Abstract
The unprecedented progress in the treatment of metastatic castration-resistant prostate cancer is only beginning to be realized in patients with noncastrate disease. This slow progress in part reflects the use of trial objectives focused on time-to-event end points, such as time to metastasis and overall survival, which require long follow-up durations and large sample sizes, and has been further delayed by the use of approved therapies that are effective at the time of progression. Our central hypotheses are that progress can be accelerated, and that outcomes can be improved by shifting trial objectives to response measures occurring early that solely reflect the effects of the treatment. To test these hypotheses, a continuously enrolling multi-arm, multi-stage randomized trial design, analogous to that used in the STAMPEDE trial, has been developed. Eligibility is focused on patients with incurable disease or those with a high risk of death with any form of monotherapy alone. The primary objective is to eliminate all disease using a multimodality treatment strategy. End points include pathological complete response and an undetectable level of serum prostate-specific antigen, with recovery of serum testosterone levels. Both are binary, objective, and provide an early, quantitative indication of efficacy.
The unprecedented progress in the treatment of metastatic castration-resistant prostate cancer (mCRPC) in the past 7 years is only beginning to be realized in the treatment of patients with early stage, noncastrate disease. The traditional approach to developing drugs for prostate cancer has followed a paradigm in which therapies that are effective in patients with mCRPC are then studied in patients with prostate cancer of different non- castrate states, including ‘high risk’ localized disease in the neoadjuvant and/or adjuvant setting, biochemical recurrence after local therapy, or in noncastrate metastatic disease, using time-to-event outcome measures that include prostate-specific antigen (PSA)-defined recurrence, PSA-defined progression, radiographic progression, or death. These trials take years to complete and often provide inconclusive results owing to the long natural history and the limited number and clinical significance of the failure events on which conclusions are based. Moreover, with six life-prolonging treatments for patients with prostate cancer currently approved by the FDA1–7, a seventh designated as a breakthrough therapy for which an accelerated approval is anticipated8, and many more in development, simply too many possible combination therapies exist to study all of them with the available resources. If six to eight agents are available, the number of possible two-drug combination, for example, ranges from 15 to 28. Furtherrmore, the ongoing de-lineation of a molecularly defined disease taxonomy9,10, and the approval and ongoing clinical testing of a variety of targeted agents further highlights the need to design and complete informative trials in a more timely manner.
A new strategy that provides a more rapid and definitive readout of treatment success or failure is urgently needed, so that only the most promising therapeutic approaches are investigated further. In this Review, we describe how this can be achieved using a multimodality clinical trial platform that involves the enrolment of patients with noncastrate disease across a continuum of risk, and shifts the objectives from time-to-event outcomes, for which therapy is given to delay or prevent outcomes that occur late in the course of disease, to response indicators that solely indicate the effects of treatment that occur early and reflect ‘no evidence of disease’ or a ‘complete elimination of disease’ state11. Each response indicator, approached in a biomarker context, should be reproducibly and reliably measurable and, in itself, clinically meaningful as an indicator of treatment efficacy.
Limitations of traditional measures
The difficulties in evaluating drugs in patients with prostate cancer have long been recognized. These difficulties are in part caused by the fact that the traditional measures of response used to assess the effects of drugs in other tumour types, such as the RECIST criteria, do not apply to changes in serum PSA levels or osseous metastases, which are the most-common manifestations of the disease. Measurable disease, for which changes in size can be quantitated objectively, is infrequent. To address this challenge, a group of clinical investigators came together to form the Prostate Cancer Working Group 2 (PCWG2), in order to focus on aspects of drug development related to the treatment of patients with mCRPC11. Among the group’s recommendations were: to consider each disease manifestation — such as serum PSA level, the primary tumour, lymph nodes, bone, or visceral metastases, and other symptoms — independently; to avoid the grouped categorizations of different disease manifestations into broad overall response categorizations, such as complete response, partial response or stable disease; to separate treatment outcomes into early measures of response, classified as the control, relief, or elimination of each disease manifestation present at the start of therapy, and later time-to-event measures of progression, such as the delay or prevention of future manifestations11.
The indications for use of currently approved drugs for mCRPC align with the PCWG2 outcomes paradigm, although none were approved based on an early indicator of a response, such as a decline in serum PSA level or tumour shrinkage. To date, the combination of mitoxantrone and prednisone remains the only drug regimen approved based on an early response end point: the relief or palliation of pain12. All of the other approved drugs were licensed based on their demonstrated ability to delay the onset of disease manifestations such as skeletal-related events, symptomatic skeletal events, or death. Progression-free survival (PFS) has not, by itself, been an end point on which approval decisions have been made. However, with the development of an analytically valid bone-scan-based assay, which has shown strong correlation with overall survival outcomes, radiographic PFS based on this validated assay and RECIST 1.1 criteria for soft-tissue disease, when present, is now included as a regulatory end point to support approval decisions13,14.
High-risk localized disease.
The clinical activity of androgen-deprivation therapy (ADT) in patients with advanced-stage localized and/or metastatic disease was first described in 1944 (REF. 15), when the ability to assess the extent of disease using imaging was virtually nonexistent and trial design methodologies were in their infancy. Considerable resources have since been invested in trials in the neoadjuvant and/or adjuvant setting that enrolled patients with tumours classified as locally advanced or high-risk. These trials are costly, both in terms of the large number of patients needed to show the protocol-defined level of benefit and the time required to do so. The conclusions of a critical review show that the majority of trials that have influenced clinical practice demonstrated a survival benefit (delaying or preventing all-cause or prostate-cancer-specific mortality)16. Unfortunately, before 2014, few treatments had been demonstrated to have this level of efficacy (TABLE 1).
Table 1 |.
Cohort sizes and time to publication, and clinical implications of selected phase III trials
| Treatment approach | Number of patients* | Number of centres | Start of accrual | Accrual duration (months) | Months from start of accrual to publication | Supported changes in clinical practice?‡ | Ref. |
|---|---|---|---|---|---|---|---|
| High-risk localized disease | |||||||
| RTOG 0521 (RTfollowed by ADT ± docetaxel) | 282 vs 281 | 12§ | December 2005 | 43.6 | 113.1 | No | 23 |
| GETUG12 (ADT ± docetaxel + estramustine followed by RP/RTfollowed by ADT) | 207 vs 206 | 26 | November 2002 | 48.8 | 149.2 | No | 21 |
| TAX - 3501(RP followed by ADT ± docetaxel) | 70 vs 68 | 108 | December 2005 | 20.8 | 91.6 | No | 69 |
| SWOG S9921 (RP followed by ADT ± mitoxantrone) | 480 vs 481 | NR | October 1999 | 86.2 | 206.3 | No | 24 |
| Locally-advanced disease | |||||||
| RT ± GnRH | 987 vs 992 | 212 | October 1994 | 77.4 | 199.8 | Yes | 108 |
| ADT ± RT | 603 vs 602 | 78 | March 1995 | 124.0 | 189.8 | Yes | 109 |
| ADT ± RT | 436 vs 439 | 47 | February 1996 | 81.3 | 153.2 | Yes | 18 |
| Biochemically recurrent disease | |||||||
| Salvage RT ± anti-androgen | 384 vs 376 | 19§ | March 1998 | 59.5 | 225.2 | Yes | 34 |
| Metastatic castration-sensitive disease | |||||||
| Intergroup (intermittent versus continuous ADT) | 770 vs 765 | 18§ | May 1995 | 158.7 | 213.3 | No | 66 |
| Bilateral orchiectomytflutamide (Ml) | 667 vs 669 | 9§ | December 1989 | 56.5 | 105.4 | No | 37 |
| GETUG 15 (ADT ± docetaxel) | 192 vs 193 | 29§ | October 2004 | 50.0 | 97.9 | No | 49,50 |
| CHAARTED (ADT ± docetaxel) | 397 vs 393 | 15§ | July 2006 | 76.4 | 108.7 | Yes | 44–46 |
| STAMPEDE (ADT ± docetaxel) | 727 vs 1,090 | 125 | October 2005 | 89.1 | 121.5 | Yes | 47,48 |
| LATITUDE (ADT ± abiraterone) | 597 vs 602 | 235 | February 2013 | 21.8 | 31.2 | Yes | 51 |
| STAMPEDE (ADT ± abiraterone) | 500 vs 502 | 116 | November 2011 | 25.9 | 66.1 | Yes | 52 |
ADT, androgen deprivation therapy; GnRH, gonadotrophin-releasing hormone; NR, not reported; RP, radical prostatectomy; RT, radiotherapy.
First number represents the number of patients assigned to the experimental arm, followed by the number assigned to the control arm.
Refers to study results that lead to incorporation within National Comprehensive Cancer Network guidelines.
Estimated, based upon number of investigators.
As an example, the results of two international multicentre trials17,18 collectively showed a statistically significant, clinically meaningful improvement in disease-specific and overall survival outcomes for the combination of localized radiotherapy and ADT, relative to ADT or radiotherapy alone. Both results were practice-changing17,18, and while each study was sufficiently powered to answer the clinical question asked, a total of 2,080 patients and 6–12 years of follow-up monitoring were needed to do so.
The findings of these trials contrast with the outcomes of trials addressing the question of whether the addition of docetaxel to ADT, which was originally reported to be effective in patients with mCRPC in 1999 (REFs. 19,20), would improve outcomes relative to ADT alone in patients with high-risk localized disease. GETUG-12, a trial involving men with high-risk localized disease, was launched in 2002, and was designed to determine whether neoadjuvant docetaxel in combination with ADT would lead to a 54% reduction in risk of relapse relative to ADT alone in men who had undergone radical prostatectomy21. The study adopted a composite definition of relapse, which included biochemical recurrence, the development of metastatic disease, or symptoms of metastatic disease. However, 2 years later, when the findings of two definitive phase III trials — TAX-327 (REF. 1) and SWOG-9916 (REF. 22) — indicated the survival benefit of docetaxel plus prednisone versus mitoxantrone plus prednisone, which lead to FDA approval of docetaxel for mCRPC, the true efficacy of docetaxel was found to have been overestimated in GETUG-12. The planned statistical analysis was subsequently modified to increase both the sample size and follow-up duration in order that a sufficient number of relapse events could be captured. Ultimately, 13 years after trial activation, an absolute 12% difference in relapse-free survival was found. A close inspection of the data also revealed that most of the relapse events were PSA-defined recurrences that, ultimately, were not necessarily clinically significant21. Furthermore, at the time of reporting, no survival advantage was detected, which was postulated to reflect a low number of deaths observed during follow-up monitoring. Also of note was the observation that 46% of the patients in the control arm never had any disease recurrence. Docetaxel would, therefore, have been an unnecessary overtreatment of these patients that could not have improved their outcomes21. The authors concluded that “longer follow-up will be needed to establish whether this benefit translates into improved metastasis-free, and ultimately, overall survival” (REF. 21); however, the real question is how long can patients continue to wait.
