Abstract
Objective
Recruitment of immunological competent cells to the vessel wall is a crucial step in formation of abdominal aortic aneurysms (AAA). Innate immunity effectors (e.g., macrophages), as well as mediators of adaptive immunity (e.g., T cells), orchestrate a local vascular inflammatory response. Interleukin-10 (IL-10) is an immune-regulatory cytokine with a crucial role in suppression of inflammatory processes. We hypothesized that an increase in systemic IL-10-levels would mitigate AAA progression.
Approach and Results
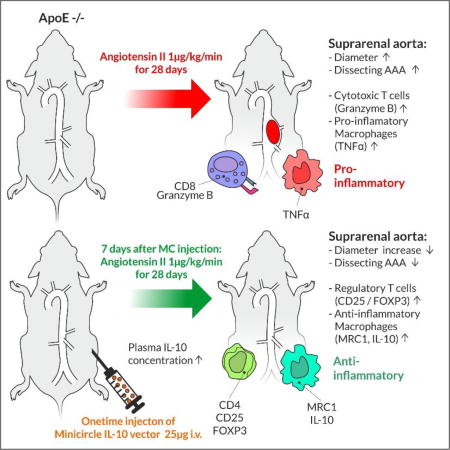
Using a single intravenous injection protocol, we transfected an IL-10 transcribing non-immunogenic minicircle (MC) vector into the Angiotensin II (AngII)-ApoE−/− infusion mouse model of AAA. IL-10 MC transfection significantly reduced average aortic diameter measured via ultrasound at day 28 from 166.1 ± 10.8 % (control) to 131.0 ± 5.8 % (IL-10 transfected). Rates of dissecting AAA were reduced by IL-10 treatment, with an increase in freedom from dissecting AAA from 21.5% to 62.3%. Using flow cytometry of aortic tissue from MC IL-10 treated animals, we found a significantly higher percentage of CD4+/CD25+/Foxp3+ regulatory T cells, with fewer CD8+/GranzymeB+ cytotoxic T cells. Furthermore, isolated aortic macrophages produced less TNF-α, more IL-10 and were more likely to be MRC1- positive alternatively-activated macrophages. These results concurred with gene expression analysis of LPS-stimulated and AngII-primed human peripheral blood mononuclear cells.
Conclusions
Taken together, we provide an effective gene therapy approach to AAA in mice by enhancing anti-inflammatory and dampening pro-inflammatory pathways through minicircle-induced augmentation of systemic IL-10 expression.
Keywords: abdominal aortic aneurysm, vascular inflammation, cytokines, in vivo transfection
Graphical abstract
Introduction
Human abdominal aortic aneurysm (AAA) is defined as a pathologic dilatation that exceeds the normal diameter by 50%, or that has an antero-posterior diameter of over 30 mm. AAAs are a significant cause of morbidity and mortality in the United States and worldwide. The average incidence in Western civilizations is 0.4–0.67%1. Although mortality numbers are slowly decreasing due to better endovascular and open surgical repair techniques as well as better risk factor management2, AAAs still accounted for up to 10,000 deaths per year in the US in 2014, or approximately 3.1 deaths per 100,000 people3.
Infiltration of monocytes/macrophages, polymorphonuclear leukocytes (PMNs), and T- and B-lymphocytes mark the early phases of aneurysm development4. With further progression, a variety of cytokines, leukotrienes and immunoglobulins attract additional inflammatory cells and establish a local vascular inflammatory response. Matrix degeneration with disruption of local collagen and elastin follows, accompanied by smooth muscle cell (SMC) apoptosis in the aortic media, and ultimately leading to AAA expansion and rupture5, 6.
With vascular inflammation being one of the major initial hallmarks of AAA formation, mechanisms that dampen the immune response might be leveraged to prevent progressive tissue damage. Interleukin-10 (IL-10) is a well described anti-inflammatory cytokine, with a crucial role in suppression of inflammatory processes in diseases such as environmental allergy, asthma or inflammatory bowel disease. There is evidence that the immune-regulatory capacities of IL-10 might also be crucial in aneurysm development, as the IL-10-1082 'A' polymorphism (associated with lower IL-10 production7) is more common in patients with AAA8, and patients with this polymorphism are at an increased risk of developing AAA9. IL-10 plasma concentrations in patients with AAA are significantly decreased compared to matched patients with coronary artery disease10. Also, IL-10-knockout mice show increased susceptibility to Angiotensin II (AngII)-induced aortic aneurysm and aortic rupture11. To our knowledge, no studies have to-date been conducted utilizing transgenic IL-10 animals.
IL-10 treatment has already been successfully performed in inflammatory bowel disease and glomerulosclerosis in rodents. However, recent studies have encountered major pitfalls. Intravenous or intraperitoneal injections of recombinant IL-10 need to be performed close to the time point of disease induction, or be repeated daily, due to IL-10’s short plasma half-life12–14. In humans, outcomes in the treatment of inflammatory bowel disease did not demonstrate efficacy15. While disease-specific reasons for these limited results may exist, one major drawback is the lack of sufficient IL-10 bioavailability at the tissue of interest16.
To increase IL-10 bioavailability and to overcome IL-10 delivery issues, here we applied a non-viral minicircle transfection approach to the AngII-infusion-ApoE−/− AAA model. Minicircles (MC) are episomal DNA vectors lacking a bacterial plasmid backbone. Due to their small size compared to standard plasmids, they display higher transfection efficiency and survival rate of the target cells. In addition, MC constructs sustain a higher expression rate of their harboring transgenes over a longer period of time. This, combined with their low immunogenicity, makes them intriguing vectors for gene therapy17–19.
Material and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Mouse model
B6.129P2-Apoetm1Unc/J male mice (ApoE−/−) were transfected via intravenous injection of 25µg MC into the retro-orbital venous plexus at a concentration of 25µg/100µl containing either a transgenic transcript for murine IL-10 or GFP (control). MC GFP was chosen as a control vector against MC IL10 to adjust for the effects of actual translation of proteins from minicircle DNA to protein in transfected cells. Based on this approach, the experiment was performed as shown in Supp. figure 1, showing that MC GFP did not alter aneurysm formation unexpectedly and randomly compared to AngII + PBS treatment alone. Seven days after transfection, aortic aneurysm formation was induced using continuous subcutaneous angiotensin II (AngII) – infusion. Osmotic pumps (model 2004, Alzet) containing either AngII (1 µg/kg/min, Sigma-Aldrich) or saline were implanted in ApoE−/− male mice (C57BL/6J background) as previously described20. Pump implantation occurred at 3–6 months of age (mean age 168 ± 7.6 days, total n=78), mice were age-matched before starting the experiment.
Suprarenal aortic diameter was followed via ultrasound measurements on a VisualSonics Vevo2100 over 28 days as previously described21–23 and validated24. Diameter was assessed using the leading edge-to-leading edge method25. Lesions were classified as dissecting AAA if intramural hematoma was visible by ultrasound, by histology or at necropsy. Bioluminescence imaging (BLI) was performed with the Xenogen In Vivo Imaging System on days 1, 3, 9, and 15 after transfection. D-Luciferin (Promega) was administered intraperitoneally at a dose of 375 mg/kg of body weight.
Murine plasma was collected with a standardized method via mandibular vein and centrifuged at 15 min at 3000 rpm at 4°C. ELISA for IL-10 was performed according to the manufacturer’s instructions (Quantikine ELISA Mouse IL-10 Immunoassay).
