Abstract
The melanocortin-2-receptor (MC2R), also known as the ACTH receptor, is a critical component of the hypothalamic–pituitary–adrenal axis. The importance of MC2R in adrenal physiology is exemplified by the condition familial glucocorticoid deficiency (FGD), a potentially fatal disease characterised by isolated cortisol deficiency. MC2R mutations cause ~25% of cases. The discovery of a MC2R accessory protein MRAP, mutations of which account for ~20% of FGD, has provided insight into MC2R trafficking and signalling. MRAP is a single transmembrane domain accessory protein highly expressed in the adrenal gland and essential for MC2R expression and function. Mouse models helped elucidate the action of ACTH. The Mc2r-knockout (Mc2r−/−) mice was the first mouse model developed to have adrenal insufficiency with deficiencies in glucocorticoid, mineralocorticoid and catecholamines. We recently reported the generation of the Mrap−/− mice which better mimics the human FGD phenotype with isolated glucocorticoid deficiency alone. The adrenal glands of adult Mrap−/− mice were grossly dysmorphic with a thickened capsule, deranged zonation and deranged WNT4/beta-catenin and sonic hedgehog (SHH) pathway signalling. Collectively, these mouse models of FGD highlight the importance of ACTH and MRAP in adrenal progenitor cell regulation, cortex maintenance and zonation.
Keywords: ACTH, MRAP, adrenal, stem cells, MC2R
Hypothalamo–pituitary–adrenal axis
The hypothalamo–pituitary–adrenal (HPA) axis dictates the production of glucocorticoids secreted from the adrenal gland. Parvocellular neurosecretory neurons within the hypothalamic paraventricular nucleus (PVN) secrete corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) (1) into the hypophyseal portal circulation, and these act on anterior pituitary corticotroph cells to trigger secretion of adrenocorticotropic hormone (ACTH) (2, 3). ACTH, a 39 amino acid peptide, is produced by cleavage of its precursor protein, pro-opiomelanocortin (POMC). Other cleavage products of POMC include α-, β-, γ-melanocyte-stimulating hormones (MSH), β-endorphin, N-terminal peptide of pro-opiomelanocortin, lipotrophins and Met-enkephalin, corticotropin-like intermediate peptide (CLIP) (reviewed in 4, 5). ACTH is released into the circulation to act on peripheral sites, mainly the adrenal glands to stimulate glucocorticoid hormone production. Glucocorticoids have a negative feedback on the release of CRH and AVP at the hypothalamus and ACTH at the pituitary, thus providing tight regulation of cortisol production.
ACTH receptor/melanocortin-2-receptor
The ACTH receptor (also known as the melanocortin-2-receptor (MC2R)), cloned in 1992 (6), is a critical component of the HPA axis and a member of the melanocortin receptor family. Other members are MC1R, MC3R, MC4R and MC5R – the functions of which were recently reviewed elsewhere (4). MC2R is unique in that it only binds ACTH, whereas MC1R, MC3R, MC4R and MC5R bind the melanocortins α-MSH,β-MSH and γ-MSH. In the adrenal gland, MC2R is expressed in all zones of the adrenal cortex. Its principal site of action is the zona fasciculata (ZF) in the generation of glucocorticoids in response to ACTH, although action on the zona glomerulosa (ZG) and zona reticularis (ZR) have been implicated in a number of physiological and disease states (7). In the case of the ZG, ACTH can acutely stimulate aldosterone production and there is now growing interest in the role of ACTH/MC2R in primary hyperaldosteronism (8, 9).
ACTH resistance syndromes and FGD
Much of what we know about MC2R has been through the study of the ACTH resistance syndrome, FGD. FGD is a rare autosomal recessive condition clinically characterised by isolated glucocorticoid deficiency in the presence of normal mineralocorticoid function. FGD was first reported in two siblings diagnosed with Addison’s disease without hypoaldosteronism (10). Patients with FGD usually present in the neonatal period or early childhood with symptoms of hypocortisolaemia such as hypoglycaemia, failure to thrive, recurrent infections, collapse and seizures along with severe hyperpigmentation due to the extra-adrenal action of excessive plasma ACTH on MC1R in the skin melanocytes (11). Biochemically, FGD patients present with very high plasma ACTH levels often greater than 1000 pg/mL paired with very low or absent serum cortisol concentrations, hence the term ACTH resistance. Aldosterone levels are usually unaffected although derangements have been reported in a subset of patients (11, 12, 13). With the identification of additional FGD causative genes such as steroidogenic acute regulatory protein (STAR), minichromosome maintenance 4 (MCM4), nicotinamide nucleotide transhydrogenase (NNT), thioredoxin reductase 2 (TXNRD2), cytochrome p450scc (CYP11A1), glutathione peroxidase 1 (GPX1), peroxiredoxin 3 (PRDX3) and sphingosine 1-phosphate lyase (SGPL1), additional phenotypes including permanent or evolving mineralocorticoid deficiency have been reported, which has been recently reviewed elsewhere (14).
Loss-of-function mutations in MC2R and FGD type 1
The first loss-of-function missense mutation in MC2R, S74I, was identified in 1993 after candidate-gene sequencing of two siblings with FGD (15). Following this, numerous loss-of-function mutations have been identified scattered along the whole receptor (16), cementing the importance of MC2R in HPA biology. MC2R gene mutations account for approximately 25% of all FGD cases (17, 18) and are referred to as FGD type I (OMIM#202200). The majority of missense mutations result in a misfolded protein and its retention in the endoplasmic reticulum while some genetic defects affect ligand binding, signal transduction or lead to a truncated protein product (4, 19). Patients harbouring MC2R mutations have been shown to have tall stature (16, 20, 21, 22) along with advanced or dissociated bone age (23). The mechanism for this phenomenon remains unclear but is not due to the insulin-like growth factor 1–growth hormone axis as this is normal (20). One potential explanation is that the phenotype is due to high plasma ACTH levels acting on MCRs in bone (24, 25). In support of this, ACTH has been shown to directly increase chondroprogenitor cell proliferation in vitro (26) and promote chondrocyte differentiation although the target MCR responsible for this action is uncertain (24). Interestingly, growth velocity falls after the introduction of glucocorticoid replacement, during which plasma ACTH levels often remain high. Another clinical feature, absent adrenarche, has also been described (27) and, in conjunction with findings that have linked polymorphisms in MC2R with age of onset of adrenarche, highlights the importance of ACTH in the regulation of adrenarche in children (28). The adrenal glands have been reported to be small in FGD, with histopathology showing the absence of fasciculata or reticularis cells together with disorganisation of zona granulosa (ZG) cells (16). Recent data in mouse models show that PKA signalling overactivation drives the differentiation of a reticularis-like zone in mice, supportive of the importance of ACTH in adrenal androgen regulation (29).
