Abstract
Exposure- and risk-based assessments for chemicals used indoors or applied to humans (i.e., in near-field environments) necessitate an aggregate exposure pathway framework that aligns chemical exposure information from use sources to internal dose and eventually to their potential for health effects. Such a source-to-effect continuum is advocated to balance the complexity of human exposure and the insufficiency of relevant data for thousands of existing and emerging chemicals. Here, we introduce the Risk Assessment, IDentification And Ranking-Indoor and Consumer Exposure (RAIDAR-ICE) model, which establishes an integrated framework to evaluate human exposure due to indoor use and direct application of chemicals to humans. As a model evaluation, RAIDAR-ICE faithfully reproduces exposure estimates inferred from biomonitoring data for 37 chemicals with direct and indirect near-field sources. RAIDAR-ICE generates different rankings for 131 chemicals based on different exposure- and risk-based assessment metrics, demonstrating its versatility for diverse chemical screening goals. When coupled with a far-field RAIDAR model, the near-field RAIDAR-ICE model enables assessment of aggregate human exposure. Overall, RAIDAR-ICE is a powerful tool for high-throughput screening and prioritization of human exposure to neutral organic chemicals used indoors.
Graphical Abstract

Introduction
Approximately 30 000 of the estimated 100 000 chemicals in global commerce are manufactured and marketed in volumes greater than 1 t per year.(1) Mandated chemical evaluations for ecological and human health occur in different decision-making contexts, including prioritization, screening-level, and more comprehensive assessments for chemicals of high concern.(2,3) A considerable fraction of chemicals undergoing evaluations is present in consumer products, articles, or building materials that are used indoors or proximate to consumers, constituting “near-field” human exposure pathways. For some chemicals, exposure occurs through “direct” application to the body, such as the use of pharmaceuticals, personal care products (PCPs) and cosmetics.(4) Meanwhile, “indirect”, environmentally mediated, human exposure to chemicals used indoors occurs through processes such as vaporization of plasticizers and flame-retardants from articles and building materials,(5) or spraying of air fresheners to indoor air, among numerous others. In addition, humans are also exposed to chemicals via “far-field” pathways which involve food, drinking water, and outdoor air that are contaminated by chemicals released either directly to outdoor environments or initially to the indoors with subsequent transfer to the outdoors.(6−9)
The large number of chemicals requiring evaluation compared to the dearth of exposure data challenges traditional single-chemical evaluation practice and thus warrants high-throughput (HT) screening and prioritization approaches.(2,10,11) Fate and exposure models such as ACC-HUMAN,(12) CoZMoMAN,(13) USETox,(14) and RAIDAR(15,16) include far-field human exposure pathways and have been used for several years and in some cases for thousands of organic chemicals.(16–18) By comparison, HT near-field human exposure models are few;(17,18) however, recent advances have created the potential for rapid quantification of human indoor and consumer exposures.(19–22) For example, provided with emission rates and properties of chemicals, the process-based Indoor Chemical Exposure Classification/Ranking Model (ICECRM)(19) can rapidly estimate indirect human exposure to thousands of chemicals in the indoor environment. The human activity-based Stochastic Human Exposure and Dose Simulation–High-Throughput (SHEDS–HT) model(20) generates probabilistic population distributions of direct and indirect human exposures to thousands of consumer product ingredients and pesticides based on usage pattern and product composition information. Other models such as ConsExpo(23) and the Consumer Exposure Model(24) can also aid in chemical-by-chemical consumer exposure assessments. Except for ICECRM, the aforementioned exposure models do not account for key toxicokinetic processes such as metabolism (biotransformation) and excretion in the human body. Therefore, they can neither derive an internal dose (e.g., concentrations in blood), nor address the potential for adverse effects associated with the exposure using internal dose information directly. Physiologically based toxicokinetic (PBTK) models support the calculation of internal doses from external exposure estimates; however, these models are less frequently applied because of their extensive data requirements, in particular external exposure source data. Frameworks such as the Aggregate Exposure Pathway concept(25) outline the need for models to quantify the “source-to-dose” continuum for a direct comparison of exposures with toxicological data. The 2017 National Academy of Sciences, Engineering and Medicine report “Using 21st Century Science to Improve Risk-Related Evaluations”(26) further advocates for the development and evaluation of aggregate exposure models with chemical use information and (bio)monitoring data for exposure-based evaluations and to compare exposure values with toxicity (or bioactivity) values for risk-based evaluations.
In this paper, we introduce the Risk Assessment, IDentification And Ranking-Indoor and Consumer Exposure (RAIDAR-ICE) model as a versatile, efficient tool for screening and prioritization of neutral organic chemicals based primarily on near-field direct and indirect exposure pathways and associated potential human health concerns. The model development aims to balance the complexity of processes related to assessing human exposure and potential risk with data availability at a screening-level for thousands of chemicals. We describe and evaluate RAIDAR-ICE and then present case study assessment applications. The first case study demonstrates the capacity of RAIDAR-ICE to quantify direct and indirect human exposure from near-field sources in a single consistent framework. The second case study ranks chemicals based on different exposure- and risk-based metrics, linking near-field chemical use scenarios and subsequent exposure with estimates of bioactivity derived from HT in vitro bioassays. The third case study demonstrates the feasibility of a comprehensive aggregate exposure assessment by combining RAIDAR-ICE with the RAIDAR model to include far-field human exposure pathways. We conclude with recommendations for improving the modeling framework.
The RAIDAR-Indoor and Consumer Exposure (RAIDAR-ICE) Model
Model description
RAIDAR-ICE builds onto the ICECRM mass balance framework.(19) Both models contain an indoor chemical fate module, which describes a generic, evaluative indoor environment consisting of seven indoor compartments (indoor air, polyurethane foam, carpet, vinyl floor, and organic films on vertical, up-facing, and down-facing surfaces). In addition, the models include a PBTK model (Supporting Information (SI) Text S1) composed of compartments for (i) hand skin surface, (ii) skin surface on the body excluding hand skin, and (iii) the human body excluding skin surface (Figure 1). This toxicokinetic model describes the adsorption, metabolism (biotransformation) and elimination (exhalation, renal excretion, fecal egestion, skin desquamation) processes of chemicals and is parametrized here for a representative adult male. Whereas ICECRM considers only human exposure to chemicals released into physical compartments of the simulated indoor environment and subsequent indirect exposures, RAIDAR-ICE additionally includes the capacity for direct chemical applications to the body within the mass balance equations.
Figure 1.
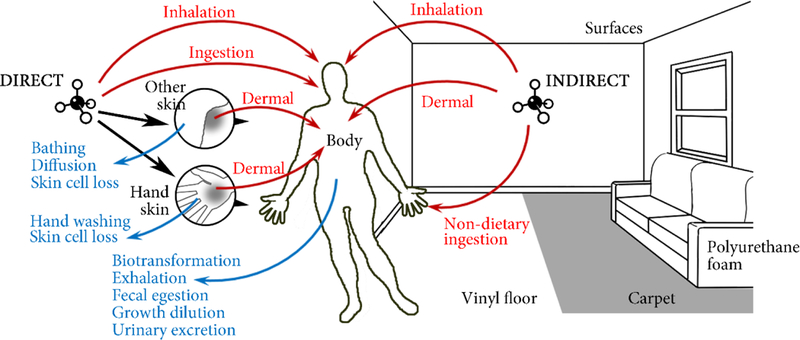
Conceptual representation of the RAIDAR-ICE model indicating two mode-of-entry scenarios, that is, application of a chemical to human compartments (“direct”) and emissions to indoor compartments (“indirect”). Red arrows depict direct (i.e., inhalation, ingestion and dermal permeation) and indirect (i.e., inhalation, nondietary ingestion and dermal permeation) exposure pathways. Blue arrows depict processes eliminating a chemical from human compartments. Calculations of D-values are tabulated in SI Table S2. Mass balance of chemicals in human compartments is given in SI Text S1. Far-field exposure pathways (not shown) can also be included for quantification of aggregate human exposure.