Construction and amplification of MC-IL-10 plasmids
For minicircle amplification, a single colony E. coli was grown at 37°C overnight and subsequently spun down. The pellet was re-dissolved with broth (pH 7.0) containing 1% L-(+)-arabinose and incubated at 30°C for 2 hours. MC constructs were isolated from the culture using plasmid purification kits (Qiagen, Venlo, Netherlands). Primers (cactagtcgcgcccggggag/gacccatgggggcccgcc) were used to PCR minicircle DNA with attB/attP elements and obtain DNA fragment A. The CMV promoter, multiple cloning site and poly A elements were PCR-amplified from pcDNA3.1/zeo(+) (Invitrogen) with primers aattgcatgaagaatctgct/ctggttctttccgcctcaga to obtain DNA fragment B. Ligation of DNA fragment A and DNA fragment B resulted in vector C. The IL-10 coding sequence was synthesized and subcloned into the KpnI/XhoI site of vector C, resulting in pMC-IL10. For detailed methods please see Huang et al.18 and Supp. Figure 5. Similar minicircles bearing transcripts for green fluorescent protein and firefly luciferase were used for control purposes26.
Tissue harvesting
Mice were sacrificed with an inhalation overdose of isoflurane (Vet One). Aortas were immediately flushed via the left ventricle with ice-cold PBS and then dissected from fat and connective tissue from the renal arteries to the diaphragm (via Microscope, Leica). Specimens were snap-frozen in liquid nitrogen and stored at −80°C.
Histological staining
Histological staining after harvesting was performed in the correlating suprarenal region of the AAA. Using already evaluated techniques24, aortic tissue was cut in 7-µm-thick serial sections and subsequently stained with haematoxylin and eosin (Sigma-Aldrich) and Picrosirius red (Sigma-Aldrich) according to standard protocols. All histological analyses were obtained at room temperature using a Keyence BZ-9000 microscope with BZ2 Analyzer software.
Flow cytometry
Flow cytometry analysis for markers of T-cell and macrophage polarization was performed in suprarenal aortic tissue at day 7 after AngII-infusion. Control mice were injected with saline instead of MC-GFP to avoid interference with flow cytometry, while untreated animals received neither AngII nor MC IL-10 treatment. Aortic tissue was digested with Liberase (27 WU/ml) and DNase I (0.1 %) in DMEM media. Cells were counted and prepared for flow cytometry and 1×106 cells were used for staining. For intra- and extra-cellular staining, cells were fixed and permeabilized using BD Cytofix/Cytoperm™ Fixation. Fluorophore-conjugated monoclonal antibodies against mouse CD3, CD4, CD8, CD25, CD45, FoxP3, GranzymeB, F4/80, IL10, MRC1, TNF-α and viablitiy (1:100; BD Bioscience, Biolegend, eBioscience) were used. Cells were assayed using a LSRII flow cytometer (BD Biosciences) and further analyzed with FlowJo software (Tree Star, Ashland, OR, USA).
Human PBMC experiments
Human PBMCs were isolated from the blood of healthy volunteers using a standard Ficoll protocol. Subsequently, cells were treated with AngII (100uM) for 24 hours followed by LPS induction (100ng/ml) for an additional 24 hours. IL-10 (20ng/ml) or control treatment (PBS) was performed in combination with AngII priming and before LPS stimulation. Cells were harvested using Qiazol and RNA was isolated using the Qiagen miRNeasy kit. RNA was quantified by Nanodrop (Thermo Fisher Scientific). mRNA was reverse transcribed using the SuperScript III RT kit (Thermo Fisher Scientific) according to the manufacturer’s instructions and expression levels were quantified with standard qRT-PCR (TaqMan) with the following assays: GAPDH (internal control), TNF, IL6, TGFB1, MRC1, CD163, FOXP3, GZMB, IL10 (all Thermo Fisher Scientific). Amplification was performed using a Quantstudio 12K Flex Real-Time PCR system (Thermo Fisher Scientific). Results were normalised to GAPDH.
Statistical analysis
All calculations were made with GraphPad Prism 6.01. Normal distribution was tested by Kolmogorov-Smirnov-Test and led to pairwise testing with Students unpaired t-test. Otherwise the Mann-Whitney rank sum test was performed. For multiple comparisons, post-hoc Fisher's Least Significant Difference (LSD) tests were calculated after one-way repeated measures analysis of variance. For curve comparisons of Kaplan-Meier survival curves a Log-rank (Mantel-Cox) test was performed. For in vitro experiments, obvious outliers were removed using the Robust regression and Outlier removal method (ROUT) with a coefficient Q of 2%27. A p value of <0.05 was considered statistically significant. Data are presented as mean ± SEM or median ± interquartile range if data were not normally distributed or not interval scaled.
Results
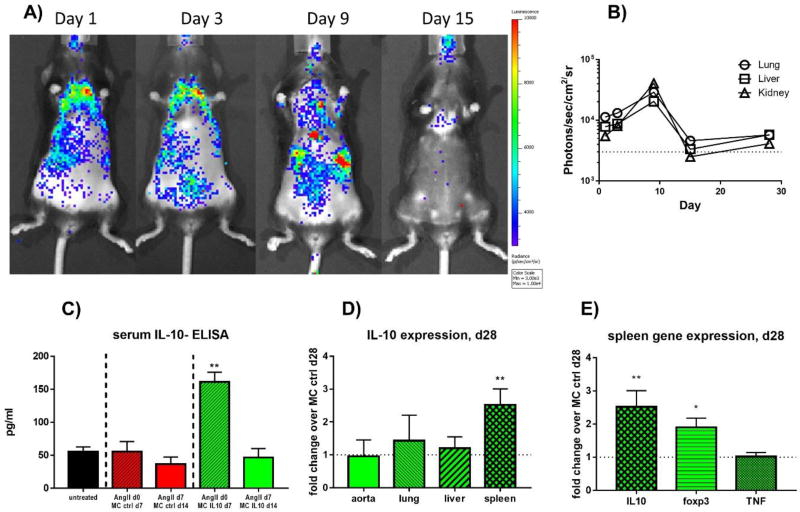
To evaluate transfection efficacy of our intravenous MC injection approach, we first utilized minicircles expressing a firefly luciferase reporter construct (MC-Luc). MC-Luc was injected and subsequent bioluminescence imaging (BLI) was performed at days 1, 3, 9, and 15. We found a noticeable increase in luciferase activity in regions corresponding to the lungs at 1–3 days after transfection, with increasing activity in the liver and kidney regions over the course of the first 9 days (Fig. 1A). By 15 days after transfection, the luciferase signal had significantly dropped, indicating a considerable loss of activity (Fig. 1B).
Figure 1. Vector expression and efficiency in vivo.
Bioluminescence imaging (BLI) was performed at days 1, 3, 9, and 15 after intravenous injection of MC-Luc. Subsequently, BLI was performed with the Xenogen In Vivo Imaging System with administration of D-Luciferin at a dose of 375 mg/kg of body weight.
A) Localization and intensity of bioluminescence.
B) Quantification of bioluminescence per organ
C) IL-10 plasma levels of mice at d7 after transfection with MC IL-10; n=5 per group, ** = p≤0.01.
D) Gene expression analysis (q-rtPCR) of IL-10 after 28days of AngII treatment in homogenized tissue
E) Gene expression of splenic tissue for IL-10, foxp3 and TNF at d28 after AngII infusion.