Melanocortin-2-receptor accessory protein (MRAP) mutations and FGD type 2
Genetic studies of FGD patients with a normal MC2R identified variants in the C21orf61 gene. These variants would result in a truncation or complete absence of the protein product, which was found to be highly expressed in the adrenal gland and adipose tissue (17). In vitro studies revealed that this protein interacted with MC2R and was the adrenal specific factor necessary for the transport of the receptor to the plasma membrane and for receptor signalling (17, 30, 31, 32). The protein was therefore renamed melanocortin 2 receptor accessory protein (MRAP). It is now known that mutations in MRAP cause approximately 20% of all FGD cases and these are termed FGD type 2 (33, 34). In comparison with FGD type 1, patients with MRAP mutations present earlier with more severe disease. Tall stature is not seen, which is probably a reflection of commencement of glucocorticoid replacement at an earlier age (21). To date, two FGD type 2 patients have been reported as requiring fludrocortisone treatment, although it is unclear if this is a transient phenomenon (35).
MRAP is a single transmembrane domain protein which is highly evolutionarily conserved in the N-terminal and transmembrane regions (17). Human MRAP has two isoforms produced by alternative splicing, MRAP-α (19 kDa) and MRAP-β (11.5 kDa), which differ in the C-terminus. The functional difference between the two isoforms is unclear although differences have been reported in ACTH-binding capacity and cAMP generation (36). Interestingly, MRAP can form unique antiparallel transmembrane homodimers, which together with its paralogue MRAP2 are as yet the only group of proteins known to do so in eukaryotic cells. These MRAP homodimers form multimers with MC2R (37, 38, 39). The orientation of the dimers is critical to function (37, 38, 39); moreover, these antiparallel homodimers are made early on in the biogenesis of the protein, are maintained and stable, and in a heterologous cell based system have a half-life of 2 h (40). Although MRAP in vitro binds and modulates the function of other MCRs (30), it is unclear what physiological relevance this has at present.
Mouse models of FGD
The first mouse model of FGD was generated by Chida et al. in 2007 (41). The Mc2r KO mouse model was the last member of the melanocortin receptor family to be knocked out in mice. We recently generated a novel Mrap KO mouse model which, like FGD, has isolated glucocorticoid deficiency. Both models are discussed in more detail below.
Mc2r-knockout mouse model
A mouse model of MC2R deficiency was generated by replacing the whole coding region of Mc2r with a neomycin-resistance gene cassette (41), leading to complete absence of Mc2r transcript in Mc2r−/− mice. On a C57BL/6J background, the majority of Mc2r−/− mice did not survive 48 h after birth. Genotyping of 129 living mice at 4 weeks of age identified that only 9 (7%) were Mc2r−/−. It is worth noting that on a B6/Balbc mix background approximately half Mc2r−/− mice survived to adulthood, suggesting genetic modifiers at play (42). Detailed analysis demonstrated that Mc2r−/− mice failed to produce glucocorticoids and developed profound neonatal hypoglycaemia resulting in high mortality levels during the first days of life. Moreover, pups born to homozygous Mc2r-null parents died before postnatal day 0.5 due to lung failure, highlighting the importance of glucocorticoids in foetal lung maturation (41). In addition to glucocorticoid deficiency, Mc2r−/− mice also had significantly lower serum levels of aldosterone and catecholamines (41). Epinephrine levels were significantly reduced in Mc2r−/− animals, whilst dopamine and norepinephrine concentrations were unchanged. Examination of the adrenals of surviving adult mice (never replaced with glucocorticoids) revealed dysmorphic glands with gross hypoplasia of the ZF. Moreover, lipid droplets within the ZF cells were markedly reduced or absent, but cell nuclei were normal. However, the precise cell identity of such ‘ZF’ cells is not known. Interestingly, the adrenal capsule in the Mc2r-null mice was thickened compared to Mc2r+/+ littermates. The majority of naturally occurring MC2R loss-of-function mutations are missense mutations, many with residual function (4, 19). Location and type of reported MC2R mutations are summarised in a recent review (4). As a demonstration of this, patients with missense MC2R mutations have been known to present later on in childhood (21). Several more severe cases including homozygous nonsense or frame shift mutations in MC2R have been described to have hyponatraemia and/or disruption of renin-angiotensin-aldosterone system (12, 13, 35). The majority of these are transient though permanent fludrocortisone replacement has been described in several children (35). Hence, although the complete KO of Mc2r in mice is not fully representative of human FGD type 1, the model has nevertheless highlighted crucial actions of ACTH and/or glucocorticoids during development.
Mrap-knockout mouse model
We recently reported a Mrap KO (Mrap−/−) mouse model created by targeting the first coding exon of Mrap, which led to complete absence of transcript and protein in homozygote mice (43). Intercrossing heterozygote (Mrap+/−), also on a C57BL/6J background, demonstrated high neonatal mortality. Out of 325 mice generated from breeding Mrap+/− mice, only three Mrap−/− mice (<1%) survived until weaning (43). The new-born pups born from heterozygous parents with normal adrenal function died before postnatal day 1 and morphologically showed immature lungs and lack of hepatic glycogen stores (43). Glucocorticoid treatment of pregnant Mrap+/− dams rescued the phenotype, resulting in Mrap−/− mice born at the expected ratio of 25%. This indicated that the observed high mortality in homozygous Mrap−/− newborns was likely to be due to glucocorticoid deficiency rather than a direct effect of Mrap deletion. Moreover, this phenotype highlights the importance of foetal rather than maternal glucocorticoids in pre-partum neonatal adaptation in the Mrap−/− mouse.
