Abstract
Background
The purpose of this study was to describe the clinical characteristics and clinical outcomes for Chinese patients with type A intramural hematoma (IMH).
Methods and Results
We studied 90 patients with Stanford type A acute aortic syndrome who presented to our institution from 1998 to 2005 and evaluated the presentation, management, and clinical outcomes of acute IMH by comparing these patients with those diagnosed with classical aortic dissection (AD). A total of 34 patients had IMH and they tended to be older (69.7 ± 12.4 versus 60.5 ± 16.2 years; p = 0.006). The development of pericardial effusion was more frequent in patients with IMH than in patients with AD. They were also less likely to receive surgery as compared to AD patients (26.5% versus 73.2%; p < 0.0001). Overall mortality of IMH was not significantly higher than that of classic AD (29.4% versus 21.4%; p = 0.45). For IMH patients, the mortality rate with medical treatment was 32%. Ten (40%) of the 25 medically treated patients developed adverse outcomes. However, no independent predictors of adverse outcomes were identified in the study. In follow‐up imaging studies of 15 patients who survived IMH without surgical repair, 14 patients showed complete resolution of IMH and 1 progressed into classical AD.
Conclusion
Acute type A IMH in Chinese patients showed a high mortality rate with medical treatment. It has a highly unpredictable course with no reliable clinical and anatomical predictors. Surgical therapy should be the treatment of choice for Chinese patients with acute IMH, especially those who are younger and have less comorbidities. © 2011 Wiley Periodicals, Inc.
Keywords: aorta, hematoma, medical therapy, surgery, mortality
Aortic intramural hematoma (IMH) is recognized as a variant form of aortic dissection (AD) and accounts for 5%–30% of acute aortic syndromes.1, 2, 3, 4 For Stanford type A IMH, it is associated with inherent risk of progression2, 3, 4, 5 to typical aortic dissection, aortic rupture, and cardiac tamponade. Hence, it is generally considered a surgical emergency as if it was typical type A AD, with expeditious surgery indicated. In a meta‐analysis6 of series published up to 1999 which comprised of mostly Western patients, mortality of type A IMH treated medically was high. Subsequent studies7, 8 involving white patients also showed frequent progression to serious complications and high mortality with medical treatment and favored early surgery as the treatment of choice in white patients.
In contrast, studies9, 10, 11 from the Far East (Korea and Japan) have shown a more benign clinical course for medically treated type A IMH with complete resolution of IMH seen in some cases and suggested an alternative strategy of medical treatment, serial imaging, and timed surgery. It is unknown whether the results of prior Asian studies are applicable to the Chinese population. This has important implications as the treatment strategy (surgical versus medical therapy) may differ and will affect the clinical outcome. Data on the clinical features and natural history of type A IMH in Chinese patients are very limited. Therefore, the aim of this study was to describe the clinical characteristics and clinical outcomes for Chinese patients with type A IMH and also, compare the clinical data directly with Chinese patients with type A AD.
Methods
Study Population
The study sample comprised of 90 consecutive Chinese patients diagnosed with Stanford type A acute aortic syndrome at Queen Mary Hospital, a tertiary referral center in Hong Kong from 1998 to 2005. Data were collected retrospectively on demographic characteristics, presenting signs and symptoms, results of imaging studies, therapeutic modality, hospital course, and short‐term and long‐term clinical outcome. Clinical data were retrieved from medical records, including the most recent clinic visit. Our database identified 34 patients with type A IMH and 56 patients with type A AD. All consecutive patients underwent diagnostic imaging studies either by contrast‐enhanced computer tomography (CT) or transesophageal echocardiography (TEE) within 48 h after onset of initial symptoms.