Another example is provided by RTOG-0521 (REF. 23), a trial of similar design to GETUG-12 (REF. 21), which used radiotherapy as the definitive treatment of the primary tumour, seeking to show a 7% absolute 4-year survival benefit with a planned enrolment of 600 patients, with 78 death events projected to occur over 5 years of accrual and 4 years of additional follow-up monitoring. The final results, reported 10 years after enrolment of the first patient, revealed a modest 4% improvement in overall survival from 89% to 93%, which was only statistically significant with a one-sided P value; owing to the low number of death events among patients in the control arm (59 in total, of which only 23 were attributed to prostate cancer), this finding is unlikely to change clinical practice.
The findings of SWOG-9921, a study comparing the efficacy of ADT alone to that of ADT plus mitoxantrone in the adjuvant setting, which started in 1999, were reported at the 2017 ASCO Genitourinary Cancers Symposium24. This trial was stopped by the data safety monitoring committee after three incidences of leukaemia were documented among patients in the mitoxantrone arm. In the final analysis, >900 patients were enrolled over a 7-year period and monitored for an additional 10 years, only to demonstrate that the addition of mitoxantrone to ADT did not improve overall survival24.
The long disease trajectories and low event rates inherent in trials involving patients with noncastrate prostate cancer have now made overall survival a challenging end point at best. This problem is further compounded by the increasing availability of therapies that are effective when disease progression occurs on protocol, particularly when given to patients in the control arms of these trials, which can blunt the effects of the experimental regimen on survival outcomes. The use of serum PSA-based surrogate end points has been proposed25, but is not currently accepted, in part owing to uncertainty as to the source of the PSA increase, such as a local recurrence that might still be cured using additional therapy delivered to the primary site, compared with a nonlocalized recurrence that might not reflect the presence of lethal disease. To further address this need, the Intermediate Clinical Endpoints in Cancer of the Prostate (ICECaP) working group was established in order to identify potential surrogate markers of overall survival by pooling raw data from 28,905 individual patients with localized prostate cancer from 43 ongoing or completed clinical trials. The analysis demonstrated a strong association between metastasis-free survival and overall survival26,27, and this surrogate will likely be accepted as a clinical trial end point for accelerated or potentially full regulatory approvals. The results of the ICECaP analysis provide a major step forward, but still fail to address the likelihood that metastasis-free survival, as an end point, will be affected by patients receiving treatment at the time of biochemical (PSA) recurrence that invariably occurs before the development of detectable meta stases, once again blunting the potential effects of treatment on the primary outcome.
Biochemical recurrence and metastasis-free survival.
Concerns similar to those surrounding studies involving patients with high-risk localized disease also apply to trials enrolling patients with biochemical recurrence after definitive localized therapy. Overall, around 15% of localized prostate cancers recur following treatment28, with the frequency varying as a function of disease aggressiveness and extent at the time of diagnosis — ranging from 50% to 80% in patients with highrisk disease29. In this scenario, the first objective is to identify patients in whom an increase in serum PSA level is solely a result of localized disease recurrence, who might therefore still be curable with additional therapy applied directly to the prostate bed following radical prostatectomy or to the prostate itself following primary radiation therapy. To do so, a range of predictive nomograms have been developed that incorporate features of the disease at diagnosis, time to recurrence, serum PSA kinetics, and/or newer biologically-based disease profiles30–33. However, many of these nomograms were based on data from small retrospective series, all of which involve slightly different patient populations, and have not been prospectively and externally validated.
Trials in this context also use time-to-metastasis and overall survival as end points, and although the required follow-up durations are shorter, the influence of additional systemic treatment given at the time of bio chemical relapse (be it approved or investigational, such as the addition of short-term ADT to salvage radio therapy in the RTOG-9601 (REF. 34) and GETUG-16 (REF. 35)), before either of these end points is achieved, can obviate the ability to determine whether intervention has had a favourable influence on patient outcomes.
Noncastrate metastatic disease.
Trials designed to improve the outcomes of patients presenting with metastatic disease began in the pre-PSA era with end points that were also primarily time-to-event based. Most trials used overall survival as the primary end point and asked the question regarding whether or not the addition of a first-generation anti-androgen to standard ADT was superior, in terms of efficacy, to ADT alone36,37. At the time, metastatic disease was largely detected using plain radiography and radionuclide bone scans. The results varied; some trials demonstrated superiority of the combination therapy and others not36,37.
Ultimately, the findings of a large meta-analysis that included data from a total of 27 trials and 8,257 patients revealed a statistically insignificant 1.8% absolute difference in overall survival at 5 years38, an observation supported by other meta-analyses that differed in terms of the studies included and agents examined38–43. Here again, the lack of an intermediate end point of efficacy and the sobering final results have reduced enthusiasm for the development of treatments of metastatic non castrate disease.
Renewed interest in trials involving patients with noncastrate disease began with the ChemoHormonal Therapy versus Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer (CHAARTED)44–46, and the Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy (STAMPEDE)47 studies, which were launched in 2005 and 2006, respectively. Both trials were designed to investigate whether the addition of docetaxel to ADT would be superior to ADT alone; however, ADT was given continuously in CHAARTED44–46, and for a minimum of 2 years in STAMPEDE47. CHAARTED, with results presented publicly in 2014 (REF. 44) and published in 2015 (REF. 45), revealed a significant delay in time to progression and an improvement in overall survival for patients who received the chemohormonal combination (HR for death 0.61, 95% CI 0.47–0.80; P <0.001).
The CHAARTED study44–46 achieved its stated primary end point; however, the observed effect was only statistically significant in patients with high-volume meta static disease (defined as ≥4 lesions detected on bone scan or the presence of visceral disease). In the initial report, the point estimate in subgroup analyses of groups of patients with low-volume disease appeared comparable to that of patients in the high-volume disease cohort and to that of the entire cohort, despite not reaching statistical significance45. With longer follow-up monitoring and a higher number of recorded deaths, as reported at ESMO 2016 (REF. 46), the trend towards potential benefit from docetaxel was lost, with an HR of 1.04. Similar results were seen in the STAMPEDE trial, which were first presented in 2015 (REF. 47) and published in 2016 (REF. 48), and also showed a significant improvement in overall survival following chemo hormonal combination therapy in patients with castration-sensitive prostate cancer, ranging from high-risk, localized, advanced-stage disease (stage T3 or T4, Gleason score 8–10 and serum PSA >40 ng/ml) to meta static or relapsed disease with high-risk features (HR for death 0.78, versus ADT alone, 95% CI 0.66–0.93; P = 0.006). A notable improvement in failure-free and overall survival outcomes was observed across the trial population, with no evidence of the treatment having a heterogeneous effect in patients with metastatic disease47.
A third trial designed to investigate the same question in patients with metastatic noncastrate prostate cancer, GETUG-15 (REF. 49), enrolled patients with a more-favourable risk profile than that of those enrolled in CHAARTED44–46, and showed no difference in out-comes, despite the trial design allowing up to 50% more chemotherapy to be administered (9 cycles versus 6 cycles in both STAMPEDE48 and CHAARTED44–46). At present, the variations in the results from the subgroups of patients with high-volume and lower-volume disease are an area of active debate. To the STAMPEDE investigators48, the survival benefit for both groups is clear, given that the trial was designed for the cohort as a whole and the primary end point was met. By contrast, the position of ASCO is that the question of benefit for patients in the low-volume disease subgroup remains open (Michael Morris, personal communication).
Both CHAARTED44–46 and STAMPEDE48 are landmark trials with results that dramatically changed clinical practice, although they required 9 years from initiation to the first public presentation of the results, and 10 years to the first peer-reviewed publication. For GETUG-15, 4 years were required to accrue sufficient follow-up data, and 9 years passed before the first report was published49, with an additional 2 years for an update that included a reclassification of disease burden in accordance with the definitions used in CHAARTED50. Such timelines are simply too long in the current therapeutic landscape. A similar survival benefit was reported for patients in the abiraterone plus ADT and prednisone arm (versus placebo plus ADT and prednisone) of the LATITUDE trial51, which included patients with metastatic noncastrate prostate cancer and in STAMPEDE48,52, which included patients with both metastatic and non-metastatic disease. These findings raise the new question as to which patients are most likely to benefit from either treatment approach, and under what circumstances should the combination approach be considered standard.
A focus on early measures of response
Shifting the primary trial objective from time-to-event measures that occur late in the course of disease to response measures that occur early and that solely reflect the effects of the treatments is central to achieving the goal of more rapid and informative drug develop ment in noncastrate disease states. To achieve this goal, we applied the same control/relieve/eliminate paradigm developed in PCWG2 (REF. 11) to individual disease manifestations that occur across non castrate disease states including the primary tumour and, separately, to sites of metastatic spread such as the pelvic lymph nodes, retro peritoneal lymph nodes, and bone. Our central hypo thesis is that the demonstrated level of efficacy of currently available and approved systemic therapies, used as part of a multimodality therapeutic approach, is sufficient to begin to systematically ask the question as to whether all disease can be completely eliminated in patients who present with tumours that range from locally advanced, high-risk disease to low-volume metastatic disease. This approach is not only an informative method for identifying successful strategies for further investigation in larger cohorts of patients, but is also a necessary prerequisite to the establishment of a paradigm for cure.