The severity of this phenotype is consistent with the human data whereby FGD type 2 patients present soon after birth (21), thought to be due to the severity of the mutations in MRAP that lead to complete disruption or absence of protein (21). Naturally occurring mutations in MRAP (location and type) have recently been reviewed (4).
Adult Mrap−/− mice of both genders exhibited a grossly dysmorphic adrenal cortex with the glucocorticoid synthesis pathway severely downregulated and unable to produce glucocorticoids in the presence of high plasma ACTH levels. Surprisingly, unlike the Mc2r−/− mice, circulating aldosterone and catecholamine levels were unaffected (41, 43). The enzyme phenylethanolamine N-methyltransferase (PNMT), which is responsible for conversion of norepinephrine to epinephrine, appeared to be unaffected in Mrap−/− mice even though PNMT expression is known to be dependent on glucocorticoid action. In contrast, PNMT is markedly reduced in Mc2r−/− (41). As both knockout models are on a C57BL/6J background, one obvious difference is that the Mrap−/− mice received relatively high doses of glucocorticoids between E17.5 until weaning. This could contribute to the discrepancy in PNMT between Mc2r−/− and Mrap−/− mice, especially in light of the fact that prenatal exposure to glucocorticoids has been shown to lead to increased PNMT mRNA by RT-PCR and elevated plasma epinephrine in adult rats (44). The discrepancy between aldosterone levels in Mc2r−/− and Mrap−/− mice could be due to a number of possibilities, such as Mrap-independent aldosterone production (although in vitro the MC2R is non-functional in the absence of MRAP and MRAP protein expression closely mirrors that of MC2R in the ZG), differences in salt intake between the two models (both rodent lines are fed ad libitum on a standard chow diet although, the salt content may differ) and finally alterations of the renin–angiotensin–aldosterone system have also been reported in animals exposed to glucocorticoids prenatally (45). Determining the mechanism of such differences would further dissect adrenal ACTH action. Importantly, however, the absence of mineralocorticoid and catecholamine deficiency in Mrap−/− mice makes it a unique model for studying FGD and isolated glucocorticoid deficiency.
Similar to the Mc2r−/− mouse model, the Mrap−/− adrenal capsule is thickened, which in the Mrap−/− mice was shown to be due to an increased cell number, rather than hyperplasia. This is of particular interest as the adrenal capsule and the subcapsular region are known to contain adrenocortical stem/progenitor cells capable of dividing, migrating centripetally and differentiating into mature steroid-producing cell types (46, 47, 48). In keeping with this, the absence of MRAP and therefore ACTH signalling resulted in small adrenals with grossly deranged cortex zonation (discussed in subsequent sections).
Other MRAP mouse models: transgenic MRAP adipose tissue overexpression mouse model
Prior to its renaming in 2005, MRAP was first identified as a putative novel membrane protein selectively expressed during adipogenic conversion of 3T3-L1 cells and called FALP (fat tissue-specific low molecular weight protein) (49). The protein expressed in both brown and white fat tissue and highly expressed during adipogenesis (49, 50). More recently, the importance of MRAP in energy balance was demonstrated using transgenic mice overexpressing MRAP in a fat-specific manner, under the control of the aP2 (adipocyte fatty acid-binding protein) promoter (51). These mice were shown to be protected against diet-induced obesity and diabetes, through enhancement of ACTH-induced lipolysis. Therefore, this demonstrated for the first time the emerging importance of MRAP in metabolism.
ACTH and adrenocortical renewal and zonation – contribution of Mrap and Mc2r KO mouse models
Adrenocortical renewal and regeneration
The adrenal gland has a great capacity to respond to changes, renew and regenerate. For example, activation of the HPA axis leads to expansion of the ZF and increased expression of CYP11B1 and glucocorticoid production, whilst suppression by dexamethasone leads to the contraction/atrophy of the ZF, and reduced CYP11B1 and glucocorticoid production (52). This is not specific to the ZF and activation or inhibition of the renin–angiotensin–aldosterone system (i.e. triggered by a diet low in sodium or treatment with ACE inhibitors, respectively) results in similar changes in ZG morphology, CYP11B2 expression and aldosterone production (53). This together with the appearance of compensatory growth of the contralateral adrenal gland following unilateral adrenalectomy (54) demonstrates the dynamic nature of the adrenal gland. Apart from remodelling, the adrenal gland can also regenerate from residual adrenal capsular and adherent ZG cells in enucleation studies (55, 56). Regeneration arises from cell proliferation and differentiation and is associated with a thickened adrenal cortex (57).
Role of ACTH in adrenal progenitor cell differentiation and maintenance
ACTH administration can induce ZF hyperplasia, with no effect on the ZG (58). More recently, the localisation of Mc2r and Mrap to the undifferentiated zone (layer of cyp11b1/b2 negative cells located between the ZG and ZF) in the rat adrenal (59, 60), suggested a role for ACTH in the differentiation of progenitor cells towards the ZF phenotype in vivo. However, the data from remodelling experiments provides somewhat conflicting evidence for the exact role of ACTH in adrenal progenitor cell differentiation and maintenance. For example, some studies show that treatment with dexamethasone blocks ZF proliferation and compensatory growth of the contralateral gland following unilateral adrenalectomy (54, 61), whilst other studies do not show this (62, 63). Hypophysectomy, with the complete removal of the pituitary gland, blocks adrenal gland regeneration following enucleation (64) but does not completely block compensatory growth (52, 65), and ACTH has even been shown to inhibit this process (65). However, overall there is agreement that hypophysectomy reduces the extent of compensatory growth suggesting the presence of a pituitary factor. One possible factor is pituitary-derived N-POMC, derived from the POMC cleavage product pro-γ-MSH. Neutralising antibodies against pro-γ-MSH inhibit both adrenal regeneration and compensatory growth. However, pro-γ-MSH has no direct mitogenic activity (66); hence, it has been suggested that further processing of pro-γ-MSH to peptides such as N-POMC with mitogenic activity is required (67). Evidence of the need for pituitary factors, including ACTH, comes from work on POMC−/− animals that have complete absence of the POMC peptide (68, 69). POMC−/− mice have adrenal glands that fail to proliferate postnatally leading to atrophic adrenals which become undetectable with age (70). These adrenal glands from POMC−/− animals can be rescued in size and function, following transplantation into a WT recipient animal with physiological levels of all POMC peptides. Interestingly, N-POMC cannot restore adrenal growth in POMC-null mice (71). However, high-dose replacement of ACTH over several days restores POMC−/− adrenal weight, morphology and corticosterone secretion (68), but it has been suggested this is due to hypertrophy of the ZF rather than full regeneration of the adrenal gland (72).