Diagnosis and Clinical Outcomes
A typical double channel aorta with dissecting membrane or intimal tear was an imaging criterion for diagnosis of AD by CT or TEE. Exclusion of dissecting flap or intimal disruption was a prerequisite for diagnosis of IMH by CT or TEE. Regional thickening of the aortic wall ≥ 0.7 cm in a circular or crescent shape or evidence of intramural accumulation of blood in TEE and a high attenuation area along the aortic wall without enhancement after contrast injection in CT were considered diagnostic of IMH. In‐hospital mortality was defined as death related to complications of AD or IMH which included aortic rupture, cardiac tamponade, cerebrovascular accident, and end‐organ hypoperfusion during the initial hospitalization. It was further subdivided into medical mortality and surgical mortality depending on the initial treatment modality received at presentation.
Subgroup analysis was performed on the medically treated IMH patients to identify predictors of adverse outcomes which were defined as those who died during hospitalization, developed early progression into classic AD requiring surgery, and developed cardiac tamponade requiring urgent pericardiocentesis.
Statistical Analysis
Numerical values are expressed as mean ± SD. Statistical analysis of the difference between groups was assessed by Student unpaired t test or Fisher's exact test as appropriate. Multivariate analyses were performed with an enter regression model, in which each entered variable had a p value < 0.05 based on univariate analysis. Analyses were performed with SPSS software, version 10.0 (Chicago, Ill., USA). A p value < 0.05 was considered statistically significant. All investigations were carried out in accordance with the Declaration of Helsinki.
Results
As shown in Table 1, patients with IMH tended to be older than those with classic AD (p = 0.006). Male preponderance was noted for both IMH and AD groups. No significant differences were observed between both groups with respect to conventional risk factors like hypertension, diabetes mellitus, and ischemic heart disease. However, there was no patient with Marfan syndrome in the IMH group (p = 0.003).
Table 1.
Clinical characteristics of patients with classic AD and IMH
| Classic AD (n = 56) | IMH (n = 34) | p | |
|---|---|---|---|
| Age, yr (mean±SD) | 60.5±16.2 | 69.7±12.4 | 0.006* |
| Sex (female:male), %, n | 32.1:67.9 (18:38) | 47.1:52.9 (16:18) | 0.18 |
| Hypertension, %, n | 59 (33) | 65 (22) | 0.66 |
| Marfan syndrome, %, n | 21.4(12) | 0 (0) | 0.003* |
| Diabetes, %, n | 3.6 (2) | 8.8 (3) | 0.4 |
| Ischemic heart disease, %, n | 5.4 (3) | 8.8 (3) | 0.67 |
| Chest pain, %, n | 75 (42) | 70.6 (24) | 0.8 |
| Back pain, %, n | 64.3 (36) | 64.7 (22) | 1.0 |
| Abdominal pain, %, n | 23.2 (13) | 26.5 (9) | 0.8 |
| Stroke, %, n | 21.4 (12) | 0 (0) | 0.003* |
| Mean systolic blood pressure, mm Hg | 133.1±42 | 129.9±48.4 | 0.74 |
| Hypotension, %, n | 26.8 (15) | 44.1(15) | 0.10 |
| Pulse deficit, %, n | 26.8 (15) | 11.8 (4) | 0.11 |
| Abnormal ECG†, %, n | 53.6 (30) | 47 (16) | 0.66 |
| Widened superior mediastinum on CXR, %, n | 76.8 (43) | 91.2 (31) | 0.09 |
| Pleural effusion, %, n | 14.3 (8) | 17.6 (6) | 0.77 |
| Pericardial effusion, %, n | 23.2 (13) | 64.7 (22) | 0.0001* |
| Cardiac tamponade, %, n | 12.5 (7) | 23.5 (8) | 0.24 |
Abnormal ECG † =, ST‐elevation,
ST‐depression, T‐inversion, AF, Lt.BBB, LVH.
for p< 0.05
Chest pains or back pains were the 2 most common presenting symptoms for both the IMH and AD groups. Patients with AD were more likely to present with symptoms of cerebrovascular accident than those with IMH (p = 0.003). No significant differences were noted between both groups at presentation for physical signs such as systolic blood pressure, hypotension, abnormal electrocardiogram, and chest x‐ray findings.