AngII-treatment began at day 7 after MC transfection, to ensure active vector expression during aneurysm induction. We also evaluated IL-10 plasma levels of mice at 7 and 14 days after injection with MC IL-10 or MC control, confirming expression and production of IL-10 in a similar fashion to MC-Luc. We found a significant increase in plasma IL-10 levels in MC IL-10-treated mice (vs. matched controls) at day 7 after transfection (161.3 ± 14.6 pg/ml vs. 55.5 ± 15.4 pg/ml, p < 0.01, n=5, Figure 1C). At day 14 after MC injection, no differences could be found for IL-10 expression in MC IL-10 vs. MC control (46.8 ± 13.4 pg/ml vs. 36.8 ± 10.6 pg/ml, p = n.s., n=4/5). To further evaluate the long-term effects of MC IL-10 injection regarding tissue levels of IL-10, expression in peripheral tissues were quantified with qRT-PCR at day 28 after AngII infusion. Here, IL-10 did not show significantly higher expression in aortic tissue, lung tissue or hepatic tissue in comparison to MC control (Figure 1D). Interestingly however, splenic IL-10 expression was significantly higher in MC IL-10 than in controls (2.52 ± 0.5-fold increase vs. MC control, p<0.01, n=10). This was accompanied by higher splenic Foxp3 expression at day 28 after AngII (1.9 ± 0.3-fold, p<0.05, n=10, Fig. 1E), but not increased TNF levels.
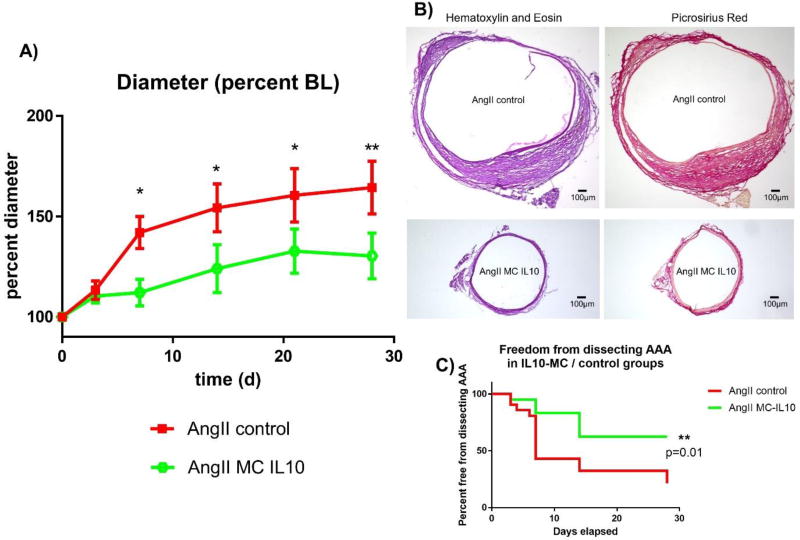
Subcutaneous AngII pump implantation at day 7 after transfection led to significant suprarenal aortic aneurysm formation in ApoE −/− mice as previously described20, with 78% of the mice having a diameter of more than 150% of baseline in the control group. Aneurysm formation was attenuated in mice that had been injected with MC IL-10, as only 10% of these animals had a diameter increase of ≥150% (average diameter 166.1 ± 10.8 % in control vs. 131.0 ± 5.8 % in IL-10 treated animals, p < 0.01, n = 9 vs. 10, Figure 2A). Differences in diameter remained throughout the time course of aneurysm formation, starting at day 7 (Fig. 2A). These findings were supported by H&E and Picrosirius Red stainings, which showed a relevant decrease in AAA diameter after MC IL-10 treatment (Figure 2B).
Figure 2. Aortic diameter and rate of dissecting AAAs after MC IL-10 transfection.
Ultrasound measurements of ApoE−/− mice after subcutaneous infusion of AngII (1 µg/kg/min).
A) Aortic diameter in percent of baseline diameter over a time course of 28 days; n = 15 vs. 15, * = p≤0.05, ** = p≤0.01 vs. AngII MC control.
B) Representative H&E and Picrosirius Red staining of suprarenal aortic sections harvested d28 after AngII infusion.
C) Kaplan-Meier curve for freedom from dissecting AAA; n = 15 vs. 15, ** = p≤0.01 vs. AngII MC control.
In the murine ApoE−/− AngII infusion AAA model, aneurysm formation is typically driven by intramural hematoma or myointimal dissection of the aortic wall. Therefore, in addition to aneurysm size, the presence of a ‘dissecting AAA’ is an important aspect of AAA formation28. In concert with the significantly decreased diameter after MC IL-10 treatment, we found improved dissecting AAA-free survival in animals transfected with the IL-10 vector (21.5 % vs. 62.3 % at day 28 in AngII vs. AngII MC-IL10, p = 0.01, n = 41, Fig. 2C).
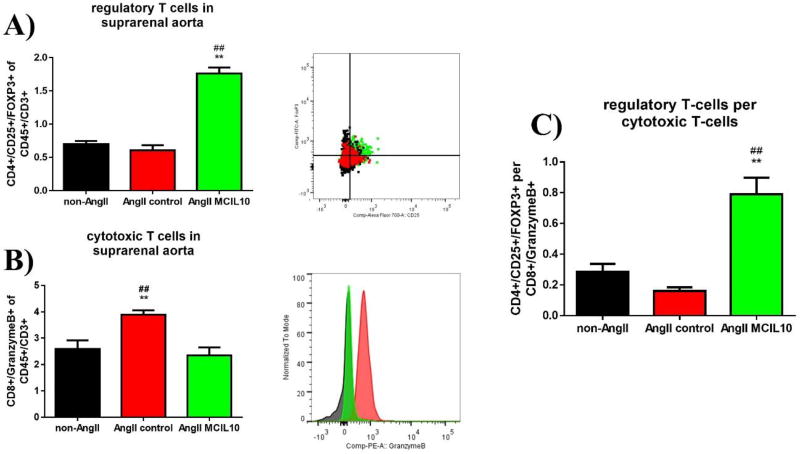
Next, we evaluated the number and polarization of CD45+ / CD3+ lymphocytes and F4/80+ macrophages in suprarenal aortic tissue 7 days after aneurysm induction via flow cytometry. AngII MC IL-10-treated animals showed a significantly higher percentage of regulatory T cells (Treg cells) than AngII-control or non-AngII-treated animals (CD4+/CD25+/Foxp3+, 1.8 ± 0.1 % of CD45+/CD3+ vs. 0.6 ± 0.08 % in AngII-control vs. 0.7 ± 0.05 % in non-AngII-animals, p < 0.01 vs. AngII-control and non-AngII, n=5/6/5 per group, Fig. 3A). In contrast, AngII-control treated animals had an increased percentage of cytotoxic T cells compared to AngII MC IL-10 or non-AngII treated mice (CD8+ / GranzymeB+, 3.9 ± 0.2 % of CD45+/CD3+ vs. 2.3 ± 0.3 % in AngII-control and 2.6 ± 0.3 % in non-AngII, p < 0.01 vs. AngII MC IL-10 and non-AngII, n= 6/5/5 per group, Fig. 3B). As a result, the Treg cell to cytotoxic T cell ratio (CD4+/CD25+/FOXP3+ per CD8+/GranzymeB+) was highly and significantly increased in the AngII MC IL-10-treated animals (Fig. 3C).
Figure 3. T lymphocyte presence and differentiation in aortic tissue.