The new RAIDAR-ICE model also inherits concepts from the RAIDAR model.(15,16) RAIDAR describes chemical fate and transport in the outdoor environment of an evaluative region, as well as chemical bioaccumulation and biomagnification in aquatic, terrestrial and agricultural food webs. It calculates human exposure through consumption of drinking water and contaminated food, as well as inhalation of outdoor air. Most notably, the calculated human exposure is combined with user-defined toxicity (hazard) data to derive risk-based assessment metrics (see below for further details).
RAIDAR-ICE is a steady-state model formulated in fugacity notation.(27) Chemical concentration in a compartment (C, in mol m–3) is expressed as the product of a fugacity (f, in Pa) and a fugacity capacity (Z, in mol m–3 Pa–1) that quantifies the ability of a compartment to store a chemical. For each chemical, fugacity capacities of compartments are functions of its equilibrium partition coefficients between air, water and octanol (KOW, KAW, and KOA). Fluxes representing chemical transport and loss in the indoor environment and in a human are expressed as the product of an f and a D-value (in mol Pa–1 h–1) that is essentially a contaminant transport or transformation rate parameter. Z-values and D-values in the RAIDAR-ICE fate module are identical to those in ICECRM, whereas those in the human toxicokinetic module are detailed in SI Tables S1 and S2. The model is coded in Visual Basic for Applications (VBA) with a graphic user interface in Excel.
Model parameterization and application
Chemical-specific inputs to RAIDAR-ICE include molar mass, equilibrium partition coefficients (KOW, and either KAW or KOA, are required), degradation half-lives in air (HLAir) and surface compartments, and the biotransformation half-life in human. These inputs can be obtained from databases and in silico calculations for chemical properties (e.g., the Estimation Programs Interface (EPI) Suite(28)) and for biotransformation half-lives (e.g., refs (29 and 30)). If biotransformation half-lives are not entered, a value corresponding to negligible biotransformation is used.
Figure 1 demonstrates two exposure scenarios. The first is an “indirect” scenario, in which a chemical is actively or passively emitted indoors and then absorbed by a human. Three exposure routes are included: (i) inhalation of gaseous and particle-bound chemicals in indoor air, (ii) nondietary ingestion of indoor dust and through object-hand-mouth contact, and/or (iii) dermal absorption directly from indoor air. This scenario requires inputs of steady-state chemical emission rates (ER, e.g., ng h–1) to individual environmental compartments. The second is a “direct” scenario, in which a chemical is intentionally applied to the skin of hands or the rest of the body, and then absorbed via (i) inhalation, (ii) ingestion, and (iii) dermal permeation. This scenario requires a chemical application rate (AR, e.g., ng h–1), which is calculated from a user-defined frequency of applications (in day–1), the amount of a product per application (in g or mL), and the concentration of a chemical in the product (in ng g–1 or ng mL–1). That is, AR is dependent on the chemical-product combination. Direct chemical inhalation and ingestion rates can also be considered as model input. The user can model human exposure to chemicals occurring via either or both of the two general scenarios depending on chemical-specific use.
In some cases, not all of the chemical applied to the skin surface remains there long enough to completely penetrate through the stratum corneum.(31) To avoid potential overestimation of exposures, RAIDAR-ICE includes the user option to estimate a unitless scaling factor (SFlag; 0 < SFlag ≤ 1), defined as the fraction of the emitted or applied amount expected to enter the skin compartments (SI Text S2 and Figure S1). SFlag is calculated based on a user-defined chemical leave-on duration (in min) and a model-derived lag time(31) describing the minimum duration required for a chemical to reach full steady-state absorption (in min) (SI Text S2). Only the completely absorbed fraction participates in subsequent calculations; the remaining fraction (i.e., 1 – SFlag) is assumed to be removed from the system through wash-off.
Exposure metrics
Three exposure metrics of typical interest are calculated. The first is an individual intake rate (iR; mgchem kg–1BW d–1) that we define as the rate of chemical crossing an absorption (e.g., epithelial) barrier and entering the body.(16) It can be calculated based on inputs of either a realistic or an arbitrary unit (e.g., 1 ng h–1) ER and/or AR. The total iR is the sum of all pathway-specific iRs for the same chemical. The second metric is an individual intake fraction (iF; unitless) that normalizes the intake rate by ER and/or AR from all sources.(32) This iF is associated with the amount of chemical absorbed into the human body, which differs slightly from iF definitions in other models.(19,21) The third metric is a steady-state whole-body concentration (CH, e.g., mmolchem kg–1BW or ngchem kg–1BW) accounting for other toxicokinetic processes. RAIDAR-ICE also estimates (i) a steady-state blood concentration (CB) from CH using a user-defined or model-calculated volume of distribution (VD, LBlood kgBW–1), and (ii) parent chemical concentrations in urine (mgchem L–1) eliminated via renal excretion. RAIDAR-ICE calculates expected concentrations if estimates for realistic ERs and/or ARs are provided; otherwise, RAIDAR-ICE outputs “unit emission rate-based” concentrations (CU) by assuming a consistent unit ER and/or AR (1 ng h–1) for all chemicals to compare chemicals for exposure potential.
RAIDAR-ICE provides two tiers for exposure estimates. Tier 0 recognizes uncertainty in chemical absorption data and conservatively assumes 100% chemical absorption for all exposure pathways. This approach provides consideration for chemicals that come in contact with the body and may have effects associated at the outer boundary. Tier 1 considers chemical- and route-specific differences in absorption by using (i) an absorption efficiency of EA = 70% for inhalation of gaseous chemicals, (ii) particle-size-specific deposition fractions for inhalation of particle-bound chemicals (the deposited fractions can be completely absorbed),(19) (iii) a dietary absorption efficiency of ED = (1.05 + 2 × 10–9 × KOW)−1 for gastrointestinal ingestion,(33) and (iv) dermal absorption efficiency either calculated from KOW and molar mass(34) or entered by the user.
Risk-based metrics
Traditional toxicity data or in vitro bioactivity data(35) can be input into RAIDAR-ICE as hazard “thresholds” if risk-based chemical assessment objectives are desired. These threshold data can be in the form of either an ingestion rate (iRT; mgchem kg–1BW d–1), such as no observed adverse effect level (NOAEL), threshold of toxicological concern (TTC), oral equivalent dose (OED), or a steady-state blood concentration (CT; mmolchem L–1Blood). In accordance with RAIDAR,(14,32)RAIDAR-ICE calculates a critical emission or application rate (ERC or ARC) denoting the ER or AR that yields human exposure corresponding to the toxicity or bioactivity thresholds. RAIDAR-ICE then calculates the ratio of actual ER or AR to the critical ERC or ARC, expressed as a unitless risk assessment factor (RAF).(15) The RAF is conceptually similar to a “risk quotient” used in environmental risk assessment; a higher RAF suggests a higher concern of a chemical. If a bioactivity threshold is used instead of a toxicity threshold, the ratio can be referred to as a “bioactivity quotient”. When ranking and comparing chemicals based on risk, potential bioactivity, or critical emission/application rates, it is best to use a consistent health assessment end point for all chemicals.