The development of pericardial effusion (p = 0.0001) were more frequent in patients with IMH than in patients with AD, but the incidence of cardiac tamponade were not significantly different for both groups.
Surgical intervention was recommended for all patients in both IMH and AD groups. As shown in Table 2, patients with acute IMH were less likely to receive surgery as compared with the AD group (26.5% versus 73.2%; p < 0.0001). Old age, multiorgan failure at presentation, comorbidities (malignancy, pulmonary disease, previous stroke, and poor premorbid status) and patients' refusal were major reasons for selecting medical treatment in patients from both groups. Medical treatment for patients with IMH or AD was general supportive care with antihypertensive medications to lower systolic blood pressure ≤ 120 mm Hg.
Table 2.
Treatment and clinical outcomes (in‐hospital mortality) of patients with AD and IMH
| Classic AD ( n = 56) | IMH ( n = 34) | p | |
|---|---|---|---|
| Medical treatment, %, n/N | 26.8 (15/56) | 73.5 (25/34) | <0.0001* |
| Surgical therapy, %, n/N | 73.2 (41/56) | 26.5 (9/34) | <0.0001* |
| Overall in‐hospital mortality, %, n/N | 21.4 (12/56) | 29.4 (10/34) | 0.45 |
| Medical mortality, %, n/N | 53.3 (8/15) | 32 (8/25) | 0.2 |
| Surgical mortality, %, n/N | 9.7 (4/41) | 22.2 (2/9) | 0.3 |
for p< 0.05
The overall in‐hospital mortality for IMH patients was 29.4%, which was not significantly higher than that of AD patients (21.4%; p = 0.45). There was no significant difference in the operative mortality between the 2 groups (22.2% in IMH versus 9.7% in AD; p = 0.3). For patients receiving medical therapy, the in‐hospital mortality of patients with IMH was lower than patients with AD but the difference was not significant (32% in IMH versus 53.3% in AD; p = 0.2). As shown in Figure 1, patients with classic AD who had undergone surgical intervention have a better survival rate as compared to AD patients who were treated medically (p = 0.001). This survival benefit with surgical intervention was however, not observed for patients with IMH.
Figure 1.
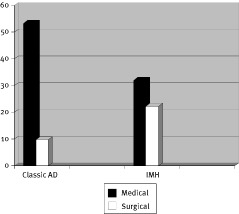
In‐hospital mortality rates of patients with AD and IMH
In the IMH group, medical treatment was selected for 25 patients. Six patients succumbed during hospitalization due to sudden aortic rupture. Two patients developed progression to classic AD within 2 wks of presentation and were treated with urgent surgical intervention in which 1 died. Another 2 patients had cardiac tamponade and were successfully stabilized with pericardiocentesis and medical therapy. One subsequently died during hospitalization due to aortic rupture and the other is still surviving after 5 y of initial presentation despite refusing surgery.
Fifteen of the 25 medically treated patients were discharged from hospital without any complications within 30 d of presentation. All have continued to survive without surgical repair with a mean follow‐up of 30 ± 20.8 mo. Follow‐up CT scans were available to evaluate the progression of the IMH. Fourteen patients showed complete resolution of IMH with 5 developing aneurysmal dilatation of the aorta (Figure 2). One octogenarian patient progressed into classic AD on follow‐up CT scan at 24 mo (Figure 3) and is still surviving despite refusing surgery.
Figure 2.
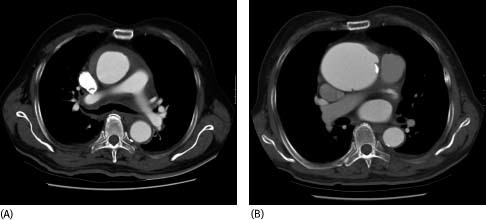
Serial CT scans performed initially (A) and 4 mo later (B) which showed resolution of IMH and aneurysmal dilatation of ascending aorta
Figure 3.
