | 9 (12.0) | 16 (5.6) | 0.05 |
| Dyspnea | 28 (37.3) | 102 (35.4) | 0.76 |
| NYHA Functional Class | |||
| 1 | 12 (16.0) | 100 (34.7) | 0.01 |
| 2 | 43 (57.3) | 123 (42.7) | |
| 3 | 20 (26.7) | 63 (21.9) | |
| 4 | 0 (0.0) | 2 (0.7) |
Etiologies of liver disease are not mutually exclusive. IQR – Interquartile Range; SD – Standard Deviation, MELD – Model for End-Stage Liver Disease; CTP - Child-Turcotte-Pugh; NYHA – New York Heart Association.
One hundred and sixty-one (44.4%) of the main comparator group had evidence of restrictive or obstructive ventilatory defects noted on spirometry or intracardiac shunting. After eliminating these subjects from the comparator group, there were no major changes from the prior comparisons discussed above (Table 2).
Table 2.
Baseline Characteristics of Patients with HPS Compared to those without HPS and Intrinsic Lung Disease
| HPS (N=75) |
No HPS or Lung Disease (N=127) |
P Value |
|
|---|---|---|---|
| Age, Median (IQR), years | 56 (50, 61) | 58 (52, 63) | 0.12 |
| Sex, N (%) | 0.03 | ||
| Female | 32 (42.7) | 36 (27.6) | |
| Male | 43 (57.3) | 92 (72.4) | |
| Race/Ethnicity, N (%) | 0.01 | ||
| Non-Hispanic White | 61 (81.3) | 81 (63.8) | |
| Black | 2 (2.7) | 12 (9.4) | |
| Hispanic | 12 (16.0) | 26 (20.5) | |
| Other | 0 (0.0) | 8 (6.3) | |
| Body Mass Index (BMI), Mean (SD) | 31.1 (7.0) | 30.3 (7.2) | 0.40 |
| Liver Disease Etiology, N (%)* | |||
| Alcohol | 31 (41.3) | 43 (33.9) | 0.29 |
| Autoimmune Hepatitis | 3 (4.0) | 4 (4.7) | 1.00 |
| Cryptogenic | 3 (4.0) | 10 (7.9) | 0.38 |
| Hepatitis C | 34 (45.3) | 57 (44.9) | 0.95 |
| Non-alcoholic Fatty Liver Disease | 8 (10.7) | 20 (15.7) | 0.31 |
| Primary Biliary Cholangitis | 8 (10.7) | 4 (3.1) | 0.03 |
| Primary Sclerosing Cholangitis | 4 (5.3) | 8 (6.3) | 1.00 |
| HCC, N (%) | 20 (26.7) | 45 (35.4) | 0.20 |
| Liver Disease Manifestations, N (%) | |||
| Ascites | 57 (76.0) | 78 (61.4) | 0.03 |
| Gastrointestinal bleeding (Variceal) | 27 (36.0) | 37 (29.1) | 0.31 |
| Hepatic Encephalopathy | 47 (62.7) | 63 (49.6) | 0.07 |
| Hepatic Hydrothorax | 9 (12.0) | 12 (9.4) | 0.57 |
| Liver Disease Severity, Median (IQR) | |||
| MELD Score, (N=198) | 14 (12, 17) | 13 (10, 16) | 0.02 |
| MELD-Na Score, (N=198) | 15 (12, 19) | 14 (10, 17) | 0.01 |
| CTP Score, (N=202) | 7 (6, 8) | 6 (4, 7) | <0.001 |
| Laboratory Studies, Median (IQR) | |||
| Albumin, g/dL (N=201) | 3.1 (2.5, 3.4) | 3.2 (2.8, 3.7) | 0.02 |
| Bilirubin, Total, mg/dL (N=201) | 2.5 (1.6, 3.7) | 1.6 (1.0, 2.9) | <0.001 |
| Creatinine, mg/dL (N=201) | 1.0 (1.0, 1.1) | 1.0 (1.0, 1.2) | 0.05 |
| INR (N=199) | 1.4 (1.2, 1.6) | 1.3 (1.1, 1.5) | 0.01 |
| Sodium, mEq/L (N=201) | 138 (134, 141) | 139 (136, 141) | 0.23 |
| Signs and Symptoms, N (%) | |||
| Clubbing | 9 (12.0) | 5 (3.4) | 0.04 |
| Dyspnea | 28 (37.3) | 26 (20.5) | 0.01 |
| NYHA Functional Class | |||
| 1 | 12 (16.0) | 53 (41.7) | 0.001 |
| 2 | 43 (57.3) | 55 (43.3) | |
| 3 | 20 (26.7) | 19(15.0) | |
| 4 | 0 (0.0) | 0 (0.0) |
Etiologies of liver disease are not mutually exclusive. IQR – Interquartile Range; SD – Standard Deviation, MELD – Model for End-Stage Liver Disease; CTP - Child-Turcotte-Pugh, NYHA – New York Heart Association.
