Abstract
Background
The incidence of ischemic stroke in young patients is increasing and associated with unfavorable prognosis due to high risk of recurrent cardiovascular events. In many young patients the cause of stroke remains unknown, referred to as cryptogenic stroke. Neuroimaging frequently suggests a proximal source of embolism in these strokes. We developed a comprehensive step-by-step echocardiography protocol for a prospective study with centralized reading to characterize preclinical cardiac changes associated with cryptogenic stroke.
Methods and study design
SECRETO (Searching for Explanations for Cryptogenic Stroke in the Young: Revealing the Etiology, Triggers, and Outcome; NCT01934725) is an ongoing multicenter case–control study enrolling patients (target n = 600) aged 18–49 years hospitalized due to first-ever ischemic stroke of undetermined etiology and age- and sex-matched controls (target n = 600). A comprehensive assessment of cardiovascular risk factors and extensive cardiac imaging with transthoracic and transesophageal echocardiography, electrocardiography and neurovascular imaging is performed. Transthoracic and transesophageal echocardiograms will be centrally read, following an extensive protocol particularly emphasizing the characteristics of left atrium, left atrial appendage and interatrial septum.
Conclusions
A detailed assessment of both conventional and unconventional vascular risk factors and cardiac imaging with transthoracic and transesophageal echocardiography are implemented in SECRETO, aiming to establish indirect and direct risk factors and causes for cryptogenic stroke and novel pathophysiological brain–heart pathways. This may ultimately enable more personalized therapeutic options for these patients.
Keywords: transthoracic echocardiography, transesophageal echocardiography, stroke, cardiac sources of embolism, patent foramen ovale, risk factors
Introduction
The incidence of ischemic stroke in young patients is increasing globally (1). Stroke at younger ages is associated with increased morbidity and mortality, in particular with respect to recurrent cardiovascular events (2). Cardiac sources of embolism underlie 15–40% of all ischemic strokes and include a wide range of cardiac conditions (3). However, the cause of stroke in 30–50% of younger adults remains unknown, also termed as cryptogenic stroke (4, 5, 6). In many young patients with cryptogenic stroke, neuroimaging pattern suggests a proximal source of embolism. Notably, the risk of recurrent stroke in these patients was comparable to those with established major source of cardioembolism (7).
The etiology of cardioembolic sources of ischemic stroke may be classified as major, minor or uncertain (3, 8). Major cardioembolic causes include atrial fibrillation or flutter, recent or previous myocardial infarction with apical akinesia, left ventricular (LV) aneurysm, dilated cardiomyopathy, reduced LV ejection fraction (<35%), LV or left atrial appendage (LAA) thrombus, rheumatic and prosthetic heart valve disease, infective endocarditis, intracardiac tumors, congenital heart diseases, Takotsubo-cardiomyopathy and atherosclerosis in the ascending aorta and aortic arch, while minor or uncertain causes are as patent foramen ovale (PFO), mitral valve prolapse, mitral annular calcification, degenerative aortic stenosis, atrial septum aneurysm, spontaneous contrast on the echocardiogram, aortic aneurysm, false tendon in the LV, moderate to severe left atrium (LA) enlargement and LV hypertrophy (3, 4, 5, 6, 7, 8).
In particular, PFO has gained much attention recently due to positive results from trials comparing transcatheter PFO closure with medical therapy in the secondary stroke prevention (9). Currently, stroke in a patient with PFO is classified as cryptogenic if no other cause is identifiable in stroke classification systems. An European Expert Panel recently proposed that when PFO is believed likely to be implicated in a cryptogenic stroke, the event should be classified as PFO-related stroke (10). The panel also stressed that research should continue to verify additional risk factors and identify new high-risk phenotypes for PFO-related stroke, comprising an array of clinical, biochemical and cardiovascular imaging characteristics. In view of these considerations, an extensive assessment of cardiovascular risk factors and cardiac imaging is required to elucidate direct and indirect causes of cryptogenic stroke and subsequently offer these patients a more personalized therapeutic management. In the present paper, we present a step-by-step transthoracic (TTE) and transesophageal echocardiography (TEE) performance protocol for the multicenter international case–control study, Searching for Explanations for Cryptogenic Stroke in the Young: Revealing the Etiology, Triggers, and Outcome (SECRETO; NCT01934725) (11).