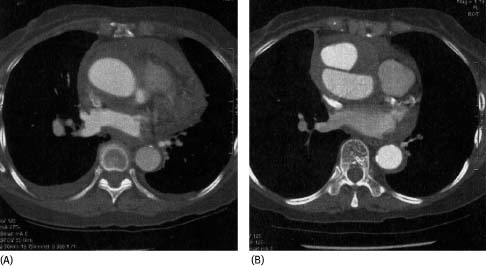
Serial CT scans performed initially (A) and 24 mo later (B) which showed late progression of IMH into type A AD
Table 3 compares the clinical characteristics of the medically treated IMH patients who had good outcomes versus those with adverse outcomes. Univariate analysis showed that those who suffered adverse outcomes tended to be older, had a lower blood pressure and hemoglobin level, but a bigger aortic diameter at presentation (p < 0.05). Hematoma thickness was almost similar for both groups. Both groups also had concomitant involvement of the descending aorta (>60%) with either IMH or AD. By multivariate analysis, no independent predictors of adverse outcomes were identified.
Table 3.
Clinical characteristics of medically treated patients with IMH (good outcomes versus adverse outcomes)
| Good outcomes (n = 15) | Adverse Outcomes (n = 10) | p | |
|---|---|---|---|
| Age, yr (mean±SD) | 66.3±12.2 | 79.7±11.6 | 0.01* |
| Sex (female: male), %, n | 33.3:66.7 | 70:30 | 0.1 |
| 5:10 | 7:3 | ||
| Hypertension, %, n | 71.4 (11) | 60 (6) | 0.7 |
| Diabetes, %, n | 13.3 (2) | 10 (1) | 1.0 |
| Ischemic heart disease, %, n | 0 (0) | 10 (1) | 0.4 |
| Pleural effusion, %, n | 26.7 (4) | 10 (1) | 0.6 |
| Pericardial effusion, %, n | 53.3 (8) | 70 (7) | 0.7 |
| Cardiac tamponade, %, n | 0 (0) | 40 (4) | 0.02* |
| Mean systolic blood pressure, mm Hg | 161.4±54 | 124.8±35.9 | 0.07* |
| Mean hemoglobin, g/dL | 14.3±2.8 | 11±1.5 | 0.002* |
| Mean creatinine, μmol/L | 159.7±50.3 | 154.4±46.7 | 0.8 |
| Maximum aortic diameter, cm | 4.15±0.42 | 4.97±0.8 | 0.003* |
| Maximum hematoma thickness, mm | 11.6±6.4 | 11.5±5.7 | 0.95 |
| Concomitant involvement of descending aorta, %, n | 66.7 (10) | 90 (9) | 0.35 |
for p< 0.05
Discussion
The current study on a Chinese population with type A IMH demonstrates an unfavorable clinical outcome (32% in‐hospital mortality) especially when treated medically. Our findings suggest the prognosis of type A IMH is not as benign as previously thought in Asian patients. Although prior Asian series9, 10, 11 have reported low death rates in type A IMH with medical therapy, a significant proportion11, 12, 13 of patients (up to 50%) go on to develop serious complications and required urgent surgical intervention.
A possible “Asian factor” has been put forth to explain the benign prognosis of medically treated IMH in prior Asian series. We think this is not likely to be the case as the clinical characteristics of Chinese patients are comparable to those reported by prior Asian series.9, 10, 11, 12, 13 Similar to the Song et al. study,10 our cohort of IMH patients had a higher incidence of pericardial effusion when compared to patients with AD. No definitive explanation is available to account for this observation. It is possible that patients with IMH were much older and more prone to periaortic bleeding or related to intrinsic differences in aortic pathology and the timing of imaging studies during acute presentation for both conditions.