The feasibility of achieving this objective is based on the demonstration that rigorously defined patho logical complete responses (pCRs) are now being achieved in men with locally advanced prostate cancer who are treated with next-generation inhibitors of androgen signalling, such as abiraterone, in the neoadjuvant setting53. In our own experience, an undetectable serum PSA level following recovery of serum testosterone levels can be achieved using a planned multimodality approach in selected patients with low-volume metastatic disease that is incurable with any single therapeutic modality54. Such multimodality approaches are not only the standard-of-care treatment for many types of cancer including urothelial55 and germ-cell tumours56,57, but have also been shown to cure a proportion of patients with these tumours who present with metastatic disease. Effective systemic therapies used earlier in the natural history of a disease, when required to treat micro metastases or visible metastatic disease in order to improve a patient’s outcome, can also lower the risks of morbidity and/or disease-specific mortality by reducing the risk of progression to mCRPC.
All trials designed to evaluate the efficacy of drugs and/or different therapeutic approaches in patients with detectable disease in any location, be it local, regional, biochemically recurrent, or metastatic, incorporate out-come measures to assess the effects of treatment. Each measure, regardless of how it is defined, is a biomarker of a response, or a change from baseline as a result of treatment. The challenge in patients with prostate cancer is to show that each defined response indicator can be measured reproducibly and quantitatively, and that the observed change from baseline following treatment is clinically meaningful, providing the justification to continue the development of the drug and/or overall therapeutic approach, and ultimately, to show that the response indicator does reflect a clinical benefit to the patient or patient group. If researchers are able to test and reject the null hypothesis that low-volume metastatic disease cannot be completely eliminated by treatment, the clinical benefit of various experimental approaches can then be truly established.
pCR and minimal residual disease in patients with advanced-stage and/or high-risk localized disease.
The efficacy of ADT has been studied extensively in the neoadjuvant setting using a range of early outcome measures. In some trials, the primary end point was a pCR, representing the complete elimination of disease, although the definition of pCR and how it was determined varied between trials, or was not explicitly defined. Furthermore, most trials that reported pCR did not have a central pathology review by expert genitourinary pathologists. Overall, however, pCRs in these trials have been rare (TABLE 2). Other reported outcome measures that have been used in trials include tumour regression (assessed clinically or using MRI), surgical margin rates, and findings of the post-therapy pathological analysis of tumour specimens, but were notable for a lack of standardization in how the end points were defined, assessed, and reported. Equally important, however, was that the systemic therapies available at the time had limited efficacy in terms of elimination of the primary tumour. A renewed level of interest in the concept of pCR has emerged with the potential as a surrogate for clinical outcomes, as demonstrated in patients with breast cancer58,59, as well as the introduction of more-efficacious second-generation inhibitors of androgen-receptor signalling. These agents include abiraterone, which suppresses circulating androgen levels to an order of magnitude lower than those routinely obtained using conventional ADT60, and enzalutamide, which has demonstrably superior activity against bicalutamide-resistant cell lines61 and in patients62,63, compared with first-generation ADT. Both agents have been shown to prolong overall survival when used either before or after chemotherapy in patients with mCRPC, and both are approved by the FDA for these indications4–7.
Table 2 |.
Selected clinical trials involving neoadjuvant ADT with pCR rates reported
| Study | n | Predefined study entry criteria in manuscript | Treatment arm(s) | pCR rate | MRD (≤ 5 mm) | MRD (<0.25 cm3) | pCR defined? | MRD defined? | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T-stage | Gleason score | Number of cores | Baseline PSA | Imaging criteria | ||||||||
| Labrie et al. (1997)110 | 161 | Yes | No | No | No | No | Leuprolide + flutamide for 3 months | 7% | NA | NA | No | No |
| Van Der Kwast et al.(1999)111 | 47 | No | No | No | No | No | Leuprolide + flutamide for 3 months | 0% | NA | NA | No | No |
| Leuprolide + flutamide for 6 months | 9% | |||||||||||
| G leave et al. (2001)112 | 547 | No* | No | No | No | No | Leuprolide + flutamide for 3 months | 5% | NA | NA | Yes | No |
| Leuprolide + flutamide for 8 months | 9% | |||||||||||
| Klotz et al. (2003)113 | 213 | Yes | No | No | Yes | No | Cyproterone for 3 months | 0% | NA | NA | No | No |
| TAPS (2014)114 | 35 | Yes | Yes | No | No | No | Goserelin + dutasteride for 3 months | 0% | NR | 17%‡ | No | Yes |
| Goserelin + bicalutamide + dutasteride for 3 months | 10% | NR | 20%‡ | |||||||||
| Goserelin + bicalutamie + dutasteride + ketoconazole for 3 months | 8% | NR | 23%‡ | |||||||||
| NeoAbi (2014)53 | 56 | Yes | Yes | No | Yes | No | LHRH agonist for 6 months + abiraterone for 3 months | 4% | 0% | 44% | No | Yes |
| LHRH agonist for 6 months + abiraterone for 6 months | 10% | 14% | 52% | |||||||||
| NeoEnza (2015)115 | 48 | Yes | Yes | Yes | Yes | No | Enzalutamide for 6 months | 0% | 0% | 36% | Yes | Yes |
| Enzalutamide + dutasteride + leuprolide for 6 months | 4% | 13% | 74% | |||||||||
| MDACC (2016)116§ | 65 | Yes | Yes | No | Yes | No | Abiraterone + enzalutmide + LHRH agonist for 6 months | 2% | NR | NR | No | Yes |
| Abiraterone + LHRH agonists for 6 months | 5% | |||||||||||
| 30 | Yes | Yes | Yes | Yes | Yes | Leuprolide + enzalutamide | 10% | 55% | NR | NA | NA | |
| Leuprolide + enzalutamide + abiraterone | 15% | 50% | ||||||||||
ADT, androgen-derpivation therapy; LHRH, luteinizing-hormone releasing hormone; NA, not available; NR, not reported; pCR, pathological complete response; PSA, prostate specific antigen
Patients were stratified for Gleason score and baseline serum PSA level
MRD defined as <0.2 cm3 in this study
Full study not reported, data were obtained from abstract.
In the past 3 years, investigators at the Dana-Farber Cancer Institute applied an approach used in a series of neoadjuvant trials involving patients with breast cancer65 to develop and later report on the performance of two rigorously defined pathological end points for use in a series of single-arm and randomized phase II neo adjuvant trials involving patients with high-risk localized prostate cancer. The end points were pCR, reflecting the complete elimination of all disease in the gland, and minimal residual disease (MRD), defined either as a residual cancer burden (RCB; a calculation of tumour volume corrected for cellularity) of ≤0.25 cm3 of tumour, or 1–5 mm of viable tumour. In the first proof-of- concept trial53, patients were randomly assigned to receive either leuprolide alone or leuprolide plus abiraterone for 12 weeks, followed by another 12 weeks of combination therapy for all patients. The number of patients in each of the arms ranged from 28–30 with a higher pCR rate of 10% versus 4% following 24 weeks versus 12 weeks of treatment with abiraterone53. In a subsequent study that is currently ongoing, researchers evaluated the efficacy of more-extensive suppression of androgen levels with enzalutamide, abiraterone, and ADT, with a pCR rate approaching 12% (Mary-Ellen Taplin, personal communication). An additional 30% of patients met the MRD end point. Long-term follow-up monitoring is needed to evaluate the association between pathological response in the gland and subsequent outcomes, such as serum PSA recurrence, metastasis, and survival.
Biochemical recurrence.
The end point of an un-detectable serum PSA level with serum testosterone levels similar to pretreatment levels can be regarded as an equivalent to a ‘no-evidence-of-disease’ status and has long been used as an early surrogate for ‘cure’ after radical prostatectomy for localized disease. Notably, such an out-come is rarely achieved with ADT alone, whether given on an intermittent basis (shown to be equivalent to continuous therapy) for patients with biochemically recurrent disease66 or to those with visible metastases observed on imaging67. We have long explored the value of this end point in trials involving patients with biochemically recurrent disease with a rapid (≤9 month) serum PSA doubling time. These patients require therapy based on their proven risk of developing detectable metastases and ultimately dying of prostate cancer. Completed studies involving this end point include our phase II study of the efficacy of docetaxel, ADT, and rapid androgen cycling68. Other studies include the phase III TAX3503 trial (), which is currently in the follow-up period and was designed to compare the efficacy of ADT for 18 months, with or without six cycles of docetaxel69, and the phase II AbiCure trial, in which patients were randomized to receive either degarelix alone, abiraterone acetate plus prednisone alone, or the combination of degarelix and abiraterone acetate plus prednisone (). The cohort size in each study accounts for the fact that serum testosterone levels do not return to noncastrate levels in 10–15% of patients, thus rendering these patients unable to meet the primary end point. In these single-modality studies, approximately 5–20% of patients had no evidence of disease at 18 months68,69, which demonstrates the feasibility of biochemical recurrence as an end point in trials involving patients with rising PSA levels alone, or with overt metastatic disease, although few of the responses were durable.
Metastatic noncastrate prostate cancer.
ADT, as a standard of care, results in median failure-free and overall survival durations of 11 months and 42 months, respectively, with sites of metastatic disease and initial Gleason scores remaining statistically significant prognostic indicators of both progression-free and overall survival70. Baseline serum PSA levels are strongly associated with failure-free survival70, while in the SWOG-9346 trial71 patients who achieved a PSA nadir of ≤4 ng/ml had longer survival durations than those with a serum PSA nadir >4 ng/ml. As discussed earlier, the added efficacy provided by addition of docetaxel to ADT has been definitively established in patients with high-volume disease45, but is debatable for patients with lower-volume disease, with similar results seen with abiraterone plus prednisone in LATITUDE51 and STAMPEDE48.
Definitive management of the primary lesion in the prostate has not been regarded as the standard of care for patients with metastatic disease; however, emerging data indicate that radical prostatectomy in this setting is feasible and safe72,73, and that both radical prostatectomy and radiotherapy to the prostate might even confer a survival benefit74–76. In a small study cohort, patients with low-volume metastatic disease who underwent radical prostatectomy had a significantly longer time to development of castration resistance and clinical progression compared with those who did not undergo radical prostatectomy, in addition to extended cancer-specific, but not overall, survival72. Furthermore, a contemporary analysis of the National Cancer Database indicates that the overall survival outcomes of men with metastatic prostate cancer treated with radiotherapy plus ADT are superior to those of patients treated with ADT alone76, even after adjusting for age, year, ethnicity, comorbidity score, serum PSA level, Gleason score, T stage, N stage, previous chemotherapy, treating facility, and insurance status. Collectively, a large body of retrospective evidence indicates a survival benefit following radical management of the primary tumour, even in the metastatic setting; this principle is currently being actively explored and validated in a number of clinical trials77 (TABLE 3).
