Abstract
Background:
The ventriculophasic response (VR) refers to shortening of atrial cycle length during heart block when a QRS complex is interposed between P waves. No formal quantitative definition has heretofore been proposed, nor have its potential clinical correlations been studied.
Hypothesis:
We hypothesized that VR is present in selected patients who are distinguished by clinical features from those who lack VR.
Methods:
Pacing devices were temporarily programmed to VVI mode at 30 ppm as electrocardiogram and intracardiac electrograms were recorded at 50 mm/sec paper speed. We measured the percentage decrease in a P‐P interval (A‐A interval on the atrial electrogram) containing a QRS, compared to the preceding P‐P interval. Left ventricular ejection fraction (LVEF) was measured by echocardiogram.
Results:
Shortening of P‐P interval was observed chiefly when the interposed QRS occurred early in the anticipated P‐P interval (as judged by the preceding P‐P interval). P‐P shortening of 0% to 3% occurred randomly. Defining VR as being a >3% P‐P interval shortening when an interposed QRS occurred in the first 60% of the anticipated P‐P interval, we found that VR was present in 28 (55%) of our patients. It was quite reproducible, was more common in women (81% vs 37% of men; P = 0.004), and positively correlated with LVEF (r = 0.41, P = 0.004). It did not correlate with age, diabetes, or β‐blocker use.
Conclusions:
Using our newly derived definition of VR, we found the phenomenon was present in 55% of our patients. It was reproducible and more commonly seen in women and patients with LVEF ≥40%. © 2011 Wiley Periodicals, Inc.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
The ventriculophasic response (VR) is a phenomenon seen in patients with heart block. It refers to shortening of the P‐P interval when a QRS complex is interposed (Figure 1). The phenomenon was first described by Erlanger and Blackman in 1910.1 Subsequent studies provided few insights into its mechanism or its clinical implications. In 1955, Rosenbaum and Lepeschkin hypothesized that shortening of the P‐P interval likely involved the autonomic nervous system, the mechanical influence of the contracting ventricle, and/or fluctuations in the blood supply to the sinus node.2 No investigators attempted to distinguish how much P‐P interval shortening represented meaningful sinus acceleration vs mere random variation of heart rate. None offered a semiquantitative definition of the VR or examined its potential clinical implications. In our study, we sought to derive a semiquantitative definition of this phenomenon to more precisely define it and to delineate some of its potential clinical correlations.
Figure 1.
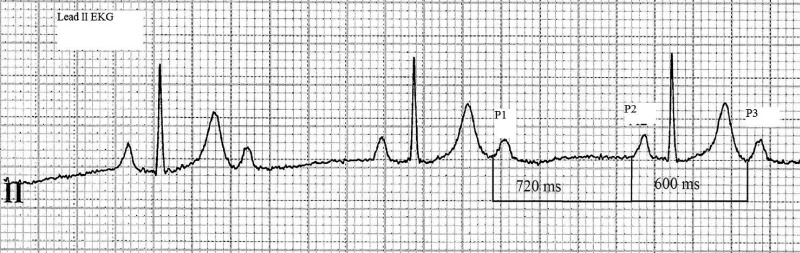
The ventriculophasic response. The tracing recorded in a patient with 2:1 second‐degree heart block demonstrates that 2 P waves with no interposed QRS complex (P1‐P2) have a longer P‐P interval (720 milliseconds) than 2 P‐waves with interposed QRS (P2‐P3 = 600 milliseconds). EKG = electrocardiogram.
Methods
The institutional review board of our hospital waived the requirement for informed consent. We analyzed 51 patients with complete heart block. Each was treated with either an implantable cardioverter defibrillator or a pacemaker. Their mean age was 77 ± 11 years. Twenty‐one (41%) were women, 18 (35%) had diabetes mellitus (DM), 27 (53%) had coronary artery disease (CAD), and 32 (63%) were taking β‐blockers. Echocardiograms were available in 48 patients. The mean time interval between the tracing recording and the echocardiogram was 18.6 ± 23.3 months. The mean left ventricular ejection fraction (LVEF) was 45% ± 18%. No significant clinical events had occurred in any patients between the echocardiogram and the day of the clinic visit.
Each patient was evaluated while seated in a chair at a routine clinic visit. The pacer function of their device was temporarily programmed to the ventricular‐inhibited mode at 30 pulses per minute as surface electrocardiogram (ECG), intracardiac atrial electrogram, and marker channels were continuously recorded at 50 mm/sec. We excluded tracings with atrial fibrillation and sections of recordings that contained premature depolarizations.
