Abstract
Background:
Cognitive training is beneficial in various clinical settings, though its perioperative feasibility and impact remain unknown. The objective of this pilot study was to determine the feasibility of home-based cognitive prehabilitation prior to major surgery in older adults.
Methods:
Sixty-one patients were enrolled, randomized, and allocated to either a home-based, preoperative cognitive training regimen or no training prior to surgery. Outcomes included postoperative delirium incidence (primary outcome; assessed with the 3D-Confusion Assessment Method), perioperative cognitive function based on NIH Toolbox measures, hospital length of stay, and physical therapy session participation. Reasons for declining enrollment were reported, as were reasons for opting out of the training program.
Results:
Postoperative delirium incidence was 6/23 (26%) in the prehabilitation group compared to 5/29 (17%) in the control group (P=0.507). There were no significant differences between groups in NIH Toolbox cognitive function scoring, hospital length of stay, or physical therapy participation rates. Study feasibility data were also collected and reported. The most common reasons for declining enrollment were lack of computer access (n=19), time commitment (n=9), and feeling overwhelmed (n=9). In the training group, only 5/29 (17%) included patients were able to complete the prescribed seven days of training, and 14/29 (48%) opted out of training once home. Most common reasons were feeling overwhelmed (n=4) and computer difficulties (n=3).
Conclusions:
Short-term, home-based cognitive training prior to surgery is unlikely to be feasible for many older patients. Barriers to training include feeling overwhelmed, technical issues with training, and preoperative time commitment.
Keywords: Cognitive Dysfunction, Cognitive Reserve, Delirium, Feasibility Studies, Neuropsychological Tests
INTRODUCTION
Postoperative delirium is a significant public health concern, affecting 10–70% of older patients presenting for surgery.1, 2 In addition to the relatively high incidence, postoperative delirium is associated with increased mortality,3 increased hospital length of stay,3 persistent cognitive decline after surgery,4 and elevated healthcare costs.5 The psychological sequelae of delirium are often distressing for both patients and families, and unfortunately, there is a paucity of evidence-based strategies that consistently and effectively reduce delirium risk.6
Cognitive prehabilitation, prior to surgery, may serve as a candidate strategy for mitigating delirium. Cognitive training exercises have been shown to improve cognitive function and functional status in community-dwelling older adults, and benefits may last for several months to years.7 Training exercises have also improved performance in attention,8, 9 short-term memory,9 and visuospatial processing;9, 10 all of which are implicated as clinical features of delirium.11, 12 Thus, there is neuroscientific plausibility that cognitive training may improve cognitive function and mitigate delirium risk.
An initial step in studying cognitive prehabilitation involves studying the feasibility of such training programs in older surgical patients. At this early stage, it seems reasonable to begin addressing these questions in the home-based setting, which might offer the most practical strategy for prehabilitation. As such, the aim of this pilot study is to assess the feasibility of implementing a home-based preoperative cognitive training program for surgical patients at high-risk for delirium and associated outcomes. We tested the two-fold hypothesis that (1) home-based cognitive prehabilitation will be feasible for older surgical patients and that (2) such training will mitigate delirium risk. Results will inform future trial design on a larger scale.
MATERIALS AND METHODS
This was a prospective, single-blinded, randomized controlled trial conducted at Michigan Medicine. The study was approved by the University of Michigan Medical School Institutional Review Board (HUM00119087, date of approval: 10/6/2016) and registered with clinicaltrials.gov (, registered 11/15/2016). All study procedures took place from March 2017 to May 2018, and the trial is compliant with the Consolidated Standards of Reporting Trials (CONSORT)13.
