Abstract
To date, the precise mechanism of atrial fibrillation (AF) as a possible cause of reflux disease remains uncertain, although some possibilities can be postulated. Inflammation and vagal stimulation may have a key role linking these 2 common diseases. There is some evidence in the form of case reports and limited observational studies reporting that reflux disease, and more specifically esophagitis, can cause paroxysmal AF, and various mechanisms have been proposed. Some studies have demonstrated that acid suppressive therapy by proton pump inhibitors (PPIs) may help ameliorate symptoms associated with AF and also facilitate conversion to normal sinus rhythm in a subset of patients. Further prospective studies are needed to determine if a true causal mechanism exists between the two and assess whether the mechanism is dependent on a specific subtype of AF. In addition, the response of AF‐related symptoms to PPI therapy and the potential for PPI therapy to reduce the development of AF merits further investigation. Clin. Cardiol. 2011 DOI: 10.1002/clc.21969
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia affecting more than 2.2 million people in the United States. AF is strongly age dependent, affecting 4% of individuals >60 years old and 8% of persons older than 80 years. Approximately 25% of individuals age 40 years and older will develop AF during their lifetime.1 AF is often associated with other cardiovascular diseases, including diabetes, hypertension, heart failure, ischemic heart disease, valvular diseases, and other cardiomyopathies. In 10% to 15% of the cases, AF occurs in the absence of any such comorbidities and is determined as lone AF.1 However, in recent years other factors playing a role in the genesis of AF have gained attention including obesity, sleep apnea, alcohol abuse and other intoxications, exercise, latent hypertension, genetic factors, acid reflux disease, and local or systemic inflammation.2., 3., 4., 5., 6., 7. Identification, awareness, and understanding regarding these less‐common risk factors will help guide appropriate treatment, avoid unnecessary interventions, and favor a good prognosis.
Gastroesophageal reflux disease (GERD) or acid reflux disease is the most common gastrointestinal diagnosis recorded during visits to outpatient clinics.8 There is some evidence in the form of case reports and limited observational studies reporting that reflux disease, and more specifically esophagitis, can cause and maintain paroxysmal AF (PAF), and several possible mechanisms have been proposed such as inflammation, autoimmune, and autonomic stimulation. Some studies have demonstrated that acid suppressive therapy by proton pump inhibitors (PPIs) may help ameliorate symptoms associated with AF and also facilitate conversion to normal sinus rhythm in a subset of patients. The present article's aim is to provide insight into the relationship between AF and reflux disease and review the current literature noting their association.
Inflammation and AF
Many studies have documented a relationship between circulating markers of inflammation (including C‐reactive protein and interleukins) and AF, especially in relationship to incidence, defibrillation, recurrence, and prognosis.9., 10. Whether the initiation of AF activates direct inflammatory reactions or the presence of preexisting inflammation promotes AF is not completely understood, and both mechanisms may be operating. For example, rapid atrial activation accompanied with calcium accumulation may result in calcium overload and apoptotic loss of atrial myocytes with consequent low‐grade inflammatory response as part of structural remodeling.11 Alternatively, in patients with triggering atrial foci, systemic inflammation with increased circulating C‐reactive protein may predispose to AF via the classic complement pathway activation and atrial tissue damage, or by binding to phosphocholine, which may contribute to membrane dysfunction by the alterations in sodium and calcium handling.12
Autonomic System and AF
Several observations suggest that the autonomic nervous system plays an important role in initiation and or maintenance of AF in humans. Sympathetic stimulation often initiates AF in patients with structural heart disease, whereas lone AF patients more frequently have PAF in the setting of increased vagal tone.13., 14. Although both sympathetic and parasympathetic components play a role in AF, the cholinergic component appears to be important for spontaneous initiation of AF. In the experimental setting, for example, electrical stimulation of the left atrial ganglionic plexi or the autonomic nerve endings with retrograde activation of the ganglia induces spontaneous firing from pulmonary veins followed by AF.15
Mechanism of AF With Acid Reflux
To date, the precise mechanism of AF as a possible cause of GERD remains uncertain, although some possibilities can be postulated (Figure 1). Chronic inflammation and sympathovagal imbalance remains the cornerstone in the etiopathogenesis.16., 17., 18. Acid reflux causes a local inflammatory process that may alter the autonomic innervations of the esophageal mucosa, and may also penetrate the esophageal wall and affect the adjacent vagal nerves due to the close juxtaposition of the esophagus and atria, especially the left atrium, where most triggers associated with atrial fibrillation have been described, affecting myelination and thus propagation of stimuli.19 Inflammation of the esophageal mucosa affects local receptors that may induce afferent‐efferent reflex mechanisms of the cardiac rhythm, which can lead to secondary stimulation of the vagal nerves inducing AF.20 This relationship has been shown to be particularly strong among younger patients and athletes who have been found to have increased vagal tone.7 Propagation of the local inflammatory process through the esophageal wall may also cause local pericarditis or atrial myocarditis. Frustaci et al identified myocarditis in 66% of patients by atrial myocardial biopsies taken from 12 patients with lone AF.21
Figure 1.