Model Evaluation and Case Study Applications
We evaluate RAIDAR-ICE model performance and demonstrate the application of the tool to screen and prioritize chemicals used indoors in a series of case studies based on some possible exposure and health assessment objectives. We also illustrate combining RAIDAR-ICE and RAIDAR models for aggregate human exposure assessment.
Case 1: Indirect and direct near-field exposures to chemicals used indoors
We calculate Tier 1 iRs for 37 chemicals (SI Table S3), including seven chemicals in PCPs (i.e., a direct scenario), 20 pesticides/antimicrobials that can be in contact with human skin (i.e., a direct scenario), and 10 plasticizers that can be absorbed into human bodies after vaporization into indoor air (i.e., an indirect scenario). Records in the Chemical/Product Categories Database (CPCat)(36) indicate that these chemicals are predominantly used and released indoors. These chemicals are selected because their exposure rates have previously been inferred from biomonitoring data in the U.S. National Health and Nutrition Examination Survey (NHANES),(17)with which we can evaluate the performance of RAIDAR-ICE. Whereas some of them can also be found in a few products/articles used outdoors, we ignore the contribution of infiltration from outdoor sources in this case study.
We first estimate ARs (for chemicals in PCPs and pesticides/antimicrobials) and ERs (for the plasticizers) as model inputs. The ARs are calculated in accordance with Isaacs et al.(20) and are product-chemical-combination-specific. Briefly, each chemical is mapped to one of 254 “product categories” defined in Isaacs et al.(20) and assigned category-specific product usage information, including use frequency, prevalence of its use in the general population, amount of product used per application, and the fraction contacting human skin. For simplification, we make two conservative assumptions: (i) when a chemical matches multiple product categories with different product application rates (i.e., a product of the frequency of application and the amount of a product per application), it is assigned to the category with the largest product application rate, and (ii) when a chemical is assigned to a product category, it is present at the same concentration in all products in this category. After the assignment of product categories, we gather chemical-specific weight fraction (i.e., concentration in product) from the Consumer Product Chemical Profiles database (CPCPdb)(37) available on the USEPA’s CompTox Chemistry Dashboard. For a few chemicals, CPCPdb records ranges of the chemical weight fraction observed in multiple products. The maximum and minimum of these ranges, as well as their average, are used for our calculations. With the information above, we calculate an “apparent” AR for each chemical (SI Table S3). The apparent ARs are then converted to the absorbed ARs by considering the duration of application (SI Table S3).
Emission of plasticizers from an article surface is treated as a diffusive process. For each chemical, the ER is calculated based on an air-side mass transfer coefficient, the mass concentration of total suspended particles in indoor air, a typical article surface area, and the fugacity in the air immediately adjacent to the article surface (f0) (see notes below SI Table S3). The f0 is assumed to be equal to the fugacity in the article, which is in turn calculated from a chemical weight fraction (collected from the CPCPdb) and a fugacity capacity (Z-value) of the article material calculated from a material-air partition coefficient. Note that the calculated fugacity is limited by a chemical’s vapor pressure, i.e., a theoretical maximum fugacity which applies when the chemical weight fraction is too large and thus a thermodynamically separated pure phase exists.(38,39) The calculated ERs are listed in SI Table S3.
Figure 2 compares the RAIDAR-ICE iRs against inferred NHANES iRs.(17) Averages of our modeled iRs are significantly correlated with the inferred medians (r2 = 0.52, p < 0.01, regression coefficient = 0.88 log unit). The Spearman’s rank correlation coefficient between modeled iRs and inferred medians is 0.68 (p < 0.01), indicating that rankings based on model predictions and observations are quite similar for the 37 chemicals. Of the 37 chemicals, the modeled and inferred iRs agree with each other within 1 order of magnitude for 16 chemicals, and 2 orders of magnitude for 31 chemicals. When attempting to reproduce inferred NHANES exposure, these RAIDAR-ICE calculations are in slightly better agreement than exposure estimates derived from SHEDS-HT (r2= 0.39, p < 0.01, regression coefficient = 2.5 log unit),(20) although we emphasize that the two studies share only 27 chemicals because we focused on chemicals used indoors. In Figure 2, most markers are situated above the diagonal line (representing ideal agreement), indicating that the model often overpredicts the inferred NHANES exposure. The overprediction is not surprising because the model inputs of ERs and ARs are associated with conservative assumptions. It should further be noted that the inferred exposure rates are also highly uncertain.
Figure 2.
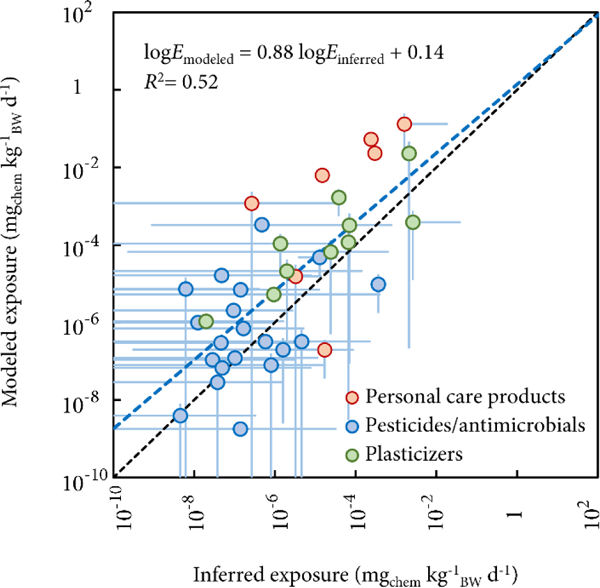
Comparison between RAIDAR-ICE modeled intake rates (based on averages of the reported ranges of chemical weight fractions) and the median intake rates inferred from NHANES biomonitoring data. Vertical error bars denote the ranges of modeled intake rates based on the maximum and minimum of chemical weight fractions reported in the Consumer Product Chemical Profiles database (CPCPdb). Horizontal error bars denote the 5th and 95th percentiles of the inferred values. Blue dashed line indicates a log–log regression between the modeled and inferred intake rates.
