Abstract
Background
The 24-h urinary output of 5-hydroxyindoleacetic acid (5-HIAA) is used to monitor disease progression and treatment responses of neuroendocrine neoplasms (NENs). Several conditions are required for 5-HIAA assay, involving urine collection/preservation and food/drug restrictions.
Aim
To evaluate the correlation between 5-HIAA concentration in a spot urine sample and the output in a 24-h urine collection, and whether spot urine specimens can replace 24-h collection.
Methods
Patients with NENs or symptoms suggestive of NENs were asked to provide a separate spot urine at the end of the 24-h urine collection for 5-HIAA assessment. The upper reference limit for 24-h urinary 5-HIAA was 40 µmol/24 h. 5-HIAA measurements in spot urine samples were corrected for variation in urine flow rate by expressing results as a ratio to creatinine concentration.
Results
We included 136 paired urinary samples for 5-HIAA assessment from 111 patients (100 NENs). The correlation between 5-HIAA values measured in 24-h and spot urines was r = +0.863 (P < 0.001) and r = +0.840 (P < 0.001) including only NEN patients. Using the 24-h urinary 5-HIAA as reference method, the AUC on ROC analysis for spot urinary 5-HIAA was 0.948 (95% CI, 0.914–0.983; P < 0.001), attaining a sensitivity of 83% and specificity of 95% using 5.3 mol/mmol as cut-off for the spot urine. The AUC among NEN patients alone was 0.945 (95% CI, 0.904–0.987; P < 0.001).
Conclusions
The ratio of 5-HIAA to creatinine in a spot urine could replace the measurement of 5-HIAA output in a 24-h urine collection, especially for follow-up of patients with known elevated 5-HIAA levels.
Keywords: 5-HIAA, 5-hydroxyindoleacetic acid, carcinoid syndrome, neuroendocrine tumours, carcinoid heart
Introduction
The incidence and prevalence of neuroendocrine neoplasms (NENs) has increased in recent years (1, 2). Although improvements in diagnostic techniques and treatment modalities have led to improved survival and health-related quality of life in patients with NENs (3, 4, 5), the clinical management of this heterogeneous family of tumours remains challenging. The identification of accurate markers to define disease status and therapeutic efficacy is a critical requirement for accurate diagnosis and effective management, particularly in the long-term follow-up of patients whose life expectancy may exceed 10 years (2). Indeed, NENs are usually slow-growing tumours and the majority are asymptomatic or cause few symptoms until they are large or have metastasised. However, up to 40% of patients, mainly with midgut carcinoids, present with features of the carcinoid syndrome (CS) (6), and undergo episodes of diarrhoea and flushing and occasionally asthma. Carcinoid heart disease has been described in approximately 60% of patients with CS, typically inducing abnormalities of the right side of the heart, although in many cases patients remain asymptomatic for a prolonged period (7, 8), and many patients also undergo mesenteric fibrosis (9, 10).
Serotonin is one of the most important biomarkers related to the development of CS, mesenteric fibrosis and carcinoid heart disease. Serotonin is produced in large amounts by NEN cells, most commonly in patients with NENs in the small intestine with multiple liver metastases, and less frequently in patients with lung NENs. More rarely, patients with direct tumour drainage into the central circulation such as ovarian carcinoids (11), or with widespread peritoneal disease, may also present with CS. Serotonin is a tryptophan-derived biogenic amine which is synthesised and stored mainly in enterochromaffin cells of the gastrointestinal tract and also in dense granules of platelets (storage only), and in the serotoninergic neurons of the central nervous system. Serotonin remains free in the plasma and is converted into the metabolite 5-hydroxyindolacetic acid (5-HIAA) by the enzymes monoamine oxidase and aldehyde dehydrogenase.
Serotonin may be assayed either free in plasma or in platelets, but it is unstable and specimens require special handling. By contrast, 5-HIAA is much more stable and can be assayed in serum and plasma (12, 13, 14, 15), but measurement of excretion in a 24-h urine specimen is still the most widely used and recommended assay for the diagnosis of CS and follow-up of NENs with CS (16, 17, 18). However, several conditions are required for optimal urinary 5-HIAA assay, particularly involving urine collection and preservation, and food and drug-related issues (18). Urine should be collected and measured in plastic containers, and while 5-HIAA is relatively stable, it tends to degrade during and after a 24-h urine collection unless preservatives such as acetic acid are added to the sample. The sample should also be stored in a refrigerator until analysis and should be protected from light. Furthermore, collecting all the urine passed over 24 h can be challenging to the patient; despite most patients being given written instructions, many specimens are demonstrably either over- or under-collected. Consumption of foods rich in dietary tryptophan (nuts, coffee, banana, chocolate, tea, pineapple, etc.) (19, 20) may lead to a false-positive result, while certain medications may give false-positive or -negative results (18). Therefore, patients should abstain from these foods and drugs for 3 days prior to and during the urinary collection (18). Finally, unless patients are provided with a collection container at a previous out-patient visit, the return of the collection will require additional travel and inconvenience.