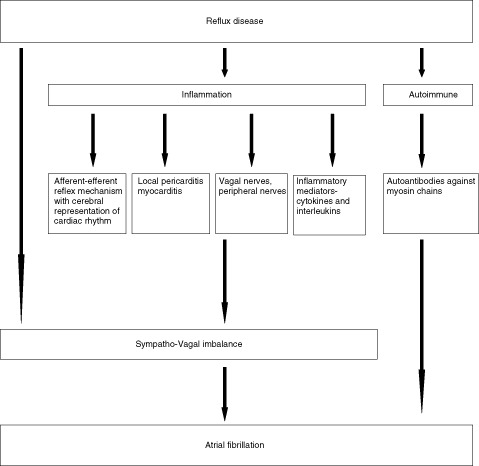
Mechanism of gastroesophageal reflux disease and atrial fibrillation.
Heart rate variability studies with continuous electrocardiogram (ECG) monitoring have shown that stimulation of the esophagus by acid can alter the balance between vagal and sympathetic activity and can trigger dysrhythmias.22 Reflux disease may also lead to a release of inflammatory mediators, which may affect the atrial myocardium and other cardiac conduction pathways.23 Recurrent acid secretion induces mucosal inflammation and secretion of interleukin (IL)‐1B and IL‐6. These inflammatory cytokines play a pivotal role in the pathogenesis of AF.24., 25. Finally, there is evidence to say that chronic GERD may induce an autoimmune response that contributes to AF. Autoantibodies against myosin heavy chain have been detected in the sera of patients with lone AF.26
Hiatal Hernia
Hiatal hernias (10% in patients younger than 40 years to 70% in patients older than 70 years) may predispose to GERD or worsen existing GERD in a few individuals. A hiatal hernia acts as a fluid trap for gastric acid reflux and increases the frequency of transient relaxation of the lower esophageal sphincter, thereby increasing the acid contact time in the esophagus, thus worsening GERD. The association between atrial tachyarrhythmias and hiatal hernia was historically reported in 1979 by Landmark et al27 and later by Schilling et al in 1998.28 Although there are a number of possible explanations for this phenomenon, the exact mechanism for this potential relationship between hiatal hernia‐GERD and atrial arrhythmias has not been previously explored. One plausible explanation is based on mechanical interactions: atrial arrhythmias may be induced by a mechanical effect on the left atrial wall that is related to the passage of food (28). However, the effect seems to be purely mechanical. Atrial arrhythmias may be induced by a mechanical effect on the left atrial wall that is related to the passage of food.28 A large hiatal hernia may also cause compression of the left atrium and may result in an area of relative ischemia and anatomical block resulting in reentry and arrhythmias.28 However, this hypothesis could not be confirmed as clear distortion of the left atrium on echocardiography and has not been documented in any reports.29 There are no clear data supporting repair of hiatal hernias in the treatment of AF in such patients other than incidental reports.28
The question that remains then is whether this is comorbidity or coincidence. Patients with a hiatal hernia are usually obese and have all the risk factors of metabolic syndrome that increase the predilection for AF. Also, hiatal hernia may worsen GERD symptoms and potentially worsen the mechanism described above. Further studies regarding the risk of hiatal hernia in AF and hiatal hernia repair on AF outcomes is warranted.