Contribution of Mc2r and Mrap KO mouse models to understanding of adrenal stem/progenitor cell differentiation and maintenance
The MC2R and MRAP KO mouse models add to our existing knowledge of the role of ACTH in adrenal gland stem/progenitor cell differentiation and gland maintenance. Assessment of the adrenal glands of Mrap−/− mice at embryonic day 17.5 shows that the adrenal sizes are comparable to Mrap+/+ mice, derived from intercrosses of heterozygous mice with normal HPA activity. However, when assessed at 8 weeks of age the gland is significantly reduced in size with a thickened adrenal capsule (43). One major issue is the separation of the effects of absent ACTH action and glucocorticoid deficiency that are both present in adult Mrap−/− mice. After lifetime glucocorticoid replacement the capsule reduced in thickness but still remained significantly increased compared to wild-type littermate animals suggesting that both glucocorticoid deficiency and absent ACTH action contribute to the expansion of the stem cell niche in the adrenal gland, in keeping with some of the data described above. Treatment of WT control mice with glucocorticoid resulted in biochemically undetectable plasma ACTH coupled with corticosterone within the normal range. These mice have a thickened capsule and thus taken together this is highly suggestive that the absence of ACTH action is a key contributor to capsule thickening.
The Mrap−/− mouse model also introduces some novel concepts in the growing landscape of molecular pathways and factors involved in adrenal stem/progenitor cell determination and differentiation. In fact this field is rapidly gaining pace with the identification of new factors required for the adrenal homeostasis and zonation such as RSPO3, EZH2 and ZNRF3 (73, 74, 75) and (reviewed in 47, 53). The WNT4/β-catenin and SHH pathways have been described to be key drivers of ZG identity (76).
Cells that express WNT/β-catenin, which co-express SHH and CYP11B2, normally reside in the subcapsular region (41, 42, 43). It has been shown that cAMP/PKA, activated by ACTH signalling in the adrenal gland, represses the WNT/beta-catenin pathway to allow lineage conversion and correct adrenal cortex zonation (46). Consistent with this is the absence of ZF in the adrenals of Mrap−/− mice, whilst ZG is intact and functional. However, the Mrap−/− mice identifies a concentric zone of cells between the ZG and adrenal medulla that are negative for Cyp11b2, Cyp11b1 and 20-αHSD and hence not terminally differentiated ZG, ZF or ZR/foetal X-zone cells respectively. These cells are WNT4/β-catenin positive, but negative for the downstream canonical targets of WNT signalling (LEF1 and DAB2), suggesting that canonical WNT signalling is not active in these cells. Another option is that these cells were once LEF1- and DAB2-positive and have subsequently lost some of ZG features. The precise origin, fate and signalling potential of these cells are currently being investigated.
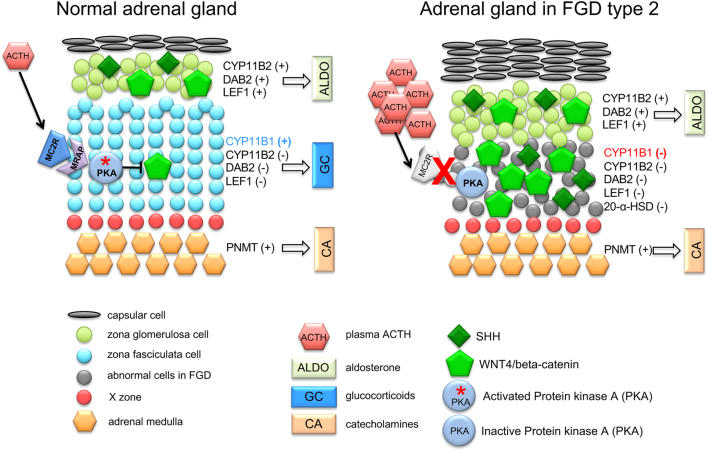
SHH signalling is another key pathway regulating the differentiation of progenitor cells into steroidogenic cells (77, 78). SHH-positive cells in the stem cell niche, which are located in the subcapsular region in mice and in the undifferentiated zone in rats, have been shown through lineage tracing to differentiate into all cortical cell populations in mice. The majority of SHH-descendants become ZG cells first and then transition to ZF cells during centripetal migration (48, 77). In SHH KO mice, the adrenal capsule is reduced to a single cell layer, suggesting that SHH could act as a capsule cell mitogen/chemoattractant for noncapsule mesenchymal cells or to maintain capsule progenitors (77). In the absence of MRAP, we see thickened capsule, upregulated SHH expression and ectopic SHH expression throughout the cortex, no longer restricted to the subscapular region. Together with the co-expression of WNT4 and CYP11B2, this is suggestive that these cells have acquired ZG features. Glucocorticoid treatment only partially attenuated this phenotype. Interestingly, the levels of Gli1, which is a canonical target of SHH, were unchanged despite high SHH expression. Determining the reason for this dissociation between high SHH and Gli1 which is unchanged as well as the co-ordination with other factors involved with capsule thickness such as FGF signalling will be investigated in the future (Fig. 1).
Figure 1.