Thus, collecting a 24-h urine specimen for 5-HIAA is cumbersome and prone to errors that may affect the accuracy of the diagnosis, and certainly it is inconvenient for patients. Using a ‘spot’ (random) urine sample for the assessment of 5-HIAA and relating it to the concentration of creatinine could be a convenient and more acceptable assessment of 5-HIAA production. The aim of the present study was to evaluate the correlation between the 5-HIAA concentration in a spot urine sample with the 24-h urine collection and to explore whether this could be an alternative assessment technique.
Methods and patients
The study was carried out at the European Neuroendocrine Tumor Society (ENETS) Centre of Excellence Multidisciplinary Group for NETs, Oxford University Hospitals, NHS Trust, between January 2016 and January 2017.
Patients with NENs or symptoms suggestive of CS for whom a 24-h urine collection for 5-HIAA assessment was recommended were asked to provide a separate spot urine taken at the end of their collection period. A total of 115 patients participated in the study. Four patients were excluded because the 24-h urine collection was not performed or partially completed, as reported by the patients. The mean age of the population was 65 years (range 20–89 years, s.d. 14.3) and 57% (63/111) were male. Of the 111 patients, 100 had a diagnosis of NEN. The diagnosis of NEN was based on histological evidence where available, otherwise the diagnosis was based on the characteristic appearances on cross-sectional imaging (computed tomography and/or magnetic resonance) and functional (111In-Octreotide scan) imaging. Written instructions were given about the conditions required for optimal 24-h urine collection and preservation for 5-HIAA assessment, and regarding food restrictions and treatments (18) being avoided for 3 days prior to and during the 24-h urine collection period. Patients were provided with a container for the 24-h urine collection to which acetic acid had been added as preservative, and a small 12 mL container for the spot urine collection. No extra samples were collected for the study; therefore, the data analysis was registered and approved as a service evaluation using the Oxford University Hospitals NHS Foundation Trust governance register as CSS-BIO-5 5435. All the patients asked to participate gave their informed consent.
Analyses of urinary 5-HIAA were performed centrally at the Department of Clinical Biochemistry, John Radcliffe Hospital, Oxford University Hospitals NHS Trust. Both 24-h and spot urinary specimens were protected from light, processed and frozen on the day of receipt, and the spot urine specimens were acidified. Laboratory staff were blinded to the study endpoints.
Urinary 5-HIAA excretion was analysed by a mass spectrometric method (21) and expressed as µmol/24 h. The coefficient of variation (CV) was 7.6% at 20.1 mmol/L and 4.6% at 130 mmol/L. The upper limit of normal (ULN) used for 24 h urinary 5-HIAA was 40 µmol/24 h (22). 5-HIAA measurements in the spot urine were corrected for variation in urine flow rate by expressing results as a ratio to creatinine concentration. Creatinine was measured by an enzymatic method (Abbott UK) with CV 0.86–0.98% at 7.2 mmol/L and 0.57–0.86% at 21.8 mmol/L.
Data are presented as mean, standard deviations (s.d.) and median values or interquartile range (IQR, 25th and 75th percentile). Correlations between 5-HIAA measured in 24-h and spot urine collections were tested using Spearman’s correlation coefficient (r). A P value <0.05 was considered statistically significant. A scatter plot graph was plotted to show correlations. As urinary 5-HIAA values were not normally distributed, the values were plotted using a logarithmic scale. Using 24-h urinary 5-HIAA as the ‘gold standard’ with values above 40 µmol/24 h as abnormal, the sensitivity and specificity of the spot urine 5-HIAA was evaluated and receiver operator characteristic (ROC) curve analysis was performed to assess the overall accuracy.
All statistical analyses were performed with SPSS statistical software version 23.0 (SPSS Inc.).
Results
A total of 136 paired urinary samples for 5-HIAA assessment from 100 patients with NENs and 11 patients without a NEN diagnosis were included in the study. It was possible to determine the exact anatomical location of the primary tumour in 73/100 patients: most were small intestine (n = 51), pancreatic (n = 10) or lung NENs (n = 5), with the remaining tumours in the ovary (n = 2), appendix (n = 3), rectum (n = 1) or Meckel’s diverticulum (n = 1). Hepatic metastases were found in 62/100 patients and 60% were on somatostatin analogues.