What could have accounted for the seemingly worse prognosis in medically treated IMH Chinese patients? Unlike patients in the Song et al.10 and Kaji et al. studies,11 not all our patients received care in the intensive care setting and blood pressure control may not be optimal. In addition, follow‐up imaging study during initial hospital admission was not routinely arranged and hence progression of IMH may not be detected with subsequent failure of delivery of timed surgery.
In our study, the mortality rate for surgically treated IMH patients (22.2%) was not significantly lower than those treated conservatively. This may be explained by selection bias of the sickest patients for surgery. One of the patients developed a rupture of the aorta just before the operation and was in cardiogenic shock with massive hemopericardium while the other suffered perioperative myocardial infarction during the operation culminating in the 2 deaths for surgically treated IMH patients.
Several predictors of progression of IMH and adverse outcomes have been identified in many studies.7, 8, 14, 15, 16, 17 In our study, 10 (40%) of the 25 medically treated IMH patients developed adverse outcomes and by univariate analysis were found to be older, had a lower blood pressure and hemoglobin level, but a bigger aortic diameter at presentation. However, no independent predictor of adverse outcomes was identified by multivariate analysis in the study. A study by von Kodolitsch et al.7 had shown that regardless of the aortic diameter, IMH of the ascending aorta is at high risk of early progression and undelayed surgical repair should be performed.
Our study had several limitations. The sample size was relatively small and limited by the single center nature of analysis. Lack of statistical significance in several of the analyses in Tables 2 and 3 was most likely a type II statistical error. Also, normalized aortic diameter values with body surface area or age were not taken into consideration when analyzing the CT films.
Unlike classical type A AD,18 the optimal therapy for type A IMH continues to be debated and it has been managed differently in the East and West. Adding to the controversy, the International Registry of Aortic Dissection registry19 had paradoxically shown a higher mortality with surgical therapy than medical treatment. Based on the findings of our study, surgical therapy should be the treatment of choice in Chinese patients with acute type A IMH especially those who are younger and with less comorbidities. If medical treatment is chosen, serial imaging studies at close intervals and aggressive antihypertensive therapy in the intensive care setting with surgical backup is absolutely necessary. Further studies are certainly needed to better understand the natural history of acute type A IMH, to identify more reliable clinical and anatomical predictors of progression and to elucidate the appropriate treatment strategy.
References
- 1. Yamada T, Tada S, Harada J: Aortic dissection without intimal rupture: Diagnosis with MR imaging and CT. Radiology 1988; 168: 347–352. [DOI] [PubMed] [Google Scholar]
- 2. Mohr‐Kahaly S, Erbel R, Kearney P, Puth M, Meyer J: Aortic intramural hemorrhage visualised by transesophageal echocardiography: Findings and prognostic implications. J Am Coll Cardiol 1994; 23: 658–664. [DOI] [PubMed] [Google Scholar]
- 3. Nienaber CA, von Kodolitsch Y, Petersen B, Loose R, Helmchen U, et al.: Intramural hemorrhage of the thoracic aorta. Diagnostic and therapeutic implications. Circulation 1995; 92: 1465–1472. [DOI] [PubMed] [Google Scholar]
