Abstract
Deficits in DNA damage repair pathways are the root cause of several human cancers. In mammalian cells, DNA double-strand break repair is carried out by multiple mechanisms, including homologous recombination (HR). PALB2 (Partner and Localizer of BRCA2), which is an essential factor for HR, binds to the breast cancer susceptibility 1 (BRCA1) protein at DNA double-strand breaks. At the break site, PALB2 also associates with breast cancer susceptibility 2 (BRCA2) protein, to form a multi-protein complex that facilitates HR. The BRCA1-PALB2 interaction is mediated by association of predicted helical coiled-coil regions in both proteins. PALB2 can also homodimerize through the formation of a coiled coil, by self-association of helical elements at the N-terminus of the PALB2 protein, and this homodimerization has been proposed to regulate the efficiency of HR. We have produced a segment of PALB2 (PALB2cc), which forms α-helical structures, and which assembles into stable homodimers or heterodimers with a PALB2-interacting segment of BRCA1 (BRCA1cc). The three-dimensional structure of the homodimer formed by PALB2cc was determined by solution NMR spectroscopy. This PALB2cc homodimer is a classical anti-parallel coiled-coil leucine zipper. NMR chemical shift perturbation studies were used to study dimer formation for both the PALB2cc homodimer and the PALB2cc:BRCA1cc heterodimer. Mutation of residue Leu24 of PALB2cc significantly reduces its homodimer stability, but has a more modest effect on the stability of the heterodimer formed between PALB2cc and BRCA1cc. We show that mutation of Leu24 leads to genomic instability and reduced cell viability after treatment with agents that induce DNA double-strand breaks. These studies may allow the identification of distinct mutations of PALB2 which selectively disrupt homodimeric versus heterodimeric interactions, and reveal the specific role of PALB2 homodimerization in HR.
Keywords: DNA damage, homologous recombination, PALB2, BRCA1, NMR structure determination, coiled coil
INTRODUCTION
Repair of DNA double-strand breaks in mammalian cells can be achieved by end-joining pathways or by homologous recombination (HR) 1, 2. Whereas end-joining pathways are considered to have mutagenic potential, HR has high fidelity of repair, because it restores the broken DNA using homologous sequence as a template. HR-mediated repair requires pairing of DNA sequences, which is achieved by strand invasion of a resected broken DNA end into a homologous DNA duplex. Strand invasion is catalyzed by RAD51, which is loaded onto DNA around the break site by BRCA2 3. Recruitment of BRCA2 to DNA double-strand breaks is therefore essential for cellular viability, as it enables efficient HR-mediated repair of breaks that arise during DNA replication and as a result of exposure to exogenous mutagens.
PALB2 (Partner and Localizer of BRCA2) is an essential factor for normal association of BRCA2 with broken DNA ends 4. Cells that lack PALB2 show a profound defect in HR and cell cycle checkpoint defects, and individuals who inherit bi-allelic mutations in the PALB2 gene are affected by a severe form of Fanconi Anemia, equivalent to other genetic mutations that prevent repair of DNA damage 5-7 In mice, PALB2 is essential for normal development, and conditional inactivation of Palb2 leads to tumorigenesis 8, 9. Mutations affecting the PALB2 gene have likewise been linked to cancer predisposition in humans 10-12. Determining the cellular activities of PALB2 is therefore of considerable importance for human health, and for a full understanding of the DNA damage response.
Whereas the structural basis for how PALB2 interacts with BRCA2 has been revealed 13, the specific molecular mechanisms by which PALB2 binds other repair factors are not fully characterized. PALB2 is recruited to DNA damage sites partly through direct interaction with DNA, or RPA-coated DNA 14,15, but stable recruitment of PALB2 to break sites has been shown to depend on its interaction with BRCA1 16, 17. BRCA2, PALB2 and BRCA1 therefore form a multiprotein complex at DNA double-strand break sites that enables normal HR 18. Several lines of evidence suggest that the BRCA1-PALB2 interaction is mediated by association of predicted helical coiled-coil regions in both proteins 19. Mutations affecting these predicted coiled-coil motifs in BRCA1 and PALB2 disrupt their ability to interact in vitro 16, 20, and mutagenesis of residues in the predicted coiled-coil region of PALB2 causes HR and male fertility defects in gene-targeted mice 21.
Previous work has additionally shown that PALB2 forms homo-oligomers through association of predicted coiled coil sequences at the N-terminus of the protein 14, 22. Homo-oligomerization of PALB2 has been proposed to have a regulatory role, potentially by sequestering PALB2 in a form that has a reduced ability to mediate HR. Deletion of the coiled-coil region from PALB2 increases the proportion of monomeric PALB2, which was shown to stimulate RAD51 filament formation in vitro 14. Coimmunoprecipitation experiments additionally showed that the N-terminal region of PALB2 can disrupt BRCA1-PALB2 heterodimers, by formation of a homo-oligomeric form of PALB2. Size-exclusion chromatography suggested that PALB2 forms a mixture of oligomers, but the stoichiometry of PALB2 homo-oligomers, and their relative stability versus PALB2-BRCA1 heterodimers has not previously been determined. In support of a role for PALB2 homo-oligomers in HR, ionizing radiation-induced DNA damage was reported to increase PALB2 self-association 22. On the other hand, Zhang et. al. generated a PALB2 fusion protein which can interact with BRCA1, but which cannot self-associate, to test the importance of homodimerization for DNA repair. Their study indicated that only heterodimerization of PALB2 with BRCA1 was needed for RAD51 foci formation and normal cell growth in the presence of the DNA damaging agent mitomycin C 23. The importance of PALB2 homo-oligomerization is therefore still unclear, and warrants further study.
In order to better understand the nature of oligomeric interactions formed by PALB2, we have tested the ability of polypeptide segments of PALB2 that include the predicted coiled-coil motif to form stable helices and to self-associate. Using circular dichroism (CD) and Nuclear Magnetic Resonance (NMR) spectroscopy, we have characterized fragments of PALB2 that form stable α-helical structures and assemble into stable homodimers. The three-dimensional structure of a homodimer of one of these homodimeric segments of PALB2 was determined by solution-state NMR spectroscopy. The PALB2 homodimer interaction is mediated by an anti-parallel coiled-coil structure, stabilized by key Leu and other hydrophobic residues at the dimer interface. Mutation of residue Leu24 in this segment of PALB2 significantly reduces the PALB2 homodimer affinity, but has a smaller effect on interactions between PALB2cc and the PALB2cc-interaction segment of BRCA1. This work suggests novel approaches for designing PALB2 molecules which disrupt its homodimeric interaction but not its heterodimeric interaction with BRCA1.
EXPERIMENTAL PROCEDURES
Preparation of Protein Samples.
Constructs of the mouse PALB2 coiled-coil domain (residues 1-60, referred to here as PALB2cc), mutant [L24A]-PALB2cc, and the mouse BRCA1 coiled-coil domain (residues 1337-1387, referred to here as BRCA1cc) were cloned, expressed, and purified following the standard protocols of the Northeast Structural Genomics Consortium 24. A complete description of the methods used in this work is presented in Supplemental Material. Uniformly 13C,15N- and 5% 13C, U-15N-enriched PALB2cc and [L24]-PALB2cc samples for NMR studies were prepared using standard protocols 24, and concentrated to 1.2-1.5 mM in 95% H2O and 5% 2H2O solution containing 20 mM MES buffer at pH 6.5, 200 mM NaCl, 10 mM DTT, and 5 mM CaCl2 (except where otherwise specified). NMR studies were carried out using 4-mm Shigemi, or 1.7-mm micro, NMR tubes.