In total, 78/136 (57.3%) measurements of 5-HIAA from 24-h urine collection were >40 µmol/24 h. The median concentration of 5-HIAA measured in the 24-h urine collection was 50.5 µmol/24 h (IQR 26.75–145.5). The median concentration of 5-HIAA measured in the spot urine was 4.9 mol/mmol (IQR 2.26–16.4).
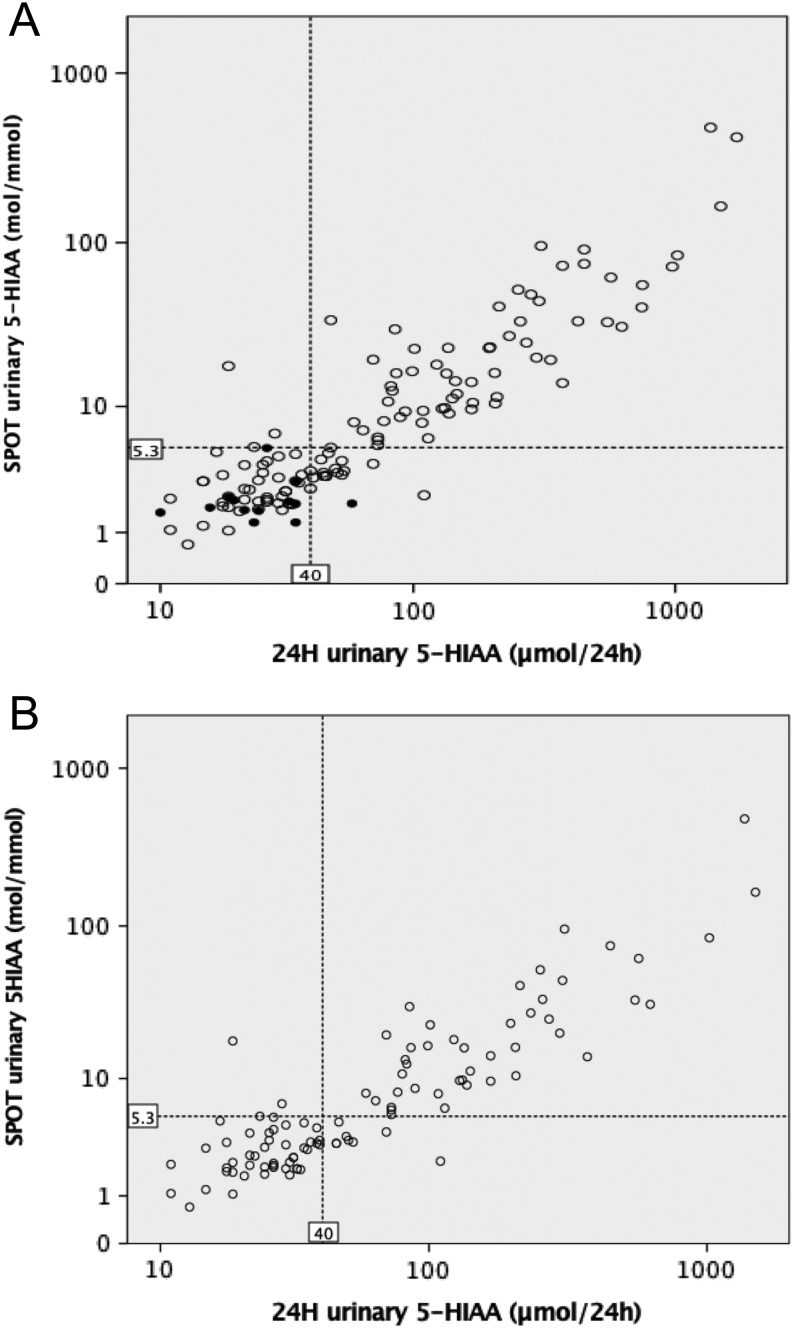
Spearman’s correlation between the 5-HIAA values measured in the 24 h urine and the spot urine was r = +0.863 (P < 0.001) (Fig. 1A). Including only the values of one sample per patient in the group of patients with NENs, the correlation was r = +0.840 (P < 0.001) (Fig. 1B).
Figure 1.
Correlation between the 5-HIAA values measured in the 24-h urine and the spot urine for the total number of measurements in patients with and without a NEN diagnosis (A) and including one sample per patient from the group of patients with NEN (B). 5-HIAA values are plotted using logarithmic (log10) scale. The upper normal range for 24-h urinary 5-HIAA of 40 µmol/24 h and the suggested cut-off for the spot urinary 5-HIAA of 5.3 mol/mmol are shown by dashed lines. The white and black circles represent the measurements of patients, respectively, with and without a NEN diagnosis.