At day 7 after aneurysm induction (d14 after transfection with MC) aortic tissue was harvested, lysed and analyzed via flow cytometry to characterize T cell differentiation. A and B include representative flow cytometry graphs and histograms; n=4/5/4; n=5/6/5
A) Percentage of CD4+/CD25+/Foxp3 of CD45+/CD3+ cells in untreated animals, AngII-infused controls and AngII-infused/MC-IL10 transfected animals; ** = p≤0.01 vs. AngII, ## = p≤0.01 vs. untreated.
B) Percentage of CD8+/GranzymeB+ of CD45+/CD3+ cells; ** = p≤0.01 vs. AngII MC IL-10, ## = p≤0.01 vs. untreated.
C) Ratio of CD4+/CD25+/Foxp3 over CD8+/GranzymeB+ cell (Treg to cytotoxic T cell ratio), ** = p≤0.01 vs. AngII, ## = p≤0.01 vs. untreated.
We did not find a significant difference in absolute cell number in aortic tissue (CD4+ or CD8+ or CD3+/CD45+ or F4/80+ cells of single cells) between the three experimental groups, indicating that there was a difference in cell polarization, rather than cell number (Supp. Figure 2).
Peripheral blood quantification did not reveal significant differences in T cell polarization regarding Treg and cytotoxic T cells. CD4+ cells were significantly more present in peripheral blood in AngII-treated animals, independent of MC IL-10 treatment (CD4+ of single cells, 2.2 ± 0.3 % in non-AngII-animals vs. 3.5 ± 0.4% in AngII-control vs. 3.7 ± 0.4 % in AngII MC IL-10, p < 0.01 vs. non-AngII, n=5/6/5 per group, Supp. Fig. 3D)
F4/80-positive macrophages in suprarenal aortic tissue were more prone to Tumor necrosis factor alpha (TNF-α) production (38.6 ± 5.3 percent of F4/80+ cells) without MC IL-10 application than after transfection with IL-10 (20.0 ± 3 percent of F4/80+, p < 0.05, n= 4/5/4 per group, Fig. 4A), and also showed decreased IL-10 signal (3.9 ± 0.5 % of F4/80+ cells vs. 8.6 ± 1.6 % of F4/80+ cells, p < 0.01 vs. AngII-control, n= 4/5/4 per group, Fig. 4B). Notably, the increased TNF-α/IL-10 ratio in AngII-treated control animals was reversed with IL-10 transfection (Fig. 4C). Intriguingly, suprarenal aortic macrophages showed increased differentiation into the more anti-inflammatory M2-like phenotype with MC IL-10 treatment (19.4 ± 1.2 % of Mannose Receptor C type 1 (MRC1)+ over F4/80+ cells vs. 11.6 ± 1.9 % in AngII control, p < 0.05 vs. control, n= 4/5/4 per group, Fig. 4D).
Figure 4. Macrophage presence and differentiation in aortic tissue.
At day 7 after aneurysm induction (d14 after transfection with MC) aortic tissue was harvested, lysed and analyzed via flow cytometry to characterize macrophage differentiation markers. A, B, D include representative flow cytometry graphs and histograms; n=4/5/4
A) Percentage of TNF-α+ of F4/80+ cells in untreated, AngII-infused, and AngII-infused/MC-IL10 transfected animals; * = p≤0.05 vs. AngII MC-IL10, # = p≤0.05 vs. untreated.
B) Percentage of IL-10+ of F4/80+ cells; ** = p≤0.01 vs. AngII
C) Ratio of TNF-α+ over IL-10+ cells; * = p≤0.05 vs. AngII MC-IL10, # = p≤0.05 vs. untreated.
D) Percentage of MRC1+ of F4/80+ cells; * = p≤0.05 vs. AngII
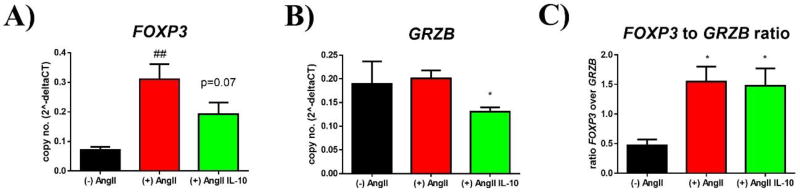
To further investigate translational potential in human cells, we primed human PBMCs with AngII or AngII/IL-10 before stimulation with LPS and evaluated expression of inflammatory markers. There was a significant reduction in inflammatory gene expression with AngII/IL-10 vs. AngII alone for all markers except FOXP3 (0.07 ± 0.01 vs. 0.31 ± 0.05 vs. 0.19 ± 0.04 relative expression vs. GAPDH in cells not primed with AngII vs. AngII primed cells vs. AngII/IL-10 treated cells, p = 0.07 vs. (+) AngII, n = 9/18/19, Fig. 5A). Granzyme B (GRZB)-expression was lower in AngII/IL-10 treated cells (0.19 ± 0.05 vs. 0.2 ± 0.02 vs. 0.14 ± 0.01 04 relative expression vs. GAPDH in cells with (−) AngII vs. (+) AngII vs. (+) AngII/IL-10, p < 0.05 vs. (+) AngII, n = 9/18/19, Fig. 5B), resulting in no significant change of FOXP3 to GRZB ratio in AngII (+) vs. AngII (+) / IL-10 (0.47 ± 0.1 vs. 1.55 ± 0.25 vs. 1.48 ± 0.3 –fold, p = n.s. vs. (+) AngII, n = 9/18/19, Fig 5C).
Figure 5. T lymphocyte differentiation in human PBMCs after AngII priming and stimulation.
Human PBMCs were isolated and primed with AngII or AngII/IL-10 for 24 hours before cells were activated with LPS (24h) to further evaluate gene expression of T cell characterizing markers; n=9/18/19
A) Relative expression of FOXP3 over GAPDH in unprimed, AngII-primed, and AngII/IL-10 primed PBMCs; ## = p≤0.01 vs. untreated; p=.0.07 in AngII IL-10 vs. AngII.
B) Relative expression of GranzymeB (GZMB) in unprimed, AngII, AngII/IL-10 PBMCs; * = p≤0.05 vs. AngII.
C) Ratio (relative expression) of FOXP3 over GRZB; * = p≤0.05 vs. AngII.