Model illustrating the morphological alterations and pathway deregulation in the adrenal gland of FGD type 2. In the normal adrenal cortex, activation of SHH and WNT4 signalling results in driving the expression of CYP11B2, DAB2 and LEF1 leading to a zona glomerulosa (ZG) lineage. Activation of PKA due to the action of ACTH on MC2R/MRAP complex suppresses WNT4 resulting in inhibition of CYP11B2, DAB2 and LEF1 and differentiation into ZF cells. In FGD type 2 (Mrap−/− mice) adrenal glands, the lack of PKA activation results in complete absence of ZF and WNT4/SHH accumulation outside the ZG, where WNT4 expression in such cells do not lead to a functional ZG identity. Morphologically the ZG is expanded and is able to secrete aldosterone. The adrenal medulla in FGD type 2 is morphologically intact and secretes both epinephrine and norepinephrine.
In conclusion, the mouse models of FGD have helped elucidate the action of ACTH and glucocorticoids in adrenal development, progenitor cell renewal and zonation. Further studies will focus on the role of ACTH signalling in physiology as well as how this can be altered in disease states.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
The work reviewed was funded by The Medical Research Council UK (MRC/Academy of Medical Sciences Clinician Scientist Fellowship Grant G0802796 to L F Chan).
References
- 1.Sawchenko PE, Swanson LW. Localization, colocalization, and plasticity of corticotropin-releasing factor immunoreactivity in rat brain. Federation Proceedings 1985. 44 (1 Pt 2) 221-227. [PubMed] [Google Scholar]
- 2.Aguilera G, Harwood JP, Wilson JX, Morell J, Brown JH, Catt KJ. Mechanisms of action of corticotropin-releasing factor and other regulators of corticotropin release in rat pituitary cells. Journal of Biological Chemistry 1983. 258 . [PubMed] [Google Scholar]
- 3.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 1981. 213 . ( 10.1126/science.6267699) [DOI] [PubMed] [Google Scholar]
- 4.Novoselova TV, Chan LF, Clark AJL. Pathophysiology of melanocortin receptors and their accessory proteins. Best Practice and Research. Clinical Endocrinology and Metabolism 2018. 32 . ( 10.1016/j.beem.2018.02.002) [DOI] [PubMed] [Google Scholar]
- 5.Harno E, Gali Ramamoorthy T, Coll AP, White A. POMC: the physiological power of hormone processing. Physiological Reviews 2018. 98 . ( 10.1152/physrev.00024.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science 1992. 257 . ( 10.1126/science.1325670) [DOI] [PubMed] [Google Scholar]
- 7.Clark AJ, Forfar R, Hussain M, Jerman J, McIver E, Taylor D, Chan L. ACTH antagonists. Frontiers in Endocrinology 2016. 7 101 ( 10.3389/fendo.2016.00101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Ghorayeb N, Bourdeau I, Lacroix A. Role of ACTH and other hormones in the regulation of aldosterone production in primary aldosteronism. Frontiers in Endocrinology 2016. 7 72 ( 10.3389/fendo.2016.00072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funder JW. The potential of ACTH in the genesis of primary aldosteronism. Frontiers in Endocrinology 2016. 7 40 ( 10.3389/fendo.2016.00040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shepard TH, Landing BH, Mason DG. Familial Addison’s disease; case reports of two sisters with corticoid deficiency unassociated with hypoaldosteronism. AMA Journal of Diseases of Children 1959. 97 . ( 10.1001/archpedi.1959.02070010156002) [DOI] [PubMed] [Google Scholar]
- 11.Turan S, Hughes C, Atay Z, Guran T, Haliloglu B, Clark AJ, Bereket A, Metherell LA. An atypical case of familial glucocorticoid deficiency without pigmentation caused by coexistent homozygous mutations in MC2R (T152K) and MC1R (R160W). Journal of Clinical Endocrinology and Metabolism 2012. 97 E771–E774. ( 10.1210/jc.2011-2414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin L, Hindmarsh PC, Metherell LA, Alzyoud M, Al-Ali M, Brain CE, Clark AJ, Dattani MT, Achermann JC. Severe loss-of-function mutations in the adrenocorticotropin receptor (ACTHR, MC2R) can be found in patients diagnosed with salt-losing adrenal hypoplasia. Clinical Endocrinology 2007. 66 . ( 10.1111/j.1365-2265.2006.02709.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan LF, Metherell LA, Krude H, Ball C, O’Riordan SMP, Costigan C, Lynch SA, Savage MO, Cavarzere P, Clark AJL. Homozygous nonsense and frameshift mutations of the ACTH receptor in children with familial glucocorticoid deficiency (FGD) are not associated with long-term mineralocorticoid deficiency. Clinical Endocrinology 2009. 71 . ( 10.1111/j.1365-2265.2008.03511.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maharaj A, Maudhoo A, Chan LF, Novoselova T, Prasad R, Metherell LA, Guasti L. Isolated glucocorticoid Deficiency: genetic causes and animal models. Journal of Steroid Biochemistry and Molecular Biology 2019. 189 . ( 10.1016/j.jsbmb.2019.02.012) [DOI] [PubMed] [Google Scholar]
- 15.Clark AJ, McLoughlin L, Grossman A. Familial glucocorticoid deficiency associated with point mutation in the adrenocorticotropin receptor. Lancet 1993. 341 . ( 10.1016/0140-6736(93)90208-x) [DOI] [PubMed] [Google Scholar]
- 16.Clark AJ, Weber A. Adrenocorticotropin insensitivity syndromes. Endocrine Reviews 1998. 19 . ( 10.1210/edrv.19.6.0351) [DOI] [PubMed] [Google Scholar]
- 17.Metherell LA, Chapple JP, Cooray S, David A, Becker C, Ruschendorf F, Naville D, Begeot M, Khoo B, Nurnberg P, et al Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nature Genetics 2005. 37 . ( 10.1038/ng1501) [DOI] [PubMed] [Google Scholar]