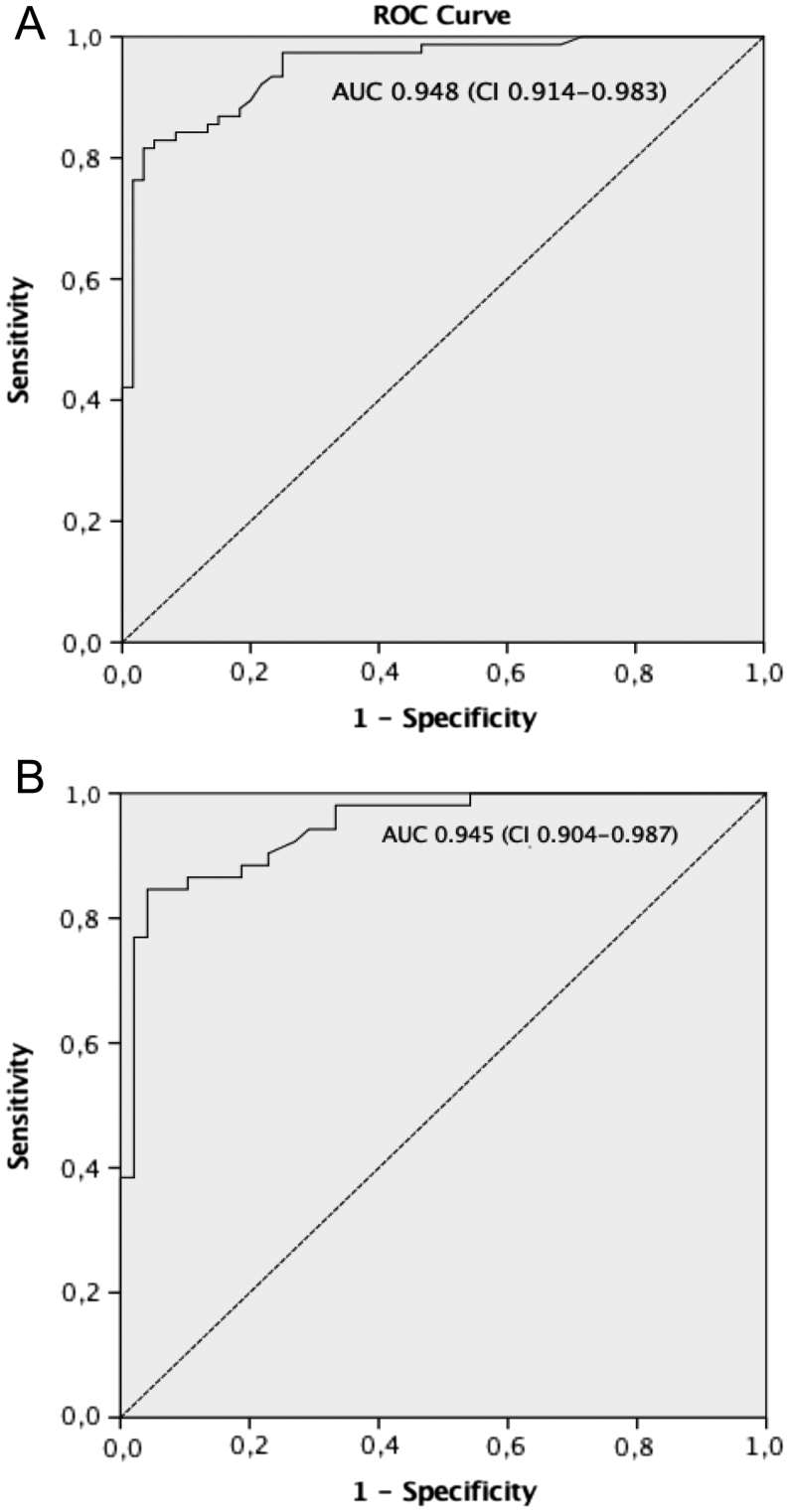
Using the 24-h urine collection as a gold standard for 5-HIAA assessment with an upper normal value of 40 µmol/24 h, the ROC curve analysis for spot urinary 5-HIAA gave an area under the curve (AUC) of 0.948 (95% CI, 0.914–0.983; P < 0.001) (Fig. 2A). Considering only the patients with NEN and including only a single sample per patient, the AUC was 0.945 (95% confidence interval, 0.904–0.987; P < 0.001) (Fig. 2B).