Patients with HPS had a median room air SpO2 of 97% when sitting and supine (IQR 95 – 99% and IQR 96 – 99%, respectively) which were statistically (but not clinically) significantly lower than all others and those without lung disease (Table 3). Patients with HPS had significantly lower median PaO2 (80.2 mm Hg, IQR 72.4 – 87.5 mm Hg) and lower median partial pressure of carbon dioxide in arterial blood (PaCO2) (33.2 mm Hg, IQR 30.0 – 36.0 mm Hg) than all others and those without lung disease. Additionally, patients with HPS had significantly higher A-a gradients, by definition, compared to all-comers without HPS and the no lung disease group without HPS (p<0.001 for both comparisons). Some patients in the “all comers” group had elevated A-a gradients, however these were not considered HPS because of 1) the lack of intrapulmonary vascular dilatation, 2) the presence of significant restrictive or obstructive lung disease and/or 3) the presence of an intracardiac shunt.
Table 3.
Pulse Oximetry and Arterial Blood Gas Measurements of Patients with HPS, All Comers and No Lung Disease (LD)
| HPS (N=75) |
All Comers (N=288) |
P Value |
No LD (N-127) |
P Value | |
|---|---|---|---|---|---|
| Pulse Oximetry, Median (IQR) | |||||
| Supine % | 97 (96, 99) | 98 (96, 99) | 0.02 | 98 (97, 99) | <0.001 |
| Sitting % | 97 (95, 99) | 98 (96, 99) | 0.02 | 98 (97, 99) | <0.001 |
| Arterial Blood Gas, Median (IQR) | |||||
| pH | 7.5 (7.4, 7.5) | 7.4 (7.4, 7.5) | 0.06 | 7.4 (7.4, 7.5) | 0.01 |
| PaO2 , mmHg | 80.2 (72.4, 87.5) | 89.2 (81.4, 97.4) | <0.001 | 93.0 (85.0, 99.0) | <0.001 |
| PaCO2 , mmHg | 33.2 (30.0, 36.0) | 35.0 (32.0, 39.0) | <0.001 | 35.0 (32.1, 39.0) | 0.002 |
| A-a gradient, mmHg | 25.8 (20.5, 40.0) | 15.7 (9.3, 23.9) | <0.001 | 12.4 (7.3, 18.0) | <0.001 |
IQR – Interquartile Range; PaO2 - Partial Pressure of Oxygen in Arterial Blood; PaCO2 - Partial Pressure of Carbon Dioxide in Arterial Blood; A-a gradient - Alveolar-Arterial Gradient
Performance of Pulse Oximetry for Detecting HPS
The c-statistic for SpO2 for discriminating HPS in all comers was 0.59 (95% CI 0.51 – 0.66) (Figure 2). The discrimination of HPS in did not appreciably change in patients when stratified by severity of liver disease (data not shown). A SpO2 cutoff of < 96% has been considered sufficiently sensitive to detect HPS, specifically severe HPS (PaO2 < 60 mm Hg). The American Association for the Study of Liver Diseases (AASLD) and the International Liver Transplant Society (ILTS) practice guidelines suggest proceeding to CE and/or ABG only if SpO2 is below this threshold, so that many centers do not perform CE or ABG in LT candidates with oxygen saturations above this level. However, this threshold had suboptimal sensitivity (28%, 95% CI 18 – 38%) to identify those with HPS in our cohort (Table 4). A cutoff of SpO2 < 96% had a NPV of 82% (95% CI 77 – 86%), meaning that 18% of patients with SpO2 ≥ 96% actually had HPS, almost identical to the underlying prevalence of HPS (20.7%).