Table 3 |.
Selected ongoing studies in metastatic noncastrate prostate cancer
| Study | Study arms | Phase | Estimated cohort size | Identifier | Primary end point |
|---|---|---|---|---|---|
| Systemic therapy in mCSPC | |||||
| ARCHES | ADT + enzalutamide versus ADT + NSAA | III | 1,100 | Radiographic PFS | |
| MRC STAMPEDE (Arm J) | ADT + abiraterone + enzalutamide versus ADT | III | 1,800 | OS | |
| TITAN | ADT + apalutamide versus ADT | III | 1,000 | OS and radiographic PFS | |
| ARASENS | ADT+docetaxel + ODM-201 versus ADT + docetaxel | III | 1,300 | OS | |
| Treatment of the primary lesion in mCSPC | |||||
| PEACE1 | ADT ± docetaxel ± localized radiotherapytabiraterone | III | 916 | OS and PFS | |
| MDACC | Best systemic therapytsurgery/radiotherapy | II | 180 | PFS | |
| JHU | ADT + NSAA + docetaxel + radiotherapy+surgery | II | 33 | Safety | |
| MRC STAMPEDE (Arm H) | ADT + radiotherapy versus ADT | III | 1,250 | OS | |
| Treatment ofmetastatic lesions in mCSPC | |||||
| Ghent | Observation versus radiotherapy to metastatic sites (≤ 3 sites) | II | 54 | ADT-free survival | |
| University of Florida | SBRT or stereotactic hypofractionated radiotherapy | II | 48 | PFS | |
ADT, androgen-deprivation therapy; mCSPC, metastatic castration-sensitive prostate cancer; NSAA, non-steroidal anti-androgen; OS, overall survival; PFS, progression-free survival; SBRT, stereotactic body radiotherapy.
Similarly, radiotherapy delivered to bone metastases in patients with low-volume disease is not an established standard of care, although data from several centres78 highlight the potential benefits of effective control of individual osseous lesions in patients with low-volume disease. Such control is achieved most frequently with stereotactic body radiotherapy (SBRT), defined by the American Society for Radiation Oncology (ASTRO) as “external beam radiotherapy used to deliver a high dose of radiation very precisely to an extracranial target within the body, as a single dose or a small number of fractions” (REF. 79). Local control rates obtainable with this approach are high: in one series of patients with prostate cancer and 1–2 bone metastases receiving single-fraction SBRT, the 12-month local control rate (defined as a lack of progression on follow-up imaging) was 95.5%, with follow-up durations of 6–77 months80. Repeat SBRT has also been used in patients with minimal bone and/or lymph-node only recurrences in an effort to delay the need to start ADT. The results of these studies are some-what difficult to interpret owing to differences in trial design, outcomes, and the triggers used to indicate a need for systemic therapy81. More importantly, the efficacy of SBRT is currently being evaluated further in a number of ongoing trials (TABLE 3).
Multimodality therapy.
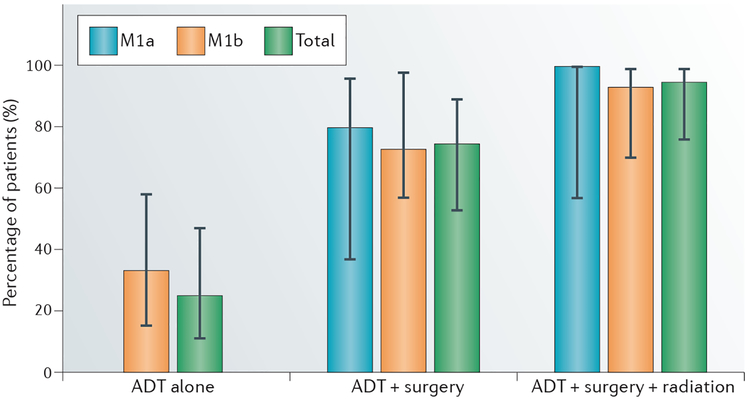
In 2016, we reported the out-comes of a pilot experience of multimodality treatment in patients with low-volume metastatic disease, defined as <10 bone metastases, based on 99mTc radionuclide bone scans and/or nonregional lymph-node involvement54. Patients were treated for 6–12 months with ADT followed by radical prostatectomy with lymph-node dissection of all gross nodal disease and SBRT to bone metastases. The report included a total of 20 patients, of whom four (20%) met the primary end point of a PSA response (serum PSA <0.05 ng/ml) with noncastrate serum testosterone levels at 20 months after the initiation of treatment. In two of these patients, serum PSA remained undetectable at 27 months and 46 months, respectively54. This pilot experience established the feasibility and safety of the multimodality approach and provides an approximate baseline response rate for the design of future trials. The most-important finding was that each treatment modality was required and contributed to the outcome; indeed, the frequency at which an undetectable serum PSA level was achieved increased as each modality was applied, ultimately leading to 95% of patients having undetectable serum PSA levels during the course of treatment (FIG. 1).
Figure 1 |. Percentage of patients with an undetectable serum PSA level following multimodality therapy.
The percentage of patients with an undetectable serum prostate-specific antigen (PSA) level during the treatment phase increased with the addition of each component of multimodality therapy54. Patients responses to treatment were assessed using measurements of serum PSA levels; the frequency of patients in whom serum PSA levels were undetectable after androgen-deprivation therapy (ADT) alone, ADT plus surgery, and/or ADT plus surgery plus radiotherapy is shown. M1a, extrapelvic nodal disease; M1b, bone metastasis. Reproduced with permission obtained from Elsevier © O’Shaughnessy, M. J. et al. A pilot study of a multimodal treatment paradigm to accelerate drug evaluations in early stage metastatic prostate cancer. Urology 102, 164–172 (2017).
A new approach to drug development
To test the hypothesis that a multimodality treatment strategy that includes systemic therapy, radiotherapy to visible metastases and radical surgery can eliminate disease, MetaCURE, a continuously enrolling Multi-Arm Multi-Stage (MAMS) randomized trial design has been developed, analogous to that used in the STAMPEDE trial82, which enables the efficacy of several approaches to be studied in parallel, and new treatment arms to be added at any time without the need for a new protocol (BOX 1, FIG. 2). Both trials are predominantly enrolling patients with newly diagnosed disease across a range of noncastrate clinical states.
Box 1 |. MetaCure: advantages and future directions.
Eligibility
The broad range of eligibility, along with a randomized design reduces the uncertainties associated with clinical staging based on the specific imaging modalities used to assess both the primary tumour and the presence or absence of metastatic disease
The inclusion of a wider range of disease states enhances accrual, and trials can be directed at biologically defined subsets of patients, and be completed more rapidly
Treatment
The ability to add new experimental arms on a continuous basis eliminates the need for new trials, thus enabling promising new approaches to be evaluated earlier and reducing the amount of resources required to conduct a clinical trial
Results
Early readouts of success or failure using a binary end point that solely reflects the effects of treatment
Reduces the number of patients needed to show benefit, and reduces the uncertainty associated with time-to-event measures and confounding the effects of post-protocol therapy on outcomes
Enables the results of competing treatments to be placed into the context of other findings
Secondary/exploratory
Provides a standardized clinical framework to prospectively ask questions regarding the performance of imaging and molecular biomarkers with an appropriate level of statistical power
The standardized trial framework, including acquisition and storage of clinical samples, enables specific biological questions to be prioritized and investigated using retrospective and/or prospective studies, thus substantially shortening the time required for validation
Provides an objective platform enabling the prioritization of approaches and biomarkers for investigation in larger clinical trial cohorts
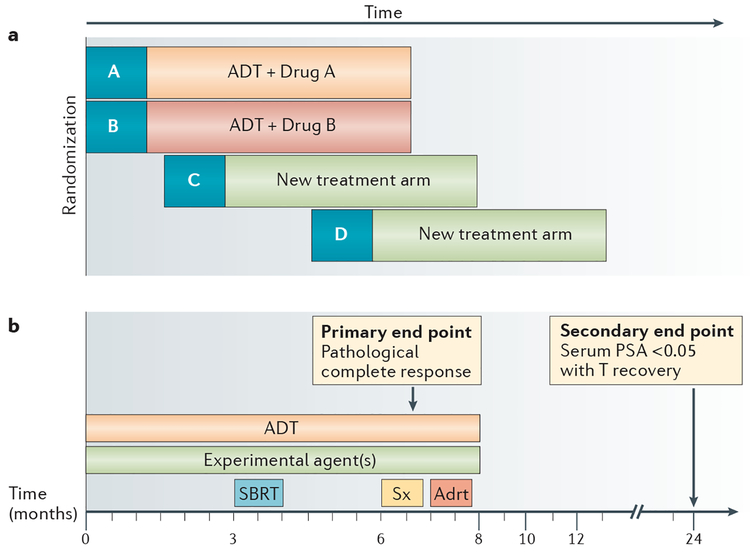
Figure 2 |. Multimodality treatment schema for multiarm, multistage trials in patients with advanced-stage prostate cancer.
a | General study schematic, in which treatment arms can be added in the future, with existing arms serving as a contemporary control. b | Binary trial end points include pathological complete response for patients with prostate cancer in situ and undetectable serum prostate-specific antigen (PSA) levels following testosterone recovery; these end points are measured at 6 and 24 months after randomization, respectively. Failure to achieve an undetectable serum PSA level with testosterone recovery at the conclusion of active treatment will be used as a futility end point. ADT, androgen-deprivation therapy; adrt, adjuvant radiotherapy to prostate bed; SBRT, stereotactic body radiation therapy; Sx, surgery (radical prostatectomy plus pelvic lymph node dissection with or without retroperitoneal lymph node dissection).