Study design
SECRETO is an international multicenter prospective case–control study of young adults (aged 18–49 years) presenting with a documented first-ever ischemic stroke of undetermined etiology. Patients are included after standardized diagnostic procedures: brain MRI, imaging of intracranial and extracranial vessels, TTE and TEE, ECG and screening for coagulopathies. Stroke-free healthy control participants are recruited and matched with index patients by age (within 5 years), sex and ethnicity in a 1:1 fashion, as per the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines at each study site. As the incidence of ischemic stroke is approximately 10/100,000 per year in this age group (12), an international consortium has been established to recruit a sufficient number of patients in a reasonable time. Recruitment of subjects began in 2013 and is expected to be completed by the end of 2020 aiming to enroll a total of 600 patients and 600 control subjects. As of March 2019, 17 European centers are participating in SECRETO. The SECRETO study was approved by local ethical committtees in all recruiting centers. Written informed consent was obtained and will be obtained from all study subjects prior to study enrollment.
Objectives
The principal aim of SECRETO is to document new risk factors and pathogenic pathways leading to currently cryptogenic stroke in the young. Specifically regarding the investigations on proximal sources of embolism, we aimed to (1) develop a standardized echocardiography performance protocol exceeding state-of-the-art; (2) to compare cardiac structural and functional findings in a case-control study to establish novel cardiac sources for embolism and (3) to use these data to explore cardiac structural and functional characteristics associated with recurrent thromboembolic events in a long-term follow-up. The cardiac imaging data can be further cross-linked with clinical risk factor data, neuroimaging findings, blood markers of thrombosis and hemostasis and genetic profiles. Finally, systems and precision medicine approaches can be applied to identify clusters of clinical and cardio-anatomical, and biological characteristics to construct more holistic explanatory models for cryptogenic stroke.
Measures and clinical data at baseline
At baseline during hospitalization for the index stroke and at follow-up visit anthropometrics (height, weight, waist and hip circumference), blood pressure and heart rate are measured. Clinical data including an extensive review of neurological symptoms at presentation, diagnostic work-up, treatments, conventional and unconventional stroke risk factor information are collected using structured questionnaires. Brain and neurovascular imaging are subject to blinded central reading. A standard 12-lead ECG is obtained on admission, and after the acute phase.
Echocardiography
A step-by-step performance protocol was developed for TTE and TEE to allow their accurate and blinded central reading (SS) at Echo Core Lab in University of Bergen, Norway.
The protocol is based upon standard recommendations for the use of TTE and TEE (3). Particular emphasis will be placed on the measures of left and right atrium, atrial septum, LAA, the anatomical shape and size of PFO and its relation with aortic root and other adjacent structures, as well as interatrial and intrapulmonary (delayed) shunts. TTE may be the only cardiac imaging in cases with very severe stroke, receptive aphasia, or swallowing difficulties.
Transthoracic echocardiography
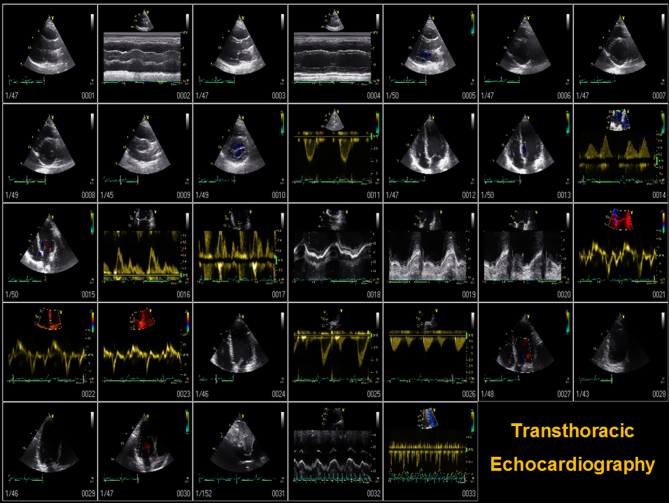
Each study is labeled with a study ID and date. Age, height and weight are entered to calculate body surface area. A sweep speed of 100 mm/s for all M-mode and Doppler recording are used. A loop of three RR intervals of all images in patients with sinus rhythm and five RR intervals in patients with atrial fibrillation is recorded. The order and modalities used to obtain the required TTE images are presented in Figs 1 and 2. Quantitative assessment of right ventricle, LV dimension and volumes are performed according to current international guidelines on quantitative echocardiography (13). LV systolic function is assessed by biplane Simpson’s ejection fraction (13). Diastolic function is assessed according to current European guidelines (14).