Dots representing chemical exposure under both direct (chemicals in PCPs and pesticides/antimicrobials) and indirect (plasticizers) scenarios are evenly distributed on either side of the diagonal (Figure 2), indicating that the model’s performance does not differ between the two exposure scenarios. However, a closer inspection of Figure 2 shows that dots representing chemicals in PCPs depart farther from the diagonal than those representing pesticides/antimicrobials and plasticizers. The chemicals in PCPs investigated here possess a log KOW between 2 and 4; dermal permeation overwhelmingly dominates human exposure to these chemicals. To understand such a deviation, we analyze the extent to which the modeled iRs for chemicals in PCPs are sensitive to individual inputs (SI Table S4). The sensitivity analysis indicates that our modeled iRs are proportional to the provided ARs (i.e., S = 1), moderately sensitive to KOW (|S|<1) and almost insensitive to KOA and degradation half-life in air (|S| < 0.02 for most PCPs). That is, uncertainty can be proportionally propagated from the input ARs to the modeled iRs; this is because direct application of PCPs onto the human skin is the starting point of model calculation. In addition, the calculated sensitivities are negative with respect to KOW (SI Table S4), indicating that an increase in hydrophobicity decreases aggregate iRs, and in particular dermal permeation iRs. This is because increasing KOW not only decreases maximum chemical flux through the skin (i.e, the rate of transdermal absorption),(34) it also increases the relative “elimination” rates of chemicals from skin via bathing and cell loss. As such, the sensitivity analysis points to two reasons likely responsible for the more noticeable departure from observations for chemicals in PCPs. First, the ARs used here are calculated based on category-specific usage patterns rather than detailed product-specific information, which can be a source of uncertainty. Second, the model description of processes that are governed by partitioning (in particular KOW), for example, absorption and loss of chemicals on the human skin, can be uncertain.
Case 2: Screening and prioritizing chemicals based on exposure and risk-based metrics
In this case, we compare the rankings of 131 chemicals included in the U.S. EPA ToxCast Program based on four RAIDAR-ICE metrics: Tier 1 iF, whole-body concentration based on a consistent unit emission rate, critical emission rate, and bioactivity quotient (Figure 3). The 131 chemicals (SI Table S5) were identified to belong to use categories “indoor emissions” or “passive indoor emissions” according to Shin et al.(40) thus have confirmed indoor uses. Most (>80%) of these chemicals have a log KOA between 4 and 10, a log KOW lower than 5, and degradation half-lives in indoor air ranging from 2.6 to 300 h (SI Table S5). The list also includes a few highly recalcitrant and hydrophobic organochlorine pesticides identified as persistent organic pollutants, e.g., mirex (log KOA = 11.6, log KOW = 7.4, and HLAir = 10 000 h) and dichlorodiphenyltrichloroethane (DDT; log KOA = 10.3, log KOW = 6.8, and HLAir = 510 h) (SI Table S5). Note that we calculate here only exposure based on near-field sources although we recognize that substantial exposure to some of these chemicals also occurs via the far-field pathways. Instead of considering realistic modes of emission or direct application, we assume all chemicals are emitted 100% to indoor air for simplification in this demonstration.
Figure 3.
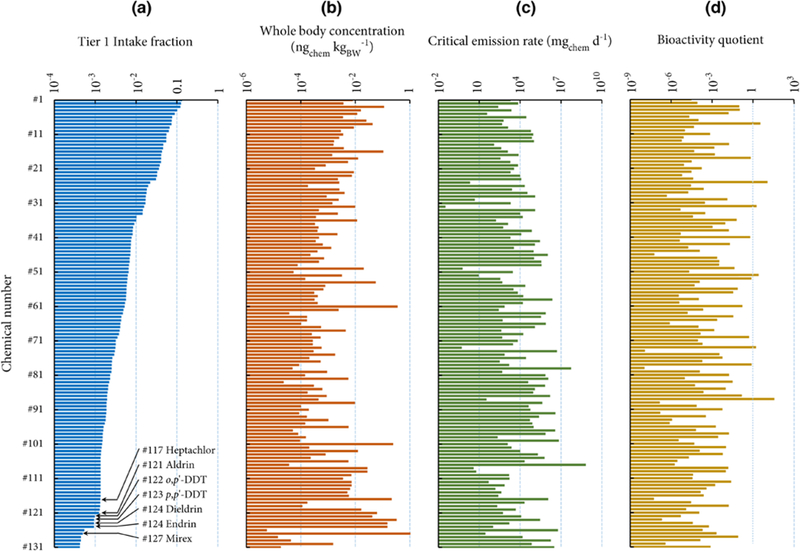
(a) Tier 1 intake fractions, (b) unit emission rate-based whole-body concentrations, (c) critical emission rates, and (d) bioactivity quotients of 131 chemicals released into indoor air, ranked by the Tier 1 intake fractions. Higher concern is technically associated with chemicals with higher Tier 1 intake fractions, unit emission rate-based whole-body concentrations, and bioactivity quotients, but those with lower critical emission rates. Names of the chemicals are given in SI Table S5. Organochlorine pesticides identified as persistent organic pollutants are labeled.
As shown in Figure 3a, the iFs span over 3 orders of magnitude, from 4.2 × 10–4 [di(2-ethylhexyl)adipate] to 0.13 (benzidine). These iFs have been previously evaluated by Shin et al.,(40) in which maxima of estimates derived from three models(19,21,22) were demonstrated to correlate well with exposure levels inferred from biomonitoring data. Our calculated iFs correlate significantly with those by Shin et al.(40) (r2 = 0.54, p < 0.01; data not shown). SI Figure S2 shows the relative contribution of individual near-field exposure routes to the calculated iFs. Chemicals with high iFs are associated with higher dermal permeation, while chemicals with low iFs tend to be primarily inhaled. The inhalation iF is quite similar for all chemicals, irrespective of partition coefficients and degradation half-lives, because the fate of these chemicals in indoor air is largely governed by physical processes(6) (e.g., ventilation(22) and dust removal(19)), which are similar for all compounds investigated here. By contrast, dermal permeation is more variable, spanning over 2 orders of magnitude, and is more sensitive to the chemical’s properties. Nondietary ingestion contributes little to human exposure for these chemicals and this assumed use scenario.
As shown in Figure 3b, RAIDAR-ICE ranks the 131 chemicals based on a “unit emission rate-based whole-body concentration (CU)”, which accounts for toxicokinetic differences between chemicals. For the chemicals considered, CU ranges from 6.0 × 10–6 to 1.7 ngchem kgBW–1. Persistent and lipophilic chemicals tend to have higher CU values because they are slowly biotransformed and eliminated. For example, despite their low iFs persistent organochlorine pesticides, e.g., mirex (ranking 1), p,p’-DDT (ranking 2), endrin (ranking 3), and dieldrin (ranking 4), exhibited the highest biological concentrations. Notably, mirex now ranks first with respect to CU (Figure 3b), although it ranked 127 in terms of iF (Figure 3a). Rankings based on CU and iFs are poorly correlated (Spearman’s rank correlation coefficient = 0.17).
For the third ranking exercise, the 131 chemicals are compared using the critical emission rate (ERC), which additionally considers toxicity or bioactivity threshold data (Figure 3c). Here, ERC is calculated using OEDs derived from the U.S. EPA ToxCast in vitro bioactivity data (SI Table S5);(40) a lower ERC corresponds to “higher concern”. The OEDs are extrapolated from concentrations leading to biological responses in in vitro assays with cells or isolated proteins, instead of individual-level adverse end points (e.g., neurotoxicity) or specific mechanisms of action (e.g., mutagenicity). Therefore, bioactivity is not necessarily indicative of the potential for an adverse effect.(35) Figure 3c shows that ERC spans approximately 10 orders of magnitude for these 131 chemicals, from 3.9 μg d–1 (imazalil, chemical #32) to 660 kg d–1 (methyl laurate, chemical #107). The Spearman’s rank correlation coefficient between ERC and CU is 0.52 (p < 0.01), indicating that chemicals with higher biological concentrations are more likely to reach the threshold of biological activity in the human body.