GERD‐AF Connection: The Available Data So Far
There have been several studies to date on atrial fibrillation and GERD (Table 1). Historically, the association between gastrointestinal symptoms and cardiac dysrhythmias has been described under gastrocardiac syndromes. However, the association between acid reflux and AF was first reported by Tougas et al 1997.22 In their study, neurocardiac response to esophageal stimulation and the effects of electrical and mechanical esophageal stimulation on the power spectrum of beat‐to‐beat heart rate variability in 14 male volunteers was examined. Heart rate variability was compared at rest and during esophageal stimulation using either electrical or mechanical stimuli. The study reported that distal esophageal stimulation decreased heart rate (both electrical and mechanical) (P < 0.005), and markedly altered heart rate variability (P < 0.001). Both electrical and mechanical esophageal stimulation increased the absolute and normalized area of the high‐frequency band within the power spectrum (P < 0.001), while simultaneously decreasing the low‐frequency power (P < 0.005). This study concluded that esophageal stimulation, whether electrical or mechanical, appeared to amplify respiratory‐driven cardiac vago‐afferent modulation while decreasing sympathetic modulation.
Table 1.
Studies to Date on Atrial Fibrillation and Acid Reflux Disease
| Study Authors | Nature of the Study | No. of Patients | Methodology | Results | Conclusion |
|---|---|---|---|---|---|
| Tougas et al, 199722 | Observational, prospective. | Fourteen healthy volunteers | Heart rate variability was compared at rest and during esophageal stimulation, using either electrical or mechanical stimuli. | Decreased heart rate (both electrical and mechanical) (P < 0.005), and altered heart rate variability (P < 0.001). Increased absolute and normalized area of the high‐frequency band within the power spectrum (P < 0.001). Decreasing the low‐frequency power (P < 0.005). | Esophageal stimulation (electrical or mechanical) appears to amplify respiratory‐driven cardiac vagoafferent modulation while decreasing sympathetic modulation. |
| Weigl et al, 200330 | Observational, retrospective. | Eighteen patients with paroxysmal AF. | Upper endoscopic examination showed that 14 patients had esophagitis and the other 4 had Barrett esophagus. All patients received daily or twice‐daily PPI therapy. | Decrease or disappearance of at least 1 PAF‐related symptom occurred in 14 of 18 patients (78%) after PPI therapy. In 2 of the remaining 4 patients, GERD‐related symptoms persisted. Antiarrhythmic drugs were discontinued in 5 patients, and none had to be increased in dosage or newly prescribed. ECG showed sinus rhythm in all patients. | GERD should be investigated as a potential pathogenetic mechanism in lone PAF. PPI therapy reduces not only GERD‐related but also PAF‐related symptoms. |
| Cuomo et al, 200631 | Observational, prospective. | Thirty‐two patients with GERD and dysarrhtyhmias and 9 with GERD. | Power spectrum analysis of heart rate variability was obtained with both its LF (sympathetic modulation) and HF (vagal modulation) components. Hourly mean esophageal pH and LF/HF ratio (esophagus‐heart) were correlated. A 3‐month regimen of esomeprazole 40 mg/day was prescribed. | In 8 (56%) of the 32 patients with dysrhythmia and in none with GERD only, a significant correlation between esophageal pH and LF/HF ratio was observed. A significant reduction of cardiac symptoms after PPI therapy was observed only in these patients (13/16 vs 4/11, P < 0.01). | Subgroup of dysrhythmic patients in whom the esophageal acid stimulus elicited cardiac autonomic reflexes. In these patients, acid suppression seems to improve GERD and cardiac symptoms. |
| Gerson et al, 200632 | Observational, prospective. | All 3 patients had heartburn, acid regurgitation, and palpitations occurring at least weekly and reported a potential association between the palpitations and GERD symptoms. | The patients underwent simultaneous 24‐hour esophageal ambulatory pH and Holter monitoring off of antireflux therapy for at least 7 days to investigate a potential relationship between GERD symptoms and the presence of cardiac arrhythmic events. | All of the patients reported a reduction in arrhythmia symptoms on PPI. | Atrial arrhythmia and reflux should have a trial of aggressive acid suppressive therapy. |
| Bunch et al, 200834 | Observational, prospective. | A total of 5288 residents (age 25–74 years) of Olmsted County, Minnesota, to assess the presence and frequency of GERD from 1988–1994. | A self‐report questionnaire was mailed to a random sample. | GERD was not associated with risk for AF (HR: 0.81, 95% CI: 0.68–0.96, P = 0.014) after adjustment for other risk factors. Patients with more frequent GERD had a slightly higher nonsignificant AF risk. Esophagitis increased the risk for AF (HR: 1.94, 95% CI: 1.35–2.78, P < 0.001), but the association did not persist when accounting for other risk factors. | No association was found with the presence of GERD or the frequency of symptoms and AF. Patients with esophagitis were more likely to develop AF. |
