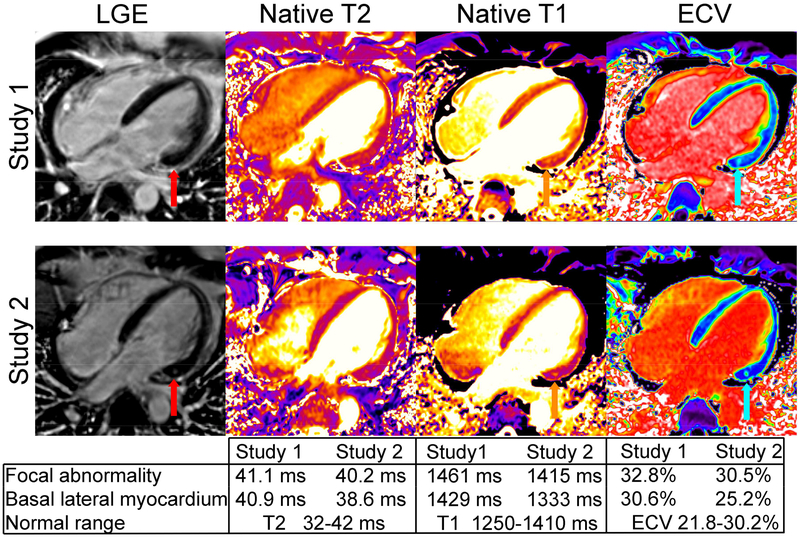
In March, 2015, a 34 year-old previously healthy male healthcare worker was evacuated from Sierra Leone to the National Institutes of Health (NIH) Clinical Research Center for the management of severe Ebola virus disease (EVD) (1). The patient arrived 7 days after initial symptom onset with high fever and asthenia. His clinical course was complicated by severe meningoencephalitis and sequential organ failure despite meticulous attention to fluid and electrolyte status and absence of hypotension. Daily bedside echocardiograms during peak illness did not reveal gross myocardial dysfunction, although cardiac ejection fractions were not specifically assessed. On days 17–18 of clinical illness the patient developed QTc prolongation of up to 600 milliseconds that resolved with discontinuation of propofol administration. Cardiac MRI (CMR) on clinical illness day 32, performed in the setting of improved clinical status and following resolution of viremia, showed mild diffuse left ventricular hypokinesis, an ejection fraction of 51% (normal 55–75%), and abnormalities in T1 and extracellular volume fraction (ECV) localized in the basal lateral wall. These findings were consistent with myocardial inflammation or edema (Figure). Repeat CMR 11 weeks later showed persistent mild diffuse left ventricular hypokinesis (LVEF 52%). Gadolinium-enhanced imaging revealed a new small focus of atypical enhancement in the basal wall consistent with fibrosis and resolution of abnormalities previously seen in the surrounding myocardium. This is the first reported CMR in EVD. Mild myocardial dysfunction and subtle findings of edema or inflammation with evidence of progression to fibrosis suggest the presence of myocarditis. Gold standard diagnostic criteria for viral myocarditis require endomyocardial biopsy (EMB) using standardized histopathological (Dallas criteria) and immunohistochemical diagnostic criteria (2). CMR, however, is a valuable noninvasive modality for detection of myocarditis with high specificity (91%) and positive predictive value (91%) when two of three CMR characteristics listed in the Lake Louise criteria are present (2). Lake Louise criteria include detection of early capillary leakage on the basis of T1-weighted early gadolinium enhancement, detection of necrosis and fibrosis on the basis of late gadolinium enhancement (LGE), and detection of edema or hyperemia by T2-weighted imaging (2). In our patient, mild myocardial dysfunction with evidence of myocardial edema or inflammation (T1 and ECV abnormalities) in the basal lateral wall of the left ventricle during initial CMR, and evidence of evolution to fibrosis (LGE) in the basal wall on follow-up CMR (Figure) are highly specific for myocarditis. While EMB has not been reported in EVD, post-mortem immunohistochemistry examination in fatal cases shows abundant viral antigen in the endocardium, endothelium, and extracellular in the subendocardium (3). Myocarditis due to viruses other than Ebola, prominently enteroviruses, ranges in clinical severity from mild infection to progressive dilated cardiomyopathy, cardiogenic shock, and fatal arrhythmias. Whether QT prolongation in our patient was related to myocarditis is not possible to discern. However, QT prolongation is frequently observed in infectious and non-infectious inflammatory conditions, was the most common EKG finding among 186 patients with myocarditis (~25% of cases), and predicted a poor clinical outcome, including cardiac death, and may be attributable to inflammatory cytokines (4). The contribution of viral myocarditis to myocardial dysfunction and predisposition to fatal arrhythmias in the acute setting of EVD or myocardial dysfunction in long-term survivors requires further evaluation.
Figure 1. Cardiac MRI studies were performed following recovery from severe Ebola virus disease on clinical illness day 32 (study 1) and 11 weeks later (study 2).
Study 1 showed mildly reduced ejection fraction (LVEF 52%). Late gadolinium enhancement (LGE) was initially unremarkable (red arrow, study 1). There was evidence of mild edema or inflammation of the midwall of the basal inferolateral segment of the left ventricle with a focal abnormal spot on native T1 and ECV (orange and cyan arrows) but also milder abnormalities in surrounding myocardium excluding the spot (see table). Quantitative maps of myocardial T2 showed a non-significant elevation in mid-myocardial T2 in the interventricular septum. On study 2, the T1 and ECV abnormalities normalized except for a sub-gram region localized to the region of most severe abnormality seen on the initial study. Interval development of fibrosis was evident on LGE images (red arrow on study 2). LVEF remained mildly abnormal (51%).
Acknowledgments:
The Intramural Research Programs of National Institute of Allergy and Infectious Diseases and National Heart, Lung, and Blood Institute, National Institutes of Health, supported this work. The content of this publication does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Arai: Research agreements with Siemens and Bayer.
References:
- 1.Chertow DS, Nath A, Suffredini AF, Danner RL, Reich DS, Bishop RJ, Childs RW, Arai AE, Palmore TN, Lane HC, Fauci AS, Davey RT. Severe Meningoencephalitis in a Case of Ebola Virus Disease: A Case Report. Ann Intern Med 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollack A, Kontorovich AR, Fuster V, Dec GW. Viral myocarditis--diagnosis, treatment options, and current controversies. Nat Rev Cardiol 2015; 12: 670–680. [DOI] [PubMed] [Google Scholar]
- 3.Martines RB, Ng DL, Greer PW, Rollin PE, Zaki SR. Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg viruses. J Pathol 2015; 235: 153–174. [DOI] [PubMed] [Google Scholar]
- 4.Lazzerini PE, Capecchi PL, Laghi-Pasini F. Long QT Syndrome: An Emerging Role for Inflammation and Immunity. Front Cardiovasc Med 2015; 2: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]