Figure 1.
Echocardiography performance protocol showing the order of imaging planes and recommended TEE transducer angle °.
Figure 2.
The number and order of imaging planes in conventional 2D, M-mode, tissue, spectral and color Doppler TTE.
Following TTE, supine brachial blood pressure is measured on the left arm (phase 1 and 5 Korotkoff sound) using an appropriately sized cuff and a regularly calibrated aneroid blood pressure sphygmomanometer. The measurements are recorded and provided along with the echocardiography images.
Transesophageal echocardiography
Prior to TEE, patients should have been fasting for at least 6 h. Benzodiazepines (diazepam or midazolam) are the common sedative agents used during TEE. The patient is examined awake under a conscious sedative state and is able to respond to verbal or tactile stimuli. Xylocaine spray is used for local pharyngeal anesthesia. Any jaw prosthesis or artificial teeth that are not fixed are removed before the probe insertion. The patient is placed in the left lateral decubitus position during probe insertion. In the mid-line of the mouth the probe is slowly advanced to the sophagus. In the final study report, any difficulties during probe insertion, procedure-related complications as well as image quality and heart rhythm are documented. A standardized TEE protocol includes adequate visualization of all cardiac chambers, with emphasis on both atria, LAA, interatrial septum, heart valves and the thoracic aorta (8). The overall TEE parameters implemented in the SECRETO study are summarized in Box 1 and in Figs 1 and 3. The suggested degrees to assess cardiac structures may vary to some extent according to the position of the heart in the chest in individual patients.
Figure 3.
(A and B) Upper-esophageal 5-chamber view 0° showing aortic valve (B color Doppler), (C) mid-esophageal 4-chamber view 0°, (D) color Doppler across the tricuspid and (E) mitral valve, (F and G) mid-esophageal 2-chamber view showing mitral valve, (H) 2-chamber simultaneous X-plane view showing left atria appendage (LAA) without thrombus, (I) short-axis view of LAA combined with color Doppler, (J) zoomed image with color Doppler flow in LAA and left upper pulmonary vein [LUPV] at 27°, (K) pulsed-wave velocity in LAA, (L) pulse wave velocity in LUPV, (M and N) mid-esophageal image display of atria, inter-atrium septum, Chiari network (arrow) and a patent foramen ovale (PFO) tunnel (red lines) at 100°. (O) Mid-esophageal view showing right atrium filled with agitated saline bubbles, (P) short-axis view of atria, atrial septum and a cross-sectional view of aortic valve at 40° (also called procedural view), (Q) long-axis view showing mitral and aortic valve at 125°, (R) simultaneous x-plane view of aortic valve, (S) color Doppler flow across mitral (left) and aortic valve (right), (T) dimensions at aortic annulus (1), aortic root (2), sinotubular junction (3) and ascending aorta (4) in long-axis view 122°, and (U) a simultaneous view of descending aorta, cross-sectional (left) and longitudinal view (right) at 180°.