| Kunz et al, 200935 | Retrospective. | There were 163627 patients of which 7992 (5%) had AF and 47845 (29%) had GERD. | Database containing all healthcare encounters for patients who received ambulatory care in the National Capitol Region military healthcare system between January 1, 2001 and October 28, 2007. | The presence of GERD increased the RR of a diagnosis of AF (RR: 1.39, 95% CI: 1.33–1.45). In sensitivity analyses, this relationship persisted after adjustment for cardiovascular disease risk factors (RR: 1.19, 95% CI: 1.13–1.25) and diagnoses known to be strongly associated with AF (RR: 1.08, 95% CI: 1.02–1.13). | GERD is associated with an increased risk of a diagnosis of AF. |
| Shimazu et al, 201136 | Retrospective. | There were 188 subjects treated for GERD as outpatients | Patients were classified by the frequency scale for symptoms of GERD (F scale). | Total scores on the F scale were significantly greater in patients with AF (P = 0.019) compared to other subjects. Univariate and multivariate analysis of the prevalence of GERD demonstrated AF alone showed a significant (P < 0.001) correlation with GERD. | AF was an independent risk factor for GERD. |
Abbreviations: AF, atrial fibrillation; CI, confidence interval; ECG, electrocardiogram; GERD, gastroesophageal reflux disease; HF, high frequency; HR, hazard ratio; LF, low frequency; PAF, paroxysmal atrial fibrillation; PPI, proton pump inhibitor; RR, relative risk.
Since the Tougas et al. study, there have been several random reports and observational studies designed to assess the association of reflux disease and AF. Weigl et al in 200330 conducted a pilot study on 89 patients, 18 (6 women, age 39–69 years) of whom had lone PAF. Upper endoscopic examination showed 14 patients had esophagitis and the other 4 had Barrett's esophagus. All patients received daily or twice‐daily PPI therapy. On follow‐up visit therapy with a PPI, not only the GERD symptoms subsided, but remarkably also AF‐related symptoms in 14 of the 18 patients. A follow‐up ECG showed sinus rhythm in all patients. Decrease or disappearance of at least 1 AF‐related symptom occurred in 14 of 18 patients (78%) after PPI therapy. Antiarrhythmic drugs were discontinued in 5 patients (28%), and none had to be increased in dosage or needed newly prescribed antiarrythmics. The study concluded that in a subgroup of patients with lone‐AF, appropriate treatment of underlying esophagitis may be beneficial and less harmful than standard therapy. Limitations of this study included the low number of patients, retrospective design, and the lack of a control group. As all patients were informed about the diagnosis and treatment of reflux esophagitis, there could have been a placebo effect of PPIs that contributed to the improvement of PAF in a subgroup of patients.
Another study by Cuomo et al in 200631 investigated establishing a relationship between esophageal acid exposure and neurocardiac function in patients with symptoms of GERD and dysrhythmias, and also to verify whether acid‐suppressive therapy was able to improve GERD and cardiac symptoms. Thirty‐two patients (13 women and 19 men, age 20–69 years) with a combination of dysrhythmias and GERD, and 9 patients (5 women and 4 men, age 43–58 years) with GERD only underwent simultaneous 24‐hour pH and ECG monitoring. Power spectrum analysis of heart rate variability was obtained with both low‐frequency (LF) (sympathetic modulation) and high‐frequency (HF) (vagal modulation) components. Hourly mean esophageal pH and LF/HF ratio (esophagus‐heart) were correlated. A 3‐month regimen of esomeprazole 40 mg/day was prescribed. In 18 (56%) of the 32 patients with dysrhythmias and in none with GERD only, a significant correlation between esophageal pH and LF/HF ratio was observed. A significant reduction of cardiac symptoms after PPI therapy was observed only in these patients (13/16 vs 4/11, P < 0.01). The study concluded that there could be a subgroup of patients with heart dysrhythmias in whom esophageal acid stimulus elicited cardiac autonomic reflexes, and acid suppression in this subgroup may improve both GERD and cardiac symptoms. Although this study demonstrated a reduction or disappearance of the dysrhythmias in patients with a cardiac response to esophageal acid exposure, it had some limitations. The evaluation of cardiac symptoms with a simple clinical questionnaire and the absence of a control group treated with placebo did not add definitive value to the results.