Figure 2.
ROC curve for spot urine 5-HIAA. The 24 h urine collection for 5-HIAA assessment (ULN 40 µmol/24) was used as the reference method. (A) ROC curve analysis for the total number of urinary 5-HIAA measurements (136 measurements). (B) ROC curve analysis including one sample per patient among patients with a diagnosis of NEN (100 measurements). The area under the ROC curve (AUC) and 95% confidence interval (CI) are reported.
Based on the ROC analysis of 5-HIAA levels from patients both with NEN and without a diagnosis of NEN, a sensitivity of 83% and specificity of 95% was attained using 5.3 mol/mmol as cut-off point for the spot urine (Table 1A). Of patients with NENs, using the above cut-off point for the spot urine, a sensitivity of 85% and specificity of 94% was found (Table 1B). Eight patients with NEN had levels of spot urine 5-HIAA below 5.3 mol/mmol (median 3.4, range 2.3–4.8 mol/mmol) in the presence of slightly increased 24-h urinary 5-HIAA (median 52, range 46–110, µmol/24 h). Of those patients, seven had small intestine NENs and one patient had an NEN of unknown primary site.
Table 1.
Sensitivity and specificity of the spot urinary 5-HIAA at different cut-off levels for the assessment of 5-HIAA, using the 5-HIAA measured in the 24-h urine collection as reference method (ULN 40 µmol/24).
| Cut-off value of spot urine 5-HIAA | Sensitivity (%) | Specificity (%) |
|---|---|---|
| (A) | ||
| 1.95 | 100 | 28 |
| 3.3 | 95 | 75 |
| 3.5 | 89 | 80 |
| 4.2 | 85 | 87 |
| 5.3 | 83 | 95 |
| 5.7 | 80 | 97 |
| 8.7 | 70 | 98 |
| 18.2 | 42 | 100 |
| (B) | ||
| 1.95 | 100 | 24 |
| 3.3 | 94 | 69 |
| 3.5 | 89 | 75 |
| 4.2 | 86 | 82 |
| 5.3 | 85 | 94 |
| 5.7 | 83 | 96 |
| 8.7 | 67 | 98 |
| 18.2 | 38 | 100 |
(A) Sensitivity and specificity results of the analysis of the total number of urinary 5-HIAA measurements (136). (B) Sensitivity and specificity results of the analysis of urinary 5-HIAA measurements in the group of patients with NEN (100).
Discussion
Depending on the site of origin, NENs can give rise to excessive synthesis, storage and release of serotonin, which can cause CS and carcinoid heart disease, representing a major cause of morbidity and mortality for patients with NENs (22, 23). The metabolite of serotonin, 5-HIAA, is the most frequently requested assay in the clinical setting of the CS, with a published sensitivity of 70% and a specificity of 90% (16, 17).
Serum and plasma samples for 5-HIAA measurement correlate well with urine 5-HIAA (12, 13, 14, 15), and both tests are increasingly regarded as a tool for diagnosing and the monitoring of patients with NENs (18, 24). At present, the European Neuroendocrine Tumour Society (18), the UK and Ireland Neuroendocrine Tumour Society (25) and the North American Neuroendocrine Tumour Society (26) all recommend urinary 5-HIAA assessment for the diagnosis and management of patients with syndromic NENs. Interestingly, it has been recently found that patients with NENs without CS symptoms may still secrete substantial quantities of 5-HIAA (27). The utility of 5-HIAA as a prognostic marker of survival is under debate (28, 29, 30, 31, 32).
This study shows, for the first time, that a spot urine 5-HIAA concentrations related to creatinine concentrations has a very high correlation compared with the current standard measurement of 5-HIAA in the 24-h urine collection. A cut-off of 5-HIAA measured in spot urine of 5.3 mol/mmol showed a high sensitivity and specificity compared with the ULN value of 5-HIAA measured in the 24-h urine collection. Two previous studies have suggested that a collection interval of less than 24 h might be sufficient to give a representative picture of serotonin levels. In 26 patients with metastatic carcinoid, Zuetenhorst and colleagues found an overnight collection interval (~8 h) to be highly correlated to 24 h 5-HIAA excretion values (33). More recently, a significant correlation between 24 h and overnight urinary 5-HIAA levels was found in a cohort of 34 patients with NENs (34).
Measuring 5-HIAA in a spot urine would have several advantages. First, it eliminates errors related to over-collection and under-collection of urine. It is not uncommon that patients report not having collected all the urine required for various reasons. For example, the 24 h urine collection can be unreliable in case of severe diarrhoea, often seen in patients with CS. Secondly, because of the shorter delay in the specimen arriving in the laboratory, measuring 5-HIAA in a spot urine assay would be less affected by errors related to incorrect exposure of the urine bottle (and for some patients more than one bottle) to temperatures >8°C and excessive light.