Study Design and Procedures
Participants were recruited from preoperative clinic locations at least one week prior to scheduled surgery, and informed written consent was obtained from all participants prior to study enrollment. Inclusion criteria included age ≥ 60 years old, scheduled non-cardiac, non-major vascular, or non-intracranial surgery, and daily access to computer and internet use prior to surgery. Exclusion criteria included severe pre-existing cognitive impairment (preoperative delirium, Mild Cognitive Impairment, dementia, and/or not having capacity to provide informed consent), severe auditory or visual impairment, emergency surgery, no daily computer and internet access, or concurrent participation in cognitive training exercise. These eligibility criteria were designed to maximize the ability to detect efficacy and feasibility specific to cognitive training while minimizing relevant perioperative confounders (i.e., altered cerebral blood flow with cardiac and neurosurgical procedures). After enrollment, demographic data were obtained, and baseline cognitive function assessments were performed (see below). Participants were then randomized to the interventional group (cognitive training) or control group (no training) in a block-stratified manner based on age and gender under biostatistician guidance (AT). Assignment was concealed in a randomization packet, and participants were instructed to open the packet after leaving the clinic office. A member of the team (PEV) remained unblinded and contacted participants after this initial visit to confirm treatment assignment and coordinate training. The PI was chosen for this role to maintain a consistent presence and resource for participants during study duration. To minimize bias, the PI was not involved with perioperative assessments, and statistical analysis was performed separately by the team statistician (AT).
After the initial baseline study visit, participants were requested to train from their home computer for at least seven days, if possible, prior to surgery. Adaptive training for seven days, with sessions lasting approximately 20 minutes per day, has led to appreciable changes in neural and cognitive function.14 Thus, seven days was chosen as a target goal for participants based on this research and the need to pilot an intervention that would be only minimally demanding for participants preparing for surgery. Follow-up assessments were then performed immediately prior to surgery and through the afternoon of postoperative day three. The training battery, outcomes, and assessments are described in the following sections.
Cognitive Training Battery
This study utilized an adaptive, computer-based cognitive training battery that specifically targets executive function, attention, working memory, and visuospatial processing (BrainHQ, Posit Science Corporation, San Francisco, CA USA), which are cognitive domains particularly affected by delirium.11, 12 The specific games chosen were Divided Attention, Double Decision, Card Shark, Juggle Factor, and Eye for Detail (see table, Supplemental Digital Content 1, for detailed game and training information).
Outcomes and Assessments
The primary outcome was delirium incidence from the postanesthesia care unit (PACU) (assessment beginning least two hours after the end of surgery) through postoperative day three. Delirium screening was performed with the 3-minute diagnostic assessment (3D-Confusion Assessment Method, CAM), which has demonstrated high inter-rater reliability and validity compared to DSM-IV based criteria with expert panel adjudication.15 After PACU discharge, delirium assessments were then performed twice daily – morning and afternoon – through postoperative day three. Any positive screen during this timeframe was counted towards delirium incidence in each group. All investigators performing delirium assessments (ARD, BK, MZ, MJC, AMM) were trained in 3D-CAM administration.
Multiple secondary outcomes were included in this study. Cognitive function was separately assessed throughout the study period using three tests from the NIH Toolbox Cognition Battery – Flanker Inhibitory Control and Attention Test, List Sorting Working Memory Test, and the Pattern Comparison Processing Speed Test. Collectively, these tests assess executive function, attention, working memory, and processing speed, which mirror the cognitive domains targeted with training as previously described. These tests have been validated in adults up to the age of 85,16 and they were administered via iPad to participants at baseline, the morning of surgery, and at the conclusion of the postoperative day three afternoon visit. Fully Corrected T-Scores were used for analysis, which adjust for age, gender, race, ethnicity, and educational attainment.17 The purpose of this testing was to assess for sustained, transferred, cognitive gains afforded by training in the intervention group. Testing was completed after enrollment (baseline), on the morning of surgery (after training completion), and after the first two postoperative days, which coincides with the highest incidence of delirium.3 Finally, hospital length of stay was calculated for each group, as was the proportion of physical therapy sessions successfully completed compared to the total number prescribed. As cognitive impairment is associated with reduced functional status,18 improved cognition may bolster engagement with physical therapy. As a final secondary outcome, assessing length of ICU stay was planned (), though this was deferred as very few patients required ICU admissions in the study.