Sinus‐rate alterations were measured as demonstrated in Figure 2. All P‐P intervals were measured as the A‐A intervals on the atrial electrogram. We also measured A‐V intervals as the interval between an interposed QRS and its preceding A‐wave on the atrial electrogram. All values were expressed in milliseconds. The shortening of the P‐P interval containing a QRS was expressed as being the percentage shortening of an A‐A interval containing a QRS compared to the preceding A‐A interval.
Figure 2.
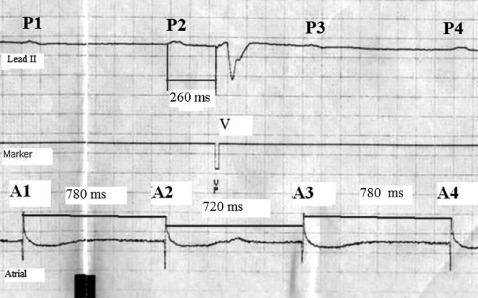
Measurement of the P‐P interval shortening when a QRS is interposed. The upper channel is electrocardiogram lead II. It demonstrates 4 P waves (P1‐P4). Between the second and the third P waves, there is an interposed paced QRS complex (V). The middle channel, the marker channel, denotes the stimulus artifact of the paced QRS complex (VP). The bottom channel is the atrial electrogram, which demonstrates the 4 atrial events (A1‐A4) that correspond to the 4 surface P waves. The P‐P interval shortening was calculated by comparing A2‐A3 to A1‐A2: P‐P interval shortening = 100 × [(A1‐A2) − (A2‐A3)]/A1‐A2. In this example, P‐P shortening = 100 × [(60)/780] = 7.7%.
All statistical analysis was performed using SPPS software version 17 (SPSS Inc., Chicago, IL). Variables were expressed as means with standard deviation. Comparisons between groups of variables were made using the Student t test when the variables were normally distributed and the Friedman test when they were not. The differences between frequencies were tested with χ 2 test or Fisher exact test as appropriate. Correlations between variables were tested with the Spearman test. A P value of <0.05 was considered to indicate statistical significance.
Results
Quantification of VR
We analyzed 1 tracing from each patient. These 51 tracings contained 185 instances in which measurement of potential VR was possible. Lepeskin and Rosembaum had suggested that the magnitude of VR might be influenced by the timing of the QRS complex inside the P‐P interval.2 We analyzed this potential relationship by expressing the timing of the QRS (the A‐V interval index) as a percentage of the preceding A‐A interval. For the example shown in Figure 2, A‐V interval index was calculated as follows: 100 × [(A2‐V)/(A1‐A2)] = 100 × (260/780) = 33% (eg, this QRS occurred at 33% of the anticipated P‐P interval).
For all 185 measurements, we plotted the shortening of the P‐P interval containing a QRS (expressed as percentage of the preceding P‐P interval) against the A‐V interval index (Figure 3). This plot revealed that P‐P shortening ≤3% occurred regardless of the timing of the interposed QRS, but P‐P shortening >3% occurred chiefly when the A‐V interval index was ≤60% of the anticipated A2‐A3. Among the 131 instances when an interposed QRS occurred in the first 60% of the anticipated P‐P interval, 68 (52%) demonstrated P‐P interval shortening >3%, whereas only 5 (9%) of the 54 instances of later occurring QRS complexes were associated with P‐P shortening >3% (P < 0.001). These observations suggest that P‐P interval shortening >3% surrounding an interposed QRS represents an appropriate cutoff point that distinguishes meaningful, nonrandom P‐P interval shortening from random heart‐rate variation. Further analysis, demonstrated a weak but statistically significant inverse relationship between the A‐V interval index and the magnitude of P‐P shortening, with P‐P interval shortening tending to increase as the QRS occurs earlier (r = −0.33, P < 0.001).
Figure 3.