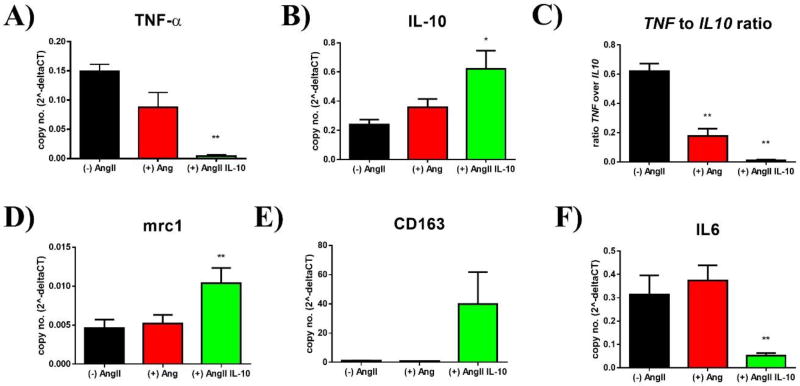
Looking at macrophage markers revealed beneficial effects for (+) AngII/IL-10 vs. (+) AngII alone. TNF-α (TNF) expression dropped significantly with MC IL-10 treatment (0.15 ± 0.01 vs. 0.09 ± 0.03 vs. 0.004 ± 0.002; p < 0.05 vs. (+) AngII, n = 9/18/19, Fig. 6A). IL-10 expression was increased in (+) AngII / IL-10 (0.24 ± 0.03 vs. 0.36 ± 0.06 vs. 0.62 ± 0.12; p < 0.05 vs. (+) AngII, n = 9/15/11, Fig. 6B), resulting overall in a significantly decreased TNF-α to IL-10 ratio (0.62 ± 0.05 vs. 0.18 ± 0.07 vs. 0.01 ± 0.01; p < 0.01 vs. (+) AngII and vs. (−) AngII, n = 6/14/11, Fig. 6C). Markers of alternatively activated M2-macrophages, such as MRC1 (0.005 ± 0.001 vs. 0.005 ± 0.001 vs. 0.01 ± 0.002; p < 0.05 vs. (+) AngII, n = 9/15/17, Fig. 6D) and CD163 (1.04 ± 0.16 vs. 0.8 ± 0.09 vs. 39.92 ± 21.79; p < 0.01 vs. (+) AngII, n = 9/18/11, Fig. 6E) were significantly up-regulated. Conversely, IL-6 expression was reduced in cells that were treated with IL-10 (0.32 ± 0.08 vs. 0.37 ± 0.06 vs. 0.05 ± 0.01; p < 0.01 vs. (+) AngII and vs. (−) AngII, n = 6/15/14, Fig. 6F).
Figure 6. Macrophage differentiation in human PBMCs after AngII priming and stimulation.
Human PBMCs were isolated and primed with AngII or AngII/IL-10 for 24 hours before cells were activated with LPS (24h) to further evaluate macrophage activation (n=9/18/19)
A) Relative expression of TNF-α (TNF) over GAPDH in unprimed, AngII-primed, and AngII/IL-10 primed PBMCs; ** = p≤0.01 vs. AngII.
B) Relative expression of IL-10 in unprimed, AngII, and AngII/IL-10 PBMCs; * = p≤0.05 vs. AngII.
C) Ratio (relative expression) of TNF over IL-10; ** = p≤0.01 vs. AngII, ## = p≤0.01 vs. unprimed
D) Relative expression of MRC1 in unprimed, AngII, and AngII/IL-10 PBMCs; ** = p≤0.01 vs. AngII.
E) Relative expression of CD163 in unprimed, AngII, and AngII/IL-10 PBMCs; ** = p≤0.01 vs. AngII.
F) Relative expression of IL-6 in unprimed, AngII, and AngII/IL-10 PBMCs; ** = p≤0.01 vs. AngII.
Discussion
Here, we show that systemic transfection with a non-immunogenic delivery method using IL-10 expressing minicircles is feasible and effective in a murine model of AAA disease. Plasma IL-10 levels were significantly increased at 1 week after transfection in Apo E−/− mice, and IL-10 induction significantly decreased aneurysm size and likelihood of suprarenal dissecting AAA formation. These beneficial effects upon aneurysm development were accompanied by a significant increase in Treg cells and a decrease in cytotoxic T cells. Furthermore, local macrophages were more likely to differentiate into the alternatively activated M2-macrophages and express less TNF-α as well as more IL-10. Human PBMCs with IL-10 treatment after AngII-incubation showed ameliorated expression of differentiation markers Granzyme B (GZMB), TNF-α, MRC1, CD163 and IL-6, indicating possible translational potential in the application of IL-10.
One major IL-10-induced anti-inflammatory process in tissue is the induction of CD4+ CD25+ FOXP3+ Treg cells29. In general, their mechanism of action also involves production of Interleukin-10 (IL-10) itself29, 30, providing a self-amplifying feedback loop, which we also identified. The induction of a subset of more anti-inflammatory macrophages by Treg cells31, particularly the so-called ‘deactivated’ M2c-macrophages which are believed to be important for tissue remodelling and matrix deposition, is also regulated by IL-1032, 33. In AAA disease, M2-macrophages are present in the later phases of development34 and are involved in healing and prevention of further AAA growth. By contrast, activation and clonal expansion of CD8+ cytotoxic T cells induces cell damage and apoptosis potentially worsening AAA disease, if not properly regulated35, 36. As IL-10 has been shown to contribute to CD8+ cytotoxic T cell (CTL) expansion under certain conditions, we monitored the CD8+ GranzymeB+ CTL amount in tissue and calculated the ratio of Treg / CTL ratio to further characterize the impact of IL-10 on the T cell population in the aortic wall.
Using the AngII-induced AAA model, we observed that systemic overexpression of IL-10 via single timepoint MC transfection led to aortic cell polarization into Treg cells and anti-inflammatory M2-macrophages expressing MRC1. These changes in adaptive and innate immune cell differentiation were associated with a clear reduction in aneurysm formation. We did not find a significant change in CD3+/CD45+, CD4+, CD8+ or macrophage cell number in aortic tissue after MC injection. Therefore, our results suggest a polarization effect, rather than a recruitment. Considering the limited expression period of the MC-delivered plasmids of, at most, 2 weeks with regard to IL-10 plasma levels (when compared to the four-week period of aneurysm development), it could be that this treatment might have sparked self-amplifying cascades and cell-cell interactions that maintain the anti-inflammatory response. At 28 days after aneurysm induction, only splenic tissue showed increased expression of IL-10 (Figure 1D). This was accompanied by higher Foxp3 expression, possibly indicating pronounced Treg polarization (Figure 1E). This is especially intriguing, as flow cytometry analysis of spleens at day 7 after AngII showed significantly fewer CD4+ cells and a tendency towards fewer Foxp3-positive Treg cells (Supp. Figure 4 A and D). This might indicate an initial mobilization of CD4+ cells from the spleen in response to AngII, a redistribution process to non-lymphoid tissue known to occur after recognition of certain antigens37. Subsequently, splenic IL-10 and Foxp3 expression might increase again over the next 3 weeks if MC IL-10 expression had successfully induced anti-inflammatory processes in the aorta.
In patients with AAA, both the number and function of Treg cells as well as the expression of FOXP3 are significantly decreased in peripheral blood38, 39, suggesting that the lack of Treg immunomodulation may accelerate AAA formation. Indeed, adoptive transfer of Treg cells to AngII-treated ApoE−/− mice reduced AAA incidence and severity in a dose-dependent manner40. Further, direct treatment with Treg cells decreased macrophage number and the expression of pro-inflammatory cytokines in local aortic tissue. After Treg treatment in vitro, macrophages had a more pronounced M2-like phenotype, consistent with previous reports31, 33. As such, the beneficial effects of Treg cells are likely mediated via direct cell-cell contact as well as paracrine effects.
Yodoi et al.41 demonstrated that expansion of Foxp3+ Treg cells by IL-2 complex treatment resulted in a marked decrease in the incidence and mortality of AAA, and that this effect could be reversed by genetic depletion of Foxp3+ Treg cells, further underlining the importance of Treg cells in the prevention of aneurysm disease. Moreover, Zhou and colleagues39 further clarified the importance of IL-10 in mediating the beneficial effects of Treg cells in AAA formation. Transfusion of Treg cells from WT animals suppressed macrophage activation and reduced lesion macrophage and T cell counts in the murine AngII AAA model. However, this was not the case in mice that were infused with Treg cells from IL-10−/− animals, suggesting that the presence of IL-10 is imperative for cell-cell interaction of Treg cells with the surrounding tissue. IL-10 has also been shown to be a crucial regulator of inflammation-matrix interaction, as Treg cells from IL-10 competent mice suppress MMP-9 and MMP-13 activity more strongly than Treg cells from IL-10 −/− mice39. Notably, IL-10−/− mice show increased elastin degradation11 and, Treg treatment inhibits apoptosis of aortic wall cells in the AngII-induced aneurysm model40. Our data support a critical role for the mediator IL-10 in prevention of AAA formation, and suggest that a transient increase in IL-10 might be sufficient to induce a critical mass of anti-inflammatory Treg cells and regulatory macrophages at the site of lesion, thereby possibly decreasing AAA growth by positively modulating the inflammatory response, with potential downstream effects on extracellular matrix degradation and apoptosis via IL-10.