From a laboratory point of view, measuring 5-HIAA levels in small urine containers means that they are easier to protect from light as spot urines are easier to keep covered. In addition, it saves staff time for weighing the 24-h urine collection and recording it. Finally, spot urine measurements for 5-HIAA would represent a less expensive option. All these considerations become more important considering that the number of requests for urinary 5-HIAA have increased over time; for instance, in our laboratory these have increased from 471 in 2008 to 1074 in 2018, and we now analyse approximately 20 samples per week.
Ultimately, using a spot urine specimen for 5-HIAA measurement will avoid the necessity for troublesome and an unpleasant 24-h urine collection. For most patients, such collections are time consuming and embarrassing, and a simple urine spot assay would increase patient satisfaction and potentially compliance in obtaining specimens for 5-HIAA assessment. Furthermore, as patients are increasingly seen and monitored in ‘Centres of Excellence’, where assessment and treatment facilities can be maximised, they may need to travel long distances and bring bulky urine containers, especially when they have to be specifically returned, which is an additional patient burden. Indeed, the decreased compliance to the standard urinary 5-HIAA assay for follow-up monitoring is likely to reflect the impact on quality of life resulting from a 24-h urine collection (35). This patient-centric issue is especially important in a slow-growing disease for which patients may have many years of follow-up.
This study has some limitations. Due to the nature of the study, only a small number of patients without a diagnosis of NEN were included. Additionally, the majority of the patients with NEN were on treatment with somatostatin analogues which had likely contributed to decrease the urinary 5-HIAA levels. Thus, we were unable to test the diagnostic sensitivity and specificity of spot urinary 5-HIAA per se and its utility in determining the prognosis and the severity of the CS. However, using the 24-h urinary 5-HIAA as reference method for plotting the ROC curve analysis, we found that the spot urinary 5-HIAA has excellent favourable accuracy characteristics, although in general urinary 5-HIAA is not an entirely reliable prognostic factor (28, 29, 30, 31, 32).
In conclusion, this study shows that the ratio of 5-HIAA to creatinine concentration in a spot urine correlates well with 5-HIAA output in a 24-h urine specimen. Using spot urine samples for 5-HIAA quantification is desirable and would be beneficial to both patients and clinicians. We suggest using the spot urine assay for 5-HIAA analysis, in particular for follow-up in patients with known elevated 5-HIAA levels.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any agency in the public, commercial or not-for-profit sector.
Acknowledgement
The authors thank the patients for participating in the study.
References
- 1.Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015. 121 . ( 10.1002/cncr.29099) [DOI] [PubMed] [Google Scholar]
- 2.Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncology 2017. 3 . ( 10.1001/jamaoncol.2017.0589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003. 97 . ( 10.1002/cncr.11105) [DOI] [PubMed] [Google Scholar]
- 4.Shen C, Dasari A, Chu Y, Halperin DM, Zhou S, Xu Y, Shih YT, Yao JC. Clinical, pathological, and demographic factors associated with development of recurrences after surgical resection in elderly patients with neuroendocrine tumors. Annals of Oncology 2017. 28 . ( 10.1093/annonc/mdx164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyar Cetinkaya R, Aagnes B, Myklebust TÅ, Thiis-Evensen E. Survival in neuroendocrine neoplasms: a report from a large Norwegian population-based study. International Journal of Cancer 2018. 142 . ( 10.1002/ijc.31137) [DOI] [PubMed] [Google Scholar]