A 3‐patient case series by Gerson et al 200632 concluded that a potential causal relationship exists between GERD and atrial arrhythmias. Arrhythmias and pH (esophageal acid exposure) were significantly correlated by simultaneous Holter and 24‐hour esophageal pH monitoring in each patient, and their symptoms diminished with omeprazole therapy. The strengths of this case series was that 24‐hour pH and Holter monitoring were used, allowing for more detailed quantification and comparison of reflux and arrhythmic events. In addition, this series demonstrated a relationship between GERD and atrial arrhythmias in patients with nonerosive GERD. However, lack of a substantial patient population could have resulted in random beneficial findings in these 3 patients. Also, there were no data on the type of atrial fibrillation associated with GERD. Two other single‐case studies also have reported that treatment with PPI not only was beneficial in reducing acidic GERD but in substantially reducing the frequency of AF episodes.29., 33.
On the contrary, conflicting results were reported by a large study by Bunch et al in 2008,34 who examined the potential association between GERD and AF and surveyed the presence and frequency of GERD and long‐term risk of AF. A self‐report questionnaire was mailed to a random sample of 5288 residents (age 25–74 years) of Olmsted County, Minnesota, to assess the presence and frequency of GERD from 1988 to 1994. The average age was 53 ± 17 years, and 2571 subjects (49%) were men. GERD was not associated with risk for AF (hazard ratio [HR]: 0.81, 95% confidence interval [CI]: 0.68–0.96, P = 0.014) after adjustment for other risk factors. Patients with more frequent GERD had a slightly higher nonsignificant AF risk. Esophagitis increased the risk for AF (HR: 1.94, 95% CI: 1.35–2.78, P < 0.001), but the association did not persist when accounting for other risk factors. The authors concluded that no association was found with the presence or GERD or the frequency of symptoms and AF. However, patients with esophagitis were more likely to develop AF. The study strengths include a large random community sample. The survey used was a subjective tool to assess for GERD rather than a more objective tool such as endoscopic diagnosis. AF diagnosis was based on International Classification of Diseases‐9th Revision (ICD‐9) codes from hospital dismissal summaries, the electrocardiogram database, and review of the inpatient and outpatient medical records, which means that there is a chance that subclinical or asymptomatic AF could have been undetected. This study neither provides insight into the effect of reflux on different types of AF, nor does it provide information on the effect of PPI in AF. Another recent major study by Kunz et al in 200935 reported that there was an association between AF and GERD, and that acid suppressive therapy with PPIs may provide a potential simple means of controlling symptomatic paroxysms of AF, particularly if accompanied by symptoms of reflux esophagitis. This study was performed in 163627 patients, of whom 7992 (5%) had AF and 47845 (29%) had GERD. GERD increased the relative risk (RR) of a diagnosis of AF (RR: 1.39, 95% CI: 1.33–1.45). In sensitivity analyses, this relationship persisted after adjustment for cardiovascular risk factors (RR: 1.19, 95% CI: 1.13–1.25) and diagnoses known to be strongly associated with AF (RR: 1.08, 95% CI: 1.02–1.13).19 The strength of the study was that it had a large patient population. Limitations to this study included the dependence on ICD‐9 code accuracy. In addition, due to the cross‐sectional nature of the data, patients were not followed over time to evaluate if this finding was causative or correlative. Another recent multicenter questionnaire survey by Shimazu et al in 201136 enrolled 188 consecutive subjects (110 males and 78 females; mean age, 60.4 ± 0.9 years) treated as outpatients. Patients were classified by the frequency scale for symptoms of GERD (F scale). The cutoff value for a diagnosis of GERD was set at 8.0 points. Total scores on the F scale were significantly greater in female subjects (P = 0.004) and in patients with AF (P = 0.019) compared to the other subjects. Univariate and multivariate analysis of the prevalence of GERD demonstrated that GERD was not related to gender, hypertension, dyslipidemia, or coronary artery disease, and AF alone showed a significant (P < 0.001) correlation with GERD. This multicenter questionnaire survey demonstrated that AF was an independent risk factor for GERD. The study had a few drawbacks. It was a retrospective study where the questionnaire responses were not correlated with the endoscopic findings. This study had no data on the effect of gastric acid on various types of AF.