- 18.Weber A, Clark AJ. Mutations of the ACTH receptor gene are only one cause of familial glucocorticoid deficiency. Human Molecular Genetics 1994. 3 . ( 10.1093/hmg/3.4.585) [DOI] [PubMed] [Google Scholar]
- 19.Chung TT, Webb TR, Chan LF, Cooray SN, Metherell LA, King PJ, Chapple JP, Clark AJ. The majority of adrenocorticotropin receptor (melanocortin 2 receptor) mutations found in familial glucocorticoid deficiency type 1 lead to defective trafficking of the receptor to the cell surface. Journal of Clinical Endocrinology and Metabolism 2008. 93 . ( 10.1210/jc.2008-1744) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elias LL, Huebner A, Metherell LA, Canas A, Warne GL, Bitti ML, Cianfarani S, Clayton PE, Savage MO, Clark AJ. Tall stature in familial glucocorticoid deficiency. Clinical Endocrinology 2000. 53 . ( 10.1046/j.1365-2265.2000.01122.x) [DOI] [PubMed] [Google Scholar]
- 21.Chung TT, Chan LF, Metherell LA, Clark AJ. Phenotypic characteristics of familial glucocorticoid deficiency (FGD) type 1 and 2. Clinical Endocrinology 2010. 72 . ( 10.1111/j.1365-2265.2009.03663.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imamine H, Mizuno H, Sugiyama Y, Ohro Y, Sugiura T, Togari H. Possible relationship between elevated plasma ACTH and tall stature in familial glucocorticoid deficiency. Tohoku Journal of Experimental Medicine 2005. 205 . ( 10.1620/tjem.205.123) [DOI] [PubMed] [Google Scholar]
- 23.Clark AJ, Weber A. Molecular insights into inherited ACTH resistance syndromes. Trends in Endocrinology and Metabolism 1994. 5 . ( 10.1016/1043-2760(94)90079-5) [DOI] [PubMed] [Google Scholar]
- 24.Bohm M, Grassel S. Role of proopiomelanocortin-derived peptides and their receptors in the osteoarticular system: from basic to translational research. Endocrine Reviews 2012. 33 . ( 10.1210/er.2011-1016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong Q, Sridhar S, Ruan L, Ding KH, Xie D, Insogna K, Kang B, Xu J, Bollag RJ, Isales CM. Multiple melanocortin receptors are expressed in bone cells. Bone 2005. 36 . ( 10.1016/j.bone.2005.01.020) [DOI] [PubMed] [Google Scholar]
- 26.Yeh JK, Evans JF, Niu QT, Aloia JF. A possible role for melanocortin peptides in longitudinal growth. Journal of Endocrinology 2006. 191 . ( 10.1677/joe.1.06729) [DOI] [PubMed] [Google Scholar]
- 27.Weber A, Clark AJ, Perry LA, Honour JW, Savage MO. Diminished adrenal androgen secretion in familial glucocorticoid deficiency implicates a significant role for ACTH in the induction of adrenarche. Clinical Endocrinology 1997. 46 . ( 10.1046/j.1365-2265.1997.1580969.x) [DOI] [PubMed] [Google Scholar]
- 28.Lappalainen S, Utriainen P, Kuulasmaa T, Voutilainen R, Jaaskelainen J. ACTH receptor promoter polymorphism associates with severity of premature adrenarche and modulates hypothalamo-pituitary-adrenal axis in children. Pediatric Research 2008. 63 . ( 10.1203/PDR.0b013e3181659c14) [DOI] [PubMed] [Google Scholar]
- 29.Dumontet T, Sahut-Barnola I, Septier A, Montanier N, Plotton I, Roucher-Boulez F, Ducros V, Lefrancois-Martinez AM, Pointud JC, Zubair M, et al PKA signaling drives reticularis differentiation and sexually dimorphic adrenal cortex renewal. JCI Insight 2018. 3 e98394 ( 10.1172/jci.insight.98394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan LF, Webb TR, Chung TT, Meimaridou E, Cooray SN, Guasti L, Chapple JP, Egertova M, Elphick MR, Cheetham ME, et al MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. PNAS 2009. 106 . ( 10.1073/pnas.0809918106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webb TR, Chan L, Cooray SN, Cheetham ME, Chapple JP, Clark AJ. Distinct melanocortin 2 receptor accessory protein domains are required for melanocortin 2 receptor interaction and promotion of receptor trafficking. Endocrinology 2009. 150 . ( 10.1210/en.2008-0941) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooray SN, Almiro Do Vale I, Leung KY, Webb TR, Chapple JP, Egertova M, Cheetham ME, Elphick MR, Clark AJ. The melanocortin 2 receptor accessory protein exists as a homodimer and is essential for the function of the melanocortin 2 receptor in the mouse y1 cell line. Endocrinology 2008. 149 . ( 10.1210/en.2007-1463) [DOI] [PubMed] [Google Scholar]
- 33.Metherell LA, Chan LF, Clark AJ. The genetics of ACTH resistance syndromes. Best Practice and Research. Clinical Endocrinology and Metabolism 2006. 20 . ( 10.1016/j.beem.2006.09.002) [DOI] [PubMed] [Google Scholar]
- 34.Chan LF, Clark AJ, Metherell LA. Familial glucocorticoid deficiency: advances in the molecular understanding of ACTH action. Hormone Research 2008. 69 . ( 10.1159/000111810) [DOI] [PubMed] [Google Scholar]
- 35.Guran T, Buonocore F, Saka N, Ozbek MN, Aycan Z, Bereket A, Bas F, Darcan S, Bideci A, Guven A, et al Rare causes of primary adrenal insufficiency: genetic and clinical characterization of a large nationwide cohort. Journal of Clinical Endocrinology and Metabolism 2016. 101 . ( 10.1210/jc.2015-3250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy S, Rached M, Gallo-Payet N. Differential regulation of the human adrenocorticotropin receptor [melanocortin-2 receptor (MC2R)] by human MC2R accessory protein isoforms alpha and beta in isogenic human embryonic kidney 293 cells. Molecular Endocrinology 2007. 21 . ( 10.1210/me.2007-0041) [DOI] [PubMed] [Google Scholar]