- 6.Halperin DM, Shen C, Dasari A, Xu Y, Chu Y, Zhou S, Shih YT, Yao JC. Frequency of carcinoid syndrome at neuroendocrine tumor diagnosis: a population-based study. Lancet: Oncology 2017. 18 . ( 10.1016/S1470-2045(17)30110-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grozinsky-Glasberg S, Grossman AB, Gross DJ. Carcinoid heart disease: from pathophysiology to treatment – ‘something in the way it moves’. Neuroendocrinology 2015. 101 . ( 10.1159/000381930) [DOI] [PubMed] [Google Scholar]
- 8.Davar J, Connolly HM, Caplin ME, Pavel M, Zacks J, Bhattacharyya S, Cuthbertson DJ, Dobson R, Grozinsky-Glasberg S, Steeds RP, et al Diagnosing and managing carcinoid heart disease in patients with neuroendocrine tumors: an expert statement. Journal of the American College of Cardiology 2017. 69 . ( 10.1016/j.jacc.2016.12.030) [DOI] [PubMed] [Google Scholar]
- 9.Druce M, Rockall A, Grossman AB. Fibrosis and the carcinoid syndrome – from causation to future therapy. Nature Reviews: Endocrinology 2009. 5 . ( 10.1038/nrendo.2009.51) [DOI] [PubMed] [Google Scholar]
- 10.Laskaratos FM, Walker M, Wilkins D, Tuck A, Ramakrishnan S, Phillips E, Gertner J, Megapanou M, Papantoniou D, Shah R, et al Evaluation of clinical prognostic factors and further delineation of the effect of mesenteric fibrosis on survival in advanced midgut neuroendocrine tumours. Neuroendocrinology 2018. 107 . ( 10.1159/000493317) [DOI] [PubMed] [Google Scholar]
- 11.Preda VA, Chitoni M, Talbot D, Reed N, Grossman AB. Primary ovarian carcinoid: extensive clinical experience with an under-recognised uncommon entity. International Journal of Gynecological Cancer 2018. 28 . ( 10.1097/IGC.0000000000001215) [DOI] [PubMed] [Google Scholar]
- 12.Degg TJ, Allen KR, Barth JH. Measurement of plasma 5-hydroxyindoleacetic acid in carcinoid disease: an alternative to 24-h urine collections? Annals of Clinical Biochemistry 2000. 37 . ( 10.1258/0004563001899780) [DOI] [PubMed] [Google Scholar]
- 13.Miller AG, Brown H, Degg T, Allen K, Keevil BG. Measurement of plasma 5-hydroxyindoleacetic acid by liquid chromatography tandem mass spectrometry–comparison with HPLC methodology. Journal of Chromatography: B, Analytical Technologies in the Biomedical and Life Sciences 2010. 878 . ( 10.1016/j.jchromb.2010.01.010) [DOI] [PubMed] [Google Scholar]
- 14.Tellez MR, Mamikunian G, O’Dorisio TM, Vinik AI, Woltering EA. A single fasting plasma 5-HIAA value correlates with 24-hour urinary 5-HIAA values and other biomarkers in midgut neuroendocrine tumors (NETs). Pancreas 2013. 42 . ( 10.1097/MPA.0b013e318271c0d5) [DOI] [PubMed] [Google Scholar]
- 15.Adaway JE, Dobson R, Walsh J, Cuthbertson DJ, Monaghan PJ, Trainer PJ, Valle JW, Keevil BG. Serum and plasma 5-hydroxyindoleacetic acid as an alternative to 24-h urine 5-hydroxyindoleacetic acid measurement. Annals of Clinical Biochemistry 2016. 53 . ( 10.1177/0004563215613109) [DOI] [PubMed] [Google Scholar]
- 16.Feldman JM. Urinary serotonin in the diagnosis of carcinoid tumors. Clinical Chemistry 1986. 32 . [PubMed] [Google Scholar]
- 17.Meijer WG, Kema IP, Volmer M, Willemse PH, de Vries EG. Discriminating capacity of indole markers in the diagnosis of carcinoid tumors. Clinical Chemistry 2000. 46 . [PubMed] [Google Scholar]
- 18.Oberg K, Couvelard A, Delle Fave G, Gross D, Grossman A, Jensen RT, Pape UF, Perren A, Rindi G, Ruszniewski P, et al ENETS Consensus Guidelines for standard of care in neuroendocrine tumours: biochemical markers. Neuroendocrinology 2017. 105 . ( 10.1159/000472254) [DOI] [PubMed] [Google Scholar]
- 19.Kema IP, Schellings AM, Meiborg G, Hoppenbrouwers CJ, Muskiet FA. Influence of a serotonin- and dopamine-rich diet on platelet serotonin content and urinary excretion of biogenic amines and their metabolites. Clinical Chemistry 1992. 38 . [PubMed] [Google Scholar]
- 20.Mashige F, Matsushima Y, Kanazawa H, Sakuma I, Takai N, Bessho F, Ohkubo A. Acidic catecholamine metabolites and 5-hydroxyindoleacetic acid in urine: the influence of diet. Annals of Clinical Biochemistry 1996. 33 . ( 10.1177/000456329603300106) [DOI] [PubMed] [Google Scholar]
- 21.Kroll CA, Magera MJ, Helgeson JK, Matern D, Rinaldo P. Liquid chromatographic-tandem mass spectrometric method for the determination of 5-hydroxyindole-3-acetic acid in urine. Clinical Chemistry 2002. 48 . [PubMed] [Google Scholar]