Finally, the ERC can be compared with an estimate of the actual emission rate (ERA) to derive a unitless risk assessment factor (RAF), or in this case referred to as a bioactivity quotient (BQ; Figure 3d) because the OEDs used as effect thresholds reflect bioactivity.(40) The ERA estimates for 131 chemicals are obtained by dividing their assumed national emissions by the number of exposed U.S. households (both data from ref (40)). The BQs span approximately 10 orders of magnitude for these 131 chemicals with most chemicals having a BQ < 1. Six chemicals exhibit BQs > 1, suggesting prioritization for further scrutiny of the results and possibly more comprehensive assessment (Figure 3d). All six chemicals are also in a prioritized list by Shin et al.(40) using multiple exposure models; notably, both studies highlight the potential risk of triphenyl phosphate because of its large estimated ERA (linked to its production volume in the U.S.) and relatively low minimum OEDs. This indicates that RAIDAR-ICE is compatible with existing approaches using multiple exposure models.
In summary, RAIDAR-ICE efficiently discriminates diverse chemicals based on exposure- and risk-based assessment metrics; however, chemical rankings can be very different if distinct metrics are selected. The metric selected is based on the assessment objectives and decision-making context. Exposure-based assessment metrics, that is, Tier 1 iF and the whole-body concentration, characterize an “emission-to-intake” relationship and thus are suitable for evaluating the likelihood of human contact with chemicals. Oftentimes, Tier 1 iF is employed to inform external human exposure (e.g., daily intake) and relative exposure potential, whereas a blood or body concentration evaluates chemicals whose toxicological effects are associated with internal cumulative exposure since it includes absorption, biotransformation and passive elimination processes.(41) Modeled blood concentrations can be directly compared with biomonitoring observations for model evaluation.(41) Calculated ERC provides theoretical emission guidance for setting putative use thresholds for chemicals prior to commercialization or for evaluating a proposed change to the use of a chemical already on the market.(16) Risk-based assessment metrics, that is, BQ or RAF, link estimated exposure with hazard, and thus gauge the potential for biological activity or adverse outcomes respectively resulting from human contact with chemicals.(42) They can be used for prioritizing chemicals for higher-tiered assessment, while providing insights for risk reduction measures.(43) For example, a chemical’s ranking based on the RAF (or BQ) will be lower if its actual use and emission are lowered, although such a restriction does not affect the rankings based on the other metrics in Figure 3.
Case 3: Coupled near- and far-field exposure modeling
The RAIDAR-ICE calculations in Case 2 (release of chemicals to indoor air only) indicate that 30–99% of indoor emissions can be ventilated out to the far-field environment (data not shown), where the chemicals then contaminate food chains, outdoor air and fresh water and are taken up by humans through diet, inhalation and drinking water. The exposure of single consumers from the far-field environment arises from not only their own use of these chemicals, but also the use by their neighbors, and even the entire regional population. Therefore, it is of interest to know the extent ventilated fractions contribute to human exposure from the far-field sources, and by extension, the relative exposures from near- and far-field sources. Here, we couple the RAIDAR-ICE and RAIDAR models to calculate and compare population Tier 1 iFs of the 131 chemicals in Case 2 from near- and far-field environments. We first assume a constant, unit emission rate to indoor air in RAIDAR-ICE and calculate ventilation fluxes (in ng h–1) for the 131 chemicals from a single house (SI Figure S3). These ventilation fluxes, multiplied with the assumed number of houses in the region (assumed to be 1/4 of the population in the RAIDAR region), then serve as input to the air compartment in RAIDAR (SI Figure S3). A simplifying assumption underlying this illustrative case is that air exchange is the single vehicle transporting contamination from indoor to outdoor environments, although we recognize that indoor-released chemicals can also reach the outdoor environment via other routes such as sewage. RAIDAR-ICE and RAIDAR generate respective near- and far-field iRs for the modeled individual, the combination of which across the modeled region yields population iRs. The population iRs are then normalized by the summed regional emission rate to derive population iFs (SI Figure S3).
Figure 4 shows the relative contribution of inhalation of indoor air, dermal permeation, diet and other routes (nondietary ingestion, inhalation of outdoor air and drinking water) to the aggregate iFs. Overall, dietary exposure from the far-field environment contributes little to the aggregate iFs of most of the 131 chemicals. Nevertheless, ignoring dietary exposure would underestimate the total iFs by almost a factor of 10 for mirex (chemical no. 127), DDT (chemical nos. 122 and 123) and dieldrin and endrin (chemical nos. 124), even though all emissions are assumed indoors in these simulations. SI Figure S4 shows the dependence of the dietary contribution on the partition coefficients of chemicals. Dietary exposure matters for persistent (normalized biotransformation half-life >100 h) chemicals with a log KOA between 6 and 12 and a log KOW between 5 and 8 (SI Figure S4). These chemicals have a strong tendency to partition from air into organic phases in surface media and organisms, thus being most bioaccumulative among all investigated chemicals. Our identified range of partition coefficients agrees well with the range of chemicals of highest “environmental bioaccumulation potential” by Czub and McLachlan.(44) By contrast, most investigated chemicals are too water-soluble (log KOW < 5; SI Figure S4) to substantially bioaccumulate. Likewise, Ernstoff et al.(7) observed that 65 of 69 evaluated chemical ingredients in shampoo (all with −1 < log KOW < 6.5) have higher product intake fractions (PiF) during the use phase (via direct application and inhalation) than during the waste disposal phase (via inhalation of outdoor air and ingestion of water and food items); the difference can be up to a factor of 104. This illustrative case highlights that far-field exposure routes can still make a substantial contribution to aggregate human exposure even when the chemicals are released indoors. A modeling framework that integrates both near- and far-field exposure routes is thus essential for a comprehensive understanding of aggregate human exposure.
Figure 4.
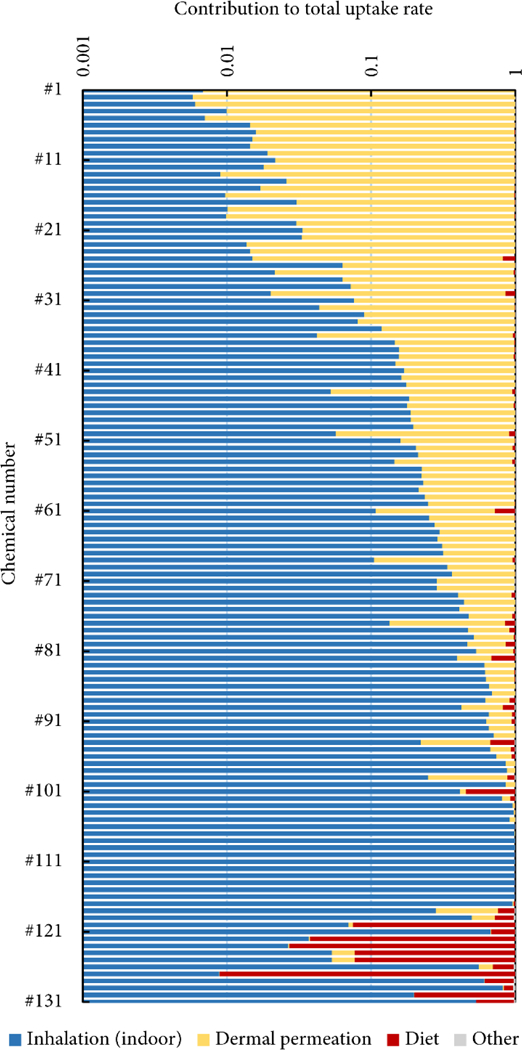
Contributions of near- and far-field routes to total human intake rate of 131 chemicals.