Discussion
A number of complex physiological interrelationships between the upper gastrointestinal and cardiovascular systems has been documented in the medical literature, although only a few have clinical importance. Whatever the type of interplay, the mechanism is ordinarily designated as autonomic stimulation and inflammation, a term that may represent oversimplification of the involved physiology. Although almost all of the clinically useful information on this subject of reflux disease and AF has been derived from retrospective observational studies and scattered single case reports, there are enough data to suggest that autonomic activation and inflammation can play a major role between the heart and GERD. Almost all of the studies, with the exception of the study by Bunch et al, identified an association between reflux disease and AF.22., 30., 31., 32., 34., 35., 36.
It appears logical that autonomic activation and inflammation play an important role in the pathogenesis of a few patients if not all patients with PAF, due to the close proximity of the left atrium and the esophagus. Needless to say, careful attention should be paid to those subjects at risk. It can also be argued that reflux disease is a usual phenomena seen in patients with metabolic syndrome who also are at risk for AF, and that these 2 diseases may mutually exist without any association. An important question is how to differentiate patients at risk of PAF from reflux disease compared to those who are not. Unfortunately, there are no data currently on ways to risk‐stratify these patients. Another hypothesis is that AF from reflux disease may exist as a continuous spectrum of disease, with no association with mild reflux at 1 end and a strong association in patients with severe reflux and evidence of esophagitis at the other.
The association between esophagitis and PAF is uniformly reported in all studies so far.22., 30., 31., 32., 34., 35., 36. Genetic contributions to AF have been suggested by a number of recent studies, and in particular, lone AF has a substantial genetic basis.37., 38., 39. It is possible for genetic basis to play a role in reflux‐induced PAF in a subset of patients with lone AF; however, there are no studies to validate this theory.
Based on the evidence so far, can we conclude that reflux disease is a risk factor for AF? It is still premature to jump to that conclusion, though there is some data reporting the association between esophageal acid exposure and AF. It is unclear if the extent of esophageal acid exposure has a graded effect on the sympathovagal system and the type of AF. Generally, lone, paroxysmal AF is treated with anti‐arrhythmics, systemic anticoagulation and, occasionally, with catheter ablation with the primary goal of pulmonary vein islolation. In patients in whom paroxysmal AF with associated esophagitis is suspected, a trial of acid suppressive therapy with a PPI may lead to a marked reduction in the frequency of AF episodes, or even complete disappearance of paroxysmal AF events. Under PPI treatment, a subgroup of AF patients could be defined in whom paroxysmal AF can be mitigated by anti‐esophagitis treatment. Concerning persistent AF, it is speculated that some patients may initially also have PAF induced by esophagitis. Possibly, PPIs in these patients may facilitate cardioversion and maintenance of sinus rhythm. In patients with esophagitis‐induced PAF, PPIs may also prevent the development of permanent AF.
Clinicians should be aware of the possible AF‐reflux association, and identification and appropriate treatment of GERD, especially esophagitis in patients especially lone AF, may help minimize the use of anti‐arrhythmic agents, which often have a complex side effect profile and the potential for pro‐arrhythmic effects. Further prospective studies are needed to determine if a true causal mechanism exists between these two common diagnoses, as well as to assess whether the mechanism is dependent on a specific sub‐type of AF. In addition, the response of AF‐related symptoms to PPI therapy and the potential for PPI therapy to reduce the development of AF merits further investigation.