NMR Data Collection and Solution Structure Calculations.
All NMR data for resonance assignments and structure determination were collected at 20 °C using Bruker AVANCE II 600 MHz and 800 MHz NMR spectrometers equipped with 5-mm cryoprobes. Complete 1H, 13C, and 15N resonance assignments for wt-PALB2cc were determined using conventional triple-resonance NMR methods 25 (as summarized in Figures S1 and S2] and deposited, together with raw tripleresonance NMR spectra (fid’s), in the Biological Magnetic Resonance Data Bank (BMRB accession number 27534). Resonance assignments were validated using the Assignment Validation Suite (AVS) software package 26. The dimer interface in PALB2cc was identified using 13C filtered NOESY experiments 27 on a 1:2 sample of 13C,15N enriched : unlabeled (natural abundance) protein. Residual dipolar coupling (RDC) data were collected for two bond vectors, 15N-1HN and 15N-13C’, in a single alignment medium containing acrylamidopropyltrimethylammonium chloride + 50% acrylamide (see Supplemental Material for details). The PALB2cc homodimer structure was calculated from these NMR data using CYANA Version 3.0 28-30, and the 20 models out of 100 with the lowest target function were refined by restrained molecular dynamics in explicit water using CNS Version 1.1 31. All structure calculations included both intra- and interchain NOE data, as well as RDC data. Structural statistics and global structure quality factors, including Verify3D 32, ProsaII 33, PROCHEC34, and MolProbity 35 raw and statistical Z-scores, were computed using the Protein Structure Validation Suite (PSVS Version 1.5) software package 36. Values for the global goodness of fit of the final structure ensembles with the NOESY peak list data were determined using the RPF analysis program 37. The final refined ensemble of 20 models has been deposited in the Protein Data Bank (code 6e4h), and the corresponding NOESY spectra (fid’s) and NOESY peak lists were deposited in the Biological Magnetic Resonance Data Bank (BMRB accession number 27534).
Rotational Correlation Time Measurements and NMR Titration Studies.
Rotational correlation times at 20 °C were determined from 15N relaxation measurements, as described elsewhere 38, Samples were prepared for these NMR measurements in the same buffer at pH 6.5 used for NMR assignments and structure determination. Heterodimer NMR titration studies were carried out using 15N-enriched PALB2cc, and various amounts of unenriched BRCA1cc, in the same buffer at pH 6.5. Rotational correlation time measurements and NMR titration studies were carried out using a Bruker 600 MHz Avance II NMR System equipped with a 1.7-mm tripleresonance microcryoprobe 39, with 30 – 50 μL sample volumes.
Circular dichroism (CD) spectroscopy.
CD measurements were performed in solution using the same buffer at pH 6.5 used for the NMR studies, with an Aviv 62DS CD spectrometer (Aviv Associates). Wavelength scans (200 to 260 nm) were performed on 15 μM protein solutions. Thermal denaturation profiles were obtained by measuring the ellipticity at 222 nm (θ222) as a function of temperature. Protein solutions (15 μM) were measured at 0.5 degree intervals starting at 0 °C, with an equilibration time of 2 min and an acquisition time of 0.5 min. The apparent melting temperature (Tm) was estimated from the maximum of the first derivative of Θ222 with respect to temperature. Thermal denaturation curves used to estimate Tm values were fully reversible.
Analytical Gel Filtration with Multiple Angle Static Light Scattering (AGF-MALS).
Wild-type or mutant PALB2cc were prepared at protein concentration of 3 mg/ml in 20 mM MES at pH 6.5, containing 200 mM NaCl, 5 mM CaCl2, 10 mM DTT, 1 x protease inhibitors, and 0.02% NaN3. 30 μl samples were injected onto an analytical gel filtration column (PROTEIN KW-Shodex, Kawasaki, Japan), and the effluent monitored by refractive index (black trace; Optilab rEX) and 90° static light scattering (miniDAWN TREOS, Wyatt Technology) detectors. Data were analyzed to determine shape-independent molecular mass, as described elsewhere 40.
Cell culture and transduction.
Mammalian expression plasmids encoding FLAG-tagged coiled-coil domain (amino acid residues 1-60) of mouse PALB2 (WT and L24A) were constructed in the pMX-PIE expression vector. Resting B lymphocytes were isolated from mouse spleens and cultured with LPS (25 μg/ml, Sigma) and IL-4 (5 ng/ml, Sigma) as described 41. Mouse embryonic fibroblasts (MEFs) and B lymphocytes expressing pMX-PIE vector, WT-PALB2cc and [L24A]-PALB2cc were generated by retroviral transduction. Retrovirus was packaged using BOSC cells and incubated with MEFs or B lymphocytes overnight. Transduced MEFs were selected by 2 μg/ml puromycin while transduced B lymphocytes were not drug selected. Successful transduction of cells was confirmed by western blot using mouse monoclonal anti-FLAG (Sigma, catalog number F1804) antibody.
Preparation of metaphase chromosomes and analysis of chromosome aberrations.
24 hours after transduction of mouse B lymphocytes, cells were mock-treated or treated with 2 μM olaparib overnight. Dividing cells were arrested with 10 μl/ml colcemid for one hour. Metaphase fixation and telomere PNA-FISH was performed as previously described 42.
Immunofluorescent detection of RAD51 foci.
Transduced MEFs were grown on sterile coverslips overnight and treated with 10 Gy of ionizing radiation to induce DNA double-strand breaks. After 4 hours recovery at 37°C, cells were fixed using 2% paraformaldehyde followed by treatment with 0.5% Triton X-100. Antibodies used were mouse monoclonal anti-FLAG (Sigma, catalog number F1804) and rabbit polyclonal anti-RAD51 (Santa Cruz, catalog number sc-8349). DAPI was used for nuclei counterstaining. Stained cells were imaged using a Nikon Eclipse E800 epifluorescence microscope.
RESULTS
PALB2cc homodimer formation.
Oligomerization of PALB2 is mediated by a coiled coil motif 43, corresponding to amino acid residues 9 to 44 in the N-terminal region of the protein 14, 16, 17, 22, 23, 24, 44, 45. Several different constructs corresponding to this N-terminal region of PALB2 were cloned and expressed. Some of these constructs provided good expression and solubility, including the PALB2 construct spanning residues 1 to 60. This segment of PALB2 is referred to here as PALB2cc. Backbone amide circular dichroism (CD) analysis (Figure 1A) demonstrates that PALB2cc is highly helical (55%), and exhibits a highly-cooperative thermal folding/unfolding equilibrium. The oligomerization state of PALB2cc was next characterized, using both estimates of rotational correlations times τc from 15N NMR relaxation measurements (Figure 1B) and analytical gel filtration with static light scattering (Figure 1C). These measurements firmly establish that PALB2cc forms a homodimer in solution under the conditions used in this study.
Figure 1. Biophysical and NMR measurements reveal an antiparallel coiled-coil structure of PALB2cc.