The designs of both trials involve a prespecified intermediate activity analysis to determine whether or not a given approach is worthy of further investigation83–85. Differences between our platform and that of STAMPEDE82 include the intermediate activity end point used (early response indicators that are binary and occur early, as opposed to time-to-event measures that occur later), and the sample sizes enrolled to inform the decision to proceed (30–40 per arm for MetaCURE, in contrast to STAMPEDE82, which incorporates three to four efficacy stages that require >100 events to be observed in the control arm for the first stage of comparative analysis). An additional difference is that the next step for a successful initial experimental arm in MetaCURE is validation in an independent phase III trial, while STAMPEDE48 enables the seamless continuation of enrolment until the phase III question is answered, without the need to design a new trial.
A randomized design is used to avoid imbalances in treatment assignment as a result of the rapidly changing diagnostic and therapeutic landscape. Two binary end points are used: pCR and MRD in the prostate for patients with the primary tumour in situ at the start of therapy, and undetectable serum PSA levels with physiological levels of serum testosterone after completion of treatment for those with metastatic disease who are effectively incurable with any single-modality approach. Both end points are objective, occur early, result solely from treatment, and provide an early quantitative readout of efficacy, thus enabling the results obtained with each treatment approach to be viewed in the context of others and prioritized for additional investigation.
In MetaCURE, new treatments will be entered over time, and treatment cohorts that have completed accrual are monitored as outlined in the statistical plan. For each new treatment, the same end points, patient accrual goals, and efficacy boundaries will be retained. The benefit of integrating new treatments is that the existing treatments can be used as concurrent controls, thus ensuring that stage migration has not occurred over time. A fundamental difference with STAMPEDE85, is that, although patients are randomized to different treatments, the response rates between randomized groups will not be formally tested.
Eligibility.
We propose to include patients with prostate cancer of different clinical states, ranging from very-high-risk localized disease (defined as a minimum of four tumour-positive biopsy cores with Gleason score 8–10 disease, or Gleason 4 + 3 disease with either serum PSA levels >20 ng/ml or evidence of stage T3 disease on MRI), with or without regional lymph-node involvement in the pelvis, to those with a limited number of detect able extrapelvic metastases in the retro peritoneum and/or bone. The lower and upper limits of this continuum have been defined with recognition of the uncertainty in the accuracy of clinical staging using standard imaging modalities, such as CT and radionuclide bone scans. However, the accuracy of staging is anticipated to improve with increasing use of commercially available assays that enable profiling of the primary tumour. We particularly use the term ‘low-volume metastatic’ as opposed to ‘oligometastatic’ to describe those with a limited number of metastases because, as PET tracers become increasingly available, patients who were previously considered to have oligometastatic disease, with three or fewer sites detectable on imaging, will likely be found to harbour more metastases and will no longer meet the current criteria.
Issues addressed by MetaCURE
Differences in eligibility for the ‘same’ named popu lation.
The limitations of the TNM staging classification in determining risk in patients with clinically localized disease have resulted in a range of risk stratification schema based on parameters that reflect the extent of disease, including: T-stage using digital rectal examination; number of tumour-positive biopsy cores, and the percentage of cancer present within each core; in addition to measures of tumour aggressiveness, such as Gleason score and baseline serum PSA level. The reported criteria for classification of high-risk and very-high-risk localized disease overlap considerably, without a definite consensus among guidelines provided by different groups, such as the AUA, EAU, and NCCN (TABLE 4). Some guidelines describe dichotomized variables while others are nomogram-based and include continuous variables, all of which have been combined to generate a final score. These variations lead to substantial heterogeneity in 5-year PFS rates ranging from 50% to 80%, depending on the guidelines used29. This recognition of the range of outcomes led to efforts, such as that of the International Society of Urological Pathology, to revise the Gleason scoring system into grade groups that reflect the distinction in prognosis between men with high-risk Gleason score 8 disease relative to that of those with Gleason score 9–10 disease86.
Table 4 |.
Classifications and definitions of high-risk localized prostate cancer
| Source | Serum PSA >20ng/ml | Gleason 8–10 | Gleason pattern 5 | ≥5 cores with Gleason score 8–10 | ≥cT2 | cT3a | cT3-cT4 | cN+ | Other |
|---|---|---|---|---|---|---|---|---|---|
| NCCN guidelines (high risk)117 | X | X | - | - | - | X | - | - | - |
| NCCN guidelines (very high risk)117 | - | - | X | X | - | X | - | - | |
| AUA (high risk)118 | X | X | - | - | X(T2c) | - | - | - | - |
| EAU-ESTRO-SIOG119 | X | X | - | - | X(T2c) | - | - | - | Any serum PSA level, any Gleason score, cT3b -T4orcN + - locally advanced |
| RTOG/NRG (groups 1 – 4)120 | - | X | - | - | - | - | X | X | On the basis of patients enrolled in RTOG phase III trials |
| JHU (very high risk)121–122 | - | - | X | X | - | - | - | - | Or multiple NCCN-defined high-risk features |
| EMPaCT(high risk)123 | X | X | - | - | - | - | X | - | Patients with high-risk disease are substratified into three subgroups* |
| UCSF-CAPRA124 | - | - | - | - | - | - | - | - | On the basis of serum PSA, Gleason score,T-stage, percentage positive biopsy and age‡ |
| MSKCCNomogram125 | - | - | - | - | - | - | - | - | On the basis of Gleason score, clinicalT stage and serum PSA level‡ |
| Decipher87 | - | - | - | - | - | - | - | - | RNA-based classifier |
| OncoType GenomeProstate Score88 | - | - | - | - | - | - | - | - | 17-gene qRT-PCR based assay |
AUA, American Urological Association; CAPRA, Cancer of the Prostate Risk Assessment; EAU, European Urological Association; EMPaCT, European Multicenter Prostate Cancer Clinical and Translational Research Group; ESTRO, European Society for Radiotherapy and Oncology; JHU, Johns Hopkins University; MSKCC, Memorial Sloan Kettering Cancer Center; NCCN, National Comprehensive Cancer Network; PSA, prostate-specific antigen; qRT-PCR, quantitative reverse-transcriptase polymerase chain reaction; RTOG, Radiation Therapy Oncology Group; SIOG, International Society of Geriatric Oncology; UCSF, University of California, San Francisco.
Patients with high-risk disease are substratified into three subgroups: a good prognosis subgroup (characterized by a single high-risk factor); an intermediate-prognosis subgroup (serum PSA>20 ng/ml and stage cT3–4 disease); and a poor-prognosis subgroup (Gleason score 8–10 in combination with at least one other high-risk factor).
On the basis of a nomogram.
Genomic testing is also becoming widely adopted. Examples include the RNA-based Decipher genomic classifier87, which revealed a strong correlation between genomics-based risk score and the presence of high-risk pathological features; the Oncotype Genomic Prostate Score88, which is designed to identify patients at risk of biochemical recurrence or disease progression; or the PAM50 classifier89, which enables the identification of luminal subtypes among patients with early stage prostate cancer with varying risks of biochemical recurrence. Several of these genomic classifiers have been demonstrated to provide prognostic information beyond what can be obtained using clinical or pathological investigations alone90–92.
Imaging extraprostatic disease.
The specific imaging modalities used to determine the extent of primary disease and to detect metastases varies widely between centres. This variability in part reflects the availability of specific PET imaging tracers, differences in image interpretation, and in how they are reported once the images have been analysed. The preoperative identification and localization of pelvic nodal disease is important from both a prognostic and therapeutic perspective93. Detection of pelvic nodal disease using conventional CT and/or MRI has limited sensitivity and specificity94,95. However, this situation is changing rapidly with the increasing availability of 11C-choline PET, 18F-fluciclovine, and prostate-specific membrane antigen (PSMA)-directed tracers labelled with 18F or 68Ga. These tracers are currently the most frequently used among patients with biochemically recurrent disease in order to determine the source and location of the PSA, and to better inform the decision to recommend the delivery of salvage therapy to the prostate or prostate bed and/or systemic therapy. The role of PET in the detection of nodal disease remains an active area of investigation96.
Radionuclide-based bone imaging remains the standard imaging approach for determining the extent of osseous metastases, despite limited levels of sensitivity. A key limitation of this technique is that the radio nuclides only localize to areas of bone formation that occur in response to the tumour and not to the tumour itself, in addition to poor specificity owing to accumulation of radionuclides in degenerative, traumatic, and/or inflammatory lesions. 18F-sodium fluoride PET imaging generally enables more lesions to be detected, relative to conventional bone scintigraphy, but is also similarly limited by nonspecific uptake in nontumour involved areas97. CT is useful in determining the morphological changes that distinguish between lytic, blastic, and mixed lesions but is not advisable for routine detection of bone metastases98. Contrast-enhanced whole-body MRI is proving to be a sensitive detection method that not only enables secondary changes in bone structure to be detected, but also changes in the bone marrow, in the absence of a discernible change in the patient’s bone structure99. Detection rates associated with contemporary PET imaging agents vary as a function of the tracer selected, and the serum PSA thresholds at which imaging is performed. Choline is currently considered the least-sensitive imaging agent and, at the Advanced Prostate Cancer Consensus Conference in March 2017, PSMA–PET and whole- body MRI were considered to provide comparable levels of perfromance100.
Interventions
The multimodal approach includes a period of induction with systemic therapy followed by surgery, and SBRT to any detectable osseous metastases, potentially followed by adjuvant radiotherapy to the prostate bed and regional lymph nodes according to pathological findings during surgery. This approach is based on a previous pilot experience demonstrating that each treatment modality contributes to the ‘no-evidence-of-disease’ state54. ADT and a second-generation androgen receptor signalling pathway inhibitor, such as abiraterone, enzalutamide, or apalutamide, will serve as the systemic therapy backbone, to which additional agents, either those that target androgen receptor signalling or alternative pathways, can be added to future arms. The choice of the systemic backbone agent is supported by the results of both LATITUDE51 and STAMPEDE48 showing that more-complete inhibition of the androgen-receptor signalling axis prolongs survival, relative to ADT alone. Systemic therapy is discontinued after 8–12 months in patients with undetectable serum PSA levels, and patients’ serum testosterone levels are then monitored until testosterone levels return to noncastrate levels. The first stage of the protocol will begin with examinations of the role of intensification of androgen suppression, with future arms focusing on tumours with deficient DNA-damage repair mechanisms.