Figure 2:
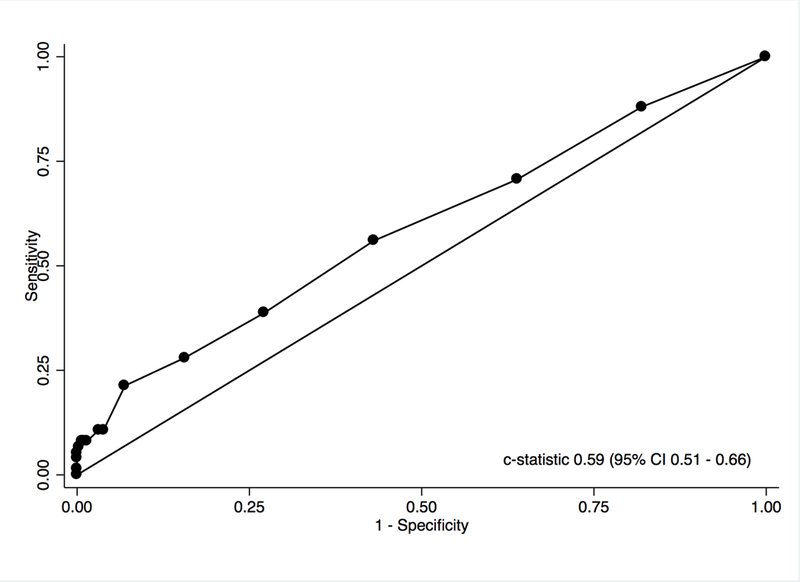
ROC Curve-Performance spO2 by Pulse Oximetry for the Discrimination of HPS in All Comers
Table 4:
Pulse Oximetry Performance Characteristics in All Comers, Patients without Significant Liver Disease and HPS with Severe Hypoxemia
A: Performance Characteristics in All Comers
B: Performance Characteristics in Patients without Lung Disease
C: Performance Characteristics in HPS with PaO2 < 60 mm Hg and All Comers
| SpO2 Cutoff | Sensitivity (95% CI) |
Specificity (95% CI) |
NPV (95% CI) |
PPV (95% CI) |
|---|---|---|---|---|
| < 96% | 0.28 (0.18 – 0.38) | 0.84 (0.80 – 0.89) | 0.82 (0.77 – 0.86) | 0.32 (0.21 – 0.43) |
| ≤ 94% | 0.21 (0.12 – 0.31) | 0.93 (0.90 – 0.96) | 0.82 (0.78 – 0.86) | 0.44 (0.28 – 0.61) |
| ≤ 97% (Optimal) | 0.56 (0.45 – 0.67) | 0.57 (0.51 – 0.63) | 0.83 (0.78 – 0.88) | 0.25 (0.19 – 0.32) |
| Cohort | Sensitivity (95% CI) |
Specificity (95% CI) |
NPV (95% CI) |
PPV (95% CI) |
|---|---|---|---|---|
| < 96% | 0.28 (0.18 – 0.38) | 0.91 (0.86 – 0.96) | 0.68 (0.61 – 0.75) | 0.66 (0.49 – 0.82) |
| ≤ 94% | 0.21 (0.12 – 0.31) | 0.96 (0.93 – 0.99) | 0.67 (0.61 – 0.74) | 0.76 (0.58 – 0.94) |
| ≤ 97% (Optimal) | 0.56 (0.45 – 0.67) | 0.68 (0.60 – 0.76) | 0.72 (0.64 – 0.80) | 0.51 (0.40 – 0.61) |
| Cohort | Sensitivity (95% CI) |
Specificity (95% CI) |
NPV (95% CI) |
PPV (95% CI) |
|---|---|---|---|---|
| < 96% | 0.71 (0.38 – 1.00) | 0.83 (0.79 – 0.87) | 0.99 (0.98 – 1.00) | 0.08 (0.01 – 0.14) |
| ≤ 94% | 0.71 (0.38 – 1.00) | 0.91 (0.88 – 0.94) | 0.99 (0.98 – 1.00) | 0.14 (0.03 – 0.25) |
| ≤ 88% (Optimal) | 0.71 (0.38 – 1.00) | 0.99 (0.98 – 1.00) | 0.99 (0.99 – 1.00) | 0.62 (0.29 – 0.96) |
A SpO2 threshold of 94% had a similar sensitivity (21%, 95%CI 12 – 31%) and NPV (82%, 95% CI 78 – 86%), having little impact on the likelihood of having HPS after performance of the test. The optimal cutoff of SpO2 to detect HPS derived from the current data was 97%, which still only had a sensitivity of 56% (95% CI 45 – 67%) and NPV of 83% (95% CI 78 – 88%) (i.e., 17% of patients with SpO2 ≥ 96% had HPS). These estimates were similar in the subsample in which patients with lung disease were excluded (Table 4).