Recent work highlights the special considerations that need to be taken into account regarding the role of aortic dissection in the AngII-AAA model28, 42. Inflammatory cell infiltration tends to localize to sites of aortic dissection43. It is believed that classical activation of macrophages induces vascular dissection, thereby promoting AAA formation at least in part by an IL-6 dependent mechanism44. Our work suggests that re-adjusting the macrophage activation cascades using IL-10 treatment suppresses IL-6 as well as TNF-α expression and promotes MRC1 and CD163 transcription. The IL-10-induced increase in Treg cells and the shift from classical to alternatively activated macrophages in aortic tissue is therefore likely to be an important mechanism in the prevention of dissecting AAA. We assume that our anti-inflammatory treatment primarily suppresses both micro- and macro-ruptures, as these hemorrhagic events are thought to drive AAA growth in the AngII-model. This is further underscored by the early separation of our AAA diameter and dissecting AAA-free survival curves (Fig. 2 A and B) at day 7 with a subsequently similar growth rate.
In PBMCs from healthy controls stimulated with AngII, we observed minimal impact on inflammatory gene expression in the absence of IL-10 treatment. Our cells of interest, mainly monocytes/macrophages and T cells, all express receptors for AngII41, 45, 46 and should therefore be susceptible to AngII priming. However, effects of AngII on leukocytes appear to be context-sensitive45, 47 and are difficult to duplicate in vitro48. We acknowledge that our understanding of AngII-induced effects on mononuclear cells is not complete, nevertheless, IL-10 treatment was beneficial after PBMCs were primed with AngII, supporting our main hypothesis that IL-10 initiates an anti-inflammatory and self-amplifying polarization towards Treg cells and M2-macrophages.
There are limitations to our study. We applied the MC IL-10 vector systemically, once, and did not seek to control the site of expression nor the effect duration. It is possible that observed effects might be even stronger, and display less off-target impact if a more specific approach to the vasculature or immune cells had been chosen49, 50. Additionally, we cannot definitively conclude whether systemic or local increases of IL-10 are more important to prevent AAA formation, as the cellular changes we induced in aortic tissue were observed after systemic IL-10 delivery with an initial increase of plasma IL-10. With regard to clinical applicability and the known methodological shortcomings of cell-specific transfections, we deliberately chose this approach, intending to maintain translational potential and seeking to prove that MCs are feasible non-immunogenic vectors for the treatment of AAAs in this mouse model. We did not seek to provide deeper mechanistic insights as the interaction of IL-10 with lymphocytes and myeloid cells has been extensively studied before. While we do not provide hemodynamic data (such as blood pressure) in these animals, and therefore cannot exclude differences in MC-IL10 treated vs. non-MC-IL-10 treated animals, a recent publication suggests no prognostic value of hemodynamic markers on aneurysm growth51. Also, initial characterization of the model did not show any alteration in arterial blood pressure after AngII infusion20. The recently published prevention of blood pressure increase by continuous IL-10 infusion has only been shown in combination with significantly higher doses of AngII (90ng/min) and vastly higher IL-10 levels (0.5ng/min) when compared to our plasma IL-10 concentration52.
In summary, we provide evidence that a one-time application of an IL-10 transcribing minicircle vector successfully limits aneurysm formation in a mouse model of AAA, while increasing regulatory T cells and alternatively activated macrophages in the aortic wall. IL-10 treatment of PBMCs partially recapitulated the anti-inflammatory effects of IL-10, indicating possible translational potential in humans.
Supplementary Material
Highlights.
Application of a non-viral, non-immunogenic vector (minicircle) to systemically overexpress Interleukin-10 (IL-10) is feasible in mice and increases plasma IL-10 significantly 7 days after injection.
After minicircle IL-10 injection, aneurysm growth and size as well as rate of dissecting AAA decreased in a murine abdominal aortic aneurysm model (Angiotensin II - ApoE−/− infusion).
These relevant effects were accompanied by higher amounts of beneficial Treg cells and alternatively activated, anti-inflammatory macrophages in local aortic tissue. Furthermore, local pro-inflammatory immune response was dampened with lower TNF-α production and fewer cytotoxic T cells.
Human PBMCs with IL-10 treatment after AngII - incubation show ameliorated expression of differentiation markers Granzyme B (GZMB), TNF-α, MRC1, CD163 and IL-6, indicating possible translational potential in the application of IL-10.
Acknowledgments
We thank Prof. Mark Kay, Stanford, for kindly providing the technical background regarding the MC technology.
Sources of Funding:
This work was supported by research grants from the National Institute of Health (R01 HL105299 and R01 HL122939 to P.S. Tsao, R01 AI085575 and R01 HL093172 to J.C. Wu), the Department of Veterans Affairs (1I01BX002641-01A to P.S. Tsao), the Deutsche Forschungsgemeinschaft (AD 492/1-1 to M. Adam, WA 3583/2-1 to M.U. Wagenhäuser, RA 2179/1-1 to U. Raaz), the Boehringer Ingelheim Fonds and the International Scholarship for Medical Students, Medical School of the University Erlangen, Germany (both to I. N. Schellinger), the Banyu Fellowship Program by Banyu Life Science Foundation International (to K. Toyama) and the Stanford Cardiovascular Institute (to J.M. Spin).
Abbreviations
- AAA
abdominal aortic aneurysm
- AngII
Angiotensin II
- BLI
Bioluminescence imaging
- CTL
Cytotoxic T cell
- FOXP3
forkhead box P3
- GRZB
Granzyme B
- IL-10
Interleukin-10
- IL-6
Interleukin-6
- MC
Minicircle
- MRC1
Mannose Receptor, C type 1
- PBMC
Peripheral Blood Mononuclear Cell
- SMC
Smooth muscle cell
- TNFa
Tumor necrosis factor alpha
- Treg
Regulatory T cell.
Footnotes
Disclosures
None.
References
- 1.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nature reviews. Cardiology. 2011;8:92–102. doi: 10.1038/nrcardio.2010.180. [DOI] [PubMed] [Google Scholar]
- 2.Schermerhorn ML, Bensley RP, Giles KA, Hurks R, O'Malley AJ, Cotterill P, Chaikof E, Landon BE. Changes in abdominal aortic aneurysm rupture and short-term mortality, 1995–2008: A retrospective observational study. Annals of surgery. 2012;256:651–658. doi: 10.1097/SLA.0b013e31826b4f91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. Centers for disease control and prevention, national center for health statistics. Underlying cause of death 1999–2015 on cdc wonder online database, released december, 2016. Data are from the multiple cause of death files, 1999–2015, as compiled from data provided by the 57 vital statistics jurisdictions through the vital statistics cooperative program. Accessed at http://wonder.Cdc.Gov/ucd-icd10.Html on oct 4, 2017 7:14:35 am.