Surgery.
In the proposed meta CURE platform, all patients will undergo radical prostatectomy with a thorough pelvic and, if applicable, retroperitoneal lymph-node dissection after 6–8 months of systemic therapy, building on the safety and feasibility demonstrated in research by our group54 and others72,73. Clinically, this approach offers the potential to eliminate disease that, in patients in whom systemic therapy does not result in a pCR in the primary tumour, could be a persistent source of metastases101 or localized disease symptoms. The removal of any residual primary disease is also necessary in order to best assess the neuroendocrine differentiation end point that might be achieved with combinations of systemic therapy. Finally, this protocol will provide ample amounts of both primary tumour and lymph-node tissue samples, thus enabling molecular interrogation of predictors of responses, which might help to identify those who are most likely to benefit from surgery and enable the antineoplastic activity of drug combinations to be evaluated.
Radiotherapy.
Radiotherapy will have multiple roles in our proposed study paradigm. Firstly, SBRT will be used to treat any radiographically evident, minimally osseous disease, with the caveat that such disease must be safely encompassed within three radiation isocentres — to balance between maximizing treatable metastatic lesions versus minimizing the risk of overexposing patients to radiation. Radiotherapy will be delivered in 1–5 fractions. Secondly, conventionally fractionated radiotherapy delivered to the prostate bed and/or pelvic nodal basins will be given as an adjuvant therapy in patients with pathological features suggestive of high-risk disease following prostatectomy (such as positive margins or pelvic nodes), or as part of salvage therapy in patients with biochemically recurrent disease owing to the established role of salvage radiotherapy in providing durable local disease control in the majority of these patients102,103.
Outcomes.
Two binary end points are used in Meta CURE: a common end point of pCR in the prostate for all patients undergoing radical prostatectomy, and undetectable serum PSA levels following recovery of serum testosterone levels, reflecting the complete elimination of all macroscopic and microscopic disease present at the start of treatment. Both binary end points are unequivocal, can be reached early in the course of treatment, and result solely from treatment. Taken together, the use of such end points eliminates the uncertainty associated with trying to interpret changes in serum PSA levels and tumour regression at unmeasurable disease sites, in addition to that surrounding the clinical significance of the reported outcomes of many time-to-event based studies, and the effects of the available and approved post-protocol therapies that can dilute the influence of a treatment on overall survival outcomes. Both end points also provide a quantitative measure that enables the performance of competing strategies to be ranked and prioritized for further study in trials with larger cohorts of patients. We will also be able to prospectively explore the clinical and/or prognostic value of MRD in the prostate, as observed previously in other disease types, such as breast cancer104.
Conclusions
The advantages of a platform that functions as a master trial, in which additional treatment arms can be added without the need to develop a new protocol, are manifold. The continuous control arm enables a higher percentage of enrolled patients to undergo treatment using an experimental approach — that is, to receive treatment regimens that are likely to meet the criteria for superiority to become the new standard approach without the need for a new trial82,85. Another advantage is the inclusion of patients with low-volume disease of a wide range of stages and using a common end point for an effect of treatment: such a control arm also eliminates the uncertainties associated with differences in stage classification based on the various imaging modalities and criteria used to assess both the primary tumour and the presence or absence of metastatic disease.
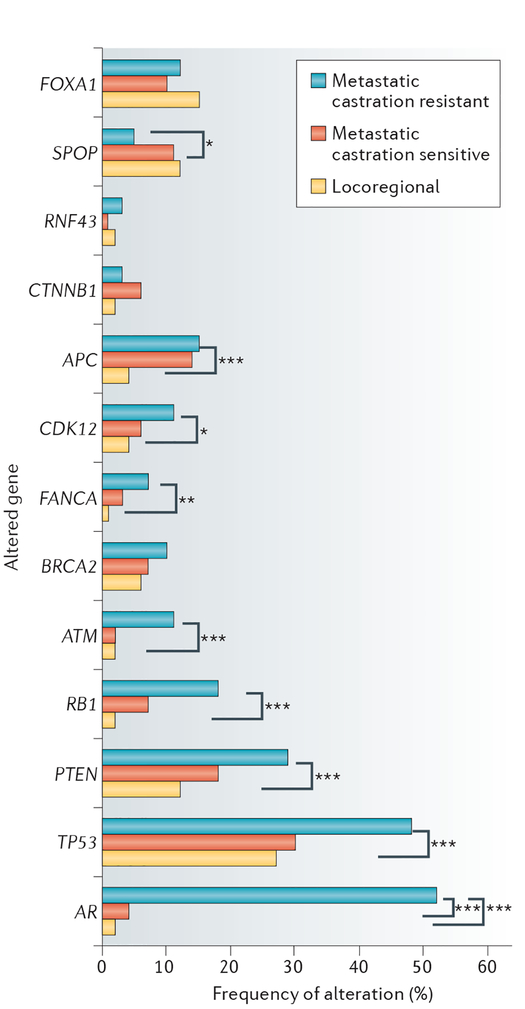
As a platform to enhance scientific understanding of prostate cancer biology, Meta CURE will provide a standardized clinical framework that enables the prospective investigation of different imaging-related and biomarker-related questions; with the use of an acquisition algorithm designed to promote consistent and standardized collection of tissue and blood samples, biological questions can be prioritized and examined using retrospective and/or prospective designs that enable clinical validation timelines to be shortened. The inclusion of patients with a wider range of clinical characteristics enables trials directed at biologically defined subsets of patients that, comparatively, will be completed more rapidly, at a time when the disease is less heterogeneous and more likely to be sensitive to targeted agents. Molecular profiles of prostate cancers of different disease states show the increasing level of complexity and tumour heterogeneity acquired as their disease progresses and is exposed to a wider range of systemic therapies (FIG. 3). The frequency of genomic alterations in the clinically localized tumours examined as part of The Cancer Genome Atlas cohort9 was approximately 27% versus 90% in the SU2C dataset105 which involved prospective whole-exome and transcriptome sequencing of bone or soft-tissue tumour biopsy samples from patients with mCRPC, although these two series are not directly comparable. In the MSK–IMPACT series, in which tumours across different clinical states were examined, a markedly lower rate of alterations in androgen receptor signalling pathways was observed in both the localized and noncastrate metastatic states9,10, potentially predicting increased sensitivity to therapies targeting androgen receptor signalling. In addition to interrogation of the baseline molecular features of these cancers, the MetaCure platform will facilitate a systemic and prospective study of MRD in prostatectomy specimens to enable further understanding of the mechanisms of disease and treatment resistance.
Figure 3 |. Frequency of genetic alterations in different disease states.
Locoregional and metastatic castration-sensitive prostate cancers (CSPCs) have a similar genomic landscape, in contrast to CSPCs, which have a higher rate of genomic alterations. This figure illustrates the frequencies of alterations in selected genes across different disease states in the MSK–IMPACT dataset107. P values are represented (Fisher’s exact test).
Using binary end points that occur early in the course of disease and solely reflect the effects of treatment provides a more-rapid readout of success or failure, thus enabling the results of different treatment strategies to be placed in the context of others. Such an approach also provides an objective platform to prioritize areas for investigation in large-scale trials. Use of binary end points also substantially reduces the time and number of patients needed to show benefit, thus eliminating the uncertainty and lack of clinical significance often associated with time-to-event outcome-based studies. Importantly, the confounding effects of the post-protocol choice of therapy on patient outcomes will also be eliminated. If a relationship between achieving a pCR or MRD in the primary tumour and improved long-term disease-free survival outcomes can be demonstrated, this approach will set the stage for the development of trials designed to establish these outcomes as end points for registration, analogous to what has been established from the neoadjuvant studies conducted in patients with breast cancer, which showed such outcomes to be reliable surrogate indicators of overall survival104 that can lead to accelerated approval106. Most importantly, the approach to trial design presented herein provides an opportunity to capitalize on the latest advances in drug development, molecular and diagnostic taxonomy, and imaging to accelerate the identification of the optimal therapeutic strategies for men with noncastrate prostate cancer.
Key points.
The increasing number of both approved and experimental therapies available mandates the use of new trial designs for patients with noncastrate prostate cancer, which provide expedited readouts of efficacy
Trials based on time-to-event end points, such as progression to metastatic disease and overall survival, require large numbers of patients with long follow-up durations, and might provide inconclusive results owing to use of post-protocol interventions
To accelerate progress, a multi-arm, multistage, multimodality trial platform involving delivery of systemic therapy, radiotherapy to detectable metastases, and radical surgery was developed that enables new arms to be added at any time
The objective is to eliminate all disease using binary quantitative end points that occur early and solely reflect the effects of treatment, such as pathological complete response and undetectable serum prostate-specific antigen levels after testosterone recovery
Acknowledgements
The authors gratefully acknowledge financial support from the Department of Defense Prostate Cancer Research Program (PC121111 and PC131984), the NIH/NCI (Cancer Center Support Grant P30-CA008748, P50-CA92629 SPORE in Prostate Cancer), the Prostate Cancer Foundation, and the Sidney Kimmel Center for Prostate and Urologic Cancers.