| Box 1: Variables assessed by transesophageal echocardiography in SECRETO. |
| Aortic annulus diameter (cm) |
| Aortic sinus diameter (cm) |
| Sinotubular junction diameter (cm) |
| Ascending aortic diameter (cm) |
| Aortic arch atheromatosis (plaque thickness ≥4 mm) |
| Descending aortic wall thickness (mm) |
| Aortic valve cusps (numbers and morphology) |
| Aortic valve calcification |
| Tumors |
| Vegetation/infective endocarditis, peri-valvular edema/inflammation |
| Atrial septal defect |
| Patent foramen ovale (PFO) |
| Spontaneous left-to-right shunt assessed by color Doppler imaging |
| Shift of shunt direction after Valsalva maneuver |
| PFO diameter (mm) |
| PFO tunnel length (mm) |
| Assessment of delayed intrapulmonary shunt with bubble study |
| Atrial septal aneurysm (ASA) ≥10 mm |
| Hypertrophy of interatrial septum (lipomatous hypertrophy) |
| Prominent Eustachian valvea |
| Left atrial thrombus/mass |
| Left atrial appendage (LAA) thrombus |
| Slow left atrial appendage flow velocity (peak velocity ≤30 cm/s) |
| Ridge between left upper pulmonary vein and LAA and pectinate muscle |
| Spontaneous echo contrast |
| Mitral annular calcification and leaflet thickness |
| Etiology of mitral regurgitation (functional, degenerative, mixed) |
| Mitral regurgitation grade (I-IV): Quantitative assessment with PISA, vena contracta, regurgitant volume, EROA |
| Aortic valve calcification: mild moderate severe |
| Aortic regurgitation: mild moderate severe |
| Tricuspid regurgitation: mild moderate severe |
| aDefined as valve thickness ≥1 mm with at least 10 mm protrusion within the right atrium as measured from the border of inferior vena cava. |
TEE with bubble study with and without Valsalva for the diagnosis of PFO
PFO is diagnosed by TEE with color Doppler imaging demonstrating spontaneous left-to-right shunt (from high pressure chamber to low pressure chamber) or the shift of shunt direction to right-to-left during Valsalva maneuver, confirmed by bubble study when microbubbles particles are visualized in the left atrial chamber in any single frame during first 3–5 cycles. An intravenous cannula is inserted in the right antecubital vein. For bubble studies, administration of intravenous agitated saline is used to assess atrial septal defects. Prior to the TEE procedure, the patients are given (sufficient) instruction to perform a successful Valsalva maneuver. The Valsalva maneuver is initiated when the contrast is arrived in the right atrium and to release on command after a total of 10–15 cardiac cycles. The size of PFO may be quantified by the number of microbubbles appearing in the LA (3–10 microbubbles as small shunt, 11–30 microbubbles as moderate shunt and >30 bubbles as a large shunt) (15). ASA is defined according to Olivares classification: Type 1R if the bulging is only in the right atrium. Type 2L if the bulging is only in the LA. Type 3RL if the major excursion bulges to the right atrium and the lesser excursion bulges toward the left. Type 4LR if the maximal excursion of the ASA is toward the LA with a lesser excursion toward the right atrium. Type 5 if the ASA movement is bidirectional and equidistant to both atria during the cardiorespiratory cycle (16).
Spontaneous echo contrast is identified as a dynamic and slowly swirled smoke-like signal. LAA slow flow velocity is defined as peak velocity ≤30 cm/s. The LAA is carefully inspected for the presence of thrombus using multiplane imaging from 0° to 180°. Furthermore, normal variants of cardiac structures and artifacts in right atrium (lipomatous hypertrophy of atrial septum, pectinate muscles, Chiari network, Eustachian valve, catheters and pacemaker leads), LA (the ridge between left upper pulmonary vein and LAA) and ventricles (trabeculation, false chorda, papillary muscle, catheter and pacemaker leads, moderate band in the right ventricle), which are essentially not related to embolic events, are distinguished from cardiac masses (17).
Discussion
The incidence of ischemic stroke in younger patients is increasing, and risk factors are changing over time. Among the most likely explanations for this worrying observation is the increasing prevalence of cardiometabolic risk factors, mainly obesity in young individuals in populations (18), leading to early development of hypertension and diabetes mellitus and vascular aging (19). Contemporary neuroimaging suggests proximal source of embolism in a large proportion of young patients with cryptogenic stroke, thus more comprehensive studies, searching for underlying causes of ischemic stroke in the heart or the proximal aorta are spoken for. The SECRETO multicenter study includes a step-by-step echocardiography protocol to explore the cardiac structural and functional risk factors as novel causal factors in cryptogenic ischemic stroke in the young.