Discussion
Merits of the RAIDAR-ICE model
Case 1 demonstrates that RAIDAR-ICE is capable of modeling both direct and indirect human exposure scenarios within a single coherent and compatible framework. Such a holistic framework is useful because it provides directly comparable results for chemicals in different application categories and use scenarios. Case 2 illustrates the application of the model for different assessment objectives (e.g., exposure- or risk-based), and how chemical rankings, and ostensibly priorities for more comprehensive assessment, change depending on assessment metrics selected. Unlike a number of existing exposure models, e.g., Shin et al.(21) and Wenger et al.,(22)which sort chemicals based on human external exposure alone, RAIDAR-ICE establishes a continuum from emissions to blood and urine concentrations. This facilitates comparisons of exposure predictions directly with toxicity or bioactivity data, thus informing chemical risk assessment and risk reduction measures. Case 3 reconciles near- and far-field exposure models to achieve a systematic and harmonized evaluation of aggregate human exposure. Whereas a few previous efforts have integrated multiple exposure routes,(7,45,46) most of them largely rely on empirical transfer factors, that is, chemical mass fractions moving from one compartment to another. For many chemicals, these factors are missing or inadequate, which limits the application range and predictive capacity of these models. Instead, both RAIDAR-ICE and RAIDAR are mechanistic (process-based) models and link fate, human exposure and toxicokinetics with properties of chemicals and the associated products/articles. This facilitates the use of the RAIDAR models for HT applications to a wide range of emerging chemicals while the internal exposure metrics (i.e., blood or urine concentrations) enable model evaluation with measured biomonitoring data. The internal exposure calculations also serve as a proxy for the concentration at sites of possible toxicological action, providing a quantitative exposure bridge with molecular initiating events of the adverse outcome pathway (AOP)(47) and related toxicological frameworks.(25)
Limitations and recommendations
Earlier work has demonstrated that chemical emission rates are the greatest contributors to the overall uncertainty in far-field human exposure modeling.(16) We expect estimates of actual ER and AR to also be a primary source of uncertainty in model input for the near-field RAIDAR-ICE model calculations pertaining to actual exposure- and risk-based estimates. Accurate emission and application information is often not readily available for most chemicals. For example, it is difficult to determine whether the calculated iRs based on algorithmically simplistic calculations of ARs and ERs overestimate or underestimate the actual exposure to chemicals. Using category-based usage patterns aims to have the calculated ARs and ERs represent reasonable maxima. However, even chemicals explicitly made for indoor use can be released into the environment throughout the lifecycle, for example, during industrial processing, use phase and waste disposal.(48) The ARs and ERs estimated for the use phase alone therefore constitute only a fraction of total lifecycle emissions. Future research can address the uncertainty from ARs and ERs by refining and improving methods for estimating ARs and ERs in a mechanistic, lifecycle-based manner.
Additional RAIDAR-ICE model evaluation efforts are encouraged. Comprehensive model evaluations require spatially and temporally consistent multimedia monitoring and biomonitoring data with chemical use and human behavior information. A recent review(49) has compiled measurements of semivolatile organic compounds in a wide range of articles and indoor compartments, which can serve as a valuable starting point for further model evaluations. Meanwhile, values of key parameters, for example, exchange rate between indoor and outdoor air,(6) can be further examined and refined to ensure greater representation of variability. For example, new work indicates that diet dominates human exposure to polychlorinated biphenyl congener 153 among the general Swedish population while indoor sources contribute more than 80% of its total regional emission, that is, ventilation from the indoor to outdoor environments plays a critical role in human exposure via far-field pathways.(9) In this sense, a comprehensive understanding of human chemical exposure via multiple relevant vectors helps improve environmental and health management decision-making, which in turn necessitates a combination of near- and far-field models.(45)
Finally, the application domain of RAIDAR-ICE can be expanded. The current version of RAIDAR-ICE does not discriminate between neutral and disassociated species for ionizable organic chemicals (IOCs), which may be a source of the less satisfactory performance in reproducing the inferred exposure to some chemicals, for example, PCPs. This warrants inclusion of algorithms to better address the properties and behavior of IOCs in the indoor environment and in humans. Furthermore, RAIDAR-ICE is a steady-state model and this condition may not always be satisfied, for example, when a chemical has episodic, intermittent use. Whereas a calculated iF is independent from whether an application/emission is episodic or continuous,(50) calculated iRs can be overestimated in cases of highly degradable chemicals. A dynamic version of RAIDAR-ICE or correction algorithms are necessary to expand the capacity of this framework to address a more extensive range of chemical use and exposure scenarios.
Supplementary Material
Acknowledgement
The research has been funded in part by the U.S. Environmental Protection Agency through contract EP-12-D-000404 and by the American Chemistry Council Long-Range Research Initiative program (Contract Number 6459) to ARC Arnot Research and Consulting. The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the U.S. EPA. This publication has not been formally reviewed by the American Chemistry Council. We appreciate helpful comments from Daniel Vallero and Paul Price (U.S. EPA) on a previous draft of the manuscript. L.L. acknowledges support through a MITACS Elevate postdoctoral fellowship.