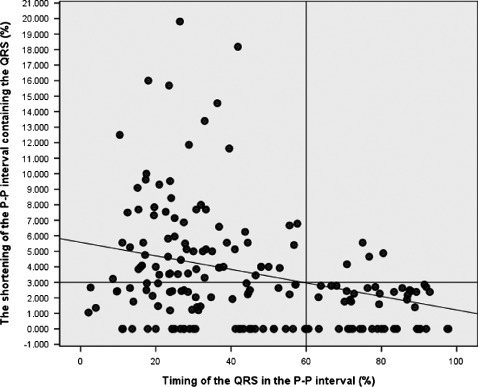
The shortening of the P‐P interval containing a QRS vs the timing of the interposed QRS. P‐P shortening ≤3% occurs randomly, whereas shortening more than 3% is seen predominantly when the QRS occurs in the first 60% of the anticipated P‐P interval. See text for details.
Based on the foregoing observations, we propose the following definition of the VR: VR is present during heart block when an interposed QRS, occurring in the first 60% of the anticipated P‐P interval, results in a >3% shortening of the P‐P interval surrounding the QRS compared to the preceding P‐P interval. That is, VR is present when P2‐V is less than 60% of A1‐A2 and A2‐A3 is >3% shorter than A1‐A2 (Figure 2).
Reproducibility of VR
Using our definition, we divided our patients into those who had VR present and those who did not. Each patient had an average of 3.3 ± 1.1 analyzable intervals on their tracing. For 23 patients (45%), there was no VR present on any of the measurements on their tracings. Tracings in these “VR‐negative” patients contained between 2 and 7 analyzable instances (that is, moments when there were no extra systoles and the A‐V interval index of the interposed QRS was <60%). Among these, there were 7 analyzable instances in 1 patient (4%), 4 instances in 3 (13%), 3 instances in 11 (48%) and 2 instances in 8 (35%). Twenty‐eight (55%) of our patients had VR present, by our proposed definition, at least once on their tracing. Among these 28 “VR‐positive” patients, 6 (21%) had VR present only once on their tracing, 11 (39%) had VR present in 2 instances, 7 (25%) had VR present in 3 instances, 2 (7%) had VR present in 4 instances, and 2 (7%) had VR present in 5 instances. The 6 patients in whom VR was present only once on their tracing had between 2 and 4 instances in which potential VR could be measured (1 patient had 4 instances, 2 patients had 3 instances, and 3 patients had 2 instances). Thus, the within‐tracing reproducibility of VR, defined as being present at least twice in a given tracing, was 79%.
Clinical Correlations of VR
We divided our study group into 2 subgroups: VR positive (n = 28) and VR negative (n = 23). The VR‐positive group included the patients who had at least 1 VR present on their tracing. The VR‐negative group included patients in whom no VR was present among any of the analyzable intervals on their tracing. A comparison of the characteristics of the 2 groups is presented in the Table 1. VR was more prevalent in women (81%) than men (37%) (P = 0.004). There was no significant relationship between VR and the presence of DM, CAD, or β‐blocker therapy. Echocardiograms were available for 48 patients. Among VR‐positive subjects, LVEF was ≥40% in 81% as opposed to 45% of those with no VR (P = 0.012). The mean LVEF was significantly greater in those with VR (49.9% ± 16.7%) compared to those without VR (38.9% ± 18.3%; P = 0.037). Omitting from this analysis the 6 VR‐positive patients in whom VR was present only once did not change these results.
Table 1.
Comparison of the Characteristics of the 2 Ventriculophasic Response Groups
| Parameter | VR Present, n = 28 | VR Absent, n = 23 | P Value |
|---|---|---|---|
| Age, y | 77.2 ± 12.5 | 78.2 ± 11.9 | 0.7 |
| Women, no. (%) | 17 (60.7) | 4 (17.4) | 0.004 |
| Coronary artery disease, no. (%) | 12 (42.8) | 15 (65) | 0.1 |
| Diabetes mellitus, no. (%) | 7 (25) | 11 (47.8) | 0.09 |
| β‐Blocker therapy, no. (%) | 15 (53) | 17 (73) | 0.1 |
| LVEF ≥40%, no. (%) | 21 (81) | 10 (45) | 0.01 |
| Mean LVEF, % | 49.9 ± 16.7 | 38.9 ± 18.3 | 0.03 |
Abbreviations: LVEF, left ventricular ejection fraction; VR, ventriculophasic response.
As shown in Figure 4, there was a significant positive correlation between the maximum VR value on each tracing and LVEF; the magnitude of VR decreased as left ventricular function declined (r = 0.41, P = 0.004). Although P‐P interval shortening ≤3% was seen randomly at all values of LVEF, shortening >3% was mainly seen when LVEF was ≥40%.
Figure 4.