PFO is a common finding in the general population with a prevalence of approximately 25% and may well be an innocent bystander in a patient with cryptogenic stroke (10). Stroke in a patient with PFO is classified as cryptogenic if no other cause is identifiable, according to existing stroke classification systems. Neuroimaging pattern and patient characteristics are typically unhelpful in the assessment of PFO’s clinical relevance (10). Acute evidence of a deep venous thrombosis or pulmonary embolism, recent prolonged travel and immobilization or Valsalva maneuver preceding the ischemic stroke event, are considered to increase the probability of paradoxical embolism due to a right-to-left shunt (17, 20). The differentiation between an incidental and a culprit PFO for index stroke will be evaluated based on risk calculators such as the risk of paradoxical embolism (RoPE) score (21), clinical assessment based on a multidisciplinary approach and multimodality imaging. Importantly, due to remaining uncertainty in the causality of PFO, a European expert panel stressed the need to identify new high-risk PFO phenotypes and search additional risk factors that would help in prediction of PFO-related events (10).
A patient presenting with the previously mentioned risk factors for paradoxical embolism may also have other factors that increase the risk of thrombosis, such as genetic or acquired pro-thrombotic factors. Similarly, a sudden rise in pulmonary arterial pressure due to acute pulmonary embolism caused by deep venous thrombosis in the lower extremities may contribute to the migration of the thrombus across the atrial septum to the systemic circulation (20). It is, however, of utmost importance to study cardiac characteristics also in patients without PFO, in particular, because embolic cortical infarct patterns are similar in patients with and without PFO (10). Moreover, a patient-level meta-analysis of randomized trials suggests that also subcortical infarcts, traditionally not considered embolic, may indeed have an embolic origin (22).
Of note, previous stroke etiology studies have not included a structured, comprehensive cardiac evaluation strategy or centralized core laboratory analysis of echocardiography (17). In SECRETO, it is mandatory to (1) minimize inter-operator variability and (2) allow standardized central reading of multiple exploratory measures. Therefore, we aimed to develop a comprehensive step-by-step TEE protocol to characterize potentially pathogenic changes associated with cryptogenic stroke. In particular, a standardized TEE with thorough evaluation of both atria, LAA, interatrial septum aneurysm and defect and the anatomical shape and size of PFO is applied. All these factors have been linked to PFO-associated strokes in retrospective studies (23, 24).
Poor performance of Valsalva may hamper identification of PFO. However, we complement echocardiography studies with a prior transcranial Doppler ultrasound bubble test to quantify right-to-left shunt. Transcranial Doppler (TCD) is being done systematically at selected sites. To achieve the maximal accuracy in PFO diagnosis, the combined use of different techniques is warranted (10). However, as the majority of right-to-left shunts is represented by PFO, PFO-related parameters are better evaluated by standardized step-by-step TEE compared with TTE. Further, whether 3D echocardiography has incremental clinical and prognostic value over 2D echocardiography should be explored in future studies. Finally, TEE is a semi-invasive modality and its use in healthy volunteers is an ethical consideration. However, this is counter-balanced by the fact that complications related to TEE are rare. In a study population that included patients with a heart disease, the complication risk attributable to TEE was 0.2–0.5% (25). Complication risk of TEE is similar to that of gastroscopy and is usually associated with concomitant diseases of upper gastrointestinal tract. TEE will not be performed if any contraindication is found, including difficulty in swallowing, any upper gastrointestinal tract disease, severe cervical spinal arthrosis or a known risk of bleeding, coagulation abnormality or thrombocytopenia. Therefore, in healthy subjects without contraindications for the investigation or cardiac disease, the health risks associated with TEE are considered minimal. TEE may be therefore applied following individual agreement, and after approval by the local ethics committee.
Conclusion
Cardioembolic stroke is a syndromal disorder which includes multiple cardiac conditions, and may have devastating implications if occurred in early life. A detailed assessment of both conventional and unconventional vascular risk factors and cardiac imaging with TTE and TEE are implemented in SECRETO. Particularly, a standardized step-by-step TEE imaging aiming to the evaluation of both atria, LAA, Eustachian valve, Chiari network, interatrial septum aneurysm and defect and the anatomical shape and size of PFO, are implemented in the post-stroke screening work-up. The goal of SECRETO is to identify novel risk factors and cardiac imaging characteristics of cryptogenic stroke to advance current understanding, and ultimately enable more personalized therapeutic options for these patients.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
The participating centers are funded by Finnish Medical Foundation, the Academy of Finland and Helsinki University Central Hospital.