Lastly, additional and exploratory outcomes were analyzed that related to training metrics. For all eligible patients, the most commons reasons for declined enrollment were collected and reported. Additionally, for participants allocated to training but then elected not to train, the primary reason was reported. An exploratory post-hoc analysis was also conducted to determine the correlation between the Brain Activity Quotient (i.e., overall training progress made with BrainHQ, reported as a continuous measure) compared to perioperative NIH Toolbox scoring.
Statistical Analyses
A univariate analysis was conducted to compare those randomized to the control and treatment groups in regard to patient demographics and procedure characteristics. Continuous data elements were analyzed to determine their distribution with the Shapiro-Wilk test, and analysis proceeded with Student’s t-tests or Mann-Whitney U tests, as appropriate. Categorical data were analyzed using a chi-squared or Fisher’s Exact tests, as appropriate.
To assess changes in cognitive function across each time point between groups, a series of linear repeated measures generalized estimating equation (GEE) models were constructed. An autoregressive correlation structure was used given that correlations may weaken over time. This GEE-based strategy can incorporate longitudinal data over time in the setting of partially missing data.19 An interaction term was created between group and time to determine whether significant interactions were present over the study period. If interaction terms were non-significant, they were removed from respective models.
All analysis was conducted using SAS version 9.3 (SAS Institute, Cary, NC) and SPSS version 21 (IBM Inc., Chicago, IL). A P-value of <0.05, with two-tailed hypothesis testing, was considered statistically significant for all analyses unless otherwise specified. Bonferroni-correction was used for test families: namely, the GEE models (three hypotheses tested) and exploratory, post-hoc correlation analyses (nine hypotheses tested). As the BrainHQ Quotient data followed a non-parametric distribution, Spearman’s rank-order correlation was used.
Power and sample size
Given the feasibility nature of this study, the sample size represents a convenience sample, and power calculations were deferred. By having a relatively small group of participants, detailed analysis can be conducted for determining digital literacy, barriers to enrollment, and barriers to training program completion.
RESULTS
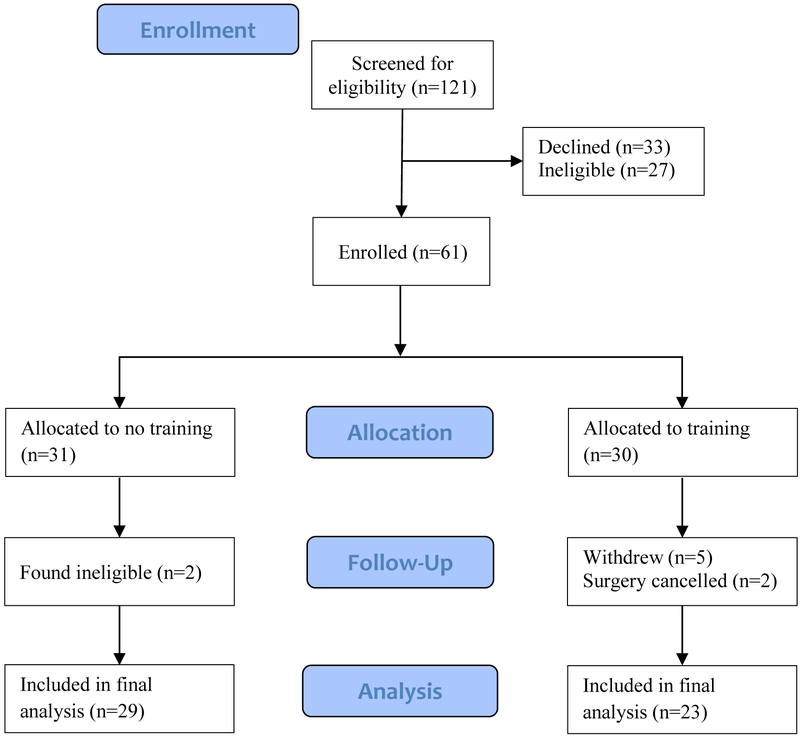
The study flow diagram is presented in Figure 1. Enrollment took place between April 2017 and April 2018. In total, 121 patients were screened for eligibility, and 61 patients were randomly allocated to either the control or prehabilitation group. The most common non-medical reasons for declining trial enrollment were lack of computer access, time commitment, and feeling overwhelmed preoperatively (see figure, Supplemental Digital Content 2). The proportion of participants that withdrew from the trial was significantly higher in the prehabilitation group (5/30, 16.7%) compared to the control group (0/31, 0%, P=0.024). Reasons for withdrawing included anxiety (n=3), home computer/technical issues (n=1), and not finding the games enjoyable (n=1). Data from 29 control group participants and 23 prehabilitation participants were included in the final analysis (unless otherwise indicated). Lastly, baseline characteristics are presented in Table 1.