- 37.Sebag JA, Hinkle PM. Melanocortin-2 receptor accessory protein MRAP forms antiparallel homodimers. PNAS 2007. 104 . ( 10.1073/pnas.0708916105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooray SN, Chung TT, Mazhar K, Szidonya L, Clark AJ. Bioluminescence resonance energy transfer reveals the adrenocorticotropin (ACTH)-induced conformational change of the activated ACTH receptor complex in living cells. Endocrinology 2011. 152 . ( 10.1210/en.2010-1053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malik S, Dolan TM, Maben ZJ, Hinkle PM. Adrenocorticotropic hormone (ACTH) responses require actions of the Melanocortin-2 receptor accessory protein on the extracellular surface of the plasma membrane. Journal of Biological Chemistry 2015. 290 . ( 10.1074/jbc.M115.668491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maben ZJ, Malik S, Jiang LH, Hinkle PM. Dual topology of the Melanocortin-2 receptor accessory protein is stable. Frontiers in Endocrinology 2016. 7 96 ( 10.3389/fendo.2016.00096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chida D, Nakagawa S, Nagai S, Sagara H, Katsumata H, Imaki T, Suzuki H, Mitani F, Ogishima T, Shimizu C, et al Melanocortin 2 receptor is required for adrenal gland development, steroidogenesis, and neonatal gluconeogenesis. PNAS 2007. 104 . ( 10.1073/pnas.0706953104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chida D, Sato T, Sato Y, Kubo M, Yoda T, Suzuki H, Iwakura Y. Characterization of mice deficient in melanocortin 2 receptor on a B6/Balbc mix background. Molecular and Cellular Endocrinology 2009. 300 . ( 10.1016/j.mce.2008.10.027) [DOI] [PubMed] [Google Scholar]
- 43.Novoselova TV, Hussain M, King PJ, Guasti L, Metherell LA, Charalambous M, Clark AJL, Chan LF. MRAP deficiency impairs adrenal progenitor cell differentiation and gland zonation. FASEB Journal 2018. 32 fj201701, 274RR ( 10.1096/fj.201701274RR) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen P, Khurana S, Peltsch H, Grandbois J, Eibl J, Crispo J, Ansell D, Tai TC. Prenatal glucocorticoid exposure programs adrenal PNMT expression and adult hypertension. Journal of Endocrinology 2015. 227 . ( 10.1530/JOE-15-0244) [DOI] [PubMed] [Google Scholar]
- 45.Waddell BJ, Bollen M, Wyrwoll CS, Mori TA, Mark PJ. Developmental programming of adult adrenal structure and steroidogenesis: effects of fetal glucocorticoid excess and postnatal dietary omega-3 fatty acids. Journal of Endocrinology 2010. 205 . ( 10.1677/JOE-09-0459) [DOI] [PubMed] [Google Scholar]
- 46.Drelon C, Berthon A, Sahut-Barnola I, Mathieu M, Dumontet T, Rodriguez S, Batisse-Lignier M, Tabbal H, Tauveron I, Lefrancois-Martinez AM, et al PKA inhibits WNT signalling in adrenal cortex zonation and prevents malignant tumour development. Nature Communications 2016. 7 12751 ( 10.1038/ncomms12751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lerario AM, Finco I, LaPensee C, Hammer GD. Molecular mechanisms of stem/progenitor cell maintenance in the adrenal cortex. Frontiers in Endocrinology 2017. 8 52 ( 10.3389/fendo.2017.00052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freedman BD, Kempna PB, Carlone DL, Shah M, Guagliardo NA, Barrett PQ, Gomez-Sanchez CE, Majzoub JA, Breault DT. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Developmental Cell 2013. 26 . ( 10.1016/j.devcel.2013.07.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu A, Choi KL, Wang Y, Permana PA, Xu LY, Bogardus C, Cooper GJ. Identification of novel putative membrane proteins selectively expressed during adipose conversion of 3T3-L1 cells. Biochemical and Biophysical Research Communications 2002. 293 . ( 10.1016/S0006-291X(02)00354-6) [DOI] [PubMed] [Google Scholar]
- 50.Menssen A, Haupl T, Sittinger M, Delorme B, Charbord P, Ringe J. Differential gene expression profiling of human bone marrow-derived mesenchymal stem cells during adipogenic development. BMC Genomics 2011. 12 461 ( 10.1186/1471-2164-12-461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X, Saarinen AM, Campbell LE, De Filippis EA, Liu J. Regulation of lipolytic response and energy balance by melanocortin 2 receptor accessory protein (MRAP) in adipocytes. Diabetes 2018. 67 . ( 10.2337/db17-0862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dallman MF. Control of adrenocortical growth in vivo. Endocrine Research 1984-1985. 10 . ( 10.1080/07435808409036499) [DOI] [PubMed] [Google Scholar]
- 53.Yates R, Katugampola H, Cavlan D, Cogger K, Meimaridou E, Hughes C, Metherell L, Guasti L, King P. Adrenocortical development, maintenance, and disease. Current Topics in Developmental Biology 2013. 106 . ( 10.1016/B978-0-12-416021-7.00007-9) [DOI] [PubMed] [Google Scholar]
- 54.Engeland WC, Ennen WB, Elayaperumal A, Durand DA, Levay-Young BK. Zone-specific cell proliferation during compensatory adrenal growth in rats. American Journal of Physiology. Endocrinology and Metabolism 2005. 288 E298–E306. ( 10.1152/ajpendo.00307.2004) [DOI] [PubMed] [Google Scholar]
- 55.Bland ML, Desclozeaux M, Ingraham HA. Tissue growth and remodeling of the embryonic and adult adrenal gland. Annals of the New York Academy of Sciences 2003. 995 . ( 10.1111/j.1749-6632.2003.tb03210.x) [DOI] [PubMed] [Google Scholar]
- 56.Feuerstein B, Streeten DH. Recovery of adrenal function after failure resulting from traumatic bilateral adrenal hemorrhages. Annals of Internal Medicine 1991. 115 . ( 10.7326/0003-4819-115-10-785) [DOI] [PubMed] [Google Scholar]
- 57.Skelton FR. Adrenal regeneration and adrenal-regeneration hypertension. Physiological Reviews 1959. 39 . ( 10.1152/physrev.1959.39.1.162) [DOI] [PubMed] [Google Scholar]