References
- 1.Bejot Y, Delpont B, Giroud M. Rising stroke incidence in young adults: more epidemiological evidence, more questions to be answered. Journal of the American Heart Association 2016. 5 e003661 ( 10.1161/JAHA.116.003661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aarnio K, Siegerink B, Pirinen J, Sinisalo J, Lehto M, Haapaniemi E, Nave AH, Kaste M, Tatlisumak T, Putaala J. Cardiovascular events after ischemic stroke in young adults: a prospective follow-up study. Neurology 2016. 86 . ( 10.1212/WNL.0000000000002689) [DOI] [PubMed] [Google Scholar]
- 3.Pepi M, Evangelista A, Nihoyannopoulos P, Flachskampf FA, Athanassopoulos G, Colonna P, Habib G, Ringelstein E, Sicari R, Zamorano JL, et al. Recommendations for echocardiography use in the diagnosis and management of cardiac sources of embolism: European Association of Echocardiography (EAE) (a registered branch of the ESC). European Journal of Echocardiography 2010. 11 . ( 10.1093/ejechocard/jeq045) [DOI] [PubMed] [Google Scholar]
- 4.Yesilot Barlas N, Putaala J, Waje-Andreassen U, Vassilopoulou S, Nardi K, Odier C, Hofgart G, Engelter S, Burow A, Mihalka L, et al. Etiology of first-ever ischaemic stroke in European young adults: the 15 Cities Young Stroke Study. European Journal of Neurology 2013. 20 . ( 10.1111/ene.12228) [DOI] [PubMed] [Google Scholar]
- 5.Rolfs A, Fazekas F, Grittner U, Dichgans M, Martus P, Holzhausen M, Bottcher T, Heuschmann PU, Tatlisumak T, Tanislav C, et al. Acute cerebrovascular disease in the young: the Stroke in Young Fabry Patients study. Stroke 2013. 44 . ( 10.1161/STROKEAHA.112.663708) [DOI] [Google Scholar]
- 6.Li L, Yiin GS, Geraghty OC, Schulz UG, Kuker W, Mehta Z, Rothwell PM. & Oxford Vascular Study. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurology 2015. 14 . ( 10.1016/S1474-4422(15)00132-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Majander N, Aarnio K, Pirinen J, Lumikari T, Nieminen T, Lehto M, Sinisalo J, Kaste M, Tatlisumak T, Putaala J. Embolic strokes of undetermined source in young adults: baseline characteristics and long-term outcome. European Journal of Neurology 2018. 25 . ( 10.1111/ene.13540) [DOI] [PubMed] [Google Scholar]
- 8.Saric M, Armour AC, Arnaout MS, Chaudhry FA, Grimm RA, Kronzon I, Landeck BF, Maganti K, Michelena HI, Tolstrup K. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. Journal of the American Society of Echocardiography 2016. 29 . ( 10.1016/j.echo.2015.09.011) [DOI] [PubMed] [Google Scholar]
- 9.Ntaios G, Papavasileiou V, Sagris D, Makaritsis K, Vemmos K, Steiner T, Michel P. Closure of patent foramen ovale versus medical therapy in patients with cryptogenic stroke or transient ischemic attack: updated systematic review and meta-analysis. Stroke 2018. 49 . ( 10.1161/STROKEAHA.117.020030) [DOI] [PubMed] [Google Scholar]
- 10.Pristipino C, Sievert H, D'Ascenzo F, Louis Mas J, Meier B, Scacciatella P, Hildick-Smith D, Gaita F, Toni D, Kyrle P, et al. European position paper on the management of patients with patent foramen ovale. General approach and left circulation thromboembolism. European Heart Journal 2018. 25 ehy649 ( 10.1093/eurheartj/ehy649) [DOI] [PubMed] [Google Scholar]