(A) Amide CD spectra indicate a helical structure of PALB2cc. (B) Rotational correlation time (τc) at 20 °C versus molecular mass plotted for known monomeric proteins (blue) and PALB2cc monomer (red triangle) demonstrate a dimeric structure. (C) AGF-MALS data indicate a molecular mass of 18 ± 2 kDa PALB2cc (upper dashed red horizontal line). Compared with the expected molecular mass for the single chain including an affinity tag 7.9 kDa, these data further demonstrate the dimeric structure. (D) Ensemble of the 20 lowest-energy NMR-derived conformers. (E) Expanded view of the dimer interface. Side-chains that mediate important interactions are shown as ball-and-sticks, and are labeled. (F) Coiled-coil helical wheel representation of PALB2cc dimer. Labeled residues at “a” and “d” coiled-coil positions form a predominantly hydrophobic interaction interface.
PALB2cc forms a stable antiparallel coiled coil in solution.
The solution NMR structure of dimeric PALB2cc was determined using 15N- and 13C-edited NOESY, X-filtered NOESY, backbone chemical shift, and both 15N-1HN and 15N-13C’ RDC data (Figure 1D, E). Structural statistics and quality scores for the resulting solution NMR structure are summarized in Table 1.
Table 1.
Summary of NMR data and structure statistics for PALB2cc homodimer.
| conformationally-restricting NOE-based distance restraints | |
| total | 3142 |
| intra-residue [i=j] | 803 |
| sequential [∣i - j∣ = 1] | 1027 |
| medium range [1 ∣i - j∣ < 5] | 1029 |
| long range [∣i - j∣ ≥ 5] (interchain) | 303 |
| hydrogen bond restraints | 100 |
| dihedral angle restraints | 164 |
| residual dipolar couplings | 51 |
| number of conformationally-restricting restraints per restrained residue | 56.2 |
| residual restraint violationsa | |
| average number of distance restraint violations per structure | |
| 0.1 – 0.2 Å | 12.7 |
| 0.2 – 0.5 Å | 3.65 |
| > 0.5 Å | 0 |
| maximum distance restraint violation | 0.36 Å |
| average number of dihedral restraint violations per structure | |
| 1 – 10° | 14.25 |
| > 10° | 0 |
| maximum dihedral restraint violation | 5.10° |
| root-mean-square deviations of superimposed coordinatesa | |
| backbone atoms (for well-ordered residues 11 – 39) | 0.3 Å |
| heavy atoms (for well-ordered residues 11 – 39) | 0.6 Å |
| Ramachandran statistics (for well-ordered residues 11 – 39, Molprobity)a | |
| most favored regions (%) | 100 |
| additional allowed regions (%) | 0 |
| disallowed regions (%) | 0 |
| Global quality scoresa | Raw / Z-score |
| Verify3D | 0.14 / −5.14 |
| ProsaII | 0.82 / 0.70 |
| Procheck (φ-ψ)b | 0.70 / 3.07 |
| Procheck (all)b | 0.21 / 1.24 |
| MolProbity Clash | 15.6 / −1.16 |
| RPF Scoresc | |
| recall, precision, F-measure, DP-Score | 0.925, 0.971, 0.948, 0.766 |
Values were calculated using PSVS Version 1.5. Averaged distance violations were calculated using the sum over r −6.
Ordered residue range: residues 11 – 39.
RPF scores reflect the goodness of fit of the final ensemble of structures (including disordered residues) to the NMR data.
The structure was determined from 3,142 NOE-based distance restraints, including 303 interchain restraints derived from X-filtered NOESY and iterative NOESY assignment, 51 RDC values, and 164 dihedral restraints derived from backbone chemical shift data46 These provided 56.2 restraints per residue, including 5.9 interchain restraints per residue. The average backbone root mean square deviation of ordered residues in the final ensemble is 0.3 Å. Backbone dihedral angle analysis indicated that 100% of the well-ordered residues fall in the most favored regions of the Ramachandran plot 35. Overall, the structure quality scores, including RPF scores 37 comparing the structure with the unassigned NOESY peak lists (summarized in Table 1), indicate a high quality solution NMR structure for the dimeric PALB2cc.
The dimerization of the PALB2cc is mediated by hydrophobic interactions. The amino acid residues at the center of each chain show a characteristic heptad repeat pattern with hydrophobic residues located in the a and d positions and charged residues in the e and g positions (Figure 1F). The highly conserved hydrophobic residues, including Leu21, Leu24, Thr31 and Leu35 at the a/d positions of the two α-helices, form an extensive hydrophobic interface in which their aliphatic side chains interact with each other in a ‘knobs-into-holes’ manner (Figure S3). Numerous long-distance NOEs are observed between Hδ methyl protons in the side chain of residue Leu21 and protons of residues Tyr28, Thr31 and Leu32 (Figure S4). These residues are located more than 10 A apart within each α -helical chain of PALB2cc, and thus are too far apart to generate intramolecular NOEs. These definitive, long-distance intermolecular NOEs demonstrate unambiguously that the dimer is antiparallel.
Residue Leu24 is critical for PALB2 homodimerization.
Based on the NMR structure of the PALB2cc homodimer, we designed a key mutation expected to disrupt the dimer structure. Residue Leu24 is located in the middle of the dimeric interface (Figure 1D) and is highly conserved in many species (Figure S5). This residue was replaced by alanine. Analytical gel filtration with static light scattering experiments demonstrate that this mutant PALB2cc is predominantly monomeric in solution at room temperature (Figure 2A). Thermal transition temperatures for wt- and [L24A]-PALB2cc demonstrate that the L24A mutation significantly destabilizes PALB2cc, with Tm for wt- and mutant of higher than 50 °C and ~ 20 °C, respectively (Figure 2B). These thermal transition curves were fully reversible. Interestingly, monomeric PALB2cc retains some helical structure, as CD spectra of [L24A]-PALB2cc exhibit two negative peaks at 209 and 222 nm (Figure 2C). The [1H-15N]-HSQC spectrum of [L24A]-PALB2cc further confirms that [L24A]-PALB2cc retains some ordered structure (Figure S6). Taken together, these data confirm that interfacial residue Leu24 is critical for PALB2cc homodimer formation.
Figure 2. Mutation of residue Leu24 to Ala disrupts the PALB2cc homodimer.

(A) AGF-MALS traces demonstrate that [L24A]-PALB2cc is monomeric in solution. Comparison of (B) CD melting curves and (C) CD wavelength scans of wt (top) and [L24A]- PALB2cc (bottom) confirm the disruption of the homodimeric structure by this interfacial mutation. (D) Expanded view of the dimer interface. Side-chains that mediate important packing interactions are shown as spheres, and the Leu24 side-chain is colored red.
NMR titration experiments were next used to determine the homodimeric dissociation constants Kd for wt- and [L24A]-PALB2cc. Qualitative features of complex affinity were assessed based on whether monomers and complexes are in slow-exchange on the NMR time scale, where separate resonance frequencies are measured for atoms in the monomer and in the complex, or in fast-exchange on the NMR timescale, where interconversion between monomer and complex states is sufficiently rapid that NMR resonance frequencies are population-weighted averages of the frequencies in the monomer and complex states. Generally speaking, slow-exchange dynamics are observed for longer-lived complexes, and tighter binding affinities, while fast-exchange dynamics indicate shorter-lived complexes and weaker binding affinities. In the slow-exchange limit, dimer dissociation constants Kd’s were determined from relative intensities of NMR resonances assigned to monomer and dimer states, respectively, measured in titration experiments, while in the fast-exchange limit Kd’s were estimated by fitting titration data to population- weighted chemical shift curves47, 48. Consistent with the solution NMR structure and analytical gel filtration data, these NMR data demonstrate that the L24A mutation disrupts the PALB2cc homodimer. The homodimer dissociation constants estimated from NMR titration data are 100-fold different, with Kdhomo being ~ 80 ± 10 μM and ~ 7000 M ± 2000 μM, for the wt and L24A mutant, respectively. Hence, interfacial interactions involving residue Leu24 in the coiled-coil structure (Figure 2D) contribute significantly to stabilizing the PALB2cc homodimer.