Figure 1.
CONSORT flow diagram presented.
Table 1.
Participant Characteristics
| Training Group n=23 |
Non-Training Group n=29 |
|
|---|---|---|
| Age--years, mean (+/− SD) | 66 (±4.9) | 68 (±5.4) |
| Male sex-- no. (%) | 10 (43) | 15 (52) |
| Education (college or higher) | 5 (22) | 12 (41) |
| Comorbidities—no. (%) | ||
| CAD | 4 (14) | 4 (16) |
| CVD* | 1 (4.3) | 1 (3.4) |
| HTN | 13 (57) | 16 (55) |
| COPD | 2 (8.7) | 4 (14) |
| CKD | 1 (4.3) | 2 (6.9) |
| DM | 3 (13) | 5 (17) |
| Type of surgery-- n (%) | ||
| Gastrointestinal | 12 (52) | 8 (28) |
| Urologic | 6 (26) | 6 (21) |
| Spine | 4 (17) | 12 (41) |
| Hepatobiliary | 1 (4.3) | 3 (10) |
Stroke, TIA, or carotid artery stenosis history. CAD = coronary artery disease, CVD = cerebrovascular disease, HTN = hypertension, COPD = chronic obstructive pulmonary disease, CKD = chronic kidney disease, CM = diabetes mellitus. All patients were of non-Hispanic ethnicity.
Outcomes
Spanning from the postanesthesia care unit through postoperative day three, the incidence of delirium (primary outcome) was 6/23 (26%) in the prehabilitation group vs. 5/29 (17%) in the control group (P=0.507; see figure, Supplemental Digital Content 3, for additional delirium outcome data). For the cognitive function test results, the previously described GEE modeling strategy was used to analyze attention (Flanker Inhibitory Control and Attention Test), working memory (List Sorting Working Memory), and processing speed (Pattern Comparison Processing Speed Test) over time (baseline, preoperative morning, postoperative day three) between groups. For all tests, the group and time interaction terms were non-significant and thus removed from each model. There was no significant difference in estimated mean score between groups for any of the tests (Table 2).
Table 2.
Estimated Mean Score Differences – NIH Toolbox Tests
| 95% Confidence Interval (Mean Difference) | ||||
|---|---|---|---|---|
| Test | Estimated Mean Score Difference (β) | Lower Limit | Upper Limit | P Value |
| Flanker Inhibitory Control Test | 2.77 | −1.69 | 7.23 | 0.223 |
| List Sorting Working Memory Test | −0.94 | −5.29 | 3.41 | 0.672 |
| Pattern Comparison Processing Speed | 4.21 | −3.25 | 11.7 | 0.268 |
For GEE modeling, the non-training group serves as the reference control. No group-time interaction terms were significant for any of the tests. Results reflect the mean difference in fully corrected T-scores between groups for each respective test. Standardized scores have a mean of 50 with standard deviation of 10, and scores are adjusted for age, gender, race/ethnicity, and educational attainment (see manuscript text for additional information and references). All available scores were incorporated from both groups (non-training group, n = 29 participants; training group, n = 23 participants)
For the final secondary outcomes, hospital length of stay was 6.8 days in the prehabilitation group compared to 6.4 days in the control group (P=0.696). In terms of physical therapy sessions, after excluding attempted sessions for which patients were unavailable (i.e., off the floor) or not yet medically cleared (for cognitively unrelated reasons), physical therapy participation was 100% in both groups.