References
- 1. Lloyd‐Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. [DOI] [PubMed] [Google Scholar]
- 2. Issac TT, Dokainish H, Lakkis NM. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. J Am Coll Cardiol. 2007;50:2021–2028. [DOI] [PubMed] [Google Scholar]
- 3. Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. [DOI] [PubMed] [Google Scholar]
- 4. Ettinger PO, Wu CF, De La Cruz C Jr, et al. Arrhythmias and the “Holiday Heart”: alcohol‐associated cardiac rhythm disorders. Am Heart J. 1978;95:555–562. [DOI] [PubMed] [Google Scholar]
- 5. Mukamal KJ, Tolstrup JS, Friberg J, et al. Alcohol consumption and risk of atrial fibrillation in men and women: the Copenhagen City Heart Study. Circulation. 2005;112:1736–1742. [DOI] [PubMed] [Google Scholar]
- 6. Brugada R, Tapscott T, Czernuszewicz GZ, et al. Identification of a genetic locus for familial atrial fibrillation. N Engl J Med. 1997;336:905–911. [DOI] [PubMed] [Google Scholar]
- 7. Turagam MK, Velagapudi P, Kocheril AG. Atrial fibrillation in athletes. Am J Cardiol. 2012;109:296–302. [DOI] [PubMed] [Google Scholar]
- 8. Shaheen NJ, Hansen RA, Morgan DR, et al. The burden of gastrointestinal and liver diseases. Am J Gastroenterol. 2006;101:2128–2138. [DOI] [PubMed] [Google Scholar]
- 9. Aviles RJ, Martin DO, Apperson‐Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108: 3006–3010. [DOI] [PubMed] [Google Scholar]
- 10. Hatzinikolaou‐Kotsakou E, Tziakas D, Hotidis A, et al. Relation of C‐reactive protein to the first onset and the recurrence rate in lone atrial fibrillation. Am J Cardiol. 2006;97:659–661. [DOI] [PubMed] [Google Scholar]
- 11. Mihm MJ, Yu F, Carnes CA, et al. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–180. [DOI] [PubMed] [Google Scholar]
- 12. Engelmann MDM, Svendsen JH. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur Heart J. 2005;26:2083–2092. [DOI] [PubMed] [Google Scholar]
- 13. Allessie MA, Boyden PA, Camm JA, et al. Pathophysiology and prevention of atrial fibrillation. Circulation. 2001;103:769–777. [DOI] [PubMed] [Google Scholar]
- 14. Huang JL, Wen ZC, Lee WL, et al. Changes of autonomic tone before the onset of paroxysmal atrial fibrillation. Int J Cardiol. 1998;66:275–283. [DOI] [PubMed] [Google Scholar]
- 15. Zhou J, Scherlag B, Edwards J, et al. Gradient of atrial refractoriness and inducibility of atrial fibrillation due to stimulation of ganglionated plexi. J Cardiovasc Electrophysiol. 2007;18:83–90. [DOI] [PubMed] [Google Scholar]
- 16. Chen PS, Tan AY. Autonomic nerve activity and atrial fibrillation. Heart Rhythm. 2007;4:S61–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bajwa A, Hollerbach S, Kamath MV, et al. Neurocardiac response to esophageal electric stimulation in humans: effects of varying stimulation frequencies. Am J Physiol. 1997;272:R896–R901. [DOI] [PubMed] [Google Scholar]
- 18. Pappone C, Oral H, Santinelli V, et al. Atrio‐esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation. 2004;109:2724–2726. [DOI] [PubMed] [Google Scholar]
- 19. Smith KJ, Hall SM. Factors directly affecting impulse transmission in inflammatory demyelinating disease: Recent advances in our understanding. Curr Opin Neurol. 2001;14:289–298. [DOI] [PubMed] [Google Scholar]
- 20. Newton M, Kamm MA, Soediono PO, et al. Esophageal epithelial innervation in health and reflux esophagitis. Gut. 1999;44:317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frustaci A, Chimenti C, Bellocci F, et al. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. [DOI] [PubMed] [Google Scholar]