The PALB2cc:BRCA1cc heterodimer is more stable than the PALB2cc homodimer.
Next, we used NMR spectroscopy to probe the interactions between PALB2cc and BRCA1cc. 15N-enriched PALB2cc was titrated with unenriched BRCA1cc. These results are illustrated in Fig. 3. In this titration, some 15N-1H correlation peaks in the two-dimensional [1H-15N]-HSQC spectrum of PALB2cc became weaker or disappeared (Figure 3A). These changes were accompanied by the appearance of the 15N-1H resonances of PALB2cc in the complex with BRCA1cc. Estimates of rotational correlation time τc based on 15N-relaxation T1/T2 measurements demonstrate that the resonances associated with this complex have a τc corresponding to a heterodimer (Figure 3B). NMR titration experiments demonstrate that conformational exchange between homodimeric PALB2cc and the PALB2cc:BRCA1cc complex is slow on the NMR timescale (Figure 3C-E), as resonances of the homodimer decreased in intensity as resonances from the heterodimer increase in intensity. The dissociation constant Kdhetero for this heterodimeric complex, estimated from these NMR data, is ~ 6 ± 3 μM, about 10-fold more stable than the PALB2cc homodimer.
Figure 3. Formation of PALB2cc:BRCA1cc heterodimer and effect of [L24A]-PALB2cc mutation on heterodimerization.

(A) [1H-15N]-HSQC NMR data demonstrate formation of PALB2cc:BRCA1cc heterodimer. Peaks from 15N-enriched PALB2cc (20 μM, blue peaks) appear at new frequencies upon complex formation (red peaks) by addition of 2-fold molar excess of unlabeled BRCA1cc (40 μM). (B) Rotational correlation time (τe) at 20 °C versus molecular mass plotted for known monomeric proteins (blue) and the PALB2cc:BRCA1cc complex (red triangle) demonstrate that the 15N labeled PALB2cc forms a dimeric complex with unlabeled BRCA1cc. (C-E). Titration of 20 μM 15N-PALB2cc with 20, 24, and 36 μM unlabeled BRCA1cc reveals that the equilibrium exchange between 15N-PALB2cc homodimer (blue peaks in each panel) and 15N-PALB2cc:BRCA1cc heterodimer (red peaks) is slow on the NMR chemical shift timescale. The Kd estimated from these data is ~ 6 ± 3 μM. (F) [1H-15N]-HSQC NMR data demonstrate formation of [L24A]-PALB2cc:BRCA1cc heterodimer. Titration of 20 μM 15N-[L24A]-PALB2cc with 40 μM unlabeled BRCA1cc also results in spectral changes indicating complex formation. In this case, the equilibrium between 15N-PALB2cc homodimer (blue peaks) and the 15N-PALB2cc:BRCA1cc heterodimer (red peaks) is fast on the NMR chemical shift timescale, with an estimated Kd of 90 ± 20 μM. All NMR titration and 15N relaxation data were obtained at 600 MHz and 20 °C.
Residue Leu24 contributes less to PALB2 interactions with BRCA1.
Compared to the PALB2 homodimer, the heterodimeric PALB2cc:BRCA1cc complex is less disrupted by the L24A mutation in PALB2. As shown for the wt PALB2cc:BRCA1cc complex, titration of 15N-[L24A]-PALB2cc with unlabeled BRCA1cc can be used to detect complex formation (Figure 3F). In this case, the equilibrium exchange is fast on the NMR chemical shift timescale. NMR titration data provide an estimate of the heterodimer dissociation constant for the [L24A]-PALB2cc:BRCA1cc complex Kdhetero ~ 90 ± 20 μM, about 10-fold weaker than corresponding Kdhetero ~ 6 ± 3 μM for the wt-PALB2cc:BRCA1cc complex. Hence, as illustrated in Figure 4, these NMR titration data demonstrate that (i) the heterodimeric PALB2cc:BRCA1cc complex is significantly more stable than the homodimeric PALB2cc complex, and (ii) the L24A mutation in the PALB2 homodimer interface has a significant (~ 100-fold) effect on the homodimer stability, but a more modest effect (~ 10-fold) on the PALB2cc:BRCA1cc heterodimer stability. These results indicate that it may be possible to identify PALB2 mutations that selectively disrupt homodimeric versus heterodimeric interactions.
Figure 4. Schematic representation of homo- and heterodimer formation in wt and L24A PALB2cc.
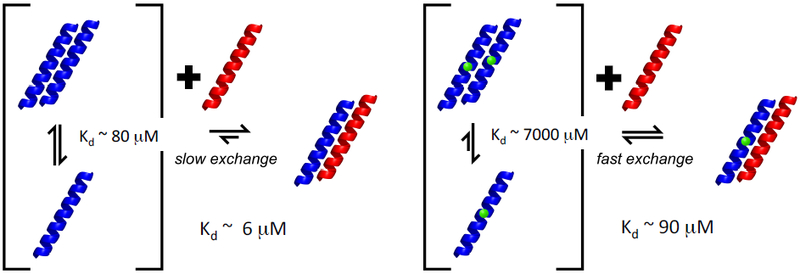
The homo- and heterodimer dissociation constants determined from NMR are illustrated for the wt- and L24A mutants (denoted with green highlight) of PALB2cc (blue coil) interacting with BRCA1cc (red coil). The heterodimeric complex PALB2cc:BRCA1cc is illustrated hypothetically as a coiled-coil structure, and the monomeric form of BRCA1cc is illustrated as a helix for convenience, when in fact NMR data indicate it is not ordered in the absence of the heterodimeric interaction.
Mutation of residue Leu24 impairs the DNA damage repair activity of PALB2.
To investigate the effect of the L24A substitution on PALB2 function in cells, peptides containing normal and mutant versions of the PALB2 coiled-coil motif were expressed in mouse B lymphocytes and embryonic fibroblasts (MEFs). Peptides containing the PALB2 coiled-coil motif normally have a dominant-negative effect on PALB2 function when overexpressed, based on their ability to associate with endogenous PALB2 and BRCA1 and prevent them from associating with their normal cellular binding partners 14. As reported previously, overexpression of a FLAG-tagged peptide containing residues 1-60 from PALB2 (i.e. PALB2cc) induced substantial genomic instability in WT B cells (Figure 5A). In contrast, an equivalent peptide carrying the L24A substitution induced a significantly lower level of genomic instability. Replacement of Leu24 with alanine therefore appears to prevent PALB2cc peptides from interfering with normal PALB2 activity, supporting the idea that residue Leu24 is important for PALB2 coiled-coil interactions and for its function in DNA damage repair.
Figure 5. Functional impact of the L24A mutation in PALB2.