References
- 1.Muir DC; Howard PH Are there other persistent organic pollutants? A challenge for environmental chemists. Environ. Sci. Technol. 2006, 40 (23), 7157–7166, DOI: 10.1021/es061677a [DOI] [PubMed] [Google Scholar]
- 2.Egeghy PP; Vallero DA; Hubal EAC Exposure-based prioritization of chemicals for risk assessment.Environ. Sci. Policy 2011, 14 (8), 950– 964, DOI: 10.1016/j.envsci.2011.07.010 [DOI] [Google Scholar]
- 3.Bonnell MA; Zidek A; Griffiths A; Gutzman D Fate and exposure modeling in regulatory chemical evaluation: new directions from retrospection. Environ. Sci. Process. Impacts 2018, 20, 20– 31, DOI: 10.1039/C7EM00510E [DOI] [PubMed] [Google Scholar]
- 4.Daughton CG; Ternes TA Pharmaceuticals and personal care products in the environment: agents of subtle change?. Environ. Health Perspect. 1999, 107 (Suppl 6), 907– 938, DOI: 10.1289/ehp.99107s6907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weschler CJ; Nazaroff WW Semivolatile organic compounds in indoor environments. Atmos. Environ.2008, 42 (40), 9018–9040, DOI: 10.1016/j.atmosenv.2008.09.052 [DOI] [Google Scholar]
- 6.Hellweg S; Demou E; Bruzzi R; Meijer A; Rosenbaum RK; Huijbregts MAJ; McKone TE Integrating human indoor air pollutant exposure within life cycle impact assessment. Environ. Sci. Technol.2009, 43 (6), 1670– 1679, DOI: 10.1021/es8018176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernstoff AS; Fantke P; Csiszar SA; Henderson AD; Chung S; Jolliet O Multi-pathway exposure modeling of chemicals in cosmetics with application to shampoo. Environ. Int. 2016, 92–93, 87– 96, DOI: 10.1016/j.envint.2016.03.014 [DOI] [PubMed] [Google Scholar]
- 8.Csiszar SA; Meyer DE; Dionisio KL; Egeghy P; Isaacs KK; Price PS; Scanlon KA; Tan Y-M;Thomas K; Vallero D Conceptual framework to extend life cycle assessment using near-field human exposure modeling and high-throughput tools for chemicals. Environ. Sci. Technol. 2016, 50 (21), 11922–11934, DOI: 10.1021/acs.est.6b02277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L; Arnot JA; Wania F Revisiting the contributions of far- and near-field routes to aggregate human exposure to polychlorinated biphenyls (PCBs). Environ. Sci. Technol. 2018, 52 (12), 6974– 6984, DOI: 10.1021/acs.est.8b00151 [DOI] [PubMed] [Google Scholar]
- 10.Egeghy PP; Sheldon LS; Isaacs KK; Özkaynak H; Goldsmith M-R; Wambaugh JF;Judson RS; Buckley TJ Computational exposure science: an emerging discipline to support 21st-century risk assessment. Environ. Health Perspect. 2016, 124 (6), 697– 702, DOI: 10.1289/ehp.1509748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egeghy PP; Judson R; Gangwal S; Mosher S; Smith D; Vail J; Hubal EAC The exposure data landscape for manufactured chemicals. Sci. Total Environ. 2012, 414, 159– 166, DOI: 10.1016/j.scitotenv.2011.10.046 [DOI] [PubMed] [Google Scholar]
- 12.Czub G; McLachlan MS A food chain model to predict the levels of lipophilic organic contaminants in humans. Environ. Toxicol. Chem. 2004, 23 (10), 2356– 2366, DOI: 10.1897/03-317 [DOI] [PubMed] [Google Scholar]
- 13.Breivik K; Czub G; McLachlan MS; Wania F Towards an understanding of the link between environmental emissions and human body burdens of PCBs using CoZMoMAN. Environ. Int. 2010, 36 (1),85– 91, DOI: 10.1016/j.envint.2009.10.006 [DOI] [PubMed] [Google Scholar]
- 14.Rosenbaum RK; Bachmann TM; Gold LS; Huijbregts MA; Jolliet O; Juraske R; Koehler A;Larsen HF; MacLeod M; Margni M USEtox—the UNEP-SETAC toxicity model: recommended characterisation factors for human toxicity and freshwater ecotoxicity in life cycle impact assessment. Int. J. Life Cycle Assess. 2008, 13 (7), 532, DOI: 10.1007/s11367-008-0038-4 [DOI] [Google Scholar]
- 15.Arnot JA; MacKay D; Webster E; Southwood JM Screening level risk assessment model for chemical fate and effects in the environment. Environ. Sci. Technol. 2006, 40 (7), 2316– 2323, DOI: 10.1021/es0514085 [DOI] [PubMed] [Google Scholar]
- 16.Arnot JA; Brown TN; Wania F; Breivik K; McLachlan MS Prioritizing chemicals and data requirements for screening-level exposure and risk assessment. Environ. Health Perspect. 2012, 120 (11),1565, DOI: 10.1289/ehp.1205355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wambaugh JF; Setzer RW; Reif DM; Gangwal S; Mitchell-Blackwood J; Arnot JA; Joliet O;Frame A; Rabinowitz J; Knudsen TB High-throughput models for exposure-based chemical prioritization in the ExpoCast project. Environ. Sci. Technol. 2013, 47 (15), 8479–8488, DOI: 10.1021/es400482g [DOI] [PubMed] [Google Scholar]
- 18.Mitchell J; Arnot JA; Jolliet O; Georgopoulos PG; Isukapalli S; Dasgupta S; Pandian M;Wambaugh J; Egeghy P; Cohen Hubal EA; Vallero DA Comparison of modeling approaches to prioritize chemicals based on estimates of exposure and exposure potential. Sci. Total Environ. 2013, 458–460, 555– 567, DOI: 10.1016/j.scitotenv.2013.04.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X; Arnot JA; Wania F Model for screening-level assessment of near-field human exposure to neutral organic chemicals released indoors. Environ. Sci. Technol. 2014, 48 (20), 12312– 12319, DOI: 10.1021/es502718k [DOI] [PubMed] [Google Scholar]
- 20.Isaacs KK; Glen WG; Egeghy P; Goldsmith M-R; Smith L; Vallero D; Brooks R; Grulke CM;Özkaynak H SHEDS-HT: An integrated probabilistic exposure model for prioritizing exposures to chemicals with near-field and dietary sources. Environ. Sci. Technol. 2014, 48 (21), 12750–12759, DOI: 10.1021/es502513w [DOI] [PubMed] [Google Scholar]
- 21.Shin H-M; McKone TE; Bennett DH Intake fraction for the indoor environment: a tool for prioritizing indoor chemical sources. Environ. Sci. Technol. 2012, 46 (18), 10063–10072, DOI: 10.1021/es3018286 [DOI] [PubMed] [Google Scholar]
- 22.Wenger Y; Li D; Jolliet O Indoor intake fraction considering surface sorption of air organic compounds for life cycle assessment. Int. J. Life Cycle Assess. 2012, 17 (7), 919–931, DOI: 10.1007/s11367-012-0420-0 [DOI] [Google Scholar]
- 23.Delmaar JE; Schuur AG ConsExpo Web: Consumer Exposure models model documentation (RIVM Report 2017–0197); National Institute for Public Health and the Environment: Bilthoven, The Netherlands,2018. [Google Scholar]
- 24.ICF Consulting Consumer Exposure Model (CEM) Version 2.0 User Guide; USEPA Office of Pollution Prevention and Toxics: Washington D.C, 2012. [Google Scholar]
- 25.Teeguarden JG; Tan Y-M; Edwards SW; Leonard JA; Anderson KA; Corley RA; Kile ML;Simonich SM; Stone D; Tanguay RL Completing the link between exposure science and toxicology for improved environmental health decision making: The aggregate exposure pathway framework. Environ. Sci. Technol. 2016, 50, 4579–4586,DOI: 10.1021/acs.est.5b05311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Academy of Sciences Engineering and Medicine Using 21st Century Science to Improve Risk-Related Evaluations; Washington, DC, 2017. [PubMed] [Google Scholar]