- 22.Møller JE, Pellikka PA, Bernheim AM, Schaff HV, Rubin J, Connolly HM. Prognosis of carcinoid heart disease: analysis of 200 cases over two decades. Circulation 2005. 112 . ( 10.1161/CIRCULATIONAHA.105.553750) [DOI] [PubMed] [Google Scholar]
- 23.Askew JW, Connolly HM. Carcinoid valve disease. Current Treatment Options in Cardiovascular Medicine 2013. 15 . ( 10.1007/s11936-013-0265-2) [DOI] [PubMed] [Google Scholar]
- 24.Corcuff JB, Chardon L, El Hajji Ridah I, Brossaud J. Urinary sampling for 5HIAA and metanephrines determination: revisiting the recommandations. Endocrine Connections 2017. 6 R87–R98. ( 10.1530/EC-17-0071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramage JK, Ahmed A, Ardill J, Bax N, Breen DJ, Caplin ME, Corrie P, Davar J, Davies AH, Lewington V, et al UK and Ireland Neuroendocrine Tumour Society Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut 2012. 61 . ( 10.1136/gutjnl-2011-300831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strosberg JR, Halfdanarson TR, Bellizzi AM, Chan JA, Dillon JS, Heaney AP, Kunz PL, O’Dorisio TM, Salem R, Segelov E, et al The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas 2017. 46 . ( 10.1097/MPA.0000000000000850) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirakhur B, Pavel ME, Pommier RF, Fisher GA, Phan AT, Massien C, Liyanage N, Lowenthal SP, Vinik AI. Biochemical responses in symptomatic and asymptomatic. Patients with neuroendocrine tumors: pooled analysis of 2 phase 3 trials. Endocrine Practice 2018. 24 . ( 10.4158/EP-2018-0296) [DOI] [PubMed] [Google Scholar]
- 28.Turner GB, Johnston BT, McCance DR, McGinty A, Watson RG, Patterson CC, Ardill JE. Circulating markers of prognosis and response to treatment in patients with midgut carcinoid tumours. Gut 2006. 55 . ( 10.1136/gut.2006.092320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Formica V, Wotherspoon A, Cunningham D, Norman AR, Sirohi B, Oates J, Chong G. The prognostic role of WHO classification, urinary 5-hydroxyindoleacetic acid and liver function tests in metastatic neuroendocrine carcinomas of the gastroenteropancreatic tract. British Journal of Cancer 2007. 96 . ( 10.1038/sj.bjc.6603699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Horst-Schrivers AN, Post WJ, Kema IP, Links TP, Willemse PH, Wymenga AN, de Vries EG. Persistent low urinary excretion of 5-HIAA is a marker for favourable survival during follow-up in patients with disseminated midgut carcinoid tumours. European Journal of Cancer 2007. 43 . ( 10.1016/j.ejca.2007.07.025) [DOI] [PubMed] [Google Scholar]
- 31.Zandee WT, Kamp K, van Adrichem RC, Feelders RA, de Herder WW. Limited value for urinary 5-HIAA excretion as prognostic marker in gastrointestinal neuroendocrine tumours. European Journal of Endocrinology 2016. 175 . ( 10.1530/EJE-16-0392) [DOI] [PubMed] [Google Scholar]
- 32.Tirosh A, Nilubol N, Patel D, Kebebew E. Prognostic utility of 24-hour urinary 5-HIAA doubling time in patients with neuroendocrine tumors. Endocrine Practice 2018. 24 . ( 10.4158/EP-2018-0022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuetenhorst JM, Korse CM, Bonfrer JMG, Peter E, Lamers CBHW, Taal BG. Daily cyclic changes in the urinary excretion of 5-hydroxyindoleacetic acid in patients with carcinoid tumors. Clinical Chemistry 2004. 50 . ( 10.1373/clinchem.2004.032151) [DOI] [PubMed] [Google Scholar]
- 34.Gedde-Dahl M, Thiis-Evensen E, Tjølsen AM, Mordal KS, Vatn M, Bergestuen DS. Comparison of 24-h and overnight samples of urinary 5-hydroxyindoleacetic acid in patients with intestinal neuroendocrine tumors. Endocrine Connections 2013. 2 . ( 10.1530/EC-12-0077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan DL, Moody L, Segelov E, Metz DC, Strosberg JR, Pavlakis N, Singh S. Follow-up for resected gastroenteropancreatic neuroendocrine tumours (GEP-NETs): a practice survey of the Commonwealth Neuroendocrine collaboration (CommNETs) and the North American Neuroendocrine Tumor Society (NANETS). Neuroendocrinology 2018. 107 . ( 10.1159/000488394) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a