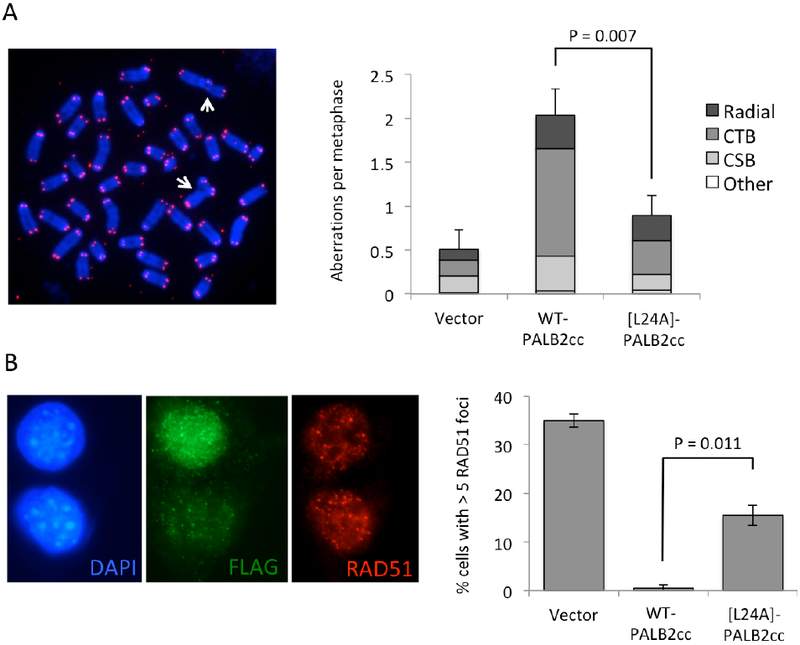
(A) Analysis of genomic instability in mouse B lymphocytes overexpressing PALB2 coiled-coil peptides. Left, example of metaphase chromosome spread showing two radial chromosomes (white arrows). Right, average frequencies of radial chromosomes, chromatid breaks (CTD), chromosome breaks (CSB) and all other chromosome aberrations in metaphase chromosome spreads. Error bars show standard deviation of the mean total number of aberrations, based on three experiments. At least 50 metaphases were scored per experiment. (B) Detection of RAD51 foci in irradiated mouse embryonic fibroblasts. Left, immunofluorescence for RAD51 in nuclei exposed to 10 Gy of ionizing radiation. Nuclear DNA is stained with DAPI, and cells overexpressing PALB2 coiled-coil peptides were identified by staining for FLAG. Right, percentage of either all cells (vector control), or cells staining positive for FLAG (others), that showed greater than five RAD51 foci. Error bars show standard deviation of the means from two separate experiments in which at least 50 nuclei were scored. P values (two-tailed) were calculated using Student’s t-test.
PALB2-mediated localization of BRCA2 to double-strand breaks is essential for normal RAD51 recruitment to damaged DNA 4. Whereas ionizing radiation induced the formation of RAD51 foci in the nuclei of approximately 35% of control cells, very few cells expressing dominant negative PALB2cc peptide showed RAD51 foci formation (Figure 5B). Cells overexpressing a PALB2cc peptide carrying the L24A mutation showed significantly higher levels of RAD51 foci formation than was seen in cells expressing the wt-PALB2cc construct. This indicates that Leu24 is critical for complex formation by the PALB2 coiled-coil motif. Disruption of this residue alone is sufficient to block much of the dominant negative effect of PALB2cc peptides. Cells showing low levels of FLAG co-staining were excluded from this analysis, so that only cells with high levels of peptide expression were counted. The L24A mutation, which was revealed by CD and NMR spectroscopy to be disruptive for both PALB2cc homodimerization and PALB2cc-BRCA1cc heterodimerization, is therefore also disruptive for normal PALB2 complex formation in cells. Although our results based on biophysical characterizations indicate that the L24A mutation has a greater effect on PALB2 homodimerization versus heterodimerization, at this stage we cannot distinguish whether the cellular phenotypes associated with expression of [L24A]-PALB2cc arise by disruption of homodimeric or heterodimeric PALB2 complexes.
DISCUSSION
The homodimer formed by coiled-coil regions of PALB2 has a classic leucine zipper structure (Figure 1), with antiparallel orientation. In this solution NMR structure, the flanking regions of the peptide construct studied are largely disordered, perhaps adopting short-lived transient structures (Figure 1D). Key residues at the dimer interface include Leu17, Leu21, Leu24, Tyr28, Thr31, and Leu35. This homodimer is disrupted by the Leu24 to Ala single-site mutant. Significantly, our CD data also demonstrate that the resulting monomeric form of the PALB2cc peptide retains significant helical structure (Figure 2C). This PALB2cc region also forms a heterodimeric complex with the BRCA1cc region; however, the structure and relative domain orientations of this heterodimer are not yet determined.
Homodimeric PALB2cc has a dissociation constant (Kd) of ~ 80 μM, which is weaker than that of the corresponding PALB2cc:BRCA1cc heterodimer (~ 6 μM). Hence, at equilibrium, these polypeptide fragments energetically prefer the heterodimeric form. However, this preference corresponds to only a few kilocalories in free energy, and in the context of full-length proteins and the cellular environment, this equilibrium could be significantly different. Both homodimer and heterodimer formation are impaired by the L24A mutation; however, the homodimer dissociation constant is significantly more sensitive to this mutation than that of the heterodimer. This result suggests it may be possible to design mutants of PALB2 or BRCA1 that preferentially disrupt the PALB2 homodimer relative to the PALB2:BRCA1 heterodimer.
The six amino acid residues (Leu17, Leu21, Leu24, Tyr28, Thr31 and Leu35) that occupy particularly prominent sites at the PALB2 coiled-coil homodimer interface (Figure 1D-F) all show 100% conservation in the PALB2 sequences from human, mouse, cow and horse 21. Leu17, Leu21, Tyr28 and Thr31 are additionally conserved in Xenopus laevis (Figure S5). Several of these residues have been the subject of earlier studies aimed at understanding the importance of the PALB2 coiled-coil region. Zhang et al. generated point mutant versions of PALB2 in which either Leu21 or Leu24 was replaced with proline (PALB2-L21P and PALB2-L24P) 45. Neither of these mutant PALB2 mutant proteins was able to interact with BRCA1, nor were they able to rescue HR in cells in which endogenous PALB2 was knocked down. These results do not, however, provide specific insight into the contributions of residues Leu21 and Leu24 to PALB2 activity, as insertion of proline residues into an α-helical region will likely cause disruption of the entire helix structure 49. Indeed, based on our structural studies it is likely that these proline mutants would disrupt both the homodimeric and heterdimeric complexes. Foo et al. also reported a PALB2 missense mutation in which proline replaces Leu35 50. The L35P variant is associated with breast cancer, and prevents stable association of PALB2 with BRCA1. L35 is a key interface residue in our PALB2 homodimer structure, and its mutation to a proline residue would also be expected to cause a broader disruption of the entire helical region. Accordingly, based on our experimental structure of the PALB2cc coiled-coil homodimer, none of these previous studies distinguish the roles of residues Leu21, Leu24, or Leu35 in specific dimeric interactions, as replacing any of these Leu residues with Pro is expected to disrupt the overall structures of both the homodimeric PALB2 and heterodimeric PALB2:BRCA1 coiled-coil complexes.
Several other reports have suggested that genetic changes affecting the PALB2 coiled-coil region have the potential to cause disease. Four different splice or frameshift mutations within the region of the PALB2 gene encoding the coiled-coil motif were identified in a patient study of pathogenic PALB2 variants 51. Sequencing of 1,250 individuals from the USA further revealed that a PALB2 variant in which arginine replaces Lys18 is present in 0.89% of the population 52. Based on in silico analysis, this relatively-common PALB2 variant is predicted to have the potential to be pathogenic by altering the function of the protein. According to our solution NMR structure, the K18R mutation is not predicted to directly alter interface contacts in the PALB2 homodimer, and immunoprecipitation experiments have demonstrated that this mutation has little effect on PALB2 homodimerization or association with BRCA1 50.