- 4.Thomas M, Gavrila D, McCormick ML, Miller FJ, Jr, Daugherty A, Cassis LA, Dellsperger KC, Weintraub NL. Deletion of p47phox attenuates angiotensin ii-induced abdominal aortic aneurysm formation in apolipoprotein e-deficient mice. Circulation. 2006;114:404–413. doi: 10.1161/CIRCULATIONAHA.105.607168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunieda T, Minamino T, Nishi J, Tateno K, Oyama T, Katsuno T, Miyauchi H, Orimo M, Okada S, Takamura M, Nagai T, Kaneko S, Komuro I. Angiotensin ii induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation. 2006;114:953–960. doi: 10.1161/CIRCULATIONAHA.106.626606. [DOI] [PubMed] [Google Scholar]
- 6.Ailawadi G, Moehle CW, Pei H, Walton SP, Yang Z, Kron IL, Lau CL, Owens GK. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. The Journal of thoracic and cardiovascular surgery. 2009;138:1392–1399. doi: 10.1016/j.jtcvs.2009.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV. An investigation of polymorphism in the interleukin-10 gene promoter. European journal of immunogenetics : official journal of the British Society for Histocompatibility and Immunogenetics. 1997;24:1–8. doi: 10.1111/j.1365-2370.1997.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 8.Bown MJ, Lloyd GM, Sandford RM, Thompson JR, London NJ, Samani NJ, Sayers RD. The interleukin-10-1082 'a' allele and abdominal aortic aneurysms. Journal of vascular surgery. 2007;46:687–693. doi: 10.1016/j.jvs.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 9.McColgan P, Peck GE, Greenhalgh RM, Sharma P. The genetics of abdominal aortic aneurysms: A comprehensive meta-analysis involving eight candidate genes in over 16,700 patients. International surgery. 2009;94:350–358. [PubMed] [Google Scholar]
- 10.Kadoglou NP, Papadakis I, Moulakakis KG, Ikonomidis I, Alepaki M, Moustardas P, Lampropoulos S, Karakitsos P, Lekakis J, Liapis CD. Arterial stiffness and novel biomarkers in patients with abdominal aortic aneurysms. Regulatory peptides. 2012;179:50–54. doi: 10.1016/j.regpep.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Ait-Oufella H, Wang Y, Herbin O, Bourcier S, Potteaux S, Joffre J, Loyer X, Ponnuswamy P, Esposito B, Dalloz M, Laurans L, Tedgui A, Mallat Z. Natural regulatory t cells limit angiotensin ii-induced aneurysm formation and rupture in mice. Arterioscler Thromb Vasc Biol. 2013;33:2374–2379. doi: 10.1161/ATVBAHA.113.301280. [DOI] [PubMed] [Google Scholar]
- 12.Grool TA, van Dullemen H, Meenan J, Koster F, ten Kate FJ, Lebeaut A, Tytgat GN, van Deventer SJ. Anti-inflammatory effect of interleukin-10 in rabbit immune complex-induced colitis. Scandinavian journal of gastroenterology. 1998;33:754–758. doi: 10.1080/00365529850171710. [DOI] [PubMed] [Google Scholar]
- 13.Rachmawati H, Beljaars L, Reker-Smit C, Bakker HI, Van Loenen-Weemaes AM, Lub-De Hooge MN, Poelstra K. Intravenous administration of recombinant human il-10 suppresses the development of anti-thy 1-induced glomerulosclerosis in rats. PDA journal of pharmaceutical science and technology / PDA. 2011;65:116–130. [PubMed] [Google Scholar]
- 14.Duchmann R, Schmitt E, Knolle P, Meyer zum Buschenfelde KH, Neurath M. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. European journal of immunology. 1996;26:934–938. doi: 10.1002/eji.1830260432. [DOI] [PubMed] [Google Scholar]
- 15.Buruiana FE, Sola I, Alonso-Coello P. Recombinant human interleukin 10 for induction of remission in crohn's disease. Cochrane database of systematic reviews (Online) 2010:CD005109. doi: 10.1002/14651858.CD005109.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marlow GJ, van Gent D, Ferguson LR. Why interleukin-10 supplementation does not work in crohn's disease patients. World journal of gastroenterology : WJG. 2013;19:3931–3941. doi: 10.3748/wjg.v19.i25.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z-Y, Riu E, He C-Y, Xu H, Kay MA. Silencing of episomal transgene expression in liver by plasmid bacterial backbone DNA is independent of cpg methylation. Molecular therapy : the journal of the American Society of Gene Therapy. 2008;16:548–556. doi: 10.1038/sj.mt.6300399. [DOI] [PubMed] [Google Scholar]
- 18.Huang M, Chen Z, Hu S, Jia F, Li Z, Hoyt G, Robbins RC, Kay MA, Wu JC. Novel minicircle vector for gene therapy in murine myocardial infarction. Circulation. 2009;120:S230–S237. doi: 10.1161/CIRCULATIONAHA.108.841155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lijkwan MA, Hellingman AA, Bos EJ, van der Bogt KE, Huang M, Kooreman NG, de Vries MR, Peters HA, Robbins RC, Hamming JF, Quax PH, Wu JC. Short hairpin rna gene silencing of prolyl hydroxylase-2 with a minicircle vector improves neovascularization of hindlimb ischemia. Human gene therapy. 2014;25:41–49. doi: 10.1089/hum.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daugherty A, Manning MW, Cassis LA. Angiotensin ii promotes atherosclerotic lesions and aneurysms in apolipoprotein e-deficient mice. The Journal of clinical investigation. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maegdefessel L, Azuma J, Toh R, Deng A, Merk DR, Raiesdana A, Leeper NJ, Raaz U, Schoelmerich AM, McConnell MV, Dalman RL, Spin JM, Tsao PS. Microrna-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Science translational medicine. 2012;4:122ra122. doi: 10.1126/scitranslmed.3003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maegdefessel L, Azuma J, Toh R, Merk DR, Deng A, Chin JT, Raaz U, Schoelmerich AM, Raiesdana A, Leeper NJ, McConnell MV, Dalman RL, Spin JM, Tsao PS. Inhibition of microrna-29b reduces murine abdominal aortic aneurysm development. The Journal of clinical investigation. 2012;122:497–506. doi: 10.1172/JCI61598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maegdefessel L, Spin JM, Raaz U, et al. Mir-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nature communications. 2014;5:5214. doi: 10.1038/ncomms6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azuma J, Maegdefessel L, Kitagawa T, Dalman RL, McConnell MV, Tsao PS. Assessment of elastase-induced murine abdominal aortic aneurysms: Comparison of ultrasound imaging with in situ video microscopy. Journal of biomedicine & biotechnology. 2011;2011:252141. doi: 10.1155/2011/252141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurtelschmid M, Bjorck M, Wanhainen A. Comparison of three ultrasound methods of measuring the diameter of the abdominal aorta. The British journal of surgery. 2014;101:633–636. doi: 10.1002/bjs.9463. [DOI] [PubMed] [Google Scholar]