- 22. Tougas G, Kamath M, Watteel G, et al. Modulation of neurocardiac function by esophageal stimulations in humans. Clin Sci. 1997;92:167–174. [DOI] [PubMed] [Google Scholar]
- 23. Rieder F, Cheng L, Harnett KM, et al. Gastroesophageal reflux disease‐associated esophagitis induces endogenous cytokine production leading to motor abnormalities. Gastroenterology. 2007;132:154–165. [DOI] [PubMed] [Google Scholar]
- 24. Yamashita T, Sekiguchi A, Iwasaki YK, et al. Recruitment of immune cells across atrial endocardium in human atrial fibrillation. Circ J. 2010;74:262–270. [DOI] [PubMed] [Google Scholar]
- 25. Osmancik P, Peroutka Z, Budera P, et al. Changes in cytokine concentrations following successful ablation of atrial fibrillation. Eur Cytokine Netw. 2010;21:278–284. [DOI] [PubMed] [Google Scholar]
- 26. Maixent JM, Paganelli F, Scaglione J, et al. Antibodies against myosin in sera of patients with idiopathic paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 1998;9:612–617. [DOI] [PubMed] [Google Scholar]
- 27. Landmark K, Storstein O. Ectopic atrial tachycardia on swallowing. Report on favourable effect of verapamil. Acta Med Scand. 1979;205:251–254. [PubMed] [Google Scholar]
- 28. Schilling RJ, Kaye GC. Paroxysmal atrial flutter suppressed by repair of a large paraesophageal hernia. Pacing Clin Electrophysiol. 1998;21:1303–1305. [DOI] [PubMed] [Google Scholar]
- 29. Duygu H, Ozerkan F, Saygi S, et al. Persistent atrial fibrillation associated with gastroesophageal reflux accompanied by hiatal hernia. Anadolu Kardiyol Derg 2008;8:164–165. [PubMed] [Google Scholar]
- 30. Weigl M, Gschwantler M, Gatterer E, et al. Reflux esophagitis in the pathogenesis of paroxysmal atrial fibrillation: results of a pilot study. South Med J. 2003;96:1128–1132. [DOI] [PubMed] [Google Scholar]
- 31. Cuomo R, De Giorgi F, Adinolfi L, et al. Esophageal acid exposure and altered neurocardiac function in patients with GERD and idiopathic cardiac dysrhythmias. Aliment Pharmacol Ther. 2006;24:361–370. [DOI] [PubMed] [Google Scholar]
- 32. Gerson LB, Friday K, Triadafilopoulos G. Potential relationship between gastroesophageal reflux disease and atrial arrhtyhmias [published correction appears in J Clin Gastroenterol 2006;40:958]. J Clin Gastroenterol. 2006;40:828–832. [DOI] [PubMed] [Google Scholar]
- 33. Budzynski J, Klopocka M, Pulkowski G, et al. Gastroesophageal acid reflux as a causative factor of paroxysmal atrial fibrillation [in Polish]. Kardiol Pol. 2005;62:52–54. [PubMed] [Google Scholar]
- 34. Bunch TJ, Packer DL, Jahangir A, et al. Long‐term risk of atrial fibrillation with symptomatic gastroesophageal reflux disease and esophagitis. Am J Cardiol. 2008;102:1207–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kunz JS, Hemann B, Edwin Atwood J, et al. Is there a link between gastoesophageal reflux disease and atrial fibrillation? Clin Cardiol. 2009;32:584–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shimazu H, Nakaji G, Fukata M, et al. Relationship between atrial fibrillation and gastroesophageal reflux disease: a multicenter questionnaire survey. Cardiology. 2011;119:217–223. [DOI] [PubMed] [Google Scholar]
- 37. Darbar D, Herron KJ, Ballew JD, et al. Familial atrial fibrillation is a genetically heterogeneous disorder. J Am Coll Cardiol 2003;41:2185–2192. [DOI] [PubMed] [Google Scholar]
- 38. Ellinor PT, Yoerger DM, Ruskin JN, et al. Familial aggregation in lone atrial fibrillation. Hum Genet. 2005;118: 179–184. [DOI] [PubMed] [Google Scholar]
- 39. Fox CS, Parise H, D'Agostino RB Sr, et al. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA 2004;291:2851–2855. [DOI] [PubMed] [Google Scholar]