Prehabilitation Metrics
As previously mentioned, only 5/29 (17%) patients randomized to training (and had surgery as scheduled) were able to train for seven days prior to surgery. Furthermore, 14/29 patients (48%) opted out of the training schedule once home. Of the 15 patients who engaged in the training exercises, the median number of days trained was 6 (IQR: 5 – 7), and the average daily training session lasted for a median of 20 minutes (IQR: 11 – 23). For patients that elected not to train, the primary reason was recorded and reported (see figure, Supplemental Digital Content 2). Common problems encountered during training were feeling overwhelmed and experiencing computer-related difficulties.
Post-hoc Exploratory Correlations
A post-hoc, exploratory analysis was conducted to analyze correlations between training progress, defined as maximum the Brain Activity Quotient (n) achieved, and Fully Corrected T-scores on each Toolbox test. The Brain Activity Quotient reflects the overall gains made with training, with higher scores reflecting further progress (score range: 0 – 2624). This analysis resulted in nine correlations (Brain HQ quotient score correlated with baseline, preoperative, and postoperative day three Fully Corrected T-scores for all three tests). If training progress (i.e., maximum Brain Activity Quotient) correlated with post-training test scores, this suggests that more training led to improved perioperative cognitive performance. If, however, training progress correlated just as well with baseline (pre-training) scores, this suggests that those with higher baseline cognitive ability were inherently able to train more rigorously (compared to those with lower baseline scores). Correlation was only significant for processing speed, and correlation strength was approximately equal across all three time points (see table, Supplemental Digital Content 4). There were correlation trends with training progress and attention testing on the morning of surgery (ρ = 0.528, P=0.014, unadjusted) and postoperative day three (ρ = 0.690, P=0.006, unadjusted) and working memory testing on the morning of surgery (ρ = 0.580, P=0.006, unadjusted), though correlations did not reach statistical significance after Bonferonni-correction.
DISCUSSION
This was a pilot study of cognitive prehabilitation prior to major surgery in older patients. There was no significant difference in delirium incidence or cognitive performance in either group throughout the study period. This is perhaps not surprising given the limited training time achieved. Those enrolled in the prehabilitation arm were more likely to withdraw from the study, and most patients were not able to train for the prescribed seven days. Time constraints, anxiety, and inconsistent computer access were common reasons for both declining enrollment and experiencing difficulty with home training. Additionally, there were no differences in hospital length of stay or physical therapy participation in either group. Post-hoc exploratory analysis revealed possible trends in training progress and perioperative attention and working memory scores, though these correlations were not statistically significant after correction for multiple comparisons. Overall, home-based, unsupervised cognitive training over a short timeframe appears unlikely to improve perioperative cognitive function. In fact, compressed training efforts may exacerbate negative experiences (e.g., anxiety, apprehension) given the high opt-out rate (48%) and higher withdrawal rate compared to the non-training group.
Important themes have become apparent with this investigation. In general, patients initially expressed enthusiasm for participating in cognitive prehabilitation. More than 60 patients were enrolled in just over one year, and more patients likely would have enrolled if computer and internet access were made consistently available. Indeed, lack of computer access was the most common reason for study ineligibility. Problems with home computer and internet access were also among the most cited reasons for experiencing difficulty with training. Thus, providing consistent and reliable access to training platforms (e.g., computer, tablet) may improve enrollment, compliance, and efficacy in future studies. Patients also frequently conveyed feeling overwhelmed prior to surgery, and this was commonly listed as a reason for declining enrollment and reporting trouble adhering to the training schedule. Time commitment was an additional, related factor, which also hindered enrollment and training participation.