We then determined the utility of pulse oximetry to screen for HPS with PaO2 < 60 mm Hg, which was present in 1.9% (7) of all-comers and 3.5% of patients without lung disease or intracardiac shunt (Table 4). The c-statistic for SpO2 for discriminating HPS with PaO2 < 60 mm Hg in all-comers was 0.76 (95% CI 0.46 – 1.00), which was quantitatively better than in the overall HPS population but not significantly different from chance (p=0.09). All of the cutoffs showed similar NPVs (1%), which was similar to the pre-test probability of severe HPS in all comers.
Discussion
Twenty-one percent of this well-characterized, prospective cohort of patients being evaluated for LT at three academic centers had HPS; the prevalence was more than 37% after the exclusion of LT candidates with restrictive or obstructive lung disease and/or intracardiac shunting. Approximately 2% of patients had HPS with significant hypoxemia, making them eligible for MELD exception points under the current liver allocation system in the US. We found that pulse oximetry essentially performed no better than chance (i.e., a “coin flip”) in the discrimination of patients with HPS from all comers (which is how pulse oximetry is used in clinical practice for LT evaluation), and in those without significant lung disease. More importantly, while SpO2 below certain cutoffs was somewhat useful in suggesting the presence of HPS with severe hypoxemia (as demonstrated by the PPV), a SpO2 above any proposed cutoff 1) did not rule out HPS, 2) did not substantially change the pre-test probability of HPS, and 3) would miss detecting most patients with HPS in a LT evaluation clinic. For instance, 18% of patients with a SpO2 ≥ 96% had HPS, and while a SpO2 ≤ 94% greatly increased the likelihood of identifying those with HPS (from 19% to 44% in all comers), 18% of those with a SpO2 above this cutoff still had HPS. Therefore, pulse oximetry was not useful in “ruling out” HPS. Unfortunately, small sample sizes limited the precision of the test characteristics in the subgroup of patients with HPS and severe hypoxemia (PaO2 < 60 mmHg), though none of the proposed or derived cut points greatly increased the pre-test probability of identifying those with severe HPS in this group.