Scanning alanine mutagenesis of triplets of amino acid residues revealed sequence requirements within the PALB2 coiled-coil region that are essential to support homologous recombination 21. PALB2 protein in which each of residues 24 - 26 were replaced by alanine was unable to support homologous recombination. Knock-in mice expressing PALB2 with this triplealanine mutation (Palb2CC6/CC6) were viable, but showed a male fertility defect 21. Cells from these mice were hypersensitive to mitomycin C (MMC), indicating a defect in DNA repair. These results were interpreted as evidence for an essential role for residue segment 24 – 26 in stabilizing heterodimers of PALB2 with BRCA1. Notably, one of these residues (Leu24) was identified in the present study as a key interface residue for homodimer formation by coiled-coil regions from PALB2. These results are therefore also consistent with the possibility that PALB2 homodimerization is essential for normal repair of DNA damage.
Deletion of the coiled-coil motif from BRCA1, which prevents BRCA1 from forming a heterodimer with PALB2, results in severely reduced HR efficiency and hypersensitivity to chemotherapy agents in human cell lines 53. This observation matches earlier observations that the integrity of the BRCA1 coiled-coil motif is necessary for HR activity 16. Residues Leu21, Tyr28 and Leu35 were all predicted to occupy the ‘a’ position in the heptad repeat of the PALB2 coiled-coil region when it associates with a coiled-coil region from BRCA1 16. PALB2 protein in which any of residues Leu21, Tyr28 or Leu35 is replaced with alanine was additionally shown to be non-functional for sustaining HR in cellular reporter assays, and was not able to complement mitomycin C hypersensitivity of PALB2-deficient cells. This deficiency in HR correlated with a failure of L21A, Y28A or L35A PALB2 to interact with BRCA1 in co-IP assays. The structural analysis presented in this study reveals that these same residues are present at the dimer interface for PALB2 homodimerization. In fact, the predicted model for the BRCA1:PALB2 heterodimer interface 16 turns out to be largely consistent with our homodimer structure. This evidence supports the idea that competition between formation of PALB2 homodimeric coiled coils versus heterodimeric PALB2:BRCA1 coiled coils may influence the efficiency of HR 14.
The ability of PALB2 to regulate HR is modulated by DNA damage-induced phosphorylation of several residues. Ionizing radiation induces phosphorylation of residues Ser59, Ser157, and Ser37654-56. Of these, Ser59 is part of the PALB2 constructs that we used in this study. We found that Ser59 does not participate in the coiled-coil interactions, but instead lies on the C-terminal side of the coiled-coil α-helix, within an unstructured region. The importance of Ser59 phosphorylation was underscored by the demonstration that ATR-dependent phosphorylation of Ser59 regulates the interaction of PALB2 with BRCA1, and further contributes to recruitment of PALB2 to DNA damage site 44. The interaction of human PALB2 with BRCA1 is also influenced by CRL3-KEAP1-mediated ubiquitylation of PALB2 at Lys20, Lys25 or Lys3057. The corresponding residues in mouse PALB2, Lys20, Lys25, and Arg30, are within the coiled-coil region. Although the sidechain of residue Lys-25 forms an intrachain salt bridge with Glu-27, none of these proposed ubiquitylation sites are key interface residues of the PALB2 coiled-coil homodimer interaction. It is possible, however, that ubiquitylations and phosphorylations of sites within and around the PALB2 coiled-coil region selectively affect heterodimerization of PALB2 with BRCA1, while leaving intact the interface for PALB2 homodimerization. The solution NMR structure of the PALB2 homodimer described here provides the basis for further studies of the structure-function relationships of the coiled-coil motifs of PALB2 and BRCA1 in HR.
Supplementary Material
Figure S1. Triple resonance NMR connectivity map and summary of other NMR data used to determine resonance assignments and secondary structure for PALB2cc.
Figure S2. Assigned [1H-15N]-TROSY-HSQC spectrum of PALB2cc.
Figure S3. Highly-conserved hydrophobic residues Leu17, Leu21, Leu24, Thr31 and Leu35 form an extensive hydrophobic interface.
Figure S4. Long-distance intermolecular NOEs demonstrate unambiguously that the dimer is antiparallel.
Figure S5. Multiple Sequence Alignment for N-terminal region of PALB2cc.
Figure S6. The [1H-15N]-HSQC spectrum of [L24A]-PALB2cc confirms that this variant retains some ordered structure
ACKNOWLEDGMENTS
We thank Drs. J. Prestegard and H.W. Lee for providing residual dipolar coupling data, and Dr. J. Everett for assistance in molecular graphics.
Funding Sources
This work was supported by NIH grants U54-GM094597 (to G.T.M) and R01-GM120574 (to G.T.M.), and R01-CA190858 (to S.F.B.). NMR facilities used in this study were supported in part by a NIH shared instrumentation grant, 1S10-OD018207-01 (to G.T.M.). F.S. and N.D. were supported in part by the Jerome and Lorraine Aresty Chair Endowment.
ABBREVIATIONS
- PALB2
Partner and localizer of BRCA2
- HR
Homologous recombination
- BRCA1
Breast cancer susceptibility 1
- BRCA2
Breast cancer susceptibility 2
- CD
circular dichroism
- NMR
nuclear magnetic resonance
- ATR
Ataxia telangiectasia related
Footnotes
The authors declare no competing financial interest. G.T.M. is a founder of Nexomics Biosciences, Inc.