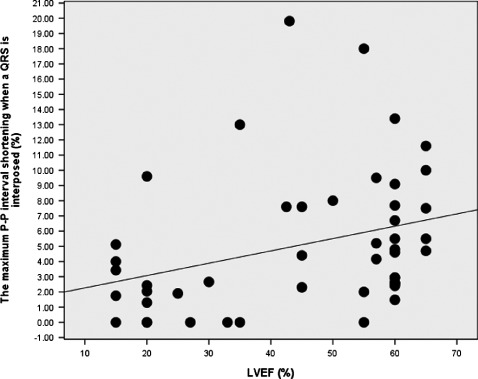
Relationship between P‐P shortening and left ventricular ejection fraction (LVEF). The maximum value of P‐P shortening on each tracing decreases as the LVEF declines (r = 0.41, P = 0.004). Values ≤3% are seen at all values of LVEF, whereas P‐P shortening >3% is mainly observed when LVEF is ≥40%.
Discussion
Investigators have long observed that ventricular systoles that occur in patients with second‐ and third‐degree heart block may transiently affect the prevailing sinus rate. Brief sinus acceleration (P‐P interval shortening) in response to a QRS complex that is interposed between 2 sinus P waves has been termed the VR. For more than a century, VR has been an electrocardiographic curiosity, loosely defined, poorly understood, and largely devoid of any documented clinical significance. Most of the research on this phenomenon has been performed by observing capture and escape complexes that occur in patients with heart block. No investigators have sought to rigorously define VR by quantifying the observed response or to describe its clinical correlations. Our study is the first to examine VR in patients who have received permanent pacemakers for heart block. Careful analysis has allowed us to derive a semiquantifiable definition of the response.
Based on our observations, we propose that VR be defined as being present during heart block when an interposed QRS, occurring in the first 60% of the anticipated P‐P interval, results in a >3% shortening of the P‐P interval surrounding the QRS compared to the preceding P‐P interval (Figure 2). To our knowledge, no cutoff value to differentiate VR from random variations in heart rate has been heretofore derived. We observed that P‐P interval shortening ≤3% occurs regardless of the timing of an interposed QRS between its surrounding P waves (Figure 3). Such minimal P‐P interval shortening appears to represent random variation of sinus rate. Thus, our data indicate that >3% shortening of P‐P interval surrounding an interposed QRS represents meaningful, nonrandom P‐P interval shortening when a QRS is interposed. Our data also indicate that one should not measure potential VR when the interposed QRS occurs in the last 40% of the anticipated P‐P interval because the P‐P interval surrounding such late QRS complexes rarely shortens. Based on the definition of VR that we have derived, 28 (55%) of our patients demonstrated VR on at least 1 of the analyzable intervals on their tracings. Among them, the phenomenon was reproduced in ≥2 instances in 79%.
Some of our findings echo what previous investigators have observed. The proportion of subjects with VR in our group (55%) is very similar to the 50% prevalence reported by Parsonnet and Miller.3 Rosenbaum and Lepeschkin, in an analysis similar to ours, plotted the length of the P‐P interval against the Q‐P interval in 1 patient. Unlike our analysis, they did not normalize the Q‐P coupling interval to the heart rate (eg, A1‐A2 interval in Figure 2). They observed that Q‐P intervals longer than 0.8 seconds generated a P‐P interval longer than the P‐P intervals surrounding it, and Q‐P intervals of approximately 0.5 seconds generated shorter P‐P intervals. The last finding parallels our observation that the P‐P interval shortens chiefly when the interposed QRS occurs in the first 60% of the anticipated P‐P interval.
There are several aspects of our analysis that differ from previous investigations of VR. First, we studied patients with chronic heart block, taking advantage of their permanent pacer devices to study VR “on demand.” This approach has not been heretofore employed. It afforded the opportunity to measure intracardiac atrial intervals, which may be more accurately measured than surface P waves (particularly when P waves merge with QRS complexes and T waves). Second, we normalized all the key intervals to the ambient instantaneous heart rate by expressing P‐P interval shortening and the timing of the interposed QRS (the A‐V interval index) as a percentage of the anticipated P‐P interval (that is the P‐P interval preceding a QRS complex, A1‐A2 in Figure 2). Third, we made all measurements at paper speed of 50 mm/sec, which permits more accurate measurements than at 25 mm/sec (the paper speed used in previous studies of VR).