- 27.Mackay D Multimedia environmental models: the fugacity approach. CRC press: 2001. [Google Scholar]
- 28.U.S. Environmental Protection Agency Estimation Programs Interface (EPI) Suite for Microsoft® Windows, ver. 4.1. Released October, 2011; U.S. Environmental Protection Agency: Washington, DC, 2011. [Google Scholar]
- 29.Arnot JA; Brown TN; Wania F Estimating screening-level organic chemical half-lives in humans.Environ. Sci. Technol. 2014, 48 (1), 723– 730, DOI: 10.1021/es4029414 [DOI] [PubMed] [Google Scholar]
- 30.Papa E; Sangion A; Arnot JA; Gramatica P Development of human biotransformation QSARs and application for PBT assessment refinement. Food Chem. Toxicol. 2018, 112, 535– 543, DOI: 10.1016/j.fct.2017.04.016 [DOI] [PubMed] [Google Scholar]
- 31.Tibaldi R; ten Berge W; Drolet D Dermal absorption of chemicals: estimation by IH SkinPerm. J. Occup. Environ. Hyg. 2014, 11 (1), 19–31, DOI: 10.1080/15459624.2013.831983 [DOI] [PubMed] [Google Scholar]
- 32.Bennett DH; McKone TE; Evans JS; Nazaroff WW; Margni MD; Jolliet O; Smith KR Peer reviewed: Defining intake fraction. Environ. Sci. Technol. 2002, 36 (9), 206A– 211A, DOI: 10.1021/es0222770 [DOI] [PubMed] [Google Scholar]
- 33.Arnot JA; Mackay D Policies for chemical hazard and risk priority setting: Can persistence, bioaccumulation, toxicity, and quantity information be combined?. Environ. Sci. Technol. 2008, 42 (13),4648–4654, DOI: 10.1021/es800106g [DOI] [PubMed] [Google Scholar]
- 34.Brown TN; Armitage JM; Egeghy P; Kircanski I; Arnot JA Dermal permeation data and models for the prioritization and screening-level exposure assessment of organic chemicals. Environ. Int 2016, 94,424– 435, DOI: 10.1016/j.envint.2016.05.025 [DOI] [PubMed] [Google Scholar]
- 35.Dix DJ; Houck KA; Martin MT; Richard AM; Setzer RW; Kavlock RJ The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicol. Sci. 2007, 95 (1) 5–12, DOI: 10.1093/toxsci/kfl103 [DOI] [PubMed] [Google Scholar]
- 36.Dionisio KL; Frame AM; Goldsmith M-R; Wambaugh JF; Liddell A; Cathey T; Smith D; Vail J;Ernstoff AS; Fantke P; Jolliet O; Judson RS Exploring consumer exposure pathways and patterns of use for chemicals in the environment. Toxicol. Rep. 2015, 2, 228–237,DOI: 10.1016/j.toxrep.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldsmith MR;Grulke CM; Brooks RD; Transue TR; Tan YM; Frame A; Egeghy PP;Edwards R; Chang DT; Tornero-Velez R; Isaacs K; Wang A; Johnson J; Holm K; Reich M;Mitchell J; Vallero DA; Phillips L; Phillips M; Wambaugh JF; Judson RS; Buckley TJ;Dary CC Development of a consumer product ingredient database for chemical exposure screening and prioritization. Food Chem. Toxicol. 2014, 65, 269– 279, DOI: 10.1016/j.fct.2013.12.029 [DOI] [PubMed] [Google Scholar]
- 38.Xu Y; Liu Z; Park J; Clausen PA; Benning JL; Little JC Measuring and predicting the emission rate of phthalate plasticizer from vinyl flooring in a specially-designed chamber. Environ. Sci. Technol 2012,46 (22), 12534– 12541, DOI: 10.1021/es302319m [DOI] [PubMed] [Google Scholar]
- 39.Little JC; Weschler CJ; Nazaroff WW; Liu Z; Cohen Hubal EA Rapid methods to estimate potential exposure to semivolatile organic compounds in the indoor environment. Environ. Sci. Technol.2012, 46 (20), 11171–11178, DOI: 10.1021/es301088a [DOI] [PubMed] [Google Scholar]
- 40.Shin H-M; Ernstoff A; Arnot JA; Wetmore BA; Csiszar SA; Fantke P; Zhang X; McKone TE;Jolliet O; Bennett DH Risk-based high-throughput chemical screening and prioritization using exposure models and in vitro bioactivity assays. Environ. Sci. Technol. 2015, 49 (11), 6760–6771, DOI: 10.1021/acs.est.5b00498 [DOI] [PubMed] [Google Scholar]
- 41.Judson R; Houck K; Martin M; Knudsen T; Thomas RS; Sipes N; Shah I; Wambaugh J;Crofton K In vitro and modelling approaches to risk assessment from the U.S. Environmental Protection Agency ToxCast Programme. Basic Clin. Pharmacol. Toxicol. 2014, 115 (1), 69– 76, DOI: 10.1111/bcpt.12239 [DOI] [PubMed] [Google Scholar]
- 42.Wetmore BA; Wambaugh JF; Ferguson SS; Sochaski MA; Rotroff DM; Freeman K;Clewell HJ; Dix DJ; Andersen ME; Houck KA; Allen B; Judson RS; Singh R; Kavlock RJ;Richard AM; Thomas RS Integration of dosimetry, exposure, and high-throughput screening data in chemical toxicity assessment. Toxicol. Sci 2012, 125 (1), 157–174, DOI: 10.1093/toxsci/kfr254 [DOI] [PubMed] [Google Scholar]
- 43.Rotroff DM; Wetmore BA; Dix DJ; Ferguson SS; Clewell HJ; Houck KA; LeCluyse EL;Andersen ME; Judson RS; Smith CM; Sochaski MA; Kavlock RJ; Boellmann F; Martin MT;Reif DM; Wambaugh JF; Thomas RS Incorporating human dosimetry and exposure into high-throughput in vitro toxicity screening. Toxicol. Sci 2010, 117 (2), 348– 358, DOI: 10.1093/toxsci/kfq220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Czub G; McLachlan MS Bioaccumulation potential of persistent organic chemicals in humans. Environ. Sci. Technol. 2004, 38 (8), 2406– 2412, DOI: 10.1021/es034871v [DOI] [PubMed] [Google Scholar]
- 45.Fantke P; Ernstoff AS; Huang L; Csiszar SA; Jolliet O Coupled near-field and far-field exposure assessment framework for chemicals in consumer products. Environ. Int. 2016, 94, 508–518, DOI: 10.1016/j.envint.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 46.Shin HM; McKone T; Bennett D Model framework for integrating multiple exposure pathways to chemicals in household cleaning products. Indoor air 2017, 27 (4), 829– 839, DOI: 10.1111/ina.12356 [DOI] [PubMed] [Google Scholar]
- 47.Ankley GT; Bennett RS; Erickson RJ; Hoff DJ; Hornung MW; Johnson RD; Mount DR;Nichols JW; Russom CL; Schmieder PK Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010, 29 (3), 730–741,DOI: 10.1002/etc.34 [DOI] [PubMed] [Google Scholar]
- 48.Li L; Wania F Occurrence of single- and double-peaked emission profiles of synthetic chemicals. Environ. Sci. Technol. 2018, 52 (8), 4684–4693, DOI: 10.1021/acs.est.7b06478 [DOI] [PubMed] [Google Scholar]
- 49.Lucattini L; Poma G; Covaci A; de Boer J; Lamoree MH; Leonards PEG A review of semi-volatile organic compounds (SVOCs) in the indoor environment: occurrence in consumer products, indoor air and dust. Chemosphere 2018, 201, 466–482, DOI: 10.1016/j.chemosphere.2018.02.161 [DOI] [PubMed] [Google Scholar]
- 50.Nazaroff WW Inhalation intake fraction of pollutants from episodic indoor emissions. Build. Environ. 2008,43 (3), 269–277, DOI: 10.1016/j.buildenv.2006.03.021 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