REFERENCES
- [1].Kass EM, and Jasin M (2010) Collaboration and competition between DNA double strand break repair pathways, FEBS Lett 584, 3703–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bunting SF, and Nussenzweig A (2013) End-joining, translocations and cancer, Nat Rev Cancer 13, 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jensen RB, Carreira A, and Kowalczykowski SC (2010) Purified human BRCA2 stimulates RAD51-mediated recombination, Nature 467, 678–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Xia B, Sheng Q, Nakanishi K, Ohashi A, Wu J, Christ N, Liu X, Jasin M, Couch FJ, and Livingston DM (2006) Control of BRCA2 cellular and clinical functions by a nuclear partner, PALB2, Mol Cell 22, 719–729. [DOI] [PubMed] [Google Scholar]
- [5].Reid S, Schindler D, Hanenberg H, Barker K, Hanks S, Kalb R, Neveling K, Kelly P, Seal S, Freund M, Wurm M, Batish SD, Lach FP, Yetgin S, Neitzel H, Ariffin H, Tischkowitz M, Mathew CG, Auerbach AD, and Rahman N (2007) Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer, Nat Genet 39, 162–164. [DOI] [PubMed] [Google Scholar]
- [6].Xia B, Dorsman JC, Ameziane N, de Vries Y, Rooimans MA, Sheng Q, Pals G, Errami A, Gluckman E, Llera J, Wang W, Livingston DM, Joenje H, and de Winter JP (2007) Fanconi anemia is associated with a defect in the BRCA2 partner PALB2, Nat Genet 39, 159–161. [DOI] [PubMed] [Google Scholar]
- [7].Kim H, and D’Andrea AD (2012) Regulation of DNA cross-link repair by the Fanconi anemia/BRCA pathway, Genes Dev 26, 1393–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bowman-Colin C, Xia B, Bunting S, Klijn C, Drost R, Bouwman P, Fineman L, Chen X, Culhane AC, Cai H, Rodig SJ, Bronson RT, Jonkers J, Nussenzweig A, Kanellopoulou C, and Livingston DM (2013) Palb2 synergizes with Trp53 to suppress mammary tumor formation in a model of inherited breast cancer, Proc Natl Acad Sci U S A 110, 8632–8637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Huo Y, Cai H, Teplova I, Bowman-Colin C, Chen G, Price S, Barnard N, Ganesan S, Karantza V, White E, and Xia B (2013) Autophagy opposes p53-mediated tumor barrier to facilitate tumorigenesis in a model of PALB2-associated hereditary breast cancer, Cancer discovery 3, 894–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Erkko H, Xia B, Nikkila J, Schleutker J, Syrjakoski K, Mannermaa A, Kallioniemi A, Pylkas K, Karppinen SM, Rapakko K, Miron A, Sheng Q, Li G, Mattila H, Bell DW, Haber DA, Grip M, Reiman M, Jukkola-Vuorinen A, Mustonen A, Kere J, Aaltonen LA, Kosma VM, Kataja V, Soini Y, Drapkin RI, Livingston DM, and Winqvist R (2007) A recurrent mutation in PALB2 in Finnish cancer families, Nature 446, 316–319. [DOI] [PubMed] [Google Scholar]
- [11].Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, Parsons DW, Lin JC, Palmisano E, Brune K, Jaffee EM, Iacobuzio-Donahue CA, Maitra A, Parmigiani G, Kern SE, Velculescu VE, Kinzler KW, Vogelstein B, Eshleman JR, Goggins M, and Klein AP (2009) Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene, Science 324, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rahman N, Seal S, Thompson D, Kelly P, Renwick A, Elliott A, Reid S, Spanova K, Barfoot R, Chagtai T, Jayatilake H, McGuffog L, Hanks S, Evans DG, Eccles D, Breast Cancer Susceptibility C, Easton DF, and Stratton MR (2007) PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene, Nat Genet 39, 165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Oliver AW, Swift S, Lord CJ, Ashworth A, and Pearl LH (2009) Structural basis for recruitment of BRCA2 by PALB2, EMBO Rep 10, 990–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Buisson R, and Masson JY (2012) PALB2 self-interaction controls homologous recombination, Nucleic Acids Res 40, 10312–10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Murphy AK, Fitzgerald M, Ro T, Kim JH, Rabinowitsch AI, Chowdhury D, Schildkraut CL, and Borowiec JA (2014) Phosphorylated RPA recruits PALB2 to stalled DNA replication forks to facilitate fork recovery, The Journal of cell biology 206, 493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sy SM, Huen MS, and Chen J (2009) PALB2 is an integral component of the BRCA complex required for homologous recombination repair, Proc Natl Acad Sci U S A 106, 7155–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang F, Ma J, Wu J, Ye L, Cai H, Xia B, and Yu X (2009) PALB2 links BRCA1 and BRCA2 in the DNA-damage response, Curr Biol 19, 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Moynahan ME, and Jasin M (2010) Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis, Nat Rev Mol Cell Biol 11, 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Burkhard P, Stetefeld J, and Strelkov SV (2001) Coiled coils: a highly versatile protein folding motif, Trends Cell Biol 11, 82–88. [DOI] [PubMed] [Google Scholar]
- [20].Foo TK, Tischkowitz M, Simhadri S, Boshari T, Zayed N, Burke KA, Berman SH, Blecua P, Riaz N, Huo Y, Ding YC, Neuhausen SL, Weigelt B, Reis-Filho JS, Foulkes WD, and Xia B (2017) Compromised BRCA1-PALB2 interaction is associated with breast cancer risk, Oncogene. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Simhadri S, Peterson S, Patel DS, Huo Y, Cai H, Bowman-Colin C, Miller S, Ludwig T, Ganesan S, Bhaumik M, Bunting SF, Jasin M, and Xia B (2014) Male fertility defect associated with disrupted BRCA1-PALB2 interaction in mice, J Biol Chem 289, 24617–24629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sy SM, Huen MS, Zhu Y, and Chen J (2009) PALB2 regulates recombinational repair through chromatin association and oligomerization, J Biol Chem 284, 18302–18310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang F, Bick G, Park JY, and Andreassen PR (2012) MDC1 and RNF8 function in a pathway that directs BRCA1-dependent localization of PALB2 required for homologous recombination, J Cell Sci 125, 6049–6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Acton TB, Xiao R, Anderson S, Aramini J, Buchwald WA, Ciccosanti C, Conover K, Everett J, Hamilton K, Huang YJ, Janjua H, Kornhaber G, Lau J, Lee DY, Liu G, Maglaqui M, Ma L, Mao L, Patel D, Rossi P, Sahdev S, Shastry R, Swapna GV, Tang Y, Tong S, Wang D, Wang H, Zhao L, and Montelione GT (2011) Preparation of protein samples for NMR structure, function, and small-molecule screening studies, Methods Enzymol 493, 21–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Baran MC, Huang YJ, Moseley HN, and Montelione GT (2004) Automated analysis of protein NMR assignments and structures, Chemical reviews 104, 3541–3556. [DOI] [PubMed] [Google Scholar]
- [26].Moseley HN, Sahota G, and Montelione GT (2004) Assignment validation software suite for the evaluation and presentation of protein resonance assignment data, J Biomol NMR 28, 341–355. [DOI] [PubMed] [Google Scholar]
- [27].Stuart A, Borzilleri K, Withka J, and Palmer A (1999) Compensating for Variations in 1 H– 13 C Scalar Coupling Constants in Isotope-Filtered NMR Experiments, Journal of the American Chemical Society. [Google Scholar]
- [28].Guntert P, Mumenthaler C, and Wuthrich K (1997) Torsion angle dynamics for NMR structure calculation with the new program DYANA, J Mol Biol 273, 283–298. [DOI] [PubMed] [Google Scholar]
- [29].Schmidt E, and Guntert P (2015) Automated structure determination from NMR spectra, Methods Mol Biol, 2230–2237_2216. [DOI] [PubMed] [Google Scholar]
- [30].Herrmann T, Guntert P, and Wuthrich K (2002) Protein NMR structure determination with automated NOE assignment using the new software CANDID and the torsion angle dynamics algorithm DYANA, J Mol Biol 319, 209–227. [DOI] [PubMed] [Google Scholar]