Our analysis is the first to derive a semiquantitative definition of VR and to examine its clinical correlations. The VR‐positive patients had a significantly higher mean LVEF and a smaller prevalence of LVEF depressed to <40%. There was a significant positive correlation between the LVEF and VR. The fact that VR increases as LVEF increases suggests that mechanical aspects of ventricular contraction might have a role in the physiology of VR, as suggested many years ago by Rosenbaum and Lepeschkin.2 Perhaps a more efficient ventricular emptying provided by healthier ventricles increases the VR by affecting baroreceptors. One of the possible mechanisms for VR cited by previous investigators is the Bainbridge reflex: a sudden rise in the right atrial pressure may cause abrupt vagal inhibition that transiently accelerates the sinus‐node firing rate.2 This hypothesis may warrant further study. We found no correlation between VR and age, the use of β‐blockers, or the presence of DM or CAD. It is unclear why VR was more frequently present in women than men. Parsonnet and Miller noted a similar pattern. In their patients, VR was observed in 53% of women and 43% of men. This may represent a chance observation, or perhaps there is some difference in female physiology that amplifies VR. In our analysis, the 6 patients with only 1 VR instance were not excluded from the VR‐positive group, as we felt that even 1 instance of VR might be physiologically and/or clinically meaningful compared to those patients who demonstrated no evidence of VR. Omitting these 6 patients from the between‐group analysis did not alter any of the results discussed previously and summarized in the Table 1.
Our study has a few potential limitations. First, the study group was small, although it was larger than in all previous investigations into VR. Second, we analyzed paced beats. Previous studies examined escape or conducted beats. It is not known whether different types of interposed QRS complexes generate different degrees of VR. Third, the echocardiogram recordings were obtained at a fairly long interval from the VR analysis. Although this might impact the correlation between LVEF and VR, all of our patients appeared to be clinically unchanged in the time between the 2 studies.
Conclusion
Based on our observations, we have derived a new semiquantitative definition of the VR. By this definition, we found VR to be present in 55% of our patients with heart block. It was more prevalent in women, although we cannot explain why. Greater VR was seen in people with better left ventricular function, which suggests that ventricular contraction and/or emptying influence the response. Other phenomena related to heart‐rate fluctuations, such as heart‐rate variability,4 baroreceptor sensitivity,5 or heart‐rate turbulence (HRT), have been shown to have prognostic significance, predominantly in survivors of myocardial infarction. Heart‐rate turbulence (HRT) is a measure of the autonomic response after a single ventricular premature contraction. It correlates significantly with baroreflex sensitivity and has been shown to portend prognosis after myocardial infarction.6 It is possible that VR also reflects autonomic nervous tone changes initiated by a ventricular contraction in the setting of advance heart block. At least 1 investigation has suggested that VR may share a common mechanism with HRT.7 One might speculate that VR may confer prognostic information akin to HRT.7 We propose this new definition of VR in hopes that it will facilitate future investigation into its physiologic mechanisms, clinical correlations, and perhaps its prognostic significance.
Acknowledgements
The authors acknowledge and thank Dr. Ramona Dadu for her assistance with preparation of the manuscript.
References
- 1. Erlanger J, Blackman JR. Further studies in the physiology of heart block in mammals: chronic auriculo‐ventricular heart block in the dog. Heart. 1910;1:177–182. [Google Scholar]
- 2. Rosenbaum MB, Lepeschkin E. The effect of ventricular systole on auricular rhythm in auriculoventricular block. Circulation. 1955;11:240–261. [DOI] [PubMed] [Google Scholar]
- 3. Parsonnet AE, Miller R. Heart block: the influence of ventricular systole upon the auricular rhythm in complete and incomplete heart block. Am Heart J. 1944;27:676–687. [Google Scholar]
- 4. Cripps TR, Malik M, Farrell TG, et al. Prognostic value of reduced heart rate variability after myocardial infarction: clinical evaluation of a new analysis method. Br Heart J. 1991;65:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farrell TG, Odemuyiwa O, Bashir Y, et al. Prognostic value of baroreflex sensitivity testing after acute myocardial infarction. Br Heart J. 1992;67:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barthel P, Schneider R, Ing D, et al. Risk stratification after acute myocardial infarction by heart rate turbulence. Circulation. 2003;108:1221–1226. [DOI] [PubMed] [Google Scholar]
- 7. Schmidt G, Malik M, Barthel P, et al. Heart‐rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet. 1999;353:1390–1396. [DOI] [PubMed] [Google Scholar]