Footnotes
Competing interests
H.I.S. declares that he is a member of the board of directors of Asterias Biotherapeutics, has served as a compensated consultant of Blue Earth Diagnostics, Sanofi Aventis, and WCG Oncology, and has served as an uncompensated consultant of Ferring Pharmaceuticals, Janssen Research & Development, LLC and Medivation. M.Y.T., M.J.O., S.M.M. and H.A.V. declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tannock IF et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med 351, 1502–1512 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Kantoff PW et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med 363, 411–422 (2010). [DOI] [PubMed] [Google Scholar]
- 3.de Bono JS et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376, 1147–1154 (2010). [DOI] [PubMed] [Google Scholar]
- 4.de Bono JS et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med 364, 1995–2005 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan CJ et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med 368, 138–148 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scher HI et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med 367, 1187–1197 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Beer TM et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med 371, 424–433 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mateo J et al. DNA-repair defects and olaparib in metastatic prostate cancer. N. Engl. J. Med 373, 1697–1708 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell 163, 1011–1025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abida W et al. Genomic characterization of primary and metastatic prostate cancer (PC) using a targeted next-generation sequencing assay [abstract]. J. Clin. Oncol 34 (Suppl.), 254 (2016). [Google Scholar]
- 11.Scher HI et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol 26, 1148–1159 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. [No authors listed.].NOVANTRONE® mitoXANTRONE for injection concentrate. U.S. Food and Drug Administration; https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/019297s030s031lbl.pdf(2008). [Google Scholar]
- 13.Morris MJ et al. Correlation between radiographic progression-free survival (rPFS) and overall survival (OS): Results from PREVAIL [abstract]. J. Clin. Oncol 34 (Suppl.), 182 (2016). [Google Scholar]
- 14.Morris MJ et al. Radiographic progression-free survival as a response biomarker in metastatic castration-resistant prostate cancer: COU-AA-302 results. J. Clin. Oncol 33,1356–1363. (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huggins C The Treatment of Cancer of the Prostate (The 1943 Address in Surgery before the Royal College of Physicians and Surgeons of Canada). Can. Med. Assoc. J 50, 301–307 (1944). [PMC free article] [PubMed] [Google Scholar]
- 16.Chang AJ, Autio KA, Roach M 3rd, Scher HI High-risk prostate cancer-classification and therapy. Nat. Rev. Clin. Oncol 11, 308–323 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mason MD et al. Final report of the intergroup randomized study of combined androgen-deprivation therapy plus radiotherapy versus androgen-deprivation therapy alone in locally advanced prostate cancer. J. Clin. Oncol 33, 2143–2150 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Widmark A et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet 373, 301–308 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Friedland D et al. A phase II trial of docetaxel (Taxotere) in hormone-refractory prostate cancer: correlation of antitumor effect to phosphorylation of Bcl-2. Semin. Oncol 26, 19–23 (1999). [PubMed] [Google Scholar]
- 20.Picus J & Schultz M Docetaxel (Taxotere) as monotherapy in the treatment of hormone-refractory prostate cancer: preliminary results. Semin. Oncol 26, 14–18 (1999). [PubMed] [Google Scholar]
- 21.Fizazi K et al. Androgen deprivation therapy plus docetaxel and estramustine versus androgen deprivation therapy alone for high-risk localised prostate cancer (GETUG 12): a phase 3 randomised controlled trial. Lancet Oncol 16, 787–794 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Petrylak DP et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med 351, 1513–1520 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Sandler HM et al. A phase III protocol of androgen suppression (AS) and 3DCRT/IMRT versus AS and 3DCRT/IMRT followed by chemotherapy (CT) with docetaxel and prednisone for localized, high-risk prostate cancer (RTOG 0521) [abstract]. J. Clin. Oncol 33 (Suppl), LBA5002 (2015). [Google Scholar]
- 24.Glode LM et al. Adjuvant androgen deprivation (ADT) versus mitoxantrone plus prednisone (MP) plus ADT in high-risk prostate cancer (PCa) patients following radical prostatectomy: A phase III intergroup trial (SWOG S9921) [abstract]. J. Clin. Oncol 35 (Suppl.), 2 (2017). [Google Scholar]
- 25.Royce TJ et al. Surrogate end points for all-cause mortality in men with localized unfavorable-risk prostate cancer treated with radiation therapy versus radiation therapy plus androgen deprivation therapy: a secondary analysis of a randomized clinical trial. JAMA Oncol 3, 652–658 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ICECaP Working Group et al. The development of Intermediate Clinical Endpoints in Cancer of the Prostate (ICECaP). J. Natl Cancer Inst. 107, djv261 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie W et al. Metastasis-free survival (MFS) is a surrogate for overall survival (OS) in localized prostate cancer (CaP) [abstract]. Ann. Oncol 27 (Suppl. 6), 717O (2016). [Google Scholar]
- 28.Pound CR et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 281, 1591–1597 (1999). [DOI] [PubMed] [Google Scholar]
- 29.Yossepowitch O et al. Radical prostatectomy for clinically localized, high risk prostate cancer: critical analysis of risk assessment methods. J. Urol 178, 493–499 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Briganti A et al. Prediction of outcome following early salvage radiotherapy among patients with biochemical recurrence after radical prostatectomy. Eur. Urol 66, 479–486 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Brockman JA et al. Nomogram predicting prostate cancer-specific mortality for men with biochemical recurrence after radical prostatectomy. Eur. Urol 67, 1160–1167 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katz MS et al. Predictors of biochemical outcome with salvage conformal radiotherapy after radical prostatectomy for prostate cancer. J. Clin. Oncol 21, 483–489 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Karlin JD et al. Identifying appropriate patients for early salvage radiotherapy after prostatectomy. J. Urol 190, 1410–1415 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Shipley WU et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N. Engl. J. Med 376, 417–428 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carrie C et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol 17, 747–756 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Crawford ED et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N. Engl. J. Med 321, 419–424 (1989). [DOI] [PubMed] [Google Scholar]
- 37.Eisenberger MA et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N. Engl. J. Med 339, 1036–1042 (1998). [DOI] [PubMed] [Google Scholar]
- 38.Prostate Cancer Trialists’ Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet 355, 1491–1498 (2000). [PubMed] [Google Scholar]
- 39.Bennett CL et al. Maximum androgen-blockade with medical or surgical castration in advanced prostate cancer: a meta-analysis of nine published randomized controlled trials and 4128 patients using flutamide. Prostate Cancer Prostatic Dis. 2, 4–8 (1999). [DOI] [PubMed] [Google Scholar]
- 40.Bertagna C, De Gery A, Hucher M, Francois JP & Zanirato J Efficacy of the combination of nilutamide plus orchidectomy in patients with metastatic prostatic cancer. A meta-analysis of seven randomized double-blind trials (1056 patients). Br. J. Urol 73, 396–402 (1994). [DOI] [PubMed] [Google Scholar]
- 41.Caubet JF et al. Maximum androgen blockade in advanced prostate cancer: a meta-analysis of published randomized controlled trials using nonsteroidal antiandrogens. Urology 49, 71–78 (1997). [DOI] [PubMed] [Google Scholar]
- 42.Schmitt B et al. Combined androgen blockade with nonsteroidal antiandrogens for advanced prostate cancer: a systematic review. Urology 57, 727–732 (2001). [DOI] [PubMed] [Google Scholar]
- 43.Seidenfeld J et al. Relative effectiveness and cost-effectiveness of methods of androgen suppression in the treatment of advanced prostate cancer. Evid. Rep. Technol. Assess 4, 1–246 (1999). [PMC free article] [PubMed] [Google Scholar]
- 44.Sweeney C et al. Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): an ECOG-led phase III randomized trial [abstract]. J. Clin. Oncol 32, (Suppl), LBA2 (2014). [Google Scholar]
- 45.Sweeney CJ et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl. J. Med 373, 737–746 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sweeney C et al. Long term efficacy and QOL data of chemohormonal therapy (C-HT) in low and high volume hormone naïve metastatic prostate cancer (PrCa): E3805 CHAARTED trial [abstract]. Ann. Oncol 27 (Suppl. 6), 720PD (2016). [Google Scholar]
- 47.James ND et al. Docetaxel and/or zoledronic acid for hormone-naïve prostate cancer: first overall survival results from STAMPEDE () [abstract]. J. Clin. Oncol 33, (Suppl.), 5001 (2015). [Google Scholar]
- 48.James ND et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 387, 1163–1177 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gravis G et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol 14, 149–158 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Gravis G et al. Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur. Urol 70, 256–262 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Fizazi K et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N. Engl. J. Med 377, 352–360 (2017). [DOI] [PubMed] [Google Scholar]
- 52.James ND et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N. Engl. J. Med 377, 338–351 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taplin ME et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. J. Clin. Oncol 32, 3705–3715 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Shaughnessy MJ et al. A pilot study of a multimodal treatment paradigm to accelerate drug evaluations in early stage metastatic prostate cancer. Urology 102, 17–172 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sternberg CN et al. M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for advanced transitional cell carcinoma of the urothelium. J. Urol 139, 461–469 (1988). [DOI] [PubMed] [Google Scholar]
- 56.Hendry WF et al. Metastatic nonseminomatous germ cell tumors of the testis: results of elective and salvage surgery for patients with residual retroperitoneal masses. Cancer 94, 1668–1676 (2002). [DOI] [PubMed] [Google Scholar]
- 57.Eggener SE et al. Pathologic findings and clinical outcome of patients undergoing retroperitoneal lymph node dissection after multiple chemotherapy regimens for metastatic testicular germ cell tumors. Cancer 109, 528–535 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Symmans WF et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J. Clin. Oncol 35, 1049–1060 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berruti A et al. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: a meta-regression of 29 randomized prospective studies. J. Clin. Oncol 32, 3883–3891 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Attard G et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J. Clin. Oncol 26, 4563–4571 (2008). [DOI] [PubMed] [Google Scholar]
- 61.Tran C et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324, 787–790 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shore ND et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol 17, 153–163 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Penson DF et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE trial. J. Clin. Oncol 34, 2098–2106 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Scher HI et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet 375, 1437–1446 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Symmans WF et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol 25, 4414–4422 (2007). [DOI] [PubMed] [Google Scholar]