- [31].Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, and Warren GL (1998) Crystallography & NMR system: A new software suite for macromolecular structure determination, Acta Crystallogr D Biol Crystallogr 54, 905–921. [DOI] [PubMed] [Google Scholar]
- [32].Luthy R, Bowie JU, and Eisenberg D (1992) Assessment of protein models with three dimensional profiles, Nature 356, 83–85. [DOI] [PubMed] [Google Scholar]
- [33].Sippl MJ (1993) Recognition of errors in three-dimensional structures of proteins, Proteins 17, 355–362. [DOI] [PubMed] [Google Scholar]
- [34].Laskowski R, Macarthur M, Moss D, and Thornton J (1993) PROCHECK: a program to check the stereochemical quality of protein structures, Journal of Applied Crystallography. [Google Scholar]
- [35].Lovell SC, Davis IW, Arendall WB 3rd, de Bakker PI, Word JM, Prisant MG, Richardson JS, and Richardson DC (2003) Structure validation by Calpha geometry: phi,psi and Cbeta deviation, Proteins 50, 437–450. [DOI] [PubMed] [Google Scholar]
- [36].Bhattacharya A, Tejero R, and Montelione GT (2007) Evaluating protein structures determined by structural genomics consortia, Proteins 66, 778–795. [DOI] [PubMed] [Google Scholar]
- [37].Huang YJ, Powers R, and Montelione GT (2005) Protein NMR recall, precision, and F-measure scores (RPF scores): structure quality assessment measures based on information retrieval statistics, J Am Chem Soc 127, 1665–1674. [DOI] [PubMed] [Google Scholar]
- [38].Rossi P, Swapna GV, Huang YJ, Aramini JM, Anklin C, Conover K, Hamilton K, Xiao R, Acton TB, Ertekin A, Everett JK, and Montelione GT (2010) A microscale protein NMR sample screening pipeline, Journal of biomolecular NMR 46, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Monleon D, Colson K, Moseley HN, Anklin C, Oswald R, Szyperski T, and Montelione GT (2002) Rapid analysis of protein backbone resonance assignments using cryogenic probes, a distributed Linux-based computing architecture, and an integrated set of spectral analysis tools, Journal of structural and functional genomics 2, 93–101. [DOI] [PubMed] [Google Scholar]
- [40].Xiao R, Anderson S, Aramini J, Belote R, Buchwald WA, Ciccosanti C, Conover K, Everett JK, Hamilton K, Huang YJ, Janjua H, Jiang M, Kornhaber GJ, Lee DY, Locke JY, Ma LC, Maglaqui M, Mao L, Mitra S, Patel D, Rossi P, Sahdev S, Sharma S, Shastry R, Swapna GVT, Tong SN, Wang DY, Wang HA, Zhao L, Montelione GT, and Acton TB (2010) The high-throughput protein sample production platform of the Northeast Structural Genomics Consortium, Journal of Structural Biology 172, 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bunting SF, Callen E, Wong N, Chen HT, Polato F, Gunn A, Bothmer A, Feldhahn N, Fernandez-Capetillo O, Cao L, Xu X, Deng CX, Finkel T, Nussenzweig M, Stark JM, and Nussenzweig A (2010) 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks, Cell 141, 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Misenko SM, and Bunting SF (2014) Rapid analysis of chromosome aberrations in mouse B lymphocytes by PNA-FISH, J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lupas A (1996) Coiled coils: new structures and new functions, Trends Biochem Sci 21, 375–382. [PubMed] [Google Scholar]
- [44].Buisson R, Niraj J, Rodrigue A, Ho CK, Kreuzer J, Foo TK, Hardy EJ, Dellaire G, Haas W, Xia B, Masson JY, and Zou L (2017) Coupling of Homologous Recombination and the Checkpoint by ATR, Mol Cell 65, 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang F, Fan Q, Ren K, and Andreassen PR (2009) PALB2 functionally connects the breast cancer susceptibility proteins BRCA1 and BRCA2, Mol Cancer Res 7, 1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Shen Y, and Bax A (2015) Protein structural information derived from NMR chemical shift with the neural network program TALOS-N, Methods Mol Biol 1260, 17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Akerud T, Thulin E, Van Etten RL, and Akke M (2002) Intramolecular dynamics of low molecular weight protein tyrosine phosphatase in monomer-dimer equilibrium studied by NMR: a model for changes in dynamics upon target binding, J Mol Biol 322, 137–152. [DOI] [PubMed] [Google Scholar]
- [48].Lee HW, Wylie G, Bansal S, Wang X, Barb AW, Macnaughtan MA, Ertekin A, Montelione GT, and Prestegard JH (2010) Three-dimensional structure of the weakly associated protein homodimer SeR13 using RDCs and paramagnetic surface mapping, Protein science : a publication of the Protein Society 19, 1673–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Richardson JS, and Richardson DC (1988) Amino acid preferences for specific locations at the ends of alpha helices, Science 240, 1648–1652. [DOI] [PubMed] [Google Scholar]
- [50].Foo TK, Tischkowitz M, Simhadri S, Boshari T, Zayed N, Burke KA, Berman SH, Blecua P, Riaz N, Huo Y, Ding YC, Neuhausen SL, Weigelt B, Reis-Filho JS, Foulkes WD, and Xia B (2017) Compromised BRCA1-PALB2 interaction is associated with breast cancer risk, Oncogene 36, 4161–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Antoniou AC, Casadei S, Heikkinen T, Barrowdale D, Pylkas K, Roberts J, Lee A, Subramanian D, De Leeneer K, Fostira F, Tomiak E, Neuhausen SL, Teo Z, Khan S, Aittomaki K, Moilanen JS, Turnbull C, Seal S, Mannermaa A, Kallioniemi A, Lindeman GJ, Buys SS, Andrulis IL, Radice P, Tondini C, Manoukian S, Toland AE, Miron P, Weitzel JN, Domchek SM, Poppe B, Claes KB, Yannoukakos D, Concannon P, Bernstein JL, James PA, Easton DF, Goldgar DE, Hopper JL, Rahman N, Peterlongo P, Nevanlinna H, King MC, Couch FJ, Southey MC, Winqvist R, Foulkes WD, and Tischkowitz M (2014) Breast-cancer risk in families with mutations in PALB2, N Engl J Med 371, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nguyen-Dumont T, Hammet F, Mahmoodi M, Tsimiklis H, Teo ZL, Li R, Pope BJ, Terry MB, Buys SS, Daly M, Hopper JL, Winship I, Goldgar DE, Park DJ, and Southey MC (2015) Mutation screening of PALB2 in clinically ascertained families from the Breast Cancer Family Registry, Breast Cancer Res Treat 149, 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Anantha RW, Simhadri S, Foo TK, Miao S, Liu J, Shen Z, Ganesan S, and Xia B (2017) Functional and mutational landscapes of BRCA1 for homology-directed repair and therapy resistance, Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ahlskog JK, Larsen BD, Achanta K, and Sorensen CS (2016) ATM/ATR-mediated phosphorylation of PALB2 promotes RAD51 function, EMBO Rep 17, 671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Guo Y, Feng W, Sy SM, and Huen MS (2015) ATM-dependent Phosphorylation of the Fanconi Anemia Protein PALB2 Promotes the DNA Damage Response, J Biol Chem 290, 27545–27556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, Shiloh Y, Gygi SP, and Elledge SJ (2007) ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage, Science 316, 1160–1166. [DOI] [PubMed] [Google Scholar]
- [57].Orthwein A, Noordermeer SM, Wilson MD, Landry S, Enchev RI, Sherker A, Munro M, Pinder J, Salsman J, Dellaire G, Xia B, Peter M, and Durocher D (2015) A mechanism for the suppression of homologous recombination in G1 cells, Nature 528, 422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Triple resonance NMR connectivity map and summary of other NMR data used to determine resonance assignments and secondary structure for PALB2cc.
Figure S2. Assigned [1H-15N]-TROSY-HSQC spectrum of PALB2cc.
Figure S3. Highly-conserved hydrophobic residues Leu17, Leu21, Leu24, Thr31 and Leu35 form an extensive hydrophobic interface.
Figure S4. Long-distance intermolecular NOEs demonstrate unambiguously that the dimer is antiparallel.
Figure S5. Multiple Sequence Alignment for N-terminal region of PALB2cc.
Figure S6. The [1H-15N]-HSQC spectrum of [L24A]-PALB2cc confirms that this variant retains some ordered structure