These results are in contrast to older studies supporting the use of pulse oximetry as a screening test for HPS. In a prior study of 200 prospectively enrolled patients with cirrhosis and portal hypertension, a SpO2 of ≤ 94% detected all patients with HPS and a PaO2 of < 60 mmHg.(18) However, in this study, the definition of HPS included a PaO2 of 70 mmHg or less (hypoxemia) and contrast echocardiogram and lung perfusion scan suggestive of shunting through intrapulmonary vascular dilatations. Additionally, the study was designed to evaluate the utility of pulse oximetry for the determination of hypoxemia, not specifically from HPS, and hence patients with pulmonary etiologies of hypoxemia (chronic obstructive pulmonary disorder, sarcoidosis, pulmonary hypertension and hepatic hydrothorax) were included. In a follow up study, 127 liver transplant candidates were evaluated in whom a SpO2 threshold of 96% had a high sensitivity for the detection of cirrhotics with PaO2 < 60 mm Hg.(3) HPS was defined as the presence of intrapulmonary vascular dilatations on contrast echocardiography, and A-a gradient greater than 20 and lack of significant findings on chest radiograph and PFTs. Of note, the A-a gradient utilized in the study did not take into account age-specific cut points and the performance of the pulse oximetry measurement was undertaken within 72 hours of the performance of the contrast enhanced echocardiography and arterial blood gas. Given that our study utilized the currently accepted standardized definition of HPS and all study sites assessed patients in the same manner with a standardized approach to obtaining all study related measurements and procedures, it is not unexpected that our results would deviate from those of other observational data.
Though not based on data utilizing the current ERS Task Force of Lung-Liver Disease recommended criteria for the diagnosis of HPS, as used in the current study, the previously discussed data, coupled with the low cost, non-invasive nature, and lack of specific operator expertise required for the performance of pulse oximetry has led the AASLD and ILTS to recommend the use of pulse oximetry as a reasonable screening modality for HPS in LT candidates, though the ILTS practice guidelines emphasize that ABG is necessary to diagnose HPS.(10, 12) Further, no specific oxygen saturation threshold was suggested by the AASLD to trigger further testing, but screening with pulse oximetry was determined to be a Grade 1A recommendation (strong recommendation, high level of evidence). The ILTS determined screening using the 96% screening threshold to be Grade 1C (strong recommendation, low level of evidence).
Our study differs from prior studies in its use of the an age-adjusted A-a gradient, PaO2 and CE to define HPS (the current clinical standard as endorsed by the ERS Task Force of Lung-Liver Disease and suggested by the International Liver Transplant Society Guidelines), careful phenotyping of lung function and elimination of those with obstructive or restrictive lung disease, centralized determination of intrapulmonary vascular dilatation versus intracardiac shunting, inclusion of a large sample size, and multicenter design. There are several reasons why pulse oximetry may not be adequate for screening LT candidates for HPS. Firstly, pulse oximetry overestimates oxygen saturation in patients with advanced liver disease. Up to 98% of cirrhotic patients in one study were found to have had an overestimation of the oxyhemoglobin as determined by pulse oximetry.(29) With a range of bias from 1–10%, a cirrhotic patient with profound hypoxemia may easily be misclassified based on SpO2. Though the rationale for pulse oximetry overestimation is not well understood, it may depend partly on concentrations of carboxyhemoglobin, the degree of peripheral vasodilation present in liver disease, and/or on the basis of reduced metabolism of vasodilators. Secondly, SpO2 is normal in most patients with HPS (> 96% in 75% of our HPS patients) given that these patients may have a relatively preserved PaO2 in the setting of an increased A-a gradient, due to lower PCO2 from baseline hyperventilation and respiratory alkalosis present in cirrhotics.(17) Thirdly, cirrhotics also have alterations in their oxyhemoglobin dissociation curve. Dispersion of PaO2 values are influenced by 3 distinct factors of importance in the cirrhotic: 1) alteration of enzyme activity in the 2,3 DPG phosphatase pathway, 2) perturbation of plasma ions in the setting of altered sodium and water excretion, and 3) administration of various therapeutic interventions such as diuretics and beta blockers which may influence the position of the dissociation curve or alter the affinity for hemoglobin.(16) Lastly, anemia, hypotension and other concomitant comorbidities may also lead to poor correlation between pulse oximetry and PaO2 in patients with cirrhosis.