- 58.McEwan PE, Lindop GB, Kenyon CJ. Control of cell proliferation in the rat adrenal gland in vivo by the renin-angiotensin system. American Journal of Physiology 1996. 271 E192–E198. ( 10.1152/ajpendo.1996.271.1.E192) [DOI] [PubMed] [Google Scholar]
- 59.Gorrigan RJ, Guasti L, King P, Clark AJ, Chan LF. Localisation of the melanocortin-2-receptor and its accessory proteins in the developing and adult adrenal gland. Journal of Molecular Endocrinology 2011. 46 . ( 10.1530/JME-11-0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guasti L, Cavlan D, Cogger K, Banu Z, Shakur A, Latif S, King PJ. Dlk1 up-regulates Gli1 expression in male rat adrenal capsule cells through the activation of beta1 integrin and ERK1/2. Endocrinology 2013. 154 . ( 10.1210/en.2013-1211) [DOI] [PubMed] [Google Scholar]
- 61.Phillips R, Crock C, Funder J. Effects of mineralocorticoids and glucocorticoids on compensatory adrenal growth in rats. American Journal of Physiology 1985. 248 E450–E456. ( 10.1152/ajpendo.1985.248.4.E450) [DOI] [PubMed] [Google Scholar]
- 62.Engeland WC, Dallman MF. Compensatory adrenal growth is neurally mediated. Neuroendocrinology 1975. 19 . ( 10.1159/000122456) [DOI] [PubMed] [Google Scholar]
- 63.Grizzle WE, Dunlap NE. Aldosterone blocks adrenal compensatory hypertrophy in the rat. American Journal of Physiology 1984. 246 E306–E310. ( 10.1152/ajpendo.1984.246.4.E306) [DOI] [PubMed] [Google Scholar]
- 64.Estivariz FE, Carino M, Lowry PJ, Jackson S. Further evidence that N-terminal pro-opiomelanocortin peptides are involved in adrenal mitogenesis. Journal of Endocrinology 1988. 116 . ( 10.1677/joe.0.1160201) [DOI] [PubMed] [Google Scholar]
- 65.Dallman MF, Engeland WC, Holzwarth MA, Scholz PM. Adrenocorticotropin inhibits compensatory adrenal growth after unilateral adrenalectomy. Endocrinology 1980. 107 . ( 10.1210/endo-107-5-1397) [DOI] [PubMed] [Google Scholar]
- 66.Lowry PJ, Silas L, McLean C, Linton EA, Estivariz FE. Pro-gamma-melanocyte-stimulating hormone cleavage in adrenal gland undergoing compensatory growth. Nature 1983. 306 . ( 10.1038/306070a0) [DOI] [PubMed] [Google Scholar]
- 67.Estivariz FE, Iturriza F, McLean C, Hope J, Lowry PJ. Stimulation of adrenal mitogenesis by N-terminal proopiocortin peptides. Nature 1982. 297 . ( 10.1038/297419a0) [DOI] [PubMed] [Google Scholar]
- 68.Coll AP, Challis BG, Yeo GS, Snell K, Piper SJ, Halsall D, Thresher RR, O’Rahilly S. The effects of proopiomelanocortin deficiency on murine adrenal development and responsiveness to adrenocorticotropin. Endocrinology 2004. 145 . ( 10.1210/en.2004-0491) [DOI] [PubMed] [Google Scholar]
- 69.Karpac J, Ostwald D, Bui S, Hunnewell P, Shankar M, Hochgeschwender U. Development, maintenance, and function of the adrenal gland in early postnatal proopiomelanocortin-null mutant mice. Endocrinology 2005. 146 . ( 10.1210/en.2004-1290) [DOI] [PubMed] [Google Scholar]
- 70.Karpac J, Kern A, Hochgeschwender U. Pro-opiomelanocortin peptides and the adrenal gland. Molecular and Cellular Endocrinology 2007. 265–266 . ( 10.1016/j.mce.2006.12.035) [DOI] [PubMed] [Google Scholar]
- 71.Coll AP, Fassnacht M, Klammer S, Hahner S, Schulte DM, Piper S, Tung YC, Challis BG, Weinstein Y, Allolio B. Peripheral administration of the N-terminal pro-opiomelanocortin fragment 1–28 to Pomc-/- mice reduces food intake and weight but does not affect adrenal growth or corticosterone production. Journal of Endocrinology 2006. 190 . ( 10.1677/joe.1.06749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karpac J, Czyzewska K, Kern A, Brush RS, Anderson RE, Hochgeschwender U. Failure of adrenal corticosterone production in POMC-deficient mice results from lack of integrated effects of POMC peptides on multiple factors. American Journal of Physiology. Endocrinology and Metabolism 2008. 295 E446–E455. ( 10.1152/ajpendo.00762.2007) [DOI] [PubMed] [Google Scholar]
- 73.Vidal V, Sacco S, Rocha AS, da Silva F, Panzolini C, Dumontet T, Doan TM, Shan J, Rak-Raszewska A, Bird T, et al The adrenal capsule is a signaling center controlling cell renewal and zonation through Rspo3. Genes and Development 2016. 30 . ( 10.1101/gad.277756.116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mathieu M, Drelon C, Rodriguez S, Tabbal H, Septier A, Damon-Soubeyrand C, Dumontet T, Berthon A, Sahut-Barnola I, Djari C, et al Steroidogenic differentiation and PKA signaling are programmed by histone methyltransferase EZH2 in the adrenal cortex. PNAS 2018. 115 E12265–E12274. ( 10.1073/pnas.1809185115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Basham KJ, Rodriguez S, Turcu AF, Lerario AM, Logan CY, Rysztak MR, Gomez-Sanchez CE, Breault DT, Koo BK, Clevers H, et al A ZNRF3-dependent Wnt/beta-catenin signaling gradient is required for adrenal homeostasis. Genes and Development 2019. 33 . ( 10.1101/gad.317412.118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Finco I, Lerario AM, Hammer GD. Sonic hedgehog and WNT signaling promote adrenal gland regeneration in male mice. Endocrinology 2018. 159 . ( 10.1210/en.2017-03061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.King P, Paul A, Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. PNAS 2009. 106 . ( 10.1073/pnas.0909471106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guasti L, Paul A, Laufer E, King P. Localization of Sonic hedgehog secreting and receiving cells in the developing and adult rat adrenal cortex. Molecular and Cellular Endocrinology 2011. 336 . ( 10.1016/j.mce.2010.11.010) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a