- 4. Vilacosta I, San Roman JA, Ferreiros J, Aragoncillo P, Mendez R, et al.: Natural history and serial morphology of acute intramural hematoma: A variant of aortic dissection. Am Heart J 1997;134: 495–507. [DOI] [PubMed] [Google Scholar]
- 5. Ide K, Uchida H, Otsuji H, Nishimine K, Tsushima J, et al.: Acute aortic dissection with intramural hematoma: Possibility of transition to classic dissection or aneurysm. J Thorac Imaging 1996; 11: 46–52. [DOI] [PubMed] [Google Scholar]
- 6. Maraj R, Rerkpattanapipat P, Jacobs LE, Makornwattana P, Kotler MN: Meta‐analysis of 143 reported cases of aortic intramural hematoma. Am J Cardiol 2000; 86: 664–668. [DOI] [PubMed] [Google Scholar]
- 7. von Kodolitsch Y, Csosz SK, Koschyk DH, Schalwat I, Loose R, et al.: Intramural hematoma of the aorta: Predictors of progression to dissection and rupture. Circulation 2003; 107(8): 1158–1163. [DOI] [PubMed] [Google Scholar]
- 8. Evangelista A, Dominguez R, Sebastia C, Salas A, Permanyer‐Miralda G, et al.: Prognostic value of clinical and morphologic findings in short term evolution of aortic intramural hematoma. Eur Heart J 2004; 25(1): 81–87. [DOI] [PubMed] [Google Scholar]
- 9. Song JK, Kim HS, Song JM, Kang DH, Ha JW, et al.: Outcomes of medically treated patients with aortic intramural hematoma. Am J Med 2002; 113: 181–187. [DOI] [PubMed] [Google Scholar]
- 10. Song JK, Kim HS, Kang DH, Lim TH, Song MG, et al.: Different clinical features of aortic intramural hematoma versus dissection involving the ascending aorta. J Am Coll Cardiol 2001; 37: 1604–1610. [DOI] [PubMed] [Google Scholar]
- 11. Kaji S, Akasaka T, Horibata Y, Nishigami K, Shono H, et al.: Long term prognosis of patients with type A aortic intramural hematoma. Circulation 2002; 106((suppl I)): I248–252. [PubMed] [Google Scholar]
- 12. Motoyoshi N, Moizumi Y, Komatsu T, Tabayashi K: Intramural hematoma and dissection involving ascending aorta: The clinical features and prognosis. Eur J Cardiothorac Surg 2003; 24(2): 237–242. [DOI] [PubMed] [Google Scholar]
- 13. Moizumi Y, Komatsu T, Motoyoshi N, Tabayashi K: Clinical features and long term outcome of type A and type B intramural hematoma of the aorta. J Thorac Cardiovasc Surg 2004;127(2): 421–427. [DOI] [PubMed] [Google Scholar]
- 14. Kaji S, Nishigami K, Akasaka T, Hozumi T, Takagi T, et al.: Predictors of progression or regression of type A aortic intramural hematoma by computed tomography. Circulation 1999; 100(suppl II): II281–286. [DOI] [PubMed] [Google Scholar]
- 15. Nishigami K, Tsuchiya T, Shono H, Horibata Y, Honda T: Disappearance of aortic intramural hematoma and its significance to the prognosis. Circulation 2000; 102((suppl III)): III243–247. [DOI] [PubMed] [Google Scholar]
- 16. Song JM, Kim HS, Song JK, Kang DH, Hong MK, et al.: Usefulness of the initial non‐invasive imaging study to predict the adverse outcomes in the medical treatment of acute type A aortic intramural hematoma. Circulation 2003; 108(suppl 1): II324–328. [DOI] [PubMed] [Google Scholar]
- 17. Song JK, Kang SJ, Song JM, Kang DH, Song H, et al.: Factors associated with in‐hospital mortality in patients with acute aortic syndrome involving the ascending aorta. Int J Cardiol (Epub ahead of print; May 29, 2006). [DOI] [PubMed] [Google Scholar]
- 18. Tsai TT, Nienaber CA, Eagle KA: Acute aortic syndromes. Circulation 2005; 112: 3802–3813. [DOI] [PubMed] [Google Scholar]
- 19. Evangelista A, Mukherjee D, Mehta RH, O'Gara PT, Fattori R, et al.; International Registry of Aortic Dissection (IRAD) Investigators: Acute intramural hematoma of the aorta: A mystery in evolution. Circulation 2005; 111: 1063–1070. [DOI] [PubMed] [Google Scholar]