A diagnosis of HPS with any severity of oxygenation abnormality is associated with more than a doubling in overall mortality.(2, 4, 5, 30) Therefore, a diagnosis of HPS in a cirrhotic should lead to the consideration of LT evaluation. Currently, the documentation of HPS with significant hypoxemia qualifies LT candidates for receipt of MELD exception points in the US. This policy was intended to appropriately prioritize them for LT, considering the increased risk of mortality and the concern that profound hypoxemia portends worsened post transplant outcomes.(7) Interestingly, we have recently shown that this policy actually confers a significantly higher chance of LT and a significantly lower overall risk of death (improved survival) for patients who receive HPS exception when compared to other LT candidates without HPS exception.(7) Given the poor performance of pulse oximetry in these patients and the increased risk of death associated with HPS, regardless of the degree of oxygen defect, we would therefore advocate for routine screening of LT candidates with CE and ABG (see Figure 3). Spirometry is also important to rule out other causes of abnormal oxygenation in these patients. The frequency of re-evaluation of patients after initial evaluation for HPS is currently undefined.
Figure 3:
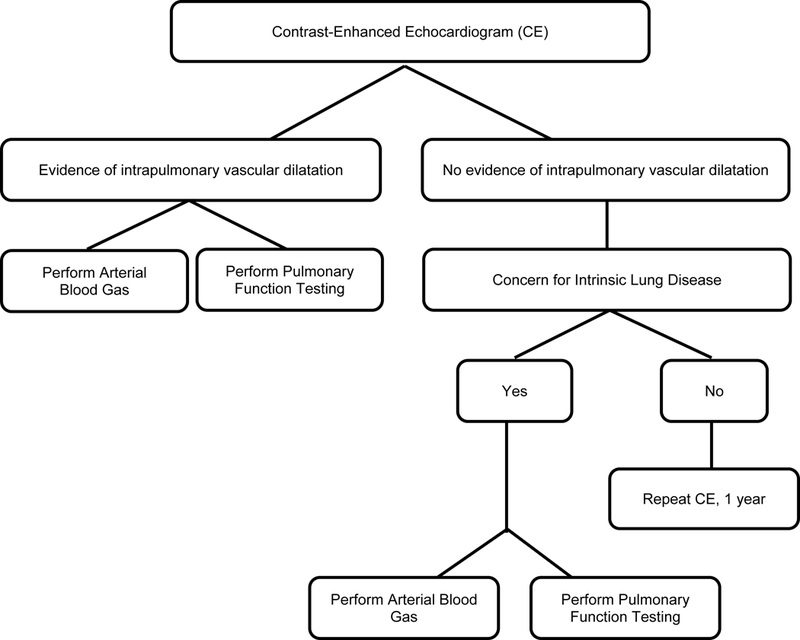
Staged Algorithm for Diagnosis of HPS
There are a number of strengths of our study, including the use of a large prospective multicenter cohort of patients with portal hypertension, utilization of standardized, research-grade technical assessments overseen by respective core laboratories (Pulmonary Function Core and Echocardiography Core), and blinded assessment of CE by a central laboratory with clearly defined criteria for determination of intrapulmonary shunting.
Our study also had a number of limitations. We did not perform serial assessments of SpO2, making the utility of multiple assessments of oxygenation over time unknown. Different pulse oximeters were used at the study sites, although this reflects clinical practice and the results did not change significantly when analyzed by site (data not shown). Additionally, there were only 7 patients with PaO2 of <60 mmHg, limiting our sample size for the examination of the precision of pulse oximetry performance in patients with HPS and severe hypoxemia.