A recent meta-analysis by Lampit et al. provides complementary insight into training barriers and expected effect sizes of training regimens.20 An analysis of 52 studies revealed a consistent and mild-moderate effect size (Hedge’s g = 0.2–0.3) of computerized cognitive training on cognitive function with a minimum cumulative training period of four hours. Importantly, home-based training was found to be ineffective, which supports our preliminary findings. As described above, technological difficulties, feeling overwhelmed, and time constraints may hinder self-directed, home-based training. The meta-analysis also demonstrated that training more than three times per week is not only ineffective – but likely worsens performance – compared to training ≤3 times per week. Training for >60 minutes was also not found to be more beneficial compared to a shorter training window (i.e., 30–60 minutes). Thus, attempts to augment training “dose” in a short time window may exacerbate anxiety without improving cognitive function; in fact, such attempts might worsen performance based on data from Lampit et al. These findings are important for informing future perioperative study design. Given the short period of time that often occurs between scheduling surgery and the surgical date, it may be challenging to implement an effective minimum training time (≥4 hours20) with a flexible schedule that allows for rest and minimizes stress. If such studies are to be designed, patients should be included who have a relatively large time interval (weeks, if not months) prior to surgery. Furthermore, participants should be given consistent, reliable access to training platforms, programs should be designed for short-term gains,21 and training should be supervised. Overall, these logistical challenges – coupled with an expected small effect size20 – question the feasibility and effectiveness of cognitive prehabilitation programs for surgical patients.
This study had notable strengths and limitations. The trial was performed in a pragmatic, real-world perioperative setting. Aside from the training intervention, participants engaged in regular preoperative activities and habits. Perioperative care proceeded per routine clinical care, standards, and guidelines without additional intervention. Thus, the data obtained come from a highly generalizable surgical setting. Additionally, cognitive outcomes were obtained using validated, reliable measures. The 3D-CAM has demonstrated high reliability and validity compared DSM-based expert assessment,15, 22_ENREF_30 and our group has experience with these delirium assessment strategies.23 Cognitive function was assessed using NIH Toolbox measures, which have been nationally standardized across diverse populations,16 and normative Toolbox data are available for the patient population studied in this trial (i.e., older adults in the United States). Fully Corrected T-Score data were presented, which account for age, gender, race, ethnicity, and prior educational attainment, adding to analytical rigor. In terms of study limitations, this was a small feasibility study not powered for a particular outcome. In this context, the conclusions drawn from this study are limited. Additional limitations relate to the 3D-CAM instrument. The 3D-CAM was initially validated in medical patients,15 and validation studies are currently ongoing for the immediate postoperative setting (e.g., PACU) (, 24). Additionally, previous 3D-CAM inter-rater reliability data were derived from medical patients;15 as such, further validation and inter-rater reliability data are needed for surgical patients, and these analyses were not conducted for this preliminary study. These points should be considered for follow-up, large-scale prehabilitation studies, where validated perioperative delirium instruments should be used. Lastly, 11 patients inadvertently broke the blind to study team members despite reminders, and additional research team members were rotated in attempt to maintain assessment blinding. This is informative for future trial design, as multiple study team members may need to be available to perform assessments should participants break blinding.
In conclusion, this feasibility study did not provide evidence to suggest that home-based, unsupervised cognitive prehabilitation positively impacts postoperative cognitive function. In fact, enrollment in such a program over a short time-frame may worsen preoperative anxiety and apprehension. To achieve meaningful gains in cognitive function, without adding undue stress, prehabilitation likely needs to occur over a relatively long timeframe, with consistent supervision, and with allotted time for breaks to prevent stress and help consolidate learning.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the Michigan Institute of Clinical and Health Research for database support (UL1TR002240), The Hospital Elder Life Program for 3D-CAM permissions (Confusion Assessment Method. © 1988, 2003, Hospital Elder Life Program. All rights reserved. Adapted from: Inouye SK et al. Ann Intern Med. 1990; 113:941–8), the Department of Anesthesiology, University of Michigan Medical School, and Ms. Betsy Huang, BS, for assistance with trial coordination.
Funding: PEV is supported by National Institutes of Health Grant K23GM126317. The study was also supported by the Department of Anesthesiology, University of Michigan Medical School (Ann Arbor, MI, USA)
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
Previous Presentations: The methodology of this trial was presented as a poster presentation at the Foundation for Anesthesia Education and Research 2017 Medical Student Anesthesia Research Fellowship Symposium (MSARF Fellow: Abhijit R. Das).