- 66.Crook JM et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N. Engl. J. Med 367, 895–903 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hussain M et al. Intermittent versus continuous androgen deprivation in prostate cancer. N. Engl. J. Med 368, 1314–1325 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rathkopf D et al. Phase II trial of docetaxel with rapid androgen cycling for progressive noncastrate prostate cancer. J. Clin. Oncol 26, 2959–2965 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morris MJ et al. Efficacy analysis of a phase III study of androgen deprivation therapy (ADT) +/− docetaxel (D) for men with biochemical relapse (BCR) after prostatectomy [abstract]. J. Clin. Oncol 33 (Suppl.), 5011 (2015). [Google Scholar]
- 70.James ND et al. Survival with newly diagnosed metastatic prostate cancer in the “docetaxel era”: data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019). Eur. Urol 67, 1028–1038 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Hussain M et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162). J. Clin. Oncol 24, 3984–3990 (2006). [DOI] [PubMed] [Google Scholar]
- 72.Heidenreich A, Pfister D & Porres D Cytoreductive radical prostatectomy in patients with prostate cancer and low volume skeletal metastases: results of a feasibility and case-control study. J. Urol 193, 832–838 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Sooriakumaran P et al. A multi-institutional analysis of perioperative outcomes in 106 men who underwent radical prostatectomy for distant metastatic prostate cancer at presentation. Eur. Urol 69, 788–794 (2016). [DOI] [PubMed] [Google Scholar]
- 74.Antwi S & Everson TM Prognostic impact of definitive local therapy of the primary tumor in men with metastatic prostate cancer at diagnosis: a population-based, propensity score analysis. Cancer Epidemiol 38, 435–441 (2014). [DOI] [PubMed] [Google Scholar]
- 75.Culp SH, Schellhammer PF & Williams MB Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur. Urol 65, 1058–1066 (2014). [DOI] [PubMed] [Google Scholar]
- 76.Rusthoven CG et al. Improved survival with prostate radiation in addition to androgen deprivation therapy for men with newly diagnosed metastatic prostate cancer. J. Clin. Onco 34, 2835–2842 (2016). [DOI] [PubMed] [Google Scholar]
- 77.Moschini M, Soria F, Briganti A & Shariat SF The impact of local treatment of the primary tumor site in node positive and metastatic prostate cancer patients. Prostate Cancer Prostatic Dis. 20, 7–11 (2017). [DOI] [PubMed] [Google Scholar]
- 78.Ost P et al. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: a multiinstitutional analysis. Eur. Urol 69, 9–12 (2016). [DOI] [PubMed] [Google Scholar]
- 79.Seung SK et al. American College of Radiology (ACR) and American Society for Radiation Oncology (ASTRO) practice guideline for the performance of stereotactic radiosurgery (SRS). Am. J. Clin. Oncol 36, 310–315 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ost P et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur. Urol 67, 852–863 (2015). [DOI] [PubMed] [Google Scholar]
- 81.Berkovic P et al. Salvage stereotactic body radiotherapy for patients with limited prostate cancer metastases: deferring androgen deprivation therapy. Clin. Genitourin. Cancer 11, 27–32 (2013). [DOI] [PubMed] [Google Scholar]
- 82.James ND et al. STAMPEDE: Systemic Therapy for Advancing or Metastatic Prostate Cancer — a multiarm multi-stage randomised controlled trial. Clin. Oncol 20, 577–581 (2008). [DOI] [PubMed] [Google Scholar]
- 83.James ND et al. Systemic therapy for advancing or metastatic prostate cancer (STAMPEDE): a multiarm, multistage randomized controlled trial. BJU Int 103, 464–469 (2008). [DOI] [PubMed] [Google Scholar]
- 84.Parmar MK et al. Speeding up the evaluation of new agents in cancer. J. Natl Cancer Inst 100, 1204–1214 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sydes MR et al. Flexible trial design in practice — stopping arms for lack-of-benefit and adding research arms mid-trial in STAMPEDE: a multi-arm multi-stage randomized controlled trial. Trials 13, 168 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Epstein JI et al. A contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur. Urol 69, 428–435 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Den RB et al. Decipher correlation patterns post prostatectomy: initial experience from 2342 prospective patients. Prostate Cancer Prostatic Dis. 19, 374–379 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cullen J et al. A biopsy-based 17-gene genomic prostate score predicts recurrence after radical prostatectomy and adverse surgical pathology in a racially diverse population of men with clinically low- and intermediate-risk prostate cancer. Eur. Urol 68, 123–131 (2015). [DOI] [PubMed] [Google Scholar]
- 89.Feng FY et al. Luminal and basal subtyping of prostate cancer [abstract]. J. Clin. Oncol 35 (Suppl.), 3 (2017). [Google Scholar]
- 90.Cooperberg MR et al. Combined value of validated clinical and genomic risk stratification tools for predicting prostate cancer mortality in a high-risk prostatectomy cohort. Eur. Urol 67, 326–333 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klein EA et al. A genomic classifier improves prediction of metastatic disease within 5 years after surgery in node-negative high-risk prostate cancer patients managed by radical prostatectomy without adjuvant therapy. Eur. Urol 67, 778–786 (2015). [DOI] [PubMed] [Google Scholar]
- 92.Ross AE et al. Tissue-based genomics augments post-prostatectomy risk stratification in a natural history cohort of intermediate- and high-risk men. Eur. Urol 69, 157–165 (2016). [DOI] [PubMed] [Google Scholar]
- 93.Touijer KA, Mazzola CR, Sjoberg DD, Scardino PT & Eastham JA Long-term outcomes of patients with lymph node metastasis treated with radical prostatectomy without adjuvant androgen- deprivation therapy. Eur. Urol 65, 20–25 (2014). [DOI] [PubMed] [Google Scholar]
- 94.Evangelista L, Guttilla A, Zattoni F, Muzzio PC & Zattoni F Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: a systematic literature review and meta-analysis. Eur. Urol 63, 1040–1048 (2013). [DOI] [PubMed] [Google Scholar]
- 95.Hovels AM et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin. Radiol 63387–395 (2008). [DOI] [PubMed] [Google Scholar]
- 96.Maurer T et al. Diagnostic efficacy of 68gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J. Urol 195, 1436–1443 (2016). [DOI] [PubMed] [Google Scholar]
- 97.Kulshrestha RK, Vinjamuri S, England A, Nightingale J & Hogg P The role of 18F-sodium fluoride PET/CT bone scans in the diagnosis of metastatic bone disease from breast and prostate cancer. J. Nucl. Med. Technol 44, 217–222 (2016). [DOI] [PubMed] [Google Scholar]
- 98.Vargas HA et al. Bone metastases in castration- resistant prostate cancer: associations between morphologic CT patterns, glycolytic activity, and androgen receptor expression on PET and overall survival. Radiology 271, 220–229 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Robertson NL et al. Combined whole-body and multi-parametric prostate MRI as a single-step approach for the simultaneous assessment of local recurrence and metastatic disease after radical prostatectomy. J. Urol 198, 65–70 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gillessen S et al. Management of patients with advanced prostate cancer: the report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur. Urol 10.1016/j.eururo.2017.06.002 (2017). [DOI] [PubMed] [Google Scholar]
- 101.Kim MY et al. Tumor self-seeding by circulating cancer cells. Cell 139, 1315–1326 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abdollah F et al. Impact of adjuvant radiotherapy on survival of patients with node-positive prostate cancer. J. Clin. Oncol 32, 3939–3947 (2014). [DOI] [PubMed] [Google Scholar]
- 103.Bolla M et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet 380, 2018–2027 (2012). [DOI] [PubMed] [Google Scholar]
- 104.Cortazar P et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384, 164–172 (2014). [DOI] [PubMed] [Google Scholar]
- 105.Robinson D et al. Integrative clinical genomics of advanced prostate cancer. Cell 161, 1215–1228 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for industry pathological complete response in neoadjuvant treatment of high-risk early-stage breast cancer: use as an endpoint to support accelerated approval. U.S. Food and Drug Administration; http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM305501.pdf (2014) [Google Scholar]
- 107.Abida W et al. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may impact clinical decision making. JCO Precis. Oncol 10.1200/PO.17.00029 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jones CU et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N. Engl. J. Med 365, 107–118 (2011). [DOI] [PubMed] [Google Scholar]
- 109.Warde P et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet 378, 2104–2111 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Labrie F et al. Neoadjuvant hormonal therapy: the Canadian experience. Urology 49, 56–64 (1997). [DOI] [PubMed] [Google Scholar]
- 111.van der Kwast TH et al. Prolonged neoadjuvant combined androgen blockade leads to a further reduction of prostatic tumor volume: three versus six months of endocrine therapy. Urology 53, 523–529 (1999). [DOI] [PubMed] [Google Scholar]
- 112.Gleave ME et al. Randomized comparative study of 3 versus 8-month neoadjuvant hormonal therapy before radical prostatectomy: biochemical and pathological effects. J. Urol 166, 500–507 (2001). [PubMed] [Google Scholar]
- 113.Klotz LH et al. Long-term followup of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. J. Urol 170, 791–794 (2003). [DOI] [PubMed] [Google Scholar]
- 114.Mostaghel EA et al. Targeted androgen pathway suppression in localized prostate cancer: a pilot study. J. Clin. Oncol 32, 229–237 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Montgomery B et al. Neoadjuvant enzalutamide prior to prostatectomy. Clin. Cancer Res 23, 2169–2176 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Efstathiou E et al. Neoadjuvant enzalutamide (ENZA) and abiraterone acetate (AA) plus leuprolide acetate (LHRHa) versus AA+ LHRHa in localized high-risk prostate cancer (LHRPC) [abstract]. J. Clin. Oncol 34 (Suppl.), 5002 (2016). [Google Scholar]
- 117.National Comprehensive Cancer Network. Prostate Cancer Guidelines (Version 2.2017). https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.(2017).
- 118.Thompson I et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J. Urol 177, 2106–2131 (2007). [DOI] [PubMed] [Google Scholar]
- 119.Mottet N et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur. Urol 71, 618–629 (2017). [DOI] [PubMed] [Google Scholar]
- 120.Roach M et al. Four prognostic groups predict long-term survival from prostate cancer following radiotherapy alone on Radiation Therapy Oncology Group clinical trials. Int. J. Radiat. Oncol. Biol. Phys 47, 609–615 (2000). [DOI] [PubMed] [Google Scholar]
- 121.Narang AK et al. Very high-risk localized prostate cancer: outcomes following definitive radiation. Int. J. Radiat. Oncol. Biol. Phys 94, 254–262 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sundi D et al. Identification of men with the highest risk of early disease recurrence after radical prostatectomy. Prostate 74, 628–636 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Joniau S et al. Stratification of high-risk prostate cancer into prognostic categories: a European multi- institutional study. Eur. Urol 67, 157–164 (2015). [DOI] [PubMed] [Google Scholar]
- 124.Cooperberg MR et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J. Urol 173, 1938–1942 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stephenson AJ et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J. Clin. Oncol 27, 4300–4305 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]