- 11.Putaala J, Martinez-Majander N, Saeed S, Yesilot N, Jäkälä P, Nerg O, Tsivgoulis G, Numminen H, Gordin D, von Sarnowski B, et al. Searching for Explanations for Cryptogenic Stroke in the Young: revealing the Triggers, Causes, and Outcome (SECRETO): rationale and design. European Stroke Journal 2017. 2 . ( 10.1177/2396987317703210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Putaala J, Metso AJ, Metso TM, Konkola N, Kraemer Y, Haapaniemi E, Kaste M, Tatlisumak T. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki Young Stroke Registry. Stroke 2009. 40 . ( 10.1161/STROKEAHA.108.529883) [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography 2015. 28 1.e14–39.e14. ( 10.1016/j.echo.2014.10.003) [DOI] [PubMed] [Google Scholar]
- 14.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography 2016. 29 . ( 10.1016/j.echo.2016.01.011) [DOI] [PubMed] [Google Scholar]
- 15.Hahn RT, Abraham T, Adams MS, Bruce CJ, Glas KE, Lang RM, Reeves ST, Shanewise JS, Siu SC, Stewart W, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Journal of the American Society of Echocardiography 2013. 26 . ( 10.1016/j.echo.2013.07.009) [DOI] [PubMed] [Google Scholar]
- 16.Olivares-Reyes A, Chan S, Lazar EJ, Bandlamudi K, Narla V, Ong K. Atrial septal aneurysm: a new classification in two hundred five adults. Journal of the American Society of Echocardiography 1997. 10 . ( 10.1016/S0894-7317(97)70027-0) [DOI] [PubMed] [Google Scholar]
- 17.Saeed S, Gerdts E. Echocardiography in Ischemic Stroke in Young Adults in Ischemic Stroke in the Young, ch 10. Eds Tatlisumak T. & Thomassen L. Oxford, UK: Oxford University Press, 2017. [Google Scholar]
- 18.George MG, Tong X, Bowman BA. Prevalence of cardiovascular risk factors and strokes in younger adults. JAMA Neurology 2017. 74 . ( 10.1001/jamaneurol.2017.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saeed S, Waje-Andreassen U, Fromm A, Øygarden H, Kokorina MV, Naess H, Gerdts E. Early vascular aging in young and middle-aged ischemic stroke patients: the Norwegian Stroke in the Young Study. PLoS ONE 2014. 9 e112814 ( 10.1371/journal.pone.0112814) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellensen VS, Saeed S, Geisner T, Haaverstad R. Management of thromboembolism-in-transit with pulmonary embolism. Echo Research and Practice 2017. 4 K47–K51. ( 10.1530/ERP-17-0043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kent DM, Ruthazer R, Weimar C, Mas JL, Serena J, Homma S, Di Angelantonio E, Di Tullio MR, Lutz JS, Elkind MSV, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology 2013. 81 . ( 10.1212/WNL.0b013e3182a08d59) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kent DM, Dahabreh IJ, Ruthazer R, Furlan AJ, Reisman M, Carroll JD, Saver JL, Smalling RW, Jüni P, Mattle HP, et al. Device closure of patent foramen ovale after stroke: pooled analysis of completed randomized trials. Journal of the American College of Cardiology 2016. 67 . ( 10.1016/j.jacc.2015.12.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuchlenz HW, Saurer G, Weihs W, Rehak P. Persisting eustachian valve in adults: relation to patent foramen ovale and cerebrovascular events. Journal of the American Society of Echocardiography 2004. 17 . ( 10.1016/j.echo.2003.12.003) [DOI] [PubMed] [Google Scholar]
- 24.Goel SS, Tuzcu EM, Shishehbor MH, de Oliveira EI, Borek PP, Krasuski RA, Rodriguez LL, Kapadia SR. Morphology of the patent foramen ovale in asymptomatic versus symptomatic (stroke or transient ischemic attack) patients. American Journal of Cardiology 2009. 103 . ( 10.1016/j.amjcard.2008.08.036) [DOI] [PubMed] [Google Scholar]
- 25.Hilberath JN, Oakes DA, Shernan SK, Bulwer BE, D'Ambra MN, Eltzschig HK. Safety of transesophageal echocardiography. Journal of the American Society of Echocardiography 2010. 23 ; quiz 1220. ( 10.1016/j.echo.2010.08.013) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a