In summary, we found that pulse oximetry was no more accurate than a coin flip in detecting the presence of HPS. Reduced SpO2 was specific for HPS, but was not sensitive, limiting its utility as a screening test for all LT candidates. Use of CE and arterial blood gas sampling, either in a staged or simultaneous algorithm, in all candidates for LT is the most prudent approach to the detection and appropriate prioritization of patients with HPS for whom LT is curative. Future studies are necessary to determine the desired frequency of this testing in patients who are either eligible or listed for LT
Grant Support:
This work was funded by the National Institutes of Health (R01–113988, UM1-HL116886, K24 HL103844, K23 DK090209, K08 DK098272).
Abbreviations
- ABG
arterial blood gas
- CE
contrast-enhanced echocardiography
- HPS
hepatopulmonary syndrome
- LT
liver transplant
- MELD
Model for End-Stage Liver Disease
- PaO2
partial pressure of oxygen
- PVCLD2
Pulmonary Vascular Complications of Liver Disease 2
- SpO2
oxygen saturation
Footnotes
Conflict of Interest Statement: None of the authors have any relevant financial conflicts of interest with respect to this manuscript.
References
- 1.Rodriguez-Roisin R, Krowka MJ, Herve P, Fallon MB, Committee ERSTFP-HVDS. Pulmonary-Hepatic vascular Disorders (PHD). Eur Respir J 2004;24:861–880. [DOI] [PubMed] [Google Scholar]
- 2.Schenk P, Schoniger-Hekele M, Fuhrmann V, Madl C, Silberhumer G, Muller C. Prognostic significance of the hepatopulmonary syndrome in patients with cirrhosis. Gastroenterology 2003;125:1042–1052. [DOI] [PubMed] [Google Scholar]
- 3.Arguedas MR, Singh H, Faulk DK, Fallon MB. Utility of pulse oximetry screening for hepatopulmonary syndrome. Clin Gastroenterol Hepatol 2007;5:749–754. [DOI] [PubMed] [Google Scholar]
- 4.Fallon MB, Krowka MJ, Brown RS, Trotter JF, Zacks S, Roberts KE, Shah VH, et al. Impact of hepatopulmonary syndrome on quality of life and survival in liver transplant candidates. Gastroenterology 2008;135:1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arguedas MR, Abrams GA, Krowka MJ, Fallon MB. Prospective evaluation of outcomes and predictors of mortality in patients with hepatopulmonary syndrome undergoing liver transplantation. Hepatology 2003;37:192–197. [DOI] [PubMed] [Google Scholar]
- 6.Swanson KL, Wiesner RH, Krowka MJ. Natural history of hepatopulmonary syndrome: Impact of liver transplantation. Hepatology 2005;41:1122–1129. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg DS, Krok K, Batra S, Trotter JF, Kawut SM, Fallon MB. Impact of the hepatopulmonary syndrome MELD exception policy on outcomes of patients after liver transplantation: an analysis of the UNOS database. Gastroenterology 2014;146:1256–1265 e1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krowka MJ, Mandell MS, Ramsay MA, Kawut SM, Fallon MB, Manzarbeitia C, Pardo M Jr, et al. Hepatopulmonary syndrome and portopulmonary hypertension: a report of the multicenter liver transplant database. Liver Transpl 2004;10:174–182. [DOI] [PubMed] [Google Scholar]
- 9.OPTN/UNOS policies and bylaws. (Accessed May 22, 2017, at https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf-nameddest=Policy_09).