- 26.Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z, Robbins RC, Kay MA, Longaker MT, Wu JC. A nonviral minicircle vector for deriving human ips cells. Nature methods. 2010;7:197–199. doi: 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Motulsky H, Brown R. Detecting outliers when fitting data with nonlinear regression - a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics. 2006;7:123. doi: 10.1186/1471-2105-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trachet B, Fraga-Silva RA, Piersigilli A, Tedgui A, Sordet-Dessimoz J, Astolfo A, Van der Donckt C, Modregger P, Stampanoni MFM, Segers P, Stergiopulos N. Dissecting abdominal aortic aneurysm in ang ii-infused mice: Suprarenal branch ruptures and apparent luminal dilatation. Cardiovascular research. 2015;105:213–222. doi: 10.1093/cvr/cvu257. [DOI] [PubMed] [Google Scholar]
- 29.Saraiva M, O'Garra A. The regulation of il-10 production by immune cells. Nature reviews. Immunology. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 30.Steinke JW, Lawrence MG. T-cell biology in immunotherapy. Annals of Allergy, Asthma & Immunology. 2014;112:195–199. doi: 10.1016/j.anai.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJC, John S, Taams LS. Cd4+cd25+foxp3+ regulatory t cells induce alternative activation of human monocytes/macrophages. Proceedings of the National Academy of Sciences. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez FO, Gordon S. The m1 and m2 paradigm of macrophage activation: Time for reassessment. F1000prime reports. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu G, Ma H, Qiu L, Li L, Cao Y, Ma J, Zhao Y. Phenotypic and functional switch of macrophages induced by regulatory cd4+cd25+ t cells in mice. Immunology and cell biology. 2011;89:130–142. doi: 10.1038/icb.2010.70. [DOI] [PubMed] [Google Scholar]
- 34.Rateri DL, Howatt DA, Moorleghen JJ, Charnigo R, Cassis LA, Daugherty A. Prolonged infusion of angiotensin ii in apoe−/− mice promotes macrophage recruitment with continued expansion of abdominal aortic aneurysm. The American Journal of Pathology. 2011;179:1542–1548. doi: 10.1016/j.ajpath.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindeman JHN, Abdul-Hussien H, van Bockel JH, Wolterbeek R, Kleemann R. Clinical trial of doxycycline for matrix metalloproteinase-9 inhibition in patients with an abdominal aneurysm: Doxycycline selectively depletes aortic wall neutrophils and cytotoxic t cells. Circulation. 2009;119:2209–2216. doi: 10.1161/CIRCULATIONAHA.108.806505. [DOI] [PubMed] [Google Scholar]
- 36.Henderson EL, Geng Y-J, Sukhova GK, Whittemore AD, Knox J, Libby P. Death of smooth muscle cells and expression of mediators of apoptosis by t lymphocytes in human abdominal aortic aneurysms. Circulation. 1999;99:96–104. doi: 10.1161/01.cir.99.1.96. [DOI] [PubMed] [Google Scholar]
- 37.Bronte V, Pittet Mikael J. The spleen in local and systemic regulation of immunity. Immunity. 2013;39:806–818. doi: 10.1016/j.immuni.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin M, Zhang J, Wang Y, Wang S, Böckler D, Duan Z, Xin S. Deficient cd4+cd25+ t regulatory cell function in patients with abdominal aortic aneurysms. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:1825–1831. doi: 10.1161/ATVBAHA.109.200303. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Y, Wu W, Lindholt JS, Sukhova GK, Libby P, Yu X, Shi G-P. Regulatory t cells in human and angiotensin ii-induced mouse abdominal aortic aneurysms. Cardiovascular research. 2015;107:98–107. doi: 10.1093/cvr/cvv119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meng X, Yang J, Zhang K, An G, Kong J, Jiang F, Zhang Y, Zhang C. Regulatory t cells prevent angiotensin ii–induced abdominal aortic aneurysm in apolipoprotein e knockout mice. Hypertension. 2014;64:875–882. doi: 10.1161/HYPERTENSIONAHA.114.03950. [DOI] [PubMed] [Google Scholar]
- 41.Yodoi K, Yamashita T, Sasaki N, Kasahara K, Emoto T, Matsumoto T, Kita T, Sasaki Y, Mizoguchi T, Sparwasser T, Hirata K-i. Foxp3+ regulatory t cells play a protective role in angiotensin ii–induced aortic aneurysm formation in mice. Hypertension. 2015;65:889–895. doi: 10.1161/HYPERTENSIONAHA.114.04934. [DOI] [PubMed] [Google Scholar]
- 42.Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin ii-infused, apolipoprotein e-deficient mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:1621–1626. doi: 10.1161/01.ATV.0000085631.76095.64. [DOI] [PubMed] [Google Scholar]
- 43.Cao RY, Amand T, Ford MD, Piomelli U, Funk CD. The murine angiotensin ii-induced abdominal aortic aneurysm model: Rupture risk and inflammatory progression patterns. Frontiers in Pharmacology. 2010;1:9. doi: 10.3389/fphar.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tieu BC, Lee C, Sun H, LeJeune W, Recinos A, 3rd, Ju X, Spratt H, Guo D-C, Milewicz D, Tilton RG, Brasier AR. An adventitial il-6/mcp1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. The Journal of clinical investigation. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yanagitani Y, Rakugi H, Okamura A, Moriguchi K, Takiuchi S, Ohishi M, Suzuki K, Higaki J, Ogihara T. Angiotensin ii type 1 receptor–mediated peroxide production in human macrophages. Hypertension. 1999;33:335–339. doi: 10.1161/01.hyp.33.1.335. [DOI] [PubMed] [Google Scholar]
- 46.Crowley SD, Song Y-S, Sprung G, Griffiths R, Sparks M, Yan M, Burchette JL, Howell DN, Lin EE, Okeiyi B, Stegbauer J, Yang Y, Tharaux P-L, Ruiz P. A role for angiotensin ii type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin ii–dependent hypertension. Hypertension. 2010;55:99–108. doi: 10.1161/HYPERTENSIONAHA.109.144964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim H-S, Tharaux P-L, Coffman TM. Stimulation of lymphocyte responses by angiotensin ii promotes kidney injury in hypertension. American Journal of Physiology - Renal Physiology. 2008;295:F515–F524. doi: 10.1152/ajprenal.00527.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keidar S, Heinrich R, Kaplan M, Hayek T, Aviram M. Angiotensin ii administration to atherosclerotic mice increases macrophage uptake of oxidized ldl: A possible role for interleukin-6. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21:1464–1469. doi: 10.1161/hq0901.095547. [DOI] [PubMed] [Google Scholar]
- 49.Mayer CR, Geis NA, Katus HA, Bekeredjian R. Ultrasound targeted microbubble destruction for drug and gene delivery. Expert opinion on drug delivery. 2008;5:1121–1138. doi: 10.1517/17425247.5.10.1121. [DOI] [PubMed] [Google Scholar]
- 50.Sapet C, Laurent N, Bertosio E, Bertuzzi M, Sicard F. Targeted transfection of cells/tissue in vivo. 2012 [Google Scholar]
- 51.Prins PA, Hill MF, Airey D, Nwosu S, Perati PR, Tavori H, M FL, Kon V, Fazio S, Sampson UK. Angiotensin-induced abdominal aortic aneurysms in hypercholesterolemic mice: Role of serum cholesterol and temporal effects of exposure. PLoS One. 2014;9:e84517. doi: 10.1371/journal.pone.0084517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lima VV, Zemse SM, Chiao CW, Bomfim GF, Tostes RC, Clinton Webb R, Giachini FR. Interleukin-10 limits increased blood pressure and vascular rhoa/rho-kinase signaling in angiotensin ii-infused mice. Life sciences. 2016;145:137–143. doi: 10.1016/j.lfs.2015.12.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.