REFERENCES
- 1.Radtke FM, Franck M, Lorenz M, et al. Remifentanil reduces the incidence of post-operative delirium. J Int Med Res 2010;38: 1225–32. [DOI] [PubMed] [Google Scholar]
- 2.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma 2008;65: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson TN, Raeburn CD, Tran ZV, et al. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg 2009;249: 173–8. [DOI] [PubMed] [Google Scholar]
- 4.Inouye SK, Marcantonio ER, Kosar CM, et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement 2016;12: 766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leslie DL, Marcantonio ER, Zhang Y, et al. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008;168: 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American geriatrics society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc 2015;63: 142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rebok GW, Ball K, Guey LT, et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc 2014;62: 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verhaeghen P, Cerella J, Basak C. A working memory workout: how to expand the focus of serial attention from one to four items in 10 hours or less. J Exp Psychol Learn Mem Cogn 2004;30: 1322–37. [DOI] [PubMed] [Google Scholar]
- 9.Kundu B, Sutterer DW, Emrich SM, et al. Strengthened effective connectivity underlies transfer of working memory training to tests of short-term memory and attention. J Neurosci 2013;33: 8705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stepankova H, Lukavsky J, Buschkuehl M, et al. The malleability of working memory and visuospatial skills: a randomized controlled study in older adults. Dev Psychol 2014;50: 1049–59. [DOI] [PubMed] [Google Scholar]
- 11.Meagher DJ, Moran M, Raju B, et al. Phenomenology of delirium. Assessment of 100 adult cases using standardised measures. Br J Psychiatry 2007;190: 135–41. [DOI] [PubMed] [Google Scholar]
- 12.Yang FM, Marcantonio ER, Inouye SK, et al. Phenomenological subtypes of delirium in older persons: patterns, prevalence, and prognosis. Psychosomatics 2009;50: 248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357: 1191–4. [PubMed] [Google Scholar]
- 14.Buschkuehl M, Hernandez-Garcia L, Jaeggi SM, et al. Neural effects of short-term training on working memory. Cogn Affect Behav Neurosci 2014;14: 147–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcantonio ER, Ngo LH, O’Connor M, et al. 3d-cam: Derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med 2014;161: 554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weintraub S, Dikmen SS, Heaton RK, et al. Cognition assessment using the NIH Toolbox. Neurology 2013;80: S54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casaletto KB, Umlauf A, Beaumont J, et al. Demographically corrected normative standards for the English version of the NIH Toolbox Cognition Battery. J Int Neuropsychol Soc 2015;21: 378–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bickel H, Gradinger R, Kochs E, et al. High risk of cognitive and functional decline after postoperative delirium. A three-year prospective study. Dement Geriatr Cogn Disord 2008;26: 26–31. [DOI] [PubMed] [Google Scholar]
- 19.Hanley JA, Negassa A, Edwardes MD, et al. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol 2003;157: 364–75. [DOI] [PubMed] [Google Scholar]
- 20.Lampit A, Hallock H, Valenzuela M. Computerized cognitive training in cognitively healthy older adults: a systematic review and meta-analysis of effect modifiers. PLoS Med 2014;11: e1001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lampit A, Hallock H, Moss R, et al. The timecourse of global cognitive gains from supervised computer-assisted cognitive training: a randomised, active-controlled trial in elderly with multiple dementia risk factors. J Prev Alzheimers Dis 2014;1: 33–39. [DOI] [PubMed] [Google Scholar]
- 22.Vasunilashorn SM, Guess J, Ngo L, et al. Derivation and validation of a severity scoring method for the 3-minute diagnostic interview for Confusion Assessment Method--defined delirium. J Am Geriatr Soc 2016;64: 1684–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avidan MS, Maybrier HR, Abdallah AB, et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet 2017;390: 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui V, Tedeschi CM, Kronzer VL, et al. Protocol for an observational study of delirium in the post-anaesthesia care unit (PACU) as a potential predictor of subsequent postoperative delirium. BMJ Open 2017;7: e016402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.