- 10.Krowka MJ, Fallon MB, Kawut SM, Fuhrmann V, Heimbach JK, Ramsay MA, Sitbon O, et al. International Liver Transplant Society Practice Guidelines: Diagnosis and Management of Hepatopulmonary Syndrome and Portopulmonary Hypertension. Transplantation 2016;100:1440–1452. [DOI] [PubMed] [Google Scholar]
- 11.Martin P, DiMartini A, Feng S, Brown R Jr., Fallon M Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 2014;59:1144–1165. [DOI] [PubMed] [Google Scholar]
- 12.Roberts DN, Arguedas MR, Fallon MB. Cost-effectiveness of screening for hepatopulmonary syndrome in liver transplant candidates. Liver Transpl 2007;13:206–214. [DOI] [PubMed] [Google Scholar]
- 13.Sinex JE. Pulse oximetry: principles and limitations. Am J Emerg Med 1999;17:59–67. [DOI] [PubMed] [Google Scholar]
- 14.Krowka MJ. Hepatopulmonary syndrome: monitoring at your fingertip. Dig Dis Sci 2011;56:1599–1600. [DOI] [PubMed] [Google Scholar]
- 15.Hoerning A, Raub S, Neudorf U, Muntjes C, Kathemann S, Lainka E, Stehling F, et al. Pulse oximetry is insufficient for timely diagnosis of hepatopulmonary syndrome in children with liver cirrhosis. J Pediatr 2014;164:546–552 e541–542. [DOI] [PubMed] [Google Scholar]
- 16.Clerbaux T, Detry B, Geubel A, Veriter C, Liistro G, Horsmans Y, Frans A. The oxyhemoglobin dissociation curve in liver cirrhosis. Chest 2006;129:438–445. [DOI] [PubMed] [Google Scholar]
- 17.Henriksen JH, Bendtsen F, Moller S. Acid-base disturbance in patients with cirrhosis: relation to hemodynamic dysfunction. Eur J Gastroenterol Hepatol 2015;27:920–927. [DOI] [PubMed] [Google Scholar]
- 18.Abrams GA, Sanders MK, Fallon MB. Utility of pulse oximetry in the detection of arterial hypoxemia in liver transplant candidates. Liver Transpl 2002;8:391–396. [DOI] [PubMed] [Google Scholar]
- 19.Arguedas MR, Drake BB, Kapoor A, Fallon MB. Carboxyhemoglobin levels in cirrhotic patients with and without hepatopulmonary syndrome. Gastroenterology 2005;128:328–333. [DOI] [PubMed] [Google Scholar]
- 20.DuBrock HM, Krowka MJ, Forde KA, Krok K, Patel M, Sharkoski T, Sprys M, et al. Clinical impact of intrapulmonary vascular dilatation in liver transplant candidates. Chest 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33:464–470. [DOI] [PubMed] [Google Scholar]
- 22.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, Edwards E, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med 2008;359:1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crapo RO, Jensen RL, Hegewald M, Tashkin DP. Arterial blood gas reference values for sea level and an altitude of 1,400 meters. Am J Respir Crit Care Med 1999;160:1525–1531. [DOI] [PubMed] [Google Scholar]
- 24.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–338. [DOI] [PubMed] [Google Scholar]
- 25.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159:179–187. [DOI] [PubMed] [Google Scholar]
- 26.Abrams GA, Jaffe CC, Hoffer PB, Binder HJ, Fallon MB. Diagnostic utility of contrast echocardiography and lung perfusion scan in patients with hepatopulmonary syndrome. Gastroenterology 1995;109:1283–1288. [DOI] [PubMed] [Google Scholar]
- 27.Pepe MS. The Statistical Evaluation of Medical Tests for Classification and Prediction: Oxford University Press, 2004. [Google Scholar]
- 28.Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, Harper AM, et al. OPTN/SRTR 2015 Annual Data Report: Liver. Am J Transplant 2017;17 Suppl 1:174–251. [DOI] [PubMed] [Google Scholar]
- 29.Abrams GA, Nanda NC, Dubovsky EV, Krowka MJ, Fallon MB. Use of macroaggregated albumin lung perfusion scan to diagnose hepatopulmonary syndrome: a new approach. Gastroenterology 1998;114:305–310. [DOI] [PubMed] [Google Scholar]
- 30.Krowka MJ. Hepatopulmonary syndrome and portopulmonary hypertension: implications for liver transplantation. Clin Chest Med 2005;26:587–597, vi. [DOI] [PubMed] [Google Scholar]


